Abstract
Normal liver sinusoidal endothelial cells (LSECs) promote quiescence of hepatic stellate cells. Prior to fibrosis LSECs undergo capillarization, which is permissive for hepatic stellate cell activation, the proximate event in hepatic fibrosis. The aims of this study were to elucidate the nature of and mechanisms leading to capillarization, and to determine how LSECs promote hepatic stellate cell quiescence and why “capillarized LSECs” lose control of HSC activation. The contribution of bone marrow endothelial progenitor cells to capillarization was identified using rats transplanted with transgenic EGFP+ bone marrow. Shotgun proteomics and informatics were used to identify the LSEC mediator that maintains HSC quiescence. The study shows that capillarization is due to repair of injured LSECs by bone marrow endothelial progenitors that engraft but fail to fully mature. Lack of maturation of bone marrow derived LSECs is due to cell autonomous pathways that inhibit the nitric oxide pathway. We identify HB-EGF as the signal that maintains hepatic stellate cell quiescence and show that immature LSEC are unable to shed HB-EGF from the cytosolic membrane. Conclusion: chronic liver injury can recruit bone marrow progenitors of LSECs that engraft and fail to fully differentiate, which creates an environment that is permissive for hepatic fibrosis. Elucidation of these early events in the fibrotic process will provide new targets for treatment of hepatic fibrosis.
Keywords: liver sinusoidal endothelial cell, nitric oxide, thrombospondin-1, HB-EGF, hepatic stellate cell
Prior to fibrosis, LSECs undergo capillarization. Capillarization has traditionally been defined morphologically as a loss of the unique, characteristic ultrastructure of LSECs: the presence of non-diaphragmed fenestrae grouped in sieve plates and development of an organized basement membrane 1. In addition to the characteristic morphological change, “capillarized” LSECs, which are present in pre-fibrotic and fibrotic liver, lose the capacity to maintain HSC quiescence under pressure of external signals that promote HSC activation 2. The changes in morphology and function described in capillarization in chronic liver injury have been assumed to be a de-differentiation of the injured LSEC.
Little is known about how chronic liver injury affects LSECs. Acute liver damage caused by monocrotaline, partial hepatectomy or dimethylnitrosamine, results in substantial loss of LSECs, which elicits the recruitment of sinusoidal endothelial cell progenitor cells (sprocs) for LSEC repair 3–6. Bone marrow (BM) sprocs only engraft after injury has damaged LSECs and denuded sinusoids 3. Fusion of sprocs with resident LSECs does not occur 3. BM suppression impairs recovery from liver injury, whereas infusion of bone marrow or of sprocs promotes liver regeneration 3, 5. The contribution of BM sprocs in chronic liver injury has not previously been characterized.
There are two main reasons to consider sprocs a sub-set of the general population of endothelial progenitor cells. First, there is a two-fold increase in circulating endothelial progenitor cells that persists for at least 72 hours after partial hepatectomy 5. In the first 6 hours 90% of the circulating endothelial progenitor cells are CXCR7+, but by 24 hours fewer than 5% of the circulating endothelial progenitor cells are CXCR7+. Only CXCR7+ LSEC progenitors are found in the liver after partial hepatectomy 6. Thus only a subset of circulating endothelial progenitor cells engraft in the liver. Second and perhaps more importantly, the LSEC is an unusual endothelial cell in that it is both CD31+ and CD45+ 3, 7. Hence, sprocs are defined as CD133+CD31+CD45+, which is contrary to the conventional description of endothelial progenitor cells.
Since the key, proximate event in hepatic fibrosis is the activation of hepatic stellate cells (HSCs), the ability of LSECs in the normal liver to promote HSC quiescence is a critical gatekeeper function that prevents fibrosis 8, 9. However, how LSECs maintain HSC quiescence in normal liver, why the LSEC changes phenotype prior to fibrosis, and how this change in the LSEC leads to HSC activation remained unanswered 2, 4, 10, 11. Understanding how LSECs maintain HSC quiescence in normal liver and how this function is lost in fibrosis may provide novel targets for the therapy of fibrosis.
Therefore, we initiated studies to investigate the nature of capillarization, how BM sprocs, contribute to this process, and why “capillarized LSECs” lose control of HSC activation. Surprisingly, we discovered that capillarization is due to incomplete differentiation of BM-derived LSECs that are recruited to the injured liver. The study identifies aberrant signaling pathways that prevent LSEC differentiation in fibrosis, discovers that HB-EGF is the LSEC signal that promotes HSC quiescence, and identifies why capillarized LSECs are unable to utilize HB-EGF to promote HSC quiescence.
MATERIAL AND METHODS
Rats.
All protocols were reviewed and approved by the Animal Care and Use Committee at the University of Southern California. This study followed the guidelines outlined in the Office of Laboratory Animal Welfare “Public Health Service Policy on Humane Care and Use of Laboratory Animals” (2015). Lewis male rats were obtained from Harlan Corp (Placentia, CA). Breeding pairs of Lew-Tg(CAG-EGFP)ys Lewis rats were obtained from the National Institutes of Health (NIH) Rat Resource and Research Center at the University of Missouri. The chronic liver injury was induced by thioacetamide (TAA) treatment (200 mg/kg intra-peritoneally, twice weekly, for three weeks).
BM transplantation (BMT).
Recipients rats underwent 1,000-cGy total body irradiation and were injected via the tail vein with 5 × 107 BM cells from Lew-Tg(CAG-EGFP)ys Lewis rat. BM was allowed to engraft for two months before use.
Cell Isolation.
LSECs were isolated by collagenase perfusion, iodixanol density gradient centrifugation, and centrifugal elutriation as previously described 12, 13.
Cell Sorting.
After isolation, LSECs from BMT rats were separated into GFP- and GFP+ cells using BD SORP FACSAria I/II cell sorters (BD Biosciences, San Jose, CA).
Intrahepatic Sproc Quantification.
As previously described 5, intrahepatic sprocs were isolated from LSECs isolates by immunomagnetic selection for CD133 using the autoMACs Pro-separator (Miltenyi Biotec). CD133+ LSECs were counted on a hemocytometer and compared to the total number of LSECs isolated from each animal.
Scanning Electron Microscopy (SEM).
LSECs cultured on fibronectin-coated Thermanox® plastic coverslips (Thermo Fisher Scientific, Rochester, NY) were fixed with 2% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA), treated with 1% tannic acid (Electron Microscopy Sciences) and postfixed in 1% osmium tetroxide (Ted Pella, Redding, CA). After sequential dehydration with graded alcohols, samples were dried with hexamethyldisilazane (Ted Pella), sputter-coated with 10-nm gold, and examined via a JSM-6390LV scanning electron microscope (JEOL, Tokyo, Japan).
Fifteen random LSEC pictures per experiment were analyzed. Porosity was determined by Image J software (version 1.51r; NIH, USA). Total LSEC surface area and the open fenestrated areas were measured, and the porosity was calculated as percent of open area.
Effect of ADAMTS13 on the Nitric Oxide (NO) Pathway.
CTR LSECs were plated on fibronectin-coated in 10% FBS-DMEM, at 37°C/5%CO2. Non-adherent cells were removed and LSECs were incubated for 15 min with FBS-free DMEM, anti-human CD36 Antibody (0.01μg/mL - STEMCELL™ Technologies, cat#60084) or Isotype control (0.01μg/mL - Santa Cruz Biotechnology, Cat# sc-3877). Then, cells were and harvested for cGMP measurement.
Effect of TGFβ Receptor I on NO pathway and on LSEC fenestration.
Cirrhotic LSECs were plated on fibronectin-coated in 10% FBS DMEM, at 37°C/5%CO2. Non-adherent cells were removed and LSECs were incubated with 10% FBS-DMEM supplemented with 40ng/mL rmVEGF164 (R&D System, cat#493-MV-005/CF) or 40ng/mL rmVEGF164 plus 10μM SB431542 (STEMCELL™ Technologies, cat#72232). After 24h of shear stress on an orbital shaker at 37°C/5%CO2, cells were harvested for cGMP measurement, SEM analysis and gene expression of ADAMTS13 and TSP-1.
cGMP Measurement.
We used the Cyclic GMP ELISA Kit (Cayman Chemical, cat#581021) according to manufacturer’s instructions and final cGMP quantification was normalized to total protein.
Gene Expression Assay.
RNA was isolated using RNeasy Plus Mini Kit (Qiagen, cat# 74134) and converted to cDNA using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, cat# 368814). For the qPCR reactions we used RT2 SYBR Green/ROX qPCR Mastermix (Qiagen, cat# 330521). All reactions used 40 cycles of amplification on StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific). Primer sequences and respective Tms are listed in Supporting Table S1. β-actin was used as the housekeeping gene.
Immunoblotting.
Protein of freshly isolated LSECs was purified using triple lysis buffer (TLB: 50mM Tris base, 150mM NaCl, 3mM sodium azide, 12mM sodium deoxycholate, 0.1% SDS, 1% Nonidet-P40) supplemented with 2mM PMSF, 1mM sodium orthovanadate and 1% protease inhibitors cocktail (Santa Cruz sc-24948). 50μg of total protein was used from each sample per lane and resolved on NuPAGE™ Novex™ 10% Bis-Tris Protein Gels. Proteins were transferred onto 0.45μm nitrocellulose membranes via electroblotting using Trans-Blot® SD Semi-Dry (Bio-rad). Membranes were blocked using NAP-BLOCKER™. Blots were probed with primary antibody overnight followed by IRDye secondary antibodies and analyzed using LI-COR Image Studio™. Analyses were normalized to β-actin. All antibodies and dilutions used are listed in Supporting Table S2.
Shotgun Proteomics and Data Analysis.
Proteins (100μg) were processed and analyzed using an LC/MS system consisting of an Eksigent NanoLC Ultra 2D (Dublin, CA) and Thermo Fisher Scientific LTQ Orbitrap XL (San Jose, CA). The resulting fragments were detected in Orbitrap with resolution 7500. Proteome Discoverer 1.4 (Thermo Fisher Scientific) was used to identify proteins.
Data availability.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository [1] with the dataset identifier < PXD009010>.” [1] and also for general PRIDE reference, please use: Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, O’Kelly G, Schoenegger A, Ovelleiro D, Perez-Riverol Y, Reisinger F, Rios D, Wang R, Hermjakob H. The Proteomics Identifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013 Jan 1;41(D1):D1063-9. doi: 10.1093/nar/gks1262. Epub 2012 Nov 29. PubMed PMID:23203882.
Co-Cultures of LSEC and HSC with HB-EGF Neutralization.
HSC were plated in the wells at a density of 130,000/cm2. LSECs were plated at a density of 400,000 cells/cm2 on collagen-coated Transwell inserts (Costar, Fisher Scientific, Pittsburgh, PA). Cells were co-cultured for three days with or without anti-human HB-EGF (10ng/mL - R&D System, cat#AF-259) in 10%FBS-DMEM. The percentage of alpha-smooth muscle (α-SMA) positive cells were obtained by counting the number of α-SMA positive cells in 15 randomly selected fields.
α-SMA Staining.
HSCs were fixed in 10% formalin/PBS, then permeabilized with 0.1% Triton X-100. Cells were incubated with 1:1000 anti-α-SMA (Sigma, cat#A2547) or isotype control (Sigma, cat#M5409) and with secondary antibody (Sigma, cat#T7657).
Statistical analysis.
All experiments were performed in triplicates and statistical analysis was done using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). Data is presented as mean ± s.e.m. Statistical significance was assumed at p < 0.05, and p values were indicated as follows: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. All statistical tests and results are summarized in the Supporting Table S3.
RESULTS
What is capillarization?
Cirrhosis was induced with thioacetamide (TAA) in rats that had been transplanted with bone arrow from Lew-Tg(CAG-EGFP)ys rats (BMT rats) to allow tracking of GFP+ bone marrow (BM)-derived cells. After induction of cirrhosis with TAA, LSECs were isolated from BMT-TAA and BMT-control rats, sorted by GFP positivity, and plated for scanning electron microscopy analysis.
In TAA-induced cirrhosis in BMT rats, 65% of LSECs were BM-derived (i.e. GFP+), whereas fewer than 5% were BM-derived LSECs in control rats (Fig. 1A). The engraftment of BM sprocs, therefore, indicates that the fibrotic stimulus is injurious to LSECs. In addition to the presence of BM-derived LSECs, the overall number of sprocs (CD133+ LSECs) in the liver increased from 1.5% to 9.4% in cirrhotic liver (Fig. 1B), further indicating increased LSEC injury. Since most BM-derived LSECs do not express CD133, an endothelial progenitor cell marker 14–16, after engraftment, we conclude that BM-derived LSECs cells had begun differentiation.
Fig. 1. Capillarization is due to engraftment of BM-derived LSECs that fail to completely differentiate.
(A) In control rats (BMT-CTR), fewer than 5% of LSECs were BM-derived (GFP+, •), whereas 65% of LSECs were BM-derived in cirrhosis (****P<0.0001). (B) The number of sprocs (CD133+) increased during cirrhosis (***P<0.001). (C) Representative images of LSECs isolated from wild-type control rats (CTR, upper left), and from resident LSECs (GFP-, lower left) and BM-derived LSECs (GFP+, lower right) from cirrhotic liver. Inserts are magnified portions of the cytoplasm. Resident LSECs from cirrhotic liver had fenestrae organized in sieve plates (arrowheads) comparable to control rat (CTR) whereas BM-derived LSECs lacked fenestration. (D) Calculation of porosity confirms that capillarization is only observed in BM-derived LSECs isolated from cirrhotic liver (***P<0.001). Scale bars, 5μm. Data are mean ± s.e.m. See Supporting Table S3 for numbers of replicates and details of statistical analysis.
The characteristic ultrastructural feature of LSECs, fenestrae organized in sieve plates (Fig. 1C, top panel, arrowheads), is (near)-absent in capillarization. Fig. 1C (bottom left panel) demonstrates that fenestration was fully preserved in tissue-resident (i.e., liver-derived) LSECs in cirrhotic liver (Fig. 1, bottom left panel), but absent in BM-derived LSECs in cirrhosis (Fig. 1C, bottom right panel, single arrowhead; Fig. 1D). Thus, capillarization is not a de-differentiation of resident LSECs, but incomplete differentiation of engrafted BM sprocs to LSECs in fibrotic liver. In contrast, engrafted BM-derived LSECs after partial hepatectomy in normal liver do fenestrate 5.
To confirm in another model of chronic liver injury that “capillarization” of LSECs is due to engrafted BM-derived LSECs that fail to fenestrate, studies in fatty liver showed normal fenestration in resident LSECs (Supporting Fig. S1B, left panel), but capillarization in BM-derived LSECs (Supporting Fig. S1B, right panel).
Why don’t BM-derived LSECs fully differentiate in the cirrhotic liver?
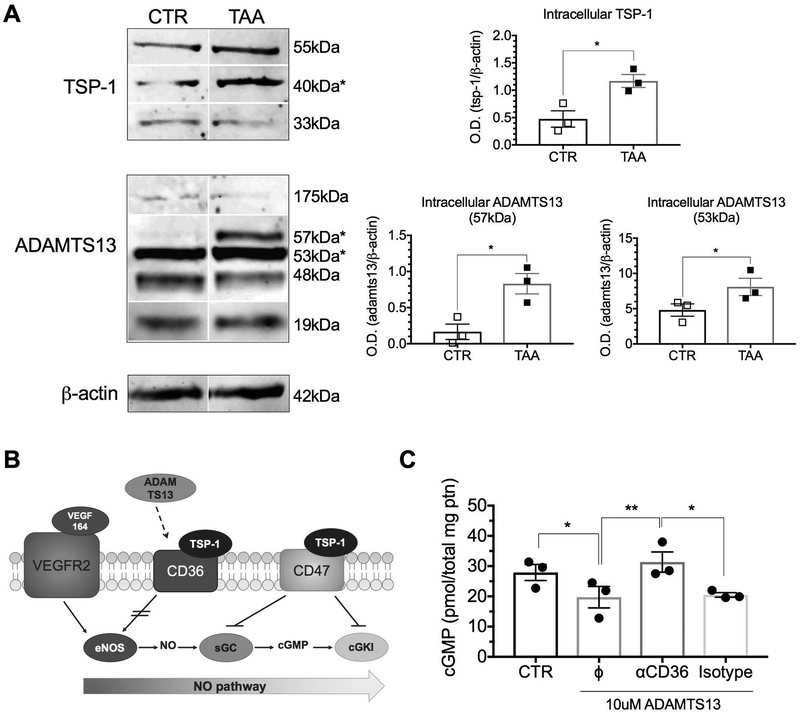
In cirrhosis, lack of LSEC fenestration is due to decreased eNOS activity in the VEGF-eNOS-soluble guanylate cyclase (sGC)-cGMP pathway and fenestration can be induced by treatment with a sGC activator 9, 17, 18 However, the intracellular signaling cascade suppressing eNOS activity in hepatic fibrosis was not previously characterized. Thrombospondin (TSP)-1 is a glycoprotein that is induced in hepatic fibrosis 19 and can modulate a variety of cellular functions. TSP-1 is known to inhibit sGC, cGMP-dependent protein kinase I (cGKI), and nitric oxide (NO) synthase activation via CD47 and CD36 receptors 20. ADAMTS13, which has several TSP-1 repeat domains, has been identified as a possible ligand for CD36, with unknown biological significance 21. TSP-1 and ADAMTS13 protein expression was highly upregulated in LSECs from cirrhotic liver compared to control LSECs (Fig. 2a). We hypothesized that ADAMTS13 might have an effect analogous to that of TSP-1 on CD36 (Fig. 2B). Indeed, ADAMTS13 downregulated LSEC production of cGMP, whereas a CD36 antibody that inhibits binding of ADAMTS13 to CD36 blocked this effect and restored cGMP production (Fig. 2C). Of note, CD47 and CD36 gene and protein expression by LSECs were not altered in cirrhosis (Supporting Fig. S2). Therefore, TSP-1 and ADAMTS13 produced by LSECs, and possibly by neighboring cells, directly downregulate the NO pathway and consequently LSEC fenestration.
Fig. 2. Expression of NO pathway inhibitors is increased in cirrhotic LSECs.
(A) Western blot analysis demonstrated increased expression of both TSP-1 and ADAMTS13 in cirrhotic LSECs compared to CTR LSECs (***P<0.001). (B) The effect of ADAMTS13 binding to CD36 was previously unknown. We propose that it is similar to TSP-1, which inhibits the NO pathway by inhibiting eNOS phosphorylation. (C) cGMP production was decreased when CTR LSECs were stimulated with ADAMTS13. LSEC cGMP levels were restored to control levels when ADAMTS13 binding to CD36 was blocked with the CD36 antibody FA6–152 (*P<0.05, **P<0.01). Data are mean ± s.e.m. Unprocessed original scans of blots are shown in Supporting Fig. S6. See Supporting Table S3 for numbers of replicates and details of statistical analysis.
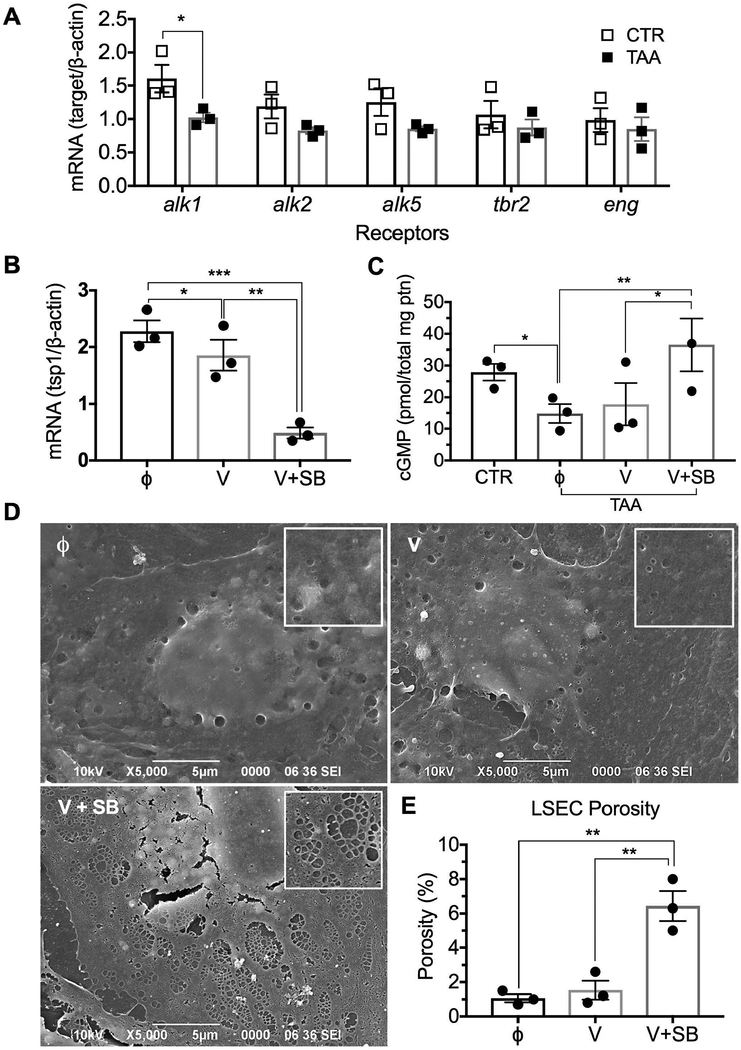
Next, we sought to identify the signaling that could modulate TSP-1 and/or ADAMTS13 expression in LSECs from cirrhotic liver. Transforming growth factor (TGF) β, which is increased during fibrosis, is known to stimulate TSP-1 expression in other cell types 22. Furthermore, the inhibition of TGFβ signaling via the ALK5 receptor favors fetal LSEC maturation, indicating the importance of this growth factor in LSEC developmental biology 23. Hence, we analyzed gene expression of TGFβ receptors in LSECs from normal and cirrhotic rats. Although TGFβ receptors ALK2, ALK5, TGFβRII and endoglin were not different in control versus cirrhotic LSECs (Fig. 3A), ALK1 was significantly lower in cirrhotic LSECs. We therefore postulated that when TGFβ signaling preferentially occurs via ALK5, TSP-1 expression increases and the NO pathway is inhibited. Conversely, when TGFβ signaling shifts towards ALK1, this should release the inhibition of the NO pathway. To verify this hypothesis, control LSECs were cultured for 24h in the presence of VEGF164 with and without the inhibitor SB431542 (a competitive antagonist of the TGFβ ALK4/5/7 kinase receptors) to block the effect of autocrine TGFβ (Supporting Fig. S3A). Indeed in the presence of SB431542, when TGFβ signaling was shifted towards ALK1, TSP-1 production was downregulated (Fig. 3B). In contrast, ADAMTS13 was not downregulated under these conditions, by ADAMTS13 itself, or by TGFβ (Supporting Fig. S3B and S3C). The NO pathway was examined using the same conditions in cirrhotic LSECs. As expected, cGMP levels in untreated cirrhotic LSECs were significantly lower than in control LSECs (Fig. 3C). However, when ALK5 was inhibited by SB431542, cGMP levels in cirrhotic LSECs were restored to that of control LSECs. In response to normalization of cGMP levels, cirrhotic LSECs also developed fenestration with normal porosity in vitro (Fig. 3D and 3E). Taken together this suggests that TSP-1 expression is increased in cirrhotic LSECs due to a shift to ALK5 signaling and this shift inhibits the NO pathway, which prevents the development of fenestration. This is the first report that normal fenestration organized in sieve plates could be induced in vitro in LSECs and it further supports the concept that lack of fenestration is due to incomplete differentiation.
Fig. 3. A shift in TGFβ signaling from the ALK1 to the ALK5 receptor in cirrhotic LSECs inhibits the NO pathway by upregulating TSP-1 expression.
(A) Control LSECs expressed higher levels of ALK1 than cirrhotic LSECs. (B) Expression of TSP-1 by control LSECs was downregulated by an inhibitor of the TGFβ ALK4/5/7 kinase receptors (SB431542) to block stimulation of ALK5 by autocrine TGFβ. (C) cGMP was, as expected, lower in cirrhotic LSECs than in control. A 24h incubation with VEGF164 plus an inhibitor of the TGFβ ALK4/5/7 kinase receptors increased cGMP production in cirrhotic LSECs. (D) Scanning electron microscopy demonstrated few fenestrae in cirrhotic LSECs treated without (Φ) or with VEGF (V), but normal fenestration was induced overnight in cirrhotic LSECs cultured in the presence of VEGF and the inhibitor of the TGFβ ALK4/5/7 kinase receptors (V + SB). Inserts are magnified portions of the cytoplasm. (E) Measurements of LSEC porosity of the groups described in Fig. 3D. Data are mean ± s.e.m. See Supporting Table S3 for numbers of replicates and details of statistical analysis.
Why can’t capillarized LSECs maintain HSC quiescence?
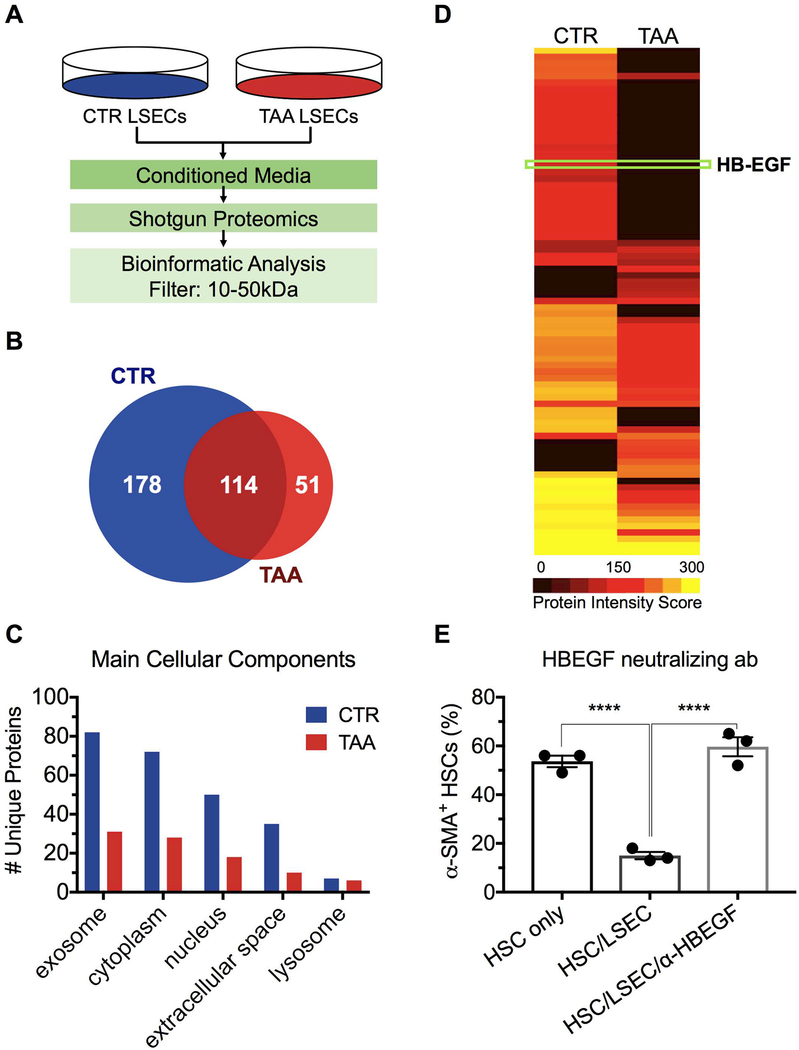
Conditioned medium from control LSECs mimicked the ability of control LSECs to prevent HSC activation in co-culture, demonstrating that the ability to maintain HSC quiescence is mediated by a secreted factor (Supporting Fig. S4). The activity of conditioned medium from control LSECs was degraded with heating to 60°C for 30 minutes, demonstrating that the factor that maintains HSC quiescence is a protein. Using molecular weight cutoff filters, we determined that the protein is between 10 and 50kDa. Proteins present in the conditioned medium of LSECs from control and cirrhotic liver were identified using a shotgun proteomics approach (Fig. 4A). An in-solution digest was performed, peptides identified, and corresponding proteins determined by mass spectrometry for each individual sample. The list of proteins from the samples was compared to identify unique and common proteins: 178 proteins, all with MW between 10 and 50 kDa, were identified only in conditioned medium from LSECs from control liver and 40 of these proteins were determined to be extracellular proteins (Fig. 4C). One of these proteins, Heparin-Binding EGF-Like Growth Factor (HB-EGF) (Fig. 4D), has been shown to promote HSC quiescence; also, fibrosis is exacerbated in HB-EGF knockout mice 24, 25. Thus HB-EGF is sufficient to promote HSC quiescence. We performed co-culture studies of LSECs and HSCs in the presence and absence of neutralizing antibody to HB-EGF and demonstrated that the presence of the antibody completely blocked the ability of LSECs to maintain HSC quiescence (Fig. 4E). Thus HB-EGF is necessary to mediate the promotion of HSC quiescence by LSECs. As HB-EGF is both necessary and sufficient to account for the ability of LSECs to maintain HSC quiescence, logic dictates that HB-EGF fully accounts for the LSEC effect on HSC quiescence. Other proteins might be capable of contributing to the effect, but are redundant (i.e. not necessary).
Fig. 4. HSC quiescence is maintained by HB-EGF released by LSECs.
(A) Conditioned medium from CTR and cirrhotic (TAA) LSEC cultures were evaluated by shotgun proteomics. 10–50 kDa proteins were analyzed. (B) and (C) CTR and TAA LSECs have 178 and 51 unique proteins, respectively, that were classified according to their subcellular location using the DAVID database. (D) Representative heat map of all proteins located in the extracellular space. HB-EGF was previously reported to induce HSC quiescence. (E) Co-culture of LSECs and HSCs in the presence of HB-EGF neutralizing antibody confirmed that HB-EGF was necessary to maintain HCS quiescence (****P<0.0001). Data are mean ± s.e.m. See Supporting Table S3 for numbers of replicates and of statistical analysis.
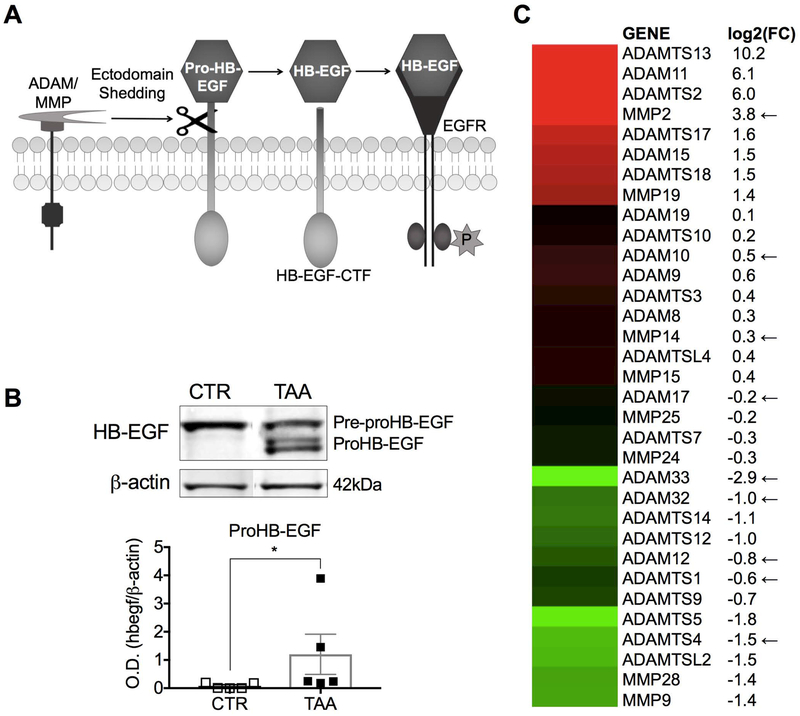
HB-EGF is synthesized as pre-proHB-EGF and is expressed at the cell surface as proHB-EGF (Fig. 5A). The release of the HB-EGF ectodomain from the cytosolic membrane requires proteolytic cleavage by metalloproteinases termed sheddases: members of the ADAM (a disintegrin and metalloproteinase), ADAMTS, and MMP (matrix metalloproteinase) families (Fig. 5A). No difference was found in HB-EGF gene expression (Supporting Fig. S5) or protein expression of pre-proHB-EGF in control vs cirrhotic LSECs (Fig. 5B). However, proHB-EGF remained trapped in the cytosolic membrane of LSECs from cirrhotic liver, whereas it was released from LSECs from control liver (Fig. 5B). Gene expression of all 34 metalloproteinases described in the LSEC transcriptome 26 was compared in LSECs from cirrhotic versus control liver (Fig. 5C). Six of the nine metalloproteinases that have been identified as HB-EGF sheddases were downregulated in LSECs from cirrhotic liver. Taken together these data demonstrate that HB-EGF is the signal that mediates the ability of LSECs to promote HSC quiescence. Immature LSECs in cirrhotic liver lose the ability to maintain HSC quiescence because of downregulation of sheddases needed to release the HB-EGF ectodomain from the cytosolic membrane.
Fig. 5. Cirrhotic LSECs fail to release HB-EGF from the cytosolic membrane.
(A) Illustration of HB-EGF ectodomain shedding. (B) Western blot analysis revealed that TAA LSECs retained proHB-EGF (*P<0.05). (C) Gene expression analysis of ADAMs and MMPs indicated downregulation of 6 of the 9 sheddases involved in HB-EGF shedding in TAA LSECs (indicated by arrows). Log2(FC) represents the fold change of TAA LSECs compared to CTR LSECs. Data are mean ± s.e.m. Unprocessed original scans of blots are shown in Supporting Fig. S6. See Supporting Table S3 for numbers of replicates and details of statistical analysis.
DISCUSSION
The current study examines events upstream of HSC activation that prevent HSC activation in normal liver and that are dysregulated in fibrotic liver disease. LSECs in normal liver act as gatekeepers that prevent activation of HSC and the aberrant LSEC phenotype, so-called capillarization, that is found prior to fibrosis is permissive for HSC activation. Here, we demonstrate that tissue-resident LSECs do not change in fibrosis, but that “capillarization” is due to repair of damaged LSECs by BM-derived sinusoidal endothelial cell progenitor cells, so-called BM sprocs, which fail to fully differentiate to fenestrated LSECs.
We used rats transplanted with transgenic EGFP+ BM (BMT rats) that were treated for three weeks with thioacetamide (TAA) to induce chronic liver injury. These rats provide an instructive model to elucidate the role of BM sprocs in sinusoidal regeneration during liver fibrosis and to determine the origin of LSEC capillarization. These studies revealed that capillarization only occurs in BM-derived LSECs. In studies of partial hepatectomy with the same model of BMT rats, BM-derived LSECs did fenestrate after engraftment, indicating that the cirrhotic environment prevents full maturation of BM-derived LSECs, and ruling out the possibility that lack of fenestration is an artifact of the model. This was further supported by demonstrating that LSECs from transgenic EGFP+ rats were normally fenestrated (Supporting Fig. S1A). This concept of incomplete differentiation of BM-derived LSECs contradicts the current thinking that capillarization is caused by LSEC de-differentiation due to liver injury.
The second major finding reported here is the signaling that inhibits full differentiation of engrafted BM sprocs to LSECs, i.e., the TGFβ, TSP-1 and ADAMTS13 pathways. TGFβ is a profibrogenic cytokine important for fibrosis progression 27. Typically, TGFβ signals via ALK5. TGFβ signaling in endothelial cells may also occur through ALK1, resulting in regulation via a fine balance between ALK5 and ALK1 signaling 28. Our results indicate a shift in TGFβ signaling from the ALK1 to the ALK5 receptor in the immature LSEC. TGFβ/ALK5 signaling has been described as detrimental to differentiation of fetal LSECs 23. We showed that when TGFβ/ALK5 signaling was inhibited and cGMP in cirrhotic LSECs reached levels comparable to control LSECs, that cirrhotic LSECs fenestrated in vitro, supporting the participation of TGFβ/ALK1 signaling in LSEC differentiation. Inhibition of TGFβ/ALK5 signaling also downregulated TSP-1, a known modulator of the NO pathway 20. Given that impairment of the eNOS/sGC/cGMP pathway plays a central role in LSEC capillarization 29 and that TSP-1 is known to impair this pathway, the finding that capillarized LSECs produce high levels of TSP-1 demonstrates that variations in TSP-1 production directly control LSEC differentiation. The key contribution of TSP-1 to this process was confirmed by the finding that downregulation of TGFβ/Alk5 induction of TSP-1 in cirrhotic LSECs induced normal fenestration organized in sieve plates in vitro, something that has never previously been accomplished. Although it is unclear if the LSEC defenestration that occurs in vitro is directly linked to the inability to form fenestrae in vivo in immature LSECs in fibrosis, there is one commonality of these processes: it has been shown that the interaction of TSP-1 with CD47 accelerates in vitro defenestration of LSECs, albeit unrelated to TGF-β receptor inhibition 30.
In addition to TSP-1, we also detected upregulation of ADAMTS13 in immature LSECs. ADAMTS13 is a ligand of CD36 but does not bind to CD47 21. Binding of some ligands to CD36 limits eNOS activation through alterations in calcium signaling and inhibition of the fatty acid translocase activity of CD36 31, 32. This study is the first demonstration that binding of ADAMTS13 to CD36 downregulates eNOS activity. Fig. 6 outlines the signaling pathways described above.
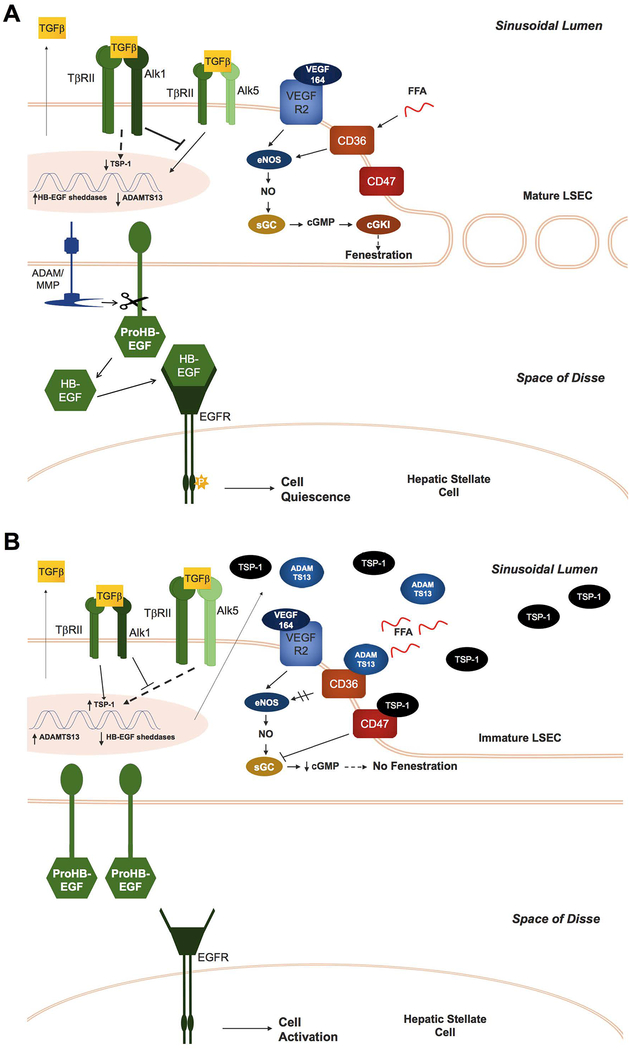
Fig. 6. Proposed signaling pathways that regulate LSEC phenotype and crosstalk between LSECs and HSCs in normal and fibrotic liver.
(A) In normal liver, LSECs express high levels of ALK1 receptors, favoring TGFβ/ALK1 signaling, which shifts signaling away from the TGFβ/ALK5 pathway and thereby downregulates TSP-1 gene expression. Furthermore, LSECs in normal liver shed HB-EGF from the cytosolic membrane, which maintains HSC quiescence. (B) In fibrotic liver, increased levels of TGFβ and lower expression of the ALK1 receptor favor TGFβ/ALK5 signaling in BM-derived LSECs, resulting in increased TSP-1 expression. TSP-1 binds to CD47 and CD36, which inhibits both sGC and cGMP-dependent protein kinase I (cGKI), as well nitric oxide synthase activation. ADAMTS13 is also upregulated and inhibits the NO pathway by binding to CD36. The consequent downregulation of the NO pathway by TSP-1 and ADAMTS13 prevents maturation of BM-derived LSECs, manifested by lack of fenestration and downregulation of key HB-EGF sheddases. Inability to release HB-EGF permits HSC activation.
Activated hepatic stellate cells also produce TSP-1 33 and ADAMTS13 34, and both are upregulated in fibrotic and cirrhotic liver 19,34. Therefore, expression by both immature LSECs and by activated hepatic stellate cells means that TSP-1 and ADAMTS13 act as autocrine signals, but could also conceivably be environmental signals that prevent maturation of BM-derived LSECs in fibrosis. In contrast to the fibrotic liver, BM-derived LSECs that engraft in normal liver after partial hepatectomy become normally fenestrated by day 14 5. TSP-1 is elevated during the first 12 hours after partial hepatectomy but is then suppressed until at least day 14 35. Taken together, elevated TSP-1 inhibits maturation of BM-derived LSECs in fibrosis, but suppression of TSP-1 after partial hepatectomy may allow engrafted BM sprocs to fully mature.
The third major group of findings in this study is the discovery of HB-EGF as the LSEC mediator that maintains HSC quiescence. In vitro and in vivo studies have previously shown that HB-EGF is a suppressor of HSC activation 25. Here, we showed that HB-EGF was expressed by LSECs from both normal and cirrhotic liver, but it was only detected in conditioned medium from normal LSECs in culture. We found that immature LSECs fail to release HB-EGF due to a deficiency in the sheddases needed to release the HB-EGF ectodomain from the cytosolic membrane. The sheddases ADAM12, ADAM17, ADAM32, ADAM33, ADAMTS1, and ADAMTS4 are downregulated in the immature LSEC 36–41, which explains why these cells are unable to maintain HSC quiescence.
Aberrant stem cell maturation has been described in developmental abnormalities 42–44 and cancer stem cells have been implicated in hematological and solid organ malignancies. The current study is among the first examples of incomplete maturation of adult stem cells in acquired, non-malignant disease. Pathology in various organs often shares common pathways and the findings of the current study could be relevant to fibrosis in other organs, e.g., lung, kidney, and in systemic sclerosis 45–47. There is extensive literature that describes repair of endothelial cell injury in various organs by BM endothelial progenitor cells. There is also a large body of work on crosstalk between endothelial cells and mural cells (pericytes, smooth muscle cells) in various organs. We speculate that the pathways discovered here may be recapitulated in fibrosis of other organs and provide targets for therapy.
Supplementary Material
Acknowledgments
Financial support: This work was supported by NIH grant R01DK100580 (L.D.D.); by a New Investigator Transition Award from the Research Center for Liver Diseases (A.M.M.); by the Research Center for Liver Diseases (NIH grant P30DK048522) Cell Separation and Culture Core, the Analytic, Metabolic, Instrumentation core, and the Proteomics Subcore; and by the USC Integrative Liver Cell Core of the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases.
Abbreviations:
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin elements
- BM
bone marrow
- BMT
bone marrow transplanted
- HB-EGF
heparin binding epidermal growth factor-like growth factor
- HSC
hepatic stellate cells
- LSEC
liver sinusoidal endothelial cells
- NO
nitric oxide
- sGC
soluble guanylate cyclase
- sprocs
sinusoidal endothelial cell progenitor cells
- TAA
thioacetamide
- TGFβ
transforming growth factor beta
- TSP-1
thrombospondin-1
REFERENCES
- 1.Schaffner F, Popper H. Capillarization of hepatic sinusoids in man. Gastroenterology 1963;44:239–242. [PubMed] [Google Scholar]
- 2.DeLeve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 2008;48:920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harb R, Xie G, Lutzko C, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology 2009;137:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Wang X, Wang L, et al. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology 2012;143:1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Wang X, Xie G, et al. Liver Sinusoidal Endothelial Cell Progenitor Cells Promote Liver Regeneration in Rats. Journal of Clinical Investigation 2012;122:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLeve LD, Wang X, Wang L. VEGF-sdf1 recruitment of CXCR7+ bone marrow progenitors of liver sinusoidal endothelial cells promotes rat liver regeneration. American Journal of Physiology-Gastrointestinal and Liver Physiology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie G, W L, W X, et al. Isolation of periportal, mid-lobular and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am J Physiol-Gastrointest Liver Physiol 2010;299:G1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14:397–411. [DOI] [PubMed] [Google Scholar]
- 9.Xie G, Wang X, Wang L, et al. Role of liver sinusoidal endothelial cell differentiation in progression and regression of rat hepatic fibrosis. Gastroenterology 2012;142:918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol 2016;65:608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrone G, Russo L, Rosado E, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. Journal of Hepatology 2013;58:98–103. [DOI] [PubMed] [Google Scholar]
- 12.DeLeve LD, Wang X, McCuskey MK, et al. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic separation lack fenestrae and sieve plates. Am J Physiol Gastrointest Liver Physiol 2006;291:G1187–9. [DOI] [PubMed] [Google Scholar]
- 13.Steffan AM, Gendrault JL, McCuskey RS, et al. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology 1986;6:830–6. [DOI] [PubMed] [Google Scholar]
- 14.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997;90:5002–5012. [PubMed] [Google Scholar]
- 15.Gehling UM, Ergun S, Schumacher U, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 2000;95:3106–12. [PubMed] [Google Scholar]
- 16.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 2000;95:952–958. [PubMed] [Google Scholar]
- 17.Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999;117:1222–8. [DOI] [PubMed] [Google Scholar]
- 18.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology 1998;114:344–351. [DOI] [PubMed] [Google Scholar]
- 19.Smalling RL, Delker DA, Zhang Y, et al. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol 2013;305:G364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isenberg JS, Roberts DD, Frazier WA. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol 2008;28:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis AK, Makar RS, Stowell CP, et al. ADAMTS13 binds to CD36: a potential mechanism for platelet and endothelial localization of ADAMTS13. Transfusion 2009;49:206–13. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Li JH, Garcia G, et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int 2004;66:605–13. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida M, Nishikawa Y, Omori Y, et al. Involvement of signaling of VEGF and TGF-beta in differentiation of sinusoidal endothelial cells during culture of fetal rat liver cells. Cell Tissue Res 2007;329:273–82. [DOI] [PubMed] [Google Scholar]
- 24.Takemura T, Yoshida Y, Kiso S, et al. Conditional loss of heparin-binding EGF-like growth factor results in enhanced liver fibrosis after bile duct ligation in mice. Biochem Biophys Res Commun 2013;437:185–91. [DOI] [PubMed] [Google Scholar]
- 25.Huang G, Besner GE, Brigstock DR. Heparin-binding epidermal growth factor-like growth factor suppresses experimental liver fibrosis in mice. Laboratory investigation 2012;92:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahani T, Covens K, Lavend’homme R, et al. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. Journal of Thrombosis and Haemostasis 2014;12:36–42. [DOI] [PubMed] [Google Scholar]
- 27.Fabregat I, Moreno-Caceres J, Sanchez A, et al. TGF-beta signalling and liver disease. FEBS J 2016;283:2219–32. [DOI] [PubMed] [Google Scholar]
- 28.Goumans MJ, Valdimarsdottir G, Itoh S, et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 2002;21:1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie G, Wang X, Wang L, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 2012;142:918–927.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatraman L, Tucker-Kellogg L. The CD47-binding peptide of thrombospondin-1 induces defenestration of liver sinusoidal endothelial cells. Liver Int 2013;33:1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer EM, Qin Y, Miller TW, et al. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 2010;88:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isenberg JS, Jia Y, Fukuyama J, et al. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem 2007;282:15404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breitkopf K, Sawitza I, Gressner AM. Characterization of intracellular pathways leading to coinduction of thrombospondin-1 and TGF-beta1 expression in rat hepatic stellate cells. Growth Factors 2005;23:77–85. [DOI] [PubMed] [Google Scholar]
- 34.Niiya M, Uemura M, Zheng XW, et al. Increased ADAMTS-13 proteolytic activity in rat hepatic stellate cells upon activation in vitro and in vivo. J Thromb Haemost 2006;4:1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi H, Sakai K, Baba H, et al. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology 2012;55:1562–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Liu B, Gao X, et al. Overexpression of sigma-1 receptor inhibits ADAM10 and ADAM17 mediated shedding in vitro. Protein Cell 2012;3:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan Y, Long J, Chen J, et al. Overexpression of soluble ADAM33 promotes a hypercontractile phenotype of the airway smooth muscle cell in rat. Exp Cell Res 2016;349:109–118. [DOI] [PubMed] [Google Scholar]
- 38.Marczok S, Bortz B, Wang C, et al. Comprehensive Analysis of Genome Rearrangements in Eight Human Malignant Tumor Tissues. PLoS One 2016;11:e0158995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Díaz B, Yuen A, Iizuka S, et al. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol 2013;201:279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu YJ, Xu Y, Yu Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene 2006;25:2452–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S, Springstead JR, Parks BW, et al. Metalloproteinase processing of HBEGF is a proximal event in the response of human aortic endothelial cells to oxidized phospholipids. Arterioscler Thromb Vasc Biol 2012;32:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steimle JD, Moskowitz IP. TBX5. Current Topics in Developmental Biology 2017;122:195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldini A, Fulcoli FG, Illingworth E. Tbx1. Current Topics in Developmental Biology 2017;122:223–243. [DOI] [PubMed] [Google Scholar]
- 44.Miranda RC. MicroRNAs and Fetal Brain Development: Implications for Ethanol Teratology during the Second Trimester Period of Neurogenesis. Frontiers in genetics 2012;3:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao Z, Lis R, Ginsberg M, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nature medicine 2016;22:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 2008;173:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Papa N, Quirici N, Soligo D, et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum 2006;54:2605–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository [1] with the dataset identifier < PXD009010>.” [1] and also for general PRIDE reference, please use: Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, O’Kelly G, Schoenegger A, Ovelleiro D, Perez-Riverol Y, Reisinger F, Rios D, Wang R, Hermjakob H. The Proteomics Identifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013 Jan 1;41(D1):D1063-9. doi: 10.1093/nar/gks1262. Epub 2012 Nov 29. PubMed PMID:23203882.