Abstract
The frequency of antimicrobial resistance has increased globally due to misuse and overuse of antibiotics, and multi-drug resistant (MDR) bacteria are now recognized as a major cause of hospital-acquired infections (HAI). Our aim was to investigate the prevalence, distribution, and antimicrobial susceptibility rates of MDR bacteria in patients with HAI from a tertiary hospital in China. We retrospectively evaluated all patients with a confirmed diagnosis of bacterial infection at a tertiary general hospital in Jining, for the period between January 2012 and December 2014. The following clinical and demographic data were collected: age, sex, specimens, treatment, microbiology results, and antibiotic resistance patterns of isolates. Bacterial identification and susceptibility testing were performed using VITEK 2 COMPACT system. We screened a total of 15,588 patients, out of which 7579 (48.6%) had an HAI. MDR showed 3223 out of 7579 isolates (42.5%). The most frequently isolated MDR bacteria in patients with HAI were extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli (n = 1216/3223, 37.7%), MDR Pseudomonas aeruginosa (n = 627/3223, 19.5%) and MDR Acinetobacter baumannii (n = 588/3223, 18.2%). MDR-HAI were more common in males (2074/3223, 64.4%) and in elderly patients (≥60 years; 1196/3223, 37.1%). Sputum was the main source of MDR isolates (2056/3223, 63.8%). Patients with MDR-HAI were predominantly distributed in different types of intensive care units. MDR strains in our study showed resistance to most current antibiotics. Overall, patients with HAI infections attributed to MDR bacteria were widely distributed in our hospital. Enhanced surveillance of MDR bacteria is critical for guiding the rational use of antibiotics and reducing the incidence of HAI.
Keywords: Multidrug-resistant bacteria, infection, distribution, intensive care unit, MDR, nosocomial infections, hospital-acquired infections, HAI
INTRODUCTION
Hospital-acquired infections (HAI), also called nosocomial infections, affect the clinical outcomes in hospitalized patients and represent a serious concern worldwide [1]. The misuse and overuse of antibiotics in medicine and agriculture has led to the development of multidrug-resistant (MDR) bacteria, which are now recognized as a major cause of nosocomial infections. In addition to a higher risk of poor clinical outcomes and death, MDR bacterial infections are associated with increased economic burden of patients [2,3]. Understanding the clinical characteristics, prevalence, and distribution of nosocomial infections caused by MDR bacteria is crucial for effective treatment.
In the strictest sense, MDR organisms are defined as those showing resistance to more than one antimicrobial agent in in vitro antimicrobial susceptibility tests. MDR Gram-positive and Gram-negative bacteria are commonly defined as ‘resistant to three or more antimicrobial classes’ [4,5]. In addition, a more accurate definition of MDR has been proposed as ‘non-susceptibility (resistant, intermediate, or non-susceptible result) to at least one agent in three or more antimicrobial categories’. In the same paper, pandrug-resistant (PDR) was defined as ‘non-susceptibility to all agents in all antimicrobial categories’ [5]. Common bacteria that show MDR/PDR are methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum beta-lactamase (ESBL)-producing bacteria, carbapenem-resistant Enterobacteriaceae (CRE), carbapenem-resistant Acinetobacter baumannii (A. baumannii, CRAB), MDR/PDR Pseudomonas aeruginosa (P. aeruginosa, MDR/PDRPA), and MDR Mycobacterium tuberculosis [6-8]. A high prevalence of nosocomial infections attributable to MDR strains has been reported in countries worldwide [8-12]. In 2014, the average MRSA detection rate across 17 Chinese hospitals was 44.6% (29.1% to 74.2%), and the detection rates of ESBL-producing Escherichia coli (E. coli, ESBLECO) and ESBL-producing Klebsiella pneumoniae (ESBLKPN) were 55.8% and 29.9%, respectively [10].
In the present study, we retrospectively evaluated the prevalence, distribution, and antimicrobial susceptibility rates of MDR strains in patients with HAI for a 3-year period, to gain an overview of the situation in a tertiary general hospital in China.
MATERIALS AND METHODS
Study design and sample
This retrospective study was conducted in a tertiary general hospital in Jining, China, for the period between January 2012 and December 2014. The hospital contains 4200 beds and is organized into 43 clinical departments. Initially, we included all inpatients with a confirmed diagnosis of bacterial infection. The diagnosis of HAI was made based on the Hospital Infection Diagnosis Standard issued by the National Health and Family Planning Commission of the People’s Republic of China (NHFPC) in 2001. Antibiotic susceptibility of bacterial isolates was determined with antimicrobial susceptibility testing. Patients tested negative for MDR strains or those with colonized bacteria were excluded from further analysis.
Clinical and demographic data of patients were obtained from the Laboratory Information System (LIS) of the hospital and included age, sex, specimens, treatment, microbiology results, and antibiotic resistance patterns of isolates.
Bacterial isolates
Bacterial isolates were collected from different sources, including blood, sputum, secretions, urine, pus, or any other site that was clinically suspected for infection based on the Technical guidelines for the prevention and control of nosocomial infections with multidrug-resistant bacteria of the People’s Republic of China. The isolates were cultured on agar plates containing 5% sheep blood (90 mm, Jnbabio, China) for 24 hours at 37°C and under 5% CO2. Bacterial identification was performed using the VITEK 2 COMPACT system (BioMerieux SA, USA). The following quality control (QC) strains were included: Staphylococcus aureus ATCC25923, Escherichia coli ATCC25922, Klebsiella pneumoniae ATCC700603, and Pseudomonas aeruginosa ATCC27853.
Antimicrobial susceptibility testing
Bacterial isolates were cultured on 5% sheep blood agar plates (90 mm, Jnbabio, China) for 24 hours at 37°C, under 5% CO2. Antimicrobial susceptibility testing was performed using the VITEK2 COMPACT system with standardized inoculum (BioMerieux, Hazelwood, MO, USA). The testing was conducted according to the Clinical and Laboratory Standards Institute (CLSI) standards (24th edition) [13].
MDR bacteria were considered to be resistant to at least three or more antibiotic classes, and predominately included the following strains: MRSA, ESBL-producing gram-negative Enterobacteriaceae such as ESBLECO and ESBLKPN; vancomycin-resistant Enterococcus faecium (VREF); MDRPA, MDR A. baumannii (MDRAB); other Gram-negative bacteria were considered to be MDR if they were resistant to carbapenem, fluoroquinolone, and cephalosporin [5].
Statistical analysis
Data was extracted from the LIS and converted into a standard format using WHONET 5.4 software (WHO Collaborating Centre for Surveillance of Antimicrobial Resistance). We used descriptive statistics for data processing and reporting.
RESULTS
Patients
We screened a total of 15 588 patients out of which 7579 (48.6%) had an HAI (Table 1). Among 7579 patients with HAI there were 4765 males/2814 females, and the median age was 55 years. The majority of patients were from the departments of neurology, neurosurgery, orthopedics and joint surgery, urology, plastic surgery, oncology, respiratory unit, gastroenterology, nephrology, and intensive care unit (ICU). All patients with positive cultures received antibiotics.
TABLE 1.
Basic characteristics of study population
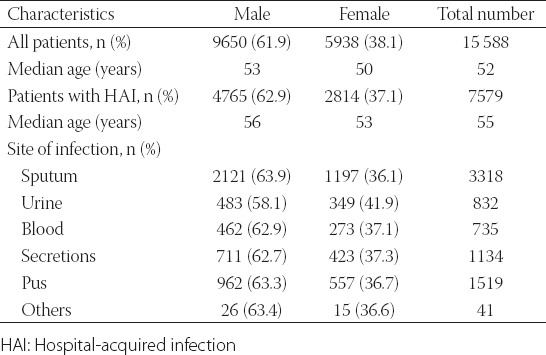
Identification of MDR strains
Out of 7579 isolates from patients with HAI, 3223 showed MDR (42.5%). The most frequently isolated MDR bacteria were ESBLECO (n = 1216/3223, 37.7%), MDRPA (n = 627/3223, 19.5%) and MDRAB [n = 588/3223, 18.2%] (Table 2).
TABLE 2.
MDR strains isolated from patients with HAI (n=7579)
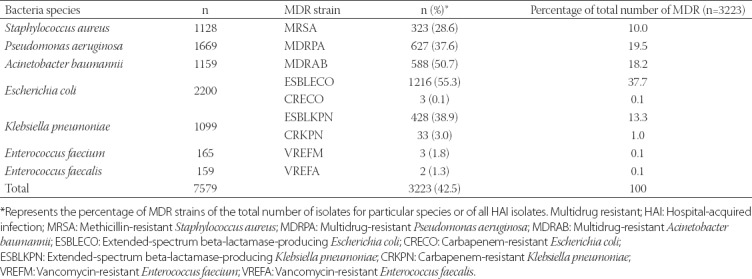
Age and gender distribution of patients infected with MDR bacteria
The distribution of patients with MDR bacterial infections (n = 3223) according to gender and age groups is shown in Table 3. Out of 3223 MDR isolates, 2074 (64.4%) were from male and 1149 (35.7%) from female patients. Elderly (≥60 years; 1196/3223, 37.1%) represented the highest proportion of patients with MDR bacterial infections, followed by middle-aged patients (40–59 years; 873/3223, 27.1%) and infants (<2 years; 330/3223, 10.2%).
TABLE 3.
Age and gender distribution of patients with MDR-HAI
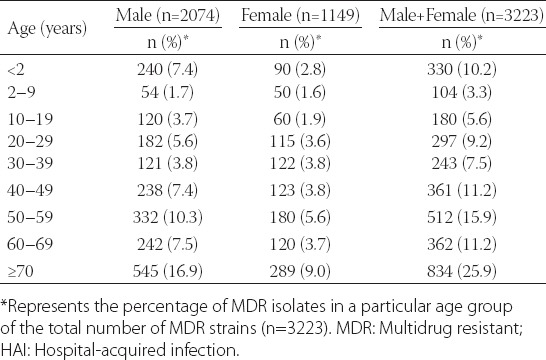
Sources of MDR bacteria
From the initial 15,588 specimens, 7579 positive isolates (48.6%) were from patients with HAI. The percentage of MDR positive isolates from a total number of sputum, secretions, blood, urine, pus, and other specimens was, respectively, 23.7% (2056/8675), 37.4% (414/1106), 5.6% (218/3892), 26.7% (369/1382), 45% (150/333) and 8% (16/200). The proportion of each specimen type in the group of isolates positive for MDR bacteria was as follows: sputum (2056/3223, 63.8%), secretions (414/3223, 12.8%), urine (369/3223, 11.4%), blood (218/3223, 6.8%), pus (150/3223, 4.6%) and other specimens (16/3223, 0.5%). The most common MDR bacteria was ESBLECO, detected in all specimen types. Sputum was the main source of MDRAB (550/2056, 26.7%), ESBLECO (476/2056, 23.1%), MDRPA (411/2056, 20.0%) and ESBLKPN (396/2056, 19.3%), followed by urine specimens (Table 4).
TABLE 4.
Sources of MDR bacteria
Distribution of MDR strains across hospital departments
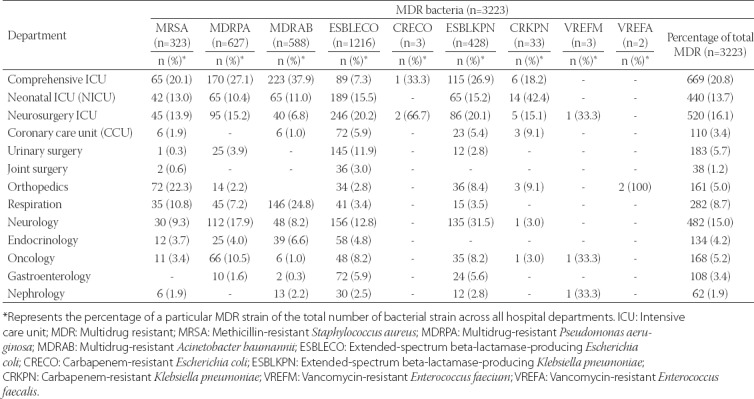
Patients with MDR bacterial infections were distributed across various hospital departments, especially in comprehensive ICU (669/3223, 20.7%), neurosurgery ICU (520/3223, 16.1%), neonatal ICU [NICU] (440/3223, 13.6%) and neurology department (482/3223, 15.0%). As shown in Table 5, MRSA, MDRPA and MDRAB were most commonly isolated from patients in comprehensive ICU. ESBLECO was distributed in almost every main department, with the highest proportion of patients with ESBLECO in neurosurgery ICU (246/1216, 20.2%), followed by NICU (189/1216, 15.5%) and neurology department (156/1216, 12.8%).
TABLE 5.
Distribution of MDR bacteria across major hospital departments
Antibiotic susceptibility of MDR strains
The susceptibility of MRSA isolates to vancomycin, tigecycline, and linezolid was 100% (Figure 1A). The resistance rate of MRSA isolates to penicillin G, cefoxitin, and oxacillin reached above 90%. MRSA isolates were resistant to 5 antimicrobial agents from 3 classes (β-lactams, macrolides, and chloramphenicols).
FIGURE 1.
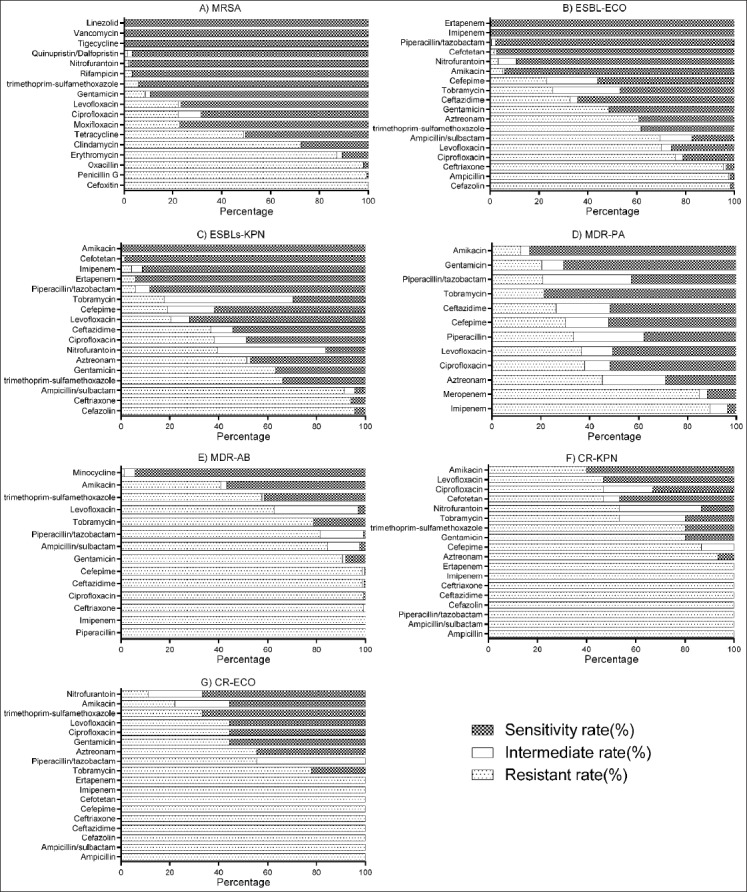
Susceptibility rates of MDR strains isolated from patients with HAI. A) The susceptibility of MRSA isolates to vancomycin, tigecycline, and linezolid was 100%. B) The susceptibility rate of ESBLECO isolates to imipenem, ertapenem, piperacillin/tazobactam, and cefotetan was above 90%. C) ESBLKPN isolates were highly susceptible to amikacin (100%), ceftriaxone (98.5%), ertapenem (94.1%), and imipenem (91.2%). D) MDRPA isolates showed high susceptibility to amikacin (84.7%), tobramycin (78.5%), and gentamicin (70.8%). E) MDRAB isolates were susceptible to minocycline (94.2%) and amikacin (56.7%). F) CRKPN isolates were susceptible to amikacin (60%) and levofloxacin (53.3%). G) CRECO isolates were susceptible to nitrofurantoin (66.7%) and trimethoprim/sulfamethoxazole (66.7%). *The susceptibility testing was not performed for VREFM and VREFA due to a very small number of resistant strains. MDR: Multidrug resistant; HAI: Hospital-acquired infection; MRSA: Methicillin-resistant Staphylococcus aureus; MDRPA: Multidrug-resistant Pseudomonas aeruginosa; MDRAB: Multidrug-resistant Acinetobacter baumanii; ESBLECO: Extended-spectrum beta-lactamase-producing Escherichia coli; CRECO: Carbapenem-resistant Escherichia coli; ESBLKPN: Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae; CRKPN: Carbapenem-resistant Klebsiella pneumoniae; VREFM: Vancomycin-resistant Enterococcus faecium; VREFA: Vancomycin-resistant Enterococcus faecalis.
The susceptibility rate of ESBLECO isolates to imipenem, ertapenem, piperacillin/tazobactam and cefotetan was above 90% (Figure 1B). ESBLECO isolates showed high resistance to cefazolin (98.4%), ampicillin (97.7%) and ceftriaxone (95.5%), and resistance to two quinolone agents, ciprofloxacin (75.9%) and levofloxacin (70.2%).
ESBLKPN isolates were highly sensitive to amikacin (100%), ceftriaxone (98.5%), ertapenem (94.1%) and imipenem [91.2%] (Figure 1C). They showed a high resistance predominately to β-lactams. The resistance rates of ESBLKPN isolates to cefazolin, ceftriaxone, and ampicillin/sulbactam were 95.6%, 94.1%, and 91.2%, respectively.
MDRPA isolates showed high susceptibility to amikacin (84.7%), tobramycin (78.5%) and gentamicin [70.8%] (Figure 1D), and high resistance to imipenem (89.1%) and meropenem (85.0%).
MDRAB isolates were sensitive to minocycline (94.2%) and amikacin [56.7%] (Figure 1E). They were highly resistant to most of the tested antibiotics, including piperacillin (100%), imipenem (100%), ceftriaxone (99.3%), ciprofloxacin (99.3%), and ceftazidime (98.7%).
CRKPN isolates were sensitive to amikacin (60%) and levofloxacin [53.3%] (Figure 1F). They also showed high resistance to most of the tested antibiotics, including 12 antimicrobial agents from 3 classes (β-lactams, aminoglycosides, and sulfonamides).
CRECO isolates were susceptible to nitrofurantoin (66.7%) and trimethoprim/sulfamethoxazole (66.7%), and resistant mainly to the antibiotics from β-lactam, aminoglycoside, and quinolone classes (Figure 1G).
DISCUSSION
Over the past decades, the frequency of antimicrobial resistance has increased globally. MDR bacteria are now recognized as a major cause of nosocomial infections, representing a serious safety concern for both health care practitioners and the patients [9,14]. The risk of MDR bacterial infection has been related to a number of factors, including long-term antimicrobial therapy, cross-transmission, length of hospital stay, and invasive procedures [15,16]. MDR bacteria can cause different types of infections, such as pneumonia, urinary tract, hematogenous and wound infection, and are associated with a high mortality/morbidity and increased medical costs [16,17]. Due to differences in environmental conditions and antibiotic use, antimicrobial susceptibility patterns often differ between geographical regions, populations and hospital types/units [9,18,19]. Therefore, understanding the distribution of MDR bacteria, especially in hospitals, is important for the control of infection as well as for the rational use of antimicrobial agents.
In the present study, we reported a high prevalence of MDR-HAI infections in a tertiary general hospital; i.e., out of 7579 bacterial isolates from patients with HAI, 3223 showed MDR (42.5%). ESBL-producing Gram-negative bacteria were the most frequently isolated MDR pathogens, with the highest detection rate observed for ESBLECO (37.7%). ESBLECO and ESBLKPN together accounted for 51.0% of all MDR isolates. Deng et al. [20] investigated antibiotic resistance of isolates from hospitalized patients with hematological disease, for a 6-year period. Among 1453 bacterial isolates, MRSA accounted for 72.8% of antibiotic resistant strains, while the detection rates of ESBLECO and ESBLKPN were 18.9% and 10.4%, respectively [20].
In our study, patients aged above 60 years represented the highest proportion of patients with MDR bacterial infections, followed by middle-aged patients (40–59 years), and infants (<2 years). Elderly patients are generally consider to be at high risk of nosocomial infections, due to a higher disease prevalence in this population, including neurological disorders, diabetes and cardiovascular diseases [21-25].
The number of MDR isolates was higher in male (64.4%) than in female patients (35.7%) in our study population. The highest percentage of MDR isolates was obtained from sputum specimens (63.8%), showing that the respiratory system was the most frequent site of infection. Generally, the prevalence of cigarette smoking is higher in males than in females, which makes them more prone to respiratory tract diseases, including infections. In addition, frequent use of extended-spectrum antimicrobial agents in long-term care facilities provides selective pressure for multidrug resistance in bacteria [26]. Further research is necessary to clarify the gender differences in the prevalence of HAI infections caused by MDR bacteria.
The risk of infections with MDR bacteria is especially high for patients admitted to the ICU, due to the underlying condition, impaired immunity, extensive antibiotic use, and exposure to invasive devices (e.g., mechanical ventilation, central venous catheters, and tracheotomy) [17,27,28]. In the current study, patients with MDR bacterial infections were distributed across various hospital departments. Consistent with the previous research, the percentage of MDR bacteria isolated from ICU patients was higher compared to patients from other units/departments.
MRSA is one of the most common causes of HAI and a leading cause of death in hospitalized patients. This is mainly due to the fact that S. aureus strains carry genes that mediate resistance to various antibiotics, including widely used anti-staphylococcal drugs [29]. We showed that MRSA isolates were primarily resistant to cephalosporins and penicillins, which suggests that the overuse of these antibiotics should be avoided. On the other hand, MRSA isolates were highly susceptible to vancomycin, tigecycline and linezolid. In most countries, vancomycin remains the first-choice treatment for MRSA infections.
Other bacteria that showed MDR in this study included Gram-negative strains such as ESBLECO, ESBLKPN, MDRPA, and MDRAB. They were all resistant to β-lactams, especially cephalosporins (i.e., cefazolin, ceftazidime, ceftriaxone, and cefepime). Therefore, control of β-lactam use appears to be essential in our hospital. In contrast, these Gram-negative bacteria were highly susceptible to amikacin, suggesting it could be a viable treatment option in patients infected by MDR bacteria. In addition, MDRAB isolates showed a high susceptibility to minocycline, which is a broad-spectrum tetracycline antibiotic. Furthermore, we showed that ESBL-producing strains (ESBLECO and ESBLKPN) were not resistant to the new-generation semisynthetic antibiotics, e.g., cefotetan, piperacillin/tazobactam, imipenem and ertapenem. The production of beta-lactamases, such as ESBLs and AmpC beta-lactamases (AmpC), is a common resistance mechanism in Gram-negative bacteria. The third-generation cephalosporins have been recognized as the main factor leading to the emergence and spread of ESBL-producing strains [30]. Still, antibiotics remain the primary means to control infections and the widespread use of the new generation antibiotics will further contribute to the emergence and spread of antibiotic resistant bacteria. Therefore, continuous surveillance of antimicrobial resistance and rational use of antibiotics is necessary to effectively control these infections.
CONCLUSION
In conclusion, patients infected with MDR bacteria were widely distributed in our hospital. MDR bacterial infection was more likely to occur among elderly and ICU patients. MDR isolates found in our hospital were resistant to most current antibiotics. Enhanced surveillance of MDR bacteria is critical for guiding the rational use of antibiotics and reducing the incidence of HAI.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Tsalik EL, Li Y, Hudson LL, Chu VH, Himmel T, Limkakeng AT, et al. Potential cost-effectiveness of early identification of hospital-acquired infection in critically ill patients. Ann Am Thorac Soc. 2016;13(3):401–13. doi: 10.1513/AnnalsATS.201504-205OC. https://doi.org/10.1513/AnnalsATS.201504-205OC. [DOI] [PubMed] [Google Scholar]
- 2.Antony HA, Parija SC. Antimalarial drug resistance:An overview. Trop Parasitol. 2016;6(1):30–41. doi: 10.4103/2229-5070.175081. https://doi.org/10.4103/2229-5070.175081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenz S, Wilson DN. Blast from the past:Reassessing forgotten translation inhibitors, antibiotic selectivity, and resistance mechanisms to aid drug development. Mol Cell. 2016;61(1):3–14. doi: 10.1016/j.molcel.2015.10.019. https://doi.org/10.1016/j.molcel.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55(Pt 12):1619–29. doi: 10.1099/jmm.0.46747-0. https://doi.org/10.1099/jmm.0.46747-0. [DOI] [PubMed] [Google Scholar]
- 5.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria:An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x. https://doi.org/10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 6.Wallinga D, Rayner G, Lang T. Antimicrobial resistance and biological governance:Explanations for policy failure. Public Health. 2015;129(10):1314–25. doi: 10.1016/j.puhe.2015.08.012. https://doi.org/10.1016/j.puhe.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Chellat MF, Raguz L, Riedl R. Targeting antibiotic resistance. Angew Chem Int Ed Engl. 2016;55(23):6600–26. doi: 10.1002/anie.201506818. https://doi.org/10.1002/anie.201506818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seligman R, Ramos-Lima LF, Oliveira Vdo A, Sanvicente C, Sartori J, Pacheco EF. Risk factors for infection with multidrug-resistant bacteria in non-ventilated patients with hospital-acquired pneumonia. J Bras Pneumol. 2013;39(3):339–48. doi: 10.1590/S1806-37132013000300011. https://doi.org/10.1590/S1806-37132013000300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubonja-Sonje M, Matovina M, Skrobonja I, Bedenic B, Abram M. Mechanisms of carbapenem resistance in multidrug-resistant clinical isolates of Pseudomonas aeruginosa from a Croatian hospital. Microb Drug Resist. 2015;21(3):261–9. doi: 10.1089/mdr.2014.0172. https://doi.org/10.1089/mdr.2014.0172. [DOI] [PubMed] [Google Scholar]
- 10.Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–14. doi: 10.1016/j.cmi.2016.01.001. https://doi.org/10.1016/j.cmi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Peretz A, Labay K, Zonis Z, Glikman D. Disengagement does not apply to bacteria:A high carriage rate of antibiotic-resistant pathogens among Syrian civilians treated in Israeli hospitals. Clin Infect Dis. 2014;59(5):753–4. doi: 10.1093/cid/ciu374. https://doi.org/10.1093/cid/ciu374. [DOI] [PubMed] [Google Scholar]
- 12.Costa Pde O, Atta EH, Silva AR. Infection with multidrug-resistant gram-negative bacteria in a pediatric oncology intensive care unit:Risk factors and outcomes. J Pediatr. 2015;91(5):435–41. doi: 10.1016/j.jped.2014.11.009. https://doi.org/10.1016/j.jped.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Institute CLS. Performance standards for antimicrobial susceptibility testing:22th informational supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2012. [Google Scholar]
- 14.Toubes E, Singh K, Yin D, Lyu R, Glick N, Russell L, et al. Risk factors for antibiotic-resistant infection and treatment outcomes among hospitalized patients transferred from long-term care facilities:Does antimicrobial choice make a difference? Clin Infect Dis. 2003;36(6):724–30. doi: 10.1086/368081. https://doi.org/10.1086/368081. [DOI] [PubMed] [Google Scholar]
- 15.Agodi A, Zarrilli R, Barchitta M, Anzaldi A, Di Popolo A, Mattaliano A, et al. Alert surveillance of intensive care unit-acquired Acinetobacter infections in a Sicilian hospital. Clin Microbiol Infect. 2006;12(3):241–7. doi: 10.1111/j.1469-0691.2005.01339.x. https://doi.org/10.1111/j.1469-0691.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- 16.Ylipalosaari P, Ala-Kokko TI, Laurila J, Ohtonen P, Syrjala H. Intensive care acquired infection is an independent risk factor for hospital mortality:A prospective cohort study. Crit Care. 2006;10(2):R66. doi: 10.1186/cc4902. https://doi.org/10.1186/cc4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aly NY, Al-Mousa HH, Al Asar el SM. Nosocomial infections in a medical-surgical intensive care unit. Med Princ Pract. 2008;17(5):373–7. doi: 10.1159/000141500. https://doi.org/10.1159/000141500. [DOI] [PubMed] [Google Scholar]
- 18.Gomila M, Del Carmen Gallegos M, Fernandez-Baca V, Pareja A, Pascual M, Diaz-Antolin P, et al. Genetic diversity of clinical Pseudomonas aeruginosa isolates in a public hospital in Spain. BMC Microbiol. 2013;13(138):1471–2180. doi: 10.1186/1471-2180-13-138. https://doi.org/10.1186/1471-2180-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werarak P, Kiratisin P, Thamlikitkul V. Hospital-acquired pneumonia and ventilator-associated pneumonia in adults at Siriraj Hospital:Etiology, clinical outcomes, and impact of antimicrobial resistance. J Med Assoc Thai. 2010;93(1):S126–38. [PubMed] [Google Scholar]
- 20.Deng Q, Li Q, Lin XM, Li YM. Epidemiology and antimicrobial resistance of clinical isolates about hospital infection from patients with hematological diseases. [Article in Chinese] Zhonghua Xue Ye Xue Za Zhi. 2012;33(12):994–9. [PubMed] [Google Scholar]
- 21.Ruscher C, Pfeifer Y, Layer F, Schaumann R, Levin K, Mielke M. Inguinal skin colonization with multidrug-resistant bacteria among residents of elderly care facilities:Frequency, persistence, molecular analysis and clinical impact. Int J Med Microbiol. 2014;304(8):1123–34. doi: 10.1016/j.ijmm.2014.08.006. https://doi.org/10.1016/j.ijmm.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 22.March A, Aschbacher R, Pagani E, Sleghel F, Soelva G, Hopkins KL, et al. Changes in colonization of residents and staff of a long-term care facility and an adjacent acute-care hospital geriatric unit by multidrug-resistant bacteria over a four-year period. Scand J Infect Dis. 2014;46(2):114–22. doi: 10.3109/00365548.2013.859392. https://doi.org/10.3109/00365548.2013.859392. [DOI] [PubMed] [Google Scholar]
- 23.Pop-Vicas A, Tacconelli E, Gravenstein S, Lu B, D'Agata EM. Influx of multidrug-resistant, gram-negative bacteria in the hospital setting and the role of elderly patients with bacterial bloodstream infection. Infect Control Hosp Epidemiol. 2009;30(4):325–31. doi: 10.1086/596608. https://doi.org/10.1086/596608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horasan ES, Ersoz G, Horoz M, Goksu M, Karacorlu S, Kaya A. Risk factors for infections caused by multidrug-resistant bacteria in patients with solid tumours. Scand J Infect Dis. 2011;43(2):107–11. doi: 10.3109/00365548.2010.534500. https://doi.org/10.3109/00365548.2010.534500. [DOI] [PubMed] [Google Scholar]
- 25.Kemp M, Holt H, Holm A, Kolmos HJ. Elderly patients are at high risk from hospital-acquired infection. [Article in Danish] Ugeskr Laeger. 2013;175(47):2874–6. [PubMed] [Google Scholar]
- 26.Marchaim D, Chopra T, Bogan C, Bheemreddy S, Sengstock D, Jagarlamudi R, et al. The burden of multidrug-resistant organisms on tertiary hospitals posed by patients with recent stays in long-term acute care facilities. Am J Infect Control. 2012;40(8):760–5. doi: 10.1016/j.ajic.2011.09.011. https://doi.org/10.1016/j.ajic.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Consales G, Gramigni E, Zamidei L, Bettocchi D, De Gaudio AR. A multidrug-resistant Acinetobacter baumannii outbreak in intensive care unit:Antimicrobial and organizational strategies. J Crit Care. 2011;26(5):453–9. doi: 10.1016/j.jcrc.2010.12.016. https://doi.org/10.1016/j.jcrc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Cornejo-Juarez P, Vilar-Compte D, Perez-Jimenez C, Namendys-Silva SA, Sandoval-Hernandez S, Volkow-Fernandez P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int J Infect Dis. 2015;31:31–4. doi: 10.1016/j.ijid.2014.12.022. https://doi.org/10.1016/j.ijid.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Otto M. Community-associated MRSA:What makes them special? Int J Med Microbiol. 2013;303(6-7):324–30. doi: 10.1016/j.ijmm.2013.02.007. https://doi.org/10.1016/ j.ijmm.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzur A, Tubau F, Pujol M, Calatayud L, Dominguez MA, Pena C, et al. Nosocomial outbreak due to extended-spectrum-beta-lactamase-producing Enterobacter cloacae in a cardiothoracic intensive care unit. J Clin Microbiol. 2007;45(8):2365–9. doi: 10.1128/JCM.02546-06. https://doi.org/10.1128/JCM.02546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


