Abstract
Triple-negative breast cancer (TNBC) is the leading cause of cancer-related death in women. Previous studies indicated that miR-361-5p was downregulated in breast cancer, however, the exact effect of miR-361-5p on TNBC requires further investigation. In the present study, we investigated whether miR-361-5p can act as a tumor suppressor by targeting required for cell differentiation 1 homolog (RQCD1) and inhibiting epidermal growth factor receptor (EGFR)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway in TNBC. The expression of miR-361-5p and RQCD1 was determined by quantitative reverse transcription PCR (qRT-PCR) and/or western blot in TNBC and the adjacent tissues. miR-361-5p mimics were constructed and transfected to TNBC cell line MDA-MB-231. Cells were divided into three groups: blank control group, miRNA mimic negative control (NC) group, and miR-361-5p mimics group. Expression of miR-361-5p, mRNA and protein expression of PI3K, Akt, EGFR, phosphorylated (p)-EGFR/PI3K/Akt, and protein expression of RQCD1 and matrix metallopeptidase 9 (MMP-9) in MDA-MB-231 were measured by qRT-PCR/western blot after transfection. Cell viability was determined by CCK-8 assay. Cell migration and invasion ability were evaluated by scratch and transwell assay, respectively. miR-361-5p target gene was determined by bioinformatics analysis and luciferase reporter assay. RQCD1 was identified as a target of miR-361-5p by TargetScan and confirmed by luciferase reporter assay. Downregulated miR-361-5p and upregulated RQCD1 were observed in TNBC tissues. Expression of EGFR, PI3K, Akt and MMP-9 was inhibited in cells treated with miR-361-5p mimics. Transfection of miR-361-5p mimics also inhibited the phosphorylation of EGFR, PI3K, and Akt. Suppressed cell viability, migration, and invasion was found in miR-361-5p mimics groups. Our results indicated that overexpression of miR-361-5p might act as a suppressor in TNBC by targeting RQCD1 to inhibit the EGFR/PI3K/Akt signaling pathway.
Keywords: miR-361-5p, RQCD1, EGFR/PI3K/Akt signaling pathway, cell migration and invasion, triple-negative breast cancer, TNBC
INTRODUCTION
Breast cancer (BC) is one of the most common cancers in women worldwide. Many factors, including primary mutations, reproductive history, lifestyle and environmental factors, have been associated with an increased risk of BC [1,2]. Familial BC accounts for a small percentage of all BC cases and occurs in patients whose family members were diagnosed with breast, ovarian, or related cancer [3]. Moreover, inherited mutations in high-risk genes, such as BRCA1, BRCA2 and TP53, are estimated to cause about 5% of all BCs [3,4].
BC is classified into several (molecular) subtypes based on the expression of classical immunohistochemistry (IHC) markers [e.g., estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2/neu)], clinicopathological tumor characteristics, and/or microarray gene expression profiling. BCs lacking expression of all three receptors (ER, PR and HER2/neu) are called triple-negative breast cancers (TNBCs); these cancers are characterized by an aggressive clinical course/outcome and currently have limited treatment options due to the absence of validated molecular targets [5].
MicroRNAs (miRNAs) are endogenous small non-coding RNAs, 22–24 nucleotides in length, important for posttranscriptional regulation of gene expression. miRNAs induce mRNA degradation or translational repression by preferentially binding to the 3’-untranslated region (UTR) of target gene [6]. Dysregulation/aberrant expression of miRNAs has been associated with cancer, where they can function as oncogenes or tumor suppressors [6]. In BC, miRNAs have been implicated in oncogenesis, metastasis and resistance to therapies [7]. For example, the members of miR-200 family regulate transcription factors zinc finger E-box-binding homeobox (ZEB)1/2, which are important for cell differentiation and tissue-specific functions. Conversely, in cancer cells including BC, ZEB1 appears to be an essential epithelial-to-mesenchymal transition (EMT) activator through the suppression of epithelial genes and miR-200 family miRNAs [8]. Another example of miRNA involvement in BC is miR-342, whose expression was positively correlated with ERα expression in human BC samples and the introduction of miR-342 into estrogen-dependent MCF-7 BC cells enhanced their sensitivity to tamoxifen-induced apoptosis. Consistently, the downregulation of miR-342 was associated with tamoxifen resistance in MCF-7 BC cells [9,10].
miR-361-5p is encoded by a gene located on chromosome X and has been implicated in several cancers, including colorectal and gastric cancer, where it was suggested to act as a tumor suppressor [11]. Ma et al. [12] investigated the role of miR-361-5p in BC, they showed a significantly decreased expression of miR-361-5p in BC compared to normal breast tissue and an association between miR-361-5p downregulation and poor prognosis of BC patients. Moreover, they confirmed both in vitro and in vivo a tumor-suppressor role of miR-361-5p in BC, by demonstrating that it could inhibit aerobic glycolysis and proliferation in BC cells through direct targeting of the promoter of glycolytic pathway, fibroblast growth factor receptor 1 (FGFR1), and suppress BC cell invasion and metastasis by targeting matrix metalloproteinase-1 (MMP-1) [12].
The epidermal growth factor receptor (EGFR)/phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt) pathway has an important role in the regulation of angiogenesis, cell apoptosis and proliferation, and its dysregulation was associated with both benign proliferative diseases and malignant tumors, as demonstrated in cholesteatoma, lung, nasopharyngeal and breast cancer, among others [13-16]. Ajiro et al. [17,18] identified required for cell differentiation 1 homolog (RQCD1), known as CCR4-NOT transcription complex subunit 9 (CNOT9), as a key component in the downstream activation from EGFR to Act in BC cells, suggesting its potential as a therapeutic target. Namely, they showed that RQCD1 is frequently upregulated in clinical BC samples and in BC cell lines, compared to normal tissue (except the testis). In a series of experiments, they further demonstrated that RQCD1 is involved in the downstream activation of Act from EGFR, most probably through its interaction with Grb10 interacting GYF protein 1 and 2 (GIGYF1/2) and growth factor receptor binding protein 10 (Grb10) [17,18]. Computational analyses identified RQCD1 as a target of miR-361-5p. In the present study, we investigated whether miR-361-5p can act as a tumor suppressor in TNBC by targeting RQCD1 and inhibiting EGFR/PI3K/Akt pathway.
MATERIALS AND METHODS
Patients
This study involved thirty patients diagnosed with TNBC by pathology. Paired breast tumor and adjacent normal tissues were obtained during surgery and stored in liquid nitrogen. All patients signed informed consent prior to study initiation. Clinicopathological data of TNBC patients are provided in Table 1. We analyzed the expression of miR-361-5p and mRNA expression of RQCD1 by quantitative reverse transcription polymerase chain reaction (qRT-PCR), and protein expression of RQCD1 by western blot in TNBC/normal tissues, as described below.
TABLE 1.
Clinicopathological data of patients with TNBC
TNBC cell line
MDA-MB-231 TNBC cell line was obtained from the BeNa Culture Collection (BNCC, China) and maintained in RPMI-1640 (Thermo Fisher Scientific, USA) supplemented with 10% fetal bovine serum (FBS, Sigma, Germany), at 37°C and with 5% CO2.
miRNA plasmids and cell transfection
MDA-MB-231 cells were seeded into 24-well plates and incubated in RPMI-1640 at 37°C with 5% CO2 for 24 hours, to reach 60–70% confluency. Cells were divided into three groups: blank control group, miRNA mimic negative control (NC) group, and miR-361-5p mimics group. miR-361-5p mimics and NC plasmids were constructed by Addgene (Watertown, USA) and transfected into cells with 1 µl Lipo6000™ Transfection Reagent (Thermo Fisher Scientific, USA). The transfection was performed at 37° with 5% CO2 for 6 hours. The transfected cells were then incubated for additional 48 hours for qRT-PCR and 72 hours for western blot. We evaluated the expression of miR-361-5p, mRNA expression of EGFR, PI3K, Akt by qRT-PCR, and protein level of EGFR, PI3K, Akt, phosphorylated (p)-EGFR/PI3K/Akt, RQCD1 and matrix metallopeptidase 9 (MMP-9) in transfected cells by western blot, as described below.
qRT-PCR
RNA was extracted from TNBC and control cells (60% confluency cultures) using total RNeasy Plant Mini Kit (Qiagen, Germany). The concentration of RNA was measured with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). Pyrobest DNA Polymerase and M-MLV Reverse Transcriptase (Thermo Fisher Scientific, USA) were used for complementary DNA (cDNA) synthesis and PCR reaction. PCR conditions for cDNA synthesis were set at 95°C for 15 minutes, and for PCR reaction at 96°C for 5 minutes (initiation), 94°C for 30 seconds (denaturation), 65°C for 30 seconds (annealing), and 75°C for 1 minute (extension) for 33 cycles. miR-361-5p expression was normalized to U6 small nuclear RNA and mRNA expression of selected proteins to the GAPDH gene. The relative quantification of RNA was performed using the 2−ΔΔCt method [19].
Western blot
RIPA lysis buffer (Thermo Fisher Scientific) was used for protein isolation from TNBC and control cells and BCA Kit (Thermo Fisher Scientific) for determination of their concentration. The protein samples (2 µl/per lane) were separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membrane (Membrane Solutions, USA), and blocked with 5% skimmed milk for 2 hours. After that, the samples were incubated with the primary antibodies anti-RQCD1, EGFR, PI3K, Akt, MMP-9, p-EGFR/PI3K/Akt, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for 1 hour at room temperature. In addition, the incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies was performed for 45 minutes at room temperature. ECL kit (Thermo Fisher Scientific) was used for the visualization and the images were analyzed by ImageJ (National Institutes of Health). Western blot images were determined semiquantitatively with QuantityOne software (Bio-Rad Laboratories, USA).
Cell Counting Kit-8 (CCK-8) assay for cell viability
The viability of MDA-MB-231 cells was assessed by CCK-8 assay (Dojindo, Japan) after transfection. Cells were divided into three groups: blank control, miRNA mimic NC, and miR-361-5p mimics group. The cells were placed in 96-well plates, and after the addition of 10 µl CCK-8 reagent they were cultured for 24 hours in RPMI-1640 at 37°C with 5% CO2. The optical density (OD) was measured at 450 nm on a microplate reader. The CCK-8 assays were carried out at 12, 24, and 48 hours of incubation.
Scratch assay
Cells were grown in 6-well plates (1×106 cells/well). A sterile 10-µL pipette tip was utilized for shape scratch. After being washed with phosphate buffered saline (PBS), cells in blank control, miRNA mimic NC, and miR-361-5p mimics group were cultured in serum-free medium. The cell migration rate were determined using a fluorescence microscope (Olympus, Japan). Each independent experiment was performed in triplicate.
Transwell assay
The invasion ability of MDA-MB-231 cells was measured by transwell assay after 6 hours of transfection. Cells were divided into blank control, miRNA mimic NC, and miR-361-5p mimics group. Transwell cell culture inserts (8-mm pore size; Falcon; BD Biosciences, USA) were placed onto 96-well plates to create separated upper and lower chambers. The upper side of the membrane was precoated with Matrigel (BD Biosciences, USA) and incubated for 1 hour at 37°C for gel formation. The membranes were hydrated with FBS 2 hours before their use. RPMI-1640 (600 µl) containing 10% FBS and 1 × 105 cells/well were added to the lower and upper chamber, respectively. After 48 hours of incubation, the number of invading cells was counted in the chambers.
Bioinformatics analysis and luciferase reporter assay
RQCD1 was predicted as a target of miR-361-5p using TargetScan (http://www.targetscan.org/vert_71/) and this was further validated by luciferase reporter assay. MDA-MB-231 cells were placed in 96-well plates and incubated in RPMI-1640 to reach 60% confluency. The co-transfection of miR-361-5p mimics (50 ng) and RQCD1-3’-untranslated region wild type [3’-UTR-WT] (50 ng), containing miR-361-5p binding site, or RQCD1-3’-UTR mutant [RQCD1-3’-UTR-MUT] (50 ng) plasmids was performed using Lipofectamine3000™ Transfection Reagent (0.2 µl) [Thermo Fisher Scientific] for 48 hours. The luciferase activity was determined using a luciferase assay kit (Thermo Fisher Scientific, USA), according to the manufacturer’s protocol, and normalized to Renilla luciferase activity.
Statistical analysis
Each experiment was performed in triplicate and data are presented as mean ± standard deviation (x ± s). Student’s t-test was used for comparison between two groups and one-way analysis of variance (ANOVA) for comparison between multiple groups. All analyses were performed with SPSS for Windows, Version 14.0. (SPSS Inc., Chicago). A value of p < 0.05 was considered statistically significant.
RESULTS
Downregulated miR-361-5p and upregulated RQCD1 in TNBC tissues
miR-361-5p and RQCD1 mRNA expression was determined by qRT-PCR, and RQCD1 protein expression was assessed by western blot in TNBC vs. normal adjacent tissues from 30 patients. As shown in Figure 1A and B, the expression of miR-361-5p was significantly downregulated in tumor tissues (n = 27/30, p < 0.01) while RQCD1 mRNA expression was upregulated (n = 28/30, p < 0.01) compared with normal tissues. The protein levels of RQCD1 were also significantly increased in tumor compared to normal tissues, as shown in Figure 1C (representative sample, p < 0.01). Overall, we observed aberrant expressions of miR-361-5p and RQCD1 in TNBC tissues.
FIGURE 1.
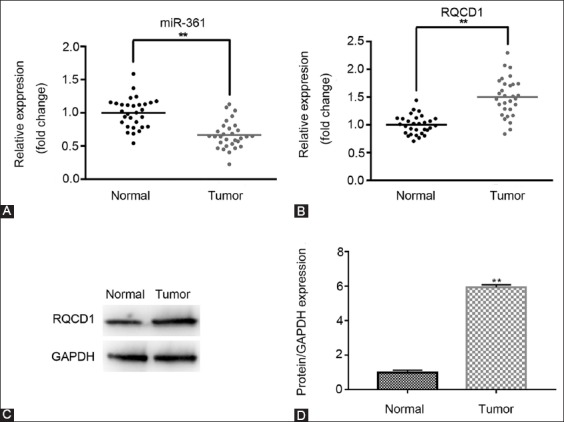
Downregulation of miR-361-5p and upregulation of RQCD1 in TNBC tissues. The expression of miR-361-5p and RQCD1 in TNBC and adjacent normal tissues was determined by qRT-PCR and western blot. (A) Relative expression of miR-361-5p in normal and tumor tissues. (B) Relative expression of RQCD1 mRNA in normal and tumor tissues. (C) Relative expression of RQCD1 protein in tissues of representative patients. **p < 0.01 vs. control group. TNBC: Triple-negative breast cancer; qRT-PCR: Quantitative reverse transcription polymerase chain reaction; RQCD1: Cell differentiation 1 homolog; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
Inhibited EGFR/PI3K/Akt signaling pathway in MDA-MB-231 cells after transfection with miR-361-5p mimics
MDA-MB-231 cells were transfected with miR-361-5p mimics/NC plasmids and after 6 hours of transfection and additional 48 and 72 hours of incubation for qRT-PCR and western blot, respectively, the mRNA and protein expression of EGFR, PI3K and Akt were assessed. As shown in Figure 2A-C, the mRNA expressions of EGFR, PI3K and Akt were significantly decreased in miR-361-5p mimics group compared to miRNA mimic NC group (p < 0.01), while there was no significant difference in the mRNA expression between NC and blank control group. Furthermore, protein expressions of RQCD1, PI3K, Akt, EGFR, p-EGFR/PI3K/Akt, and MMP-9 were markedly decreased in miR-361-5p mimics group compared to NC group, and there was no apparent difference between NC and blank control group (Figure 2D and E, p < 0.01). These results indicate that the overexpression of miR-361-5p possibly affects the EGFR/PI3K/Akt signaling pathway in TNBC by downregulating the expression of related genes.
FIGURE 2.
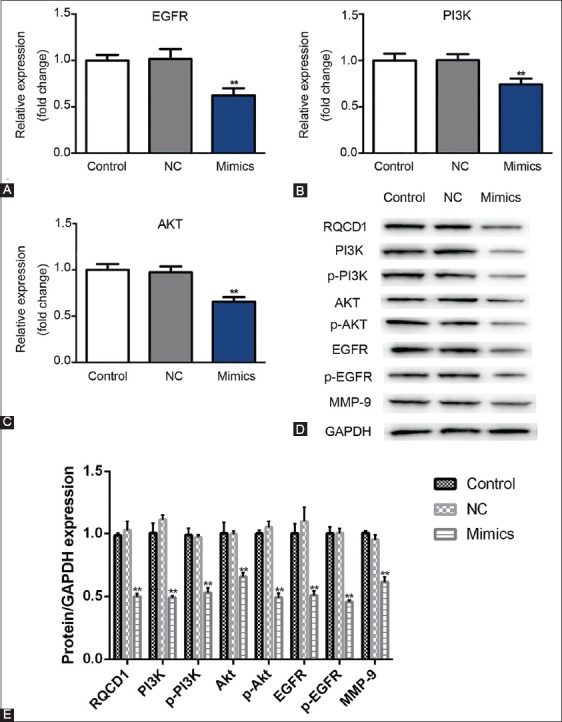
Inhibited EGFR/PI3K/Akt signaling pathway in miR-361-5p mimics-transfected MDA-MB-231 TNBC cells. After the transfection of MDA-MB-231 cells with miR-361-5p mimics/NC plasmids and 48 and 72 hours of incubation in RPMI-1640 medium for qRT-PCR and western blot, respectively, mRNA and protein expression was assessed. (A) Relative expression of EGFR mRNA. (B) Relative expression of PI3K mRNA. (C) Relative expression of Akt mRNA. (D) Western blot results for RQCD1, PI3K, p-PI3K, EGFR, p-EGFR, Akt, p-Akt, and MMP-9 protein expression. (E) Relative expression of RQCD1, PI3K, p-PI3K, EGFR, p-EGFR, Akt, p-Akt, and MMP-9 expression versus GAPDH. Control: Blank group. Negative control (NC) group: Cells treated with control plasmids. Mimics group: Cells treated with miR-361-5p mimics plasmids. **p < 0.01 vs. control group. EGFR: Epidermal growth factor receptor; PI3K: Phosphatidylinositol-4,5-bisphosphate 3-kinase; Akt: Protein kinase B; TNBC: Triple-negative breast cancer; qRT-PCR: Quantitative reverse transcription polymerase chain reaction; RQCD1: Cell differentiation 1 homolog; p: phosphorylated; MMP-9: Matrix metallopeptidase 9; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; TNBC: Triple-negative breast cancer.
Inhibited cell viability in MDA-MB-231 cells after transfection with miR-361-5p mimics
Cell proliferation ability was evaluated following 12, 24, and 48 hours of MDA-MB-231 cell transfection with miR-361-5p mimics/NC plasmids. Inhibited cell viability was observed in miR-361-5p mimics group at 24 and 48 hour incubation compared to miRNA mimic NC group. On the other hand, there was no obvious difference in cell viability between NC and blank control group (Figure 3, p < 0.01), suggesting that the overexpression of miR-361-5p had an inhibitory effect on TNBC cell proliferation.
FIGURE 3.
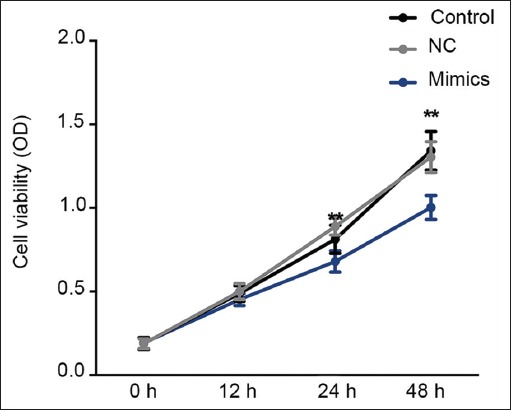
Inhibited cell viability in miR-361-5p mimics-transfected MDA-MB-231 TNBC cells. The cell viability was evaluated at 0, 12, 24, and 48 hour time point by CCK-8 assay. Inhibited cell proliferation was observed after miR-361-5p mimics transfection. Control: Blank group. Negative control (NC) group: Cells treated with control plasmids. Mimics group: Cells treated with miR-361-5p mimics plasmids **p < 0.01 vs. control group. TNBC: Triple-negative breast cancer; CCK-8: Cell Counting Kit-8 assay.
Inhibited migration and invasion of MDA-MB-231 cells after transfection with miR-361-5p mimics
The migration and invasion ability of MDA-MB-231 cells was determined by scratch and transwell assay, respectively, following 48 hours of cell transfection with miR-361-5p mimics/NC plasmids. The cell migration and invasion ability was inhibited in miR-361-5p mimics group compared to miRNA mimic NC, while there was no significant difference between NC and blank control group (Figure 4, p < 0.01). Thus, overexpression of miR-361-5p possibly inhibits migration and invasion ability of TNBC cells.
FIGURE 4.
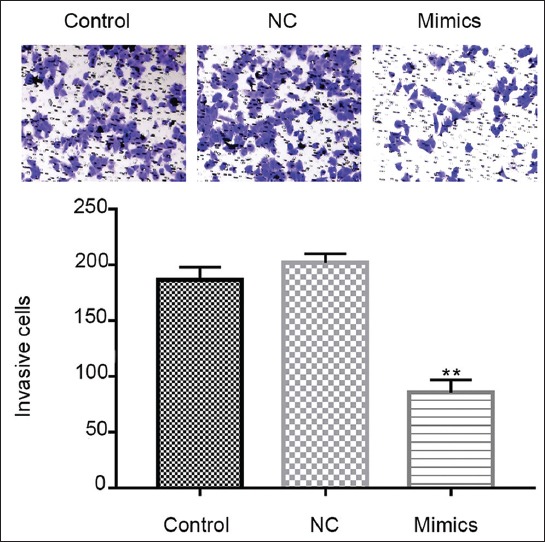
Inhibited cell migration and invasion of MDA-MB-231 after miR-361-5p mimics transfection. Transwell assay of MDA-MB-231 and the number of invasion cells. Control: Blank group. Negative control (NC) group: Cells treated with control plasmids. Mimics group: Cells treated with miR-361-5p mimics plasmids. **p < 0.01 vs. NC group.
RQCD1 was the target of miR-361-5p by luciferase reporter assay
The luciferase reporter assay was performed to validate whether the RQCD1 is a target of miR-361-5p, as predicted by TargetScan (Figure 5A). MDA-MB-231 cells were co-transfected with miR-361-5p mimics and RQCD1-3’-UTR-WT or RQCD1-3’-UTR-MUT plasmids. The lower luciferase activity observed in cells co-transfected with RQCD1-3’-UTR-WT and miR-361 mimics suggested that RQCD1 was the target of miR-361-5p (Figure 5B, p < 0.01).
FIGURE 5.
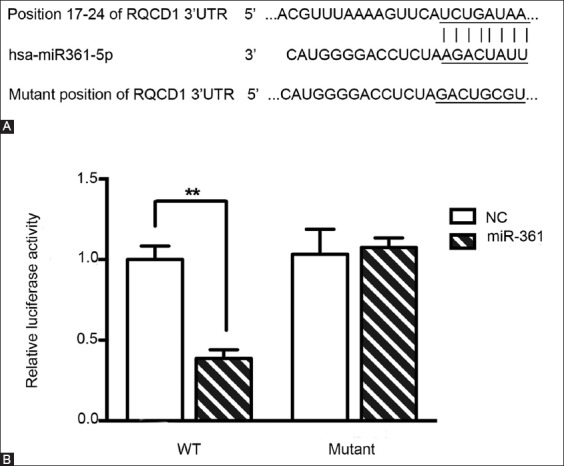
RQCD1 as a target of miR-361-5p. (A) RQCD1 was predicted to be a target of miR-361-5p by TargetScan and (B) further confirmed by luciferase reporter assay. Relative luciferase activity after co-transfection of MDA-MB-231 TNBC cells with miR-361-5p mimics and RQCD1-3’-UTR-WT or RQCD1-3’-UTR-MUT plasmids. Lower luciferase activity observed in cells co-transfected with RQCD1-3’-UTR-WT and miR-361 mimics suggested that RQCD1 was the target of miR-361-5p. Control: Blank group. Negative control (NC) group: Cells treated with control plasmids. Mimics group: Cells treated with miR-361-5p mimics plasmids **p < 0.01 versus NC group. TNBC: Triple-negative breast cancer; RQCD1: Cell differentiation 1 homolog; 3’-UTR-WT: 3’-untranslated region wild type; 3’-UTR-MUT: 3’-untranslated region mutant.
DISCUSSION
RQCD1 was demonstrated to have a key role in the downstream activation from EGFR to Act in BC cells, suggesting that it could be used as a therapeutic target [17,18]. miR-361-5p may target RQCD1 to regulate the EGFR/PI3K/Akt signaling pathway in TNBC. In this study, we first analyzed the expression levels of miR-361-5p in TNBC tissues and then investigated the effects of miR-361-5p on RQCD1 and EGFR/PI3K/Akt signaling pathway in MDA-MB-231 TNBC cells. We observed downregulation of miR-361-5p in TNBC vs. normal adjacent tissues. Similar results were shown by Ma et al. [12], who reported a significantly decreased expression of miR-361-5p in BC compared to normal breast tissue; moreover, reduced expression of miR-361-5p was associated with poor prognosis of their BC patients. However, few studies reported specific molecular targets in TNBC. Using TargetScan, we identified RQCD1 as a possible target of miR-361-5p and confirmed this result with luciferase reporter assay. Furthermore, in MDA-MB-231 cells transfected with miR-361-5p mimics we showed that mRNA expression of EGFR, PI3K and Akt and protein expression of RQCD1, PI3K, Akt, EGFR and MMP-9 were significantly decreased compared to control cells, suggesting that overexpression of miR-361-5p leads to inhibition of the EGFR/PI3K/Akt signaling pathway in TNBC cells. Finally, we observed decreased proliferation, migration, and invasion in MDA-MB-231 cells transfected with miR-361-5p mimics, which indicated a tumor suppressor role of miR-361-5p in BC. Ma et al. [12] also confirmed, in vitro and in vivo, a tumor-suppressor role of miR-361-5p in BC; i.e, miR-361-5p inhibited aerobic glycolysis and proliferation in BC cells by targeting FGFR1 and suppressed BC cell invasion and metastasis by downregulating MMP-1 [12].
BC remains a significant public health problem, even though considerable efforts have been made over the past decade to improve prevention and treatment; the therapeutic strategies are especially limited in the case of aggressive BC types such as TNBC. This may be due to the lack of therapeutic targets, and genomic/proteomic heterogeneity between TNBC molecular subtypes [20]. In most countries the incidence of BC is increasing, probably due to an increase in the number of women with familial/lifestyle risk factors for BC such as lower age of menarche, late first pregnancy, shorter periods of breastfeeding or not breastfeeding, late-onset menopause, obesity, alcohol consumption, and physical inactivity among other factors [21].
Aberrant expression of miRNAs is commonly observed in BC and other cancers, and they contribute to tumor development acting as either oncogenes or tumor suppressors [22]. For example in BC, miR-21, miR-10b, miR-125 and miR-155 were indicated to have an oncogenic role, while miR-206, miR-31, miR-146 and miR-91 were suggested to act as tumor suppressors [22]. Numerous studies demonstrated that miR-361-5p functions as a tumor-suppressor by targeting different genes in different cancer types, for example, in BC it inhibited cell proliferation and invasion by targeting FGFR1 and MMP-1 [12], while in non-small-cell lung carcinoma (NSCLC) miR-361-5p suppressed cancer progression by inhibiting signal transducer and activator of transcription 6 (STAT6) [23]. Because an individual miRNA can target multiple genes and mRNA of a single gene can be targeted by different miRNAs [24], it is necessary to consider the interaction between miRNA and multiple genes/molecular pathways when investigating a possible function of a single miRNA.
Our study has several limitations. Although we were able to show that the overexpression of miR-361-5p in TNBC cells inhibited the proliferation, migration and invasion ability, we did not investigate changes in molecular factors involved in these processes, such as alterations in the levels of proliferation-related factors. Moreover, the inhibition of EGFR/PI3K/Akt signaling pathway is associated with cell apoptosis [25,26]; thus, the assessment of miR-361-5p effect on cell apoptosis in TNBC using apoptosis assays could provide additional insights into the underlying mechanisms of tumor-suppressive role of miR-361-5p.
CONCLUSION
We showed that miR-361-5p is downregulated in TNBC tissues. We further demonstrated that the overexpression of miR-361-5p inhibits proliferation, migration and invasion in TNBC cells, probably by downregulating RQCD1 and inhibiting the EGFR/PI3K/Akt signaling pathway. Our results confirm a tumor-suppressive role of miR-361-5p in TNBC, suggesting its use as a therapeutic target.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Bodai BI, Tuso P. Breast cancer survivorship:A comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19(1):48–97. doi: 10.7812/TPP/14-241. https://doi.org/10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray JM, Rasanayagam S, Engel C, Rizzo J. State of the evidence 2017:An update on the connection between breast cancer and the environment. Environ Health. 2017;16(1):94. doi: 10.1186/s12940-017-0287-4. https://doi.org/10.1186/s12940-017-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans DG, Graham J, O'Connell S, Arnold S, Fitzsimmons D. Familial breast cancer:Summary of updated NICE guidance. BMJ. 2013;346:f3829. doi: 10.1136/bmj.f3829. https://doi.org/10.1136/bmj.f3829. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Ung MH, Cantor S, Cheng C. Computational investigation of homologous recombination DNA repair deficiency in sporadic breast cancer. Sci Rep. 2017;7(1):15742. doi: 10.1038/s41598-017-16138-2. https://doi.org/10.1038/s41598-017-16138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hon JD, Singh B, Sahin A, Du G, Wang J, Wang VY, et al. Breast cancer molecular subtypes:From TNBC to QNBC. Am J Cancer Res. 2016;6(9):1864–72. [PMC free article] [PubMed] [Google Scholar]
- 6.Lan H, Lu H, Wang X, Jin H. MicroRNAs as potential biomarkers in cancer:Opportunities and challenges. Biomed Res Int. 2015;2015:125094. doi: 10.1155/2015/125094. https://doi.org/10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Schooneveld E, Wildiers H, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015;17:21. doi: 10.1186/s13058-015-0526-y. https://doi.org/10.1186/s13058-015-0526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang J, Ahmad A, Sarkar FH. The role of microRNAs in breast cancer migration, invasion and metastasis. Int J Mol Sci. 2012;13(10):13414–37. doi: 10.3390/ijms131013414. https://doi.org/10.3390/ijms131013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He YJ, Wu JZ, Ji MH, Ma T, Qiao EQ, MA R, et al. miR-342 is associated with estrogen receptor-αexpression and response to tamoxifen in breast cancer. Exp Ther Med. 2013;5(3):813–8. doi: 10.3892/etm.2013.915. https://doi.org/10.3892/etm.2013.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young J, Kawaguchi T, Yan L, Qi Q, Liu S, Takabe K. Tamoxifen sensitivity-related microRNA-342 is a useful biomarker for breast cancer survival. Oncotarget. 2017;8(59):99978–89. doi: 10.18632/oncotarget.21577. https://doi.org/10.18632/oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma F, Song H, Guo B, Zhang Y, Zheng Y, Lin C, et al. MiR-361-5p inhibits colorectal and gastric cancer growth and metastasis by targeting staphylococcal nuclease domain containing-1. Oncotarget. 2015;6(10):17404–16. doi: 10.18632/oncotarget.3744. https://doi.org/10.18632/oncotarget.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma F, Zhang L, Ma L, Zhang Y, Zhang J, Guo B, et al. MiR-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J Exp Clin Cancer Res. 2017;36(1):158. doi: 10.1186/s13046-017-0630-1. https://doi.org/10.1186/s13046-017-0630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Ren H, Ren J, Yin T, Hu B, Xie S, et al. The role of EGFR/PI3K/Akt/cyclinD1 signaling pathway in acquired middle ear cholesteatoma. Mediators Inflamm. 2013;2013:651207. doi: 10.1155/2013/651207. https://doi.org/10.1155/2013/651207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Su W, Zhang S, Hu Y, Liu J, Zhang X, et al. Epidermal growth factor receptor and AKT1 gene copy numbers by multi-gene fluorescence in situ hybridization impact on prognosis in breast cancer. Cancer Sci. 2015;106(15):642–9. doi: 10.1111/cas.12637. https://doi.org/10.1111/cas.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XL, Zhang XT, Meng J, Zhang HF, Zhao Y, Li C, et al. ING5 knockdown enhances migration and invasion of lung cancer cells by inducing EMT via EGFR/PI3K/Akt and IL-6/STAT3 signaling pathways. Oncotarget. 2017;8(33):54265–76. doi: 10.18632/oncotarget.17346. https://doi.org/10.18632/oncotarget.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Zhang G, Miao XB, Deng XB, Wu Y, Liu Y, et al. Cancer stem-like cell properties are regulated by EGFR/AKT/β-catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma. FEBS J. 2013;280(9):2027–41. doi: 10.1111/febs.12226. https://doi.org/10.1111/febs.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajiro M, Nishidate T, Katagiri T, Nakamura Y. Critical involvement of RQCD1 in the EGFR-akt pathway in mammary carcinogenesis. Int J Oncol. 2010;37(5):1085–93. doi: 10.3892/ijo_00000760. [DOI] [PubMed] [Google Scholar]
- 18.Ajiro M, Katagiri T, Ueda K, Nakagawa H, Fukukawa C, Lin ML, et al. Involvement of RQCD1 overexpression, a novel cancer-testis antigen, in the Akt pathway in breast cancer cells. Int J Oncol. 2009;35(4):673–81. [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. https://doi.org/10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Kong P, Chen L, Yu M, Tao J, Liu J, Wang Y, et al. miR-3178 inhibits cell proliferation and metastasis by targeting Notch1 in triple-negative breast cancer. Cell Death Dis. 2018;9(11):1059. doi: 10.1038/s41419-018-1091-y. https://doi.org/10.1038/s41419-018-1091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howell A, Anderson AS, Clarke RB, Duffy SW, Evans DG, Garcia-Closas M, et al. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014;16(5):446. doi: 10.1186/s13058-014-0446-2. https://doi.org/10.1186/s13058-014-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vimalraj S, Miranda PJ, Ramyakrishna B, Selvamurugan N. Regulation of breast cancer and bone metastasis by microRNAs. Disease Mark. 2013;35(5):369–87. doi: 10.1155/2013/451248. https://doi.org/10.1155/2013/451248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Bao C, Kong R, Xing X, Zhang Y, Li S, et al. MicroRNA-361 5p suppresses cancer progression by targeting signal transducer and activator of transcription 6 in non-small cell lung cancer. Mol Med Rep. 2015;12(5):7367–73. doi: 10.3892/mmr.2015.4411. https://doi.org/10.3892/mmr.2015.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granados López AJ, López JA. ultistep model of cervical cancer:Participation of miRNAs and coding genes. Int J Mol Sci. 2014;15(9):15700–33. doi: 10.3390/ijms150915700. https://doi.org/10.3390/ijms150915700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Jiang H, Wang L, Chen X, Wu K, Zhang S, et al. Increased MIR31HG lncRNA expression increases gefitinib resistance in non-small cell lung cancer cell lines through the EGFR/PI3K/AKT signaling pathway. Oncol Lett. 2017;13(5):3494–500. doi: 10.3892/ol.2017.5878. https://doi.org/10.3892/ol.2017.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Xia D, Luo JD, Wang P. Exogenous p27KIP1 expression induces anti-tumour effects and inhibits the EGFR/PI3K/Akt signalling pathway in PC3 cells. Asian J Androl. 2009;11(6):669–77. doi: 10.1038/aja.2009.51. https://doi.org/10.1038/aja.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]


