Abstract
Postoperative delirium (POD) is a common complication associated with increased resource utilization, morbidity and mortality. Our institution screens all postsurgical patients for postoperative delirium. The study aim was to perform an automated interrogation of the electronic health records to estimate the incidence of and identify associated risk factors for POD following total joint arthroplasty (TJA). Adult patients who underwent TJA with a multimodal analgesia protocol, including peripheral nerve blockade, from 2008 through 2012, underwent automated chart review. POD was identified by routine nursing assessment and administrative billing codes. Of 11,970 patients, 181 (1.5%) were identified to have POD. Older age (odds ratio, 95% CI 2.20, 1.80–2.71 per decade, p < 0.001), dementia (7.44, 3.54–14.60, p < 0.001), diabetes mellitus (1.70, 1.1.5–2.47, p = 0.009), renal disease (1.68, 1.03–2.65, p = 0.039), blood transfusions (2.04, 1.14–3.52, p = 0.017), and sedation during anesthesia recovery (1.76, 1.23–2.51, p = 0.002) were associated with POD. Anesthetic management was not associated with POD risk. Patients who developed POD required greater healthcare resources. Dementia is strongly associated with POD. The association between POD and transfusions may reflect higher acuity patients or detrimental effect of blood. Postoperative sedation should be recognized as a warning sign of increased risk.
Keywords: Delirium, surgery, outcomes, total joint arthroplasty
INTRODUCTION
Postoperative delirium (POD) is a common complication associated with increased resource utilization, morbidity, and mortality [1]. Unfortunately, POD is underdiagnosed during routine care [2], which places limitations on retrospective studies reliant on administrative billing codes [3]. In addition, episodes of delirium that occur during a period of hospitalization for the treatment of any medical disorder are underreported, even when specifically diagnosed [4]. Reported rates of POD effecting elective total joint arthroplasty (TJA) vary widely [5] which impedes efforts to investigate the true rates and risk factors associated with this complication. Routine structured assessments of hospitalized patients with diagnostic tools such as the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) increase the recognition of delirium [6,7] and have been a useful method for identifying POD cases in patients in the ICU [3]. Our institution has a robust electronic health record (EHR) system that allow for detailed automated data abstraction of large patient cohorts [8]. The aim of this study is to interrogate the EHR of a previously described cohort [8] of 11,970 patients who underwent elective TJA to determine the incidence of POD as detected by routine nursing assessment supplemented by administrative billing codes. A secondary aim is to assess patient and procedural characteristics for potential associations with POD.
MATERIALS AND METHODS
This study was approved by the Mayo Clinic Institutional Review Board and, consistent with Minnesota Statute 144.295, only patients who provided authorization for research use of their medical records were included.
Study design
This study is a retrospective analysis of a previously described cohort [8] of adult patients from a single institution from January 1, 2008 to December 31, 2012, who underwent elective total knee or hip arthroplasty under neuraxial (spinal or epidural) or general anesthesia supplemented by a peripheral nerve block and multimodal analgesia. The EHR of these patients was automatically interrogated to identify episodes of POD by searching for positive records of CAM-ICU score, clinical documentation in the medical record, and billing (ICD-9) codes.
The multimodal analgesic protocol used at our institution has been previously described [9]. The protocol consists of preoperative analgesic medications and continuous peripheral nerve blockade. Preoperative analgesic medications (single doses that were not repeated postoperatively) included a combination of 400 mg celecoxib, gabapentin on an age-based dosing regimen (age: 18–59 years, 600 mg; 60–69 years, 300 mg; and ≥70 years, none), and sustained-release oxycodone on an age-based dosing regimen (age: 18–59 years, 20 mg; 60–74 years, 10 mg; and ≥75 years, none). The choice of neuraxial or general anesthesia was based on the preference of the patient and anesthesiologist.
Variables assessed
Medical, surgical, and anesthesia records were abstracted using previously described automated electronic search strategy of the EHR [10]. We obtained patient demographic variables and comorbidities that are components of the Charlson Comorbidity Index, that could have an association with POD [11]; perioperative factors including type of surgery, surgical duration, blood transfusions, type of anesthesia, perioperative analgesic administration; and the clinical course in the postanesthesia care unit (PACU) including episodes of nursing-diagnosed respiratory depression (episodes of apnea, hypopnea, desaturation, or “pain-sedation” mismatch – episodes of severe pain in the setting of moderate to deep sedation, see Gali et al. [12] for definitions) and lowest Richmond Agitation-Sedation Scale (RASS) score [13]. Furthermore, we recorded whether patients required ICU admission, hospital length of stays, and discharge disposition (discharge to home or care facility, or death).
Delirium assessment
The presence of POD was detected using the CAM-ICU or International Classification of Diseases-9-Clinical Modification (ICD-9-CM) coding. In our practice, CAM-ICU has been applied to all hospitalized surgical patients upon admission, every nursing shift, and if there were interim mental status changes. Administrative databases were reviewed to identify ICD-9 codes for delirium (780.09, 293.0, 292.81, 290.11, 290.3, 291.0, 291.1, R41.0, and F05).
Statistical analysis
The primary endpoint was a binary variable indicating POD. Univariate analyses and multivariable logistic regression analysis were performed to assess for patient and perioperative variables and the development of POD. To assess if isoflurane, the most fat-soluble volatile anesthetic used in our practice, was associated with POD, a secondary analysis limited to the subset of patients receiving general anesthesia was performed. In all cases, two-tailed p < 0.05 was considered statistically significant. Data were analyzed with JMP version 10.0 software (SAS Institute Inc., Cary, NC).
RESULTS
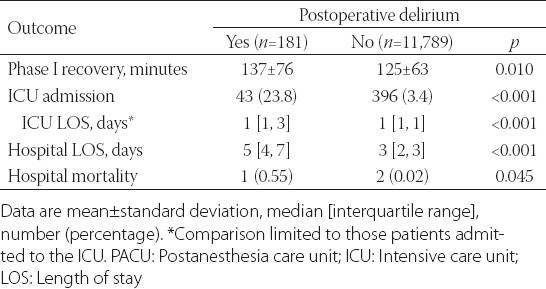
Out of 11,970 patients, 181 (1.5%, 15 per 1,000 cases; 95% confidence interval [95% CI], 13–18) developed POD. Seventy-seven cases were identified with ICD-9 codes and the remainder with CAM-ICU assessments. Patient and perioperative characteristics and their association with POD are summarized in Table 1. Multimodal analgesic components gabapentin and sustained-release oxycodone, administration of midazolam or ketamine, and total perioperative intravenous opioid dose were not associated with POD risk. Even though there were higher rates of POD in patients undergoing general anesthesia, an association was not found following multivariable analysis (Table 1). Isoflurane was used in 4,918 (74.1%) of the general anesthetic cases. Of the 123 POD cases which occurred after general anesthesia, isoflurane was used in 94 (76.4%) cases. Multivariable analysis limited to the general anesthetic cases did not find an association between isoflurane and POD, odds ratio 1.18, 95% CI 0.75, 1.91, p = 0.480. The postoperative course is summarized in Table 2 with patients who developed POD having higher rates of ICU admissions, and longer PACU, ICU, and hospital lengths of stay.
TABLE 1.
Patient and perioperative characteristics and their association with postoperative delirium
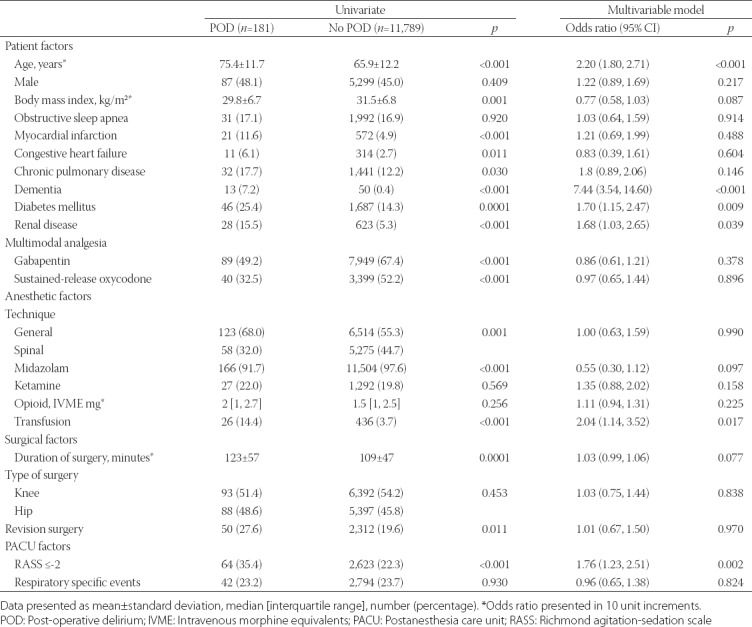
TABLE 2.
Length of Phase I recovery and hospital course in patients who experienced delirium versus not following elective total joint arthroplasty
DISCUSSION
In this study, we were able to employ an automated search strategy to identify cases of POD in a large surgical cohort undergoing elective TJA surgery. Using this method, we were able to calculate an incidence of 15 cases of POD per 1,000 surgeries. A systematic review of four studies with 738 subjects showed a range of reported POD from 0% to 10% of cases [14]. The study that reported a rate of 10% was a prospective study by Jankowski et al. [15], which originated from our institution. There are several methodological differences between these studies which explain the discrepancy in rates. First, the timeframe (2003–2005) of the previous study predated the introduction of an opioid-sparing multimodal analgesic pathway into our practice, which included peripheral nerve blocks [9]. Some evidence suggests that peripheral nerve blocks may decrease POD with orthopedic procedures [16]; on the other hand, higher doses of opioids have been associated with POD following cardiac surgery [17]. Furthermore, our institution has adopted other strategies to reduce the rate of POD [18], which could also have yielded a lower recorded rate. Unfortunately, the opioid administered in the Jankowski study [15] was not reported but, in our cohort, intraoperative opioid administration was low (Table 1). Another important difference is that the Jankowski study included only patients 65 years or older while the median age in the current cohort is 68, and advanced age was found to be an important risk factor for POD. A post hoc analysis of our data found the rate of POD in the subset of patients 65 years or older to be 23 (95% CI 20–27) cases per 1,000 surgeries. Finally, cases of POD in the Jankowski study were identified prospectively, while our relied on retrospective analysis. The routine clinical application of CAM-ICU does increase the number of recognized POD [6,7,19]. However, while CAM-ICU as a standardized approach for the assessment of delirium has high sensitivity and specificity when used in the ICU setting, a more recent validation of CAM-ICU in non-critically ill hospitalized patients reported high specificity, but lower sensitivity [20]. The lower sensitivity may result in missing true positive events, especially mild cases [20], and therefore may in part explain our low rate of POD.
In addition to age, we found associations between POD and diabetes mellitus, kidney disease, and dementia - which had the strongest association and is consistent with other studies [21-23]. Anesthetic management variables were not associated with increased rates of POD in multivariable analysis. Interestingly, univariate analysis suggested that patients at higher risk of POD were less likely to be administered midazolam, sustained-release oxycodone, and gabapentin. This may reflect the age-based dosing regimen of these drugs, where by protocol older, higher-risk, patients are less likely to be administered these medications [9,8]. In addition, choice of anesthetic (general versus neuraxial) was not associated with POD in multivariable analysis, a finding consistent with the literature [24]. Blood transfusions were found to be associated with POD. However, the literature regarding perioperative blood transfusions and POD is mixed [25], and our study design cannot determine if transfusions are deleterious in this regard (e.g. pro-inflammatory effect) or if patients at higher risk for POD are also at higher risk for needing blood transfusions. Finally, we observed that higher levels of sedation in the PACU were associated with subsequent development of POD. Not surprisingly, we observed that patients who developed POD required greater resources during their hospitalization; however, it is unclear if the higher rates of ICU admissions among patients who developed POD were contributory to the development of this complication.
Our study has all inherited limitations of retrospective design. The routine clinical use of the CAM as a means to ascertain delirium in our practice has not been validated by research criteria, and the overall incidence of detected delirium obtained appears to be lower than that reported from prospective investigations in earlier time periods. However, any underreporting of delirium, if present across the entire cohort, should not bias our conclusions regarding individual risk factors for delirium.
In conclusion, an automated interrogation of the EHR of a large cohort of patients undergoing elective TJA surgery was able to identify cases of POD; however, this rate was lower compared to previous prospective trials conducted at our institution. Nevertheless, important risk factors for POD were able to be identified, namely older age, dementia, blood transfusions, and increased sedation during anesthetic recovery. Recognition of these factors could identify higher risk patients and help direct anti-POD measures appropriately.
ACKNOWLEDGMENTS
Internal funding from the Mayo Clinic Department of Anesthesiology and Perioperative Medicine.
DECLARATION OF INTERESTS
Dr. Weingarten currently serves as a consultant to Medtronic in the role as chairman of the Clinical Endpoint Committee for the Prodigy Trial. Dr. Weingarten has received unrestricted investigator-initiated grants from Merck for research unrelated to current study. Other authors declare no conflict of interests.
REFERENCES
- 1.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–62. doi: 10.1001/jama.291.14.1753. https://doi.org/10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Milisen K, Foreman MD, Wouters B, Driesen R, Godderis J, Abraham IL, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23–9. doi: 10.3928/0098-9134-20021101-07. https://doi.org/10.3928/0098-9134-20021101-07. [DOI] [PubMed] [Google Scholar]
- 3.Katznelson R, Djaiani G, Tait G, Wasowicz M, Sutherland AM, Styra R, et al. Hospital administrative database underestimates delirium rate after cardiac surgery. Can J Anaesth. 2010;57(10):898–902. doi: 10.1007/s12630-010-9355-8. https://doi.org/10.1007/s12630-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 4.van Zyl LT, Davidson PR. Delirium in hospital:An underreported event at discharge. Can J Psychiatry. 2003;48(8):555–60. doi: 10.1177/070674370304800807. https://doi.org/10.1177/070674370304800807. [DOI] [PubMed] [Google Scholar]
- 5.Scott JE, Mathias JL, Kneebone AC. Incidence of delirium following total joint replacement in older adults:A meta-analysis. Gen Hosp Psychiatry. 2015;37(3):223–9. doi: 10.1016/j.genhosppsych.2015.02.004. https://doi.org/10.1016/j.genhosppsych.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Bigatello LM, Amirfarzan H, Haghighi AK, Newhouse B, Del Rio JM, Allen K, et al. Effects of routine monitoring of delirium in a surgical/trauma intensive care unit. J Trauma Acute Care Surg. 2013;74(3):876–83. doi: 10.1097/TA.0b013e31827e1b69. https://doi.org/10.1097/TA.0b013e31827e1b69. [DOI] [PubMed] [Google Scholar]
- 7.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients:Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–9. doi: 10.1097/00003246-200107000-00012. https://doi.org/10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Weingarten TN, Jacob AK, Njathi CW, Wilson GA, Sprung J. Multimodal analgesic protocol and postanesthesia respiratory depression during phase I recovery after total joint arthroplasty. Reg Anesth Pain Med. 2015;40(4):330–6. doi: 10.1097/AAP.0000000000000257. https://doi.org/10.1097/AAP.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 9.Hebl JR, Dilger JA, Byer DE, Kopp SL, Stevens SR, Pagnano MW, et al. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33(6):510–7. https://doi.org/10.1016/j.rapm.2008.04.008; https://doi.org/10.1097/00115550-200811000-00002. [PubMed] [Google Scholar]
- 10.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart:A non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28(11):42–44-5. [PubMed] [Google Scholar]
- 11.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. https://doi.org/10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Gali B, Whalen FX, Schroeder DR, Gay PC, Plevak DJ. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110(4):869–77. doi: 10.1097/ALN.0b013e31819b5d70. https://doi.org/10.1097/ALN.0b013e31819b5d70. [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation scale:Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44. doi: 10.1164/rccm.2107138. https://doi.org/10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 14.Bin Abd Razak HR, Yung WY. Postoperative delirium in patients undergoing total joint arthroplasty:A Systematic review. J Arthroplasty. 2015;30(8):1414–7. doi: 10.1016/j.arth.2015.03.012. https://doi.org/10.1016/j.arth.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Jankowski CJ, Trenerry MR, Cook DJ, Buenvenida SL, Stevens SR, Schroeder DR, et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesth Analg. 2011;112(5):1186–93. doi: 10.1213/ANE.0b013e318211501b. https://doi.org/10.1213/ANE.0b013e318211501b. [DOI] [PubMed] [Google Scholar]
- 16.Kinjo S, Lim E, Sands LP, Bozic KJ, Leung JM. Does using a femoral nerve block for total knee replacement decrease postoperative delirium? BMC Anesthesiol. 2012;12:4. doi: 10.1186/1471-2253-12-4. https://doi.org/10.1186/1471-2253-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkhart CS, Dell-Kuster S, Gamberini M, Moeckli A, Grapow M, Filipovic M, et al. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2010;24(4):555–9. doi: 10.1053/j.jvca.2010.01.003. https://doi.org/10.1053/j.jvca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. https://doi.org/10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients:Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703. https://doi.org/10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 20.Neufeld KJ, Hayat MJ, Coughlin JM, Huberman AL, Leistikow NA, Krumm SK, et al. Evaluation of two intensive care delirium screening tools for non-critically ill hospitalized patients. Psychosomatics. 2011;52(2):133–40. doi: 10.1016/j.psym.2010.12.018. https://doi.org/10.1016/j.psym.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 21.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit:Occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591–8. doi: 10.1034/j.1600-0579.2003.00201.x. https://doi.org/10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly:Risk factors and outcomes. Ann Surg. 2009;249(1):173–8. doi: 10.1097/SLA.0b013e31818e4776. https://doi.org/10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 23.Visser L, Prent A, van der Laan MJ, van Leeuwen BL, Izaks GJ, Zeebregts CJ, et al. Predicting postoperative delirium after vascular surgical procedures. J Vasc Surg. 2015;62(1):183–9. doi: 10.1016/j.jvs.2015.01.041. https://doi.org/10.1016/j.jvs.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium:A systematic review with meta-analysis. J Alzheimers Dis. 2010;22(Suppl3):67–79. doi: 10.3233/JAD-2010-101086. https://doi.org/10.3233/JAD-2010-101086. [DOI] [PubMed] [Google Scholar]
- 25.van der Zanden V, Beishuizen SJ, Swart LM, de Rooij SE, van Munster BC. The effect of treatment of anemia with blood transfusion on delirium:A systematic review. J Am Geriatr Soc. 2017;65(4):728–37. doi: 10.1111/jgs.14564. https://doi.org/10.1111/jgs.14564. [DOI] [PubMed] [Google Scholar]


