Abstract
This is a phase I study of 7-hydroxystaurosporine (UCN-01) and fludararbine monophosphate (FAMP) in relapsed lymphoma. UCN-01 alone was administered in cycle 1 and with FAMP in cycles 2–6. FAMP was escalated in cohorts from 1 to 5 days. UCN–01 and FAMP pharmacokinetics and apoptosis of malignant lymphocytes was evaluated. Eighteen patients were enrolled. Standard FAMP with UCN-01 was tolerated without dose-limiting toxicity (DLT) and those seen were common to either agent alone. One patient died due to Stevens-Johnson syndrome Seven of 18 patients responded. No pharmacological effect of UCN-01 by FAMP was noted. Lymphocytosis occurred in 15 of 18 patients following UCN-01 to paradoxically increase circulating tumor cells. UCN-01 induced apoptosis in six of eight patients with chronic lymphocytic leukemia (CLL). UCN-01 does not increase FAMP toxicity. Transient lymphocytosis followed by apoptosis occurs with UCN-01. Mobilization from tissue reservoirs may play a role in the induction of cell death in malignant lymphocytes.
Keywords: Cell death, CLL, flow cytometry, protein kinase inhibitor
Introduction
We are interested in identifying agent(s) that may decrease the threshold for apoptosis induced by cytotoxic agents. The novel staurosporine analog, 7-hydroxystaurosporine (UCN- 01: NSC 638850), appears to increase cytotoxic-induced apoptosis, and thus may decrease the threshold for apoptosis [1–3]. UCN-01 is a non-specific protein kinase inhibitor that regulates signal transduction, and is synergistic in vitro with a number of chemotherapy agents [4]. Thus, UCN-01 may augment the activity of chemotherapy in drug-resistant patients. UCN-01 as a protein kinase inhibitor shows some selectivity for protein kinase C and checkpoint regulation of cyclin-dependent kinases and 3-phosphoinositide-dependent protein kinase-1 (PDK1) [4–7]. In vitro, UCN-01 down-regulates anti-apoptosis proteins and induces apoptosis in chronic lymphocytic leukemia (CLL) lymphocytes [6,7]. UCN-01 also increases chemotherapy-induced apoptosis and is synergistic in vitro with fludarabine monophosphate (FAMP) in human tumor cell lines [8]. This formed the basis of the rationale for this study. To begin to assess this in patients, we wished to ascertain the tolerance of UCN-01 and FAMP in patients with relapsed indolent lymphoid malignantcies. One objective for this study was to look for evidence of in vivo apoptosis associated with UCN-01 alone and with FAMP in CLL and other lymphomas. As a phase I study, we also wished to determine the safety and tolerability of fludarabine with a fixed dose of UCN-01. Other objectives included the determination of UCN-01 pharmacokinetics when combined with FAMP and the assessment of anti-tumor activity.
Materials and methods
Patient cohort
Twenty patients were screened and 18 adult patients were eligible and enrolled for this study. Of the two patients excluded, one was found to have lung cancer and the other unacceptable thrombocytopenia. Their age, sex, and diagnosis are shown in Table I. Eligibility required confirmation of the presence of a relapsed lymphoma or CLL, adequate organ function as evidenced by an absolute neutrophil count (ANC) > 1000 per μL and a platelet count of > 50 000 per μL, the need for systemic treatment, and informed consent. Nine patients had a diagnosis of CLL/small lymphocytic lymphoma (SLL), two had mantle cell lymphoma (MCL), six had follicular lymphoma (FL), and one had lymphoplasmacytic lymphoma (LPL). All diagnoses were confirmed in the Laboratory of Pathology, Clinical Center, National Institutes of Health. The clinical protocol was approved by the National Cancer Institute Insitutional Review Board and all patients signed informed consent.
Table I.
Patient demographics: histology, age, sex, and treatment.
| Patient | Histology | Age | Sex | Cycles UCN/Flu |
Days Flu |
Response to UCN/Flu |
Prior therapy and response |
|---|---|---|---|---|---|---|---|
| 1 | CLL | 49 | M | 4 | 1 | SD | Fludarabine PR, chlorambucil PD |
| 2 | CLL | 59 | M | 2 | 2 | PD | Fludarabine PR, CVP PR, CVP SD, 506U78 PD |
| 3 | Lymphoplasmacytic lymphoma | 55 | M | 3 | 3/4 | PD | 2CDA?, rituximab PD, mitoxantrone/fludarabine PD; rituximab PD, Dex/Adria/Vcr PD |
| 4 | Follicular lymphoma | 48 | M | 7 | 5 | PR | EPOCH PR |
| 5 | CLL | 51 | M | 3 | 5 | PR | Pred/Vcr/Mitox/Cytox SD, carboplatin SD; Dex/Vcr/ Bleo/Etop/Ara-C/carboplatin SD; fludarabine X2 SD, BL22? |
| 6 | CLL | 59 | M | 3 | 5 | SD | Fludarabine X 7 PR, rituximab NE, fludarabine PR |
| 7 | Follicular lymphoma | 66 | F | 4 | 5 | PR | ProMACE/CytaBOM PR; Dex/mitoxantrone/ fludarabine PR; rituximab NE, ESHAP PD; EPOCH?, methotrexate/Etop/Ifos SD |
| 8 | Follicular lymphoma | 75 | M | 2 | 5 | PR | CHOP CR, fludarabine PR, rituximab PR |
| 9 | Transformed follicular | 66 | F | 1 | 0 | PD | CVP PD, CHOP X 2?, CVP/rituximab PD, XRT |
| 10 | CLL | 48 | M | 1 | 0 | SD | Fludarabine PD, CVP?, rituximab PD |
| 11 | Follicular lymphoma | 54 | M | 3 | 5 | PR | ProMACE/CytaBOM PR |
| 12 | Mantle cell lymphoma | 57 | F | 1 | 0 | PD | EPOCH-rituximab/vaccine CR |
| 13 | Follicular lymphoma | 43 | M | 3 | 5 | SD | ProMACE PR; idiotype vaccine PD, CVP PD; rituximab X2 PR, rituximab PD, fludarabine PR |
| 14 | SLL | 58 | M | 1 | 0 | PR | CHOP/Rituxan PR, mitoxantrone/fludarabine?; BEAM?, rituximab PR, dexamethasone SD; mitoxantrone/fludarabine SD; rituximab/ apolizumab CR, ABMT PR |
| 15 | CLL | 57 | M | 2 | 5 | SD | Prednisone/fludarabine CR, rituximab PR, CVP SD |
| 16 | CLL | 51 | M | 7 | 5 | SD | Fludarabine/Cytoxan CR, Campath SD, CHOP MR; rituximab X 2 SD, Cytoxan/fludarabine SD, rituximab SD; Cytoxan/fludarabine/rituximab SD, rituximab SD |
| 17 | CLL | 70 | F | 6 | 5 | CR | Fludarabine PR, dexamethasone SD; Cytoxan/ fludarabine/rituximab?, chlorambucil SD |
| 18 | Mantle cell lymphoma | 55 | M | 3 | 5 | SD | CHOP PR, ICE SD, XRT CR |
CLL, chronic lymphocytic leukemia; UCN, 7-hydroxystaurosporine; Flu, fludararbine monophosphate; SD, stable disease; PD, progressive disease; PR, partial response; CR, complete response; CVP, cyclophosphamide, vincrisitne, prednisone; 2CDA, cladribine; Dex, dexamethasone; Vcr, vincristine; EPOCH, etoposide, vincristine, doxorubicin, cyclophosphamide, prednisone; Pred, prednisone; Mitox, mitoxantrone; Cytox, Cytoxan; Bleo, bleomycin; Etop, etoposide; Ara-C, cytarabine; ProMACE, prednisone, methotrexate, doxorubicin, cyclophosphamide, and etopside; CytaBOM, cytarabine, bleomycin, vincristine, methotrexate; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, cisplatin; Ifos, ifosfamide; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; XRT, X-radiotherapy; BEAM, carmustine, etoposide, cytarabine, melphalan; ABMT, autologous bone marrow transplant; MR, mixed response; ICE, ifosfamide, carboplatin, etoposide.
Sites of disease involvement
All of nine patients with CLL/SLL had peripheral blood and marrow involvement with diffuse lymphadenopathy; additional involvement of the spleen and the mediastinum was present in five and one patient, respectively. The malignant cells from all patients with CLL showed evidence of light chain restriction, dim CD20 positivity, and expression of CD5, CD23, and CD38. The six patients with FL had multiple sites of lymph node involvement, including mediastinal lymphadenopathy in 2/6, and tonsillar enlargement, pulmonary nodules, a plural effusion, and skin nodule involvement in one patient each. Transformation to diffuse large B-cell lymphoma (DLBCL) occurred in one patient. Immunophenotypic analysis in FL was consistent with the presence of a CD 10-positive light chain restricted B-cell lymphoproliferation expressing bcl-2. One patient with mantle cell lymphoma presented with splenomegaly, bone marrow disease, and leukemia, while the other had diffuse lymphadenopathy and bone marrow disease. Cases of MCL showed expression of cyclin D1 and were negative for CD23 expression. The single patient with LPL presented with bilateral pleural effusion, bone marrow disease, and retroperitoneal and pelvic lymph node involvement with unilateral axillary and inguinal lymphadenopathy, and was characterized with immunoglobulin M (IgM) kappa expressing B-cells with a corresponding serum IgM gammopathy.
Drug treatment schedule
The schedule consisted of a single cycle of UCN-01 alone followed by a maximum of six cycles of UCN-01 combined with FAMP. The first cycle dose of UCN-01 was fixed at 135 mg/m2 continuous intravenous (CIV) infusion over 72 h. On cycles 2–6, UCN-01 was administered at 67.5 mg/m2 CIVX36 h (days 1–2) due to its long half-life, with an escalating FAMP dosage. FAMP was administered at 25 mg/m2/day with the number of treatment days escalated in single patient cohorts. FAMP was given for 1, 2, 3, 4, or 5 days (beginning on day 1) if no dose-limiting toxicity (DLT) was observed. Intra-patient FAMP escalation was allowed. Supportive care consisted of the use of allopurinol to prevent tumor lysis syndrome and Bactrim DS to prevent Pneumocystis jiroveci pneumonia. Filgrastim (5–10 μg per kg) was permitted. UCN-01 was supplied by Kyowa Hakko Kogyo, Tokyo, Japan. The clinical product was manufactured by Ben Venue Laboratories, Inc., under a National Cancer Institute (NCI) contract managed by the Cancer Therapy Evaluation Program (CTEP). The Investigational New Drug Application (IND) for UCN-01 (NSC 638850) is held by NCI CTEP, and the formulated product is supplied by CTEP. FAMP (Fludara; Berlex Laboratories) was purchased from commercially available sources.
Response criteria
Complete response (CR) was defined as the disappearance of all signs and symptoms of lymphoma/leukemia for a period of at least 1 month. Patients with greater than 75% reduction in initial tumor bulk whose measurable disease did not change over the last two treatments, and negative biopsies, were considered to be in CR. Lymph nodes up to 1.5 cm in size were considered to be within normal limits. Partial response (PR) was defined as a 50% or greater decrease in the sum of the products of the longest perpendicular diameters of all measured lesions lasting for a period of at least 4 weeks. No individual lesions may increase in size and no new lesions may appear. Stable disease (SD) was defined as tumor measurements not meeting the criteria of CR, PR, or PD. Progression (PD) was an increase of 50% or more in the sum of the products of the longest perpendicular diameters of measured lesions compared to the smallest previous measurements, or the appearance of any new lesion(s). Complete blood counts (CBCs), chemistries, and computed tomography (CT) scans were used to monitor the patient before, during, and after treatment.
Toxicity criteria
The National Cancer Insititute (NCI) Common Toxicity Criteria version 2.0 was used for toxicity and adverse event reporting (http://ctep:info.nih.gov).
Response statistics
For this phase I trial, a conservative version of design 2A from Simon et al. was used to determine patient safety at each dose [9]. For preliminary response data, single stage design with the following parameters was followed. A 25% response rate was considered to be low (p0), and a response rate of at least 60% (p1) was considered to be of interest. Of the 11 patients treated at the maximum tolerated dose (MTD), if 0–4 of 11 patients responded, it would be concluded that the combination did not merit further consideration. If five or more patients responded, the combination would be considered for phase II trials. It was determined that 20 patients would be required to fulfill all of these objectives.
Plasma sample collection
Blood samples for UCN-01 pharmacokinetic analysis were collected using an indwelling heparin lock. Plasma samples during cycle 1 were collected at the following time points: 0, 12, 24, 48, and 72 h after the start of the infusion and 2, 12, 24, 48 h, and 2 weeks after completion of the infusion. Samples were also collected during cycle 2 at 0, 12, 24, and 36 h after the start and 2, 12, 24, 48 h, and 2 weeks after completion of the infusion. Blood samples were collected in tubes containing heparin, centrifuged (2400 rpm, 10 min, 4°C), and stored at −80°C until analysis.
Sample analysis
The analytical method adapted from Buaer et al. for UCN-01 detection was based on a high-performance liquid chromatography (HPLC) assay with ultraviolet (UV) detection [10]. Briefly, 0.25 mL aliquots of plasma were extracted with 1 mL of acetonitrile, containing an internal standard (umbelliferone). The precipitated protein fraction was removed by centrifugation and the clear supernatant transferred to a glass tube and evaporated to dryness under a stream of air at 40°C. The residues were reconstituted in 0.25 mL of mobile phase, i.e. acetonitrile/0.05 M pH 4.15 ammonium acetate, 20:80 (vol/vol). Separations were achieved on a Waters Nova-Pak® phenyl (3.9 mm internal diameter × 150 mm, 4 μm particle size; Waters, Milford, MA) utilizing a gradient elution from 80% 0.01 M aqueous ammonium acetate to 50% acetonitrile. The gradient run time was 18 min at a flow rate of 1 mL/min. UV detection of UCN-01 was performed at 295 nm. Under these conditions, the retention times were approximately 9.6 min (UCN-01) and 3.9 min (umbelliferone). The lower limit of quantitation was established to be 0.2 μg/mL for 0.25 mL plasma samples.
Pharmacokinetic analysis
Plasma UCN-01 concentration versus time profiles were analyzed by non-compartmental analysis (NCA) using WinNonlin Pro v. 5.0 (Pharsight, Mountain View, CA). The peak concentration (Cmax) and time to peak (tmax) were determined by visual inspection of the concentration-time profiles. The terminal rate constant (λz) was determined by linear regression of the terminal linear portion of the log concentration-time curve. λz was evaluated using at least three concentration time points of the terminal phase. The terminal half-life (t1/2) was calculated by 0.693/λz. The area under the concentration versus time curve (AUC) was calculated by linear-trapezoidal interpolation from time zero to the last measurable time concentration (Ct). Total clearance (CLtot) and volume of distribution (Vλz) were calculated as dose/AUCc- α and dose/ (AUCc-_α*λz), respectively.
Pharmacokinetic statistical evaluation
Pharmacokinetic (PK) data are presented as median values (range), unless otherwise stated. Comparison between the cycle-1 CLtot (without FAMP) and the cycle-2 CLtot (with FAMP) were achieved using a paired t-test. All statistical evaluations were performed on the NCSS 2001 software package (J. L. Hintze, East Kaysville, UT).
Apoptosis flow cytometry method
Neoplastic B cells were analyzed for annexin V-fluorescein isothiocyanate (FITC) staining (Caltag Burlington, CA) according to the manufacturer’s protocol as described, within 12 h of collection [11]. Erythrocytes were lysed by incubating with lysing solution (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM ehtylenediaminetetraacetic acid [EDTA]) for 10 min at room temperature at a ratio of 1:9 (sample volume:lysing solution volume). After incubation, cells were pelleted by centrifugation (500 × g for 15 min at room temperature), the media was aspirated, and the cells washed twice in a phosphate buffered saline (PBS) solution containing 0.1% NaN3 t Cells were costained with annexin V-FITC and a combination of antibodies designed to identify the patient’s tumor cells (e.g. CD19 and CD5 in chronic lymphocytic leukemia) for 30 min at room temperature. A positive control (freshly thawed peripheral blood lymphocytes) and negative control (fresh whole blood) were run for each apoptosis analysis. Four-color cytometry was performed with a BD (Becton-Dickinson, San Jose, CA) FACScan or FACSCalibur flow cytometer. The sensitivity of fluorescent detectors was set and monitored using Calibrite beads (Becton-Dickinson) according to the manufacturer’s recommendations. Daily quality control/quality assurance (QC/QA) consisted of an ongoing program of determining the linearity of the instrument response on a daily basis using Quantibrite® microbreads and accompanying software from Becton-Dickinson Bioscience (San Jose, CA). Data (collected in list mode) were analyzed with CellQuest software (BD). At least 5000 lymphocytes were acquired per tube.
Results
Patient characteristics
Eighteen patients were entered in the study between August 1999 and November 2004. The off-study date for the last patient was December 2005. Thus, the duration of patient accrual was approximately 5 years and patient follow-up was for 6 years. Table I gives the patient characteristics. Half of the patients had CLL/SLL. FL was the second most common tumor type entered in the study. All nine patients with CLL/SLL had peripheral blood involvement.
Previous chemotherapy treatment
FAMP had been used as first-line treatment in seven of the nine patients with CLL and all nine received FAMP alone or in combination with other agents before entry in this trial. The average number of cycles of FAMP was 8.5 per patient (range 5–20). The patients were heavily pretreated with a median of four prior regimens, with a range of 1–9. The six patients with FL had been treated with standard regimens containing doxorubicin (Table I).
Number of treatment cycles
All patients received one cycle of UCN-01, and 11 of 18 patients were treated at the highest dose of FAMP (25 mg/m2 daily for 5 days). Of the 18 patients treated, four received only cycle 1 of UCN-01 alone and did not receive FAMP. Three patients received two cycles; six patients, three cycles; two patients, four cycles; one patient, six cycles; and two patients received seven cycles. A total of 18 cycles of UCN-01 alone and 38 cycles of UCN-01 and FAMP were given. Eleven patients received cycles 2–7 consisting of 5 days of FAMP with UCN-01.
Toxicities
The toxicities of treatment are described in Tables II and III. Grade 2 and grade 3 toxicities of UCN-01 alone are shown in Table II while Table III contains a summary of grade 2 and grade 3 toxicities for 32 cycles given at dose level 5. The toxicities seen in this study consisted primarily of the known toxicities for the administration of UCN-01 by itself in the phase I trial and for known toxicities of FAMP, without significant overlap. Hyperglycemia was a common toxicity previously noted in the phase I study of UCN-01, as were nausea, hypoxemia, and headache, and were grade 2 or grade 3 [12–14]. Hypocalcemia (grade 2) was observed in six patients (33%). All of these toxicities were self-limiting and none were dose-limiting. Three patients developed tumor lysis syndrome, a grade 3 toxcity in Common Terminology Criteria for Adverse Events (CTCAE) v2. All three had grade 4 hyperuricemia and two had renal dysfunction (serum blood urea nitrogen [BUN] peaked at 66 and 40 mg/dL and creatinine peaked at 2.1 mg/dL). It was of further interest to note grade 4 and grade 5 toxicities that occurred at the maximum combined dose of UCN-01 and FAMP (dose level 5). A patient with CLL experienced a grade 4 rise in cardiac troponin I levels and other circulatory cardiac complaints, with a grade 2 rise in creatine phosphokinase (CPK). In a patient with FL, a grade 4 dyspnea and shortness of breath was associated with grade 3 hypoxemia and grade 2 hemoglobin toxicity. One of the two patients with grade 4 neutropenia developed a grade 3 febrile neutropenia. As anticipated, grade 4 (five patients) or grade 5 leukopenia (one patient), anemia (two patients), and thrombocytopenia (two patients) were seen, and with two patients being given platelet transfusions, expected toxicities with the use of FAMP alone.
Table II.
Combined grade 2 and 3 toxicities of cycle 1 UCN-01 alone*.
| Toxicity type | Grade 2 or 3 | Toxicity type | Grade 2 or 3 |
|---|---|---|---|
| Amylase | 2(11%) | Hypoxia | 6(33%) |
| Anorexia | 3(l6%) | Inf w/o neut | 2(11%) |
| Chest pain | 3(l6%) | Leukopenia | 3(16%) |
| Creatinine | 4(22%) | Lymphopenia | 5(27%) |
| Dyspnea-SOB | 2(l1%) | Myalgia | 2(11%) |
| Fatigue | 3(l6%) | Nausea | 8(44%) |
| Headache | 8(44%) | Neutropenia | 4(22%) |
| Hemoglobin | 6(33%) | Rash/desquamation | 2(11%) |
| Hyperglycemia | 12(67%) | SGOT (AST) | 3(16%) |
| Hypermagnesemia | 3(16%) | Stomatitis | 3(16%) |
| Hypoalbuminemia | 2(11%) | Sup/vnt arrh | 2(11%) |
| Hypocalcemia | 6(33%) | Trans prbcs | 3(16%) |
| Hyponatremia | 2(11%) | Tumor lysis | 3(16%) |
| Hypotension | 4(2%) | Vomiting | 4(22%) |
SOB, shortness of breath; Inf w/o neut, infection without neutropenia; SGOT (AST), serum glutamic oxaloacetic transaminase (aspartate transaminase); Sup/vnt arrh, supraventricular arrhythmia; Trans prbcs, transfused packed red blood cells; SGPT, serum glutamic pyruvic transaminase; CPK, creatine phosphokinase.
Not included in the above table are the following grade 3 toxicities that occurred one time during cycle 1: isolated elevated SGPT and one episode of febrile neutropenia. Single episodes of grade 2 toxicities included an elevated bicarbonate level, circulation cardiac-other, constipation, an elevated CPK, depression, dyspepsia, pruritus, and taste disturbance.
Table III.
Summary of combined grade 2 and grade 3 toxicities for 32 cycles given at dose level 5*.
| Toxicity type | Grade 2 or 3 | Toxicity type | Grade 2 or 3 |
|---|---|---|---|
| Constipation | 3 | Hypokalemia | 1 |
| Dyspnea-SOB | 4 | Inf w/o neutropenia | 1 |
| Fatigue | 2 | Inf + 3/4 neutropenia | 2 |
| Febrile neutropenia | 1 | Leukopenia | 13 |
| Hem + 3/4 cyto | 1 | Lymphopenia | 15 |
| Hemoglobin | 7 | Nausea | 3 |
| Hyperglycemia | 7 | Neutropenia | 8 |
| Hypermagnesemia | 5 | Platelets | 5 |
| Hypoalbuminemia | 2 | Stomatitis | 1 |
| Hypocalcemia | 5 | Trans prbcs | 7 |
SOB, shortness of breath; Hem, hemoglobin; cyto, cytopenia; Inf. w/o, infection without; Trans prbcs, transfused packed red blood cells.
Not included in the above table are the following grade 2 toxicities that occurred one time during dose level 5: anorexia, diarrhea, hyperkalemia, hypotension, photosensitivity, vomiting, and weight loss. One grade 3 rash/desquamation event was noted.
The most serious toxicity occurred in a patient who developed a transient grade 1 rash on cycle 1. On cycle 2 (first combined cycle), he developed a diffuse rash. Although therapy was discontinued, including Bactrim, the rash worsened, and the patient developed Stevens-Johnson syndrome characterized by diffuse desquamation of the skin, hypotension, and respiratory distress. Despite aggressive treatment with steroids, intravenous immunoglobulin, and antibiotics, the patient’s condition deteriorated and he developed multi-organ failure and died. Skin rashes were noted in two other patients. One patient developed a grade 2 rash on cycle 1 (UCN-01 alone), and did not receive further therapy. A second patient developed a grade 2 rash on cycle 1 and a grade 3 rash on the second combined cycle, and did not receive further therapy. Neither of these two patients had any further significant clinical sequelae.
Responses
There were one CR and six PRs for an overall response (OR) of 38%. Responses were confined to patients with CLL/SLL and FL. Seven patients maintained stable disease (38%) and four patients (22%) had progressive disease. The duration of the CR was 7 months, while the duration of the PRs was an average of 2 months (range 1–3 months). SD was documented for 12 months in one patient, while the other six demonstrated stability of 1–3 months. As of the completion of this report, all patients have died. The cause of death was attributed to treatment and/or progression of disease.
Pharmacokinetics
Evaluable UCN-01 pharmacokinetic profiles were obtained from 15 patients during cycle 1. All patients received the same dose, and high inter-individual variability was observed in pharmacokinetic parameters, summarized in Table IV. The concentration-time profiles of UCN-01 were characterized by Cmax occurring at the end of infusion (tmax) followed by a slow decline in plasma concentration, and hence a very long median terminal t1/2 of 361 h. Overall, the CLtot was extremely low (5–89 mL/m2.h) with a small volume of distribution (3–18 L), similar to previously obtained clinical values [12]. At the dose investigated (45 mg/m2/day of UCN-01 × 3 days in cycle 1 and 45 mg/m2/day of UCN-01 × 1.5 days in cycle 2), cycle 2 pharmacokinetics obtained from five evaluable patients indicate that there is no intra-individual difference in UCN-01 CLtot between cycle 1 and cycle 2 (p = 0.27). This suggests no apparent effect in UCN-01 elimination due to co-administration with FAMP. This finding suggests that FAMP (25 mg/m2 × 5 days) and UCN-01 are safely tolerated.
Table IV.
UCN-01 pharmacokinetic parameter estimates (cycle 1) after 72 h IV infusion at 45 mg/m2/day*.
| Cmax (μg/mL) | AUC (0-∞) (μg.h/mL) | CLtot (mL/m2.h) | VλZ (L) | t1/2 (h) | |
|---|---|---|---|---|---|
| n | 15 | 15 | 15 | 15 | 15 |
| Median | 16.7 | 7258 | 23.7 | 7.4 | 360.6 |
| (Range) | (7.9–37.5) | (1089.8–14 348.9) | (4.8–89.0) | (3.2–17.5) | (32.7–856.2) |
Pharmacokinetic parameters were calculated using non-compartmental analysis. Abbreviations used in this table are defined in the text.
Post-UCN-01 lymphocytosis
Leukocytosis was seen in the first cycle with UCN-01 alone. A 2.0–2.7-fold increase in the absolute lymphocyte count (ALC) was seen in the first three of four patients with CLL during the first cycle of UCN-01 alone. This prompted us to take a closer look at the ALC in all treated subjects. These data are summarized in Figure 1 (log plot), which shows these data for six patients. Linear plots were examined for the remaining nine patients with available ALCs. Lymphocytosis appeared to peak by day 4 and decreased thereafter. The amplitude of this paradoxical lymphocytosis appears to be related to the initial ALC. Although this response was still evident in patients with an ALC between 6,000 and 10, 000 cells per μL, it was difficult to observe this event if the ALC was below 2000 cells per μL whole blood (data not shown).
Figure 1.
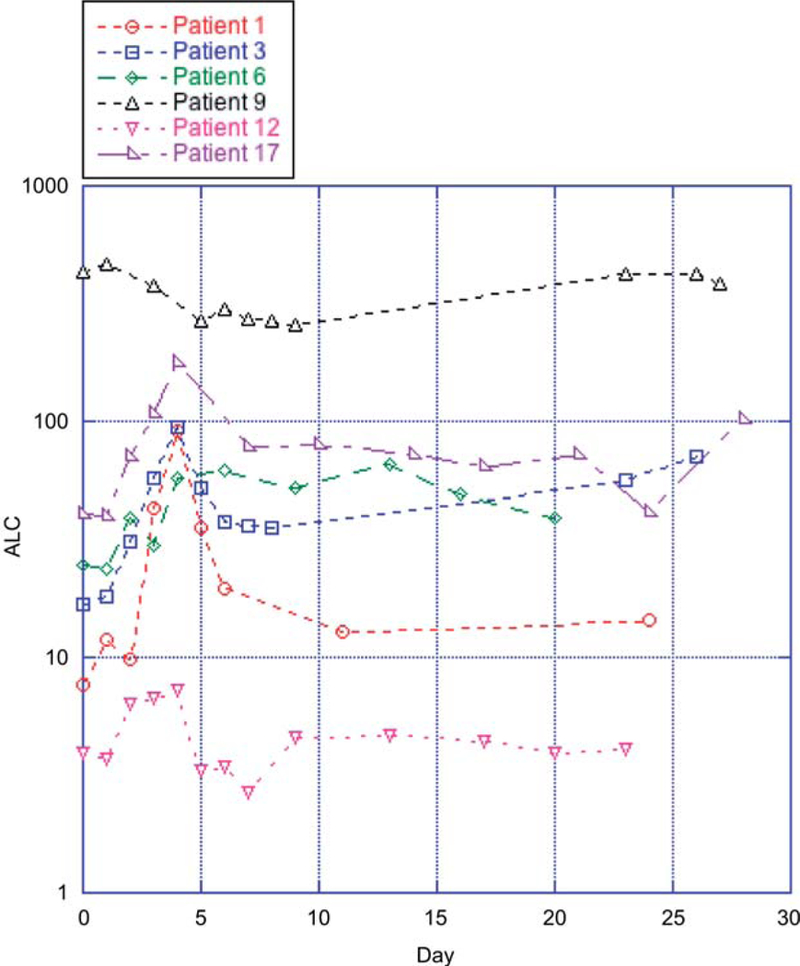
Absolute lymphocyte count [(WBC × % lymphocyte differential) ALC] in patients with definite lymphocytosis after receiving UCN-01.
Apoptosis
Peripheral blood samples were examined in eight patients with CLL. The serial results from six of these patients showed that both the maximum percentage and day of annexin V-positive cells were variable. Peak values of apoptosis 8%, 15%, 21%, 22%, 22%, and 28% occurred on days 28, 4, 30, 9, 10, and 28, respectively, of cycle 1 (data not shown). Figure 2 is a representative example of the flow cytometric analysis of apoptosis seen in two patients, numbers 10 and 17 in this study. Their baseline ALCs were below 5000 cells per μL and did not increase to more than 10 000 cells per μL. Patient 17 is shown in Figure 1.
Figure 2.
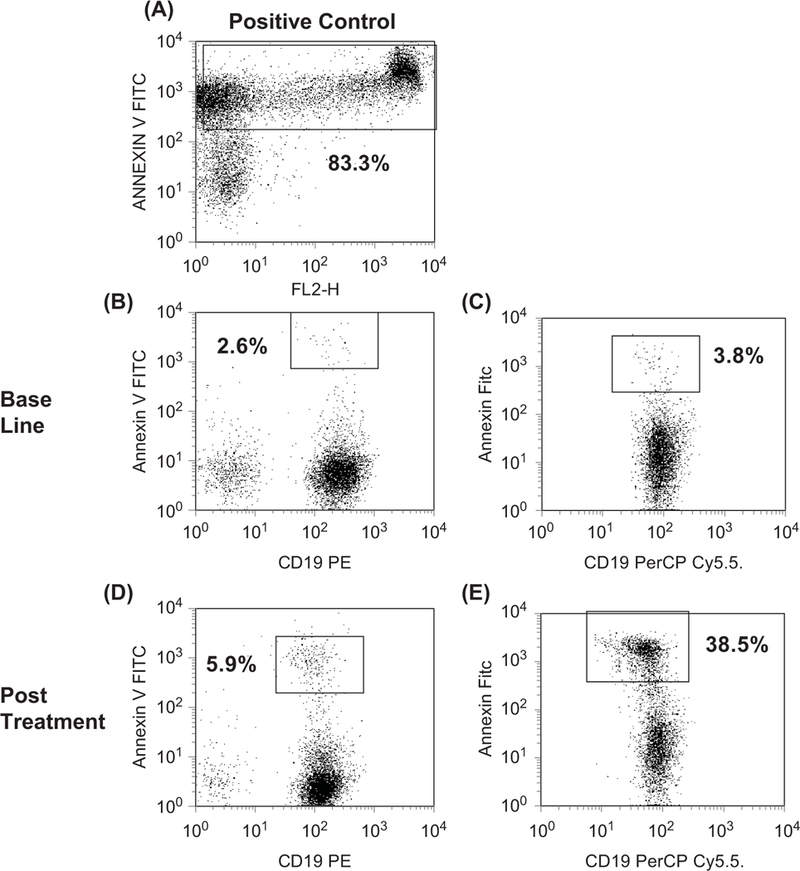
Detection of apoptosis by annexin V staining in B cells. (A) Annexin V staining (y-axis) in positive control. (B) Low level (2.6%) apoptotic B cells (in box) staining positive for CD19 (x-axis) and annexin V (y-axis). (C) Low level (3.8%) apoptotic B cells (in box) staining positive for CD19 (x-axis) and annexin V (y-axis). (D) Moderate level (5.9%) apoptotic B cells (in box) staining positive for CD19 (I-axis) and annexin V (y-axis). (E) High level (38.9%) apoptotic B cells (in box) staining positive for CD19 (x-axis) and annexin V (y-axis). Patient 10 is shown in (B) and (D) while patient 17 is shown in (C) and (E).
Discussion
Our major interest in UCN-01 was that in initial in vitro studies it appeared to increase cytotoxic-induced apoptosis, and thereby decrease the threshold for apoptosis [1,2,15]. Prior to this study, Monks et al. in the Developmental Therapeutics Program (NCI) had performed synergy studies with UCN-01 and several cytotoxic agents in five human cell lines [8]. A 100-fold increase in synergy was noted for the combination of UCN-01 and FAMP. Because FAMP is very active in indolent lymphomas and yet does not produce cures, it seemed reasonable to pair these two drugs. Also of importance is that the synergistic effects of UCN-01 were observed at a non-cytotoxic concentration range between 3 and 10 nM [8]. These studies also showed that the interaction of UCN-01 and FAMP is not schedule-dependent, with synergy being equally potent whether cells are pre-incubated with FAMP or added simultaneously with UCN-01 [8].
Also prior to this study, a phase I study of UCN-01 had determined that DLT (> grade 3) occurred at the 53 mg/m2/ day dose level and was defined by nausea/vomiting, hyperglycemia, and transient hypoxia (original reports). A MTD of 42.5 mg/m2/day was identified as the projected phase II dose [12]. At the 42.5 dose level, 1/9 had grade 4 hyperglycemia (complicated by steroids), and no patients had dose-limiting (> grade 3) nausea/vomiting or pulmonary toxicity. Other minor and reversible toxicities seen at the MTD included grade 1/2 headache (6/9), hypotension (7/9), low-grade temperature (7/9), tumor pain (1/9), myalgias (4/9), anorexia (9/9), fatigue (9/9), and nausea/vomiting (9/9). None of the patients had significant hematopoietic toxicity. Including all dose levels, the observed toxicities included headache, myalgias, transient and asymptomatic hypotension, nausea and vomiting, elevated hepatic transaminases, hyperglycemia, and asymptomatic oxygen desaturation.
Patient selection for the present study was based on the presence of a low-grade indolent, chronic lymphoid neoplasm or MCL. We required that patients had received at least one prior regimen, but did not place a limit on the number of prior regimens or the type of prior regimens. Our previous studies with EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, prednisone) and 9-AC (9-aminocaptothecin) indicated that the chemotherapy status (i.e. response to last administered chemotherapy) is an important clinical endpoint for predicting drug resistance, and is a more biologically relevant endpoint than simply the number of prior regimens [16]. Furthermore, to assess the differential efficacy of this treatment in resistant and sensitive patients, the patient population should contain both types of patients. We did not exclude patients who had received prior FAMP treatment, because if UCN-01/FAMP showed significant activity in this group, it would be an important indication that UCN-01 may be modulating drug resistance (i.e. apoptosis). Second, many patients with indolent lymphomas would have received FAMP in the past, and their exclusion from the study would significantly impede accrual.
This study was a phase I pharmacokinetic study. Following one cycle of UCN-01 alone, patients received UCN-01 and FAMP beginning on cycle 2. We approached the development of UCN-01 as a modulating agent and not as a ‘conventional’ chemotherapy agent, and hence designed this protocol around the concept that the dose of the modulating agent should be kept constant. Because we did not know whether there would be a biological or PK interaction between UCN-01 and FAMP, the first few patients on this trial received lower doses (days) of FAMP. We planned a maximum of five FAMP dose levels based on the standard schedule of daily bolus × 5 consecutive days [17–21]. The standard full dose of single-agent FAMP as used in the pivotal trial was 25 mg/m2 IV daily for 5 days [22]. In developing the combination of FAMP and UCN-01, we believed it would be preferable, if full-dose FAMP is not tolerated, to administer fewer days of the standard daily dose than to administer 5 days of a reduced standard daily dose. Thus, the dose levels of FAMP were based on increasing number of days of full daily dose FAMP. A conservative version of design 2A from Simon et al. was used to test patients at the various dose levels of FAMP [9].
In 2001, Sausville et al. reported a phase I trial of a 72 h continuous infusion of UCN-01 in 47 patients with refractory neoplasms [12]. That study produced a recommended phase II study 3-day infusion dose of 42.5 mg/m2/day and confirmed the previously noted unusually high binding of the drug to α1-acidic glycoprotein as the cause of the prolonged drug half-life [12–14]. UCN-01 causes hyperglycemia due to peripheral insulin resistance. Hyperglycemia is seen when the insulin-like growth factor-1 pathway is inhibited [23]. Based on the observation that protein kinase C activation results in insulin receptor signaling inhibition, inhibition of protein kinase C must be acting on an alternative pathway to produce hyperglycemia. This study provided the basis for future studies of combined drugs. Another phase I single-agent study by Dees et al. reported on the results of a short infusion of UCN-01 in 24 patients with refractory solid tumors and saw no objective responses [24]. They did note grade 1–2 nausea, vomiting, hyperglycemia, and hypotension. There have been several reported studies of UCN-01 in combination with other chemotherapy agents. In 2006, Hotte et al. reported a phase I trial of UCN-01 and topotecan in 33 patients with solid tumors [25]. This led to a phase II study in 29 patients with recurrent ovarian cancer [26]. Three patients (10%) had a PR with 3.3 months to progression and a median survival of 9.7 months. Grade 3–4 neutropenia (79%), anemia (41%), thrombocytopenia (14%), hyperglycemia (10%), and pain (10%) were noted. This combination was not felt to have significant anti-tumor activity for ovarian cancer. Kortmansky et al. reported on the combined use of UCN-01 and fluorouracil in 35 patients with advanced solid tumors and established an upper dose for fluorouracil, keeping the UCN- 01 dose constant [27]. They too noted difficulties with hyperglycemia and began to screen for diabetes in their patient population. Lara et al. did a similar study using escalating doses of cisplatin and a fixed dose of UCN-01 in 10 patients with advanced malignant solid tumors and had to terminate this study due to unacceptable toxicity [28]. Edelman et al. reported the combined use of UCN-01 and carboplatin in 23 patients with advanced solid tumors but saw no responses [29].
Some of the toxicity seen in this study was not unexpected. Hyperglycemia had previously been noted [12–14,23,30,31]. Compared to FAMP by itself, there was also an increase in nausea and headache with UCN-01 by itself. Although tumor lysis syndrome has been reported with FAMP used alone [32–35], it occurred in this study at the higher combined dose level in this small patient sample. The in vivo apoptosis was expected from previous in vitro studies, and was confirmed in this study. In those few patients studied in cycle 2, the degree of apoptosis was maintained (data not shown). Future studies should include a quantitative analysis of the markers of apoptosis [36,37]. The transient lymphocytosis was unexpected, and was similar to that seen sometimes with the use of steroids [38] and more recently with the phosphoinositide-3 (PI-3) kinase inhibitor CAL-101 where 60% of patients demonstrated an increase in lymphocyte count associated with decreasing lymph node size, suggesting a mobilization event [39]. Its mechanism is likely related to the inhibition of kinase-mediated homing and attachment to lymph node stromal structures, in particular disruption of stromal chemokines acting on CCL receptors [40,41]. And more recently the PI-3 kinase inhibitors of Syk and BTK have been noted to be associated with lymphocytosis with a decrease in lymph node size. The rapid nodal changes reported by Friedberg et al., using fostamatinib disodium, were not seen with UCN-01 [42]. The increased ALC seen by Zent et al. using everolimus was greater than that seen for UCN-01 in this study [43].
In summary, these findings suggest that UCN-01 does not significantly increase the toxicity of FAMP and that it induces both apoptosis of the malignant cells and a transient lymphocytosis. We believe that these results will be useful in understanding the behavior of other experimental tyrosine kinase inhibitors.
Supplementary Material
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- [1].Akinaga S, Gomi K, Morimoto M, Tamaoki T, Okabe M. Antitumor activity of UCN-01, a selective inhibitor of protein kinase C, in murine and human tumor models. Cancer Res 1991;51:4888–4892. [PubMed] [Google Scholar]
- [2].Wang Q, Worland PJ, Clark JL, Carlson BA, Sausville EA. Apoptosis in 7-hydroxystaurosporine-treated T lymphoblasts correlates with activation of cyclin-dependent kinases 1 and 2. Cell Growth Differ 1995;6:927–936. [PubMed] [Google Scholar]
- [3].Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood 2000;96: 393–397. [PubMed] [Google Scholar]
- [4].Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin nCancer Res 2007;13:1955–1960. [DOI] [PubMed] [Google Scholar]
- [5].Sato S, Fujita N, Tsuruo T. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine). Oncogene 2002;21:1727– 1738. [DOI] [PubMed] [Google Scholar]
- [6].Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst 2000; 92:376–387. [DOI] [PubMed] [Google Scholar]
- [7].Senderowicz AM. The cell cycle as a target for cancer therapy: basic and clinical findings with the small molecule inhibitors flavopiridol and UCN-01. Oncologist 2002;7(Suppl. 3):12–19. [DOI] [PubMed] [Google Scholar]
- [8].Monks A, Harris ED, Vaigro-Wolff A, et al. UCN-01 enhances the in vitro toxicity of clinical agents in human tumor cell lines. Invest New Drugs 2000;18:95–107. [DOI] [PubMed] [Google Scholar]
- [9].Simon R, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 1997;89: 1138–1147. [DOI] [PubMed] [Google Scholar]
- [10].Bauer KS, Lush RM, Rudek MA, et al. A high-performance liquid chromatography method using ultraviolet and fluorescence detection for the quantitation of UCN-01, 7-hydroxystaurosporine, from human plasma and saliva. Biomed Chromatogr 2000;14:338–343. [DOI] [PubMed] [Google Scholar]
- [11].Dive C, Gregory CD, Phipps DJ, et al. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta 1992;1133: 275–285. [DOI] [PubMed] [Google Scholar]
- [12].Sausville EA, Arbuck SG, Messmann R, et al. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J Clin Oncol 2001;19:2319–2333. [DOI] [PubMed] [Google Scholar]
- [13].Fuse E, Tanii H, Takai K, et al. Altered pharmacokinetics of a novel anticancer drug, UCN-01, caused by specific high affinity binding to alpha1-acid glycoprotein in humans. Cancer Res 1999;59: 1054–1060. [PubMed] [Google Scholar]
- [14].Fuse E, Hashimoto A, Sato N, et al. Physiological modeling of altered pharmacokinetics of a novel anticancer drug, UCN-01 (7-hydroxystaurosporine), caused by slow dissociation of UCN-01 from human alpha1-acid glycoprotein. Pharm Res 2000;17:553–564. [DOI] [PubMed] [Google Scholar]
- [15].Sampath D, Cortes J, Estrov Z, et al. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood 2006;107:2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gutierrez M, Chabner BA, Pearson D, et al. Role of a doxorubicin- containing regimen in relapsed and resistant lymphomas: an 8-year follow-up study of EPOCH. J Clin Oncol 2000;18:3633–3642. [DOI] [PubMed] [Google Scholar]
- [17].Hiddemann W, Unterhalt M, Pott C, et al. Fludarabine single-agent therapy for relapsed low-grade non-Hodgkin ‘ s lymphomas: a phase II study of the German Low-Grade Non-Hodgkin’s Lymphoma Study Group. Semin Oncol 1993;20:28–31. [PubMed] [Google Scholar]
- [18].Hochster HS, Kim KM, Green MD, et al. Activity of fludarabine in previously treated non-Hodgkin’s low-grade lymphoma: results of an Eastern Cooperative Oncology Group study. J Clin Oncol 1992;10: 28–32. [DOI] [PubMed] [Google Scholar]
- [19].Redman JR, Cabanillas F, Velasquez WS, et al. Phase II trial of fludarabine phosphate in lymphoma: an effective new agent in low-grade lymphoma. J Clin Oncol 1992;10:790–794. [DOI] [PubMed] [Google Scholar]
- [20].Puccio CA, Mittelman A, Lichtman SM, et al. A loading dose/continuous infusion schedule of fludarabine phosphate in chronic lymphocytic leukemia. J Clin Oncol 1991;9:1562–1569. [DOI] [PubMed] [Google Scholar]
- [21].Keating MJ. Fludarabine phosphate in the treatment of chronic lymphocytic leukemia. Semin Oncol 1990;17:49–62. [PubMed] [Google Scholar]
- [22].Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000;343:1750–1757. [DOI] [PubMed] [Google Scholar]
- [23].Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials— early lessons. J Mammary Gland Biol Neoplasia 2008;13:471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dees EC, Baker SD, O’Reilly S, et al. A phase I and pharmacokinetic study of short infusions of UCN-01 in patients with refractory solid tumors. Clin Cancer Res 2005;11:664–671. [PubMed] [Google Scholar]
- [25].Hotte SJ, Oza A, Winquist EW, et al. Phase I trial of UCN-01 in combination with topotecan in patients with advanced solid cancers: a Princess Margaret Hospital Phase II consortium study. Ann Oncol 2006;17:334–340. [DOI] [PubMed] [Google Scholar]
- [26].Welch S, Hirte HW, Carey MS, et al. UCN-01 in combination with topotecan in patients with advanced recurrent ovarian cancer: a study of the Princess Margaret Hospital Phase II consortium. Gynecol Oncol 2007;106:305–310. [DOI] [PubMed] [Google Scholar]
- [27].Kortmansky J, Shah MA, Kaubisch A, et al. Phase I trial of the cyclin-dependent kinase inhibitor and protein kinase C inhibitor 7-hydroxystaurosporine in combination with fluorouracil in patients with advanced solid tumors. J Clin Oncol 2005;23:1875–1884. [DOI] [PubMed] [Google Scholar]
- [28].Lara PN Jr, Mack PC, Synold T, et al. The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res 2005;11:4444–4450. [DOI] [PubMed] [Google Scholar]
- [29].Edelman MJ, Bauer KS Jr, Wu S, et al. Phase I and pharmacokinetic study of 7-hydroxystaurosporine and carboplatin in advanced solid tumors. Clin Cancer Res 2007;13:2667–2674. [DOI] [PubMed] [Google Scholar]
- [30].Wang Q, Fan S, Eastman A, et al. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst 1996;88:956–965. [DOI] [PubMed] [Google Scholar]
- [31].Sparreboom A, Chen H, Acharya MR, et al. Effects of alpha1-acid glycoprotein on the clinical pharmacokinetics of 7-hydroxystaurosporine. Clin Cancer Res 2004;10:6840–6846. [DOI] [PubMed] [Google Scholar]
- [32].Ramachandran A, Majumdar G. Acute tumour lysis syndrome after oral fludarabine in a patient with chronic lymphocytic leukaemia. Hematol J 2004;5:528–529. [DOI] [PubMed] [Google Scholar]
- [33].Dizdar O, Yurekli BS, Purnak T, Aksu S, Haznedaroglu IC. Tumor lysis syndrome associated with fludarabine treatment in chronic lymphocytic leukemia. Ann Pharmacother 2004;38:1319–1320. [DOI] [PubMed] [Google Scholar]
- [34].Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy for chronic lymphocytic leukemia (CLL): case report and review of the literature. Am J Hematol 2003;72:212–215. [DOI] [PubMed] [Google Scholar]
- [35].Cheson BD, Frame JN, Vena D, Quashu N, Sorensen JM. Tumor lysis syndrome: an uncommon complication of fludarabine therapy of chronic lymphocytic leukemia. J Clin Oncol 1998;16:2313–2320. [DOI] [PubMed] [Google Scholar]
- [36].Wang L, Abbasi F, Jasper GA, et al. Variables in the quantification of CD4 in normals and hairy cell leukemia patients. Cytometry B Clin Cytom 2011;80:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jasper GA, Arun I, Venzon D, et al. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytometry B Clin Cytom 2011;80:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shaw RK, Boggs DR, Silberman HR, Frei E. A study of prednisone therapy in chronic lymphocytic leukemia. Blood 1961;17:182–189. [Google Scholar]
- [39].Flinn IW, Byrd JC, Furman RR, et al. Evidence of clinical activity in a phase 1 study of CAL-101, an oral P110d isoform-selective inhibitor of phosphatidylinositol 3-kinase, in patients with relapsed or refractory B-cell malignancies. Blood 2009;114(Suppl. 1): Abstract 922. [Google Scholar]
- [40].Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node micro-environment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011;117:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hoellenriegel J, Meadows SA, Wierda WG, et al. Phosphoinositide 3’-kinase (PI3K) delta inhibition with CAL-101 blocks B-cell receptor (BCR) signaling and the prosurvival actions of nurslike cells (NLC), in chronic lymphocytic leukemia. Blood 2010;116(Suppl. 1): Abstract 48. [Google Scholar]
- [42].Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non- Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010;115:2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zent CS, LaPlant BR, Johnston PB, et al. The treatment of recurrent/ refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer 2010;116:2201–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


