Abstract
Objective
To determine the safety and clinical efficacy of two anti-angiogenic agents, bevacizumab and lenalidomide, with docetaxel and prednisone.
Patients and methods
Eligible patients with metastatic castration-resistant prostate cancer enrolled in this open-label, phase II study of lenalidomide with bevacizumab (15 mg/kg), docetaxel (75 mg/m2) and prednisone (10 mg daily). Docetaxel and bevacizumab were administered on day 1 of a 3-week treatment cycle. To establish safety, lenalidomide dosing in this combination was escalated in a conventional 3 + 3 design (15, 20 and 25 mg daily for 2 weeks followed by 1 week off). Patients received supportive measures including prophylactic pegfilgrastim and enoxaparin. The primary endpoints were safety and clinical efficacy.
Results
A total of 63 patients enrolled in this trial. Toxicities were manageable with most common adverse events (AEs) being haematological, and were ascertained by weekly blood counts. Twenty-nine patients (46%) had grade 4 neutropenia, 20 (32%) had grade 3 anaemia and seven (11%) had grade 3 thrombocytopenia. Despite frequent neutropenia, serious infections were rare. Other common non-haematological grade 3 AES included fatigue (10%) and diarrhoea (10%). Grade 2 AES in of patients included anorexia, weight loss, constipation, osteonecrosis of the jaw, rash and dyspnoea. Of 61 evaluable patients, 57 (93%), 55 (90%) and 33 (54%) had PSA declines of >30, >50 and >90%, respectively. Of the 29 evaluable patients, 24 (86%) had a confirmed radiographic partial response. The median times to progression and overall survival were 18.2 and 24.6 months, respectively.
Conclusions
With appropriate supportive measures, combination angiogenesis inhibition can be safely administered and potentially provide clinical benefit. These hypothesis-generating data would require randomized trials to confirm the findings.
Keywords: prostate cancer, angiogenesis inhibition, combinationation therapy, metastatic castration resistant prostate cancer, docetaxel coimbination
Introduction
Up until 2010, docetaxel with prednisone was the only treatment that had been found to have a survival benefit in patients with metastatic castration-resistant prostate cancer (mCRPC), which annually claims more than 300 000 lives worldwide [1–3]. Recent advances in the treatment of mCRPC have revolutionized treatment algorithms [4]. Despite their impact on overall survival (OS), sipuleucel-T and Ra-223 have unknown impact in symptomatic patients or those with visceral metastasis, respectively. Abiraterone and enzalutamide have favourable toxicity profiles, but they share mechanisms of resistance that probably diminish the benefits of sequential use [5–7].
Given that current clinical studies are focusing on using many of these new treatments earlier in the disease process (at the non-metastatic stage or as neoadjuvant therapy), it is likely that future populations of patients with mCRPC may have disease with greater inherent androgen resistance based on earlier exposure to modern antiandrogens [7]. In this context, taxane-based chemotherapy remains a very relevant treatment, and efforts to maximize its benefit should continue. The therapeutic potential of docetaxel is exemplified by the significant clinical impact of limited docetaxel (for six cycles) when added to androgen deprivation therapy in patients with castration-sensitive, metastatic disease: a median improvement in OS of 13.6 months compared with androgen deprivation therapy alone (57.6 vs 44.0 months; hazard ratio [HR] 0.61; P < 0.001). [8] These findings have been supported by similar outcomes in the STAMPEDE trial [9].
Subsequently, numerous docetaxel-based combination studies failed to improve on the benefits of docetaxel and prednisone [10]. Despite a strong preclinical rationale with preliminary clinical data, the list of failed studies includes several phase Ill trials which combined docetaxel with a single angiogenesis inhibitor, including bevacizumab, lenalidomide and vascular endothelial growth factor (VEGF)-trap (aflibercept) [11–13]. The toxicity for these combination regimens could have curtailed treatment exposure, limiting clinical benefit compared with docetaxel alone. Another possible explanation for the failure of these trials was that, over time, resistance mechanisms, such as upregulation of proangiogenesis factors, ultimately circumvented the benefits of these anti-angiogenic therapies, thereby limiting their potential clinical benefit [14]. An earlier phase II trial suggested that an approach using a combination of antiangiogenic agents could limit such treatment resistance. The study evaluated the combination of thalidomide, bevacizumab, docetaxel and prednisone which resulted in a median progression-free survival (PFS) of 18.3 months and a median OS of 28.2 months.[15]
In the present study, patients with mCRPC were treated with docetaxel, prednisone, bevacizumab and lenalidomide. Lenalidomide was substituted for thalidomide in this regimen because of the reduced side effect profile, namely it is not associated with fatigue and neuropathy. Patients were also given broad supportive measures (growth factor and anticoagulation) in an attempt to mitigate morbidity and treatment-limiting toxicities.
Patients and Methods
Study Population
Eligible patients were required to have progressive mCRPC as defined by the Prostate Cancer Clinical Trials Working Group 2 (PCWG2) [16]. Patients were aged ≥18 years with Eastern Cooperative Oncology Group performance status of ≤2, and adequate organ and bone marrow functions. Previous use of chemotherapy or anti-angiogenic therapy for mCRPC was an exclusion criterion. Patients with brain metastases, congestive heart failure, unstable angina, history of hypertensive encephalopathy, proteinuria ≥2 g/24 h, non-healing ulcers, bleeding diathesis and peripheral neuropathy ≥grade 2 were not eligible for enrolment. Patients on active treatment for venous thromboembolism, and those with abdominal fistula or gastrointestinal perforation within the previous 6 months were also not eligible.
The protocol was approved by the National Cancer Institute’s Institutional Review Committee and written informed consent was obtained from all patients. The protocol was registered with the US National Clinical Trials Registry (NCT00942578).
Study Design and Statistical Methods
After a brief dose escalation portion, the study was designed to be a single-arm, open-label phase II study of lenalidomide and bevacizumab with docetaxel and prednisone in patients with mCRPC. The initial dose escalation portion was planned to enroll three to six patients at each of 15, 20 and 25 mg of lenalidomide. (Dose-limiting toxicity was defined as a ≥grade 3 non-haematological toxicity related to lenalidomide.) Then, 45 planned patients were to be enrolled at 25 mg lenalidomide to exclude 25% of patients with grade 4 non-haematological toxicity attributable to that dose level of lenalidomide. See the supplemental statistical methods in Supporting Information Appendix S1 for more details.
The primary objectives were safety of the treatment regimen and clinical efficacy, including response rate and time to progression (TTP) using PCWG2 criteria. [16] TTP was determined from the on-study date until the date of progression or last follow-up without progression, while survival was determined from the on-study date until the date of death or last follow-up. Patients who did not progress but were removed from treatment for adverse effects, preference and other reasons had follow-up for TTP censored at that time. Additional objectives included evaluation of OS and the impact of changes in immune cells, circulating endothelial cells (CECs) and genotype on outcomes.
All P values are two-tailed and, except as noted in the supplemental statistical methods, are presented without any adjustments for multiple comparisons.
Drug Administration
All patients received i.v. docetaxel 75 mg/m2 and bevacizumab 15 mg/kg on day 1 of each 21-day cycle. Lenalidomide was taken orally for the first 14 days of each cycle, while 10 mg prednisone was taken daily throughout the cycle. All patients received pegfilgrastim 6 mg s.c. on day 2, enoxaparin 1 mg/kg/day s.c. starting on day 1 for thromboembolic prophylaxis, and continued androgen deprivation therapy. Oral dexamethasone 8 mg was administered at 12, 3 and 1 h before docetaxel infusion, except in cases where i.v. infusions were required because of patient non-compliance. Patients receiving bisphosphonate before study enrolment were allowed to continue the drug. Because of potential concerns about the development of osteonecrosis of the jaw, bisphosphonate treatment was initiated after cycle 5 among patients with bone metastases who were not on bisphosphonate therapy before study enrolment. (Treatment modifications are described in the Supporting Information Appendix S2.)
Results
Patient Characteristics
In the dose escalation portion of the study, cohorts of three patients were treated with lenalidomide at 15, 20 and 25 mg daily, respectively, in combination with docetaxel, bevacizumab and prednisone. In the second part of the study, a total of 43 patients received a 25-mg dose of lenalidomide, and 11 patients in the expansion cohort received the 15-mg lenalidomide dose in the combination regimen. Patient characteristics for all 63 patients are shown in Table 1.
Table 1.
Baseline characteristics (N = 63).
| Median (range) age (years) | 65.6 (51.3–82.4) |
| Gleason score, n | |
| ≤6 | 4 |
| 7 | 15 |
| 8 | 15 |
| 9 | 23 |
| 10 | 6 |
| Median (range) PSA (ng/mL) | 90.36 (0.14–3 520) |
| Median (range) alkaline phosphatase, U/L | 436.5 (53–956) |
| Median (range) lactate dehydrogenase, U/L | 206 (127–2 305) |
| Median (range) haemoglobin (g/dL) | 11.8 (7.4–14.8) |
| ECOG performance status, n | |
| 0 | 10 |
| 1 | 50 |
| 2 | 3 |
| Patients requiring opiates for pain relief, n | 49 |
| Location of disease, n | |
| Bone only | 24 |
| Bone and lymph nodes | 27 |
| Bone and visceral | 7 (3 hepatic) |
| Lymph node alone | 3 |
| Visceral alone | 2 (bladder and lung) |
ECOG, Eastern Cooperative Oncology Group.
Clinical Response, Time to Progression and Overall Survival
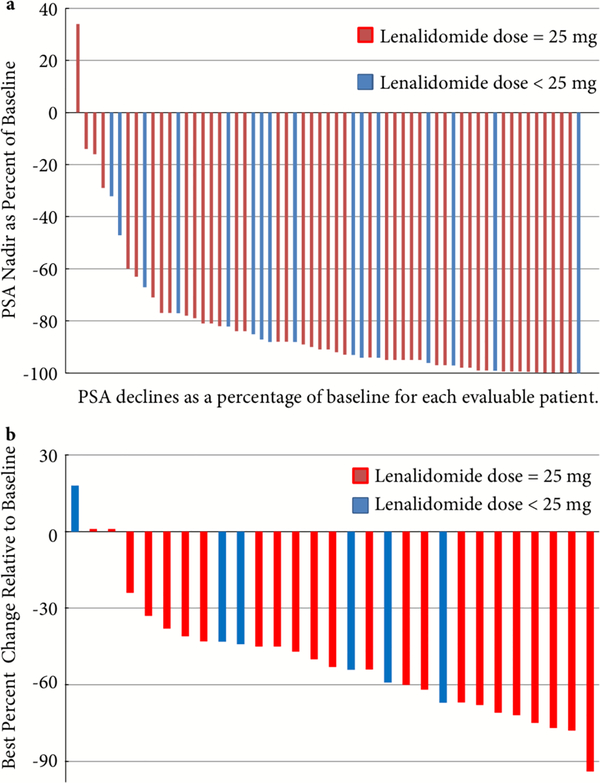
Of 61 evaluable patients, 57 (93%) had confirmed PSA declines >30%, of which 55 (90%) and 33 (54%) had confirmed PSA declines >50 and >90%, respectively (Fig. 1A) In addition, 29 patients were evaluable for radiographic responses based on Response Evaluation Criteria In Solid Tumors (RECIST) 1.1. Twenty-four patients (84%) met the criteria for confirmed partial (>30%) radiographic response (Fig. 1B).
Fig. 1.
Clinical responses. (a) Maximum PSA declines and (b) radiographic responses.
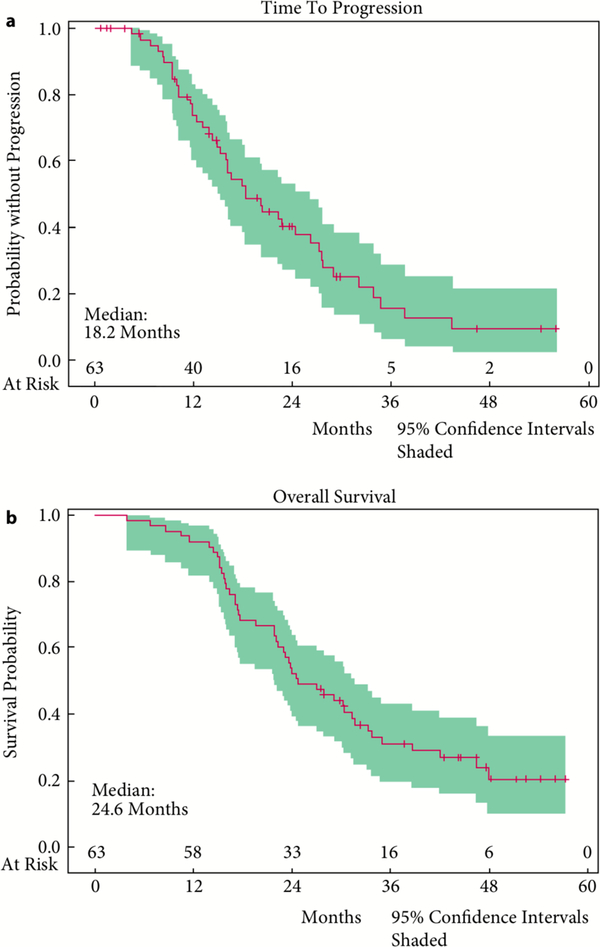
With a median time of potential follow-up of 47.5 months, the median TTP for all 63 enrolled patients was 18.2 months and the median OS was 24.6 months (Fig. 2). There were no significant differences noted with regard to lenalidomide dose levels and thus we reported these results in a combined fashion. There was no clear association between magnitude of PSA decline or rapidity of PSA decline and survival.
Fig. 2.
Clinical outcomes. (a)Time to progression and (b) Overall survival.
Toxicity
The dose escalation of lenalidomide did not reveal any dose-limiting toxicities, making 25 mg the recommended phase II dose in this combination. For all patients enrolled in the study, the most common adverse events (AEs) were haematological (Table 2). There were 29 patients (46%) with grade 4 neutropenia (ascertained by weekly complete blood counts). Non-heme grade 4 toxicities were seen in eight patients (13%), with neutropenic fever the most common (n = 4; 6%). Twenty patients (32%) and seven patients (11%) had grade 3 anaemia and thrombocytopenia, respectively. Although one patient had sepsis, most other infections were common respiratory infections, including five that were grade 3. Other frequent grade 3 AES included fatigue (10%) and diarrhoea (10%). Additional grade 2 AES experienced by >10% of the patients included anorexia, weight loss, constipation, osteonecrosis of the jaw, rash and dyspnoea. Clinically significant AES seen in a minority of patients included arrhythmia (n = 2), transient ischaemic attack (n = 2), thromboembolic event (n = 2) and rectal fistula (n = 1). There was one sudden death of unknown aetiology during the study in a patient with multiple cardiac risk factors including diabetes, hypertension and hyperlipidaemia. Nonetheless, attribution to study drugs could not be excluded. Forty-five of 61 patients (73.8%) required dose reductions or discontinuations of lenalidomide, bevacizumab or docetaxel (Table 3). Lenalidomide and docetaxel dose reductions were most frequently attributable to cytopenias, while bevacizumab discontinuation was often related to bleeding or tissue ulceration. Only one patient required docetaxel discontinuation.
Table 2.
Adverse events of interest.
| Grade 2, n(%) | Grade 3, n(%) | Grade 4, n(%) | |
|---|---|---|---|
| Haematological* | |||
| Anaemia | 14 (22) | 20 (32) | 0 |
| Lymphopenia | 1 (2) | 0 | 0 |
| Neutropenia | 11 (17) | 32 (50) | 29 (46) |
| Thrombocytopenia | 3 (14) | 7 (11) | 0 |
| Total haematological adverse events | 25 (40) | 45 (71) | 29 (46) |
| Constitutional | |||
| Fatigue | 18 (29) | 6 (11) | 0 |
| Weight loss | 8 (13) | 1 (2) | 0 |
| Infection | |||
| Febrile neutropenia | n/a | 9 (14) | 4 (6) |
| Respiratory | 14 (22) | 5 (8) | 0 |
| Tooth/gum infection | 4 (6) | 0 | 0 |
| Skin | 6 (10) | 3 (5) | 0 |
| UTI | 6 (10) | 2 (3) | 0 |
| Infection: other | 10 (16) | 5 (8) | 1 (2) |
| Cardiopulmonary | |||
| Arrythmia | 2 (3) | 0 | 0 |
| Dyspnoea | 8 (13) | 2 (3) | 0 |
| Hypoxia | 1 (2) | 0 | 0 |
| Thromboembolic event | 2 (3) | 0 | 0 |
| Gastrointestinal/digestive | |||
| Anorexia | 9 (14) | 1 (2) | 0 |
| Constipation | 9 (14) | 0 | 0 |
| Diarrhoea | 12 (19) | 6 (10) | 0 |
| Rectal Fistula | 1 (2) | 0 | 0 |
| Neurological | |||
| Dizziness | 2 (3) | 0 | 0 |
| Neuropathy | 2 (3) | 2 (3) | 0 |
| Syncope | n/a | 1 (2) | 0 |
| Transient ischaemic attack | 2 (3) | 0 | 0 |
| Dermatological | |||
| Palmar plantar erythrodysesthesia | 2 (3) | 1 (2) | 0 |
| Pruritis | 3 (14) | 0 | 0 |
| Rash | 9 (14) | 0 | 0 |
| Skin ulceration | 2 (3) | 0 | 0 |
| Dental/oral | |||
| Osteonecrosis of Jaw | 10 (16) | 3 (5) | 0 |
| Oral Mucositis | 5 (8) | 1 (2) | 0 |
| Oral Pain | 3 (14) | 0 | 0 |
| Renal/electrolytes | |||
| Dehydration | 1 (2) | 2 (3) | 0 |
| Haematuria | 0 | 1 (2) | 0 |
| Hypokalaemia | 1 (2) | 1 (2) | 1 (2) |
| Hypophosphataemia | 2 (3) | 2 (3) | 0 |
| Total non-heme adverse events | 61 (97) | 52 (83) | 8 (13) |
Adverse events determined using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0.
Haematological toxicities acquired from weekly complete blood counts.
Table 3.
Dose modifications and discontinuations.
| n/N | % | |
|---|---|---|
| Participants requiring dose reduction and/or discontinuation | 45/61 | 73.8 |
| Lenalidomide only dose reduction and/or discontinuation | 12/61 | 19.7 |
| Bevacizumab only dose reduction and/or discontinuation | 6/61 | 9.8 |
| Docetaxel only dose reduction and/or discontinuation | 1/61 | 1.6 |
| >1 medications requiring dose reduction and/or discontinuation | 26/61 | 42.6 |
| Participants requiring dose reduction for one or more medications | 35/61 | 57.4 |
| Lenalidomide dose reduction | 34/61 | 55.7 |
| Bevacizumab dose reduction | N/A | N/A |
| Docetaxel dose reduction | 19/61 | 31.1 |
| Participants requiring one or more medications discontinued | 21/61 | 34.4 |
| Lenalidomide discontinued | 12/61 | 19.7 |
| Bevacizumab discontinued | 16/61 | 26.2 |
| Docetaxel discontinued | 1/61 | 1.6 |
Genetic Analysis
Inter-individual variation in the gene expression and plasma levels of VEGF has been attributed to single nucleotide polymorphisms (SNPs) in the VEGF gene [17]; therefore, the VEGF −634G>C SNP (rs2010963) was evaluated in 54 patients as it has been associated with a more aggressive phenotype of prostate cancer, altered-VEGF binding affinity, bevacizumab toxicity, and altered response to thalidomide-based therapy [18–20]. Cereblon (encoded by CRBN) is considered to be the target of lenalidomide, and SNPs in CRBN (rs1714327G>C, rs1705814T>C, and rs1672753G>A) have been associated with lenalidomide efficacy [21–23].
Twenty-four patients were found to have at least one VEGF-634 C allele (CC or CG), while 30 patients were carriers of the GG genotype. The median TTP for patients with the C allele (CC or CG) was 15.5 months, compared with 22.2 months for patients without the C allele (GG; P = 0.014 unadjusted; P = 0.027 adjusted; Fig. S1). According to a multivariate Cox analysis, after univariate analyses had shown that albumin (2.4–3.4 g/DL vs > 3.5 g/DL) and Halabi predicted survival (< 8 months vs ≥ 8 months) were the only clinical factors to consider for inclusion in modelling, the VEGF-634 C allele retained its significance (P < 0.009, HR 0.41; 95% CI for HR 0.21–0.80) after adjusting for albumin (P = 0.006; HR 0.28; Cl for HR 0.19–0.76). Univariate analysis of OS also favoured patients who had the VEGF-634G (CG or GG) allele, with median OS of 25.7 vs 17.3 months (P = 0.037 unadjusted; P = 0.073 adjusted), although significance was not retained for CC or CG vs GG (P = 0.19), with medians of 26.2 vs 23.2 months. Cox model analysis for OS failed to show an association of the VEGF-634 C allele when other clinical factors were taken into consideration (data not shown). None of the CRBN SNPs were associated with TTP or OS (all P > 0.20; data not shown).
Immune Analysis
Immune subsets were evaluated by flow cytometry to assess for T-cells, regulatory T -cells, myeloid-derived suppressor cells and cd14+monocytes. Associations were seen with high expression of markers linked with T-cell exhaustion and dysfunction [24]. Forty-nine patients were evaluable for T-cell PD-I expression at baseline and those whose PD-I expression was lower than the median value had greater TTP (medians 27.6 vs 16.1 months; P = 0.007). Similarly, individuals with lower Tim-3 expression than the median at baseline had better TTP (median 22.3 vs 15.2 months; P = 0.031 [Fig. S2]). In addition, patients who had increases at 3 months in CD45+CD14+ HLA-DRhigh monocytes were associated with longer survival than patients who had declines (P = 0.028). This population of cells has been associated with higher tumour necrosis factor production which could assist in an anti-tumour response [25].
Circulating Endothelial Cells
While baseline CECs were not predictive of TTP or OS, post-treatment changes in CECs were associated with improved OS. Patients with a decrease in CECs after 3 months of therapy had improved OS compared to those (n = 20) with increases in CECs (P = 0.048; Fig. S3).
Discussion
Findings from the present study suggest that simultaneous treatment with two angiogenesis inhibitors can be safely combined with standard docetaxel and prednisone in mCRPC, with the potential for clinical benefit. One of the noteworthy aspects of previous phase Ill trials of docetaxel with angiogenesis inhibitors was the risk of increased toxicity. In the MAINSAIL trial (docetaxel ± lenalidomide) investigators noted substantial toxicity and postulated that this limited treatment in patients randomized to lenalidomide [12]. The most striking toxicities limiting treatment were neutropenia and pulmonary embolism. Similarly, CALGB 90401 (docetaxel ± bevacizumab) and the VENICE trial (docetaxel ± aflibercept) attributed increased treatmentrelated deaths primarily to infections [11,13]. The present study had one possible treatment-related death, but this was in a patient with multiple cardiac risk factors (diabetes, hypertension, hyperlipidaemia) who experienced sudden death. Furthermore, the study protocol necessitated supportive measures (daily anticoagulation with low molecular weight heparin and granulocyte colony-stimulating factor), which might have minimized the treatment toxicity that was seen in other studies. It is important to note that, although the incidence of grade 3 and 4 neutropenia was higher in the present trial than in previous studies, this was probably attributable to ascertainment bias because this protocol required weekly complete blood counts, while the other studies evaluated blood counts less frequently [11]. Furthermore, the trial allowed treatment holidays, in which patients may hold therapy to recover from toxicities and then continue as long as they had not met progression criteria. This strategy may minimize the burden of chronic toxicities and maximize drug efficacy. Nonetheless, as with many combination chemotherapy regimens, toxicities were seen with this regimen and do require management considerations.
The potential short-term clinical impact in the present trial can be shown by substantial PSA declines (≥80% in 75% of patients), confirmed partial responses in 24 of 29 patients (83%) with evaluable disease as per RECIST 1.1, and a median TTP >18 months. Compared with previous phase Ill docetaxel plus angiogenesis inhibitor trials, the proportion of patients with 50% PSA declines is substantially greater in the present trial (90% in the present study vs 59–70% in previous studies) [11–13]. The objective response rate is also greater than the only other study to use RECIST 1.1 (MAINSAIL, responses <25% in both arms) and compares favourably with the chemotherapy-naïve abiraterone (36%) and enzalutamide (59%) trials [5,6,12]. Although TTP was substantially longer in the present study compared with that reported in the other docetaxel/anti-angiogenic combination trials, progression criteria varied across all the studies making definitive comparisons difficult [11–13]. (The use of PSA progression parameters in other trials could have limited drug exposure and thus clinical benefit.) The criteria used in the present study, however, are similar to those used in trials evaluating abiraterone and enzalutamide in chemotherapy-naïve patients, and the 18.2-month TTP seen in the present trial compares favourably with those antiandrogen agents [5,6].
The survival data from the present trial also compare favourably with the previous trials although the difference is not as substantial as intermediate surrogates (e.g. PFS or responses). The median OS in the present study was 24.6 months compared with 22.6 months in the CALGB 90 401 trial, 22.1 months in the VENICE study and 17.1 months in the MAINSAIL trial [11–13]. Although caution should be taken with such comparisons, recent analyses have suggested that patients with more aggressive mCRPC may benefit most from chemotherapy, perhaps providing an explanation for the relatively prolonged OS seen in the present study [26].
The OS in the present trial, however, is shorter than previously reported for the combination angiogenesis trial performed here at the National Cancer Institute (NCI; docetaxel, bevacizumab, thalidomide; OS 28.2 months) [15]. Although lenalidomide was developed as a next-generation version of thalidomide, the exact antineoplastic mechanisms for both agents remain undefined; it is possible that the substitution of lenalidomide for thalidomide may have compromised some anti-cancer activity. The principal toxicities of thalidomide (fatigue and neurotoxicity) were substantially reduced in the present study, and thus in combination with docetaxel and bevacizumab, lenalidomide appears to be more tolerable. In addition, it is unclear why the PFS was similar between these two trials (≈18 months) but OS was shorter in the lenalidomide trial compared with the thalidomide trial (24.6 vs 28.2 months), given that both study populations had similar characteristics. Possible explanations include cumulative toxicity that was not captured within the follow-up timeframe of the present trial, treatment selection of particularly malignant/aggressive clones in patients treated in the lenalidomide trial, or a pro-angiogenesis rebound that has been postulated in previous trials [11,27].
The correlative studies provide some hypothesis-generating data. Tim-3 and PD-I expression on T-cells has previously been associated with T-cell exhaustion/dysfunction [24]. That could explain why patients with lower levels of expression of Tim-3 and PD-I had better responses to therapy, especially given the postulated immune effects of docetaxel, lenalidomide and bevacizumab [28–30]. Analysis of the VEGF-634G>C polymorphism suggested an association between clinical benefit (PFS) and patients with the C allele (CC or CG). Further evaluation is required to determine whether they can be reliable predictive markers of response.
Although the clinical responses seen in the present trial are compelling, the study has shortcomings inherent to single-institution, non-randomized trials. While toxicity was improved with the addition of supportive care measures, it is unclear if such trends would hold true in a larger, multicentre trial. Similarly, the survival trends reported in the present study should also be considered in that context. Additionally, the criteria for assessing disease progression differed between the present trial and several previous trials of docetaxel plus angiogenesis inhibitors, and it is not certain how that could have influenced the survival data.
Several antiangiogenic phase Ill trials have failed to show a survival benefit in mCRPC [11–13]. Toxicity was a critical obstacle in those trials. Two trials at the NCI have suggested the potential clinical benefits of using multiple angiogenesis inhibitors in combination with docetaxel, perhaps enhancing outcomes by suppressing compensatory pro-angiogenic factors. Moreover, the use of supportive measures in both NCI trials appears to mitigate the toxicity to a large degree. Despite the negative survival data from previous trials, preclinical and clinical evidence indicate the importance of angiogenesis in mCRPC biology, thus developing newer agents with improved or broader antiangiogenic properties could be considered for future investigations. Alternatively, given the sharp PSA declines and high proportion of objective responses, a similar docetaxel regimen with multiple anti-angiogenic agents could enhance the benefits produced in the CHAARTED study [8] when given earlier in the disease process, for a short course. Despite the development of modern antiandrogens, such as enzalutamide and abiraterone, emerging resistance patterns to those agents should serve as a reminder that docetaxel continues to have a substantial therapeutic role in mCRPC, and building on that regimen should remain a priority in future studies, which could include docetaxel and combination angiogenesis inhibitors with appropriate supportive measures.
Supplementary Material
Appendix S1 Supplemental statistical methods.
Appendix S2 Treatment modifications.
Fig. S1 Time to Progression Based on VEGF-634 Polymorphism.
Fig. S2 PD-I T-cell expression and time to progression. Fig. S3 Changes in circuting endothelial cells at 3 months and overall survival.
Fig. S3 Changes in circuiting endothetical cells at 3 months and overall survival.
Table S1 Comparison of docetaxel, anti-angiogenesis combination trials.
Abbreviations:
- mCRPC
metastatic castration-resistant prostate cancer
- OS
overall survival
- HR
hazard ratio
- VEGF
vascular endothelial growth factor
- PFS
progression-free survival
- TTP
time to progression
- RECIST
Response Evaluation Criteria In Solid Tumors
- AE
adverse event
- SNP
single nucleotide polymorphism
- CEC
circulating endothelial cell
- NCI
National Cancer Institute
Footnotes
Conflict of Interest
None declared.
References
- 1.Petrylak DP, Tangen CM, Hussain MH et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–20 [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12 [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108 [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Loblaw DA, Oliver TK et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol 2014; 32: 3436–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer TM, Armstrong AJ, Rathkopf DE et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonarakis ES, Lu C, Wang H et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371: 1028–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeney CJ, Chen YH, Carducci M et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James ND, Sydes MR, Mason MD et al. Docetaxel and/or zoledronic acid for hormone-naïve prostate cancer: first overall survival results from STAMPEDE (NCT00268476). J Clin Oncol 2015; 33 (suppl): abstr 5001) [Google Scholar]
- 10.Antonarakis ES, Eisenberger MA. Phase Ill trials with docetaxel-based combinations for metastatic castration-resistant prostate cancer: time to learn from past experiences. J Clin Oncol 2013; 31: 1709–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly WK, Halabi S, Carducci M et al. Randomized, double-blind, placebo-controlled phase Ill trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: cALGB 90401. J Clin Oncol 2012; 30: 1534–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrylak DP, Vogelzang NJ, Budnik N et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2015; 16: 417–25 [DOI] [PubMed] [Google Scholar]
- 13.Tannock IF, Fizazi K, Ivanov S et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol 2013; 14: 760–8 [DOI] [PubMed] [Google Scholar]
- 14.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA 2007; 104: 17069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning YM, Gulley JL, Arlen PM et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28: 2070–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher HI, Halabi S, Tannock I et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain L, Vargo CA, Danesi R et al. The role of vascular endothelial growth factor SNPs as predictive and prognostic markers for major solid tumors. Mol Cancer Ther 2009; 8: 2496–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panoilia E, Schindler E, Samantas E et al. A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients. Cancer Chemother Pharmacol 2015; 75: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etienne-Grimaldi MC, Formento P, Degeorges A et al. Prospective analysis of the impact of VEGF-A gene polymorphisms on the pharmacodynamics of bevacizumab-based therapy in metastatic breast cancer patients. Br J Clin Pharmacol 2011; 71: 921–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen NF, Vogel U, Klausen TW et al. Vascular endothelial growth factor (VEGF) gene polymorphisms may influence the efficacy of thalidomide in multiple myeloma. Int J Cancer 2012; 131: E636–42 [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Ando H, Suzuki T et al. Identification of a primary target of thalidomide teratogenicity. Science 2010; 327: 1345–50 [DOI] [PubMed] [Google Scholar]
- 22.Sardnal V, Rouquette A, Kaltenbach S et al. A G polymorphism in the CRBN gene acts as a biomarker of response to treatment with lenalidomide in low/int-l risk MDS without del(5q). Leukemia 2013; 27: 1610–3 [DOI] [PubMed] [Google Scholar]
- 23.Iskierka-Jazdzewska E, Stepien A, Canzian F et al. (eds). Cereblon (CRBN) gene polymorphisms predict clinical response and progression-free survival in multiple myeloma patients treated with lenalidomide: a pharmacogenetic study of immense consortium. Washington, D.C.: 56th Annual Meeting and Exposition of the American Society of Hematology; 2014 [DOI] [PubMed] [Google Scholar]
- 24.Fourcade J, Sun Z, Benallaoua M et al. Upregulation of Tim-3 and PD-I expression is associated with tumor antigen-specific CD8+T cell dysfunction in melanoma patients. J Exp Med 2010; 207: 2175–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belge KU, Dayyani F, Horelt A et al. The proinflammatory CD 14 + CD 16 + DR++ monocytes are a major source of TNF. J Immunol 2002; 168: 3536–42 [DOI] [PubMed] [Google Scholar]
- 26.Chi KN, Higano CS, Blumenstein BA et al. Phase 111 SYNERGY trial: docetaxel +/− custirsen and overall survival in patients with metastatic castration-resistant prostate cancer and poor prognosis. J Clin Oncol 2015; 33 (abstract 5009) [Google Scholar]
- 27.Bagri A, Berry L, Gunter B et al. Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Clin Cancer Res 2010; 16: 3887–900 [DOI] [PubMed] [Google Scholar]
- 28.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res 2008; 14: 3536–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorgun G, Calabrese E, Soydan E et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 2010; 116: 3227–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Yuan J, Righi E et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 2012; 109: 17561–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplemental statistical methods.
Appendix S2 Treatment modifications.
Fig. S1 Time to Progression Based on VEGF-634 Polymorphism.
Fig. S2 PD-I T-cell expression and time to progression. Fig. S3 Changes in circuting endothelial cells at 3 months and overall survival.
Fig. S3 Changes in circuiting endothetical cells at 3 months and overall survival.
Table S1 Comparison of docetaxel, anti-angiogenesis combination trials.