Abstract
A quantitative analysis by confocal fluorescence microscopy of the entry into HEK293 and MCF-7 cells by fluorescein-labeled octaarginine (1) and by three octa-Adp derivatives (2 – 4, octamers of the β-Asp-Arg-dipeptide, derived from the biopolymer cyanophycin) is described, including the effects of the membrane dye R18 and of DMSO on cell penetration.
Keywords: guanidinium-rich cell penetrating peptides (CPPs), octaarginine, octa-Adp, cyanophycin, quantitative CPP analysis, membrane dye R18, DMSO, confocal fluorescence microscopy
Introduction
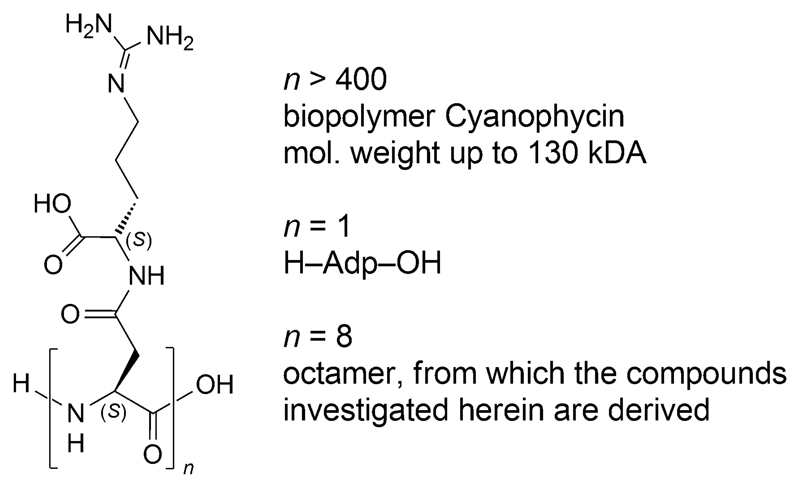
We have recently studied[1] a novel type of guanidinium-rich peptides derived from the biopolymer cyanophycin, which consists of polyaspartic-acid, to the side chains of which arginine residues are attached (Figure 1).2 Unlike in-vitro cell-penetrating octaarginine derivatives,3 such as 1, which turn out to be highly toxic upon intravenous administration to mice, octa-Adp derivatives with a free carboxylic-acid group in the side chains, such as the fluorescein-labeled FAM-Adp8 (2; Figure 2), have been found to be neither cell-penetrating nor toxic.[1]
Figure 1.
The biopolymer cyanophycin, its building block H-Adp-OH, and an octameric section; Adp stands for the β-Asp-Arg dipeptide moiety.
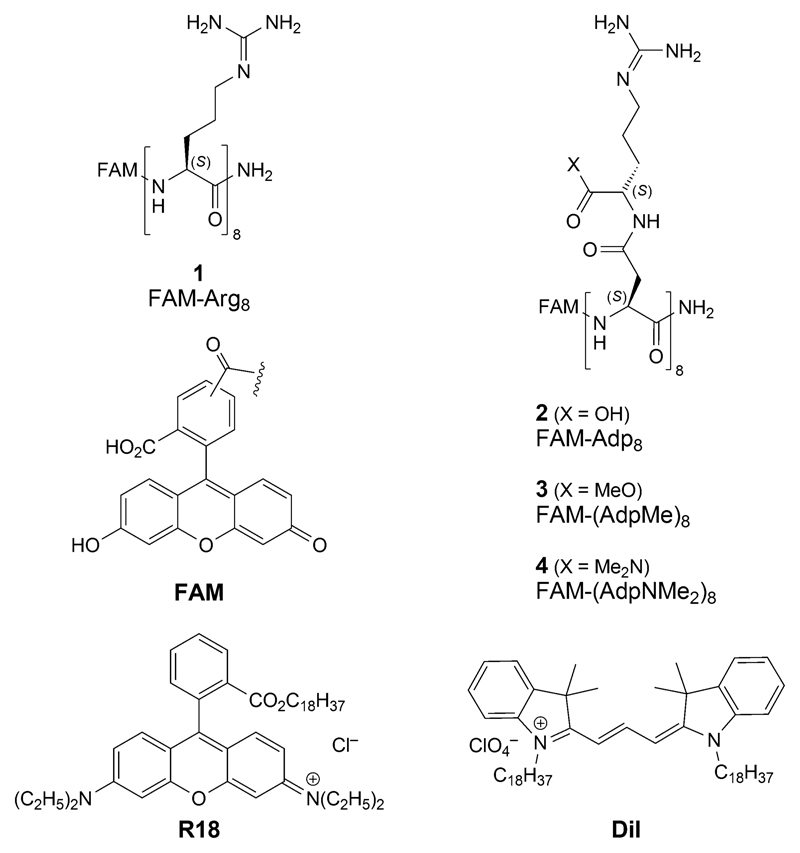
Figure 2.
Fluorescein-labeled FAM-octaarginine amide 1, the Adp analogs 2 – 4, and cationic membrane-imaging dyes R18 and Dil.
These properties reverse[1] upon esterification (→ 3, FAM-(AdpMe)84 or conversion of the carboxylic-acid group into an amide group group[8] (→ 4, FAM-(AdpNMe2)8).
In our previous paper,[1] we have described cell penetration only qualitatively. In view of the surprising properties of the Adp-derivatives 2 – 4, we have now performed a quantitative cell-penetration analysis in comparison with FAM-octaarginine 1. In particular, we wondered whether the cell-wall marker dye R18 (frequently used for fluorescence microscopy studies; Figure 2) and the solvent DMSO (widely employed for peptide dissolution) influence the cell penetration5 into mammalian cells. To further elucidate potentially varying peptide penetrations and their underlying mechanisms, we chose to study the effects onto two different cell lines with differences in their outer plasma-membrane net charge, namely HEK293 and MCF-7 cells, the latter being more negatively charged.
Materials and Methods
Peptide Syntheses
All peptides were synthesized as described by M. Grogg et al.[8]
Cell Culture
Human embryonic kidney cells (HEK293, DSMZ, Germany) were cultured in DMEM medium containing 1 g/l glucose and supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (all ThermoFischer Scientific, Switzerland), and 1% non-essential amino acids (TPP, Switzerland). The human breast cancer cell line MCF-7 (ATCC, LGC Standards, France) was cultured in DMEM medium containing 1 g/l glucose and supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (all ThermoFischer). Both cell lines were kept below 80% confluence at 37 °C and 5% CO2 in a humidified atmosphere.
μ-Slide Experiments
For peptide permeation experiments, cells were seeded into μ-slide wells (Ibidi, Switzerland). For this, cells were treated with 0.05% trypsin for 5 min and seeded into the μ-slides to form a cell layer at 20% confluency. To promote cell adhesion allowing for multiple washing steps, HEK293 cells were plated into wells functionalized with poly-l-lysine. After culture in the μ-wells for 24 h, the medium was discarded and the cells were washed once with Hank’s balanced salt solution (HBSS) containing calcium and magnesium (ThermoFischer Scientific, Switzerland). Peptides were administered at 2 μm in HBSS for 10 min at 37 °C in the dark, whereas the following conditions and controls were run in parallel, i.e. one condition per well: i) negative control including a membrane stain at 1 μm (R18, ThermoFischer Scientific, Switzerland), ii) peptide (2 μm) in HBSS, iii) peptide (2 μm) and membrane stain (1 μm), and iv) peptide (2 μm), membrane stain (1 μm), and dimethyl sulfoxide (DMSO; cell culture grade, Axon Lab, Switzerland) at a final concentration of 1% (v/v). All four peptides are very well soluble in water. Peptide stock solutions were prepared in ultra-pure water (molecular biology grade, ThermoFischer Scientific, Switzerland) at 1 mm and stored at −20 °C until use and were stable for up to 6 months as verified by HPLC measurements (data not shown). The R18 stock solution was prepared at 1 mm in ethanol (analytical grade, Sigma, Switzerland) and stored at −20 °C. Tested peptides were FAM-Arg8 (1), FAM-Adp8 (2), FAM-(AdpMe)8 (3), and FAM-(AdpNMe2)8 (4).6 Subsequent to incubation with the peptide, cells were very gently washed five times with HBSS and imaged right after.
Image Acquisition
Images were acquired using a confocal microscope (Visitron, Germany) built on a Nikon Ti2 body and a spinning disk confocal unit (CSU-W1) equipped with a live cell imaging chamber set to 37 °C and a 5% CO2 atmosphere. Images were taken with a 60 × /1.2 NA water immersion objective. The peptides’ FAM-labels were excited at λ = 488 nm (Toptica diode laser iBeam smart) and recorded with a sCMOS camera (Photometrics Prime 965B, Photometrics, USA). Filter sets were a multiband 405/488/561/640 dichroic and a 525/50 nm emission filter. R18-fluorescence was excited at λ = 561 nm (Cobold Jive DPSS laser), filter sets were a multiband 405/488/561/640 dichroic and a 630/75 nm emission filter. The R18 signal was chosen for focus adjustment as well as to prevent bleaching of the FAM molecules. Focus adjustment for conditions lacking the R18 dye was based on bright-field images.
Data Analysis
Data was extracted by ImageJ. Single cells were manually mapped in the bright field images using the polygon selection tool and single-cell fluorescence intensities extracted from multiple fluorescence images containing the FAM-signals. Background signals display the mean of six individual cell free areas from the respective FAM-negative control images. Cell fluorescence intensities were background corrected and normalized to the mean value of the FAM-negative control using MS Excel. Data plotting as well as statistics using a one-way ANOVA on ranks (non-parametric Kruskal–Wallis test) were conducted using Prism7 (Version 7.0b, GraphPad, USA).
Results
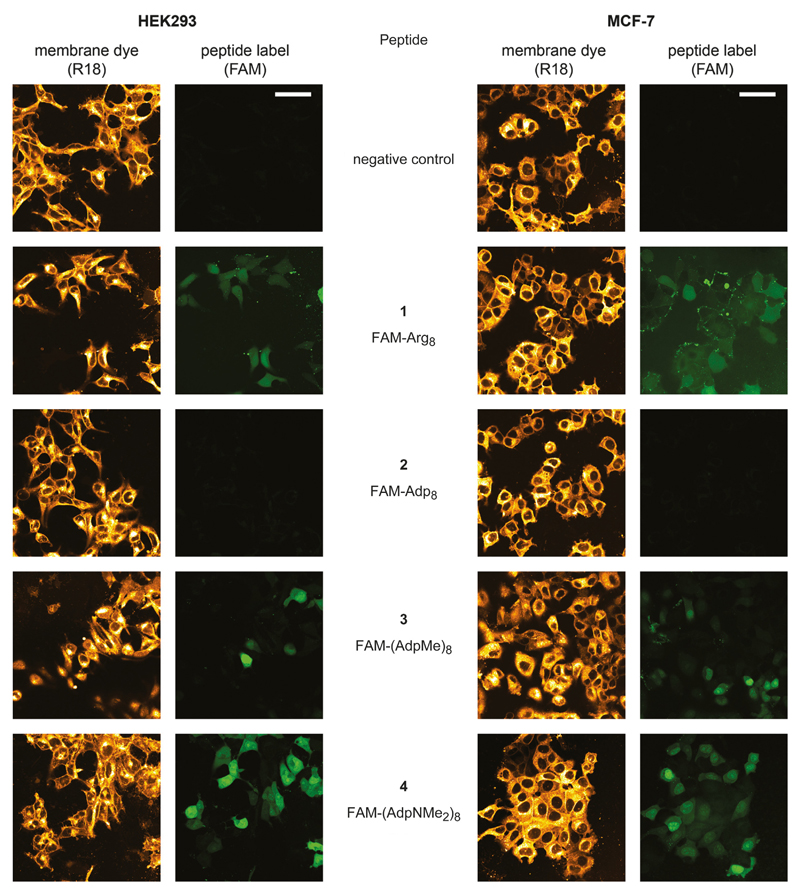
To evaluate cell permeability, we monitored the permeation efficiency of the FAM-labeled peptide derivatives 1 – 4 by confocal fluorescence microscopy (CFM). We tested the permeation across cell lipid bilayers into HEK293 and MCF-7 cells. FAM-labeled octaarginine (1) is known to permeate well into mammalian cells; we have used this compound as a reference in an investigation of enantiomeric and diastereomeric l/d-mixed octaarginines[9] and thus compound 1 now also served as a reference to the permeation characteristics of the cyanophycin derivatives, with acid-, ester- and amide-groups (2, 3, and 4, respectively) in the side chains of octa-Adp. For practical imaging purposes, cell organelle localization, and comparability to the previous study, when we had employed Dil,[9][10] we have now used R18 instead as cationic fluorescent lipophilic membrane stain to mark the plasma membranes of the cells (for molecular formulae of these markers see Figure 2).
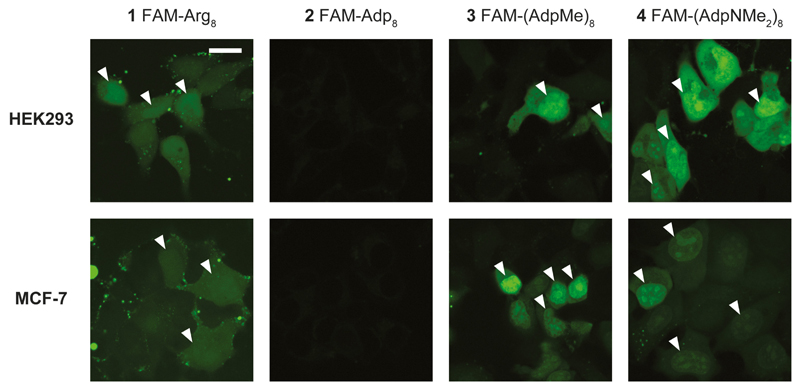
Whereas FAM-Arg8 (1) proved cell permeation, thereby confirming the previous results, FAM-Adp8 (2) showed no cell permeation at all (Figure 3). In contrast, its derivatives FAM-(AdpMe)8 (3) and especially FAM-(AdpNMe2)8 (4) showed mediocre to high cell permeability. Generally, cell permeation of FAM-octaarginine was characterized by a higher degree of compartmentalization compared to the two octa-Adp derivatives 3 and 4, which gave rise to a very homogeneous cell loading, accompanied by additionally higher concentrations in the nuclear region (Figure 4). It should be noted that peptide permeation did hardly occur in areas of full cell confluence independent of the peptide and cell line (data not shown). Cells must consequently prevail in a proliferative state for successful peptide permeation.
Figure 3.
Qualitative cell permeability assessment of the tested FAM-labeled peptides 1 – 4 for HEK293 (left) and MCF-7 (right) cells compared to its respective negative control. The employed membrane dye R18 depicts cell morphology. All peptides were administered at a final concentration of 2 μm, R18 at 1 μm. Scale bars: 50 μm.
Figure 4.
Magnified view onto peptide permeability into cells and cellular compartments, respectively. Octa-Adp derivatives 3 and 4 show homogeneous cell loading accompanied by higher peptide concentrations in the nuclei (marked by white triangles). In contrast, the higher peptide concentration is hardly visible for the octa-Arg (1) (nuclei edges marked by white triangles), no permeation again for the octa-Adp derivative 2. Compound concentrations were identical to those given in Figure 3. Scale bar: 20 μm.
Next, we aimed at evaluating the peptide permeability in more depth and quantitatively investigated the peptide penetration under varying conditions. Among others, these conditions considered dimethyl sulfoxide (DMSO), which is a vastly chosen polar solvent for the preparation of target molecule stock solutions and has as well been used in our previous studies on membrane permeation.[9][10] DMSO is, however, highly cell permeable[11] and thus may affect peptide permeation per se; likewise, the permeation characteristics of the peptides can be affected by the membrane dye, see the discussion section, below. Our tested conditions thus included the administration of the pure FAM-peptides, the FAM-peptides in combination with the membrane dye (Figure 3), and finally the FAM-peptides in combination with both, the membrane dye and DMSO, all compared to a negative control, which contained membrane dye only.
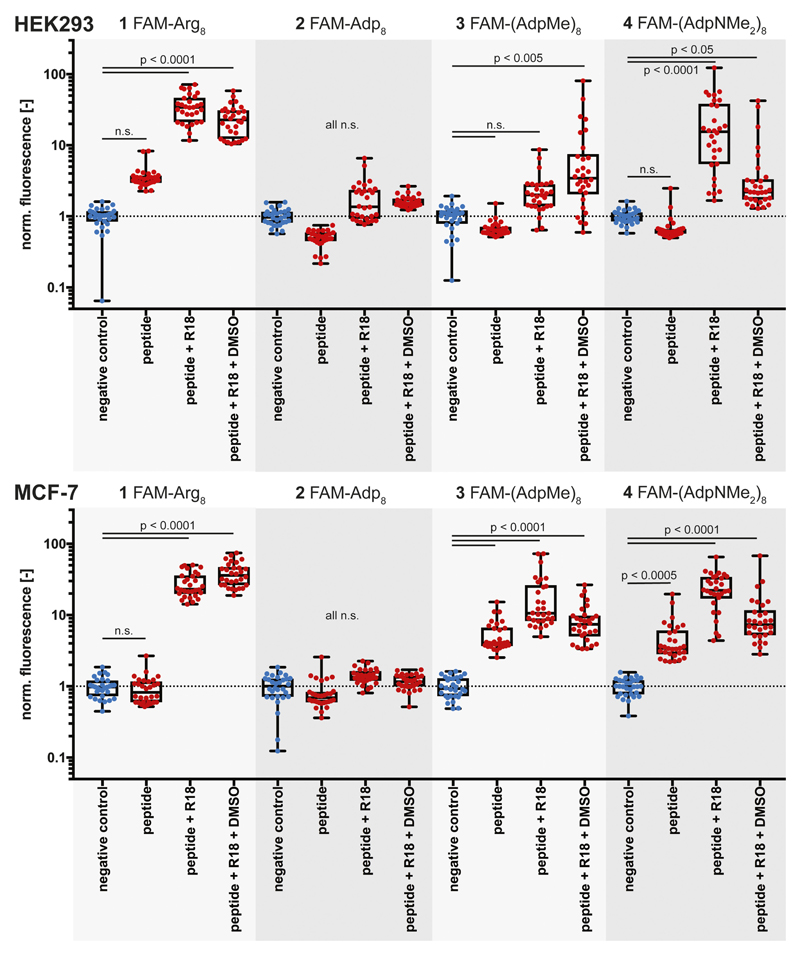
In Figure 5, all single cell data sets including the statistical evaluation are depicted. As already illustrated by the fluorescence-microscopy images (Figure 3), FAM-octaarginine 1 permeates strongest. The octameric section of cyanophycin, i.e. FAM-Adp8 peptide 2 with free carboxylic acid groups in the side chains hardly shows any cell permeation. The corresponding octamers with methyl ester (3) and dimethylamide (4) groups, however, penetrate into HEK293 and MCF-7 cells, whereby generally higher efficiencies are observed for the cancer cell line. Interestingly, all conditions without the membrane stain reveal substantially lower permeation rates compared to other conditions. Only the MCF-7 cell line exhibits significant permeation of the peptides FAM-(AdpMe)8 and FAM-(AdpNMe2)8 under conditions lacking the membrane dye.
Figure 5.
Quantitative single cell data for cell penetrating peptide permeation of 1 – 4 under all studied conditions. Top part: data represent the results from tests with HEK293 cells. Bottom part: data result from tests with the MCF-7 cells. All indicated significances (peptide alone, peptide + R18, peptide + R18 + DMSO) are in relation to the respective negative control. Concentrations of peptides were 2 μm, of membrane stain R18 1 μm, and DMSO at a final concentration of 1%. The negative control contained 1 μm R18. ncells = 30/condition, n.s. = not significant.
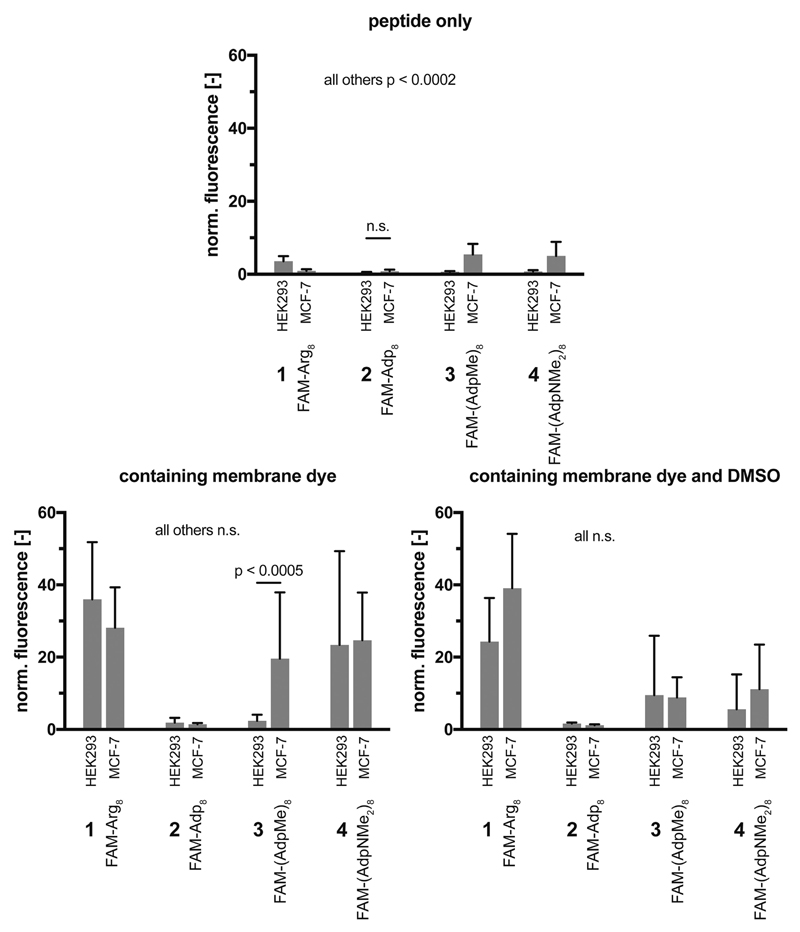
An alternative representation of these data directly compares the tested conditions for each peptide and reveals partially varying permeation efficiencies for the two cell lines (Figure 6). Although permeation rates for conditions without membrane dye are comparably low, the peptides with methylester (3) and N,N-dimethylamide groups (4) in the side chains tend to show higher permeation rates into MCF-7 cells (vice versa for FAM-Arg8, 1). The most significant difference was prevalent for the derivative containing methylester groups (FAM-(AdpMe)8, 3) under conditions containing peptide and membrane stain. All other conditions do not demonstrate significantly different permeation behaviors. Interestingly, it can be concluded from Figures 5 and 6 that the permeability of the Adp-derivative 4, which was increased in the presence of the membrane dye R18, decreased by addition of DMSO.
Figure 6.
Direct quantitative comparison of peptide permeation into HEK293 and MCF-7 cells for the four peptides 1 – 4 under various conditions. All administered concentrations are identical to those given in the caption of Figure 3; ncells = 30/condition, n.s. = not significant.
Conclusions and Discussions
In conclusion, our detailed investigation of HEK293 and MCF-7 cell penetration has i) confirmed previous results with oligo-arginine derivatives and it has ii) clearly demonstrated that the cyanophycin-derived octa-Adp peptide 2 is essentially non-cell-penetrating under all conditions tested; it has iii) shown that the ester- and amide-derivatives 3 and 4 permeate the cell walls; it has iv) provided an example for decreased cell-permeability caused by DMSO; and it has v) established that the membrane marker R18 can actually boost cell entry, especially for FAM-Arg8 and FAM-(AdpNMe2)8.
The lacking cell permeability of the Adp8-derivative 2 has been interpreted[1] as resulting from an ‘internal neutralization’ by salt formation between the guanidinum- and the carboxylate-groups in the side chains under physiological conditions (pH 7.4). This would prevent the interaction of the cationic guanidinium groups with anionic phosphate groups on the cell surfaces,7 considered to be an important first step of cell penetration by guanidinium-rich cell penetrating peptides (CPPs).[4 –6][9] Interestingly, the cell-permeabilities of the three Adp-peptides 2 – 4 are in line with their toxicities determined by mouse tail-vein injection: 2 and all other peptides derived from cyanophycin with ‘free’ carboxylic acid groups in the side chains are non-toxic, while the derivatives 3 and 4 with ester and amide groups in the side chains have toxicities comparable with those of octaarginines.[1]
Effects of DMSO on the properties of phospholipid bilayers have been extensively studied for decades.[11] [14][15 – 21] The subjects of these investigations were planar bilayers, unilamellar and multilamellar vesicles (liposomes), lipid membranes, and living cell membranes. The methods of detection of DMSO effects were X-ray diffraction, vapor pressure and electrochemical measurements, differential scanning calorimetry, and molecular dynamics simulations have also been reported. Closest to our results is perhaps the report, in which the effect of DMSO on plasma-membrane permeability to water and Ca2+ ions was studied with Chinese hamster lung fibroblast cells (DC-3F) in the presence of the permeabilization marker Yo-Pro-1: at low DMSO concentration the membranes of the cells exhibit undulations, with intermediate DMSO doses the cells become permeable to H2O and Ca2+, and with higher DMSO concentrations even the Yo-Pro-1 marker enters the cells.[18]
A look at the formulae of the cell-wall-markers R18 and Dil in Figure 2 reveals that these molecules with one or two C18H37 chains, respectively, and a positive charge will perfectly fit into cell membranes, spanning a monolayer section with their lipophilic aliphatic chain and engaging in interactions with the negatively charged phosphate groups. This must have an influence on the properties of the bilayer; the question is whether the alteration(s) of the bilayer property is significant and actually detectable with the method used. Such possible effects are often not verified in cell permeation studies.8 In the case described herein, the cell-wall-marker dye R18 significantly intensified permeation for the peptides 1, 3, and 4, under all applied conditions. There is a vast body of published papers describing various effects of such type of dyes on the properties of lipid bilayers.9 Emphasizing the role of R18, a rhodamine derivative, we should like to give a brief overview in the following section.
The localization of R18 in model membranes and in biological membranes has been extensively studied.[24 – 26] It was shown that this fluorescent cell-wall marker, if added externally, is first incorporated in the outer leaflet and can then move into the inner leaflet of the phospholipid bilayer, by what is called a ‘lipid flip-flop’ process, known to be accelerated by the membrane potential.[27][[28] The dye R18 further has a specific affinity for the Ld phase of the bilayer.[24][26] As a π-system with delocalized positive charge, R18 may act as a transporter of molecules with likewise delocalized anionic charges into and across phospholipid bilayers.[29] Dramatic effects of R18 and other membrane markers have been observed in investigations of viral membrane fusion, all the way to inactivation of the PR8 influenza virus.[30] Finally, fluorescent lipid probes have been shown to generate light-induced artifacts under laser irradiation of confocal fluorescence microscopy experiments.[31 – 34]
In summary, and in agreement with our experimental result, we must state that these fluorescent dyes are by no means ‘innocent’ labels, and that ‘caution is needed when using direct labeling of biological membranes’.[30]
Acknowledgements
The authors gratefully acknowledge peptide syntheses by M. Grogg. Financial supports from the European Research Council (ERC Consolidator Grant No. 681587) and from the NCCR Molecular Systems Engineering to P. S. D. are acknowledged.
Footnotes
Oligo-arginine derivatives may be considered the ‘gold standard’ of cell-penetrating peptides (CPPs). For recent review articles by experts in the field see Refs. [4 – 6] and extensive citations in Ref. [1].
An Arg methylester, as in 3, was attached to make poly (disulfide)s cell-permeable, as described by the Matile group.[7]
We have not found a systematic quantitative analysis of such effects on cell entry of FAM-labeled guanidinium-rich CPPs in the literature.
The peptide samples employed for preparing the solutions were the 1-, 2-, 3-, and 4- 8 CF3CO2H salts; the concentrations given herein refer to the actual peptide content. For a discussion of this correction see footnote 5 in Ref. [1].
In line with this argumentation is the observation that the two Adp-peptides 3 and 4 with ester and amide groups, respectively, showed augmented permeation into the MCF-7 cancer cells, as compared to the HEK cells: cancer cells are known to exhibit a more negative net charge on the outer leaflet of the plasma membrane due to a deficit in maintaining negatively charged phosphatidylserines within the inner leaflet;[12][13] see also the discussion in Ref. [1].
In our first investigations of cell penetrating oligo-β-arginines with 3T3 mouse fibroblast cells, with human foreskin keratinocytes (HFKs), and with HeLa cells no cell-wall or nuclear markers were used.[22][23]
For a comprehensive guide to fluorescent probes visit thermofisher.com/bioprobes.
Author Contribution Statement
All experiments, image and data analyses were carried out by F. K. P. S. D., and D. S. supervised the project. The research was conceived by F. K. and D. S. The manuscript was written by F. K., P. S. D., D. S., and P. W.
References
- [1].Grogg M, Hilvert D, Ebert M-O, Beck AK, Seebach D, Kurth F, Dittrich PS, Sparr C, Wittlin S, Rottmann M, Mäser P. Cell Penetration, Herbicidal Activity, and in-vivo-Toxicity of Oligoarginine Derivatives and of Novel Guanidinium-Rich Compounds Derived from the Biopolymer Cyanophycin. Helv Chim Acta. 2018;101:e1800112. doi: 10.1002/hlca.201800112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frommeyer M, Wiefel L, Steinbüchel A. Features of the biotechnologically relevant polyamide family “cyanophycins” and their biosynthesis in prokaryotes and eukaryotes. Crit Rev Biotechnol. 2016;36:153–164. doi: 10.3109/07388551.2014.946467. [DOI] [PubMed] [Google Scholar]
- [3].Sallam A, Kast A, Przybilla S, Meiswinkel T, Steinbüchel A. Biotechnological Process for Production of β-Dipeptides from Cyanophycin on a Technical Scale and Its Optimization. Appl Environ Microbiol. 2009;75:29–38. doi: 10.1128/AEM.01344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Futaki S, Nakase I. Cell-Surface Interactions on Arginine-Rich Cell-Penetrating Peptides Allow for Multiplex Modes of Internalization. Acc Chem Res. 2017;50:2449–2456. doi: 10.1021/acs.accounts.7b00221. [DOI] [PubMed] [Google Scholar]
- [5].Vargas JR, Geihe Stanzl E, Teng NNH, Wender PA. Cell-Penetrating, Guanidinium-Rich Molecular Transporters for Overcoming Efflux-Mediated Multidrug Resistance. Mol Pharmaceutics. 2014;11:2553–2565. doi: 10.1021/mp500161z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Copolovici DM, Langel K, Eriste E, Langel Ü. Cell-Penetrating Peptides: Design, Synthesis, and Applications. ACS Nano. 2014;8:1972–1994. doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- [7].Morelli P, Bartolami E, Sakai N, Matile S. Glycosylated Cell-Penetrating Poly(disulfide)s: Multifunctional Cellular Uptake at High Solubility. Helv Chim Acta. 2018;101:e1700266. [Google Scholar]
- [8].Grogg M, Hilvert D, Beck AK, Seebach D. Syntheses of Cyanophycin Segments for Investigations of Cell-Penetration. Synthesis. 2018 doi: 10.1055/s-0037-1610202. [DOI] [Google Scholar]
- [9].Purkayastha N, Eyer K, Robinson T, Dittrich PS, Beck AK, Seebach D, Kolesinska B, Cadalbert R. Enantiomeric and Diastereoisomeric (Mixed) L/D-Octaarginine Derivatives – A Simple Way of Modulating the Properties of Cell-Penetrating Peptides. Chem Biodiversity. 2013;10:1165–1184. doi: 10.1002/cbdv.201300180. [DOI] [PubMed] [Google Scholar]
- [10].Kolesinska B, Eyer K, Robinson T, Dittrich PS, Beck AK, Seebach D, Walde P. Interaction of β3/β2-Peptides, Consisting of Val-Ala-Leu Segments, with POPC Giant Unilamellar Vesicles (GUVs) and White Blood Cancer Cells (U937) – A New Type of Cell-Penetrating Peptides, and a Surprising Chain-Length Dependence of Their Vesicle- and Cell-Lysing Activity. Chem Biodiversity. 2015;12:697–732. doi: 10.1002/cbdv.201500085. [DOI] [PubMed] [Google Scholar]
- [11].Yu Z-W, Quinn PJ. Dimethyl sulphoxide: A review of its applications in cell biology. Bioscience Rep. 1994;14:259–281. doi: 10.1007/BF01199051. [DOI] [PubMed] [Google Scholar]
- [12].Riedl S, Zweytick D, Lohner K. Membrane-active host defense peptides – Challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids. 2011;164:766–781. doi: 10.1016/j.chemphyslip.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190–194. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- [14].Yu Z-W, Quinn PJ. Solvation effects of dimethyl sulfoxide on the structure of phospholipid bilayers. Biophys Chem. 1998;70:35–39. doi: 10.1016/s0301-4622(97)00100-2. [DOI] [PubMed] [Google Scholar]
- [15].Yamashita Y, Kinoshita K, Yamazaki M. Low concentration of DMSO stabilizes the bilayer gel phase rather than the interdigitated gel phase in dihexadecylphosphatidylcholine membrane. Biochim Biophys Acta. 2000;1467:395–405. doi: 10.1016/s0005-2736(00)00237-6. [DOI] [PubMed] [Google Scholar]
- [16].Sum AK, de Pablo JJ. Molecular Simulation Study on the Influence of Dimethylsulfoxide on the Structure of Phospholipid Bilayers. Biophys J. 2003;85:3636–3645. doi: 10.1016/S0006-3495(03)74781-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Westh P. Preferential interaction of dimethyl sulfoxide and phosphatidyl choline membranes. Biochim Biophys Acta. 2004;1664:217–223. doi: 10.1016/j.bbamem.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [18].de Ménorval M-A, Mir LM, Fernández ML, Reigada R. Effects of Dimethyl Sulfoxide in Cholesterol-Containing Lipid Membranes: A Comparative Study of Experiments In Silico and with Cells. PLoS ONE. 2012;7:e41733. doi: 10.1371/journal.pone.0041733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gurtovenko AA, Anwar J. Modulating the Structure and Properties of Cell Membranes: The Molecular Mechanism of Action of Dimethyl Sulfoxide. J Phys Chem B. 2007;111:10453–10460. doi: 10.1021/jp073113e. [DOI] [PubMed] [Google Scholar]
- [20].Lin J, Novak B, Moldovan D. Molecular Dynamics Simulation Study of the Effect of DMSO on Structural and Permeation Properties of DMPC Lipid Bilayers. J Phys Chem B. 2012;116:1299–1308. doi: 10.1021/jp208145b. [DOI] [PubMed] [Google Scholar]
- [21].Majdi S, Najafinobar N, Dunevall J, Lovric J, Ewing AG. DMSO Chemically Alters Cell Membranes to Slow Exocytosis and Increase the Fraction of Partial Transmitter Released. ChemBioChem. 2017;18:1898–1902. doi: 10.1002/cbic.201700410. [DOI] [PubMed] [Google Scholar]
- [22].Rueping M, Mahajan Y, Sauer M, Seebach D. Cellular Uptake Studies with β-Peptides. ChemBioChem. 2002;3:257–259. doi: 10.1002/1439-7633(20020301)3:2/3<257::AID-CBIC257>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [23].Seebach D, Namoto K, Mahajan YR, Bindschädler P, Sustmann R, Kirsch M, Ryder NS, Weiss M, Sauer M, Roth C, Werner S, et al. Chemical and Biological Investigations of β-Oligoarginines. Chem Biodiversity. 2004;1:65–97. doi: 10.1002/cbdv.200490014. [DOI] [PubMed] [Google Scholar]
- [24].Baumgart T, Hunt G, Farkas ER, Webb WW, Feigenson GW. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim Biophys Acta. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jensen EC. Use of Fluorescent Probes: Their Effect on Cell Biology and Limitations. Anat Rec. 2012;295:2031–2036. doi: 10.1002/ar.22602. [DOI] [PubMed] [Google Scholar]
- [26].Klymchenko AS, Kreder R. Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chem Biol. 2014;21:97–113. doi: 10.1016/j.chembiol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- [27].Leenhouts JM, De Kruijff B. Membrane potential-driven translocation of a lipid-conjugated rhodamine. Biochim Biophys Acta. 1995;1237:121–126. doi: 10.1016/0005-2736(95)00093-i. [DOI] [PubMed] [Google Scholar]
- [28].Melikyan GB, Deriy BN, Ok DC, Cohen FS. Voltage-Dependent Translocation of R18 and Dil Across Lipid Bilayers Leads to Fluorescence Changes. Biophys J. 1996;71:2680–2691. doi: 10.1016/S0006-3495(96)79459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rokitskaya TI, Sumbatyan NV, Tashlitsky VN, Korshunova GA, Antonenko YN, Skulachev VP. Mitochondria-targeted penetrating cations as carriers of hydrophobic anions through lipid membranes. Biochim Biophys Acta. 2010;1798:1698–1706. doi: 10.1016/j.bbamem.2010.05.018. [DOI] [PubMed] [Google Scholar]
- [30].Wunderli-Allenspach H, Güthert M, Ott S. Inactivation of PR8 Influenza Virus through the Octadecylrhodamine B Chloride Membrane Marker. Biochemistry. 1993;32:900–907. doi: 10.1021/bi00054a022. [DOI] [PubMed] [Google Scholar]
- [31].Devaux PF, Fellmann P, Hervé P. Investigation on lipid asymmetry using lipid probes: Comparison between spin-labeled lipids and fluorescent lipids. Chem Phys Lipid. 2002;116:115–134. doi: 10.1016/s0009-3084(02)00023-3. [DOI] [PubMed] [Google Scholar]
- [32].Ayuyan AG, Cohen FS. Lipid Peroxides Promote Large Rafts: Effects of Excitation of Probes in Fluorescence Microscopy and Electrochemical Reactions during Vesicle Formation. Biophys J. 2006;91:2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao J, Wu J, Shao H, Kong F, Jain N, Hunt G, Feigenson G. Phase studies of model biomembranes: Macroscopic coexistence of Lα + Lβ with light-induced coexistence of Lα + Lo Phases. Biochim Biophys Acta. 2007;1768:2777–2786. doi: 10.1016/j.bbamem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Staneva G, Seigneuret M, Conjeaud H, Puff N, Angelova MI. Making a Tool of an Artifact: The Application of Photoinduced Lo Domains in Giant Unilamellar Vesicles to the Study of Lo/Ld Phase Spinodal Decomposition and Its Modulation by the Ganglioside GM1. Langmuir. 2011;27:15074–15082. doi: 10.1021/la203101y. [DOI] [PubMed] [Google Scholar]