Abstract
Nucleated cells eliminate lesions induced by bacterial pore-forming toxins, such as pneumolysin via shedding patches of damaged plasmalemma into the extracellular milieu. Recently, we have shown that the majority of shed pneumolysin is present in the form of inactive pre-pores. This finding is surprising considering that shedding is triggered by Ca2+-influx following membrane perforation and therefore is expected to positively discriminate for active pores versus inactive pre-pores.
Here we provide evidence for the existence of plasmalemmal domains that are able to attract pneumolysin at high local concentrations. Within such a domain an immediate plasmalemmal perforation induced by a small number of pneumolysin pores would be capable of triggering the elimination of a large number of not yet active pre-pores/monomers and thus pre-empt more frequent and perilous perforation events. Our findings provide further insights into the functioning of the cellular repair machinery which benefits from an inhomogeneous plasmalemmal distribution of pneumolysin.
1. Introduction
Streptococcus pneumoniae is a potent human pathogen [1,2]. Its cholesterol-dependent cytolysin (CDC), pneumolysin (PLY) is instrumental for breaching the host's epithelial barrier and for the incapacitation of the immune system [3–5]. CDCs are secreted as soluble monomers and bind to cholesterol within eukaryotic plasmalemma [6,7]. After oligomerization, they initially form inactive pre-pores that eventually undergo a transition to active membrane-perforating pores [6,8].
The plasmalemmal lipid bilayer is believed to display an inhomogeneous distribution of different lipid species [9,10]. Aided by protein-lipid and lipid-lipid interactions, cholesterol-rich microdomains embedded in a saturated lipid environment (lipid rafts) alternate with regions of low cholesterol surrounded by unsaturated phospholipids [11–14]. Remaining beyond the resolution limit of conventional light microscopy [9], short-living and highly dynamic, lipid rafts could be stabilized by protein-ligands, which, in turn, could trigger raft coalescence [15].
While playing an important role in cellular homeostasis [4], membrane rafts are also hijacked by pathogens to harm a host cell: plasmalemmal binding of CDCs and their oligomerization is potentiated within membrane lipid rafts [2,15–19].
Nucleated cells are capable of eliminating PLY-induced lesions by shedding damaged, PLY- containing plasmalemmal patches into the extracellular milieu in form of microvesicles [1,20,21]. Recently, we have shown that the vast majority of shed, microvesicle-associated PLY is present in the form of inactive pre-pores; whereas active PLY-pores featuring a perforated membrane account for only 10% of the total PLY [22]. This finding is surprising considering that the process of shedding is triggered by Ca2+-entry from the extracellular milieu following plasmalemmal perforation and is therefore expected to positively discriminate for active pores versus inactive pre-pores [21,22].
Here, using live-cell imaging in combination with model membrane experiments under microfluidic control we have analyzed potential mechanisms that might be responsible for the preferential shedding of inactive PLY-pre-pores in the process of plasmalemmal repair. We provide evidence for the existence of distinct plasmalemmal domains that are capable to attract PLY at a high local concentration. Furthermore, we show that in artificial membranes PLY preferentially targets cholesterol-rich domains and their boundaries. It is feasible that such domains might be either permanently present within the plasmalemma of nucleated cells as a result of protein-driven lipid segregation, or are a result of PLY-induced self-association of nanoscale, lipid-driven lipid inhomogeneities such as membrane rafts. Our findings provide further insights into the functioning of the cellular repair machinery that benefits from an inhomogeneous distribution of PLY on the plasmalemma and triggers an effective antibacterial defense [1,20].
2. Results
2.1. Inhomogeneous distribution of PLY on the plasmalemma of nucleated cells
Treatment of cultured human cells of various origins with fluorescently labelled PLY revealed a strikingly inhomogeneous distribution of the toxin within the plane of the cell's plasmalemma: distinct, clearly defined sub-micrometer domains of highly concentrated PLY, alternated with domains that were virtually toxin-free (Fig. 1a, Fig. SI 1a). Remarkably aerolysin, a pore-forming toxin, secreted by Aeromonas hydrophila, which does not bind to cholesterol but instead uses a glycosylphosphatidylinositol (GPI) anchor as its plasmalemmal receptor [23–25] remained homogeneously distributed on the plasma membrane (Fig. 1b, Fig. SI 1b, Supplemental Movies 1,2).
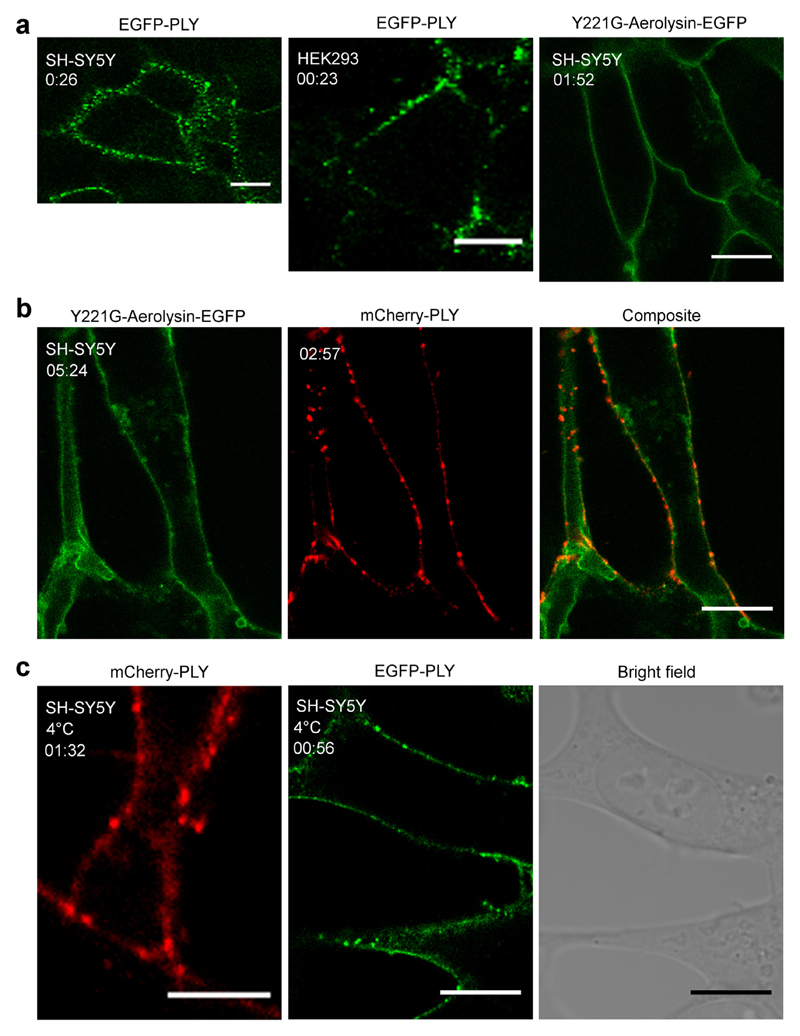
Fig. 1. Inhomogeneous binding of pneumolysin to the plasma membrane of nucleated cells.
(a) Inhomogeneous distribution of EGFP-PLY (14.6 nM) on the plasmalemma of SH-SY5Y or HEK293 cells in the presence of 2.5 mM Ca2+ contrasts with a homogeneous distribution of Y221G-Aerolysin-EGFP (113 nM). (b) Inhomogeneous distribution of mCherry-PLY (160 nM) on SH-SY5Y cells that were pre-treated with Y221G-Aerolysin-EGFP (113 nM). (c) Inhomogeneous distribution of mCherry-PLY (160 nM) or EGFP-PLY (159.5 nM) on SH-SY5Y cells at 4 °C in the presence of 2.5 mM Ca2+. Representative images are shown. (a) n = 9, (b) n = 3, (c) n = 3. Magnification bars (a, b) = 10 μm, (c, mCherry-PLY) = 5 μm, (c, EGFP-PLY) = 10 μm. Incubation time = mm:ss.
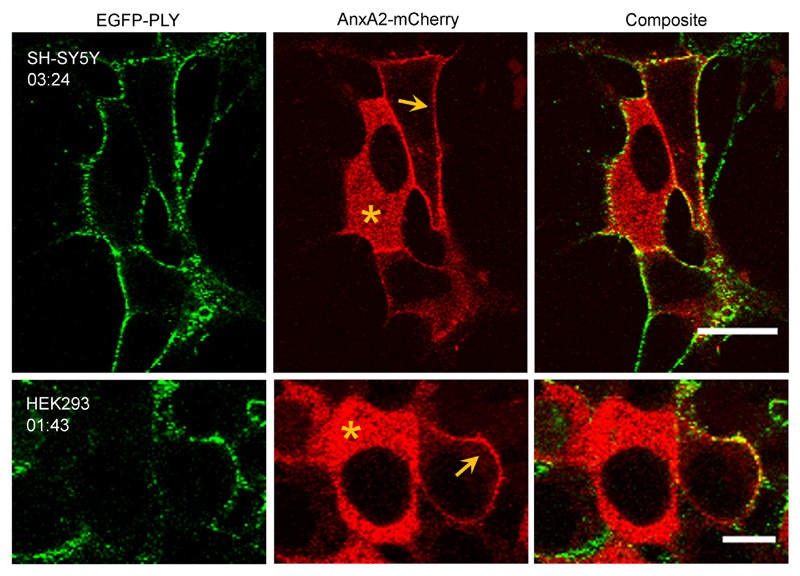
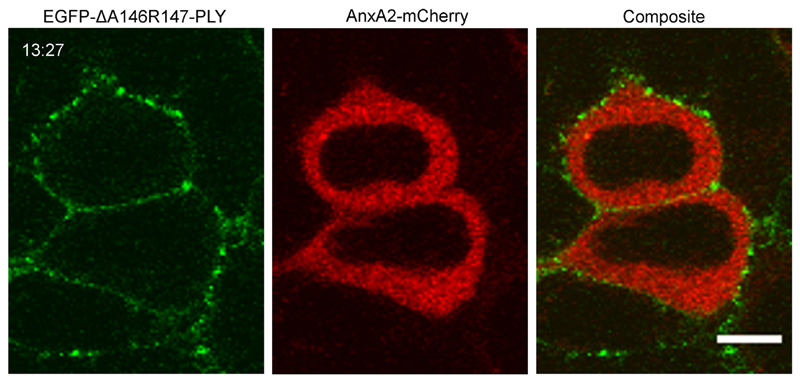
The PLY-rich plasmalemmal domains were formed at sublytic (Fig.1a) and lytic (Fig.1b) PLY concentrations alike [22]. Moreover, similar domains were observed at 4 °C, indicating that a decrease in membrane fluidity [26] of the plasmalemma did not affect PLY selective binding or the size of the domains (Fig.1c). An inhomogeneous PLY-distribution was observed in cells that were able to reseal PLY-induced plasmalemmal lesions and thus prevented uncontrolled elevation of intracellular Ca2+ (marked by the cytoplasmic localization of the Ca2+-sensor, annexin A2 (AnxA2) [1,22]) (Fig. 2, asterisks; Fig. SI 2) and in actively perforated cells (marked by the plasmalemmal localization of AnxA2 [1,22]) (Fig. 2, arrows). The inhomogeneous plasmalemmal PLY distribution was also observed for the non-lytic PLY-mutant [22] (Fig. 3, Fig. SI 2), which is capable of plasmalemmal binding but is unable to perforate the plasmalemma [27]. Thus, the segregation between PLY-rich and PLY-depleted plasmalemmal domains occurred already during the PLY-binding stage and persisted throughout the process of active pore-formation.
Fig. 2. Inhomogeneous distribution of pneumolysin on the plasmalemma of perforated and non-perforated cells.
Annexin A2-mCherry expressing SH-SY5Y and HEK293 cells were incubated with EGFP-PLY (29.1 nM) in the presence of 2.5 mM Ca2+. Note the cytosolic localization of the Ca2+ senor, AnxA2-mCherry (asterisks) in resealed cells; the plasmalemmal localization of AnxA2-mCherry (arrows) marks perforated cells. Representative images are shown. n = 4. Magnification bars: SH-SY5Y = 20 μm, HEK293 = 10 μm. Incubation time = mm:ss.
Fig. 3. Inhomogeneous membrane association of a non-lytic pneumolysin mutant.
Binding of a non-lytic mutant EGFP-ΔA146R147-PLY (35.7 nM) to the plasmalemma of AnxA2-mCherry transfected HEK293 cells in the presence of Ca2+. Representative images are shown. n = 2. Magnification bar = 10 μm. Incubation time = mm:ss.
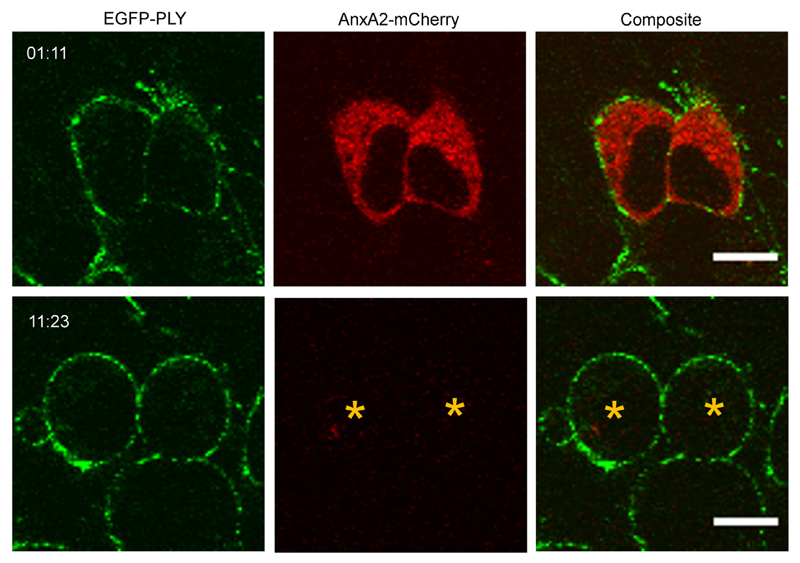
The inhomogeneous PLY-distribution was observed, both in the presence (Fig. 1) and in the absence of extracellular calcium (Fig. 4), which indicates that PLY-segregation was not driven by the Ca2+-dependent proteins of the plasmalemmal repair machinery that were responsible for the elimination of PLY-pores [1,22]. The inhomogeneous PLY-distribution persisted in lysed cells that completely lost their cytosolic content (Fig. 4, asterisks), which suggested that PLY-segregation was not an active, ATP-driven process. In the absence of extracellular Ca2+, the lysed cells rounded up (Fig. 4), which indicates that the attachment between plasma membrane and cytoskeleton was compromised. However, the inhomogeneous distribution of PLY remained, suggesting that the spatial segregation of the plasmalemma driven by its permanent attachment to static cytoskeletal elements was also not the causative agent of PLY-segregation.
Fig. 4. Inhomogeneous membrane association of pneumolysin in the absence of Ca2+.
AnxA2-mCherry transfected SH-SY5Y cells were treated with EGFP-PLY (24.4 nM) in the presence of 100 μM EGTA. The inhomogeneous PLY-distribution persisted in lysed cells that completely lost their cytosolic content (asterisks). Representative images are shown. n = 4. Magnification bar = 10 μm. Incubation time = mm:ss.
Based on this wealth of evidence, we hypothesized that PLY-segregation is driven by the intrinsic features of the plasmalemmal lipid bilayer, most likely by its inhomogeneity in the distribution of cholesterol. Therefore, we ascertained factors that determine PLY-binding and its spatial distribution on artificial lipid bilayers.
2.2. PLY preferentially binds to cholesterol embedded in an unsaturated phospholipid environment
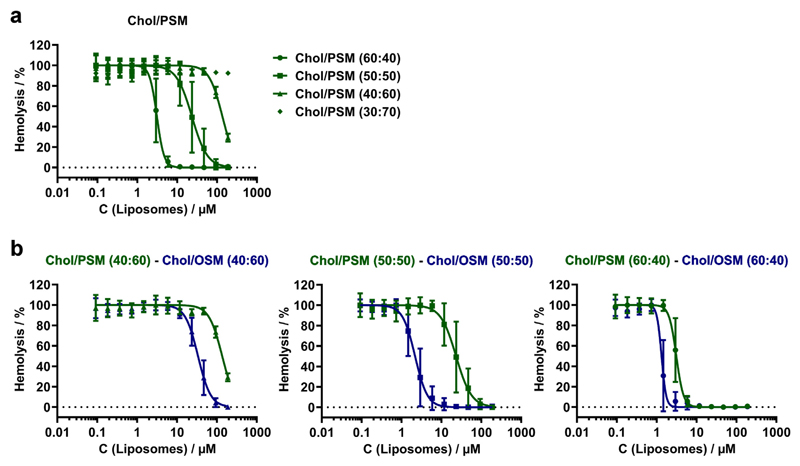
Initially we analyzed PLY-binding to liposomes composed of homogenous binary lipid mixtures using a competitive hemolytic assay, in which liposomes of different lipid composition were pre-incubated with PLY followed by the addition of human red blood cells. An inhibition of hemolytic activity was indicative of PLY-sequestration (binding) by liposomes. We confirmed that PLY binds to cholesterol in liposomal membranes using binary mixtures of cholesterol (Chol) and N-palmitoyl-sphingosylphosphorylcholine (sphingomyelin, PSM) (Fig. 5a). The highest degree of protection was achieved by liposomes that were saturated with cholesterol (60 mol/%) [28,29].
Fig. 5. Membrane cholesterol and lipid saturation determine pneumolysin binding to liposomes.
(a) Hemolytic activity was measured after pre-incubation of PLY with PSM-liposomes containing various amounts of cholesterol. (b) Pneumolysin binds with higher affinity to cholesterol in an unsaturated lipid environment (OSM, blue) compared to cholesterol in a saturated lipid environment (PSM, green). Each curve represents the results of 3 to 5 independent experiments performed in triplicates. Error bars represent SD.
However, the relative concentration of cholesterol within the liposomal bilayer rather than its overall concentration in the assay seems to be the primary determinant of PLY-binding. Please note that 0.6 μM cholesterol present within liposomes with a high relative cholesterol content (Chol:PSM = 60:40; 1.46 μM total lipid) provided complete protection against PLY-induced hemolysis, whereas a ~100 fold higher amount of cholesterol (56.25 μM) present within liposomes with a low relative cholesterol content (Chol:PSM = 30:70; 187.5 μM total lipid) provided no protection at all (Fig. 5a). Thus, it is evident that a threshold of ≥30 mol/% cholesterol had to be attained within the liposomal membrane for the efficient inhibition of hemolysis. This suggests that the lipid bilayer itself plays a modulatory role in PLY-cholesterol binding. In particular, it appears that at low relative concentrations, cholesterol is shielded from PLY by the bulky lipid head-groups [28–31].
Therefore, we tested whether the packing order of individual phospholipid molecules within the liposomal bilayer might influence the availability of cholesterol for PLY-binding. The packing order of lipids within bilayers is largely determined by the saturation of their alkyl chains. To address the effect of lipid alkyl chain saturation on PLY-cholesterol binding, we analyzed the PLY-sequestration properties of liposomes composed of cholesterol embedded in a tightly packed lipid bilayer formed by a saturated lipid N-palmitoyl-sphingosylphosphorylcholine (Chol/PSM) in comparison to that of the loosely packed lipid bilayer formed by an unsaturated N-oleoyl-sphingosylphosphorylcholine (Chol/OSM) (Fig. 5b). The direct comparison of these two lipid mixtures demonstrated that cholesterol-containing liposomes composed of unsaturated OSM sequestered PLY with considerably higher affinity than liposomes composed of saturated PSM (Fig. 5b, Table SI 1). The largest differences in the affinities of PLY-binding to cholesterol, which was embedded either in a saturated or an unsaturated lipid environment, were observed for liposomes containing 50 mol/% cholesterol with Chol/OSM binding PLY with ~11 higher efficiency than Chol/PSM (Table SI 1).
Taken together, our data suggest that binding of PLY to artificial lipid bilayers is facilitated by the high content of cholesterol and by the high content of unsaturated phospholipids, the latter being in line with a recent study showing that increased membrane fluidity positively relates to binding and oligomerization of pore forming toxins [32].
It is believed that in the plasmalemma of nucleated cells, micro-domains of high cholesterol concentration embedded in a saturated lipid environment (lipid rafts) alternate with regions of low cholesterol surrounded by unsaturated phospholipids [11,13,33]. Therefore, our results (Fig. 5, Table SI 1) suggest that the plasmalemmal lipid bilayer in vivo does not feature structural elements that would grant optimal PLY-binding, namely domains with high content of both cholesterol and unsaturated phospholipids.
2.3. PLY preferentially binds the cholesterol-rich domains in liposomes featuring segregated lipid phases
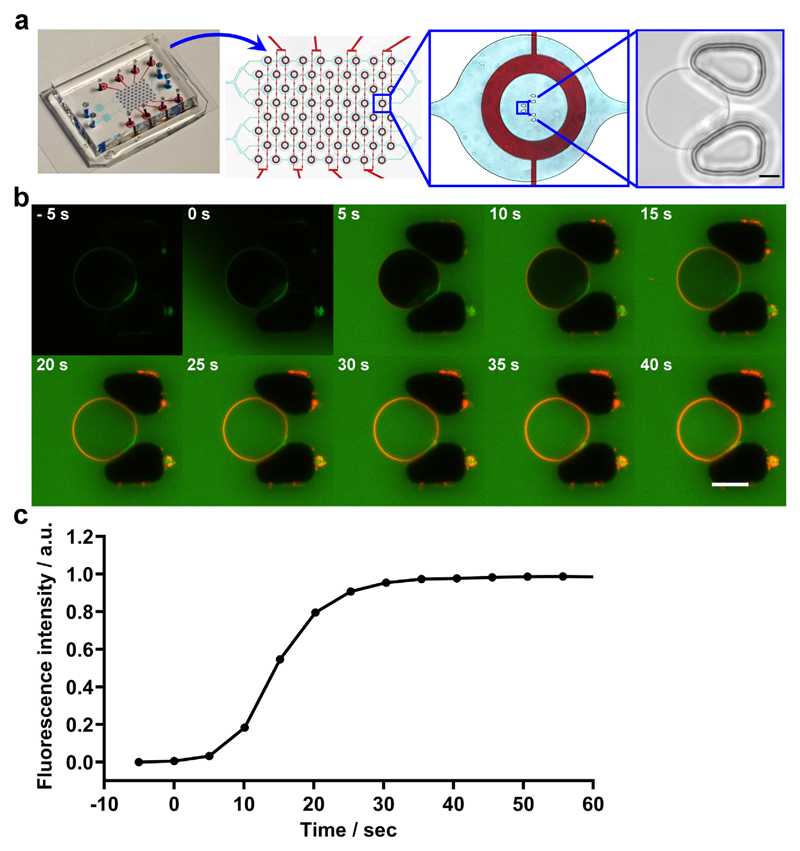
Therefore, we probed further whether PLY shows binding preferences for distinct lipid domains by laser-scanning confocal microscopy using fluorescently-labelled PLY and membranes featuring a homogenous, non-segregated lipid bilayer as well as a bilayer featuring stable segregated lipid phases that mimic an inhomogeneous distribution of natural lipids within the plasmalemma. For these experiments we employed a microfluidic chip with an improved design to enhance the trapping efficacy of individual giant vesicles [34–36]. The layout contains two separate microfluidic inlet channels; each containing 32 trap-chambers with three individual gap distances between the pillars (Fig. 6a).
Fig. 6. Homogeneous binding of pneumolysin to a giant vesicle featuring a homogeneous membrane.
(a) Overview of the microfluidic device designed to analyze individual giant vesicles. The microfluidic layer, which is used for vesicle trapping is marked by blue dye and the operation control layer is highlighted by red dye. The whole chip is 27 × 20 × 6 mm (LWH), assembled on a microscopic slide. The gaps between the pillars have a distance of 7, 8, and 10 μm and can trap giant vesicles of about 7–20 μm in diameter (bright field image). Magnification bar = 5 μm. (b) A giant vesicle composed of cholesterol/DOPC (50:50) and containing 0.05 mol/% BODIPY-PC was incubated with buffer containing 0.5% (w/v) FITC-dextran and mCherry-PLY (320 nM) in a flow-trap chamber. Magnification bar = 10 μm. (c) Changes in the FITC-dextran fluorescence intensity in the vesicle's lumen (normalized to background FITC-dextran intensity) denote the vesicle's permeabilization by PLY. n = 4.
Incubation of giant vesicles composed of cholesterol and unsaturated di-oleoyl-phosphatidylcholine (DOPC) (50:50), which do not feature segregated lipid phases [37] with mCherry-PLY showed homogenous PLY-distribution on the vesicles' membrane (Fig. 6b). The homogenous PLY-distribution was observed during the initial PLY-binding phase and persisted after the formation of active PLY-pores and perforation of the vesicle's membrane (Fig. 6b,c). This is in stark contrast to the inhomogeneous PLY-distribution on the membranes of nucleated cells (Fig. 1-4).
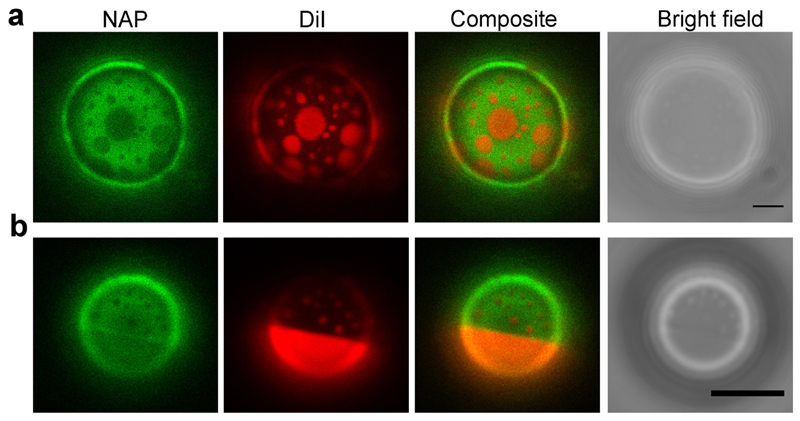
We next assessed PLY-binding to liposomes featuring phase-separated domains. In these experiments liposomes containing DOPC/dipalmitoyl-phosphatidylcholine (DPPC)/cholesterol (30:30:40) and DOPC/PSM/cholesterol (30:30:40) were selected to mimic the lipid composition of the plasma membrane of eukaryotic cells in terms of cholesterol concentration, the ratio of saturated versus unsaturated lipids and the presence of (mostly unsaturated) natural phosphatidylcholine and (mostly saturated) natural sphingomyelin. The mixture of DOPC/DPPC/cholesterol (30:30:40) segregates into liquid-ordered (lo) domains that are enriched in saturated DPPC and cholesterol and liquid disordered (ld) domains that are rich in unsaturated DOPC but depleted in cholesterol [38]. The DOPC/PSM/cholesterol (30:30:40) mixture forms lo domains rich in saturated PSM as well as cholesterol and ld domains which are rich in DOPC but are depleted of cholesterol [39]. Correspondingly, both types of vesicles featured distinct domains that were alternatively marked by Naphtho[2,3-a]pyrene (NAP) and [1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine iodide] (DiI) (Fig. 7). We confirmed that at our experimental conditions NAP selectively labelled the lo, cholesterol-rich domain, whereas DiI labelled the ld, cholesterol-depleted domain, which is in line with a previous report [35] (Supplemental Information, Fig. SI 3, Fig. SI 4).
Fig. 7. Vesicles composed of ternary lipid mixtures show separated lipid phases that are marked by either NAP or DiI.
(a) Top view on the upper hemisphere of a giant vesicle composed of DOPC/DPPC/cholesterol (30:30:40) containing 0.2 mol/% NAP and 0.05 mol/% DiI. (b) Top view on the upper hemisphere of a giant vesicle composed of DOPC/PSM/cholesterol (30:30:40) containing 0.2 mol/% NAP and 0.05 mol/% DiI. Representative images are shown. n = 5. Magnification bar = 5 μm.
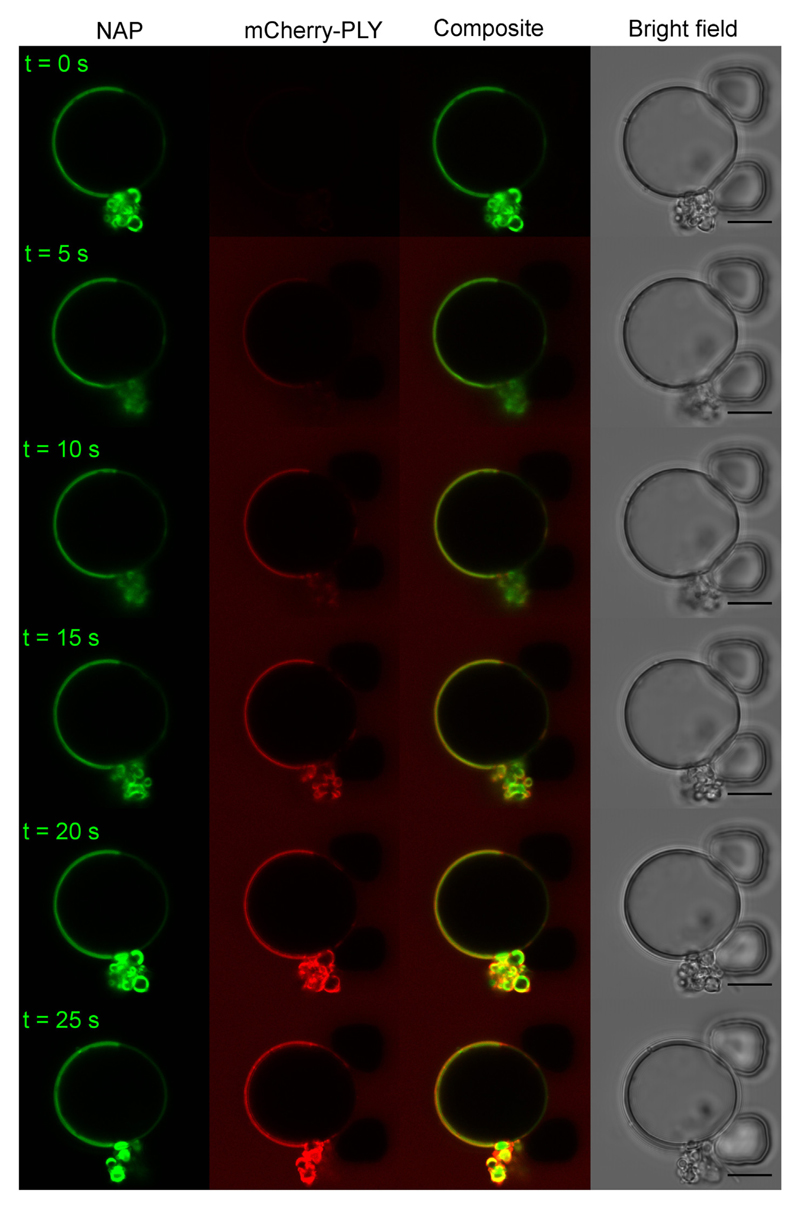
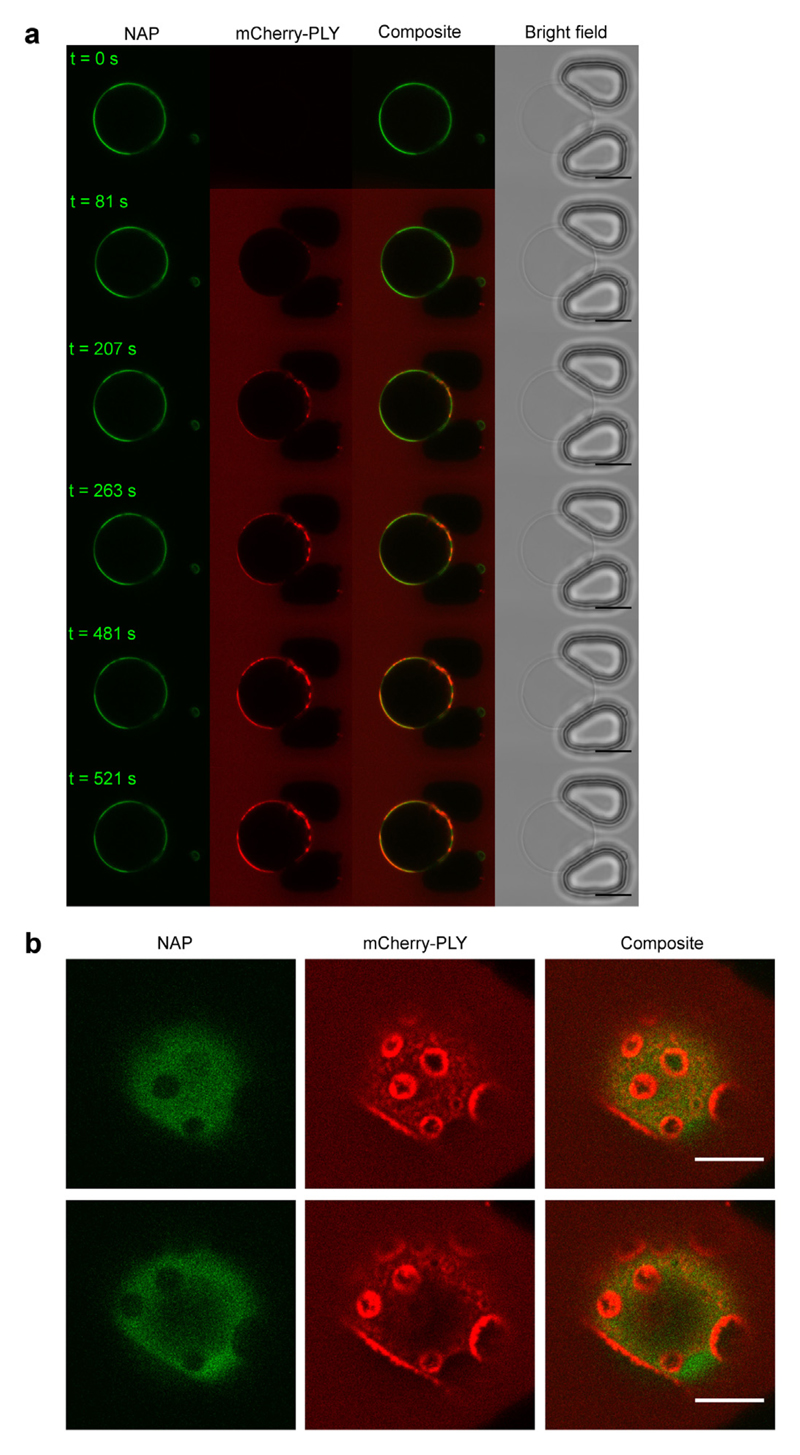
Similar to the inhomogeneous binding to the plasmalemma of nucleated cells, PLY formed distinct domains on phase-separated DOPC/DPPC/cholesterol (30:30:40) giant vesicles (Fig. 8) and displayed a clear preference for the lo phase that was rich in cholesterol and saturated DPPC (Fig. 8). Likewise, PLY showed a clear preference for the sphingomyelin- and cholesterol-rich lo phase on DOPC/PSM/cholesterol (30:30:40) vesicles (Fig. 9a,b) (Fig. SI 5, Supplemental Movie 3). Therefore, our data suggests, that if both, lo domains containing saturated phospholipids and an elevated amount of cholesterol and domains containing unsaturated phospholipids with a lower amount of cholesterol are present next to each other, PLY binding preference is dominated by the higher membrane content of its plasmalemmal receptor cholesterol. Thus, the elevated content of cholesterol in lo domains counterbalances the unfavorable presence of saturated lipids (Figs. 7, 8 and 5).
Fig. 8. Pneumolysin preferentially binds to the DPPC/cholesterol enriched phase on giant vesicles that feature a phase separated lipid bilayer.
Single-plane confocal images of a vesicle composed of DOPC/DPPC/cholesterol (30:30:40) containing 0.2 mol/% NAP treated with mCherry-PLY (3.2 μM, red). NAP denotes the cholesterol-rich, lo domain (green). Representative images are shown. n = 3. Magnification bar = 10 μm.
Fig. 9. Pneumolysin preferentially binds to boundaries between cholesterol-rich and cholesterol-depleted phases of giant vesicles composed of phosphati-dylcholine and sphingomyelin.
(a) Single-plane confocal images of a vesicle composed of DOPC/PSM/cholesterol (30:30:40) containing 0.2 mol/% NAP and treated with mCherry-PLY (3.2 μM, red). NAP denotes the cholesterol-rich, lo domain (green). Magnification bar = 10 μm. (b) Top view on the upper hemisphere of the giant vesicle shown in (a), at ~12 min PLY incubation time. Note the enhanced accumulation of PLY at the phase boundaries. n = 3. Magnification bar = 5 μm.
Moreover, after prolonged incubation (5–10 min) with DOPC/PSM/cholesterol (30:30:40) vesicles, i.e. the vesicles that most accurately mimic the lipid composition of the plasma membrane of eukaryotic cells in terms of cholesterol concentration and the presence of unsaturated natural phosphatidylcholines and saturated natural sphingomyelins, PLY preferentially accumulated in submicrometer domains at the border between lo and ld phases (Fig. 9b, Supplemental Movie 4) mirroring its distribution on the plasmalemma of nucleated cells (Fig. 1).
3. Discussion
We show that binding of the pore-forming toxin pneumolysin (PLY) to human cells occurs within distinct, clearly defined plasmalemmal areas that alternate with virtually toxin-free regions. The segregation between PLY-rich and PLY-depleted plasmalemmal domains occurs during the PLY-binding stage and persists through the process of active pore-formation. We further show that PLY-segregation is not driven by the Ca2+-dependent repair proteins that are responsible for the elimination of PLY-pores. In addition, the inhomogeneous PLY-distribution is not affected by the disruption of cytoskeleton-plasmalemmal contacts. Therefore, PLY-segregation is most likely driven by the intrinsic properties of the plasmalemmal lipid moieties and their interactions within the plasmalemmal lipid bilayer.
In experiments with artificial, protein-free liposomes, we have confirmed cholesterol as a major binding partner of PLY and shown that PLY-cholesterol binding occurs most efficiently within bilayers formed by unsaturated phospholipids, whereas the environment of saturated lipids decreases PLY-cholesterol binding affinity. Liposomes featuring a homogenous lipid bilayer showed a homogenous PLY-distribution suggesting that the distinct PLY-domains observed in cultured cells cannot be attributed to the intrinsic properties of the toxin.
However, PLY-binding to liposomes featuring stable phase-separated liquid ordered (lo) and liquid disordered (ld) domains showed inhomogeneous toxin distribution, similar to that observed in cultured cells. On liposomes, the majority of the toxin was associated with cholesterol-rich lo domains. Moreover, in DOPC/PSM/cholesterol (30:30:40) vesicles, PLY accumulated at the border between lo and ld phases after initial binding to the cholesterol/sphingomyelin-rich phase. The border regions between the cholesterol-rich/saturated lipid phase and the cholesterol-depleted/unsaturated lipid phase are the most likely places where PLY might encounter domains of relatively high cholesterol concentration surrounded by unsaturated lipids, - i.e. the conditions required for its most favorable binding to cholesterol. Packing defects [40] that may arise at the interface of lipid domains were shown to facilitate binding of another pore-forming toxin, Equinatoxin-II to coexisting lipid phases of monolayers by enhancing exposure of its receptor [41].
Thus, it is feasible that in vivo, PLY preferentially targets cholesterol-rich domains (or their boundaries) that are either permanently present within the plasmalemma of nucleated cells as a result of protein-driven lipid segregation, or PLY is capable of triggering the formation of stable cholesterol-rich platforms by stabilizing and promoting self-association and fusion of nanoscale, highly dynamic lipid inhomogeneities such as membrane rafts [9,13,42].
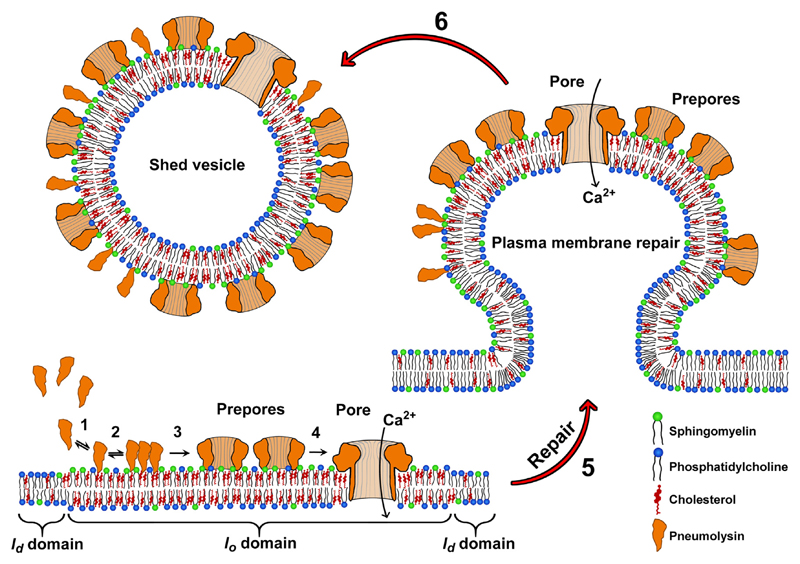
Our findings suggest that nucleated cells that are under attack by invading bacterial pathogens might benefit from the intrinsic inhomogeneity of their plasmalemmal lipid bilayer. Whereas cholesterol-rich domains represent high affinity targets for PLY-binding and therefore would promote a plasmalemma-perforating activity, the particular properties of the segregated lipid bilayer and the unique characteristics of the plasmalemmal repair processes might counter-balance the negative effects of increased PLY-binding: a high local PLY-concentration within domains enables plasmalemmal repair mechanisms [1,20] that are triggered by the formation of a small number of active pores to eliminate a large number of PLY-pre-pores, PLY-arcs and PLY-ring oligomers [22] and therefore pre-empt more extensive cellular damage (Fig. 10).
Fig. 10. Cellular microvesicle shedding triggered by binding of pneumolysin to cholesterol-rich plasmalemmal domains.
1: Soluble PLY-monomers bind to cholesterol/SM rich plasmalemmal domains. 2: Oligomerization of PLY 3: Formation of arcs or ring pre-pores. 4: Transition into active pores. This transition may comprise an early as well a late prepore state [5]. At this stage, arc and ring oligomers may be present in pre-pore or pore transition-states alongside with PLY monomers. 5: Calcium dependent plasma membrane repair, triggered in response to the formation of an active pore. 6: Release of microvesicle-containing pre-pores and yet unassembled PLY-mono- and oligomers in addition to the vesiculation-triggering pore.
4. Methods
4.1. Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), N-palmitoyl-D-erythro-sphingosylphosphorylcholine (PSM), N-oleoyl-D-erythro-sphingosylphosphorylcholine (OSM) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Cholesterol, FITC-dextran (70 kDa), Dithiothreitol (DTT), Dulbecco's PBS and bovine serum albumin (BSA) come from Sigma-Aldrich (Munich, Germany). Naphtho[2,3-a]pyrene (NAP) was supplied by TCI Deutschland GmbH and [1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine iodide] (DiI) was from Ana Spec Inc. (Fremont, CA, USA). Poly(vinylalcohol) (PVA), fully hydrolyzed, 145 kDa was from Merck KGaA (Darmstadt, Germany). BODIPY-PC was from Thermo Fischer Scientific (Molecular Probes) (Thermo Scientific, Reinach, Switzerland).
4.2. Protein expression and purification
Recombinant plasmid constructs containing the mCherry-tagged PLY were produced as follows: The mCherry sequence was amplified from the original pN1-mCherry construct from Clontech Laboratories (Mountain View, USA) by using the primers CAGGCTAGCATGGTGAG CAAGGGCGAGGAG (mCherry-fwd) and CACGGATCCCTTGTACAGCTCGTCCATG (mCherry-rev), double digested by NheI and BamHI and ligated (N-terminally to the toxin sequence) into the equally digested pET28a-PLY-vector (containing the PLY-sequence of Streptococcus pneumoniae strain D39 as described [22]). The primers were synthesized and positive clones were sequenced by Microsynth (Balgach, Switzerland), the restriction enzymes were from Fermentas (Fisher Scientific, Wohlen, Switzerland) and all other reagents used in the cloning experiments were purchased from Promega (Dübendorf, Switzerland). The pET28a-mCherry-PLY-vector was transformed into BL21 (DE3) pLysS competent cells (Promega, Switzerland). Protein expression followed a modified protocol as described [22]. Briefly, protein expression was induced by addition of 1 mM IPTG (Sigma-Aldrich) at an OD = 0.6 for 3 h at 37 °C. Cells were harvested by centrifugation (3500 × g, 30 min, 4 °C). Lysis employed usage of Lysozyme and DNAse I (Sigma-Aldrich) as described by the manufacturer and 10 × 20 s sonication steps with 15 s breaks (on ice) (BANDELIN sonoplus uw 2070, BANDELIN electronic GmbH & Co. KG, Berlin, Germany). Following ultracentrifugation (70.000 × g, 1 h, 4 °C) the protein was purified from the supernatant with Protino® Ni-NTA 1 ml columns (MACHEREY-NAGEL, Oensingen, Switzerland) employing an ÄKTAprime plus (GE heathcare, Little Chalfont, UK) machine according to the manufacturer's protocols. After dialysis against PBS (MWCO 12 kDa, overnight, 4 °C, Sigma-Aldrich) endotoxin was removed by Pierce® High-Capacity Endotoxin Removal Spin Column, 0.5 ml according to the manufacturer's instructions (Thermo Scientific, Rockford, USA). Protein purity (≥95%) was confirmed by SDS-PAGE and Western blotting (1F11 mouse anti pneumolysin IgG, Santa Cruz Biotechnology, Dallas, TX, USA), WesternBright™ ECL detection kit, Advansta Inc., CA, USA). Wild type PLY and EGFP-PLY were expressed and purified as described previously [22], but employed the Ni-NTA columns and endotoxin removal as described above. PLY-constructs were freshly activated in the presence of 1 mM DTT prior to experiments and used immediately. The activity of PLY-constructs is similar to the activity of wild-type PLY (Supplemental Information, Fig. S1 5).
4.3. Mammalian cell culture and transfections
Human embryonic kidney cells (HEK293) were cultured in DMEM (Dulbecco's modified Eagle's medium, Gibco, Life Technologies, Paisley, UK) and human neuroblastoma SH-SY5Y cells were cultured in MEM (1×, GlutaMax, Gibco, Life Technologies, Paisley, UK) medium, both supplemented with 10% heat-inactivated FBS (Fetal Bovine Serum. Gibco, Life Technologies, Paisley, UK) and 1% penicillin / streptomycin (Gibco, Life Technologies, Paisley, UK) as previously described [43]. Cells were grown in 5% CO2 at 37 °C to 80–90% confluence. Following transient transfections via electroporation (BioRad) with the coding sequence of human Annexin A2, cloned into the Living Colors Fluorescent protein vector pEYFP-N1 (Takara Bio Europe/Clontech, Saint-Germain-en-Laye, France) [44], cells were seeded on glass coverslips and grown for 48 h, until 60–80% confluence [22]. The transfection rate typically reached 70–90%. Live cell imaging experiments were performed in Tyrode's buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, pH = 7.4) containing either 2.5 mM CaCl2 or 100 μM EGTA.
4.4. Preparation of liposomes and giant vesicles
Lipid stocks were handled in solution (chloroform/methanol, 1:1 v/v) and mixed in molar ratio. Liposomes were prepared by extrusion, using a 200 nm polycarbonate membrane and a LiposoFast-Basic extrusion system as described by the manufacturer (Avestin Europe GmbH, Mannheim, Germany). Giant vesicles were prepared employing a modified protocol [45,46]. Briefly, a thin film of poly(vinyl alcohol) (PVA) is prepared by drying a 5% (w/v) PVA solution in ultrapure water at 80 °C on a glass slide. The film is dried for 5 min at 40–50 °C and the lipid mixture was deposited on top until the solvent evaporated. The films were then thoroughly dried (60 min, RT, vacuum) and vesicles were swollen in PBS pH 7.4 buffer at 50–60 °C for 1 h.
4.5. PLY-sequestration by liposomes
Liposomes (≈90 nM–188 μM) of various lipid composition were pre-incubated with 16 nM mCherry-PLY at RT in the presence of 2.5 mM DTT. Following 15 min pre-incubation, hemolysis was initiated by the addition (1:1 v/v) of 2% hRBCs. The mixture (200 μl) containing 1% hRBCs and 8 nM PLY (sufficient to induce full lysis) was then incubated at 37 °C for 30 min. Pelleting and determination of hemolysis was performed as described [22] and normalized hemolytic data were analyzed by nonlinear regression of (symmetrical) sigmoidal dose-response curve fitted with variable hill slope using GraphPad Prism 7 (Supplemental Information, Table SI 1).
4.6. Microfluidic experiments employing giant vesicles
An established microfluidic chip-design [34] was modified by implementing three hydrodynamic traps per chamber to increase the probability of vesicle loading (gap distance between the pillars: 7, 8, and 10 μm). The microfluidic device is based on a two-layer-design with two separate microfluidic compartments (bottom layer) providing an array of 32 microchambers each. Eight separate pressure channels (top layer) enable the actuation of a valve for complete spatial isolation of the trapped vesicles in four chambers in parallel. The device is operated using nitrogen at a pressure of 200 kPa in combination with a custom-made pressure valve control instrument. Chip fabrication followed an established protocol as described [34,36]. Reagents were loaded using a syringe-pump system (Nanojet syringe pump, Chemyx, Stafford, TX, USA) and Teflon tubes. Ultrapure water was filled into the fluid layer and pressure control openings by centrifugation (700 ×g, 5 min, RT) to avoid air pockets [34,36]. Before vesicle trapping, the chip was mounted onto the microscope temperature controlled stage holder and the channels of the bottom layer were coated by 4% (w/v) BSA in PBS at a flow of 10 μl/min for 10 min. Giant vesicles were then trapped from PBS solution at 2 μl/min and experiments were performed at 0.5 μl/min, which is equivalent to a flow-rate of 0.125 μl/min per chamber. This did not apply any observable shear stress on the liposomes [35].
4.7. Microscopy
Live-cell imaging was performed in a round coverslip recording chamber (Multi Channel Systems MCS GmbH, Reutlingen, Germany) and microfluidic chips were mounted on the stage of an inverted microscope (Zeiss LSM 880), equipped with a Plan APO 63×/1.4 Oil DIC M27 objective (Carl Zeiss Microscopy GmbH, Jena, Germany). The 458 nm line of an argon laser (Lasos Lasertechnik GmbH, Jena, Germany) (BP 465 nm–550 nm) was used for NAP, and a 561 nm diode pumped solid state (DPSS) laser (BP 578 nm–696 nm) was used for DiI or mCherry respectively (MBS 458/561). For excitation of BODIPY-PC or TopFluor-cholesterol the 488 nm line of the argon laser and beam splitter MBS 488/561 (BP 493 nm–556 nm) was used instead. The LSM 880 was equipped with a temperature controlled incubation chamber. Image processing included the Zeiss ZEN 2 software (Carl Zeiss Microscopy GmbH 1997–2014) and ImageJ/Fiji. Images have been contrast enhanced to optimize presentation. Vesicle CLSM experiments were performed in PBS at T = 28 ± 1 °C.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbalip.2018.04.010.
Acknowledgements
We gratefully acknowledge funding from the Novartis Foundation for Medical-Biological Research (16B100 to E.B·B), the Swiss National Science Foundation (SNF 31003A_159414, to A.D.), the Gebert Rüf Foundation (to A.D.), the European Research Council (ERC Consolidator Grant No. 681587, HybCell, to P.S.D.) and the University of Bern (UniBE Initiator Grant to P.D.). We would also like to thank Christoph Bärtschi for constructing the pressure valve control instrument required to operate the microfluidic device and the clean room facility FIRST at ETH Zurich as well as Benoît Zuber and Ioan Iacovache for providing Y221G-Aerolysin-EGFP and for many fruitful discussions.
Abbreviations
- SM
sphingomyelin
- PC
phosphatidylcholine
- PSM
N-palmitoyl- sphingosylphosphorylcholine
- OSM
N-oleoyl-sphingosylphosphorylcholine
- DOPC
di-oleoyl phosphatidylcholine
- DPPC
di-palmitoyl phosphatidylcholine
- PFT
pore-forming toxin
- CDC
cholesterol dependent cytolysin
- NAP
Naphtho[2,3-a]pyrene
- DiI
Dioctadecyl-tetramethylindo-carbocyanine
- hRBC
human red blood cell
- MWCO
molecular weight cut-off
- CLSM
confocal laser scanning microscopy
- BP
bandpass
- MBS
main beam splitter
Footnotes
Transparency document
The Transparency document associated with this article can be found, in online version.
Author contributions
P.D. and S.B. performed and analyzed in vitro experiments. P.D., H.W., V.S.B., R.K. and E.B.B. performed and analyzed cell culture experiments. R.S. and P.D. generated the mCherry-PLY-construct. P.D., A.D. and E.B.B. designed the study and wrote the paper. P.S.D., A.D. and E.B.B. coordinated the study. All authors analyzed and discussed the results and commented on the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- [1].Babiychuk EB, Draeger A. Defying death: cellular survival strategies following plasmalemmal injury by bacterial toxins. Semin Cell Dev Biol. 2015;45:39–47. doi: 10.1016/j.semcdb.2015.10.016. [DOI] [PubMed] [Google Scholar]
- [2].Peraro MD, van der Goot FG. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Micro. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- [3].Lukoyanova N, Hoogenboom BW, Saibil HR. The membrane attack complex, perforin and cholesterol-dependent cytolysin superfamily of pore-forming proteins. J Cell Sci. 2016;129:2125–2133. doi: 10.1242/jcs.182741. [DOI] [PubMed] [Google Scholar]
- [4].Helen MM, Timothy JM, David HD. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 2008;8:497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- [5].van Pee K, Neuhaus A, D'Imprima E, Mills DJ, Kühlbrandt W, Yildiz Ö. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of pneumolysin. eLife. 2017;6:e23644. doi: 10.7554/eLife.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tilley SJ, Orlova EV, Gilbert RJC, Andrew PW, Saibil HR. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell. 2005;121:247–256. doi: 10.1016/j.cell.2005.02.033. [DOI] [PubMed] [Google Scholar]
- [7].Nöllmann M, Gilbert R, Mitchell T, Sferrazza M, Byron O. The role of cholesterol in the activity of pneumolysin, a bacterial protein toxin. Biophys J. 2004;86:3141–3151. doi: 10.1016/S0006-3495(04)74362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gilbert RJC, Rossjohn J, Parker MW, Tweten RK, Morgan PJ, Mitchell TJ, Errington N, Rowe AJ, Andrew PW, Byron O. Self-interaction of pneumolysin, the pore-forming protein toxin of Streptococcus pneumoniae. J Mol Biol. 1998;284:1223–1237. doi: 10.1006/jmbi.1998.2258. [DOI] [PubMed] [Google Scholar]
- [9].Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- [10].Simons K, Van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- [11].Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van Meer G, Hoetzl S. Sphingolipid topology and the dynamic organization and function of membrane proteins. FEBS Lett. 2010;584:1800–1805. doi: 10.1016/j.febslet.2009.10.020. [DOI] [PubMed] [Google Scholar]
- [13].Kraft ML. Plasma membrane organization and function: moving past lipid rafts. Mol Biol Cell. 2013;24:2765–2768. doi: 10.1091/mbc.E13-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kinoshita M, Suzuki KGN, Matsumori N, Takada M, Ano H, Morigaki K, Abe M, Makino A, Kobayashi T, Hirosawa KM, Fujiwara TK, et al. Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs. J Cell Biol. 2017;216:1183. doi: 10.1083/jcb.201607086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lafont F, Abrami L, van der Goot FG. Bacterial subversion of lipid rafts. Curr Opin Microbiol. 2004;7:4–10. doi: 10.1016/j.mib.2003.12.007. [DOI] [PubMed] [Google Scholar]
- [16].Waheed AA, Shimada Y, Heijnen HFG, Nakamura M, Inomata M, Hayashi M, Iwashita S, Slot JW, Ohno-Iwashita Y. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts) Proc Natl Acad Sci U S A. 2011;98:4926–4931. doi: 10.1073/pnas.091090798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nelson LD, Johnson AE, London E. How interaction of Perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of Perfringolysin O-lipid raft interaction. J Biol Chem. 2008;283:4632–4642. doi: 10.1074/jbc.M709483200. [DOI] [PubMed] [Google Scholar]
- [18].Gonzalez MR, Bischofberger M, Pernot L, van der Goot Y, Frêche B. Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci. 2008;65:493–507. doi: 10.1007/s00018-007-7434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abrami L, van der Goot FG. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J Cell Biol. 1999;147:175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jimenez AJ, Perez F. Plasma membrane repair: the adaptable cell life-insurance. Curr Opin Cell Biol. 2017;47:99–107. doi: 10.1016/j.ceb.2017.03.011. [DOI] [PubMed] [Google Scholar]
- [21].Romero M, Keyel M, Shi G, Bhattacharjee P, Roth R, Heuser JE, Keyel PA. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ. 2017;24:798–808. doi: 10.1038/cdd.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wolfmeier H, Radecke J, Schoenauer R, Koeffel R, Babiychuk VS, Drücker P, Hathaway LJ, Mitchell TJ, Zuber B, Draeger A, Babiychuk EB. Active release of pneumolysin prepores and pores by mammalian cells undergoing a Streptococcus pneumoniae attack. BBA-Gen Subjects. 2016;1860:2498–2509. doi: 10.1016/j.bbagen.2016.07.022. [DOI] [PubMed] [Google Scholar]
- [23].Iacovache I, De Carlo S, Cirauqui N, Dal Peraro M, van der Goot FG, Zuber B. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the poreformation process. Nat Commun. 2016;7 doi: 10.1038/ncomms12062. 12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Degiacomi MT, Iacovache I, Pernot L, Chami M, Kudryashev M, Stahlberg H, van der Goot FG, Dal Peraro M. Molecular assembly of the aerolysin pore reveals a swirling membrane-insertion mechanism. Nat Chem Biol. 2013;9:623–629. doi: 10.1038/nchembio.1312. [DOI] [PubMed] [Google Scholar]
- [25].Abrami L, Velluz M-C, Hong Y, Ohishi K, Mehlert A, Ferguson M, Kinoshita T, Gisou van der Goot F. The glycan core of GPI-anchored proteins modulates aerolysin binding but is not sufficient: the polypeptide moiety is required for the toxin-receptor interaction. FEBS Lett. 2002;512:249–254. doi: 10.1016/s0014-5793(02)02274-3. [DOI] [PubMed] [Google Scholar]
- [26].Los DA, Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochim Biophys Acta Biomembr. 2004;1666:142–157. doi: 10.1016/j.bbamem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [27].Kirkham L-AS, Kerr AR, Douce GR, Paterson GK, Dilts DA, Liu D-F, Mitchell TJ. Construction and immunological characterization of a novel nontoxic protective pneumolysin mutant for use in future pneumococcal vaccines. Infect Immun. 2006;74:586–593. doi: 10.1128/IAI.74.1.586-593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ali MR, Cheng KH, Huang J. Assess the nature of cholesterol–lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. Proc Natl Acad Sci. 2007;104:5372–5377. doi: 10.1073/pnas.0611450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang J, Feigenson GW. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huang J, Buboltz JT, Feigenson GW. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim Biophys Acta Biomembr. 1999;1417:89–100. doi: 10.1016/s0005-2736(98)00260-0. [DOI] [PubMed] [Google Scholar]
- [31].Huang J. Model membrane thermodynamics and lateral distribution of cholesterol: from experimental data to Monte Carlo simulation. Meth Enzymol. 2009;455:329–364. doi: 10.1016/S0076-6879(08)04212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rojko N, Anderluh G. How lipid membranes affect pore forming toxin activity. Acc Chem Res. 2015;48:3073–3079. doi: 10.1021/acs.accounts.5b00403. [DOI] [PubMed] [Google Scholar]
- [33].Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- [34].Robinson T, Kuhn P, Eyer K, Dittrich PS. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics. 2013;7 doi: 10.1063/1.4816712. 044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sturzenegger F, Robinson T, Hess D, Dittrich PS. Membranes under shear stress: visualization of non-equilibrium domain patterns and domain fusion in a microfluidic device. Soft Matter. 2016;12:5072–5076. doi: 10.1039/c6sm00049e. [DOI] [PubMed] [Google Scholar]
- [36].Eyer K, Kuhn P, Hanke C, Dittrich PS. A microchamber array for single cell isolation and analysis of intracellular biomolecules. Lab Chip. 2012;12:765–772. doi: 10.1039/c2lc20876h. [DOI] [PubMed] [Google Scholar]
- [37].Veatch SL, Keller SL. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. BBA-Mol Cell Res. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- [39].Veatch SL, Keller SL. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys Rev Lett. 2005;94 doi: 10.1103/PhysRevLett.94.148101. 148101. [DOI] [PubMed] [Google Scholar]
- [40].Cannon B, Hermansson M, Györke S, Somerharju P, Virtanen JA, Cheng KH. Regulation of calcium channel activity by lipid domain formation in planar lipid bilayers. Biophys J. 2003;85:933–942. doi: 10.1016/S0006-3495(03)74532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Barlič A, Gutiérrez-Aguirre I, Caaveiro JMM, Cruz A, Ruiz-Argüello M-B, Pérez-Gil J, González-Mañas JM. Lipid phase coexistence favors membrane insertion of Equinatoxin-II, a pore-forming toxin from Actinia equina. J Biol Chem. 2004;279:34209–34216. doi: 10.1074/jbc.M313817200. [DOI] [PubMed] [Google Scholar]
- [42].Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Atanassoff AP, Wolfmeier H, Schoenauer R, Hostettler A, Ring A, Draeger A, Babiychuk EB. Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of Streptolysin O-induced Plasmalemmal damage. PLoS One. 2014;9:e89743. doi: 10.1371/journal.pone.0089743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Monastyrskaya K, Babiychuk EB, Hostettler A, Rescher U, Draeger A. Annexins as intracellular calcium sensors. Cell Calcium. 2007;41:207–219. doi: 10.1016/j.ceca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [45].Weinberger A, Tsai F-C, Koenderink GH, Schmidt TF, Itri R, Meier W, Schmatko T, Schröder A, Marques C. Gel-assisted formation of giant unilamellar vesicles. Biophys J. 2013;105:154–164. doi: 10.1016/j.bpj.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Drücker P, Pejic M, alla H-J, Gerke V. Lipid segregation and membrane budding induced by the peripheral membrane binding protein Annexin A2. J Biol Chem. 2013;288:24764–24776. doi: 10.1074/jbc.M113.474023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.