Abstract
Introduction:
CC-002 is a prospective cooperative group study conducted by NRG Oncology to evaluate whether a pre-operative GA-GYN score derived from a predictive model utilizing components of an abbreviated geriatric assessment (GA) is associated with major post-operative complications in elderly women with suspected ovarian, fallopian tube, primary peritoneal or advanced stage papillary serous uterine (GYN) carcinoma undergoing primary open cytoreductive surgery.
Methods:
Patients 70 years or older with suspected advanced gynecologic cancers undergoing evaluation for surgery were eligible. A GA-GYN score was derived from a model utilizing the GA as a pre-operative tool. Patients were followed for six weeks post-operatively or until start of chemotherapy. Post-operative events were recorded either directly as binary occurrence (yes or no) using CTCAE version 4.0.
Results:
There were 189 eligible patients, 117 patients with primary surgical intervention and 37 patients undergoing interval cytoreduction surgery. The association between higher GA-GYN score and major postoperative complications in patients undergoing primary surgery was not significant (p=0.1341). In a subgroup analysis of patients with advanced staged malignant disease who underwent primary cytoreductive surgery, there was a trend towards an association with the GA-GYN score and post-operative complications.
Conclusion:
The pre-operative GA-GYN score derived from a predictive model utilizing components of an abbreviated geriatric assessment was not predictive of major post-operative complications in elderly patients undergoing primary open cytoreductive surgery. However, there was an association between GA-GYN score and post-operative complications in a subgroup of patients with advanced staged malignant disease.
Keywords: Gynecologic cancers, NRG-CC002, post-operative outcomes
INTRODUCTION
The older population in the US is growing and by the year 2050, will comprise 20% of the total population [1]. Increasing age is associated with increasing rates of cancer, with those over age 65 years accounting for 60% newly diagnosed malignancies and 70% of all cancer deaths [1]. Rates of gynecologic cancers increase with age; the median age at diagnosis for ovarian cancer was 63 years with 40.8% of diagnosed cases in women 65 years and older. Of those cases diagnosed in the elderly, half were diagnosed in women over the age of 75 years [2].
Older patients are less likely to receive surgery and/or receive adjuvant chemotherapy, all of which may contribute to lower survival rates [3]. However, if elderly patients can tolerate primary cytoreductive surgery, they may have rates of initial response to chemotherapy, overall survival (OS) and progression free survival (PFS) that are similar to those of their younger counterparts [4–6].
Prospective randomized trials have reported cytoreductive surgery performed after neoadjuvant chemotherapy to be associated with decreased complications and similar disease-free and OS outcomes compared to up-front surgery [7]. While it is straightforward both to identify both ill older patients who will simply not tolerate surgery and the healthiest older patients who will likely do well with primary surgery, there is a large intermediate group who may have significantly less morbidity from surgery delayed until after neoadjuvant chemotherapy. A tool to categorize postoperative risk would assist in the decision regarding the timing of surgery and, potentially, the advisability of surgery at all. In addition, a reliable predictor of postoperative risks would allow resources for mitigating those risks to be targeted where they are most needed. A number of risk classifications aimed at predicting surgical outcomes have been developed, but none are completely reliable, and none are validated for major gynecologic oncology operations [8–11].
The Cancer and Aging Research Group (CARG) developed a brief self-administered geriatric assessment tool (GA) (Table 1 and 2) for use in patients with cancer who were 65 years and older receiving physician-choice chemotherapy [12]. This tool uses validated measures of geriatric assessment across all domains and underwent feasibility evaluation to determine rate of patient completion of the questionnaire, patient satisfaction, and length of time required for completion [13, 14]. The CARG geriatric assessment tool was found to be acceptable to patients and required approximately 20 minutes total to complete both in the initial feasibility studies and in the cooperative group setting [13].
Table 1.
Components in the Brief Geriatric Assessment Tool
| Domain | Measure |
|---|---|
| Functional Status | Activities of Daily Living [Subscale of MOS Physical Health] |
| Instrumental Activities of Daily Living [Subscale of the OARS] | |
| NRG self-reported Performance Rating Scale | |
| NRG Physician-rated Performance Rating Scale | |
| Number of falls in last 6 months | |
| Comorbidity |
Physical Health Section [Subscale of the OARS] |
| Psychological | Mental Health Inventory-17 |
| Social Activity | MOS Social Activity Survey |
| Social support | MOS Social Support Survey: Emotional/Information and Tangible Subscales |
| Nutrition | Body Mass Index |
| Percent unintentional weight loss in last 6 months |
Table 2.
Risk Factors Included in GA-GYN Score
| Value | |
|---|---|
| Need for assistance in taking medications (item from IADL). | 1 |
| Limited in walking one block (item from ADL). | 2 |
| Decreased social activity at least sometimes due to physical/emotional health (item from social activity survey). | 1 |
| Number of falls in the last 6 months ≥ 1. | 3 |
| Fair or worse Hearing. | 2 |
| Age >=72 | 2 |
| Hemoglobin <10 g/dl. | 3 |
| Creatinine clearance <34 ml/min. | 3 |
The CARG GA score has been shown to be a much better predictor of chemotherapy toxicity than ECOG/KPS performance status in a cohort of 500 elderly patients undergoing chemotherapy for a variety of different cancers [14]. We hypothesize that the predictive model might identify oncology patients who are vulnerable to any treatment toxicity including surgical complications [13]. The risk factors and their scores identified by the predictive model were used in the current study and modified for gynecologic oncology patients.
CC-002 is a prospective cooperative group study conducted by the National Clinical Trials Network (NCTN) group NRG Oncology to evaluate whether a GA-GYN score (Table 2) derived from the CARG GA tool would be associated with major post-operative complications in elderly women with suspected ovarian, fallopian tube, primary peritoneal or advanced stage papillary serous uterine carcinoma undergoing primary open cytoreductive surgery.
METHODS
Eligible patients included women 70 years or older with suspected primary peritoneal, ovarian, fallopian tube or advanced staged uterine carcinomas. Patients were included irrespective of their performance status. Patients were excluded for planned minimally invasive cytoreductive surgery (laparoscopy/robotic surgery).
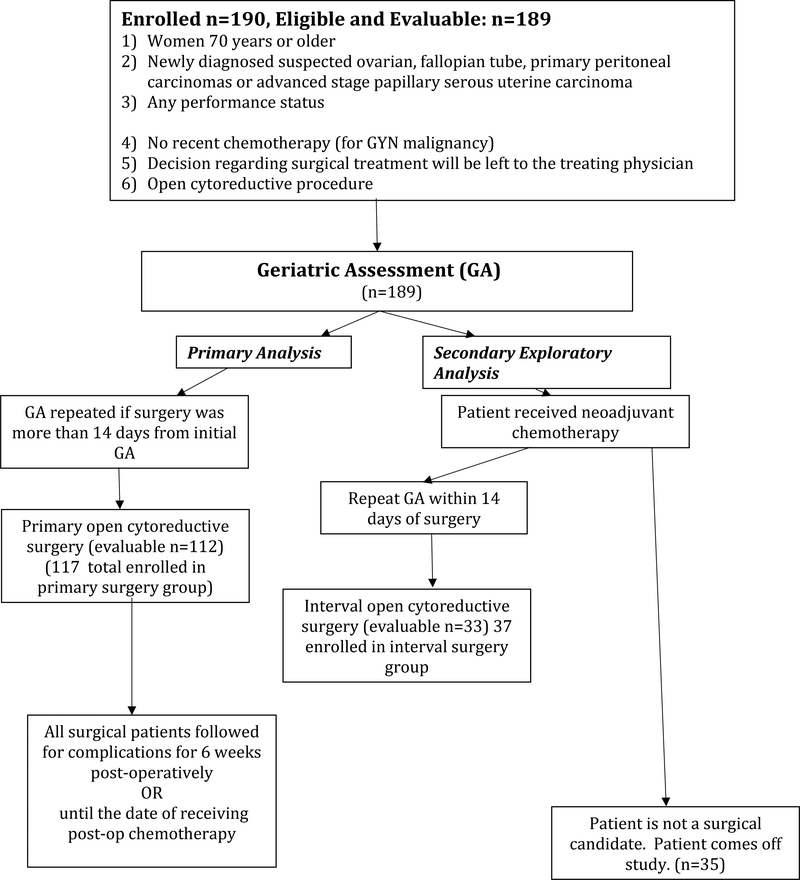
Patients were enrolled on the study prior to chemotherapy or cytoreductive surgery, and given the abbreviated geriatric assessment. The decision regarding timing and extent of surgical treatment was left to the treating surgeon. If neoadjuvant chemotherapy was given, the abbreviated geriatric assessment was repeated prior to interval cytoreductive surgery, Figure 1 (study flow diagram). The protocol specifies that the surgeon would be blinded to the results of the questionnaire from the geriatric assessment, but there was no protocol-defined enforcement or monitoring of this blinding.
Figure 1:
CC-002 Flow Diagram
The abbreviated geriatric assessment encompassed the following domains (measures in parentheses): functional status (activities of daily living [ADL], instrumental activities of daily living [IADL] [Subscale of the Multidimensional Functional Assessment Questionnaire: Older American Resources and Services (OARS)), NRG physician-rated performance status, self-reported performance status and number of falls in last 6 months); comorbid medical conditions (physical health section subscale of the OARS), psychological state (Mental Health Inventory-17); social support; nutritional status (BMI, percent unintentional weight loss in past six months), Table 1.
As part of the pre-treatment assessments, blood work was collected at study entry, and re-collected within 14 days of primary or interval surgery, or on the day of enrollment for patients not undergoing surgical treatment (albumin, serum creatinine, fasting glucose, [drawn the AM of surgery, optional for those not undergoing surgery] and CBC).
A GA-GYN score was calculated by summation of the risk factors’ scores in Table 2. A higher score indicates higher vulnerability.
Patients were followed postoperatively for complications for 6 weeks or until the start or resumption of chemotherapy, whichever was sooner. Post-operative complications were defined as the occurrence of an event outlined in The American College of Surgeons’ National Surgical Quality Improvement Program meeting severity criteria of grade 3 or higher using CTCAE version 4.0.
Study design and statistical analyses:
The primary objective was to determine whether the pre-operative GA-GYN score, performed within 14 days prior to surgery, is associated with major post-operative complications in elderly patients undergoing primary open cytoreduction surgery.
Secondary objectives were included: 1-to explore associations between individual variables of the preoperative geriatric assessment and major post-operative complications in patients undergoing open primary cytoreduction surgery, and 2- to assess the association between the preoperative GA-GYN score and cytoreducibility defined by extent of residual disease in patients undergoing open primary cytoreduction surgery.
A sample size of 100 patients was planned to provide 90% power to detect a 15 percent increase of the complication rate from 40%. This complication rate of 40% was determined by the overall complication rate in the Preoperative Assessment of Cancer in Elderly Patient (PACE) studies [15, 16].
To ensure that the primary objective would have adequate power, this trial had a total sample size of 228 eligible patients, presuming 44% of patients in this study would have primary surgery. The primary surgery number was estimated from data from the Gynecologic Oncology group trial 273.
Exact conditional logistic regression, Monte-Carlo based exact Spearman correlation coefficient test, Monte-Carlo based exact Wilcoxon rank sum (or Kruskal-Wallis) tests and Wilcoxon signed rank test were used in the statistical analyses. All tests were two-sided, unless stated as one-sided test, and the significance level was 0.05. No adjustment of multiple testing was made for subgroup, secondary, and exploratory analyses. SAS* 9.4® was used in the statistical analyses.
RESULTS
NRG CC-002 was open to accrual from February 10th, 2015 to November 2nd, 2015. Data cutoff for analysis was January 31st, 2017. 190 patients were enrolled to the study. One patient was ineligible with 189 patients eligible and evaluable.
Patient characteristics are listed in Table 3. Patient ages ranged from ages 70 years to 89 years. Performance status (PS) was greatly favorable (majority 0 or 1) in all surgical groups: primary surgery, interval surgery or no surgery.
Table 3.
Demographics of all Patients Stratified by Treatment
| Primary Surgery (n=117) | Interval Surgery (n=37) | No Surgery (n=35) | Total (n=189) | |||||
|---|---|---|---|---|---|---|---|---|
| Age Group (years) | N | % | N | % | N | % | N | % |
| 70–74 years | 50 | 42.7 | 14 | 37.8 | 9 | 25.7 | 73 | 38.6 |
| 75–79 years | 43 | 36.8 | 13 | 35.1 | 14 | 40.0 | 70 | 37.0 |
| 80–84 years | 18 | 15.4 | 8 | 21.6 | 6 | 17.1 | 32 | 16.9 |
| 85–89 years | 6 | 5.1 | 2 | 5.4 | 6 | 17.1 | 14 | 7.4 |
| Stage of Disease | ||||||||
| I | 14 | 12 | 0 | 0 | 0 | 0 | 14 | 7.4 |
| II | 8 | 6.8 | 0 | 0 | 0 | 0 | 8 | 4.2 |
| III | 58 | 49.6 | 24 | 64.9 | 0 | 0 | 82 | 43.4 |
| IV | 10 | 8.5 | 13 | 35.1 | 0 | 0 | 23 | 12.2 |
| Benign/No Cancer | 26 | 22.2 | 0 | 0 | 26 | 13.8 | ||
| No surgery | 0 | 0 | 0 | 0 | 35 | 100 | 35 | 18.5 |
| Race | ||||||||
| Asian | 1 | .9 | 1 | 2.7 | 0 | 0 | 2 | 1.1 |
| African American | 4 | 3.4 | 1 | 2.7 | 5 | 14.3 | 10 | 5.3 |
| Caucasian | 109 | 93.2 | 35 | 94.6 | 30 | 85.7 | 174 | 92.1 |
| Not Reported | 3 | 2.6 | 0 | 0 | 0 | 0 | 3 | 1.6 |
| Baseline Performance Status at Study Entry | ||||||||
| 0 | 80 | 68.4 | 15 | 40.5 | 12 | 34.3 | 107 | 56.6 |
| 1 | 29 | 24.8 | 16 | 43.2 | 14 | 40.0 | 59 | 31.2 |
| 2 | 7 | 6.0 | 4 | 10.8 | 8 | 22.9 | 19 | 10.1 |
| 3 | 1 | 0.9 | 2 | 5.4 | 1 | 2.9 | 4 | 2.1 |
| Baseline Number of Medications at Study Entry | ||||||||
| 0 | 8 | 6.8 | 4 | 10.8 | 1 | 2.9 | 13 | 6.9 |
| 1–5 | 60 | 51.3 | 15 | 40.5 | 13 | 37.1 | 88 | 46.6 |
| 6–10 | 30 | 25.6 | 15 | 40.5 | 11 | 31.4 | 56 | 29.6 |
| 11–15 | 16 | 13.7 | 3 | 8.1 | 6 | 17.1 | 25 | 13.2 |
| 16–20 | 1 | 0.9 | 0 | 0 | 3 | 8.6 | 4 | 2.1 |
| 21+ | 2 | 1.7 | 0 | 0 | 1 | 2.9 | 3 | 1.6 |
Among the 189 eligible patients, 79.9% of patients completed treatment per protocol criteria, 2.6 % of patients came off study secondary to disease progression during primary treatment, 1.6% came off study due to adverse events or death, 2.6% withdrew from the study, 12.2% was off study due to other reasons, and one patient came off study for unknown reason. 56.1% of patients were planned to receive further treatment after completion of the study protocol.
The pre-operative GA-GYN score ranged from 0–10 in patients undergoing primary surgery, with a median score of 3. The highest scores, indicating most vulnerability, occurred in patients with stage III disease, see Table 4.
Table 4.
Distributions of GA-GYN Score and Disease Stage (Primary Open Surgery)
| Pre-operative GA-GYN score | ||||||
|---|---|---|---|---|---|---|
| Stage of disease | Total | 0–1 | 2–3 | 4–5 | 6–10 | Missing |
| I | 13 | 0 | 5 | 5 | 1 | 2 |
| II | 8 | 0 | 6 | 1 | 1 | 0 |
| III | 57 | 8 | 22 | 10 | 9 | 8 |
| IV | 9 | 0 | 5 | 3 | 1 | 0 |
| Benign/no cancer | 24 | 1 | 7 | 6 | 8 | 2 |
| Not Reported | 1 | 0 | 0 | 0 | 0 | 1 |
| Total | 112 | 9 | 45 | 25 | 20 | 13 |
Out of the 189 eligible patients, 154 patients underwent surgery, with 117 patients having primary surgical intervention and 37 patients undergoing interval cytoreduction surgery. One-hundred-forty-five patients had a laparotomy (open approach) with 112 undergoing primary open surgical intervention and 33 patients undergoing open interval cytoreduction. The most common reason for not undergoing primary surgery was provider preference for neoadjuvant chemotherapy in 21 out of the 37 patients. Thirty-five patients did not undergo any surgery; reasons for not undergoing surgery were not recorded in this study and chemotherapy receipt was unknown given these patients were not followed after initial assessment. However, it was found that these patients were older and had poorer scores on the geriatric assessment than when compared to patients undergoing surgery. The mean pre-operative GA-GYN scores among patients undergoing primary surgery, interval surgery and no surgery was 3.7, 4.6 and 6.2, respectively.
50% of the African-American patients enrolled did not have surgery (five out of the thirty-five patients) and 17% of the Caucasian patients enrolled did not have surgery (the remaining thirty patients). The absolute numbers were too small to make a significant conclusion, but the larger percentage of African-American patients not having surgery at all may reflect a treatment disparity in this group.
Of the 145 patients undergoing primary surgery, 17.2% (90% confidence interval: 12.3% - 23.2%) experienced major post-operative complications, with the majority occurring in patients with stage III disease. In primary open surgery patients, 20 (17.9%; 90% CI: 12.2 – 24.9%) patients had major post-operative complications, and 10 out of these 20 patients who had major post-operative complications had stage III disease; in interval open cytoreduction surgery patients, 5 (15.2%, 90% CI: 6.2% - 29.3%) patients had major post-operative complications, and all of the 5 patients who had major post-operative complications had stage III disease. Age alone was not associated with post-operative complications.
In regards to the primary objective, the association between the pre-operative GA-GYN score and the major postoperative complications in patients undergoing primary surgery was not significant (one-sided p=0.1341) at 0.05 significance level. A larger than expected percentage of patients undergoing primary surgery had benign disease, 24 out of 112 eligible patients (laparotomy only), 21.4%. There was concern that inclusion of patients with benign disease would decrease power of the primary objective, as they were unlikely to have had extensive operations. Therefore, two retrospective unplanned subgroup analyses were performed in the 88 patients with malignant disease who underwent open primary cytoreduction surgery and the 66 patients with stage 3 or 4 malignant disease who underwent open primary cytoreduction surgery.
For the subgroup analysis of 88 patients with malignant disease (all stages) each 1-point increase in the GA-GYN score produced an odds ratio of 1.195 (90% CI: 0.963 – 1.488) of having major grade 3 post-operative complications. For the subgroup analysis of 66 patients with stage 3 or 4 malignant disease undergoing open cytoreduction surgery, the estimated odds ratio of 1.290 (90% CI: 1.006 – 1.674) of having major post-operative complications for each 1-unit increase of pre-operative GA-GYN score (summarized in Table 5). The subgroup analysis of patients with advanced staged disease undergoing primary open cytoreductive surgery suggests an association of the GA-GYN score and grade 3 post-operative complications at a 0.05 significance level.
Table 5.
Subgroup Analysis for the Primary Objective
| Exact Conditional Logistic Regression One-Sided P-value | Exact Odds Ratio of 1-Unit increase of GA-GYN score | 90% Confidence Interval | Number of Observation Included in the Analysis | |
|---|---|---|---|---|
| Malignant disease: Pre-operative GA-GYN score and major post-operative complications | 0.0893 | 1.195 | 0.963 – 1.488 | 77 including 17 major post-operative complications |
| Stage 3/4 disease: Pre-operative GA-GYN score and major post-operative complications | 0.0456 | 1.290 | 1.006 – 1.674 | 58 including 11 major post-operative complications |
Secondary objectives included assessment of the association between individual pre-operative geriatric assessment variables and major post-operative complications in the primary surgery group and assessment of the association between the pre-operative GA-GYN score and cytoreducibility in the primary surgery group.
There were four individual variables within the domains of IADL, Mental Health Inventory and MOS Social Support which all had a test result of p-value less than 0.05 for their associations with post-operative complications. Specifically, ability to use the phone, feeling depressed or hopeless and lack of social support for transportation to receive medical care were each significantly associated with post-operative complications after open primary cytoreduction surgery. There was no association found between cytoreducibility, defined as complete vs. optimal vs. suboptimal cytoreduction, and the pre-operative GA-GYN score for patients undergoing primary surgery.
There was no association found between the GA-GYN score and post-operative complications in the 37 patients (all with malignant disease) who underwent interval cytoreduction after neoadjuvant chemotherapy. Post-operative complications, grade 3 and higher percentage events were similar in both surgical groups. Patients who underwent primary surgery (n=117), 21.4% had a grade 3 or higher post-operative adverse event compared to patients who underwent interval cytoreduction (n=37), 21.6% had a grade 3 or higher adverse post-operative event.
For patients who had neoadjuvant chemotherapy, the geriatric assessment was given twice, prior to initiation of chemotherapy and prior to interval cytoreduction surgery. There was no significant difference in the GA-GYN scores, indicating neoadjuvant chemotherapy did not have an effect on the score. This may suggest that the GA-GYN score in this setting reflects underlying issues related to aging rather than just debility due to disease. There was a strong association found (two-sided p value 0.0051) between the GA-GYN score and surgery choice (primary cytoreduction, interval cytoreduction, no surgery). This association suggests an agreement between surgeon perception of vulnerability and the GA-GYN score. This may make the score a useful way to “index” debility related to aging and allow better cross-trial comparisons.
Relationships of surgery type (i.e., primary, interval and no surgery) with baseline laboratory and chemistry variables were explored, and the test results for baseline measures for serum albumin, blood platelet, blood hematocrit and blood hemoglobin values all had a p-value less than 0.05 implying that the baseline measures for these four variables were different among surgery type groups at 0.05 significance level, respectively; patients who had the primary surgery tended to have higher baseline lab values for serum albumin, hematocrit, and hemoglobin in comparison to patients undergoing interval surgery and patients undergoing no surgery. In the primary open surgery group, there was a significant association of major post-operative complications with pre-operative serum albumin and blood hemoglobin at a significance level of 0.05, respectively. Lab values from patients undergoing primary open surgery and their association with post-operative complications are listed in Table 6.
Table 6.
Pre-Operative Labs for Primary Open Surgery Patients
| Major post-operative complications | ||||
|---|---|---|---|---|
| Endpoint | Yes | No | All | P-value1 |
| Albumin (g/dl), serum | ||||
| Mean | 3.6 | 3.89 | 3.84 | 0.0309 |
| Hemoglobin (g/dl), blood | ||||
| Mean | 11.87 | 12.75 | 12.59 | 0.0286 |
| Creatinine (mg/dl), serum | ||||
| Mean | 0.93 | 0.89 | 0.9 | 0.4676 |
| Creatinine clearance (ml/min) | ||||
| Mean | 49.12 | 50.75 | 50.46 | 0.6355* |
| Age (years) | ||||
| Mean | 77.89 | 76.13 | 76.44 | 0.1123 |
| Range | 70.32 – 87.38 | 70.02 – 89.59 | 70.02 – 89.59 | |
Exact conditional simple logistic regression
Likelihood ratio test of simple logistic regression
DISCUSSION
The primary objective of this study was to evaluate whether a GA-GYN score derived from the brief geriatric assessment tool developed by Hurria et al [13,14] would be associated with major post-operative complications in elderly women with suspected ovarian, fallopian tube, primary peritoneal or advanced stage papillary serous uterine carcinoma undergoing primary open cytoreductive surgery.
There was a significant issue with missing data; specifically number of falls was missing in approximately 12% in the group of 112 eligible primary surgeries. The absence of this data point likely affected the GA-GYN score since this was a heavily weighted item in the score, Table 2. Another major issue was the large percentage of benign patients enrolled in the primary surgery group. Approximately 14% of patients out of all patients enrolled and 21% of all primary surgery patients had benign disease; This likely accounted for the low post-operative complication rate with subsequent non-association between the GA-GYN score and post-operative complications in the primary surgery group.
Overall, this study provided valuable insight to treatment patterns of elderly women with advanced staged malignancy. If surgeons chose neoadjuvant chemotherapy as primary treatment the majority reason stated was provider preference, not unresectable disease or poor performance status. In the primary surgery arm, 93% of patients had a NRG/GOG performance status of 0 or 1, and post-operative complication rate was lower than expected when compared to other surgical studies at 17.9% [15, 16], which may reflect a change in patient selection patterns.
A modified version of the CARG geriatric assessment tool was used in this study. This tool has been associated with chemotherapy toxicity in the elderly population [12–14]. The ongoing goal of using this tool was to incorporate a uniform geriatric assessment measurement of elderly populations across cooperative group studies. This study demonstrated the feasibility of using this geriatric assessment tool in a busy surgical practice and did find an association between the GA-GYN score and post-operative complications in elderly women with advanced staged malignancies undergoing primary surgery
A major goal of this study was to identify a tool to categorize postoperative risk which would then assist in the decision regarding the advisability of surgery in the older patient population. There was a significant association of the GA-GYN score and post-operative complications in older patients with advanced staged disease and a statistically significant association between the GA-GYN score and surgery choice. This study was successful in identifying a pre-operative tool, predictive of post-operative complications in older women with advanced staged GYN malignancies. There was also a suggested agreement between surgeon perception of patient vulnerability and the GA-GYN score given the correlation between score and surgery choice. The GA-GYN score, by extrapolation, is in agreement with provider and patient choice of surgery (or no surgery) and can be predictive of post-operative complications in a sub-set of older patients.
NRG CC-002 is the first prospective surgical study in elderly women with advanced staged gynecologic cancers and successfully evaluated a pre-operative geriatric assessment tool in this population. Surgeons should consider usage of the GA-GYN tool as a predictor for surgical toxicity in older women with advanced staged gynecologic malignancies. This tool could also aid in choice of surgery in this patient population. Future studies should strive to continue incorporation of uniform geriatric assessment tools within and across cooperative groups.
Acknowledgments
This study was supported by National Cancer Institute grants to NRG Oncology (1 U10 CA180822), NRG Operations (U10CA180868) and NCORP (UG1CA189867). The following Gynecologic Oncology institutions participated in this study: University of Iowa/Holden Comprehensive Cancer Center; University of Oklahoma Health Sciences Center, University of Pennsylvania/Abramson Cancer Center, Abington Memorial Hospital, Maine Medical Center-Scarborough Campus, Memorial Sloan Kettering Cancer Center, University of Virginia Cancer Center, Duke University Medical Center, Dartmouth-Hitchcock Medical Center/Norris Cotton Cancer Center, Women’s Cancer Center of Ne3vada, Hartford Hospital, University of Alabama at Birmingham Cancer Center, Froedtert and the Medical College of Wisconsin, Washington University School of Medicine, UT Southwestern/Simmons Cancer Center-Dallas, Rush University Medical Center, Mayo Clinic, Women and Infants Hospital, Avera Cancer Institute, Parkview Hospital Randallia, Wayne State University/Karmanos Cancer Institute, Henry Ford Hospital, Saint Joseph’s Hospital and Medical Center, UCLA/Jonsson Comprehensive Cancer Center, and UC San Diego Moores Cancer Center.
Footnotes
CONFLICTS OF INTEREST
Dr. Robert Mannel served on Advisory Boards for Tesaro and Clovis.
Dr. Mitchell Edelson states that his spouse is an employee of Merck and received stock as an employee.
Dr. Susan Modesitt reports that her institution received grant funding from NRG.
Dr. Anthony Evans reports his institution received standard payment for institutional support from the NRG.
All other co-authors have no conflicts of interest to declare.
SAS and all other SAS Institute Inc. products or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® Indicates USA registration.
REFERENCES
- 1.Berger NA, Savvides P, Koroukian S, Kahana E, Deimling G, Rose J, et al. Cancer in the Elderly. Transactions of the American Clinical and Climatological Association 2006; 117:147–156. [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- 3.Hightower RD, Nguyen HN, Averette HE, Hoskins W, Harrison T, Steren A. National survey of ovarian carcinoma: Patterns of care and related survival for older patients. Cancer 1994; 73:377–383. [DOI] [PubMed] [Google Scholar]
- 4.Eisenher EL, Tew WP, Levine DA, Lichtman SM, Brown CL, Aghajanian C, et al. Response and outcomes in elderly patients with stages IIIC-IV ovarian cancer receiving platinum-taxane chemotherapy. Gynecol Oncol 2007; 106(2):381–387. [DOI] [PubMed] [Google Scholar]
- 5.Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced stage ovarian cancer. Obstet Gynecol 2012; 120(4):871–881. [DOI] [PubMed] [Google Scholar]
- 6.Wright J, Doan T, McBride R, Jacobson J, Hershman D. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer 2008; 98(7):1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363:943–953. [DOI] [PubMed] [Google Scholar]
- 8.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, et al. An analysis of patients with bulky advanced stage ovarian, tubal and peritoneal carcinoma treated with primary debulking surgery during an identical time period as the randomized EORTC-NCIC trial of PDS vs. neoadjuvant chemotherapy. Gynecol Oncol 2012; 124(1):10–14. [DOI] [PubMed] [Google Scholar]
- 9.Zighelboim I, Kizer N, Taylor NP, Case AS, Gao F, Thaker PH, et al. “Surgical Apgar Score” predicts postoperative complications after cytoreduction for advanced ovarian cancer. Gynecol Oncol 2010; 116(3):370–373. [DOI] [PubMed] [Google Scholar]
- 10.Aletti GD, Eisenhauer EL, Santillian A, Axtell A, Aletti G, Holschneider C, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol 2011; 120(1):23–28. [DOI] [PubMed] [Google Scholar]
- 11.Aletti SD, Dowdy SC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007; 197(6):676. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Cirrincione CT, Muss HB, Kornblith AB, Barry W, Artz A, et al. Implementing a Geriatric Assessment in Cooperative Group Clinical Cancer Trials: CALGB 360401. J Clin Oncol 2011; 29:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurria A, Togawa K, Mohile S, Owusu C, Klepin H, Gross C, et al. Predicting Chemotherapy Toxicity in Older Adults with Cancer: A Prospective Multicenter Study. J Clin Oncol 2011; 29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, et al. Developing a Cancer Specific Geriatric Assessment. Cancer 2005; 104:1998–2005. [DOI] [PubMed] [Google Scholar]
- 15.Audisio R, Gennari R, Sunouchi K, Nair HR, Sestini A, Pope D, et al. Preoperative Assessment of Cancer in Elderly Patients: A Pilot Study. Support Cancer Ther 2003; 1:55–60. [DOI] [PubMed] [Google Scholar]
- 16.Audisio RA, Pope D, Ramesh H, Gennari R, van Leeuwen B, West C, et al. Shall we operate? Preoperative assessment in elderly patients can help. Crit Rev Oncol Hematol 2008; 65:156–163. [DOI] [PubMed] [Google Scholar]