We have previously described a Korean family afflicted with spinocerebellar ataxia type 2 (SCA2) parkinsonism in which genetic analysis revealed CAG expansion of 40 repeats in the ATXN2 gene.1 The affected members presented with levodopa‐responsive parkinsonism without cerebellar ataxia. Some showed motor fluctuation and dyskinesia, further mimicking idiopathic Parkinson’s disease (PD). Herein, we report a member of this family who developed jaw‐opening and lingual‐protrusion dystonia as the chief presentation.
Case report
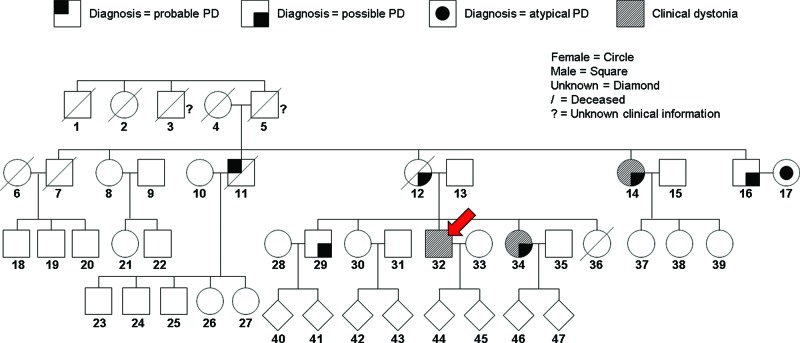
The patient, no. 32 in the pedigree (Fig. 1), presented to the clinic at age 58. He reported involuntary jaw opening that persisted for 2 months, which led to disabling difficulties in swallowing and speech. A sense of his leg dragging and a gait disturbance accompanied the symptoms. There was no history of exposure to antipsychotics or other medications.
Figure 1. Pedigree of the Spinocerebellar Ataxia Type 2 Korean Family. Adopted and revised from the pedigree in Kim et al.1 The red arrow indicates no. 32, the proband.
Upon examination, oromandibular dystonia was found to be prominent. Involuntary jaw opening and tongue protrusion continued with fluctuation, causing difficulty in speech and chewing, but he did not lose weight. These involuntary movements were reported to fluctuate with no particular diurnal pattern, and no definite triggering or suppressive factor was identified. No sensory trick was found. Systemic evaluation revealed bilateral hyper‐reflexive deep‐tendon reflex, mildly stooped posture, and parkinsonian gait. There was no sign of lower motor lesion, including weakness, muscle atrophy, or fasciculation. Ataxia, dysmetria, and extraocular movement abnormality were not observed upon careful examination. Dystonia was absent in other parts of the body.
Dopaminergic agents, anticholinergics, baclofen, and clonazepam were ineffective or not consistently effective for his oromandibular dystonia. A maximum 600 mg daily dosage of levodopa, 3 mg of pramipexole, 1 mg of rasagiline, 6 mg of biperiden, 300 mg of amantadine, 30 mg of baclofen, and 1.5 mg of clonazepam was serially administered over 4 years. The patient committed suicide at age 62.
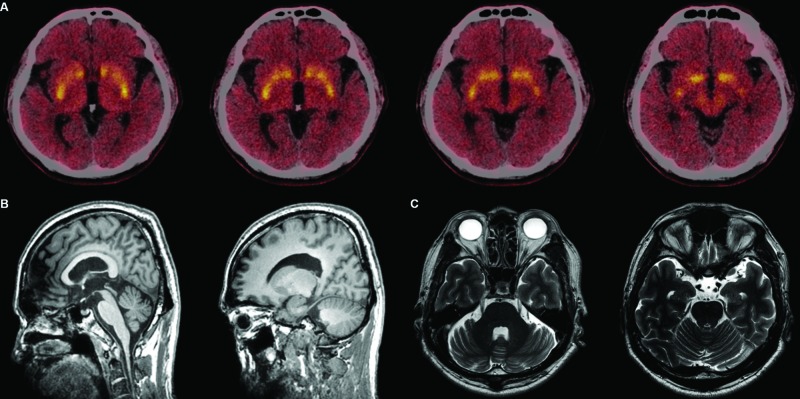
The initial differential diagnosis of his presentation included Wilson’s disease, X‐linked dystonia parkinsonism, Fahr disease, and neurodegeneration with brain iron accumulation before it was revealed that he was a member of the SCA2 family. Magnetic resonance imaging was unremarkable, with no focal atrophy or iron accumulation. Decreased uptake in the anterior part of both striata was revealed by [18F]‐N‐(3‐fluoropropyl)‐2β‐carbon ethoxy‐3β‐(4‐iodophenyl) nortropane ([18F]‐FP‐CIT) positron emission tomography (Fig. 2). Both horizontal and vertical saccadic velocity were normal using video‐oculography. Because of his family history, the patient was tested for SCA2 with a positive result, and the same CAG expansion of 40 as in other affected family members.
Figure 2. Structural and Functional Brain Imaging of the Patient. (A) Reduced dopamine transporter density in the anterior parts of both striata as shown in [18F]-FP-CIT positron emission tomography performed at age 59. (B,C) Brain magnetic resonance imaging at age 59 showed no definite cerebellar atrophy.
Discussion
This is the first case of SCA2 that presented with lingual‐protrusion dystonia as the chief and initial complaint, accompanied by subtle parkinsonian features. This case demonstrates that intrafamilial heterogeneity exists within the single SCA2 kindred. In the previous report on this family, we suggested that SCA2 parkinsonism be considered in the diagnostic workup of familial PD even without cerebellar ataxia. This case supports broadening the indication of the workup.
As detailed in Kim et al.,1 the other affected family members had levodopa‐responsive parkinsonism without cerebellar ataxia. No sign of cerebellar dysfunction was found on careful follow‐up examination and video‐oculography. Structural and functional brain imaging in this family showed no cerebellar atrophy but indicated presynaptic dopaminergic degeneration with a spared post‐synaptic dopaminergic system in the striatum. Sequencing was performed in patients 12 and 29. The expanded allele with 40 CAG repeats had four CAA interruptions ((CAG)8CAA(CAG)8CAA(CAG)8CAA(CAG)4CAA(CAG)8) whereas the normal allele with 22 CAG repeats showed two CAA interruptions ((CAG)8CAA(CAG)4CAA(CAG)8). LRRK2, VPS35, EIF4G1, SNCA, and ATXN3 were all negative.1 Notably, subjects 14 and 34 (Fig. 1) showed dystonia and blepharospasm during the long course of the disease, but their symptoms remained mild until death or last follow‐up.
While dystonia is observed in patients with parkinsonism, it is typically not the initial or main presentation, especially in the oromandibular area.2 The prevalence of dystonia in SCA2 ranges from 14% to 18.1% but is as high as 61% in some reports.3–5 Jaw‐opening dystonia has been reported in SCA2 patients,6 but only one case of lingual‐protrusion dystonia is described in SCA2. That patient had a 27‐year history of cerebellar symptoms and was already wheelchair‐bound when she developed jaw‐opening and lingual‐protrusion dystonia.3
The mechanism of oromandibular dystonia in this SCA2 patient remains unclear. In contrast to the parkinsonian symptoms of other family members, dystonia in this patient remained unresponsive to dopaminergic treatment, even though there was a severe decrease in dopamine transporter (DAT) ligand binding in the anterior part of the striatum (Fig. 2). This finding implies that presynaptic dopaminergic denervation was less likely to be related to dystonia in this patient. Cerebellar pathophysiology may have contributed to the dystonia via interconnection with basal ganglia,7 although there was no overt sign of cerebellar dysfunction either clinically or radiologically.
With regard to imaging studies of the dopaminergic system, several reports have described prominent striatal DAT loss in SCA2 patients without parkinsonism.8–10 Similarly, autopsies of two SCA2 patients revealed significant nigral degeneration.11 Another autopsy study suggested that the combined degeneration of the subthalamic nucleus (STN) may be responsible for the lack of clinical parkinsonism in typical SCA2 and SCA3 patients despite presynaptic dopaminergic deficits. An autopsy of the only patient with parkinsonism in the study revealed sparing of the STN, in contrast to the other patients.9 Considering the efficacy of STN deep brain stimulation in refractory dystonia,12 there is also a possibility that sparing of the STN in certain SCA2 patients may play a role in the development of dystonia as well as parkinsonism.
This case report emphasizes prominent intrafamilial heterogeneity in clinical manifestations of a large SCA2 parkinsonism family in Korea. Modifying gene or epigenetic factors altering the phenotype of SCA2 should be further investigated.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflicts of Interest: The authors report no conflict of interest.
Ethics Statement: This study was reviewed by the authors' institutional ethics committee and was considered exempted from further review.
References
- 1.Kim YE, Jeon B, Farrer MJ, Scott E, Guella I, Park SS, et al. SCA2 family presenting as typical Parkinson’s disease: 34 year follow up. Parkinsonism Relat Disord. 2017;40:69–72. doi: 10.1016/j.parkreldis.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Tolosa E, Compta Y. Dystonia in Parkinson’s disease. J Neurol. 2006;253(Suppl. 7):VII7–13. doi: 10.1007/s00415-006-7003-6. [DOI] [PubMed] [Google Scholar]
- 3.Markovic V, Dragasevic‐Miskovic NT, Stankovic I, Petrovic I, Svetel M, Kostić VS. Dystonia in patients with spinocerebellar ataxia type 2. Mov Disord Clin Pract. 2016;3:292–295. doi: 10.1002/mdc3.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boesch SM, Muller J, Wenning GK, Poewe W. Cervical dystonia in spinocerebellar ataxia type 2: clinical and polymyographic findings. J Neurol Neurosurg Psychiatry. 2007;78:520–522. doi: 10.1136/jnnp.2006.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo PH, Gan SR, Wang J, Lo RY, Figueroa KP, Tomishon D, et al. Dystonia and ataxia progression in spinocerebellar ataxias. Parkinsonism Relat Disord. 2017;45:75–80. doi: 10.1016/j.parkreldis.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antenora A, Peluso S, Saccà F, De Michele G, Filla A. Jaw‐opening oromandibular dystonia associated with spinocerebellar ataxia type 2. Mov Disord Clin Prac. 2014;1:121–122. doi: 10.1002/mdc3.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wullner U, Reimold M, Abele M, Bürk K, Minnerop M, Dohmen BM, et al. Dopamine transporter positron emission tomography in spinocerebellar ataxias type 1, 2, 3, and 6. Arch Neurol. 2005;62:1280–1285. doi: 10.1001/archneur.62.8.1280. [DOI] [PubMed] [Google Scholar]
- 9.Schols L, Reimold M, Seidel K, Globas C, Brockmann K, Hauser TK, et al. No parkinsonism in SCA2 and SCA3 despite severe neurodegeneration of the dopaminergic substantia nigra. Brain. 2015;138:3316–3326. doi: 10.1093/brain/awv255. [DOI] [PubMed] [Google Scholar]
- 10.Varrone A, Salvatore E, De Michele G, Barone P, Sansone V, Pellecchia MT, et al. Reduced striatal [123I]FP-CIT binding in SCA2 patients without parkinsonism. Ann Neurol. 2004;55:426–430. doi: 10.1002/ana.20054. [DOI] [PubMed] [Google Scholar]
- 11.Durr A, Smadja D, Cancel G, Lezin A, Stevanin G, Mikol J, et al. Autosomal dominant cerebellar ataxia type I in Martinique (French West Indies). Clinical and neuropathological analysis of 53 patients from three unrelated SCA2 families. Brain. 1995;118((Pt 6)):1573–1581. doi: 10.1093/brain/118.6.1573. [DOI] [PubMed] [Google Scholar]
- 12.Ostrem JL, San Luciano M, Dodenhoff KA, Ziman N, Markun LC, Racine CA, et al. Subthalamic nucleus deep brain stimulation in isolated dystonia: a 3-year follow-up study. Neurology. 2017;88:25–35. doi: 10.1212/WNL.0000000000003451. [DOI] [PubMed] [Google Scholar]