Abstract
BRAF mutations occur infrequently in non–small-cell lung cancer, and therefore, our understanding of the natural history of tumors harboring these mutations remains limited. In this retrospective study, we report the outcomes, treatment responses, and co-occurring mutations in a series of patients with BRAF-mutated non–small-cell lung cancer. In our cohort, survival rates at 2 and 5 years were 56% and 13%, respectively, suggesting more favorable outcomes in a subset of patients.
Background:
BRAF mutations occur in 1% to 4% of non–small-cell lung cancer (NSCLC) cases. Previous retrospective studies have reported similar outcomes for BRAF-mutated NSCLC as compared with wild-type tumors without a known driver mutation or tumors harboring other mutations. However, select cases of prolonged survival have also been described, and thus, the natural history of BRAF-mutated NSCLC remains an area of ongoing study. The aim of this series was to describe the natural history, clinical outcomes, and occurrence of co-mutations in patients with BRAF-mutated NSCLC.
Patients and Methods:
Patients with BRAF-mutated NSCLC seen at Stanford University Medical Center from January 1, 2006 through July 31, 2015 were reviewed. The Kaplan-Meier method was used to calculate median overall survival, and the generalized Wilcoxon test was used to compare median survivals across subgroups of patients.
Results:
Within a cohort of 18 patients with BRAF-mutated NSCLC, V600E mutations were most common (72%; 13/18). Clinicopathologic features were similar between patients with V600E versus non-V600E mutations, although there was a trend toward more patients with non-V600E mutations being heavy smokers (80% vs. 31%; P = .12). Co-occurring mutations in TP53 were identified most commonly (28%; 5/18). The median overall survival for the entire cohort was 40.1 months, and the median survival from the onset of metastases (n = 16) was 28.1 months. Survival rates at 2 and 5 years from the onset of metastases were 56% and 13%, respectively.
Conclusion:
The clinical behavior of BRAF-mutated NSCLC is variable, but favorable outcomes can be seen in a subset of patients.
Keywords: BRAF, Next-generation sequencing, Non-small cell lung cancer, Overall survival, V600E
Introduction
An expanded understanding of the molecular pathways and mutations that underlie tumorigenesis in non–small-cell lung cancer (NSCLC) has led to changes in management of the disease for many patients. NSCLC that harbors an activating mutation in epidermal growth factor receptor (EGFR), for example, is now routinely treated first-line with targeted inhibitors such as erlotinib, gefitinib, afatinib, or osimertinib, based on studies demonstrating superior progression-free survival compared with standard therapy.1,2 Translocations in anaplastic lymphoma kinase (ALK) are similarly amenable to first-line targeted therapy with inhibitors such as crizotinib, ceritinib, and alectinib.3 Although treatment with genotype-directed therapy in NSCLC results in improved survival, targetable mutations in EGFR and ALK are present in only a subset of cases of NSCLC.4 As a result, there remains an ongoing need to better understand the pathogenesis of other driver mutations in NSCLC as well.
V-Raf murine sarcoma viral oncogene homolog (BRAF) encodes a serine-threonine kinase within the MAPK signaling pathway.5,6 Mutations in BRAF lead to downstream pathway activation in experimental cancer models and have been identified as drivers in multiple cancer subtypes including melanoma.7 BRAF V600 mutations, in particular, are characterized by an amino acid substitution for valine at position 600 within exon 15 that encodes the protein kinase domain.5 Although uncommon, BRAF mutations occur in approximately 1% to 4% of NSCLC and are postulated to act as oncogenic drivers in this setting.8–11 In contrast to other cancer subtypes where V600 mutations predominate, V600 and non-V600 mutations occur with similar incidence in NSCLC.12,13
With respect to clinicopathologic features including age, gender, race, smoking history, and stage of disease, patients with BRAF-mutated NSCLC present similarly to patients whose tumors do not harbor a known oncogenic driver.14 However, patients with V600 versus non-V600-mutated NSCLC may differ, with multiple studies reporting nonsmokers to be more prevalent among those with BRAF V600-mutated NSCLC.15,16 With respect to survival, some previous studies have suggested that the outcomes of patients with BRAF-mutated NSCLC are similar to those of patients with tumors harboring other driver mutations or wild-type tumors without a known driver.10,14–16 However, worse survival has also been reported for patients with V600E mutations in particular.8
Although our ability to detect BRAF mutations has improved with next-generation sequencing, the goal of detection is ultimately to translate this information into more effective treatment decisions. In an effort to move toward this goal, a recent series of clinical trials evaluated the efficacy of targeted therapy for patients with BRAF V600E-mutated NSCLC. In the first of these studies, previously-treated patients receiving dabrafenib, an oral BRAF inhibitor, had a response rate of 33% and a median duration of response lasting 9.6 months.17 In 2 subsequent trials, combination dabrafenib plus trametinib (MEK inhibitor) was similarly effective in the first-and later-line settings, with a response rate of 64% and a median survival of 24.6 months in previously-untreated patients in particular.18,19 These results led to United States Food and Drug Administration approval of dabrafenib plus trametinib for any line of treatment in patients with metastatic BRAF V600E-mutated NSCLC.
The emergence of BRAF-targeted therapy represents an important milestone in the management of patients who have previously had limited treatment options, especially in the second-line setting.20,21 However, our understanding of the natural history of BRAF-mutated NSCLC continues to be relatively limited. Furthermore, case reports have described prolonged survival in select patients with BRAF-mutated NSCLC, which raises questions about the course of the disease overall.21–23 In this study, we therefore aimed to describe the natural history, outcomes, and occurrence of co-mutations in patients with BRAF-mutated NSCLC who were seen at our institution.
Patients and Methods
Patients with known BRAF-mutated NSCLC seen at Stanford University Medical Center from January 1, 2006 through July 31, 2015 were identified by two authors (SH, DW) using an institutional review board-approved chart review protocol within the Stanford Cancer Institute Research Database (SCIRDB), which includes data from the Stanford Cancer Registry. The Stanford Cancer Registry collects demographic (address, gender, race, age), diagnostic (type of cancer, stage of disease at diagnosis), and treatment-related (surgery, radiation, or chemotherapy in the first-line setting) information on individual cases. Patients with NSCLC were identified using 3 diagnostic criteria: (1) they were recorded within the Stanford Cancer Registry as having a primary lung cancer diagnosis; (2) they had confirmed problem list and encounter International Classification of Diseases, Ninth Revision diagnostic codes of lung cancer from the EPIC clinical reporting database; and (3) they had completed at least 1 visit to a provider in the medical oncology (thoracic) or thoracic surgery groups at Stanford University Medical Center. Among patients identified by these criteria as having lung cancer, the subset of patients with BRAF results were then selected if they had: (1) positive BRAF-related molecular testing; (2) full text search of pathology reports containing the term ‘BRAF’; or (3) full text search of progress notes containing the term ‘BRAF.’
Data on patients confirmed by manual chart review to have BRAF-mutated NSCLC was then collected retrospectively through detailed review of the medical record. The clinical course of each patient was reviewed through December 31, 2017 or until loss of follow-up or death, whichever occurred first. The median duration of follow-up among all patients in this study was 31.6 months (range, 0.8–132.4 months).
Collected demographic data included date of birth, age at diagnosis, gender, race/ethnicity, and smoking history. Pathology reports were reviewed to identify tumor histology, and molecular results were reviewed to identify the type of BRAF mutation as well as co-occurring mutations or exon deletions in other genes. Variants of unknown significance were not considered unless they occurred in BRAF. Mutations in BRAF were defined as V600E or non-V600E according to whether or not the mutation substituted glutamic acid for valine at position 600. Mutations were compared to the Catalogue Of Somatic Mutations in Cancer (COSMIC) (http://cancer.sanger.ac.uk/cosmic) and My Cancer Genome (https://www.mycancergenome.org) databases. Imaging reports and clinical documentation were reviewed to identify stage, sites of metastases, clinical course, and treatment response.
The date of diagnosis was defined as the date on which biopsy or resection confirmed the pathologic diagnosis of NSCLC. Because this was a retrospective study done outside the confines of a clinical trial, and given the potential limitations of using Response Evaluation Criteria In Solid Tumours-based progression in other forms of driver-mutated NSCLC, treatment duration was used as a clinically relevant proxy of disease stability.24 In this case, treatment duration for each line of systemic therapy administered for metastatic disease was defined as the time from the start of therapy to the date of discontinuation if there was evidence of clinical or radiographic progression. In cases in which a treatment holiday was initiated without evidence of progression, treatment duration was defined as the time from the start of therapy to the date on which the holiday ended owing to progression. Date of death was determined by chart review or review of public obituary records. Overall survival (OS) was defined as the time from diagnosis to death or to the date of last follow-up for patients still alive or lost to follow-up. Survival from metastatic disease was defined as the time from first radiographic development of metastases to death or last follow-up.
The median OS was calculated using the Kaplan-Meier method. The generalized Wilcoxon test was used to compare the median survivals of patients with de novo versus recurrent metastases. To compare clinicopathologic characteristics between patients with V600E versus non-V600E mutations, the Fisher exact test was used for categorical variables and the Mann-Whitney U test was used for continuous variables. All categorical variables were dichotomized. A 2-sided P-value < .05 defined statistical significance. Statistical calculations were performed using SPSS software (IBM Corp, Armonk, NY).
Results
Demographics
A total of 657 patients were identified in the SCIRDB as having a problem list diagnosis of NSCLC within the time period of interest. From this cohort, 18 (2.7%) patients with BRAF-mutated NSCLC were identified, including 1 who has been described previously in detail (Table 1).23 Nearly 75% of patients in this cohort were male. Caucasian race was most common, although Asian and Hispanic patients together comprised 44% of the cohort, partially reflecting the ethnic diversity of patients seen at our center. Although the majority of patients reported a smoking history, the number of heavy smokers (>15 pack-years) was nearly equal to the number of never-smokers. Biopsy identified adenocarcinoma in all cases, except for 1 that showed poorly-differentiated histology (data not shown).
Table 1.
Clinicopathologic Characteristics
| Characteristics | Total, N =18 (%) | V600E, N =13 (%) | Non-V600E, N =5 (%) | P Valuea |
|---|---|---|---|---|
| Age, y | ||||
| Median | 66.5 | 69 | 61 | .29 |
| Gender | ||||
| Male | 13 (72) | 9 (69) | 4 (80) | 1.0 |
| Female | 5 (28) | 4 (31) | 1 (20) | |
| Ethnicity | ||||
| Caucasian | 8 (44) | 6 (46) | 2 (40) | 1.0 |
| African-American | 1 (6) | 0 (0) | 1 (20) | |
| Asian | 5 (27) | 4 (31) | 1 (20) | |
| Hispanic | 3 (17) | 2 (15) | 1 (20) | |
| Other | 1 (6) | 1 (8) | 0 (0) | |
| Smoking status | ||||
| > 15 ppy | 8 (44) | 4 (31) | 4 (80) | .12 |
| 0–15 ppy | 3 (17) | 3 (23) | 0 (0) | |
| Never | 7 (39) | 6 (46) | 1 (20) | |
| Stage (at diagnosis) | ||||
| I | 2 (11) | 2 (15) | 0 (0) | .58 |
| II | 1 (6) | 1 (8) | 0 (0) | |
| III | 4 (22) | 2 (15) | 2 (40) | |
| IV | 11 (61) | 8 (62) | 3 (60) |
Abbreviations: ppy=pack-years.
P value for the comparison of V600E versus non-V600E subgroups.
BRAF Mutations
BRAF mutations were detected by commercially available multiplex primer-extension based methods (12 patients) or by next-generation sequencing (6 patients). In all but 3 patients for whom tumor molecular testing was done by multiplex primer-extension based methods, Clinical Laboratory Improvement Amendmentsbased SNaPhot testing was used. This panel tests for mutations in the following genes: APC, BRAF, CTNNB1, EGFR, IDH1, IDH2, KRAS, NOTCH1, NRAS, PIK3CA, PTEN, TP53, and, in some cases, DNMT3A, MYD88, SF3B1, and ERBB2/HER2 as well. Next-generation sequencing was performed using the Foundation Medicine panel or the Stanford Solid Tumor Actionable Mutation Panel (STAMP) that is available at our institution. Molecular testing was performed at the time of diagnosis or, for those with initially early-stage disease, at the time of first metastases in 8 (44%) patients. Molecular testing in the remaining patients was performed in the second-line or later setting. Of note, 1 patient in our cohort had primer-based extension sequencing performed on pleural fluid at the time of initial diagnosis followed by next-generation sequencing of lymph node tissue obtained at progression. In this patient, a BRAF mutation was detected only by next-generation sequencing, raising the possibility that the mutation arose at a later time point in tumor evolution or that it was initially missed owing to differential sensitivity of the assays and/or variability of the tissue samples (fluid vs. nodal tissue).
V600E mutations (1799T>A) accounted for 13 (72%) of 18 BRAF mutations identified. The most common non-V600E mutation was G469A (1406G>C; n = 2). Other mutations included G466V (not reported), K601E (1801A>G), and V600_W604delinsR deletion/insertion, each of which occurred in single patients. V600_W604delinsR deletion/insertion mutations lead to deletion of valine, lysine, serine, arginine, and threonine residues with substitution of arginine.
With respect to age (median, 69 vs. 61 years), gender (69% vs. 80% male), ethnicity (46% vs. 40% Caucasian), and stage at presentation (77% vs. 60% advanced stage [IIIB/IV]), V600E and non-V600E groups were similar (P < .05 for all variables) (Table 1). Most patients in the non-V600E group (80%) reported a heavy smoking history compared with the majority of patients (69%) in the V600E group who were light (0–15 pack-years) or never-smokers (P = .12).
Co-occurring Mutations
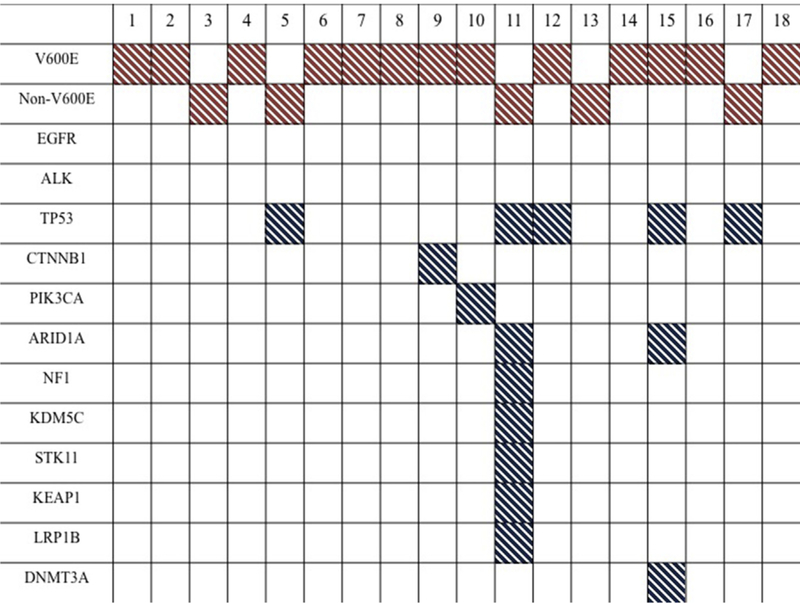
No co-occurring mutations in EGFR, ALK, or KRAS were identified in any patient with BRAF-mutated NSCLC. The most common co-occurring mutation was TP53 in 5 of the 8 patients whose tumors harbored secondary genomic alterations. Each TP53 mutation was unique, with the following mutations identified in 5 patients: I195T, C242F (725 G>T), R248L (743 G>T), E298X (892 G>T), and splice site 559þ1 G>C. Among 6 patients who had next-generation sequencing results available, the majority was found to have at least 1 other genomic mutation. Aside from mutations involving TP53, most mutations occurring in other genes were identified in single patients only. One patient’s tumor harbored alterations in multiple genes including mutations in BRAF, TP53, NF1, ARID1A, and KDM5C and exon deletions in STK11 (exons 3–5), KEAP1 (exons 3–6), and LRP1B (exons 16–30) (Figure 1).
Figure1.
Genomic Alterations Found in Each patient’s Tumor are Shown. No Co-Occurring Mutations in EGFR, ALK, or KRAS Were Seen, Although Mutations in TP53 Co-Occurred With BRAF Mutations in 28% of Patients. Most Patients had Only a Few Secondary Genomic Alterations Detected, With the Exception of 1 Patient (Case #11) Whose Tumor had Mutations And/or Exon Deletions in Multiple Genes as Identified by Next-Generation Sequencing. In the figure, BRAF Mutations are Highlighted in Red, and Other Mutations are Highlighted in Blue
Outcomes
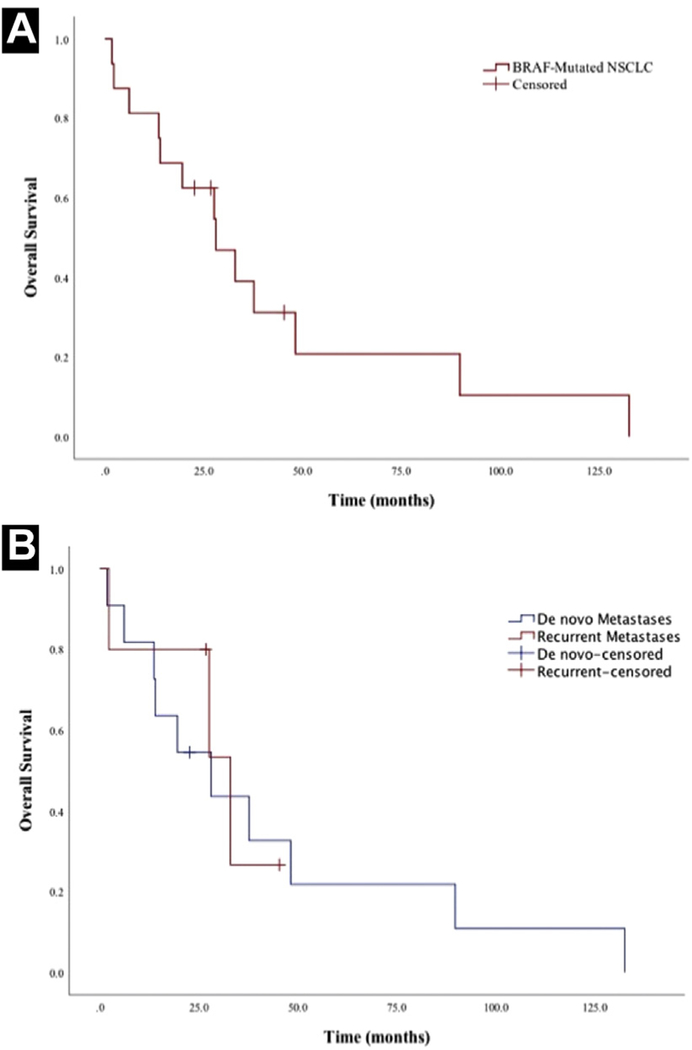
The median OS for the entire cohort, including 2 patients diagnosed with early-stage disease, was 40.1 months (95% confidence interval [CI], 24.7–55.5 months) (Table 2). The median survival from the onset of metastatic disease for patients (n = 16) who developed distant metastases (de novo or at progression) was 28.1 months (95% CI, 13.3–42.9 months) (Figure 2A). Among those patients with metastatic disease, the 2- and 5-year survival rates from the onset of metastases were 56% and 13%, respectively. To ensure that the timing of onset of metastatic disease was not a driver of these findings, median survival was calculated separately for patients with de novo metastases versus metastases at recurrence only. In this analysis, median OS was 28.1 months (95% CI, 6.7–49.5 months) for patients with de novo metastases versus 33.0 months (95% CI, 7.5–58.5 months) for patients with metastases at recurrence (P = .76) (Figure 2B). Among all patients, common initial sites of metastases seen recurrently in more than 1 patient were the cervical lymph nodes, brain, bone, liver, adrenal glands, axillary lymph nodes, and subpectoral lymph nodes. Four patients initially had intrathoracic metastases only.
Table 2.
Survival Outcomes by Patient
| Stage | BRAF Mutation | Overall Survival, mos | Survival After Metastasis, mos | |
|---|---|---|---|---|
| 1 | IA | V600E | 90.0a | 45.4a |
| 2 | IIIB | V600E | 8.9 | 2.3 |
| 3 | IV | G469A | 37.7 | 37.7 |
| 4 | IIIB | V600E | 40.3a | 26.8a |
| 5 | IIIA | G469A | 41.7 | 27.7 |
| 6 | IV | V600E | 14.0 | 14.0 |
| 7 | IV | V600E | 132.6 | 132.6 |
| 8 | IIA | V600E | 66.2 | NA |
| 9 | IV | V600E | 1.8 | 1.8 |
| 10 | IV | V600E | 48.2 | 48.2 |
| 11 | IIIA | G466V | 40.1 | 33.0 |
| 12 | IA | V600E | 37.0a | NA |
| 13 | IV | V600_W604delinsR | 22.7a | 22.7a |
| 14 | IV | V600E | 28.1 | 28.1 |
| 15 | IV | V600E | 13.7 | 13.7 |
| 16 | IV | V600E | 89.8 | 89.8 |
| 17 | IV | K601E | 6.2 | 6.2 |
| 18 | IV | V600E | 19.6 | 19.6 |
Denotes patients who had no recorded date of death beyond their last follow-up encounter or who were still alive as of December 31, 2017.
Figure 2.
A, Median Overall Survival Calculated by the Kaplan-Meier Method was 28.1 Months From the Onset of Metastatic Disease for Those Patients With Either de Novo or Recurrent Metastases (n =16). B, Median Overall Survival was 28.1 for Patients With de Novo Metastases Versus 33.0 Months for Patients With Metastases at the Time of Recurrence Only
Abbreviation: NSCLC = non–small-cell lung cancer.
Subset survival analyses were also done to compare outcomes between subgroups of patients harboring different tumor mutations. With respect to BRAF, the median OS from the time of metastases for patients with V600E mutations was 28.1 months (95% CI, 6.7–49.5 months) versus 33.0 months (95% CI, 10.8–55.2 months) for patients with non-V600E mutations, a difference that was not statistically significant (P = .91). Patients with co-occurring mutations in TP53 had a shorter median OS from the time of metastases (13.7 months; 95% CI, 0–34.8 months) compared with those with wild-type TP53 (37.7 months; 95% CI, 11.1–64.3 months), although this did not reach statistical significance (P =.23). This does not include 1 patient with a co-occurring TP53 mutation who presented with early-stage disease and has had no evidence of metastatic progression to date.
Patients who developed metastatic disease received a median number of 3 lines of systemic therapy (range, 0–7 lines), and the treatment regimens received in each line of therapy are shown in Supplemental Table 1 (in the online version). In the first-line setting, systemic platinum-based combination chemotherapy was used most commonly (75%; 12/16). Among the 4 patients who did not receive platinum-based chemotherapy in the first-line setting, one was a never-smoker who initially received empiric erlotinib, 2 underwent radiation therapy only, and 1 died shortly after diagnosis without any record of receiving systemic therapy. Two patients followed induction platinum-based chemotherapy with maintenance erlotinib despite being EGFR wild-type, reflecting standard of care at the time of diagnosis. Both had progression after starting erlotinib, requiring a change in therapy approximately 3 and 9 months later, respectively. The median duration of first-line chemotherapy was 8.4 months (range, 1.3–22.5 months) (Table 3). The median durations of second- and third-line chemotherapy were 5.6 and 5.8 months, respectively, with few patients receiving chemotherapy beyond this.
Table 3.
Duration of Treatment on First- and Later-lines of Therapy
| No. Patients (%) | Treatment Duration, mos,Median (Range) | |
|---|---|---|
| Metastatic first-line | ||
| Chemotherapya | 12 (67) | 8.4 (1.3–22.5) |
| BRAF-targeted therapy | 0 (0) | – |
| Other targeted therapyb | 1 (6) | 33.7 (33.7) |
| Metastatic second-line | ||
| Chemotherapy | 7 (39) | 5.6 (2.8–75.8) |
| BRAF -targeted therapyc | 6 (33) | 5.8 (1.1–9.8) |
| Other targeted therapy | 0 (0) | – |
| Metastatic third-line | ||
| Chemotherapy | 3 (17) | 5.8 (1.7–7.1) |
| BRAF -targeted therapyd | 2 (11) | 3.0 (0.7–5.3) |
| Other targeted therapyd | 2 (11) | 4.4 (2.8–6.0) |
| Immunotherapy | 3 (17) | 4.3 (0.6–8.0) |
| Metastatic later-lines | ||
| Chemotherapy | 3 (17) | 1.6 (1.2–3.6) |
| BRAF -targeted therapye | 3 (17) | 1.7 (0.3–45.3) |
| Other targeted therapye | 0 (0) | – |
| Immunotherapy | 2 (11) | 0.8 (0.5–1.1) |
Two patients received erlotinib maintenance therapy following induction chemotherapy (see text for details).
First-line targeted therapy was administered empirically with erlotinib in a never-smoking patient prior to tumor molecular testing.
Second-line targeted therapies included dabrafenib monotherapy (n = 4) and combination dabrafenib plus trametinib (n = 2).
Third-line targeted therapies included erlotinib (n = 2; both EGFR wild-type), dabrafenib (n = 1), and trametinib (n = 1).
Later-line targeted therapies included vemurafenib (n = 1) and combination dabrafenib plus trametinib (n = 2).
A single patient (case #7) who received empiric erlotinib monotherapy in the first-line setting remained on this therapy for 33.7 months with very slow-growing disease. BRAF-directed targeted therapy was used exclusively in the second-line or later setting. In the second-line setting, in particular, 4 patients received dabrafenib monotherapy and 2 patients received combination dabrafenib plus trametinib. Median duration of second-line targeted BRAF therapy was 5.8 months (range, 1.1–9.8 months), with similar results for those receiving dabrafenib monotherapy versus combination dabrafenib plus trametinib (6.1 vs. 5.5 months) (Table 3). Only 1 patient with a non-V600E mutation received second-line targeted therapy, and the duration of time on therapy was short (1.1 months). In the third-line setting, 2 patients received targeted therapy with erlotinib, and 2 others received dabrafenib and trametinib monotherapy, respectively, for durations of 5.3 and 0.7 months each. The patient receiving trametinib monotherapy had a non-V600E mutation (K601E). Duration of targeted therapy beyond the third-line setting was generally very short, aside from 1 patient who received vemurafenib monotherapy for 45.3 months.
Programmed death-ligand 1 checkpoint inhibitor therapy was used uncommonly in the third-line setting or later, with all patients having previously received both chemotherapy and BRAF-directed targeted therapy. Treatment duration was < 2 months in all but 1 case.
Patterns of Progression
All 4 patients diagnosed with stage III NSCLC eventually developed distant disease, with a median time to onset of 9.4 months (range, 5.4–13.4 months). One of 3 patients with stage I to II disease later developed metastases.
Consistent with the wide range of OS seen, history and progression in this cohort were variable. Three patients died within 12 months of diagnosis. However, more than one-half of patients (56%; 9/16) who developed metastatic disease survived > 24 months beyond the onset of metastases. The clinicopathologic features of these patients and the treatments received were heterogeneous with respect to BRAF mutation (V600E vs. non-V600E), co-occurring mutations, smoking history, initial TNM staging at diagnosis, and receipt of BRAF-directed targeted therapy (see Supplemental Table 2 in the online version).
Among patients surviving > 24 months, 5 had periods of slow growing disease marked by prolonged holidays off systemic therapy or prolonged periods of stability on the same treatment regimen. This included 1 patient who developed a biopsy-proven bone metastasis several years after definitive radiation therapy for stage IA disease. After undergoing localized radiation to the bone, he has had no other recurrence off therapy for 45 months. Two patients surviving > 5 years had intrathoracic metastases with stable to slow growing disease for years on systemic therapy (respectively, 45.3 months on vemurafenib and 75.8 months on pemetrexed, carboplatin, and bevacizumab followed by maintenance pemetrexed and bevacizumab). In 2 remaining patients, slow disease growth was managed primarily with intermittent radiation therapy. This included 1 patient who survived for 37.7 months after initially presenting at diagnosis with symptomatic brain metastases and who was treated over that time period with intermittent radiotherapy for localized recurrences and only limited, periodic cycles of systemic chemotherapy owing to poor tolerance. Another patient initially required no systemic chemotherapy after developing metastatic progression to the adrenal gland and was managed over 18.3 months with intermittent localized radiotherapy alone.
Discussion
BRAF mutations occur infrequently in NSCLC, and therefore, our understanding of the natural history of BRAF-mutated NSCLC is still evolving. In this single-center, retrospective series, we describe the natural disease course of patients with BRAF-mutated NSCLC who were seen at our institution.
Previous retrospective studies of BRAF-mutated NSCLC have reported mixed results with respect to survival outcomes. In one of the earliest published cohorts, Marchetti et al reported shorter disease-free survival (15.2 vs. 52.1 months) and OS (29.3 vs. 72.4 months) for patients with BRAF V600E mutations compared with BRAF wild-type controls.8 The majority of patients in this cohort, however, had early-stage disease and underwent surgical resection. In contrast, Cardarella et al reported a median OS of 15.2 months for patients with BRAF-mutated NSCLC who presented with stage IV disease or developed metastases at a later date, which is shorter than the 28.1 month median survival from the time of metastases in our cohort.10
Subsequent retrospective studies of BRAF-mutated NSCLC have mostly described better survival outcomes than these earlier studies. Villaruz et al reported a median OS of 56 months for patients with BRAF-mutated NSCLC with stage IV or recurrent disease.14 Litvak et al reported a 3-year OS rate of 24% for patients with BRAF V600-mutated, advanced stage NSCLC, a figure that was not significantly different than the survival rate of 38% for EGFR-mutated NSCLC.15 Notably, however, 94% of patients in this cohort with EGFR-mutated NSCLC benefitted from targeted therapy versus only 50% of patients with BRAF-mutated NSCLC. Finally, Tissot et al reported a trend toward improved survival in BRAF-mutated NSCLC with a median OS of 22.1 months versus months for NSCLC without a known driver mutation.16
Early pathologic studies suggested that BRAF-mutated NSCLC might have a more aggressive phenotype given the association with papillary or micropapillary histology.25 However, our findings, which compare similarly with the other recent retrospective studies described above, suggest that these patients can have better out-comes from a clinical perspective than the underlying pathology might portend, especially considering that no patients in our series received first-line BRAF-directed targeted therapy and very few received combined BRAF and MEK inhibition in the second- or later-line setting. Furthermore, the 2-year and 5-year survival rates in our cohort compare favorably with what has been historically reported for NSCLC, although comparisons across studies are made with caution.26,27 It is worth noting that nearly one-half of patients surviving > 2 years from the onset of metastases had recurrent rather than de novo metastatic disease. Although the difference in survival for these 2 subgroups did not reach statistical significance in our small sample, prior studies of KRAS- and EGFR-mutated NSCLC have identified de novo metastases to be a poor prognostic factor.28 Additionally, not all patients in our series underwent tumor molecular testing upfront at the time of diagnosis. Therefore, it possible that there was a bias toward identifying BRAF mutations in patients who lived long enough to undergo tumor molecular testing.
We had previously described in case report format 1 of the patients in this cohort who achieved a long survival in the setting of slow-growing disease.23 Although we cannot conclude from our study that median survival of BRAF-mutated NSCLC is longer than that of other molecular subtypes of lung cancer, we did identify multiple patients whose natural history was marked by prolonged courses of disease stability. This included 2 patients who survived > 5 years after the onset of metastatic disease and another who had oligometastatic spread to the bone and is still living several years later without further progression. Notably, each of these patients survived for several years beyond the time at which a BRAF mutation was identified by molecular testing.
The clinicopathologic features of our cohort were similar to that described in prior studies of BRAF-mutated NSCLC with respect to age, histology, and stage.14–16 Our cohort was more ethnically diverse than most prior studies, with Caucasian race comprising 44% of the cohort, compared with 87% to 94% in earlier studies.10,14,15 Kinno et al presented a larger cohort of Asian patients in their study, although the majority of these patients had early-stage disease.11 The trend toward more heavy smokers in the group of patients with non-V600E mutations matches that of previous reports.15
The majority of BRAF mutations identified in this cohort have been described previously in NSCLC, with the exception of V600_W604delinsR, which, to our knowledge, has been reported in papillary thyroid carcinoma.29,30 Additionally, most prior studies evaluated only for a limited set of co-occurring mutations (ie, EGFR, ALK, KRAS) in BRAF-mutated tumors.8,10,14–16,31 With the advantage of having some patients whose tumors were analyzed by next-generation sequencing, we found that mutations in TP53 were a common co-occurring genomic alteration. This was not reported in a previous meta-analysis but is consistent with a recent abstract that reported the co-occurrence of BRAF and TP53 mutations in 74% of patients.32,33 Although the difference in survival from the time of metastases between patients with and without concomitant TP53 mutations did not reach statistical significance, the numerically shorter survival in patients with TP53 mutations was notable. Worse outcomes have been reported in patients whose tumors harbor TP53 mutations in addition to other drivers such as EGFR, and thus, further research should evaluate whether this is indeed true of BRAF-mutated NSCLC as well.34
Limitations of this study include its descriptive, hypothesis-generating design and focus on a single-center experience. The small sample size, which is secondary to the low frequency of BRAF mutations in general, limits the power to detect statistically significant findings, and the results reported here require validation in larger cohorts. Although several patients in this series received programmed death-ligand 1 therapy, most did so late in their disease. Therefore, conclusions regarding the efficacy of immunotherapy in BRAF-mutated NSCLC are limited and remain an important area of future research given the lack of consensus recommendations to guide the sequence of therapy as it pertains to combined chemoimmunotherapy and targeted agents.
Conclusion
In conclusion, we describe the natural history, outcomes, and co-occurring mutations in patients with BRAF-mutated NSCLC. The natural history and clinical behavior of BRAF-mutated NSCLC was variable, but multiple patients in this series achieved prolonged survival with periods of slow disease growth, both with and without the benefit of BRAF-directed targeted inhibitors. Together with the recent emergence of targeted therapy options, this data suggests that the presence of a BRAF mutation should not be considered a uniformly poor prognostic factor.
Supplementary Material
Clinical Practice Points.
The natural history of BRAF-mutated NSCLC is variable, but disease control may be achieved in a subset of patients, and further studies should identify factors that might predict more favorable outcomes.
The clinical characteristics of patients with V600E versus non-V600E mutations are similar, with the exception of a heavy smoking history, which is more common in patients with BRAF non-V600E mutations.
Next-generation sequencing identifies TP53 as the most commonly co-mutated gene alongside BRAF.
Acknowledgments
The Stanford Cancer Institute Research Database (SCIRDB) is supported by the NCI Cancer Center Support Grant 5P30CA124435 and Stanford NIH/NCRR CTSA Award Number UL1 RR025744. The authors also thank the patients whose history was included in this work as well as the physicians and staff who provided care to these patients.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental tables accompanying this article can be found in the online version at https://doi.org/10.1016/j.cllc.2018.10.003.
References
- 1.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361:947–57. [DOI] [PubMed] [Google Scholar]
- 2.Soria JC, Ohe Y, Vansteenkiste J, et al. , FLAURA Investigators. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378:113–25. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368:2385–94. [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014; 311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pao W, Girard N. New driver mutations in non-small cell lung cancer. Lancet Oncol 2011; 12:175–80. [DOI] [PubMed] [Google Scholar]
- 6.Cooper WA, Lam DC, O’Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis 2013; 5(Suppl 5):S479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417:949–54. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small cell lung cancer harboring BRAF mutations. J Clin Oncol 2011; 29:3574–9. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Sonobe M, Takahashi T, et al. Clinical significance of BRAF gene mutations in patients with non-small cell lung cancer. Anticancer Res 2011; 31: 4619–23. [PubMed] [Google Scholar]
- 10.Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013; 19:4532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol 2014; 25:138–42. [DOI] [PubMed] [Google Scholar]
- 12.Kim DW, Haydu LE, Joon AY, et al. Clinicopathological features and clinical outcomes associated with TP53 and BRAF-non-V600 mutations in cutaneous melanoma patients. Cancer 2017; 123:1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011; 29:2046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 2015; 121:448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litvak AM, Paik PK, Woo KM, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancer. J Thorac Oncol 2014; 9:1669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tissot C, Couraud S, Tanguy R, Bringuier PP, Girard N, Souquet PJ. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016; 91:23–8. [DOI] [PubMed] [Google Scholar]
- 17.Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicenter, open-label, phase 2 trial. Lancet Oncol 2016; 17:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutated metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016; 17:984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017; 18:1307–16. [DOI] [PubMed] [Google Scholar]
- 20.Barlesi F, Mazieres J, Merlio JP, et al. Biomarkers France contributors. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016; 387:1415–26. [DOI] [PubMed] [Google Scholar]
- 21.Baik CS, Myall NJ, Wakelee HA. Targeting BRAF-mutant non-small cell lung cancer: from molecular profiling to rationally designed therapy. Oncologist 2017; 22:786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman JM, Gray JE. BRAF V600E mutations: a series of case reports in patients with non-small cell lung cancer. Cancer Genet 2015; 208:351–4. [DOI] [PubMed] [Google Scholar]
- 23.Myall NJ, Neal JW, Cho-Phan CD, et al. Long-term survival of a patient with non-small-cell lung cancer harboring a V600E mutation in the BRAF oncogene. Clin Lung Cancer 2016; 17:e17–21. [DOI] [PubMed] [Google Scholar]
- 24.Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst 2012; 104:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yousem SA, Nikiforova M, Nikiforov Y. The histopathology of BRAF-V600E-mutated lung adenocarcinoma. Am J Surg Pathol 2008; 32:1317–21. [DOI] [PubMed] [Google Scholar]
- 26.Wang T, Nelson RA, Bogardus A, Grannis FW Jr. Five-year lung cancer survival: which advanced stage non-small cell lung cancer patients attain long-term survival? Cancer 2010; 116:1518–25. [DOI] [PubMed] [Google Scholar]
- 27.Giroux Leprieur E, Lavole A, Ruppert AM, et al. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology 2012; 17: 134–42. [DOI] [PubMed] [Google Scholar]
- 28.Yu HA, Sima CS, Hellman MD, et al. Differences in the survival of patients with recurrent versus de novo metastatic KRAS-mutant and EGFR-mutant lung adenocarcinomas. Cancer 2015; 121:2078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadow PM, Heinrich MC, Corless CL, Fletcher JA, Nosé V. Absence of BRAF, NRSA, KRAS, HRAS mutations, and RET/PTC gene rearrangements distinguishes dominant nodules in Hashimoto thyroiditis from papillary thyroid carcinomas. Endocr Pathol 2010; 21:73–9. [DOI] [PubMed] [Google Scholar]
- 30.Barollo S, Pezzani R, Christiani A, et al. Prevalence, tumorigenic role, and biochemical implications of rare BRAF alterations. Thyroid 2014; 24:809–19. [DOI] [PubMed] [Google Scholar]
- 31.Brustugun OT, Khattak AM, Trømborg AK, et al. BRAF-mutations in non-small cell lung cancer. Lung Cancer 2014; 84:36–8. [DOI] [PubMed] [Google Scholar]
- 32.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013; 24:2371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kron A, Riedel R, Michels S, et al. Impact of co-occurring genomic alterations on overall survival of BRAF V600E and non-V600E mutated NSCLC patients: results of the Network Genomic Medicine. Ann Oncol 2017; 28(Suppl 5):v460–96, Abstract 1300PD. [Google Scholar]
- 34.Aggarwal C, Davis CW, Mick R, et al. Influence of TP53 mutation on survival in patients with advanced EGFR-mutant non-small-cell lung cancer. JCO Precis Oncol 2018, Published online August 31. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.