Abstract
Objectives:
All Alzheimer’s disease (AD) clinical trials, including those enrolling patients with Mild Cognitive Impairment (MCI), require dyadic participation. The purpose of this study was to elucidate how people with MCI and their study partners decide whether to enroll in clinical trials.
Design:
Mixed methods interview study.
Participants:
We interviewed patient participants with a consensus research diagnosis of MCI and their study partners.
Measurements:
Interviews examined how dyads decide whether to enroll in a clinical trial and whether AD biomarker testing affects willingness to enroll.
Results:
Though most MCI patients and study partners would decide in partnership whether to enroll in a clinical trial, agreement was lower among non-spousal, compared to spousal, dyads. Deterrents to enrollment included concerns about patient safety and inconvenience, especially for study partners. Motivators to enrollment included altruism, the desire to contribute to research, hope for patient benefit, and the desire to learn more about the patient’s condition. When asked open-ended questions about motivators to enroll in trials, few patients cited access to biomarker testing specifically, though most expressed a desire to undergo biomarker testing when asked directly.
Conclusions:
Spousal and non-spousal MCI dyads may approach clinical trial decisions differently. Future research should investigate how AD biomarker testing affects participants’ willingness to enroll in trials.
Keywords: recruitment, clinical trials, prodromal Alzheimer’s disease
1. Introduction
In an effort to test promising candidate therapies at an earlier disease stage, Alzheimer’s disease (AD) clinical trials enroll patients who meet criteria for Mild Cognitive Impairment (MCI). MCI is defined as cognitive performance below that expected for a person’s age and education, which elicits complaint but does not affect activities of daily living and does not meet criteria for dementia.(1)
Although people with MCI are functionally independent, MCI clinical trials require dyadic enrollment of a patient and a study partner. In trials enrolling patients with AD dementia, both the patient and the study partner, most often the patient’s primary caregiver, are critical to enrollment decisions,(10–14) with study partners playing an increasingly important role as disease progresses.(15) Little is known, however, about how patients with MCI, in conjunction with their spouses, family members or friends, decide whether to enroll in these studies.
Many MCI trials exclusively enroll patients meeting AD biomarker criteria, a concept referred to as MCI due to AD(16) or prodromal AD.(17) MCI patients with biomarker evidence of AD are at increased risk for progression to AD dementia.(6, 7) While this added information is of value to some patients and families, it also risks stigma as well as other negative psychosocial and medicolegal ramifications.(8, 9) Enrolling in prodromal AD trials may involve two complex decisions for people with MCI and their partners: whether to participate in a study of a potentially risky therapy and whether to undergo AD biomarker testing.
2. Methods
2.1. Participants.
From June 24, 2016 to April 20, 2017, we performed a mixed methods interview study. We interviewed patients with a consensus research diagnosis of MCI and their study partners from the University of California, Irvine Alzheimer’s Disease Research Center (ADRC) longitudinal cohort study. Eligible patients were age 50 years and older with presumed underlying etiology of AD. All eligible patients were required to have a qualified study partner age 18 and older who was also willing to participate. To ensure patients had not converted to dementia, we only enrolled those within six months of their most recent ADRC study visit. By design, patients were sampled in order to ensure similar numbers of spousal and non-spousal dyads, thereby allowing for the analysis of potential differences in clinical trial decision-making among these groups. All participants signed an informed consent and received a $25 gift card to a retail store as compensation for their time.
2.2. Data collection.
An investigator trained in qualitative data collection (CGC) performed separate interviews with patients and study partners using an interview guide. We conducted in-person interviews at the medical center whenever possible and telephone interviews to accommodate participants as needed. We completed telephone interviews with eight participants, including five study partners and three patient participants.
Prior to data collection, we read participants a primer on clinical trials that reviewed relevant concepts such as randomization, placebo-control, and blinding. This was followed by structured questions to identify 1) who makes the decision whether to enroll in a trial, 2) motivating factors and deterrents to clinical trial enrollment, and 3) the impact of biomarker testing on trial decision-making.
Who makes the decision?
We asked participants to identify which member of the dyad would make the decision whether to enroll in a clinical trial using a single forced-choice question. Participants selected from: the patient would make the decision without the study partner’s input; the patient would make the decision with the study partner’s input; the patient and study partner would make the decision in partnership; the study partner would make the decision with the patient’s input; the study partner would make the decision without the patient’s input.
Motivating factors and deterrents to clinical trial enrollment.
We asked participants open-ended questions regarding motivating factors and deterrents to clinical trial enrollment. Specifically, we asked participants to describe in their own words “what would you hope to get out of being in a clinical trial, were you to enroll in one” and the “reasons or things that would make you choose to not enroll in a clinical trial.”
Impact of biomarker testing on trial decision-making.
We read participants a description of the difference between general MCI clinical trials that enroll all patients meeting clinical criteria and prodromal AD trials that enroll only MCI patients who meet AD biomarker criteria. Amyloid positron emission tomography (PET) imaging was described as one biomarker test used to identify MCI patients with elevated Aβ who are at increased risk for AD and eligible for prodromal AD trials. Following this description, we asked participants to confirm their understanding by describing the difference between general MCI and prodromal AD trials. If participants were unable to describe the difference, we provided clarification and proceeded with the interview.
We asked both patients and study partners if the patient had ever undergone amyloid imaging and, if so, what were the results. We then asked both members of the dyad whether they would want the patient with MCI to undergo amyloid imaging.
We presented participants with six clinical trial vignettes varied by drug risk (low, medium, high) and biomarker inclusion criteria (MCI trial vs. prodromal AD trial) and asked them to rate their willingness to enroll (6-point Likert scale ranging from “extremely unlikely” to “extremely likely”). Vignettes were based on current or previous trials in MCI or prodromal AD. Trial aspects held constant included the length of the study (2-years), the number of visits (6 per year), the treatment mode of administration (oral), the placebo randomization allocation (1:1), and the required procedures (magnetic resonance imaging [MRI], PET imaging, and cognitive testing). The low-risk scenario tested a vitamin supplement with no known side effects. The medium-risk scenario tested a drug with 10% risk of upset stomach and dizziness. The high-risk scenario tested a drug with 10% risk of swelling or bleeding in the brain. We presented the trial scenarios in identical order for every participant. Vignettes were presented in order of ascending risk, asking first about MCI trials and then trials that only enrolled individuals who meet biomarker criteria for prodromal AD.
Covariates.
During interviews, participants were asked to complete the Research Attitude Questionnaire (RAQ (18)). Additional covariates, such as patient and partner demographics and patient performance on the Mini-Mental State Examination (MMSE (19)), were taken from the most recent ADRC longitudinal visit.
2.3. Data analyses.
Interviews were audio-recorded and transcribed verbatim. To analyze qualitative data, two of us (CGC and JDG) scrutinized each interview transcript independently. We used the separate open-ended questions as a priori datasets for theme identification.(20) We used a template document to identify themes of responses for each question, identifying replication of specific responses and categories of responses among participants. We used a consensus-building exercise to establish agreement on the number and definition of themes for each open-ended question. We used subsequent independent reviews of the transcripts to code each participant’s data for the presence or absence of the identified themes and another consensus-building exercise to address any discordance in coding. We used McNemar’s test to compare the frequency of thematic comments between participant types (patient vs. study partner) and Fisher’s Exact Test to compare between dyad types (spousal vs. non-spousal). Comparisons by dyad type were stratified by patient or partner. No adjustments for multiple comparisons were performed.
Quantitative data was managed using Research Electronic Data Capture (REDCap).(21) We calculated kappa statistics(22) to assess the level of agreement between patients and study partners as to who would make the decision to enroll in a clinical trial. We modeled the distribution of responses using multinomial regression models, adjusted for dyad type. We used likelihood ratio tests to assess the effect of dyad type. We used logistic regression to assess the relationship among trial risk level, AD biomarker inclusion criteria (MCI trial vs. prodromal AD trial), and a participant’s very or extremely high likelihood to enroll (answering 5 or 6 on the Likert scale). Covariates identified a priori as potential confounding factors were age, years of education, dyad type, and MMSE score at interview. We built a similar model to assess the relationship for study partners. In partner analyses, the MMSE score for the partner’s corresponding patient was included as a potential confounding factor.
3. Results
3.1. Participants.
We interviewed 25 patients and 24 study partners. One participant completed the interview twice, once as the patient with MCI and once as a study partner to another patient. Because qualitative responses from this individual were very similar, we included quantitative data from both interviews but qualitative responses from only the patient interview for this participant. Interview data were removed for two dyads for whom the patients’ diagnoses of cancer affected responses. Thus, we included 23 patients and 22 study partners in the final quantitative analyses and 23 patients and 21 study partners in the final qualitative analyses.
Table 1 describes the demographics of the participants whose quantitative data were included in analyses. Most participants, including patients and study partners, were Caucasian, well-educated, and had high scores on the RAQ. Most patients were male, while most partners were female. More patients with a non-spousal study partner were female, compared to patients with a spousal study partner. Non-spousal study partners were younger than spousal study partners.
Table 1.
Characteristics of participants interviewed
| Spousal Patients (n=13) |
Non-Spousal Patients (n=10) |
Spousal Partners (n=13) |
Non-Spousal Partners (n=9) |
|
|---|---|---|---|---|
| Female, n (%) | 0 (0) | 7 (70) | 13 (100) | 7 (78) |
| Non-Hispanic white, n (%) | 12 (92) | 7 (70) | 12 (92) | 5 (56) |
| Age, mean (SD) [Range] | 76.9 (9.2) [57– 88] |
79.7 (10.0) [66– 96] |
72.3 (9.5) [56– 82] |
58.3 (12) [36– 74] |
| Education, mean (SD) [Range] | 16.8 (1.9) [14– 20] |
15.8 (2.2) [12– 18] |
15.8 (1.6) [12– 18] |
15.9 (2.4) [12– 20] |
| RAQ, mean (SD) [Range] | 31.3 (2.5) [27– 35] |
32.1 (3.4) [26– 35] |
30.1 (4.3) [22– 35] |
30.2 (2.8) [26– 34] |
| MMSE, mean (SD) [Range] | 28.6 (1.4) [26– 30] |
27.6 (1.4) [26– 30] |
3.2. Who makes the decision?
Most patients (n=14) and study partners (n=17) indicated they would make the decision whether to enroll in a clinical trial in partnership (Figure 1). Four participants indicated they would make the decision on their own with no input from a partner; all were patients with a non-spousal study partner. Using cutoffs and corresponding nomenclature proposed by (22), agreement between patients and study partners was fair overall (κ=0.35; 95% CI: −0.044, 0.72), moderate for spousal dyads (κ=0.46, 95% CI: −0.072, 0.99), and fair for non-spousal dyads (κ=0.20, 95% CI: −0.16, 0.56). A likelihood ratio test (LRT) for the effect of dyad type indicated that the distributions of responses were significantly different in the patient model (χ2 LRT: 10.921; df=4, p=0.0275) but not in the study partner model (χ2 LRT: 6.258; df=3, p=0.10).
Figure 1.
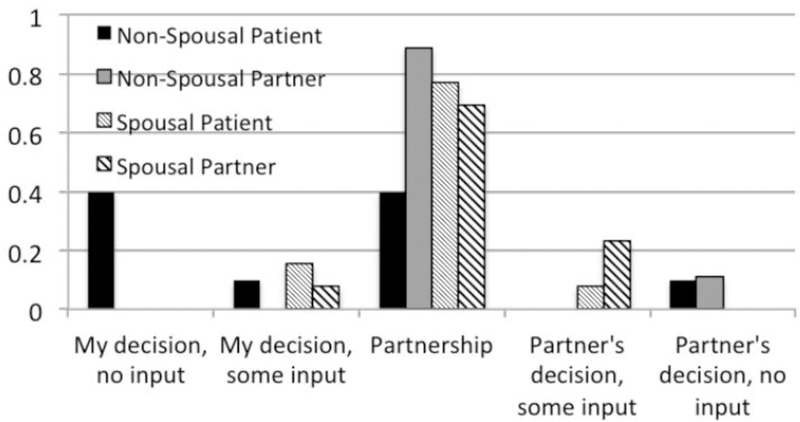
Participant responses when asked how the dyad would decide whether to enroll in a clinical trial.
3.3. Motivating factors and deterrents to clinical trial enrollment.
When asked open-ended questions regarding why they would decide to enroll in a clinical trial, patients and partners both most frequently cited altruism, the desire to advance research, and the desire for the patient to benefit directly through participation (Table 2). Nearly a third of participants (14 of 44) endorsed a desire to learn more about the patient’s condition through clinical trial participation. No differences were seen in the frequency of specific themes between patients and partners.
Table 2.
Motivating factors to clinical trial enrollment, n
| Themes/example quotes | Spousal patients (n=13) |
Non– Spousal patients (n=10) |
Spousal partners (n=12) |
Non– Spousal partners (n=9) |
|---|---|---|---|---|
|
Altruism “I guess on a bigger plane, a higher plane, you know it would help other people as well who might have the problem.” (69-year-old non– spousal study partner) |
9 | 7 | 7 | 5 |
|
Desire to help advance research “That a greater understanding of MCI is made…and that from that there are interventions or medication or an indication that further research is needed based on the study” (36-year-old non-spousal partner) |
6 | 9 | 4 | 4 |
|
Hope for direct patient benefit • Access to treatment “Well, from a personal standpoint, I would hope that the drug, presuming it’s a drug that’s used on me, would effectively stop or reverse mild cognitive disorder.” (79-year-old patient with spousal partner) • Access to experts “Just the feeling that someone has an eye on you, someone who is knowledgeable with the field, someone you can turn to with a question.” (82-year-old spousal partner) |
7 4 3 |
5 2 0 |
7 4 2 |
5 4 0 |
|
To learn more about the patient’s neurological condition “Well, more knowledge about my state of cognitive impairment. So, I see it as an educational process…So it’s just self- knowledge and to see how I’m doing, which is a lot of value.” (70-year-old patient with spousal partner) |
4 | 4 | 2 | 4 |
|
Desire for social support/sense of community “Having some support group I think would be very important.” (82-year-old spousal partner) |
1 | 0 | 2 | 0 |
|
Due to a family history Alzheimer’s disease/dementia “Because of our family history I’m very interested in what develops along the lines of dementia.” (53-year-old non-spousal partner) |
0 | 2 | 0 | 0 |
|
Find the idea of participating interesting “I find it very interesting and think that I would enjoy that process…I just like science” (69– year-old non-spousal partner) |
0 | 1 | 1 | 1 |
|
To gain access to results from the trial “I would hope there would be enough time where the results of the testing would be available to the participants, you know, instead of normal consequences. I don’t know. It seems to me that there’s a long gap between when something is started and when it’s actually in the public’s use.” (78-year-old patient with a non-spousal partner) |
0 | 2 | 0 | 0 |
The most frequently cited deterrent to participation was potential physical risk to the patient (Table 3). This included potential treatment side effects, risks related to invasive procedures such as blood draws and lumbar punctures, and concern over the patient’s condition worsening as a result of participating. After physical risks, the most frequently cited deterrents to participation were related to inconvenience. These included time commitment, transportation, and the study partner requirement. Ten study partners, compared to 4 participants, cited time commitment as a deterrent (χ2 McNemar: 2.286; df= 1, p= 0.131). Six of nine non-spousal study partners, compared to five of twelve spousal study partners, noted inconvenience as a deterrent to enrollment (Fisher exact test; p=0.39). Other barriers to enrollment included issues related to trust, both in the research team and in companies sponsoring trials, and concerns regarding confidentiality. Some participants communicated they would not enroll in a clinical trial that was not “worth their while” or interesting. One dyad focused on the negative social implications of being “labeled” and one study partner expressed concerns about the potential emotional impact of the MCI diagnosis on the patient if she were to enroll. Several participants stated they could think of no deterrents to clinical trial enrollment, with two study partners commenting they would be in support of whatever the patient decided to do.
Table 3.
Deterrents to clinical trial enrollment, n
| Themes/example quotes | Spousal patients (n=13) |
Non– Spousal Patients (n=10) |
Spousal partners (n=12) |
Non– Spousal partners (n=9) |
|---|---|---|---|---|
|
Physical risks to the patient • Treatment side effects “Risks of the medicine doing some very harmful things to you potentially. The potential harm to your health. I can die on my own.” (85-year-old patient with a spousal partner) • Invasive procedures “I don’t want to do a spinal tap. I don’t want to do anything which I feel might cause me some physical risk.” (80-year– old patient with a spousal partner) • Potential to worsen condition “For example, if there was a risk that maybe, because of the clinical trial, [patient’s] MCI would get worse, we probably wouldn’t want to do that.” (66-year-old spousal partner) |
10 7 3 1 |
3 2 1 1 |
6 5 2 2 |
2 1 1 0 |
|
Inconvenience associated with participating • Time commitment “If it was inconvenient time-wise, um, to my schedule, because [patient’s] schedule is much more flexible than ours. But if there was a lot of involvement of participation to be available, you know, for an extended amount of time over time, I would not be able to participate due to my work and personal obligations.” (58-year-old non-spousal partner) • Location/transportation “This particular distance is too far. It’s about 20 miles I think. I mean, to me that’s far if I have to drive myself; I don’t drive a far distance anymore and it just so happens that my daughter got one of your people, the transportation department to come and pick me up, which if fine. Otherwise, I wouldn’t come.” (86-year-old patient with a non-spousal partner) • Study partner conflict “Maybe if my partner, in this case my spouse, either one of us could have some kind of conflict.” (83-year-old patient with a spousal partner) |
5 4 2 1 |
1 0 1 0 |
5 5 1 0 |
6 5 3 0 |
|
Distrust • In research team “Perhaps my relationship with people like your yourself, [researcher], that I wouldn’t be comfortable with. I’m comfortable with you. Would I be comfortable with everybody? I don’t know, maybe not.” (80-year-old patient with a spousal partner) • Confidentiality “If I read anything about information being leaked out to other people. Or if I read that people that were handling it were not licensed.” (82-year-old spousal partner) • In drug company “I don’t know if the people sponsoring the tests, a drug company, has a strong desire for the success of their tests, so you would want to make sure that, if you’re a neutral organization doing the testing, that the pharmaceutical company sponsoring the test is treating the data fairly I guess.” (83-year-old patient with a spousal partner) |
4 3 0 1 |
2 1 2 0 |
3 2 1 1 |
0 0 0 0 |
|
No deterrents “I don’t think there would be anything that I’d choose not to enroll. If it’s something that he wants to do, and um, he’s the one that has the disease and I would go along with that to be his partner if that’s what he desires.” (76-year-old spousal partner) |
1 (8) | 4 (40) | 1 (8) | 1 (11) |
|
Not worthwhile/lack of interest ”If I didn’t feel it was worthwhile, I wouldn’t bother to do it.” (82-year-old spousal partner) |
2 (15) | 0 (0) | 2 (17) | (11) |
|
Risk related to diagnosis: • Labeling “…I don’t know about all trials, but if you don’t make certain trials, they put classification on you…and it kind of stays with you for future even though they say they don’t reveal it to anybody.” (74-year-old patient with non-spousal partner) • Emotional distress “…How much of a toll it would have on her considering her age and physical ability; other individuals talking about MCI or being tested or the greater risk of developing AD, just like the emotional and psychological impact that would have. It could be depressing.” (36-year-old non-spousal partner) |
0 (0) 0 (0) 0 (0) |
1 (10) 1 (10) 0 (0) |
0 (0) 0 (0) 0 (0) |
2 (22) 1 (11) 1 (11) |
|
No access to trial results “…Not having the advantage of knowing your test results when you’re going through all of the different, the PET scan or the MRI or whatever, and not having a clue what the reports were on those.” (57-year-old spousal partner) |
0 (0) | 0 (0) | 1 (8) | 1 (11) |
|
Others will participate instead “Well, there’s always in the back of your mind - well, she can always get other study participants if she doesn’t get one…you’ll always be able to get more other people.” (51– year-old non-spousal partner) |
0 (0) | 0 (0) | 0 (0) | 1 (11) |
|
Cost “I’m not going to pay $50 for every time I come in to see a doctor to participate in a study…” (84-year-old patient with a spousal partner) |
1 (7) | 0 (0) | 0 (0) | 0 (0) |
3.4. Impact of biomarker testing on the trial decision-making.
None of the patients underwent amyloid imaging as part of the study from which they were recruited. Three study partners indicated that the patient had otherwise undergone amyloid imaging. One indicated that the scan was positive and two were unsure of the results. One patient was uncertain whether she had undergone amyloid imaging. When asked specifically, nearly all patients (20/23) and study partners (19/22) indicated they would want the patient to undergo amyloid imaging and learn the result.
Overall, participants’ willingness to enroll in MCI and prodromal AD trials decreased as level of risk increased (Figure 2). Table 3 reports the odds ratios for patient and study partner willingness to enroll in prodromal AD trials, compared with MCI trials, for low, medium, and high risk trials. None were significant. Tests for an interaction effect between trial type and risk level were not statistically significant for patients (χ2 LRT: 1.2; df=2, p=0.55) or study partners (χ2 LRT: 0.04; df=2, p=0.98).
Figure 2.
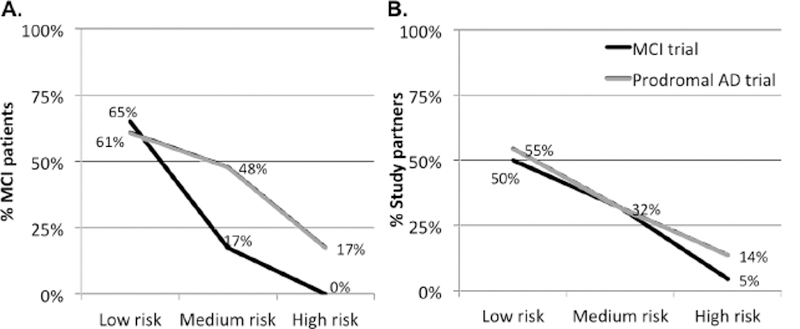
The proportion of patients (A) and study partners (B) responding they would be “extremely likely” to participate in trial vignettes of three different risk strata, dependent upon whether the trial did not (MCI trial) or did (Prodromal AD trial) enroll only individuals meeting amyloid PET biomarker inclusion criteria.
4. Discussion
We believe this is the first study to assess the level of involvement of the MCI study partner in trial enrollment decisions. Studies in AD dementia found that caregivers play a prominent role in trial decisions, even in very mild disease.(14) Here, we find that in MCI, a diagnostic syndrome in which patients remain functionally independent, the decision to enroll in a trial involves the study partner a majority of the time. In fact, in most cases, both patients and study partners indicated that the decision-making process would be an equal partnership between the two members of the dyad. This suggests that, as in AD dementia, recruitment to MCI trials must necessarily involve recruiting two individuals, not one, and that study partners may be in a position to drive the decision to enroll, or to not enroll.(13)
A few patients with MCI indicated that they would make trial decisions autonomously, without the input of their study partner. Given that MCI patients demonstrate impairment in complex functions that require higher order cognitive function,(23, 24) they may be at risk for decision errors. In one previous study, MCI patients performed worse than controls in a research risk assessment task(25) and 40–50% of MCI patients may lack capacity to provide informed consent for trials.(26) Notably, patients who lack insight into their own impairment may be at greatest risk for short-term progression to AD dementia.(27) Unfortunately, in this study, we did not assess risk assessment or capacity to provide consent, and therefore are not able to assess the extent to which those MCI patients indicating autonomous decision-making would be at increased risk for errors in trial decisions. Further research is needed to minimize this risk and instruct the informed consent process in MCI trials.
Individuals who indicated that they would make trial decisions autonomously were exclusively MCI patients who lacked a spouse. Spousal dyads are substantially overrepresented in AD dementia research(28, 29) and previous studies found differences between spousal and non-spousal AD caregivers in availability (e.g., being retired vs. working), research attitudes, and willingness to participate in trials.(28, 30, 31) Here, we found that agreement about how the trial enrollment decision would be made was lower among non-spousal dyads, compared to spousal MCI dyads. We did not find differences in research attitudes between the groups, potentially because all participants were already enrolled in a natural history study. Like AD dementia trials, MCI trials consistently require dyadic enrollment. Thus, the lower agreement among non-spousal dyads may indicate an increased risk for skewed participation rates toward spousal dyads, potentially putting trial generalizability at risk. Moreover, in AD dementia trials, underrepresented racial and ethnic patients are more likely to be members of non-spousal dyads.(28) Here, the sample was mostly Caucasian, though the prevalence of non-Caucasians was higher in the non-spousal group when compared to the spousal group. If MCI trials, like AD dementia trials, are biased toward spousal dyads, this could also have implications to trial diversity.(32)
Most trials enrolling MCI patients now require AD biomarker testing to confirm inclusion criteria, so called prodromal AD trials. Just as a diagnosis of AD carries risk of stigma and distress,(8, 33) knowledge of increased risk for AD based on biomarker testing may carry similar risks.(34) Alternatively, the prognostic ambiguity of an MCI diagnosis may lead to anxiety and frustration,(8, 9, 35) and AD biomarker information may alleviate anxiety and spur future planning.(36–38) In fact, in one focus group study, patients with MCI and their family members indicated that among the most important reasons to enroll in prodromal AD trials was to gain access to AD biomarker testing.(39) Though most MCI patients and study partners in this study indicated a desire for the patient to undergo AD biomarker testing, few specifically noted access to biomarker testing as a motivator to enroll in their responses to open-ended questions. Several participants (14/44) did, however, indicate that the desire to learn more about the patient’s MCI was a motivating factor to enroll. In MCI patients, and to a lesser extent in MCI study partners, we observed a trend toward greater willingness to enroll in prodromal AD trials, compared to trials that did not involve biomarker testing, though this effect was limited to trials with at least moderate risk (Figure 2 and Table 4). Research with larger sample sizes will be necessary to confirm whether the observed trends represent true effects and to further clarify attitudes toward and desire to undergo biomarker testing among MCI patients, both in the setting of trials and more broadly.
Table 4.
Relative likelihood of participating in varying risk prodromal vs. MCI trials.
| Trial risk |
Patients | Study partners |
|---|---|---|
| Low | OR=1.70; 95% CI: 0.401, 7.237, p = 0.470 |
OR=1.37; 95% CI: 0.288, 6.534, p = 0.692 |
| Medium | OR=1.54; 95% CI: 0.422, 5.585, p = 0.515 |
OR=1.64; 95% CI: 0.410, 6.596; p = 0.483 |
| High | OR=4.8; 95% CI: 0.859, 27.055, p = 0.074 |
OR=1.68; 95% CI: 0.224, 12.561; p = 0.615 |
Estimated OR obtained from a logistic regression model; P-value based upon a Wald test of the null hypothesis that the true OR is equal to 1.
Participants in this sample also noted altruism, the desire to advance research, and potential patient benefit as motivators for trial enrollment. Concerns about patient safety were the most consistent deterrents. Additionally, barriers related to convenience such as time commitment and study location were important to study partners, particularly non-spousal partners. These preliminary data may help instruct trial design to accommodate schedules of working partners—for example requiring shorter study visits, offering weekend or evening visits, or providing visit location or modality options. These accommodations may facilitate recruitment, especially of non-spousal dyads. For example, of the five study partners who were interviewed by phone in this study, four were non-spouses.
Limitations.
The generalizability of these data is limited by the small sample size and possible sample bias due to recruiting from an ongoing research study. Nevertheless, these research participants would in fact be eligible to enroll in MCI trials, adding ecological validity to results. Although an effort was made enroll a similar number of spousal and non-spousal dyads, small sample size also limited the power to detect differences between the groups (e.g., patient vs. partner; spouse vs. non-spouse). We presented trial vignettes in a consistent order to all participants, rather than randomizing the order of presentation. This decision was made in an effort to ensure that participants understood the difference between prodromal AD and MCI trials, but may have introduced the potential confound of an order effect. Collection of data by phone may have altered data quality, especially for individuals with MCI. Each of the three individuals with MCI interviewed by phone, for example, required further clarification of the MCI and prodromal AD concepts. Future studies employing mixed methods for data collection should consider implications to data integrity and potentially study power.
Conclusions.
These data provide initial insight to the decision-making experience of a population increasingly being enrolled in AD clinical trials. Further research on this topic is critical to instruct successful recruitment of MCI dyads to these clinical trials and to ensure participants are fully informed and protected when deciding to participate in research.
Acknowledgements
We thank the MCI participants and study partners who participated in this study as well as Megan Witbracht and Shirley Sirivong for their assistance with participant recruitment and Drs. Malcolm Dick and Aimee Pierce for assistance with the neuropsychological and diagnostic aspects of the project.
Sources of support
This work was supported by a pilot grant from the UC Irvine Alzheimer’s Disease Research Center, NIA AG016573. Ms. Ryan is supported by NIA AG000096.
Dr. Grill has received research funding from Biogen Idec, Eli Lilly & Company, Genentech, the Alzheimer’s Disease Cooperative Study, and the Alzheimer’s Therapeutic Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest disclosures
Ms. Cox has nothing to disclose.
Ms. Ryan has nothing to disclose.
Dr. Gillen has nothing to disclose.
References
- 1.Petersen RC, Smith GE, Waring SC, et al. : Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308 [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Roberts RO, Knopman DS, et al. : Mild cognitive impairment: ten years later. Arch Neurol 2009; 66:1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser PJ, Kester A, Jolles J, et al. : Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology 2006; 67:1201–1207 [DOI] [PubMed] [Google Scholar]
- 4.Malek-Ahmadi M: Reversion From Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Alzheimer Dis Assoc Disord 2016; [DOI] [PubMed]
- 5.Grill JD, Apostolova LG, Bullain S, et al. : Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther 2017; 9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villemagne VL, Pike KE, Chetelat G, et al. : Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 2011; 69:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolk DA, Sadowsky C, Safirstein B, et al. : Use of Flutemetamol F 18-Labeled Positron Emission Tomography and Other Biomarkers to Assess Risk of Clinical Progression in Patients With Amnestic Mild Cognitive Impairment. JAMA neurology 2018; [DOI] [PMC free article] [PubMed]
- 8.Garand L, Lingler JH, Conner KO, et al. : Diagnostic labels, stigma, and participation in research related to dementia and mild cognitive impairment. Res Gerontol Nurs 2009; 2:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauthier S, Leuzy A, Racine E, et al. : Diagnosis and management of Alzheimer’s disease: past, present and future ethical issues. Prog Neurobiol 2013; 110:102–113 [DOI] [PubMed] [Google Scholar]
- 10.Black BS, Taylor HA, Rabins PV, et al. : Study partners perform essential tasks in dementia research and can experience burdens and benefits in this role. Dementia (London) 2016; [DOI] [PMC free article] [PubMed]
- 11.Black BS, Wechsler M,Fogarty L: Decision making for participation in dementia research. Am J Geriatr Psychiatry 2013; 21:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlawish J, Kim SY, Knopman D, et al. : The views of Alzheimer disease patients and their study partners on proxy consent for clinical trial enrollment. Am J Geriatr Psychiatry 2008; 16:240–247 [DOI] [PubMed] [Google Scholar]
- 13.Karlawish JH, Casarett D, Klocinski J, et al. : How do AD patients and their caregivers decide whether to enroll in a clinical trial? Neurology 2001; 56:789–792 [DOI] [PubMed] [Google Scholar]
- 14.Karlawish JH, Casarett DJ,James BD: Alzheimer’s disease patients’ and caregivers’ capacity, competency, and reasons to enroll in an early-phase Alzheimer’s disease clinical trial. J Am Geriatr Soc 2002; 50:2019–2024 [DOI] [PubMed] [Google Scholar]
- 15.Karlawish JH, Casarett D, Propert KJ, et al. : Relationship between Alzheimer’s disease severity and patient participation in decisions about their medical care. J Geriatr Psychiatry Neurol 2002; 15:68–72 [DOI] [PubMed] [Google Scholar]
- 16.Albert MS, DeKosky ST, Dickson D, et al. : The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois B, Feldman HH, Jacova C, et al. : Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014; 13:614–629 [DOI] [PubMed] [Google Scholar]
- 18.Rubright JD, Cary MS, Karlawish JH, et al. : Measuring how people view biomedical research: Reliability and validity analysis of the Research Attitudes Questionnaire. J Empir Res Hum Res Ethics 2011; 6:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE,McHugh PR: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198 [DOI] [PubMed] [Google Scholar]
- 20.Ryan GW,Bernard HR: Techniques to identify themes. Field Methods 2003; 15:85–109. [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landis JR,Koch GG: The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174 [PubMed] [Google Scholar]
- 23.Griffith HR, Belue K, Sicola A, et al. : Impaired financial abilities in mild cognitive impairment: a direct assessment approach. Neurology 2003; 60:449–457 [DOI] [PubMed] [Google Scholar]
- 24.Okonkwo OC, Wadley VG, Griffith HR, et al. : Awareness of deficits in financial abilities in patients with mild cognitive impairment: going beyond self-informant discrepancy. Am J Geriatr Psychiatry 2008; 16:650–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jefferson AL, Carmona H, Gifford KA, et al. : Clinical research risk assessment among individuals with mild cognitive impairment. Am J Geriatr Psychiatry 2012; 20:878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jefferson AL, Lambe S, Moser DJ, et al. : Decisional capacity for research participation in individuals with mild cognitive impairment. J Am Geriatr Soc 2008; 56:1236–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Therriault J, Ng KP, Pascoal TA, et al. : Anosognosia predicts default mode network hypometabolism and clinical progression to dementia. Neurology 2018; 90:e932–e939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grill JD, Raman R, Ernstrom K, et al. : Effect of study partner on the conduct of Alzheimer disease clinical trials. Neurology 2013; 80:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grill JD, Monsell S,Karlawish J: Are Patients Whose Study Partners Are Spouses More Likely to be Eligible for Alzheimer’s Disease Clinical Trials? Dement Geriatr Cogn Disord 2012; 33:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cary MS, Rubright JD, Grill JD, et al. : Why are spousal caregivers more prevalent than nonspousal caregivers as study partners in AD dementia clinical trials? Alzheimer Dis Assoc Disord 2015; 29:70–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grill JD,Karlawish J: Addressing the challenges to successful recruitment and retention in Alzheimer’s disease clinical trials. Alzheimers Res Ther 2010; 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson JL, Ryan L, Silverberg N, et al. : Obstacles and opportunities in Alzheimer’s clinical trial recruitment. Health Aff (Millwood) 2014; 33:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werner P,Heinik J: Stigma by association and Alzheimer’s disease. Aging Ment Health 2008; 12:92–99 [DOI] [PubMed] [Google Scholar]
- 34.Stites SD, Karlawish J, Harkins K, et al. : Awareness of Mild Cognitive Impairment and Mild Alzheimer’s Disease Dementia Diagnoses Associated With Lower Self-Ratings of Quality of Life in Older Adults. J Gerontol B Psychol Sci Soc Sci 2017; 72:974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beard RL,Neary TM: Making sense of nonsense: experiences of mild cognitive impairment. Sociol Health Illn 2013; 35:130–146 [DOI] [PubMed] [Google Scholar]
- 36.Carpenter BD, Xiong C, Porensky EK, et al. : Reaction to a dementia diagnosis in individuals with Alzheimer’s disease and mild cognitive impairment. J Am Geriatr Soc 2008; 56:405–412 [DOI] [PubMed] [Google Scholar]
- 37.Vanderschaeghe G, Schaeverbeke J, Vandenberghe R, et al. : Amnestic MCI Patients’ Perspectives toward Disclosure of Amyloid PET Results in a Research Context. Neuroethics 2017; 10:281–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grill JD, Cox CG, Kremen S, et al. : Patient and caregiver reactions to clinical amyloid imaging. Alzheimers Dement 2017; 13:924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence V, Pickett J, Ballard C, et al. : Patient and carer views on participating in clinical trials for prodromal Alzheimer’s disease and mild cognitive impairment. Int J Geriatr Psychiatry 2014; 29:22–31 [DOI] [PubMed] [Google Scholar]


