Abstract
Recent advances in circuit manipulation technologies have enabled the association of distinct neural circuits with complex social behaviors. The brain areas identified through historical anatomical characterizations as mediators of sexual and parental behaviors can now be functionally linked to adult social behaviors within a unified circuit. In vivo electrophysiology, optogenetics and chemogenetics have been used to follow the processing of social sensory stimuli from perception by the olfactory system to valence detection by the amygdala and mesolimbic dopamine system to integration by the cerebral and cerebellar cortices under modulation of hypothalamic neuropeptides. Further, these techniques have been able to identify the distinct functional changes induced by social as opposed to non-social stimuli. Together this evidence suggests that there is a distinct, functionally coupled circuit that is selectively activated by social stimuli. A unified social circuit provides a new framework against which synaptopathic autism related mutations can be considered and novel pharmacotherapeutic strategies can be developed.
There are many regions of the brain that both from birth and throughout development become particularly sensitive to the presentation of social information. These regions include both areas of the limbic system and striatum that are conserved throughout mammalian evolution and specialized cortical areas that have evolved to facilitate human social interactions. It is unknown, though, if these regions constitute an individually defined social circuit or are simply the recruitment of independent circuitry for the processing of social information. Historically, connectivity within a circuit was assessed through histological and surgical interrogation. However, recent advances in optogenetic and chemogenetic strategies in conjunction with in vivo single unit and local field potential recording during social behaviors has empowered direct assessment of the social circuit (See Table 1 for a description of current technologies in circuit characterization). In this paper, we will identify the brain areas activated by social stimuli and critical for the production of social behavior and review the evidence that these areas are functionally connected. Identification of a functionally connected circuit for the processing of social stimuli provides a framework for understanding how and where autism associated mutations give rise to social communicative impairments.
Table 1.
Novel technologies used to characterize the social circuit.
| Technique | Description | Example Use |
|---|---|---|
| Optogenetics | Light-based activation or inhibition of neuronal populations genetically induced to express light-sensitive ion channels. | Expression of inhibitory opsins in the the projects from CA1 to NAc were used to demonstrate the necessity of this projection for social recognition (Okuyama, 2016). |
| Chemogenetics | Chemical activation or inhibition of neuronal populations through the expression of genetically modified receptors and administration of an engineered ligand to modulate neuronal activity. | Inhibition of Crus 1 Purkinje cells through the expression of inhibitory DREADD receptors demonstrated the functional connectivity between this region and the parietal cortex (Stoodley, 2017). |
| Amperometry | Electrochemical technique to detect transient current changes induced by an oxidation reaction associated with the vesicular release of specific neurotransmitters. | Reduced dopamine efflux was detected in the prefrontal cortex via fixed potential amperometry in lurcher mice after electrical stimulation of cerebellar Purkinje outputs (Mittleman, 2008). |
| Calcium imaging | Fluorescent reporter of dynamic fluctuations in calcium levels related to neuronal activity that can be read via two-photon imaging,, microendoscopes or fiber photometry. | Viral mediated expression of the calcium reporter GCaMP in dopaminergic VTA neurons was used to indicate the release of dopamine in response to social stimuli. Fluorescence was detected using fiber photometry to demonstrate an increase in dopaminergic activity during social interaction (Gunaydin, 2014). |
| Multi-electrode recording | Recording of single neuron activity through the implantation of arrays of bundled electrodes that allow the isolation of individual units of activity. | In vivo multielectrode recordings in the VMH of male was used to identify two subsets of neuron populations that distinctly responded to either male conspecifics in aggressive interactions or female conspecifics in affiliative interactions (Anderson, 2012). |
| Local field potential recording | Recording of an electrophysiological signal derived from the summed electrical activity in a small volume of tissue often organized into rhythmic oscillatory patterns based on the dynamics of cellular firing. | Local field potential was recorded from the mPFC and NAc of prairie voles to demonstrate socially facilitated functional connectivity between these brain regions evident in the coherence in low frequency oscillations during mating (Amadei, 2017) |
| Electroencephalography (EEG) | Translational readout of neural activity summed across a large volume of tissue detected at the level of the skull or epidural space from which neural oscillations can be extracted and compared to human electrophysiological readouts. | Electrophysiological recording of cortical EEG signal measured from electrodes embedded in the skull demonstrated an enhancement in gamma power oscillatory activity of Shank3b mice that exhibit robust social behavioral deficits (Dhamne, 2017). |
| Cell-selective gene manipulation | Promoter driven gene manipulation to selectively modify gene expression within a discrete cell population. | Viral mediated expression of Cre dependent tetanus neurotoxin in a CA2 specific Cre genetically modified mouse line was used to inactivate the output of this region to identify a specific phenotype of social recognition (Hitti, 2014). |
Despite human social cognition being one of the key differentiating factors of our species, many of the neural contributors to this behavior have evolutionary origins. It is hypothesized that mammalian adult social behavior is an adaptation of maternal behavior and consequently utilizes much of the same circuitry (Numan and Young, 2015). The endocrine mediators of parturition and lactation also trigger the onset of centrally mediated maternal behavior and mother-infant attachment (Lee, Macbeth, Pagani, and Young, 2009). Maternal behavior requires the interaction between the limbic system and the mesolimbic dopamine system (MLDS; See Table 2 for a list of acronyms used for brain regions), and both rodent physiology and human neuroimaging studies indicate that these nodes are also activated in adult social cognition (Adolphs, 2009; Skuse and Gallagher, 2009). The ability to dissect the circuitry within rodent models facilitates the implication of specific neural substrates with much finer resolution than is possible in non-invasive human studies. Consequently, we will focus on the conserved neural circuitry of social cognition that has been elucidated based on anatomical, physiological, pharmacological and genetic manipulations that have been carried out in animal models during social behavior (Box 1). There are of course aspects of human social cognition, both critical to human social behavior and dysregulated in autism, which cannot be captured in rodent models. Many of these features, like theory of mind, are enabled by the evolutionary expansion of the human cortex (Saxe, 2006). Human social cognition, and social impairment, therefore is dependent on similar neural circuitry as in the rodent, but likely with added complexity in cortical regulation (Donaldson and Young, 2008; Goodson, 2013).
Table 2.
Acronyms for brain regions within the social circuit.
| Brain Regions | |
| AOS | Accessory olfactory system |
| BLA | Basolateral amygdala |
| BNST | Bed nucleus of the stria terminalis |
| CERBLM | Cerebellum |
| Hab | Habenula |
| HPC | Hippocampus |
| IFC | Infralimbic cortex |
| MeA | Medial amygdala |
| MLDS | Mesolimbic dopamine system |
| MOS | Main olfactory system |
| (m)PFC | (medial) Prefrontal cortex |
| MPOA | Medial preoptic area |
| NAc | Nucleus accumbens |
| PLC | Prelimibc cortex |
| PVN | Paraventricular nucleus |
| SN | Substantia nigra |
| SON | Supraoptic nucleus |
| VMH | Ventral medial hypothalamus |
| VP | Ventral pallidum |
| VTA | Ventral tegmental area |
Box 1. Behavioral Measures of Social Circuitry in Rodents.
Social investigation-
Olfactory investigation (sniffing) of a conspecific. Primary method for rodent social perception.
Social recognition-
Memory of familiar conspecific. Scored based on the amount of time subject spends sniffing conspecific. Recognition is evident if there is a decrease in the amount of time spent sniffing a familiar conspecific over a novel conspecific.
Social novelty detection-
Reciprocal of social recognition, increased olfactory investigation of a novel conspecific relative to a familiar conspecific.
Maternal behavior-
Initiation of maternal behavior (not present prior to first parturition) in rodents. Includes crouching, licking/grooming, pup retrieval. Paternal behavior in male rodents can also be assessed, behaviors include licking/grooming and pup retrieval.
Social bonding-
Formation of selective bonds in prairie voles. Bond is evident if subjects spends twice as much time huddling (side by side social contact) with partner over stranger in partner preference test.
The social circuit: an overview
The olfactory system of rodents and the visual system of humans are tuned to the detection of social stimuli. Social sensory information is detected earlier and is given greater attention than non-social stimuli. Both primary sensory and perceptual cortical areas project to the amygdala, which holds a central position in the processing of social information. The amygdala enhances the salience of social sensory stimuli and assigns an affective valence (intrinsic attractiveness). Dedicated hippocampal circuits encode representations of social stimulus-salience pairings. Both the amygdala and the hippocampus connect with the mesolimbic dopamine system, and this “reward” circuitry promotes reinforcement of the social stimuli through pairing of the stimuli with dopamine signals. Both the MLDS and the amygdala are strongly influenced by connections with the hypothalamus mediating neuromodulatory tone and behavioral outputs. The limbic components of the social circuit are reciprocally connected with higher cortical processing areas in the medial prefrontal cortex (PFC) and the cerebellum. Many of the mutations identified in autism spectrum disorder (ASD) alter the connectivity within this network and disrupt the production of social behavior in mutant mouse models.
Enhanced perception of social stimuli
Social information holds a privileged position in the sensory and perceptual mechanism of most animals, and the neural architecture underlying sensory perception is critical to the circuitry of social cognition. Enhanced attention or processing is typically given to social cues, particularly those in the dominant social sensory modality (Vuilleumier, 2015). In rodents, the dominant modality for the perception of social information is olfaction, and mice will respond distinctly to olfactory pheromones from other mice (Crawley, 2012). In humans, vision is the primary sensory modality for the perception of social information, and people visually orient to the eyes of other people (Klin, Shultz, and Jones, 2015). Evidence suggests, though, that while the projections of the olfactory system in mice are not the same as the projections of the visual system in humans, similar downstream areas are activated (Domes, Heinrichs, Glascher, Buchel, Braus, and Herpertz, 2007; Gur, Tendler, and Wagner, 2014). Selective attention to social stimuli suggests that the perception of social stimuli is mediated, at least in part, by different mechanisms than perception of inanimate stimuli (Bielsky, Hu, Szegda, Westphal, and Young, 2004; Tobin, Hashimoto, Wacker, Takayanagi, Langnaese, Caquineau, Noack, Landgraf, Onaka, Leng, Meddle, Engelmann, and Ludwig, 2010).
Social odors are first represented in either the main olfactory system (volatile odors) or the accessory olfactory system (non-volatile odors) of rodents with both regions being critical for the production of normative social behavior (Petrulis, 2009). Activation of the accessory olfactory bulb is sufficient to drive investigation of a weakly positive social odor and simultaneously inhibit investigation of a weakly aversive social odor, but fails to have an effect on non-social odors (Kunkhyen, McCarthy, Korzan, Doctor, Han, Baum, and Cherry, 2017). Mitral/tufted (M/T) neurons within the main and accessory olfactory bulbs, the output neurons of the olfactory system, show selectivity for different types of social stimuli. These neurons are differentially activated by the odors of individual animals in a reliable fashion that is modulated by familiarity with that individual (Brennan, Kaba, and Keverne, 1990; Luo, Fee, and Katz, 2003). The output of the activity of M/T neurons is gated, in part, by the neuropeptides oxytocin (OT) and vasopressin (AVP; key mediators of social behavior). Bulbar application of either neuropeptide reduces mitral cell output from the olfactory bulb (Tobin et al., 2010; Yu, Kaba, Okutani, Takahashi, and Higuchi, 1996) thereby limiting the outflow of information to downstream processing areas facilitating the behavioral effect of social recognition (Box 1) (Dluzen, Muraoka, Engelmann, and Landgraf, 1998; Wacker and Ludwig, 2012). Similarly, optogenetic stimulation of OT release promotes social olfactory exploration which facilitates social recognition by increasing the signal to noise ratio of olfactory output (Oettl, Ravi, Schneider, Scheller, Schneider, Mitre, da Silva Gouveia, Froemke, Chao, Young, Meyer-Lindenberg, Grinevich, Shusterman, and Kelsch, 2016). The neuropeptides may therefore serve as a filter for social sensory information, as opposed to non-social information, within the olfactory system(Wacker and Ludwig, 2012).
Filtering of bulbar output can also be detected at the level of local field potentials (LFP). In the olfactory bulb, the identification of odors elicits LFPs with oscillatory features in the theta, beta and gamma frequency bands (Kay, 2014). Exposure to social odors reduces power in the low frequency bands (4-8Hz) and increases power in the higher frequency bands (8-24Hz)(Binns and Brennan, 2005). Characteristic changes in LFP in response to social odors, like these, could be used as a biomarker of normative social sensory processing. Alterations in the LFP of the olfactory bulb could provide critical evidence of early processing differences in the perception of social sensory stimuli in animal models with social deficits. Both the main and accessory olfactory bulbs project to the amygdala and are functionally coupled during the perception of social stimuli. Functional connectivity between these regions is critical for the production of social behaviors in mouse models including social recognition and maternal care (Petrulis, 2009).
Affective valence assignment in the amygdala
The amygdala is critical for the impartment of affective value to sensory stimuli and transducing the association to brain areas mediating behavioral responses (Maren and Quirk, 2004). The amygdala receives numerous projections from primary sensory areas, including both direct and indirect projections from the olfactory bulbs, and associative sensory areas and thus is primed to serve as a “gateway” in the processing of sensory information (Zalla and Sperduti, 2013).
Medial Amygdala
The medial amygdala (MeA)receives convergent inputs from both the main and accessory olfactory bulbs with half of the olfactory receptive neurons selectively responding to social odors (Petrulis, 2009). This region also is innervated by projections from oxytocinergic neurons in the supraoptic nucleus of the hypothalamus (SON)(Otero-Garcia, Agustin-Pavon, Lanuza, and Martinez-Garcia, 2015). The convergence of these inputs in the MeA facilitates a neuronal response to social stimuli that corresponds with the salience of the stimuli to that individual (Bergan, Ben-Shaul, and Dulac, 2014; Ferguson, Aldag, Insel, and Young, 2001). Novel social odors or the highly salient odors of potential mates are more strongly represented in the MeA (Bergan et al., 2014; Gur et al., 2014). OT coupled with prolonged bulbar activation, though, reduces the responsiveness of the neurons to a particular odor providing a mechanism for social recognition (Gur et al., 2014). The ultimate result of medial amygdalar activation is the production of social behavior. Low-level activation of the GABA-ergic neurons within this region is sufficient to induce social grooming or mating but not asocial self-grooming (Hong, Kim, and Anderson, 2014). The behavioral effects of the MeA are primarily mediated through downstream projections to the brainstem and the hypothalamus (Keshavarzi, Sullivan, Ianno, and Sah, 2014).
Basolateral Amygdala
The basolateral amygdala (BLA) is reciprocally connected with the MLDS to modulate reactivity to social stimuli. The BLA projects to the nucleus accumbens (NAc) and the ventral palladium (VP) and receives projections from the ventral tegmental area (VTA)(Lalumiere, 2014). Glutamatergic efferents from the basomedial and BLA convey olfactory, gustatory and somatosensory information associated with social stimuli to the ventral striatum. In the initiation of maternal motivation in rats, the amygdalar projections go from being independent to paired with ventral striatal activity to promote the production of maternal behavior (Numan and Young, 2015). Disconnection of the projections from the basolateral and basomedial amygdala to the NAc and VP or suppression of neural activity in the amygdalar nuclei disrupts maternal behavior (Numan, Bress, Ranker, Gary, Denicola, Bettis, and Knapp, 2010). It is hypothesized that the NAc inhibits activity in the VP, but dopamine signaling in the accumbens disinhibits the nucleus allowing for the VP to be activated by incoming amygdalar signaling, which pairs the sensory information associated with rat pups to the motivational effects of dopamine stimulating maternal behavior (Numan and Young, 2015).
The connectivity of the amygdala within the social circuit is currently being interrogated through the use of optogenetics (Lalumiere, 2014). The heterogeneity within both the amygdala and the individual subnuclei has made it difficult to characterize distinct patterns of LFP, despite substantial characterization of single unit activity. The identification of discrete projections like those from the olfactory bulb to the MeA may facilitate the identification of signatures in the LFP indicative of integrity within the circuit. For example, stimulation of the inputs from the olfactory bulbs causes an increase in LFP in the MeA (Figure 1a) (Gur et al., 2014) and should be assessed for their integrity in animal models of social impairments. Given the centrality of the amygdala in the processing of social information, the identification of functional measures of connectivity between the amygdala and its projections, including the MLDS and the hypothalamus, may prove to be an important measure of social circuit function.
Figure 1. Measures of functional connectivity within the social circuit.
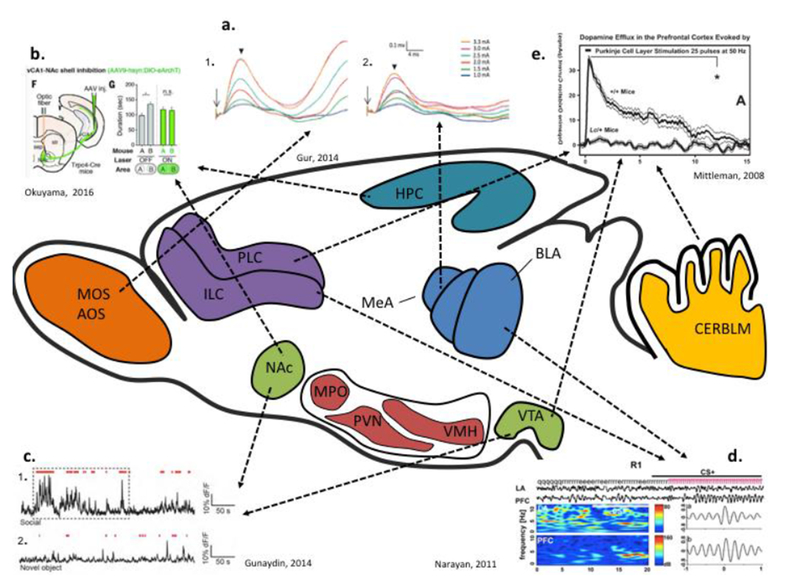
Social sensory information is primarily conveyed through the olfactory and auditory perceptual circuits in rodents both of which demonstrate enhanced sensitivity to social stimuli over non-social stimuli. The sensory circuits feed into the amygdala whose functionality is critical to the impartment of emotional valence to the perceptual stimuli. Proceeds from a. counter clockwise a) Stimulation (arrow) of the accessory olfactory bulb (1) or the main olfactory bulb (2) induces an increase in the local field potential of the MeA. This response is modulated by the presence of OT. The output nuclei of the amygdala projects to the ventral striatum, the primary reward processing circuit of the brain (Gur et al., 2014). b) Optogenetic inhibition (“on” phase vs. “off” phase) of the projections from CA1 to the NAc is sufficient to disrupt social recognition c) Projections from the VTA to the NAc are activated more strongly by novel social stimuli (1) than non-social stimuli (2). The PFC interacts at multiple points within the social circuit providing an integration point for the subcortical processors of social information (Gunaydin et al., 2014). d) The BLA and the PFC undergo theta synchronization during the presentation of fear stimuli. This effect is modulated by social experience. The cerebellum interacts with both the ventral striatum and the PFC to modulate higher-order responses to social sensory stimuli (Narayanan et al., 2011). e) Stimulation of the Purkinje cell layer evokes an increase in prefrontal dopamine in wild type mice (+/+) that is absent in the Lurcher ASD mouse model (Lc/+) (Mittleman et al., 2008). MOS-main olfactory system, AOS-accessory olfactory system, PLC-prelimbic cortex, ILC-infralimbic cortex, NAc-nucleus accumbens, MeA-medial amygdala, MPO-medial preoptic area, PVN-paraventricular nucleus, VMH-ventromedial nucleus of the hypothalamus, BLA-basolateral amygdala, VTA-ventral tegmental areas, CERBLM-cerebellum, PFC-prefrontal cortex, OT-oxytocin.
Hypothalamic modulation of behavioral response to social stimuli
Several different hypothalamic nuclei interact with nodes of the social circuit to evoke behavioral responses to social stimuli. The connections of each nucleus are unique such that each serves a distinct function in the reinforcement and production of different types of social behaviors. Several nuclei including the ventromedial nucleus of the hypothalamus (VMH) and the medial preoptic area (MPOA) mediate the production of social behavior. The paraventricular (PVN) and SON serve a unique role within social circuits through the release of OT and AVP throughout the circuit to coordinate the response to social stimuli.
Paraventricular/Supraoptic Nucleus
As discussed in the preceding sections, the axonal projections from PVN and SON innervate the olfactory bulb, the medial and lateral amygdala, the CA1 and CA2 regions of the hippocampus and the ventral striatum to influence local neuronal activity through the release of OT and AVP (Otero-Garcia et al., 2015). OT and AVP are neuropeptides synthesized primarily within these hypothalamic nuclei for independent release into both peripheral circulation and the brain that are critical for the expression of social behavior (Neumann, 2007). In the brain, central OT and AVP are locally released to mediate a number of social behaviors including maternal care, social investigation, aggression and mating inducing differential effects depending on the node of the social circuit (Lukas and Neumann, 2013). The neuropeptides bind to G-protein coupled receptors whose effect on neuronal activity is dependent on the cell type on which they are expressed (Gimpl and Fahrenholz, 2001). For example, OT increases local neuronal activity in the MeA (Gur et al., 2014) but has an inhibitory effect in the mitral cells of the olfactory bulb (Yu et al., 1996). OT modulates functional connectivity between the brain regions regulating social bonding such that coordinated activity is seen after social interactions in the MeA, BLA, medial PFC, NAc and hypothalamus (Johnson, Walum, Jamal, Xiao, Keebaugh, Inoue, and Young, 2016). The activation of oxytocinergic neurons in the hypothalamus, presumed to induce OT release, is sufficient to induce several types of social behaviors. Optical stimulation of OT release induces a social preference of a nonsalient social stimulus in mice through receptors expressed in the piriform cortex (Choe, Reed, Benavidez, Montgomery, Soares, Yim, and Choi, 2015); enhanced social exploration and recognition in rats via the olfactory cortex (Oettl et al., 2016) and social bonding with novel conspecifics (member of the same species) in prairie voles. As neuromodulators, the peptides have long-lasting effects on neural activity and thus can shift the tone within the aforementioned circuitry to respond specifically to social stimuli.
Behavioral Output Nuclei
The medial amygdala projects to a number of medial hypothalamic nuclei that mediate the production of facultative social behaviors in response to differential sensory input (Choi, Dong, Murphy, Valenzuela, Yancopoulos, Swanson, and Anderson, 2005). There is an anatomical division between the dorsal and ventral subnuclei within the MeA such that projections from the dorsal region project primarily to the medial preoptic nucleus (MPO), the ventrolateral portion of the ventromedial hypothalamic nucleus (VMH) and the ventral premammillary nucleus mediating affiliative responses; whereas the ventral portion of the MeA projects to the anterior hypothalamic nucleus and the dorsomedial portion of the ventromedial hypothalamic nucleus to induce aggressive behaviors (Choi et al., 2005). The VMH plays a critical role in the production of social behavior as evidenced by its afferent projections from the MeA and efferent projections to the bed nucleus of the stria terminalis (BNST). Neurons in this region regulate the valence of the behavioral response to a conspecific. Activation of a discrete population of VMH neurons induces aggressive behavior towards same sex conspecifics(Lin, Boyle, Dollar, Lee, Lein, Perona, and Anderson, 2011). However, activation of an overlapping but distinct population of neurons within the same area induces mating with opposite sex conspecifics, indicating the VMH can serve as a switch on social behavioral output based on the valence of the social stimuli (Anderson, 2012).
The MPOA has similar connections as the VMH receiving inputs from the MeA and projecting to the MLDS directly through the VTA and indirectly through the BNST, though mediates a distinct behavioral response. The MPOA is activated in social recognition and is critical for the expression of maternal behavior (Ferguson et al., 2001; Numan and Young, 2015) (Kohl, Babayan, Rubinstein, Autry, Marin-Rodriguez, Kapoor, Miyamishi, Zweifel, Luo, Uchida, and Dulac, 2018). MPOA neurons that project to the VTA are preferentially activated by reproductively relevant social odors over same sex or non-social odors, and activation of these neurons drives the release of dopamine in the VTA. Conversely, activation of BNST-projecting MPOA neurons is sufficient to acutely increase the expression of parental behavior through inhibition of the BNST (Tsuneoka, Tokita, Yoshihara, Amano, Esposito, Huang, Yu, Odaka, Shinozuka, McHugh, and Kuroda, 2015; Wu, Autry, Bergan, Watabe-Uchida, and Dulac, 2014). This implicates the MPOA as a transition point between sensory afferents from the MeA and the motivational efferents to the ventral striatum (McHenry, Rubinow, and Stuber, 2015).
Activation of both the VMH and the MPOA can induce specific behavioral states and thus be used to assess the functional output of the social circuit. Behaviorally appropriate activation of these nuclei indicates functional connectivity between other nodes within the social circuit. Current characterizations of activity in this region, though, are at the neuronal and not field potential level. Further work is needed to identify whether measures of averaged neural activity are also reflective of behavioral responses.
Social memory in the hippocampuss
The central role of the hippocampus in memory is well-known; however, to function distinctly within the social circuit, there must be evidence of differential patterns activation to social vs. non-social information and distinct connections with the other nodes of the circuit. Two main regions of the hippocampus, dorsal CA2 and ventral CA1, demonstrate sufficient differential responses to social vs. nonsocial stimuli to suggest they function as a distinct node within the social circuit (Kogan, Frankland, and Silva, 2000; Montagrin, Saiote, and Schiller, 2017). CA2 receives inputs from within in the hippocampus, as well as the entorhinal cortex and supramammilary nucleus of the hypothalamus serving as an input node for social information and projects to CA1, which serves as an output node to the social circuit(Hitti and Siegelbaum, 2014; Okuyama, Kitamura, Roy, Itohara, and Tonegawa, 2016).
These regions express neuropeptidergic receptors enabling coordinated regulation with the rest of the circuit by OT and AVP. Dorsal CA2 and ventral CA1 are both innervated by hypothalamic projections and express AVP-V1b and OT receptors (Cui, Gerfen, and Young, 2013; Raam, McAvoy, Besnard, Veenema, and Sahay, 2017). Interestingly, while OTR and AVP-V1a receptors are expressed throughout the brain, particularly within the social circuit, AVP-V1b receptor expression is relatively restricted to the CA2 region of the hippocampus, suggesting a unique function for these receptors in social memory (Young, Li, Wersinger, and Palkovits, 2006). Stimulation of the hypothalamic inputs to the CA2 during acquisition, similar to what may occur with AVP release, dramatically prolongs social recognition memory from 30 minutes to seven days (Smith, Williams Avram, Cymerblit-Sabba, Song, and Young, 2016). Genetic inactivation of CA2 pyramidal neurons results in specific deficits in social recognition but not spatial or contextual memory or olfaction(Hitti and Siegelbaum, 2014).
Social information in CA2 is conveyed locally within the hippocampus through direct projections to the deep layers of the CA1 region (Kohara, Pignatelli, Rivest, Jung, Kitamura, Suh, Frank, Kajikawa, Mise, Obata, Wickersham, and Tonegawa, 2014). In addition, social information is transferred to CA1 through projections from the BLA to the ventral portions of this region. Inhibition of the BLA terminals within CA1 increases olfactory social exploration whereas activation inhibits it (Felix-Ortiz and Tye, 2014). However, inhibition of the ventral CA1 neurons more broadly, while also associated with increased sniffing, inhibits social recognition (Okuyama et al., 2016). Ventral CA1 neurons contribute to social recognition through the formation of a social engram (pattern of cellular activation associated with a stimulus). Reactivation of social stimulus selective neurons extends social recognition memory beyond the time frame typically observed (Okuyama et al., 2016). Ventral CA1 projects to the NAc, OB and BLA; however, only the projections from ventral CA1 to NAc are necessary for social recognition (Figure 1b) (Okuyama et al., 2016). Interestingly, both activation and inhibition of these projections disrupt social recognition but has no effect on the recognition of novel objects or contexts (Okuyama et al., 2016). There is a convergence of the projections from the BLA and the ventral CA1 region prelimbic and infralimbic regions of the PFC, and simultaneous activation of these regions are thought to amplify socially relevant neural activity in this region (Ishikawa and Nakamura, 2003). The specificity of the deficits associated with disruption of the dorsal CA2 and the ventral CA1 region suggest distinct pathways within the architecture of the hippocampus for the memory of social information.
Reward attribution in the ventral striatum
The MLDS is hypothesized to mediate the reinforcing effects of social interactions and drive social motivation in both rodents and humans (Gunaydin and Deisseroth, 2014). Inputs into the MLDS including the amygdala, the hypothalamus, the hippocampus and the habenula provide valence to the social sensory stimuli such that stimuli can then be associated with the appropriate reinforcement cues within the striatum. The lateral habenula is bidirectionally modulated in response to either aversive or rewarding social stimuli, exciting and inhibiting the habenular projections to the MLDS respectively (Benekareddy, Stachniak, Bruns, Knoflach, von Kienlin, Kunnecke, and Ghosh, 2017; Wang, Li, Feng, Guo, Zhou, and Luo, 2017a). Projections from the amygdala and the hypothalamus effect dopaminergic tone at all levels of the mesolimbic system through connections with the BNST, the VTA and the NAc to drive dopamine signaling in response to social stimuli. Dopamine release in the striatum increases during periods of novel social investigation (Robinson, Heien, and Wightman, 2002), and the rate of release decreases as the animal habituates to the conspecific (Robinson et al., 2002). Stimulation of VTA dopaminergic neurons, which results in axonal dopamine release in the NAc, increases the amount of time a mouse spends investigating a novel conspecific, while inhibition of the same neurons decreases investigation time (Gunaydin, Grosenick, Finkelstein, Kauvar, Fenno, Adhikari, Lammel, Mirzabekov, Airan, Zalocusky, Tye, Anikeeva, Malenka, and Deisseroth, 2014). The presence of a social partner enhances endogenous neuronal firing in the NAc of mice relative to a nonsocial environment (Gunaydin et al., 2014)(Figure 1c).
Dopamine also facilitates the formation of social attachments to individual conspecifics (Coria-Avila, Manzo, Garcia, Carrillo, Miquel, and Pfaus, 2014). Rats can be conditioned to show a preference for any conspecific through pairings of a dopamine D2 receptor agonist with the individual (Cibrian-Llanderal, Rosas-Aguilar, Triana-Del Rio, Perez, Manzo, Garcia, and Coria-Avila, 2012). Further, the formation of selective social bonds in monogamous rodents like the prairie vole is dependent on the activation of D2 receptors in conjunction with OT receptors in the NAc (Liu and Wang, 2003). During mating, both neurochemicals are released in the NAc and administration of an antagonist for either receptor prevents the formation of a social bond(Gingrich, Liu, Cascio, Wang, and Insel, 2000) (Young, Lim, Gingrich, and Insel, 2001). It is hypothesized that the co-expression of both OT and dopamine D2 receptors on an overlapping population of medium spiny neurons within the accumbens enables the association of the socially salient signal, mediated by OT release, to be coupled with the rewarding signal, mediated by the dopamine release (Wang and Aragona, 2004). Dopamine neurons in the two midbrain regions, the VTA and the substantia nigra (SN) are regulated by OT in an opposing fashion. Activation of oxytocinergic neurons increases the firing of dopamine neurons in the VTA but inhibits activity in the SN based on the relative expression of receptors on the dopaminergic neurons versus local GABA neurons (Xiao, Priest, Nasenbeny, Lu, and Kozorovitskiy, 2017). Interestingly, OT and serotonin also interact within the NAc to mediate social reward, suggesting that this region plays a broad role in the integration of social and neuromodulatory reward signals(Dolen, Darvishzadeh, Huang, and Malenka, 2013).
Dopamine from the VTA is also released in the medial PFC. The postsynaptic prefrontal pyramidal neurons in turn project back to both the VTA and the NAc to regulate dopaminergic tone. Connectivity between the NAc and the medial PFC may facilitate the formation of social bonds. Mating in the prairie vole, which drives social bond formation, induces enhanced oscillatory coherence between the NAc and the PFC and the level of coherence is correlated with the expression of affiliative huddling behavior (Amadei, Johnson, Jun Kwon, Shpiner, Saravanan, Mays, Ryan, Walum, Rainnie, Young, and Liu, 2017). Measures of coherence between nodes of the social circuit have the potential to be critical readouts of the coordinated activation that is necessary to produce normative social behavior.
Feature integration in the prefrontal cortex
As suggested in the prairie vole, the PFC is connected with all of the other nodes within the social circuit, receiving input from the sensory cortices, amygdala, ventral striatum and hypothalamus. The diverse connections prime the PFC for a role in coordinating sensory perception, emotional valence and motivation in complex social cognition (Kas, Modi, Saxe, and Smith, 2014). Chemogenetic activation of the Emx positive cells in the PFC activates the lateral habenula, medial hypothalamus, and the VTA but inhibits the NAc and suppresses social preference (Benekareddy et al., 2017). Within the prelimbic portion of the PFC, distinct subpopulations project to the Nac, amygdala and VTA, however stimulation of only the NAc pathway disrupts social preference (Murugan, Jang, Park, Miller, Cox, Taliaferro, Parker, Bhave, Hur, Liang, Nectow, Pillow, and Witten, 2017). Stimulation of the NAc to mPFC projections in the prairie voles is sufficient to induce the formation of a social bond in prairie voles in the absence of the typically pre-requisite mating behavior (Amadei et al., 2017). Social investigation preferentially recruits subcortically projecting medial PFC neurons positive for D2 dopamine receptors and stimulation of this subset of neurons disrupts the production of normal social interaction (Brumback, Ellwood, Kjaerby, Iafrati, Robinson, Lee, Patel, Nagaraj, Davatolhagh, and Sohal, 2017). Stimulation of the BLA-medial PFC projections reduces social investigation of a juvenile conspecific and inhibition of the pathway increases investigation (Felix-Ortiz, Burgos-Robles, Bhagat, Leppla, and Tye, 2015). Neurons that project from the lateral amygdala to the infralimbic component of the PFC generate oscillatory activity in the theta frequency band during emotionally arousing situations (Pape, 2005). Theta activity (∼4Hz) is highly synchronized between the lateral amygdala and the medial infralimbic cortex during the recall of emotionally associated cues, suggesting a functional connectivity between the regions (Figure 1d)(Narayanan, Heiming, Jansen, Lesting, Sachser, Pape, and Seidenbecher, 2011). Genetic disruption of the serotonin synthesis pathway enhances the connection between the amygdala and the medial PFC increasing the coherence between these two regions in the delta and beta frequencies (Dzirasa, Kumar, Sachs, Caron, and Nicolelis, 2013).
Within the medial PFC (mPFC) itself, local circuit activity also plays a role in the expression of social behavior. Neurons within the mPFC respond selectively to social stimuli, with roughly a quarter of neurons increasing activity and a quarter decrease activity with social interaction time (Jodo, Katayama, Okamoto, Suzuki, Hoshino, and Kayama, 2010). Interaction with a novel social stimulus induces a greater increase in the firing of parvalbumin positive interneurons (PV+) than a novel object (Selimbeyoglu, Kim, Inoue, Lee, Hong, Kauvar, Ramakrishnan, Fenno, Davidson, Wright, and Deisseroth, 2017); however, mice with the Cntnap2 mutation, which fail to show a preference for social interaction, do not show the bias in neuronal firing. Activation of cortical pyramidal neurons inhibits the investigation of a novel conspecific and disrupts the preference for social interaction in the three-chamber arena, through the disruption of local connectivity (Yizhar, Fenno, Prigge, Schneider, Davidson, O’Shea, Sohal, Goshen, Finkelstein, Paz, Stehfest, Fudim, Ramakrishnan, Huguenard, Hegemann, and Deisseroth, 2011). Strikingly, both activating PV+ neurons and inhibiting pyramidal neurons was also sufficient to rescue the social behavioral deficits of the Cntnap2 mutant mice (Selimbeyoglu et al., 2017). The balance of excitatory to inhibitory activity is reflected in oscillations in the gamma frequency driven by reciprocal interactions between parvalbumin positive interneurons and principal cells (Featherstone, McMullen, Ward, Bang, Xiao, and Siegel, 2015). Gamma oscillations are thought to integrate the neural activity across brain structures to facilitate higher sensory processing and consequently could facilitate the organizational role of the PFC in response to social stimuli (Featherstone et al., 2015). Alterations in cortical gamma oscillation specifically in response to a social environment have been observed in an animal model of autism and should be explored more broadly in other models as a biomarker of neural response to social stimuli (Vogt, Cho, Lee, Sohal, and Rubenstein, 2015).
Cerebral and cerebellar coordination in response to social stimuli
Until recently, the role of the cerebellum in social cognition has been underappreciated relative to its traditional role in motor function. However its role in the pathophysiology of several psychiatric disorders, including autism, has been long known and its potential role in the pathogenesis of the disorder is gaining credence (Mosconi, Wang, Schmitt, Tsai, and Sweeney, 2015; Parker, Narayanan, and Andreasen, 2014; Sundberg and Sahin, 2015). Increasing evidence from mouse models of disorders characterized by social impairments has implicated the cerebellum in the pathophysiology of the impairments (Shevelkin, Ihenatu, and Pletnikov, 2014) and particularly the disruption of cerebellar Purkinje cells.
The cerebellum is connected to the cerebral cortex through a number of indirect reciprocal connections that mediate a diverse suite of functions. It is hypothesized that it is the disruption specifically of the circuits that mediate sensorimotor processing, cognition and affective functions that could give rise to social impairments (D’Mello and Stoodley, 2015). The cerebellum and prefrontal cortex exhibit coordinated neural responses to direct stimulation and oscillatory synchronization in response to certain behavioral interventions (Chen, Wang, Yang, Sui, Hu, and Hu, 2016; Watson, Becker, Apps, and Jones, 2014; Watson, Jones, and Apps, 2009). The Purkinje cells of the right Crus I lobe of the cerebellum project to the parietal association cortex. There is an inverse relationship between the activity of Purkinje cells in this area and parietal neurons, such that chemogenetic inhibition increases firing in this area and functionally results in impaired social preference and social novelty detection and reduced exploration of social olfactory cues (Stoodley, D’Mello, Ellegood, Jakkamsetti, Liu, Nebel, Gibson, Kelly, Meng, Cano, Pascual, Mostofsky, Lerch, and Tsai, 2017).
Mutations associated with disrupted social behavior have been shown to disrupt the connectivity between the cerebellum and the cerebral cortex. The Ube3 mutation alters the rhythmicity of the cerebellum interfering not only with local neuronal plasticity but also cerebral plasticity, as a result of the reciprocal connections (Cheron, Servais, Wagstaff, and Dan, 2005). Selective mutation of Tsc1, Tsc2 and Shank2 within the Purkinje cells of the cerebellum disrupts social function (Peter, Ten Brinke, Stedehouder, Reinelt, Wu, Zhou, Zhou, Boele, Kushner, Lee, Schmeisser, Boeckers, Schonewille, Hoebeek, and De Zeeuw, 2016; Reith, McKenna, Wu, Hashmi, Cho, Dash, and Gambello, 2013; Tsai, Hull, Chu, Greene-Colozzi, Sadowski, Leech, Steinberg, Crawley, Regehr, and Sahin, 2012). However, stimulation of the right Crus 1 region is sufficient to rescue the social impairments that result from Tsc1 mutation (Stoodley et al., 2017). Reduced cerebral-cerebellar connectivity, such as that generated by a decrease in Purkinje cells, can also decrease dopamine in the PFC (Figure 1e)(Mittleman, Goldowitz, Heck, and Blaha, 2008). Stimulation of the cerebellum in both Fmr1 mutant mice and Lurcher mutant mice (which show a complete developmental loss of Purkinje cells) fails to induce prefrontal dopamine release as it does in wild-type mice due to a reorganization of ventral tegmental and thalamic connections (Rogers, Dickson, McKimm, Heck, Goldowitz, Blaha, and Mittleman, 2013). The lateral cerebellar nuclei projects, via the thalamus, to the PFC, as evidenced by coordinated firing after cerebellar stimulation. Intriguingly, only activation via delta frequency oscillatory stimulation is able to rescue prefrontal dysfunction due to dopamine D1 receptor inhibition (Parker, Kim, Kelley, Nessler, Chen, Muller-Ewald, Andreasen, and Narayanan, 2017). The alteration of cerebellar-prefrontal connectivity is a plausible mechanism for autistic-like impairments; however, further characterization of the specific pathologic cerebro-cerebellar circuits is necessary (Kloth, Badura, Li, Cherskov, Connolly, Giovannucci, Bangash, Grasselli, Penagarikano, Piochon, Tsai, Geschwind, Hansel, Sahin, Takumi, Worley, and Wang, 2015).
A unified circuit for social information processing
Evidence of functional connectivity between the nodes and selectivity for the processing of social information suggests that the brain areas described above function as a distinct circuit for social information processing. Current technological advances have enabled both functional connectivity and selectivity to be rigorously investigated through the current work, as well as challenged and refined in future efforts (See Table 2). Functional connectivity has been demonstrated using techniques that enable the activation or inhibition of specific populations of neurons and quantification of the resultant neuronal activity. Most commonly, the regulation of neuronal activity has been achieved through the use of optogenetic or chemogenetic technologies combined with promoter specific neuronal population identification. For example, Amigo2 gene is primarily expressed within pyramidal neurons of the CA2 region. Cre dependent co-expression of the light sensitive cation channel, channelrhodopsin-2, enables the selective activation of CA2 pyramidal neurons upon exposure to light. The functional connectivity of this region can be assessed through readouts of neuronal activation either through electrophysiology or through the imaging calcium dynamics within populations of neurons suspected of being functionally coupled. Activation of these neurons results in neuronal spiking within the CA2 pyramidal neurons and synaptic transmission between CA2 and CA1 as measured in whole cell current clamp recordings from brains slices of these regions (Hitti and Siegelbaum, 2014). Likewise the activation of these neurons in vivo can be associated specifically with the processing of social information through behavioral constructs that test the effect of manipulating the activity of this population of neurons in relation to social or non-social stimuli. Inactivation of these neurons using similar techniques did not interfere with detection of novel non-social objects but did prevent the differentiation between novel and familiar social stimulus (Hitti and Siegelbaum, 2014). Thus through advances the ability to selectively activate and inhibit neuronal populations hypothesized to function within the social circuit, it was possible to both characterize circuit connectivity and stimulus selectivity.
Viewing social behavior as the output of a distinct circuitry provides a framework for understanding how the synaptopathic mutations associated with ASD can give rise to social impairments. Individual mutations in ASD risk genes, including Shank3, Tsc1/2, Cntnap2, Ube3a, may differentially affect different connections within the social circuit (Hulbert and Jiang, 2016; Sahin and Sur, 2015). Disruptions of specific pathways can then give rise to variations in behavioral phenotype that all cluster under the heading of social communicative impairments. For example, Shank3 mutation preferentially affects synaptic plasticity in the striatum and gives rise to corticostriatal hyperconnectivity (Peixoto, Wang, Croney, Kozorovitskiy, and Sabatini, 2016; Reim, Distler, Halbedl, Verpelli, Sala, Bockmann, Tenzer, Boeckers, and Schmeisser, 2017). Further, Shank3b mutation particularly impairs the indirect pathway striatopallidal medium spiny neurons (Wang, Li, Chen, van der Goes, Hawrot, Yao, Gao, Lu, Zang, Zhang, Lyman, Wang, Guo, Wu, Gerfen, Fu, and Feng, 2017b). It could be hypothesized based on these that children with SHANK3 mutations associated with autism would preferentially have impairments in reward attribution to social stimuli and repetitive behaviors due to striatal misconnectivity. Similar circuit relevant pathological hypotheses can also be generated for more ubiquitously expressed genes, like Tsc1 and Tsc2, based on the relative vulnerability of specific cell types to disruptions of specific cellular processes. For example, Tsc1 mutation leads to dysregulation of cellular metabolism and Purkinje cells are particularly vulnerable to metabolic insults due to their high rates of neuronal activity. On this basis, one could hypothesize that the social pathological consequences of Tsc1 mutation may be due to the enhanced effects within the cerebellar node of the social circuit. In fact, selective deletion of Tsc1 only within Purkinje cells recapitulates the social behavioral phenotypes of more pervasive mutations (Tsai et al., 2012). Based on the predicted circuit specific deficits with respect to a given mutation, driven either through expression patterns or cellular vulnerabilities, functional neuroimaging or electroencephaolography (EEG) could be used to confirm the suspected impairments and an individualized treatment approach could be adopted.
Circuit based strategies, as opposed to target based, also have the potential to shed new light on the development of novel therapeutic strategies for social impairments. The activation or inhibition of specific circuits linked to specific social behavioral components may prove more successful than global neuromodulation. Potentially, individuals with SHANK3 mutations who demonstrate functional alterations of striatum would benefit from treatment with dopamine D2 receptor or phosphodiesterase 4 modulators to compensate for the dysfunction of SHANK3 within the striatopallidal medium spiny neurons (Heckman, van Duinen, Bollen, Nishi, Wennogle, Blokland, and Prickaerts, 2016). With further refinement, circuit based alterations due to known mutations could be targeted with the therapeutic application of optogenetics or chemogenetics (Song and Knopfel, 2016). Stimulation of striatopallidal medium spiny neurons through chemogenetics ameliorates the repetitive behavioral impairments of Shank3 mutant mice (Wang et al., 2017b). Circuit level readouts of functional connectivity, like EEG, could be used as translational biomarkers for assessing treatment response (Dhamne, Silverman, Super, Lammers, Hameed, Modi, Copping, Pride, Smith, Rotenberg, Crawley, and Sahin, 2017; Modi and Sahin, 2017).
The anatomic and functional connectivity, as evidenced by the studies outlined above, between the nodes of the social circuit suggests that these areas function as a discrete circuit in the presence of social stimuli. The widespread innervation of the social circuit by neuropeptide projections provides a means for the circuit to be regulated in a coordinated fashion. However, it will be important to further assess whether the functional connectivity within the social circuit is disrupted by perturbations, which impair social cognition. Evidence of circuit dysfunction should be present both in individuals with ASD and in animal models expressing ASD associated mutations to implicate functional connectivity in the pathophysiology of ASD. If the circuitry can be implicated, assessment of the specific connections within the circuit should also be carried out to determine if certain projections are specifically vulnerable to the insults associated with autism. A better understanding of the circuitry dysregulated in ASD will empower the development of targeted treatment strategies.
Figure 2. Map of functional connectivity within the social circuit.
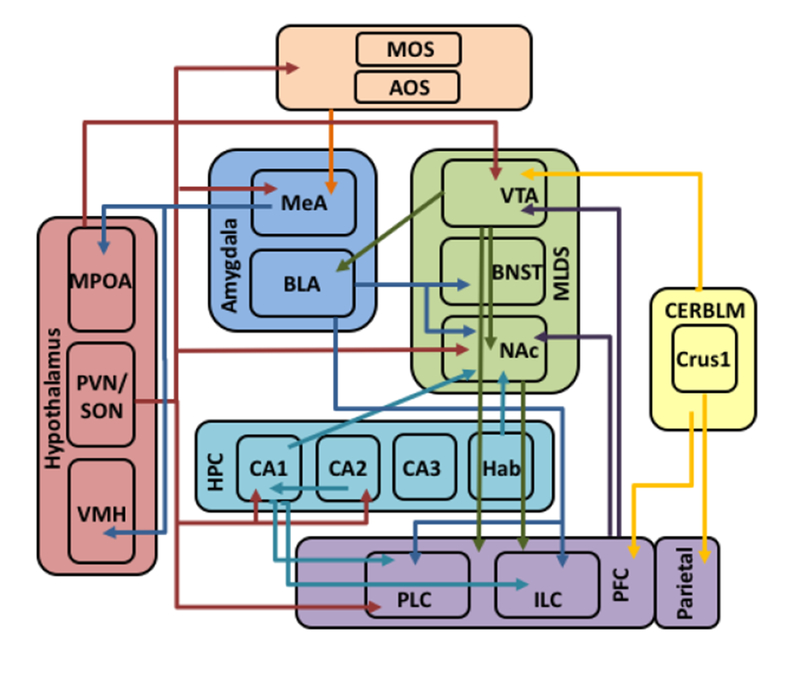
Functional connectivity within the social circuit as described in the text. Arrow colors denote the brain region from which the projections originate and arrow directions denote where the projections terminate.
Highlights:
We review a functionally coupled circuit selectively activated by social stimuli.
Adult social behavior is adapted from maternal behavior and uses similar circuitry.
Rodent olfactory and human visual systems are tuned to detect social stimuli.
Amygdala and mesolimbic dopamine system detect the valence of sensory stimuli.
Cerebrum, cerebellum, hippocampus and striatum all contribute to this network.
Mutations identified in autism alter connectivity within this social behavior circuit.
Acknowledgements:
We would like to thank Denise McGinnis for her invaluable comments on the manuscript. Sahin lab has received research funding from the US National Institutes of Health (NIH) (U01-NS082320, U01-NS092595, U54-NS092090 and U54-HD090255), US Department of Defense W81XWH–15–1-0189, Nancy Lurie Marks Family Foundation, Autism Speaks, TS Alliance, PTEN Research, Simons Foundation, Phelan-McDermid Syndrome Foundation, Tommy Fuss Center, National Ataxia Foundation, Roche, Novartis, Pfizer, Quandrant Biosciences and LAM Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
M.S. has served on the Scientific Advisory Board of Sage Therapeutics, Takeda and PTEN Research Foundation and PTEN Hamartoma Tumor Syndrome Foundation. He also serves on the professional advisory board of the Tuberous Sclerosis Alliance. He has received research funding from Roche, Novartis, Pfizer, Quandrant Biosciences and LAM Therapeutics. M.E.M has nothing to disclose.
Bibliography:
- Adolphs R (2009). The social brain: neural basis of social knowledge. Annu Rev Psychol, 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, Ryan SJ, Walum H, Rainnie DG, Young LJ, & Liu RC (2017). Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature, 546, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ (2012). Optogenetics, sex, and violence in the brain: implications for psychiatry. Biol Psychiatry, 71, 1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benekareddy M, Stachniak TJ, Bruns A, Knoflach F, von Kienlin M, Kunnecke B, & Ghosh A (2017). Identification of a Corticohabenular Circuit Regulating Socially Directed Behavior. Biol Psychiatry, [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, & Dulac C (2014). Sex-specific processing of social cues in the medial amygdala. Elife, 3, e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, & Young LJ (2004). Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology, 29, 483–493. [DOI] [PubMed] [Google Scholar]
- Binns KE, & Brennan PA (2005). Changes in electrophysiological activity in the accessory olfactory bulb and medial amygdala associated with mate recognition in mice. Eur J Neurosci, 21, 2529–2537. [DOI] [PubMed] [Google Scholar]
- Brennan P, Kaba H, & Keverne EB (1990). Olfactory recognition: a simple memory system. Science, 250, 1223–1226. [DOI] [PubMed] [Google Scholar]
- Brumback AC, Ellwood IT, Kjaerby C, Iafrati J, Robinson S, Lee AT, Patel T, Nagaraj S, Davatolhagh F, & Sohal VS (2017). Identifying specific prefrontal neurons that contribute to autism-associated abnormalities in physiology and social behavior. Mol Psychiatry, [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang YJ, Yang L, Sui JF, Hu ZA, & Hu B (2016). Theta synchronization between medial prefrontal cortex and cerebellum is associated with adaptive performance of associative learning behavior. Sci Rep, 6, 20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Servais L, Wagstaff J, & Dan B (2005). Fast cerebellar oscillation associated with ataxia in a mouse model of Angelman syndrome. Neuroscience, 130, 631–637. [DOI] [PubMed] [Google Scholar]
- Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, & Choi GB (2015). Oxytocin Mediates Entrainment of Sensory Stimuli to Social Cues of Opposing Valence. Neuron, 87, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, & Anderson DJ (2005). Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron, 46, 647–660. [DOI] [PubMed] [Google Scholar]
- Cibrian-Llanderal T, Rosas-Aguilar V, Triana-Del Rio R, Perez CA, Manzo J, Garcia LI, & Coria-Avila GA (2012). Enhaced D2-type receptor activity facilitates the development of conditioned same-sex partner preference in male rats. Pharmacol Biochem Behav, 102, 177–183. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Manzo J, Garcia LI, Carrillo P, Miquel M, & Pfaus JG (2014). Neurobiology of social attachments. Neurosci Biobehav Rev, 43, 173–182. [DOI] [PubMed] [Google Scholar]
- Crawley JN (2012). Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci, 14, 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Gerfen CR, & Young WS 3rd (2013). Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J Comp Neurol, 521, 1844–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, & Stoodley CJ (2015). Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci, 9, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamne SC, Silverman JL, Super CE, Lammers SHT, Hameed MQ, Modi ME, Copping NA, Pride MC, Smith DG, Rotenberg A, Crawley JN, & Sahin M (2017). Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, & Landgraf R (1998). The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides, 19, 999–1005. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, & Herpertz SC (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry, 62, 1187–1190. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, & Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322, 900–904. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Kumar S, Sachs BD, Caron MG, & Nicolelis MA (2013). Cortical-amygdalar circuit dysfunction in a genetic mouse model of serotonin deficiency. J Neurosci, 33, 4505–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McMullen MF, Ward KR, Bang J, Xiao J, & Siegel SJ (2015). EEG biomarkers of target engagement, therapeutic effect, and disease process. Ann N Y Acad Sci, 1344, 12–26. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, & Tye KM (2015). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience, 321, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, & Tye KM (2014). Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci, 34, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, & Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci, 21, 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, & Fahrenholz F (2001). The oxytocin receptor system: structure, function, and regulation. Physiol Rev, 81, 629–683. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, & Insel TR (2000). Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci, 114, 173–183. [DOI] [PubMed] [Google Scholar]
- Goodson JL (2013). Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology, 38, 465–478. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, & Deisseroth K (2014). Dopaminergic Dynamics Contributing to Social Behavior. Cold Spring Harb Symp Quant Biol, 79, 221–227. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, & Deisseroth K (2014). Natural neural projection dynamics underlying social behavior. Cell, 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R, Tendler A, & Wagner S (2014). Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiatry, 76, 377–386. [DOI] [PubMed] [Google Scholar]
- Heckman PR, van Duinen MA, Bollen EP, Nishi A, Wennogle LP, Blokland A, & Prickaerts J (2016). Phosphodiesterase Inhibition and Regulation of Dopaminergic Frontal and Striatal Functioning: Clinical Implications. Int J Neuropsychopharmacol, [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, & Siegelbaum SA (2014). The hippocampal CA2 region is essential for social memory. Nature, 508, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Kim DW, & Anderson DJ (2014). Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell, 158, 1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert SW, & Jiang YH (2016). Monogenic mouse models of autism spectrum disorders: Common mechanisms and missing links. Neuroscience, 321, 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, & Nakamura S (2003). Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci, 23, 9987–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Katayama T, Okamoto M, Suzuki Y, Hoshino K, & Kayama Y (2010). Differences in responsiveness of mediodorsal thalamic and medial prefrontal cortical neurons to social interaction and systemically administered phencyclidine in rats. Neuroscience, 170, 1153–1164. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, & Young LJ (2016). Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles Horm Behav, 79, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, Modi ME, Saxe MD, & Smith DG (2014). Advancing the discovery of medications for autism spectrum disorder using new technologies to reveal social brain circuitry in rodents. Psychopharmacology (Berl), 231, 1147–1165. [DOI] [PubMed] [Google Scholar]
- Kay LM (2014). Circuit oscillations in odor perception and memory. Prog Brain Res, 208, 223–251. [DOI] [PubMed] [Google Scholar]
- Keshavarzi S, Sullivan RK, Ianno DJ, & Sah P (2014). Functional properties and projections of neurons in the medial amygdala. J Neurosci, 34, 8699–8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Shultz S, & Jones W (2015). Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci Biobehav Rev, 50, 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth AD, Badura A, Li A, Cherskov A, Connolly SG, Giovannucci A, Bangash MA, Grasselli G, Penagarikano O, Piochon C, Tsai PT, Geschwind DH, Hansel C, Sahin M, Takumi T, Worley PF, & Wang SS (2015). Cerebellar associative sensory learning defects in five mouse autism models. Elife, 4, e06085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, & Silva AJ (2000). Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus, 10, 47–56. [DOI] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, Wickersham IR, & Tonegawa S (2014). Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci, 17, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, & Dulac C (2018). Functional circuit architecture underlying parental behaviour. Nature, 556, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkhyen T, McCarthy EA, Korzan WJ, Doctor D, Han X, Baum MJ, & Cherry JA (2017). Optogenetic Activation of Accessory Olfactory Bulb Input to the Forebrain Differentially Modulates Investigation of Opposite versus Same-Sex Urinary Chemosignals and Stimulates Mating in Male Mice. eNeuro, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalumiere RT (2014). Optogenetic dissection of amygdala functioning. Front Behav Neurosci, 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, & Young WS 3rd (2009). Oxytocin: the great facilitator of life. Prog Neurobiol, 88, 127–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, & Anderson DJ (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature, 470, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, & Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience, 121, 537–544. [DOI] [PubMed] [Google Scholar]
- Lukas M, & Neumann ID (2013). Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res, 251, 85–94. [DOI] [PubMed] [Google Scholar]
- Luo M, Fee MS, & Katz LC (2003). Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science, 299, 1196–1201. [DOI] [PubMed] [Google Scholar]
- Maren S, & Quirk GJ (2004). Neuronal signalling of fear memory. Nat Rev Neurosci, 5, 844–852. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Rubinow DR, & Stuber GD (2015). Maternally responsive neurons in the bed nucleus of the stria terminalis and medial preoptic area: Putative circuits for regulating anxiety and reward. Front Neuroendocrinol, 38, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, & Blaha CD (2008). Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse, 62, 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, & Sahin M (2017). Translational use of event-related potentials to assess circuit integrity in ASD. Nat Rev Neurol, 13, 160–170. [DOI] [PubMed] [Google Scholar]
- Montagrin A, Saiote C, & Schiller D (2017). The social hippocampus. Hippocampus. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Wang Z, Schmitt LM, Tsai P, & Sweeney JA (2015). The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front Neurosci, 9, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan M, Jang HJ, Park M, Miller EM, Cox J, Taliaferro JP, Parker NF, Bhave V, Hur H, Liang Y, Nectow AR, Pillow JW, & Witten IB (2017). Combined Social and Spatial Coding in a Descending Projection from the Prefrontal Cortex. Cell, 171, 1663–1677 e1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan V, Heiming RS, Jansen F, Lesting J, Sachser N, Pape HC, & Seidenbecher T (2011). Social defeat: impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS One, 6, e22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID (2007). Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans, 35, 1252–1257. [DOI] [PubMed] [Google Scholar]
- Numan M, Bress JA, Ranker LR, Gary AJ, Denicola AL, Bettis JK, & Knapp SE (2010). The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behav Brain Res, 214, 368–376. [DOI] [PubMed] [Google Scholar]
- Numan M, & Young LJ (2015). Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav, 77, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, & Kelsch W (2016). Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron, 90, 609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, & Tonegawa S (2016). Ventral CA1 neurons store social memory. Science, 353, 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Garcia M, Agustin-Pavon C, Lanuza E, & Martinez-Garcia F (2015). Distribution of oxytocin and co-localization with arginine vasopressin in the brain of mice. Brain Struct Funct, 221, 3445–3473. [DOI] [PubMed] [Google Scholar]
- Pape HC (2005). GABAergic neurons: gate masters of the amygdala, mastered by dopamine. Neuron, 48, 877–879. [DOI] [PubMed] [Google Scholar]
- Parker KL, Kim YC, Kelley RM, Nessler AJ, Chen KH, Muller-Ewald VA, Andreasen NC, & Narayanan NS (2017). Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol Psychiatry, 22, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Narayanan NS, & Andreasen NC (2014). The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci, 8, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto RT, Wang W, Croney DM, Kozorovitskiy Y, & Sabatini BL (2016). Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B(−/−) mice. Nat Neurosci, 19, 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter S, Ten Brinke MM, Stedehouder J, Reinelt CM, Wu B, Zhou H, Zhou K, Boele HJ, Kushner SA, Lee MG, Schmeisser MJ, Boeckers TM, Schonewille M, Hoebeek FE, & De Zeeuw CI (2016). Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat Commun, 7, 12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A (2009). Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus). Behav Brain Res, 200, 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raam T, McAvoy KM, Besnard A, Veenema AH, & Sahay A (2017). Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun, 8, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim D, Distler U, Halbedl S, Verpelli C, Sala C, Bockmann J, Tenzer S, Boeckers TM, & Schmeisser MJ (2017). Proteomic Analysis of Post-synaptic Density Fractions from Shank3 Mutant Mice Reveals Brain Region Specific Changes Relevant to Autism Spectrum Disorder. Front Mol Neurosci, 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith RM, McKenna J, Wu H, Hashmi SS, Cho SH, Dash PK, & Gambello MJ (2013). Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol Dis, 51, 93–103. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, & Wightman RM (2002). Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci, 22, 10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TD, Dickson PE, McKimm E, Heck DH, Goldowitz D, Blaha CD, & Mittleman G (2013). Reorganization of circuits underlying cerebellar modulation of prefrontal cortical dopamine in mouse models of autism spectrum disorder. Cerebellum, 12, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, & Sur M (2015). Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R (2006). Uniquely human social cognition. Curr Opin Neurobiol, 16, 235–239. [DOI] [PubMed] [Google Scholar]
- Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong ASO, Kauvar I, Ramakrishnan C, Fenno LE, Davidson TJ, Wright M, & Deisseroth K (2017). Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevelkin AV, Ihenatu C, & Pletnikov MV (2014). Pre-clinical models of neurodevelopmental disorders: focus on the cerebellum. Rev Neurosci, 25, 177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, & Gallagher L (2009). Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci, 13, 27–35. [DOI] [PubMed] [Google Scholar]
- Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, & Young WS (2016). Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol Psychiatry, 21, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, & Knopfel T (2016). Optogenetics enlightens neuroscience drug discovery. Nat Rev Drug Discov, 15, 97–109. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, Gibson JM, Kelly E, Meng F, Cano CA, Pascual JM, Mostofsky SH, Lerch JP, & Tsai PT (2017). Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci, 20, 1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M, & Sahin M (2015). Cerebellar Development and Autism Spectrum Disorder in Tuberous Sclerosis Complex. J Child Neurol, 30, 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, & Ludwig M (2010). An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature, 464, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, & Sahin M (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature, 488, 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneoka Y, Tokita K, Yoshihara C, Amano T, Esposito G, Huang AJ, Yu LM, Odaka Y, Shinozuka K, McHugh TJ, & Kuroda KO (2015). Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBOJ, 34, 2652–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Cho KK, Lee AT, Sohal VS, & Rubenstein JL (2015). The parvalbumin/somatostatin ratio is increased in Pten mutant mice and by human PTEN ASD alleles. Cell Rep, 11, 944–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P (2015). Affective and motivational control of vision. Curr Opin Neurol, 28, 29–35. [DOI] [PubMed] [Google Scholar]
- Wacker DW, & Ludwig M (2012). Vasopressin, oxytocin, and social odor recognition. Horm Behav, 61, 259–265. [DOI] [PubMed] [Google Scholar]
- Wang D, Li Y, Feng Q, Guo Q, Zhou J, & Luo M (2017a). Learning shapes the aversion and reward responses of lateral habenula neurons. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li C, Chen Q, van der Goes MS, Hawrot J, Yao AY, Gao X, Lu C, Zang Y, Zhang Q, Lyman K, Wang D, Guo B, Wu S, Gerfen CR, Fu Z, & Feng G (2017b). Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J Clin Invest, 127, 1978–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, & Aragona BJ (2004). Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav, 83, 319–328. [DOI] [PubMed] [Google Scholar]
- Watson TC, Becker N, Apps R, & Jones MW (2014). Back to front: cerebellar connections and interactions with the prefrontal cortex. Front Syst Neurosci, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TC, Jones MW, & Apps R (2009). Electrophysiological mapping of novel prefrontal - cerebellar pathways. Front Integr Neurosci, 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, & Dulac CG (2014). Galanin neurons in the medial preoptic area govern parental behaviour. Nature, 509, 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Priest MF, Nasenbeny J, Lu T, & Kozorovitskiy Y (2017). Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron, 95, 368–384 e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, & Deisseroth K (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature, 477, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, & Insel TR (2001). Cellular mechanisms of social attachment. Horm Behav, 40, 133–138. [DOI] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, & Palkovits M (2006). The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience, 143, 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GZ, Kaba H, Okutani F, Takahashi S, & Higuchi T (1996). The olfactory bulb: a critical site of action for oxytocin in the induction of maternal behaviour in the rat. Neuroscience, 72, 1083–1088. [DOI] [PubMed] [Google Scholar]
- Zalla T, & Sperduti M (2013). The amygdala and the relevance detection theory of autism: an evolutionary perspective. Front Hum Neurosci, 7, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]


