Abstract
Extracellular cold-inducible RNA-binding protein (CIRP) exaggerates inflammation in sepsis. Neutrophil reverse transendothelial migration (rTEM) allows neutrophils to migrate from tissues into the circulation. The phenotype of neutrophils following reverse migration is CD54hiCXCR1lo. We hypothesize that CIRP induces neutrophil rTEM in sepsis. Sepsis was induced in male C57BL/6 mice by cecal ligation and puncture (CLP), and at 5, 10, or 20 h after CLP, the frequencies of reversely migrated (RM) neutrophils were assessed in the blood by flow cytometry. Since 20 h of CLP showed highest increase in the frequency of RM neutrophils, we further assessed RM neutrophils in the blood of WT and CIRP−/− mice at this time point. The effect of CIRP on neutrophil rTEM was determined by injecting mice with recombinant mouse CIRP (rmCIRP) intratracheally (i.t.) and assessed the frequencies of RM neutrophils. The expression of neutrophil elastase (NE) and junctional adhesion molecule-C (JAM-C) in the lungs were measured by Western blot. The mean frequency of RM neutrophils in sham mice was 0.4%, while the frequencies were significantly increased to 1%, 3%, and 7% at 5, 10, and 20 h of CLP, respectively. The mean frequency of RM neutrophils in the blood of CIRP−/− mice was significantly lower than that of WT mice at 20 h of CLP. The RM neutrophils in the blood was significantly increased after administration of rmCIRP i.t. into mice in a time- and dose-dependent manners. NE expression was upregulated while JAM-C expression was downregulated in the lungs after CLP or rmCIRP administration. For the first time, we have showed that CIRP induces neutrophil rTEM in sepsis by increasing NE and decreasing JAM-C.
Keywords: CIRP, rTEM neutrophil, JAM-C, neutrophil elastase, inflammation, sepsis
Introduction
Sepsis is an overwhelming inflammatory response to infection and causes tissue injury and organ dysfunction (1). Damage-associated molecular patterns (DAMPs) interact with pattern recognition receptors expressed on the surface of immune-reactive cells, leading to the release of pro-inflammatory cytokines, chemokines and recruitment of leukocytes at the site of inflammation (2). We have discovered that extracellular cold-inducible RNA-binding protein (CIRP) is a novel DAMP (3). CIRP is a member of the cold shock protein family expressed in various cells and serves as a RNA chaperones (4, 5). CIRP is upregulated by hypoxia, hypothermia, and oxidative stress (6, 7). During sepsis and hemorrhagic shock, CIRP is translocated from the nucleus to cytoplasmic stress granules and is subsequently released into the circulation (3). Once released, CIRP acts as a DAMP to increase sepsis severity and mortality rate (3). CIRP promotes inflammatory responses by its receptors Toll-like receptor 4 (TLR4) and myeloid differentiation factor 2 (MD2) complex (3). Therefore, therapeutic targeting of CIRP protects mice from organ injuries during sepsis and organ ischemia-reperfusion (I/R) (3, 8).
Neutrophils are the most abundant leukocytes in blood to play a pivotal role in host resistance against pathogen (9). The effector function of neutrophils is mediated through phagocytosis, degranulation, reactive oxygen species (ROS), and neutrophil extracellular traps (NETs) (9–12). However, neutrophils also promote tissue damage through the release of cytokines, proteases, ROS and NETs (11, 13, 14). Although neutrophils are considered a homogenous population of terminally differentiated cells with a well-defined function, increasing evidence has demonstrated phenotypic heterogeneity and functional versatility of neutrophils, which arise due to their different migratory behaviors (11, 15).
Neutrophil migration from the vasculature into the tissue beds is an irreversible and unidirectional mechanism (16). However, recent studies have reported the ability of neutrophils to return to the bloodstream after migrating to the extravascular space through a process known as reverse transendothelial migration (rTEM) (17–19). The surface phenotypes of reversely migrated (RM) neutrophils are intercellular adhesion molecule-1 (ICAM-1 or CD54)hi and CXCR1lo, while the phenotypes of circulating and tissue resident neutrophils are ICAM-1loCXCR1hi and ICAM-1loCXCR1lo, respectively (17). Neutrophils undergoing rTEM exhibit a pro-inflammatory phenotype characterized by increased levels of superoxides and high surface ICAM-1 expression (19). We previously reported that CIRP-induced ICAM-1+ neutrophils are pro-inflammatory in terms of the increased production of inducible nitric oxide synthase (iNOS) and NETs in sepsis (20). The RM neutrophils have prolonged life-span and are associated with pulmonary inflammation following cremaster muscle ischemia-reperfusion (I/R) injury in mice (19). These results suggest that RM neutrophils may contribute to turning a local inflammation into a systemic inflammatory response.
Neutrophil rTEM predominantly depends on junctional adhesion molecule (JAM)-C expressed on the surface of endothelial cell(s) (EC) (18, 19). In murine cremaster muscle I/R injury model, a lipid chemoattractant leukotriene B4 (LTB4) was upregulated in the inflamed tissues, which led neutrophils to produce excess amount of the proteolytic enzyme neutrophil elastase (NE) (18). Thus, excessive production of NE may cause EC surface JAM-C reduction and subsequently promotes neutrophil rTEM (18, 19).
While the phenotype and function of RM neutrophils have been characterized in a sterile inflammatory condition (cremaster muscle I/R injury), their status and function in polymicrobial sepsis is not known. We therefore aim to determine the status and function of RM neutrophils in sepsis. Since CIRP as a novel DAMP is increased in sepsis to exaggerate inflammation and tissue injury, we further aim to delineate the role of CIRP for inducing neutrophil rTEM in sepsis. Our data clearly revealed the direct role of CIRP for inducing neutrophil rTEM in mice through the modulation of endothelial JAM-C levels by upregulating NE expression in the lungs. These findings point out a novel pathophysiological role of CIRP in sepsis.
Materials and Methods
Mice
C57BL/6 wild-type (WT) mice (male, 8–9 week, 21–28 g weight) were purchased from Charles River (Wilmington, MA). CIRP−/− mice (C57BL/6 genetic background) were obtained from Dr. Jun Fujita (Kyoto University, Kyoto, Japan). Mice were housed in rooms at room temperature (~23°C) with 12 h light cycles and provided standard laboratory food and water. All experiments were performed in accordance with NIH guidelines for the use of experimental animals. The animal protocol was approved by the Institutional Animal Care and Use Committees (IACUC) of the Feinstein Institute for Medical Research.
Polymicrobial sepsis
WT and CIRP−/− mice were anesthetized with 2% isoflurane inhalation. The abdomen was shaved and made sterile with 10% povidone-iodine swab. Sepsis was initiated in mice by cecal ligation and puncture (CLP) as described previously (21, 22). After a 1.5 cm midline abdominal incision the cecum was exposed and ligated at 1 cm from the tip with 4–0 silk suture. Ligated distal part of the cecum was punctured one time through and through using a 22 gauge needle and squeezed gently to extrude a small volume of fecal material. The cecum was placed back inside the abdomen, and the abdominal wound was closed in two layers with running 4–0 silk suture. Sham mice underwent only abdominal incision without ligation and puncture of the cecum. Both sham- and CLP-operated animals were resuscitated with 1 ml of normal saline subcutaneously, and then returned to their cages with access to food and water.
Administration of recombinant mouse CIRP
Recombinant mouse CIRP (rmCIRP) was prepared in-house (3). Various doses of rmCIRP or equivalent volume phosphate buffered saline (PBS) was administered into WT mice intratracheally (i.t.) as described previously (23, 24). After induction of anesthesia by 2% isoflurane inhalation, mice were placed supine and the trachea was exposed to deliver rmCIRP or PBS using a 29G × 1/2″ U-100 insulin syringe (Terumo Medical Corporation, Elkton, MD). The total volume of rmCIRP or PBS for i.t. instillation was less than 50 μl. At 20 h after injection of rmCIRP or PBS, all mice were killed by CO2 asphyxiation and blood and lung tissues were collected for various analyses.
Detection of RM neutrophils
To detect RM neutrophils, 250 μl of whole blood obtained from sham, CLP, rmCIRP-, and PBS-treated mice were taken into Falcon 15 ml conical centrifuge tubes and added 5 ml red blood cell lysis buffer (BD Biosciences, San Jose, CA). After 1–2 min of incubation at room temperature, the samples were immediately centrifuged at 450 ×g for 2 min. Supernatants were aspirated and the cell pellet was washed by suspending the cells in 5 ml fluorescence-activated cell sorting (FACS) buffer containing PBS with 2% fetal bovine serum (FBS) and centrifuged at 450 ×g for 2 min. The supernatant was discarded and the cell pellet was dissolved in 500 μl FACS buffer. Cells were stained with APC anti-mouse Ly-6G Ab (clone: 1A8; Biolegend, San Diego, CA), FITC anti-mouse CD54 Ab (clone: 3E2; BD Biosciences) and PE anti-mouse CXCR1 Abs (clone: U45–632; BD Biosciences). Unstained cells were used to establish the flow cytometer voltage setting, and single-color positive controls were used for the adjustment of the compensation. Acquisition was performed on 50,000 events using a BD LSR Fortessa flow cytometer (BD Biosciences) and data were analyzed with FlowJo software (Tree Star, Ashland, OR). RM neutrophils were defined as CD54hiCXCR1lo within Ly6G+ population.
Assessment of NE and JAM-C expression in the lungs
Lung tissues were collected from WT and CIRP−/− sham- and CLP-operated mice or from WT mice injected with PBS or rmCIRP. Proteins were extracted from liquid nitrogen crushed tissue powders by homogenizing in lysis buffer containing 10 mM Tris-HCl, pH 7.5, 120 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) using a sonicator (Sonic Dismembranator 100; Fisher Scientific, Pittsburgh, PA). Concentration of protein of each sample was determined by Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). Equal amounts of crude lung protein extracts were fractionated on Bis-Tris gels (4–12%) and transferred to 0.2 μm pore size nitrocellulose membrane. The membrane was blocked with 0.1% casein in Tris-buffered saline with 0.1% tween-20 (TBST) and incubated with anti-mouse NE Ab (Cat. No: MAB 4517-SP; R&D Systems, MN), anti-mouse JAM-C Ab (Cat.No: AT 1213; R&D Systems) or β-actin primary Ab (Cat. No.: A5441; Sigma-Aldrich, St Louis, MO). After washing the membranes with TBST buffer, they were incubated with fluorescent-labeled secondary Abs (Li-Cor Biosciences, Lincoln, NE). Bands were detected by Odyssey FC Dual-Mode Imaging system (Li-Cor Biosciences), and the intensities of bands were measured using Image J software (25).
Assessment of NE in bone marrow-derived neutrophil culture supernatants
Murine bone marrow-derived neutrophil(s) (BMDN) were isolated as described previously (20). Mice were anesthetized by 2% isoflurane inhalation and the femurs and tibias were dissected. Marrow contents form bones were flushed out with Ca++ and Mg++ free HBSS using a 25 gauge needle into a petri dish. Suspensions of cells were filtered through a Corning sterile 70 μm cell strainer (Fisher Scientific, Waltham, MA) and BMDN were purified by negative selection using the EasySep mouse neutrophil enrichment kit (Cat. No.: 19762; STEMCELL Technologies, Vancouver, Canada). The purity of the sorted neutrophils was assessed by staining the cells with APC anti-mouse Ly6G Ab (clone 1A8; Biolegend) using BD LSR Fortessa flow cytometer (BD Biosciences). A total of 5 × 105 BMDN were treated with rmCIRP (1 μg/ml) for 4 h. Supernatants were collected and concentrated by trichloroacetic acid (TCA) method as described previously (26). Protein samples were subjected to Western blot for the detection of NE in the concentrated culture supernatants using anti-mouse NE Ab (Cat. No: MAB 4517-SP; R&D Systems). For normalization blots were stained with Ponceu Red dye.
Mouse primary lung microvascular endothelial cells
C57BL/6 mouse primary lung microvascular endothelial cells (EC) were purchased from Cell Biologics (Cat. No.: C57–6011; Chicago, IL) and grown in basal medium (Cell Biologics) supplemented with 0.5 ml each of VEGF, ECGS, heparin, EGF, hydrocortisone, L-glutamine, antibiotic/antimycotic solution and 25 ml FBS. The basal medium and the supplement kit (Cat. No.: M1168) were obtained from Cell Biologics. According to the data sheet (Cell Biologics), these cells were developed from C57BL/6 mice lungs and purified using a rat anti-mouse CD31 (PECAM-1) mAb. While growing these cells, we noticed that their viability was around 90% (data not shown). We also assessed their purity for the expression of their cell surface marker CD31 using antibody anti-CD31/PECAM-1 (rat anti-mouse CD31, catalog no. 553370, BD Biosciences) by flow cytometry and noticed that over 80% of these cells expressed CD31 on their cell surface (data not shown).
Assessment of JAM-C expression on EC surface following stimulation with conditioned medium from rmCIRP-treated BMDN
A total of 5 × 105 BMDN sorted from C57BL/6 mice bone marrow total cells were plated into 12-well cell culture plate in 1.5 ml RPMI with 10% FBS medium. BMDN were treated with PBS or rmCIRP (1 μg/ml) for 4 h and then collected the culture supernatants, which served as conditioned medium to treat the cultured EC. Mouse primary lung microvascular endothelial cells or EC were plated into 12-well cell culture plate at a number 2 × 105 cells in 1.5 ml conditioned medium obtained from PBS- or rmCIRP-treated BMDN. In two separate groups of EC treated with conditioned medium of rmCIRP-stimulated BMDN, we added 1 μg/ml each of anti-mouse NE Abs (Cat. No: MAB 4517-SP; R&D Systems) or IgG isotype (Clone: RTK2071; Biolegend). The EC grown in conditioned medium and anti-mouse NE Abs or IgG isotype were incubated overnight. The EC were then collected from the plate, centrifuged to remove the supernatants and assessed surface expression of JAM-C by flow cytometry. To detect JAM-C expression on the surface of conditioned medium treated EC, the cells were washed with FACS and stained with PE anti-mouse CD31 Ab (Clone:390; Biolegend) and APC anti-mouse JAM-C Ab (Clone: 2096–28; R&D Systems). Unstained cells were used as a negative control to establish the flow cytometer voltage setting, and single-color positive controls were used for the adjustment of the compensation. Acquisition was performed on 30,000 events using a BD LSR Fortessa flow cytometer (BD Biosciences) and data were analyzed with FlowJo software (Tree Star).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Multiple groups were compared by one-way analysis of variance (ANOVA) using the Student-Newman-Keuls. The p values <0.05 were considered statistically significant. All statistical analysis were performed and graphics were created by using Sigma Plot graphing and statistical analysis software (Systat Software Inc., San Jose, CA).
Results
Neutrophil rTEM is increased in sepsis
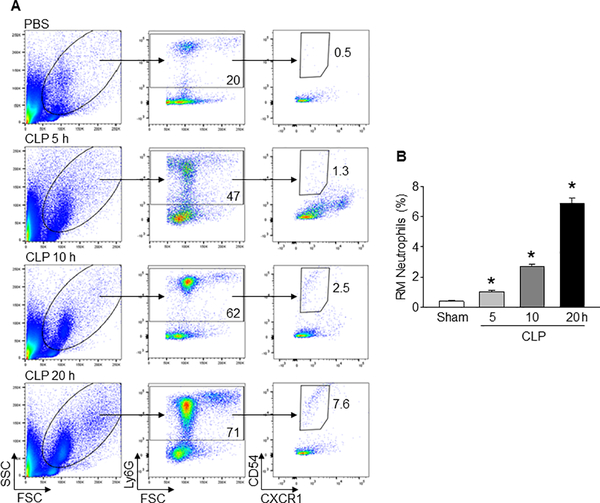
At various time intervals after CLP, we collected blood samples to assess the frequencies of RM neutrophils. We found that the frequencies of RM neutrophils were significantly increased to mean values of 1%, 2.7%, and 6.9% at 5, 10, and 20 h after CLP, respectively, as compared to sham-operated mice of only 0.4% (Fig 1A, B). The highest increase in the frequencies of RM neutrophils occurs at 20 h of CLP (Fig 1A, B). Thus, circulating RM neutrophils increase in sepsis in a time-dependent fashion.
Fig 1: Sepsis increases neutrophil rTEM in the blood.
(A) After 5, 10, and 20 h of CLP or sham operation, blood samples were collected from mice and flow cytometric detection was performed. Representative plots indicating forward and side scatter, Ly6G+, and CD54hiCXCR1lo populations are shown. (B) Bar diagram representing the mean frequencies of RM neutrophils (CD54hiCXCR1lo) within Ly6G+ populations are shown. Data are expressed as means ± SE (n = 9 mice/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. sham).
CIRP deficiency leads to the decreased neutrophil rTEM in sepsis
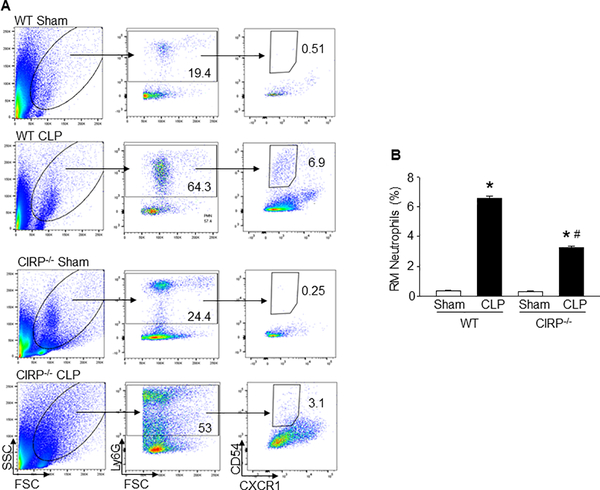
To determine the possible association between CIRP and neutrophil rTEM, we assessed the frequencies of RM neutrophils in the blood in WT and CIRP−/− mice at 20 h of CLP. As shown in Fig 2A, B, both WT and CIRP−/− mice showed significant increase in the frequencies of RM neutrophils in the blood in sepsis as compared to their respective sham-operated mice. Interestingly, we found the mean frequency of RM neutrophils in the blood of CIRP−/− mice was significantly lower than that of WT mice at 20 h of CLP (Fig 2A, B). The data show that CIRP plays a role to increase neutrophil rTEM in sepsis.
Figure 2: Decreased frequencies of blood RM neutrophils in CIRP−/− mice during sepsis.
At 20 h of CLP or sham operation, blood samples were collected from WT and CIRP−/− mice. The frequencies of RM neutrophils in the blood was detected by flow cytometry. (A) Representative dot plots of the frequencies of RM neutrophils (CD54hiCXCR1lo) in Ly6G+ populations in the blood from WT and CIRP−/− mice are shown. (B) A bar diagram showing the quantitative mean values of the frequencies of RM neutrophils in the blood from WT and CIRP−/− mice are shown. Data are expressed as means ± SE (n = 9 mice/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. respective sham mice; #p < 0.05 vs. WT CLP mice).
rmCIRP induces neutrophil rTEM in the blood
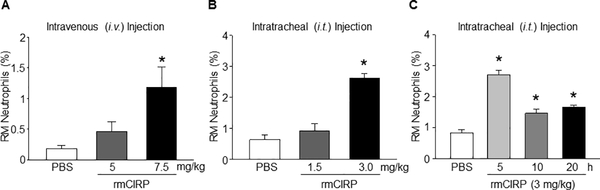
To determine the direct role of CIRP to induce neutrophil rTEM, we injected rmCIRP i.t. to WT mice, and then assessed the frequencies of RM neutrophils in the blood. We choose i.t. over intravenous (i.v.) delivery of rmCIRP in mice because i.t. delivery of rmCIRP showed highest induction of rTEM of neutrophils in the blood than those in i.v. administration of rmCIRP (Figs 3A and 3B). Because of the limited available information about the in vivo dose of rmCIRP in mice, we carried-out dose-response and time-course change studies to obtain optimum dose and time-point of the in vivo administration of rmCIRP for inducing RM neutrophils in mice. We found an increase in the frequencies of RM neutrophils by 43%, and 310% in the WT mice injected with 1.5 and 3.0 mg/kg BW rmCIRP i.t. for 5 h, respectively as compared to PBS-injected mice (Fig 3B). Similarly, by injecting WT mice with 3 mg/kg BW rmCIRP i.t., we determined the time-course change effects of rmCIRP for induction of the frequencies of RM neutrophils in the blood. We found that WT mice injected with rmCIRP i.t. significantly increased the frequencies of RM neutrophils by 231%, 74%, and 99% at 5, 10, and 20 h, respectively, in the blood as compared to PBS-injected mice (Fig 3C). These data suggest that CIRP induces neutrophil rTEM in a dose- and time-dependent manners.
Figure 3: rmCIRP induces neutrophil rTEM in the blood in mice.
(A) Various doses (5 and 7.5 mg/kg BW) of rmCIRP or equal volume of PBS (as control) were injected into WT mice via i.v. After 5 h of rmCIRP injection, blood was collected from the mice and flow cytometric detection of reversely migrated (RM) neutrophils was performed. Bar diagram representing the mean values of the frequencies of RM neutrophils treated with either PBS or at various doses of rmCIRP through i.v. injection is shown. Data are expressed as means ± SE (n = 6 mice/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. PBS-treated mice). (B) Various doses of rmCIRP (1.5 and 3.0 mg/kg BW) or equal volume of PBS (as control) were i.t. injected into WT mice. After 5 h of rmCIRP injection, blood was collected from all mice and flow cytometric detection was performed. Bar diagram representing the mean values of the frequencies of RM neutrophils treated with either PBS or at various doses of rmCIRP is shown. (C) WT mice were injected i.t. with 3.0 mg/kg BW of rmCIRP or equal volume of PBS (as control). Blood was collected from all mice at various time-points (5, 10, 20 h) after PBS or rmCIRP injection. Blood samples from PBS-injected mice at 20 h served as a control. Bar diagram representing the mean values of the frequencies of RM neutrophils treated with either PBS or rmCIRP (3 mg/kg BW) at 5, 10, and 20 h is shown. Data are expressed as means ± SE (n = 6 mice/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. PBS-treated mice).
rmCIRP increases NE and decreases JAM-C expression in the lungs
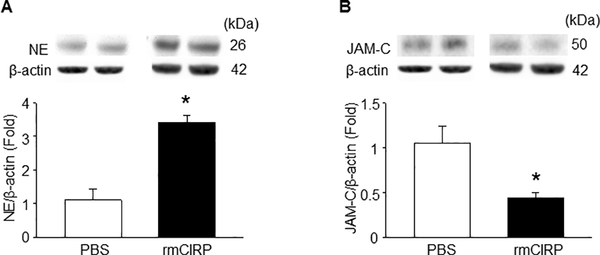
We assessed the lung expression of NE and JAM-C following treatment of mice with rmCIRP. We found that the NE expression in the lungs were significantly increased at 5 h after rmCIRP injection (Fig 4A). Correspondingly, compared with the PBS-treated mice, we found a significant decrease in the expression of JAM-C in the lungs at the same time point (Fig 4B).
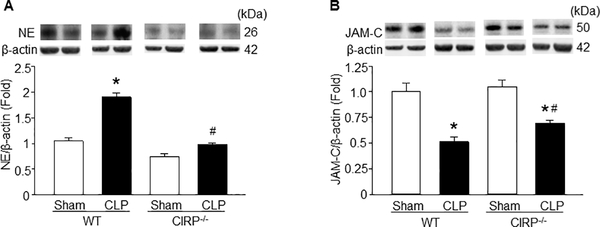
Figure 4: NE is decreased and JAM-C is increased in the lungs of CIRP−/− mice during sepsis.
Sepsis was induced in WT and CIRP−/− mice by CLP and at 5 h of CLP we harvested the lungs to assess (A) NE expression and at 20 h of CLP we harvested the lungs to assess (B) JAM-C expression at their protein levels by Western blot. Each blot was quantified by densitometry analysis. NE and JAM-C expression in each sample was normalized to β-actin expression and the mean value of sham group was standardized as one for comparison. Representative blots for NE, JAM-C and β-actin are shown. Data are expressed as means ± SE (n = 8–10 mice/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. respective sham mice; #p < 0.05 vs. WT CLP mice).
CIRP−/− mice express lower NE and higher JAM-C in the lungs in sepsis
NE decreases the expression of surface JAM-C in the EC, thereby promoting neutrophil rTEM (18, 19). We therefore assessed the expression of NE and JAM-C in the lungs in sepsis. We induced sepsis in WT and CIRP−/− mice by CLP and after 5 h we collected the lungs to assess the expression of NE. As shown in Fig 5A, NE expression in the lungs were significantly induced after CLP in WT mice, but not in CIRP−/− mice. We further assessed the expression of JAM-C in the lungs at 20 h of CLP. As shown in Fig 5B, both WT and CIRP−/− mice showed significant decrease in the levels of JAM-C, while the CIRP−/− mice had less reduction in JAM-C expression. The data suggest that the decreased NE and less reduction of JAM-C in the lungs of CIRP−/− mice in sepsis may be responsible for the decreased neutrophil rTEM in the blood in sepsis. NE expression was assessed at 5 h while the JAM-C expression was assessed at 20 h of CLP in the lungs, because at these time points we noticed optimum induction of NE and reduction of JAM-C expression (data not shown).
Figure 5: In vivo administration of rmCIRP increases NE and decreases JAM-C expression in the lungs.
WT mice were i.t. injected with rmCIRP (3 mg/kg BW) or equal volume of PBS as control and after 5 h we harvested the lungs. Total protein was extracted from lung tissues of each animal and then determined the protein levels of (A) NE and (B) JAM-C by Western blot. Each blot was quantified by densitometry analysis. NE and JAM-C expression in each sample was normalized to β-actin expression and the mean value of sham group was standardized as one for comparison. Representative blots for NE, JAM-C and β-actin are shown. Data are expressed as means ± SE (n = 8–10 mice/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. sham mice).
NE released by rmCIRP-treated BMDN decreases surface JAM-C expression on endothelial cells
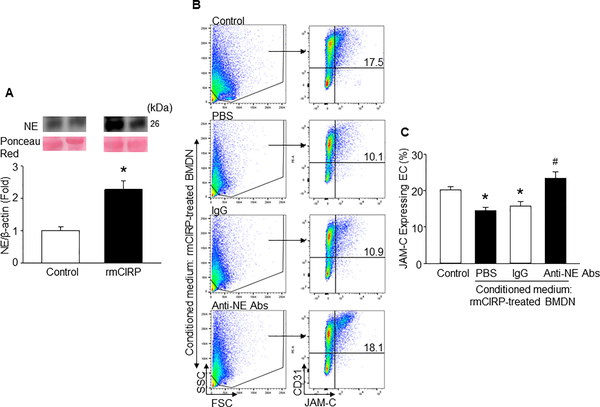
First, we stimulated BMDN with rmCIRP and assessed NE levels in the culture supernatants. In this in vitro study, the concentration of rmCIRP at 1 μg/ml was chosen from our previous study which showed that the treatment of BMDN with 1 μg/ml of rmCIRP significantly increased their ability to from neutrophil extracellular traps (NETs) (20). BMDN stimulated with rmCIRP significantly induced NE levels in the cell culture supernatants compared to PBS-treated BMDN (Fig 6A). We then collected the supernatants of BMDN treated with PBS (control), rmCIRP and rmCIRP with anti-NE Abs or isotype IgG. We separately added these supernatants to EC and assessed surface expression of JAM-C on EC. We found that EC stimulated with the conditioned medium from rmCIRP-treated BMDN decreased the surface expression of endothelial JAM-C by relative 28%, compared to the control (Fig 6B). EC treated with the conditioned medium obtained from rmCIRP plus anti-NE Abs-treated BMDN were protected from the decrease in the expression of surface JAM-C. Conversely, the EC treated with conditioned medium from rmCIRP and isotype IgG treated BMDN showed significant decreased in the surface levels of JAM-C as compared with the control (Fig 6B). The data suggest that CIRP induces neutrophils to produce NE and addition of CIRP-induced neutrophil culture supernatants to EC results in decreased surface expression of JAM-C EC.
Figure 6: CIRP-induced BMDN release NE to decrease JAM-C expression in EC.
(A) A total of 5 × 105 BMDN were stimulated by either rmCIRP (1 μg/ml) or PBS as control for 4 h. Cell culture supernatants were assessed for NE expression by Western blot. NE levels in the culture supernatants of each sample was normalized to Ponseu Red stain and the mean value of PBS-treated group was standardized as one for comparison. Representative blots for NE, and Ponseu Red are shown. Data are expressed as means ± SE (n = 6 samples/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. PBS-treated BMDN). (B) A total of 5 × 105 BMDN were stimulated with rmCIRP (1 μg/ml) for 4 h, followed by the collection of culture supernatants to serve as conditioned medium for EC stimulation. A total of 2 × 105 mouse primary lung microvascular endothelial cells were plated into cell culture plate and added 1.5 ml of the conditioned media. EC cultured in conditioned mediums were also treated with either anti-mouse NE Abs or isotype IgG each at a dose of 1 μg/ml for overnight and assessed the expression of JAM-C by flow cytometry. Data are expressed as means ± SE (n = 6 samples/group) and compared by one-way ANOVA and SNK method (*p < 0.05 vs. PBS-treated conditioned medium; #p<0.05 vs. rmCIRP-treated conditioned medium).
Discussion
The lungs are particularly susceptible to injury in sepsis, and more than 50% of patients with sepsis develop acute lung injury (ALI) (27, 28). In the animal model of CLP-induced sepsis, ALI is commonly developed (29). We previously reported that following sepsis CIRP levels were increased in the blood as well as in the lungs (3, 20). The role of CIRP on neutrophil transendothelial migration for infiltration into the lungs to cause ALI was previously demonstrated following administration of rmCIRP in mice (30). Nonetheless, the role of CIRP on neutrophil rTEM in sepsis remains unknown. Here, for the first time we identified rTEM of neutrophils into the blood, whose frequencies were significantly increased in sepsis, as well as upon CIRP treatment. We found a dose-dependent effect of rmCIRP in increasing the frequencies of RM neutrophils in the blood. However, we noticed an atypical pattern of the increase of the frequencies of RM neutrophils in the blood in the time-course experiment, representing the highest increase at 5 h after rmCIRP administration, while their frequencies decreased at later time-points. This atypical finding could be due to CIRP’s relative short half-life and/or the re-entering of RM neutrophils into tissues, giving rise to a lower frequency of RM neutrophils in the circulation at later-time point.
To elucidate the novel role of CIRP on neutrophil rTEM during sepsis, we induced sepsis in WT and CIRP−/− mice. The mean frequency of RM neutrophils in the blood of CIRP−/− mice was significantly lower than that of WT mice at 20 h of CLP. These data demonstrate that CIRP plays a major role in neutrophil rTEM, although other mechanisms may also contribute to this process. Studies demonstrated that both the concentration of chemoattractants and the dynamic regulation of surface expressions of chemokine receptors or their sensitivity to the corresponding ligands are the factors which could promote neutrophil rTEM (11, 31). Future studies on chemokines and their gradients and several chemokine receptors expressed on neutrophils in WT and CIRP−/− mice during sepsis will provide further insights into the mechanism of CIRP-dependent generation of RM neutrophils in the blood during sepsis.
The frequency of RM neutrophils in the blood could be associated with the intensity of inflammation during sepsis. Since the overall inflammation in CIRP−/− mice in sepsis was decreased compared to WT mice (3), the frequency of RM neutrophils in CIRP−/− mice in the blood during sepsis could be decreased as compared to WT mice, implicating an indirect role of CIRP on neutrophil rTEM in sepsis. To determine the direct role of CIRP on neutrophil rTEM, we administered rmCIRP into the WT mice via i.t. and found increased frequencies of rTEM of neutrophils in the blood. We stimulated BMDN with rmCIRP and found significant increase of NE levels in the supernatants, and when these NE rich supernatants were added into EC culture we found decreased expression of JAM-C on EC. This suggests the direct role of CIRP for inducing neutrophil rTEM by upregulating the expression of NE by neutrophils and downregulating the surface expression of JAM-C on EC. However, our in vivo data showed that in CIRP−/− mice the expression of JAM-C protein was increased by only 35% as compared to WT mice in sepsis, suggesting factors other than CIRP might contribute to the changes in JAM-C expression. So far, LTB4-NE axis has been shown to be the main mediator of the regulation of JAM-C expression (18), while the relationship between CIRP and LTB4-NE axis still needs further exploration.
In a pilot study, we administered rmCIRP in mice via i.v. or i.t. and found the frequency of RM neutrophils in the blood was higher in i.t. (local) administration of rmCIRP compared to its i.v. (systemic) administration. Based on these data, we chose to inject rmCIRP through i.t. in mice. For i.v. delivery, we injected up to 7.5 μg/kg BW of rmCIRP in a total volume of 150 μl, which could be disseminated in the large surface area of body’s circulatory system and thus became diluted. On the other hand, in i.t. delivery we administered same dose of rmCIRP in a relatively small volume (40 μl), retaining its original concentration intact to exhibit greater efficacies for inducing neutrophil rTEM. Along with this finding, we previously showed that the intratracheal delivery of LPS resulted in ALI in mice (24).
We previously demonstrated that TLR4 and MD2 complex serves as the receptor for CIRP, which leads to the activation of downstream transcription factor NF-κB to upregulate the expression of pro-inflammatory cytokines (3, 20). In the current study, although we found significant increase of the expression of NE by neutrophils following CIRP treatment, the mechanism of NE induction had not been demonstrated. Further study focusing the role of TLR4 pathway for CIRP-mediated induction of NE expression could be of interest.
Neutrophil rTEM caused by murine cremaster muscle I/R injury is associated with systemic inflammation and lung injury (18, 19). The RM neutrophils have a prolonged lifespan and increased ability to generate ROS (17, 24). Hence, the systemic inflammation and lung injury caused by RM neutrophils could be due to their increased ROS production. Delayed neutrophil apoptosis caused by sepsis has been previously reported (32), therefore the RM neutrophils could be the population of neutrophils which are resistant from apoptosis during sepsis (17, 33). Targeting this population of neutrophils may provide a new therapeutic strategy in sepsis.
Excessive neutrophil infiltration causes acute lung injury (ALI) (10, 11). The removal of neutrophils from inflamed tissues is beneficial (34). Thus, neutrophil rTEM may serve as a tool for the removal of neutrophils from tissues. However, since these neutrophils acquire inflammatory properties after coming back into the circulation, the RM neutrophils may disseminate/reenter into other organs to cause multiple organ damage in sepsis. The biological function of RM neutrophils needs further investigation.
The rTEM of neutrophils was demonstrated in a murine cremaster muscle I/R injury model (18, 19, 31), in which the infiltrated neutrophils into the cremaster muscle following I/R return to the circulation and then enter into the lungs to cause ALI. In the present study, we focused on lungs to study neutrophil rTEM during sepsis as lungs are affected during sepsis (29). Since sepsis-mediated organ injury is not limited to lungs (1), further studies focusing on other organs like kidney, liver, and intestine will provide a deep insight into the pathophysiological role of RM neutrophils in sepsis.
In the present study, we identified a novel role of CIRP on neutrophil rTEM in a murine model of sepsis, implicating future studies to elucidate the mechanism of CIRP on human rTEM in sepsis. We have recently identified several therapeutic tools targeting CIRP and obtained beneficial outcomes in sepsis (3, 35). Identification of the novel role of CIRP for inducing neutrophil rTEM in sepsis will be helpful to assess the efficacy of CIRP targeted therapies by assessing the frequencies of RM neutrophils in the blood in sepsis. In summary, for the first time, we have showed that CIRP induces neutrophil rTEM in sepsis by increasing NE and decreasing JAM-C.
Acknowledgements
We are thankful to the Shock Society for selecting this work for New Investigator Award at the 41st Annual Conference on Shock.
Funding
This study was supported by the National Institutes of Health (NIH) grants R35GM118337 to PW.
Footnotes
Competing interests
The authors report no conflicts of interest.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi ME: DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81(1):1–5, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al. : Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med 19(11):1489–1495, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J: A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol 137(4):899–908, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiyama H, Higashitsuji H, Yokoi H, Itoh K, Danno S, Matsuda T,Fujita J: Cloning and characterization of human CIRP (cold-inducible RNA-binding protein) cDNA and chromosomal assignment of the gene. Gene 204(1–2):115–120, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Wellmann S, Bührer C, Moderegger E, Zelmer A, Kirschner R, Koehne P, Fujita J,Seeger K: Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci 117(Pt 9):1785-1794, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Al-Fageeh MB, Smales CM: Alternative promoters regulate cold inducible RNA-binding (CIRP) gene expression and enhance transgene expression in mammalian cells. Mol Biotechnol 54(2):238–249, 2013. [DOI] [PubMed] [Google Scholar]
- 8.McGinn J, Zhang F, Aziz M, Yang WL, Nicastro J, Coppa GF, Wang P: The Protective Effect of A Short Peptide Derived From Cold-Inducible RNA-Binding Protein in Renal Ischemia-Reperfusion Injury. Shock, 49(3):269–279, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segal AW: How neutrophils kill microbes. Annu Rev Immunol 23:197–223, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan C: Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6(3):173–182, 2006. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira S, Rosowski EE, Huttenlocher A: Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16(6):378–391, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y,Zychlinsky A: Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Kolaczkowska E, Kubes P: Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13(3):159–175, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MJ, Radic M: Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 189(6):2689–2695, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvestre-Roig C, Hidalgo A, Soehnlein O: Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 127(18):2173–2181, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Nourshargh S, Alon R: Leukocyte migration into inflamed tissues. Immunity 41(5):694–707, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, et al. : Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 79(2):303–311, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S: Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity 42(6):1075–1086, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, et al. : The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12(8):761–769, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ode Y, Aziz M, Wang P: CIRP increases ICAM-1+ phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J Leukoc Biol 103(4):693:707, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker CC, Chaudry IH, Gaines HO, Baue AE: Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 94(2):331–335, 1983. [PubMed] [Google Scholar]
- 22.Aziz M, Holodick NE, Rothstein TL, Wang P: B-1a Cells Protect Mice from Sepsis: Critical Role of CREB. J Immunol 199(2):750–760, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolognese AC, Sharma A, Yang WL, Nicastro J, Coppa GF, Wang P: Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner. Cell Mol Immunol 15(1):38–47, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz M, Matsuda A, Yang WL, Jacob A, Wang P: Milk fat globule-epidermal growth factor-factor 8 attenuates neutrophil infiltration in acute lung injury via modulation of CXCR2. J Immunol 189(1):393–402, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajalingam D, Loftis C, Xu JJ, Kumar TK: Trichloroacetic acid-induced protein precipitation involves the reversible association of a stable partially structured intermediate. Protein Sci 18(5):980–993, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sevransky JE, Martin GS, Shanholtz C, Mendez-Tellez PA, Pronovost P, Brower R, Needham DM: Mortality in sepsis versus non-sepsis induced acute lung injury. Crit Care 13(5):R150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu WJ, Wan YD, Tie HT, Kan QC, Sun TW: Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: a meta-analysis. PLoS One 9(2):e90426, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M, Ochani M, Khader A, Wang P: Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care 19:53, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang WL, Sharma A, Wang Z, Li Z, Fan J, Wang P: Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci Rep 6:26571, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirano Y, Aziz M, Wang P: Role of reverse transendothelial migration of neutrophils in inflammation. Biol Chem 397(6):497–506, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC: Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med 32(7):1460–1469, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Hughes J, Johnson RJ, Mooney A, Hugo C, Gordon K, Savill J: Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. Am J Pathol 150(1):223–234, 1997. [PMC free article] [PubMed] [Google Scholar]
- 34.Selders GS, Fetz AE, Radic MZ, Bowlin GL: An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater 4(1):55–68, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F, Brenner M, Yang WL, Wang P: A cold-inducible RNA-binding protein (CIRP)-derived peptide attenuates inflammation and organ injury in septic mice. Sci Rep 8(1):3052, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]