Abstract
Cognitive impairment is a core feature of several neuropsychiatric and neurological disorders, including narcolepsy and age-related dementias. Current pharmacotherapeutic approaches to cognitive enhancement are few in number and limited in efficacy. Thus, novel treatment strategies are needed. The hypothalamic orexin (hypocretin) system, a central integrator of physiological function, plays an important role in modulating cognition. Several single- and dual-orexin receptor antagonists are available for various clinical and preclinical applications, but the paucity of orexin agonists has limited the ability to research their therapeutic potential. To circumvent this hurdle, direct intranasal administration of orexin peptides is being investigated as a prospective treatment for cognitive dysfunction, narcolepsy or other disorders in which deficient orexin signaling has been implicated. Here, we describe the possible mechanisms and therapeutic potential of intranasal orexin delivery. Combined with the behavioral evidence that intranasal orexin-A administration improves cognitive function in narcoleptic and sleep-deprived subjects, our neurochemical studies in young and aged animals highlights the capacity for intranasal orexin administration to improve age-related deficits in neurotransmission. In summary, we highlight prior and original work from our lab and from others that provides a framework for the use of intranasal orexin peptides in treating cognitive dysfunction, especially as it relates to age-related cognitive disorders.
1. Introduction
Two decades have passed since the original discovery of the orexin/hypocretin system (de Lecea et al., 1998; Sakurai et al., 1998) and its subsequent characterization as a central integrator of physiological function. Two distinct neuropeptides, orexin-A/hypocretin-1 (OxA) and orexin-B/hypocretin-2 (OxB), arise from the precursor gene prepro-orexin and act upon two different G-protein coupled receptors. The OxA peptide binds to the orexin-1/hypocretin-1 receptor (OX1) and the orexin-2/hypocretin-2 (OX2) receptor with approximately equal affinity while the OxB peptide possesses a higher affinity for the OX2 receptor (Ammoun et al., 2003; Gotter et al., 2012; Leonard and Kukkonen, 2014; Smart and Jerman, 2002). Early evidence that orexin neurons act as a central hub for ‘physiological integration’ developed from anatomical studies highlighting the projections that arise from these neurons. While orexin/hypocretin neurons are exclusively confined to the lateral hypothalamus/perifornical area, their efferents are sent to a diverse set of brain regions located in cortical, limbic, and brainstem circuits (Peyron et al., 1998). Furthermore, these orexinergic neurons receive reciprocal afferents, largely from limbic regions (Sakurai et al., 2005; Yoshida et al., 2006). Together, this heterogeneous collection of neural circuits suggests that orexin neurons have a distinct capacity to regulate endocrine, autonomic, and behavioral responses to maintain homeostasis (Li et al., 2014).
1.1. Orexins modulate cognitive function
While the orexin/hypocretin system (hereafter referred to orexin for simplicity) is chiefly labeled as a ‘physiological integrator’, mounting evidence suggests that orexins may also modulate cognitive functions including attention, wakefulness/arousal, and learning and memory. Orexin modulation of cognitive function arises from multifarious interactions within telencephalic and hindbrain regions and their neurotransmitter systems. For example, orexins can modulate attentional function through connections with dopaminergic and noradrenergic systems of the ventral midbrain and locus coeruleus, respectively (Baldo et al., 2003; España et al., 2005; Fadel and Deutch, 2002; Horvath et al., 1999; Vittoz and Berridge, 2006). Furthermore, orexins can also facilitate attentional function through excitation of basal forebrain cholinergic neurons (Fadel and Burk, 2010; Zajo et al., 2016) and modulation of glutamatergic thalamocortical synapses (Huang et al., 2006; Lambe et al., 2005; Song et al., 2006) that ultimately alter prefrontal cortical release of acetylcholine and glutamate. Importantly, orexin mediated effects on cognition are not limited to attention as OxA has also been shown to alter long-term synaptic plasticity, a presumed correlate of learning and memory, through coordinated alterations of cholinergic, glutamatergic, GABAergic, and noradrenergic transmission within the hippocampus (Selbach et al., 2004; (Selbach et al., 2010; Yang et al., 2013). Indeed, the powerful effect of orexins on synaptic plasticity may underlie their role in persisting behavioral adaptations in a variety of contexts (Baimel and Borgland, 2017; Borgland et al., 2006).
1.2. Orexin/hypocretin function degenerates during aging
Because orexins play such a vital role in maintaining physiological homeostasis, dysregulation of the orexin system can result in a multitude of cognitive and behavioral deficits. This phenomenon is most clearly exemplified in narcolepsy, which is hallmarked by a selective loss of orexin neurons (Siegel, 1999; Thannickal et al., 2009). While narcoleptic symptomatology is classically defined by disruption in the sleep/wake cycle, heuristic observations of narcoleptic patients have shown that narcoleptics display additional cognitive dysfunction including deficits in sustained attention and olfactory discrimination (Bayard et al., 2010; Naumann et al., 2006; Rieger et al., 2003). Interestingly, narcoleptic patients also show subtle similarities in the cognitive deficits, namely deficits in attention and olfactory discrimination, that arise during aging and early Alzheimer’s disease (Hüttenbrink et al., 2013; Perry and Hodges, 1999; Sarter and Turchi, 2002; Wesson et al., 2010). The observed cognitive deficits in narcoleptics that arise from the loss of orexin neurons may also correlate to age-related cognitive dysfunction. Prior work from our lab and others has shown a selective age-related loss of orexin neurons and/or their peptides and receptors (Downs et al., 2007; Kessler et al., 2011; Porkka-Heiskanen et al., 2004; Sawai et al., 2010; Terao et al., 2002; Zhang et al., 2002). Moreover, recent post-mortem analysis of brains from patients with Alzheimer’s disease suggests a selective loss of orexin neurons (Davies et al., 2015; Fronczek et al., 2012). Collectively, these findings provide convincing evidence that orexins modulate the underlying neural substrates of cognition and suggest that orexin-based therapeutics may be useful in the treatment of age-related cognitive disorders.
1.3. Intranasal administration of orexins
The orexin system exerts a powerful influence over physiological and behavioral states by interacting with systems involved in sleep/wakefulness, energy homeostasis, addiction, stress responses, and cognition. These observations have given way to a substantial interest in developing therapeutic agents that target orexin receptors (Chieffi et al., 2017). To date, there are numerous selective and non-selective orexin receptor antagonists that have been developed (Gotter et al., 2012; Roecker et al., 2016; Skudlarek et al., 2017; Smart et al., 2001; Steiner et al., 2013). Conversely, selective orexin receptor agonists with preclinical or clinical efficacy are scarce (Mieda and Sakurai, 2013; Nagahara et al., 2015; Turku et al., 2017), ultimately leading to the use of orexin peptides as the agonists of choice. While early work in canine narcolepsy models suggested that systemic delivery of orexins could have therapeutic efficacy (Fujiki et al., 2003; John et al., 2000), concerns surrounding this route of administration include peripheral degradation, poor delivery across the blood-brain-barrier, and significant peripheral side effects (Dhuria et al., 2009; Hallschmid and Born, 2008; Kastin and Akerstrom, 1999). To circumvent these issues, intranasal administration has been proposed as a feasible treatment route to target orexins and various other neuropeptides to the CNS (Dhuria et al., 2010; Hanson and Frey, 2008; Lochhead and Thorne, 2012). Intranasal administration of neuropeptides provides several benefits over systemic administration including targeted delivery to the CNS, reduced peripheral complications, and complete bypass of the blood-brain-barrier (Hanson and Frey, 2008; Lochhead and Thorne, 2012; Meredith et al., 2015; Spetter and Hallschmid, 2015). The extent and time course of peptide delivery to the CNS depends on several factors such as peptide size, lipophilicity, and transportation methods from the olfactory mucosa into the brain (Dhuria et al., 2010; Lochhead and Thorne, 2012; Meredith et al., 2015; Spetter and Hallschmid, 2015). The mechanisms of intranasal orexin delivery to the CNS are not completely understood but available data suggest that proteins transported to the CNS start at olfactory and trigeminal nerve constituents of the nasal epithelium and proceed to the olfactory bulb and sensory/spinal trigeminal regions of the pons, the CNS origins of chemosensory and somatosensory innervation, respectively, of the nasal mucosa. Once in the CNS, peptides can then diffuse to various rostral and caudal brain regions (Lochhead and Thorne, 2012; Thorne et al., 2008, 2004). Brain penetration and distribution of peptides and proteins may be affected by multiple factors, including molecular weight, tertiary structure, lipophilicity and receptor localization. However, there are no broadly-applicable predictive models, emphasizing the importance of peptide-specific descriptions of distribution patterns in understanding behavioral and physiological effects of intranasal administration.
While these studies provide a powerful framework for the therapeutic potential of intranasal OxA, studies investigating the mechanisms responsible for these behavioral observations remain limited. Accordingly, we have recently performed neurochemical and anatomical studies using intranasal OxA in young rats to assess these mechanisms (Calva et al., 2017). These studies will be examined further in the proceeding results and discussion sections by comparing the effects of intranasal OxA administration on neuronal activation between young and aged animals. The available literature on intranasal orexin administration has primarily focused on the non-selective OxA neuropeptide; therefore, the contributions of each receptor to the aforementioned behavioral and neurochemical observations cannot be determined. The scarcity of in vivo studies using OxB administration stem from a multitude of reasons. These concerns are substantiated through work that shows limited diffusion of OxB across the blood-brain-barrier due to its low-lipophilic properties and rapid metabolic degradation by inactivating peptidases (Kastin and Akerstrom, 1999). The affinity of OxB for the OX2 receptor is roughly 10-fold higher than its affinity for the OX1 receptor (Sakurai et al., 1998), making it difficult to draw receptor-specific mechanistic conclusions about physiological or behavioral responses to OxB. The development of a modified OxB peptide, [Ala11,D-Leu15]-OxB, with a reported 400-fold higher affinity for the OX2 receptor vs. the the OX1 receptor (Asahi et al., 2003) may offer a more selective tool for dissecting relative contributions of the orexin receptors to orexin peptide effects on a variety of physiological functions (but see (Putula et al., 2011) for caveats surrounding the relative selectivity and potency of this compound for the different orexin receptors in vitro). Here, we utilized immunohistochemistry to directly study the effects of intranasal [Ala11,D-Leu15]-OxB administration on measures of neuronal activation in young rats. Our studies discussed below include novel data from young animals treated with [Ala11,D-Leu15]-OxB and comparisons of the neuronal activation patterns in young and aged animals that result from intranasal administration of OxA or [Ala11,D-Leu15]-OxB. The ultimate goal of these comparative analyses was to gain further insight into potential orexin receptor-mediated effects on neuronal activation that may underlie the neurochemical and behavioral observations after intranasal orexin administration.
2. Results
2.1. Effects of intranasal [Ala11,D-Leu15]-OxB on c-Fos expression
Intranasal [Ala11,D-Leu15]-OxB administration increased neuronal activation (c-Fos expression) in cortical and basal forebrain regions (Fig. 1A). In the cortex, intranasal [Ala11,D-Leu15]-OxB administration significantly increased activation in the piriform cortex (t12 = 3.224, p = 0.0073) and the agranular insular cortex (t12 = 2.519, p = 0.0269) when compared to intranasal saline treated animals. In addition, there a was a strong trend for increased activation in the prelimbic cortex (t12 = 2.033, p = 0.0647). In the basal forebrain, intranasal [Ala11,D-Leu15]-OxB administration significantly increased c-Fos expression within the nucleus basalis/substantia innominata (t12 = 3.663, p = 0.0032) compared to intranasal saline treatment. Density measurements for c-Fos were obtained using a 0.032 mm2 area within cortical regions and a 0.1225 mm2 area within basal forebrain regions. We also stained cells in the cortex for parvalbumin (PV), a marker for fast-spiking GABAergic interneurons (Hu et al., 2014), to determine effects of intranasal [Ala11,D-Leu15]-OxB on specific neuronal populations in the cortex. Intranasal [Ala11,D-Leu15]-OxB administration did not significantly alter c-Fos expression in PV+ cells within any of the brain regions analyzed (data not shown).
Fig. 1.
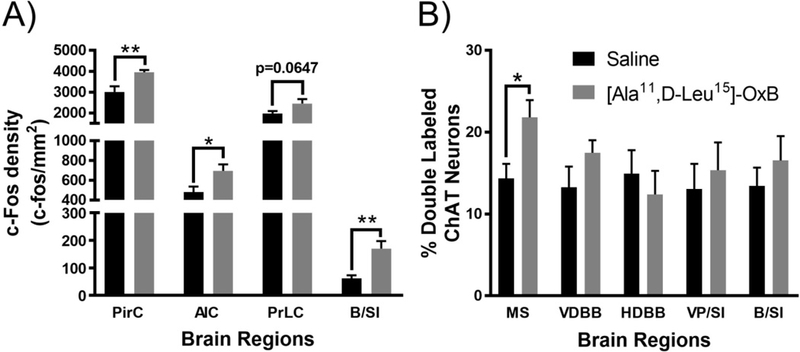
Neuronal activation (c-Fos expression density) in cortical and basal forebrain regions following intranasal [Ala11,D-Leu15]-OxB administration in young rats. (A) Single-labeled c-Fos densities in brain regions of animals treated with intranasal vehicle (saline; PirC, AIC, PrLC, B/SI, n=7 rats) or intranasal [Ala11,D-Leu15]-OxB (50 μL, 100 µM; PirC, AIC, PrLC, B/SI, n=7 rats). Intranasal [Ala11,D-Leu15]-OxB administration significantly increased c-Fos expression within the PirC, AIC, and B/SI regions compared to vehicle treated controls. There was a strong trend for increased c-Fos expression within the PrLC after intranasal [Ala11,D-Leu15]-OxB administration. Percentage of ChAT-positive neurons with c-Fos-positive nuclei within the MS, VDBB, HDBB, VP/SI, and B/SI after intranasal [Ala11,D-Leu15]-OxB (all brain regions, n=7) or intranasal vehicle (all brain regions, n=7) administration. Treatment with intranasal [Ala11,D-Leu15]-OxB significantly increased c-Fos expression within ChAT-positive neurons of the MS compared to intranasal vehicle administration. Abbreviations: Ala, Alanine; D-Leu, D-Leucine; OxB, orexin-B; PirC, piriform cortex; AIC, agranular insular cortex; B/SI, nucleus basalis/substantia innominata; PrLC, prelimbic prefrontal cortex; ChAT, choline acetyltransferase; MS, medial septum; VDBB, vertical limb of the diagonal band of Broca; HDBB, horizontal limb of the diagonal band of Broca; VP/SI, ventral pallidum/substantia innominata. Error bars represent SEM. **p < 0.01, *p < 0.05
2.1.1. Effects of intranasal [Ala11,D-Leu15]-OxB on basal forebrain cholinergic neurons
Our previous work in young animals utilized intranasal administration of the OxA peptide (Calva et al., 2017); therefore, the receptor mechanisms by which intranasal orexin administration may activate basal cholinergic neurons remains unresolved. Accordingly, we examined the effects of intranasal [Ala11,D-Leu15]-OxB administration, a selective OX2 receptor agonist, on c-Fos expression within cholinergic (ChAT+) neurons of the basal forebrain (Fig. 1B). Ultimately, we discovered that intranasal [Ala11,D-Leu15]-OxB significantly increased c-Fos expression within cholinergic neurons of the medial septum compared to intranasal saline administration (t12 = 2.704, p = 0.0192). Intranasal [Ala11,D-Leu15]-OxB administration did not significantly alter activation of cholinergic neurons within any other subdivision of the basal forebrain.
2.2. Comparative effects of intranasal orexin administration across treatment and age
In addition to using intranasal [Ala11,D-Leu15]-OxB administration in young animals, we have combined intranasal OxA administration and immunoperoxidase staining for c-Fos in aged animals. To compare the effects aging and intranasal orexin administration (i.e. OxA or [Ala11,D-Leu15]-OxB) in multiple brain regions, we constructed a heatmap using c-Fos expression ratios that were normalized to intranasal saline treated controls (Fig. 2). The range of ratios that we observed fell between 0.4 and 3.8, with lower scores lighter in color and higher scores darker in color. Scores above 1 indicate higher c-Fos expression when compared to saline treated controls and vice-versa for scores below 1. Accordingly, YOA/YS and YOB/YS groups qualitatively suggest that intranasal OxA and intranasal [Ala11,D-Leu15]-OxB administration increases c-Fos expression in young animals. Further statistical analysis on these differences from prior intranasal OxA studies and from our data presented above (Fig. 2) show that significant differences in c-Fos expression are localized to specific brain regions. Specifically, in the cortex of young animals, intranasal OxA significantly increased c-Fos expression in the piriform, agranular insular, prelimbic, and ventral orbital cortices (Calva et al., 2017). Significant increases in cortical c-Fos expression after intranasal [Ala11,D-Leu15]-OxB were limited to the piriform and agranular insular cortices. In addition, the higher ratios present in the AS/YS group suggests that aged animals exhibit higher basal levels of activation. This is confirmed with one-way ANOVA analysis of c-Fos expression in basal forebrain (BF) cholinergic neurons of young and aged animals treated with intranasal OxA (F3,28 = 27.67, p < 0.0001). Further analysis with Tukey’s multiple comparisons test revealed that intranasal OxA increased c-Fos expression in BF ChAT+ neurons of young (q28 = 6.339, p =0.0006) and aged animals (q28 = 7.532, p <0.0001). Finally, when compared to young animals, aged animals showed higher basal (q28 = 5.281, p = 0.0045) and intranasal OxA induced (q28 = 6.474, p = 0.0005) levels of c-Fos expression (Fig. 3).
Fig. 2.
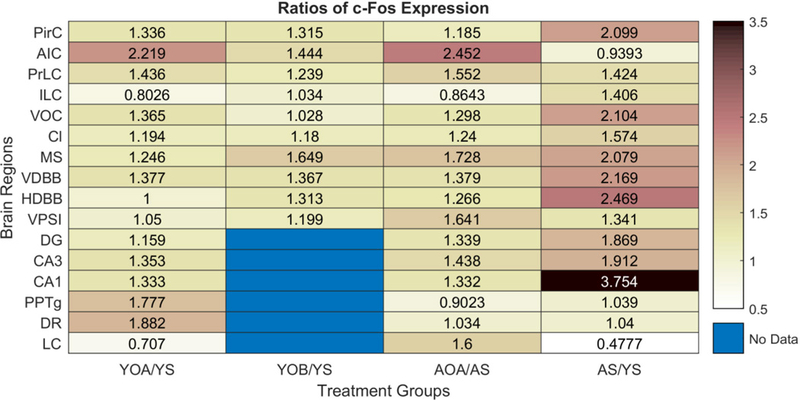
Heatmap of c-Fos expression ratios between treatment groups and age compared to young saline treated animals. The treatment groups represented in the heat map are as follows: 1) YOA/YS (YOA and YS, n=8 rats), 2) YOB/YS (YOB and YS, n=7 rats), 3) AOA/AS (AOA and AS, n=8 rats), and 4) AS/YS (AS, n=8 rats; YS, n=15 rats). The YOA/YS and AOA/AS treatment comparisons largely showed similar patterns of c-Fos expression ratios. On average, the effects of OxA treatment in aged animals were more robust than the OxA treatment in young animals. Aged animals also exhibited higher ‘basal’ c-Fos expression in most brain regions compared to young animals, as indicated by the AS/YS treatment comparison. When comparing the effects between the OxA and [Ala11,D-Leu15]-OxB treatments (i.e. YOA/YS vs. YOB/YS), the YOA/YS group shows higher c-Fos expression ratios in most brain regions. Abbreviations: YOA/YS, young-orexin-A vs. young saline; YOB/YS, young-[Ala11,D-Leu15]-orexin-B vs. young saline; AOA/AS, aged-orexin-A vs aged saline; AS/YS, aged saline vs. young saline; OxA, orexin-A; [Ala11,D-Leu15]-OxB, [Ala11,D-Leu15]-orexin-B; PirC, piriform cortex; AIC, agranular insular cortex; PrLC, prelimbic cortex; ILC, infralimbic cortex; VOC, ventral orbital cortex; Cl, claustrum; MS, medial septum; VDBB, vertical limb of the diagonal band of Broca; HDBB, horizontal limb of the diabonal band of Broca; VP/SI, ventral pallidum/substantia innominata; DG, dentate gyrus; CA3, cornu ammonis 3; CA1, cornu ammonis 1; PPTg, pedunculopontine tegmentum; DR, dorsal raphe; LC, locus coeruleus.
Fig. 3.
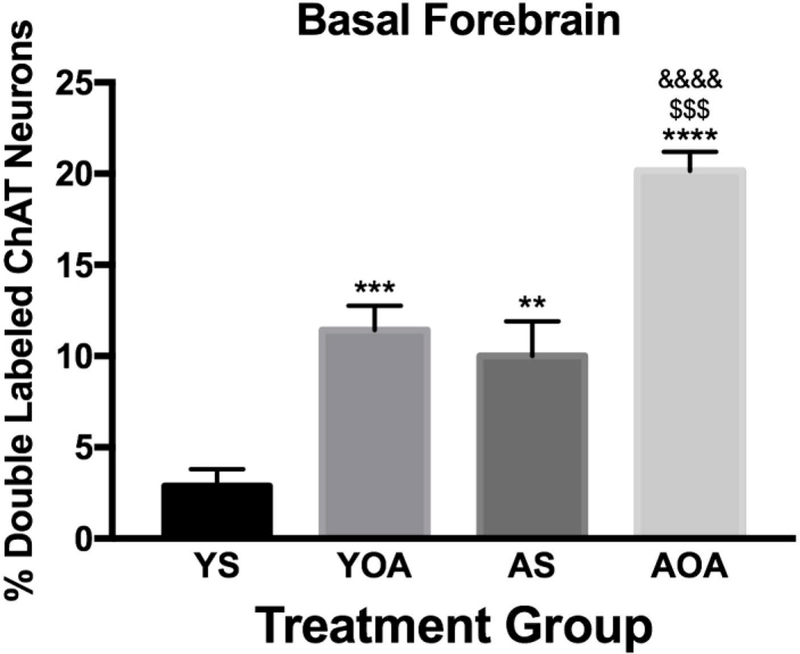
Neuronal activation (c-Fos expression density) in cholinergic neurons across all basal forebrain regions in young and aged rats administered intranasal OxA (n=8 rats) or intranasal vehicle (saline; n=8 rats). Data are represented as the percentage of double-labeled (c-Fos/ChAT) neurons relative to the total number of ChAT-positive neurons within the basal forebrain. In both young and aged animals, intranasal OxA significantly increased activation of ChAT-positive neurons of the basal forebrain. Aged animals treated with intranasal vehicle exhibited a significantly higher percentage of double-labeled neurons (c-Fos/ChAT) in the basal forebrain compared to young animals treated with vehicle. Aged animals treated with intranasal OxA exhibited a significantly higher percentage of double-labeled neurons (c-Fos/ChAT) in the basal forebrain compared to young animals treated with intranasal OxA. Treatment comparisons: ***= YS vs. YOA, **= YS vs. AS, ****= YS vs. AOA, $$$= YOA vs. AOA, &&&&= AS vs. AOA. Abbreviations: OxA, orexin-A; ChAT, choline acetyltransferase; YS, young saline; YOA, young-orexin-A; AS, aged saline; AOA, aged-orexin-A. Error bars represent SEM. **** and &&&&= p < 0.0001, *** and $$$= p < 0.001, **= p < 0.01
3. Discussion
These studies, combined with our previous studies using intranasal OxA administration in young animals highlight that intranasal orexins selectively increase neuronal activation in distinct cortical, basal forebrain, and brainstem regions. While intranasal [Ala11,D-Leu15]-OxB selectively activates the piriform and agranular insular cortices, intranasal OxA activates a broader range of cortical regions including the prelimbic and ventral orbital cortices. These studies also demonstrate that intranasal OxA activates basal forebrain cholinergic neurons in young and aged animals, which suggest a capacity for intranasal OxA to modulate cholinergic neurotransmission across the lifespan. Our understanding of the effects of intranasal orexin administration on neurotransmission and cognition will continue to evolve with continuing studies in young animals treated with intranasal [Ala11,D-Leu15]-OxB and aged animals treated with intranasal OxA.
3.1. Effects of intranasal orexin administration on cortical activation
Orexin neurons send widespread projections to the cortex that modulate various aspects of cognition, especially those related to attentional function. Correspondingly, intranasal [Ala11,D-Leu15]-OxB and intranasal OxA (Calva et al., 2017) increased c-Fos expression in the agranular insular cortex, a brain region that facilitates interoceptive attention to an organism’s physiological status (Avery et al., 2017; Craig and Craig, 2002; Hassanpour et al., 2017). Orexin’s actions in the insular cortex may help promote appropriate behavioral responses to homeostatic challenges, consistent with orexin’s role as a physiological integrator. Indeed, we have previously shown that modulation of orexin expression alters both behavioral and insular cortical cholinergic responses to food-paired stimuli in food-restricted animals (Hagar et al., 2017).
Increased c-Fos expression, after intranasal OxA or [Ala11,D-Leu15]-OxB, was also observed in the piriform cortex, suggesting that activation of this brain region is primarily mediated through the OX2 receptor. This is consistent with OX receptor mRNA expression patterns in the rat piriform cortex that indicate the selective presence of the OX2 receptor (Marcus et al., 2001). The piriform cortex plays an important role in olfactory discrimination (Bekkers and Suzuki, 2013; Stettler and Axel, 2009). Interestingly, olfactory dysfunction occurs during aging and age-related cognitive disorders (Enwere, 2004; Kovács, 2004; Mobley et al., 2014), and may serve as an early predictor for Alzheimer’s disease (Djordjevic et al., 2008; Hüttenbrink et al., 2013; Sohrabi et al., 2012). Orexin modulation of olfactory function has been demonstrated by studies that show i.c.v. administration of OxA enhances olfactory sensitivity to odors (Julliard et al., 2007; Prud’homme et al., 2009). Together, these findings suggest that OX2 receptor mediated activation of the piriform cortex by OxA or [Ala11,D-Leu15]-OxB may serve to enhance odor discrimination and olfactory function within the piriform cortex.
Orexin neurons also densely innervate the PFC where they modulate neurotransmission related to attentional processing (Fadel et al., 2005; Huang et al., 2006; Vittoz and Berridge, 2006; Zajo et al., 2016). Our prior intranasal studies indicate that intranasal OxA increases glutamatergic and cholinergic neurotransmission within the PFC of young rats (Calva et al., 2017), suggesting that intranasal OxA enhances attentional processing in the PFC. Though we observed a strong trend for increased c-Fos expression in the prelimbic PFC after intranasal [Ala11,D-Leu15]-OxB administration, our overall observations indicate that orexin-mediated enhancement of attentional function may primarily occur via the OX1 receptor. The extent to which intranasal orexin administration modulates neurotransmission and cognitive function in aged animals remains unknown.
3.2. Effects of intranasal orexin administration on basal forebrain neurotransmission
3.2.1. Effects of intranasal orexin administration on GABAergic transmission in the basal forebrain
Our previous observ ations indicate that intranasal OxA administration decreases activation of fast-spiking PV+ GABAergic interneurons in the PFC (Calva et al., 2017). These cells are the principal interneuron phenotype within the cortex and function to gate the firing of cortical pyramidal neurons (Hu et al., 2014; Kawaguchi and Kubota, 1997; Kelsom and Lu, 2013; Kim et al., 2015; Sohal et al., 2009; Xu et al., 2010). While the mechanisms driving this inhibition remain unknown, one possibility is through inhibition from basal forebrain PV+ projection neurons that preferentially synapse onto PV+ cortical interneurons (Freund and Meskenaite, 1992; Henny and Jones, 2008). Anatomical evidence suggests that the large majority of these PV+ basal forebrain neurons are GABAergic (Gritti et al., 2003; Mckenna et al., 2013). Functionally, these PV+ basal forebrain projection neurons regulate cortical gamma band oscillations (Kim et al., 2015), a putative electrophysiological correlate of attention and feature binding (Gray and Singer, 1989; Tiitinen et al., 1997). Behavioral and electrophysiological evidence suggests that activation of basal forebrain PV+ neurons is mediated primarily through the OX2 receptor (Mieda et al., 2011; Wu et al., 2002). Given the prominent role of the orexin system modulating arousal/wakefulness (Jones, 2008; Sakurai, 2002), intranasal orexins may ultimately regulate cortical activation through modulation of these PV+ neurons in the basal forebrain.
3.2.2. Effects of intranasal orexin administration on cholinergic transmission in the basal forebrain
Orexin neurons are anatomically and functionally positioned to modulate cholinergic neurotransmission. Specifically, orexin neurons modulate the basal forebrain cholinergic system (BFCS), the primary source of cholinergic neurotransmission to the cortex (Fadel and Burk, 2010; Villano et al., 2017). Several studies illustrate interactions between OxA and the basal forebrain. In particular, infusion of OxA directly into the basal forebrain modulates cholinergic-dependent attentional processing and potently increases cortical ACh release (Fadel et al., 2005; Zajo et al., 2016). Furthermore, intranasal OxA activates cholinergic neurons of the ventral pallidum/substantia innominata and vertical limb of the diagonal band, and increases cholinergic transmission in the PFC (Calva et al., 2017). In contrast, intranasal administration of [Ala11,D-Leu15]-OxB selectively activates cholinergic neurons of the medial septum (Fig. 1B). This pattern of activation is consistent with in-situ evidence in rats that describes the selective presence of the OX2 receptor in the medial septum (Marcus et al., 2001). As described above, intranasal OxA administration also enhances activation of cholinergic neurons in the basal forebrain of aged animals, indicating that intranasal OxA may be a viable therapeutic for treating age-related deficits in neurotransmission. Accordingly, we are investigating the effects of intranasal OxA administration on neurotransmission in aged animals.
3.3. Mechanisms of intranasal orexin administration
The OxA neuropeptide exerts equal affinity for both orexin receptor subtypes; therefore, the neurochemical and behavioral observations surrounding this neuropeptide cannot be attributed to one specific receptor. In addition, because of caveats surrounding the use of G-protein-coupled receptor agonists (e.g., differences in penetrance, unknown brain concentrations with systemic administration, etc.) it is difficult to say definitively what the receptor mechanisms are that mediate single-dose in vivo responses. However, the available evidence suggests that our effects following intranasal orexin are primarily mediated via the OX1 receptor, particularly those involving the basal forebrain cholinergic system. For example, systemic or intrabasalis administration of the specific OX1 receptor antagonist SB-334867 attenuates ACh release that is induced during feeding (Frederick-Duus et al., 2007). Additionally, OxA administration into the basal forebrain results in greater ACh release in the somatosensory cortex compared to OxB (Dong et al., 2006). Furthermore, the observations described above indicate that intranasal administration of the OX2 receptor agonist [Ala11,D-Leu15]-OxB results in the activation of fewer brain regions than intranasal OxA. Nevertheless, the OX2 receptor likely plays an important role in various aspects of orexin mediated neurotransmission. For example, we observed that medial septal cholinergic neurons were selectively activated by [Ala11,D-Leu15]-OxB (Fig. 1B). Furthermore, previous evidence indicates that orexins likely mediate activation of BF PV+ neurons through the OX2 receptor (Mieda et al., 2011; Wu et al., 2002). Intriguingly, in-vitro evidence indicates that BF cholinergic neurons potently excite BF PV+ and other GABAergic neurons (Yang et al., 2014), suggesting that cholinergic and GABAergic systems in the BF work in tandem to modulate cortical activity. Because intranasal OxA and [Ala11,D-Leu15]-OxB affect distinct cholinergic and GABAergic systems, these evidence indicate that intranasal orexins facilitate cognition, in part, through coordinated activation of cholinergic and GABAergic neurotransmission in the basal forebrain. These putative mechanisms that underlie the behavioral and neurochemical correlates of intranasal orexin administration are outlined in a summary figure (Fig. 4).
Fig. 4.
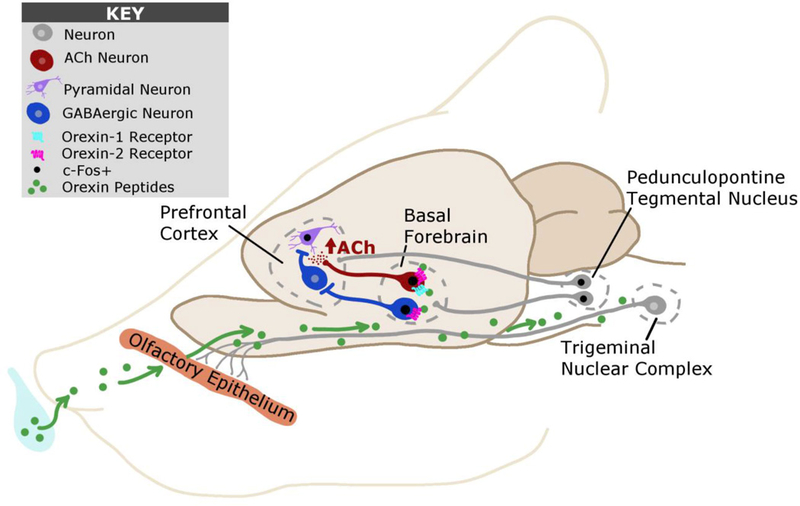
Putative mechanisms underlying intranasal orexin entry and action within the brain. Orexins administered via the intranasal route are hypothesized to enter the brain via two main mechanisms: 1) diffusion across the olfactory epithelium into olfactory and rostral brain areas and 2) extra-axonal diffusion along trigeminal sensory pathways into brainstem regions. After accessing the CNS, our data suggests that orexins activate basal forebrain cholinergic neurons via the orexin-1 or orexin-2 receptor. Excitation of these neurons by orexins ultimately increases acetylcholine efflux within the prefrontal cortex, a putative neurochemical correlate of attention. We also observed that intranasal orexin-A administration increases neuronal activation of excitatory pyramidal neurons and decreases neuronal activation of inhibitory parvalbumin-positive GABAergic interneurons within the prefrontal cortex. This dichotomy may ultimately arise from orexin-2 receptor mediated excitation of parvalbumin-positive GABAergic neurons within the basal forebrain. These inhibitory projections neurons of the basal forebrain preferentially synapse onto cortical GABAergic interneurons. Finally, we observed that intranasal orexin-A administration can activate brainstem neurons of the pedunculopontine tegmental nucleus, which may also modulate activity within the basal forebrain and/or cortex.
3.4. Therapeutic implications of intranasal orexin administration
Accumulating behavioral evidence from both animals and humans suggests that intranasal OxA administration may be useful in treating a variety of cognitive disorders. Recent rodent studies have demonstrated that intranasal OxA administration increases locomotion and food intake (Dhuria et al., 2016). Additionally, intranasal OxA administration in sleep deprived rhesus macaque monkeys improves performance in a short-term memory task and alters local cerebral glucose metabolism (Deadwyler et al., 2007). Of clinical significance, intranasal OxA administration in patients with narcolepsy has been shown to decrease the number of spontaneous wake-REM sleep transitions, improve deficits in olfactory acuity, and enhance divided attention (Baier et al., 2011, 2008; S L Weinhold et al., 2014). The subtle similarities in the cognitive deficits between narcolepsy and some forms of age-related cognitive decline, especially deficits in attention, hint at the involvement of the orexin system (Hüttenbrink et al., 2013; Perry and Hodges, 1999; Sarter and Turchi, 2002; Wesson et al., 2010). Indeed, aged animal models are associated with a reduction in orexin neurons and/or neuropeptide expression (Kessler et al., 2011; Porkka-Heiskanen et al., 2004; Terao et al., 2002). Furthermore, post-mortem examination of brains of patients with Alzheimer’s disease or dementia with Lewy bodies reveals a reduced number of orexin neurons (Fronczek et al., 2012; Kasanuki et al., 2014).
The neuronal and pharmacological mechanisms underlying the effect of orexins on cognitive function remain to be fully elucidated. Advancements may include peptide or vehicle modifications that enhance brain penetrance, such as inclusion of cyclodextrin compounds to promote bioavailability of intranasally-administered proteins and peptides (Meredith et al., 2015). Development of novel ligands such as the non-peptide OX2 receptor agonist YNT-185, which has recently shown promise in murine narcolepsy models (Irukayama-Tomobe et al., 2017; Takenoshita et al., 2018), will also facilitate advancements in this field. It will also be important to more fully examine and clarify potential negative effects of intranasal orexin on disease processes such as amyloid plaque formation (Kang et al., 2009; Liguori, 2017) prior to clinical implementation. Nonetheless, as described above intranasal orexin administration rapidly enters the brain and targets brain regions and neurotransmitter systems that mediate proper cognitive functioning. Ultimately these studies, combined with our ongoing work in aged animals, may provide mechanistic evidence for the therapeutic potential of intranasal orexin administration in treating cognitive dysfunction.
4. Experimental Procedures
4.1. Animals
Experimental methods, materials, and procedures were generally as described in our previous work examining intranasal OxA administration in young animals (Calva et al., 2017). Young (3–4 months, 250–300g) and aged (26–28 months, 450–550g) male Fisher 344/Brown Norway F1 hybrid rats (Harlan/NIA) were used for all experiments. This rat strain is used extensively for aging studies due to their reduced susceptibility to several peripheral age-related complications (e.g., intraperitoneal tumors) that are commonly observed in other strains during late life (Lipman et al., 1996; Turturro et al., 1999). Therefore, we utilized the Fisher 344/Brown Norway F1 hybrid strain in order to compare the effects of intranasal orexin administration on the neurobiological systems, including neurotransmission and neuronal activation, that change during aging. Furthermore, as a proof of concept, this strain has previously been utilized in our lab to study orexin-aging interactions (Hagar et al., 2017; Kessler et al., 2011; Stanley et al., 2012; Stanley and Fadel, 2011). Animals were kept on a 12:12 light: dark cycle (lights on at 07:00 hours) and provided ad libitum access to standard rat chow and water. All experiments commenced during the light phase of the light: dark cycle. Animal care and use practices were all performed within protocols written under the guidelines of the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of South Carolina (Animal Use Protocol #2409). All experiments were performed by the first author. In lieu of performing power analyses to determine group sizes, prior studies from our lab using comparable numbers of animals were referenced to obtain the appropriate sample sizes for each experiment. The experimenter performing intranasal administration was not blinded to the treatment condition during experimentation.
4.2. Immunohistochemistry and imaging
Upon arrival, both young and aged animals were assigned to receive intranasal administration of either vehicle (50μL of 0.9% saline) or OxA (50μL of a 100μM solution; Enzo Life Sciences, Farmingdale, NY, USA). Each animal received several days of gentle handling and habituation to intranasal saline administration prior to the test day. Briefly, each animal was loosely blanketed with a small cloth and held in a supine position so that only the animal’s snout was protruding from a small opening. No restraint or anesthesia was used during intranasal habituation or treatment. On the test day, each rat was administered 50μL of saline or OxA. Intranasal administration of the total 50μL volume was delivered in 12.5μL increments into alternating nares over a total of 2–3 minutes. All solutions were administered in four 12.5 μL portions (a total of 25 µL in each naris) over a 2-minute period. A separate set of young animals were assigned to receive either vehicle or a modified OxB peptide (50μL of a 100μM solution) serving as a potent and selective OX2 receptor agonist ([Ala11,D-Leu15]-OxB; Tocris Bioscience; Minneapolis, MN, USA). While rat-to-human conversion of doses holds many caveats, based on conversion factors suggested by Nair and Jacob (Nair and Jacob, 2016) our rat dose of 5 nmol would roughly equate to a human dose of 200 nmol. While we are not aware of any papers describing intranasal administration of [Ala11,D-Leu15]-OxB in humans, Weinhold et al. (Weinhold et al., 2014) observed enhanced wakefulness and attention in human narcoleptic patients following intranasal administration of 435 nmol OXA. Thus, given the limited comparative information available, our orexin doses seems reasonable.
Treatment group assignment for all immunohistochemistry experiments was pseudorandomized for each batch of animals such that treatment order (i.e. intranasal saline or intranasal orexin) was counterbalanced and equally represented for each batch. All animals were habituated to intranasal saline for at least 7 days prior to the treatment day. Starting on day 8, animals received their designated treatment and subsequently sacrificed under heavy isoflurane anesthesia and perfused with phosphate buffered saline and 4% paraformaldehyde 2 hours post-administration to observe optimal c-Fos expression (Kaczmarek, 1992). After 24-hour post-fixation, the brains were sectioned on a vibratome coronally at a 50 μm thickness using a 1:4 serial sectioning method. Sections not immediately used for immunohistochemistry were stored in 30% sucrose/30% ethylene glycol anti-freezing solution at −20°C until use. Single and dual-label immunohistochemistry followed similar protocols, where free-floating sections were incubated with a rabbit anti-c-Fos primary antibody (1:5000; Millipore, Billerica, MA, USA; catalog No. ABE457; RRID AB_2631318) followed by a biotinylated donkey anti-rabbit secondary antibody (1:1000; Jackson ImmunoResearch Laboratories Inc.; West Grove, PA, USA; code No. 711–065-152; RRID AB_2340593) and a horseradish peroxidase conjugated streptavidin tertiary antibody (1:1600; Jackson ImmunoResearch Laboratories Inc.; code No. 016–030-084; RRID AB_2337238). Staining for c-Fos was developed with 0.3% hydrogen peroxide and nickel-cobalt enhanced diaminobenzidine (DAB) to yield blue-black immunopositive nuclei. Dual-label staining for either choline acetyltransferase (ChAT) or parvalbumin (PV) used c-Fos stained sections that were subsequently incubated in either a goat anti-ChAT (1:3000; Millipore, Temecula, CA, USA; catalog No. AB144; RRID AB_90650) or a mouse anti-PV (1:4000; Sigma, St. Louis, MO, USA; catalog No. P3088; RRID AB_477329) primary antibody. Secondary and tertiary steps followed with incubations in either an unlabeled donkey anti-goat (1:200; Jackson ImmunoResearch Laboratories Inc.; code No. 705–005-003; RRID AB_2340384) or an unlabeled donkey anti-mouse (1:200; Jackson ImmunoResearch Laboratories Inc.; code No. 715–005-150; RRID AB_2340759) secondary antibody, followed by incubations in either a goat peroxidase anti-peroxidase (1:500; Jackson ImmunoResearch Laboratories Inc.; code No. 123–005-024; RRID AB_2338953) or a mouse peroxidase anti-peroxidase (1:500; Jackson ImmunoResearch Laboratories Inc.; code No. 223–005-024; RRID AB_2339261) tertiary antibody. Immunostaining for ChAT or PV were developed with 3% hydrogen peroxide and DAB to yield brown immunopositive cell bodies. Using a 0.15% gelatin solution, sections were mounted onto slides and allowed to dry overnight before dehydration, delipidation, and cover-slipping with DEPEX mounting medium. Histological imaging for the single-label (c-Fos) and dual-label (c-Fos + ChAT/PV) immunoperoxidase experiments were visualized using a Nikon E600 microscope fitted with a CoolSNAP digital camera (Roper Scientific, Trenton, NJ, USA) and IP Lab software (Scanalytics, Trenton, NJ, USA). During quantitative analysis of immunoperoxidase staining, experimenters were unaware of the treatment group of each animal. Images were imported into Adobe Photoshop 6.0 (Adobe Systems, San Jose, CA, USA) for minor alterations to contrast and brightness. Brain regions where photomicrographs were obtained are indicated through modified illustrations from the third edition of The Rat Brain Atlas (Paxinos and Watson, 1998).
4.3. c-Fos heatmap
A comparative heat map (Fig. 2) was generated utilizing single-label c-Fos data in order to visualize differences in region-specific neuronal activation between intranasal treatment groups (i.e. saline, OxA, or [Ala11,D-Leu15]-OxB) across young and aged animals. The treatment groups used for producing the heat map were as follows: 1) Young saline (YS), 2) Young-OxA (YOA), 3) Young [Ala11,D-Leu15]-OxB (YOB), 4) Aged saline (AS), and 5) Aged OxA (AOA). For each brain region mapped, the data were computed across the total number of animals for each group with a minimum of n=7 animals per treatment group. The scaled colorimetric data within the heat map is represented as the ratio of average c-Fos densities for each brain region between the different treatment groups. All treatment groups were normalized to saline groups to yield the resulting heat map conditions: 1) YOA/YS, 2) YOB/YS, 3) AOA/AS, 4) AS/YS. The YOA and YOB experiments were performed at different time points; therefore, these groups were normalized using the corresponding YS group. In contrast, the AS/YS ratios was calculated using the from the average c-Fos densities pooled across both YS groups. Data calculations and analyses for the heat map were performed using Microsoft Excel 2016 for Macintosh (Microsoft Corporation, Redmond, WA, USA). The data was then imported into MATLAB R2018a (MathWorks Inc., Natick, MA, USA) for generation of the colorimetric heat map.
4.4. Statistics and data analysis
For all immunohistochemistry experiments, single-labeled (c-Fos) and double-labeled (c-Fos + ChAT) positive cells were counted within the confines of a reticle fixed into the eyepiece of the microscope. Counts for each brain region was determined by the total number of immunopositive nuclei/cells from two representative sections at different levels of the rostro-caudal gradient. Single-label c-Fos data were expressed as the density of immunopositive nuclei counted within the reticle area (c-Fos nuclei/mm2). Statistical analyses of these data were analyzed by two-tailed unpaired t-tests (GraphPad Prism 7; GraphPad Software for Macintosh, La Jolla, CA, USA). Double-labeled neurons were expressed as the percentage of the total number of ChAT neurons positive for c-Fos within the reticle area (i.e. % Double Labeled Neurons). Dual-label immunoperoxidase data were analyzed by two-tailed unpaired t-tests. Significant effects of treatment condition (i.e., OxA or saline) across age were determined by one-way ANOVA followed by Tukey’s multiple comparisons test. A significance cutoff of p < 0.05 was used for all statistical measures.
Deficient orexin signaling has been implicated in several neuropsychiatric conditions, including age-related cognitive decline and narcolepsy
Intranasal orexin rapidly targets and activates brain regions and neurotransmitter systems implicated in cognitive function
These effects appear to be predominantly, but not exclusively, mediated by the orexin-1 receptor
Intranasal orexin may represent an effective, non-invasive means of enhancing cognitive function
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01AG050518; JRF) and the University of South Carolina SPARC Graduate Research Program (CBC). The authors thank Habiba Fayyaz for technical assistance and Dr. Victoria Macht for valuable assistance with artwork.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, Kukkonen JP, 2003. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther 305, 507–514. [DOI] [PubMed] [Google Scholar]
- Asahi S, Egashira SI, Matsuda M, Iwaasa H, Kanatani A, Ohkubo M, Ihara M, Morishima H, 2003. Development of an orexin-2 receptor selective agonist, [Ala11, D-Leu15]orexin-B. Bioorganic Med. Chem. Lett 13, 111–113. 10.1016/S0960-894X(02)00851-X [DOI] [PubMed] [Google Scholar]
- Avery JA, Gotts SJ, Kerr KL, Burrows K, Ingeholm JE, Bodurka J, Martin A, Kyle Simmons W, 2017. Convergent gustatory and viscerosensory processing in the human dorsal mid-insula. Hum. Brain Mapp 38, 2150–2164. 10.1002/hbm.23510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier PC, Hallschmid M, Seeck-Hirschner M, Weinhold SL, Burkert S, Diessner N, Göder R, Aldenhoff JB, Hinze-Selch D, 2011. Effects of intranasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexy. Sleep Med 12, 941–946. 10.1016/j.sleep.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Baier PC, Weinhold SL, Huth V, Gottwald B, Ferstl R, Hinze-Selch D, 2008. Olfactory dysfunction in patients with narcolepsy with cataplexy is restored by intranasal Orexin A (Hypocretin-1). Brain 131, 2734–2741. 10.1093/brain/awn193 [DOI] [PubMed] [Google Scholar]
- Baimel C, Borgland SL, 2017. Hypocretin/Orexin and Plastic Adaptations Associated with Drug Abuse. Curr Top Behav Neurosci 33, 283–304. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE, 2003. Overlapping distributions of orexin/hypocretin- and dopamine-??-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J. Comp. Neurol 464, 220–237. 10.1002/cne.10783 [DOI] [PubMed] [Google Scholar]
- Bayard S, Plazzi G, Poli F, Serra L, Ferri R, Dauvilliers Y, 2010. Olfactory dysfunction in narcolepsy with cataplexy. Sleep Med 11, 876–881. 10.1016/j.sleep.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Suzuki N, 2013. Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci 10.1016/j.tins.2013.04.005 [DOI] [PubMed]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A, 2006. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49, 589–601. [DOI] [PubMed] [Google Scholar]
- Calva CB, Fayyaz H, Fadel JR, 2017. Increased acetylcholine and glutamate efflux in the prefrontal cortex following intranasal orexin-A (hypocretin-1). J. Neurochem 10.1111/jnc.14279 [DOI] [PMC free article] [PubMed]
- Chieffi S, Carotenuto M, Monda V, Valenzano A, Villano I, Precenzano F, Tafuri D, Salerno M, Filippi N, Nuccio F, Ruberto M, Luca V. De, Cipolloni L, Cibelli G, Mollica MP, Iacono D, Nigro E, Monda M, Messina G, Messina1 A, 2017. Orexin system: The key for a healthy life. Front. Neurol 10.3389/fphys.2017.00357 [DOI] [PMC free article] [PubMed]
- Craig a. D., Craig a. D., 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3, 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Davies J, Chen J, Pink R, Carter D, Saunders N, Sotiriadis G, Bai B, Pan Y, Howlett D, Payne A, Randeva H, Karteris E, 2015. Orexin receptors exert a neuroprotective effect in Alzheimer’s disease (AD) via heterodimerization with GPR103. Sci. Rep 5, 12584 10.1038/srep12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A 95, 322–7. 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE, 2007. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J. Neurosci 27, 14239–47. 10.1523/JNEUROSCI.3878-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria SV, Fine JM, Bingham D, Svitak AL, Burns RB, Baillargeon AM, Panter SS, Kazi AN, Frey WH, Hanson LR, 2016. Food consumption and activity levels increase in rats following intranasal Hypocretin-1. Neurosci. Lett 627, 155–159. 10.1016/j.neulet.2016.05.053 [DOI] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH, 2010. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci 99, 1654–1673. 10.1002/jps.21924 [DOI] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH, 2009. Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system, Journal of pharmaceutical sciences 10.1002/jps.21604 [DOI] [PubMed]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H, 2008. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 29, 693–706. 10.1016/j.neurobiolaging.2006.11.014 [DOI] [PubMed] [Google Scholar]
- Dong H, Fukuda S, Murata E, Zhu Z, Higuchi T, 2006. Orexins increase cortical acetylcholine release and electroencephalographic activation through orexin-1 receptor in the rat basal forebrain during isoflurane anesthesia. Anesthesiology 104, 1023–1032. 10.1097/00000542-200605000-00019 [DOI] [PubMed] [Google Scholar]
- Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF, 2007. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol. Aging 28, 1286–1295. 10.1016/j.neurobiolaging.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Enwere E, 2004. Aging Results in Reduced Epidermal Growth Factor Receptor Signaling, Diminished Olfactory Neurogenesis, and Deficits in Fine Olfactory Discrimination. J. Neurosci 24, 8354–8365. 10.1523/JNEUROSCI.2751-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Reis KM, Valentino RJ, Berridge CW, 2005. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J. Comp. Neurol 481, 160–178. 10.1002/cne.20369 [DOI] [PubMed] [Google Scholar]
- Fadel J, Burk JA, 2010. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res 1314, 112–123. 10.1016/j.brainres.2009.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY, 2002. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience 111, 379–387. 10.1016/S0306-4522(02)00017-9 [DOI] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR, 2005. Stimulation of cortical acetylcholine release by orexin A. Neuroscience 130, 541–547. 10.1016/j.neuroscience.2004.09.050 [DOI] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J, 2007. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience 149, 499–507. 10.1016/j.neuroscience.2007.07.061 [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V, 1992. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc. Natl. Acad. Sci 89, 738–742. 10.1073/pnas.89.2.738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronczek R, van Geest S, Frölich M, Overeem S, Roelandse FWC, Lammers GJ, Swaab DF, 2012. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol. Aging 33, 1642–1650. 10.1016/j.neurobiolaging.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Mignot E, Nishino S, 2003. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep 26, 953–9. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Roecker AJ, Hargreaves R, Coleman PJ, Winrow CJ, Renger JJ, 2012. Orexin receptors as therapeutic drug targets. Prog. Brain Res 198, 163–188. 10.1016/B978-0-444-59489-1.00010-0 [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W, 1989. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci 86, 1698–1702. 10.1073/pnas.86.5.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE, 2003. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J. Comp. Neurol 458, 11–31. 10.1002/cne.10505 [DOI] [PubMed] [Google Scholar]
- Hagar JM, Macht VA, Wilson SP, Fadel JR, 2017. Upregulation of orexin/hypocretin expression in aged rats: Effects on feeding latency and neurotransmission in the insular cortex. Neuroscience 350, 124–132. 10.1016/j.neuroscience.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Born J, 2008. Revealing the potential of intranasally administered orexin A. Mol.Interventions 10.1124/mi.8.3.5 [DOI] [PubMed]
- Hanson LR, Frey WH, 2008. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. Bmc Neurosci 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus RC, Bodurka J, Paulus MP, Khalsa SS, 2017. The Insular Cortex Dynamically Maps Changes in Cardiorespiratory Interoception. Neuropsychopharmacology 10.1038/npp.2017.154 [DOI] [PMC free article] [PubMed]
- Henny P, Jones BE, 2008. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur. J. Neurosci 27, 654–670. 10.1111/j.1460-9568.2008.06029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, Van Den Pol AN, 1999. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol 415, 145–159. [DOI] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P, 2014. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science (80-. ) 345, 1255263–1255263. 10.1126/science.1255263 [DOI] [PubMed] [Google Scholar]
- Huang H, Ghosh P, van den Pol AN, 2006. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J. Neurophysiol 95, 1656–1668. 10.1152/jn.00927.2005 [DOI] [PubMed] [Google Scholar]
- Hüttenbrink K-B, Hummel T, Berg D, Gasser T, Hähner A, 2013. Olfactory dysfunction: common in later life and early warning of neurodegenerative disease. Dtsch. Arztebl. Int 110, 1–7, e1. 10.3238/arztebl.2013.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S, Kawabe Y, Uchida S, Nakajima R, Saitoh T, Kanda T, Vogt K, Sakurai T, Nagase H, Yanagisawa M, 2017. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci U S A 114, 5731–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Wu MF, Siegel JM, 2000. Systemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogs. Sleep Res Online 3, 23–8. [PMC free article] [PubMed] [Google Scholar]
- Jones BE, 2008. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Mol. Biophys. Mech. Arousal, Alertness, Atten 1129, 26–34. 10.1196/annals.1417.026 [DOI] [PubMed] [Google Scholar]
- Julliard AK, Chaput MA, Apelbaum A, Aimé P, Mahfouz M, Duchamp-Viret P, 2007. Changes in rat olfactory detection performance induced by orexin and leptin mimicking fasting and satiation. Behav. Brain Res 183, 123–129. 10.1016/j.bbr.2007.05.033 [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, 1992. Expression of c-fos and other genes encoding transcription factors in long-term potentiation. [Review]. Behav Neural Biol 57, 263–266. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM, 2009. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanuki K, Iseki E, Kondo D, Fujishiro H, Minegishi M, Sato K, Katsuse O, Hino H, Kosaka K, Arai H, 2014. Neuropathological investigation of hypocretin expression in brains of dementia with Lewy bodies. Neurosci Lett 569, 68–73. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, 1999. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther 289, 219–223. [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y, 1997. GABAergic Cell Subtypes and their Synaptic Connections in Rat Frontal Cortex 476–486. [DOI] [PubMed]
- Kelsom C, Lu W, 2013. Development and specification of GABAergic cortical interneurons. Cell Biosci 3, 19 10.1186/2045-3701-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler BA, Stanley EM, Frederick-Duus D, Fadel J, 2011. Age-related loss of orexin/hypocretin neurons. Neuroscience 178, 82–88. 10.1016/j.neuroscience.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, McCarley RW, 2015. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc. Natl. Acad. Sci 112, 3535–3540. 10.1073/pnas.1413625112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács T, 2004. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res. Rev 10.1016/j.arr.2003.10.003 [DOI] [PubMed]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK, 2005. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J. Neurosci 25, 5225–5229. 10.1523/JNEUROSCI.0719-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CS, Kukkonen JP, 2014. Orexin/hypocretin receptor signalling: A functional perspective. Br. J. Pharmacol 10.1111/bph.12296 [DOI] [PMC free article] [PubMed]
- Li J, Hu Z, de Lecea L, 2014. The hypocretins/orexins: integrators of multiple physiological functions. Br. J. Pharmacol 171, 332–50. 10.1111/bph.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, 2017. Orexin and Alzheimer’s Disease. Curr Top Behav Neurosci 33, 305–322. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Chrisp CE, Hazzard DG, Bronson RT, 1996. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J. Gerontol. A. Biol. Sci. Med. Sci 51, B54–B59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG, 2012. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev 64, 614–628. 10.1016/j.addr.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol 435, 6–25. 10.1002/cne.1190 [DOI] [PubMed] [Google Scholar]
- Mckenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, Yanagawa Y, Mccarley RW, Brown RE, 2013. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J. Comp. Neurol 521, 1225–1250. 10.1002/cne.23290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith ME, Salameh TS, Banks W a, 2015. Intranasal Delivery of Proteins and Peptides in the Treatment of Neurodegenerative Diseases. AAPS J 17, 780–787. 10.1208/s12248-015-9719-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T, 2011. Differential Roles of Orexin Receptor-1 and −2 in the Regulation of Non-REM and REM Sleep. J. Neurosci 31, 6518–6526. 10.1523/JNEUROSCI.6506-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Sakurai T, 2013. Orexin (Hypocretin) receptor agonists and antagonists for treatment of sleep disorders: Rationale for development and current status. CNS Drugs 10.1007/s40263-012-0036-8 [DOI] [PubMed]
- Mobley AS, Rodriguez-Gil DJ, Imamura F, Greer CA, 2014. Aging in the olfactory system. Trends Neurosci 10.1016/j.tins.2013.11.004 [DOI] [PMC free article] [PubMed]
- Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, Gouda H, Kumagai H, Fujii H, Yanagisawa M, Nagase H, 2015. Design and Synthesis of Non-Peptide, Selective Orexin Receptor 2 Agonists. J Med Chem 58, 7931–7. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann A, Bellebaum C, Daum I, 2006. Cognitive deficits in narcolepsy. J. Sleep Res 15, 329–338. 10.1111/j.1365-2869.2006.00533.x [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The Rat Brain in Stereotaxic Coordinates. Acad. Press 1–474. 10.1007/s13398-014-0173-7.2 [DOI] [PubMed]
- Perry RJ, Hodges JR, 1999. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain 122 (Pt 3, 383–404. 10.1093/brain/122.3.383 [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol a N., de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18, 9996–10015. https://doi.org/10.1.1.335.5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Alanko L, Kalinchuk A, Heiskanen S, Stenberg D, 2004. The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain. Neurobiol. Aging 25, 231–238. 10.1016/S0197-4580(03)00043-5 [DOI] [PubMed] [Google Scholar]
- Prud’homme MJ, Lacroix MC, Badonnel K, Gougis S, Baly C, Salesse R, Caillol M, 2009. Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience 162, 1287–1298. 10.1016/j.neuroscience.2009.05.043 [DOI] [PubMed] [Google Scholar]
- Putula J, Turunen PM, Jantti MH, Ekholm ME, Kukkonen JP, 2011. Agonist ligand discrimination by the two orexin receptors depends on the expression system. Neurosci Lett 494, 57–60. [DOI] [PubMed] [Google Scholar]
- Rieger M, Mayer G, Gauggel S, 2003. Attention deficits in patients with narcolepsy. Sleep 26, 36–43. [PubMed] [Google Scholar]
- Roecker AJ, Cox CD, Coleman PJ, 2016. Orexin Receptor Antagonists: New Therapeutic Agents for the Treatment of Insomnia. J. Med. Chem 59, 504–530. 10.1021/acs.jmedchem.5b00832 [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2002. Roles of orexins in the regulation of feeding and arousal. Sleep Med 3 Suppl 2, S3–9. 10.1097/00001756-200206120-00001 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M, 2005. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46, 297–308. 10.1016/j.neuron.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Sarter M, Turchi J, 2002. Age- and dementia-associated impairments in divided attention: Psychological constructs, animal models, and underlying neuronal mechanisms. Dement. Geriatr. Cogn. Disord 10.1159/000048633 [DOI] [PubMed]
- Sawai N, Ueta Y, Nakazato M, Ozawa H, 2010. Developmental and aging change of orexin-A and -B immunoreactive neurons in the male rat hypothalamus. Neurosci. Lett 468, 51–55. 10.1016/j.neulet.2009.10.061 [DOI] [PubMed] [Google Scholar]
- Selbach O, Doreulee N, Bohla C, Eriksson K., Sergeeva O., Poelchen W, Brown R., Haas H., 2004. Orexins/hypocretins cause sharp wave- and θ-related synaptic plasticity in the hippocampus via glutamatergic, gabaergic, noradrenergic, and cholinergic signaling. Neuroscience 127, 519–528. 10.1016/j.neuroscience.2004.05.012 [DOI] [PubMed] [Google Scholar]
- Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson KS, Sergeeva OA, Haas HL, 2010. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol (Oxf) 198, 277–85. [DOI] [PubMed] [Google Scholar]
- Siegel JM, 1999. Narcolepsy: A key role for hypocretins (Orexins). Cell 10.1016/S0092-8674(00)81969-8 [DOI] [PMC free article] [PubMed]
- Skudlarek JW, DiMarco CN, Babaoglu K, Roecker AJ, Bruno JG, Pausch MA, O’Brien JA, Cabalu TD, Stevens J, Brunner J, Tannenbaum PL, Wuelfing WP, Garson SL, Fox SV, Savitz AT, Harrell CM, Gotter AL, Winrow CJ, Renger JJ, Kuduk SD, Coleman PJ, 2017. Investigation of orexin-2 selective receptor antagonists: Structural modifications resulting in dual orexin receptor antagonists. Bioorganic Med. Chem. Lett 27, 1364–1370. 10.1016/j.bmcl.2017.02.012 [DOI] [PubMed] [Google Scholar]
- Smart D, Jerman JC, 2002. The physiology and pharmacology of the orexins. Pharmacol. Ther 10.1016/S0163-7258(02)00171-7 [DOI] [PubMed]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC, 2001. SB-334867-A: the first selective orexin-1 receptor antagonist. Br. J. Pharmacol 132, 1179–82. 10.1038/sj.bjp.0703953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K, 2009. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. 10.1038/nature07991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi HR, Bates KA, Weinborn MG, Johnston ANB, Bahramian A, Taddei K, Laws SM, Rodrigues M, Morici M, Howard M, Martins G, Mackay-Sim A, Gandy SE, Martins RN, 2012. Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl. Psychiatry 2, e118 10.1038/tp.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CH, Chen XW, Xia JX, Yu ZP, Hu ZA, 2006. Modulatory effects of hypocretin-1/orexin-A with glutamate and gamma-aminobutyric acid on freshly isolated pyramidal neurons from the rat prefrontal cortex. Neurosci. Lett 399, 101–105. 10.1016/j.neulet.2006.01.065 [DOI] [PubMed] [Google Scholar]
- Spetter MS, Hallschmid M, 2015. Intranasal Neuropeptide Administration To Target the Human Brain in Health and Disease. Mol. Pharm 12, 2767–2780. 10.1021/acs.molpharmaceut.5b00047 [DOI] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, 2011. Aging-related alterations in orexin/hypocretin modulation of septo-hippocampal amino acid neurotransmission. Neuroscience 195, 70–79. 10.1016/j.neuroscience.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, Mott DD, 2012. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol. Aging 33 10.1016/j.neurobiolaging.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Gatfield J, Brisbare-Roch C, Dietrich H, Treiber A, Jenck F, Boss C, 2013. Discovery and Characterization of ACT-335827, an Orally Available, Brain Penetrant Orexin Receptor Type1 Selective Antagonist. ChemMedChem 8, 898–903. 10.1002/cmdc.201300003 [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R, 2009. Representations of Odor in the Piriform Cortex. Neuron 63, 854–864. 10.1016/j.neuron.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Takenoshita S, Sakai N, Chiba Y, Matsumura M, Yamaguchi M, Nishino S, 2018. An overview of hypocretin based therapy in narcolepsy. Expert Opin Investig Drugs 27, 389–406. [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Morairty S, Freund YR, Kilduff TS, 2002. Age-related decline in hypocretin (orexin) receptor 2 messenger RNA levels in the mouse brain. Neurosci. Lett 332, 190–194. 10.1016/S0304-3940(02)00953-9 [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Nienhuis R, Siegel JM, 2009. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep 32, 993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH, 2008. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 152, 785–97. 10.1016/j.neuroscience.2008.01.013 [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH, 2004. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127, 481–496. 10.1016/j.neuroscience.2004.05.029 [DOI] [PubMed] [Google Scholar]
- Tiitinen H, May P, Näätänen R, 1997. The transient 40-Hz response, mismatch negativity, and attentional processes in humans. Prog. Neuro-Psychopharmacology Biol. Psychiatry 10.1016/S0278-5846(97)00077-8 [DOI] [PubMed]
- Turku A, Rinne MK, Boije Af Gennäs G, Xhaard H, Lindholm D, Kukkonen JP, 2017. Orexin receptor agonist Yan 7874 is a weak agonist of orexin/hypocretin receptors and shows orexin receptor-independent cytotoxicity. PLoS One 12 10.1371/journal.pone.0178526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW, 1999. Growth Curves and Survival Characteristics of the Animals Used in the Biomarkers of Aging Program. Journals Gerontol. Ser. A Biol. Sci. Med. Sci 54, B492–B501. 10.1093/gerona/54.11.B492 [DOI] [PubMed] [Google Scholar]
- Villano I, Messina A, Valenzano A, Moscatelli F, Esposito T, Monda V, Esposito M, Precenzano F, Carotenuto M, Viggiano A, Chieffi S, Cibelli G, Monda M, Messina G, 2017. Basal Forebrain Cholinergic System and Orexin Neurons: Effects on Attention. Front. Behav. Neurosci 11 10.3389/fnbeh.2017.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW, 2006. Hypocretin/Orexin Selectively Increases Dopamine Efflux within the Prefrontal Cortex: Involvement of the Ventral Tegmental Area. Neuropsychopharmacology 31, 384–395. 10.1038/sj.npp.1300807 [DOI] [PubMed] [Google Scholar]
- Weinhold SL, Goder R, Baier PC, 2014. Improvement of divided attention in narcolepsy by intranasal orexin-A. J. Sleep Res 23, 291. [DOI] [PubMed] [Google Scholar]
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC, 2014. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav. Brain Res 262, 8–13. 10.1016/j.bbr.2013.12.045 [DOI] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon R. a, Wilson D. a, 2010. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J. Neurosci 30, 505–14. 10.1523/JNEUROSCI.4622-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zhang Z, Leranth C, Xu C, van den Pol AN, Alreja M, 2002. Hypocretin increases impulse flow in the septohippocampal GABAergic pathway: implications for arousal via a mechanism of hippocampal disinhibition. J. Neurosci 22, 7754–65. https://doi.org/22/17/7754 [pii] ET - 2002/08/28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM, 2010. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J. Comp. Neurol 518, 389–404. 10.1002/cne.22229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, McKenna JT, Zant JC, Winston S, Basheer R, Brown RE, 2014. Cholinergic Neurons Excite Cortically Projecting Basal Forebrain GABAergic Neurons. J. Neurosci 34, 2832–2844. 10.1523/JNEUROSCI.3235-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE, 2006. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol 494, 845–861. 10.1002/cne.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajo KN, Fadel JR, Burk JA, 2016. Orexin A-induced enhancement of attentional processing in rats: role of basal forebrain neurons. Psychopharmacology (Berl) 233, 639–647. 10.1007/s00213-015-4139-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Sampogna S, Morales FR, Chase MH, 2002. Age-related changes in hypocretin (orexin) immunoreactivity in the cat brainstem. Brain Res 930, 206–211. 10.1016/S0006-8993(02)02240-0 [DOI] [PubMed] [Google Scholar]


