Abstract
The orexin system includes the neuropeptides orexin-A and -B and the cognate receptors of orexin-1 (OX1) and −2 (OX2) and has been indicated in a number of important physiological processes. It is generally accepted that the OX1 receptor is mainly involved in motivation and reward and the OX2 receptor in the modulation of sleep/wake cycle and energy homeostasis. A variety of OX1 selective antagonists (1-SORAs) have been disclosed in the literature and some of them have been evaluated as potential therapeutics for addiction treatment. In this review we summarize all OX1 antagonists reported thus far based on their core structure. Several dual orexin receptor antagonists (DORAs) and OX2 selective antagonist (2-SORAs) have also been recently evaluated in reward and addiction models. While DORAs may seem pharmacologically advantageous for alcohol addiction given the recent findings on the OX2 receptor in reward and alcohol consumption, 1-SORAs are the better options for other drugs of addiction such as cocaine due to the absence of the sedative effects inherently associated with dual antagonists.
Keywords: Orexin, Hypocretin, Antagonist, Selective, Addiction
1. INTRODUCTION
The orexin/hypocretin system consists of two neuropeptides, orexin A and B (also called hypocretin 1 and 2), and their binding targets, two G protein-coupled receptors (GPCRs), orexin-1 (OX1) and 2 (OX2) receptors (de Lecea et al., 1998; Sakurai et al., 1998). Both peptides and receptors are highly conserved across mammalian species (Sakurai et al., 1998). Orexin A is a 33-amino acid peptide with two disulfide bridges, whereas orexin B is a linear peptide consisting of 28 amino acids. These peptides are derived from the cleavage of the common precursor prepro-orexin, which is produced by a small population of neurons, estimated at a few thousand, located in the perifornical area (PFA), the dorsomedial hypothalamus (DMH) and the lateral hypothalamus (LH) (de Lecea et al., 1998; Sakurai et al., 1998). These orexin-producing neurons have extensive projections into many brain regions and not surprisingly, the orexin system has been indicated in a variety of physiological processes and functions, including appetite/feeding, sleep and wakefulness, energy homeostasis, stress, and reward (Baimel et al., 2015; Gotter et al., 2012; Scammell & Winrow, 2011).
Multiple lines of evidence suggest that the OX1 and OX2 receptors may play differential physiological roles. First, the orexin peptides, the orexin receptors and their mRNAs have overlapping but sometimes distinct patterns of distribution in the brain. For instance, only OX1 is found in locus coeruleus (LC), laterodorsal tegmental nucleus (LDT), pedunculopontine tegmental nucleus (PPT) and the prefrontal cortex (PFC), whereas the tuberomammillary nucleus (TMN), nucleus accumbens (NAc) and the paraventricular nucleus (PVN) only express OX2 (Lu et al., 2002; Marcus et al., 2001; Nambu et al., 1999; Peyron et al., 1998). Further, the orexin receptors have different binding affinity for the two endogenous peptides. The OX2 receptor binds both orexin A and B with high affinity; however, OX1 receptor binds orexin A with ~20 fold selectivity over orexin B as determined by competitive binding studies (Sakurai et al., 1998), and this selectivity could increase to ~100 fold when measured in intracellular calcium assays (Holmqvist et al., 2002; Sakurai et al., 1998; Smart et al., 2001). The extensive pharmacological and behavioral studies conducted on the orexin system have come to define the respective roles of these two receptors: the OX2 receptor in the modulation of sleep/wake cycle and energy homeostasis and the OX1 receptor in motivation and reward (Gotter et al., 2012; Tsujino & Sakurai, 2009).
Given the unambiguous link between the orexin system and sleep/arousal and the huge market for sleep disorder medications, the majority of research and clinical investigations on the orexin system seek to block the orexin receptors by the development of orexin antagonists (Sakurai, 2007; Winrow & Renger, 2014). Although OX2 receptor is considered the major contributor to sleep and wake regulation, double OX1/2 knock-out mice display more severe narcoleptic phenotype than individual OX1/2 receptor KO mice (Willie et al., 2003). Consistent with the genetic findings, several studies suggest that blockade of both receptors may be more effective and efficacious (Brisbare-Roch et al., 2007; Etori et al., 2014; Morairty et al., 2012). Indeed, a variety of dual antagonists of different chemotypes have been developed, several of which entered clinical development (Figure 1) (for reviews, see (Andrews et al., 2016; Boss et al., 2009; Boss & Roch, 2015; Gatfield et al., 2010; Kodadek & Cai, 2010; Lebold et al., 2013; Roecker & Coleman, 2008; Winrow & Renger, 2014)). The most successful among them is the dual antagonist suvorexant (Belsomra®) by Merck, which was approved by the FDA in 2014 for the treatment for insomnia. However, it should be noted that there are also studies that suggest selective OX2 receptor antagonists (2-SORAs) could be more efficacious (Dugovic et al., 2009; Dugovic et al., 2014), where co-administration of OX1 and OX2 antagonists actually decreased the sleep promoting effects of the OX2 antagonist. Several 2-SORAs have been evaluated clinically, including JNJ-42847922/MIN-202 which displayed favorable properties in insomnia and is being evaluated clinically for the treatment of major depressive disorder (Bonaventure et al., 2015a).
Figure 1.
Examples of orexin antagonists advanced into clinical trials
On the other hand, the addiction related orexin research has mostly centered around the OX1 receptor (Khoo & Brown, 2014; Mahler et al., 2012). The ability of orexin peptide to stimulate appetite and feeding, together with the projections of orexin neurons to brain areas that are associate with reward including ventral tegmental area (VTA), amygdala, NAc and pre-frontal cortex, promote investigations of the orexin system in other reward processes including drug addiction. The fact that OX1 selective antagonist (1-SORA) SB-334867 blocked the agonist peptide stimulated reinstatement of extinguished drug (morphine or cocaine) seeking strongly support OX1 receptor’s role in addiction (Boutrel et al., 2005; Harris et al., 2005). Subsequent studies showed that antagonism of the OX1 receptor was effective in attenuating the addiction effects of other drugs such as ethanol (Lawrence, 2010; Lawrence et al., 2006; Walker & Lawrence, 2017), and nicotine (Kenny, 2011). In addition, blockade of the OX1 receptor using 1-SORAs including SB-334867 and ACT335827 has been shown to be effective in attenuating anxiety/stress related behaviors (Beig et al., 2015).
A variety of selective OX1 receptor antagonists have been discovered and developed. Many of them have been covered in several comprehensive reviews (Andrews et al., 2016; Boss et al., 2009; Boss & Roch, 2015; Gatfield et al., 2010; Roecker & Coleman, 2008; Winrow & Renger, 2014)), including one specifically on selective orexin receptor antagonists in 2013 (Lebold et al., 2013). Since the most recent reviews several new series of 1-SORAs have been reported, including the morphinans and 1,2-disubstituted cyclopentanes (Fieldhouse et al., 2015; Nagase et al., 2017). We herein provide an up-to-date summary of the OX1 selective antagonists reported thus far, with an emphasis on the newly disclosed ones. Most of the previous reviews arranged the orexin antagonists, DORAs or SORAs, based on the companies or laboratories that they are developed in. In this review, we summarize selective OX1 antagonists based on their core structures. They can be categorized in several classes, such as diaryl ureas, pyrrolidines/piperidines, tetrahydroisoquinolines, morphinans, and cyclopentanes.
2. Diaryl Ureas
SmithKline Beecham (now GSK) first disclosed a series of small molecule antagonists of the orexin receptors based on a diaryl urea scaffold. Optimization of a hit identified in an HTS campaign led to the identification of SB-334867, (Porter et al., 2001; Smart et al., 2001), which showed good OX1 affinity/potency (Kb = 28 nM) but significantly reduced affinities against 5-HT2B (pKi = 5.4) and 5-HT2C (pKi < 5.3) than the initial hit. SB-334867 demonstrated ~50-fold selectivity against the OX2 receptor (pKi = 5.7) and was at least 100-fold selective against a panel of GPCRs and ion channels. In preliminary pharmacokinetic evaluations, SB-334867 displayed a generally favorable profile with good CNS penetration after i.v. infusion, moderate half-life (t1/2 = 0.4 h) after i.p. administration, and reasonable bioavailability (10%) (Porter et al., 2001). Subsequent reports confirmed that sustained plasma concentration of SB-334867 was observed after i.p. administration (Gotter et al., 2012).
SB-334867 has since become the most widely used tool compound to study the orexin system and was essential in elucidating the in vivo function of the OX1 and OX2 receptors (Aston-Jones et al., 2009; Aston-Jones et al., 2010; Merlo Pich & Melotto, 2014). There are over 400 published studies that used this important compound (based on a search in PubMed.gov). However, the use of SB-334867 as a pharmacological tool has several limitations. First, the target selectivity of SB-334867 is modest. It is only ~50-fold selective against the OX2 receptor. Researchers from Merck later discovered that in addition to 5-HT2B and 5-HT2C (Ki = 1.2 μM), SB-334867 also displayed micromolar affinity for several GPCRs including the adenosine A2A receptor (Ki = 0.67 μM) (Gotter et al., 2012). Second, this compound displayed some hydrolytic instability under acidic conditions, decomposing to the 2-methylbenzoxazole ring-opened product which has no affinity for the OX1 receptor (McElhinny et al., 2012). Therefore, the in vivo behavioral results, particularly those that are negative, should be analyzed and interpreted with caution in many of the earlier studies when the hydrochloride salt (SB-334867A) was employed. Finally, SB-334867 as the free base has poor aqueous solubility (Haynes et al., 2000), presumably due to its planar conformation resulting in tight packing. It is generally administered as a suspension in vehicles such as DMSO and therefore, the injection volume is a limiting factor to avoid the toxic effect of DMSO. When solutions were used they are prepared by dissolving the drug in DMSO followed by dilution (e.g. saline). However, this dilution has the potential to generate supersaturated solutions, which may lead to drug precipitation after injection (Williams et al., 2013). This drug precipitation not only may cause mechanical or chemical irritation at the injection site, it may also complicate the study results.
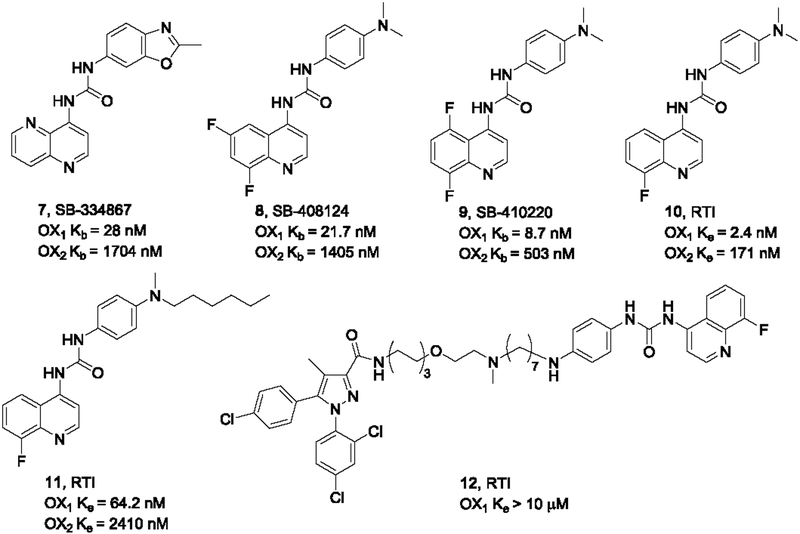
Two structurally close analogs of SB-334867 with similar OX1/OX2 selectivity profiles, SB-408124 (OX1 Kb = 21.7 nM, OX2 Kb = 1405 nM) and SB-410220 (OX1 Kb = 8.7 nM, OX2 Kb = 503 nM), were later reported by the same company (Figure 2). They are less studied, but SB-408124 appeared to share some of the selectivity issues with SB-334867, displaying affinity for several targets including 5-HT2B (Gotter et al., 2012).
Figure 2.
Diaryl urea-based selective OX1 antagonists from GSK and RTI
Perrey et al. at Research Triangle Institute (RTI) conducted further SAR studies on the diarylureas related to SB-334867 (Perrey et al., 2011). While the dimethylamino analog 10 showed significant activity at both receptors, the hexyl analog (11) was reasonably selective against the OX2 receptor, indicating steric tolerance at this position. Based on these results, bivalent ligand 12 connecting this OX1 antagonist with the CB1 receptor antagonist SR141716 were constructed (Figure 2), but it showed no activity at the OX1 receptor at concentrations up to 10 μM (unpublished results), suggesting that tolerance for bulky substitutions at this position is limited.
3. Pyrrolidines and Piperidines
3.1. Pyrrolidines
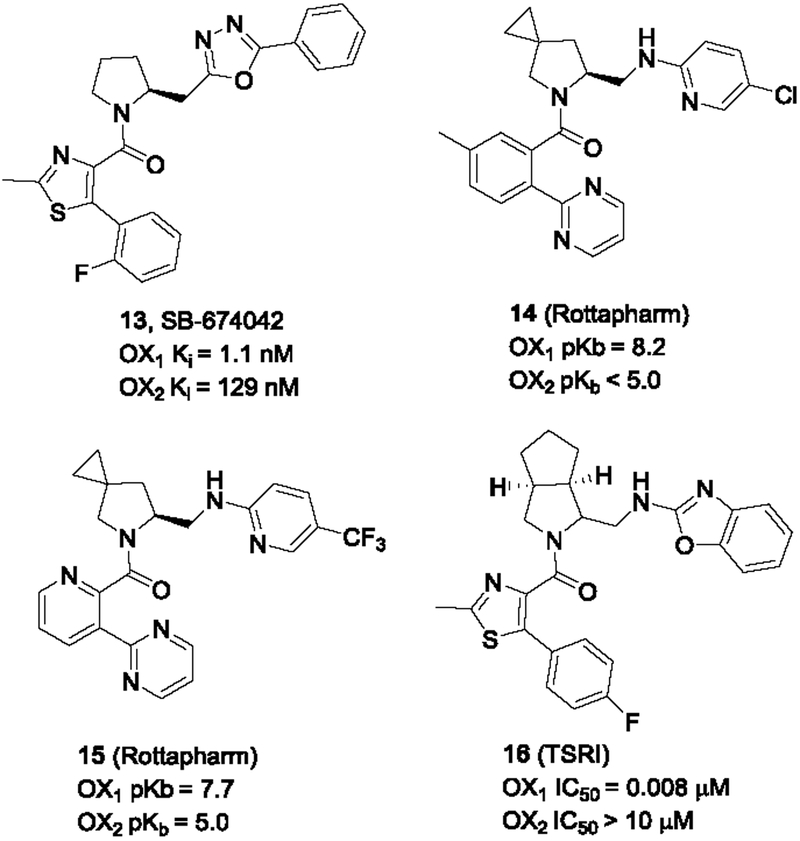
In addition to the diaryl ureas, SmithKline Beecham also developed another OX1 selective antagonist SB-674042 with a pyrrolidine core (Langmead et al., 2004). Although it had excellent OX1 affinity and good selectivity over the OX2 receptor, SB-674042 has not been widely used in the pharmacology community. This may be because of concerns on its considerable affinity for the OX2 receptor (Ki = 129 nM), and it has been reported to have poor solubility (Lebold et al., 2013). However, the tritiated version [3H]-SB-674042 has been used as an important radioligand in orexin receptor binding studies (Malherbe et al., 2009; Malherbe et al., 2010). Recently, SB-674042 and the dual antagonist suvorexant were used in the determination of the crystal structures of hOX1 receptor and investigation of mechanisms of antagonist subtype selectivity between hOX1R and hOX2R using molecular modeling (Yin et al., 2016).
Rottapharm Madaus later revealed a series of spiro analogs of the pyrrolidines. As illustrated in Figure 3, some of these compounds (e.g. 14 and 15) possessed good OX1 potency and high selectivity (> 100-fold) (Stasi et al., 2013; Stasi & Rovati, 2011). The Scripps Research Institute (TSRI) Florida group reported hexahydrocyclopenta[c]pyrrole analogs. Compounds such as 16 had excellent potency at the OX1 receptor and was inactive at the OX2 receptor at concentrations of 10 μM (Kamenecka et al., 2013).
Figure 3.
Pyrrolidines and analogs from GSK, Rottapharm and TSRI
3.2. 1,2-Substituted Piperidines
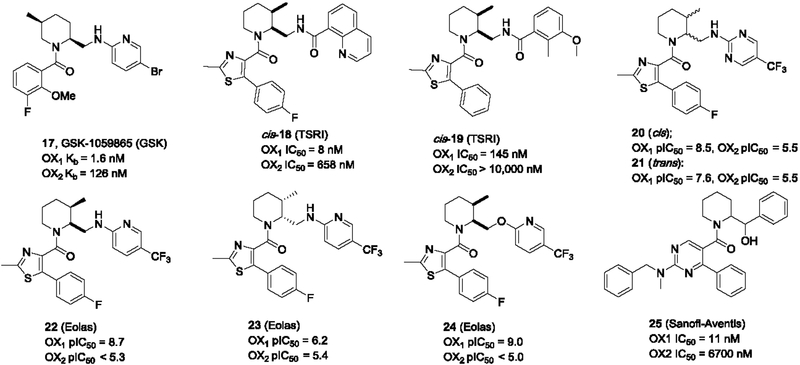
OX1 selective antagonists based on a piperidine core, the ring expanded analogs of the pyrrolidines, have been extensively studied. A wide range of piperidines have been developed by several laboratories. The first of such was GSK-1059865, a 1,2-piperidine bearing a 5-methyl group (Alvaro et al., 2009; Gozzi et al., 2011) (Figure 4). Compared to SB-649868, the most advanced piperidine-based DORA from GSK that went to phase 2 clinical trials, GSK-1059865 is less characterized. GSK-1059865 has good OX1 potency (pKB = 8.8) and selectivity over the OX2 receptor (pKB = 6.9), and displayed little affinity (< 50% inhibition at 1 μM) over a panel of 113 targets except the κ-opioid receptor (pKi = 6.5). It also had favorable pharmacokinetic properties, including brain penetration with a brain:blood ratio of 0.3 upon i.p. injection. While it did not produce significant sleep-promoting effects, GSK1059865 dose-dependently reduced expression of cocaine-induced conditioned place preference. In a later study, GSK1059865 significantly decreased ethanol drinking in a dose-dependent manner in mice exposed to chronic intermittent ethanol, but only at the highest dose in air-exposed mice (Lopez et al., 2016). The authors concluded that the OX system may be an important target system for treating disorders of compulsive reward seeking in which strong motivation is required. GSK-1059865 (10 mg/kg s.c.) also significantly attenuated the cortical glutamate release elicited by MK-801, an NMDA receptor antagonist (Aluisio et al., 2014), suggesting OX1 receptor antagonists may represent novel therapeutics for the treatment of psychiatric disorders associated with hyperactive states.
Figure 4.
Substituted 1,2-piperidines from GSK, TSRI, Eolas and Sanofi-Aventis
Jiang and co-workers at TSRI designed and synthesized a series of substituted 1,2-piperidines and identified key structural features that affect the OX1/2 selectivity profile of this series of compounds (Jiang et al., 2012). Additional substituents such as a methyl group were added to the piperidine ring at the 3, 4, 5, and 6-positions, respectively to result in 4 series of piperidines. Among them, the 3-methyl series appeared to be mostly OX1 selective such as compounds 18 and 19, whereas the other series were mostly dual antagonists. These compounds showed poor metabolic stability in mouse, rat, and human microsomes, and limited plasma and brain exposure at 10 mg/kg with i.p. administration and required a much higher dose (50 mg/kg) to obtain good exposure in the plasma and brain. This group also synthesized and tested the racemic version of SB-649868, which shared poor PK properties as the rest of the series.
Several authors from the TSRI group subsequently filed three patents on these 1,2-piperidines under a company named Eolas Therapeutics, describing additional piperidine analogs (Figure 4) (Kamenecka et al., 2013; Kamenecka et al., 2014a; Kamenecka et al., 2014b). The importance of the 3-methyl group on the piperidine for the OX1 selectivity was confirmed in these patents. In addition, the relative stereochemistry between the 2,3-subsituents were investigated, and in general, the cis-isomers were slightly more potent at the OX1 receptor than the trans isomers (e.g. 20 vs 21, Figure 4). Among the two cis-isomers, the (2S, 3R) isomer appeared to be quite active, whereas the other one (2R, 3S) is mostly inactive (22 vs. 23). This trend is apparent in a number of examples given in the patents. Further, the heteroatom on the linker at the 2-position of the piperidine ring was found to also influences the selectivity. For example, compound 24 with an oxygen-containing linker was highly potent and OX1 selective.
Sanofi-Aventis filed a patent on OX1 selective antagonists based on a series of pyrimidines as OX1 selective antagonists (Aranyi et al., 2005), which are based on a substituted 1,2-piperidine core (Figure 4). Compounds such as 25 was potent at the OX1 receptor and selective against the OX2 receptor. The stereochemistry of this series was not reported.
3.3. 1,3-Substituted Piperidines
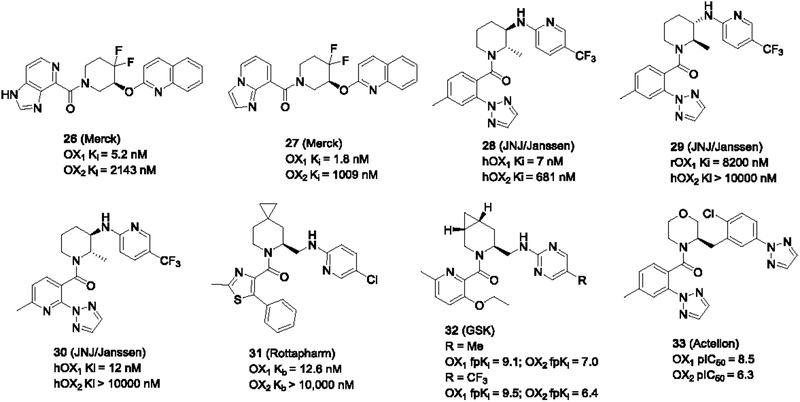
Merck has developed several series of 1,3-substituted piperidines as orexin antagonists. Compared to the earlier 1,2-piperidines, these piperidines bear the main substituents at the 3-position, instead of the 2-position. Most of these piperidines are DORAs including filorexant (MK-6096) or 2-SORAs, whereas a small set were potent and selective 1-SORAs such as compounds 26–27 (Figure 5) (Raheem et al., 2015). The difluoro groups at the 4-position in 27 were added presumably for metabolic considerations.
Figure 5.
1,3-Piperidines (Merck and JNJ/Janssen) and other piperidine-like 1-SORAs
Janssen (Johnson and Johnson, JNJ) disclosed another series of 1,3-piperidine analogs bearing a methyl group at the 2-position (Coate et al., 2014). The stereochemistry at the 2,3-positions was crucial for the OX1 affinity. As can be seen from Figure 5, compound 28 showed excellent OX1 potency and was OX1 selective, whereas 29 was mostly inactive at both receptors. The pyridinyl analog 32 that adopts the same stereochemistry as 28 also displayed excellent OX1 potency and selectivity.
3.4. Other Piperidine-like Analogs
Rottapharm Madaus reported a series of azaspiro compounds (e.g. 31) (Stasi et al., 2013; Stasi & Rovati, 2011), and GSK described a variety of azabicylco[4.1.0]heptanes (e.g. 32) (Alvaro et al., 2010). Both series can be considered substituted piperidines. These compounds showed high OX1 potency and selectivity (Figure 5). Actelion also described the morpholino analogs of piperidines that were up to 100-fold selective for the OX1 receptor (e.g. 33) (Bolli et al., 2012).
4. Tetrahydroisoquinolines
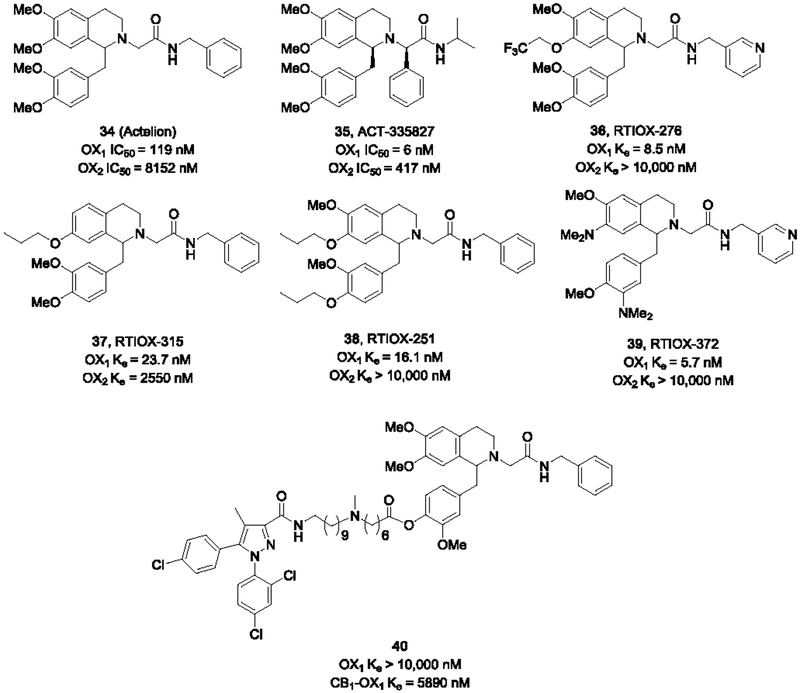
Actelion pioneered the tetrahydroisoquinoline (THIQ)-based orexin antagonist development. Their program reported a series of tetrahydroisoquinoline derivatives based on a screening hit 34, a benzylacetamide derivative of tetrahydropapaverine (Koberstein et al., 2003). Much of Actelion’s work was directed to developing DORAs, which would lead to their clinical candidate Almorexant that reached phase 3 clinical trials. However, optimization of the already OX1 selective lead compound (> 100-fold over OX2) resulted in the identification of the potent OX1 antagonist ACT-335827 (35) (Steiner et al., 2013a). While it only moderately selective against the OX2 receptor (~70-fold), ACT-335827 showed no significant off-target activities in a panel of 110 other enzymes, receptors and ion channels. This compound was also brain penetrant with pharmacologically significant levels of drug in the brain when dosed orally at 100 mg/kg. Behavioral experiments showed it decreased fear-induced startle response at 300 mg/kg and reduced stress-induced hypothermia at 300 mg/kg and tachycardia at 100 mg/kg, without affecting locomotion or blood pressure. It was also shown to decrease compulsive drinking behavior in a schedule-induced polydipsia model without affecting home cage water intake. Notably, the OX1 selective compound did not affect the lengths of wake or sleep cycles or the amount of REM or non-REM sleep.
Selective OX1 antagonists based on this THIQ core have been a long-standing interest of the RTI group. Optimization around the 7-position of the THIQ core and replacing the benzyl group with a pyridinylmethyl led to the highly potent and OX1 selective RTIOX-276 (36, OX2 Ke > 10,000 nM) (Perrey et al., 2013). The importance of the 7-substitution was confirmed in a series of mono substituted THIQs, where the 7-position analog showed good OX1 potency and selectivity (e.g. 37) and the 6-substituted analogs were generally inactive (Perrey et al., 2015a). Exploration of the 1-benzyl substitution led to RTIOX-251 (38) with a 3-methoxy-4-propxy benzyl group (Perrey et al., 2015b), which showed exceptional selectivity for OX1 as well (OX2 Ke > 10,000 nM). Despite the excellent OX1 potency and selectivity, there remained concern over the high lipophilicity of some of the compounds in this series, such as RTIOX-251 which has a clogP of 6.9. Further optimization to improve the ADME properties of this series resulted in RTIOX-372 (39) with a dimethylamino group at the 7-position (Perrey et al., 2018). This compound retained the exceptional OX1 potency and selectivity of the earlier compound, and also has much lower clogP (3.1). RTIOX-372 showed great kinetic solubility (>200 μM), good BBB permeability (Papp = 14.7 × 10−6 cm/s), and possessed little Pgp activity with an efflux ratio of 3.3.
In addition to OX2, RTIOX-276 was examined against a panel of ~50 GPCRs, including many that are commonly indicated in addiction such as MOR, DOR, KOR, D1–5 (unpublished results). Except a modest affinity for 5-HT2B (Ki = 2586 nM), RTIOX-276 did not show any significant binding for the rest of the targets at 10 μM. Similar to RTIOX-372, RTIOX-276 also showed good solubility and brain permeability in the MDCK assay (Perrey et al., 2018). RTIOX-276 reduced the conditioned place preference for cocaine in rats (Perrey et al., 2013), while RTIOX-251 was shown to block the development of locomotor sensitization to cocaine in rats (Perrey et al., 2015b). Further work showed that RTIOX-276 reduced the spontaneous dopamine transient amplitude as well as cue-evoked dopamine release, providing support for the hypothesis that orexin blockade suppresses phasic dopamine signaling and attenuates the effect of cocaine by reduction of cocaine sensitivity at dopamine terminals (Levy et al., 2017).
CB1-OX1 bivalent ligands based on these THIQs and the CB1 antagonist SR141716 have been developed by the RTI group (Perrey et al., 2014). These bivalent ligands were tested for activity against CB1 and OX1 individually and in cell lines co-expressing both receptors. While these compounds demonstrated reduced potencies in cells expressing either CB1 or OX1 receptor alone, compound 40 showed a robust enhancement in potency at both receptors in cells coexpressing both receptors, suggesting possible interactions with CB1-OX1 dimers.
5. Morphinans
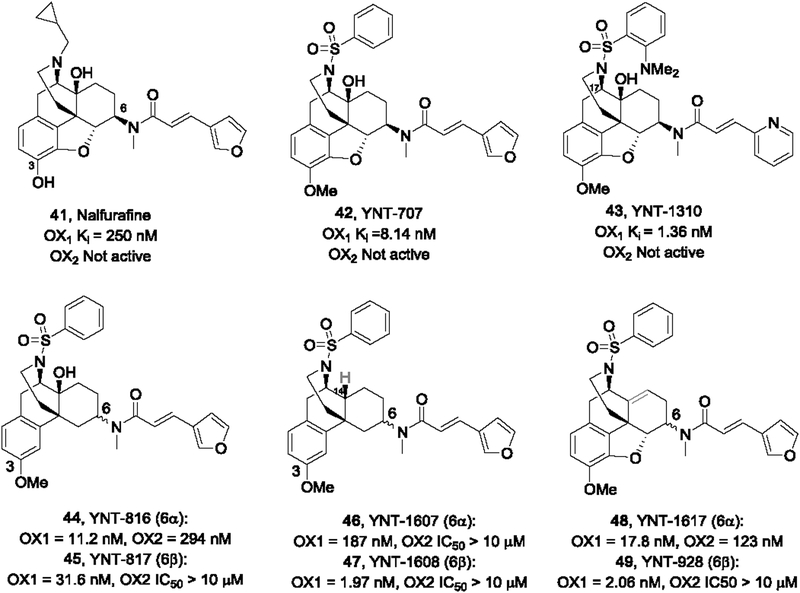
Nagase and co-workers recently described a series of orexin antagonists based on the common opioid skeleton morphinan (Nagase et al., 2017). This work was prompted by the authors’ interest in opioid research and interactions of the orexin system with the endogenous κ-opioid agonist dynorphin, such as colocalization of both peptides within the same vesicle in the orexin neuron and heterodimerization of the OX1 and KOR receptors. The authors noticed that nalfurafine (41), unlike many KOR agonist, showed neither addiction nor drug aversion, and subsequently discovered that nalfurafine had antagonistic activity for the OX1 receptor (Ki = 250 nM; OX2, not active) as well. This compound was highly active at the KOR and MOR, with high nanomolar potency at the DOR. Optimization led to the discovery of compound YNT707 (42) and YNT-1310 (43), which were exceptionally potent at the OX1 receptor and selective against OX2 and all three opioid receptors (IC50 or Ki > 10,000 nM). Docking studies based on the recently published X-ray structure of the OX1 receptor were also conducted, which suggested that a clash between the 17-o-dimethylaminobenzene group of YNT-1310 with the T111 (TM2) residue of the OX2R was most likely responsible for the observed subtype selectivity. Compound YNT-1310 upon i.p. administration significantly suppressed naloxone-precipitated body weight loss, diarrhea, and jumping behavior, indicating that the OX1R antagonist attenuated the expression of naloxone-precipitated morphine withdrawal.
In follow-up studies the same group investigated several positions for their importance for the OX1 potency and selectivity (Ohrui et al., 2018; Yamamoto et al., 2017). It was discovered that the 3-methoxy was important for OX1 activity as its removal resulted in markedly reduced potency. The stereocenter at the 6-position was also crucial for OX1 activity, as the 6α-isomer of YNT-707, YNT-1369, showed significantly reduced OX1 potency (Ki = 849 nM). However, when the 4,5-epoxy ring was removed, the 6α-isomer 44 was more potent than the 6β-isomer 45. Based on molecular modeling studies, the author postulated that the removal of the epoxy ring may facilitate both the α- and β-isomers to adopt conformations similar to the high affinity antagonist YNT-1310. When the 4-hydroxy group was replaced with a hydrogen (46, 47) or removed (48, 49), the resulting analogs all demonstrated higher potency. The authors suggested that the hydroxy group may form hydrogen bonding with the 14-amide or sulfonamide group, forming a conformation that was unfavorable in the interaction with the OX1 receptor. Again, the 6-position β-isomers were generally more potent than the corresponding α-isomers, although YNT-1617 was active at the OX2 receptor (Ki = 123 nM).
6. 1,2-Disubstituted cyclopentanes
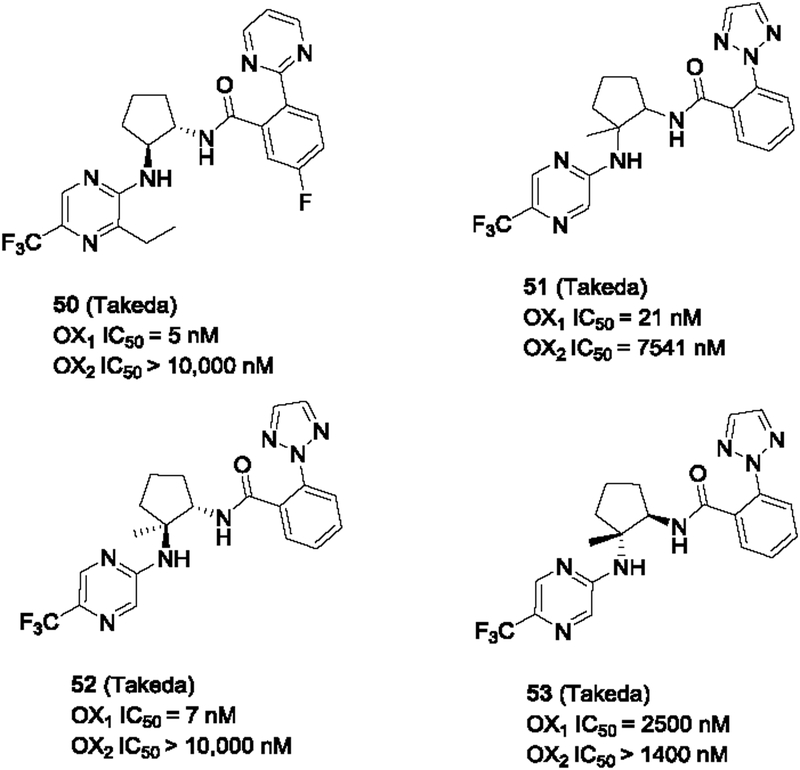
In 2015, Takeda Pharmaceuticals filed a patent claiming 1,2-substituted cyclopentanes as orexin antagonists, and many of the compounds showed excellent OX1 selectivity against the OX2 receptor (Fieldhouse et al., 2015). As shown in Figure 8, some of these compounds (e.g. 50) had IC50’s less than 10 nM and were inactive at the OX2 receptor at 10 μM. The stereochemistry of the substituents is important for their orexin antagonist activity. Of the trans-isomer of the racemic mixture 51 which displayed good OX1 potency and selectivity, the (1S, 2S) isomer (52) appeared to be the active one, whereas the (1R, 2R) isomer (53) was had potencies in the micromolar range at both receptors. These 1,2-disubstituted pentanes represent a novel class of OX1 selective antagonists, with exceptional OX1 selectivity over the OX2 receptor. It should be noted that it is apparent that these pentanes have substitution patterns that resemble the pyrrolidine and piperidine series, both in the nature of the peripheral substituents and their spatial arrangements.
Figure 8.
1,2-Disubstituted cyclopentanes as OX1 selective antagonists
7. Other OX1 antagonists
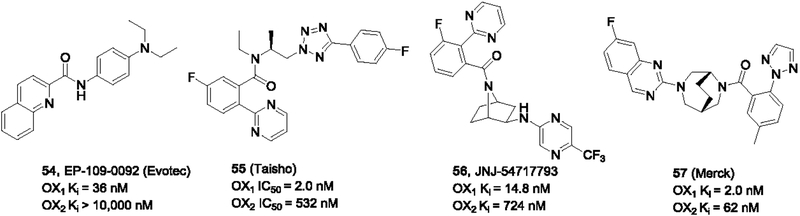
Evotec AG recently reported a series of diaryl amides including the OX1 selective antagonist EP-109–0092 (54, Figure 9) (Heifetz et al., 2012). It showed good OX1 potency and excellent selectivity over the OX2 receptor. No other studies on this compound have been reported. This compound clearly resembles SB-334867 and the related ureas in structure. The replacement of the urea functionality with the amide group may cause the compound to adopt a less planar conformation and therefore, it is possible this compound may have improved solubility than the corresponding ureas.
Figure 9.
Other antagonists with OX1 selectivity
Suzuki and co-workers from Taisho Pharmaceuticals described the design and synthesis of a series of benzamides as OX1 selective antagonists via derivatization of dual antagonists previously discovered by the same group (Futamura et al., 2017; Suzuki et al., 2015). The most selective compound 55 was highly potent at the OX1 receptor (IC50 = 2 nM) and had a selectivity index of 265 over the OX2 receptor.
Other OX1 antagonists include the bridged bicyclic compounds disclosed by JNJ/Janssen in two patents (Coate et al., 2014; Gelin et al., 2014). Some of these compounds such as JNJ-54717793 (56) showed ~50-fold OX1 selectivity over the OX2 receptor and showed promise as potential candidates for the treatment of anxiety disorders (Bonaventure et al., 2017; Bonaventure et al., 2015b; Johnson et al., 2015). Along with the diazepine-based DORAs reported by Merck, some compounds such as 57 depicted in Figure 9 showed OX1 selectivity, although only modest (Coleman et al., 2010).
Concluding Remarks and Future Directions
It is now generally accepted that OX1 is the dominating receptor and the OX2 receptor plays a lesser role in the modulation of addiction and other reward processes. However, it should be noted that many of the studies that led to these conclusions used OX1 selective antagonists with moderate selectivity against the OX2 receptor. SB334867 was by far the mostly used tool compound and is only ~50-fold selective for the OX1 receptor (OX1 Kb = 27.8 nM, OX2 Kb = 1704 nM) (Langmead et al., 2004; Porter et al., 2001; Smart et al., 2001). While GSK1059865 is more selective (~100 fold), it still has significant activity at the OX2 receptor (OX2 Kb = 126 nM) (Gozzi et al., 2011). Similarly, ACT335827 demonstrates activity at the OX2 as well (OX1 IC50 = 6 nM, OX2 IC50 = 417 nM) (Steiner et al., 2013c). Therefore, the observed effects with these OX1 antagonists may not be exclusively attributed to the OX1 receptor. Now that OX1 antagonists with significantly higher selectivity over the OX2 receptor are available, some of the key findings related to addiction and other psychiatric disorders may be re-examined with these improved tools.
Dual antagonists have begun to be evaluated in addiction related models associated with cocaine or other drugs. Similar to 1-SORAs, suvorexant attenuated cocaine self-administration, prevented cocaine-induced CPP, and reduced cocaine-evoked impulsivity (Gentile et al., 2018a; Gentile et al., 2018b). Almorexant attenuated the expression of CPP to cocaine D.L-amphetamine (Steiner et al., 2013b). Similar to SB334867, the DORA almorexant significantly reduced the effects of cocaine on dopamine signaling and decreased the motivation to take cocaine (Prince et al., 2015). Interestingly, higher doses of almorexant (25, 50, 100 mg/kg), which has higher in vitro OX1 affinity than SB-334867, were needed to achieve the same effects observed with SB-334867 (7.5, 15, 30 mg/kg) in these studies (Prince et al., 2015). While further studies are clearly needed, these results suggest that blocking both OX1 and OX2 receptors may not be more effective than 1-SORA in addiction related functions. Factors that should be carefully considered to avoid potential complications in such studies include the different pharmacodynamic and pharmacokinetic properties of DORAs and 1-SORAs from different structural classes. A straightforward strategy to best accomplish direct comparisons may be to investigate the effects of the same 1-SORA in the present or absence of a selective 2-SORA in side-by-side experiments. Without these, it may be difficult to conclude whether 1-SORAs or DORAs are more effective as addiction therapeutics.
Recent studies have indicated that the OX2 receptor also plays a role in reward processing and alcohol use. Similar to previous reports that pharmacological blockade of the OX1 receptor decreased alcohol seeking and drinking (Lawrence, 2010; Lawrence et al., 2006; Mahler et al., 2012), the selective OX2 antagonist TCS-OX2–29, although having no impact on cue-induced reinstatement of ethanol seeking, also selectively reduced self-administration of ethanol (Brown et al., 2013). Another OX2 selective antagonist JNJ-10397049 dose-dependently reduced ethanol self-administration, and also attenuated the acquisition, expression, and reinstatement of ethanol CPP and ethanol-induced hyperactivity in mice, whereas the OX1 selective SB-408124 showed no effect in these procedures (Shoblock et al., 2011). In another study, DORA almorexant as well as the 2-SORA LSN2424100 reduced breakpoints and ethanol consumption in progressive ratio operant experiments, whereas SB-334867 did not significantly affect the motivation to consume ethanol (Anderson et al., 2014). These findings strongly support the role of the OX2 receptor in the reward process and seem to suggest that dual antagonists would be the better option for these conditions. However, similar to the early OX1 selective antagonists such as SB-334867, some of the OX2 selective antagonists used in these studies have remaining OX1 activities, such as JNJ-10397049 (OX1 Kb = 1.1 μM) and LSN2424100 (OX1 Ki = 393 nM, Kb = 90.3 nM) (Fitch et al., 2014). Importantly, it should be noted that the OX2 involvement in reward is mainly observed with alcohol consumption thus far, but not the other drugs of addiction such as cocaine. In fact, OX2 selective TCS-OX2–29 failed to show any effects on cocaine seeking and self-administration (Gentile et al., 2018b; Prince et al., 2015; Smith et al., 2009). Therefore, the choice of dual or OX1 selective antagonists may depend on the specific drugs of addiction being treated. DORAs may be more pharmacologically effective for alcohol addiction, whereas 1-SORAs are more appropriate for cocaine addiction.
As sleep promoting drugs, dual orexin antagonists require administration right before bedtime. Even so, it has been reported that suvorexant causes significant next day drowsiness, and sometimes memory loss, which may significantly impair the next day activities such as driving and decision making. In fact, the FDA did not approve the higher doses (30 and 40 milligrams) of the drug largely due to concerns over this residual sedative effect. This strict administration requirement may make compliances even more challenging, when incompliance is already a significant issue in addiction treatment. Scientists have postulated that the long half-live of 12 hours may contribute to the increased risk of suvorexant to next day activity impairment. Efforts in developing dual antagonists with shorter half-lives have been reported to be underway which in theory would lead to reduced residual sedative effects. For example, Heptares Pharmaceuticals recently filed a patent on their dual antagonists that were predicted to have shorter half-lives in human (Congreve et al., 2014). However, these newer compounds will not address the sedative effects inherently associated with DORAs blocking both OX receptors. Together, these considerations would support the OX1 selective antagonists as the drugs of choice as addiction therapeutics.
A variety of OX1 selective antagonists have been reported thus far. Many of them are, in reality, the “by-products” of dual antagonist development from the pharmaceutical industry and understandably so, as sleep disorders are a significantly larger market to target than addiction. The incentives for the development of OX1 selective antagonists for the sole purpose of treating addiction may not be sufficiently high for the pharmaceutical industry. However, considering the extensive research and development that have been done in this area, many pharmaceutical companies may already have promising 1-SORA leads that can be readily developed, which would have lower preclinical development cost compared to totally new scaffolds. As the recent opioid epidemic draws more attention to drug addiction and abuse, it is expected that more resources will be allocated to addressing this pressing issue for the society. Given its critical role in reward and addiction, together with the effectiveness of orexin antagonists display against multiple drugs at various stages of addiction, the orexin system represents an important target for the development of novel and more effective addiction medications.
Figure 6.
Orexin-1 selective tetrahydroisoquinoline-based antagonists from Actelion and RTI.
Figure 7.
Morphinan-based OX1 antagonist from University of Tsukuba
Highlights.
Differential roles of OX1 and OX2 receptors are discussed
Orexin-1 selective antagonists disclosed to date are reviewed
Insights on OX1 or dual antagonists as addiction therapeutics are provided
Acknowledgements
This work is supported by the National Institute on Drug Abuse, National Institutes of Health, U.S. grants DA032837 and DA040693 to Y.Z and the Six Talent Peaks Project of Jiangsu Province, China (2016-SWYY-CXTD-008). We thank Dr. Thuy Nguyen for the proofreading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Aluisio L, Fraser I, Berdyyeva T, Tryputsen V, Shireman BT, Shoblock J, et al. (2014). Pharmacological or genetic orexin1 receptor inhibition attenuates MK-801 induced glutamate release in mouse cortex. Frontiers in neuroscience 8: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro G, Amantini D, Castiglioni E, Di Fibio R, & Pavone F (2010). (WO2010063663) N-{[(IR,4S,6R-3-(2-PYRIDINYLCARBONYL)-3-AZABICYCLO [4.1.0]HEPT-4-YL] METHYL}−2-HETEROARYLAMINE DERIVATIVES AND USES THEREOF. WO 2010/063663.
- Alvaro G, Amantini D, & stasi LP (2009). Pyridine derivatives used to treat orexin related disorders. WO 2009124956.
- Anderson RI, Becker HC, Adams BL, Jesudason CD, & Rorick-Kehn LM (2014). Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Frontiers in neuroscience 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SP, Aves SJ, Christopher JA, & Nonoo R (2016). Orexin Receptor Antagonists: Historical Perspectives and Future Opportunities. Curr Top Med Chem 16: 3438–3469. [DOI] [PubMed] [Google Scholar]
- Aranyi P, Balogh M, Batori S, Bence J, Finet M, Kaui Z, et al. (2005). Pyrimidine Derivatives as orexin receptors antagonists. WO 2005/075458.
- Aston-Jones G, Smith RJ, Moorman DE, & Richardson KA (2009). Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology 56 Suppl 1: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. (2010). Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res 1314: 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, et al. (2015). Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol 172: 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beig MI, Dampney BW, & Carrive P (2015). Both Ox1r and Ox2r orexin receptors contribute to the cardiovascular and locomotor components of the novelty stress response in the rat. Neuropharmacology 89: 146–156. [DOI] [PubMed] [Google Scholar]
- Bolli M, Boss C, Brotschi C, Heidmann B, Sifferlen T, & Williams J (2012). 2-(1,2,3-TRIAZOL-2-YL)BENZAMIDE AND 3-(1,2,3-TRIAZOL-2-YL)PICOLINAMIDE DERIVATIVES AS OREXIN RECEPTOR ANTAGONISTS. WO 2013/068935.
- Bonaventure P, Dugovic C, Shireman B, Preville C, Yun S, Lord B, et al. (2017). Evaluation of JNJ-54717793 a Novel Brain Penetrant Selective Orexin 1 Receptor Antagonist in Two Rat Models of Panic Attack Provocation. Front Pharmacol 8: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Shelton J, Yun S, Nepomuceno D, Sutton S, Aluisio L, et al. (2015a). Characterization of JNJ-42847922, a Selective Orexin-2 Receptor Antagonist, as a Clinical Candidate for the Treatment of Insomnia. J Pharmacol Exp Ther 354: 471–482. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, et al. (2015b). A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther 352: 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss C, Brisbare-Roch C, & Jenck F (2009). Biomedical application of orexin/hypocretin receptor ligands in neuroscience. J Med Chem 52: 891–903. [DOI] [PubMed] [Google Scholar]
- Boss C, & Roch C (2015). Recent trends in orexin research−−2010 to 2015. Bioorg Med Chem Lett 25: 2875–2887. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. (2005). Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102: 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. (2007). Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13: 150–155. [DOI] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, & Lawrence AJ (2013). Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol 16: 2067–2079. [DOI] [PubMed] [Google Scholar]
- Coate HR, Dvorak CA, Preville C, & Shireman BT (2014). Substituted piperidine compounds and their use as orexin receptor modulators. WO 2014/165065.
- Coleman PJ, Schreier JD, Roecker AJ, Mercer SP, McGaughey GB, Cox CD, et al. (2010). Discovery of 3,9-diazabicyclo[4.2.1]nonanes as potent dual orexin receptor antagonists with sleep-promoting activity in the rat. Bioorg Med Chem Lett 20: 4201–4205. [DOI] [PubMed] [Google Scholar]
- Congreve MS, Christopher JA, Tehan BG, Klair SS, & Aves SJ (2014). OREXIN RECEPTOR ANTAGONISTS. WO/2014/006402.
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, et al. (2009). Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther 330: 142–151. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Yun S, Bonaventure P, Shireman BT, & Lovenberg TW (2014). Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Frontiers in neuroscience 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etori K, Saito YC, Tsujino N, & Sakurai T (2014). Effects of a newly developed potent orexin-2 receptor-selective antagonist, compound 1 m, on sleep/wakefulness states in mice. Frontiers in neuroscience 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldhouse C, Glen A, Maine S, Fujimoto T, & Borbinson JS (2015). 1,2-Substituted cyclopentanes as orexin receptor antagonists. WO 2015/124932.
- Fitch TE, Benvenga MJ, Jesudason CD, Zink C, Vandergriff AB, Menezes MM, et al. (2014). LSN2424100: a novel, potent orexin-2 receptor antagonist with selectivity over orexin-1 receptors and activity in an animal model predictive of antidepressant-like efficacy. Frontiers in neuroscience 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futamura A, Nozawa D, Araki Y, Tamura Y, Tokura S, Kawamoto H, et al. (2017). Identification of highly selective and potent orexin receptor 1 antagonists derived from a dual orexin receptor 1/2 antagonist based on the structural framework of pyrazoylethylbenzamide. Bioorg Med Chem 25: 5203–5215. [DOI] [PubMed] [Google Scholar]
- Gatfield J, Brisbare-Roch C, Jenck F, & Boss C (2010). Orexin receptor antagonists: a new concept in CNS disorders? ChemMedChem 5: 1197–1214. [DOI] [PubMed] [Google Scholar]
- Gelin CF, Lebold TP, Shireman BT, & Ziff JM (2014). SUBSTITUTED 2-AZABICYCLES AND THEIR USE AS OREXIN RECEPTOR MODULATORS. WO/2014/165070.
- Gentile TA, Simmons SJ, Barker DJ, Shaw JK, Espana RA, & Muschamp JW (2018a). Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict Biol 23: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, et al. (2018b). Effects of Suvorexant, a Dual Orexin/Hypocretin Receptor Antagonist, on Impulsive Behavior Associated with Cocaine. Neuropsychopharmacology 43: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, & Winrow CJ (2012). International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev 64: 389–420. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, et al. (2011). Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PloS one 6: e16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, & Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, et al. (2000). A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96: 45–51. [DOI] [PubMed] [Google Scholar]
- Heifetz A, Morris GB, Biggin PC, Barker O, Fryatt T, Bentley J, et al. (2012). Study of human Orexin-1 and −2 G-protein-coupled receptors with novel and published antagonists by modeling, molecular dynamics simulations, and site-directed mutagenesis. Biochemistry (Mosc) 51: 3178–3197. [DOI] [PubMed] [Google Scholar]
- Holmqvist T, Akerman KE, & Kukkonen JP (2002). Orexin signaling in recombinant neuron-like cells. FEBS Lett 526: 11–14. [DOI] [PubMed] [Google Scholar]
- Jiang R, Song X, Bali P, Smith A, Bayona CR, Lin L, et al. (2012). Disubstituted piperidines as potent orexin (hypocretin) receptor antagonists. Bioorg Med Chem Lett 22: 3890–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Federici LM, Fitz SD, Renger JJ, Shireman B, Winrow CJ, et al. (2015). Orexin 1 and 2 Receptor Involvement in Co2-Induced Panic-Associated Behavior and Autonomic Responses. Depress Anxiety 32: 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenecka T, Jiang R, & Song X (2013). SUBSTITUTED PROLINES / PIPERIDINES AS OREXIN RECEPTOR ANTAGONISTS. WO2013/119639
- Kamenecka TM, He Y, Jiang R, Nguyen W, & Song X (2014a). SUBSTITUTED PROLINES / PIPERIDINES AS OREXIN RECEPTOR ANTAGONISTS. US2014/0364433.
- Kamenecka TM, He Y, Nguyen W, Jiang R, & Song X (2014b). SUBSTITUTED PROLINES / PIPERIDINES AS OREXIN RECEPTOR ANTAGONISTS. US2014/0364432.
- Kenny PJ (2011). Tobacco dependence, the insular cortex and the hypocretin connection. Pharmacol Biochem Behav 97: 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo SY, & Brown RM (2014). Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs 28: 713–730. [DOI] [PubMed] [Google Scholar]
- Koberstein R, Aissaoui H, Bur D, Clozel M, Fischli W, Jenck F, et al. (2003). Tetrahydroisoquinolines as Orexin Receptor Antagonists: Strategies for Lead Optimization by Solution-Phase Chemistry. Chimia 57: 270–275. [Google Scholar]
- Kodadek T, & Cai D (2010). Chemistry and biology of orexin signaling. Molecular bioSystems 6: 1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, & Herdon HJ (2004). Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br J Pharmacol 141: 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ (2010). Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain Res 1314: 124–129. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, & Oldfield B (2006). The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebold TP, Bonaventure P, & Shireman BT (2013). Selective orexin receptor antagonists. Bioorg Med Chem Lett 23: 4761–4769. [DOI] [PubMed] [Google Scholar]
- Levy KA, Brodnik ZD, Shaw JK, Perrey DA, Zhang Y, & Espana RA (2017). Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Moorman DE, Aston-Jones G, & Becker HC (2016). The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res 1636: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, & Saper CB (2002). Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci 22: 4568–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, & Aston-Jones G (2012). Multiple roles for orexin/hypocretin in addiction. Prog Brain Res 198: 79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P, Borroni E, Pinard E, Wettstein JG, & Knoflach F (2009). Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol 76: 618–631. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Roche O, Marcuz A, Kratzeisen C, Wettstein JG, & Bissantz C (2010). Mapping the binding pocket of dual antagonist almorexant to human orexin 1 and orexin 2 receptors: comparison with the selective OX1 antagonist SB-674042 and the selective OX2 antagonist N-ethyl-2-[(6-methoxy-pyridin-3-yl)-(toluene-2-sulfonyl)-amino]-N-pyridin-3-ylmethyl-acetamide (EMPA). Mol Pharmacol 78: 81–93. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25. [DOI] [PubMed] [Google Scholar]
- McElhinny CJ Jr., Lewin AH, Mascarella SW, Runyon S, Brieaddy L, & Carroll (2012). Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorg Med Chem Lett 22: 6661–6664. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, & Melotto S (2014). Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use. Frontiers in neuroscience 8: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morairty SR, Revel FG, Malherbe P, Moreau JL, Valladao D, Wettstein JG, et al. (2012). Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PloS one 7: e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Yamamoto N, Yata M, Ohrui S, Okada T, Saitoh T, et al. (2017). Design and Synthesis of Potent and Highly Selective Orexin 1 Receptor Antagonists with a Morphinan Skeleton and Their Pharmacologies. J Med Chem 60: 1018–1040. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, & Goto K (1999). Distribution of orexin neurons in the adult rat brain. Brain Res 827: 243–260. [DOI] [PubMed] [Google Scholar]
- Ohrui S, Yamamoto N, Saitoh T, Kutsumura N, Nagumo Y, Irukayama-Tomobe Y, et al. (2018). Essential structure of orexin 1 receptor antagonist YNT-707, Part II: Drastic effect of the 14-hydroxy group on the orexin 1 receptor antagonistic activity. Bioorg Med Chem Lett 28: 774–777. [DOI] [PubMed] [Google Scholar]
- Perrey DA, Decker AM, Li JX, Gilmour BP, Thomas BF, Harris DL, et al. (2015a). The importance of the 6- and 7-positions of tetrahydroisoquinolines as selective antagonists for the orexin 1 receptor. Bioorg Med Chem 23: 5709–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, Decker AM, & Zhang Y (2018). Synthesis and Evaluation of Orexin-1 Receptor Antagonists with Improved Solubility and CNS Permeability. ACS chemical neuroscience 9: 587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, German NA, Decker AM, Thorn D, Li JX, Gilmour BP, et al. (2015b). Effect of 1-substitution on tetrahydroisoquinolines as selective antagonists for the orexin-1 receptor. ACS chemical neuroscience 6: 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, German NA, Gilmour BP, Li JX, Harris DL, Thomas BF, et al. (2013). Substituted tetrahydroisoquinolines as selective antagonists for the orexin 1 receptor. J Med Chem 56: 6901–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, Gilmour BP, Runyon SP, Thomas BF, & Zhang Y (2011). Diaryl urea analogues of SB-334867 as orexin-1 receptor antagonists. Bioorg Med Chem Lett 21: 2980–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey DA, Gilmour BP, Thomas BF, & Zhang Y (2014). Toward the Development of Bivalent Ligand Probes of Cannabinoid CB1 and Orexin OX1 Receptor Heterodimers. ACS medicinal chemistry letters 5: 634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, et al. (2001). 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett 11: 1907–1910. [DOI] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, & Espana RA (2015). Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS chemical neuroscience 6: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheem IT, Breslin MJ, Bruno J, Cabalu TD, Cooke A, Cox CD, et al. (2015). Discovery of piperidine ethers as selective orexin receptor antagonists (SORAs) inspired by filorexant. Bioorg Med Chem Lett 25: 444–450. [DOI] [PubMed] [Google Scholar]
- Roecker AJ, & Coleman PJ (2008). Orexin receptor antagonists: medicinal chemistry and therapeutic potential. Curr Top Med Chem 8: 977–987. [DOI] [PubMed] [Google Scholar]
- Sakurai T (2007). The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8: 171–181. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585. [DOI] [PubMed] [Google Scholar]
- Scammell TE, & Winrow CJ (2011). Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol 51: 243–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, et al. (2011). Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl) 215: 191–203. [DOI] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, et al. (2001). SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol 132: 1179–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, & Aston-Jones G (2009). Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci 30: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi LP, Artusi R, Bovino C, Buzzi B, Canciani L, Caselli G, et al. (2013). Discovery, synthesis, selectivity modulation and DMPK characterization of 5-azaspiro[2.4]heptanes as potent orexin receptor antagonists. Bioorg Med Chem Lett 23: 2653–2658. [DOI] [PubMed] [Google Scholar]
- Stasi PL, & Rovati L (2011). Spiro amino compounds suitable for the treatment of inter alia sleep disorders and drug addiction. WO2011006960Rottapharm S.P.A.
- Steiner MA, Gatfield J, Brisbare-Roch C, Dietrich H, Treiber A, Jenck F, et al. (2013a). Discovery and Characterization of ACT-335827, an Orally Available, Brain Penetrant Orexin Receptor Type 1 Selective Antagonist. ChemMedChem 8: 898–903. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Lecourt H, & Jenck F (2013b). The dual orexin receptor antagonist almorexant, alone and in combination with morphine, cocaine and amphetamine, on conditioned place preference and locomotor sensitization in the rat. Int J Neuropsychopharmacol 16: 417–432. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Sciarretta C, Brisbare-Roch C, Strasser DS, Studer R, & Jenck F (2013c). Examining the role of endogenous orexins in hypothalamus-pituitary-adrenal axis endocrine function using transient dual orexin receptor antagonism in the rat. Psychoneuroendocrinology 38: 560–571. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Nozawa D, Futamura A, Nishikawa-Shimono R, Abe M, Hattori N, et al. (2015). Discovery and in vitro and in vivo profiles of N-ethyl-N-[2-[3-(5-fluoro-2-pyridinyl)-1H-pyrazol-1-yl]ethyl]-2-(2H-1,2,3-triazol −2-yl)-benzamide as a novel class of dual orexin receptor antagonist. Bioorg Med Chem 23: 1260–1275. [DOI] [PubMed] [Google Scholar]
- Tsujino N, & Sakurai T (2009). Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev 61: 162–176. [DOI] [PubMed] [Google Scholar]
- Walker LC, & Lawrence AJ (2017). The Role of Orexins/Hypocretins in Alcohol Use and Abuse. Curr Top Behav Neurosci 33: 221–246. [DOI] [PubMed] [Google Scholar]
- Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. (2013). Strategies to address low drug solubility in discovery and development. Pharmacol Rev 65: 315–499. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. (2003). Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 38: 715–730. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, & Renger JJ (2014). Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol 171: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Ohrui S, Okada T, Yata M, Saitoh T, Kutsumura N, et al. (2017). Essential structure of orexin 1 receptor antagonist YNT-707, Part I: Role of the 4,5-epoxy ring for binding with orexin 1 receptor. Bioorg Med Chem Lett 27: 4176–4179. [DOI] [PubMed] [Google Scholar]
- Yin J, Babaoglu K, Brautigam CA, Clark L, Shao Z, Scheuermann TH, et al. (2016). Structure and ligand-binding mechanism of the human OX1 and OX2 orexin receptors. Nat Struct Mol Biol 23: 293–299. [DOI] [PubMed] [Google Scholar]