Abstract
Background
The prevalence and incidence of type 1 diabetes mellitus (T1DM) in all age groups and the prevalence of metabolic syndrome in patients with T1DM in Korea were estimated.
Methods
The incidence and prevalence of T1DM between 2007 and 2013 were calculated using the Korean National Health Insurance Service (NHIS) datasets of claims. Clinical characteristics and prevalence of metabolic syndrome in individuals with T1DM between 2009 and 2013 were determined using the database of NHIS preventive health checkups.
Results
The prevalence of T1DM in Korea between 2007 and 2013 was 0.041% to 0.047%. The annual incidence rate of T1DM in Korea in 2007 to 2013 was 2.73 to 5.02/100,000 people. Although the incidence rate of typical T1DM was highest in teenagers, it remained steady in adults over 30 years of age. In contrast, the incidence rate of atypical T1DM in 2013 was higher in people aged 40 years or older than in younger age groups. Age- and sex-adjusted prevalence of metabolic syndrome in patients with T1DM was 51.65% to 55.06% between 2009 and 2013.
Conclusion
T1DM may be more common in Korean adults than previously believed. Metabolic syndrome may be a frequent finding in individuals with T1DM in Korea.
Keywords: Diabetes mellitus, type 1; Incidence; Korea; Metabolic syndrome; Prevalence
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is associated with an increased risk of cardiovascular mortality and morbidity [1,2]. Therefore, estimating the epidemiology and disease burden of T1DM and reviewing the metabolic characteristics of T1DM patients may have a significant impact on public health.
The immunologic features and epidemiology of T1DM vary considerably among Asians compared to Caucasians [3]. The lowest incidence of T1DM in the world has been reported in East Asian countries, including Korea, China, and Japan [3]. In addition to the typical autoimmune T1DM, there is also a varying incidence of non-autoimmune types of T1DM associated with insulin deficiency, such as fulminant T1DM and virus-induced T1DM, and other various forms of atypical diabetes in Asian countries, especially in older children and adults[3].
There have been reports suggesting that the number of patients with T1DM may be increasing in Asian children and adolescents [4,5,6]. Nevertheless, reports on T1DM epidemiology in Korea are insufficient, and most of them focused mainly on individuals aged less than 15 years [4,7,8]. Also, the only study covering all age groups in Korea included data for only 26 months [1]. Since T1DM has been reported to be more common in adults in Western countries than previously believed [9,10,11], more information on T1DM epidemiology including all age groups is required to confirm whether this is also the case in Korea.
Metabolic syndrome (MetS) increases the risk of cardiovascular diseases and mortality in the general population and in patients with type 2 diabetes mellitus (T2DM) [2,12,13]. Studies in Western populations have reported that MetS is common in individuals with T1DM [2,12,13] and the prevalence of MetS in T1DM may increase through weight gain as the duration of the disease increases [12]. However, the prevalence of MetS in patients with T1DM in Asian countries including Korea remains to be established.
Therefore, we determined the prevalence and incidence of T1DM at all ages in Korea, and estimated the prevalence of MetS in T1DM patients compared with age- and sex-matched T2DM patients using the Korean National Health Insurance Service (NHIS) database.
METHODS
Data sources
The NHIS datasets of claims and preventive health checkups in Korea from January 2002 to December 2015 were used. We set a 5-year washout period from 2002 to 2006 to confirm the incident cases.
The NHIS, a single-payer system for all residents in Korea, covers more than 99% of the Koreans, who are approximately 50 million individuals. Information including anonymous identification numbers, demographics, primary and secondary diagnoses, dates of hospital visits, hospitalization, procedures, and prescriptions is available from the NHIS database of claims. The diagnoses were classified using the International Classification of Disease, 10th Revision (ICD-10).
The database of NHIS preventive health checkups, consisting of more than 10 million Koreans, includes (1) employee subscribers and regional insurance subscribers who are regional householders, (2) employee subscribers' dependents and household members (40 years or older), and (3) medical aid beneficiaries who are householders (19 to 64 years old) or household members (41 to 64 years old) [14]. It contains information on demographics; smoking history; anthropometric measurements including height, weight, waist circumference (WC), and blood pressure (BP); laboratory results such as fasting plasma glucose, total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides (TGs), and routine urinalysis; and insurance claim data. Among these, data on WC, TG, HDL-C, and LDL-C were collected since 2009.
This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB File number; SMC 2016-06-133). An exemption from informed consent was granted by the board because all data were analyzed anonymously.
Definition of type 1 diabetes mellitus and study cohort
T1DM was defined as the presence of all three of the following: (1) at least one claim under ICD-10 code E10; (2) three or more claims for the prescription of insulin; and (3) at least one claim for prescription of insulin between 365 and 730 days after the first prescription of insulin. Among these, patients who had claims under ICD-10 codes E11–-14 within 730 days after the first prescription of insulin were excluded because they were more likely to have ketosis-prone T2DM [15]. Those who underwent total or partial pancreatectomy were also excluded.
The incidence date of T1DM was considered to be the date when insulin was prescribed for the first time in patients that met the criteria of T1DM. Incident cases were confirmed by reviewing data from 2002 to the incidence date. Therefore, disease-free observation periods of 5 or more years were obtained. The datasets from 2014 to 2015 were also used to determine the incidence and prevalence of T1DM between 2007 and 2013 because we had to review the information on whether insulin was prescribed between 365 and 730 days after the incidence date or whether claims under ICD-10 codes E11–14 were present within 730 days after the incidence date to define a T1DM case.
In July 2011, the Korean NHIS promoted the Type 1 Diabetes Registration Project for the Reimbursement of Consumable Materials [1,4,16]. Registered patients received financial aid for consumable materials. Patients were required to be on insulin and satisfy one of the following criteria for registration: fasting serum C-peptide levels ≤0.6 ng/mL, stimulated serum C-peptide levels ≤1.8 ng/mL, 24-hour urine C-peptide values <30 µg/day, history of diabetic ketoacidosis at diagnosis, autoantibody positivity for glutamate decarboxylase (GAD), insulin, or islet cell. Since the introduction of this program in 2011, the accuracy of the ICD-10 diagnostic codes according to the type of diabetes at diagnosis has improved. Thus, T1DM patients in 2013 were classified into two categories; typical and atypical cases based on diagnostic codes assigned in 2011 to 2012. Among the individuals that satisfied the criteria of T1DM, those who had at least one claim under ICD-10 codes E11–14 1 to 2 years before the incidence date (from −730 to −365 days) were defined as atypical T1DM because slowly progressive cases including latent autoimmune diabetes (LADA) or long-standing T2DM with insulin dependency were more likely to be included in this group than patients with typical T1DM. It is impossible to distinguish completely between slowly progressive T1DM and long-standing T2DM cases based on NHIS data. The remaining cases with no claim under ICD-10 codes E11–14 1 to 2 years before the incidence date were considered patients with typical T1DM.
Ascertainment of clinical characteristics and prevalence of metabolic syndrome in the population with type 1 diabetes mellitus
Using the database of NHIS preventive health checkups, clinical characteristics of patients with T1DM were determined including age, sex, smoking history, body weight, body mass index (BMI), WC, BP, fasting plasma glucose, total cholesterol, TG, HDL-C, LDL-C, and urine dipstick positivity for proteinuria. BMI was derived from body weight in kilograms divided by height in meters squared (kg/m2).
MetS was defined according to the 2005 revision of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria with Asian-specific cut off values for abdominal obesity (WC ≥90 cm in men or ≥80 cm in women) [17,18]. Since all patients with T1DM were using insulin and satisfied the component of fasting plasma glucose ≥100 mg/dL or use of insulin/oral diabetic agents by definition, only two additional ones among the other four components (abdominal obesity; TG ≥150 mg/dL or medication use; HDL-C <40 mg/dL in men or <50 mg/dL in women or medication use; BP ≥130/85 mm Hg or antihypertensive medication use) were needed for the diagnosis of MetS. Statin, fibrate, and ezetimibe were included as medications to define the components of hypertriglyceridemia and low HDL-C. Prevalence of MetS and its individual components in T1DM patients was calculated separately for men and women. Age and sex-adjusted prevalence of MetS was also presented after direct adjustment for the Korean population in the year 2010. For comparison, patients with T1DM and patients with T2DM were matched 1:2 by age and sex for adjustment. T2DM was defined according to the previous report [19]. Prevalence of MetS and its components was also estimated in age- and sex-matched T2DM patients and the data were compared with those for T1DM patients.
Statistical analyses
The annual prevalence of T1DM was calculated by dividing the number of T1DM cases in the NHIS database by the number of the total population in the NHIS program in a certain year. The annual incidence rate of T1DM was estimated by dividing the number of incident T1DM patients by the number of enrolled individuals who did not have T1DM from 2002 to 1 year prior to the given year. SAS software version 9.3 (SAS Institute, Cary, NC, USA) was used for statistical analyses.
RESULTS
Prevalence of type 1 diabetes mellitus
The number of total source population covered by NHIS was 49.6 million in 2007 and 51.3 million in 2013. The number of T1DM cases ranged from 20,814 to 23,932 between 2007 and 2013 (Table 1). The prevalence of T1DM was 0.043% in 2007 and 0.041% in 2010, and increased to 0.047% in 2013. The prevalence of T1DM was 1.15 to 1.18 times higher in males than in females. The mean age of the patients with T1DM was 51.36±20.1 years in 2013.
Table 1. Prevalence of type 1 diabetes mellitus in Korea according to National Health Insurance Service database of claims between 2007 and 2013.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | P for trend | |
|---|---|---|---|---|---|---|---|---|
| Total population | ||||||||
| Total number | 49,570,064 | 49,884,461 | 50,175,381 | 50,455,745 | 50,771,065 | 51,020,532 | 51,289,103 | |
| Male | 24,864,222 (50.16) | 25,016,666 (50.15) | 25,154,436 (50.13) | 25,287,443 (50.12) | 25,438,317 (50.10) | 25,542,446 (50.06) | 25,669,471 (50.05) | |
| Female | 24,705,842 (49.84) | 24,867,795 (49.85) | 25,020,945 (49.87) | 25,168,302 (49.88) | 25,332,748 (49.90) | 25,478,086 (49.94) | 25,619,632 (49.95) | |
| Age, yr | 36.08±20.39 | 36.53±20.26 | 37.07±20.54 | 37.56±20.69 | 38.02±20.74 | 38.46±20.84 | 38.94±20.96 | |
| Prevalent cases | ||||||||
| Total number | 21,131 | 21,935 | 21,497 | 20,814 | 21,551 | 23,129 | 23,932 | |
| Male | 11,363 (53.77) | 11,820 (53.89) | 11,683 (54.35) | 11,178 (53.70) | 11,587 (53.77) | 12,427 (53.73) | 12,954 (54.13) | |
| Female | 9,768 (46.23) | 10,115 (46.11) | 9,814 (45.65) | 9,636 (46.30) | 9,964 (46.23) | 10,702 (46.27) | 10,978 (45.87) | |
| Age, yr | 54.08±18.44 | 54.00±18.07 | 53.89±18.67 | 52.93±19.32 | 52.54±19.49 | 51.96±19.74 | 51.36±20.1 | |
| Prevalence (per 100,000) | ||||||||
| Total | 42.63 | 43.97 | 42.84 | 41.25 | 42.45 | 45.33 | 46.66 | <0.0001 |
| Prevalence (per 100,000) by sex | ||||||||
| Male | 45.70 | 47.25 | 46.45 | 44.20 | 45.55 | 48.65 | 50.46 | <0.0001 |
| Female | 39.54 | 40.68 | 39.22 | 38.29 | 39.33 | 42.00 | 42.85 | <0.0001 |
Values are presented as number (%) or mean±standard deviation.
Incidence of type 1 diabetes mellitus
The number of incident T1DM cases annually ranged from 1,400 to 2,489 between 2007 and 2013 (Table 2). The incidence of T1DM was calculated to be 5.02 per 100,000 people in 2007 and 2.73 per 100,000 in 2013. It showed a trend of overall increase from 2007 to 2013 in youth aged 19 years or younger whereas an overall decrease in T1DM incidence was seen in adults aged 20 years or older. The incidence rate in males was 1.21 to 1.41 times higher than that in females. The mean age of the population with incident T1DM was 49.62±21.74 years in 2013.
Table 2. Incidence of type 1 diabetes mellitus in Korea according to National Health Insurance Service database of claims between 2007 and 2013.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | P for trend | |
|---|---|---|---|---|---|---|---|---|
| Incident cases | ||||||||
| Total number | 2,489 | 2,284 | 2,124 | 1,767 | 2,102 | 1,802 | 1,400 | |
| Male | 1,375 (55.24) | 1,254 (54.90) | 1,195 (56.26) | 1,003 (56.76) | 1,202 (57.18) | 995 (55.22) | 820 (58.57) | |
| Female | 1,114 (44.76) | 1,030 (45.10) | 929 (43.74) | 764 (43.24) | 900 (42.82) | 807 (44.78) | 580 (41.43) | |
| Age, yr | 54.24±20.60 | 54.13±17.37 | 53.97±17.73 | 52.55±18.50 | 52.49±19.31 | 52.27±21.00 | 49.62±21.74 | |
| Incidence rate (per 100,000) | ||||||||
| Total | 5.02 | 4.58 | 4.23 | 3.5 | 4.14 | 3.53 | 2.73 | <0.0001 |
| Incidence rate (per 100,000) by sex | ||||||||
| Male | 5.53 | 5.01 | 4.75 | 3.97 | 4.73 | 3.90 | 3.19 | <0.0001 |
| Female | 4.51 | 4.14 | 3.71 | 3.04 | 3.55 | 3.17 | 2.26 | <0.0001 |
| Incidence rate (per 100,000) by age | ||||||||
| 0–19 yr | 1.47 | 1.28 | 1.33 | 1.42 | 1.82 | 1.82 | 1.76 | <0.0001 |
| 20 yr or older | 6.17 | 5.62 | 5.12 | 4.12 | 4.81 | 4.01 | 2.99 | <0.0001 |
Values are presented as number (%) or mean±standard deviation.
Incidence and prevalence of typical and atypical type 1 diabetes mellitus in 2013
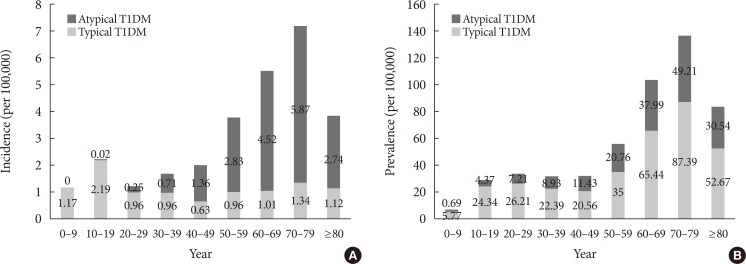
Among the total 1,400 cases of incident T1DM in 2013, 565 (40.36%) were typical and the remaining 835 (59.64%) were atypical T1DM (Table 3). The mean age of the population with incident typical T1DM (35.72±22.55 years) was lower than that of individuals with incident atypical T1DM (58.83±13.58 years). The incidence rate of typical T1DM according to age group showed a peak in the teenage group (Fig. 1A). However, of the 565 incident typical T1DM patients in 2013, 310 cases (54.9%) were aged 30 years or older. The incidence rate of atypical T1DM was highest in the age group of 70s.
Table 3. Incidence and prevalence of typical and atypical T1DM in Korea according to National Health Insurance Service database of claims in 2013.
| Incident T1DM cases | Prevalent T1DM cases | |||||
|---|---|---|---|---|---|---|
| Typical T1DM | Atypical T1DM | Total T1DM | Typical T1DM | Atypical T1DM | Total T1DM | |
| Total number | 565 | 835 | 1,400 | 16,190 | 7,742 | 23,932 |
| Male | 320 (56.64) | 500 (59.88) | 820 (58.57) | 8,779 (54.22) | 4,175 (53.93) | 12,954 (54.13) |
| Female | 245 (43.36) | 335 (40.12) | 580 (41.43) | 7,411 (45.78) | 3,567 (46.07) | 10,978 (45.87) |
| Age, yr | 35.72±22.55 | 58.83±13.58 | 49.62±21.74 | 49.48±20.78 | 55.24±17.55 | 51.36±20.10 |
| Rate (per 100,000) | ||||||
| Male | 1.25 | 1.95 | 3.19 | 34.20 | 16.26 | 50.46 |
| Female | 0.96 | 1.31 | 2.26 | 28.93 | 13.92 | 42.85 |
| Total | 1.10 | 1.63 | 2.73 | 31.57 | 15.09 | 46.66 |
Values are presented as number (%) or mean±standard deviation.
T1DM, type 1 diabetes mellitus.
Fig. 1. The incidence rate and prevalence of typical and atypical type 1 diabetes mellitus (T1DM) according to age groups in 2013. (A) The incidence rate of typical and atypical T1DM according to age groups in 2013. (B) The prevalence of typical and atypical T1DM according to age groups in 2013.
Of the 23,932 patients with prevalent T1DM in 2013, 16,190 (67.65%) had typical T1DM while the remaining 7,742 (32.35%) had atypical T1DM (Table 3). The mean age of individuals with typical T1DM was 49.48±20.78 years compared with 55.24±17.55 years for those with atypical T1DM. The prevalence of typical T1DM by age group showed a small peak in the teens and 20s, and a higher peak in 70s (Fig. 1B). The prevalence of atypical T1DM by age group had the highest value in the age group of 70s. The incidence of typical T1DM was 1.30 times higher in males than in females whereas that of atypical T1DM was 1.49 times higher in males (Table 3). Similarly, the prevalence of typical T1DM was 1.18 times higher in males than in females, and the prevalence of atypical T1DM was 1.17 times higher in males.
Clinical characteristics of patients with type 1 diabetes mellitus
Clinical characteristics of patients with prevalent T1DM covered by the database of NHIS preventive health checkups are summarized in Table 4. In 2007, among the source population of 7,878,249 who had health checkups within the national preventive health care system, 2,998 (0.038%) had prevalent T1DM. In 2013, 4,910 (0.042%) of total 11,739,899 individuals were prevalent T1DM patients. Of the 4,910 individuals with prevalent T1DM in 2013, 2,921 (59.49%) were males. The mean age of the prevalent T1DM patients in the preventive health checkup data ranged from 55.89 to 58.35 years between 2007 and 2013. The BMI and the WC showed similar values throughout the study periods; however, systolic BP, diastolic BP, and total cholesterol tended to decrease with time. Fasting glucose, TGs, and LDL-C also showed a tendency to decrease between 2009 and 2013. Although improved from the rate of 55.44% in 2009, 48.51% of T1DM patients in 2013 had LDL-C levels higher than 100 mg/dL. Urine dipstick positivity for proteinuria was observed in 13.17% to 16.91% of patients with prevalent T1DM between 2007 and 2013.
Table 4. Clinical characteristics of patients with prevalent T1DM according to national preventive health care.
| Year | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|---|---|
| Total population in national preventive health care database | |||||||
| Number | 7,878,249 | 9,751,365 | 10,490,491 | 11,447,468 | 11,687,229 | 12,144,200 | 11,739,899 |
| Men | 4,563,167 (57.92) | 5,525,658 (56.67) | 5,785,632 (55.15) | 6,293,856 (54.98) | 6,408,686 (54.83) | 6,615,411 (54.47) | 6,385,819 (54.39) |
| Age, yr | 45.89±14.34 | 45.67±14.01 | 47.11±14.13 | 47.46±13.90 | 47.66±14.00 | 48.21±13.90 | 48.38±14.16 |
| Clinical characteristics of patients with prevalent T1DM | |||||||
| Number | 2,998 | 3,843 | 4,235 | 4,220 | 4,480 | 5,198 | 4,910 |
| Men | 1,813 (60.47) | 2,323 (60.45) | 2,482 (58.61) | 2,486 (58.91) | 2,646 (59.06) | 3,081 (59.27) | 2,921 (59.49) |
| Women | 1,185 (39.53) | 1,520 (39.55) | 1,753 (41.39) | 1,734 (41.09) | 1,834 (40.94) | 2,117 (40.73) | 1,989 (40.51) |
| Age, yr | 57.30±13.31 | 56.56±13.37 | 58.35±12.72 | 57.56±13.17 | 57.44±13.64 | 56.80±13.93 | 55.89±14.35 |
| Current smoker | 683 (22.78) | 854 (22.22) | 941 (22.22) | 959 (22.73) | 1,017 (22.7) | 1,209 (23.26) | 1,114 (22.69) |
| Body weight, kg | 63.59±10.29 | 63.9±10.57 | 64.02±10.48 | 64.11±10.94 | 64.52±11.01 | 64.23±11.28 | 64.44±11.64 |
| BMI, kg/m2 | 24.12±3.23 | 24.10±3.24 | 24.29±3.3 | 24.23±3.34 | 24.27±3.34 | 24.10±3.38 | 24.05±3.44 |
| Waist circumference, cm | NA | NA | 84.49±8.94 | 84.11±9.23 | 84.13±9.39 | 83.61±9.57 | 83.34±9.75 |
| Systolic BP, mm Hg | 129.07±17.14 | 128.20±16.97 | 127.71±16.52 | 127.37±16.42 | 127.26±16.31 | 126.55±16.49 | 125.86±15.84 |
| Diastolic BP, mm Hg | 77.80±10.22 | 77.32±10.01 | 77.03±10.00 | 76.76±10.05 | 76.45±10.01 | 75.98±9.97 | 75.62±9.72 |
| Fasting glucose, mg/dL | 158.50±72.60 | 152.74±69.07 | 155.03±68.33 | 151.45±66.51 | 151.41±66.55 | 150.10±66.15 | 150.63±67.58 |
| Total cholesterol, mg/dL | 191.73±42.29 | 188.46±40.75 | 189.10±41.12 | 185.55±39.64 | 183.26±40.36 | 179.97±39.08 | 179.69±39.70 |
| Triglyceride, mg/dL | NA | NA | 121.0 (82.0–181.0) | 114.0 (79.0–169.0) | 112.0 (77.0–170.0) | 110.0 (75.0–168.0) | 108.0 (72.0–162.0) |
| HDL-C, mg/dL | NA | NA | 52.79±22.57 | 51.96±16.65 | 52.18±14.71 | 52.30±16.08 | 52.77±15.65 |
| LDL-C, mg/dL | NA | NA | 107.02±41.90 | 106.39±37.68 | 103.98±39.56 | 101.03±35.10 | 100.88±34.79 |
| LDL-C ≥100 mg/dL | NA | NA | 2,348 (55.44) | 2,321 (55.00) | 2,351 (52.48) | 2,526 (48.6) | 2,382 (48.51) |
| Proteinuria (urine dipstick positivity) | 445 (14.84) | 506 (13.17) | 716 (16.91) | 641 (15.19) | 717 (16.00) | 781 (15.03) | 690 (14.05) |
Values are presented as number (%), mean±standard deviation, or median (interquartile range).
T1DM, type 1 diabetes mellitus; BMI, body mass index; BP, blood pressure; NA, not available; HDL-C, high density lipoprotein cholesterol; LDL-C, low density
Prevalence of metabolic syndrome in patients with type 1 diabetes mellitus in comparison with that in type 2 diabetes mellitus
The age and sex-adjusted prevalence of MetS in prevalent T1DM patients was 55.06% in 2013 and 53.99% in 2009 (Table 5). When we calculated the prevalence of MetS separately for typical and atypical T1DM patients in 2013, the age and sex-adjusted prevalence of MetS in patients with prevalent atypical T1DM was 57.73%, and that in patients with prevalent typical T1DM was 53.57% (Supplementary Table 1). Although the prevalence of MetS in T1DM was 15.72% to 19.51% lower than that in prevalent T2DM between 2009 and 2013, more than half of the patients with T1DM were found to have MetS (Table 5). When we estimated the prevalence of individual components of MetS, the prevalence of hypertriglyceridemia differed the most between patients with prevalent T1DM and T2DM, showing a difference of 8.98% to 11.93%. On the other hand, the prevalence of high BP in individuals with T1DM was comparable to that in age- and sex-matched T2DM patients. Women had a higher prevalence of MetS than men throughout the study periods; however, the prevalence of MetS in women with T1DM showed a trend of decreasing over the 5 years whereas the prevalence of MetS in male T1DM patients increased from 59.87% in 2009 to 62.44% in 2013. In T1DM patients, the overall decrease in prevalence of abdominal obesity over the 5 years was noted only in women (Supplementary Fig. 1A). The prevalence of high BP showed a trend of decrease in T1DM patients overall and women with T2DM, but has been increasing since 2011 in men with T2DM (Supplementary Fig. 1B). Since 2010, the prevalence of hypertriglyceridemia has been increasing in both types of diabetes (Supplementary Fig. 1C). Trends of increase were observed for the prevalence of low HDL-C in men with T1DM and T2DM patients of both sexes (Supplementary Fig. 1D).
Table 5. Prevalence of metabolic syndrome and its components in patients with prevalent T1DM compared to those with prevalent T2DM according to national preventive health care database between 2009 and 2013.
| In patients with prevalent T1DM | In patients with age and sex-matcheda prevalent T2DM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2009 | 2010 | 2011 | 2012 | 2013 | |
| Prevalence of metabolic syndrome | ||||||||||
| Total, unadjusted | 2,890 (68.24) | 2,836 (67.20) | 3,020 (67.41) | 3,498 (67.30) | 3,313 (67.47) | 6,400 (75.56) | 6,439 (76.29) | 6,809 (75.99) | 7,990 (76.86) | 7,621 (77.61) |
| Men | 1,486 (59.87) | 1,474 (59.29) | 1,618 (61.15) | 1,881 (61.05) | 1,824 (62.44) | 3,486 (70.23) | 3,537 (71.14) | 3,740 (70.67) | 4,466 (72.48) | 4,278 (73.25) |
| Women | 1,404 (80.09) | 1,362 (78.55) | 1,402 (76.44) | 1,617 (76.38) | 1,489 (74.86) | 2,914 (83.11) | 2,902 (83.68) | 3,069 (83.67) | 3,524 (83.23) | 3,343 (83.99) |
| Total, age and sex-adjusted, %b | 53.99 | 51.65 | 51.97 | 53.50 | 55.06 | 69.71 | 70.47 | 70.73 | 73.01 | 74.17 |
| Prevalence of individual components of metabolic syndrome | ||||||||||
| Abdominal obesityc | 1,908 (45.05) | 1,848 (43.79) | 1,939 (43.28) | 2,126 (40.90) | 1,948 (39.67) | 4,096 (48.87) | 4,103 (49.30) | 4,285 (48.34) | 4,884 (47.67) | 4,689 (48.47) |
| Men | 775 (31.22) | 766 (30.81) | 832 (31.44) | 926 (30.06) | 886 (30.33) | 1,782 (36.26) | 1,850 (37.83) | 1,869 (35.79) | 2,213 (36.56) | 2,163 (37.72) |
| Women | 1,133 (64.63) | 1,082 (62.40) | 1,107 (60.36) | 1,200 (56.68) | 1,062 (53.39) | 2,314 (66.72) | 2,253 (65.63) | 2,416 (66.34) | 2,671 (63.70) | 2,526 (64.13) |
| BP ≥130/85 mm Hg or medication use | 3,071 (72.51) | 2,993 (70.92) | 3,189 (71.18) | 3,568 (68.64) | 3,349 (68.21) | 6,136 (73.20) | 6,136 (73.72) | 6,420 (72.43) | 7,464 (72.85) | 6,920 (71.53) |
| Men | 1,779 (71.68) | 1,735 (69.79) | 1,878 (70.98) | 2,095 (68.00) | 2,032 (69.57) | 3,632 (73.91) | 3,636 (74.36) | 3,823 (73.21) | 4,484 (74.08) | 4,217 (73.53) |
| Women | 1,292 (73.70) | 1,258 (72.55) | 1,311 (71.48) | 1,473 (69.58) | 1,317 (66.21) | 2,504 (72.20) | 2,500 (72.82) | 2,597 (71.31) | 2,980 (71.07) | 2,703 (68.62) |
| Triglyceride ≥150 mg/dL or medicationd use | 2,357 (55.66) | 2,287 (54.19) | 2,456 (54.82) | 2,919 (56.16) | 2,776 (56.54) | 5,418 (64.64) | 5,455 (65.54) | 5,917 (66.75) | 6,930 (67.64) | 6,610 (68.33) |
| Men | 1,270 (51.17) | 1,259 (50.64) | 1,364 (51.55) | 1,630 (52.90) | 1,562 (53.47) | 3,124 (63.57) | 3,170 (64.83) | 3,460 (66.26) | 4,083 (67.45) | 3,907 (68.13) |
| Women | 1,087 (62.01) | 1,028 (59.28) | 1,092 (59.54) | 1,289 (60.89) | 1,214 (61.04) | 2,294 (66.15) | 2,285 (66.56) | 2,457 (67.46) | 2,847 (67.90) | 2,703 (68.62) |
| HDL-C | 2,273 (53.67) | 2,286 (54.17) | 2,454 (54.78) | 2,949 (56.73) | 2,849 (58.02) | 4,767 (56.87) | 4,873 (58.55) | 5,272 (59.48) | 6,337 (61.85) | 6,153 (63.60) |
| Men <40 mg/dL or medicationd use | 1,098 (44.24) | 1,126 (45.29) | 1,245 (47.05) | 1,518 (49.27) | 1,500 (51.35) | 2,379 (48.41) | 2,485 (50.82) | 2,684 (51.40) | 3,308 (54.65) | 3,277 (57.14) |
| Women <50 mg/dL or medicationd use | 1,175 (67.03) | 1,160 (66.90) | 1,209 (65.92) | 1,431 (67.60) | 1,349 (67.82) | 2,388 (68.86) | 2,388 (69.56) | 2,588 (71.06) | 3,029 (72.24) | 2,876 (73.01) |
Values are presented as number (%).
T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; BP, blood pressure; HDL-C, high density lipoprotein cholesterol.
aPatients with T1DM and those with T2DM were matched 1:2 according to age and sex for adjustment, bDirect age and sex-adjustment was performed for the Korean population in the year 2010, cWaist circumference >90 cm in men and >80 cm in women using Asian-specific cut-off for abdominal obesity, dStatins, fibrates, and ezetimibes were included.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the epidemiology of T1DM at all ages in Korea over a period of 7 years. To date, T1DM epidemiologic studies in Korea have focused mainly on individuals under 15 years of age [4,7,8]. Recently, Song et al. [1] estimated the incidence and prevalence of T1DM at all ages in Korea, and our data correspond to their findings. However, the study of Song et al. [1] included only 26 months of data from July 2011 to August 2013 [1] whereas our data covered 7 years.
Although the incidence rate of typical T1DM in 2013 was highest in teenagers, it remained constant in adults over 30 years of age, and more than half of incident typical T1DM patients were 30 years or older. The incidence rate of atypical T1DM in 2013 was higher in people aged 40 years or older than in the younger age groups. It is now well established that T1DM may develop at any age, even in the elderly [9,11,20], and epidemiological studies in Western countries have reported that T1DM is more common in adults than previously believed [9,10,11]. In Denmark, almost 40% of patients with T1DM were estimated to develop the disease after age 30 [10]. In Italy, the incidence of T1DM between 30 and 49 years was comparable to that in the age group of 15 to 19 years [9]. When frequency of genetically-defined T1DM in the first six decades of life was analyzed in the white European population from UK Biobank, T1DM cases were distributed throughout all ages of diagnosis, and 42% of cases were diagnosed when patients were aged 31 to 60 years [11]. However, most of these studies were performed in Western populations, and there are insufficient reports on this issue in Asian countries. Japan, which has the lowest incidence rate of childhood T1DM, showed a 2-fold higher prevalence of T1DM in adulthood compared with childhood [20]. In the only previous report in Korea, Song et al. [1] noted that more than two-thirds of the T1DM patients were older than 20 years.
The high incidence and prevalence of T1DM in adults in this study may arise from the followings. First, the considerable incidence of typical T1DM in adults may be associated with fulminant T1DM, an important subtype of diabetes in East Asia [21] and a disease with adult onset [21,22]. Fulminant T1DM is defined as the development of diabetic ketosis/ketoacidosis soon after the onset of hyperglycemic symptoms, plasma glucose ≥288 mg/dL with a glycosylated hemoglobin level <8.5% at initial visit, and the negligible urinary or serum (fasting and stimulated) C-peptide level at onset [21]. In a previous report in Korea, fulminant T1DM accounted for 7.1% of all patients with newly diagnosed T1DM and 30.4% of adult-onset cases [22]. The onset age of fulminant T1DM was reported to be 43 years in males and 35 years in females [21]. Second, long-standing T2DM and LADA cases with insulin dependency may be included in the population with atypical T1DM. According to a collaborative project between Italy and Korea [20], the prevalence of LADA was similar between these two countries with different ethnicities even though the prevalence of T1DM is known to be lower in Korea [20]. Although the population with atypical T1DM may be exaggerated by long-standing T2DM with insulin dependency, even clinically it is not feasible to completely distinguish these cases. Moreover, with reference to previous reports from Western countries and Japan [9,10,20], the possibility that the prevalence of T1DM in adults in Korea is actually high should also be considered.
The time-based trends of increase in T1DM incidence was seen in children and adolescents aged 0 to 19 years, which was compatible to the reports not only in areas with high or high-intermediate rates of disease but also in Korea [4,23,24]. In contrary, in adults aged 20 years or older, the incidence showed a trend of overall decrease. In adults, T1DM incidence may have been overestimated by misclassified long-standing T2DM with insulin dependency before the introduction of the Type 1 Diabetes Registration Project for the Reimbursement of Consumable Materials in July 2011, and improvement in the accuracy of the ICD-10 diagnostic codes by the diabetes types after the promotion of this program may be associated with this trend of decrease in adults. However, given that the same trend of decrease in adult-onset T1DM incidence was reported in the United States [23], it should be considered that the T1DM incidence in adults may be actually decreasing also in Korea.
A male predilection was observed in the prevalence and incidence of both typical and atypical T1DM in the current study. In European countries, a higher incidence of T1DM in males over females, especially after puberty, has been reported [9,25,26,27,28]. Studies performed in Iraq [29], Kuwait [30], and Tunisia [31] showed a slightly higher risk for male than female subjects for the incidence of T1DM, which is similar to our results.
The BMI of the prevalent T1DM patients showed mean values around 24 kg/m2, higher than previously reported [32]. Since the risk for cardiovascular disease starts to increase at a lower BMI in the Asian population, a BMI of 23 kg/m2 is considered to be the cutoff for public health action [33]. These findings suggest that overweight and obesity may be common in Korean T1DM patients. In a study including 65 adult patients with new-onset diabetes presenting as diabetic ketoacidosis [32], 38 T1DM and 27 ketosis-prone T2DM patients were confirmed. Although the mean age of these new-onset T1DM patients was only 34.24±16.01 years, the mean BMI was 22.25±4.95 kg/m2 [32]. Mean BMI of individuals with immune-mediated T1DM with relatively preserved β-cell function and those with fulminant T1DM was 24.91±5.66 and 23.10±5.40 kg/m2, respectively, whereas that of patients with typical immune-mediated T1DM with absent β-cell function was 18.70±4.48 kg/m2 [32], suggesting that BMI of T1DM patients may vary by subtype. Also, a longer duration of diabetes, especially with intensive insulin therapy, is associated with weight gain and higher BMI [12]. The cohort of patients with prevalent T1DM from the NHIS preventive health checkup datasets from 2007 to 2013, which accounted for 14.19% to 22.47% of the total T1DM patients each year, had higher mean age than the total T1DM cases in the NHIS database of claims. Also, the proportion of atypical cases among the patients with prevalent T1DM was 32.35% in 2013 according to the database of claims, and may be higher in the cohort of T1DM from the preventive health checkup dataset. These may be linked to the high BMI values of T1DM patients in our study.
Although LDL-C levels in prevalent T1DM patients tended to improve from 2009 to 2013, nearly half of the T1DM patients still showed LDL-C levels greater than 100 mg/dL in 2013. Adults with T1DM have reported a higher risk for atherosclerotic disease than those without diabetes despite comparable lipid levels [34,35]. The recent American Association of Clinical Endocrinologists (AACE) guideline [36] recommended that diabetes patients with established cardiovascular disease should reduce their LDL-C levels to below 55 mg/dL. LDL-C goals of <70 and <100 mg/dL were recommended for diabetes patients with one or more risk factors and those with no other risk factor, respectively [36]. Considering these recommendations, lipid management in T1DM patients in Korea may need improvement.
More than half of patients with prevalent T1DM had MetS after standardization of age and sex. This was more than 1.5 times higher than the prevalence of MetS in the general population based on the 2007 National Health and Nutrition Examination Survey data using the same revised NCEP definition of MetS [37]. Although the prevalence of MetS in T1DM patients was not as high as that in age and sex-matched T2DM patients, these data suggest that MetS is also common in individuals with T1DM in Korea. Also, considering that more than half of the patients with typical T1DM as well as atypical T1DM had MetS, the high prevalence of MetS in T1DM does not seem to be affected by the possible inclusion of cases with long-standing T2DM with insulin dependency as atypical T1DM cases. Analyses of Finnish Diabetic Nephropathy Study (FinnDiane) and Diabetes Control and Complications Trial (DCCT) datasets showed that MetS is common in patients with T1DM in Western countries [12,13]. The prevalence of MetS was 38% in men and 40% in women among the participants with T1DM in the FinnDiane study, although the mean age of the study population was only 37 years [13]. In Korea, to the best of our knowledge there are no studies estimating the prevalence of MetS in T1DM patients. The prevalence of MetS in our study may be overestimated since patients on medication for dyslipidemia were considered to satisfy the criteria of both hypertriglyceridemia and low HDL-C. Actually, prevalence of hypertriglyceridemia or medication use showed a trend of increase in men and women with both types of diabetes, and the prevalence of low HDL-C or medication use also increased in men with T1DM and T2DM patients of both sexes, which may be associated with increased statin use.
In contrast, T1DM patients with good glycemic control may show subnormal TGs and upper normal or slightly elevated HDL-C levels due to the peripheral hyperinsulinemia induced by exogenous insulin injection, although atherogenic compositional changes in lipoprotein seem to be present even at these lipid concentrations [34,38,39]. Thus, direct comparison of TG or HDL-C levels in T1DM subjects under intensive insulin treatment with those in individuals without insulin treatment may require prudence, and the prevalence of hypertriglyceridemia and low HDL-C may be underestimated in insulin-treated patients with good glycemic control. In this study, among the components of MetS, the prevalence of hypertriglyceridemia differed the most between patients with T1DM and T2DM. This may be explained by changes in TG levels due to peripheral hyperinsulinemia during insulin treatment in T1DM patients.
Our study has several limitations. First, the possibility that individuals with long-standing T2DM and LADA with insulin dependency were included as atypical T1DM cases should be considered. Second, it was not feasible to clearly distinguish the subtypes of T1DM because laboratory data such as serum C-peptide levels or autoantibody positivity for GAD were not available. Third, the prevalence of MetS may be overestimated, especially in those on medications for dyslipidemia. Fourth, an index of insulin resistance like estimated glucose disposal rate [40] could not be obtained because information on glycosylated hemoglobin and hip circumference was not available. Finally, it is not shown whether a high MetS prevalence or higher BMI value in patients with T1DM is associated with a higher risk for cardiovascular diseases or other diabetic complications. Since the role of MetS in T1DM as a predictor of cardiovascular events and/or nephropathy has been inconsistently reported depending on various definitions of MetS [2,12,13], this should be explored further in subsequent studies.
In conclusion, this study explored the incidence and prevalence of T1DM in all age groups in Korea over a period of 7 years and suggested a relatively high incidence and prevalence of T1DM in adults, especially for the atypical form of T1DM. Our data also indicated that MetS may be common in individuals with T1DM in Korea, although the role of MetS as a predictor of cardiovascular events or other microvascular complications in T1DM patients should be investigated further.
ACKNOWLEDGMENTS
This work was performed by cooperation with the National Health Insurance Service (NHIS) and the Korean Diabetes Association. The National Health Information Database constructed by NHIS was used (No. NHIS-2017-4-014).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Prevalence of metabolic syndrome and its components in patients with typical and atypical T1DM according to national preventive health care database in 2013
Prevalence of individual components of metabolic syndrome in men and women with prevalent type 1 diabetes mellitus (T1DM) and those with age- and sex-matched prevalent type 2 diabetes mellitus (T2DM) between 2009 and 2013. (A) Abdominal obesity. (B) Blood pressure ≥130/85 mm Hg or medication use. (C) Triglyceride ≥150 mg/dL or medication use. (D) High density lipoprotein cholesterol <40 mg/dL in men, <50 mg/dL in women or medication use.
References
- 1.Song SO, Song YD, Nam JY, Park KH, Yoon JH, Son KM, Ko Y, Lim DH. Epidemiology of type 1 diabetes mellitus in Korea through an investigation of the national registration project of type 1 diabetes for the reimbursement of glucometer strips with additional analyses using claims data. Diabetes Metab J. 2016;40:35–45. doi: 10.4093/dmj.2016.40.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorn LM, Forsblom C, Waden J, Saraheimo M, Tolonen N, Hietala K, Groop PH Finnish Diabetic Nephropathy (FinnDiane) Study Group. Metabolic syndrome as a risk factor for cardiovascular disease, mortality, and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2009;32:950–952. doi: 10.2337/dc08-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park Y, Wintergerst KA, Zhou Z. Clinical heterogeneity of type 1 diabetes (T1D) found in Asia. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2907. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Lee CG, Lee YA, Yang SW, Shin CH. Increasing incidence of type 1 diabetes among Korean children and adolescents: analysis of data from a nationwide registry in Korea. Pediatr Diabetes. 2016;17:519–524. doi: 10.1111/pedi.12324. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z, Sun C, Wang C, Li P, Wang W, Ye J, Gu X, Wang X, Shen S, Zhi D, Lu Z, Ye R, Cheng R, Xi L, Li X, Zheng Z, Zhang M, Luo F. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997-2011. Acta Diabetol. 2014;51:947–953. doi: 10.1007/s00592-014-0590-2. [DOI] [PubMed] [Google Scholar]
- 6.Lin WH, Wang MC, Wang WM, Yang DC, Lam CF, Roan JN, Li CY. Incidence of and mortality from type I diabetes in Taiwan from 1999 through 2010: a nationwide cohort study. PLoS One. 2014;9:e86172. doi: 10.1371/journal.pone.0086172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko KW, Yang SW, Cho NH. The incidence of IDDM in Seoul from 1985 to 1988. Diabetes Care. 1994;17:1473–1475. doi: 10.2337/diacare.17.12.1473. [DOI] [PubMed] [Google Scholar]
- 8.Shin CH. Epidemiologic characteristics of type 1 diabetes in children aged 14 years or under in Korea, 1985-2000. Korean J Pediatr. 2008;51:569–575. [Google Scholar]
- 9.Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, Pinach S, Novelli G, Trovati M, Cerutti F, Pagano G Piedmont Study Group for Diabetes Epidemiology. Incidence of type 1 and type 2 diabetes in adults aged 30-49 years: the population-based registry in the province of Turin, Italy. Diabetes Care. 2005;28:2613–2619. doi: 10.2337/diacare.28.11.2613. [DOI] [PubMed] [Google Scholar]
- 10.Molbak AG, Christau B, Marner B, Borch-Johnsen K, Nerup J. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabet Med. 1994;11:650–655. doi: 10.1111/j.1464-5491.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6:122–129. doi: 10.1016/S2213-8587(17)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007;30:707–712. doi: 10.2337/dc06-1982. [DOI] [PubMed] [Google Scholar]
- 13.Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, Waden J, Ronnback M, Rosengard-Barlund M, Bjorkesten CG, Taskinen MR, Groop PH FinnDiane Study Group. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study) Diabetes Care. 2005;28:2019–2024. doi: 10.2337/diacare.28.8.2019. [DOI] [PubMed] [Google Scholar]
- 14.Lee YH, Han K, Ko SH, Ko KS, Lee KU Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using national health information database established by National Health Insurance Service. Diabetes Metab J. 2016;40:79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seok H, Jung CH, Kim SW, Lee MJ, Lee WJ, Kim JH, Lee BW. Clinical characteristics and insulin independence of Koreans with new-onset type 2 diabetes presenting with diabetic ketoacidosis. Diabetes Metab Res Rev. 2013;29:507–513. doi: 10.1002/dmrr.2421. [DOI] [PubMed] [Google Scholar]
- 16.Jin SM, Baek JH, Suh S, Jung CH, Lee WJ, Park CY, Yang HK, Cho JH, Lee BW, Kim JH. Factors associated with greater benefit of a national reimbursement policy for blood glucose test strips in adult patients with type 1 diabetes: a prospective cohort study. J Diabetes Investig. 2018;9:549–557. doi: 10.1111/jdi.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Noh J, Han KD, Ko SH, Ko KS, Park CY. Trends in the pervasiveness of type 2 diabetes, impaired fasting glucose and co-morbidities during an 8-year-follow-up of nationwide Korean population. Sci Rep. 2017;7:46656. doi: 10.1038/srep46656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guglielmi C, Palermo A, Pozzilli P. Latent autoimmune diabetes in the adults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes Metab Res Rev. 2012;28(Suppl 2):40–46. doi: 10.1002/dmrr.2345. [DOI] [PubMed] [Google Scholar]
- 21.Imagawa A, Hanafusa T. Fulminant type 1 diabetes: an important subtype in East Asia. Diabetes Metab Res Rev. 2011;27:959–964. doi: 10.1002/dmrr.1236. [DOI] [PubMed] [Google Scholar]
- 22.Cho YM, Kim JT, Ko KS, Koo BK, Yang SW, Park MH, Lee HK, Park KS. Fulminant type 1 diabetes in Korea: high prevalence among patients with adult-onset type 1 diabetes. Diabetologia. 2007;50:2276–2279. doi: 10.1007/s00125-007-0812-z. [DOI] [PubMed] [Google Scholar]
- 23.Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 2017;15:199. doi: 10.1186/s12916-017-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blohme G, Nystrom L, Arnqvist HJ, Lithner F, Littorin B, Olsson PO, Schersten B, Wibell L, Ostman J. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15-34-year age group in Sweden. Diabetologia. 1992;35:56–62. doi: 10.1007/BF00400852. [DOI] [PubMed] [Google Scholar]
- 26.Casu A, Pascutto C, Bernardinelli L, Songini M. Type 1 diabetes among Sardinian children is increasing: the Sardinian diabetes register for children aged 0-14 years (1989-1999) Diabetes Care. 2004;27:1623–1629. doi: 10.2337/diacare.27.7.1623. [DOI] [PubMed] [Google Scholar]
- 27.Kyvik KO, Nystrom L, Gorus F, Songini M, Oestman J, Castell C, Green A, Guyrus E, Ionescu-Tirgoviste C, McKinney PA, Michalkova D, Ostrauskas R, Raymond NT. The epidemiology of type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia. 2004;47:377–384. doi: 10.1007/s00125-004-1331-9. [DOI] [PubMed] [Google Scholar]
- 28.Bruno G, Cerutti F, Merletti F, Cavallo-Perin P, Gandolfo E, Rivetti M, Runzo C, Pinach S, Pagano G Piedmont Study Group for Diabetes Epidemiology. Residual beta-cell function and male/female ratio are higher in incident young adults than in children: the registry of type 1 diabetes of the province of Turin, Italy, 1984-2000. Diabetes Care. 2005;28:312–317. doi: 10.2337/diacare.28.2.312. [DOI] [PubMed] [Google Scholar]
- 29.Almahfoodh D, Alabbood M, Alali A, Mansour A. Epidemiology of type 1 diabetes mellitus in Basrah, Southern Iraq: a retrospective study. Diabetes Res Clin Pract. 2017;133:104–108. doi: 10.1016/j.diabres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Shaltout AA, Moussa MA, Qabazard M, Abdella N, Karvonen M, Al-Khawari M, Al-Arouj M, Al-Nakhi A, Tuomilehto J, El-Gammal A Kuwait Diabetes Study Group. Further evidence for the rising incidence of childhood type 1 diabetes in Kuwait. Diabet Med. 2002;19:522–525. doi: 10.1046/j.1464-5491.2002.00703.x. [DOI] [PubMed] [Google Scholar]
- 31.Zayed H. Genetic epidemiology of type 1 diabetes in the 22 Arab countries. Curr Diab Rep. 2016;16:37. doi: 10.1007/s11892-016-0736-4. [DOI] [PubMed] [Google Scholar]
- 32.Kim MK, Lee SH, Kim JH, Lee JI, Kim JH, Jang EH, Yoon KH, Lee KW, Song KH. Clinical characteristics of Korean patients with new-onset diabetes presenting with diabetic ketoacidosis. Diabetes Res Clin Pract. 2009;85:e8–11. doi: 10.1016/j.diabres.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 33.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 34.Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, D'Agostino R, Jr, Marcovina S, Dabelea D. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for diabetes in youth case-control study. Diabetes Care. 2009;32:416–420. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadwa RP, Kinney GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, Eckel RH, Rewers M. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes Care. 2005;28:1051–1056. doi: 10.2337/diacare.28.5.1051. [DOI] [PubMed] [Google Scholar]
- 36.Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah-Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(Suppl 2):1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 37.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, Choi SH, Cho SI, Park KS, Lee HK, Jang HC, Koh KK. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazumi T, Vranic M, Bar-On H, Steiner G. Portal v peripheral hyperinsulinemia and very low density lipoprotein triglyceride kinetics. Metabolism. 1986;35:1024–1028. doi: 10.1016/0026-0495(86)90038-7. [DOI] [PubMed] [Google Scholar]
- 39.Taskinen MR. Quantitative and qualitative lipoprotein abnormalities in diabetes mellitus. Diabetes. 1992;41(Suppl 2):12–17. doi: 10.2337/diab.41.2.s12. [DOI] [PubMed] [Google Scholar]
- 40.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–632. doi: 10.2337/diabetes.49.4.626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prevalence of metabolic syndrome and its components in patients with typical and atypical T1DM according to national preventive health care database in 2013
Prevalence of individual components of metabolic syndrome in men and women with prevalent type 1 diabetes mellitus (T1DM) and those with age- and sex-matched prevalent type 2 diabetes mellitus (T2DM) between 2009 and 2013. (A) Abdominal obesity. (B) Blood pressure ≥130/85 mm Hg or medication use. (C) Triglyceride ≥150 mg/dL or medication use. (D) High density lipoprotein cholesterol <40 mg/dL in men, <50 mg/dL in women or medication use.