Abstract
Objective
Nonspecific chronic low back pain (CLBP) is a frequent medical condition among middle-aged and older adults. Its detrimental consequences for functional ability and quality of life are well known. However, less is known about associations of chronological age with disability and well-being among CLBP patients. Coping with pain may be harder with advancing age due to additional age-associated losses of physical, sensory, and other resources, resulting in higher disability and lower quality of life. Alternatively, older patients may feel less impaired and report higher quality of life than younger patients because the experience of chronic pain may be better anticipated and more “normative” in old age.
Methods
We investigated an age-heterogeneous sample of 228 CLBP patients (mean age = 59.1 years, SD = 10.2 years, range 41–82 years). Our outcomes were pain intensity, pain disability (as assessed by self-reported activity restrictions and performance-based tests), and measures of quality of life (health-related quality of life: SF-12 physical and mental health; well-being: anxiety, depression, perceived control over life, affective distress).
Results
Although older patients had higher performance-based disability, they scored higher on mental health and on most measures of well-being than younger patients.
Conclusions
Our findings provide evidence for a “paradoxical” pattern of age effects in CLBP patients and are thus in line with other studies based on nonclinical samples: Although disability in CLBP patients increases with advancing age, indicators of quality of life are equal or even higher in older patients.
Keywords: Anxiety, Depression, Chronic Pain, Impairment, Mood, Psychology
Introduction
Back pain, and particularly chronic low back pain, has a high prevalence in middle adulthood and old age [1,2]. It is associated with impaired physical functioning, higher levels of subclinical anxiety, and depression (e.g., [3–5]), but also with an increased risk of clinically relevant affective and anxiety disorders [6], as well as with reduced longevity [7].
For a considerable proportion of patients with chronic low back pain, the anatomic factors causing back pain do not fully explain existing pain symptoms, and these subjects are commonly diagnosed with “nonspecific” chronic low back pain (CLBP). In contrast to patients with specific back pain, associations of structural findings with pain intensity, disability, and quality of life are weak in CLBP patients [8]. Therefore, other factors than pathophysiological influences may account for interindividual differences in pain intensity, disability, quality of life, and well-being in the remarkably heterogeneous population of CLBP patients [9,10]. However, so far most studies have investigated patients with chronic pain in general, without explicitly focusing on the specific group of CLBP patients.
Age may be one of the factors accounting for interindividual differences among samples of CLBP patients: Specifically, it could be that older patients feel less impaired and reveal higher quality of life scores because the experience of chronic pain may be better anticipated and regarded as more “normative” in old age: “When you’re this age and you have an ache, well so what? You expect to have aches when you’re this age” [11]. Alternatively, coping with pain may get harder with advancing age due to additional age-associated decline in physical, sensory, and other functions, resulting in higher disability and lower quality of life. Indeed, coping strategies are subject to age-associated change [12]. For instance, coping efforts aimed at tenaciously pursuing specific goals decline with advancing age [13–15], and age-associated decreases in resources needed for tenacious goal pursuit may be the underlying reason for this decline.
When considering age differences in nonclinical samples, disability generally increases with advancing age [16,17]. Regarding well-being and quality of life, the well-known “well-being” paradox [18–22] states that although older age is accompanied by losses of cognitive, physical, sensory, and other resources [23–26], well-being does not necessarily decline with advancing age. Although longitudinal findings cannot fully support this paradox [19,27–29], it is noteworthy that scores on (most) well-being indicators remain indeed quite high even into old and very old age [30,31].
Regarding previous studies based on clinical samples, previous findings with chronic pain patients suggest that pain intensity does not meaningfully vary as a function of age: Comparing patients with different types of chronic pain who were either younger (18–44 years), middle-aged (45–64 years), or older adults (65–85 years), Riley et al. [32] found that these groups did not significantly differ regarding pain intensity and pain unpleasantness. Similarly, Rustøen et al. [33] found no significant differences in pain intensity between different age groups (18–39 years, 40–59 years, and 60–81 years) with reported chronic pain. With regard to associations between chronological age and well-being in pain patients, one study compared age groups with chronic pain and found less emotional response (such as depression or anxiety) in older pain patients than in younger and middle-aged patient groups [32]. Rustøen et al. [33] also compared different age groups of chronic pain patients and found that the oldest group reported better mood and scored higher on quality of life than the other (younger and middle-aged) age groups. In addition, perceived control over pain seems to be higher in older pain patients [34]. However, so far most studies have relied on single well-being indicators, though well-being is a multidimensional construct that comprises various aspects and that needs to be assessed based on multiple indicators [35–40].
As stated above, previous studies were limited in that most of them focused on pain patients in general, without further differentiating between specific pain conditions such as CLBP. The aim of this study is therefore to investigate a well-defined sample of patients with CLBP regarding associations between chronological age and multiple outcomes of 1) pain intensity, 2) disability (assessed both by self-reports and performance-based tests), and 3) health-related quality of life and well-being. Unlike many previous studies that relied on only one single indicator of well-being, we follow established conceptual frameworks and empirical findings related to well-being [35–40]; consequently, we regard well-being as a multidimensional construct that needs to be assessed by multiple indicators, including affective (in our study: affective distress) and cognitive-evaluative components (perceived control over life) as well as facets of “ill-being,” “negative well-being,” or mental distress (anxiety, depression). Moreover, we build on previous studies by measuring disability both based on self-report and based on objective clinical assessments. Specifically, previous research has shown that self-reported and clinician-measured physical function are only moderately interrelated in patients with low back pain [41], so that both components may represent relatively distinct aspects of functional ability. Therefore, for a comprehensive assessment of disability, both components need to be considered.
Methods
This study is part of the research consortium “Localized and Generalized Musculoskeletal Pain: Psychobiological Mechanisms and Implications for Treatment” (“LOGIN,” subproject number 6: “Subgroups Characterized by Psychological Trauma, Mental Co-morbidity, and Psychobiological Patterns and Their Specialized Treatment”), which was funded by the German Federal Ministry of Education and Research (01EC1010A-F). First results based on data of this research project have recently been published [42–45]. More details concerning the LOGIN study design, measures, and sample can be found elsewhere [9]. Study participants provided written informed consent before study participation. The study was approved by the Ethics Research Committee of the Faculty of Medicine, University of Heidelberg (S-261/2010), and was carried out in compliance with the Helsinki Declaration.
Study Design
Patients were recruited between August 2011 and April 2014 from a tertiary care pain center at the University Hospital Heidelberg. All patients were CLBP patients and were screened consecutively for the below-mentioned inclusion criteria. For the “LOGIN” study project, different patient groups were recruited (according to spatial extent of pain and psychological factors; for further details, see [9]) with at least 30 patients per study group. Recruitment was terminated as soon as the desired group size was reached for all groups.
For the following analyses, 228 patients with nonspecific chronic back pain were included whose age ranged from 41 to 82 years (mean age = 59.1 years, SD = 10.2 years). We excluded patients with an age <40 years because of the very small size of this subsample (N = 11).
Inclusion criteria were as follows: the presence of nonspecific chronic back pain lasting for ≥45 days during the past three months and fluency in German. Exclusion criteria were 1) specific pathologies of chronic back pain (e.g., structural findings, such as spinal canal stenosis, disc herniation, spondylolisthesis, infection, malignancy, rheumatic and systematic inflammatory disorders, and fracture) as well as presence of chronic conditions that may be the cause of chronic back pain; 2) pain intensity in a leg that was equal to or higher than the intensity of back pain (sciatica pain); 3) disorders or diseases impairing sensory processing (e.g., diabetes, alcohol or substance abuse, neuropathy, and inflammatory diseases); 4) back surgery within the past three years; and 5) cognitive impairment.
All study participants underwent a thorough clinical evaluation by a study physician, consisting of a physical examination, blood tests, and, if indicated, further technical investigations (x-ray and magnetic resonance imaging) to validate the clinical diagnosis of nonspecific chronic low back pain and to rule out specific pathologies. Participants were advised not to take any medication 24 hours prior to the investigation.
A sample description is provided in Table 1. The majority of patients were female (71.5%) and married (71.5%) and most patients (65.2%) had a reported onset of pain that was more than 10 years prior to enrollment in this study. Notably, only 26.3% of the sample had an education level of >10 years in school. However, as low education is associated with worse health behaviors and represents a risk factor for poorer health [46–48], the relatively low levels of education in our clinical sample of chronic pain patients are not surprising.
Table 1.
Sample description
| Total Sample (N = 228) | |
|---|---|
| Age, M±SD | 59.1±10.2 |
| Female sex, No. (%) | 163 (71.5) |
| Partnership, No. (%) | |
| Single or separated/divorced | 48 (21.1) |
| Married | 163 (71.5) |
| Widowed | 17 (7.5) |
| Education (>10 y in school), No. (%) | 60 (26.3) |
| Opioid intake, No. (%) | |
| No | 217 (95.2) |
| Yes | 1 (0.4) |
| Yes, lower-potent opioids | 10 (4.4) |
| Onset of pain, No. (%) | |
| 1–3 mo ago | 1 (0.4) |
| 4–6 mo ago | 3 (1.3) |
| 7–12 mo ago | 5 (2.2) |
| >1–2 y ago | 11 (4.8) |
| >2–5 y ago | 21 (9.2) |
| >5–10 y ago | 38 (16.7) |
| >10 y ago | 148 (65.2) |
| Pain intensity: | |
| MPI-D pain intensity, M±SD (0–18)* | 10.1±3.9 |
| Self-reported disability: | |
| MPI-D social and leisure activities (0–48)† | 18.4±7.6 |
| MPI-D household activities, M±SD (0–30)† | 18.8±7.8 |
| MPI-D out-of-home activities, M±SD (0–30)† | 8.1±6.5 |
| MPI-D activities, M±SD (sum score, 0–108)† | 45.6±14.5 |
| Performance-based disability: | |
| Back performance scale, M±SD (0–15)* | 5.3±3.6 |
| Physical impairment scale, M±SD (0–7)* | 2.2±1.9 |
| Health-related quality of life: | |
| SF-12 Physical Health, M±SD (0–100)‡ | 36.7±9.8 |
| SF-12 Mental Health, M±SD (0–100)‡ | 45.2±11.8 |
| Well-being: | |
| Anxiety, M±SD (0–21)§ | 7.6±4.1 |
| Depression, M±SD (0–21)§ | 6.7±4.4 |
| Perceived control over life, M±SD (0–18)§ | 11.8±3.8 |
| Affective distress, M±SD (0–18)§ | 8.3±4.2 |
MPI-D = German version of the West Haven-Yale Multidimensional Pain Inventory.
Higher scores indicate higher pain intensity/higher disability.
Higher scores indicate higher activity engagement.
Higher scores indicate better physical/mental health.
Higher scores indicate higher anxiety, higher depression, higher perceived control over life, and more affective distress.
Measures
Sociodemographic Variables
Sociodemographic variables included were age, sex, marital status, and level of education. Education was dichotomized into “≤10 years of education” and “>10 years of education”, which is a common categorization for the German education system (in Germany, 10 years of school education corresponds to “mittlere Reife,” i.e., secondary school leaving certificate).
Pain Intensity
Pain intensity was assessed based on the pain intensity subscale of the German version of the West Haven-Yale Multidimensional Pain Inventory (MPI-D) [49]. This subscale consists of three items that address the intensity of individuals’ current pain and average pain during the last week, as well as the extent of suffering due to pain. The resulting sum score has a range between 0 and 18, with higher scores indicating a higher pain intensity. The MPI-D has been found to be a highly reliable and valid assessment instrument [49]. The scale’s internal consistency within our study sample was α = .87.
Disability
Subjective Disability. For the assessment of subjective disability, we used the three activity subscales of the MPI-D. These subscales measure the frequency of social and leisure activities (e.g., visiting friends; eight items, scale range = 0–48, α = .77), household activities (e.g., preparing a meal; five items, scale range = 0–30, α = .85), and out-of-home activities (e.g., working in the garden; five items, scale range = 0–30, α = .84), with higher scores indicating more frequent activity engagement. We also included the MPI-D total activity scale (α = .80), which is computed by summing up all three activity subscales.
Objective (Performance-Based) Disability. Additionally, we used two objective, performance-based measures of disability. The Back Performance Scale (BPS) is an objective clinical assessment tool to observe limitations in daily functioning caused by lower back pain. It consists of five tests of daily activities (Sock Test, Pick-up Test, Roll-up Test, Fingertip-to-Floor Test, and Lift Test) that are often impaired in back pain patients. Performances are evaluated by the observer according to operational score definitions and then summed up. Tests are combined to obtain a global performance measure of mobility-related activities requiring sagittal plane mobility (range = 0–15). Higher scores indicate higher impairment. The BPS discriminates between pain patients with different return-to-work statuses and is sensitive to change [50]. Cronbach’s α of the BPS in our study sample was 0.77.
The Physical Impairment Scale (PIS) is another simple and standardized clinical observation tool to evaluate physical impairment in patients with chronic low back pain. Unlike the BPS, the focus of PIS is rather on spinal function than on everyday activities. The assessment instrument consists of seven tests that measure lower back movement (total flexion, total extension, and average lateral flexion, measured with the inclinometer), straight leg raises, spinal tenderness, and strength (bilateral active straight leg raises, sit-ups). The measurements are transformed into binary values of either 0 or 1 according to specified cutoff values and then summed. A higher sum score indicates lower spinal function and thus higher physical impairment. The PIS is able to discriminate between pain patients and healthy controls and is meaningfully related to self-reported disability in activities of daily living [51].
Health-Related Quality of Life and Well-being
The two broad and multidimensional domains of health-related quality of life and well-being were also assessed based on multiple indicators.
Health-Related Quality of Life. Health-related quality of life was measured with the 12-Item Short Form Health Survey (SF-12) [52]. The SF-12 consists of 12 items on eight scales (“physical functioning,” “role limitations due to physical problems,” “bodily pain,” “general health,” “vitality,” “social functioning,” “role limitations due to emotional problems,” and “perceived mental health”). Items are combined and transformed, resulting in one physical and one mental health composite score, which both range from 0 (worst) to 100 (best).
Well-being. As stated above, a broad set of indicators was included to take the well-established multidimensionality of well-being [35–40] into account. To measure the severity of anxiety and depression in study participants, the Hospital Anxiety and Depression Scale (HADS-D) [53,54] was used. The HADS-D was specificially developed for patients with somatic diseases and therefore excludes physical symptoms. Each scale consists of seven items that assess anxiety and depression, respectively, via patients’ self-reports based on a four-stage response format. The range of both scales is 0–21, with higher values indicating higher anxiety/depression scores. The HADS-D has good psychometric properties, including high reliability (subscale depression: α = .88; subscale anxiety: α = .83) and validity [53].
As additional measures of quality of life, we included the MPI-D subscales perceived control over life (α = .73) and affective distress (α = .76), each consisting of three items and ranging from 0 to 18. Higher scores indicate higher perceived control and more affective distress, respectively.
Statistical Analyses
Regression analyses were computed to investigate the associations of chronological age with pain intensity, disability, health-related quality of life, and well-being. Specifically, we regressed all outcomes of pain intensity, disability, health-related quality of life, and well-being on chronological age. Gender, education (coded as a dichotomous variable: up to 10 years of formal education vs >10 years of formal education), and marital status (dichotomized as widowed/single/separated/divorced vs married) were included as additional predictors in order to control for their potentially confounding impact. Moreover, as the associations of our considered outcomes with age may be nonlinear in nature, we investigated quadratic age effects as part of the data screening. Those quadratic age terms that reached statistical significance were included in the models, whereas nonsignificant quadratic age components were removed. For better interpretability of our findings, age was centered on its mean (= 59.07 years), and standardized regression coefficients (rather than unstandardized coefficients) are reported in the following. All analyses were conducted using IBM SPSS Statistics 22.
Results
1. Age, Pain Intensity, and Disability
Pain intensity and disability were regressed on chronological age, with gender, education, and marital status included as covariates in the regression models. Results are shown in Table 2.
Table 2.
Age effects on pain intensity and disability (adjusted for gender, education, and marital status)
| Pain Intensity† | Self-Reported Disability‡ |
Performance-Based Disability† |
|||||
|---|---|---|---|---|---|---|---|
| MPI-D Pain Intensity | MPI-D Social and Leisure Activities | MPI-D Household Activities | MPI-D Out-of-Home Activities | MPI-D Activities (Sum Score) | Back Performance Scale | Physical Impairment Scale | |
| Predictors | |||||||
| Age linear | −0.08±0.12 | 0.06±0.16 | −0.13*±0.12 | −0.01±0.09 | −0.12±0.13 | 0.34***±0.12 | 0.30***±0.13 |
| Age quadratic | −0.27**±0.16 | ||||||
| R2 | 0.14 | 0.11 | 0.25 | 0.17 | 0.03 | 0.18 | 0.12 |
Reported are M±SD and standardized regression coefficients (β, ±95% symmetric confidence interval).
MPI-D = German version of the West Haven-Yale Multidimensional Pain Inventory.
P < 0.05;
P < 0.01;
P < 0.001.
Higher scores indicate higher pain intensity/higher disability.
Higher scores indicate higher activity engagement.
1.1. Pain Intensity
Age was negatively associated with pain intensity. However, this association was weak and not significant (β = –0.08, P > 0.05) (Table 2).
1.2. Subjective Disability
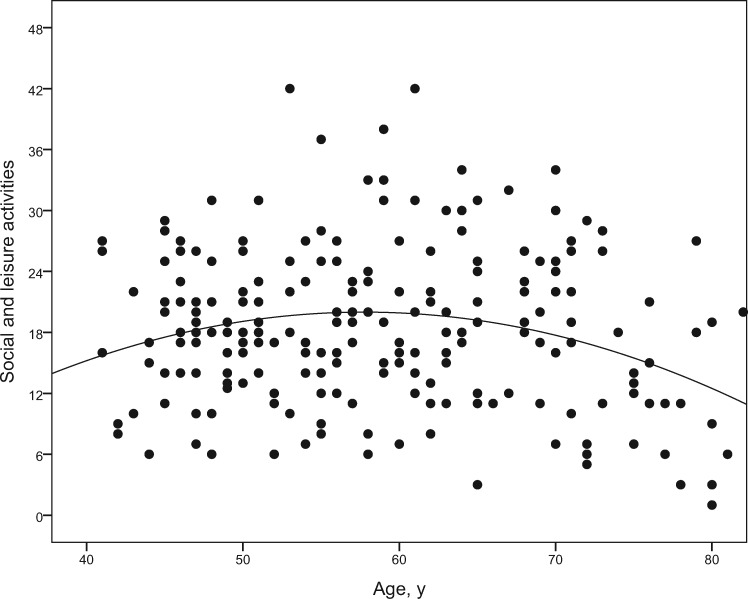
The effect of the quadratic age component on social and leisure activities reached significance (β = –0.27, P < 0.01). This effect was negative, implying that engagement is highest at around age 60 years (Figure 1), but lower before and thereafter.
Figure 1.
Association between age and social and leisure activities. Dots represent the scores of all individuals. Higher scores indicate higher engagement in social and leisure activities (scale range from 0 to 48). The curvilinear association between age (in years) and social and leisure activities is illustrated by the curve indicating that activity engagement is highest at around age 60 years, but lower before and thereafter.
Age was also significantly related with household activities, with lower activity engagement with advancing age (β = –0.13, P < 0.05). All other effects of age on subjective disability outcomes (out-of-home activities and general activities) were not significant.
1.3. Objective Disability
Age was a significant predictor of both performance-based tests of disability (BPS: β = 0.34, P < 0.001; PIS: β = 0.30, P < 0.001), with higher disability scores in older patients.
2. Age, Quality of Life, and Well-being
In a next step, we regressed outcomes of quality of life and well-being on chronological age, again controlling for gender, education, and marital status. Findings are shown in Table 3.
Table 3.
Age effects on health-related quality of life and well-being (adjusted for gender, education and marital status)
| Health-Related Quality of Life† |
Well-being‡ |
|||||
|---|---|---|---|---|---|---|
| SF-12 Physical Health | SF-12 Mental Health | Anxiety | Depression | Perceived Control over Life | Affective Distress | |
| Predictors | ||||||
| Age linear | −0.08±0.13 | 0.19**±0.13 | −0.26***±0.12 | −0.13*±0.13 | 0.20*±0.17 | −0.22**±0.13 |
| Age quadratic | −0.17*±0.16 | |||||
| R2 | 0.12 | 0.11 | 0.16 | 0.10 | 0.07 | 0.12 |
Reported are standardized regression coefficients (β, ±95% symmetric confidence interval).
P < 0.05;
P < 0.01;
P < 0.001.
Higher scores indicate better physical/mental health.
Higher scores indicate higher anxiety, higher depression, higher perceived control over life, and more affective distress.
2.1. Health-Related Quality of Life
Age was not significantly associated with physical health (β = –0.08, P > 0.05), but it was a significant predictor of mental health, which was better in older patients (β = 0.19, P < 0.01).
2.2. Well-being
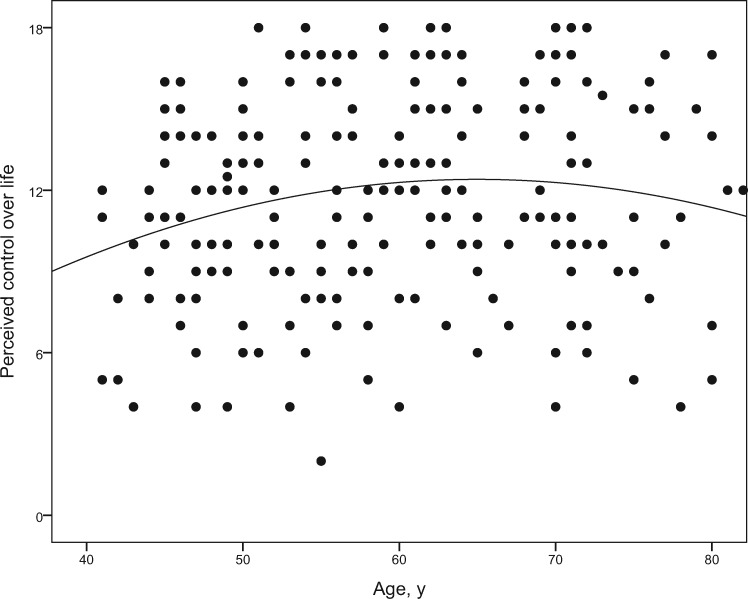
Age was significantly associated with all well-being outcomes. Patients who were older scored lower on anxiety (β = –0.26, P < 0.001), depression (β = –0.13, P < 0.05), and affective distress (β = −0.22, P < 0.01) than younger patients. For perceived control over life, both the linear (β = 0.20, P < 0.05) and the quadratic age (β = –0.17, P < 0.05) components reached significance. As shown in Figure 2, perceived control over life peaked in the age range between 60 and 70 years, and scores were lower at younger and older ages.
Figure 2.
Associations between age and perceived control over life. Dots represent the scores of all individuals. Higher scores indicate higher perceived control over life (scale range from 0 to 18). The curvilinear association between age (in years) and perceived control over life is illustrated by the curve indicating that perceived control over life peaks at an age of about 60–70 years and is lower at older ages as well as in middle adulthood.
Discussion
In this study, we investigated associations of age with outcomes of pain intensity (subjective and objective), disability, and quality of life in an age-heterogeneous sample of patients with nonspecific chronic low back pain. We found no significant association between age and pain intensity. Regarding subjective disability, age was meaningfully related to social and leisure activities, and this association was nonlinear, with the highest activity engagement at around age 60 years and lower levels both at younger and older ages. Moreover, household activity engagement was lower in older patients, whereas both out-of-home activities and general activities were not significantly related with age. A considerably different age pattern was found for objective disability indicators, which were significantly negatively associated with chronological age, with the highest disability levels in the oldest patients. However, older patients did not report worse physical health, and they even scored higher on mental health than younger patients. Similarly, age effects on well-being outcomes were in favor of the older patients: With advancing age, anxiety, depression, and affective distress levels were lower. Perceived control over life was nonlinearly associated with age, with the highest scores between 60 and 70 years and lower scores before and there after.
Our findings are in line with the “well-being paradox” [18–21], which states that although older individuals have to face cognitive and physical declines as well as other loss experiences and a higher risk of disability, their well-being is not necessarily lower compared with younger individuals, implying that objective vs subjective criteria of quality of life and of “successful aging” [55,56] can be remarkably divergent [57–60]. This paradox has originally been stated and investigated with a focus on nonclinical populations, and we were able to provide empirical support for the paradox assumption in a well-defined clinical sample of patients with nonspecific chronic back pain: Despite higher disability scores with advancing age, older patients were either not significantly different from younger patients regarding quality of life outcomes (e.g., regarding self-reported physical health) or they scored even higher than younger patients (e.g., lower anxiety, depression, and affective distress scores as well as higher self-reported mental health with advancing age).
Our findings are in line with other pain-related studies that observed that pain intensity does not vary as a function of age [32,33]. Similarly, our finding of higher quality of life scores with advancing age in chronic pain patients is in accordance with previous research that investigated chronic pain patients in general without a specific focus on nonspecific chronic low back pain [32–34]. According to Wijeratne et al. [61], such differences between age groups with chronic pain regarding psychological characteristics may imply that older pain patients represent an “aetiologically distinct subgroup.” Indeed, the source of chronic low back pain may vary as a function of chronological age [62]. Pain may also be perceived and experienced differently at different ages, like health in general [63]; however, to further address this possibility, more research using qualitative approaches and based on longitudinal study designs will be needed.
What causes the paradoxical pattern of higher disability, but also higher quality of life and well-being in older compared with younger patients with chronic pain? One possible explanation is that, given that pain may be regarded as more “normative” in old age, the negative impact of pain on quality of life may be stronger at younger ages. Moreover, coping strategies in general change with age [12,64], and so does coping with pain [34]. Older adults may be more experienced in facing critical life events and in using adequate coping strategies. As an example, the onset of hearing loss seems to affect older adults’ well-being and mental health less compared with middle-aged and younger adults [65]. Moreover, there may be a general positive age trend toward more adaptive and less maladaptive coping and defense strategies, at least until early old age [12]. In addition, the adaptive mechanisms and resources that contribute to maintenance of high quality of life in most (healthy) older adults [21,66] may also work in older adults affected by chronic pain.
This study has several strengths and limitations. Starting with the strengths of this study, we were, to our knowledge, the first to investigate the “well-being paradox” in a clinical sample of nonspecific chronic back pain patients who were diagnosed based on thorough clinical assessments. Nonspecific chronic back pain is a frequent condition in middle and late adulthood. As it is characterized, unlike other pain disorders, by an absence of structural changes, psychosocial factors (such as well-being and health-related quality of life) may be particularly relevant for these patients. Moreover, for a comprehensive assessment, a broad range of indicators was included in this study to take the multidimensionality of both disability (as assessed by self-reports and by objective, performance-based tests as well as clinical assessment instruments) and quality of life into account. Thus, in contrast to other studies, our assessment instruments were not restricted to self-reports and questionnaires.
One of this study’s limitations is that it is based on a cross-sectional study design. Therefore, the age effects we identified may as well reflect cohort differences. Longitudinal studies are thus needed to investigate whether disability and quality of life really change with advancing age in chronic pain patients (and how). Moreover, due to the various outcomes we included, multiple comparisons may have contributed to an inflation of the Type I error rate and thus to “false-positive” effects. However, most significant age effects were significant at P < 0.01 or even at P < 0.001, so that even when adjusting for multiple comparisons, these effects would have remained statistically significant. Also, all age effects that reached statistical significance were of small (but not meaningless) effect size (i.e., β > 0.10; [67]), and most of them were even close to or above a medium effect size (i.e., β = 0.30). Still, and not surprisingly, the proportions of variance accounted for in all outcomes (as indicated by the R2 scores) reveal that a remarkable amount of interindividual variability in the outcomes cannot be explained by age. Factors other than chronological age (such as personality or coping strategies) might thus additionally explain why functional ability and well-being are impaired in certain patients with chronic pain, but not in others.
In addition, our sample did not include very old individuals aged 85 years and older. Self-regulatory capacities and adaptation to adverse conditions such as pain may come to a limit in advanced old age [68–70], and even in nonclinical samples, there is evidence for marked “terminal” declines in well-being in the last years of life (e.g., [71–73]), with a particularly long and steep terminal decline period among those individuals who reach very old age [74,75]. Our finding of age differences among patients with chronic back pain that were in favor of the older individuals may therefore not be true when very old patients are considered, which requires further research.
Moreover, there may be additional factors underlying the associations between age, disability, and well-being. For instance, the strong age effects we observed with regard to objective disability outcomes may to a large extent be due to age differences in cognitive abilities, which are an important prerequisite for maintaining functional ability into old age and for preventing disability. However, for the sake of interview length and to reduce the burden for our study participants, cognitive abilities were not assessed in this study.
Finally, the participants of this study were recruited from a tertiary care center; thus results may not (or only to some extent) be representative for CLBP patients in general.
In conclusion, our findings provide empirical support for the “well-being paradox” in a well-defined clinical study sample of adults with nonspecific chronic back pain: Whereas older patients revealed higher disability scores than younger patients, both based on self-reports and on performance tests, they scored higher on several quality of life indicators. Our findings thus suggest that well-being among chronic pain patients is actually not negatively associated with chronological age. This is an important message for all those who are working with older pain patients: Restrictions in well-being due to chronic pain must not be taken for granted in older age, but should be addressed individually. With regard to additional practical implications of our study, older patients with nonspecific chronic back pain seem to be at a particularly high risk of disability and might therefore benefit most from interventions to improve or maintain functional ability, though most interventions for patients with chronic pain have so far resulted in small to moderate effects only [76,77]. On the contrary, younger patients were characterized by lower well-being scores compared with older individuals, so interventions to promote well-being in pain patients should have a particular focus on middle-aged adults.
Acknowledgments
The authors thank Beate Eisenecker, Department of General Internal Medicine and Psychosomatics, University Hospital Heidelberg, Heidelberg, Germany, for her excellent technical assistance.
Funding sources: We thank the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung; LOGIN consortium, FKZ: 01EC1010A and 01EC1010B) for supporting this research with a grant.
Conflicts of interest: None.
References
- 1. Cayea D, Perera S, Weiner DK.. Chronic low back pain in older adults: What physicians know, what they think they know, and what they should be taught. J Am Geriatr Soc 2006;5411:1772–7. [DOI] [PubMed] [Google Scholar]
- 2. Gerhardt A, Hartmann M, Blumenstiel K, Tesarz J, Eich W.. The prevalence rate and the role of the spatial extent of pain in nonspecific chronic back pain—a population-based study in the South-West of Germany. Pain Med 2014;157:1200–10. [DOI] [PubMed] [Google Scholar]
- 3. Rudy TE, Weiner DK, Lieber SJ, Slaboda J, Boston JR.. The impact of chronic low back pain on older adults: A comparative study of patients and controls. Pain 2007;1313:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hagen EM, Svensen E, Eriksen HR, Ihlebæk CM, Ursin H.. Comorbid subjective health complaints in low back pain. Spine 2006;3113:1491–5. [DOI] [PubMed] [Google Scholar]
- 5. Glombiewski JA, Hartwich-Tersek J, Rief W.. Depression in chronic back pain patients: Prediction of pain intensity and pain disability in cognitive-behavioral treatment. Psychosomatics 2010;512:130–6. [DOI] [PubMed] [Google Scholar]
- 6. Gerhardt A, Hartmann M, Schuller-Roma B, et al. The prevalence and type of axis-I and axis-II mental disorders in subjects with non-specific chronic back pain: Results from a population-based study. Pain Med 2011;128:1231–40. [DOI] [PubMed] [Google Scholar]
- 7. Zhu K, Devine A, Dick IM, Prince RL.. Association of back pain frequency with mortality, coronary heart events, mobility, and quality of life in elderly women. Spine 2007;3218:2012–8. [DOI] [PubMed] [Google Scholar]
- 8. Tesarz J, Gerhardt A, Hartmann M, Kohlmann T, Eich W.. The course of the spatial extent of pain in nonspecific chronic back pain: A prospective population-based cohort study with clinical evaluation. Clin J Pain 2016;327:580–7. [DOI] [PubMed] [Google Scholar]
- 9. Gerhardt A, Hartmann M, Tesarz J, et al. Subgroups of musculoskeletal pain patients and their psychobiological patterns—The LOGIN study protocol. BMC Musculoskelet Disord 2012;131:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loevinger BL, Shirtcliff EA, Muller D, Alonso C, Coe CL.. Delineating psychological and biomedical profiles in a heterogeneous fibromyalgia population using cluster analysis. Clin Rheumatol 2012;314:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hudak PL, Clark JP, Hawker GA, et al. “You’re perfect for the procedure! Why don’t you want it?” Elderly arthritis patients’ unwillingness to consider total joint arthroplasty surgery: A qualitative study. Med Decis Making 2002;223:272–8. [DOI] [PubMed] [Google Scholar]
- 12. Diehl M, Chui H, Hay EL, et al. Change in coping and defense mechanisms across adulthood: Longitudinal findings in a European American sample. Dev Psychol 2014;502:634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bailly N, Hervé C, Joulain M, Alaphilippe D.. Validation of the French version of Brandtstädter and Renner's Tenacious Goal Pursuit (TGP) and Flexible Goal Adjustment (FGA) scales. Revue Européenne de Psychologie Appliquée/European Review of Applied Psychology 2012;621:29–35. [Google Scholar]
- 14. Bailly N, Joulain M, Hervé C, Alaphilippe D.. Coping with negative life events in old age: The role of tenacious goal pursuit and flexible goal adjustment. Aging Ment Health 2012;164:431–7. [DOI] [PubMed] [Google Scholar]
- 15. Brandtstädter J. Adaptive resources of the aging self: Assimilative and accommodative modes of coping In: Pachana NA, ed. Encyclopedia of Geropsychology. Singapore: Springer Singapore; 2015:1–8. [Google Scholar]
- 16. Cohen-Mansfield J, Shmotkin D, Blumstein Z, et al. The old, old-old, and the oldest old: Continuation or distinct categories? An examination of the relationship between age and changes in health, function, and wellbeing. Int J Aging Hum Dev 2013;771:37–57. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs JM, Maaravi Y, Cohen A, et al. Changing profile of health and function from age 70 to 85 years. Gerontology 2012;584:313–21. [DOI] [PubMed] [Google Scholar]
- 18. Kunzmann U, Little TD, Smith J.. Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychol Aging 2000;153:511–26. [DOI] [PubMed] [Google Scholar]
- 19. Schilling OK. Cohort- and age-related decline in elder’s life satisfaction: Is there really a paradox? Eur J Ageing 2005;24:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schilling OK. Development of life satisfaction in old age: Another view on the ‘paradox.’ Soc Indic Res 2006;752:241–71. [Google Scholar]
- 21. Staudinger UM. Viele Gründe sprechen dagegen, und trotzdem geht es vielen Menschen gut: Das Paradox des subjektiven Wohlbefindens. Psychologische Rundschau 2000;514:185–97. [Google Scholar]
- 22. Herschbach P. Das “Zufriedenheitsparadox” in der Lebensqualitätsforschung. Psychother Psych Med 2002;52(03/04):141–50. [DOI] [PubMed] [Google Scholar]
- 23. Wettstein M, Wahl H-W.. Hearing In: Whitbourne SK, ed. The Encyclopedia of Adulthood and Aging. Hoboken, NJ: Wiley; 2016. [Google Scholar]
- 24. Bainbridge KE, Wallhagen MI.. Hearing loss in an aging American population: Extent, impact, and management. Annu Rev Public Health 2014;351:139–52. [DOI] [PubMed] [Google Scholar]
- 25. Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: Results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wettstein M, Wahl H-W.. Plasticity of aging In: Pachana AN, ed. Encyclopedia of Geropsychology. Singapore: Springer; 2015:1–9. [Google Scholar]
- 27. Charles ST, Reynolds CA, Gatz M.. Age-related differences and change in positive and negative affect over 23 years. J Pers Soc Psychol 2001;801:136–51. [PubMed] [Google Scholar]
- 28. Mroczek DK, Spiro A III.. Change in life satisfaction during adulthood: Findings from the veterans affairs normative aging study. J Pers Soc Psychol 2005;881:189–202. [DOI] [PubMed] [Google Scholar]
- 29. Hansen T, Slagsvold B.. The age and subjective well-being paradox revisited: A multidimensional perspective. Norsk Epidemiologi 2012;222:187–95. [Google Scholar]
- 30. Jopp D, Rott C.. Adaptation in very old age: Exploring the role of resources, beliefs, and attitudes for centenarians’ happiness. Psychol Aging 2006;212:266–80. [DOI] [PubMed] [Google Scholar]
- 31. Wettstein M, Schilling OK, Reidick O, Wahl H-W.. Four-year stability, change, and multidirectionality of well-being in very-old age. Psychol Aging 2015;303:500–16. [DOI] [PubMed] [Google Scholar]
- 32. Riley JL III, Wade JB, Robinson ME, Price DD.. The stages of pain processing across the adult lifespan. J Pain 2000;12:162–70. [Google Scholar]
- 33. Rustøen T, Wahl AK, Hanestad BR, et al. Age and the experience of chronic pain: Differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain 2005;216:513–23. [DOI] [PubMed] [Google Scholar]
- 34. Lachapelle DL, Hadjistavropoulos T.. Age-related differences among adults coping with pain: Evaluation of a developmental life-context model. Can J Behav Sci 2005;372:123–37. [Google Scholar]
- 35. Diener E, Suh EM, Lucas RE, Smith HL.. Subjective well-being: Three decades of progress. Psychological Bulletin 1999;1252:276–302. [Google Scholar]
- 36. Headey B, Holmstrom E, Wearing A.. Models of well-being and ill-being. Soc Indic Res 1985;173:211–34. [Google Scholar]
- 37. Headey B, Holmström E, Wearing A.. Well-being and ill-being: Different dimensions? Soc Indic Res 1984;142:115–39. [Google Scholar]
- 38. Ryff CD, Dienberg Love G, Urry HL, et al. Psychological well-being and ill-being: Do they have distinct or mirrored biological correlates? Psychother Psychosom 2006;752:85–95. [DOI] [PubMed] [Google Scholar]
- 39. Keyes CLM. Mental illness and/or mental health? Investigating axioms of the complete state model of health. J Consul Clin Psychol 2005;733:539–48. [DOI] [PubMed] [Google Scholar]
- 40. Westerhof GJ, Keyes CLM.. Mental illness and mental health: The two continua model across the lifespan. J Adult Dev 2010;172:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee CE, Simmonds MJ, Novy DM, Jones S.. Self-reports and clinician-measured physical function among patients with low back pain: A comparison. Arch Phys Med Rehabil 2001;822:227–31. [DOI] [PubMed] [Google Scholar]
- 42. Gerhardt A, Eich W, Treede R-D, Tesarz J.. Conditioned pain modulation in patients with nonspecific chronic back pain with chronic local pain, chronic widespread pain, and fibromyalgia. Pain 2017;1583:430–9. [DOI] [PubMed] [Google Scholar]
- 43. Tesarz J, Gerhardt A, Treede R, Eich W.. Altered pressure pain thresholds and increased wind-up in adult chronic back pain patients with a history of childhood maltreatment: A quantitative sensory testing study. Pain 2016;1578:1799–809. [DOI] [PubMed] [Google Scholar]
- 44. Gerhardt A, Eich W, Janke S, et al. Chronic widespread back pain is distinct from chronic local back pain: Evidence from quantitative sensory testing, pain drawings, and psychometrics. Clin J Pain 2016;327:568–79. [DOI] [PubMed] [Google Scholar]
- 45. Tesarz J, Gerhardt A, Leisner S, et al. Distinct quantitative sensory testing profiles in nonspecific chronic back pain subjects with and without psychological trauma. Pain 2015;1564:577–86. [DOI] [PubMed] [Google Scholar]
- 46. Anstey KJ, Butterworth P, Windsor TD, et al. The value of comparing health outcomes in cohort studies: An example of self-rated health in seven studies including 79 653 participants. Australas J Ageing 2007;264:194–200. [Google Scholar]
- 47. Lodi-Smith J, Jackson J, Bogg T, et al. Mechanisms of health: Education and health-related behaviours partially mediate the relationship between conscientiousness and self-reported physical health. Psychol Health 2010;253:305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lynch SM. Cohort and life-course patterns in the relationship between education and health: A hierarchical approach. Demography 2003;402:309–31. [DOI] [PubMed] [Google Scholar]
- 49. Flor H, Rudy TE, Birbaumer N, Streit B, Schugens MM.. Zur Anwendbarkeit des West Haven-Yale multidimensional pain inventory im deutschen Sprachraum. Schmerz 1990;42:82–7. [DOI] [PubMed] [Google Scholar]
- 50. Strand LI, Moe-Nilssen R, Ljunggren AE.. Back performance scale for the assessment of mobility-related activities in people with back pain. Physical Therapy 2002;8212:1213–23. [PubMed] [Google Scholar]
- 51. Waddell G, Somerville D, Henderson I, Newton M.. Objective clinical evaluation of physical impairment in chronic low back pain. Spine 1992;176:617–28. [DOI] [PubMed] [Google Scholar]
- 52. Ware JEJ, Kosinski M, Keller SD.. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996;343:220–33. [DOI] [PubMed] [Google Scholar]
- 53. Hermann C, Buss U, Snaith RP.. Hospital Anxiety and Depression Scale—Deutsche Version Ein Fragebogen zur Erfassung von Angst und Depressivität in der somatischen Medizin. Bern: Verlag Hans Huber; 2011. [Google Scholar]
- 54. Zigmond AS, Snaith RP.. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;676:361–70. [DOI] [PubMed] [Google Scholar]
- 55. Rowe JW, Kahn RL.. Successful aging. Gerontologist 1997;374:433–40. [DOI] [PubMed] [Google Scholar]
- 56. Rowe JW, Kahn RL.. Successful aging 2.0: Conceptual expansions for the 21st century. J Gerontol B Psychol Sci Soc Sci 2015;704:593–6. [DOI] [PubMed] [Google Scholar]
- 57. Jeste DV, Depp CA, Vahia IV.. Successful cognitive and emotional aging. World Psychiatry 2010;92:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McLaughlin SJ, Connell CM, Heeringa SG, Li LW, Roberts JS.. Successful aging in the United States: Prevalence estimates from a national sample of older adults. J Gerontol B Psychol Sci Soc Sci 2010;65B2:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Strawbridge WJ, Wallhagen MI, Cohen RD.. Successful aging and well-being: Self-rated compared with Rowe and Kahn. Gerontologist 2002;426:727–33. [DOI] [PubMed] [Google Scholar]
- 60. Martin M, Schneider R, Eicher S, Moor C.. The Functional Quality of Life (fQOL)-Model. GeroPsych 2012;251:33–40. [Google Scholar]
- 61. Wijeratne C, Shome S, Hickie I, Koschera A.. An age-based comparison of chronic pain clinic patients. Int J Geriatr Psychiatry 2001;165:477–83. [DOI] [PubMed] [Google Scholar]
- 62. DePalma MJ, Ketchum JM, Saullo T.. What is the source of chronic low back pain and does age play a role? Pain Med 2011;122:224–33. [DOI] [PubMed] [Google Scholar]
- 63. Wolff JK, Brose A, Lövdén M, et al. Health is health is health? Age differences in intraindividual variability and in within-person versus between-person factor structures of self-reported health complaints. Psychol Aging 2012;274:881–91. [DOI] [PubMed] [Google Scholar]
- 64. Brandtstädter J, Greve W.. The aging self: Stabilizing and protective processes. Dev Rev 1994;141:52–80. [Google Scholar]
- 65. Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: Results from the Nord-Trøndelag Hearing Loss Study. Psychosom Med 2004;665:776–82. [DOI] [PubMed] [Google Scholar]
- 66. Jopp D, Smith J.. Resources and life-management strategies as determinants of successful aging: On the protective effect of selection, optimization, and compensation. Psychol Aging 2006;212:253–65. [DOI] [PubMed] [Google Scholar]
- 67. Cohen J. A power primer. Psychological Bulletin 1992;1121:155–9. [DOI] [PubMed] [Google Scholar]
- 68. Baltes PB. Facing our limits: Human dignity in the very old. Daedalus 2006;1351:32–9. [Google Scholar]
- 69. Baltes PB, Smith J.. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology 2003;492:123–35. [DOI] [PubMed] [Google Scholar]
- 70. Gerstorf D, Ram N.. Limitations on the importance of self-regulation in old age. Hum Dev 2009;521:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gerstorf D, Ram N, Mayraz G, et al. Late-life decline in well-being across adulthood in Germany, the United Kingdom, and the United States: Something is seriously wrong at the end of life. Psychol Aging 2010;252:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schilling O, Wahl H-W, Wiegering S.. Affective development in advanced old age: Analyses of terminal change in positive and negative affect. Dev Psychol 2013;495:1011–20. [DOI] [PubMed] [Google Scholar]
- 73. Vogel N, Schilling OK, Wahl H-W, Beekman ATF, Penninx BWJH.. Time-to-death-related change in positive and negative affect among older adults approaching the end of life. Psychol Aging 2013;281:128–41. [DOI] [PubMed] [Google Scholar]
- 74. Gerstorf D, Ram N, Estabrook R, et al. Life satisfaction shows terminal decline in old age: Longitudinal evidence from the German Socio-Economic Panel Study (SOEP). Dev Psychol 2008;444:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gerstorf D, Ram N, Röcke C, Lindenberger U, Smith J.. Decline in life satisfaction in old age: Longitudinal evidence for links to distance-to-death. Psychol Aging 2008;231:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eccleston C, Morley SJ, Williams ACdC.. Psychological approaches to chronic pain management: Evidence and challenges. Br J Anaesth 2013;1111:59–63. [DOI] [PubMed] [Google Scholar]
- 77. Tesarz J, Leisner S, Gerhardt A, et al. Effects of eye movement desensitization and reprocessing (EMDR) treatment in chronic pain patients: A systematic review. Pain Med 2014;152:247–63. [DOI] [PubMed] [Google Scholar]