Salmonids are particularly susceptible to warming and hypoxia during development in redds. We reared Chinook salmon embryos and alevins under chronic warming and hypoxia to evaluate the effects of each stressor individually and their interaction. Warming and hypoxia affected survival, physiological performance and development with management implications for salmon conservation.
Keywords: Chinook salmon, climate change, developmental physiology, hypoxia, temperature
Abstract
Early life stages of salmonids are particularly vulnerable to warming and hypoxia, which are common stressors in hyporheic, gravel bed, rearing habitat (i.e. a ‘redd’). With the progression of global climate change, high temperatures and hypoxia may co-occur more frequently within redds, particularly for salmonid species at their southern range limit. Warming and hypoxia have competing effects on energy supply and demand, which can be detrimental to energy-limited early life stages. We examined how elevated temperature and hypoxia as individual and combined stressors affected the survival, physiological performance, growth, and development of Chinook salmon (Oncorhynchus tshawytscha). We reared late fall-run Chinook salmon from fertilization to the fry stage in a fully factorial design of two temperatures [10°C (ambient) and 14°C (warm)] and two oxygen levels [normoxia (100% air saturation, 10 mg O2/l) and hypoxia (50% saturation, 5.5 mg O2/l)]. Rearing in hypoxia significantly reduced hatching success, especially in combination with warming. Both warming and hypoxia improved acute thermal tolerance. While rearing in hypoxia improved tolerance to acute hypoxia stress, warming reduced hypoxia tolerance. Hypoxia-reared fish were smaller at hatch, but were able to reach similar sizes to the normoxia-reared fish by the fry stage. High temperature and normoxia resulted in the fastest rate of development while low temperature and hypoxia resulted in the slowest rate of development. Despite improved physiological tolerance to acute heat and hypoxia stress, hypoxia-reared embryos had reduced survival and growth, which could have larger population-level effects. These results suggest that both warming and hypoxia are important factors to address in conservation strategies for Chinook salmon.
Introduction
Increasing water temperatures resulting from climate change are predicted to be problematic for numerous species, particularly for fishes such as Pacific salmonids, which require cool, flowing, highly oxygenated water (Moyle, 2002). The Central Valley watershed of California supports the southernmost populations of Chinook salmon (Oncorhynchus tshawytscha), and is projected to see large, consistent temperature increases nearing 5°C this century (Hayhoe et al., 2004; Dettinger, 2005). In addition to warming, hypoxia (low dissolved oxygen [DO] in the environment) is rapidly becoming more prevalent globally because of climate change and anthropogenic influences, such as eutrophication from agriculture and sewage runoff (Diaz, 2001; Breitburg et al., 2018). Warming and hypoxia are likely to co-occur, as oxygen is less soluble in warmer water (Keeling et al., 2010; Helm et al., 2011). In California, the effects of climate change have been exacerbated by prolonged drought, as warming and low water flows increase water temperatures and thus the potential for hypoxia to occur (Hanak et al., 2015). While the effects of each stressor on animal physiology have been studied in depth individually, there is a greater need to study the interaction between the two stressors in environmentally relevant scenarios (Crain et al., 2008; Todgham and Stillman, 2013; Gunderson et al., 2016).
Both warming and hypoxia are common stressors within the microhabitat of salmon redds, the gravel nests where embryos and larvae develop within the streambed. Temperature and DO within redds are influenced by numerous abiotic and biotic factors including intragravel flow velocity, sedimentation, gravel size, groundwater upwelling and oxygen consumption by developing embryos or other organic matter present (Acornley, 1999; Greig et al., 2007a; Sear et al., 2014). Hypoxia within redds has been correlated with detrimental effects on survival and growth of developing salmonids in natural streams (Rubin and Glimsäter, 1996; Youngson et al., 2004; Greig et al., 2007b). In California, as with many river systems, the rearing conditions for egg and fry development are determined by water releases from dams upstream. These water releases further dictate key abiotic water parameters downstream including temperature and DO. The combination of warming and low DO as a result of low water flows is thought to have reduced the thermal tolerance, and thus survival, of Chinook salmon embryos in the Sacramento River (Martin et al., 2017).
From a physiological perspective, warming and hypoxia are likely to interact through contrasting effects on energy metabolism. Temperature is a controlling factor that determines metabolic rates in ectotherms, whereas oxygen is a limiting factor that restricts metabolic rate (Fry, 1971). Therefore, as warming increases metabolism, hypoxia limits the oxygen supply available to support increased metabolic demand (McBryan et al., 2013). The concept of oxygen and capacity limitation of thermal tolerance (OCLTT) hypothesizes that the mismatch in oxygen supply and demand can reduce thermal tolerance and affect the physiology and ecology of many species (Pörtner, 2001). The OCLTT hypothesis predicts temperature and oxygen will interact negatively to influence stress tolerance such that exposure to high temperature is expected to reduce hypoxia tolerance and hypoxia is expected to reduce thermal tolerance (McBryan et al., 2013).
Early life stages of Chinook salmon are particularly sensitive to both warming and hypoxia, as embryos and alevins are the least thermally tolerant life stages and have little to no mobility to avoid suboptimal habitat conditions (McCullough, 1999; Myrick and Cech, 2004). Embryos of oviparous fish such as salmonids have stronger energy constraints than older organisms because they possess a finite amount of energy in the form of yolk to support their development (Rombough, 2006). Under optimal conditions during development, the majority of energy is allocated towards growth. When energy supply or demand is altered, as with warming or hypoxia, there is increased competition for energy between coping with stress and continued growth and development (Sokolova, 2013). With a limited ability to increase aerobic metabolic rate above routine levels, compensatory energy partitioning may detract energy from processes necessary for development (Rombough, 2011). Therefore, the metabolic interactions between warming and hypoxia may be especially detrimental during early development.
Developing salmon are known to be sensitive to warming and hypoxia individually but are likely to experience both stressors simultaneously in their rearing environment, especially as climate change progresses and local anthropogenic impacts (e.g. drought) persist. In this study, we assessed the effects of chronic warming and hypoxia, on developing late fall-run Chinook salmon, as individual and combined stressors. We reared salmon from fertilization through the fry stage in a fully factorial design of two temperatures (10 and 14°C) and two oxygen levels (100% and 50% air saturation). Throughout development we measured hatching success, growth and developmental rate as well as tolerance to acute thermal and hypoxic stress to examine the lethal and sublethal responses to rearing in each treatment. We predicted that there would be detrimental effects of warming and hypoxia as individual stressors that would be amplified through synergistic interactions in the multiple stressor treatment due to competing effects on balancing energy supply and demand. Examining the effects of two key stressors across salmon development will further our understanding of the capacity of early life stage salmonids to cope with multiple stressors in their natural environment. Our findings can also inform water management strategies to promote egg to fry survival in highly managed, complex systems such as the Sacramento River.
Materials and methods
Fish acquisition and care
Freshly fertilized late fall-run Chinook salmon embryos were obtained from four separate breeding pairs spawned at the Coleman National Fish Hatchery (US Fish and Wildlife Service, Anderson, CA, USA). Embryos were transported to the University of California Davis Center for Aquatic Biology and Aquaculture in January 2017. Embryos were immediately transferred to their rearing treatments in one of four replicate 19 l square culture buckets. Embryos were held in floating mesh baskets affixed with plastic dividers creating individual wells to keep embryos separated in an even layer. Embryos from all four families were evenly distributed across each replicate bucket. Once alevins could sustain swimming, the baskets were removed from the culture buckets. Since early developmental stages rely on endogenous yolk reserves (Kamler, 2008), fish were not fed during the experiment. The experiment ended when fish reached the fry stage and nearly all of the yolk sac was absorbed. All fish care and protocols were reviewed and approved by the UC Davis Institutional Animal Care and Use Committee (protocol no. 19 593).
Experimental design
To assess the effects of elevated temperature and decreased oxygen as individual and combined stressors, we reared developing Chinook salmon from fertilization to the fry stage in four treatments in a fully factorial design of two temperatures [10°C (ambient) and 14°C (warm)] and two oxygen (O2) saturation levels [normoxia (100% air saturation, 10 mg O2/l) and hypoxia (50% saturation, 5.5 mg O2/l)]. Ambient temperature of 10°C was chosen as this is within the average range of temperatures in the Sacramento River when late fall-run salmon embryos are present (Bureau of Reclamation (2018), Central Valley Operations, Sacramento River Temperature Report). The warm temperature of 14°C was chosen to represent a 4°C increase of water temperatures projected with climate change and is a potentially stressful, but not lethal, temperature because embryo mortality increases around 16°C in California Chinook salmon (Myrick and Cech, 2004; Williams, 2006). Dissolved oxygen within natural redds can fluctuate widely between 2–11 mg O2/l (Coble, 1961; Peterson and Quinn, 1996). Normoxia was maintained at 100% saturation to represent optimal habitat conditions and 50% was chosen as a moderate level of hypoxia that is potentially stressful, but not lethal (Silver et al., 1963). Two different temperature treatments were maintained by placing culture and reservoir buckets in four large water bath tanks (1.2 m in diameter) containing flow through water at the corresponding treatment temperature. Each water bath (at 10°C or 14°C) held two culture buckets from the normoxia and hypoxia treatments, with two replicate water bath tanks for each temperature (n = 4 culture buckets per temperature and DO treatment combination). Oxygen saturation was manipulated using mass flow controller valves (Sierra Instruments, Monterey, CA, USA) to mix N2 gas and air to maintain low DO in hypoxic treatments or air alone for normoxic treatments. The gas mixture was bubbled into reservoir buckets using venturi injectors (one reservoir bucket for each temperature × DO treatment). Equilibrated treatment water from each reservoir was then dripped into the culture buckets holding salmon at 16 l/h to ensure high turnover. Gas mixtures were also bubbled directly into culture buckets through air stones for further mixing and adjustment of DO levels within each individual bucket. Temperature and DO were measured in each culture bucket, reservoir bucket, and water bath tank daily using a handheld meter (OxyGuard Handy Polaris 2, OxyGuard International, Farum, Denmark), summarized in Table 1.
Table 1:
Water temperature (°C) and dissolved oxygen (DO, mg/l and % saturation) in each treatment for the duration of the experiment. Water temperature was measured daily in each water bath tank and is reported as the average between the duplicate tanks for each temperature treatment (±SD). Dissolved oxygen was measured daily in each culture bucket and is reported as the average of the four replicate culture buckets per treatment (±SD).
| Treatment | Temperature (°C) | DO (mg/l) | DO % saturation |
|---|---|---|---|
| 14°C Normoxia | 14.1 ± 0.7 | 10.1 ± 0.5 | 98.2 ± 4.2 |
| 14°C Hypoxia | 14.1 ± 0.7 | 5.9 ± 3.1 | 55.3 ± 7.2 |
| 10°C Normoxia | 10.6 ± 0.9 | 10.8 ± 0.4 | 97.5 ± 3.2 |
| 10°C Hypoxia | 10.6 ± 0.9 | 5.5 ± 0.8 | 49.9 ± 7.6 |
Physiological testing occurred four times during the study period for each treatment. A stage-based sampling design was chosen to account for differences in developmental rate caused by the varying temperatures and oxygen saturation levels between treatments. Sampling took place when 50% or more of embryos in a treatment reached (i) eyed stage, when dark pigmented eyes were clearly visible, (ii) silver eyed stage, when silver pigment in eyes was visible, (iii) alevin stage, 1 day after hatching and lastly (iv) fry stage, when the yolk sac was almost completely absorbed. Development of salmon was monitored daily with visual inspections of each culture bucket. Stage was assessed at the treatment level because families were equally distributed among replicates, contributing to minimal variation in developmental timing between replicates. Hatching success was calculated as the ratio between the number of alevins 1-day post-hatch and the initial number of embryos per treatment. Upper thermal tolerance was assessed at each stage (eyed, silver-eyed, alevin and fry) as critical thermal maximum (CTMax), and hypoxia tolerance (time to loss of equilibrium) was tested for fry only. At the alevin and fry stages total length and mass were recorded.
Determination of upper thermal tolerance
Acute upper thermal tolerance was measured using critical thermal maximum (CTMax) methodology (Beitinger et al., 2000; Fangue et al., 2006). All CTMax trials were conducted in normoxic water (100% air saturation). The endpoint used to indicate CTMax differed between embryos and post-hatch stages due to the inability of embryos to exhibit loss of equilibrium, a common endpoint for fishes after hatch (Zebral et al., 2018).
Embryos
Critical thermal maximum for embryos at the eyed and silver eyed stages was defined as the temperature at which the heart stopped beating, similar to Angilletta et al. (2013). CTMax was determined in four embryos from each of four replicates per treatment (16 embryos total per treatment). Embryos were placed in individual wells of a divided plastic dish with water at their corresponding rearing temperature. The plastic dish was held in a well of an aluminum block and treatment water was circulated through the aluminum block to maintain treatment temperature. Embryos were given 1 h in the dishes before CTMax trials began (Becker and Genoway, 1979). Circulating water was then heated using a submersible heater and YSI Thermistemp Temperature Controller (YSI Incorporated, Yellow Springs, OH, USA) such that the water temperature in the dish increased at a rate of 0.3°C/min. Water was aerated using a pipette to ensure full oxygenation and circulation. Embryos were continuously monitored under a dissecting microscope and CTMax was recorded as the temperature when the heart was observed to stop beating for more than 30 s.
Alevins and fry
For alevins and fry, CTMax was determined for four fish per replicate per treatment (16 fish total per treatment). The apparatus consisted of a 37 l aquarium containing a water heater connected to a YSI Thermistemp Temperature Controller (YSI Incorporated), a submersible pump for circulation, and eight glass chambers suspended in the aquaria. Individual fish were placed in the jars for 1 h prior to the start of each trial with water at the corresponding rearing temperature. Eight fish were run at a time and jars were each continuously aerated throughout the CTMax protocol to ensure full oxygenation. After the 1 h acclimation, the heater was turned on and the water temperature increased at a rate of 0.3°C/min. Fish were closely monitored until they reached loss of equilibrium (LOE), defined as the point at which a fish could no longer swim upright or respond to a gentle physical stimulus. Temperature at LOE was recorded with a calibrated immersion thermometer (0.1°C precision, Fisher Scientific), after which individuals were immediately transferred to a fully oxygenated recovery tank with water at their rearing temperature. Temperature at LOE was included in the final dataset if the individual survived a 24 h recovery period. This protocol ensures that CTMax is not exceeded and therefore overestimated. One alevin in the 10°C normoxia treatment died during recovery, whereas 16 of the fry in the 14°C normoxia treatment that underwent the CTMax trial died during recovery. As a result, an additional 8 fish in this treatment were tested the same day to ensure an adequate sample size (n = 8 for 14°C normoxia fry).
Fry hypoxia tolerance
Acute hypoxia tolerance of salmon fry was measured using time to loss of equilibrium methodology (Anttila et al., 2015, McBryan et al., 2016). Time to LOE was determined for four fish per replicate per treatment (16 fish per treatment). Hypoxia tolerance trials were conducted in a 37 l aquarium held in a temperature-controlled water bath. The aquarium contained eight floating plastic beakers with mesh sides for individual fry and a submersible pump for water circulation. The water surface within each beaker was covered with bubble wrap to prevent surface respiration during trials. The water surface surrounding the beakers was also covered with bubble wrap to prevent diffusion of oxygen into the water during trials. DO was monitored throughout the trial using two oxygen dipping probes (PreSens Precision Sensing, Regensburg, Germany). Individual fish were placed in each beaker 30 min prior to the start of the trial to recover from handling. Fish were tested in water at the same temperature and DO level as their rearing treatment. In each trial DO of the water was reduced at a rate of 1.5–2%/min from initial oxygen levels (i.e. 100% and 50%) by bubbling in N2 gas until 8% air saturation was reached (0.9 mg O2/l at 10°C and 0.8 mg O2/l at 14°C). Oxygen was then held at 8% by manually adjusting the flow of N2. This final oxygen concentration was chosen based on pilot studies where all fish could maintain equilibrium indefinitely at 10% and there was little variation in the rapid time to LOE at 6%. Time to LOE was defined as the time (min) after DO saturation reached 8% until the fish could no longer swim upright or respond to a gentle physical stimulus. Upon achieving LOE fish were immediately transferred to fully oxygenated recovery chambers at respective rearing temperatures. Each trial was conducted with a maximum trial time of 2 h. Fish that maintained equilibrium when the 2 h trial ended were assigned a time to LOE of 120 min and transferred to recovery. Time to LOE for fish that survived a 24 h recovery period was included in the final dataset. A total of five fish did not survive recovery, three from the 14°C normoxia treatment, and one each in the 14°C hypoxia and 10°C normoxia treatments.
Body condition factor
Fish at the alevin and fry stages (n = 5 per replicate tank, n = 20 total per treatment) were euthanized in tricaine methanesulfonate (MS-222, Western Chemical, Ferndale, WA, USA), weighed, and measured for total length. Alevin mass measurements included the yolk sac. Body condition was used to compare overall size differences between treatment. Fulton’s condition factor (K) was calculated as:
where W is the wet mass in grams and L is the total length of the fish in cm.
Statistical analyses
Statistical analyses were performed using R Studio (v3.3.0, R Development Core Team, 2013). Datasets were visually inspected for assumptions of normality and homogeneity of variances using Q-Q plots and residuals vs. fitted values. All data were normally distributed and met the assumptions of the tests used unless otherwise noted. Data are reported as means ± SEM with α set at 0.05. Hatching success, time to LOE under hypoxia, and condition factor were analyzed as dependent variables using a two-way analysis of variance (ANOVA) with temperature and oxygen saturation as fixed factors. Post hoc tests for two-way ANOVA were carried out using TukeyHSD. CTMax was analyzed using a three-way ANOVA with temperature, oxygen saturation, and developmental stage as fixed factors. Since different CTMax methodologies were used for embryo stages (cardiac cessation [eyed and silver-eyed]) and post-hatch stages (LOE [alevin and fry]), a separate ANOVA was conducted for each. A type III ANOVA was used for the post-hatch stages to account for unequal sample sizes and interactions between the main factors. Post hoc tests for three-way ANOVA were carried out using a Tukey’s test (‘lsmeans’ package, Lenth, 2016). Initial models nested fish within their corresponding replicate treatment buckets; however, with no significant effects, replicate was removed as a factor to reduce models to their simplest form. Condition factor of alevins did not meet assumptions of homogeneity of variance and was log transformed.
Results
Hatching success
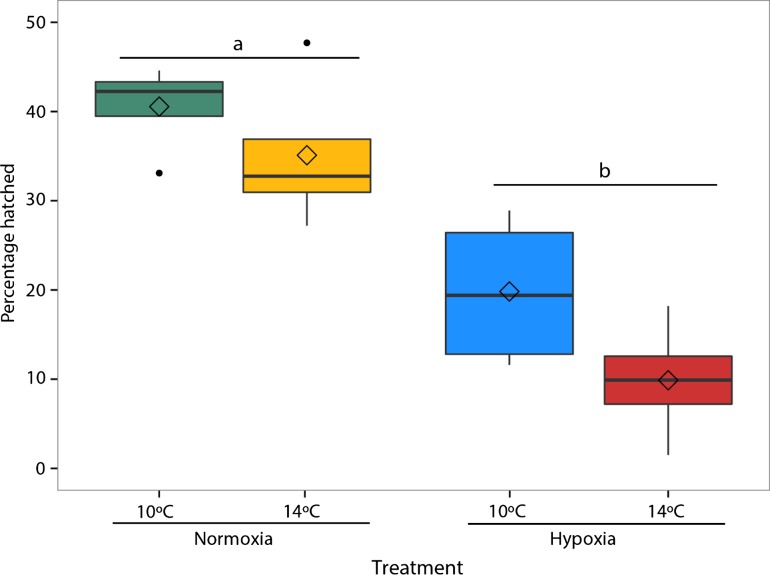
Rearing under hypoxia significantly reduced the percentage hatched (F1,12 = 37.3, P < 0.001). Percentage hatched was highest for embryos reared in normoxia with ~40% (40.5 ± 2.6) hatching success at 10°C and ~35% (35.1 ± 4.4) hatching success at 14°C (Fig. 1). At 10°C, embryos reared in the hypoxia treatment had 50% lower hatching success compared to the normoxia treatment (19.8% ± 4.4 vs. 40.5%). Although temperature did not significantly affect hatching success (F1,12 = 4.19, P = 0.06) and there was no significant interaction between temperature and oxygen (F1,12 = 0.36, P = 0.56), hatching success was lowest in the multiple stressor treatment of hypoxia and 14°C with only ~10% hatched (9.9% ± 3.4).
Figure 1:
Hatching success measured as percentage hatched in each treatment (10°C Normoxia [green], 10°C Hypoxia [blue], 14°C Normoxia [yellow], and 14°C Hypoxia [red]). The center line of the boxplots represents the median, the box represents the inter-quartile range (IQR), the whiskers extend 1.5 times IQR, black points represent values outside 1.5 the IQR, and diamonds represent the mean. Letters indicate a significant (P < 0.05) difference between dissolved oxygen treatments.
Upper thermal tolerance
Embryos
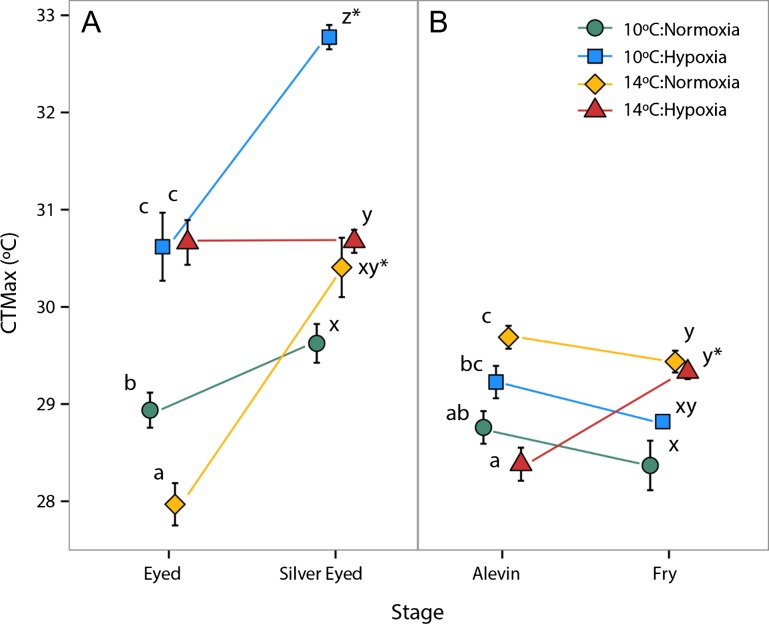
Upper thermal tolerance was highly variable across treatments and development (Fig. 2A). There was a significant two-way interaction between temperature and oxygen (F1, 120 = 8.36, P = 0.005). In addition, a significant three-way interaction (F1,120 = 36.30, P < 0.001) between the main effects of temperature (F1, 120 = 12.05, P < 0.001), oxygen saturation (F1, 120 = 145.44, P < 0.001) and developmental stage (F1, 120 = 67.1, P < 0.001) indicated salmon CTMax was dependent on the life stage and stressors. For example, eyed stage embryos reared under hypoxia at both temperatures had the highest thermal tolerance with a CTMax of 30.6°C ± 0.6 at 10°C and 30.7°C ± 0.2 at 14°C. Eyed embryos reared at 14°C in normoxia had the lowest CTMax (27.9°C ± 0.2) and 10°C normoxia reared embryos had an intermediate thermal tolerance (28.9°C ± 0.2). Thermal tolerance significantly increased at the silver eyed stage for 10°C hypoxia and 14°C normoxia treatments. The 10°C hypoxia treatment had the highest CTMax (32.8°C ± 0.1) with both hypoxia treatments again being the most thermally tolerant. Silver eyed embryos in the 10°C normoxia treatment had the lowest CTMax (29.6°C ± 0.2) and 14°C normoxia was intermediate (30.4°C ± 0.3).
Figure 2:
Critical thermal maximum (CTMax) throughout development in four rearing treatments: 10°C Normoxia (green circle), 10°C Hypoxia (blue square), 14°C Normoxia (yellow) and 14°C Hypoxia (red diamond). Average CTMax ± S.E.M. is given for n = 16 individuals per treatment (n = 15 10°C normoxia alevins, n = 8 14°C normoxia fry) at each developmental stage. Within each panel CTMax is defined as (A) the temperature at which the heart beat stopped (embryonic stages, eyed and silver eyed) and (B) the temperature at which equilibrium was lost (larval stages, alevin and fry). Letters indicate significant (P < 0.05) differences between treatments within a given developmental stage. Asterisks indicate significant (P < 0.05) differences between developmental stages within a single treatment.
Alevins and fry
There were significant interactions between temperature, oxygen saturation, and developmental stage (F1,111 = 7.68, P = 0.007) in the thermal tolerance of the post-hatch alevin and fry stages (Fig. 2B). There were significant two-way interactions between temperature and oxygen (F1,111 = 35.03, P < 0.001) and temperature and stage (F1,111 = 21.5, P < 0.001. There were also significant main effects of temperature (F1, 111 = 16.9, P < 0.001), oxygen (F1, 111 = 5.32, P = 0.02), and stage (F1, 111 = 4.53, P = 0.04) on CTMax. At the alevin stage, the normoxia and hypoxia treatments at 14°C had the highest (29.7°C ± 0.1) and lowest CTMax (28.4°C ± 0.2), respectively, with the alevins reared at 10°C having intermediate CTMax (29.2°C ± 0.2 vs. 28.8°C ± 0.2 for hypoxia and normoxia treatments, respectively). Upon reaching the fry stage CTMax significantly increased in only the 14°C hypoxia treatment (increased to 29.3°C ± 0.1) such that it was no longer significantly different from the 14°C normoxia treatment. Both 14°C treatments had the highest CTMax, while the 10°C normoxia treatment had the lowest (28.4°C ± 0.3) CTMax.
Fry hypoxia tolerance
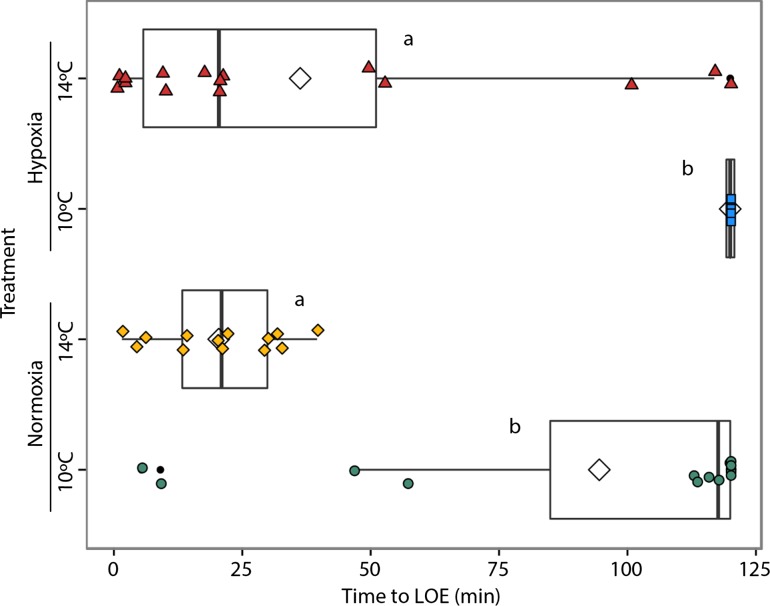
Hypoxia tolerance was only measured at the fry stage, when the fish had absorbed nearly all of the yolk sac. Rearing in hypoxia significantly increased time to LOE (F1,54 = 6.49, P = 0.014) while rearing at 14°C significantly decreased time to LOE (F1,54 = 91.74, P < 0.001) (Fig. 3). Oxygen and temperature did not significantly interact (F1,54 = 0.35, P = 0.56). Fish reared at 14°C in normoxia maintained equilibrium for ~20 min (20.4 ± 3.3) compared to ~36 min (36.3 ± 12) for fry reared at 14°C in hypoxia and ~94.5 min (±11) for fry reared at 10°C in normoxia. Fry reared at 10°C in hypoxia were the most tolerant to hypoxia and all maintained equilibrium indefinitely during the 2-h trial period at 8% air saturation (120 min).
Figure 3:
Acute hypoxia tolerance of fry was measured as the time (min) until fish lost equilibrium while held at 8% dissolved oxygen saturation. A total of n = 16 individuals per treatment (10°C Normoxia [green, n = 15], 10°C Hypoxia [blue n = 16], 14°C Normoxia [yellow, n = 13] and 14°C Hypoxia [red, n = 13]) were tested. Each test was conducted at the temperature fish were reared at and began at the dissolved oxygen saturation of the corresponding treatment. The center line of the boxplots represents the median, the box represents the inter-quartile range (IQR), the whiskers extend 1.5 times IQR, black points represent values outside 1.5 the IQR, and diamonds represent the mean. Letters indicate significant (P < 0.05) differences between treatments.
Growth
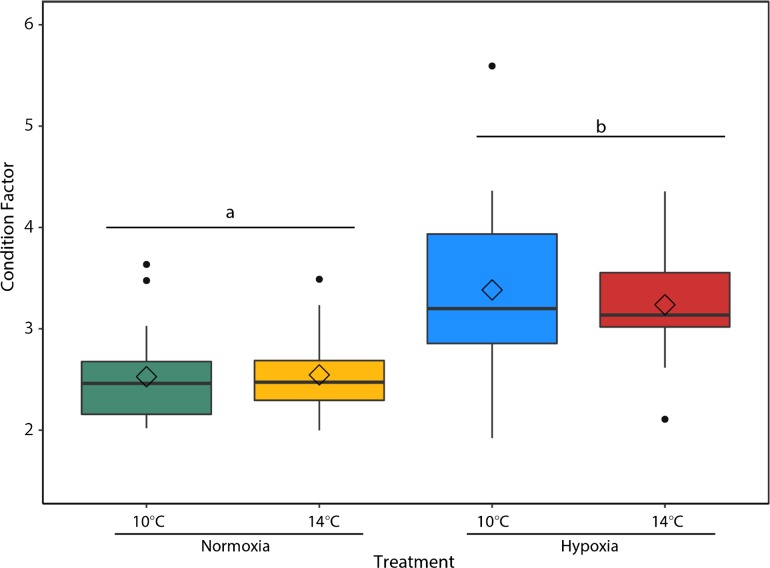
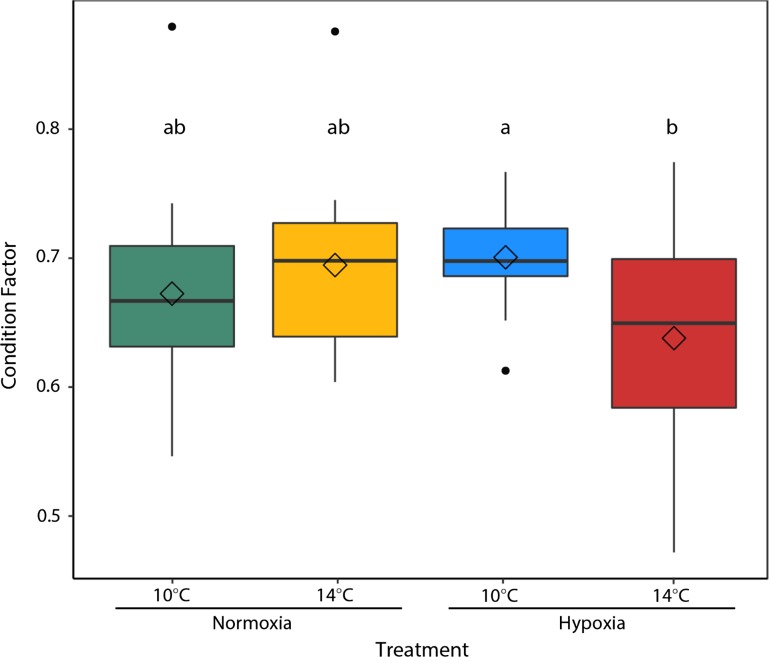
Alevins reared in hypoxia had a significantly higher Fulton’s condition factor (F1,75 = 37.51, P < 0.001) compared to alevins reared under normoxic conditions (Fig. 4). There was no significant interaction between temperature and oxygen on alevin condition factor (F1,75 = 0.39, P = 0.53). Upon reaching the fry stage there were no significant differences in condition factor between treatments. Temperature (F1,70 = 1.004, P = 0.32) and oxygen saturation (F1,70 = 0.44, P = 0.51) did not significantly affect Fulton’s condition factor, although there was a significant interaction between the two stressors (F1,70 = 7.62, P = 0.007) (Fig. 5), where warming decreased condition factor in hypoxia-reared fish.
Figure 4:
Fulton’s condition factor in post-hatch alevins was calculated as the relationship between mass and length in n = 20 individuals per treatment (10°C Normoxia [green], 10°C Hypoxia [blue], 14°C Normoxia [yellow] and 14°C Hypoxia [red]). The center line of the boxplots represents the median, the box represents the inter-quartile range (IQR), the whiskers extend 1.5 times IQR, black points represent values outside 1.5 the IQR, and diamonds represent the mean. Letters indicate a significant (P < 0.05) difference between the main effects of dissolved oxygen (Normoxia, and Hypoxia).
Figure 5:
Fulton’s condition factor in fry was calculated as the relationship between mass and length in n = 20 individuals per treatment. The center line of the boxplots represents the median, the box represents the inter-quartile range (IQR), the whiskers extend 1.5 times IQR, black points represent values outside 1.5 the IQR, and diamonds represent the mean. Letters indicate significant differences between treatments (10°C Normoxia [green], 10°C Hypoxia [blue], 14°C Normoxia [yellow], and 14°C Hypoxia [red]).
Developmental rate
Developmental rate was assessed at the treatment level because there was very little variation between replicate buckets within a treatment. Fish developed faster at 14°C (Table 2). Under normoxia, fish reared at 14°C reached each stage 7–10 days before fish reared at 10°C. Rearing in hypoxia further delayed development within each temperature. At 14°C rearing in hypoxia delayed development by 4–6 days depending on the stage, although hypoxia-reared fish hatched just one day after normoxia-reared fish. At 10°C fish reared in hypoxia reached each stage 4–10 days later than in normoxia, depending on the stage.
Table 2:
Time (days post fertilization) for 50% of individuals or more in each treatment to reach four developmental stages. Development was assessed daily in all replicate culture buckets per treatment.
| Time (days post fertilization) to reach stage | ||||
|---|---|---|---|---|
| Treatment | Eyed | Silver Eyed | Post-hatch | Fry |
| 14°C Normoxia | 17 | 26 | 35 | 64 |
| 14°C Hypoxia | 21 | 30 | 36 | 70 |
| 10°C Normoxia | 25 | 36 | 42 | 75 |
| 10°C Hypoxia | 29 | 41 | 48 | 85 |
Discussion
This study investigated how Chinook salmon development is influenced by the interaction between warming and hypoxia. Acclimation to elevated temperature and hypoxia improved acute thermal tolerance and hypoxia acclimation also improved tolerance to acute hypoxic stress, suggesting a capacity to acclimate to warming and hypoxia during early life stages. Despite improved physiological tolerance with chronic rearing under elevated temperature and hypoxia, hypoxia reduced early growth and hatching success, especially in combination with warming. Reduced growth and hatching success could lead to detrimental effects at the population level as climate change progresses.
Hatching success
The hatching process in fish embryos is a critical period during development and is strongly influenced by both temperature and oxygen (Yamagami, 1988; Korwin-Kossakowski, 2012). In the present study, warm temperature alone minimally reduced hatching compared to controls, which is not surprising given that California Central Valley Chinook salmon embryos were found to tolerate temperatures up to 16°C in laboratory studies (Myrick and Cech, 2004; Williams, 2006). Fish embryos are particularly susceptible to low DO in their environment during the critical period of hatching (Keckeis et al., 1996). Here, rearing in hypoxia markedly reduced hatching success at both temperatures (Fig. 1), with the majority of this mortality occurring within a day or two of the mean hatch date for a given treatment. The observed mortality at hatch is consistent with observations in hypoxia-reared lake trout (Garside, 1959; Carlson and Siefert, 1974) and largemouth bass (Dudley and Eipper, 1975). The mortality observed at hatch often occurred in partially hatched embryos where individuals were able to free their heads from the chorion but were unable to fully escape, suggesting the physical process of hatching was more challenging in hypoxia.
Hatching is an energetically costly process due to increased movement and oxygen consumption (Hamor and Garside, 1959; Ninness et al., 2006). With a limited capacity for anaerobic metabolism in embryos (Rombough, 2011), hatching may increase aerobic energy demand to a level that cannot be matched by energy supply under hypoxic conditions (Polymeropoulos et al., 2016). Warmer water temperatures increase the metabolic rate, and thus oxygen demand, of embryos. Combined with the additional energy required for hatching, the mismatch between energy supply and demand may have been greatest in the multiple stressor treatment of 14°C hypoxia (Pan and von Herbing, 2017), which had the lowest hatching success (Fig. 1). Low hatching success due to rearing in hypoxia and warming may be problematic for salmon populations as small changes in the survival of early life stages can have large effects on recruitment and adult population size (Trippel and Chambers, 1997). Of note, embryos reared in normoxia at 10°C had unexpectedly low hatching success for control conditions (~40%). The low percentage hatched in the control treatment was likely influenced by unusually high mortality observed in one family of embryos, possibly due to poor embryo quality.
Upper thermal tolerance
Many fish species have some degree of plasticity in thermal tolerance (Beitinger et al., 2000), such that upper thermal tolerance commonly increases with acclimation to warmer temperatures (e.g. Fangue et al. 2006; Healy and Schulte, 2012; Anttila et al., 2015). Consistent with results from other studies of warm acclimation in fishes, alevins and fry reared at 14°C under normoxia had the highest CTMax. In contrast to what would be predicted, eyed embryos (the first stage measured) reared at 14°C in normoxia had the lowest CTMax (Fig. 2). Given the high mortality of 14°C normoxia-reared fry after CTMax trials, fish reared at 14°C may have been near their thermal limit such that they were less able to allocate energy to stress tolerance mechanisms to the extent that 10°C treatments could. Thermal tolerance is often life stage specific (Komoroske et al., 2014), particularly in fishes that occupy different habitats throughout development such as Pacific salmon (McCullough, 1999; Richter and Kolmes, 2005). Salmon embryos develop in cold streams and are therefore likely to be more sensitive to warming at this stage.
Oxygen limitation of thermal tolerance hypothesizes that CTMax will be lower when exposed to environmental hypoxia. In contrast, the CTMax of 10°C hypoxia-reared embryos and alevins in the present study were consistently higher than the CTMax of 10°C normoxia-reared fish at all developmental stages. Alevins and fry reared at 10°C in hypoxia maintained a higher CTMax compared to 10°C normoxia reared fish, but had a lower CTMax than fry reared at 14°C in either oxygen treatment suggesting a stronger effect of acclimation temperature on the thermal tolerance of post-hatch stages. Although CTMax often decreases in hypoxia (e.g. Rutledge and Beitinger, 1989; Healy and Schulte, 2012; Ellis et al., 2013), CTMax has also been shown to be independent of oxygen availability (e.g. Ern et al., 2016; Motyka et al., 2017; Verberk et al., 2018). CTMax can be maintained in moderate levels of hypoxia, such as those maintained in this study, even in stenothermal species (Ern et al., 2017); however, the improvement of CTMax with acclimation to hypoxia as observed in the present study is unexpected.
The multiple stressor treatment of 14°C hypoxia had a relatively high CTMax throughout development with the exception of the alevin stage, which had the lowest CTMax for that stage. Mechanisms to cope with hypoxia include adjustments to increase oxygen uptake at the gills and improve transport to increase the supply of oxygen to tissues, as well as reductions in metabolic rate to decrease oxygen demand (Miller et al., 2008; Richards, 2009; Polymeropoulos et al., 2016). Since upper thermal tolerance can benefit from improved oxygen delivery, the mechanisms underlying long-term acclimation to hypoxia can also maintain or improve thermal tolerance (Burleson and Silva, 2011; Motyka et al., 2017) and the physiological adjustments made could have been responsible for increased upper thermal tolerance seen in this study. It should be noted that all CTMax trials were conducted in normoxic conditions, so embryos acclimated to hypoxia may have been more thermally tolerant in part because of an increased availability of oxygen during the CTMax trials, compared to acclimation conditions.
Hypoxia tolerance in fry
Within the OCLTT framework elevated temperatures are predicted to decrease tolerance to acute hypoxia (McBryan et al., 2013). Consistent with the OCLTT, the time to loss of equilibrium in hypoxia was significantly shorter in fish reared at 14°C compared to 10°C, indicating reduced hypoxia tolerance with warming (Fig. 3). Lower hypoxia tolerance at warmer temperatures has been observed in many other studies (Nilsson et al., 2010; Barnes et al., 2011; Remen et al., 2013; McDonnell and Chapman, 2015; Borowiec et al., 2016), although it varies by species (e.g. He et al., 2015). Higher temperatures are thought to reduce hypoxia tolerance by increasing metabolic rates and in turn, oxygen demand (Pörtner, 2010), and may also decrease the oxygen binding affinity of hemoglobin, thereby reducing oxygen supply (McBryan et al., 2013).
Rearing in hypoxia improved tolerance to acute hypoxia at both temperatures compared to the normoxia treatments. Improvement of hypoxia tolerance following acute (24–48 h) exposure and longer-term acclimation occurs in many fishes (e.g. Rees et al., 2001; Timmerman and Chapman, 2004; Shimps et al., 2005; Fu et al., 2011). Acclimation to hypoxia can involve a number of mechanisms such as improved oxygen uptake and transport through changes in gill morphology, concentration of red blood cells and hemoglobin, as well as alterations to cellular energy metabolism (Farrell and Richards, 2009; Borowiec et al. 2015). Additionally, there was high individual variability in hypoxia tolerance in the 14°C hypoxia and 10°C normoxia treatments. This variability may be due to the contrasting effects of warming and hypoxia on hypoxia tolerance such that individual responses to exposure to one factor that improves tolerance (i.e. low temperature or hypoxia acclimation) in combination with a factor that reduces hypoxia tolerance (i.e. elevated temperature or normoxia acclimation) are more variable than exposure to two factors that both either increase or decrease tolerance. Our results suggest that Chinook salmon fry also have the capacity to acclimate to hypoxia during chronic exposure, although the degree of improved hypoxia tolerance is temperature dependent.
Growth and development
Reduced growth and delayed development in hypoxia are compensatory responses where metabolic demand is adjusted to match the oxygen supply available (Rombough, 1988a). Despite having higher condition factors, hypoxia-reared alevins were smaller due to reduced body tissue length (data not shown) and more yolk retained at the time of hatch (Fig. 4), similar to Polymeropoulos et al. (2017) with hypoxia-reared Atlantic salmon. A reduction in size of post-hatch hypoxia-reared larvae has been observed in many other studies (Alderdice et al., 1958; Garside, 1959; Shumway et al., 1964; Marks et al. 2012). Growth is the most energetically demanding activity in early embryonic development and is almost entirely dependent on aerobic metabolism (Rombough, 2011). While the ecological significance of size at hatch is difficult to determine, alevins that are smaller at hatch may have lower chances of survival due to size selective predation pressure, decreased competitive ability, and slower swimming speeds (Mason, 1969; Pepin, 1991). Smaller salmon may be more vulnerable to predation in the Sacramento-San Joaquin Delta where predation on juvenile Chinook salmon by abundant native and non-native fish predators is high (Grossman, 2016). Given the challenges of predicting the effects of size on survival, size is best considered alongside performance (Conover and Schultz, 1997; Green and Fisher, 2004). For fish that do survive hatching in hypoxia there is a potential tradeoff between a smaller size at hatch and being more tolerant to acute thermal and hypoxic stressors. Upon reaching the fry stage there were no significant differences in condition factor between treatments (Fig. 5); however, it took hypoxia reared fry 6–10 days longer to reach the fry stage and fully absorb the yolk sac.
Developmental rate in fish embryos is highly dependent on both temperature and DO in the rearing environment (Murray and McPhail, 1987; Beacham and Murray, 1990). Both decreased temperature and hypoxia lead to slower development in many fish species (Garside, 1966; Pepin, 1991; Green and Fisher, 2004). As expected, in the present study low temperature delayed development by 7–10 days in 10°C normoxia compared to 14°C normoxia (Table 2). The developmental delay in hypoxia increased from 4 to 5 days during the embryonic stages to 6–10 days to reach the post-hatch stages, as in Geist et al. (2006), with the exception of the 14°C hypoxia treatment. The further delay of developmental rate in hypoxia may have larger phenological consequences as there may be selection against late emerging salmon (Einum and Fleming, 2000).
Low oxygen can have two opposite effects on time to hatch (Carlson and Siefert, 1974; Ciuhandu et al., 2005; Hassell et al., 2008), both of which appear to have occurred in this study, dependent on rearing temperature. Hypoxia can slow the overall rate of development extending the time to hatch (i.e. 10°C hypoxia treatment hatched 6 days after the 10°C normoxia treatment). Hypoxia can also reduce the time to hatch. Low oxygen is an important natural signal to hatch in fish embryos (Czerkies et al., 2001) and acute hypoxia can trigger hatching in mature embryos (Oppen-Berntsen et al., 1990). As embryonic development progresses, metabolic rate increases until ambient oxygen can no longer meet metabolic oxygen demand (Rombough, 1988b). Thus, hypoxia can trigger premature hatching when oxygen becomes limited before embryos are fully developed (DiMichele and Powers, 1984; Latham and Just, 1989). Given the increase in metabolic demand with warming, early oxygen limitation may explain why embryos reared at 14°C in hypoxia hatched just one day after those in 14°C normoxia, when the hypoxia treatment reached all other stages multiple days later. Similarly, precocious hatching resulting from acute hypoxia exposure was greatest at high temperature in whitefish embryos (Czerkies et al., 2001).
Conclusions
Late fall-run Chinook salmon in the Central Valley of California are listed as a Species of Concern under the federal Endangered Species Act and occupy some of the same river habitat as threatened and endangered Chinook salmon runs (i.e. threatened spring-run and endangered winter-run). A modeling study by Martin et al. (2017) suggested interactions between high temperatures, low flows, and low DO may have contributed to high embryo mortality in winter-run Chinook salmon, a run with a population of less than 1 000 estimated to be in the Sacramento River during the 2017 spawning season (Azat, 2018). Survival of wild Central Valley salmon embryos can be highly variable but is generally low, with average egg to fry survival likely below 20% (Williams, 2006). A further decrease in hatching success resulting from hypoxia, as demonstrated in this study, could potentially have large impacts on population size as a whole if hypoxia is widespread throughout the rearing habitat. Furthermore, hypoxia and warming affected many aspects of salmon development as both individual and interacting stressors in this study, suggesting both factors should be considered in the conservation and management of early life stage Chinook salmon. Exposure to warming or hypoxia during early development of fishes were found to influence long-term behavior (Ivy et al., 2016; Roussel, 2007), swimming performance (Widmer et al., 2006; Johnston et al., 2013), stress tolerance (Zambonino-Infante et al., 2013), growth and physiology (Crocker et al., 2013; Zambonino-Infante et al., 2017; Cadiz et al., 2018) and adult salmon migration timing (Jonsson and Jonsson, 2018). While current management strategies to promote embryo survival in the Sacramento River are largely focused on releases of cold water from the Shasta Dam to maintain temperatures at or below a target temperature of 56°F (~13.3°C) (National Marine Fisheries Service, 2009); this study demonstrates the need to also manage dissolved oxygen (in addition to temperature) when regulating flows as both factors influence the survival and development of salmon embryos. This study, in addition to Martin et al. (2017), suggests that in natural redds where DO is variable, the target temperature of 56°F may be too high in some cases since salmon embryo mortality can occur at lower temperatures in hypoxia. The exact mechanisms underlying the acclimation capacity at these early stages, as well as the potential for persistent or latent physiological effects of exposure to warming and hypoxia during early development warrant further investigation.
Acknowledgments
We thank the Coleman National Fish Hatchery for providing salmon embryos. Additionally, we thank D. Cocherell for assistance with equipment and facilities maintenance, as well as undergraduates G. Mukai and L. Olano, and Todgham lab members for assistance with fish care and data collection.
Funding
This work was supported by the California Agricultural Experimental Station of the University of California Davis [grant numbers CA-D-ASC-2252-H and CA-D-ASC-2253-RR to AET and CA-D-ASC-2098 to NAF], National Science Foundation Graduate Research Fellowship [grant number 1650042 AMD] and a Fly Fishers of Davis scholarship to AMD.
References
- Acornley RM. (1999) Water temperatures within spawning beds in two chalk streams and implications for salmonid egg development. Hydrol Process 13: 439–446. [Google Scholar]
- Alderdice DF, Wickett WP, Brett JR (1958) Some effects of temporary exposure to low dissolved oxygen levels on Pacific salmon eggs. J Fish Res Bd Can 15: 229–75. [Google Scholar]
- Angilletta MJ, Zelic MH, Adrian GJ, Hurliman AM, Smith CD (2013) Heat tolerance during embryonic development has not diverged among populations of a widespread species (Sceloporus undulatus). Cons Physiol 1: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila K, Lewis M, Prokkola JM, Kanerva M, Seppänen E, Kolari I, Nikinmaa M (2015) Warm acclimation and oxygen depletion induce species-specific responses in salmonids. J Exp Biol 218: 1471–1477. [DOI] [PubMed] [Google Scholar]
- Azat J. (2018) GrandTab 2018.04.09 California Central Valley Chinook Population Database Report. California Department of Fish and Wildlife.
- Barnes RK, King H, Carter CG (2011) Hypoxia tolerance and oxygen regulation in Atlantic salmon, Salmo salar from a Tasmanian population. Aquaculture 318: 397–401. [Google Scholar]
- Beacham TD, Murray CB (1990) Temperature, egg size, and development of embryos and alevins of five species of Pacific salmon: a comparative analysis. Trans Ameri Fish Soc 119: 927–945. [Google Scholar]
- Becker C, Genoway R (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fish 4: 245–256. [Google Scholar]
- Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fish 58: 237–75. [Google Scholar]
- Borowiec BG, Darcy KL, Gillette DM, Scott GR (2015) Distinct physiological strategies are used to cope with constant hypoxia and intermittent hypoxia in killifish (Fundulus heteroclitus). J Exp Biol 218: 1198–1211. [DOI] [PubMed] [Google Scholar]
- Borowiec BG, Crans KD, Khajali F, Pranckevicius NA, Young A, Scott GR (2016) Interspecific and environment-induced variation in hypoxia tolerance. Comp Biochem Physiol A Mol Integr Physiol 198: 59–71. [DOI] [PubMed] [Google Scholar]
- Breitburg D, Levin LA, Oschiles A, Grégoire M, Chavez FP, Conley DJ, Garçon V, Gilbert D, Gutiérrez D, Isensee K, et al. (2018) Declining oxygen in the global ocean and coastal waters. Science 359(6371): eaam7240 10.1126/science.aam7240. [DOI] [PubMed] [Google Scholar]
- Bureau of Reclamation (2018) Sacramento River temperature report. https://www.usbr.gov/mp/cvo/temperature.html. (last accessed 1 August 2018).
- Burleson ML, Silva PE (2011) Cross tolerance to environmental stressors: Effects of hypoxic acclimation on cardiovascular responses of channel catfish (Ictalurus punctatus) to a thermal challenge. J Therm Biol 36: 350–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadiz L, Ernande B, Quazuguel P, Servili A, Zambonino-Infante J, Mazurais D (2018) Moderate hypoxia but not warming conditions at larval stages induces adverse carry-over effects on hypoxia tolerance of European sea bass (Dicentrarchus labrax) juveniles. Mar Environ Res 138: 28–35. [DOI] [PubMed] [Google Scholar]
- Carlson AR, Siefert RE (1974) Effects of reduced oxygen on the embryos and larvae of lake trout (Salvelinus namaycush) and largemouth bass (Micropterus salmoides). J Fish Res Bd Can 31: 1393–1396. [Google Scholar]
- Ciuhandu CS, Stevens ED, Wright PA (2005) The effect of oxygen on the growth of Oncorhynchus mykiss embryos with and without a chorion. J Fish Biol 67: 1544–1551. [Google Scholar]
- Coble DW. (1961) Influence of water exchange and dissolved oxygen in redds on survival of steelhead trout embryos. Trans Ameri Fish Soc 90: 469–474. [Google Scholar]
- Conover DO, Schultz ET (1997) Natural selection and the evolution of growth rate in the early life history: what are the trade-offs? In Chambers RC, Trippel EA, eds. Early Life History and Recruitment in Fish Populations. Chapman and Hall, London, pp 305–332. [Google Scholar]
- Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11: 1304–1315. [DOI] [PubMed] [Google Scholar]
- Crocker CD, Chapman LJ, Martinez ML (2013) Hypoxia-induced plasticity in the metabolic response of a widespread cichlid. Comp Biochem Physiol B Biochem Mol Biol 166: 141–147. [DOI] [PubMed] [Google Scholar]
- Czerkies P, Brzuzan P, Kordalski K, Luczynski M (2001) Critical partial pressures of oxygen causing precocious hatching in Coregonus lavaretus and C. albula embryos. Aquaculture 196: 151–158. [Google Scholar]
- Dettinger MD. (2005) From climate-change spaghetti to climate-change distributions for 21st century California. San Francisco Estuary Watershed Sci 3: 1–14. [Google Scholar]
- DiMichele L, Powers DA (1984) Developmental and oxygen consumption rate differences between lactate dehydrogenase-B genotypes of Fundulus heteroclitus and their effect on hatching time. Physiol Zool 57: 52–56. [Google Scholar]
- Diaz RJ. (2001) Overview of hypoxia around the world. J Environ Qual 30: 275–281. [DOI] [PubMed] [Google Scholar]
- Dudley RG, Eipper AW (1975) Survival of largemouth bass embryos at low dissolved oxygen concentrations. Trans Ameri Fish Soc 104: 122–128. [Google Scholar]
- Einum S, Fleming IA (2000) Selection against late emergence and small offspring in Atlantic salmon (Salmo salar). Evolution 54: 628–639. [DOI] [PubMed] [Google Scholar]
- Ellis LE, Sacobie CFD, Kieffer JD, Benfey TJ (2013) The effects of dissolved oxygen and triploidy on critical thermal maximum in brook charr (Salvelinus fontinalis). Comp Biochem Physiol A Mol Integr Physiol 166: 426–433. [DOI] [PubMed] [Google Scholar]
- Ern R, Norin T, Gamperl AK, Esbaugh AJ (2016) Oxygen dependence of upper thermal limits in fishes. J Exp Biol 219: 3376–3383. [DOI] [PubMed] [Google Scholar]
- Ern R, Johansen JL, Rummer JL, Esbaugh AJ (2017) Effects of hypoxia and ocean acidification on the upper thermal niche boundaries of coral reef fishes. Biol Lett 13: 20170135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangue NA, Hofmeister M, Schulte PM (2006) Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J Exp Biol 209: 2859–2872. [DOI] [PubMed] [Google Scholar]
- Farrell AP, Richards JG (2009) Chapter 11 Defining Hypoxia: an integrative synthesis of the responses of fish to hypoxia In Jeffrey G, Richards APF, Colin JB, eds. Fish Physiology, Vol 27 Academic Press, London, pp 487–503. [Google Scholar]
- Fry FEJ. (1971) The effect of environmental factors on the physiology of fish In Hoar WS, Randall DJ, eds. Fish Physiology, Vol. 6 Academic Press, New York, pp 1–98. [Google Scholar]
- Fu S, Brauner CJ, Cao A, Richards JG, Peng J, Dhillon R, Wang Y (2011) The effect of acclimation to hypoxia and sustained exercise on subsequent hypoxia tolerance and swimming performance in goldfish (Carassius auratus). J Exp Biol 2011: 2080–2088. [DOI] [PubMed] [Google Scholar]
- Garside ET. (1959) Some effects of oxygen in relation to temperature on the development of lake trout embryos. Can J Zool 37: 689–698. [Google Scholar]
- Garside ET. (1966) Effects of oxygen in relation to temperature on the development of embryos of brook trout and rainbow trout. J Fish Res Bd Can 23: 1121–1134. [Google Scholar]
- Geist DR, Abernethy CS, Hand KD, Cullinan VI, Chandler JA, Groves PA (2006) Survival, development, and growth of fall Chinook salmon embryos, alevins, and fry exposed to variable thermal and dissolved oxygen regimes. Trans Ameri Fish Soc 135: 1462–1477. [Google Scholar]
- Green BS, Fisher R (2004) Temperature influences swimming speed, growth, and larval duration in coral reef fish larvae. J Exp Mar Bio Ecol 299: 115–132. [Google Scholar]
- Greig SM, Sear DA, Carling PA (2007. a) A review of factors influencing the availability of dissolved oxygen to incubating salmonid embryos. Hydrol Process 21: 313–334. [Google Scholar]
- Greig S, Sear D, Carling P (2007. b) A field-based assessment of oxygen supply to incubating Atlantic salmon (Salmo salar) embryos. Hydrol Process 21: 3087–3100. [Google Scholar]
- Grossman GD. (2016) Predation on fishes in the Sacramento-San Joaquin Delta: Current knowledge and future directions. San Francisco Estuary Watershed Sci 14, 10.15447/sfews.2016v14iss2art8. [DOI] [Google Scholar]
- Gunderson AR, Armstrong EJ, Stillman JH (2016) Multiple stressors in a changing world: The need for an improved perspective on physiological responses to the dynamic marine environment. Ann Rev Mar Sci 8: 357–378. [DOI] [PubMed] [Google Scholar]
- Hamor T, Garside ET (1959) Developmental rates of embryos of Atlantic salmon, Salmo salar L., in response to various levels of temperature, dissolved oxygen, and water exchange. Can J Zool 54: 1912–1917. [DOI] [PubMed] [Google Scholar]
- Hanak E, Mount J, Chappelle C, Lund J, Medellín-Azuara J, Moyle P, Seavy N (2015) What If California’s Drought Continues? Public Policy Institute of California, San Francisco, CA, USA. [Google Scholar]
- Hassell KL, Coutin PC, Nugegoda D (2008) Hypoxia impairs embryo development and survival in black bream (Acanthopagrus butcheri). Mar Pollut Bull 57: 302–306. [DOI] [PubMed] [Google Scholar]
- Hayhoe K, Cayan D, Field CB, Frumhoff PC, Maurer EP, Miller NL, Moser SC, Schneider SH, Cahill KN, Cleland EE, et al. (2004) Emissions pathways, climate change, and impacts on California. Procs Natl Acad Sci 101: 12422–12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Cao Z, Fu S (2015) Effect of temperature on hypoxia tolerance and its underlying biochemical mechanism in two juvenile cyprinids exhibiting distinct hypoxia sensitivities. Comp Biochem Physiol A Mol Integr Physiol 187: 232–241. [DOI] [PubMed] [Google Scholar]
- Healy TM, Schulte PM (2012) Factors affecting plasticity in whole-organism thermal tolerance in common killifish (Fundulus heteroclitus). Comp Biochem Physiol B Biochem Mol Biol 182: 49–62. [DOI] [PubMed] [Google Scholar]
- Helm KP, Bindoff NL, Church JA (2011) Observed decreases in oxygen content of the global ocean. Geophys Res Lett 38: L23602. [Google Scholar]
- Ivy CM, Robertson CE, Bernier NJ (2016) Acute embryonic anoxia exposure favours the development of a dominant and aggressive phenotype in adult zebrafish. Proc R Soc B 284: 20161868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston EF, Alderman SL, Gillis TE (2013) Chronic hypoxia exposure of trout embryos alters swimming performance and cardiac gene expression in larvae. Physiol Biochem Zool 86: 567–575. [DOI] [PubMed] [Google Scholar]
- Jonsson B, Jonsson N (2018) Egg incubation temperature affects the timing of the Atlantic salmon Salmo salar homing migration. J Fish Biol 93: 1016–1020. 10.1111/jfb.13817. [DOI] [PubMed] [Google Scholar]
- Kamler E. (2008) Resource allocation in yolk-feeding fish. Rev Fish Biol Fisher 18: 143–200. [Google Scholar]
- Keckeis H, Bauer-Nemeschkal E, Kamler E (1996) Effects of reduced oxygen level on the mortality and hatching rate of Chondrostoma nasus embryos. J Fish Biol 49: 430–440. [Google Scholar]
- Keeling RF, Körtzinger A, Gruber N (2010) Ocean deoxygenation in a warming world. Ann Rev Mar Sci 2: 199–229. [DOI] [PubMed] [Google Scholar]
- Komoroske LM, Connon RE, Lindberg J, Cheng BS, Castillo G, Hasenbein M, Fangue NA (2014) Ontogeny influences sensitivity to climate change stressors in an endangered fish. Cons Physiol 2, 10.1093/conphys/cou008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korwin-Kossakowski M. (2012) Fish hatching strategies: a review. Rev Fish Biol Fisher 22: 225–240. [Google Scholar]
- Latham KE, Just JJ (1989) Oxygen availability provided a signal for hatching in the rainbow trout (Salmo gairdneri) embryo. Can J Fish Aquat Sci 46: 55–58. [Google Scholar]
- Lenth RV. (2016) Least-squares means: the R package lsmeans. J Stat Softw 69: 1–33. [Google Scholar]
- Marks C, Kaut KP, Moore FBG, Bagatto B (2012) Ontogenetic oxygen changes alter zebra fish size, behavior, and blood glucose. Physiol Biochem Zool 85: 635–644. [DOI] [PubMed] [Google Scholar]
- Martin BT, Pike A, John SN, Hamda N, Roberts J, Lindley ST, Danner EM (2017) Phenomenological vs. biophysical models of thermal stress in aquatic eggs. Ecol Lett 20: 50–59. [DOI] [PubMed] [Google Scholar]
- Mason J. (1969) Hypoxial stress prior to emergence and competition among Coho salmon fry. J Fish Res Bd Can 26: 63–91. [Google Scholar]
- McBryan TL, Anttila K, Healy TM, Schulte PM (2013) Responses to temperature and hypoxia as interacting stressors in fish: Implications for adaptation to environmental change. Integr Comp Biol 53: 648–659. [DOI] [PubMed] [Google Scholar]
- McBryan TL, Healy TM, Haakons KL, Schulte PM (2016) Warm acclimation improves hypoxia tolerance in Fundulus heteroclitus. J Exp Biol 219: 474–484. [DOI] [PubMed] [Google Scholar]
- McCullough DA. (1999) A Review and Synthesis of Effects of Alterations to the Water Temperature Regime on Freshwater Life Stages of Salmonids, with Special Reference to Chinook Salmon. EPA, Seattle, 279. EPA 910-R-99-010. [Google Scholar]
- McDonnell LH, Chapman LJ (2015) At the edge of the thermal window: effects of elevated temperature on the resting metabolism, hypoxia tolerance and upper critical thermal limit of a widespread African cichlid. Conserv Physiol 3: cov050 10.1093/conphys/cov050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Reeb SE, Wright PA, Gillis TE (2008) Oxygen concentration in the water boundary layer next to rainbow trout (Oncorhynchus mykiss) embryos is influenced by hypoxia exposure time, metabolic rate, and water flow. Can J Fish Aquat Sci 65: 2170–2177. [Google Scholar]
- Motyka R, Norin T, Petersen LH, Huggett DB, Gamperl AK (2017) Long-term hypoxia exposure alters the cardiorespiratory physiology of steelhead trout (Oncorhynchus mykiss), but does not affect their upper thermal tolerance. J Thermal Biol 68: 149–161. [DOI] [PubMed] [Google Scholar]
- Moyle PB. (2002) Inland Fishes of California. University of California Press, Berkeley, CA. [Google Scholar]
- Murray CB, McPhail JD (1987) Effect of incubation temperature on the development of five species of Pacific salmon (Oncorhynchus) embryos and alevins. Can J Zool 66: 266–273. [Google Scholar]
- Myrick CA, Cech JJ (2004) Temperature effects on juvenile anadromous salmonids in California’s central valley: what don’t we know? Rev Fish Biol Fisher 14: 113–123. [Google Scholar]
- National Marine Fisheries Service (2009) Chinook Salmon/Sturgeon Biological Opinion. Southwest Region, June 4th.
- Nilsson GE, Ostlund-Nilsson S, Munday PL (2010) Effects of elevated temperature on coral reef fishes: loss of hypoxia tolerance and inability to acclimate. Comp Biochem Physiol A Mol Integr Physiol 156: 389–393. [DOI] [PubMed] [Google Scholar]
- Ninness MM, Stevens ED, Wright PA (2006) Energy expenditure during hatching in rainbow trout (Oncorhynchus mykiss) embryos. Can J Fish Aquat Sci 63: 1405–1413. [Google Scholar]
- Oppen-Berntsen DO, Bogsnes A, Walther TH (1990) The effects of hypoxia alkalinity and neurochemicals on hatching of Atlantic salmon (Salmo salar) eggs. Aquaculture 86: 417–430. [Google Scholar]
- Pan TF, von Herbing IH (2017) Metabolic plasticity in development: Synergistic responses to high temperature and hypoxia in zebrafish, Danio rerio. J Exp Zool Part A 327: 189–199. [DOI] [PubMed] [Google Scholar]
- Pepin P. (1991) Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Can J Fish Aquat Sci 48: 503–518. [Google Scholar]
- Peterson NP, Quinn TP (1996) Spatial and temporal variation in dissolved oxygen in natural egg pockets of chum salmon, in Kennedy Creek, Washington. J Fish Biol 48: 131–143. [Google Scholar]
- Polymeropoulos ET, Elliot NG, Frappell PB (2016) The maternal effect of differences in egg size influence metabolic rate and hypoxia induced hatching in Atlantic salmon eggs: implications for respiratory gas exchange across the egg capsule. Can J Fish Aquat Sci 73: 1173–1181. [Google Scholar]
- Polymeropoulos ET, Elliot NG, Frappell PB (2017) Hypoxic acclimation leads to metabolic compensation after reoxygenation in Atlantic salmon yolk-sac alevins. Comp Biochem Physiol A Mol Integr Physiol 213: 28–35. [DOI] [PubMed] [Google Scholar]
- Pörtner HO. (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88: 137–146. [DOI] [PubMed] [Google Scholar]
- Pörtner HO. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: Retrieved from http://www.R-project.org/. [Google Scholar]
- Rees BB, Sudradjat FA, Love JW (2001) Acclimation to hypoxia increases survival time of zebrafish, Danio rerio, during lethal hypoxia. J Exp Zool 289: 266–272. [DOI] [PubMed] [Google Scholar]
- Remen M, Oppedal F, Imsland AK, Olsen RE, Torgersen T (2013) Hypoxia tolerance thresholds for post-smolt Atlantic salmon: dependency of temperature and hypoxia acclimation. Aquaculture 416–417: 41–47. [Google Scholar]
- Richards JG. (2009) Metabolic and molecular responses of fish to hypoxia In Richards JG, Farrell AP, Brauner CJ, eds. Fish Physiology, Vol. 27 Elsevier, London, pp 443–485. [Google Scholar]
- Richter A, Kolmes SA (2005) Maximum temperature limits for Chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Rev Fish Sci 13: 23–49. [Google Scholar]
- Rombough PJ. (1988. a) Growth, aerobic metabolism, and dissolved oxygen requirements of embryos and alevins of steelhead, Salmo gairdneri. Can J Zool 66: 651–660. [Google Scholar]
- Rombough PJ. (1988. b) Respiratory gas exchange, aerobic metabolism, and effects of hypoxia during early life In Hoar WS, Randall DJ, eds. Fish Physiology, Vol. 11A Academic Press, New York, pp 59–161. [Google Scholar]
- Rombough PJ. (2006) Developmental costs and partitioning of metabolic energy In Warburton SJ, Burggren WW, Pelster B, Reiber CL, Spicer J, eds. Comparative Developmental Physiology Contributions, Tools and Trends. Oxford University Press, Oxford, pp 99–123. [Google Scholar]
- Rombough P. (2011) The energetics of embryonic growth. Resp Physiol Neurobi 178: 22–29. [DOI] [PubMed] [Google Scholar]
- Roussel JM. (2007) Carry-over effects in brown trout (Salmo trutta): hypoxia on embryos impairs predator avoidance by alevins in experimental channels. Can J Fish Aquat Sci 64: 786–792. [Google Scholar]
- Rubin JF, Glimsäter C (1996) Egg-to-fry survival of the sea trout in some streams of Gotland. J Fish Biol 48: 585–606. [Google Scholar]
- Rutledge JC, Beitinger TL (1989) The effects of dissolved oxygen and aquatic surface respiration on the critical thermal maxima of three intermittent-stream fishes. Environ Biol Fish 24: 137–143. [Google Scholar]
- Sear DA, Pattison I, Collins AL, Newson MD, Jones JI, Naden PS, Carling PA (2014) Factors controlling the temporal variability in dissolved oxygen regime of salmon spawning gravels. Hydrol Process 28: 86–103. [Google Scholar]
- Shimps EL, Rice JA, Osborne JA (2005) Hypoxia tolerance in two juvenile estuary-dependent fishes. J Exp Mar Biol Ecol 325: 146–162. [Google Scholar]
- Silver SJ, Warren CE, Doudoroff P (1963) Dissolved oxygen requirements of developing steelhead trout and Chinook salmon embryos at different water velocities. Trans Ameri Fish Soc 92: 327–343. [Google Scholar]
- Shumway DL, Warren CE, Doudoroff P (1964) Influence of oxygen concentration and water movement on the growth of steelhead trout and coho salmon embryos. Trans Am Fish Soc 93: 342–356. [Google Scholar]
- Sokolova IM. (2013) Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr Comp Biol 53: 597–608. [DOI] [PubMed] [Google Scholar]
- Timmerman CM, Chapman LJ (2004) Behavioral and physiological compensation for chronic hypoxia in the sailfin molly (Poecilia latipinna). Physiol Biochem Zool 77: 601–610. [DOI] [PubMed] [Google Scholar]
- Trippel E A, Chambers RC (1997) Early life history and recruitment: legacy and challenges in fish populations In Chambers RC, Trippel EA, eds. Early Life History and Recruitment in Fish Populations, Vol 21 Chapman and Hall, London, pp 515–549. [Google Scholar]
- Todgham AE, Stillman JH (2013) Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr Comp Biol 53: 539–544. [DOI] [PubMed] [Google Scholar]
- Verberk WCEP, Leuven RSEW, van der Velde G, Gabel F (2018) Thermal limits in native and alien freshwater peracarid Crustacea: the role of habitat use and oxygen limitation. Funct Ecol 32: 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer S, Moore FBG, Bagatto B (2006) The effects of chronic developmental hypoxia on swimming performance in zebrafish. J Fish Biol 69: 1885–1891. [Google Scholar]
- Williams JG. (2006) Central Valley salmon: A perspective on Chinook and steelhead in the Central Valley of California. San Francisco Estuary Watershed Sci 4: 1–393. [Google Scholar]
- Yamagami K. (1988) Mechanisms of hatching in fish In Hoar WS, Randall DJ, eds. The Physiology of Developing Fish: Eggs and Larvae, Fish Physiology, Vol. 11A Academic Press, San Diego, pp 447–499. [Google Scholar]
- Youngson AF, Malcolm IA, Thorley JL, Bacon PJ, Soulsby C (2004) Long-residence groundwater effects on incubating salmonid eggs: low hyporheic oxygen impairs embryo development. Can J Fish Aquat Sci 61: 2278–2287. [Google Scholar]
- Zambonino-Infante JL, Claireaux G, Ernande B, Jolivet A, Quazuguel P, Severe A, Huelvan C, Mazurais D (2013) Hypoxia tolerance of common sole juveniles depends on dietary regime and temperature at the larval stage: evidence for environmental conditioning. Proc R Soc B 280: 20123022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambonino-Infante JL, Mazurais D, Dubuc A, Queau P, Vanderplancke G, Servili A, Cahu C, Le Bayon N, Huelvan C, Claireaux G (2017) An early life hypoxia event has a long-term impact on protein digestion and growth in juvenile European sea bass. J Exp Biol 220: 1846–4851. [DOI] [PubMed] [Google Scholar]
- Zebral YD, Lansini LR, Costa PG, Roza M, Bianchini A, Robaldo RB (2018) A glyphosate-based herbicide reduces fertility, embryonic upper thermal tolerance and alters embryonic diapause of the threatened annual fish Austrolebias nigrofasciatus. Chemosphere 196: 260–269. [DOI] [PubMed] [Google Scholar]