Abstract
The glycocalyx is an information-dense network of biomacromolecules extensively modified through glycosylation that populates the cellular boundary. The glycocalyx regulates biological events ranging from cellular protection and adhesion to signalling and differentiation. Owing to the characteristically weak interactions between individual glycans and their protein binding partners, multivalency of glycan presentation is required for the high-avidity interactions needed to trigger cellular responses. As such, biological recognition at the glycocalyx interface is determined by both the structure of glycans that are present as well as their spatial distribution. While genetic and biochemical approaches have proven powerful in controlling glycan composition, modulating the three-dimensional complexity of the cell-surface ‘glycoscape’ at the sub-micrometre scale remains a considerable challenge in the field. This focused review highlights recent advances in glycocalyx engineering using synthetic nanoscale glycomaterials, which allows for controlled de novo assembly of complexity with precision not accessible with traditional molecular biology tools. We discuss several exciting new studies in the field that demonstrate the power of precision glycocalyx editing in living cells in revealing and controlling the complex mechanisms by which the glycocalyx regulates biological processes.
Keywords: glycocalyx, cell-surface engineering, glycoconjugate, glycomaterials
1. Introduction
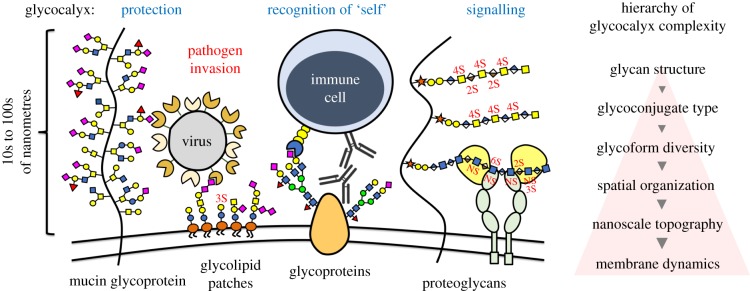
The cellular glycocalyx [1], a biological interface composed of membrane-bound glycolipids and glycoproteins, controls major biological events including protection from pathogens, regulation of immune responses and mediation of cellular communication, among others [2]. The complexity of the glycocalyx, which is the product of the combined chemical diversity of its glycan structures and their lipid and protein carriers, can be advantageous from a biological standpoint but complicates its study and manipulation (figure 1). While individual glycans provide a structural basis for affinity and selectivity in protein recognition, their nanoscale organization within glycoconjugates and across the glycocalyx dictate the localization and extent of their activity [1]. As such, the ‘glycoscape’ at the cell surface needs to be considered in its entirety to fully understand the biological processes occurring at the cell surface.
Figure 1.
Glycocalyx complexity. The cellular glycocalyx is a biological interface mediating the exchange of information between cells and their surroundings. Composed of glycolipids and glycoproteins bearing a large diversity of glycan structures, the glycocalyx is a complex and dynamic macromolecular network, which harbours both signatures of ‘self’ as well as molecular targets for opportunistic pathogens. (Online version in colour.)
One important outcome of evolution is the selection of glycans to serve as chemical determinants of ‘self’ at the surfaces of cells [3], while providing a barrier against pathogenic invasion (figure 1). The non-templated biosynthesis of glycoconjugates offers a means to rapidly generate unique molecular signatures to limit the adhesion of pathogens, which constantly adapt to exploit cell-surface glycans to enter the cell [4]. Interestingly, as the glycoscape continuously changes to minimize pathogenic threats, it must maintain its unique ‘self’ signature to avoid triggering an immune response. The centrality of glycans in these two concurrent but divergent functions raises the intriguing question of what molecular patterns define ‘self’ at the cell surface.
The mammalian glycome is constructed from a relatively small pool of building blocks (approx. 10 monosaccharides) [5]. While still affording a vast structural space of possible linear and branched glycans, only a small fraction of this space is actually occupied based on the availability and specificity of glycosyltransferase enzymes that catalyse the formation of glycosidic bonds [5]. To overcome the limitations on the diversity of biosynthetically accessible glycan structures, additional complexity can be derived from glycan presentation in glycoconjugates and the distribution of the glycoconjugates throughout the glycocalyx. The individual glycan structures can be organized within the glycocalyx to present unique molecular patterns, which are recognized by the various components of the innate and adaptive immune systems (figure 1) [6,7]. Since the most accessible terminal glycan modifications (e.g. sialic acids) are often the target of protein receptors, it is conceivable that the molecular patterns that define ‘self’ may be assembled from diverse set of glycans, as long as the terminal modifications are attached through an appropriate glycosidic linkage needed for recognition and are optimally distributed within the glycocalyx [7].
Understanding the ways in which cells use glycan presentation to maintain a specific ‘signal’ within the ‘noise’ of the evolving glycocalyx will be critical to fully account for the various biological functions of glycans. Defining how the spatial organization of glycans determines biological responses should be of great interest to the biomedical community for its potential value in the development of therapeutics that discriminate between host cells and pathogens or that harness the protective functions of the immune response for therapeutic gain [8,9].
Answering these questions will necessitate systematic cataloguing of the composition and spatial organization of the glycocalyx and correlating this information with its biological functions. Current techniques to control the composition of the glycocalyx [10,11] rely primarily on genetic [12], approaches that target the expression of specific glycan biosynthesis enzymes to influence glycan assembly, chemoenzymatic methods [13] for editing of existing cell-surface glycan structures, and metabolic strategies [14] for the introduction of non-natural monosaccharide modifications across the glycome. While powerful, these techniques result in the addition or subtraction of a specific glycan feature across entire classes of glycoconjugates and, thus, induce a global perturbation in the glycocalyx without control over cell-surface presentation and nanoscale organization.
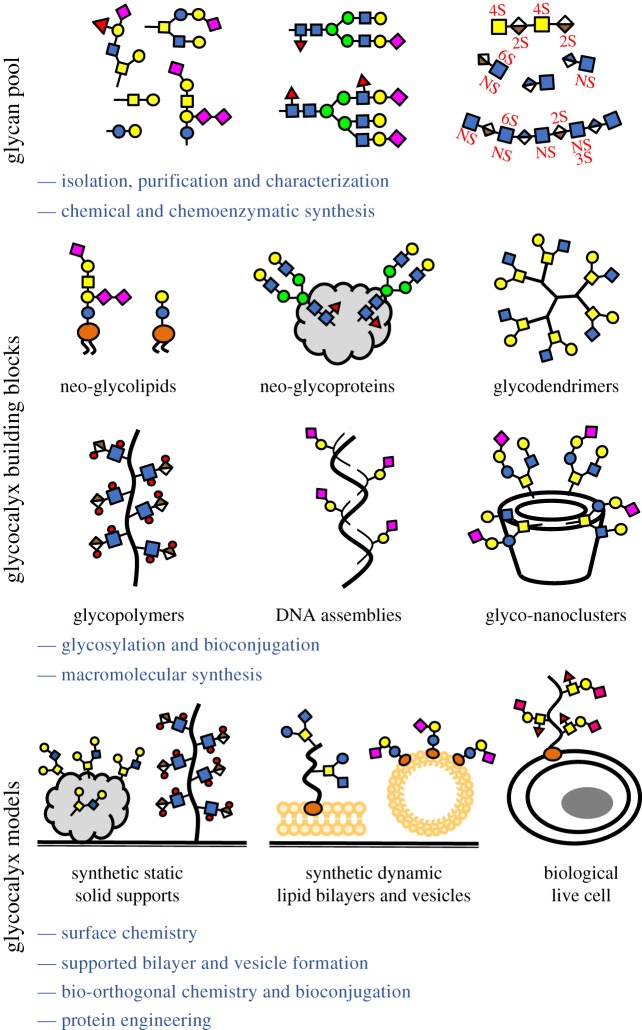
More recently, efforts to recapitulate the structural and organizational complexity of the glycocalyx have borrowed from synthetic biology; chemical tools have enabled the synthesis of nanoscale glycomaterials that mimic the various components of the glycocalyx and can be used to edit or even build synthetic glycoscapes on the surface of living cells (figure 2). Our aim is to highlight some recent advances in the field of synthetic glycobiology that enable the creation of a de novo display of glycans, glycoconjugates and mimetics in analytical platforms and on surfaces of living cells with enhanced spatial and temporal control over glycan structure and organization. Sometimes referred to as ‘precision glycocalyx editing’, this approach is becoming increasingly integrated with more traditional methods aiming to answer fundamental questions in glycobiology.
Figure 2.
Synthetic glycobiology toolkit for addressing glycocalyx complexity. An increasingly large pool of glycan structures is becoming available due to advancements in glycan isolation and purification and in chemical and chemoenzymatic methods for their synthesis. Individual glycans can be assembled into synthetic and semi-synthetic glycoconjugates, which mimic the architecture of natural glycolipids and glycoproteins. Structurally well-defined glycomimetics serve as building blocks for the de novo construction of glycocalyx models by immobilization on solid supports or by anchoring in lipid bilayers, lipid vesicles, or directly in the plasma membranes of living cells. (Online version in colour.)
In principle, the bottom-up nature of precision glycocalyx editing allows for the assembly of cellular glycoscapes composed of virtually unlimited combinations of glycan structures and macromolecular scaffolds. However, the power of this approach will ultimately lie in the ability to reliably recapitulate biologically relevant architectures found within the native glycocalyx. The glycocalyx has so far resisted detailed characterization of its composition and organization at the molecular level. However, advances in MS-based glycoproteomics, super resolution optical imaging and cryoelectron microscopy, and chemical approaches to capture and spatially correlate interactions within the glycocalyx provide hope for a more clear view of the cellular glycocalyx and its biological functions in the near future.
2. Nanoscale mimetics of macromolecular constituents of the glycocalyx
Synthetic glycomaterials have long provided a vital tool for analysing glycan protein interactions [15]. Used extensively as soluble ligands with controlled multivalent glycan presentation, they have helped reveal the mechanisms that allow glycan-binding proteins to recognize and engage glycan displays on glycoproteins to transfer biological information [16–18]. Modern macromolecular synthesis methods and protein- and nucleic acid-templated [19] material assembly strategies provide access to chemically defined one-dimensional linear glycopolymers [20], three-dimensional glycodendrimers [21] and even extended two-dimensional glycoarrays [22] where individual glycans can be presented with exquisite control over glycan valency and spatial organization at the nanometre scale (figure 2).
Among the many contributions to the field over the last three decades [23], some studies stand out as particularly powerful examples of the utility of synthetic glycomaterials in revealing the mechanisms that underpin the biological recognition of glycans. In a series of early reports, the groups of Bovin, Whitesides and Kiessling employed linear glycopolymers to investigate the effects of glycan valency and spacing on the avidity of viral haemagglutinin [24,25] and lectin [26,27] binding. Notably, the Kiessling group later developed a set of simple high-throughput assays that allowed them to delineate the relationships between the nanoscale architecture of macromolecular glycoconjugates (i.e. compact globular versus extended linear structures) and their ability to promote distinct binding events such as lectin inhibition or receptor clustering [16]. Since these pioneering studies, a wide range of glycoconjugate mimetic scaffold architectures have been proposed and investigated as inhibitors or modulators of glycan-binding receptor activities with an eye toward biomedical applications [28,29]. More recently, the predictability and programmability of the nucleic acid assembly into nanoscale materials has enabled the generation of glycomaterials with sub-nanometre precision in glycan presentation. These advanced materials have provided further insights into glycan interactions in a biological context [30,31], allowing for controlled assembly of glycan microheterogeneity [32] and the selection of optimal glycoconjugate-receptor pairs using dynamic assembly [19] and directed evolution [33,34] of glycoconjugates.
3. Static surface-supported glycocalyx models
As nanoscale glycomaterials became indispensable tools for probing the molecular interactions of glycoconjugates with glycan-binding proteins, it became evident that the properties of glycoconjugates in solution could not fully recapitulate their ensemble behaviour at surfaces. For instance, glycans functionalized with alkanethiolates forming self-assembled monolayers (SAM) on gold surfaces revealed that the modulation of surface glycan density can result in a switch in lectin-binding specificity and may act as an ‘on-off’ switch for downstream biological processes [35]. Similarly, variations in macromolecular glycoconjugate and polysaccharide architecture and surface grafting density introduce additional considerations for evaluating their recognition by glycan-binding proteins (figure 2). The immobilization of macromolecular glycoconjugates on surfaces can enhance multivalency and avidity of glycan displays [36] but also alter their molecular conformation and dynamics (e.g. mushroom to brush polymer transitions) affecting protein diffusion [37,38]. A close proximity of glycoconjugates on surfaces can also promote higher-order binding interactions, such as cross-linking of neighbouring glycoconjugates by multimeric lectins (figure 3) [39].
Figure 3.
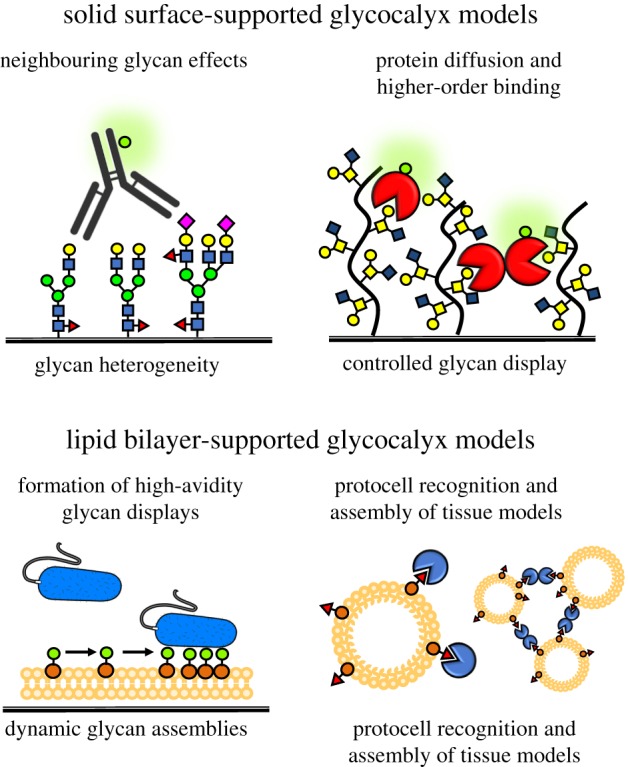
Synthetic Glycoscapes. Static glycocalyx models immobilized on solid supports offer control over glycan composition and presentation and facile integration with common analytical platforms. Glycocalyx structures anchored in model membranes can dynamically reorganize to provide optimal presentations for high-avidity binding. Lipid vesicle carriers allow for investigation of the relationships between membrane curvature and glycocalyx organization and to model glycan-mediated assembly of multicellular systems. (Online version in colour.)
An intriguing property of surface glycoconjugate displays is the possibility to generate glycocalyx models with spatially addressable structural complexity. It is increasingly recognized that changes in the microheterogeneity of glycan environments within the cellular glycocalyx can have a profound influence on cellular functions [40]. The glycan microarray provides a powerful platform for rapid profiling of protein interactions with surface-immobilized glycans [41]. Established initially as a way to present individual glycans [42], the array concept was later expanded to include well-defined synthetic glycoconjugates, such as neo-glycoproteins [43,44], glycodendrimers [45] or glycopolymers [39,46,47], allowing for presentation of glycans in a number of modes with control over scaffold geometry, glycan valency and spacing, and surface density of glycoconjugates. Most recently, glycan arrays prepared by immobilization of glycan mixtures within individual spots revealed that the presence of neighbouring glycans may result in higher avidity antibody binding to target epitope and that both density and structure of neighbouring glycans can effect recognition (figure 3) [48,49]. These findings confirmed that glycan microheterogeneity within the cellular glycocalyx should be an important consideration in the development of therapeutics targeting glycocalyx structures on pathogens as well as human cells.
One advantage of static surface-immobilized glycan displays is the ability to use advances in micro- and nanofabrication technologies and high-resolution imaging techniques to create and characterize high information-content arrays. Microcontact printing of pre-functionalized glycomaterials [50] or surface-initiated de novo glycoconjugate synthesis using beam pen lithography [51] provides two examples illustrating the emerging opportunities for building increasingly complex models of the glycocalyx on solid supports.
4. Dynamic membrane-supported glycocalyx models
Despite the convenience and utility of glycoconjugate presentation on solid surfaces, these displays fail to capture the dynamic features of the native glycocalyx. There, the lateral membrane diffusion of individual glycoconjugates components is permitted and results in a highly adaptable environment in which molecular interactions occur. This property of the glycocalyx can be critical for its ability to modulate processes such as receptor clustering, formation of the immunological synapse or mediation of host–pathogen interactions (figure 3). The supported lipid bilayer [52] serves as an excellent experimental model of the fluid plasma membrane of cells to study glycocalyx-associated phenomena [53,54]. Supported lipid bilayer experiments have provided important insights into the effects of glycolipid density and clustering on their recognition by soluble lectins [55], as well as by the more complex arrays of glycan-binding receptors on the surfaces of viruses [56] and bacteria [57]. The supported bilayer system can also be used to investigate the behaviour of macromolecular glycoconjugates in membranes. Interferometric imaging of lipid-anchored nanoscale synthetic mimetics of mucin glycoproteins, which recapitulate the characteristic long-extended architectures of mucins stemming from dense glycosylation of their core polypeptide backbones, revealed the tendency of these materials to accommodate upright orientations and project distally from the membrane surface while retaining lateral mobility in the lipid bilayer [58]. Similarly, dynamic surface presentations of hyaluronic acid and heparan sulfate (HS) glycosaminoglycan (GAG) polysaccharides in supported lipid bilayers have provided insights into biophysical behaviour, mobility and molecular recognition within sterically crowded glycocalyx environments [38]. The observed changes in GAG organization and behaviour induced by protein binding and cross-linking suggest that growth factor and cytokine activity may not be limited to receptor activation but that altered glycocalyx dynamics may also contribute to signalling events.
In an effort to create increasingly realistic and complex models of the cell surface, it has become apparent that additional features of the cell surface, such as curvature and the related capacity to undergo membrane fusion and vesicle budding, need to be considered. This is particularly relevant as the changes in glycocalyx composition and the generation of membrane curvatures and shapes are locked in an intimate reciprocal relationship [59,60]. The development of protocells with well-defined surface glycan displays offers an elegant solution to this challenge. Giant unilamellar vesicles (GUVs) serve as an artificial cell membrane upon which a synthetic glycocalyx can be constructed using cholesterol-modified glycopeptides (figure 3) [61]. The glycocalyx structures can be incorporated into the membrane by electroformation with lipids during GUV formation or by spiking of pre-formed vesicles. Interestingly, in phase-separated GUVs, the glycopeptides preferentially localized to the liquid-disordered domain, thus introducing a means by which to control the spatial organization of glycoconjugates on the protocell surface. Systematic modification of the glycopeptide scaffold using a series of orthogonal click reactions to introduce combinations of anchors, optical probes for visualization and glycans provided access to compositionally diverse materials for functional glycan-receptor pairing [62]. The platform proved to be an effective tool for studying the role of ABO blood group antigen on malarial rosetting, as it allowed for systematic presentation of the blood group antigen of interest on otherwise identical cells [63]. This approach led to the demonstration of receptor-specific malarial cell aggregation, or rosetting, by the P. falciparum parasites based on the structure of blood group antigens. Importantly, the protocell also offers an experimental system in which glycan interactions can be evaluated in the context of the assembly and organization of more complex higher-order multicellular systems [64,65]. Protocell designs based on the self-assembly of glycosylated amphiphilic block copolymers and glycodendrimersomes have also recently emerged to model pathogen interactions with the glycocalyx of host cells [66] and to rapidly evaluate the surface cross-linking and aggregation capacity of galectins in response to changes in compositional microheterogeneity of glycolipid patches [67], respectively. Together, these studies demonstrate the power of dynamic synthetic models of the cell surface for uncovering how the spatial and organizational complexity of the cellular glycocalyx influences molecular interactions at the cellular boundary. They provide a foundation for further exploration of this intricate biological interface to ultimately identify molecular patterns that constitute the markers of ‘self’ and ‘non-self’ and how these signals are maintained in the rapidly evolving glycocalyx environment.
5. Precision glycocalyx editing in living cells
While synthetic models of the cellular glycocalyx have been instrumental in developing the concepts of avidity in glycan interactions and delineating the mechanisms that underlie molecular interaction of glycans at the cell surface, ultimately these findings need to be brought into the context of cellular functions. Not surprisingly, developing approaches to control glycan presentation within the glycocalyx of living cells has been an area of intense focus [11,68]. Early work focused on applying genetic [69], enzymatic [13] and chemical [70] strategies to remove or add unique glycosylation motifs to cell-surface structures. More recently, these efforts have shifted toward the development of methods for building spatially and organizationally complex glycoscapes at the cell surface with greater precision with respect to glycan organization and localization to enable the study of complex biological processes, including signalling, differentiation and tissue morphogenesis.
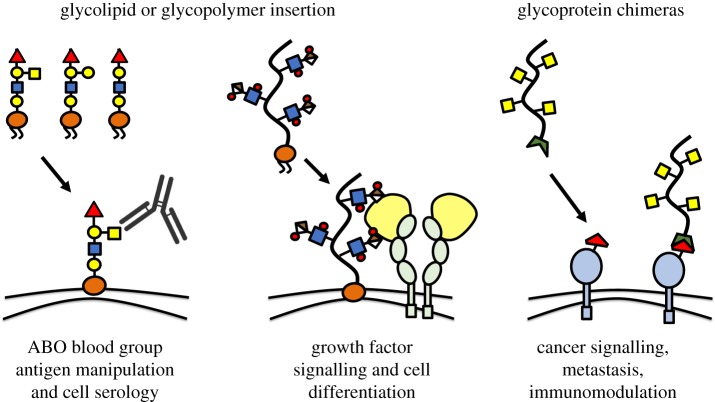
Among the examples illustrating the power of chemoenzymatic glycan engineering with exogenous glycosyltransferases are studies linking the regioselectivity of sialic acid linkages to underlying glycan structures on cell surfaces to host-specificity of Influenza A viruses [71] or demonstrating that the conversion of the CD44 glycoform into an E-selectin ligand via the addition of α-1,3-linked fucose can promote mesenchymal stem cell homing into bone marrow [72]. Chemoenzymatic glycosylation alters the structure of all acceptable enzyme substrates within the glycocalyx, limiting the ability to modify specific sets of glycoconjugates. Non-covalent modification of the cellular glycocalyx through the insertion of lipid-containing glycans into the plasma membrane overcomes this problem (figure 4). Early studies showing that glycosphingolipids with Lewis blood group antigens isolated from plasma were taken up by the membrane of human erythrocytes [73] later led to the development of a modular platform for the synthesis of synthetic glycolipid mimetics which consists of a lipid for membrane insertion, a spacer with tuneable chemical properties and a functional end group consisting of a glycan unit [74]. The glycoconjugates were used to stably remodel the surface of red blood cells with analogues of blood group determinants and verified by serological analysis [75]. In a particularly compelling example of this approach, a synthetic globotriaosylceramide analogue incorporated into the cell membrane inhibited infection by HIV-1 via both inhibition of viral entry and direct viral inhibition [76].
Figure 4.
Precision glycocalyx editing. Synthetic glycocalyx building blocks can be introduced directly to the surface of living cells. Glycolipids or lipid-functionalized glycoconjugates rapidly insert into the outer leaflet of plasma membranes. Glycocalyx editing can be used to redefine blood group antigen displays on red blood cells or to promote the association of growth factors with the cell surface and activate signalling and differentiation in stem cells. Endogenous membrane proteins engineered to present chemical groups can be joined with synthetic glycoconjugates carrying functionality with complementary reactivity. The resulting glycoprotein chimaeras can enhance the retention of the glycoconjugates at the cell surface and facilitate signalling. (Online version in colour.)
The initial studies on membrane-supported glycocalyx models (vide supra) paved the way for de novo assembly of glycocalyx structures on the surfaces of living cells [77]. Glycopolymers or polysaccharides [78] functionalized with phospholipid or cholesterol anchors can be passively inserted into the cell membrane. The use of chemically defined glycoconjugates permits the introduction of specific glycan motifs in predefined spatial arrangement and removes the limitations of chemoenzymatic glycan engineering imposed by the specificity of glycosyltransferase enzymes. The compositional complexity of the artificial glycocalyx that can be achieved using this method is not limited to a single glycoconjugate, as multiple glycoconjugates carrying distinct glycan structures and presentation can be introduced simultaneously at tuneable ratios [79]. Thus, the composition and hierarchical organization of the cellular glycoscape can be designed and rapidly constructed to meet specific experimental needs.
The precision glycocalyx editing approach has been leveraged to elucidate and control vital cellular signalling processes. For instance, sialylated glycopolymer mimetics of mucin glycoproteins were used to recapitulate the hypersialylated glycocalyx of adenocarcinoma cells. The remodelled cells showed the ability to dampen antigen-induced natural killer (NK) cell cytotoxicity by recruiting inhibitory siglec receptors into the immunological synapse [80]. Engineering the GAG component of the cellular glycocalyx to control cellular signalling via growth factors and morphogens has also been an area of active research. Liposomes modified with chondroitin sulfate (CS) GAG polysaccharides were shown to undergo fusion with the membrane of neurons. The cell-anchored CS GAGs potentiated neurotrophin signalling and neurite outgrowth in a sulfation pattern-dependent manner [78]. HS GAG-mimetic polymers with affinity for the fibroblast growth factor 2 and bone morphogenetic protein 4 have also been developed and used to edit the glycocalyx of embryonic stem cells deficient in HS GAG biosynthesis to induce differentiation toward neural [81] and mesodermal [82] germ layer cell types. In yet another application, GAG-mimetic polymers presented at the surfaces of differentiated myotubes facilitated signalling through the motoneuron-derived protein agrin and initiated the clustering of acetylcholine receptors, a process characteristic of the early stages of the neuromuscular synapse formation [83].
These initial efforts to display glycans of interest with programmable hierarchal complexity at the cell surface have also identified the need for improving material specificity and imparting temporal control over their display. One challenge associated with non-covalent glycocalyx editing is the continuous cellular uptake of the glycomaterials during cell membrane turnover. Optimization of anchoring chemistry led to the discovery that the use of a cholesterylamine anchor greatly extended the cell-surface residence time of the glycoconjugates via a recycling process, whereby internalized glycomaterials are shuttled back to the cell surface [84]. Similar effects can be achieved by integration of glycan motifs with cell-surface proteins with extended cell-surface retention (figure 4). For instance, genetic engineering of embryonic stem cells with the HaloTag protein (HTP) allowed for covalent conjugation of chloroalkane modified GAGs. This approach not only improved the surface half-life of the surface-displayed GAGs and enhanced neural differentiation of the stem cells but it also added an element of cell-specificity as only those cells stably expressing the HTP construct would undergo glycocalyx remodelling. The chemical integration of protein and glycan structures also adds another element of control to the glycocalyx editing process, where a well-defined synthetic glycoconjugate can be merged with existing receptor structures to take advantage of protein-associated biological functions. This was elegantly demonstrated by the generation of glycoprotein chimaeras using an EGF receptor engineered to present an extracellular norbornene for the covalent attachment of a synthetic mucin mimetic domain [85]. In this case, the mucin mimetic was composed of a glycopolypeptide synthesized via N-carboxyanhydride polymerization and terminated with a tetrazine handle to enable cell-surface cycloaddition conjugation with the pre-functionalized EGFR construct.
In constructing de novo glycoscapes or modulating existing glycocalyx structures on living cells, it is important to consider not only the effects on protein recognition and signalling in the new glycocalyx constructs but also their biophysical consequences. It is appreciated that bulky glycoconjugates, particularly mucins and proteoglycans, constitute a physical barrier to cells and contribute to membrane behaviour [60]. The increasing thickness, density and composition of the glycocalyx have been shown to enhance cell adhesivity and survival on soft matrices [86] and promote metastasis in vivo [87]. The mechanisms by which the glycocalyx facilitates integrin-mediated metastasis have been shown to be largely biomechanical in nature, with bulky mucin glycoproteins funnelling active integrins into clusters and applying tensile forces. Further, glycocalyx organization may force reorganization of membrane features and local curvature, as was observed in cells modified with both synthetic [59,88] and genetically encoded [89] glycoconjugates. Cells, and presumably pathogens, use the biophysical properties of the glycocalyx to regulate both molecular recognition events and adhesion as well as other glycan-independent signalling processes.
Undoubtedly, precision glycocalyx editing has become a powerful technique for elucidating the role of glycoconjugates at the surfaces of living cells in regulating biological outcomes. Despite the rapid progress in the field, many challenges still remain. In particular, approaches to cell-specifically modify the glycocalyx are limited to instances in which prior genetic manipulation of target cells is feasible. As well, the methods with which synthetic macromolecular glycomaterials can be generated still lack sequence control, such as that provided by the cellular glycosylation machinery, although promising developments in this area are beginning to emerge [90,91].
6. Convergent efforts to establish the structure of the native glycocalyx
Efforts to engineer artificial glycocalyx structures have progressed parallel to work toward better understanding the organization of the native glycocalyx, which includes careful systems-level analysis, cataloguing of the various glycocalyx components, and visualization of the glycocalyx and its interactions under physiological conditions. In the absence of methods for direct visualization of glycocalyx structures with molecular resolution, mass spectrometry (MS)-based glycoproteomics technologies [7,92] have provided the main tool for the study of the composition of glycome. High-sensitivity MS techniques combined with chemical tagging and enrichment of glycoconjugates is beginning to yield information about the interactome of the glycocalyx. The use of tandem MS approaches to profile glycans after glycosidase treatment can give a more complete understanding of the composition and structure of glycans; however, it is unable to capture the full repertoire of glyco-proteoforms. When paired with complementary bioinformatics platforms [93], chemical glycoproteomic approaches allow for the identification of both protein identity and its glycosylation state and identification of disease-associated alterations [94,95].
A more complete understanding of the molecular interactions and protein recognition events occurring within the compositionally heterogeneous environment of the cellular glycocalyx has been enabled by the development of shotgun glycan array techniques [96,97]. In this approach, entire collections of glycans harvested from cells are pooled, immobilized on a glass surface and subsequently interrogated with glycan-binding proteins of interest. Collections of glycans exhibiting biologically relevant activities are then analysed by MS and can be further fractionated to determine the identity of the active glycan structures. This strategy has been used to identify endogenous receptors for Influenza virus A [98] and to curate glycan epitopes recognized by cancer cell-specific antibodies [99]. The development of chemical tools combining metabolic oligosaccharide engineering with proximity labelling is beginning to provide information about protein interactions within the native glycocalyx with spatial resolution. Metabolic incorporation of monosaccharides modified with diazirine photoaffinity labels into cell-surface glycoconjugates allows for photo-cross-linking of glycans directly interacting with or in close proximity to proteins and receptors under investigation [100]. These approaches provide a steady stream of data rapidly generating a trove of knowledge about the composition of the glycocalyx and its interactions, which will guide future synthetic glycobiology efforts in their pursuit of revealing how this cellular interface regulates biological processes.
7. Summary and outlook
Methods by which to systematically manipulate the glycocalyx are numerous and have been instrumental in the elucidation of many structure–function relationships in glycan-mediated biological processes. Among those, the de novo glycocalyx scaffolding using synthetic glycoconjugates with tuneable architectures and functionality holds a prominent position as a particularly powerful tool to approximate the complexity and nanoscale organization of the native glycocalyx and to decipher how the cellular boundary regulates the exchange of information between the cell and its surroundings. The success of these strategies will ultimately rely on our ability to observe with molecular-level detail the native structures comprising the glycocalyx and define which material design parameters will be critical to building truly representative and functional glycocalyx models. Among the currently outstanding challenges is the development of strategies for targeting of synthetic glycomaterials to distinct membrane regions or to specific cells in multicellular systems and for manipulating the dynamics of glycocalyx displays with spatio-temporal control and in response to external stimuli. The field is now well positioned to begin to turn its focus from basic biology research toward the pursuit of the glycocalyx as a target for therapeutic intervention.
Data accessibility
This article has no additional data.
Authors' contributions
The authors contributed equally to the preparation and writing of this manuscript.
Competing interests
We declare we have no competing interests.
Funding
K.G. is supported by the Alfred P. Sloan Foundation and the Research Corporation for Science Advancement. This work was supported, in part, by the NIH Director's New Innovator Award (NICHD: 1DP2HD087954-01). S.C.P. received support from the GAANN fellowship (US Dept. of Education).
References
- 1.Weinbaum S, Tarbell JM, Damiano ER. 2007. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Bio. Eng. 9, 121–167. ( 10.1146/annurev.bioeng.9.060906.151959) [DOI] [PubMed] [Google Scholar]
- 2.Varki A, Gagneux P. 2017. Biological functions of glycans. In Essentials of glycobiology (eds Varki A, Cummings RD, Esko JD et al.), pp. 2015–2017, 3rd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- 3.Varki A. 2008. Sialic acids in human health and disease. Trends Mol. Med. 14, 351–360. ( 10.1016/j.molmed.2008.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Springer SA, Gagneux P. 2013. Glycan evolution in response to collaboration, conflict, and constraint. J. Biol. Chem. 288, 6904–6911. ( 10.1074/jbc.R112.424523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adibekian A, Stallforth P, Hecht M-L, Werz DB, Gagneux P, Seeberger PH. 2011. Comparative bioinformatics analysis of the mammalian and bacterial glycomes. Chem. Sci. 2, 337–344. ( 10.1039/C0SC00322K) [DOI] [Google Scholar]
- 6.Dam TK, Brewer CF. 2009. Lectins as pattern recognition molecules: the effects of epitope density in innate immunity. Glycobiology 20, 270–279. ( 10.1093/glycob/cwp186) [DOI] [PubMed] [Google Scholar]
- 7.Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB. 2015. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review, J. Autoimmun. 57, 1–13. ( 10.1016/j.jaut.2014.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudak JE, Bertozzi CR. 2014. Glycotherapy: new advances inspire a reemergence of glycans in medicine. Chem. Biol. 21, 16–37. ( 10.1016/j.chembiol.2013.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao H, Woods EC, Vukojicic P, Bertozzi CR. 2016. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl Acad. Sci. USA 113, 10 304–10 309. ( 10.1073/pnas.1608069113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxon E, Bertozzi CR. 2001. Chemical and biological strategies for engineering cell surface glycosylation. Annu. Rev. Cell Dev. Biol. 17, 1–23. ( 10.1146/annurev.cellbio.17.1.1) [DOI] [PubMed] [Google Scholar]
- 11.Griffin ME, Hsieh-Wilson LC. 2016. Glycan engineering for cell and developmental biology. Cell Chem. Biol. 23, 108–121. ( 10.1016/j.chembiol.2015.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esko JD, Stanley P. 2009. Glycosylation mutants of cultured cells. In Essentials of glycobiology (eds Varki A, Cummings R, Esko J et al.), chapter 46, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- 13.Lopez Aguilar A, Briard JG, Yang L, Ovryn B, Macauley MS, Wu P. 2017. Tools for studying glycans: recent advances in chemoenzymatic glycan labeling. ACS Chem. Biol. 12, 611–621. ( 10.1021/acschembio.6b01089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dube DH, Bertozzi CR. 2003. Metabolic oligosaccharide engineering as a tool for glycobiology. Curr. Opin. Chem. Biol. 7, 616–625. ( 10.1016/j.cbpa.2003.08.006) [DOI] [PubMed] [Google Scholar]
- 15.Kiessling LL, Grim JC. 2013. Glycopolymer probes of signal transduction. Chem. Soc. Rev. 42, 4476–4491. ( 10.1039/c3cs60097a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. 2002. Influencing receptor–ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 124, 14922–14933. ( 10.1021/ja027184x) [DOI] [PubMed] [Google Scholar]
- 17.Mammen M, Choi S-K, Whitesides GM. 1998. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37, 2754–2794. () [DOI] [PubMed] [Google Scholar]
- 18.Kiessling LL, Gestwicki JE, Strong LE. 2006. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. Int. Ed. 45, 2348–2368. ( 10.1002/anie.200502794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machida T, Novoa A, Gillon É, Zheng S, Claudinon J, Eierhoff T, Imberty A, Römer W, Winssinger N. 2017. Dynamic cooperative glycan assembly blocks the binding of bacterial lectins to epithelial cells. Angew. Chem. Int. Ed. 56, 6762–6766. ( 10.1002/anie.201700813) [DOI] [PubMed] [Google Scholar]
- 20.Miura Y, Hoshino Y, Seto H. 2015. Glycopolymer nanobiotechnology. Chem. Rev. 116, 1673–1692. ( 10.1021/acs.chemrev.5b00247) [DOI] [PubMed] [Google Scholar]
- 21.Turnbull WB, Stoddart JF. 2002. Design and synthesis of glycodendrimers. Rev. Mol. Biotechnol. 90, 231–255. ( 10.1016/S1389-0352(01)00062-9) [DOI] [PubMed] [Google Scholar]
- 22.Steiner K, Hanreich A, Kainz B, Hitchen PG, Dell A, Messner P, Schäffer C. 2008. Recombinant glycans on an S-layer self-assembly protein: a new dimension for nanopatterned biomaterials. Small 4, 1728–1740. ( 10.1002/smll.200701215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang ML, Godula K. 2016. Nanoscale materials for probing the biological functions of the glycocalyx. Glycobiology 26, 797–803. ( 10.1093/glycob/cww022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matrosovich MN, Mochalova LV, Marinina VP, Byramova NE, Bovin NV. 1990. Synthetic polymeric sialoside inhibitors of influenza virus receptor-binding activity. FEBS Lett. 272, 209–212. ( 10.1016/0014-5793(90)80486-3) [DOI] [PubMed] [Google Scholar]
- 25.Sigal GB, Mammen M, Dahmann G, Whitesides GM. 1996. Polyacrylamides bearing pendant α-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus: the strong inhibition reflects enhanced binding through cooperative polyvalent interactions. J. Am. Chem. Soc. 118, 3789–3800. ( 10.1021/ja953729u) [DOI] [Google Scholar]
- 26.Cairo CW, Gestwicki JE, Kanai M, Kiessling LL. 2002. Control of multivalent interactions by binding epitope density. J. Am. Chem. Soc. 124, 1615–1619. ( 10.1021/ja016727k) [DOI] [PubMed] [Google Scholar]
- 27.Kanai M, Mortell KH, Kiessling LL. 1997. Varying the size of multivalent ligands: the dependence of concanavalin A binding on neoglycopolymer length. J. Am. Chem. Soc. 119, 9931–9932. ( 10.1021/ja972089n) [DOI] [Google Scholar]
- 28.Bovin NV. 1998. Polyacrylamide-based glycoconjugates as tools in glycobiology. Glycoconj. J. 15, 431–446. ( 10.1023/A:1006963717646) [DOI] [PubMed] [Google Scholar]
- 29.Restuccia A, Fettis MM, Hudalla GA. 2016. Glycomaterials for immunomodulation, immunotherapy, and infection prophylaxis. J. Mater. Chem. B 4, 1569–1585. ( 10.1039/C5TB01780G) [DOI] [PubMed] [Google Scholar]
- 30.Gorska K, Huang K-T, Chaloin O, Winssinger N. 2009. DNA-templated homo- and heterodimerization of peptide nucleic acid encoded oligosaccharides that mimick the carbohydrate epitope of HIV. Angew. Chem. Int. Ed. 48, 7695–7700. ( 10.1002/anie.200903328) [DOI] [PubMed] [Google Scholar]
- 31.Novoa A, Winssinger N. 2015. DNA display of glycoconjugates to emulate oligomeric interactions of glycans. Beilstein J. Org. Chem. 11, 707–719. ( 10.3762/bjoc.11.81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novoa A, Machida T, Barluenga S, Imberty A, Winssinger N. 2014 PNA-encoded synthesis (PES) of a 10 000-member hetero-glycoconjugate library and microarray analysis of diverse lectins. Chembiochem 15, 2058–2065. ( 10.1002/cbic.201402280) [DOI] [PubMed] [Google Scholar]
- 33.Matsuura K, Hibino M, Yamada Y, Kobayashi K. 2001. Construction of glyco-clusters by self-organization of site-specifically glycosylated oligonucleotides and their cooperative amplification of lectin-recognition. J. Am. Chem. Soc. 123, 357–358. ( 10.1021/ja001945j) [DOI] [PubMed] [Google Scholar]
- 34.Horiya S, Bailey JK, Temme JS, Guillen Schlippe YV, Krauss IJ. 2014. Directed evolution of multivalent glycopeptides tightly recognized by HIV antibody 2G12. J. Am. Chem. Soc. 136, 5407–5415. ( 10.1021/ja500678v) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horan N, Yan L, Isobe H, Whitesides GM, Kahne D. 1999. Nonstatistical binding of a protein to clustered carbohydrates. Proc. Natl Acad. Sci. USA 96, 11 782–11 786. ( 10.1073/pnas.96.21.11782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gestwicki JE, Cairo CW, Mann DA, Owen RM, Kiessling LL. 2002. Selective immobilization of multivalent ligands for surface plasmon resonance and fluorescence microscopy. Anal. Biochem. 305, 149–155. ( 10.1006/abio.2002.5652). [DOI] [PubMed] [Google Scholar]
- 37.Meng X-L, Fang Y, Wan L-S, Huang X-J, Xu Z-K. 2012. Glycopolymer brushes for the affinity adsorption of RCA120: effects of thickness, grafting density, and epitope density. Langmuir 28, 13 616–13 623. ( 10.1021/la302389e) [DOI] [PubMed] [Google Scholar]
- 38.Migliorini E, Thakar D, Sadir R, Pleiner T, Baleux F, Lortat-Jacob H, Coche-Guerente L, Richter RP. 2014. Well-defined biomimetic surfaces to characterize glycosaminoglycan-mediated interactions on the molecular, supramolecular and cellular levels. Biomaterials 35, 8903–8915. ( 10.1016/j.biomaterials.2014.07.017) [DOI] [PubMed] [Google Scholar]
- 39.Godula K, Bertozzi CR. 2012. Density variant glycan microarray for evaluating cross-linking of mucin-like glycoconjugates by lectins. J. Am. Chem. Soc 134, 15732–15742. ( 10.1021/ja302193u) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen M, Varki A. 2014. Modulation of glycan recognition by clustered saccharide patches. Int. Rev. Cell. Mol. Biol. 308, 75–125. ( 10.1016/B978-0-12-800097-7.00003-8) [DOI] [PubMed] [Google Scholar]
- 41.Oyelaran O, Gildersleeve JC. 2009. Glycan arrays: recent advances and future challenges. Curr. Opin. Chem. Biol. 13, 406–413. ( 10.1016/j.cbpa.2009.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blixt O, et al. 2004. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl Acad. Sci. USA 101, 17 033–17 038. ( 10.1073/pnas.0407902101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Li Q, Rodriguez LG, Gildersleeve JC. 2010. An array-based method to identify multivalent inhibitors. J. Am. Chem. Soc. 132, 9653–9662. ( 10.1021/ja100608w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Campbell C, Li Q, Gildersleeve JC. 2010. Multidimensional glycan arrays for enhanced antibody profiling. Mol. BioSyst. 6, 1583 ( 10.1039/c002259d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parera Pera N, Branderhorst HM, Kooij R, Maierhofer C, van der Kaaden M, Liskamp RMJ, Wittmann V, Ruijtenbeek R, Pieters RJ. 2010. Rapid screening of lectins for multivalency effects with a glycodendrimer microarray. Chembiochem 11, 1896–1904. ( 10.1002/cbic.201000340) [DOI] [PubMed] [Google Scholar]
- 46.Huang ML, Cohen M, Fisher CJ, Schooley RT, Gagneux P, Godula K. 2015. Determination of receptor specificities for whole influenza viruses using multivalent glycan arrays. Chem. Comm. 51, 5326–5329. ( 10.1039/c4cc08613a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann K, Conde-González A, Owens M, Venturato A, Zhang Y, Geng J, Bradley M. 2017. An approach to the high-throughput fabrication of glycopolymer microarrays through thiol–ene chemistry. Macromolecule 50, 6026–6031. ( 10.1021/acs.macromol.7b00952) [DOI] [Google Scholar]
- 48.Liang C-H, Wang S-K, Lin C-W, Wang C-C, Wong C-H, Wu C-Y. 2011. Effects of neighboring glycans on antibody-carbohydrate interaction. Angew. Chem. Int. Ed. 50, 1608–1612. ( 10.1002/anie.201003482) [DOI] [PubMed] [Google Scholar]
- 49.Shivatare VS, et al. 2018. Unprecedented role of hybrid N-glycans as ligands for HIV-1 broadly neutralizing antibodies. J. Am. Chem. Soc. 140, 5202–5210. ( 10.1021/jacs.8b00896) [DOI] [PubMed] [Google Scholar]
- 50.Godula K, Rabuka D, Nam KT, Bertozzi CR. 2009. Synthesis and microcontact printing of dual end-functionalized mucin-like glycopolymers for microarray applications. Angew. Chem. Int. Ed. 48, 4973–4976. ( 10.1002/anie.200805756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bian S, Zieba SB, Morris W, Han X, Richter DC, Brown KA, Mirkin CA, Braunschweig AB. 2014. Beam pen lithography as a new tool for spatially controlled photochemistry, and its utilization in the synthesis of multivalent glycan arrays. Chem. Sci. 5, 2023 ( 10.1039/C3SC53315H) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sackmann E. 1996. Supported membranes: scientific and practical applications. Science 271, 43–48. ( 10.1126/science.271.5245.43) [DOI] [PubMed] [Google Scholar]
- 53.Richter RP, Bérat R, Brisson AR. 2006. Formation of solid-supported lipid bilayers: an integrated view. Langmuir 22, 3497–3505. ( 10.1021/la052687c) [DOI] [PubMed] [Google Scholar]
- 54.Sych T, Mély Y, Römer W. 2018. Lipid self-assembly and lectin-induced reorganization of the plasma membrane. Phil. Trans. R. Soc. B 373, 20170117 ( 10.1098/rstb.2017.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann DA, Kanai M, Maly DJ, Kiessling LL. 1998. Probing low affinity and multivalent interactions with surface plasmon resonance: ligands for concanavalin A. J. Am. Chem. Soc. 120, 10575–10582. ( 10.1021/ja9818506) [DOI] [Google Scholar]
- 56.Goronzy IN, Rawle RJ, Boxer SG, Kasson PM. 2018. Cholesterol enhances influenza binding avidity by controlling nanoscale receptor clustering. Chem. Sci. 9, 2340–2347. ( 10.1039/C7SC03236F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu X-Y, Holtz B, Wang Y, Wang L-X, Orndorff PD, Guo A. 2009. Quantitative glycomics from fluidic glycan microarrays. J. Am. Chem. Soc. 131, 13646–13650. ( 10.1021/ja902783n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godula K, Umbel ML, Rabuka D, Botyanszki Z, Bertozzi CR, Parthasarathy R. 2009. Control of the molecular orientation of membrane-anchored biomimetic glycopolymers. J Am. Chem. Soc. 131, 10263–10268. ( 10.1021/ja903114g) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aigal S, Claudinon J, Römer W. 2015. Plasma membrane reorganization: a glycolipid gateway for microbes. BBA. Mol. Cell Res. 1853, 858–871. ( 10.1016/j.bbamcr.2014.11.014) [DOI] [PubMed] [Google Scholar]
- 60.Kuo JC-H, Gandhi JG, Zia RN, Paszek MJ. 2018. Physical biology of the cancer cell glycocalyx. Nat. Phys. 14, 658–669. ( 10.1038/s41567-018-0186-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stuhr-Hansen N, Madl J, Villringer S, Aili U, Römer W, Blixt O. 2016. Synthesis of cholesterol-substituted glycopeptides for tailor-made glycocalyxification of artificial membrane systems. Chembiochem 17, 1403–1406. ( 10.1002/cbic.201600258) [DOI] [PubMed] [Google Scholar]
- 62.Stuhr-Hansen N, Vagianou C-D, Blixt O. 2017. Synthesis of BODIPY-labeled cholesterylated glycopeptides by tandem click chemistry for glycocalyxification of giant unilamellar vesicles (GUVs). Chem. Eur. J. 23, 9472–9476. ( 10.1002/chem.201702104) [DOI] [PubMed] [Google Scholar]
- 63.Vagianou C-D, Stuhr-Hansen N, Moll K, Bovin N, Wahlgren M, Blixt O. 2018. ABO blood group antigen decorated giant unilamellar vesicles exhibit distinct interactions with Plasmodium falciparum infected red blood cells. ACS Chem. Biol. 13, 2421–2426. ( 10.1021/acschembio.8b00635) [DOI] [PubMed] [Google Scholar]
- 64.Villringer S, Madl J, Sych T, Manner C, Imberty A, Römer W. 2018. Lectin-mediated protocell crosslinking to mimic cell-cell junctions and adhesion. Sci. Rep. 8, 1932 ( 10.1038/s41598-018-20230-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribeiro JP, Villringer S, Goyard D, Coche-Guerente L, Höferlin M, Renaudet O, Römer W, Imberty A. 2018. Tailor-made Janus lectin with dual avidity assembles glycoconjugate multilayers and crosslinks protocells. Chem. Sci. 9, 7634–7641. ( 10.1039/C8SC02730G) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubilis A, Abdulkarim A, Eissa AM, Cameron NR. 2016. Giant polymersome protocells dock with virus particle mimics via multivalent glycan-lectin interactions. Sci. Rep. 6, 32 414 ( 10.1038/srep32414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao Q, et al. 2018. Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc. Natl Acad. Sci. USA 115, E2509–E2518. ( 10.1073/pnas.1720055115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nischan N, Kohler JJ. 2016. Advances in cell surface glycoengineering reveal biological function. Glycobiology 26, 789–796. ( 10.1093/glycob/cww045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lowe JB, Marth JD. 2003. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 72, 643–691. ( 10.1146/annurev.biochem.72.121801.161809) [DOI] [PubMed] [Google Scholar]
- 70.Agard NJ, Bertozzi CR. 2009. Chemical approaches to perturb, profile, and perceive glycans. Acc. Chem. Res. 42, 788–797. ( 10.1021/ar800267j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rogers GN, Paulson JC. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127, 361–373. ( 10.1016/0042-6822(83)90150-2) [DOI] [PubMed] [Google Scholar]
- 72.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. 2008. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 14, 181–187. ( 10.1038/nm1703) [DOI] [PubMed] [Google Scholar]
- 73.Marcus DM, Cass LE. 1969. Glycosphingolipids with Lewis blood group activity: uptake by human erythrocytes. Science 164, 553–555. ( 10.1126/science.164.3879.553) [DOI] [PubMed] [Google Scholar]
- 74.Korchagina E, Tuzikov A, Formanovsky A, Popova I, Henry S, Bovin N. 2012. Toward creating cell membrane glyco-landscapes with glycan lipid constructs. Carbohydr. Res. 356, 238–246. ( 10.1016/j.carres.2012.03.044) [DOI] [PubMed] [Google Scholar]
- 75.Frame T, Carroll T, Korchagina E, Bovin N, Henry S. 2007. Synthetic glycolipid modification of red blood cell membranes. Transfusion. 47, 876–882. ( 10.1111/j.1537-2995.2007.01204.x) [DOI] [PubMed] [Google Scholar]
- 76.Harrison AL, Olsson ML, Jones RB, Ramkumar S, Sakac D, Binnington B, Henry S, Lingwood CA, Branch DR. 2010. A synthetic globotriaosylceramide analogue inhibits HIV-1 infection in vitro by two mechanisms. Glycoconj. J. 27, 515–524. ( 10.1007/s10719-010-9297-y) [DOI] [PubMed] [Google Scholar]
- 77.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. 2008. Noncovalent cell surface engineering: incorporation of bioactive synthetic glycopolymers into cellular membranes. J. Am. Chem. Soc. 130, 5947–5953. ( 10.1021/ja710644g) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. 2014. Directing neuronal signaling through cell-surface glycan engineering. J. Am. Chem. Soc. 136, 6794–6797. ( 10.1021/ja5005174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang ML, Purcell SC, Verespy S, Wang Y, Godula K. 2017. Glycocalyx scaffolding with synthetic nanoscale glycomaterials. Biomat. Sci. 5, 1537–1540. ( 10.1039/c7bm00289k) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hudak JE, Canham SM, Bertozzi CR. 2013. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 10, 69–75. ( 10.1038/nchembio.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang ML, Smith RAA, Trieger GW, Godula K. 2014. Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. J. Am. Chem. Soc. 136, 10 565–10 568. ( 10.1021/ja505012a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naticchia MR, Laubach LK, Tota EM, Lucas TM, Huang ML, Godula K. 2018. Embryonic stem cell engineering with a glycomimetic FGF2/BMP4 co-receptor drives mesodermal differentiation in a three-dimensional culture. ACS Chem. Biol. 13, 2880–2887. ( 10.1021/acschembio.8b00436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang ML, Tota EM, Lucas TM, Godula K. 2018. Influencing early stages of neuromuscular junction formation through glycocalyx engineering. ACS Chem. Neurosci. 9, 3086–3093. ( 10.1021/acschemneuro.8b00295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woods EC, Yee NA, Shen J, Bertozzi CR. 2015. Glycocalyx engineering with a recycling glycopolymer that increases cell survival in vivo. Angew. Chem. Int. Ed. 54, 15 782–15 788. ( 10.1002/anie.201508783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kramer JR, Onoa B, Bustamante C, Bertozzi CR. 2015. Chemically tunable mucin chimeras assembled on living cells. Proc. Natl Acad. Sci. USA 112, 12 574–12 579. ( 10.1073/pnas.1516127112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paszek MJ, et al. 2014. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325. ( 10.1038/nature13535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woods EC, Kai F, Barnes JM, Pedram K, Pickup MW, Hollander MJ, Weaver VM, Bertozzi CR. 2017. A bulky glycocalyx fosters metastasis formation by promoting G1 cell cycle progression. eLife 6, e25752 ( 10.7554/eLife.2575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arnaud J, Tröndle K, Claudinon J, Audfray A, Varrot A, Römer W, Imberty A. 2014. Membrane deformation by neolectins with engineered glycolipid binding sites. Angew. Chem. Int. Ed 53, 9267–9270. ( 10.1002/anie.201404568) [DOI] [PubMed] [Google Scholar]
- 89.Shurer CR, Colville MJ, Gupta VK, Head SE, Kai F, Lakins JN, Paszek MJ. 2017. genetically encoded toolbox for glycocalyx engineering: tunable control of cell adhesion, survival, and cancer cell behaviors. ACS Biomat. Sci. Eng. 4, 388–399. ( 10.1021/acsbiomaterials.7b00037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yilmaz G, Becer CR. 2014. Glycopolymer code based on well-defined glycopolymers or glyconanomaterials and their biomolecular recognition. Front. Bioeng. Biotechnol. 2, 1–18. ( 10.3389/fbioe.2014.00039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Q, Collins J, Anastasaki A, Wallis R, Mitchell DA, Becer CR, Haddleton DM. 2013. Sequence-Controlled multi-block glycopolymers to inhibit DC-SIGN-gp120 binding. Angew. Chem. Int. Ed. 52, 4435–4439. ( 10.1002/anie.201300068) [DOI] [PubMed] [Google Scholar]
- 92.Yang Y, Franc V, Heck AJR. 2017. Glycoproteomics: a balance between high-throughput and in-depth analysis. Trends Biotechnol. 35, 598–609. ( 10.1016/j.tibtech.2017.04.010) [DOI] [PubMed] [Google Scholar]
- 93.Palaniappan KK, Bertozzi CR. 2016. Chemical glycoproteomics. Chem. Rev. 116, 14 277–14 306. ( 10.1021/acs.chemrev.6b00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spiciarich DR, Nolley R, Maund SL, Purcell SC, Herschel J, Iavarone AM, Peehl DM, Bertozzi CR. 2017. Bioorthogonal labeling of human prostate cancer tissue slice cultures for glycoproteomics. Angew. Chem. Int. Ed. 56, 8992–8997. ( 10.1002/anie.201701424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Woo CM, Iavarone AT, Spiciarich DR, Palaniappan KK, Bertozzi CR. 2015. Isotope-targeted glycoproteomics (IsoTaG): a mass-independent platform for intact N- and O-glycopeptide discovery and analysis. Nat. Methods. 12, 561–567. ( 10.1038/nmeth.3366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song X, et al. 2010. Shotgun glycomics: a microarray strategy for functional glycomics. Nat. Methods. 8, 85–90. ( 10.1038/nmeth.1540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith DF, Cummings RD. 2013. Application of microarrays for deciphering the structure and function of the human glycome. Mol. Cell. Prot. 12, 902–912. ( 10.1074/mcp.R112.027110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Byrd-Leotis L, et al. 2014. Shotgun glycomics of pig lung identifies natural endogenous receptors for influenza viruses. Proc. Natl Acad. Sci. USA 11, E2241–E2250. ( 10.1073/pnas.1323162111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liau B, Tan B, Teo G, Zhang P, Choo A, Rudd PM. 2017. Shotgun glycomics identifies tumor-associated glycan ligands bound by an ovarian carcinoma-specific monoclonal antibody. Sci. Rep. 7, 14489 ( 10.1038/s41598-017-15123-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bond MR, Zhang H, Vu PD, Kohler JJ. 2009. Photocrosslinking of glycoconjugates using metabolically incorporated diazirine-containing sugars. Nat. Protocols 4, 1044–1063. ( 10.1038/nprot.2009.85) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.