Abstract
Interspecific brood parasitism occurs in several independent lineages of birds and social insects, putatively evolving from intraspecific brood parasitism. The cuckoo catfish, Synodontis multipunctatus, the only known obligatory non-avian brood parasite, exploits mouthbrooding cichlid fishes in Lake Tanganyika, despite the absence of parental care in its evolutionary lineage (family Mochokidae). Cuckoo catfish participate in host spawning events, with their eggs subsequently collected and brooded by parental cichlids, though they can later be selectively rejected by the host. One scenario for the origin of brood parasitism in cuckoo catfish is through predation of cichlid eggs during spawning, eventually resulting in a spatial and temporal match in oviposition by host and parasite. Here we demonstrate experimentally that, uniquely among all known brood parasites, cuckoo catfish have the capacity to re-infect their hosts at a late developmental stage following egg rejection. We show that cuckoo catfish offspring can survive outside the host buccal cavity and re-infect parental hosts at a later incubation phase by exploiting the strong parental instinct of hosts to collect stray offspring. This finding implies an alternative evolutionary origin for cuckoo catfish brood parasitism, with the parental response of host cichlids facilitating its evolution.
This article is part of the theme issue ‘The coevolutionary biology of brood parasitism: from mechanism to pattern’.
Keywords: brood parasite, cichlidae, coevolutionary arms race, host–parasite evolution
1. Introduction
Brood parasitism provides some of the best examples of coevolutionary arms races in nature. Brood parasites avoid costs associated with reproduction by exploiting the parental care of their hosts, whereas hosts are selected to avoid the loss of fitness imposed by brood parasites through evolving defences against exploitation. The study of avian brood parasite systems, in particular, has illustrated a number of mechanisms by which host defences and subsequent parasite counteradaptations can evolve [1,2]. For example, hosts recognize parasitic eggs and chicks on the basis of visual [3,4] and olfactory [5] cues and can reject parasitic eggs [6,7] and chicks [8,9]. In turn, avian brood parasites show the evolution of sophisticated behavioural repertoires [10], morphological adaptations [11], and egg and chick mimesis [12,13] to overcome host defences.
The wealth of information available on avian brood parasitism [1,2,10,14,15] is in sharp contrast with the scarcity of data on the only recognized obligatory non-avian vertebrate brood parasite, the cuckoo catfish Synodontis multipunctatus Boulenger 1898. The cuckoo catfish is endemic to African Lake Tanganyika where it coexists with many species of mouthbrooding cichlid fishes [16]. Mouthbrooding is an advanced mode of parental care in fishes in which the eggs are incubated in the buccal cavity of a parent and where hatched offspring are subsequently protected. The spawning rituals of mouthbrooding cichlids involve elaborate courtship and repeated release of small batches of eggs that are quickly collected in the buccal cavity of one or both parents (typically the female) [17]. In Lake Tanganyika, spawning by cichlids can be interrupted by groups of cuckoo catfish, which join the spawning pair of cichlids and deposit their own eggs [16]. In the subsequent mêlée, the parental cichlid frequently collects the eggs of the cuckoo catfish together with its own (e.g. Movie S2 in [18]). Catfish eggs are non-mimetic and typically smaller and rounder than the eggs of Tanganyikan mouthbrooders [19]. Cichlid and catfish eggs are subsequently incubated together in the buccal cavity of the parental cichlid where they are protected from predators. Cichlid eggs hatch within one week but remain in the buccal cavity for an additional one to two weeks until they deplete their yolk sacs and start exogenous feeding [20]. The hatching of catfish eggs precedes that of the host cichlid. Once young catfish deplete their yolk, at about 6 days post fertilization [18], they start feeding on the host embryos. By preying on the young cichlids, the catfish compromises the reproductive success of the host, often consuming the host clutch entirely. Thus, a final outcome of incubation may be a mixed brood comprising both cuckoo catfish and cichlids, but more typically just catfish [18].
While the contribution of avian systems to our understanding of brood parasitism is substantial, the opportunity to research a system with a different evolutionary origin may provide a broader understanding of how selection shapes host–parasite coevolution [14]. The catfish–cichlid system is also much more amenable to laboratory research, enabling substantial experimental manipulation [18,21,22]. Like many avian brood parasites, cuckoo catfish eliminate host progeny, though in the case of the cuckoo catfish this is achieved through direct predation [22]. Indeed, the cichlid host provides the parasite with both food and protection while incubation itself appears less critical compared with egg incubation in birds. Because of the necessity of simultaneous spawning with the host, cryptic infestation [14] is impossible and adult cuckoo catfish are always exposed to potential aggression from the host. Unlike in birds, however, where obligatory brood parasitism likely evolved from intra-specific brood parasitism [14,23], the cuckoo catfish (and its related species) perform no parental care, implying that the origin of brood parasitism in the cuckoo catfish may differ markedly from that in birds.
In a recent laboratory study, we demonstrated that females of a sympatric host cichlid Simochromis diagramma (Günther 1894) can selectively eliminate cuckoo catfish eggs by ejecting them from their buccal cavity while retaining their own brood, with rejection rates of parasite eggs extremely high (90%) [18]. In contrast to avian egg incubation, in which temperature is a limiting factor for survival of eggs and nestlings outside the nest, the aquatic environments inside and outside the mouth of a host cichlid are similar and mouthbrooding primarily protects offspring from predation [20]. In many mouthbrooding cichlids, parents frequently release their offspring from their buccal cavity to forage and collect them back into their mouth upon sighting a predator [17]. Consequently, we hypothesized that rejected cuckoo catfish eggs may have the capacity to survive and hatch in the external environment and subsequently infect their host when collected as a stray offspring by a brooding parent. We conducted three experiments that tested: (1) the ability of cuckoo catfish to develop outside the host buccal cavity; (2) whether hatched cuckoo catfish offspring actively seek a host after rejection; and (3) the propensity of host females to accept cuckoo catfish from the environment.
2. Material and methods
(a). Experimental fish
Four fish species were used in experiments and were maintained under identical conditions (water temperature 26–28°C, water conductivity 550 µS cm–1, 13 : 11 light : dark photoperiod). Cuckoo catfish eggs and early juveniles originated from 10 pairs of adults imported from Lake Tanganyika in 2012 and 40 pairs of their F1 progeny. We used in vitro fertilization [18] to produce catfish eggs. The fertilized eggs were either directly used in the experiment or incubated in plastic incubators (tumblers representing an artificial buccal cavity and made of 120 × 15 mm tubing with an inflow rate of 0.25 l min–1) to obtain experimental juveniles (see below). Juveniles were fed live Artemia sp. nauplii once each day.
A sympatric natural host of cuckoo catfish [16], the mouthbrooding Lake Tanganyika cichlid Simochromis diagramma, was obtained from a commercial seller. All adult fish (N = 72) were individually marked with Passive Integrated Transponder tags (www.oregonrfid.com), housed in three 350 l mixed sex tanks (4M:20F) and fed with dry and frozen commercial fish food. Aquaria were checked daily for the presence of recently mated (less than 24 h) females, which are readily identified by their extended buccal cavity. Brooding females were gently transferred into a 54 l treatment aquarium equipped with an air-driven sponge filter and a 150 mm ceramic cave as a refuge. There the female either underwent an experimental treatment (see below) or served as a source of experimental embryos for control replicates.
The Lake George mouthbrooding cichlid Haplochromis aeneocolor Greenwood 1973 was used as an experimental allopatric host and was obtained from a commercial seller. The allopatric host was used as a control to isolate evolved host responses resulting from the coevolution between the cuckoo catfish and its sympatric hosts. They were housed in three 350 l aquaria at a sex ratio of 6M : 20F (N = 78) and were otherwise treated in the same way as S. diagramma in terms of individual tagging, feeding, brooding female checks and subsequent experimental procedures.
Allopatric South American Sterba's corydoras Corydoras sterbai Knaack 1962 were obtained from a commercial seller and were used as a taxonomically and geographically unrelated control to the juvenile cuckoo catfish. Parental fish were housed in a 140 l aquarium where they spawned naturally. The eggs were removed from the aquarium and briefly raised on an Artemia nauplii diet until their use in the experiment (see below).
(b). Experiment 1: host and parasite egg survival outside the female buccal cavity
We experimentally tested the survival of cichlid and cuckoo catfish eggs outside their normal incubation environment; i.e. the buccal cavity of a parental cichlid. Based on our previous finding on the ability of the sympatric cichlid host S. diagramma to reject catfish eggs [18], we predicted that selection could favour cuckoo catfish to hatch and commence feeding after rejection by a host. By contrast, we predicted high pre-hatching mortality of the cichlid eggs.
A total of 1448 cuckoo catfish eggs were obtained through in vitro fertilizations (IVF) (see [18] for details on the IVF). Each IVF event involved multiple parental fish (two to five females and three to five males) to produce genetically variable offspring. Fertilized eggs were split into two groups. The treatment group eggs (599 eggs) were transferred to 64 l aquaria equipped with a 400 l h−1 power filter and 0.75 l min−1 aeration. Each egg was placed into a single cell (20 by 20 mm, 20 mm deep) of a 5 × 5 compartmentalized plastic dish on a 5 mm layer of fine sand in each compartment and observed daily. A total of 24 independent replicates (clutches) were completed, using 25 eggs per each replicate (with a single exception of 24 eggs in one replicate). In the first 10 replicates (250 eggs in total), we recorded survival to the age of 72 h (to standardize comparison with host development), time to hatching (duration of pre-hatching development) and survival to hatching (hatching success). The same data were recorded in an additional 14 clutches (349 eggs in total) but with a follow-up observation on the first day of external feeding to measure the proportion of juveniles that started to feed successfully. Control cuckoo catfish eggs originated from the same IVFs as the first 10 replicates, with 27–191 eggs per replicate (849 control eggs in total). Control eggs were placed in artificial incubators that ensured constant movement of the eggs to imitate conditions in the buccal cavity of a host. The eggs that were found not to be developing during the first inspection; i.e. 24 h after fertilization, were regarded as unfertilized. Mean fertilization rates did not differ between incubators and aquaria (p = 0.89) and were 45.6% and 44.0% in the incubators and aquaria, respectively.
Cichlid eggs were incubated using the same protocol as for catfish eggs. A total of 317 S. diagramma eggs (14 replicates) and 595 H. aeneocolor eggs (24 replicates) that originated from natural spawning (see §2a) were tested. Brooding females of each species that had spawned within the previous 6 h were gently stripped of their fertilized eggs [18]. As for the cuckoo catfish eggs, survival until hatching was scored from the eggs that were alive after 24 h, accounting for unfertilized eggs. The rate of fertilization (after 24 h) was 79.4% in S. diagramma and 73.3% in H. aeneocolor.
The survival of cuckoo catfish and host embryos outside the buccal cavity until hatching was expressed as a bivariate vector (ratio of surviving to fertilized eggs for each clutch) and differences between cuckoo catfish and sympatric and allopatric cichlids were tested using a generalized linear model (GLM) with binomial error distribution and log-link function in the glm package in the R statistical environment [24]. Given that hatching in cichlids occurred later than hatching in the cuckoo catfish (3 versus 6 days), as an additional control we tested survival over the first 3 days of incubation to accommodate this disparity in time to hatching. We also compared the hatching success of cuckoo catfish eggs between a sand substrate (treatment) and incubator (control) using a generalized mixed model with binomial error in the lme4 package [25]. This analysis included clutch ID as a random term to account for a paired design in the data, because clutches were split between the two incubation methods. Duration of pre-hatching development was tested on the same dataset, using the same GLMM procedure but with a Poisson error distribution (number of days).
(c). Experiment 2: behaviour of parasite offspring in the presence of a brooding host female
We tested the behavioural response of free-swimming cuckoo catfish offspring to the presence of a brooding cichlid female. We predicted that juvenile catfish would actively seek brooding host females to increase the probability of being collected and brooded, manifested as a positive spatial association between brooding female and the free-swimming parasite juvenile.
A 120 l aquarium (750 × 400 × 400 mm) was divided into three equally sized sections along its longitudinal axis. Both sides of the aquarium were equipped with air-driven filters and separated from the central section with transparent plastic dividers. A female S. diagramma that had recently spawned (less than 24 h) was placed in either the left or right lateral section. The dividers restricted the female from entering the central section but enabled full olfactory contact between the test fish through 30 holes (10 mm in diameter) and by positioning the divider 20 mm above the bottom of the tank. A single cuckoo catfish (4–8 days old, median = 6 days, mean total length, measured through digital imaging (95% confidence limits) = 13.5 (13.0–14.0 mm)) or a control corydoras catfish (10–20 days old, mean body size (95% confidence limits) = 13.6 (13.1–14.1 mm)) was placed in the middle of the test aquarium and covered with a transparent pot and allowed to acclimatize. After 5 min, the pot was gently removed and the catfish released. The arrangement of the tank enabled unrestricted movement of the experimental juveniles while time spent in respective sections of the test aquarium was recorded for a period of 45 min. Three individual juvenile cuckoo catfish and three corydoras catfish were tested with each of 10 host females, providing 30 cuckoo catfish replicates and 30 control corydoras replicates.
To test whether juvenile cuckoo catfish preferred to associate with the host cichlid, we used a generalized linear mixed model (GLMM) with gamma error distribution and identity-link function in the lme4 package. We tested whether juvenile cuckoo catfish associated with the brooding host female more often than control corydoras juveniles, and whether the cuckoo catfish spent more time in the preference compartment than would be expected at random (i.e. 33% of time). The analysis included female ID as a random term to account for repeated use of the same females over six successive replicates.
(d). Experiment 3: parasite re-infection of the host
We tested the potential of juvenile cuckoo catfish to re-infect brooding females of their sympatric and allopatric cichlid hosts. We predicted that small, free-swimming cuckoo catfish that were rejected by the host [18] might be able to return to the buccal cavity of a brooding host cichlid by exploiting their strong parental instinct to recover dropped or stray offspring.
Naturally spawned brooding females of both sympatric S. diagramma and allopatric H. aeneocolor were transferred to treatment aquaria (see §2a). These fish were presented with cuckoo catfish and conspecific offspring for a period of 48 h. After exposure, all offspring were gently washed out of the buccal cavity of the host to determine whether the female had accepted the experimentally exposed offspring or consumed them. In order to disentangle the effect of host brooding stage on host response, experimental exposure took place before hatching (i.e. at the egg incubation phase, with trials starting 0–1 day post fertilization in both cichlid species) or after hatching (embryo incubation phase, S. diagramma: starting 14–15 days post fertilization; H. aeneocolor: starting 8–9 days post fertilization given its more rapid development).
At the host egg incubation phase, a total of 20 S. diagramma and 20 H. aeneocolor brooding females were used. Each female was used only once. We presented 10 females of each host species with five juvenile cuckoo catfish (age 1–6 days post hatching) and an additional 10 females with four to six non-swimming embryos of their own species, obtained from a non-experimental female (age 2–8 and 2–7 days post hatching in S. diagramma and H. aeneocolor, respectively). Experimental aquaria were visually isolated from external cues for a period of 48 h. During trials, juvenile catfish were provided with 2 ml of live Artemia nauplii suspension once each day.
At the embryo incubation phase, the same protocol was used but high acceptance rates of conspecific and parasitic offspring (see §3) prompted inclusion of an additional, geographically and taxonomically unrelated control group. Thus, an additional 10 S. diagramma and 10 H. aeneocolor females were presented with five juvenile corydoras (aged 10–20 days post hatching to match the cuckoo catfish body size) following an identical protocol to that for cuckoo catfish.
Each host female was only used once at a particular incubation phase, though there was partial overlap (65%) of females used between incubation phases. To distinguish conspecific experimental offspring from the test female's own offspring at the embryo incubation phase, experimental offspring were lightly stained using a 1 h bath in Alizarin Red solution, freshly prepared before each replicate by diluting 150 mg of Alizarin Red dye in a 1 l of tank water at 26°C.
After 48 h, each experimental host cichlid female was gently netted out of the aquaria and the entire contents of her buccal cavity washed out [18]. The number of juveniles and embryos inside the buccal cavity was recorded. The offspring that remained in the aquarium (i.e. those not accepted by the experimental female) were also netted and counted. For conspecific treatments, all embryos were inspected using a binocular microscope under fluorescent light (wavelength 532 nm). Alizarin-stained individuals were identified from their fluorescently red skeletal structures [26].
To compare re-infection rates between sympatric and allopatric host species and among young stages of cuckoo catfish, conspecific control and catfish control (corydoras), we used a GLM with binomial error distribution and log-link function. Given the repeated use of a subset of females for the egg and embryo incubation phases (but while incubating different clutches), we analysed the two datasets separately. Re-infection rates were calculated as a bivariate vector (ratio of accepted offspring to offered offspring); the number of offered offspring was typically 5 but varied between 4 (5 replicates) and 6 (1 replicate). A quasi-binomial error structure was used for data from the egg incubation phase given a high incidence of zero acceptance rates. Saturated models included host species (sympatric, allopatric) and offspring species (cuckoo catfish, conspecific and corydoras in the embryo incubation phase dataset) and their interactions. Interactions between host and offspring were always non-significant and were removed from the final models. For each treatment group, the proportion of embryos accepted by a female and the proportion of host females that collected at least a single embryo (acceptor hosts) were calculated.
3. Results
(a). Experiment 1: host and parasite survival outside the female buccal cavity
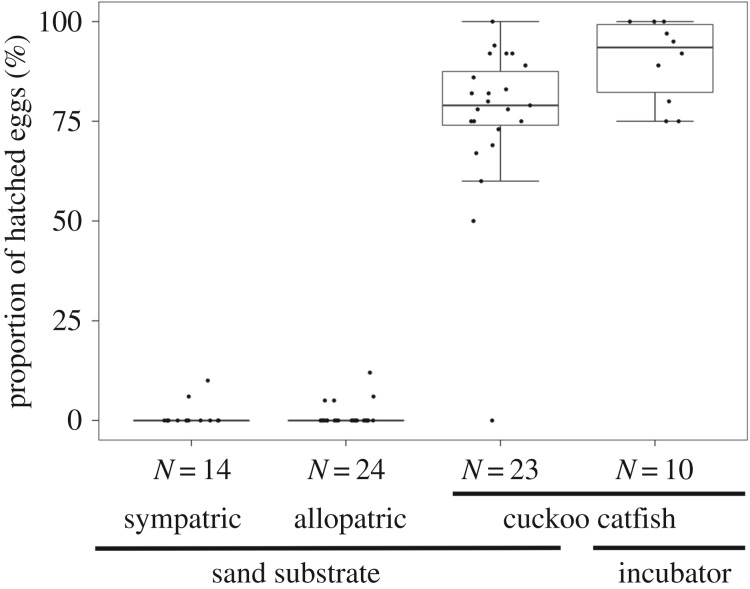
The success of incubation on a sand substrate was good for cuckoo catfish but poor in cichlids (figure 1). Hatching success in cuckoo catfish was 78%, but only 1.5% in both sympatric and allopatric hosts (GLM with binomial distribution: χ2 = 33.1, d.f. = 2, p < 0.001, N = 61 clutches). This difference remained after controlling for an unequal embryo developmental time in cuckoo catfish and cichlids; on day 3 post fertilization on a sand substrate catfish egg survival was 78.5% but only 15% for allopatric and 3.5% for sympatric host eggs (χ2 = 87.7, d.f. = 2, p < 0.001). All hatched cuckoo catfish started to feed exogenously (at day 7 post fertilization, N = 152 fish from 14 clutches), as did all cichlids (the day of first feeding not recorded).
Figure 1.
Hatching success of parasite and host eggs when incubated outside host buccal cavity. Proportion of cuckoo catfish and cichlid eggs that successfully hatched on a sand substrate and in an incubator. Median, interquartile range and non-outlier range are shown, along with replicate-specific values for clutches of 25 eggs (black circles). The number of replicates (clutches) is shown for each treatment.
There was no difference in cuckoo catfish egg survival to hatching on sand in comparison with eggs raised in an artificial incubator (GLMM with binomial error: z = 0.60, p = 0.269, n = 10 paired samples) and no difference in the time to hatching (GLMM with a Poisson error: z = 0.14, p = 0.89, n = 10). Catfish eggs typically hatched in 3–4 days (day 3: 16 clutches, day 4: 16 clutches), with a single clutch hatching on day 2. All eggs from the same clutch always hatched synchronously on the same day.
(b). Experiment 2: parasite juvenile behaviour in the presence of a brooding host female
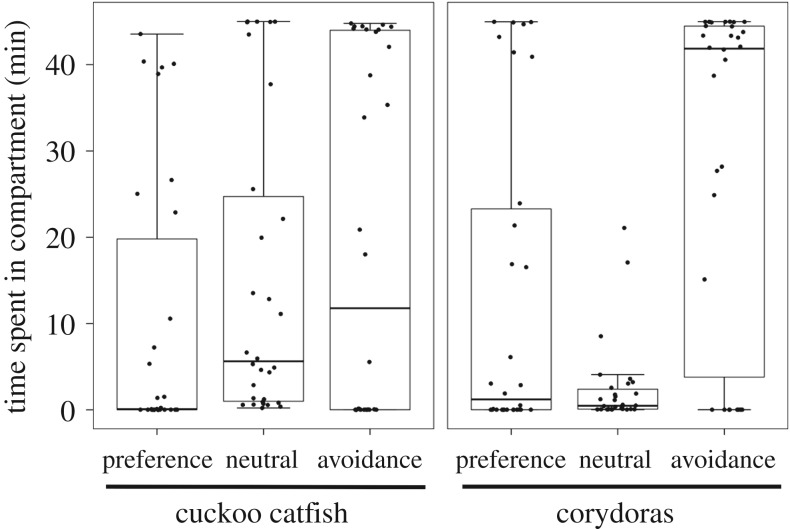
Juvenile cuckoo catfish were not attracted by brooding sympatric host females. There was no difference in association with brooding host females between the cuckoo catfish and corydoras juveniles (GLMM with gamma distribution, z = 0.60, p = 0.547, N = 30 juveniles per treatment). Time spent by cuckoo catfish juveniles in each compartment was similar, while corydoras showed a tendency to avoid the central compartment (figure 2).
Figure 2.
Catfish behaviour towards brooding host female. Median time spent in each experimental compartment (preference, neutral and avoidance zones), with interquartile range (box) and non-outlier range (whiskers) for parasitic cuckoo catfish and corydoras catfish (control). Individual values are shown as black circles.
(c). Experiment 3: parasite re-infection of the host
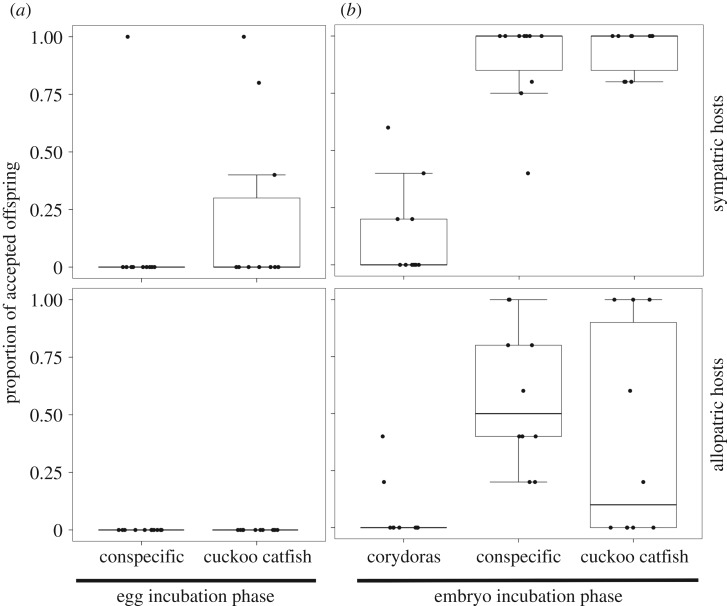
Both cichlid species accepted hatched heterospecific and conspecific offspring, although sympatric females did so at a higher rate. Host females showed a greater propensity to collect offspring at the embryo brooding stage than at the earlier egg brooding stage (figure 3).
Figure 3.
Acceptance of embryos by female cichlids at the egg (a) and embryo (b) incubation phases. Median, interquartile range and non-outlier range are shown for each tested combination of cichlid and parasite species, with replicate-specific values for each trial (consisting of four to six embryos; black circles).
While incubating their own unhatched eggs, acceptance rates of heterospecific and conspecific offspring were low, with no difference between sympatric and allopatric cichlids (GLM with quasi-binomial error, z = 0.01, p = 0.994), nor between unrelated conspecific and parasite offspring (z = 1.30, p = 0.194). Sympatric females accepted 22% of cuckoo catfish juveniles (3 out of 10 females accepted at least a single juvenile; i.e. were acceptors) and 10% of conspecific embryos (a single acceptor out of 10). No allopatric females accepted any conspecific or parasitic offspring at the egg incubation stage (figure 3a).
When incubating their own hatched embryos, the acceptance rate was significantly higher in sympatric females (GLM with binomial error, z = 3.14, p = 0.002) and differed among offspring species (z = 5.49, p < 0.001). Acceptance was low in corydoras catfish (sympatric hosts: 14% offspring collected, 40% acceptor females; allopatric hosts: 6% offspring, 20% acceptors), but high in parasitic cuckoo catfish (sympatric hosts: 94% offspring collected, 100% acceptor females; allopatric hosts: 38% offspring, 50% acceptors) and conspecific embryos (sympatric hosts: 84% offspring collected, 100% acceptor females; allopatric hosts: 58% offspring, 100% acceptors; figure 3b).
4. Discussion
We showed that the eggs and embryos of parasitic cuckoo catfish are capable of surviving at high rates outside the buccal cavities of their hosts, at least in a laboratory setting. Further, a strong parental response by both allopatric, but especially sympatric, hosts (figure 3), provided actively swimming cuckoo catfish offspring (1–6 days post-hatching) with an opportunity to parasitize hosts long after oviposition. Parental females of both tested cichlid species readily collected cuckoo catfish offspring, as well as control conspecific embryos and, to a lesser extent, offspring of geographically distinct corydoras catfish when incubating their own hatched embryos. Acceptance rate was much lower during the egg incubation phase. In contrast to our predictions, we detected no directional behavioural response by cuckoo catfish offspring to brooding host females, suggesting that they do not actively seek potential hosts.
The implications of our study are that, uniquely among all known brood parasites, cuckoo catfish have the capacity to infect hosts at two qualitatively different ontogenetic stages; as an egg and later as an actively swimming juvenile. Hence, even after rejection at the egg stage, juvenile cuckoo catfish could complete development to the free-swimming stage and return to the buccal cavity of a host, at least under the conditions imposed in this study. Indeed, the ability to reject parasitic eggs, but not to discriminate against juvenile parasites (but see [27,28]) resembles the situation seen in many avian brood parasite systems. This situation can be explained under a number of alternative hypotheses (reviewed by Grim [29]), but probably arises through low selection pressure imposed by a low frequency of occurrence of parasite offspring following frequent egg rejection [29,30].
The ability of juvenile cuckoo catfish to re-infect hosts appears to derive primarily from a parental response of the hosts to collect stray offspring, rather than from juvenile cuckoo catfish actively seeking to re-infect the host. The presence of non-swimming embryos and, notably, unrelated non-parasitic corydoras in the mouth of brooding females strongly suggests that re-infection is accomplished by the host actively collecting free-swimming juveniles. Our data also show that the motivation to collect the fish is higher when the offspring in the buccal cavity have already hatched. This finding suggests that mouthbrooding females can reference the developmental status of their broods and modify their behaviour in response. The cost of parasitism at this later stage can be either lower or higher than when the brood is infected at the time of spawning and depends on the timing of acceptance and the number and size of the accepted parasitic offspring. In many cases, parental host cichlids may be unable to distinguish their own offspring from unrelated or even heterospecific young. Mobile young stages often stray from their parents, or are displaced when predators attack a parent or brood. The inadvertent adoption of such young by unrelated parents is probably not uncommon in teleost fishes [20,21]. The costs of policing care by parents, including expelling unrelated offspring, is potentially expensive if the error rate in discriminating genetically related and unrelated young is significant. In addition, if the fitness cost to a parent of caring for small numbers of genetically unrelated offspring is trivial, the strength of selection to evolve mechanisms to discriminate and expel unrelated young may be limited. Parasite infection facilitated by the host itself is also known in the butterfly Phengaris arion whose larvae parasitize ant colonies. However, P. arion larvae manipulate the ants into carrying them to their nest using chemical and acoustic signals [31], whereas the propensity to accept offspring of other cichlid species is a general feature of many mouthbrooders [21,32,33]. Our tests demonstrated sympatric S. diagramma to be a relatively stronger acceptor than the allopatric H. aeneocolor but this finding has limited general application as the comparison only included two species. Whether cuckoo catfish similarly use behavioural, visual, olfactory or auditory signals to manipulate hosts into retrieving them is an intriguing possibility that remains to be tested.
The active compliance by hosts in their own infection by cuckoo catfish as a by-product of parental care also offers a hypothetical trajectory for the origin of this brood–parasitic relationship. While the evolution of obligatory brood parasites in birds is believed to originate from intra-specific brood parasitism (e.g. [23,34]), this scenario is not plausible in Synodontis catfishes because they belong to a lineage that lacks parental care. One scenario for the evolution of brood parasitism in cuckoo catfish could be through predation of cichlid eggs during spawning, which might eventually result in a spatial and temporal match of spawning by both the parasite and its host. The results of the present study, however, suggest an alternative evolutionary pathway, with the relationship potentially evolving through accidental incubation of ancestral cuckoo catfish juveniles by brooding cichlids, with the fitness benefits of mouthbrooding reinforcing a spatial and temporal association of the catfish with cichlid hosts. This hypothesized evolutionary pathway is analogous to the widely accepted theory for the evolution of trophically transmitted parasites from free-living species (e.g. [35]).
Cuckoo catfish eggs and juveniles showed high survival rates outside the buccal cavity of the host, potentially weakening reliance by the parasite on the host, especially in comparison with the negligible survival of cichlid embryos. Simultaneous spawning by cuckoo catfish and cichlids involves aggressive behaviour by the spawning cichlid pair, with the catfish often forced away from the spawning site [21]. Even in the confines of an aquarium setting, catfish and cichlid eggs can be swept away from the spawning arena during aggressive disputes (M. Polačik, R. Blažek 2018, personal observation). Under these conditions, some uncollected cuckoo catfish eggs, as well as the eggs rejected by a host female, may be able to survive, hatch and develop along an alternative, non-parasitic developmental pathway. Cuckoo catfish are considered an obligate brood parasite in the scientific literature (e.g. [16,18,21,22]), though evidence from the wild is indeterminate being based solely on the failure, thus far, to detect juvenile cuckoo catfish outside the care of their hosts [16]. Our own observations from captivity (M. Polačik, R. Blažek 2018, personal observation) and anecdotal information from fish hobbyists suggest that cuckoo catfish can occasionally reproduce without parasitizing cichlids, though whether outcomes in the benign environment of the aquarium necessarily translate to nature is clearly a question that needs to be addressed. Obligate brood parasitism is believed to typically evolve along a trajectory starting with facultative parasitism (e.g. [23]), and it is conceivable that cuckoo catfish have yet to complete the transition to the full, obligate brood parasitism. There is also a possibility that different populations of the cuckoo catfish, which is widespread across Lake Tanganyika [36], may express different levels of reliance on their hosts.
In conclusion, the relationship between the cuckoo catfish and mouthbrooding cichlids represents a unique example of a versatile vertebrate brood–parasitic system that is unusually amenable to experimental manipulation. We present data suggesting that cuckoo catfish offspring can complete development without exploiting a host, at least in a laboratory setting when predation is excluded. In addition, a strong parental response by mouthbrooding cichlids to collect stray offspring may facilitate re-infection of hosts by cuckoo catfish juveniles after rejection at the egg stage, and may even represent an evolutionary pathway for brood parasitism by cuckoo catfish with the strong parental instinct of host cichlids facilitating the origin of brood parasitism.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Radomil Řežucha for the help with Experiment 2 and Jiří Farkač, Adam Veleba and Jakub Žák for technical assistance with fish care.
Ethics
Research adhered to all national and institutional animal care and use guidelines (permit No. CZ62760203)
Data accessibility
Primary data available as part of the electronic supplementary material.
Authors' contributions
The study was conceived by M.P. and R.B. and designed by M.P., R.B. and M.R. Data collection: M.P. and R.B. Data analysis: M.R. Data interpretation: M.P., C.S., R.B. and M.R. Drafting ms: M.P. with important contributions made by all authors.
Competing interests
We declare we have no competing interests.
Funding
Financial support came from Czech Science Foundation (18-00682S) to M.R.
References
- 1.Soler M. 2014. Long-term coevolution between avian brood parasites and their hosts. Biol. Rev. 89, 688–704. ( 10.1111/brv.12075) [DOI] [PubMed] [Google Scholar]
- 2.Soler M. 2018. Avian brood parasitism: behaviour, ecology, evolution and coevolution. Berlin, Germany: Springer. [Google Scholar]
- 3.Yang C, Møller AP, Røskaft E, Moksnes A, Liang W, Stokke BG. 2014. Reject the odd egg: egg recognition mechanisms in parrotbills. Behav. Ecol. 25, 1320–1324. ( 10.1093/beheco/aru124) [DOI] [Google Scholar]
- 4.Noh HJ, Gloag R, Langmore NE. 2018. True recognition of nestlings by hosts selects for mimetic cuckoo chicks. Proc. R. Soc. B 285, 20180726 ( 10.1098/rspb.2018.0726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soler JJ, Pérez-Contreras T, De Neve L, Macías-Sánchez E, Møller AP, Soler M. 2014. Recognizing odd smells and ejection of brood parasitic eggs. An experimental test in magpies of a novel defensive trait against brood parasitism. J. Evol. Biol. 27, 1265–1270. ( 10.1111/jeb.12377) [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Liang W, Yang C, Cheng SJ, Hsu YC, Lu X. 2016. Egg rejection and clutch phenotype variation in the plain prinia Prinia inornata. J. Avian Biol. 47, 788–794. ( 10.5061/dryad.j4d25) [DOI] [Google Scholar]
- 7.Soler M, Ruiz-Raya F, Roncalli G, Ibáñez-Álamo JD. 2017. Relationships between egg-recognition and egg-ejection in a grasp-ejector species. PLoS ONE 12, e0166283 ( 10.1371/journal.pone.0166283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langmore NE, Hunt S, Kilner RM. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157 ( 10.1038/nature01460). [DOI] [PubMed] [Google Scholar]
- 9.Sato NJ, Tokue K, Noske RA, Mikami OK, Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69. ( 10.1098/rsbl.2009.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies NB. 2011. Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. ( 10.1111/j.1469-7998.2011.00810.x) [DOI] [Google Scholar]
- 11.Honza M, Picman J, Grim T, Novák V, Čapek M Jr, Mrlík V. 2001. How to hatch from an egg of great structural strength. A study of the common cuckoo. J. Avian Biol. 32, 249–255. ( 10.1007/s10336-015-1163-z) [DOI] [Google Scholar]
- 12.Brooke MDL, Davies NB. 1988. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630 ( 10.1038/335630a0) [DOI] [Google Scholar]
- 13.Langmore NE, Stevens M, Maurer G, Heinsohn R, Hall ML, Peters A, Kilner RM. 2011. Visual mimicry of host nestlings by cuckoos. Proc. R. Soc. B 278, 2455–2463. ( 10.1098/rspb.2010.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spottiswoode CN, Kilner RM, Davies NB. 2012. Brood parasitism. In The evolution of parental care, pp. 226–356. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Medina I, Langmore NE. 2016. The evolution of acceptance and tolerance in hosts of avian brood parasites. Biol. Rev. 91, 569–577. ( 10.1111/brv.12181) [DOI] [PubMed] [Google Scholar]
- 16.Sato T. 1986. A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323, 58 ( 10.1038/335630a0) [DOI] [PubMed] [Google Scholar]
- 17.Keenleyside MH. 1991. Cichlid fishes: behaviour, ecology and evolution (Vol. 2). Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 18.Blažek R, Polačik M, Smith C, Honza M, Meyer A, Reichard M. 2018. Success of cuckoo catfish brood parasitism reflects coevolutionary history and individual experience of their cichlid hosts. Sci. Adv. 4, eaar4380 ( 10.1126/sciadv.aar4380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MS, Hawkins MB, Stock DW, Cruz A. 2019. Early life-history features associated with brood parasitism in the cuckoo catfish, Synodontis multipunctatus (Siluriformes: Mochokidae). Phil. Trans. R. Soc. B 374, 20180205 ( 10.1098/rstb.2018.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wootton RJ, Smith C. 2015. Reproductive biology of teleost fishes. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 21.Wisenden BD. 1999. Alloparental care in fishes. Rev. Fish Biol. Fish. 9, 45–70. [Google Scholar]
- 22.Cohen MS, Hawkins MB, Knox-Hayes J, Vinton AC, Cruz A. 2018. A laboratory study of host use by the cuckoo catfish Synodontis multipunctatus. Environ. Biol. Fish. 101, 1417 ( 10.1007/s10641-018-0788-1) [DOI] [Google Scholar]
- 23.Hamilton WJ, Orians GH. 1965. Evolution of brood parasitism in altricial birds. Condor 67, 361–382. ( 10.2307/1365631) [DOI] [Google Scholar]
- 24.R Core team. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/). [Google Scholar]
- 25.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 26.Bensimon-Brito A, Cardeira J, Dionísio G, Huysseune A, Cancela ML, Witten PE. 2016. Revisiting in vivo staining with alizarin red S—a valuable approach to analyse zebrafish skeletal mineralization during development and regeneration. BMC Dev. Biol. 16, 2 ( 10.1186/s12861-016-0102-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Wang L, Chen M, Liang W, Møller AP. 2015. Nestling recognition in red-rumped and barn swallows. Behav. Ecol. Sociobiol. 69, 1821–1826. ( 10.1007/s00265-015-1994-x) [DOI] [Google Scholar]
- 28.Huo J, Yang C, Su T, Liang W, Møller AP. 2018. Russet sparrows spot alien chicks from their nests. Av. Res. 9, 12 ( 10.1186/s40657-018-0104-y) [DOI] [Google Scholar]
- 29.Grim T. 2006. The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol. Ecol. Res. 8, 785–802. [Google Scholar]
- 30.Grim T. 2011. Ejecting chick cheats: a changing paradigm? Front. Zool. 8, 14 ( 10.1186/1742-9994-8-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes MP. 2015. The biology and ecology of the large blue butterfly Phengaris (Maculinea) arion: a review. J. Insect Conserv. 19, 1037–1051. ( 10.1007/s10841-015-9820-3) [DOI] [Google Scholar]
- 32.Ribbink AJ, Marsh AC, Marsh B, Sharp BJ. 1980. Parental behaviour and mixed broods among cichlid fish of Lake Malawi. Afr. Zool. 15, 1–6. ( 10.1080/02541858.1980.11447677) [DOI] [Google Scholar]
- 33.Ochi H, Yanagisawa Y. 1996. Interspecific brood-mixing in Tanganyikan cichlids. Environ. Biol. Fish. 45, 141–149. ( 10.1007/BF00005227) [DOI] [Google Scholar]
- 34.Shaw RC, Hauber ME. 2009. Experimental support for the role of nest predation in the evolution of brood parasitism. J. Evol. Biol. 22, 1354–1358. ( 10.1111/j.1420-9101.2009.01745.x) [DOI] [PubMed] [Google Scholar]
- 35.Poulin R, Randhawa HS. 2015. Evolution of parasitism along convergent lines: from ecology to genomics. Parasitology 142, S6–S15. ( 10.1017/S0031182013001674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koblmüller S, Sturmbauer C, Verheyen E, Meyer A, Salzburger W. 2006. Mitochondrial phylogeny and phylogeography of East African squeaker catfishes (Siluriformes: Synodontis). BMC Evol. Biol. 6, 49 ( 10.1186/1471-2148-6-49) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data available as part of the electronic supplementary material.