Abstract
Insect societies play a crucial role in the functioning of most ecosystems and have fascinated both scientists and the lay public for centuries. Despite the long history of study, we are still far from understanding how insect societies have evolved and how social cohesion in their colonies is maintained. Here we suggest inquiline social parasites of insect societies as an under-exploited experimental tool for understanding sociality. We draw on examples from obligate inquiline (permanent) social parasites in wasps, ants and bees to illustrate how these parasites may allow us to better understand societies and learn more about the evolution and functioning of insect societies. We highlight three main features of these social parasite–host systems—namely, close phylogenetic relationships, strong selective pressures arising from coevolution and multiple independent origins—that make inquiline social parasites particularly suited for this aim; we propose a conceptual comparative framework that considers trait losses, gains and modifications in social parasite–host systems. We give examples of how this framework can reveal the more elusive secrets of sociality by focusing on two cornerstones of sociality: communication and reproductive division of labour. Together with social parasites in other taxonomic groups, such as cuckoos in birds, social parasitism has a great potential to reveal the mechanisms and evolution of complex social groups.
This article is part of the theme issue ‘The coevolutionary biology of brood parasitism: from mechanism to pattern’.
Keywords: social parasitism, social insects, coevolutionary arms race, sensory deception, communication, reproductive division of labour
1. The secret lives of insect societies
Insect societies represent one of the most intriguing phenomena in the natural world: they exhibit some of the most extreme examples of self-sacrifice (through altruism), conflict and cooperation [1,2]; they are among the most successful animals on the planet, dominating ecosystems globally [1,3]. It is therefore no surprise that social insects have been long-standing (and ever-expanding) models for scientists to better understand social behaviour, evolution, functioning and regulation [4]. However, we still lack a comprehensive understanding of the fundamental basis of the key facets of sociality in insects; insect societies continue to surprise us, forcing us to re-evaluate long-held beliefs. Recent work reveals that despite their ‘insect brains’ [5], these insects have complex cognitive abilities, allowing sophisticated social learning, cultural transmission and tool use [6,7]. Moreover, far from being programmed automatons [8], colony members are now known to differ widely in individual-level personalities and behavioural syndromes, which carry significant fitness impacts at both the individual and colony-level [9,10]. These examples suggest that we need some other way to unlock the secrets of insect societies. Here we discuss how social parasites could be tools to improve our understanding of sociality.
Social parasites infiltrate the societies of conspecifics or other species and exploit the socially acquired resources of those societies for their own fitness gain. They are taxonomically diverse: hundreds of species within Coleoptera, Hymenoptera and Lepidoptera show the ability to exploit social insect colonies' brood care [1,11]. In the Hymenoptera, the evolution of social parasitism has taken many guises across many lineages [12–14]; for example, the host–parasite association can be temporary or permanent, it can be facultative or obligate; there can even be variation in the life stage (larva or adult) of the parasite that exploits the host, or the caste (workers are the exploiters in slave-making ants) [11–13].
Across these guises, natural selection has equipped social parasites in diverse ways with sophisticated toolkits that enable them to invade and exploit host societies; for example, parasites need to decode their host's communication system and break the rules governing the functioning of the host society. To produce this toolkit, evolution has tinkered with the ancestral traits of the parasites' free-living (social) ancestors: a social parasite may lose, gain, retain or modify traits to enable it to better exploit the host society. Trait gains are likely to represent traits used by the parasite to manipulate the host, while losses are likely those traits that were essential to a free-living social host (such as brood care) but no longer required (and costly to otherwise maintain) by the parasite; modified traits are examples of how evolution can co-opt existing mechanisms and use them to achieve a different function, and/or exaggerate or reduce them adaptively. Here we identify some key traits and explain how such trait evolution makes social parasites excellent evolutionary experiments in nature that can be used to tease apart the ultimate and proximate factors regulating insect social life.
2. Inquiline social parasites as tools to understand insect societies
Among the various kinds of social parasites, the inquiline social parasites of the Hymenoptera (bees, wasps and ants) are particularly suited for understanding sociality. Inquilines are specialized and committed to a parasitic life and are dependent on being fully integrated into their host's colony throughout their lives [13]. There are three traits of inquilines that make them especially useful as tools for this.
The first trait is the typically close phylogenetic relationship between the social parasite and the social host species [14–16]. In most cases, obligate inquiline social parasites belong to clades that are closely related to the host clade as sister species (strict Emery's rule), or are from the same genus (relaxed Emery's rule) [14,15]. This contrasts with other kinds of social parasites such as slave-making ants, where hosts and parasites usually belong to different ant genera [15], and non-hymenopteran brood parasites such as Maculinae butterflies that exploit ant societies, where the parasite belongs to a different order (Lepidoptera). The close phylogenetic origin of many obligate inquiline social parasite–host pairs means they share many life-history traits; this shared ancestry also means the parasites are well equipped to exploit the host system efficiently, by co-opting and adapting ancestral phenotypic traits. Inquilines that are sister species to the host (i.e. strict Emery's rule) are especially valuable as they offer the opportunity to interrogate the mechanistic basis, such as molecular regulatory processes, of social traits that are subtly (but measurably) different between host and parasite.
A second trait of obligate inquilines that makes them useful tools to understand host societies is that there has been strong natural selection exerted on both the parasite and the host through coevolution, an evolutionary phenomenon that is typical of host–parasite systems [17,18]. Selection on the parasite is strong because obligate social parasites rely completely on the host colony for their fitness [12,13]: specifically, the parasite must first infiltrate a well-established host colony, deceiving and/or overcoming the host workers and queen in order to be accepted; the parasite must then become integrated (remain undetected) into the society and functionally replace the former host breeder [19]. Failure at any of these stages exerts strong selection on the parasite. Equally, parasitism usually imposes high costs on the host, because of high local prevalence (with many host colonies thus subjected to parasitism [12]) and/or severe impact of parasitism on a colony; infection by an inquiline social parasite usually leads to a drastic reduction or even to a complete loss of the host colony reproductive output [12,18]. The resulting coevolutionary arms race (19) generates a plethora of morphological, physiological and/or behavioural adaptations in both the parasite and the host [1,12,18,20] that constitutes a rich comparative toolkit for biologists to exploit in order to understand the traits and mechanisms of social living. Adaptations in the social parasite can expose how the host system works, such as chemical deception strategies, which may reveal how the communication system of the social host works; for example, the chemical signature acquired by a social parasite during usurpation reveals the key components of the nest-mate recognition mechanism used by the host [21]. Counter-adaptations that evolve in the host to defend against parasitism are informative as well: social parasites can use exploitation mechanisms that are not necessarily part of the normal host social life or exaggerate host mechanisms, and the counter defences used by the host can expose social traits that could be less evident if focusing solely on the host system. For example, hosts from parasitized populations can evolve a bigger body size to defend against the social parasite, suggesting that body size is an adaptive defence trait in the host species [22]. Similarly, hosts can evolve more complex chemical signatures to prevent parasite chemical integration [23]. The nature of these changes can highlight the most relevant features for the nest-mate recognition process in the host species [23,24]. The strength of the coevolutionary arms race exposes these traits. This is especially so in obligate social parasite–host systems, as opposed to facultative social parasites where there will be selection on the parasite to retain traits of their free-living/social ancestor, such that they can exploit the alternative reproductive options as either free-living (social) or parasitic species. The intimate relationship also implies that inquiline social parasites and their hosts share the same environment for most of their life, which permits the comparison of social traits and their underlying mechanisms, while controlling for the influence of environment [25]. This is more difficult in other social parasite–host systems, such as Maculinea butterflies and slave-making ants, where the overlap between the environment and ecology of hosts and parasites is limited.
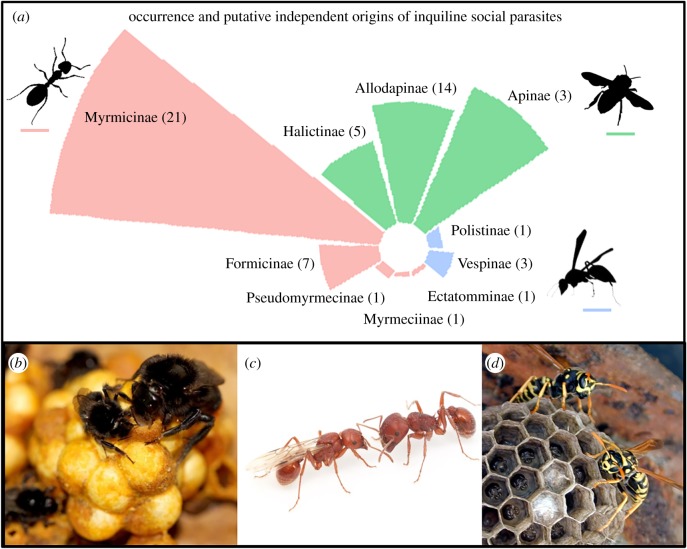
Finally, inquiline social parasitism has evolved at least 50 times across Hymenoptera social lineages (figure 1a). Indeed, its prevalence and diversity are probably underestimated, as inquilines usually have small and fragmented populations, are often phenotypically cryptic and/or are rarely noted/collected as they are typically represented by a single female in a colony, as most (to our knowledge) have lost the worker caste. The 50-plus independent origins of obligate inquilinism provide replicate natural experiments across the ant, wasp and bee clades (figure 1a). Their scattered phylogenetic distribution (figure 1, electronic supplementary material and references therein) means inquilines afford robust comparative analyses within and between host–parasite clades; this is not the case for slave-makers, for example, which are absent in wasp and bee clades.
Figure 1.
Occurrence and putative independent origins of inquiline social parasites. (a) Area of each slice is proportional to the number of species of known inquiline social parasites within the subfamily. The number in parentheses reports the minimum number of independent origins of inquilinism, as evaluated from the literature ([12,13,15,16,26–29], see electronic supplementary material, table S1). Where data were not available, the number of genera showing at least one case of the inquiline social parasites is reported, under the assumption that origins of inquiline social parasitism occurred independently in different genera. Thus, the figure provides a conservative estimate of the independent origins of social parasitism. (b–d) Exemplary representatives of obligate inquiline social parasites in: (b) bumblebees (Bombus rupestris, right, and its host Bombus lapidarius, photo by Luca Franzini); (c) Pogonomyrmex anergismus, left, and its host Pogonomyrmex barbatus (photo by Elizabeth Cash); (d) Polistes sulcifer, left, and its host Polistes dominula (photo by Rita Cervo). (Online version in colour.)
The ultimate power of inquilines, therefore, comes in their replicated evolution across a phylogeny of social hosts with contrasting life histories and evolutionary origins. The identification of common evolutionary patterns in social parasites, across the phylogeny, has the potential to reveal general secrets to social living, irrespective of specific life history (e.g. carnivorous wasps and ants, versus herbivorous bees). These three main features make obligate inquiline social parasites a fruitful group of model systems through which we can better understand some of the cornerstones of insect societies.
3. A predictive comparative framework for gaining insights into sociality
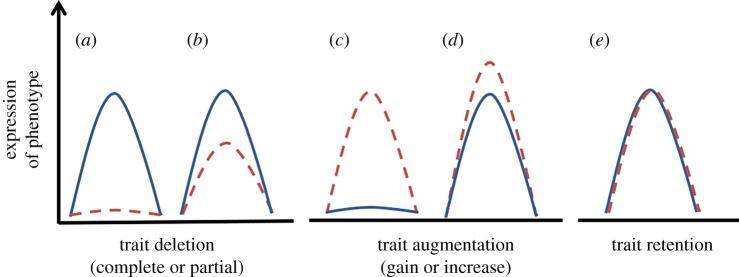
Inquiline social parasites evolve from social host lineages, through losses (and/or reduction) of many social traits and gains (and/or augmentation) of specialist parasite traits [25]. Comparisons of some of these traits in social parasites and their closely related social hosts may help us better understand what is essential to sociality [30]. Previously, we had suggested a conceptual model for the phenotypic evolution of social parasitism from a social ancestor [25] which provides a useful framework for testing empirical hypotheses on the evolution of social parasitism [31–33]. We suggested that the specialized phenotypes of social parasites could arise through the gain of new traits (putatively traits required for a parasitic but not a free-living life—‘phenotype shift’ model) and/or loss of ancestral traits (putatively traits required by a free-living life history—‘phenotype deletion’ model). The rationale for this was that social parasites have, broadly, lost (or reduced) traits important for social function (the ‘deleted’ component of the ancestral phenotype) and gained (or augmented) specific parasitic traits by modifying ancestral traits or evolving new ones (phenotypic ‘shift’ from the ancestral state). Empirical tests of these models by comparing transcriptomes and genomes of social parasites and their hosts (as a molecular representation of the physical phenotype) [31–33] recently confirmed that social parasites evolve via a combination of the two processes [25]. The focus of these models (and tests thereof), however, was to understand how social parasitism itself evolves and is maintained. Here we adapt this framework to exploit comparative host–parasite systems in order to address the counterpart question: how does sociality evolve and how is it maintained? Comparisons of trait phenotype and the underlying mechanisms in social parasites and their hosts can help us interrogate the society and find out what is important in sociality. Many traits lost (unexpressed) or reduced in social parasites but retained (expressed) in their social hosts are candidates of importance for a free-living social insect; although traits may be lost for many reasons (unconnected with sociality), the phylogenetic spread, repeated evolutionary events and diversity of host–parasite systems in the Hymenoptera (see §2) provide a powerful comparative approach. The identification of the same candidate (lost) traits across different host–parasite comparative pairs and across the phylogeny of the Hymenoptera would provide compelling evidence for social traits of importance (figure 2). These traits may be behavioural, morphological and/or physiological (examples discussed below). Recent advances in molecular methodologies make the quantification of trait losses/reductions, gains/augmentations and other modifications in social parasites relative to their hosts more tractable than before as they allow the phenotype to be studied (and quantified) at a basic level (the genome), allowing comparisons of the building blocks (and their regulators) underpinning the different phenotypic traits; this includes the expression of genes, their translation into proteins, the epigenetic marks that regulate trait expression in a direct comparison between social parasites and their hosts [34,35]. With a focus on the use of social parasites as tools to understand sociality, we now unpack and expand the concepts of phenotypic ‘deletions' and ‘shifts’ [25] to include some of the specific modes by which a trait might change (or not) in the evolution of social parasites from the social ancestors, using their extant social host as a putative representation of that ancestral social state (figure 2 and table 1). Comparative analyses of many closely related parasite–host partners, across the phylogeny of the Hymenoptera, is potentially a powerful approach to understanding a social (as opposed to parasitic) life strategy while controlling for any phylogenetic or ecological effects (see §2).
Figure 2.
A predictive framework for understanding sociality via inquiline social parasites. The comparative framework considers trait deletions, gains and modifications in parasite–host systems and is adapted from [25]. Bell curves describe the expression of a single phenotype in the host species (blue, solid lines) and the social parasite species (red, dashed lines). The phenotype ‘deletion’ model (a,b) exposes traits required for free-living social organisms that are lost and downregulated in the social parasite. The phenotype ‘augmentation model’ (c,d) can be represented by trait gain, a shift to a novel phenotypic trait, a modification of trait function or the condition under which the trait is expressed. The phenotype ‘retention’ model (e) exposes traits that are likely to be essential for the integration into the host social life. The three models are not necessarily mutually exclusive, as suggested by [25,31–33]. (Online version in colour.)
Table 1.
Comparisons of host–parasite traits identify patterns of parasite trait evolution: putatively ancestral traits may be reduced or completely lost (deletion) or exaggerated (augmentation); new traits that are not found in the host may evolve (gain), or traits may be unmodified (retention). Through comparative analyses of host–parasite pairs, these different types of trait evolution can provide insights into host sociality. The kinds of process (trait retention, modification, loss and gain) are reported together with key examples from the literature and the possible insights they provide into the ultimate and proximate mechanisms of host sociality. Here we focus on what can be learned in terms of traits lost, gained and/or modified during the transition to the parasitic lifestyle; however, it is important to acknowledge that both host and parasite adaptations through loss/gain/modification can occur also for particular life history aspects that are not related to host–parasite interactions nor to sociality. For example, social parasites may retain traits that are not related to sociality, such as thermoregulation and grooming. Similarly, social parasites will modify/acquire new traits also for other selective pressures, such as different environmental requirements (e.g. different overwintering habits [25]). The power of these analyses, therefore, lies in analysing replicated events of change in host–parasite pairs across the phylogeny of Hymenoptera, where social parasites have evolved.
| change in trait | key examples in the literature | insights into host society |
|---|---|---|
| deletion model | ||
|
complete deletion (figure 2a) the trait is no longer present |
— loss of nest founding behaviour and worker caste [1,12] — loss of wax production and pollen collecting apparatus on the hind leg in bumblebees [36] — loss of multiple mating in ants and wasps [30,37] |
complete and partial losses and/or reduction expose traits required for free-living social organisms that are not beneficial for a parasitic lifestyle, or that would actively reduce the fitness of the parasite. As reduction/loss is likely matched by reduction/loss of the regulatory mechanisms, host–parasite comparisons for these traits have the potential to reveal the mechanistic pathways (e.g. hormonal, molecular) underlying these social traits |
|
partial deletion and reduction (figure 2b) the expression of the trait is reduced (e.g. performed behaviour at a lower rate) or its value is reduced (e.g. a less pronounced morphological feature) |
— reduced expression of colony maintenance activities, such as cell building, thermoregulation, colony defence [38] — reduced antibiotic production in Acromyrmex insinuator social parasites [39] |
|
| augmentation model | ||
|
gain (figure 2c): a new trait has evolved de novo |
— usurping restlessness in a paper-wasp social parasite [40] — appeasement pheromone in the brood of paper-wasp social parasite [41] |
this trait is important for parasitic lifestyle. It might reveal unexpected host traits (e.g. sensory biases that can be exploited by the parasite) that would otherwise have gone unnoted if the host was studied alone |
|
increase (figure 2d) trait expression is increased (e.g. performed behaviour at a higher rate) or trait value is increased (e.g. a more pronounced morphological feature) |
— increased development of physical weaponry, such as sting, mandibles and size [12,38] — enlarged glands for secretion of semiochemicals (Dufour gland, venom glands and Van der Vecht organ in bumblebee and/or wasp parasites) [36,42] — increased rate of performance of stroking behaviour in paper-wasp parasites, to obtain chemical integration [12] |
augmented mandibles and stings in parasites help confirm the importance of these weapons in dominance interactions and conflict resolutions by the host; enlarged glands support their role in chemical integration. Parasites are likely to be exploiting these traits, and there is selection for them to invest more heavily in them than the host because of their critical importance in infiltrating (and controlling) host societies |
|
functional shift the trait remains the same but it acquires a different function in the social parasite |
— Acromyrmex insinuator parasite workers acquire the role of suppressing host reproduction [43] | this discloses how host social traits can be co-opted for new functions by social parasites, thus suggesting alternative use of old traits |
| retention model | ||
|
retention (figure 2e) the trait is maintained |
— dominance behaviour: bumblebees, paper-wasps [12,44] | such traits might be essential for the integration into host social life. Dominance, for example, is often crucial in the host society to maintain reproductive control. It helps corroborate any putative mechanism identified from the host system |
(a). Phenotypic deletions and reductions
The first class of phenotypic changes that may inform us about sociality are the ‘phenotypic deletions' [25] (figure 2a,b): we use this term to include ancestral traits that have been reduced in their expression, as well as those that have been completely lost (or unexpressed) in the social parasites. Such traits are putatively important for a social life, but not a parasitic one, and thus are extremely valuable in revealing facets of social living that are likely to be specific to social function rather than phylogenetic signal. Complete loss of a trait implies there is strong selection against that trait, and this will only occur if there are high costs in retaining the trait (e.g. costly chemical cues that might also increase the risk of predation). A good example of a social trait that parasites have lost to varying degrees is the investment in the worker caste. Here we explore this trait with examples of how the varying degrees of worker loss in social parasites might inform us about sociality.
Most inquilines have completely lost their worker caste; complete deletions may be informative for understanding the molecular machinery underpinning a diversity of social traits, because of the shared genetic toolkit of conserved genes that underpins much of our understanding of social evolution [45,46]. The absence of a worker caste in the social parasite may be more correctly viewed as an absence in the expression of the ancestral worker toolkit because the (conserved) machinery is likely to be present in the parasite's genome, but the right genes are just not switched on. This makes for a very useful comparative model: genes that are important components of the worker genetic toolkit in the host should be entirely unexpressed in the parasite genome.
Incipient inquilines, which represent the early stages of social parasite evolution, often express some level of worker traits, for example by still producing a worker caste, albeit vastly reduced. In these cases, partial deletion (or ‘down-regulation’) of a trait can occur, and the way in which the trait is ‘down-played’ can tell us different things about sociality. One way of ‘down-playing’ the worker caste is simply to invest less in its production: this could be a reduction in the number of workers produced, in their size or in their diversity; for example, the incipient inquiline ant Acromyrmex insinuator, which parasitizes its sister species leaf-cutting ant Acromyrmex echinatior [47], produces very few workers and those that are produced are small (as opposed to medium or large [43]); a comparison between host and social parasites has the potential to expose the proximate factors regulating the production of worker caste in the host. Another way that social parasites down-play investment in workers is by producing workers that are in some way functionally substandard; i.e. workers that show reduced participation in nest maintenance or colony defence [43]; a good example of the latter is the reduced size of the metapleural gland in A. insinuator workers and their associated inability to control infection in the colony [39] (table 1). The comparison of the machinery in functional (host) and sub-functional (parasite) workers has the potential to reveal how these specific host worker traits are regulated, at the molecular and/or at the behavioural level (e.g. the absence or inactivity of odorant binding receptors in the parasite workers but not host workers could indicate candidate mechanisms for social regulation of worker behaviours).
(b). Phenotypic augmentation and gain
The second class of phenotypic change that can inform us about sociality is ‘phenotypic augmentation’ (termed ‘shift’ in [25], figure 2c,d and table 1). Here we use this as a collective term that describes the gain of an entirely new trait as well as trait augmentation, through an increase in trait expression. It makes sense to lump trait gain and augmentation together, as they are traits that are likely to be especially useful for a parasitic lifestyle. However, from an ultimate perspective, it is important to note that they should be interpreted in quite different ways. Gains can be de novo evolution of an entirely new phenotype, with a new function (which, for example, enables the parasite to infiltrate or deceive the host), along with a de novo set of underlying machinery. Thus, we do not expect any a priori evolutionary relationship between the host and the parasite with respect to such traits. An example is the emergence of a novel behaviour by the parasite that benefits a specific stage in its life cycle such as the hyper-activity or ‘restlessness’ behaviour that appears to be essential for Polistes sulcifer, the obligate inquiline parasite of the paper-wasp Polistes dominula, in successfully usurping host colonies. Such de novo phenotypic evolution is likely to be underpinned by novel molecular machinery (e.g. new gene functions that allow expression of new behaviours/chemicals) [48,49]. Although these are specifically parasite traits (i.e. absent in the host), these sorts of comparisons could provide insights into how the parasite deceives the host: decoding the devious ways by which the parasite circumvents the nest-mate recognition system of the host (e.g. [19,21]) or suppresses a worker revolt (e.g. [50–52]) can provide us with key insights into the fortress defences of the society.
Trait augmentation is likely to be via the co-option of an ancestral trait (and the associated underlying machinery that regulates it) into a form that benefits the parasite at one or more stage(s) in its life cycle. Examples of this are the evolution of morphological adaptations required for usurpation such as large mandibles or stronger stings [12] or enlarged glands for the secretion of semiochemicals [42]. Behavioural traits can also be augmented; e.g. elevated frequency of gastral stroking by the social parasite P. sulcifer, which is thought to enable the parasite to chemically mark the nest and become chemically integrated into the colony (table 1) [12]. Paired comparisons of host and (augmented) parasite traits could provide many varied insights into sociality; for example, it may be reasonable to assume that an exaggerated version of a host trait may be underpinned by an exaggerated (or upregulated) version of the machinery underpinning it—comparisons of the molecular and physiological underpinnings of the paired traits may help reveal the key processes that regulate that trait in the social species.
A third category of phenotypic augmentation is much more elusive, but potentially one of the most interesting and valuable: this is the co-option of ancestral host traits for a completely new function in the parasite. A. insinuator provides another good example here of where the function of a phenotype has been changed: the workers have acquired a new function in regulating and suppressing host reproduction [43]; although the mechanism by which they do this is unknown, a paired comparison with host workers could reveal simultaneously the mechanistic basis of host worker function (e.g. brood care) and parasite worker function (i.e. suppressing host reproduction) through the upregulation of (for example) transcription in the host or parasite, respectively. Alternatively, the function of a specific trait may be modified; venom volatiles are, for example, used by the host P. dominula as alarm pheromones to alert colony mates and respond to a threat, while they are instead used by the parasite P. sulcifer to disrupt the colony's social cohesion, acting as propaganda pheromones [53]. Such traits have the potential to disclose how new mechanisms can be used by parasites to exploit host social life by re-wiring old traits, thus suggesting similar pathways to obtain different outputs.
(c). Phenotypic retention
Finally, it should be noted that the absence of change can also be informative. Indeed, trait retention (figure 2e and table 1) might suggest that the retained trait is essential for the integration into the host social life, such as, for example, the maintenance of physical dominance behaviour and/or dominance signalling system in social parasites of bumblebee and wasp small societies [44].
4. Empirical examples of inquiline social parasites as tools to understand sociality
Using the framework suggested in figure 2, we explain how the mechanisms and evolutionary processes that underpin the structure and function of a society can be unravelled through the study of social parasites and their hosts. We focus on two exemplar pillars of social evolution—reproductive division of labour and complex communication systems. However, a similar conceptual approach could be applied to other key traits of societies, such as immune defence and conflict resolution, to better understand the mechanisms and evolution of sociality.
(a). Intercepting the regulation of reproductive division of labour
Reproductive division of labour, whereby some individuals specialize in reproduction while others help maintain the colony and provision and defend the brood, is an essential feature that characterizes and maintains the trade-off between cooperation and conflict in societies [54]. Such a division of labour is expressed to different degrees depending on the social complexity of a specific species. Castes are a fascinating social trait, with such extreme morphological specializations in some species that the respective phenotypes can be easily mistaken for different species. Castes are the vehicle for the evolutionary commitment to helping (non-reproductive) behaviour and self-sacrificial behaviour in colony defence. The evolution of castes, and ultimately lifetime commitment to a reproductive or non-reproductive role, results in societies that are more efficient in task performance [54] and that have fewer individuals competing to be reproductive.
A comprehensive understanding of the evolution of insect societies requires uncovering the proximate and ultimate mechanisms that regulate reproductive and non-reproductive phenotypes. Obligate inquiline social parasites might be of help for two main reasons. First, social parasites have been under strong selective pressure to exploit the host's reproductive division of labour (specifically, the host worker caste). Second, the change in relatedness within the colony after usurpation by a parasite makes the sociogenetic structure, and the consequent payoffs for each party, quite easy to calculate [50]. Parasites need to secure the maximum help they can from the host species, which means suppressing host queen reproduction and, at least in primitive eusocial species where workers retain fertility, also suppressing host worker reproduction, so that all the parasite's energy is directed toward maximizing personal fitness. By contrast, the host colony should try to recover at least some fitness; in primitively eusocial species this means that helpers should (if they can detect the parasite) divert their efforts from brood rearing (as it no longer delivers indirect fitness) and instead invest in their own personal reproduction. This situation is clearly less easy when investigating reproductive choices in facultative social parasites that retain the ability to pursue alternative strategies, i.e. found their own colony, or when investigating reproductive choice within the host societies, where fitness payoffs are influenced by the relatedness.
The perfect inquiline should be able to completely suppress host worker reproduction and should invest nothing in worker behaviours/traits. The predictions of the deletion and augmentation models are clear-cut. Parasites should ‘delete’ their worker traits/phenotype (e.g. behaviours, morphological traits, gene expression, chemical cues): those traits that are expressed by host workers but omitted by parasites (figure 2a,b) might be among those specific to a social life, and allow the generation of a refined list of specific traits to be placed centre stage in the study of sociality. However, instances of ‘imperfect’ inquiline behaviours are more common, and although the predictions and hypotheses are less clear-cut than for the ‘perfect’ inquiline, they provide us with some unexpected twists in the makings of a social life. Indeed, there is variation in the ability of social parasites in suppressing host reproduction. Four out of five social parasite bumblebees and two out of four social parasite wasps examined fully suppress worker reproduction [51]. Thus, by looking at how and to what degree inquilines manage to exploit their host's reproductive division of labour we can gain insights into the proximate mechanisms regulating it [44].
An example of the usefulness of obligate social parasites comes from social parasites of Polistes wasps. In the primitively eusocial societies of these paper-wasps, workers can rapidly switch their behavioural phenotype, by developing ovaries in just a few days when given the social opportunity, and starting to lay eggs [55]. Empirical evidence first suggested that the presence of a fertile queen in the nest is the main regulator (or suppressor) of worker reproduction: when the queen is removed, workers start to develop ovaries and eventually one of the workers mates and fully replaces the previous queen [56]. However, the physical presence of the queen itself is not sufficient: the main factor regulating workers in refraining from reproduction is the presence of the main breeder's eggs [51,57]. If eggs are experimentally and periodically removed from the nest, workers develop ovaries and start to lay eggs, even if the queen is still present and active in the nest. Thus, the presence of the eggs of the dominant breeder appears to be a key factor in regulating the reproductive behaviour of workers. Testing this hypothesis within the framework of social parasite–host interaction, however, revealed a more complex story. The social parasite P. sulcifer is able to properly integrate into the host colony, both behaviourally and chemically [12,58,59]. Polistes sulcifer females dominate host workers, and, being as fertile as the previous queen, fill the nest with their eggs. Surprisingly, in contrast with queenright colonies, host workers in parasitized colonies often develop ovaries and lay eggs even though the parasite has filled all vacant cells with eggs [51]. This suggests that the presence of an active egg-layer is not enough to regulate worker reproductive behaviour in these simple societies. What we are missing remains to be seen; the framework predictions of the deletion and augmentation models, however, can help formulate clear hypotheses to be tested. For example, under the deletion model (figure 2a,b), the social parasite has lost the worker traits she no longer needs; but given that queen and worker traits arise through facultative expression of shared genes, and genes can have pleiotropic effects, it may be challenging for selection to perfectly disentangle the two suites of ancestral social phenotypic traits. The parasite may therefore have ‘deleted’ traits that permit successful regulation of worker reproduction; comparison of behaviours and/or chemical cues produced by the reproductive hosts and parasites, and/or expressed by the eggs of each species, may reveal key traits expressed by the host that are omitted by the parasite. Conversely, under the augmentation model (figure 2c,d), the parasite may have evolved a mode to regulate worker reproduction that is entirely different from that of the host (e.g. a new chemical cue) but that is also suboptimal, such that workers can sometimes subvert it. There will be very strong selection for host workers to evolve strategies to subvert reproductive suppression by the parasite, given that parasitism otherwise results in zero fitness for hosts. This is likely to result in a co-evolutionary arms race between the parasite (in suppressing her host workers) and workers (in subverting their parasite queen); under this scenario a dynamic environment of phenotype shifting by the parasite (in response to the host) is plausible, especially in the incipient stages of social parasite evolution. Evidence of host rebellion has been detected in many other social parasite–hosts systems; in Temnothorax ants parasitized host workers kill up to two-thirds of the female pupae of the slave-making parasite Protomognathus americanus [52]. Such rebellions have the potential to uncover mechanisms of regulation of reproductive division of labour that could go unnoted if only the host system is studied.
Another example of how imperfect parasitism can help us better understand sociality is in species that still invest in worker behaviours/traits or even in some cases a worker caste. Inquilines are committed to a life of parasitism and are well adapted to exploit the socially acquired resources of their host, which include workers and their functions. However, in the early stages of inquiline evolution, the worker phenotype may not have been completely deleted. In the leaf-cutting ant inquiline A. insinuator, a worker caste is often produced, although these parasite workers are less good at their job (such as brood rearing) than their host counterparts [43]. Comparisons of the host and parasite workers allow the identification of worker traits that allow optimal functioning of the society, e.g. in immunity or foraging efficiency [39]. Imperfect deletion (figure 2b) may be a first step in the evolution of social parasitism, followed later by a shift in phenotype (figure 2c); alternatively, evolving a new (or modifying an ancestral) phenotype (figure 2d) may be easier than perfectly deleting the ‘right’ part of a social phenotype. The type of model that best explains social parasitism may therefore give insights into the complexity of the social system: in the less complex societies, such as in the primitively eusocial Polistes species, a simple deletion of a suite of (worker) traits may work quite well, while in a more complex society, as in attine ants, a shift to a new phenotype may be easier to evolve.
(b). Breaking the host communication system
Another key pillar of an insect society is the sophisticated communication system that regulates, through multimodal communication channels, all the social activities in the colony, such as labour division, colony defence, resource gathering and brood care [1,60]. Social insects rely on a very complicated and multimodal signalling network, made by complex blends of different chemical compounds, integrated with vibrational and acoustic signals (pulses of variable amplitude and frequency) as well as visual ones (patterns of shapes and colours on several insect body parts [61,62]). A major topic of current research aims at elucidating whether all or only some components of these complex signals play a communicative role and how they evolved across different social lineages [60,63].
Since inquilines, by definition, infiltrate and live in host colonies, they need to break the host communication code in order to get accepted into a host colony and exploit the services of the host workers [19,21]. Here, we provide some iconic examples of studies on insect social parasite communication strategies that shed light on how host communication systems work, focusing first on how inquilines break the host nest-mate recognition code and then on how they manage to reduce host defences. In both cases, we draw on examples where ancestral host traits are lost, gained or modified in social parasites and explain how they can be used to better understand communication systems in social insects.
Eusocial insects have developed an efficient nest-mate recognition system that allows each colony member to discriminate nest-mates from foreigners [64]. Such nest-mate recognition is usually based on the complex blend of hydrocarbons that covers the insect's cuticle (CHCs), which primarily acts as a barrier against water loss and entry of pathogens [65]. These complex mixtures of long saturated and long unsaturated carbon chains—with or without methyl groups attached—have also evolved a role in communication, in particular acting as a colonial chemical signature and thus being responsible for making social insect colonies closed social units [66]. Social parasites have evolved an amazing array of strategies to overcome this efficient host barrier against intruders and successfully penetrate the host fortress [19,21]. First, social parasites can ‘copy’ the host recognition code (chemical mimicry, i.e. active production of host signature, and camouflage, i.e. passive acquisition of host signature). Given that non-nest-mate hosts (i.e. conspecifics from other colonies) cannot do this (i.e. they cannot usually infiltrate a non-natal colony), this is an example of an acquired plastic trait in the social parasite (as predicted under the augmentation model (figure 2c,d), allowing the parasite the extraordinary ability to facultatively match her chemical profile to that of her chosen host nest. Aside from being a remarkable feat of evolution, this parasitic trait is useful in revealing which facets of the host's chemical signature are fundamental in nest-mate recognition through a comparison of the parasite's chemical signature immediately before and after successful infiltration of the host colony [53,67]. Moreover, the study of these strategies has the potential to suggest additional putative key features of the host recognition system. Indeed, by studying the chemical host-specificity of bumblebee social parasites, Martin and co-workers [24] found that the diversity of alkene isomer patterns in the chemical profiles of the host species is mimicked by cuckoo bees, thus revealing the importance of isomer diversity among species in bumblebee recognition codes.
Another strategy used by many inquiline social parasites to subvert host recognition systems is chemical insignificance. First discovered in newly emerged ants [21], chemical insignificance, i.e. low levels of CHCs on the cuticle, has been found in many different species of social parasites [21,68]. These findings highlight the importance of the quantitative features of chemical cues for the recognition processes and have stimulated studies to define the lower limits of nest-mate recognition cues needed to elicit a behavioural reaction in social insects [69–71]. Such findings have supported the hypothesis of a threshold mechanism in the host chemical recognition system. Interestingly, chemical insignificance is not always obtained through a general under-production of CHCs, but rather through a selective reduction of specific classes of compounds that, owing to their structure/shape (i.e. double bonds or methyl groups), play an important role in communication [60,72–74]. Examples of chemical blends that are uninformative for the host have been reported for larvae of the paper-wasp social parasite P. sulcifer that are reared alongside host larvae [75], and for the eggs of the social parasite Vespa dybowskii laid in the host comb [76]. In both these cases, immature brood is covered by a cuticular profile with a lower proportion of branched compounds compared with host eggs/larvae; this signature does not trigger rejection by the hosts and instead allows immature parasite brood to be tolerated in host colonies. Such neutral, uninformative profiles of parasite immature brood have confirmed and reinforced the relevant role that different cuticular compounds have in conveying information concerning the bearer's identity. Chemical insignificance of parasite brood is a prediction of the deletion model (figure 2a,b): the parasite has lost traits that it no longer needs (presumably because they are costly), but equally, there has also been strong selection for the loss of traits that would otherwise reduce its fitness. Chemical cues of nest-mate identity in the brood are exactly these: a social parasite that produces brood that lack the parasite brood chemical odour will do better than a social parasite that retains it.
Another extraordinary evolved trait of social parasites is their ability to produce chemicals that appease, disperse, distract or repel host defenders [19]. Propaganda and appeasement allomones produce powerful effects on the host, ranging from panic responses in defending workers to provoking host workers to attack each other by acting as nest-mate recognition disruptors [44,60,77,78]. Usually, such substances (often acetates) are already present as defensive secretions in Hymenoptera (alarm pheromones) and they are likely to be exploited by social parasites to achieve different results. These can be obtained by under- or over-producing some compounds of a pre-existing host signal. This is a modified prediction of the augmentation model (figure 2c,d): although the parasites are using the same basic ancestral host traits, they have ‘shifted’ in the context in which they are triggered, and in their function. In this sense, the chemicals per se are not an innovation; instead the innovation in the social parasite is the behavioural conditions under which the chemical pathways are activated. Discerning how the sensory perception pathways in the host and parasite differ and yet produce the same chemicals has the potential to reveal the mechanisms underpinning complex communication systems in the evolution of sociality. Although the chemical composition of such allomones and their effect on hosts are reported for ants, bees and wasps [44,60,77,78], their physiological mechanism is unknown [79]. Certainly, a more careful evaluation of these impressive allomones could represent a useful tool to better understand different behavioural actions usually adopted by many social insects as a reaction to an incoming danger.
Overall, the broad suite of formidable strategies used by social parasites to bypass the host recognition barrier represent remarkable natural experiments that provide us with insights into the secrets of host communication systems. Future research should extend the study to the other sensory channels used by social insects to communicate, such as vibrational and visual communication.
5. Conclusion
Here we have argued that inquiline social parasites not only represent fascinating natural phenomena, but also natural, replicated evolutionary experiments that might provide insights into the insect societies that they have evolved to exploit. Because of their strong and intimate relationship with the host species, their phylogenetic proximity to host species, as well as their abundance across the main Hymenoptera social lineages, inquiline social parasites represent promising (albeit under-exploited) model systems to better understand the functioning of insect societies. We have provided a conceptual comparative framework that considers trait loss, gains and modifications in parasite–host systems, in order to use inquiline social parasite as tools to better understand insect societies. We have also supplied empirical examples of how this simple framework provides complementary hypotheses to test, by focusing on two cornerstones of sociality: reproductive division of labour and communication.
The potential of social parasites as tools in this respect is likely to reach far beyond these two aspects [80], but we hope that these topics provide exemplars to initiate more interest in using social parasites as models within this conceptual framework to better understand the mechanisms and evolution of the more elusive secrets of social living, such as immune defence and conflict resolution. Equally, although we focus on inquilines, there is huge potential to exploit other guises of social parasites of insects (from slave-makers to temporary facultative social parasites), as well as social parasites from across the animal kingdom (such as cuckoos in cooperative breeding birds) [11,18,81], as tools to unlock the secrets of living in societies.
Supplementary Material
Acknowledgements
We thank the editors of this special issue for inviting us to contribute.
Data accessibility
The dataset used for figure 1 can be accessed in the electronic supplementary material, table S1.
Authors' contributions
A.C. conceived the manuscript and all the authors participated in the writing.
Competing interests
We declare we have no competing interests.
Funding
Research was supported by funds from the University of Florence (to R.C.) and by the Marie Sklodowska-Curie Action, grant no. 706208 SocParPhenoEvol, to A.C.
References
- 1.Wilson EO. 1971. The insect societies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Gadau J, Fewell JH. 2009. Organization of insect societies: from genome to socio-complexity. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Fittkau EJ, Klinge H. 1973. On biomass and trophic structure of the Central Amazonian rain forest ecosystem. Biotropica 5, 2–14. ( 10.2307/2989676) [DOI] [Google Scholar]
- 4.Kennedy P, et al. 2017. Deconstructing superorganisms and societies to address big questions in biology. Trends Ecol. Evol. 32, 861–872. ( 10.1016/j.tree.2017.08.004) [DOI] [PubMed] [Google Scholar]
- 5.Healy SD, Rowe C. 2007. A critique of comparative studies of brain size. Proc. R. Soc. B 272, 1865–1875. ( 10.1098/rspb.2006.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alem S, Perry CJ, Zhu X, Loukola OJ, Ingraham T, Søvik E, Chittka L. 2016. Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol. 14, e1002564 ( 10.1371/journal.pbio.1002564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loukola OJ, Perry CJ, Coscos L, Chittka L. 2017. Bumblebees show cognitive flexibility by improving on an observed complex behavior. Science 355, 833–836. ( 10.1126/science.aag2360) [DOI] [PubMed] [Google Scholar]
- 8.Nowak MA, Tarnita CE, Wilson EO. 2010. The evolution of eusociality. Nature 466, 1057 ( 10.1038/nature09205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinter-Wollman N. 2012. Personality in social insects: how does worker personality determine colony personality? Curr. Zool. 58, 580–588. ( 10.1093/czoolo/58.4.580) [DOI] [Google Scholar]
- 10.Jandt JM, Bengston S, Pinter-Wollman N, Pruitt JN, Raine NE, Dornhaus A, Sih A. 2014. Behavioural syndromes and social insects: personality at multiple levels. Biol. Rev. 89, 48–67. ( 10.1111/brv.12042) [DOI] [PubMed] [Google Scholar]
- 11.Barbero F, Patricelli D, Witek M, Balletto E, Casacci LP, Sala M, Bonelli S. 2012. Myrmica ants and their butterfly parasites with special focus on the acoustic communication. Psyche (Stuttg.) 2012, 725237. ( 10.1155/2012/725237) [DOI] [Google Scholar]
- 12.Cervo R. 2006. Polistes wasps and their social parasites: an overview. Ann. Zool. Fenn. 43, 531–549. [Google Scholar]
- 13.Buschinger A. 2009. Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol. News 12, 219–235. [Google Scholar]
- 14.Lowe RM, Ward SA, Crozier RH. 2002. The evolution of parasites from their hosts: intra- and interspecific parasitism and Emery's rule. Proc. R. Soc. Lond. B 269, 1301–1305. ( 10.1098/rspb.2002.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang MH, Dornhaus A. 2008. A meta-analysis of ant social parasitism: host characteristics of different parasitism types and a test of Emery's rule. Ecol. Entomol. 33, 589–596. ( 10.1111/j.1365-2311.2008.01005.x) [DOI] [Google Scholar]
- 16.Smith JA, Chenoweth LB, Tierney SM, Schwarz MP. 2013. Repeated origins of social parasitism in allodapine bees indicate that the weak form of Emery's rule is widespread, yet sympatric speciation remains highly problematic. Biol. J. Linn. Soc. 109, 320–331. ( 10.1111/bij.12043) [DOI] [Google Scholar]
- 17.Dawkins R, Krebs JR. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511. ( 10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 18.Brandt M, Foitzik S, Fischer-Blass B, Heinze J. 2005. The coevolutionary dynamics of obligate ant social parasite systems – between prudence and antagonism. Biol. Rev. Camb. Phil. Soc. 80, 251–267. ( 10.1017/S1464793104006669) [DOI] [PubMed] [Google Scholar]
- 19.Nash DR, Boomsma JJ. 2008. Communication between hosts and social parasites. In Sociobiology of communication: an interdisciplinary perspective (eds P d'Ettorre, DP Hughes), pp. 55–79. Oxford, UK: Oxford University Press. ( 10.1093/acprof:oso/9780199216840.003.0004) [DOI] [Google Scholar]
- 20.Grüter C, Jongepier E, Foitzik S. 2018. Insect societies fight back: the evolution of defensive traits against social parasites. Phil. Trans. R. Soc. B 373, 20170200 ( 10.1098/rstb.2017.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenoir A, d'Ettorre P, Errard C, Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. ( 10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 22.Ortolani I, Cervo R. 2010. Intra-specific body size variation in Polistes paper wasps as a response to social parasite pressure. Ecol. Entomol. 35, 352–359. ( 10.1111/j.1365-2311.2010.01187.x) [DOI] [Google Scholar]
- 23.Lorenzi MC, Azzani L, Bagnères AG. 2014. Evolutionary consequences of deception: complexity and informational content of colony signature are favored by social parasitism. Curr. Zool. 60, 137–148. ( 10.1093/czoolo/60.1.137) [DOI] [Google Scholar]
- 24.Martin SJ, Carruthers JM, Williams PH, Drijfhout FP. 2010. Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J. Chem. Ecol. 36, 855–863. ( 10.1007/s10886-010-9805-3) [DOI] [PubMed] [Google Scholar]
- 25.Cini A, Patalano S, Segonds-Pichon A, Busby GBJ, Cervo R, Sumner S. 2015. Social parasitism and the molecular basis of phenotypic evolution. Front. Genet. 6, 32 ( 10.3389/fgene.2015.00032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter JM, Perera EP. 2006. Phylogenetic relationships among yellowjackets and the evolution of social parasitism (Hymenoptera: Vespidae, Vespinae). Am. Mus. Novit. 3507, 1 ( 10.1206/0003-0082(2006)3507[1:PRAYAT]2.0.CO;2) [DOI] [Google Scholar]
- 27.Hines HM, Cameron SA. 2010. The phylogenetic position of the bumble bee inquiline Bombus inexspectatus and implications for the evolution of social parasitism. Insect. Sociaux 57, 379–383. ( 10.1007/s00040-010-0094-1) [DOI] [Google Scholar]
- 28.Tierney SM, Smith JA, Chenoweth L, Schwarz MP. 2008. Phylogenetics of allodapine bees: a review of social evolution, parasitism and biogeography. Apidologie 39, 3–15. ( 10.1051/apido:2007045) [DOI] [Google Scholar]
- 29.Gibbs J, Albert J, Packer L. 2012. Dual origins of social parasitism in North American Dialictus (Hymenoptera: Halictidae) confirmed using a phylogenetic approach. Cladistics 28, 195–207. ( 10.1111/j.1096-0031.2011.00373.x) [DOI] [PubMed] [Google Scholar]
- 30.Sumner S, Hughes WOH, Pedersen JS, Boomsma JJ. 2004. Ant parasite queens revert to mating singly. Nature 428, 35 ( 10.1038/428035a) [DOI] [PubMed] [Google Scholar]
- 31.Smith CR, et al. 2015. How do genomes create novel phenotypes? Insights from the loss of the worker caste in ant social parasites. Mol. Biol. Evol. 32, 2919–2931. ( 10.1093/molbev/msv165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alleman A, Feldmeyer B, Foitzik S. 2018. Comparative analyses of co-evolving host-parasite associations reveal unique gene expression patterns underlying slavemaker raiding and host defensive phenotypes. Sci. Rep. 8, 1951 ( 10.1038/s41598-018-20262-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aumer D, Mumoki FN, Pirk CWW, Moritz RFA. 2018. The transcriptomic changes associated with the development of social parasitism in the honeybee Apis mellifera capensis. Sci. Nat. 105, 22 ( 10.1007/s00114-018-1552-2) [DOI] [PubMed] [Google Scholar]
- 34.Ellegren H. 2014. Genome sequencing and population genomics in non-model organisms. Trends Ecol. Evol. 29, 51–63. ( 10.1016/j.tree.2013.09.008) [DOI] [PubMed] [Google Scholar]
- 35.Bonasio R. 2015. The expanding epigenetic landscape of non-model organisms. J. Exp. Biol. 218, 114–122. ( 10.1242/jeb.110809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher RM, Sampson B. 1992. Morphological specializations of the bumble bee social parasite Psithyrus ashtoni (Cresson) (Hymenoptera: Apidae). Can. Entomol. 124, 69–77. ( 10.4039/Ent12469-1) [DOI] [Google Scholar]
- 37.Loope KJ, Lopez-Osorio F, Dvořák L. 2017. Convergent reversion to single mating in a wasp social parasite. Am. Nat. 189, E138–E151. ( 10.1086/691405) [DOI] [PubMed] [Google Scholar]
- 38.Reed HC, Akre RD. 1983. Colony behavior of the obligate social parasite Vespula austriaca (Panzer) (Hymenoptera: Vespidae). Insect. Soc. 30, 259–273. ( 10.1007/BF02223984) [DOI] [Google Scholar]
- 39.Sumner S, Hughes WOH, Boomsma JJ. 2003. Evidence for differential selection and potential adaptive evolution in the worker caste of an inquiline social parasite. Behav. Ecol. Sociobiol. 54, 256–263. ( 10.1007/s00265-003-0633-0) [DOI] [Google Scholar]
- 40.Ortolani I, Turillazzi S, Cervo R. 2008. Spring usurpation restlessness: a wasp social parasite adapts its seasonal activity to the host cycle. Ethology 114, 782–788. ( 10.1111/j.1439-0310.2008.01525.x) [DOI] [Google Scholar]
- 41.Elia M, Khalil A, Bagnères AG, Lorenzi MC. 2018. Appeasing their hosts: a novel strategy for parasite brood. Anim. Behav. 146, 123–134. ( 10.1016/j.anbehav.2018.10.011) [DOI] [Google Scholar]
- 42.Petrocelli I, Turillazzi S. 2013. Comparative morphology of Van der Vecht's organ in Polistes social parasites: host ecology and adaptation of the parasite. Biol. J. Linn. Soc. 109, 313–319. ( 10.1111/bij.12053) [DOI] [Google Scholar]
- 43.Sumner S, Nash DR, Boomsma JJ. 2003. The adaptive significance of inquiline parasite workers. Proc. R. Soc. Lond. B 270, 1315–1322. ( 10.1098/rspb.2003.2362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lhomme P, Hines HM. 2018. Reproductive dominance strategies in insect social parasites. J. Chem. Ecol. 22, 1–3. ( 10.1007/s10886-018-0971-z) [DOI] [PubMed] [Google Scholar]
- 45.Toth AL, et al. 2007. Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 318, 441–444. ( 10.1126/science.1146647) [DOI] [PubMed] [Google Scholar]
- 46.Toth AL, Rehan SM. 2017. Molecular evolution of insect sociality: an eco-evo-devo perspective. Annu. Rev. Entomol. 62, 419–442. ( 10.1146/annurev-ento-031616-035601) [DOI] [PubMed] [Google Scholar]
- 47.Sumner S, Aanen DK, Delabie J, Boomsma JJ. 2004. The evolution of social parasitism in Acromyrmex leaf-cutting ants: a test of Emery's rule. Insect. Sociaux 51, 37–42. ( 10.1007/s00040-003-0723-z) [DOI] [Google Scholar]
- 48.Sumner S. 2014. The importance of genomic novelty in social evolution. Mol. Ecol. 23, 26–28. ( 10.1111/mec.12580) [DOI] [PubMed] [Google Scholar]
- 49.Ferreira PG, Patalano S, Chauhan R, Ffrench-Constant R, Gabaldón T, Guigó R, Sumner S. 2013. Transcriptome analyses of primitively eusocial wasps reveal novel insights into the evolution of sociality and the origin of alternative phenotypes. Genome Biol. 14, R20 ( 10.1186/gb-2013-14-2-r20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green JP, Cant MA, Field J. 2014. Using social parasitism to test reproductive skew models in a primitively eusocial wasp. Proc. R. Soc. B 281, 20141206 ( 10.1098/rspb.2014.1206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cini A, Nieri R, Dapporto L, Monnin T, Cervo R. 2014. Almost royal: incomplete suppression of host worker ovarian development by a social parasite wasp. Behav. Ecol. Sociobiol. 68, 467–475. ( 10.1007/s00265-013-1661-z) [DOI] [Google Scholar]
- 52.Achenbach A, Foitzik S. 2009. First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evolution 63, 1068–1075. ( 10.1111/j.1558-5646.2009.00591.x) [DOI] [PubMed] [Google Scholar]
- 53.Bruschini C, Cervo R. 2011. Venom volatiles of the paper wasp social parasite Polistes sulcifer elicit intra-colonial aggression on the nest of the host species Polistes dominulus. Insect. Sociaux 58, 383–390. ( 10.1007/s00040-011-0155-0) [DOI] [Google Scholar]
- 54.Robinson GE. 1992. Regulation of division-of-labor in insect societies. Annu. Rev. Entomol. 37, 637–665. ( 10.1590/S0066-782X2009001300016) [DOI] [PubMed] [Google Scholar]
- 55.Reeve HK. 1991. Polistes. In The social biology of wasps (eds Ross KG, Matthews RW), pp. 99–148. Ithaca, NY: Cornell University Press. [Google Scholar]
- 56.Strassmann JE, Fortunato A, Cervo R, Turillazzi S, Damon JM, Queller DC, Strassmann J, Queller DC. 2004. The cost of queen loss in the social wasp Polistes dominulus (Hymenoptera: Vespidae). J. Kansas Entomol. Soc. 77, 343–355. ( 10.2317/E-15.1) [DOI] [Google Scholar]
- 57.Liebig J, Monnin T, Turillazzi S. 2005. Direct assessment of queen quality and lack of worker suppression in a paper wasp. Proc. R. Soc. B 272, 1339–1344. ( 10.1098/rspb.2005.3073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cini A, Bruschini C, Poggi L, Cervo R. 2011. Fight or fool? Physical strength, instead of sensory deception, matters in host nest invasion by a wasp social parasite. Anim. Behav. 81, 1139–1145. ( 10.1016/j.anbehav.2011.02.017) [DOI] [Google Scholar]
- 59.Cini A, Bruschini C, Signorotti L, Pontieri L, Turillazzi S, Cervo R. 2011. The chemical basis of host nest detection and chemical integration in a cuckoo paper wasp. J. Exp. Biol. 214, 3698–3703. ( 10.1242/jeb.059519) [DOI] [PubMed] [Google Scholar]
- 60.d'Ettorre P, Moore AJ. 2008. Chemical communication and the coordination of social interactions in insects. In Sociobiology of communication: an interdisciplinary perspective (eds P d'Ettore & DP Hughes), pp. 81–96. Oxford, UK: Oxford University Press. [Google Scholar]
- 61.Hunt JH, Richard FJ. 2013. Intracolony vibroacoustic communication in social insects. Insect. Sociaux 60, 403–417. ( 10.1007/s00040-013-0311-9) [DOI] [Google Scholar]
- 62.Cervo R, Cini A, Turillazzi S. 2015. Visual recognition in social wasps. In Social recognition in invertebrates (eds L Aquiloni, E Tricarico), pp. 125–145. Cham, Switzerland: Springer. [Google Scholar]
- 63.Martin SJ, Drijfhout FP. 2009. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J. Chem. Ecol. 35, 368–374. ( 10.1007/s10886-009-9612-x) [DOI] [PubMed] [Google Scholar]
- 64.Blomquist GJ, Bagnères AG (eds). 2010. Insect hydrocarbons biology, biochemistry, and chemical ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 65.Howard RW, Blomquist GJ. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393. ( 10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- 66.van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons biology, biochemistry, and chemical ecology, (eds GJ Blomquist, AG Bagnères), pp. 222–243. Cambridge, UK: Cambridge University Press ( 10.1017/CBO9780511711909.012) [DOI] [Google Scholar]
- 67.Breed MD. 1998. Chemical cues in kin recognition: criteria for identification, experimental approaches, and the honey bee as an example. In Pheromone communication in social insects: ants, wasps, bees and termites (eds Vander Meer R, Breed M, Espelie K, Winston M), pp. 57–78. Boulder, CO: Westview Press. [Google Scholar]
- 68.Lorenzi MC. 2006. The result of an arms race: the chemical strategies of Polistes social parasites. Ann. Zool. Fenn. 43, 550–563. [Google Scholar]
- 69.Cini A, Gioli L, Cervo R. 2009. A quantitative threshold for nest-mate recognition in a paper social wasp. Biol. Lett. 5, 459–461. ( 10.1098/rsbl.2009.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ichinose K, Lenoir A. 2010. Hydrocarbons detection levels in ants. Insect. Sociaux 57, 453–455. ( 10.1007/s00040-010-0103-4) [DOI] [Google Scholar]
- 71.Cappa F, Bruschini C, Cipollini M, Pieraccini G, Cervo R. 2014. Sensing the intruder: a quantitative threshold for recognition cues perception in honeybees. Naturwissenschaften 101, 149–152. ( 10.1007/s00114-013-1135-1) [DOI] [PubMed] [Google Scholar]
- 72.Dani FR, Jones GR, Corsi S, Beard R, Pradella D, Turillazzi S. 2005. Nestmate recognition cues in the honey bee: differential importance of cuticular alkanes and alkenes. Chem. Senses 30, 477–489. ( 10.1093/chemse/bji040) [DOI] [PubMed] [Google Scholar]
- 73.Lucas C, Pho DB, Jallon JM, Fresneau D. 2005. Role of cuticular hydrocarbons in the chemical recognition between ant species in the Pachycondyla villosa species complex. J. Insect Physiol. 51, 1148–1157. ( 10.1016/j.jinsphys.2005.06.003) [DOI] [PubMed] [Google Scholar]
- 74.Cristina LM, Cervo R, Bagnères AG. 2011. Facultative social parasites mark host nests with branched hydrocarbons. Anim. Behav. 82, 1143–1149. ( 10.1016/j.anbehav.2011.08.011) [DOI] [Google Scholar]
- 75.Cervo R, Dani FR, Cotoneschi C, Scala C, Lotti I, Strassmann JE, Queller DC, Turillazzi S. 2008. Why are larvae of the social parasite wasp Polistes sulcifer not removed from the host nest? Behav. Ecol. Sociobiol. 62, 1319–1331. ( 10.1007/s00265-008-0560-1) [DOI] [Google Scholar]
- 76.Martin SJ, Takahashi J, Ono M, Drijfhout FP. 2008. Is the social parasite Vespa dybowskii using chemical transparency to get her eggs accepted? J. Insect Physiol. 54, 700–707. ( 10.1016/j.jinsphys.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 77.Zimma BO, Ayasse M, Tengö J, Ibarra F, Schulz C, Francke W. 2003. Do social parasitic bumblebees use chemical weapons? (Hymenoptera, Apidae). J. Comp. Physiol. A 189, 769–775. ( 10.1007/s00359-003-0451-x) [DOI] [PubMed] [Google Scholar]
- 78.Bruschini C, Cervo R, Stefano T. 2010. Pheromones in social wasps Vitam Horm. 83, 447–492 ( 10.1016/S0083-6729(10)83019-5) [DOI] [Google Scholar]
- 79.Akino T. 2008. Chemical strategies to deal with ants: a review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol. News 11, 173–181. [Google Scholar]
- 80.Manna TJ, Hauber ME. 2016. Recognition, speciation, and conservation: recent progress in brood parasitism research among social insects. Curr. Opin. Behav. Sci. 12, 1–5. ( 10.1016/j.cobeha.2016.07.005) [DOI] [Google Scholar]
- 81.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used for figure 1 can be accessed in the electronic supplementary material, table S1.