Abstract
Obligate brood-parasitic cheats have fascinated natural historians since ancient times. Passing on the costs of parental care to others occurs widely in birds, insects and fish, and often exerts selection pressure on hosts that in turn evolve defences. Brood parasites have therefore provided an illuminating system for researching coevolution. Nevertheless, much remains unknown about how ecology and evolutionary history constrain or facilitate brood parasitism, or the mechanisms that shape or respond to selection. In this special issue, we bring together examples from across the animal kingdom to illustrate the diverse ways in which recent research is addressing these gaps. This special issue also considers how research on brood parasitism may benefit from, and in turn inform, related fields such as social evolution and immunity. Here, we argue that progress in our understanding of coevolution would benefit from the increased integration of ideas across taxonomic boundaries and across Tinbergen’s Four Questions: mechanism, ontogeny, function and phylogeny of brood parasitism. We also encourage renewed vigour in uncovering the natural history of the majority of the world's brood parasites that remain little-known. Indeed, it seems very likely that some of nature’s brood parasites remain entirely unknown, because otherwise we are left with a puzzle: if parental care is so costly, why is brood parasitism not more common?
This article is part of the theme issue ‘The coevolutionary biology of brood parasitism: from mechanism to pattern’.
Keywords: brood parasitism, coevolution, Tinbergen, co-citation network
1. Introduction
Parental care is a key aspect of the life history of many animals [1], including our own species. It is perhaps not surprising then that we find it hard to forget the sight of a small bird devoting its parental attention to a noisy and monstrously large parasitic cuckoo chick that is so clearly, to our eyes, an imposter in the nest. This reproductive strategy of having one's offspring reared by another species—brood parasitism—has fascinated naturalists and other curious minds for centuries [2]. For example, Confucian texts from the sixth century BC explained the reproductive habits of common cuckoos Cuculus canorus (the eponymous brood-parasitic bird) as an opportunity for hosts to pay homage to an exemplary ruler [3]. In the light of evolutionary theory (e.g. [4]), however, we now know that the ‘exemplary ruler’ is a cheat, parasitizing the parental investment of host species. Such cheats have been of particular scientific interest as striking and tractable examples of coevolution, the process through which two or more species reciprocally affect each other's evolution [4]: we can readily identify real selection pressures in the wild, and test them with field experiments. The hallmarks of coevolution are its dynamism and its capacity to generate novelty, as each party experiences continually changing selection from a nimble and ever-changing partner [5]. Our appreciation for its power to shape beautiful adaptations in antagonists and their victims comes in no small part from studies of brood parasites and their hosts [6].
Where do we find brood parasites in nature? Parental care strategies evolve when the fitness benefits to parents of caring for their young outweigh the costs in terms of energy and residual reproductive value [7]. These costs expose parents to cheating, because individuals that can achieve the benefits of parental care without paying the concomitant costs are favoured by natural selection. It follows then that we might expect obligate brood parasitism to evolve wherever we see parental care.
Parental care is particularly prevalent in birds, and avian brood parasitism has received the lion's share of research effort into brood parasites (for reviews, see [6,8–10]). Obligate interspecific brood parasitism is found in approximately 1% of all birds, has evolved independently seven times and can be found on every continent except Antarctica [6]. Evolutionary transitions to brood parasitism in birds vary from very ancient (e.g. approx. 26 Myr ago in Indicator honeyguides [11]) to an order of magnitude more recent (e.g. the black-headed duck Heteronetta atricapilla, Molothrus cowbirds [12]). Typically, avian brood parasites lay their eggs in the nests of host species to take advantage of both incubation and chick-rearing behaviour. They may exploit the behaviour of a single pair of hosts (parents) or of a unit of cooperatively breeding hosts (parents plus helpers) (e.g. [13], and see [14] in this issue). Parasites have a suite of adaptations across the life stages that allow successful exploitation of hosts: adult females track the nesting progress of hosts and lay eggs at the appropriate time to ensure optimal development, eggshells often mimic the colour and pattern of host eggs to avoid host detection and parasite chicks are adept at winning the preferential care of host parents, sometimes with specialized adaptations to kill foster siblings outright [15].
Among non-avian vertebrates, brood parasitism is known only from a single fish [16]. Cuckoo catfish (Synodontis multipunctatus) take advantage of cichlid hosts that provide care by mouthbrooding developing young. Cuckoo catfish biology remains poorly known, but this issue includes new studies that show the species is tractable for experimental research (see [17,18], this issue). Why is brood parasitism not known from the many other vertebrate clades that provide costly parental care, such as mammals, amphibians and reptiles? We might speculate that viviparity and extended gestation greatly limit opportunities for inserting foreign young into another's brood, and for deceiving carers that another species is kin. But this does not satisfactorily explain why, for example, care-giving frogs or crocodilians (birds' closest reptilian relatives) seem not to experience brood parasitism [19–21]. It is tempting to wonder whether examples may exist that have yet to be detected.
The other major taxonomic group where hosts are co-opted into raising offspring of other species is the insects (reviewed in [22,23] in this issue). Brood-parasitic insects include some beetles, butterflies, true bugs and both social and solitary-living hymenoptera (ants, bees and wasps). Brood parasitism in insects is typically defined by whether the parasite exploits resources acquired by solitary parents (‘brood parasites’, or ‘kleptoparasites’), or by societies that care collectively for their young (‘social parasites’) (see [23], this issue, for discussion). In the latter case, the brood parasite often remains in the host nest and uses the host's workers to provision her offspring. For example, in the obligate slave-making ant Polyergus breviceps, the invading queen kills off the resident host queen and uses chemical manipulation to ensure that the host workers care for her brood of future queens and males. Female ‘kleptoparasites’, by contrast, tend to lay their egg/s and leave. For example, cuckoo wasps (Chrysidinae) parasitize solitary bee and wasp species by laying their eggs in the host's nest chamber, such that the parasitic larvae consume the stored resources that had been intended for the host brood, and sometimes also the host egg itself. In this special issue, we take an inclusive approach and define interspecific brood parasitism as any case in which one species usurps the resources intended for parental care by another species, regardless of whether the costs are borne by host parents or cooperative groups (or indeed, the brood; see Cotter et al. [24] in this issue), or whether the adult brood parasite leaves or remains in the host nest. Brood parasitism can also occur within a species, where one female exploits the efforts of a conspecific [25], or it can be facultative across species; however, the research in this special issue focuses on obligate parasites, because these have the most potential to influence the evolution of another species.
There has thus been long-standing interest in brood parasitism, both as a fascinating natural history phenomenon and as a window into coevolution. Yet, there is still a great deal that is unknown about when, why and how brood parasitism evolves, and the extent to which it drives evolution in host species. In particular, we lack a comprehensive understanding of how ecology and evolutionary history constrain or facilitate these adaptations, via the mechanisms that shape or respond to selection. This special issue aims to illustrate the diverse ways in which current research is addressing gaps in our knowledge of brood parasitism, to bring together examples of interspecific brood parasitism from across the animal kingdom and to consider how research on brood parasitism may benefit from, and in turn perhaps help to inform, related topics such as social evolution and immunity.
2. Taxonomic boundaries to brood parasitism research
An understanding of brood parasitism in any one system often requires study of system-specific traits, which can obscure general insights across taxa. For example, avian brood parasites manipulate their hosts primarily in the visual or auditory sensory domains (so far as is known), while insect brood parasites must subvert predominantly chemical communication systems to usurp host resources (but see [26] for an example of acoustic mimicry in ants). This difference is at least partly responsible for a tendency towards different types of research programmes in the two taxanomic groups. The visual and acoustic signals of avian parasites are amenable to manipulation in the field, such that much research has focused on understanding how parasites deceive. Evidence of this lies in the many field experiments that add model eggs to nests, or use vocal playbacks of nestlings begging, to understand host responses to alien eggs and chicks (e.g. [27–29]). Chemical signals in insects are less readily manipulated in this way, and insect nests are often less accessible in situ. Much research into the brood parasitism of insect societies also focuses on defence mechanisms of hosts and counter-adaptations of the brood parasite. Yet these questions are naturally addressed in the context of social living and indirect fitness (e.g. [22,23,30] in this issue); questions that are rarely considered in avian systems ([31] and [30] in this issue).
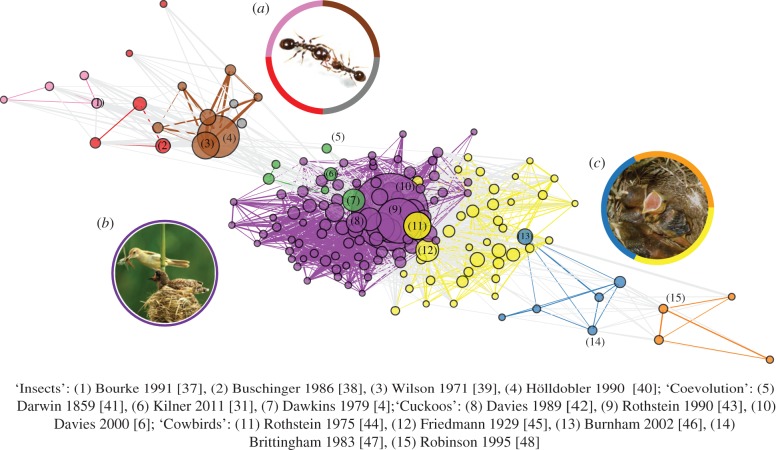
To visualize how these and other differences affect the cross-pollination of ideas and theory across taxonomic boundaries (e.g. [32]), we used the bibliometrix package [33] in R (version 3.5.2 [34]) to construct a co-citation network [35,36]. Looking simply at which papers are cited most often, or cited by other papers within a search-set, can give an idea of how connected a research topic is, but it is less effective at identifying how sub-topics are connected. Co-citation networks, on the other hand, are built by measuring which publications are cited together by the papers within a search-set. The more that papers are cited together, the more likely they represent key ideas or concepts of a research topic (or sub-topic) [35,36]. First, we searched Scopus for all journal articles published with ‘brood parasitism’ or ‘social parasitism’ in the title, abstract or keywords. We focused on obligate parasitism, and therefore excluded papers using ‘intraspecific’ or ‘conspecific’ as search terms. We also excluded journals unrelated to biology. Of 1933 articles meeting these criteria, 45.7% (883) included ‘bird’, ‘aves’ or ‘avian’ in the title, abstract or keywords, and 33.6% (650) included ‘insect’, ‘hymenopt*’, ‘lepidopt*’, ‘coleopt*’, ‘beetle’, ‘butterfly’, ‘ant’, ‘wasp’ or ‘bee’. It is likely that this simple search did not capture all papers published on brood parasitism, as the use of taxonomic keywords can be inconsistent. Nevertheless, we chose not to bias the search by including ‘cuckoo’ or ‘inquiline’, for example, as search terms. We next used these two taxonomic groupings of papers (n = 1533) to be confident that we were capturing appropriate papers to build the co-citation network. Here, we plot the top 10% of articles that were cited most commonly with others for visual clarity (figure 1). As expected, we can see clear subfields of brood parasitism research that largely align with taxonomic groupings identified by assigning each publication to the main taxa it described (figure 1). Where co-citation occurred between subfields (grey lines in figure 1), these involved a handful of review papers comparing insects and birds (e.g. [31,49]), reviews of the well-studied common cuckoo [6,50] or early work on arms races and coevolution [4,51].
Figure 1.
Co-citation network of brood parasitism publications. The top 10% of co-cited documents from a Scopus search are plotted using a Fruchterman layout; nodes represent co-cited documents (scaled by betweenness centrality) with key publications labelled (first author and year published given below, all plotted publications listed in Supplementary Table 1), and edges represent co-citations (thickness indicates frequency, only edges > 5 are plotted). Node and edge colour represent communities assigned by a walktrap clustering algorithm, and pale grey edges represent links among these communities. Inset photos show the main taxa associated with the coloured co-citation communities: (a) host Temnothorax longispinosus (right) defends nest from slave-maker T. americanus (left) (S. Foitzik); (b) Eurasian reed warbler, Acrocephalus scirpaceus, feeds a common cuckoo Cuculus canorus chick (W. LiQiang/Shutterstock); (c) Shiny cowbird Molothrus bonariensis begs in the nest with chalk-browed mockingbird Mimus saturninus host nest-mates (R. Gloag). The green community to the left represents coevolutionary theory and integrative reviews.
3. Integrating brood parasitism research
Taking an integrative approach to address key questions in biology is not new, but it is currently experiencing a renaissance [52–54]. In part, this is because addressing questions from multiple perspectives should provide a more comprehensive understanding of what can, and cannot, evolve [53,55]. As well as asking both proximate and ultimate questions about the same suite of traits, different taxa can also provide different windows into the same strategy. Despite the obvious life-history differences, there are many points of comparison between brood parasitism in different systems, and comparative work has a rich potential to identify general principles. For example, Kilner & Langmore's 2011 review [31] integrating coevolutionary studies of brood-parasitic birds and insects was able to derive general hypotheses about why host defences differ so markedly across both taxa. They proposed that the relative balance of strategy facilitation (whereby one form of defence promotes another) and strategy blocking (whereby one form of defence relaxes selection on an another) may explain this diversity, and predicted which general ecological conditions should drive different coevolutionary trajectories in both birds and insects. Nevertheless, these ideas remain untested; we hope this special issue will increase researcher dialogue across taxonomic boundaries. We have brought together research and reviews on brood parasitism in birds, insects and fish that address complementary questions. These studies cover three key themes that are common to brood parasitism, regardless of the study system.
(a). Adaptations for (and against) deception
All brood parasites must deceive their host to successfully usurp resources, either by avoiding detection during nest invasion (e.g. both common cuckoos and cuckoo wasps time parasitism events for when hosts are less likely to be active at the nest, see [24], this issue) or by avoiding recognition if detected (e.g. many insect brood parasites rely on acquiring chemical signatures of their hosts to reduce aggression; see reviews in this issue [22–24]). Understanding which adaptations arise requires knowledge of reciprocal adaptations in host defence, as these alter and determine the strength of selection acting on brood parasites (e.g. cuckoo finch Anomalospiza imberbis hosts appear sensitive to higher-level pattern features of alien eggs, implying that selection acts on parasites to mimic these, see [56] in this issue). Similarly, elucidating the mechanisms that underpin such adaptations is critical, as these can constrain the direction of evolutionary pathways of both parasite (see contributions by Litman [23] and Cotter et al. [24] in this issue) and host (see Yang et al. [57] and Spottiswoode & Busch [58] in this issue). New technologies and modelling approaches have led to a recent surge in research in the mechanisms underlying brood-parasite and host coevolution, such as sensory systems, cognition, development and genetics. In this special issue, these advances are highlighted by Kaur et al. [59], who demonstrate how studies of gene expression can provide clues as to how parasites manipulate host defence, while Stoddard et al. [56] apply new mathematical models of pattern matching to reveal new depth in egg recognition by avian hosts, and Hanley et al. [60] use visual modelling to show surprising sensory biases in whether hosts decide to reject a foreign egg. This new wave of mechanistic research has enabled a step-change in our understanding of how parasite and host adaptations coevolve.
(b). Diversity and predictability of coevolution
Coevolution between brood parasites and hosts occurs across a variety of degrees of phylogenetic distance; a parasite and its host can come from either a different order (e.g. birds: cuculiform cuckoos versus passeriform hosts, insects: Maculinea butterflies versus Myrmica ant hosts), a different family (e.g. birds: icterid cowbirds versus parulid warbler hosts, insects: cuckoo wasps targeting solitary bees) or different genera within the same family (e.g. Vidua finches versus estrildid finch hosts; inquiline ants that parasitize sister species [22,61]). Brood parasitism is also diverse in its degree of specialism, with some parasites specializing on a single host species (as in Vidua finches, and many inquilines), and others (such as the brown-headed cowbird Molothrus ater, and Maculinea butterflies) using multiple host species. There is often also variation within generalist species across a parasite's range, such that a host species is heavily parasitized in one locale, but little or never targeted in another, setting the ecological stage for possible geographical mosaics of coevolution that may help to explain otherwise puzzling variation in coevolutionary sophistication [62]. Insights into predictability of brood-parasitic systems may then be gained by comparing the different evolutionary routes by which parasites arise from non-parasitic ancestors across taxonomic groups, the extent to which parasites and hosts vary ecologically across populations and how divergent coevolution among such populations may drive diversification [49]. Research at the coevolutionary interface between ecology and evolution is becoming more important as environments change (e.g. [63]). In this issue, for example, Suhonen et al. [64] use a comparative approach to identify bumblebees, ants and wasps that may play host to brood parasites, many of which are species of conservation concern. Tartally et al. [65] examine the spatial mosaic in host use across Europe by brood-parasitic Maculinea butterflies, and shed light on the role of host switches and local extinctions in the regional persistence of this spectacular genus.
(c). Windows into social evolution
Brood parasitism in any taxonomic group is a derived behaviour of parental care. Therefore, understanding how cheating by brood parasites evolves requires knowledge of the costs and benefits of providing parental care (see [24], this issue), and who pays these costs (see [30], this issue). Any social behaviour is vulnerable to a cheater phenotype, and answering the question of what keeps costly social behaviours, especially social cooperation, evolutionarily stable is of broad relevance in biology. Can brood parasitism provide insight into the evolution of other life-history strategies? In this special issue, Cini et al. [22] consider this for sociality, Gloag & Beekman [30] for inclusive fitness and Riehl & Feeney [14] for cooperative breeding. These studies focus on the brood parasites of social insects and/or birds, but brood-parasitic cuckoo catfish may provide new avenues for similar work if we can experimentally modify the amount of care, or paternity certainty, of cichlid host males (a point argued by Polacik et al. [17] in this issue). In the final paper of this special issue, Cotter et al. [24] use the concept of host defences as a social good to ask whether viewing brood parasitism through the lens of social immunity can help to inform our understanding of social defences.
4. Tinbergen's ‘cuckoos’
Over 50 years ago, Tinbergen [66] published his landmark paper that provided a framework for integrative studies into behaviour. Here, he suggested that to fully comprehend how and why a trait evolves, we must address ‘Four questions’ regarding: (i) the mechanisms that facilitate the trait, (ii) the developmental environment that alters expression of the trait, (iii) the fitness consequences of a trait, and (iv) the similarities and differences of the trait across a phylogeny (also see [67]). Arguably, research into brood-parasite evolution has focused mostly on fitness consequences (that is, ‘Question Three’), a bias that is not unusual in the study of animal behaviour ([53,68]). This has led to great advances in our understanding of the requisite adaptations of brood parasites and counter-adaptations for host defences (e.g. birds [8], ants [69], bees [70], wasps [71]). Comparatively less attention has been given to understanding these adaptations from a mechanistic viewpoint, the role of the developmental environment in shaping adaptations, or how they vary across species and time, although recent research trends suggest this is changing. Questions of development in particular are becoming ever more timely, alongside our increasing appreciation for the role of phenotypic plasticity and learning in the evolutionary process [72–74], and in the context of the pressing need to understand and predict how populations will respond to rapid environmental change [75]. For brood parasitism research, therefore, the time seems ripe to revisit Tinbergen's proposed framework. The studies and reviews in this special issue all cover one or more of Tinbergen's Four Questions (table 1); for example, McClelland et al. [76] demonstrate how combining analyses of mechanisms across species sheds light on the traits that may make brood-parasitic birds successful; Cohen et al. [18] examine the ontogeny of brood-parasitic catfish and non-parasitic congeners to show that advanced development in this system is not an adaptation for parasitism, as we might expect if we only compared it against its host; and Medina & Langmore [77] link field experiments with evolutionary comparative analyses across hosts of brood-parasitic birds to test how population density influences fitness. We hope this encourages future research that integrates mechanism, development and phylogeny with the fitness consequences of traits to understand brood parasitism evolution.
Table 1.
Contributions to this special issue according to Tinbergen's Four Questions framework [54] for integrative studies (summaries of each question from [55]) and examples of broad questions in each category that inform our understanding of the coevolutionary biology of brood parasitism. Note that several contributions address more than one question and so appear more than once in the table.
| ‘4 Questions’ | Special issue contributions | Example research questions: |
|---|---|---|
|
(1) Mechanism “How does it work?” |
Stoddard et al. [56] Yang et al. [57] Kaur et al. [59] Hanley et al. [60] McClelland et al. [76] |
What cognitive rules do hosts use to distinguish kin from non-kin?
What molecular mechanisms underpin parasite adaptations? How are host defences constrained by sensory mechanisms? |
|
(2) Development “How does it develop?” |
Cohen et al. [18] Kaur et al. [59] McClelland et al. [76] |
Does rearing environment influence plasticity of defences? Do parasites learn to recognise suitable hosts during development via imprinting? How do brood parasites overcome developmental constraints? |
|
(3) Function “What is it for?” |
Polacik et al. [17] Litman [23] Yang et al. [57] Spottiswoode & Busch [58] Kaur et al. [59] Tartally et al. [65] Medina & Langmore [77] |
What adaptations are necessary for parasites to succeed?
How do parasites differ in morphology, behaviour and physiology to non-parasites? Why do counter-adaptations used by hosts to defend against parasites vary? Does hosting a brood parasite affect life-time reproductive success? |
|
(4) Evolution “How did it evolve?” |
Riehl & Feeney [14] Cohen et al. [18] Cini et al. [22] Gloag & Beekman [30] Cotter et al. [24] Suhonen et al. [64] McClelland et al. [76] Medina & Langmore [77] |
How readily can parasites switch hosts? Are the outcomes of coevolutionary arms’ races predictable? Can inclusive fitness theory predict the evolution of parasitism? What are the evolutionary origins of brood parasitism? |
5. Conclusion
For many of us, brood parasitism is the perfect marriage of natural history and evolutionary biology. Yet, at present, natural history is arguably more limiting to our efforts to understand coevolution than either ideas or methods. This is because the best way to test our current understanding is to validate it in diverse natural systems. In the case of birds, a recent explosion of studies in previously little-known systems has demonstrated this truth, by challenging some long-standing ideas (for example, that chick rejection cannot evolve [78–80]), and supporting others (for example, the role of maternal inheritance in the faithful transmission of parasitic specialization [81–83]). The systems enjoying most new attention are tropical and south-temperate species in Asia, Australasia, Africa and South America, where selection pressures are often quite different from those of the classic avian systems of the northern hemisphere owing in part to longer reproductive lives and opportunities for learning that likely shift the costs and benefits of defensive decisions in any one breeding attempt. New natural history has similar potential in non-avian systems. For example, the past decade has seen the discovery of several new species of inquilines of Neotropical attine fungus-growing ants [84,85], including one in the process of speciating from its host [86]. These have provided new opportunities to test theories of inquiline evolution [86]. Looking ahead, we hope that adventurous biologists continue to uncover the natural history of the many brood parasites about which tantalizingly little remains known, and perhaps to even discover brood parasitism for the first time in new taxa.
Supplementary Material
Acknowledgements
We thank Mason Youngblood for discussion about bibliometric analyses, and Nick Davies and Unni Pulliainen for providing feedback on the introduction, the contributors to this special issue for engaging with the task of thinking broadly about their work and the reviewers who improved the quality of this special issue. Finally, we are grateful to Helen Eaton, Senior Commissioning Editor at Philosophical Transactions B, for her patience and support during the preparation of this issue.
Data accessibility
Search results used for the bibliometric analysis are available in the electronic supplementary material.
Authors' contributions
R.T. conceived and carried out the bibliometric analysis; all authors wrote the manuscript.
Competing interests
We have no competing interests.
Funding
R.T. was supported by an Independent Research Fellowship from the Natural Environment Research Council (NE/K00929X/1) and a start-up grant from the Helsinki Institute of Life Science (HiLIFE), University of Helsinki. R.G. was supported by a University of Sydney Postdoctoral Fellowship. C.N.S. was supported by a BBSRC David Phillips Research Fellowship (BB/J014109/1). S.J.P. was funded by a Research Project Grant from The Leverhulme Trust (RPG-2018-332).
References
- 1.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Schulze-Hagen K, Stokke BG, Birkhead TR. 2008. Reproductive biology of the European Cuckoo Cuculus canorus: early insights, persistent errors and the acquisition of knowledge. J. Ornithol. 150, 1–16. ( 10.1007/s10336-008-0340-8) [DOI] [Google Scholar]
- 3.Lai C. 1998. Messenger of spring and morality: cuckoo lore in Chinese sources. J. Am. Orient. Soc. 118, 530–542. ( 10.2307/604785) [DOI] [Google Scholar]
- 4.Dawkins R, Krebs JR. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511. ( 10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 5.Thompson JN. 2013. Relentless evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 6.Davies NB. 2000. Cuckoos, cowbirds and other cheats. London, UK: Poyser. [Google Scholar]
- 7.Klug H, Bonsall MB. 2010. Life history and the evolution of parental care. Evolution 64, 823–835. ( 10.1111/j.1558-5646.2009.00854.x) [DOI] [PubMed] [Google Scholar]
- 8.Soler M. 2014. Long-term coevolution between avian brood parasites and their hosts. Biol. Rev. 89, 688–704. ( 10.1111/brv.12075) [DOI] [PubMed] [Google Scholar]
- 9.Feeney WE, Welbergen JA, Langmore NE. 2014. Advances in the study of coevolution between avian brood parasites and their hosts. Annu. Rev. Ecol. Evol. Syst. 45, 227–246. ( 10.1146/annurev-ecolsys-120213-091603) [DOI] [Google Scholar]
- 10.Soler M. 2017. Avian brood parasitism: behaviour, ecology, evolution and coevolution. Berlin, Germany: Springer. [Google Scholar]
- 11.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 12.Sorenson MD, Payne RB. 2002. Molecular genetic perspectives on avian brood parasitism. Integr. Comp. Biol. 42, 388–400. ( 10.1093/icb/42.2.388) [DOI] [PubMed] [Google Scholar]
- 13.Feeney WE, Medina I, Somveille M, Heinsohn R, Hall ML, Mulder RA, Stein JA, Kilner RM, Langmore NE. 2013. Brood parasitism and the evolution of cooperative breeding in birds. Science 342, 1506–1508. ( 10.1126/science.1240039) [DOI] [PubMed] [Google Scholar]
- 14.Feeney WE, Riehl C. 2019. Monogamy without parental care? Social and genetic mating systems of avian brood parasites. Phil. Trans. R. Soc. B 374, 20180201 ( 10.1098/rstb.2018.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies NB. 2011. Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. ( 10.1111/j.1469-7998.2011.00810.x) [DOI] [Google Scholar]
- 16.Sato T. 1986. A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323, 58–59. ( 10.1038/323058a0) [DOI] [PubMed] [Google Scholar]
- 17.Polačik M, Reichard M, Smith C, Blažek R. 2019. Parasitic cuckoo catfish exploit parental responses to stray offspring. Phil. Trans. R. Soc. B 374, 20180412 ( 10.1098/rstb.2018.0412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen MS, Hawkins MB, Stock DW, Cruz A. 2019. Early life-history features associated with brood parasitism in the cuckoo catfish, Synodontis multipunctatus (Siluriformes: Mochokidae). Phil. Trans. R. Soc. B 374, 20180205 ( 10.1098/rstb.2018.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crump ML. 1996. Parental care among the amphibia. Adv. Study Behav. 25, 109–144. ( 10.1016/S0065-3454(08)60331-9) [DOI] [Google Scholar]
- 20.Brown JL, Morales V, Summers K. 2009. Tactical reproductive parasitism via larval cannibalism in Peruvian poison frogs. Biol. Lett. 5, 148–151. ( 10.1098/rsbl.2008.0591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gans C. 1996. An overview of parental care among the Reptilia. Adv. Study Behav. 25, 145–160. ( 10.1016/S0065-3454(08)60332-0) [DOI] [Google Scholar]
- 22.Cini A, Sumner S, Cervo R. 2019. Inquiline social parasites as tools to unlock the secrets of insect sociality. Phil. Trans. R. Soc. B 374, 20180193 ( 10.1098/rstb.2018.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litman JR. 2019. Under the radar: detection avoidance in brood parasitic bees. Phil. Trans. R. Soc. B 374, 20180196 ( 10.1098/rstb.2018.0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotter SC, Pincheira-Donoso D, Thorogood R. 2019. Defences against brood parasites from a social immunity perspective. Phil. Trans. R. Soc. B 374, 20180207 ( 10.1098/rstb.2018.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon BE, Eadie JM. 2008. Conspecific brood parasitism in birds: a life-history perspective. Annu. Rev. Ecol. Evol. Syst. 39, 343–363. ( 10.1146/annurev.ecolsys.39.110707.173354) [DOI] [Google Scholar]
- 26.Barbero F, Thomas JA, Bonelli S, Balletto E, Schönrogge K. 2009. Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323, 782–785. ( 10.1126/science.1163583) [DOI] [PubMed] [Google Scholar]
- 27.York JE, Davies NB. 2017. Female cuckoo calls misdirect host defences towards the wrong enemy. Nat. Ecol. Evol. 1, 1520–1525. ( 10.1038/s41559-017-0279-3) [DOI] [PubMed] [Google Scholar]
- 28.Kilner RM, Noble DG, Davies NB. 1999. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature 397, 667–672. ( 10.1038/17746) [DOI] [Google Scholar]
- 29.Strausberger BM, Rothstein SI. 2009. Parasitic cowbirds may defeat host defense by causing rejecters to misimprint on cowbird eggs. Behav. Ecol. 20, 691–699. ( 10.1093/beheco/arp042) [DOI] [Google Scholar]
- 30.Gloag R, Beekman M. 2019. The brood parasite's guide to inclusive fitness theory. Phil. Trans. R. Soc. B 374, 20180198 ( 10.1098/rstb.2018.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. Camb. Philos. Soc. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
- 32.Youngblood M, Lahti D. 2018. A bibliometric analysis of the interdisciplinary field of cultural evolution. Palgrave Commun. 4, 120 ( 10.1057/s41599-018-0175-8) [DOI] [Google Scholar]
- 33.Aria M, Cuccurullo C. 2017. bibliometrix: an R-tool for comprehensive science mapping analysis. J. Informetr. 11, 959–975. ( 10.1016/j.joi.2017.08.007) [DOI] [Google Scholar]
- 34.R Core Team. 2018. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- 35.Small H. 1973. Co-citation in the scientific literature: a new measure of the relationship between two documents J. Am. Soc. Inf. Sci. 24, 265–269. ( 10.1002/asi.4630240406) [DOI] [Google Scholar]
- 36.Trujillo CM, Long TM. 2018. Document co-citation analysis to enhance transdisciplinary research. Sci. Adv. 4, e1701130 ( 10.1126/sciadv.1701130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourke AFG, Franks NR.. 1991. Alternative adaptations, sympatric speciation and the evolution of parasitic, inquiline ants. Biol. J. Linn. Soc. 43, 157–178. ( 10.1111/j.1095-8312.1991.tb00591.x) [DOI] [Google Scholar]
- 38.Buschinger A. 1986. Evolution of social parasitism in ants. Trends Ecol. Evol. 1, 155–160. ( 10.1016/0169-5347(86)90044-3) [DOI] [PubMed] [Google Scholar]
- 39.Wilson EO. 1971. The insect societies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 40.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 41.Darwin CR. 1859. On the origin of species. London, UK: John Murray. [Google Scholar]
- 42.Davies NB, Brooke M.. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 58, 225–236. ( 10.2307/4996) [DOI] [Google Scholar]
- 43.Rothstein SI. 1990. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508. [Google Scholar]
- 44.Rothstein SI. 1975. Evolutionary rates and host defenses against avian brood parasitism. Am. Nat. 109, 161–176. ( 10.1086/282984) [DOI] [Google Scholar]
- 45.Friedmann H. 1929. The cowbirds: a study in the biology of social parasitism. Springfield, IL: CC Thomas. [Google Scholar]
- 46.Burnham K, Anderson D.. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer-Verlag. [Google Scholar]
- 47.Brittingham MC, Temple SA.. 1983. Have cowbirds caused forest songbirds to decline? Bioscience 33, 31–35. ( 10.2307/1309241) [DOI] [Google Scholar]
- 48.Robinson SK, Thompson FR, Donovan TM, Whitehead DR, Faaborg J.. 1995. Regional forest fragmentation and the nesting success of migratory birds. Science 267, 1987–1990. ( 10.1126/science.267.5206.19878787) [DOI] [PubMed] [Google Scholar]
- 49.Davies NB, Bourke AFG, Brooke M. 1989. Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 4, 274–278. ( 10.1016/0169-5347(89)90202-4) [DOI] [PubMed] [Google Scholar]
- 50.Wyllie I. 1981. The cuckoo. New York, NY: Universe Books. [Google Scholar]
- 51.Thompson JN. 1994. The coevolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 52.Wake MH. 2003. What is ‘integrative biology’? Integr. Comp. Biol. 43, 239–241. ( 10.1093/icb/43.2.239) [DOI] [PubMed] [Google Scholar]
- 53.Aubin-Horth N. 2016. Using an integrative approach to investigate the evolution of behaviour. Evol. Appl. 9, 166–180. ( 10.1111/eva.12300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDougall-Shackleton SA. 2011. The levels of analysis revisited. Phil. Trans. R. Soc. B 366, 2076–2085. ( 10.1098/rstb.2010.0363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stearns SC, Magwene P. 2003. The naturalist in a world of genomics. Am. Nat. 161, 171–180. [DOI] [PubMed] [Google Scholar]
- 56.Stoddard MC, Hogan BG, Stevens M, Spottiswoode CN. 2019. Higher-level pattern features provide additional information to birds when recognizing and rejecting parasitic eggs. Phil. Trans. R. Soc. B 374, 20180197 ( 10.1098/rstb.2018.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C, Liang W, Møller A. 2019. Egg retrieval versus egg rejection in cuckoo hosts. Phil. Trans. R. Soc. B 374, 20180200 ( 10.1098/rstb.2018.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spottiswoode CN, Busch R. 2019. Vive la difference! Self/non-self recognition and the evolution of signatures of identity in arms races with parasites. Phil. Trans. R. Soc. B 374, 20180206 ( 10.1098/rstb.2018.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaur R, Stoldt M, Jongepier E, Feldmeyer B, Menzel F, Bornberg-Bauer E, Foitzik S. 2019. Ant behaviour and brain gene expression of defending hosts depend on the ecological success of the intruding social parasite. Phil. Trans. R. Soc. B 374, 20180192 ( 10.1098/rstb.2018.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanley D, López AV, Fiorini VD, Reboreda JC, Grim T, Hauber ME. 2019. Variation in multicomponent recognition cues alters egg rejection decisions: a test of the optimal acceptance threshold hypothesis. Phil. Trans. R. Soc. B 374, 20180195 ( 10.1098/rstb.2018.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang MH, Dornhaus A. 2008. A meta-analysis of ant social parasitism: host characteristics of different parasitism types and a test of Emery's rule. Ecol. Entomol. 33, 589–596. ( 10.1111/j.1365-2311.2008.01005.x) [DOI] [Google Scholar]
- 62.Thompson JN. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 63.Péron G, Altwegg R, Jamie GA, Spottiswoode CN. 2016. Coupled range dynamics of brood parasites and their hosts responding to climate and vegetation changes. J. Anim. Ecol. 85, 1191–1199. ( 10.1111/1365-2656.12546) [DOI] [PubMed] [Google Scholar]
- 64.Suhonen J, Ilvonen JJ, Nyman T, Sorvari J. 2019. Brood parasitism in eusocial insects (Hymenoptera): role of host geographical range size and phylogeny. Phil. Trans. R. Soc. B 374, 20180203 ( 10.1098/rstb.2018.0203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tartally A, et al. 2019. Patterns of host use by brood parasitic Maculinea butterflies across Europe. Phil. Trans. R. Soc. B 374, 20180202 ( 10.1098/rstb.2018.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tinbergen N. 1963. On aims and methods of ethology. Z. Tierpsychol. 20, 410–433. ( 10.1111/j.1439-0310.1963.tb01161.x) [DOI] [Google Scholar]
- 67.Bateson P, Laland KN. 2013. Tinbergen's four questions: an appreciation and an update. Trends Ecol. Evol. 28, 712–718. ( 10.1016/j.tree.2013.09.013) [DOI] [PubMed] [Google Scholar]
- 68.Bolhuis JJ, Verhulst S. 2009. Tinbergen's legacy: function and mechanism in behavioral biology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 69.Buschinger A. 2009. Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol. News 12, 219–235. [Google Scholar]
- 70.Cardinal S, Straka J, Danforth BN. 2010. Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism. Proc. Natl Acad. Sci. USA 107, 16 207–16 211. ( 10.1073/pnas.1006299107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cervo R. 2006. Polistes wasps and their social parasites: an overview. Ann. Zool. Fennici 43, 531–549. ( 10.2307/23736760) [DOI] [Google Scholar]
- 72.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 73.Mason PA. 2016. On the role of host phenotypic plasticity in host shifting by parasites. Ecol. Lett. 19, 121–132. ( 10.1111/ele.12555) [DOI] [PubMed] [Google Scholar]
- 74.Whiten A. 2017. A second inheritance system: the extension of biology through culture. Interface Focus 7, 20160142 ( 10.1098/rsfs.2016.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McClelland SC, Jamie GA, Waters K, Caldas L, Spottiswoode CN, Portugal SJ. 2019. Convergent evolution of reduced eggshell conductance in avian brood parasites. Phil. Trans. R. Soc. B 374, 20180194 ( 10.1098/rstb.2018.0194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medina I, Langmore NE. 2019. Host density predicts the probability of parasitism by avian brood parasites. Phil. Trans. R. Soc. B 374, 20180204 ( 10.1098/rstb.2018.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lotem A. 1993. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature 362, 743–745. ( 10.1038/362743a0) [DOI] [Google Scholar]
- 79.Langmore NE, Hunt S, Kilner RM. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160. ( 10.1038/nature01460) [DOI] [PubMed] [Google Scholar]
- 80.Sato NJ, Tokue K, Noske RA, Mikami OK, Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69. ( 10.1098/rsbl.2009.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Punnett RC. 1933. Inheritance of egg-colour in the ‘parasitic’ cuckoos. Nature 132, 892–893. ( 10.1080/0300443032000088212) [DOI] [Google Scholar]
- 82.Spottiswoode CN, Stryjewski KF, Quader S, Colebrook-Robjent JFR, Sorenson MD. 2011. Ancient host specificity within a single species of brood parasitic bird. Proc. Natl Acad. Sci. USA 108, 17 738–17 742. ( 10.1073/pnas.1109630108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fossøy F, et al. 2016. Ancient origin and maternal inheritance of blue cuckoo eggs. Nat. Commun. 7, 10272 ( 10.1038/ncomms10272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rabeling C, Bacci M Jr. 2010. A new workerless inquiline in the Lower Attini (Hymenoptera: Formicidae), with a discussion of social parasitism in fungus-growing ants. Syst. Entomol. 35, 379–392. ( 10.1111/j.1365-3113.2010.00533.x) [DOI] [Google Scholar]
- 85.De Souza DJ, Soares IMF, Della Lucia TMC. 2007. Acromyrmex ameliae sp. n. (Hymenoptera: Formicidae): a new social parasite of leaf-cutting ants in Brazil. Insect Sci. 14, 251–257. ( 10.1111/j.1744-7917.2007.00151.x) [DOI] [Google Scholar]
- 86.Rabeling C, Schultz TR, Pierce NE, Bacci M. 2014. A social parasite evolved reproductive isolation from its fungus-growing ant host in sympatry. Curr. Biol. 24, 2047–2052. ( 10.1016/j.cub.2014.07.048) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Search results used for the bibliometric analysis are available in the electronic supplementary material.