Abstract
In arms races with parasites, hosts can evolve defences exhibiting extensive variability within populations, which signals individual identity (‘signatures’). However, few such systems have evolved, suggesting that the conditions for their evolution are uncommon. We review (a) polymorphic egg markings that allow hosts of brood-parasitic birds to recognize and reject parasitic eggs, and (b) polymorphic tissue antigens encoded in the major histocompatibility complex (MHC), which present self- and pathogen-derived peptides to T cells of the immune system. Despite the profound differences between these systems, they share analogous features: (i) self/non-self discrimination by a highly specific recognition system (bird eyes and T-cell antigen receptor, respectively), which antagonists may escape by evolving evasion or mimicry; (ii) a self substrate upon which diversifying selection can act (eggs, and MHC molecules); (iii) acquired knowledge of self (resulting in acceptance of own eggs, and immune tolerance); and (iv) fitness costs associated with attack on self or lack of parasite detection. We suggest that these features comprise a set of requirements for parasites to drive the evolution of identity signatures in hosts, which diminish the likelihood of recognition errors. This may help to explain the variety of trajectories arising from arms races in different antagonistic contexts.
This article is part of the theme issue ‘The coevolutionary biology of brood parasitism: from mechanism to pattern’.
Keywords: negative frequency-dependent selection, host/parasite arms races, avian egg pattern diversification, brood parasites, major histocompatibility complex, T-cell antigen presentation
1. Introduction
Individuals of the same species often have detectably distinct phenotypes, as a result of selection to signal individual identity [1]. This commonly arises from the benefits of signalling identity to conspecifics, such as kin or members of a social group. However, selection for individual distinctiveness may also arise from the benefits of signalling identity to self, to avoid costs of exploitation by other species [2]. This review compares how antagonistic coevolution has shaped individual distinctiveness in two different types of host defence against parasites: the rejection of eggs by the hosts of avian brood parasites, and the recognition of pathogens by the adaptive immune system of vertebrates.
Hosts can enhance their ability to recognize a mimetic enemy by signalling their identity to themselves [2]. The more reliably they can signal their own identity, the greater the probability that they can detect an informative difference between their own phenotype and that of an antagonistic mimic. Initially, hosts' phenotypes may evolve away from those of parasitic mimics in a directional manner [3]. When mimics catch up, selection may drive the hosts to diversify their phenotypes among individuals, sometimes to the point that hosts evolve individually distinct phenotypes that distinguish most individuals within a population from one another. This hypothesis was first proposed exactly 100 years ago by Charles Swynnerton [2, p. 145], inspired by his studies of brood parasitism in the birds of present-day Zimbabwe: ‘I doubt whether [mimicry] would always end the matter, for, when a Cuckoo's egg became indistinguishable from its host's, variation in the latter would still afford the means of distinguishing it from the Cuckoo's, and it is even imaginable that a race may in some cases have taken place between the host's eggs and those of the overtaking Cuckoo.’ Swynnerton clearly perceived the evolutionary outcome: ‘High distinctiveness might sometimes have been the result.’ [2, p. 145]. Such genetically-based signals of identity are often termed ‘signatures’ [1,4,5], which we apply to denote any biological system that exhibits sufficient diversity to make genetic or phenotypic identity of two unrelated individuals within a population a rare event. Selection for signatures also occurs in social settings where individuals signal their identity towards conspecifics [1], and is distinct from other forms of negative frequency-dependent selection in that it involves very high variation within a population, rather than a small number of discrete morphs, each pursuing a particular strategy [6]. However, other systems of parasite defence have evolved less inter-individual genetic and phenotypic diversity in hosts, prompting the question of which essential features are shared by those defensive systems that exhibit signature-like individual distinctiveness.
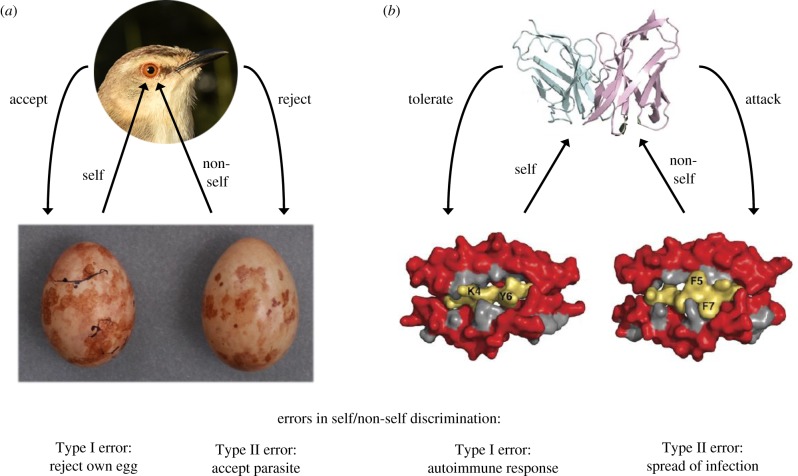
Here, we argue that self/non-self discrimination is a key mechanism that drives individual distinctiveness in two biological systems that have been shaped by antagonistic coevolution with parasites. The first type of distinctiveness is found in the unique colours and patterns of bird eggs in many species that are subject to brood parasitism (figure 1a); the second comprises the polymorphic antigen-presenting molecules of the major histocompatibility complex (MHC) of the vertebrate adaptive immune system (figure 1b). Both systems have evolved a high degree of individual diversity within populations. MHC molecules are present in all vertebrate species except the jawless fish [9] and are involved in cellular adaptive immune defences against various viral, bacterial and fungal parasites. Unlike bird egg signatures, the molecular biology of MHC proteins is well-understood thanks to research on their roles in transplant rejection, immune responses and immune-related diseases [10]. As in brood parasites and their hosts, the adaptive immune system of vertebrates is in an evolutionary arms race with parasites; hosts win an interaction if they clear the infection, and parasites win if they succeed in replicating and spreading at a cost to the host (i.e. infectious disease). In both systems, defensive action on the part of the host has to minimize the risks of mistaken attack against self (a false hit, analogous to a ‘Type I’ error in statistics) and of failure to respond effectively to dangerous non-self (a miss, or ‘Type II’ error), by failing to detect a parasite or by detecting it but misidentifying it as self.
Figure 1.
Functionally analogous components of self/non-self discrimination in parasite defence mechanisms that exhibit signature polymorphism. Bird eggs (a) are compared with MHC-associated T-cell antigens (b). The recognition system is shown at the top; the polymorphic component used for self/non-self discrimination is shown at the bottom, alongside its foreign counterpart. Recognition is achieved by visual colour and pattern detection (in a) or by measurement of receptor signal strength based on molecular binding affinities or kinetics (in b) and translated into a binary decision to mount a defence, or not. In both systems, the capacity for self/non-self discrimination is acquired through a learning process, which involves recognition of self, before it is tested by confrontation with non-self. The discrimination is subject to error that imposes a fitness cost; the error rate is reduced by signature-like individual variability in the self component. Panel (a) shows a tawny-flanked prinia Prinia subflava host female comparing an own egg to a parasitic cuckoo finch Anomalospiza imberbis egg; photos by C.N.S., egg photos previously published in [7]. Panel (b) was modified from [8]; single-letter amino acid codes show amino acid residues in the two peptides that are important for self/non-self discrimination. (Online version in colour.)
We review these two systems with respect to (a) the defensive function of host signatures; (b) how hosts learn their own signatures, and so how they distinguish self from non-self; (c) the genetic basis of host signatures; and (d) the drivers of, and constraints on, their diversification. We then propose a minimum set of analogous features in these mechanisms that drives signatures of individual identity to evolve in antagonistic coevolution.
2. Visual signatures of identity in hosts of avian brood parasites
(a). Defensive function of host egg signatures
Avian brood parasites exploit the parental care of other individuals by laying their eggs in other birds' nests, leaving the host parents to bear the cost of raising their young [11]. Hosts may be conspecific or heterospecific, but here we focus on the latter, since conspecific brood parasites may experience qualitatively different selection pressures (see §4d). Hosts defend themselves by visually recognizing and rejecting foreign eggs from their nests, and this is specifically an adaptation to parasitism since species without an evolutionary history of parasitism lack such discrimination [12]. In turn, host discrimination selects for parasitic mimicry of host eggs with respect to visual traits such as colour and pattern [11], and stronger discrimination selects for better mimicry [13]. In response to better mimicry, selection can favour hosts with an enhanced ability to discriminate small differences between eggs [14,15], but an alternative or complementary route to enhanced discrimination is to lay eggs that differ in appearance from those of the parasite [2,4]. Such diversification of egg phenotypes has evolved in unrelated groups of hosts of at least five of the seven independently evolved clades of brood parasites [16,17]. In some hosts, this variation comprises simple alternative colour morphs (typically blue versus white) [18,19], whereas in others there is near-continuous, signature-like variation in egg colour and pattern markings [15,20,21]. There is compelling evidence that this variation can be an adaptation specifically to brood parasitism, since formerly parasitized populations lose variation when they escape parasitism [22], and since field experiments confirm that such variation facilitates host recognition and rejection of parasitic eggs [7,23,24] (note that some species unsuitable for brood parasitism also show a degree of pattern variation among females; this may strengthen the shell when calcium is limiting [25]). Correspondingly, some parasites have evolved at least partial mimicry of within-host variation [15,19,26], but hosts retain the upper hand: not only do signatures reduce the proportion of the host population potentially available for exploitation by any one parasitic female, but parasites appear unable to behaviourally target the subset they best mimic, resulting in frequent mismatches and rejection by hosts (e.g. [7,27,28]). This emphasizes the efficacy of signatures in aiding host defence.
At least three further features of egg signatures may enhance their defensive function. First, high consistency within clutches laid by the same host female should facilitate detection of a mimic [4,23,29]. Second, low correlation between different traits comprising egg signatures should maximize individual variation and hence a signature's information content, and so make it more difficult to mimic [15,21], in common with other signals of individual identity in nature [5,30]. Third, egg signatures should be recognizable by their hosts, which may not always equate to maximum signature variability [31].
(b). How do hosts know their own egg signatures?
A host parent needs to recognize its own eggs, so that it does not accidentally identify self as non-self and thus reject them [32–34]. Evidence from several host–parasite systems suggests that host parents do directly recognize eggs, rather than detecting a parasitic egg through its discordant appearance relative to the rest of the clutch, although the two mechanisms may also be used simultaneously (e.g. [35,36]). Importantly, hosts must actively acquire this knowledge through a learning process (analogous to acquired tolerance of self in immunological parlance), because eggshell appearance is a product of the mother's genotype. When variation in egg appearance is autosomally inherited, it is the product of a unique genotype inherited by the mother from both her parents. Therefore, it is hard to envisage a mechanism through which hosts could innately recognize their own eggs. Rather, self-recognition appears to be learnt from the first time a female breeds, such that inexperienced host females often fail to reject non-mimetic eggs, but improve with age [34,37]. This template can be memorized, since experienced females can reject foreign eggs without any of their own present for comparison [20,24,34]. In some species, males also reject eggs [38], and thus must also learn their mate's individual egg appearance. Comparison between a learnt template of ‘self’ and potential non-self is not the only factor involved in decision-making, since the threshold corresponding to whether a given visual difference results in egg rejection is often modulated by other cues of parasitism risk, such as the sight of adult parasites in the vicinity (e.g. [39,40]) and cues of risk socially learnt from conspecifics [41].
In summary, birds appear to be initially naive to their own egg appearance, and then to learn a template of their phenotype. They then compare potentially foreign eggs to this template to recognize parasitic eggs, and use this information, supplemented by additional cues of parasitism risk, to decide whether to reject an egg from the nest.
(c). What is the genetic basis of host egg signatures of identity?
While it is well-established that egg phenotypes in birds are strongly genetically based and consistent within an individual's lifetime [16] (see [42] for evidence of limited environmental effects), their precise genetic basis remains poorly understood. The biochemical basis of egg signatures (and parasitic forgeries) appears to be simple, since all egg coloration arises from just two kinds of porphyrin pigment [43,44]. Yet the diversity of pattern traits, and the lack of correlation between different components of egg phenotypes (colour and different pattern traits) [21], suggests that multiple genetic loci are involved in controlling their deposition. Current evidence generally points to these loci being positioned on the autosomes in hosts (but see [45]), as expected if they are under selection for high variability among host individuals; autosomal inheritance is also most likely to give rise to coevolutionary oscillations continuously generating signature-like variability [46]. The only study to date directly testing the genetic basis of egg phenotypes in a host species (village weavers Ploceus cucullatus) showed that inheritance of egg background colour was consistent with two alleles at each of two autosomal loci on different chromosomes (i.e. oligogenic control), whereas egg spotting pattern was likely to be under polygenic control [47]. Thus, the limited empirical evidence available suggests that the inheritance of defensive signatures in hosts is (i) autosomal, (ii) genetically unlinked (so that the inheritance of different aspects of patterning is uncorrelated) and (iii) polygenic.
(d). Host egg signatures: drivers and constraints
Excessively sensitive discrimination can lead to a host mistakenly identifying any slightly aberrant host egg of its own as parasitic and rejecting it (a ‘Type I’ error), which trades off against the risk of mistakenly accepting a parasitic egg (a ‘Type II’ error) [32,34]. The benefits of signatures in reducing the risk of Type II errors are well-established, and reviewed in §2a. The risk of Type I errors appears to vary among species, but many host species commonly reject their own eggs by accident, together with or instead of a parasitic egg [20,39,48], sometimes in the absence of any parasitic egg [49,50], and more often when the parasitic egg is a good mimic of the host clutch [39]. Egg signatures should therefore reduce the risk of both types of error, by driving apart the distributions of host and parasitic phenotypes, reducing the area of danger where they overlap.
However, egg signatures cannot diversify without limit. Mechanistic constraints arise because of the limited number of pigments involved in depositing egg colours and patterns [43]. Moreover, the shell gland (where colours and patterns are deposited on the egg) is likely limited in the range of spatial variation in pigment deposition (i.e. pattern) that it can produce, although this process is very poorly understood. In addition, natural selection may also temper the diversification of signatures if they carry costs. Such costs likely differ between host species depending on their natural history and that of the parasite, and thus may determine a host's eventual balance of strategies [15].
First, the risk of both types of errors may arise from imperfections in the learning process through which hosts know their own phenotype. Memory is costly [51], and learning imperfect because successive clutches will be non-identical [34]. Moreover, if a host is parasitized on its first breeding attempt, its learnt template will arise from a mix of host and parasitic eggs. Costs of a mixed clutch may be low if parasitic eggs are typically outnumbered by host eggs, such that the host's learnt template includes its own phenotype [52]. However, learning errors might be common in the hosts of species such as the cuckoo finch Anomalospiza imberbis, which commonly replace all host eggs in a clutch with their own [35]; at worst this could lead to zero lifetime reproductive success if it means that hosts reject all of their own eggs in future. Whether such costs may be elevated or reduced by more extreme signatures, and so shape their diversification, remains unknown.
Second, extreme phenotypes may not succeed in reducing the risk of errors if sensory processing constrains decision-making. Certain egg pattern traits may be highly distinctive and so objectively different from a parasite's egg, but difficult for birds to recognize, suggesting that an optimal signature is not necessarily the most complex [31].
Third, even if extreme phenotypes lower the risk of both types of error, signature diversification may be tempered by other sources of selection on egg appearance. For example, novel phenotypes may become susceptible to mimicry by other parasite species or by forms of the same parasite species (host-races or ‘gentes’) that have adapted to other hosts [26]. Alternatively, novel phenotypes may be poorly camouflaged or have poor thermal or photoprotective properties, which may account for the rapid loss of particular phenotypes when populations escape parasitism [53].
Finally, signatures only evolve when a parasitic egg is detected by the host, allowing recognition and discrimination to occur. If a parasitic egg is not detected at all (see §4), then mistaken acceptance of non-self can occur without favouring signature evolution, since there is no opportunity for discrimination favouring mimicry [54].
3. Molecular signatures of identity in the major histocompatibility complex
(a). Defensive function of major histocompatibility complex-encoded antigen-presenting molecules
The MHC gene region encodes glycoproteins with important functions in the presentation of antigens—unique structures of particular infectious agents—to T lymphocytes (T cells) of the immune system. MHC glycoproteins bind oligopeptides, which arise from the breakdown of pathogen-derived proteins by host cells (reviewed in [55]). The peptides occupy a single binding site on MHC molecules, and are thus displayed on the surface of the host cell (called an antigen-presenting cell) for inspection by T lymphocytes [56].
Individual T lymphocytes express unique receptors for antigens (TCR, for ‘T-cell antigen receptor’), as a result of unique rearrangements of TCR gene elements within each T cell as it develops [57]. TCRs that bind well enough to the complex of an MHC glycoprotein with a particular antigenic peptide trigger activation of T cells, enabling them to participate in immune defence. Thus, MHC molecules provide a mechanism that enables T cells, collectively, to detect evidence of infection (in the form of MHC-associated foreign peptides) on the surfaces of other host cells, and to initiate immune responses.
The ability of MHC molecules to bind diverse peptides is critical for antigen presentation. Any one MHC protein can bind tens of thousands of different peptides [58–60]. Moreover, the processing of particular protein antigens typically yields several peptides capable of binding any one MHC molecule. Two types of intermolecular contact control MHC/peptide binding: 2–4 side chains in the bound peptide interact with ‘specificity pockets’ within the peptide binding groove [61]; other peptide side chains do not interact and may therefore vary extensively. In addition, irrespective of its amino acid sequence, the peptide is tethered in the peptide-binding groove by conserved hydrogen bonds. Thus, MHC molecules can present a substantial proportion of possible peptides to T cells [62]. Additional factors besides MHC binding limit the presentation of MHC-bound antigens to a smaller number of ‘immunodominant’ peptides [63,64].
In summary, MHC proteins allow the immune system to detect a diversity of possible peptides. Importantly, however, none of these mechanisms allows MHC proteins to distinguish between peptides derived from pathogens, which are present only during infections (‘non-self’), and peptides arising all the time from the normal turnover of proteins within uninfected host cells throughout the body (‘self’).
(b). How does the immune system know its own major histocompatibility complex signatures?
Thus, the immune system (specifically its TCRs) is continuously confronted with self peptide/MHC complexes in the absence of infection, and must distinguish them from novel peptides that appear when pathogens invade. Moreover, the process of TCR gene rearrangement, which unfolds differently in each developing T cell, regularly produces TCRs that can recognize self peptide/MHC protein complexes with high affinity. T cells with such TCRs are at risk of becoming inappropriately activated by self peptides, triggering ‘autoimmune’ responses to uninfected, healthy tissues, rather than to pathogens. This poses a danger as the immune defences activated by T-cell responses damage not only pathogens, but also normal host tissues.
Several mechanisms normally ensure that T cells remain tolerant to self. Developing T cells are first confronted with MHC-associated self peptides on antigen-presenting cells as soon as they express TCRs for the first time, in the thymus [65]. At this stage of development, strongly self-reactive T cells are killed, removing their potential to cause autoimmune damage. The only T cells that survive and enter the circulation are those whose TCRs engage self peptide/MHC ligands with a low level of self-reactivity, which does not trigger the activation of damaging immune defences [66]. Only if an encounter with an MHC-presented foreign peptide results in a stronger TCR signal will the T cell be activated to fight infection. The unresponsiveness of T cells to self is reinforced by other means: to become fully activated, T cells must receive further proinflammatory signals from antigen-presenting cells; in the absence of these additional signals, they become unresponsive [67]. Moreover, self-reactive ‘regulatory’ T cells dampen the responses of effector T cells [66,68].
In summary, T cells possess some reactivity to MHC-presented self peptides, comprising a signature of self. Activation by non-self antigens involves a comparison between baseline signalling by self and stronger signalling by non-self, supplemented by additional cues that signal inflammation and tissue damage.
(c). What is the genetic basis of major histocompatibility complex signatures of identity?
The genetic basis of MHC glycoproteins is very well understood. They differ between individuals of any one vertebrate species, both owing to the presence of gene loci coding for distinct members of the MHC family, and from the presence of multiple alleles at each locus [69,70]. Broadly, MHC molecules can be sub-divided into two ‘classes’, class I and class II, which differ in their structure and geometry of peptide binding, their expression patterns, their intracellular site of peptide loading, and their ability to stimulate distinct T-cell subsets [55,71]. In the human MHC (also called ‘HLA’, for human leucocyte antigen), there are three loci, called HLA-A, -B, and -C, which code for polymorphic MHC class I molecules [69]. Hundreds of allelic variants at these loci exist in human populations worldwide [72]. Most individuals are, as a result, heterozygous and can express up to six different MHC class I gene products. Their main function is to enable the detection of viral peptides by killer T cells for immune surveillance [55,71]. In addition, there are three pairs of polymorphic MHC class II genes, HLA-DR, -DP, and –DQ. Class II molecules are αβ heterodimers, in which one or both chains exhibit allelic polymorphism [69,72]. Moreover, a second expressed HLA-DR β chain locus is found in many people [70,73]. Thus, a heterozygous human can create at least six MHC class II heterodimers. Their main function is to allow helper T cells to cooperate with other immune cells that present peptides acquired from specific pathogens [55,71].
The extent of MHC polygeny differs. For example, the best-studied bird species, the domestic chicken Gallus gallus domesticus, in common with many vertebrate clades other than mammals, expresses only one predominant MHC class I and one predominant MHC class II locus [74,75]. However, the presence of multiple alleles is a shared feature of the MHC. In outbred populations, MHC polymorphism is so extensive that identity of MHC alleles is very rare between any two genetically unrelated individuals, and any one individual is very likely to be heterozygous at most or all MHC loci.
Most polymorphisms of MHC class I and class II alleles map to within and near the peptide-binding groove [61] and affect the specificity of peptide binding. Individuals with different HLA genotypes will therefore present different sets of self and foreign peptides to their T cells [58,59], and HLA-heterozygotes will present a more diverse repertoire than homozygotes. Thus, MHC diversity creates an immunological signature of individual identity by which developing T cells learn how to tolerate self, while simultaneously individualizing the specificity of immune responses to pathogens.
(d). Major histocompatibility complex signatures of identity: drivers and constraints
Genetic studies strongly support active balancing selection as the driver of multiple alleles being maintained with distinct peptide-binding grooves, since there is a preponderance of coding over non-coding substitutions in the corresponding exons [76]. Moreover, gene conversion appears to have distributed short ‘cassettes’ of amino acid substitutions across multiple MHC variants, both within and between loci [77]. It is less clear whether balancing selection favours rare alleles (to which parasites are less likely to have adapted than to frequent ones), MHC-heterozygotes over homozygotes (with a broader representation of peptide repertoires in heterozygotes), alleles with a distinct peptide-binding repertoire (which reduce the scope for mutational escape from immune recognition), or whether a combination of these mechanisms operates [78,79]. In any case, these mechanisms may not explain fully why MHC molecules have diversified to the extent of identity signatures, whereas the extent of balanced polymorphism in other immune-related genes has stopped short of this (see below).
As in defence against brood parasites, immune defence via MHC-restricted T-cell recognition can result in either a Type I error (mistaken attack against self) or a Type II error (a failure to respond effectively to a pathogen). Given their fitness costs, MHC diversification should be favoured to the extent that it reduces such errors. Here, a Type I error involves the activation of T cells by self peptide/MHC protein complexes (despite the ‘learning’ of self via the tolerance mechanisms described above), leading to autoimmune diseases, such as multiple sclerosis, type 1 diabetes, several types of arthritis, and others [71]. In these diseases, self-reactive T cells are activated and contribute to chronic inflammation and tissue destruction. Autoimmune diseases carry a fitness cost, especially during reproductive age. In most vertebrate species, autoimmunity is rare, as tolerance mechanisms supervene; in humans, the incidence of autoimmunity has risen in recent decades, possibly owing to recent changes in their microbiota and other environmental influences [80].
Interestingly, MHC alleles are strongly associated with susceptibility or resistance to autoimmune diseases [10,81]. Homozygosity for a risk allele is associated with greater susceptibility than heterozygosity. One possible explanation (among others) is that MHC polymorphism limits autoimmune disease risk by decreasing the risk of Type I errors, and that this is one of the drivers of MHC diversification.
A Type II error in MHC-restricted T-cell antigen recognition would be to fail to mount an immune response to an invading pathogen, owing to a lack of antigen presentation by MHC molecules, for example. A very rare, extreme case highlights the importance of antigen presentation in immune defence: bare lymphocyte syndrome is a monogenic defect in MHC class II expression, which causes profound immunodeficiency [82].
There is evidence that Type II errors in antigen presentation may occur, but their frequency is reduced by MHC diversification. For example, pathogens with a small genome, such as human immunodeficiency virus 1 (HIV-1), generate relatively few MHC-presented peptides, and not all of them elicit responses that are equally protective [83]. Within an infected individual, HIV-1 readily generates mutants in which one of its antigenic peptides loses the ability to bind the host's MHC class I proteins and thereby becomes invisible to virus-specific killer T cells [84]. Different MHC alleles are associated with faster or slower progression of HIV-1 infection; the more protective alleles present a broader repertoire of viral peptides [85], or present peptides with sequences that cannot evolve away from T-cell recognition without a cost to the replicative fitness of the virus [86]. The example of HIV highlights the role of mutational escape from MHC-restricted antigen recognition as a mechanism of Type II error. The impact of the error on the host can be reduced if presentation by another MHC variant, encoded at another locus or as a co-dominantly expressed distinct allele at the same locus, can compensate for the problem.
In a second example of Type II errors, MHC allelic polymorphism has strong effects on death or survival upon viral infection in chickens, which have only one major expressed MHC class I and class II locus each [87]. Third, vertebrate species that exhibit low MHC diversity owing to recent population bottlenecks are vulnerable to infection, although the MHC effect can be hard to disentangle from other effects of inbreeding and genetic drift [88]. An interesting example is provided by the transmissible facial tumour diseases of the Tasmanian devil, Sarcophilus harrisii, which arise from the transfer of tumour cells between conspecifics [89]. Transmissibility of tumours in other species is prevented by recognition of MHC differences (alloreactivity); the devil facial tumour diseases evade these responses owing to reduced MHC protein expression, limited host MHC diversity (reducing the scope for tumour rejection by alloreactivity), or both. The tumour is lethal, so the fitness cost is considerable. In sum, these examples illustrate that MHC polygeny and allelic polymorphism can have considerable benefits to the host, by reducing the likelihood that infectious agents can escape from MHC-dependent recognition by T cells by mutation (i.e. Type II error).
These examples concern pathogens that escape detection by the immune system. However, a further strategy for microbes to evade MHC-dependent T-cell immunity can be to mimic self, which is more directly analogous to visual mimicry by brood parasites. Examples of T-cell cross-reactivity between self and non-self have been observed [90], which may play some role in autoimmunity [91]. This suggests that some pathogens may gain survival benefits by mimicking MHC signatures of self, as the host can then defend against such pathogens only at the cost of triggering autoimmune disease. MHC polymorphism mitigates against such mimicry, because it provides the opportunity to present other peptides from the same pathogen to T cells, reducing the likelihood of successful mimicry.
In summary, these considerations suggest that the selective benefits that drive MHC diversification (comprising both polygeny and allelic polymorphism) may accrue from reducing the likelihood of mutational escape or pathogen mimicry of self (thus reducing both variants of Type II error), and autoimmunity (Type I error). All these mechanisms improve the fidelity of self/non-self discrimination in pathogen defences, but their relative contributions remain poorly defined.
Sexual selection may reinforce these benefits of MHC diversity [92]. Vertebrates actively avoid mating with close relatives, which reduces the likelihood of MHC homozygosity below the levels expected by chance. Biological mechanisms supporting inbreeding avoidance include the use of polymorphic olfactory receptors that are genetically linked to the MHC, or olfactory cues related to MHC-dependent microbial colonization [93]. Other signatures of identity that support inbreeding avoidance are not linked to the MHC, such as mouse urinary peptides. In any case, these mechanisms reduce the likelihood of producing offspring with reduced immune fitness owing to MHC homozygosity. Thus, social kin recognition mechanisms can maximize the benefits of immunological signatures of identity.
As with bird egg signatures, constraints on diversification arise because MHC polygeny and allelic polymorphism cannot increase without limit. There are energetic costs involved in increasing the number of MHC loci and expressing their gene products; this may account for the lack of MHC polygeny in birds whose genome is reduced in size owing to the energy demands of flight [94]. In addition, there may be immunological trade-offs: for example, thymic selection of developing T cells imposes considerable attrition on the T-cell repertoire [95], which may become excessive if the number of MHC loci or overall antigen diversity exceeds a critical limit. Biochemical constraints may limit the polymorphisms that can diversify the peptide-binding groove without compromising MHC protein assembly, maturation, peptide capture, and interaction with co-receptors on T cells; alleles that cannot accomplish these tasks are not functional (e.g. [96]). Lastly, polymorphisms in MHC genes should be favoured by selection only when they diversify peptide binding. This is clearly seen in the enrichment of coding polymorphisms within and near the peptide binding groove of MHC molecules, whereas the rest of the molecule remains rather non-polymorphic. Further constraints apply to specific alleles: novel alleles would be selected against if they conferred an excessive risk of autoimmune disease, or if they were associated with poor immunity to prevalent, virulent pathogens, or with excessive immune-related pathology.
4. When do signatures of identity evolve in antagonistic coevolution?
Despite the profound functional and molecular differences between the two systems of parasite defence described above, both have evolved signatures of identity in the context of host/parasite defence. Has their evolution been driven by analogous underlying mechanisms? Here we consider (a) shared features and (b) striking differences between the two systems, and then compare and contrast them with (c) other defensive systems that have not evolved signatures of identity, or (d) that have evolved such signatures for other biological reasons.
(a). Key functional analogies between host defence systems with identity signatures
Figure 1 and table 1 show that both systems perform a comparison between a complex pattern that defines self (host egg markings; molecular surfaces of self peptide/MHC protein complexes) and a distinct but similarly complex and genetically-determined pattern that identifies the parasite as non-self. In both systems, signature polymorphism has evolved in the self component that is subject to this comparative evaluation.
Table 1.
Analogous functional components of host defence systems showing individual signatures of identity.
| host defence against avian brood parasites | MHC adaptive immunity against microbial pathogens | |
|---|---|---|
| (i) recognition system for self/non-self discrimination | visual, by host parent | protein/protein, by T cell receptor (TCR) |
| (ii) self component on which diversifying selection can act | egg colours and patterns | MHC glycoproteins |
| (iii) acquired knowledge of self | learning of first clutch | T-cell tolerance mechanisms (thymic and peripheral) |
| (iv) fitness costs of recognition errors, reduced by diversification | Type I error: misidentify own egg as parasitic; outcome is rejecting own egg |
misidentify own cell as infected or exposed to pathogen; outcome is autoimmune response |
| Type II error: detect but fail to recognize parasitic egg; outcome is accepting parasitic egg and so raising parasitic chick |
fail to detect pathogen, or detect but fail to distinguish pathogen from self; outcome is failure to activate the T-cell, and so spread of infection |
The two systems share specific parallels. The recognition of self and non-self is carried out, in both cases, by a versatile and exquisitely discriminating recognition system of the host (table 1, condition (i)): the visual system of the host parent, and the repertoire of TCRs, respectively. This performs a comparison between a self component (on which diversifying selection can act; condition (ii)) and a potentially non-self component (if present), applying knowledge of self acquired during ontogeny, which enables non-rejection of the unique and unpredictable signature of self (‘learning’ in behaviour, ‘tolerance’ in immunology; condition (iii)). After appropriate signal processing, the complex pattern recognition event is translated into a binary decision: to accept the detected pattern as self, or to reject it as foreign. If it is accepted, no further action is taken; if rejected, the carrier of the foreign pattern is subject to a functional response to eliminate it. The thresholds for this distinction are flexible and can be modified by additional environmental cues (e.g. a female seeing a brood parasite nearby; detection of inflammatory stimuli by innate immune receptors; tuning of tolerance thresholds by microbial influences).
Importantly, this decision is capable of error (figure 1 and table 1, condition (iv)), for two reasons. First, the ability of the recognition system to deliver a decision to accept or reject the pattern is not hardwired, but acquired through an imperfect learning process (learning by the female of her first clutch of eggs; selection and peripheral tolerance mechanisms of T cells). Second, parasites evolve counter-adaptations that make the discrimination task more difficult. Depending on the thresholds for self/non-self recognition and the discriminating power of the recognition system, it is possible to trigger either an erroneous rejection of self (Type I error: ejection of host eggs; autoimmune disease) or an erroneous acceptance of non-self (Type II error: parasite success, whether by mimicry or evasion). Both types of error may carry a significant fitness cost to the host.
In both systems, signatures of identity reduce Type I and/or Type II errors in self/non-self discrimination. Visual signatures on eggs reduce the ability of a brood parasite to mimic the eggs of different host individuals within a population with equal accuracy, thus reducing both the likelihood of failure to recognize an intruding brood parasite, and the likelihood of mistakenly ejecting a host egg. MHC polymorphism, similarly, diversifies the presentation of foreign antigens and reduces possibilities for mimicry or escape, and may well also diminish the risk of autoimmune disease. Thus, the evolution of signature polymorphism should be favoured to the extent that diversification improves fitness by reducing these errors.
(b). Differences between host defence systems with identity signatures
Despite sharing signatures of identity, the two systems obviously differ in many important ways, which appear to be incidental to signatures having evolved. First, there seems to be no requirement for a specific evolutionary mechanism to drive diversification. In avian hosts of brood parasites, rare-allele advantage may be a major diversifying mechanism, perhaps enhanced by heterozygote advantage, since brood parasites will be most successful by mimicking the most common egg appearances in a population. MHC polymorphism, in contrast, may be driven more by heterozygote or divergent-allele advantage, reinforced by sexual selection. While it is unlikely that there is an analogous mechanistic link between egg phenotype and sexual selection in birds, other mechanisms of inbreeding avoidance would have the same effect in maximizing egg signature diversity in daughters. In both systems, however, contributions from alternative diversifying mechanisms have not been ruled out.
Second, there are substantial differences between each kind of parasitic threat. For example, brood parasites have generation times on the same order of magnitude as the generation times of their hosts, whereas the microbial parasites that are subject to MHC-restricted T-cell surveillance have much shorter generation times than their hosts. This allows greater scope for microbial parasite variation in the arms race with their hosts than is the case for brood parasites. Moreover, misidentifying a brood parasite as self (Type II error) directly affects the host's reproductive success but likely has a weaker effect on adult survival, whereas microbial parasites generally affect the health of the infected individual, with indirect consequences for reproductive success. Similarly, misidentifying self as a brood parasite (Type I error) primarily affects reproductive success, whereas autoimmune disease affects the individual whose T cells erroneously respond to self, which may or may not affect future reproductive success, depending on host life-history and the age of disease onset.
The two systems may also differ in the relative importance of potential host discrimination errors that lead to parasite success. For example, parasites can evade host defences by escape (i.e. avoiding detection; common in fast-evolving viruses, such as HIV-1) and by mimicry (i.e. avoiding recognition as non-self; common in the bird world). Put another way, Type II errors can arise either because the host fails to detect a parasite, or because it detects it but fails to recognize it as being distinct from self. In T-cell immunity, opportunities for escape and for mimicry can both be reduced by increasing MHC diversity. If a pathogen has successfully escaped presentation by one MHC variant, or if it is a close mimic of self in the context of that MHC variant, it might still be detected by the host's T cells, as long as peptides from the same pathogen can still be presented by a second MHC variant that is present. Moreover, the second MHC variant would reduce the chance of successful mimicry, because it will generally present other peptides from the pathogen as well as a self peptides differing from the first, thus offering a second chance at self/non-self discrimination. By contrast, in avian brood parasitism, egg recognition can only occur if an egg is detected, such that mimetic parasites have selected for signature diversification, but cryptic parasites have not. In the latter case of avian parasites successfully escaping host detection (known only from the dark eggs of Chalcites cuckoos), there is no selection for avoiding host recognition [54]. Thus, in T-cell immunity, both parasite escape and parasitic mimicry select for signature diversification, since both can be rendered less likely by MHC diversification, whereas in birds, only parasitic mimicry selects for signature diversification.
(c). Other host defence systems in which signatures have, or have not, evolved
If self/non-self discrimination is at the heart of the evolution of signature polymorphism in host/parasite interactions, the essential shared features summarized in table 1 should be consistently present in anti-parasite defences that have evolved signature polymorphism, and this should not be limited to visual or molecular signatures. This appears to be the case in the hosts of many insect brood parasites, whose parasite recognition systems perform an olfactory comparison between their own complex hydrocarbon signatures and those of potential parasites. Parasites mimic these signatures, which reduces recognition (e.g. [97]). Diversifying selection has acted on the self (host signatures), and hosts acquire knowledge of their own signatures through learning [98]. The parallels between visual and olfactory signature diversification in birds and insects respectively are thoroughly reviewed by Kilner & Langmore [99].
Conversely, other systems of parasite defence have not evolved the level of polymorphism that establishes signatures of identity. We find that these systems lack one or more of the minimal feature set shared by systems that do exhibit signature polymorphism. For example, while some hosts of brood-parasitic birds discriminate against parasitic chicks with mismatched begging calls, selecting for acoustic mimicry by parasites (reviewed by [100]), there are at least two good reasons why signature-like diversification of chick calls has not evolved as a defence. First, there is no genetically stable self component on which diversifying selection can act (i.e., condition (ii) in table 1 missing), because a chick is a product of both parents' genotypes and need not be consistent within or between broods, given recombination and changes in paternity; by contrast, an egg is the product solely of a mother's genotype and so remains consistent in her lifetime regardless of the embryo's paternity. Second, parasitic chicks can flexibly acquire their host mimicry through learning [101], such that parasitic mimicry is not genetically based, unlike egg and MHC signatures, undermining the effectiveness of genetically based signatures as a defence.
Similarly, in the immune system, polymorphism has remained limited in defence systems that do not share the above features driving the evolution of identity signatures. For example, antibodies provide an alternative system of adaptive defences against non-self, based on clonal diversity achieved by gene rearrangement of individual lymphocytes (as for TCRs), and with potential for immune escape by pathogens, as in HIV infection. The antibody repertoire does have some self-reactivity but, unlike T-cell recognition of MHC-bound antigen, antibodies recognize native antigens that are not associated with a self molecule, so there is no self component that could be diversified by selection (i.e., lack of condition (ii) in table 1). As another example, innate immune cells express toll-like receptor 4, a sensor of lipopolysaccharides on the outer membranes of Gram-negative bacteria, which activates host inflammatory (innate immune) defences when engaged. There is some evidence that this receptor also detects endogenous ligands released by necrotic cells, for example [102], but in this case, there is no explicit comparison between self and non-self by a flexible recognition system, thus meeting neither condition (i) nor (ii) in table 1. Moreover, knowledge of self is implicit and hardwired into the receptor structure, rather than being acquired in ontogeny, violating condition (iii) in table 1. Polymorphism in the toll-like receptor 4 gene is rather more limited, compared to that at MHC gene loci, such as HLA-DRB1, encoding one polypeptide chain of the most abundant human MHC class II molecule (dozens rather than hundreds of single-nucleotide polymorphisms at greater than 1% frequency, as compared by USCS genome browser, https://genome.ucsc.edu/, using GRCh38/hg38 assembly, dbSNP150, accessed 22 August 2018).
Interestingly, some ant brood parasites avoid host detection by presenting a less complex hydrocarbon signature than their hosts (a phenomenon termed ‘chemical insignificance’), rather than by mimicking their hosts [103]. There is evidence that hosts in this instance adapt by reducing the complexity and diversity of their own hydrocarbons—the opposite of what happens to bird egg signatures [99]. Such chemical crypsis might be considered to be analogous to the visual crypsis of Chalcites cuckoo eggs (see §4a and [99]), except that a comparison with self is still performed. This example is also consistent with the requirements for the evolution of signatures listed in table 1: the hosts' olfactory systems meet condition (i); hydrocarbons provide a consistent self component that potentially might be diversified, meeting condition (ii); knowledge of self is acquired by a learning process, meeting condition (iii); but in this case, errors in discrimination are worsened by increasing the complexity of hydrocarbon signatures that generates diversity (opposite to condition (iv) in table 1). The resultant fitness cost explains why, in this setting, diversity seems to have diminished in response to the parasite.
Together, the foregoing examples are consistent with the hypothesis that each of features of host/parasite interactions listed in table 1 must be present in order for parasites to drive the evolution of identity signatures in their hosts. Further comparative studies and mathematical modelling are warranted to test the generality of this proposed explanation.
(d). Host defence and kin recognition
Identity signatures have not only evolved in order to aid host defences against parasites. They exist, for example, in the context of the recognition of kin and individuals in animals [1] and in the context of self incompatibility in flowering plants [104], which are both settings in which coevolution with parasites is not obviously involved. The selective advantages of genetic polymorphism underpinning these signatures arise from, for example, avoiding inbreeding, helping kin, or recognizing individuals in social interactions (reviewed in [1]). However, these benefits may interact with those of host defences against parasites.
First, kin recognition can enable sexual selection to avoid potential mates who are related enough to risk a reduction in MHC diversity (§3d), and so enhance or help to maintain identity signatures as a defence against parasites.
Second, kin recognition can serve as a defensive mechanism in its own right, in cases of parasitism by conspecifics. For example, colonial tunicates, such as Botryllus schlosseri, can fuse circulatory systems between conspecifics and are thus at risk of parasitism by their neighbours' stem cells. Importantly, in any one fusion attempt, it is not predictable which of the two individuals would lose a contest for stem cell niches, and so unrelated individuals may mutually benefit more from preventing this risk than they might gain from trying to parasitize their fusion partner. Stem cell parasitism is avoided by the recognition of foreignness at sites of physical contact between adjacent colonies that differ at a single polymorphic gene locus, BHF (Botryllus histocompatibility factor, formerly Fu/HC), that signals kinship and is directly involved in the fusion process [105]. Intriguingly, the fusion rejection reaction that prevents circulatory exchange between BHF-incompatible colonies is an inflammatory response by immunocytes, used by Botryllus for defence against microbial parasites. It is not known whether BHF polymorphism solely evolved to prevent germ cell colonization by unrelated tunicates, or whether it also acts as a defence against microbial non-self. If the latter, then kin recognition would again enhance identity signatures as a defence against heterospecific parasites, since distinctiveness is always beneficial.
Third, kin recognition can be a double-edged sword in cases of conspecific parasitism where it is predictable which party will pay the cost of parasitism. In birds, egg signatures in colonial weavers Ploceus spp. aid in detecting conspecific brood parasitism [24,48], as well as heterospecific brood parasitism by the diederik cuckoo Chrysococcyx caprius. Signature diversification in weavers is more likely to be driven by the heterospecific parasites, because in the context of conspecific parasitism, the benefits of individual distinctiveness when an individual acts as a host would probably be outweighed by its costs when it acts as a parasite [106,107]. Thus, in weavers, kin recognition is unlikely to enhance diversification of identity signatures.
In summary, selection for individual distinctiveness from signalling kinship can work in concert with its benefits in defence against heterospecific parasites, enhancing signature diversity, as in the MHC or possibly in tunicates. However, signalling kinship through individual distinctiveness can also carry costs when acting as a conspecific brood parasite, such that kin recognition might also sometimes oppose (or at least fail to enhance) the diversification of identity signatures.
5. Conclusion
We have considered the role of host/parasite interactions as evolutionary drivers of signatures of identity, by which we mean genetically-based traits (with oligogenic and/or allelic diversity) that vary sufficiently to distinguish most individuals of a population, and so allow an assessment of individual identity or kinship. This represents a puzzle, because the extensive diversity needed to establish signatures has evolved in only a small fraction of parasite/host interactions. Inspired by a comparison between two superficially divergent systems, we have proposed a minimal set of features that may enable the evolution of signatures of identity in host/parasite interactions, based on functional analogies between highly divergent host defence mechanisms that involve self/non-self discrimination, combined with fitness benefits that emerge from host diversification.
In summary, signature polymorphism seems to evolve in host components that are used as the basis for self/non-self discrimination by a flexible, highly specific, but imperfect recognition system. Signature polymorphism then evolves if it reduces the likelihood of errors in self/non-self discrimination that carry a fitness cost. These features appear to be present in the two systems with signature polymorphism that we have surveyed, whereas signature polymorphism has not evolved in examples of coevolutionary systems that share only some of these features, or none. These examples are consistent with the hypothesis that these shared features define a set of necessary and sufficient conditions for the evolution of signature polymorphism in host/parasite interactions. Mathematical modelling and formal comparative analyses may shed further light on the validity of our proposal.
These proposed conditions for evolution of polymorphic host defences against parasites are highly specific and uncommon. Sufficiently sophisticated receptor and signal processing systems are effectively confined to the sensory apparatus and central nervous system, and to adaptive immune receptors and the lymphocytes that express these receptors. Of the latter, only T cells focus antigen recognition on MHC molecules as a distinct self component that enables comparison with non-self. These considerations may help to explain why signature polymorphism is comparatively rare in antagonistic settings in the natural world. For immunologists, they may help to illuminate how self/non-self discrimination, polymorphic immune response genes, and the recognition of danger [108] are interrelated. For ecologists, they may help to shed light on the variety of trajectories arising from arms races in different antagonistic contexts. In brood parasitism research, in particular, we hope that comparisons with immune systems may help to inspire advances in our mechanistic understanding of host defences, particularly the genetic basis of host egg phenotypes, and the cognitive processes underpinning how they are used in adaptive decision-making.
Acknowledgements
We thank Sidney Sussex College, Cambridge, for providing a friendly environment that encouraged this interdisciplinary collaboration, Jim Kaufman, Tanmay Dixit and three anonymous referees for helpful comments on earlier drafts, and Bruce Lyon for helpful discussion.
Data accessibility
This article has no additional data.
Authors' contributions
C.N.S. and R.B. conceived the study, wrote the paper and gave final approval for publication.
Competing interests
C.N.S. is a guest editor of this special issue. R.B. has no competing interests.
Funding
C.N.S. was supported by a BBSRC David Phillips Research Fellowship (BB/J014109/1). R.B. was supported by an Arthritis Research UK Senior Research Fellowship (ref. 18543) at the early stages of writing this paper.
References
- 1.Tibbetts EA, Dale J. 2007. Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537. ( 10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 2.Swynnerton CFM. 1918. Rejections by birds of eggs unlike their own: with remarks on some of the cuckoo problems. Ibis 6, 127–154. ( 10.1111/j.1474-919X.1918.tb00774.x) [DOI] [Google Scholar]
- 3.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: The Clarendon Press. [Google Scholar]
- 4.Davies NB, Brooke M de L. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 58, 225–236. ( 10.2307/4996) [DOI] [Google Scholar]
- 5.Beecher MD. 1982. Signature systems and kin recognition. Am. Zool. 22, 477–490. ( 10.1093/icb/22.3.477) [DOI] [Google Scholar]
- 6.Sheehan MJ, Tibbetts EA. 2009. Evolution of identity signals: frequency-dependent selection benefits of distinctive phenotypes used for individual recognition. Evolution 63, 3106–3113. ( 10.1111/j.1558-5646.2009.00833.x) [DOI] [PubMed] [Google Scholar]
- 7.Spottiswoode CN, Stevens M. 2010. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676. ( 10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. 2007. How a single T cell receptor recognizes both self and foreign MHC. Cell 129, 135–146. ( 10.1016/j.cell.2007.01.048) [DOI] [PubMed] [Google Scholar]
- 9.Flajnik MF, Kasahara M. 2001. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity 15, 351–362. ( 10.1016/S1074-7613(01)00198-4) [DOI] [PubMed] [Google Scholar]
- 10.Trowsdale J, Knight JC. 2013. Major histocompatibility complex genomics and human disease. Annu. Rev. Genomics Hum. Genet. 14, 301–323. ( 10.1146/annurev-genom-091212-153455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies NB. 2000. Cuckoos, cowbirds and other cheats London, UK: T & A D Poyser. [Google Scholar]
- 12.Davies NB, Brooke M de L. 1989. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J. Anim. Ecol. 58, 207–224. ( 10.2307/4995) [DOI] [Google Scholar]
- 13.Stoddard MC, Stevens M. 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc. R. Soc. B 277, 1387–1393. ( 10.1098/rspb.2009.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooke M de L, Davies NB. 1988. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632. ( 10.1038/335630a0) [DOI] [Google Scholar]
- 15.Spottiswoode CN, Stevens M. 2011. How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. R. Soc. B 278, 3566–3573. ( 10.1098/rspb.2011.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilner RM. 2006. The evolution of egg colour and patterning in birds. Biol. Rev. 81, 383–406. ( 10.1017/S1464793106007044) [DOI] [PubMed] [Google Scholar]
- 17.Langmore NE, Spottiswoode CN. 2012. Visual trickery in avian brood parasites. In Host manipulation by parasites (eds Hughes DP, Brodeur J, Thomas F), pp. 95–115. Oxford, UK: Oxford University Press. [Google Scholar]
- 18.Vernon CJ. 1987. On the Eastern Green-backed Honeyguide. Honeyguide 33, 6–12. [Google Scholar]
- 19.Yang C, et al. 2010. Coevolution in action: disruptive selection on egg colour in an avian brood parasite and its host. PLoS ONE 5, e10816 ( 10.1371/journal.pone.0010816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahti DC, Lahti AR. 2002. How precise is egg discrimination in weaverbirds? Anim. Behav. 63, 1135–1142. ( 10.1006/anbe.2002.3009) [DOI] [Google Scholar]
- 21.Caves EM, Stevens M, Iversen ES, Spottiswoode CN. 2015. Hosts of avian brood parasites have evolved egg signatures with elevated information content. Proc. R. Soc. B 282, 20150598 ( 10.1098/rspb.2015.0598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahti DC. 2005. Evolution of bird eggs in the absence of cuckoo parasitism. Proc. Natl Acad. Sci. USA 102, 18 057–18 062. ( 10.1073/pnas.0508930102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahti DC. 2006. Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution 60, 157–168. ( 10.1111/j.0014-3820.2006.tb01090.x) [DOI] [PubMed] [Google Scholar]
- 24.Victoria JK. 1972. Clutch characteristics and egg discriminative ability of the African village weaverbird Ploceus cucullatus. Ibis 114, 367–376. ( 10.1111/j.1474-919X.1972.tb00834.x) [DOI] [Google Scholar]
- 25.Gosler AG, Higham JP, Reynolds SJ. 2005. Why are birds’ eggs speckled? Ecol. Lett. 8, 1105–1113. ( 10.1111/j.1461-0248.2005.00816.x) [DOI] [Google Scholar]
- 26.Caves EM, Stevens M, Spottiswoode CN. 2017. Does coevolution with a shared parasite drive hosts to partition their defences among species? Proc.R. Soc. B 284, 20170272 ( 10.1098/rspb.2017.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter HC. 1961. Parasitism of the masked weaver Ploceus velatus arundinaceus. Ostrich 32, 55–63. ( 10.1080/00306525.1961.9633074) [DOI] [Google Scholar]
- 28.Antonov A, Stokke BG, Fossøy F, Ranke PS, Liang W, Yang CC, Moksnes A, Shykoff J, Røskaft E. 2012. Are cuckoos maximizing egg mimicry by selecting host individuals with better matching egg phenotypes? PLoS ONE 7, e31704 ( 10.1371/journal.pone.0031704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskát C, Avilés JM, Bán M, Hargitai R, Zölei A. 2008. Experimental support for the use of egg uniformity in parasite egg discrimination by cuckoo hosts. Behav. Ecol. Sociobiol. 62, 1885–1890. ( 10.1007/s00265-008-0618-0) [DOI] [Google Scholar]
- 30.Dale J, Lank DB, Reeve HK. 2001. Signaling individual identity versus quality: a model and case studies with ruffs, queleas, and house finches. Am. Nat. 158, 75–86. ( 10.1086/320861) [DOI] [PubMed] [Google Scholar]
- 31.Stoddard MC, Kilner RM, Town C. 2014. Pattern recognition algorithm reveals how birds evolve individual egg pattern signatures. Nat. Commun. 5, 4117 ( 10.1038/ncomms5117) [DOI] [PubMed] [Google Scholar]
- 32.Davies NB, Brooke M de L, Kacelnik A. 1996. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc. R. Soc. Lond. B 263, 925–931. ( 10.1098/rspb.1996.0137) [DOI] [Google Scholar]
- 33.Rothstein SI. 1974. Mechanisms of avian egg recognition: possible learned and innate factors. Auk 91, 796–807. ( 10.2307/4084731) [DOI] [Google Scholar]
- 34.Lotem A, Nakamura H, Zahavi A. 1995. Constraints on egg discrimination and cuckoo–host co-evolution. Anim. Behav. 49, 1185–1209. ( 10.1006/anbe.1995.0152) [DOI] [Google Scholar]
- 35.Stevens M, Troscianko J, Spottiswoode CN. 2013. Repeated targeting of the same hosts by a brood parasite compromises host egg rejection. Nat. Commun. 4, 2475 ( 10.1038/ncomms3475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyon BE. 2007. Mechanism of conspecific egg recognition in defenses against brood conspecific brood parasitism: American coots (Fulica americana) know their own eggs. Behav. Ecol. Sociobiol. 61, 455–463. ( 10.1007/s00265-006-0273-2) [DOI] [Google Scholar]
- 37.Molina-Morales M, Martínez JG, Martín-Gálvez D, Dawson DA, Burke T, Avilés JM. 2014. Cuckoo hosts shift from accepting to rejecting parasitic eggs across their lifetime. Evolution 68, 3020–3029. ( 10.1111/evo.12471) [DOI] [PubMed] [Google Scholar]
- 38.Lee JW, Kim DW, Yoo JC. 2005. Egg rejection by both male and female vinous-throated parrotbills Paradoxornis webbianus. Integr. Biosci. 9, 211–213. ( 10.1080/17386357.2005.9647273) [DOI] [Google Scholar]
- 39.Davies NB, Brooke M de L. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284. ( 10.1016/S0003-3472(88)80269-0) [DOI] [Google Scholar]
- 40.Feeney WE, Troscianko J, Langmore NE, Spottiswoode CN. 2015. Evidence for aggressive mimicry in an adult brood parasitic bird, and generalized defences in its host. Proc. R. Soc. B 282, 20180795 ( 10.1098/rspb.2015.0795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorogood R, Davies NB. 2016. Combining personal with social information facilitates host defences and explains why cuckoos should be secretive. Sci. Rep. 6, 19872 ( 10.1038/srep19872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avilés JM, Stokke BG, Moksnes A, Røskaft E, Møller AP. 2007. Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behav. Ecol. Sociobiol. 61, 475–485. ( 10.1007/s00265-006-0275-0) [DOI] [Google Scholar]
- 43.Hanley D, Grim T, Cassey P, Hauber ME. 2015. Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Biol. Lett. 11, 20150087 ( 10.1098/rsbl.2015.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Igic B, Cassey P, Grim T, Greenwood DR, Moskát C, Rutila J, Hauber ME. 2011. A shared chemical basis of avian host–parasite egg colour mimicry. Proc. R. Soc. B 279, 1068–1076. ( 10.1098/rspb.2011.1718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gosler AG, Barnett PR, Reynolds SJ. 2000. Inheritance and variation in eggshell patterning in the great tit Parus major. Proc. R. Soc. Lond. B 267, 2469–2473. ( 10.1098/rspb.2000.1307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takasu F. 2005. A theoretical consideration on co-evolutionary interactions between avian brood parasites and their hosts. Ornithol. Sci. 4, 65–67. ( 10.2326/osj.4.65) [DOI] [Google Scholar]
- 47.Collias EC. 1993. Inheritance of egg-colour polymorphisms in the village weaver (Ploceus cucullatus). Auk 110, 683–692. ( 10.2307/4088624) [DOI] [Google Scholar]
- 48.Jackson WM. 1998. Egg discrimination and egg-color variability in the northern masked weaver: the importance of conspecific versus interspecific parasitism. In Parasitic birds and their hosts: studies in coevolution (eds Rothstein SI, Robinson SK), pp. 407–418. New York, NY: Oxford University Press. [Google Scholar]
- 49.Marchetti K. 1992. Costs to host defence and the persistence of parasitic cuckoos. Proc. R. Soc. Lond. B 248, 41–45. ( 10.1098/rspb.1992.0040) [DOI] [PubMed] [Google Scholar]
- 50.Stokke BG, et al. et al. 2016. Disappearance of eggs from nonparasitized nests of brood parasite hosts: the evolutionary equilibrium hypothesis revisited. Biol. J. Linn. Soc. 118, 215–225. ( 10.1111/bij.12733) [DOI] [Google Scholar]
- 51.Dukas R. 1999. Costs of memory: ideas and predictions. J. Theor. Biol. 197, 41–50. ( 10.1006/jtbi.1998.0856) [DOI] [PubMed] [Google Scholar]
- 52.Lotem A. 1993. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature 362, 743–745. ( 10.1038/362743a0) [DOI] [Google Scholar]
- 53.Lahti DC. 2008. Population differentiation and rapid evolution of egg color in accordance with solar radiation. Auk 125, 796–802. ( 10.1525/auk.2008.07033) [DOI] [Google Scholar]
- 54.Langmore NE, Stevens M, Maurer G, Kilner RM. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468. ( 10.1016/j.anbehav.2009.06.003) [DOI] [Google Scholar]
- 55.Neefjes J, Jongsma ML, Paul P, Bakke O. 2011. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836. ( 10.1038/nri3084) [DOI] [PubMed] [Google Scholar]
- 56.Huppa JB, Davis MM. 2003. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983. ( 10.1038/nri1245) [DOI] [PubMed] [Google Scholar]
- 57.Rudolph MG, Stanfield RL, Wilson IA. 2006. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 24, 419–466. ( 10.1146/annurev.immunol.23.021704.115658) [DOI] [PubMed] [Google Scholar]
- 58.Engelhard VH. 2007. The contributions of mass spectrometry to understanding of immune recognition by T lymphocytes. Int. J. Mass Spectrom. 259, 32–39. ( 10.1016/j.ijms.2006.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rammensee HG, Friede T, Stevanoviic S. 1995. MHC ligands and peptide motifs: first listing. Immunogenetics 41, 178–228. ( 10.1007/BF00172063) [DOI] [PubMed] [Google Scholar]
- 60.Vita R, et al. 2015. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 43, D405–D412. ( 10.1093/nar/gku938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stern LJ, Wiley DC. 1994. Antigenic peptide binding by class I and class II histocompatibility proteins. Structure 2, 245–251. ( 10.1016/S0969-2126(00)00026-5) [DOI] [PubMed] [Google Scholar]
- 62.Schaeffer EB, Sette A, Johnson DL, Bekoff MC, Smith JA, Grey HM, Buus S. 1989. Relative contribution of ‘determinant selection’ and ‘holes in the T-cell repertoire’ to T-cell responses. Proc. Natl Acad. Sci. USA 86, 4649–4653. ( 10.1073/pnas.86.12.4649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim A, Sadegh-Nasseri S. 2015. Determinants of immunodominance for CD4 T cells. Curr. Opin. Immunol. 34, 9–15. ( 10.1016/j.coi.2014.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yewdell JW. 2006. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity 25, 533–543. ( 10.1016/j.immuni.2006.09.005) [DOI] [PubMed] [Google Scholar]
- 65.Mouchess ML, Anderson M. 2014. Central tolerance induction. Curr. Top. Microbiol. Immunol. 373, 69–86. ( 10.1007/82_2013_321) [DOI] [PubMed] [Google Scholar]
- 66.Hogquist KA, Jameson SC. 2014. The self-obsession of T cells: how TCR signaling thresholds affect fate ‘decisions’ and effector function. Nat. Immunol. 15, 815–823. ( 10.1038/ni.2938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valdor R, Macian F. 2013. Induction and stability of the anergic phenotype in T cells. Semin. Immunol. 25, 313–320. ( 10.1016/j.smim.2013.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campbell DJ. 2015. Control of regulatory T cell migration, function, and homeostasis. J. Immunol. 195, 2507–2513. ( 10.4049/jimmunol.1500801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beck S, Trowsdale J. 2000. The human major histocompatibility complex: lessons from the DNA sequence. Annu. Rev. Genomics Hum. Genet. 1, 117–137. ( 10.1146/annurev.genom.1.1.117) [DOI] [PubMed] [Google Scholar]
- 70.Stewart CA, et al. 2004. Complete MHC haplotype sequencing for common disease gene mapping. Genome Res. 14, 1176–1187. ( 10.1101/gr.2188104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yewdell JW, Bennink JR. 1990. The binary logic of antigen processing and presentation to T cells. Cell 62, 203–206. ( 10.1016/0092-8674(90)90356-J) [DOI] [PubMed] [Google Scholar]
- 72.Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. 2015. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 43, D423–D431. ( 10.1093/nar/gku1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andersson G. 1998. Evolution of the human HLA-DR region. Front. Biosci. 3, 739–745. [DOI] [PubMed] [Google Scholar]
- 74.Kaufman J. 2015. What chickens would tell you about the evolution of antigen processing and presentation. Curr. Opin. Immunol. 34, 35–42. ( 10.1016/j.coi.2015.01.001) [DOI] [PubMed] [Google Scholar]
- 75.Parker A, Kaufman J. 2017. What chickens might tell us about the MHC class II system. Curr. Opin. Immunol. 46, 23–29. ( 10.1016/j.coi.2017.03.013) [DOI] [PubMed] [Google Scholar]
- 76.Hughes AL. 2002. Natural selection and the diversification of vertebrate immune effectors. Immunol. Rev. 190, 161–168. ( 10.1034/j.1600-065X.2002.19012.x) [DOI] [PubMed] [Google Scholar]
- 77.Ohta T. 1999. Effect of gene conversion on polymorphic patterns at major histocompatibility complex loci. Immunol. Rev. 167, 319–325. ( 10.1111/j.1600-065X.1999.tb01401.x) [DOI] [PubMed] [Google Scholar]
- 78.Spurgin LG, Richardson DS. 2010. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc. R. Soc. B 277, 979–988. ( 10.1098/rspb.2009.2084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Froeschke G, Sommer S. 2012. Insights into the complex associations between MHC class II DRB polymorphism and multiple gastrointestinal parasite infestations in the striped mouse. PLoS ONE 7, e31820 ( 10.1371/journal.pone.0031820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bach JF. 2018. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat. Rev. Immunol. 18, 105–120. ( 10.1038/nri.2017.111) [DOI] [PubMed] [Google Scholar]
- 81.Dendrou CA, Petersen J, Rossjohn J, Fugger L. 2018. HLA variation and disease. Nat. Rev. Immunol. 18, 325–339. ( 10.1038/nri.2017.143) [DOI] [PubMed] [Google Scholar]
- 82.Krawczyk M, Reith W. 2006. Regulation of MHC class II expression, a unique regulatory system identified by the study of a primary immunodeficiency disease. Tissue Antigens 67, 183–197. ( 10.1111/j.1399-0039.2006.00557.x) [DOI] [PubMed] [Google Scholar]
- 83.Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13, 46–53. ( 10.1038/nm1520) [DOI] [PubMed] [Google Scholar]
- 84.Klenerman P, Wu Y, Phillips R. 2002. HIV: current opinion in escapology. Curr. Opin. Microbiol. 5, 408–413. ( 10.1016/S1369-5274(02)00339-9) [DOI] [PubMed] [Google Scholar]
- 85.Crawford H, et al. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 81, 8346–8351. ( 10.1128/JVI.00465-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Troyer RM, et al. et al. 2009. Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response. PLoS Pathog. 5, e1000365 ( 10.1371/journal.ppat.1000365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaufman J. 2000. The simple chicken major histocompatibility complex: life and death in the face of pathogens and vaccines. Phil. Trans. R. Soc. Lond. B 355, 1077–1084. ( 10.1098/rstb.2000.0645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Radwan J, Biedrzycka A, Babik W. 2010. Does reduced MHC diversity decrease viability of vertebrate populations? Biol. Conserv. 143, 537–544. ( 10.1016/j.biocon.2009.07.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hansen VL, Miller RD. 2018. A ‘devil’ of a problem. Elife 7, e39976 ( 10.7554/eLife.39976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lang HL, et al. 2002. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat. Immunol. 3, 940–943. ( 10.1038/ni835) [DOI] [PubMed] [Google Scholar]
- 91.Rose NR. 2017. Negative selection, epitope mimicry and autoimmunity. Curr. Opin. Immunol. 49, 51–55. ( 10.1016/j.coi.2017.08.014) [DOI] [PubMed] [Google Scholar]
- 92.Jan Ejsmond M, Radwan J, Wilson AB. 2014. Sexual selection and the evolutionary dynamics of the major histocompatibility complex. Proc. R. Soc. B 281, 20141662 ( 10.1098/rspb.2014.1662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brennan PA. 2004. The nose knows who's who: chemosensory individuality and mate recognition in mice. Horm. Behav. 46, 231–240. ( 10.1016/j.yhbeh.2004.01.010) [DOI] [PubMed] [Google Scholar]
- 94.Wright NA, Gregory TR, Witt CC. 2014. Metabolic ‘engines’ of flight drive genome size reduction in birds. Proc. R. Soc. B 281, 20132780 ( 10.1098/rspb.2013.2780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faro J, Velasco S, Gonzalez-Fernandez A, Bandeira A. 2004. The impact of thymic antigen diversity on the size of the selected T cell repertoire. J. Immunol. 172, 2247–2255. ( 10.4049/jimmunol.172.4.2247) [DOI] [PubMed] [Google Scholar]
- 96.Doebele RC, Pashine A, Liu W, Zaller DM, Belmares M, Busch R, Mellins ED. 2003. Point mutations in or near the antigen-binding groove of HLA-DR3 implicate class II-associated invariant chain peptide affinity as a constraint on MHC class II polymorphism. J. Immunol. 170, 4683–4692. ( 10.4049/jimmunol.170.9.4683) [DOI] [PubMed] [Google Scholar]
- 97.Nash DR, Als TD, Maile R, Jones GR, Boomsma JJ. 2008. A mosaic of chemical coevolution in a large blue butterfly. Science 319, 88–90. ( 10.1126/science.1149180) [DOI] [PubMed] [Google Scholar]
- 98.Howard RW, Blomquist GJ. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393. ( 10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- 99.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
- 100.Jamie GA, Kilner RM. 2017. Begging call mimicry by brood parasite nestlings: adaptation, manipulation and development. In Avian brood parasitism (ed. Soler M.), pp. 517–538, 1st edn Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 101.Langmore NE, Maurer G, Adcock GJ, Kilner RM. 2008. Socially acquired host-specific mimicry and the evolution of host races in Horsfield's Bronze-Cuckoo Chalcites basalis. Evolution 62, 1689–1699. ( 10.1111/j.1558-5646.2008.00405.x) [DOI] [PubMed] [Google Scholar]
- 102.Lee KM, Seong SY. 2009. Partial role of TLR4 as a receptor responding to damage-associated molecular pattern. Immunol. Lett. 125, 31–39. ( 10.1016/j.imlet.2009.05.006) [DOI] [PubMed] [Google Scholar]
- 103.Lenoir A, D'Ettorre P, Errard C, Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. ( 10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 104.Heslop-Harrison J. 1975. Incompatibility and the pollen–stigma interaction. Annu. Rev. Plant Physiol. 26, 403–425. ( 10.1146/annurev.pp.26.060175.002155) [DOI] [Google Scholar]
- 105.Voskoboynik A, et al. 2013. Identification of a colonial chordate histocompatibility gene. Science 341, 384–387. ( 10.1126/science.1238036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crozier RH. 1986. Genetic clonal recognition abilities in marine invertebrates must be maintained by selection for something else. Evolution 40, 1100–1101. ( 10.1111/j.1558-5646.1986.tb00578.x) [DOI] [PubMed] [Google Scholar]
- 107.Field J, Accleton C, Foster WA. 2018. Crozier's Effect and the acceptance of intraspecific brood parasites. Curr. Biol. 28, 3267–3272. ( 10.1016/j.cub.2018.08.014) [DOI] [PubMed] [Google Scholar]
- 108.Matzinger P. 1994. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045. ( 10.1146/annurev.iy.12.040194.005015) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.