Abstract
The cuckoo catfish, Synodontis multipunctatus, is the only known obligate brood parasite among fishes, exploiting the parental care of mouthbrooding cichlids endemic to Lake Tanganyika. Comparisons of this system to brood parasitism in birds may reveal broader principles that underlie the evolution of this life-history strategy in vertebrates. However, little is known about the features of the cuckoo catfish that enable this species to successfully parasitize cichlids. Here, we examine early ontogeny of the cuckoo catfish and compare it to that of its cichlid hosts as well as a non-parasitic congener. We found that cuckoo catfish embryos develop and hatch in advance of host embryos, and begin feeding on cichlid young just as they start to hatch. Overall timing of ontogeny in the cuckoo catfish was found to be similar to that of the substrate-spawning congener Synodontis lucipinnis, suggesting that more rapid development of the cuckoo catfish relative to cichlids is not a unique adaptation to brood parasitism. However, we found that cuckoo catfish progeny exhibit extensive morphological differences from S. lucipinnis, which may represent adaptations to brood parasitism. These life-history observations reveal both similarities and differences between the cuckoo catfish system and brood parasitism in other lineages.
This article is part of the theme issue ‘The coevolutionary biology of brood parasitism: from mechanism to pattern’.
Keywords: brood parasitism, cuckoo catfish, Synodontis, morphology
1. Background
Obligate interspecific brood parasitism, in which individuals of one species are completely dependent on individuals of another for parental care, is found in approximately 100 species of birds [1] but only a single species of fish, the cuckoo catfish, Synodontis multipunctatus [2–4]. The rarity of obligate brood parasitism among fishes is not owing to a lack of parental care systems; it is estimated that roughly one-quarter of teleost families have species providing some form of early offspring care [5–9]. Understanding the features that allow the cuckoo catfish to successfully use this unique life-history strategy may reveal why it is uncommon among fishes, as well as identify general features of brood parasitism systems in vertebrates.
The cuckoo catfish and its mouthbrooding cichlid hosts are endemic to Lake Tanganyika in Africa [3]. While as many as 2000 species of cichlids make up a large portion of the fish fauna in the East African Rift lakes, non-cichlid fishes account for about 30% of the fish diversity, and many of these species are catfishes [10–16]. The evolutionary success of cichlids is owing in part to their extensive levels of parental care. Mouthbrooding of young is widespread among African cichlids, and this form of oral incubation is one of the most advanced parental care systems found in fishes [3,17,18]. The offspring of maternal mouthbrooding species are nourished by large yolk sacs, and use the buccal cavity of their mother for protection until they become independent several weeks after fertilization [19]. While providing parental care helps increase the likelihood of survival of the young, it is generally considered costly to the carer, and this investment can be exploited by brood parasites. Cuckoo catfish disrupt the spawning activities of cichlids and breed concurrently with them. The catfish lay their eggs and the female mouthbrooder picks them up to be incubated along with her own eggs. Even though host and parasite eggs are fertilized at the same time, the catfish develop faster and devour the host young (often completely eliminating the host brood) while still in the mouth of the cichlid mother [3,4,7,20,21].
Previous studies on brood parasitism in both insects and birds have repeatedly shown that hosts and parasites are locked in coevolutionary arms races involving host defences and parasite counter-defences [22–27]. In different avian brood parasitism systems, parasite young express a variety of early life-history features that enable them to escape nest rejection and successfully exploit parental investment from their hosts (reviewed in [28]). For the eggs of parasites, these features include greater shell density and thickness [29], mimetic patterning to match host eggs [30], cryptic patterning to avoid host detection [31] and non-mimetic traits, such as larger size, that may be attractive to host parents [32]. Parasitic young often hatch in advance of host young, owing in part to internal incubation prior to laying [33], and in part to elevated levels of maternally derived yolk and nutrients [34]. Hatchling parasites can also exhibit morphological specializations, such as bill hooks for killing host young [35–37], mouth markings that match begging host hatchlings [38], palatal papillae to induce increases in host feeding intensity [39], wide and colourful gapes to elicit host care [40] and gape-coloured skin patches on the wing to simulate a larger gape [41]. Finally, avian brood parasite hatchlings express behaviours that increase the amount of host care they receive, such as eviction of host eggs [42,43], killing host hatchlings with bill hooks [35–37] and begging behaviour [44].
Two recent studies on the cuckoo catfish system examined adult host responses to parasitism [20,21]. However, the ontogenetic features of the cuckoo catfish that enable this species to parasitize mouthbrooding cichlids have not been described. The cuckoo catfish is a fractional spawner [4,45], which may allow the parasite to synchronize spawning with its hosts, but the details of host and parasite early life history are unknown.
Apart from the cuckoo catfish, no other members of the Synodontis genus are reported to exhibit brood parasitic behaviour. Molecular studies of Synodontis endemic to Lake Tanganyika have placed the cuckoo catfish and its closest sister species (S. granulosus) in one clade, and the remaining species in a second clade [15,46]. Synodontis lucipinnis is a substrate-spawning species in the second clade and is readily available in the aquarium trade [47]. This species is a good candidate for comparison to the cuckoo catfish as it is sympatric, not a brood parasite and readily spawns in the aquarium.
Here, we present the results of the first ontogenetic study designed to examine features of the cuckoo catfish that enable successful parasitism of cichlid hosts. To better understand the nature of host–parasite interactions between hatchling catfish and cichlids, we compared early ontogeny of the cuckoo catfish to one sympatric host and one allopatric host, from fertilization to the onset of cuckoo catfish depredation of host young. We predicted that, as in avian brood parasitism systems, the parasite would exhibit more rapid development than host species. Next, to determine if aspects of cuckoo catfish ontogeny are unique to its parasitic life history, we compared development of this species to that of the non-parasitic congener S. lucipinnis. We predicted that the cuckoo catfish would develop faster than the substrate-spawning congener that has not co-evolved to parasitize cichlids. Finally, we compared cuckoo catfish and S. lucipinnis head skeleton morphology and tooth development at the onset of feeding. We predicted that the cuckoo catfish mouthpart morphology would be modified in ways not found in S. lucipinnis that would permit depredation of host cichlid young.
2. Material and methods
(a). Study species and husbandry
Synodontis multipunctatus, the cuckoo catfish, is endemic to Lake Tanganyika, as are the mouthbrooding cichlid host species Ctenochromis horei, and the non-parasitic, substrate-spawning S. lucipinnis [2,47,48]. Ctenochromis horei is a natural host of the cuckoo catfish and is parasitized in the wild [3]. The cuckoo catfish is a generalist brood parasite that infects different species of mouthbrooding cichlids, and in a laboratory setting will parasitize cichlids that are not native to Lake Tanganyika [4,20,21,49], such as Metriaclima zebra from Lake Malawi [50]. Cuckoo catfish were kept in aquaria with C. horei and a domestic albino strain of M. zebra. We chose these host species to determine whether natural sympatric hosts expressed defences to parasitism not found in allopatric hosts that have not co-evolved with the cuckoo catfish. Breeding adults were maintained at 24–26°C on a 14 : 10 light : dark cycle, and were fed daily in the midafternoon to satiety. We performed bi-weekly water changes with dechlorinated tap water and maintained a steady pH of 8.0–9.0. Terracotta pots were placed in the aquaria and served as territorial display and spawning areas for cichlids (see [21] for more detailed information on aquarium maintenance methods).
Embryos were kept at 26.6°C in 250 ml plastic beakers suspended in a Fisher Scientific ISOTEMP 2150 re-circulating incubator. Gentle agitation of the embryos was provided by an independent aeration system in a method similar to those used in cichlid incubators [51]. Water in the beakers was replaced daily with fresh water from the aquaria of the parent fishes. Cuckoo catfish were fed frozen Daphnia, while S. lucipinnis were fed live young Artemia to satiety. The different diets were used because of the differences in gape size between species, but all growth and morphometric measurements were performed at ages prior to the onset of exogenous feeding. Cichlid embryos maintained their yolks through the duration of the study and did not require feeding. Species were raised in separate beakers except in the case of the depredation study (see §2d).
(b). Breeding colony design and egg collection
Populations of cuckoo catfish and cichlids were housed in 110 l (77 × 32 × 47 cm), 208 l (122 × 32 × 53 cm), 284 l (122 × 47 × 53 cm) and 473 l (184 × 47 × 59 cm) aquariums. Because the host species are polygynous, the cichlids were maintained at a 3 : 1 ratio (female to male), while the catfish were maintained at a 1 : 1 sex ratio. We used a ratio of host to parasite of 2 : 1 (cichlids to cuckoo catfish), which we found previously to produce good breeding results. For developmental comparisons, fertilized eggs from the cuckoo catfish, C. horei, and M. zebra were obtained following observed spawning events and approximate fertilization times were therefore known within one hour. Female cichlids carrying eggs (identified by the presence of a noticeably distended buccal cavity) were removed from the aquarium and the eggs were collected by gently holding the female's mouth open in a small volume of aquarium water while irrigating the oral cavity with a transfer pipette. Synodontis lucipinnis adults were kept in 20 l aquaria at 1 : 1 female to male ratios, and their embryos were obtained by siphoning aquarium substrate for eggs every three hours. Approximate fertilization times in this species were therefore known within three hours.
(c). Growth measurements
Live embryos were temporarily immobilized in 3% methylcellulose for measurement and photography, and subsequently returned to their incubator. Specimens were photographed using a Zeiss Stemi SV11 stereomicroscope equipped with a Zeiss AxioCam HRc camera. Cichlid host egg size was measured as the length along the animal–vegetal axis of the egg. Catfish egg size was measured as the diameter of the egg. To determine notochord length, we took straight-line measurements from the rostrum to the caudal tip of the notochord. Measurements were made using AxioVision 4 software (Zeiss). Each measurement was made in triplicate and each data point was taken as the mean of the three measures. Notochord lengths were recorded daily from 2 to 6 days post-fertilization (dpf) for the cuckoo catfish and S. lucipinnis, as both species hatch by 2 dpf. Notochord lengths were recorded daily from 3 to 6 dpf for C. horei, and unhatched specimens on 3 dpf and 4 dpf were dechorionated with forceps prior to measurement. A notochord is present in cichlids at 2 dpf, but we did not take measurements at this stage because we could not remove the chorion without damaging the embryo. Mean notochord lengths were calculated from six individuals of each species on each day, with the exception of the 2 dpf cuckoo catfish, for which only a single individual was available.
(d). Depredation study
When newly fertilized cuckoo catfish eggs were raised along with the cichlid eggs with which they were found, we did not observe depredation until 6 dpf. To observe the onset of feeding by the parasite, cuckoo catfish and host cichlid young were raised separately until 6 dpf. Cuckoo catfish were then placed in an arena (250 ml beakers filled with 50 ml of water) with M. zebra young at 6 dpf. These arenas were used to approximate the constricted environment host and parasite hatchlings experience naturally in the buccal cavity. In each arena, one cuckoo catfish was placed with a mix of 5–10 hatched and unhatched cichlid young. Interactions between the host and parasite young were observed every 30 min.
(e). Morphometric analysis
Cuckoo catfish and S. lucipinnis specimens were fixed in 4% paraformaldehyde, stained with Alcian Blue for cartilage and Alizarin Red for mineralized tissue, and then cleared with KOH/glycerol solutions using a protocol modified from [52] (electronic supplemental material, S1). To compare craniofacial morphology at the onset of exogenous feeding (6 dpf), we photographed the ventral aspect of 20 individuals from both species and measured the width of the jaw in triplicate, and took the mean of the three measures for each data point. Notochord length was measured as described above. The cuckoo catfish has a larger overall body size compared to S. lucipinnis, which could potentially explain absolute differences in jaw size. We calculated the ratio of jaw width to notochord length for each individual, and compared this ratio between cuckoo catfish and S. lucipinnis with the Wilcoxon Rank-Sum Test in the software R, v. 3.3.3, using the wilcoxon.test function [53]. To examine how jaw width covaried with notochord length in the cuckoo catfish and S. lucipinnis at 6 dpf, we performed an analysis of covariance (ANCOVA) in R. We treated jaw width as the response variable, species as a factor, and notochord length as a covariate. We ran models using the ‘aov’ command in R. Model one was jaw width ∼ notochord × species and model two was jaw width ∼ notochord + species, followed by an ANOVA comparing the two models using the ‘anova’ command. We then used the ‘lm’ command to compare the regression lines for both species. To compare the sequence of tooth appearance, we counted the number of oral teeth in cuckoo catfish and S. lucipinnis young from 5 to 12 dpf. Because acetic acid in the Alcian Blue stain can de-mineralize small bones and teeth, animals used for tooth counts were stained with Alizarin Red alone. We examined a single individual from either species at each time point.
3. Results
(a). Early host–parasite ontogeny and depredation
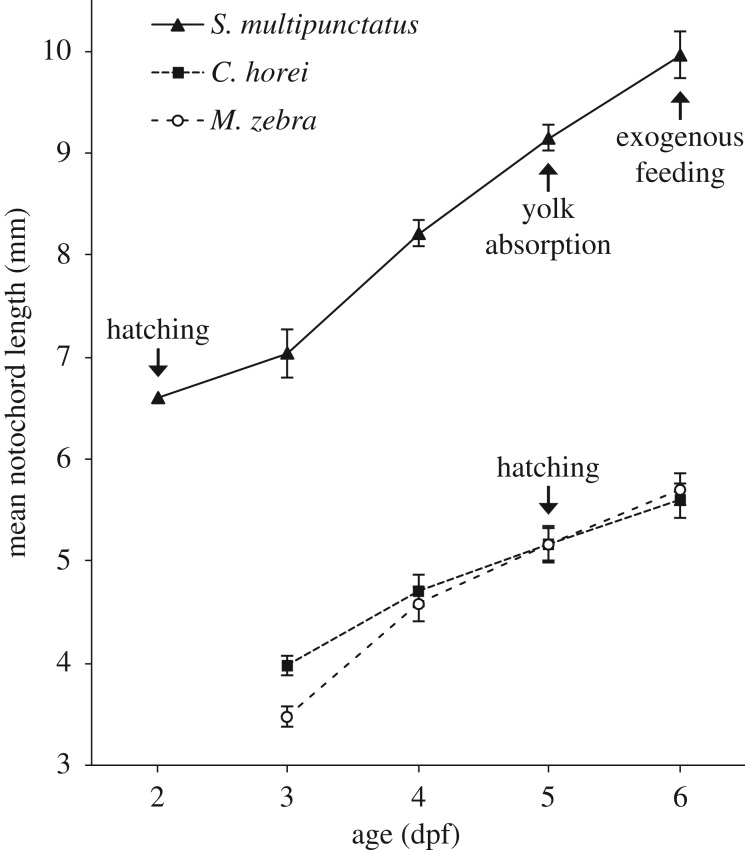
We found that cuckoo catfish young reached each observed life-history stage in advance of mouthbrooding cichlid host young (figure 1). Early development of the natural sympatric host C. horei and allopatric host M. zebra proceeds along similar trajectories of embryo size and hatching time. Cuckoo catfish young hatch 3 days in advance of the cichlid hosts (2 dpf versus 5 dpf) and maintain a larger size than the host young. The cuckoo catfish hatchlings exhaust their yolk and begin feeding exogenously at 6 dpf, while yolk absorption in the host young occurs around 21 dpf (data not shown).
Figure 1.
Early growth of the cuckoo catfish (S. multipunctatus) compared to its mouthbrooding cichlid hosts. Notochord length of the cuckoo catfish (solid line), C. horei cichlid host (narrow-spaced dashes) and M. zebra cichlid host (wide-spaced dashes) from 2 days post-fertilization (dpf) to 6 dpf. Arrows indicate the timing of key life-history events. Note that cichlid hatching occurs around the same time as the onset of exogenous feeding in the cuckoo catfish. Cichlid yolk absorption and exogenous feeding occur around 21 dpf (not shown). Error bars indicate ±1 s.d. and there are no error bars for 2 dpf cuckoo catfish because the datum is from a single specimen.
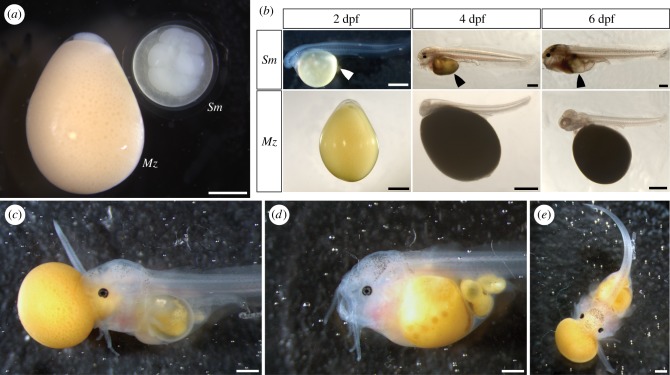
There is conspicuous disparity in egg size and shape between the cuckoo catfish and its cichlid hosts (figure 2a). The eggs of C. horei have a mean length of 4.7 mm (n = 140, standard deviation 0.18) and the eggs of M. zebra have a mean length of 4.4 mm (n = 99, standard deviation 0.23). By contrast, eggs of the cuckoo catfish have a mean diameter of 2.8 mm (n = 29, standard deviation 0.1). The cichlid eggs are opaque, teardrop-shaped, and have a rich yellow hue, while cuckoo catfish eggs are transparent, spherical, and have a pale yellow coloration. A notochord is first observable in the cuckoo catfish at 1 dpf, while in C. horei and M. zebra the notochord is first observable at 2 dpf (figure 2b). Between 5 dpf and 6 dpf the yolk of cuckoo catfish hatchlings was totally absorbed (figure 2b).
Figure 2.
Early development and depredation of host cichlid hatchlings by cuckoo catfish young. (a) Eggs of the cuckoo catfish (denoted Sm) and M. zebra (denoted Mz) shortly after fertilization. (b) Developmental sequence from 2 dpf through 6 dpf. Arrowheads indicate the location of the diminishing yolk sac of the cuckoo catfish. The M. zebra shown at 4 dpf was manually removed from its chorion prior to measuring and photographing. (c) Cuckoo catfish at 6 dpf eating a newly hatched M. zebra cichlid by biting and latching on to the head. (d) Cuckoo catfish after complete ingestion of a cichlid hatchling. (e) Cuckoo catfish consuming a second cichlid shortly after consumption of the first. 1 mm scale bars are represented by horizontal white or black lines in the bottom right of photos.
The cuckoo catfish begin feeding exogenously at 6 dpf (figure 2c). All cuckoo catfish hatchlings we observed were successful at eating host young only after biting and holding on to the head of a hatched cichlid (n = 20). Cuckoo catfish young were unable to bite unhatched cichlids or other parts of hatched cichlids. Host young attempt to escape by writhing and thrashing their tail, and were sometimes successful in freeing themselves. After latching on to the head, the cuckoo catfish ingests the whole of the cichlid by first sucking in the head, followed by the yolk, and finally the rest of the body. Total ingestion of the first cichlid hatchling by a cuckoo catfish took six hours (figure 2d). Immediately after ingesting one cichlid, cuckoo catfish are able to prey on a second cichlid (figure 2e). In instances when multiple cuckoo catfish were placed together in an arena, the young would cannibalize one another after the cichlid hosts were eaten.
(b). Comparison to non-parasitic congener
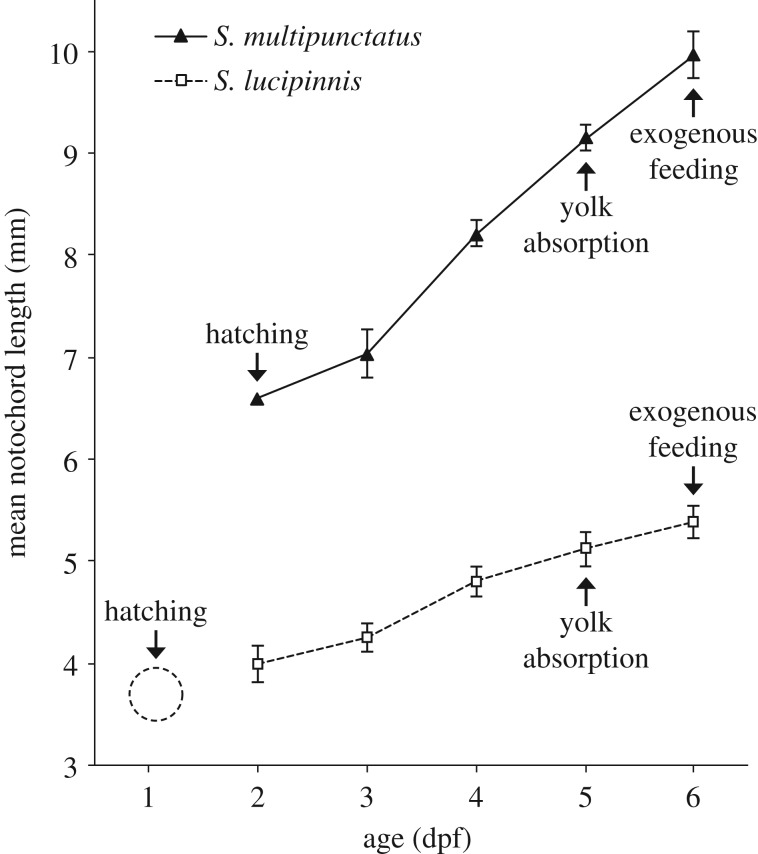
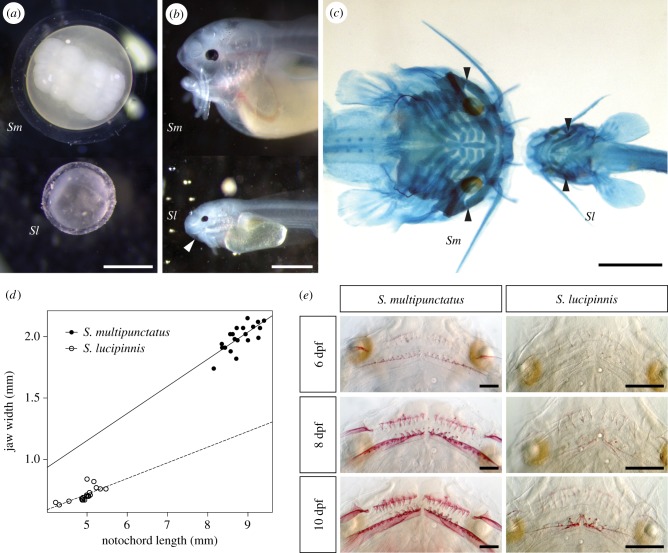
The cuckoo catfish hatches at 2 dpf while S. lucipinnis hatches a day earlier (figure 3). Both species deplete their yolk and begin exogenous feeding by 6 dpf. Shortly after fertilization, the eggs of the cuckoo catfish and S. lucipinnis are similar in colour and shape but they are different in size (figure 4a). Synodontis lucipinnis eggs are about half the size of cuckoo catfish eggs, having a mean diameter of 1.5 mm (n = 3, standard deviation 0.008). Differences in mouth position are evident by 4 dpf, when cuckoo catfish young have a terminal mouth while S. lucipinnis young have a subterminal mouth (figure 4b).
Figure 3.
Early growth of the cuckoo catfish (S. multipunctatus) compared to a non-parasitic congener. Notochord length of the cuckoo catfish (solid line) and congeneric S. lucipinnis (dashed line) plotted from 2 dpf to 6 dpf. Arrows indicate the timing of key life-history events. Hatching of S. lucipinnis occurs around 1 dpf, but no notochord length measurements at this stage were obtained. Therefore, placement of the dashed circle for S. lucipinnis hatching is for illustration purposes only. Error bars indicate ±1 s.d., and there are no error bars for 2 dpf cuckoo catfish because the datum is from a single specimen.
Figure 4.
Morphological features associated with brood parasitism in cuckoo catfish young. (a) The eggs of the cuckoo catfish (Sm) and the non-parasitic congener S. lucipinnis (Sl) shortly after fertilization. (b) Lateral view of 4 dpf hatchlings shows the terminal position of the mouth in the cuckoo catfish and a subterminal mouth position in S. lucipinnis. White arrowhead indicates mouth position. (c) Ventral view of 6 dpf young stained for cartilage with Alcian Blue shows the wider mouth of the cuckoo catfish compared to S. lucipinnis. Black arrowheads indicate jaw margin. (d) Jaw width plotted against notochord length for 20, 6 dpf cuckoo catfish and S. lucipinnis young. Solid line indicates trendline for cuckoo catfish, and dashed line indicates trendline for S. lucipinnis. (e) Comparison of oral jaw teeth between the cuckoo catfish and S. lucipinnis young at 6, 8 and 10 dpf stained with Alizarin Red to reveal mineralized structures. Scale bars denote 1 mm (a–c) and 0.2 mm (e).
Skeletal staining of bones and cartilage in 6 dpf S. lucipinnis and cuckoo catfish young reveals differences in head shape between these species (figure 4c). The cuckoo catfish has a wider jaw compared to S. lucipinnis (figure 4d). The cuckoo catfish has a mean jaw width of 1.99 mm (n = 20, standard deviation 0.11), while S. lucipinnis has a mean jaw width of 0.71 mm (n = 20, standard deviation 0.05). However, mean body size is also larger in the cuckoo catfish (8.8 mm, n = 20, standard deviation 0.35) compared to S. lucipinnis (4.9 mm, n = 20, standard deviation 0.3). To determine whether cuckoo catfish have a larger jaw width relative to body size compared to S. lucipinnis, we compared the ratio of jaw width to notochord length between these species. The cuckoo catfish has a larger median jaw width to notochord length ratio (0.23) compared with S. lucipinnis (0.14). A Wilcoxon Rank-Sum Test revealed that the ratios from either species come from distinct distributions (W = 400, n1 = 20, n2 = 20, p = 2.377 × 10−8).
Next, we sought to examine how jaw width covaries with body size in the two catfish species at 6 dpf. An ANCOVA model (notochord × species) showed a significant effect of notochord length (p < 0.001) and species (p < 0.001), but no significant interaction between them (p = 0.125). A second model (notochord + species) showed that species had a significant effect on jaw width (p < 0.001). An ANOVA revealed that removing the interaction term did not significantly affect the fit of the model (p = 0.125). We fit linear regressions separately for each species (figure 4d).
By examining the appearance of mineralized structures from 5 dpf to 12 dpf, we found that cuckoo catfish have more teeth, a wider toothed region on the dentary and a greater number of teeth on the dentary compared with the premaxilla than does S. lucipinnis (figure 4e and table 1). For more detailed sequences of head skeleton ossification see electronic supplementary material, table S1 and figures S1–S10.
Table 1.
Summary of the pattern of mineralized teeth in juvenile cuckoo catfish (S. multipunctatus) and the congeneric S. lucipinnis from 5 dpf to 12 dpf. PMAX = premaxillary teeth; DENT = dentary teeth. Data are counts from of a single specimen for each species at each age.
| days post-fertilization (dpf) | S. multipunctatus | S. lucipinnis |
|---|---|---|
| 5 | 24 oral teeth (10 PMAX, 14 DENT) | 8 oral teeth (8 PMAX, 8 DENT) |
| 6 | 40 oral teeth (16 PMAX, 24 DENT) | 14 oral teeth (8 PMAX, 6 DENT) |
| 7 | 44 oral teeth (16 PMAX, 28 DENT) | 24 oral teeth (12 PMAX, 12 DENT) |
| 8 | 64 oral teeth (24 PMAX, 40 DENT) | 24 oral teeth (12 PMAX, 12 DENT) |
| 9 | 68 oral teeth (28 PMAX, 40 DENT) | 28 oral teeth (14 PMAX, 14 DENT) |
| 10 | 72 oral teeth (32 PMAX, 40 DENT) | 32 oral teeth (18 PMAX, 14 DENT) |
| 11 | 90 oral teeth (40 PMAX, 50 DENT) | no data |
| 12 | 90 oral teeth (40 PMAX, 50 DENT) | 40 oral teeth (20 PMAX, 20 DENT) |
4. Discussion
(a). Host–parasite ontogeny and cichlid depredation
Our ability to observe cichlid spawning and cuckoo catfish parasitism events in real-time allowed us to track host–parasite interactions from the onset of development. We found striking differences in the size and visual appearance between host and parasite eggs, such that a human observer would not mistake one for the other. To bypass host defences, such as egg rejection by adults, many avian brood parasites lay eggs that mimic the size, shape, pattern or colour of their host (summarized in [54]). In the cuckoo catfish system, visual egg mimicry does not seem to be used by the parasite, and instead, the parasite eggs resemble in shape and colour the eggs of other non-parasitic Synodontis species, although the eggs of the cuckoo catfish are larger. However, we cannot rule out the possibility that cuckoo catfish eggs mimic other aspects of host eggs, such as olfactory and tactile cues. Recent work has shown that at least some host species are able to reject cuckoo catfish eggs [20]. Determining the extent to which different mouthbrooding hosts exhibit egg rejection behaviour and the relevant sensory modalities used by rejecting hosts to detect parasites merits further research.
We found that cuckoo catfish develop faster than, and hatch in advance of, C. horei and M. zebra host young. The timing of yolk depletion in the cuckoo catfish coincides with hatching in the slower-developing host cichlid young (between 5 dpf and 6 dpf). The more-developed cuckoo catfish are then able to consume the newly hatched cichlids. The timing of cichlid hatching is important because cuckoo catfish young appear unable to eat the cichlid young until they have hatched and exposed their heads. If host hatching occurred at a point any later than 5 dpf, the cuckoo catfish would have to consume one another or material pumped through the buccal cavity of the brooding adult to survive, which could decrease the overall survivorship of the catfish brood. Some species of brood parasitic birds show a similar pattern to the cuckoo catfish, wherein hatching earlier than their hosts helps facilitate removal of host young by the parasitic offspring [33,55,56]. While many avian parasites simply eject host young from the nest, the cuckoo catfish removes its host's brood by devouring them.
A previous study described a sequence of host progeny ingestion similar to our findings, whereby the parasite ingests the host body and yolk, and an analysis of cuckoo catfish stomach contents revealed that they also consume benthic materials [3]. However, we found that after the cichlid food source was depleted, the cuckoo catfish began to eat one another. Because the design of our depredation study did not allow cuckoo catfish young to leave the arena, or have the choice to consume benthic materials, the prevalence of cannibalism in a natural setting remains unknown.
Based on our finding that allopatric and sympatric hosts share a similar ontogeny and growth rate, there do not appear to be any adaptations in developmental timing used by young cichlids to escape parasitism. However, we did find that the sympatric host C. horei has larger eggs than the allopatric host M. zebra. Whether host egg size has evolved in response to cuckoo catfish parasitism is an interesting question that merits further investigation. While developmental defences on the part of the host may be lacking, adult mouthbrooders have behavioural defences to cuckoo catfish parasitism [20]. Two recent studies both found lower rates of parasitism among sympatric host species from Tanganyika when compared with allopatric host species [20,21]. Sympatric host species exhibit higher rates of egg rejection compared to naive allopatric hosts, and both natural and non-natural adult hosts with prior exposure to cuckoo catfish parasitism demonstrate a learned component and behavioural response to adult parasites [20].
(b). Comparison to non-parasitic congener
We found that the cuckoo catfish egg diameter is almost twice that of S. lucipinnis. To determine how these egg sizes compared to those of other non-parasitic members of the genus, we reviewed the literature for congeners with reported egg diameters (table 2) [57–62]. We found mean egg diameter values for seven additional Synodontis species, including the largest member of the genus, ranging from 1.0 mm to 1.6 mm. While the mean diameter of S. lucipinnis eggs (1.5 mm) falls within this range, the eggs of the cuckoo catfish are not typical of the genus, having a mean diameter of 2.8 mm. Further studies are needed to assess the significance of egg size in cuckoo catfish brood parasitism. This larger egg size might increase the frequency with which mouthbrooding hosts take up parasite eggs. Another possibility is that the larger egg size is necessary to produce predatory young big enough to eat cichlid young.
Table 2.
Mean egg diameter of the cuckoo catfish (S. multipunctatus) and non-parasitic congeners.
| species | mean egg diameter (mm) | locality | reference |
|---|---|---|---|
| S. multipunctatus | 2.8 | Lake Tanganyika | this study |
| S. nigrita | 1.6 | West Africa | [60] |
| S. lucipinnis | 1.5 | Lake Tanganyika | this study |
| S. nigromaculatus | 1.4 | East Africa | [58] |
| S. eupterus | 1.3 | West Africa | [61] |
| S. serratus | 1.2 | West Africa | [59] |
| S. schall | 1.2 | West Africa | [62] |
| S. ocellifer | 1.0 | West Africa | [57] |
| S. velifer | 1.0 | West Africa | [57] |
Our results show that key features of ontogenetic timing that allow cuckoo catfish young to depredate host cichlid embryos are also present in S. lucipinnis. Hatching occurs earlier than cichlids in both catfish species (1 dpf in S. lucipinnis and 2 dpf in the cuckoo catfish), and yolk absorption is completed at the same time in both catfish species (6 dpf). Both species also begin exogenous feeding at 6 dpf. This suggests that S. lucipinnis is not prevented from depredating cichlid young by limits imposed by their ontogeny. The timing of ontogenetic events in S. lucipinnis and size at hatching are similar to that reported for S. nigromaculatus [58].
It is therefore likely that the overall timing of the ontogeny of the cuckoo catfish did not evolve expressly for brood parasitism, but instead follows the ontogenetic sequence expected for the genus Synodontis. Thus, the early hatching time of the cuckoo catfish relative to its hosts represents an exaptation to brood parasitism.
We found extensive gross morphological differences between young cuckoo catfish and S. lucipinnis, particularly in head shape. When comparing S. lucipinnis side by side with cichlid young at 6 dpf, it appears that the catfish is too small to grasp and consume the cichlids (M. Brent Hawkins 2007 personal observation). Not only are cuckoo catfish young larger than those of S. lucipinnis, the head width of the parasite is also proportionally larger. The larger body size and wider head of the cuckoo catfish may be the key characteristics that allow this species to avoid gape limitation and eat cichlid hatchlings. These differences in craniofacial morphology established during embryogenesis may be related to diet later in life. Food resources acting as the selective agents on feeding morphology have been shown in many vertebrates, from Darwin's finches [63] to East African cichlids [51,64,65]. Synodontis lucipinnis have variable diets consisting of algae and small invertebrates from 6 dpf onward, while at about two to three weeks of age the cuckoo catfish leave the mouth of the adult cichlid and switch their feeding from cichlid young to small invertebrates, with specialized feeding on gastropods (Neothauma tanganyicense) for the remainder of their lives [66,67]. Future studies should investigate how relative jaw width changes over ontogeny in the cuckoo catfish and S. lucipinnis, to see if the relative jaw widths of these two species remain divergent into adulthood, or if they converge as they reach adulthood.
The overall pattern of ossification is similar in both species of Synodontis, but there is substantial divergence in the time of appearance of mineralized structures and number and position of teeth. In general, skeletal elements ossify earlier in the cuckoo catfish than in the non-parasitic congener. While timing of ossification is the factor with the most apparent difference between these two species, the much more interesting and pertinent difference is in structures that appear simultaneously in both species: the oral teeth. Both S. lucipinnis and the cuckoo catfish possess Alizarin-positive oral teeth at 5 dpf, but the cuckoo catfish has far more (24 opposed to 8). Another interesting observation is that the cuckoo catfish has a greater number of dentary teeth than premaxillary teeth (14 opposed to 10), which is in contrast to the condition in S. lucipinnis where equal numbers of teeth (4) are on each jaw element. Both of these trends continue through 14 dpf. The tooth-bearing region of the dentary bone is also comparatively larger in the cuckoo catfish than in S. lucipinnis. Having a large jaw, coupled with an increased number of dentary teeth spread out over a larger area of the jaw, may allow the cuckoo catfish to grip the head of the host cichlid hatchlings.
In certain cases, the young of avian brood parasites bear morphological adaptations of the mouthparts that allow them to eliminate host offspring and monopolize parental investment. Chicks of the Greater Honeyguide, indicator, hatch with prominent bill hooks, which they use to attack and kill host hatchlings [35,36]. These bill hooks are not related to the trophic modality of the adult, and they are no longer detectable by 30 days after hatching. A similar behaviour is suspected to occur in the striped cuckoo, Tapera naevia, whose hatchlings also have bill hooks and are frequently found with dead host young [37].
It is an open question as to whether the morphological features of the young cuckoo catfish represent adaptations to brood parasitism that emerged in an evolutionary arms race with cichlid young, or if they represent an exaptation that allowed the species to depredate host young. Distinguishing between these possibilities might be possible by examining the juvenile morphology of S. granulosus, the closest extant relative of the cuckoo catfish. Synodontis granulosus adults closely resemble the cuckoo catfish, but this species is not reported to be a brood parasite. If the 6 dpf morphology of non-parasitic S. granulosus were similar to the cuckoo catfish, it would suggest that the morphology arose prior to the origin of brood parasitism.
(c). Brood parasitism in birds and fishes
Like avian brood parasites, the cuckoo catfish express features in early ontogeny that enable them to successfully parasitize their hosts. Several of these early life-history features are shared between cuckoo catfish and avian brood parasites. In both systems, parasitic young develop faster than and hatch in advance of host offspring. This allows avian parasites to eliminate host young, either by eviction or stabbing, but it is unclear if earlier hatching is advantageous in the case of the cuckoo catfish. Parasitic hatchlings are larger than host hatchlings in both bird and catfish systems. In the case of avian parasites, larger hatchlings are able to create a larger begging stimulus. For cuckoo catfish, the larger size of the parasitic young enables them to feed on host progeny. Finally, specializations of mouthpart morphology are found in the hatchlings of the cuckoo catfish as well as some avian brood parasites. Striped cuckoo and honeyguide hatchlings develop sharp bill hooks that they use to kill host young, while cuckoo catfish young have a wide jaw and expanded dentition used to latch onto and eat cichlid hatchlings whole.
We have also identified key differences between the cuckoo catfish and avian brood parasitism systems. While visual egg mimicry and cryptic egg colouration are prevalent among avian brood parasites, the eggs of the cuckoo catfish do not visually resemble those of their cichlid hosts and are not camouflaged. A conspicuous egg may in fact be advantageous for the cuckoo catfish, as successful brood parasitism is dependent on mouthbrooding host females locating and taking up parasitic eggs. Another difference between avian and cuckoo catfish brood parasitism is the nature of the resource directly exploited by parasitic young. Avian brood parasites beg for food from host parents, while cuckoo catfish consume their foster siblings. In avian systems, this can result in a trade-off regarding the fate of host young, as parasites compete with host young for feedings, but also need their assistance to produce a begging stimulus. Such a trade-off may not be in play in the cuckoo catfish system, as parasitized mouthbrooding cichlids are frequently found carrying only a few, large cuckoo catfish juveniles that have consumed the entire host brood.
While early host–parasite interactions between hatchling cuckoo catfish and cichlids seem to be limited to depredation, interactions between young cuckoo catfish and host adults may be more complex. Do cuckoo catfish manipulate adult hosts to provide protection in the buccal cavity even after the host brood is eliminated? Is there a cuckoo catfish analogue to the begging behaviour seen in avian brood parasites? Many questions remain to be investigated in the cuckoo catfish system.
5. Conclusion
The cuckoo catfish system provides a unique opportunity to investigate vertebrate brood parasitism in a laboratory setting. This laboratory-based model complements field studies of brood parasitism, and allows the examination of host–parasite interactions across ontogeny, control of variables such as host to parasite ratio and host size, and the use of recording equipment to observe adult host–parasite interactions over long time scales. Using this system, we have identified parallels between cuckoo catfish and avian brood parasitism, such as the rapid development of the parasite compared to the host. We have also identified differences, such as visual egg mimicry, which is absent in the cuckoo catfish but vital to the success of certain avian brood parasites. Further study of this system may yield more parallels and reveal general features that underlie the evolution of brood parasitism.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful for our many undergraduate research assistants for their hard work and dedication over the years in helping to maintain the fish populations. We thank the National Science Foundation's Research Experiences for Undergraduates (REU) program and the University of Colorado for the numerous funding opportunities available to support undergraduate research, specifically the Undergraduate Research Opportunities Program (UROP). Lastly, we would like to acknowledge the reviewers of this article.
Ethics
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in these studies involving animals were in accordance with the ethical standards of the University of Colorado's Institutional Animal Care and Use Committee (IACUC).
Data accessibility
Additional data available at as part of the electronic supplementary material.
Authors' contributions
M.S.C., M.B.H. and D.W.S.: contributions to design of study, acquisition of data, analysis and interpretation of data, drafting of article, revisions and final approval. A.C. (deceased 15 May 2018): contributions to design of study, acquisition of data, analysis and interpretation of data and revisions to initial drafts. However, he could not give final approval owing to his death four months before final submission.
Competing interests
We have no competing interests.
Funding
Funding for this research was provided to M.B.H. by the National Science Foundation's Research Experiences for Undergraduates (REU) program and the Undergraduate Research Opportunities Program (UROP) of the University of Colorado at Boulder. M.S.C. was provided funding by the University of Colorado Graduate School and the University of Colorado Department of Ecology and Evolutionary Biology.
References
- 1.Ortega CP. 2004. Caregiving brood parasitism among Birds. In Encyclopedia of animal behavior (ed. Bekoff M.), pp. 177–180. Westport, CT: Greenwood Press. [Google Scholar]
- 2.Boulenger GA. 1898. Report on the fishes recently obtained by Mr. J.E.S. Moore in Lake Tanganyika. Proc. Zool. Soc. Lond. 1898, 494–497. [Google Scholar]
- 3.Sato T. 1986. A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323, 58–59. ( 10.1038/323058a0) [DOI] [PubMed] [Google Scholar]
- 4.Wisenden BD. 1999. Alloparental care in fishes. Rev. Fish Biol. Fish. 9, 45–70. ( 10.1023/A:1008865801329) [DOI] [Google Scholar]
- 5.Breder C Jr, Rosen D. 1966. Modes of reproduction in fishes. Garden City, NY: Natural History Press. [Google Scholar]
- 6.Blumer LS. 1982. A bibliography and categorization of bony fishes exhibiting parental care. Zool. J. Linnean Soc. 75, 1–22. ( 10.1111/j.1096-3642.1982.tb01939.x) [DOI] [Google Scholar]
- 7.Wootton RJ, Smith C. 2014. Reproductive biology of teleost fishes. New York, NY: John Wiley & Sons. [Google Scholar]
- 8.Crawford SS, Balon EK. 1996. Cause and effect of parental care in fishes. Adv. Study Behav. 25, 53–107. ( 10.1016/S0065-3454(08)60330-7) [DOI] [Google Scholar]
- 9.Pandian TJ. 2010. Sexuality in fishes. New York, NY: CRC Press. [Google Scholar]
- 10.Danley PD, Kocher TD. 2001. Speciation in rapidly diverging systems: lessons from Lake Malawi. Mol. Ecol. 10, 1075–1086. ( 10.1046/j.1365-294X.2001.01283.x) [DOI] [PubMed] [Google Scholar]
- 11.Barlow GW. 2000. The cichlid fishes: nature's grand experiment in evolution. Cambridge, MA: Perseus Publishing. [Google Scholar]
- 12.Lande R, Seehausen O, van Alphen JJM. 2001. Mechanisms of rapid sympatric speciation by sex reversal and sexual selection in cichlid fish. Genetica 112–113, 435–443. ( 10.1023/A:1013379521338) [DOI] [PubMed] [Google Scholar]
- 13.Kocher TD. 2004. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288–298. ( 10.1038/nrg1316) [DOI] [PubMed] [Google Scholar]
- 14.Sturmbauer C, Husemann M, Danley P. 2011. Explosive speciation and adaptive radiation of East African cichlid fishes. In Biodiversity hotspots (eds Zachos FE, Habel JC), pp. 333–362. Berlin, Germany: Springer. [Google Scholar]
- 15.Koblmuller S, Sturmbauer C, Verheyen E, Meyer A, Salzburger W. 2006. Mitochondrial phylogeny and phylogeography of East African squeaker catfishes (Siluriformes: Synodontis). BMC Evol. Biol. 6, 49 ( 10.1186/1471-2148-6-49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snoeks J. 2000. How well known is the ichthodiversity of the large East African lakes? In Advances in ecological research - ancient lakes: biodiversity, ecology, and evolution (eds Rossiter A, Kawanabe H), pp. 17–38. New York, NY: Academic Press. [Google Scholar]
- 17.Keenleyside MH. 1979. Diversity and adaption in fish behavior. Berlin, Germany: Springer. [Google Scholar]
- 18.Ochi H, Rossiter A, Yanagisawa Y. 2001. Biparental mouthbrooding of the catfish Phyllonemus filinemus in Lake Tanganyika. Ichthyol. Res. 48, 225–229. ( 10.1007/s10228-001-8140-7) [DOI] [Google Scholar]
- 19.Balon EK. 1977. Early ontogeny of Labeotropbeus Ahl, 1927 (Mbuna, Cichlidae, Lake Malawi), with a discussion on advanced protective styles in fish reproduction and development. Environ. Biol. Fishes 2, 147–176. ( 10.1007/BF00005370) [DOI] [Google Scholar]
- 20.Blažek R, Polačik M, Smith C, Honza M, Meyer A, Reichard M. 2018. Success of cuckoo catfish brood parasitism reflects coevolutionary history and individual experience of their cichlid hosts. Sci. Adv. 4, 4380 ( 10.1126/sciadv.aar4380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MS, Hawkins MB, Knox-Hayes J, Vinton AC, Cruz A. 2018. A laboratory study of host use by the cuckoo catfish Synodontis multipunctatus. Environ. Biol. Fishes 101, 1417–1425. ( 10.1007/s10641-018-0788-1) [DOI] [Google Scholar]
- 22.Davies NB, Bourke AFG, Brooke MDL. 1989. Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 4, 274–278. ( 10.1016/0169-5347(89)90202-4) [DOI] [PubMed] [Google Scholar]
- 23.Dawkins R, Krebs JR. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511. ( 10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 24.Langmore NE, Hunt S, Kilner RM. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157 ( 10.1038/nature01460) [DOI] [PubMed] [Google Scholar]
- 25.Rothstein SI. 1990. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508. ( 10.1146/annurev.es.21.110190.002405) [DOI] [Google Scholar]
- 26.Shokri BN, Staines M, Vo C, Puiu N, da Silva CRB, Harrington J, Wilkinson S, Pratt K, Schwarz MP. 2017. Sex ratios in a socially parasitic bee and implications for host–parasite interactions. J. Insect Behav. 30, 130–137. ( 10.1007/s10905-017-9603-7) [DOI] [Google Scholar]
- 27.Soler M. 2018. Avian brood parasitism: behaviour, ecology, evolution and coevolution. Berlin, Germany: Springer. [Google Scholar]
- 28.Spottiswoode CN, Kilner RM, Davies NB. 2012. Brood parasitism. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M), pp. 226–356. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Picman J, Pribil S. 1997. Is greater eggshell density an alternative mechanism by which parasitic cuckoos increase the strength of their eggs? J. Ornithol. 138, 531–541. ( 10.1007/bf01651384) [DOI] [Google Scholar]
- 30.Brooke MDL, Davies NB. 1988. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632. ( 10.1038/335630a0) [DOI] [Google Scholar]
- 31.Langmore NE, Stevens M, Maurer G, Kilner RM. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468. ( 10.1016/j.anbehav.2009.06.003) [DOI] [Google Scholar]
- 32.Alvarez F. 2000. Response to Common Cuckoo Cuculus canorus model egg size by a parasitized population of Rufous Bush Chat Cercotrichas galactotes. Ibis 142, 683–686. ( 10.1111/j.1474-919X.2000.tb04470.x) [DOI] [Google Scholar]
- 33.Birkhead TR, Hemmings N, Spottiswoode CN, Mikulica O, Moskát C, Bán M, Schulze-Hagen K. 2011. Internal incubation and early hatching in brood parasitic birds. Proc. R. Soc. B 278, 1019–1024. ( 10.1098/rspb.2010.1504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hargitai R, Moskát C, Bán M, Gil D, López-Rull I, Solymos E. 2010. Eggshell characteristics and yolk composition in the common cuckoo Cuculus canorus: are they adapted to brood parasitism? J. Avian Biol. 41, 177–185. ( 10.1111/j.1600-048X.2009.04818.x) [DOI] [Google Scholar]
- 35.Spottiswoode CN, Koorevaar J. 2011. A stab in the dark: chick killing by brood parasitic honeyguides. Biol. Lett. 8, 241–244. ( 10.1098/rsbl.2011.0739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedmann H. 1955. The honey-guides. Bull. US Nat. Mus. 208, 1–292. ( 10.5479/si.03629236.208.1) [DOI] [Google Scholar]
- 37.Morton ES, Farabaugh SM. 1979. Infanticide and other adaptations of the nestling Striped Cuckoo Tapera naevia. Ibis 121, 212–213. ( 10.1111/j.1474-919X.1979.tb04965.x) [DOI] [Google Scholar]
- 38.Hauber ME, Kilner RM. 2007. Coevolution, communication, and host chick mimicry in parasitic finches: who mimics whom? Behav. Ecol. Sociobiol. 61, 497–503. ( 10.1007/s00265-006-0291-0) [DOI] [Google Scholar]
- 39.Soler M, Martinez JG, Soler JJ, Møller AP. 1995. Preferential allocation of food by magpies Pica pica to great spotted cuckoo Clamator glandarius chicks. Behav. Ecol. Sociobiol. 37, 7–13. ( 10.1007/bf00173893) [DOI] [Google Scholar]
- 40.Kilner RM, Noble DG, Davies NB. 1999. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature 397, 667 ( 10.1038/17746) [DOI] [Google Scholar]
- 41.Tanaka KD, Ueda K. 2005. Horsfield's hawk-cuckoo nestlings simulate multiple gapes for begging. Science 308, 653 ( 10.1126/science.1109957) [DOI] [PubMed] [Google Scholar]
- 42.Anderson MG, Moskát C, Bán M, Grim T, Cassey P, Hauber ME. 2009. Egg eviction imposes a recoverable cost of virulence in chicks of a brood parasite. PLoS ONE 4, e7725 ( 10.1371/journal.pone.0007725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenner E. 1788. XIV. Observation on the natural history of the cuckoo. By Mr. Edward Jenner. In a letter to John Hunter, Esq. F. R. S. Phil. Trans. R. Soc. Lond. 78, 219–237. ( 10.1098/rstl.1788.0016) [DOI] [Google Scholar]
- 44.Davies NB, Kilner RM, Noble DG. 1998. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proc. R. Soc. Lond. B 265, 673–678. ( 10.1098/rspb.1998.0346) [DOI] [Google Scholar]
- 45.Cruz A, Knox J, Pawlowski S. 2004. Caregiving-brood parasitism in freshwater fish. In Encyclopedia of animal behavior (ed. Bekoff M.), pp. 180–182. Westport, CT: Greenwood Press. [Google Scholar]
- 46.Day JJ, Wilkinson M. 2006. On the origin of the Synodontis catfish species flock from Lake Tanganyika. Biol. Lett. 2, 548–552. ( 10.1098/rsbl.2006.0532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright JJ, Page LM. 2006. Taxonomic revision of Lake Tanganyikan Synodontis (Siluriformes: Mochokidae). Florida Mus. Nat. Hist. Bull. 46, 99–154. [Google Scholar]
- 48.Günther A. 1894. Descriptions of the reptiles and fishes collected by Mr. E. Coode-Hore on Lake Tanganyika. Proc. Zool. Soc. Lond. 1893, 628–632. [Google Scholar]
- 49.Loiselle PV. 1998. Egg holders: how Synodontis catfish can use cichlids from two continents to hatch their young. In Aquarium fish magazine, pp. 5–6. Irvine, CA: Fancy Publications. [Google Scholar]
- 50.Boulenger GA. 1899. A revision of the African and Syrian fishes of the Family Cichlida.–Part II.1. Proc. Zool. Soc. Lond. 67, 98–144. ( 10.1111/j.1469-7998.1899.tb06852.x) [DOI] [Google Scholar]
- 51.Albertson RC, Streelman JT, Kocher TD. 2003. Genetic basis of adaptive shape differences in the cichlid head. J. Hered. 94, 291–301. ( 10.1093/jhered/esg071) [DOI] [PubMed] [Google Scholar]
- 52.Miyake T, Hall BK. 1994. Development of in vitro organ culture techniques for differentiation and growth of cartilages and bones from teleost fish and comparisons with in vivo skeletal development. J. Exp, Zool 268, 22–43. ( 10.1002/jez.1402680105) [DOI] [Google Scholar]
- 53.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 54.Feeney WE, Welbergen JA, Langmore NE. 2014. Advances in the study of coevolution between avian brood parasites and their hosts. In Annual review of ecology, evolution, and systematics, vol. 45 (ed. Futuyma DJ.), pp. 227–246. Palo Alto, CA: Annual Reviews. [Google Scholar]
- 55.Payne RB. 1977. The ecology of brood parasitism in birds. Annu. Rev. Ecol. Syst. 8, 1–28. ( 10.1146/annurev.es.08.110177.000245) [DOI] [Google Scholar]
- 56.Davies N. 2000. Cuckoos, cowbirds and other cheats. London, UK: T & A. D. Poyser Ltd. [Google Scholar]
- 57.Ofori-Danson PK. 1992. Ecology of some species of catfish Synodontis (Pisces: Mochocidae) in the Kpong Headpond in Ghana. Environ. Biol. Fishes 35, 49–61. ( 10.1007/BF00001157) [DOI] [Google Scholar]
- 58.Van der Waal B. 1986. Note on artificial fertilization and early development of Synodontis nigromaculatus (Pisces: Mochokidae). South Afr. J. Zool. 21, 269–271. ( 10.1080/02541858.1986.11447994) [DOI] [Google Scholar]
- 59.Abdel-Latif A-F. 1984. Lake Nasser (Egypt). In Status of African reservoir fisheries (Etat Des Pêcheries Dans Les Réservoirs d'Afrique) (eds Kapetsky JM, Petr T), pp. 193–246. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 60.Olele NF, Etim L. 2011. Some aspects of the biology of Synodontis nigrita (Curvier and Valencienes, 1864) in Onah lake, Asaba, Nigeria. ARPN J. Agric. Biol. Sci. 6, 56–63. [Google Scholar]
- 61.Shinkafi B, Mamman T. 2012. Gonadosomatic index, fecundity and egg size of (Synodontis) S. eupterus (Boulenger) in River Rima, north western Nigeria. In 26th annual conference of the fisheries society of Nigeria (FISON), pp. 135–143. Minna, Nigeria. [Google Scholar]
- 62.Albaret J-J. 1982. Reproduction et fécondité des poissons d'eau douce de Côte d'Ivoire. Revue d'hydrobiologie tropicale 15, 347–371. [Google Scholar]
- 63.Grant PR, Grant BR. 1997. Genetics and the origin of bird species. Proc. Natl Acad. Sci. USA 94, 7768–7775. ( 10.1073/pnas.94.15.7768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albertson RC, Streelman JT, Kocher TD. 2003. Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc. Natl Acad. Sci. USA 100, 5252–5257. ( 10.1073/pnas.0930235100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Streelman JT, Danley PD. 2003. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 18, 126–131. ( 10.1016/S0169-5347(02)00036-8) [DOI] [Google Scholar]
- 66.Coulter GW. 1991. Lake Tanganyika and its life. Oxford, UK: Oxford University Press.
- 67.Poll M. 1953. Poissons non Cichlidae. In Resultats scientifiques de l'exploration hydrobiologique du lac Tanganika (1946–1947), volume 3, pp. 1–251. Brussels, Belgium: Institut Royal des Sciences Naturelles de Belgique. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data available at as part of the electronic supplementary material.