Abstract
Brood parasitism is a specialized form of parasitism in which the offspring of a parasite develops on the food provisions gathered by a host species for its own young. Obligate brood parasitic lineages have lost the ability to acquire provisions for their young and thus rely entirely on the location of an appropriate host to serve as a food-provider. Solitary bees provide some of the most fascinating examples of brood parasitism in animals. Most solitary bees build and provision their own nests. Some, however, usurp the nests of other species of bees. These brood parasites, or ‘cuckoo’ bees, deposit their eggs on the pollen provisions collected by a host bee for her own offspring. The provisions stored by the host bee are not sufficient to sustain the development of both the host's larva and that of the brood parasite and the parasite must kill the offspring of its host in order to obtain enough nourishment to complete its development. As a consequence, there is fierce competition between the host bee seeking to protect her nest from attack and the brood parasite seeking to avoid detection by the host in order to successfully deposit her eggs in an appropriate nest. In this paper, I review the behaviours that allow brood parasitic bees to escape detection by their hosts. Identifying these behaviours, and placing them within the general context of strategies employed by brood parasitic bees to parasitize the nests of their hosts, is key to understanding how brood parasitic lineages may have evolved from nest-building ancestors, decrypting the selective pressures that drive evolutionary transitions from one strategy to another and, more broadly, revealing how similar selective pressures in widely divergent lineages of animals have given rise to remarkably convergent behaviours.
This article is part of the theme issue ‘The coevolutionary biology of brood parasitism: from mechanism to pattern’.
Keywords: bee nests, cleptoparasitism, cuckoo bees, Hymenoptera, solitary bees
1. Brood parasitism: a definition
The manner in which insects attempt to assure a source of nourishment for their offspring varies widely from one lineage to another. Some insects simply locate an appropriate food source and deposit their eggs on or near it. Many butterflies, for example, seek out a suitable host plant and lay their eggs either singly or in clutches upon the leaves or stems; upon eclosion, the emerging caterpillars find themselves directly on their food source [1]. Similarly, saproxylic beetles deposit their eggs on decomposing wood [2], blowflies on carrion [3], lice directly on the body of an appropriate host [4] and most dragonflies into a body of freshwater, where the predaceous larvae seek out their own prey [5]. An alternative strategy, one exhibited most notably by the majority of aculeate Hymenoptera, is one in which the adult insect not only seeks out an appropriate food source but actually gathers and centralizes these provisions in a nest, where they are stored for future offspring. Eggs of such insects are deposited on the food store in the nest, which, in addition to being provisioned, has the advantage of providing a first line of defence against predators and, in principal, competitors for those provisions.
A nest, offering both shelter and a source of food, thus represents a rich resource and many insects rely entirely on the nests built by other species, and more specifically their contents, as a means of assuring the development of their own young. Brood predators, such as blister beetles (Meloidae), as well as various types of parasites including ectoparasites, such as Varroa destructor Anderson and Trueman, 2000; endoparasites, including the bee fungal parasite Nosema; and parasitoids, such as the majority of bee flies (Bombyliidae) and the ichneumonid genus Grotea, all target developing brood in the nests of various species of Hymenoptera [6–9]. Brood parasitism, however, is a specialized form of parasitism in which the parasite targets not the brood of a host but rather the provisions stored by the host for its own offspring. The offspring of the parasite develops on the provisions gathered by a host species for its own young and the adult parasite never comes into contact with its own offspring. This life-history strategy is perhaps most well documented in birds, where a female cuckoo lays her egg in a host nest and then disappears, leaving the host parents to nourish her offspring in her place [10]. Yet brood parasitism is widespread in insects and has evolved multiple times independently in diverse lineages including solitary bees [11–13], solitary wasps [14] and lycaenid butterflies [15–17].
In agreement with other authors (e.g. Michener [11]), a distinction is made here between brood parasitism (also sometimes called ‘cleptoparasitism’ when used in reference to solitary bees and wasps) and social parasitism, in which a parasite enters the colony of a social species, replaces the queen and then uses the worker caste of the host species to rear her own offspring. For example, bumblebees belonging to the subgenus Bombus (Psithyrus) are social parasites of other species of Bombus [11]. Psithyrus queens invade a suitable host nest, either kill or replace the host queen and, using a combination of behavioural dominance and chemical signalling, manipulate host workers into rearing her own offspring [18]. In some cases, the host queen may even remain in the nest alongside the parasitic queen but becomes submissive, adopting the role of a worker [19]. A social parasite thus either literally or functionally replaces the head of a social insect colony, remains in the host's nest and uses the host queen's workers to rear her own offspring. By contrast, a brood parasite, targeting the provisions gathered by the host for her own young, deposits an egg in a host nest but never occupies the nest herself. In this review, brood parasitism thus explicitly concerns those cases in which an adult parasite deposits an egg on the provisions gathered by a host species and then leaves the host's nest, leaving her offspring to develop on the appropriated food source. Although both social parasitism and brood parasitism most probably evolved in response to a similar set of selective pressures, namely competition for limited resources, the resulting strategies for appropriating the resources assembled by a host, as well the social nature of the hosts themselves, are entirely different. Thus social parasitism, described almost exclusively from the social Hymenoptera and reviewed extensively elsewhere [20–23], will not be discussed here. Ectoparasitism, endoparasitism and parasitoidism have also been excluded, as they concern an appropriation not of resources gathered by a host for its young but rather of the brood itself. Finally, only interspecific obligate brood parasites will be considered, thus excluding cases of intraspecific resource robbing and/or facultative parasitism, both of which have been reported in multiple lineages of bees, wasps and ants [24].
As a consequence of their unique life-history strategy, brood parasitic insects have evolved a suite of morphological, behavioural and physiological adaptations that allow them to fly under the radar, so to speak, escaping detection by a host and increasing the probability of survival by their offspring. In this article, I focus on the strategies exhibited by obligately brood parasitic bees to avoid detection by their hosts, and present some of the remarkable evolutionary convergences related to detection avoidance in brood parasitic lineages ranging from bees to vertebrates.
2. Brood parasitic strategies in solitary bees
Solitary bees build nests in which their young develop. Most species excavate their nests in the ground. Others build their nests in soft wood, pre-existing cavities or simply mounted on the external surfaces of trees, stones, buildings or other structures. In certain species, for example, in members of the family Megachilidae, nests may be made with a diversity of extraneous material, including leaves, flower petals, glandular secretions, plant fibres and plant resins (figure 1) [11]. Regardless of the final form, all nests have a single function: to provide food and shelter for developing bee larvae. Nests typically contain a series of between one and ten compartments, or brood cells, each of which is provisioned by the adult female with a mixture of pollen and nectar. After the female has provisioned a cell, she deposits a single egg on the pollen mass within. The brood cell is then closed and the egg is left to develop on the pollen mass. Upon eclosion, a succession of larval instars feed on the mixture of pollen and nectar, usually without ever coming into contact with the adult female (although see [25], for examples of both social species and the solitary forms of social species that open nest cells to inspect developing brood). After metamorphosis, the adult bee chews or pushes its way out of the nest.
Figure 1.
Bee nests are diverse in form. (a) Cross-section of the snail shell nest of Osmia bicolor, showing the yellow pollen mass in the innermost whorl of the shell, a single larva developing on the pollen and a nest plug of pebbles and other debris. (b) Cross-section of the nest of Andrena vaga, excavated in sand. Shown are the round yellow pollen mass and the egg of A. vaga deposited on top. (c) Cross-section of the nest of Megachile alpicola, built in a hollow plant stem. The nest is built from multiple layers of leaves cut from plants. The yellow-brown pollen mass is visible at the right end of the cell, upon which a single larva is seen. (d) Excavated nest cell of Colletes cunicularius. This nest cell, built in the sand, is lined with secretions produced by the Dufour's gland of the nesting female. When these secretions dry, they form a thin, cellophane-like layer that waterproofs the cell. The pollen mass, visible through the lining, is seen to the left of the photo. (e) Anthidiellum strigatum, finalizing a nest built from plant resin. All photos: Entomologie/Botanik, ETH Zürich/Albert Krebs.
Not all solitary bees, however, are nest building. So-called brood parasites, or cuckoo bees, locate the nest of an appropriate host bee using a combination of visual and olfactory cues and deposit an egg in a single cell of the host's nest [26]. The offspring of the parasite then feeds and completes its development on the provisions gathered by the host bee for its own offspring. In solitary bees, brood parasitic lineages have evolved independently from nest-making ancestors approximately 18 times: three independent origins are known in the family Apidae [12,27], five in Megachilidae [13,28], approximately nine in Halictidae [11,29,30] and at least one in Colletidae [31,32]. Over the course of evolutionary time, morphological structures associated with nesting behaviour, no longer under positive selection in brood parasitic lineages, have been lost. For example, the scopa, a specialized brush of hairs used for pollen collection in most nest-building bees, as well the pygidial and basitibial plates, associated with the construction of nest cells, are absent or reduced in obligate brood parasites (figure 2) [11]. These lineages have thus entirely lost the ability to gather provisions or even build their own nests. As a consequence, their reproductive success is inextricably tied to their ability to locate an appropriate host nest and successfully deposit their eggs inside.
Figure 2.

Brood parasitic bees most often exhibit reduced pilosity compared with nesting bees and have lost other structures associated with nesting behaviour, such as the pollen-collecting scopa and pygidial and basitibial plates. (a) Sleeping female Ammobatoides abdominalis; (b) Stelis signata; (c) sleeping male and female Coelioxys afra; (d) female Melecta luctuosa; (e) female Epeoloides coecutiens; (f) Nomada fabriciana, lurking outside the nest of Andrena bicolor. All photos: Entomologie/Botanik, ETH Zürich/Albert Krebs.
The caveat: the provisions in each brood cell are only sufficient to sustain the growth of a single larva. In other words, the host's offspring and the offspring of the brood parasite cannot both complete their life cycle on a single pollen mass [24]. Both the host bee and the brood parasite compete to assure the survival of their own offspring, the host bee by defending her nest from marauding brood parasites and the brood parasite by invading a suitable nest, depositing an egg inside and assuring the destruction of the offspring of the host bee, all without discovery by the host. In order to usurp the contents of the host's brood cells, brood parasites have evolved three principal strategies that allow them to eliminate the competition for resources presented by the host's larva and successfully deposit their own eggs within [13]. These strategies are outlined in detail because they are intimately related to the means by which different lineages of brood parasitic bees manage to escape detection by their hosts.
In the first strategy, the adult female parasite enters a host nest after it has been closed by the host female and destroys the offspring of the host. She then deposits her own egg in the nest cell and closes the nest behind her. The megachilid species Euaspis basalis (Ritsema, 1874), for example, attacks the stem nests of Megachile (Callomegachile) [33]. The parasite seeks out an appropriate host bee in the process of building a nest. When the nest has been completed and the host departed, E. basalis attacks. The parasite chews an opening in the resin plug closing the nest entrance and digs her way to the back of the nest, ploughing through the pollen provisions, demolishing the cell partitions and destroying the host eggs and larvae. When she reaches the far end, E. basalis then works her way back toward the entrance of the stem nest. Using the pollen already present in the nest, she forms new pollen masses, deposits an egg of her own on each mass and rebuilds the partitions between the brood cells. She then closes the nest entrance, leaving her eggs to develop inside. In addition to E. basalis, this strategy is known in the genus Hoplostelis (Megachilidae, Anthidiini), the subgenus Stelis (Dolichostelis) (Megachilidae, Anthidiini), and the genus Exaerete (Apidae, Euglossini) [34–36].
In the second strategy, as in the first, the adult female parasite attacks an appropriate nest after it has been closed by the host bee. In this case, however, the parasitic larva and not the adult parasite kills the host's offspring. While most bee larvae are sedentary and rather passive, these ‘hospicidal’, or host-killing, larvae are highly aggressive and endowed with powerful, sharp, often sickle-shaped mandibles used to kill the host's offspring [37]. Such larvae typically eclose faster than the host larvae, allowing them time to seek out and destroy the host egg or larva in the nest cell (figure 3). Thorp [41] reported on the interactions between the soil-nesting bee Anthophora edwardsii Cresson, 1878 (Apidae, Anthophorini) and its brood parasite, Melecta separata callura (Cockerell, 1926) (Apidae, Melectini). Anthophora edwardsii builds nests at the ends of burrows it digs in the soil; after nests are completed, the burrow is backfilled, likely to hide its location. Individuals of adult female M. s. callura were observed cruising in the vicinity of a nesting aggregation of A. edwardsii. Upon locating a nest already provisioned and closed by a host, the parasite re-excavated the burrow dug by the host, opened a small hole in the closure of the nest cell located at the end of the burrow, oviposited through the opening and placed her egg on either the roof or upper walls of the host cell [41]. Upon exiting, she closed the hole in the nest closure and backfilled the underground tunnel leading to the nest [41]. The first larval instar, highly mobile and equipped with massive, elongate mandibles, thrashed its way through the provisions inside the nest cell until it encountered the host's egg, which it pierced and destroyed [41]. In cases where a single nest cell is parasitized multiple times, the first hospicidal larva to emerge also kills other brood parasitic eggs or larvae present in the same cell [41]. This strategy is known in Dioxys pomonae Cockerell, 1910 (Megachilidae, Dioxyini) [42], the apid tribes Melectini, Rhathymini, Ericrocidini and Osirini [39,43] and the apid genus Coelioxoides [44].
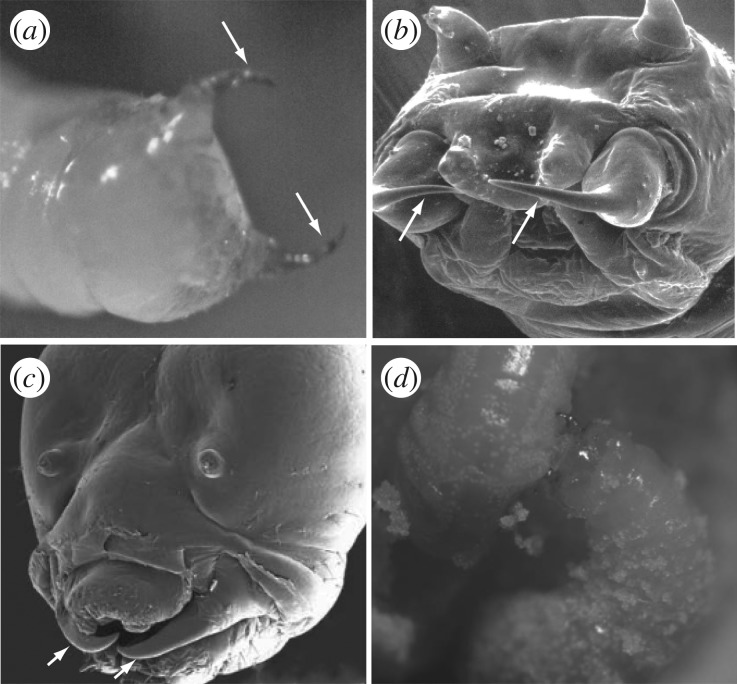
Figure 3.
At least one larval instar in each of the species shown is armed with sickle-shaped mandibles (indicated by white arrows) used for killing its host. Each of these species represents an independent origin of brood parasitism in bees. (a) Third larval instar of the megachiline bee Coelioxys chichimeca (photo Steve Thurston [38], courtesy of the American Museum of Natural History); (b) head of first larval instar of the melectine bee Xeromelecta californica (photo Jerome G. Rozen [39], courtesy of the American Museum of Natural History); (c) head of fifth larval instar of the anthidiine bee Stelis ater (photo Margaret A. Rozen [40], courtesy of the American Museum of Natural History); (d) fifth larval instar of Stelis ater (right) attacking the fourth instar larva of its osmiine host, Osmia chalybea (photo Jerome G. Rozen and H. Glenn Hall [40], courtesy of the American Museum of Natural History).
In the third strategy, the female parasite attacks a nest that is still open, i.e. still in the process of being provisioned by the host bee. As in the second strategy, her hospicidal larvae destroy the host's offspring, as well as any other brood parasitic eggs or larvae that may be present in the nest. Rozen et al. [45] reported on the behaviour of the brood parasitic bee Protepeolus singularis Linsley and Michener, 1937 (now Leiopodus singularis) attacking nest cells of Diadasia olivacea (Cresson, 1878). Brood cells of D. olivacea were built at the end of burrows dug in the soil; open burrow entrances were concealed under plants or other objects. To locate a host nest, the parasite appeared to observe either the activities of the nesting host bee or the comings and goings of other conspecific parasites attacking nearby nests. When a host was away foraging, the parasite entered the open nest cell and deposited an egg in the cell wall of the nest cell and departed. The female D. olivacea subsequently returned to complete construction of its nest, apparently unaware that the nest had been parasitized. The parasite's first larval instar, armed with tapered, sharpened mandibles, searched out and destroyed the host's egg. This strategy is known in certain members of the megachilid tribe Dioxyini, the genera Coelioxys and Radoszkowskiana and the subgenus Stelis (Stelis), as well as in the apid tribes Isepeolini (likely), Protepeolini, Ammobatini, Ammobatoidini, Epeolini, Nomadini, Townsendiellini, Biastini, Neolarrini, Hexepeolini, Brachynomadini and Caenoprosopidini [11,38,45–47].
3. Detection avoidance
The reproductive success of a parasite depends on its ability to deposit an egg in the nest of an appropriate host without being driven off by the host itself or having its egg detected. Detection avoidance strategies in solitary bees thus differ depending on whether the brood parasite targets a nest that has been closed by the host or a nest that is still in the process of being provisioned. In the case of the former, the principal strategy is to avoid the host female either by waiting until she has completed her nest or by targeting a nest in which a female has completed one nest cell in a linear series but before she has returned to begin provisioning the next cell [48]. In the case of the latter, not only does the parasite try to avoid detection by the host female, she has also evolved a suite of other strategies that allow her to conceal evidence of her visit to a host nest, as well as to hide her egg so that the host, in the course of returning to provision her nest, does not discover it.
(a). Consequences of encountering a host female
Brood parasites often lurk at nesting sites, on the alert for nests that may be suitable to parasitize. In return, female solitary bees actively defend their nests against attacking brood parasites, or even those simply patrolling in the vicinity of a nesting site. For parasites targeting closed host nests, the probability of detection by a host bee is particularly elevated in those cases where hosts nest in large aggregations and where many nests in various stages of construction may be available simultaneously. Shuckard [49], for example, described the behaviour of Anthophora acervorum (Linnaeus, 1758) (now Anthophora plumipes), a soil-nesting bee, repelling an attack by the genus Melecta, a brood parasitic genus attacking closed nest cells: ‘This section [Anthophora] is subject to the parasitism of the genus Melecta, whose incursions are very repugnant to them, and which they exhibit in very fierce pugnacity, for if they catch the intruder in her invasion they will draw her forth and deliver battle with great fury.’ Similarly, Thorp [41] observed that individuals of Melecta separata callura, in flight near a nesting aggregation of A. edwardsii, were commonly attacked and often driven off by nesting bees, regardless of whether the parasite was detected at the entrance of a nest burrow or not. It is worth mentioning that brood parasitic bees are more heavily armoured than non-parasitic bees and often exhibit a suite of morphological adaptations that have likely evolved to protect them from the attacks of their hosts. They often exhibit a thickened cuticle compared with non-parasitic bees, as well as spines, lamellae and carinae that protect vulnerable body parts [11]. In many lineages, the sting is more powerful than that seen in non-parasitic bees [11].
In brood parasitic bees that attack open nest cells, encountering a host female is a more common occurrence because the parasite targets a nest still frequented by a host. When the host female leaves the nest to gather a fresh load of pollen, the parasite must enter the nest of the host, deposit her egg in the nest cell and leave before the host female returns. Torchio [50] described the nest defence behaviour of three species of Osmia, O. lignaria propinqua Cresson, 1864, O. montana montana Cresson, 1864 and O. californica Cresson, 1864, against invasions by the anthidiine parasite Stelis montana Cresson, 1864. After returning from foraging and finding S. montana inside their nests, all three species aggressively attacked the parasite and physically dragged her from the nest. After driving out the parasite, both O. lignaria propinqua and O. montana montana re-entered their nests and remained in the entryway with their heads blocking the opening. Osmia californica chewed through the pollen provisions in its nest, thereby destroying any Stelis eggs deposited in the nest in her absence.
These examples demonstrate that, in at least some cases, the host reacts violently to an incursion by a parasite. Detection of the intruder leads either to the eviction of the parasite from the nest or to the destruction of her egg, underlying the importance of concealing their presence from the host. One of the primary advantages of attacking nests that have already been closed by a host is that the host bee is no longer present in the nest, a principal means of avoiding detection by the host. Depending on the amount of time elapsed between nest closure and deposition of the parasite's egg, however, the provisions remaining in a closed nest cell may be more or less depleted, meaning that the parasite's offspring may not have the entire pollen mass at its disposal. The evolution of a behavioural strategy in which open nest cells are attacked is advantageous because the developing parasite is assured access to all of the provisions stored by the host. It engenders, however, a series of other challenges, namely the successful attack of a nest still frequented by a host. Many of the mechanisms used by brood parasitic bees to escape detection are employed principally by those lineages attacking open nest cells.
(b). Chemical mimicry
Certain brood parasites, namely those attacking open nest cells, appear to use chemical mimicry to conceal their visits from nesting hosts. In some species, this technique is employed to hide olfactory evidence of a visit that may be left when a parasite enters a nest cell, deposits an egg within and then leaves before the host returns [50]. In other cases, a foraging bee returns to find a parasite nearby or inside her nest but does not appear to react to it in any way, suggesting that the brood parasite is not immediately perceived as a threat.
Despite the aggressive response displayed by each of the three species of Osmia mentioned in the section above upon discovering S. montana in their nests, none of the three species appeared to notice that a parasite had visited her nest while away foraging if she did not find the parasite in her nest when she returned. The reason may be a curious behaviour reported by Torchio [50]. The three Osmia were provided with trap nests consisting of cylindrical holes bored in wooden blocks; finished nests consisted of a linear series of between one and eleven brood cells. All three species built their nest partitions and their nest plugs with masticated plant material; under greenhouse conditions, the Osmia harvested and chewed bits of leaves collected during visits to both Malva rotundifolia L. (Malvaceae) and Oenothera hookeri Torr. & A. Gray (Onagraceae) for use in constructing both nest partitions and closures. Stelis montana was observed visiting the same plants visited by the Osmia, sometimes alongside the same individuals they parasitized. The parasite was observed chewing the edges of leaves and gathering the juice secreted by the plant. It then flew to a nearby perch and proceeded to slather the plant juices over the surface of its entire body. Other individuals of S. montana were also observed entering open host nests, removing great mouthfuls of pollen–nectar mixture and then immediately exiting the nest. The parasite alighted on a nearby rest and then rubbed the pollen mixture over all body surfaces. Both behaviours were attributed by the author as a strategy by which the parasite created a sort of olfactory camouflage to conceal evidence of its visit to a host's nest. This hypothesis was supported by the observation that the behaviour of the Osmia upon entering a nest cell previously visited by a parasite was not statistically different from behaviour exhibited by those entering a nest cell not visited by a parasite: the individual entered the cell and deposited her pollen load, without any apparent hesitation [50].
The behaviour of nesting bees to the presence of parasites, however, is not always hostile. In another account, Rust & Thorp [47] described the interactions between another host–parasite pair, Osmia nigrifrons Cresson, 1878 and Stelis chlorocyanea (Cockerell, 1925). Large aggregations of O. nigrifrons, a mud-nesting bee, were reported from the inside surface of a wide-diameter drain pipe. Stelis would repeatedly enter nest cells to find those at a suitable stage of construction to parasitize. If, once inside a nest cell, she came face to face with a working Osmia, the Stelis would immediately fly out of the nest. But if she entered the cell and encountered the posterior end of the host, the parasite would simply crawl over the metasoma of the Osmia. In neither case was the Osmia reported to have reacted aggressively toward the Stelis. Furthermore, both host and parasite were observed resting in the vicinity of the nesting aggregation, just millimetres from one another, without any reaction from either bee. Perhaps the Stelis was somehow able to conceal the threat that it posed to the Osmia, either by masking its presence chemically or simply by way of its physical resemblance to its host.
In other cases, brood parasitic species may mask their odours not by using extraneous material but rather by using chemical compounds that they produce themselves. The apid genus Nomada is most often a brood parasite of Andrena but some species are also known to attack the nests of other ground-nesting species belonging to the families Andrenidae, Melittidae, Halictidae and Apidae ([51] and references therein). Both Andrena and Melitta are ground-nesting bees, building their nest cells at the ends of secondary tunnels branching off of a main tunnel [11,52]. In both genera, nest cells are lined with secretions produced by the Dufour's gland of the nesting female [11,52]. The principal function of these secretions is to waterproof the contents of the brood cell but they may also serve as an olfactory cue to signal the presence of a suitable nesting site to conspecifics, namely for those Andrena nesting in large aggregations [53]. Given that brood parasites also use chemical cues when seeking out host nests, it is also likely that these secretions may alert parasites to the presence of an appropriate host nest [53]. Tengö & Bergström [53] found that the principal component of the male cephalic secretions in five out of eight species of Nomada was identical to that of the principal component of the Dufour's gland secretions of females of their main host species belonging either to the genus Andrena or to Melitta. Female Nomada encounter the cephalic secretions of conspecific males during mating. Contact with these secretions may help the female Nomada recognize a suitable host nest [53]. Encounters between Nomada and Andrena were reported by these authors as entirely non-aggressive, despite the fact that these genera do not resemble one another in size, shape or colour, and it was also proposed that the cephalic gland secretions encountered by female Nomada during copulation may also provide a sort of olfactory camouflage that protects her from attack by defensive host females [53].
Antennal grabbing, in which male Nomada stroke the antenna of female Nomada during copulation, was recently reported as a mechanism by which pheromones may be transferred from males to females [54]. This transfer of pheromones may render females unattractive to the copulatory attempts of other males [54,55], as has been suggested for other bees [50]. It may, however, also provide a chemical cue for females searching for nests to parasitize or may allow the female to pass unnoticed within the nest of a parasite by mimicking the odour of the host or her nest [54].
(c). Egg-hiding
In lineages attacking closed nest cells, the eggs of the brood parasite are less prone to discovery by the host female because the host no longer frequents the inside of the nest cell. In multiple lineages of both apid and megachilid brood parasites attacking closed nest cells, including the apid tribes Melectini, Rhathymini and Ericrocidini, as well as the megachilid species Dioxys pomonae and Hoplostelis bilineolata (Spinola, 1841), eggs are deposited either directly on the provisions themselves or unhidden along the cell wall [34,39,42]. If such nest cells are opened, the egg of the brood parasite is often partially or entirely visible in the cell.
In brood parasitic species attacking open nest cells, however, the risk is greater that the egg deposited by the parasite will be encountered by a returning host female. In order to reduce the chances that an egg is discovered, some species hide their eggs either within the nest cell or within the nest cell wall of their host. Rozen & Michener [56] reported on the behaviour of two species of the ammobatine subgenus Sphecodopsis (Pseudodichroa) on the colletid genus Scrapter. Scrapter is a soil-nesting bee that uses glandular secretions to coat the insides of its brood cells; these secretions dry as a waterproof, cellophane-like lining [11]. Sphecodopsis (Pseudodichroa), a brood parasite of open nests, deposits its eggs in a slit made in the wall of the host's nest cell. The egg is inserted perpendicularly to the cell wall and is embedded in the soil surrounding the nest such that the tip of the egg is flush with the inner lining of the nest cell. A flange located at the anterior end of the egg is made flush with the lining inside the cell, serving either to maintain the impermeability of the nest or possibly as a further means of visually concealing the presence of the egg in the cell [56]. The presence of such a flange is thus far unknown in the eggs of nest-building bees.
Torchio & Burdick [57] detailed the behaviour of another brood parasite, the epeoline Epeolus compactus Cresson, 1878, a brood parasite of Colletes kincaidii (Cockerell, 1898). Epeolus compactus used the paired, spined projections on sternite 6 to cut a U-shaped opening either in the upper portion of a side wall of an open cell or through the cell cap of a completed cell abutting onto an adjacent cell still being provisioned; in the case of the latter, the parasite's larva emerged into the still open adjacent cell. After egg deposition, the parasite added a few drops of Dufour's gland secretions to the area where the tip of the egg was inserted through the cell lining. These secretions dissolved the cut edges of the cell lining and filled the space around the egg. When this substance hardened, a continuous impermeable layer was formed between the cell lining and the egg, thus anchoring the egg to the cell wall and preventing the entry of moisture into the cell [57]. Thus the Dufour's gland secretions applied by E. compactus likely serve a role analogous to that of the apical flange seen in the eggs of Sphecodopsis (Pseudodichroa). A slightly different strategy is seen in species of Isepeolus, whose eggs are flattened and display a flange running along the length of the egg. These physical attributes serve to further conceal the egg, which is deposited by Isepeolus along the inner surface of the cell lining of its host, species of Colletes.
In a fascinating example from the apid tribe Hexepeolini, members of the genus Hexepeolus deposit their eggs into open host nests, partially embedding them longitudinally along the inner surface of the cell wall such that the long axis of the egg is parallel to the cell wall [58]. The exposed surface of the egg is rough, being ornamented with small ‘papillae’, in contrast to the typical chorionic surface of the egg, which is smooth and soft [59]. The presence of these papillae, similar in texture to the inner surface of the cell wall, is suspected to help hide the egg from the returning host [59]. Other lineages of brood parasites also deposit eggs with external chorionic ornamentation that may serve as camouflage when embedded within a brood cell of a host. Such lineages include other tribes within the subfamily Nomadinae, the tribes Protepeolini and Isepeolini and the megachilid genus Coelioxys [48].
(d). Deposition of small eggs
Brood parasites attacking open nest cells also tend to have eggs that are relatively small compared with the eggs of both nesting bees and brood parasitic bees attacking closed nest cells. Iwata & Sakagami [60] proposed the use of an ‘egg index’, a quantitative means by which to assess the relative size of a bee's egg. The egg index is equivalent to the ratio between the length of the egg and the distance between the outer margins of the tegulae of the adult female. They also proposed a qualitative interpretation of this index, whereby certain values corresponded to either dwarf, small, medium, large or giant eggs [60]. They observed that most brood parasitic bees had either dwarf- or small-sized eggs, while solitary nest-building bees had, on average, medium-sized eggs [60]. They hypothesized that having relatively small eggs allowed a parasitic female to produce more eggs from a given amount of resources, and thus to have many small eggs ready to deposit whenever a host nest at a suitable stage of development was located. In some cases, namely in those instances where the brood parasitic egg ecloses before that of its host, the rate of embryonic development associated with a smaller egg may actually be faster than that of a larger egg, a clear advantage given that the first egg to eclose is the one most likely to survive, especially in nest cells parasitized with multiple eggs [44]. Rozen [61] proposed another explanation, namely that small eggs may also be an evolutionary adaptation to facilitate egg hiding by brood parasites attacking open nest cells. In a later study, Rozen [48] found that the eggs of brood parasites attacking open nest cells were statistically smaller than those of parasites attacking closed nest cells or those of nest-building bees, providing evidence that the relatively small eggs seen in parasites of open nest cells may, in fact, be an adaptation related to their parasitic strategy.
4. Evolutionary convergences with other brood parasitic lineages
The need to avoid detection by a host female has led to the evolution of a series of behavioural and biological adaptations in multiple lineages of brood parasitic bees. These adaptations, including the use of chemical mimicry and at least three strategies related to egg-hiding, have evolved multiple times independently and thus represent remarkable examples of evolutionary convergences. These parallelisms extend well beyond bees, however, and have also appeared in other lineages, some of which are phylogenetically close to the bees, including the apoid wasps [14], and others of which are more distant, including brood parasitic lineages of fish and birds [62,63].
The tribe Nyssonini (Hymenoptera, Crabronidae), for example, is a lineage of obligately brood parasitic wasps. An account of their behaviour was given by O'Neill [14], based largely on observations of the genus Nysson. Generally described as ‘inconspicuous as a means of avoiding detection by host females', this genus parasitizes the nests of multiple genera of the crabronid tribe Gorytini [14]. Upon the discovery of a suitable host nest, Nysson removes the temporary nest closure used by the host to protect her nest in her absence. The parasite enters the nest, still in the process of being provisioned by the host, and hides her egg in an inconspicuous location on one of the prey items in the nest. When the host returns, she fails to discover the hidden egg of the parasite and deposits an egg of her own within and eventually permanently closes the nest. The egg of the parasite ecloses before that of the host and, using its mandibles, kills the host's offspring [14]. Both the egg-hiding behaviour and the presence of a hospicidal larva represent evolutionary convergences shared with bees.
Another example of a striking evolutionary parallelism related to brood parasitism is related to the interaction between a species of brood parasitic catfish and its host, a mouthbrooding cichlid fish [62]. In observations made in Lake Tanganyika, female cichlids deposited their eggs in the sand on the lake bottom. The females immediately gathered the eggs in their mouths, along with sperm released into the water by the males. Fertilization of the eggs took place in the mouths of the females. After several weeks of gestation, the eggs hatched in the mouths of the females and were then freed into the water. During the egg-laying sequence, the cuckoo catfish, Synodontis multipunctatus Boulenger, 1898, interrupted the female cichlid and took advantage of the interruption to deposit her own eggs alongside those of the cichlid host. The cichlid then resumed her egg collection, inadvertently picking up the eggs of the parasite as well as her own. Once inside the mouth of the host, the catfish eggs hatched before those of the host and preyed on the fertilized cichlid eggs in the host's mouth [62,63]. In many instances, the offspring that eventually emerged from the cichlid's mouth belonged almost exclusively to the brood parasite [63]. Thus, unbeknownst to the host female, the parasite 'hides' her eggs alongside those of the host in order to take advantage of the protection and other resources offered by the host's mouth. The offspring of the parasite develop on the resources provided by the female for her own offspring, although in this case not only is the yolk sac consumed by the parasite but the host's young as well.
Brood parasitism in birds is similar to brood parasitism in bees, with one major difference. In bees, the parasitic larva develops on the provisions collected by the host female for her own offspring but never comes into contact with the host. If the cuckoo bee successfully manages to hide her egg from a nesting female, her offspring's chances of survival are favourable. As a consequence, concealment strategies in bees are largely related to egg hiding. In most birds, however, the host bird progressively feeds the young of the cuckoo (although see [64] for details regarding the behaviour of brood parasitic black-headed ducks, Heteronetta atricapilla (Merrem, 1841), whose fledglings leave the nest almost immediately after hatching and are not fed by their host). Not only must the parasite lay an egg in the host's nest without being noticed, the cuckoo's offspring must also be accepted as one of the host's own nestlings. In some lineages of brood parasitic birds, detection avoidance strategies are related to concealing the parasite's egg among the eggs of the host. In others, physical aspects of the chick resemble the host's own chicks [10].
The common cuckoo, Cuculus canorus Linnaeus, 1758, produces eggs that are coloured and patterned to mimic those of its host [10,65]. Different populations of the cuckoo are specialized on different hosts and the eggs laid by females in each population are mimetic with those of the host on which they are specialized [65]. As in bees, discovery of the parasite's egg or eggs by a host female may lead to the destruction of the cuckoo egg, thus the more the cuckoo's egg resembles that of its host, the greater the chances that the egg will be allowed to stay in the nest; patterns of mimicry in cuckoo eggs are stronger in populations whose hosts are more discriminating [65,66]. While the concealment strategy of brood parasitic bees is to physically hide the egg within the host nest, that of birds is for the egg to be seen and accepted by the host. In another interesting parallel to the bees, the cuckoo chick also destroys the host's offspring, by rolling either the eggs or the host chick out of the nest [67].
5. Conclusion
Brood parasitic bees have evolved a suite of adaptations that allow them to deposit their eggs in the nests of their hosts without detection. These adaptations, which include the use of chemical masking to conceal evidence of a visit, strategic placement of eggs, egg camouflage in the form of chorionic ornamentation and the production of small or dwarf eggs, have multiple independent evolutionary origins in bees. Behavioural similarities, especially those related to concealing the presence of eggs from a host, are seen in other, unrelated lineages of obligate brood parasites including wasps, fish and birds, representing remarkable evolutionary convergences as a result of a shared life-history strategy and hinting at the enormous selective pressure on obligate brood parasitic lineages to avoid detection by a host.
Acknowledgements
Many thanks to the Biosystematics Group and the Institute of Agricultural Sciences at the Swiss Federal Institute of Technology in Zürich (ETHZ) and the American Museum of Natural History for image use, as well as to Rose Thorogood, Christophe Praz and one anonymous reviewer for comments that helped to improve an earlier version of this manuscript.
Data accessibility
This article has no additional data.
Competing interests
I declare that I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Ligue Suisse pour la Protection de la Nature (LSPN). 1987. [Diurnal butterflies and their biotopes: species, threats, protection], vol. 1 Basel, Switzerland: Ligue Suisse pour la Protection de la Nature. (In French.) [Google Scholar]
- 2.Cálix M, et al. 2018. European red list of saproxylic beetles. Brussels, Belgium: IUCN. [Google Scholar]
- 3.Gullan PJ, Cranston PS. 1994. The insects – an outline of entomology. London, UK: Chapman and Hall. [Google Scholar]
- 4.Clayton DH, Bush SE, Johnson KP. 2015. Coevolution of life on hosts. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Kalkman VJ, Clausnitzer V, Dijkstra K-D, Orr AG, Paulson DR, van Tol J. 2008. Global diversity of dragonflies. Hydrobiologia 595, 351–363. [Google Scholar]
- 6.Yeates DK. 1997. The evolutionary pattern of host use in the Bombyliidae (Diptera): a diverse family of parasitoid flies. Biol. J. Linn. Soc. 60, 149–185. ( 10.1111/j.1095-8312.1997.tb01490.x) [DOI] [Google Scholar]
- 7.Eiri DM, Suwannapong G, Endler M, Nieh JC. 2015. Nosema ceranae can infect honey bee larvae and reduces subsequent adult longevity. PLoS ONE 10, e0126330 ( 10.1371/journal.pone.0126330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nazzi F, Le Conte Y.. 2016. Ecology of Varroa destructor, the major ectoparasite of the western honeybee, Apis mellifera. Annu. Rev. Ent. 61, 417–432. ( 10.1146/annurev-ento-010715-023731) [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Flórez AF. 2014. A new species of Grotea Cresson (Hymenoptera, Ichneumonidae, Labeninae) from Colombia . Zookeys 389, 27–33. ( 10.3897/zookeys.389.6066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorenson MD, Payne RB. 2002. Molecular genetic perspectives on avian brood parasitism. Integr. Comp. Biol. 42, 388–400. ( 10.1093/icb/42.2.388) [DOI] [PubMed] [Google Scholar]
- 11.Michener CD. 2007. The bees of the world. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 12.Cardinal S, Straka J, Danforth BN. 2010. Comprehensive phylogeny of apid bees reveals evolutionary origins and antiquity of cleptoparasitism. Proc. Natl Acad. Sci. USA 107, 16 207–16 211. ( 10.1073/pnas.1006299107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litman JR, Praz CJ, Danforth BN, Griswold TL, Cardinal S. 2013. Origins, evolution, and diversification of cleptoparasitic lineages in long-tongued bees. Evolution 67, 2982–2998. ( 10.1111/evo.12161) [DOI] [PubMed] [Google Scholar]
- 14.O'Neill KM. 2001. Solitary wasps: behavior and natural history. Ithaca, NY: Cornell University Press. [Google Scholar]
- 15.Elmes GW, Thomas JA, Wardlaw JC. 1991. Larvae of Maculinea rebeli, a large-blue butterfly, and their Myrmica host ants: wild adoption and behaviour in ant-nest. J. Zool. Lond. 223, 447–460. ( 10.1111/j.1469-7998.1991.tb04775.x) [DOI] [Google Scholar]
- 16.Elmes GW, Wardlaw JC, Thomas JA. 1991. Larvae of Maculinea rebeli, a large-blue butterfly, and their Myrmica host ants: patterns of caterpillar growth and survival. J. Zool. Lond. 224, 79–92. ( 10.1111/j.1469-7998.1991.tb04789.x) [DOI] [Google Scholar]
- 17.Sielezniew M, Stankiewicz AM. 2007. Differences in the development of the closely related myrmecophilous butterflies Maculinea alcon and M. rebeli (Lepidoptera: Lycaenidae). Eur. J. Entomol. 104, 433–444. ( 10.14411/eje.2007.063) [DOI] [Google Scholar]
- 18.Ayasse M, Jarau S. 2014. Chemical ecology of bumble bees. Annu. Rev. Entomol. 259, 299–319. ( 10.1146/annurev-ento-011613-161949) [DOI] [PubMed] [Google Scholar]
- 19.Goulson D. 2010. Bumblebees – behavior, ecology and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 20.Cervo R. 2006. Polistes wasps and their social parasites: an overview. Ann. Zool. Fenn. 43, 531–549. [Google Scholar]
- 21.Buschinger A. 2009. Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol. News 12, 219–235. [Google Scholar]
- 22.Lhomme P, Hines HM. 2018. Reproductive dominance strategies in insect social parasites. J. Chem. Ecol. 44, 838–850. ( 10.1007/s10886-018-0971-z) [DOI] [PubMed] [Google Scholar]
- 23.Gibbs J, Albert J, Packer L. 2011. Dual origins of social parasitism in North American Dialictus (Hymenoptera: Halictidae) confirmed using a phylogenetic approach. Cladistics 28, 195–207. ( 10.1111/j.1096-0031.2011.00373.x) [DOI] [PubMed] [Google Scholar]
- 24.Wcislo WT. 1987. The roles of seasonality, host synchrony, and behaviour in the evolutions and distributions of nest parasites in Hymenoptera (Insecta), with special reference to bees (Apoidea). Biol. Rev. 62, 515–542. ( 10.1111/j.1469-185X.1987.tb01640.x) [DOI] [Google Scholar]
- 25.Plateaux-Quénu C. 2008. Subsociality in halictine bees. Insect. Sociaux 55, 335–346. ( 10.1007/s00040-008-1028-z) [DOI] [Google Scholar]
- 26.Cane JH. 1983. Olfactory evaluation of Andrena host nest suitability by kleptoparasitic Nomada bees (Hymenoptera, Apoidea). Anim. Behav. 31, 138–144. ( 10.1016/S0003-3472(83)80181-X) [DOI] [Google Scholar]
- 27.Bossert S, Murray EA, Almeida EAB, Brady SG, Blaimer BB, Danforth B. 2018. Combining transcriptomes and ultraconserved elements to illuminate the phylogeny of Apidae. Mol. Phylogenet. Evol. 130, 121–131. ( 10.1016/j.ympev.2018.10.012) [DOI] [PubMed] [Google Scholar]
- 28.Trunz V, Packer L, Vieu J, Arrigo N, Praz CJ. 2016. Comprehensive phylogeny, biogeography and new classification of the diverse bee tribe Megachilini: can we use DNA barcodes in phylogenies of large genera? Mol. Phylogenet. Evol. 103, 245–259. ( 10.1016/j.ympev.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 29.Rozen JG., Jr 2000. Systematic and geographic distributions of Neotropical cleptoparastic bees, with notes on their modes of parasitism. In Ann. IV Meeting about Bees, Ribeirão Preto, Brazil (eds G Bitondi, K Hartfelder), pp. 204–210. São Paulo, Brazil: University of São Paulo, Faculty of Philosophy, Science and Letters of Ribeirão Preto. [Google Scholar]
- 30.Danforth BN, Eardley CD, Packer L, Walker K, Pauly A, Randrianambinintsoa FJ. 2008. Phylogeny of Halictidae with an emphasis on endemic African Halictinae. Apidologie 39, 86–101. ( 10.1051/apido:2008002) [DOI] [Google Scholar]
- 31.Perkins RCL. 1899. Hymenoptera Aculeata [except ants]. In Hymenoptera Aculeata (eds RCL Perkins, A Forel). In Fauna hawaiiensis (ed. D Sharp), vol. 1, pp. 1–115. London, UK: Cambridge University Press. [Google Scholar]
- 32.Daly HV, Magnacca KN.. 2003. Hawaiian Hylaeus (Nesoprosopis) bees (Hymenoptera: Apoidea). Insects of Hawaii, vol. 17. Honolulu, HI: University of Hawaii Press. [Google Scholar]
- 33.Iwata K. 1976. Evolution of instinct: comparative ethology of Hymenoptera. New Delhi, India: Amerind Publishing Company. [Google Scholar]
- 34.Bennett FD. 1966. Notes on the biology of Stelis (Odontostelis) bilineolata (Spinola), a parasite of Euglossa cordata (Linnaeus) (Hymenoptera: Apoidea: Megachilidae). J. NY Entomol. Soc. 74, 72–79. [Google Scholar]
- 35.Parker FD, Cane JH, Frankie GW, Vinson SB.. 1987. Host records and nest entry by Dolichostelis, a kleptoparasitic anthidiine bee (Hymenoptera: Megachilidae). Pan-Pac. Entomol. 63, 172–177. [Google Scholar]
- 36.Bennett FD. 1972. Observations on Exaerete spp. and their hosts Eulaema terminata and Euplusia surinamensis (Hymen., Apidae, Euglossinae) in Trinidad. J. NY Entomol. Soc. 80, 118–124. [Google Scholar]
- 37.Rozen JG. 1989. Morphology and systematic significance of first instars of the cleptoparasitic bee tribe Epeolini (Anthophoridae: Nomadinae). Am. Mus. Novit. 2957, 1–19. [Google Scholar]
- 38.Rozen JG Jr, Vinson SB, Coville R, Frankie G. 2010. Biology and morphology of the immature stages of the cleptoparasitic bee Coelioxys chichimeca (Hymenoptera: Apoidea: Megachilidae). Am. Mus. Novit. 3679, 1–26. ( 10.1206/697.1) [DOI] [Google Scholar]
- 39.Rozen JG., Jr 1991. Evolution of cleptoparasitism in anthophorid bees as revealed by their mode of parasitism and first instars (Hymenoptera: Apoidea). Am. Mus. Novit. 3029, 1–36. [Google Scholar]
- 40.Rozen JG, Hall HG. 2011. Nesting and developmental biology of the cleptoparasitic bee Stelis ater (Anthidiini) and its host, Osmia chalybea (Osmiini) (Hymenoptera: Apoidea). Am. Mus. Novit. 3707, 1–38. ( 10.1206/3707.2) [DOI] [Google Scholar]
- 41.Thorp RW. 1969. Ecology and behavior of Melecta separata callura (Hymenoptera: Anthophoridae). Am. Midl. Nat. 82, 338–345. ( 10.2307/2423782) [DOI] [Google Scholar]
- 42.Rozen JG Jr, Favreau MS. 1967. Biological notes on Dioxys pomonae pomonae and on its host, Osmia nigrobarbata (Hymenoptera: Megachilidae). J. NY Entomol. Soc. 75, 197–203. [Google Scholar]
- 43.Rozen JG Jr, Melo GAR, Aguiar AJC, Alves-dos-Santos I. 2006. Nesting biologies and immature stages of the tapinotaspidine bee genera Monoeca and Lanthanomelissa and of their osirine cleptoparasites Protosiris and Parepeolus (Hymenoptera: Apidae: Apinae). Am. Mus. Novit. 3501, 1–60. ( 10.1206/0003-0082(2006)501[0001:nbaiso]2.0.co;2) [DOI] [Google Scholar]
- 44.Alves-dos-Santos I, Melo GAR, Rozen JG. 2002. Biology and immature stages of the bee tribe Tetrapediini (Hymenoptera: Apidae). Am. Mus. Novit. 3377, 1–45. () [DOI] [Google Scholar]
- 45.Rozen JG Jr, Eickwort KR, Eickwort GC. 1978. The bionomics and immature stages of the cleptoparasitic bee genus Protepeolus (Anthophoridae, Nomadinae). Am. Mus. Novit. 2640, 1–24. [Google Scholar]
- 46.Rozen JG Jr, Özbek H. 2005. Egg deposition of the cleptoparasitic bee Dioxys cincta (Hymenoptera: Apoidea: Megachilidae). J. NY Entomol. Soc. 78, 221–226. ( 10.2317/0411.14.1) [DOI] [Google Scholar]
- 47.Rust RW, Thorp RW. 1973. The biology of Stelis chlorocyanea, a parasite of Osmia nigrifrons. J. Kans. Entomol. Soc. 46, 548–562. [Google Scholar]
- 48.Rozen JG., Jr 2003. Eggs, ovariole numbers, and modes of parasitism of cleptoparasitic bees, with emphasis on Neotropical species (Hymenoptera: Apoidea). Am. Mus. Novit. 3413, 1–36. () [DOI] [Google Scholar]
- 49.Shuckard WE. 1866. British bees. London, UK: Lovell Reeve and Co. [Google Scholar]
- 50.Torchio PF. 1989. Biology, immature development and adaptive behavior of Stelis montana, a cleptoparasite of Osmia (Hymenoptera: Megachilidae). Ann. Entomol. Soc. Am. 82, 616–632. ( 10.1093/aesa/82.5.616) [DOI] [Google Scholar]
- 51.Eickwort GC, Abrams J. 1980. Parasitism of sweat bees in the genus Agapostemon by cuckoo bees in the genus Nomada (Hymenoptera: Halictidae, Anthophoridae). Pan-Pac. Entomol. 56, 144–152. [Google Scholar]
- 52.Celary W. 2006. Biology of the solitary ground-nesting bee Melitta leporina (Panzer, 1799) (Hymenoptera: Apoidea: Melittidae). J. Kans. Entomol. Soc. 79, 136–145. ( 10.2317/0022-8567(2006)79[136:BOTSGB]2.0.CO;2) [DOI] [Google Scholar]
- 53.Tengö J, Bergström G. 1977. Cleptoparasitism and odor mimetism in bees: do Nomada males imitate the odor of Andrena females? Science 196, 1117–1119 ( 10.1126/science.196.4294.1117) [DOI] [PubMed] [Google Scholar]
- 54.Schindler M, Hofmann MM, Wittmann D, Renner SS. 2018. Courtship behaviour in the genus Nomada – antennal grabbing and possible transfer of male secretions. J. Hymenopt. Res. 65, 47–59. ( 10.3897/jhr.65.24947) [DOI] [Google Scholar]
- 55.Cazier MA, Linsley EG. 1963. Territorial behavior among males of Protoxaea gloriosa (Fox) (Hymenoptera: Andrenidae). Can. Entomol. 95, 547–556. ( 10.4039/Ent95547-5) [DOI] [Google Scholar]
- 56.Rozen JG Jr, Michener CD. 1968. The biology of Scrapter and its cuckoo bee, Pseudodichroa (Hymenoptera: Colletidae and Anthophoridae). Am. Mus. Novit. 2335, 1–13. [Google Scholar]
- 57.Torchio PF, Burdick DJ. 1988. Comparative notes on the biology and development of Epeolus compactus Cresson, a cleptoparasite of Colletes kincaidii Cockerell (Hymenoptera: Anthophoridae, Colletidae), Ann. Entomol. Soc. Am. 81, 626–636. ( 10.1093/aesa/81.4.626) [DOI] [Google Scholar]
- 58.Rozen JG., Jr 1994. Biologies of the bee genera Ancylandrena (Andrenidae: Andreninae) and Hexepeolus (Apidae: Nomadinae), and phylogenetic relationships of Ancylandrena based on its mature larva (Hymenoptera: Apoidea). Am. Mus. Novit. 3108, 1–19. [Google Scholar]
- 59.Rozen JG Jr, Roig-Alsina A, Alexander BA. 1997. The cleptoparasitic bee genus Rhopalolemma, with reference to other Nomadinae (Apidae), and biology of its host Protodufourea (Halictidae: Rophitinae). Am. Mus. Novit. 3194, 1–28. [Google Scholar]
- 60.Iwata K, Sakagami SF. 1966. Gigantism and dwarfism in bee eggs in relation to the modes of life, with notes on the number of ovarioles. Jap. J. Ecol. 16, 4–16. [Google Scholar]
- 61.Rozen JG., Jr 1994. Biology and immature stages of some cuckoo bees belonging to Brachynomadini, with descriptions of two new species (Hymenoptera: Apidae: Nomadinae). Am. Mus. Novit. 3089, 1–23. [Google Scholar]
- 62.Sato T. 1986. A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323, 58–59. ( 10.1038/323058a0) [DOI] [PubMed] [Google Scholar]
- 63.Blažek R, Polačik M, Smith C, Honza M, Meyer A, Reichard M. 2018. Success of cuckoo catfish brood parasitism reflects coevolutionary history and individual experience of their cichlid hosts. Sci. Adv. 4, eaar4380 ( 10.1126/sciadv.aar4380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.del Hoyo J, Elliott A, Sargatal J. 1992. Handbook of the birds of the world, vol. 1: ostrich to ducks. Barcelona: Lynx Edicions. [Google Scholar]
- 65.Brooke M de L, Davies NB. 1988. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632. ( 10.1038/335630a0) [DOI] [Google Scholar]
- 66.Stoddard MC, Stevens M. 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Phil. Trans. R. Soc. B 277, 1387–1393. ( 10.1098/rspb.2009.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jenner E. 1788. Observations on the natural history of the cuckoo. Phil. Trans. R. Soc. Lond. 78, 219–23710. ( 10.1098/rstl.1788.0016) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.