Abstract
Background
Postpartum haemorrhage (PPH) is the leading cause of maternal mortality worldwide. Prophylactic uterotonic agents can prevent PPH, and are routinely recommended. The current World Health Organization (WHO) recommendation for preventing PPH is 10 IU (international units) of intramuscular or intravenous oxytocin. There are several uterotonic agents for preventing PPH but there is still uncertainty about which agent is most effective with the least side effects. This is an update of a Cochrane Review which was first published in April 2018 and was updated to incorporate results from a recent large WHO trial.
Objectives
To identify the most effective uterotonic agent(s) to prevent PPH with the least side effects, and generate a ranking according to their effectiveness and side‐effect profile.
Search methods
We searched the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (24 May 2018), and reference lists of retrieved studies.
Selection criteria
All randomised controlled trials or cluster‐randomised trials comparing the effectiveness and side effects of uterotonic agents with other uterotonic agents, placebo or no treatment for preventing PPH were eligible for inclusion. Quasi‐randomised trials were excluded. Randomised trials published only as abstracts were eligible if sufficient information could be retrieved.
Data collection and analysis
At least three review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We estimated the relative effects and rankings for preventing PPH ≥ 500 mL and PPH ≥ 1000 mL as primary outcomes. Secondary outcomes included blood loss and related outcomes, morbidity outcomes, maternal well‐being and satisfaction and side effects. Primary outcomes were also reported for pre‐specified subgroups, stratifying by mode of birth, prior risk of PPH, healthcare setting, dosage, regimen and route of administration. We performed pairwise meta‐analyses and network meta‐analysis to determine the relative effects and rankings of all available agents.
Main results
The network meta‐analysis included 196 trials (135,559 women) involving seven uterotonic agents and placebo or no treatment, conducted across 53 countries (including high‐, middle‐ and low‐income countries). Most trials were performed in a hospital setting (187/196, 95.4%) with women undergoing a vaginal birth (71.5%, 140/196).
Relative effects from the network meta‐analysis suggested that all agents were effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment. The three highest ranked uterotonic agents for prevention of PPH ≥ 500 mL were ergometrine plus oxytocin combination, misoprostol plus oxytocin combination and carbetocin. There is evidence that ergometrine plus oxytocin (RR 0.70, 95% CI 0.59 to 0.84, moderate certainty), carbetocin (RR 0.72, 95% CI 0.56 to 0.93, moderate certainty) and misoprostol plus oxytocin (RR 0.70, 95% CI 0.58 to 0.86, low certainty) may reduce PPH ≥ 500 mL compared with oxytocin. Low‐certainty evidence suggests that misoprostol, injectable prostaglandins, and ergometrine may make little or no difference to this outcome compared with oxytocin.
All agents except ergometrine and injectable prostaglandins were effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment. High‐certainty evidence suggests that ergometrine plus oxytocin (RR 0.83, 95% CI 0.66 to 1.03) and misoprostol plus oxytocin (RR 0.88, 95% CI 0.70 to 1.11) make little or no difference in the outcome of PPH ≥ 1000 mL compared with oxytocin. Low‐certainty evidence suggests that ergometrine may make little or no difference to this outcome compared with oxytocin meanwhile the evidence on carbetocin was of very low certainty. High‐certainty evidence suggests that misoprostol is less effective in preventing PPH ≥ 1000 mL when compared with oxytocin (RR 1.19, 95% CI 1.01 to 1.42). Despite the comparable relative treatment effects between all uterotonics (except misoprostol) and oxytocin, ergometrine plus oxytocin, misoprostol plus oxytocin combinations and carbetocin were the highest ranked agents for PPH ≥ 1000 mL.
Misoprostol plus oxytocin reduces the use of additional uterotonics (RR 0.56, 95% CI 0.42 to 0.73, high certainty) and probably also reduces the risk of blood transfusion (RR 0.51, 95% CI 0.37 to 0.70, moderate certainty) when compared with oxytocin. Carbetocin, injectable prostaglandins and ergometrine plus oxytocin may also reduce the use of additional uterotonics but the certainty of the evidence is low. No meaningful differences could be detected between all agents for maternal deaths or severe morbidity as these outcomes were rare in the included randomised trials where they were reported.
The two combination regimens were associated with important side effects. When compared with oxytocin, misoprostol plus oxytocin combination increases the likelihood of vomiting (RR 2.11, 95% CI 1.39 to 3.18, high certainty) and fever (RR 3.14, 95% CI 2.20 to 4.49, moderate certainty). Ergometrine plus oxytocin increases the likelihood of vomiting (RR 2.93, 95% CI 2.08 to 4.13, moderate certainty) and may make little or no difference to the risk of hypertension, however absolute effects varied considerably and the certainty of the evidence was low for this outcome.
Subgroup analyses did not reveal important subgroup differences by mode of birth (caesarean versus vaginal birth), setting (hospital versus community), risk of PPH (high versus low risk for PPH), dose of misoprostol (≥ 600 mcg versus < 600 mcg) and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
Authors' conclusions
All agents were generally effective for preventing PPH when compared with placebo or no treatment. Ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination may have some additional desirable effects compared with the current standard oxytocin. The two combination regimens, however, are associated with significant side effects. Carbetocin may be more effective than oxytocin for some outcomes without an increase in side effects.
Plain language summary
Which drug is best for reducing excessive blood loss after birth?
What is the issue?
The aim of this Cochrane Review was to find out which drug is most effective in preventing excessive blood loss at childbirth and has the least side effects. We collected and analysed all the relevant studies to answer this question (date of search: 24 May 2018).
Why is this important?
Excessive bleeding after birth is the most common reason why mothers die in childbirth worldwide. Although most women will have moderate bleeding at birth, others may bleed excessively, and this can pose a serious risk to their health and life. To reduce excessive bleeding at birth, the routine administration of a drug to contract the uterus (uterotonic) has become standard practice across the world.
Different drugs given routinely at birth have been used for reducing excessive bleeding. They include oxytocin, misoprostol, ergometrine, carbetocin, injectable prostaglandins and combinations of these drugs, each with different effectiveness and side effects. Some of the side effects identified include: vomiting, high blood pressure and fever. Currently, oxytocin is recommended as the standard drug to reduce excessive bleeding. We analysed all the available evidence to compare the effectiveness and side‐effect profiles for each drug.
What evidence did we find?
We found 196 studies involving 135,559 women. We compared seven uterotonic agents against each other and against women receiving no uterotonic. Studies were conducted across 53 countries. In most studies women were giving birth normally and in a hospital.
The analysis suggests that all drugs are effective for preventing blood loss that equals or exceeds 500 mL when compared with no routine uterotonic treatment. Compared with oxytocin (the standard recommended drug), the three best drugs for this outcome were a combination of ergometrine plus oxytocin, carbetocin, and a combination of misoprostol plus oxytocin. We found the other drugs misoprostol, injectable prostaglandins, and ergometrine may make little or no difference to this outcome compared with oxytocin.
All drugs except ergometrine and injectable prostaglandins are effective for preventing blood loss that equals or exceeds 1000 mL when compared with no treatment. Ergometrine plus oxytocin and misoprostol plus oxytocin make little or no difference in this outcome compared with oxytocin. It is uncertain whether carbetocin and ergometrine alone make any difference to this outcome. However, misoprostol is less effective in preventing blood loss that equals or exceeds 1000 mL compared with oxytocin.
Misoprostol plus oxytocin reduces the use of additional uterotonics and probably also reduces the risk of blood transfusion when compared with oxytocin. Carbetocin, injectable prostaglandins and ergometrine plus oxytocin may also reduce the use of additional uterotonics but the certainty of the evidence is low. No meaningful differences could be detected between all agents for maternal deaths or severe birth complication as these are rare in such studies.
The two combinations of drugs were associated with important side effects. When compared with oxytocin, women receiving misoprostol plus oxytocin combination are more likely to suffer vomiting and fever. Women receiving ergometrine plus oxytocin are also more likely to suffer vomiting and may make little or no difference to the risk of hypertension, however the certainty of the evidence was low for this outcome.
The analyses gave similar results irrespective of whether women were giving birth normally or by caesarean, in a hospital or in the community, were at high or low risk for bleeding excessively after birth, whether they received a high or a low dose of misoprostol and whether they received a bolus or an infusion of oxytocin or both.
What does this mean?
All agents were generally effective for preventing excessive bleeding when compared with no uterotonic drug treatment. Ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination may have some additional benefits compared with the current standard oxytocin. The two combination drugs, however, are associated with significant side effects that women might find disturbing compared with oxytocin. Carbetocin may have some additional benefits compared with oxytocin and appears to be without an increase in side effects.
Summary of findings
Summary of findings for the main comparison. PPH >= 500 mL.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: PPH ≥ 500 mL Setting: hospital or community setting | |||||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |||
| Carbetocin | 0.75 (0.58 to 0.98) | ⊕⊕⊕⊝ MODERATE a | 0.59 (0.31 to 1.12) | ⊕⊕⊝⊝ LOW b | 0.72 (0.56 to 0.93) |
⊕⊕⊕⊝ MODERATE c | 145 per 1000 | 104 per 1000 | 41 fewer per 1000 (from 64 fewer to 10 fewer) | ||
| Vaginal birth: 122 per 1000 | Vaginal birth:87 per 1000 | Vaginal birth: 34 fewer per 1000 (from 54 fewer to 9 fewer) | |||||||||
| Caesarean birth: 604 per 1000 | Caesarean birth: 435 per 1000 | Caesarean birth: 169 fewer per 1000 (from 266 fewer to 42 fewer) | |||||||||
| Misoprostol | 1.08 (0.94 to 1.24) | ⊕⊕⊝⊝ LOW d | 1.07 (0.83 to 1.39) | ⊕⊝⊝⊝ VERY LOW e | 1.08 (0.96 to 1.22) | ⊕⊕⊝⊝ LOW f | 145 per 1000 | 157 per 1000 | 12 more per 1000 (4 fewer to 32 more) | ||
| Vaginal birth:122 per 1000 | Vaginal birth:132 per 1000 | Vaginal birth:10 more per 1000 (4 fewer to 27 more) | |||||||||
| Caesarean birth: 604 per 1000 | Caesarean birth: 652 per 1000 | Caesarean birth: 48 more per 1000 (18 fewer to 133 more) | |||||||||
| Injectable prostaglandins | 0.84 (0.26 to 2.71) | ⊕⊕⊝⊝ LOW g | 1.08 (0.72 to 1.62) | ⊕⊝⊝⊝ VERY LOW e | 1.05 (0.73 to 1.51) | ⊕⊕⊝⊝ LOW f | 145 per 1000 | 152 per 1000 | 7 more per 1000 (39 fewer to 74 more) |
||
| Vaginal birth: 122 per 1000 | Vaginal birth:128 per 1000 | Vaginal birth:6 more per 1000 (33 fewer to 62 more) | |||||||||
| Caesarean birth: 604 per 1000 | Caesarean birth: 634 per 1000 | Caesarean birth: 30 more per 1000 (163 fewer to 308 more) | |||||||||
| Ergometrine | 1.31 (0.86 to 1.99) | ⊕⊝⊝⊝ VERY LOW h | 0.96 (0.70 to 1.31) | ⊕⊕⊝⊝ LOW i | 1.09 (0.85 to 1.39) | ⊕⊕⊝⊝ LOW j | 145 per 1000 | 158 per 1000 | 13 more per 1000 (22 fewer to 57 more) |
||
| Vaginal birth: 122 per 1000 | Vaginal birth: 133 per 1000 | Vaginal birth: 11 more per 1000 (18 fewer to 48 more) |
|||||||||
| Caesarean birth: 604 per 1000 | Caesarean birth: 610 per 1000 | Caesarean birth: 6 more per 1000 (91 fewer to 236 more) | |||||||||
| Ergometrine plus oxytocin | 0.72 (0.57 to 0.91) | ⊕⊕⊕⊝ MODERATE k | 0.69 (0.54 to 0.90) | ⊕⊕⊝⊝ LOW b | 0.70 (0.59 to 0.84) | ⊕⊕⊕⊝ MODERATE c | 145 per 1000 | 101 per 1000 | 44 fewer per 1000 (59 fewer to 23 fewer) | ||
| Vaginal birth: 122 per 1000 | Vaginal birth: 85 per 1000 | Vaginal birth: 37 fewer per 1000 (50 fewer to 20 fewer) | |||||||||
| Caesarean birth: 604 per 1000 | Caesarean birth: 423 per 1000 | Caesarean birth: 181 fewer per 1000 (248 fewer to 97 fewer) | |||||||||
| Misoprostol plus oxytocin | 0.71 (0.59 to 0.85) | ⊕⊕⊝⊝ LOW l | 0.79 (0.35 to 1.77) | ⊕⊕⊝⊝ LOW i | 0.70 (0.58 to 0.86) | ⊕⊕⊝⊝ LOW m | 145 per 1000 | 101 per 1000 | 44 fewer per 1000 (61 fewer to 20 fewer) | ||
| Vaginal birth: 122 per 1000 | Vaginal birth: 85 per 1000 | Vaginal birth: 37 fewer per 1000 (51 fewer to 17 fewer) | |||||||||
| Caesarean birth: 604 per 1000 | Caesarean birth: 423 per 1000 | Caesarean birth: 181 fewer per 1000 (254 fewer to 85 fewer) | |||||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin groups in the network meta‐analysis.The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. * No included studies or there are no event in included studies to estimate the baseline risk ** Absolute risk with uterotonic cannot be estimated in the absence of absolute risk with oxytocin ***Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin CI: Confidence interval; RR: Risk ratio. | |||||||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||||||
a Direct evidence downgraded ‐1 due to severe unexplained statistical heterogeneity
b Indirect evidence downgraded ‐2 due to multiple limitations in study design and severe unexplained statistical heterogeneity
c Network evidence downgraded ‐1 due to moderate certainty direct evidence (no intransitivity, incoherence, or imprecision)
d Direct evidence downgraded ‐2 due to multiple crucial limitations in study design
e Indirect evidence downgraded ‐3 due to multiple crucial limitations in study design, severe unexplained statistical heterogeneity and serious imprecision
f Network evidence downgraded ‐2 due to low certainty direct evidence (no intransitivity or incoherence, network estimate remains imprecise)
g Direct evidence downgraded ‐2 due to multiple limitations in study design and serious imprecision
h Direct evidence downgraded ‐3 due to multiple crucial limitations in study design and serious imprecision
i Indirect evidence downgraded ‐2 due to severe unexplained statistical heterogeneity, multiple limitations in study design and serious imprecision
j Network evidence downgraded ‐2 due to low certainty indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
k Direct evidence downgraded ‐1 due to multiple limitations in study design
l Direct evidence downgraded ‐2 due to multiple limitations in study design and strong suspicion of publication bias
m Network evidence downgraded ‐2 due to low certainty direct and indirect evidence (no intransitivity, incoherence, or imprecision)
Summary of findings 2. PPH >= 1000 mL.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: PPH ≥ 1000 mL Setting: hospital or community setting | |||||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |||
| Carbetocin | 0.73 (0.45 to 1.19) |
⊕⊕⊝⊝ LOW a | 0.30 (0.13 to 0.72) |
⊕⊕⊝⊝ LOW b | 0.87 (0.62 to 1.21) |
⊕⊝⊝⊝ VERY LOW c | 37 per 1000 | 32 per 1000 | 5 fewer per 1000 (from 14 fewer to 8 more) | ||
| Vaginal birth: 30 per 1000 | Vaginal birth: 26 per 1000 | Vaginal birth: 4 fewer per 1000 (11 fewer to 6 more) | |||||||||
| Caesarean birth: 33 per 1000 | Caesarean birth: 116 per 1000 | Caesarean birth: 17 fewer per 1000 (from 51 fewer to 28 more) | |||||||||
| Misoprostol | 1.26 (1.11 to 1.43) |
⊕⊕⊕⊕ HIGH | 1.23 (0.92 to 1.64) |
⊕⊕⊕⊝ MODERATE d | 1.19 (1.01 to 1.42) |
⊕⊕⊕⊕ HIGH e | 37 per 1000 | 44 per 1000 | 7 more per 1000 (0 fewer to 16 more) | ||
| Vaginal birth: 30 per 1000 | Vaginal birth: 36 per 1000 | Vaginal birth: 6 more per 1000 (0 fewer to 13 more) | |||||||||
| Caesarean birth: 133 per 1000 | Caesarean birth: 158 per 1000 | Caesarean birth: 25 more per 1000 (1 more to 56 more) | |||||||||
| Injectable prostaglandins | 1.43 (0.20 to 10.31) |
⊕⊝⊝⊝ VERY LOW f | 0.74 (0.31 to 1.72) |
⊕⊝⊝⊝ VERY LOW g | 0.88 (0.41 to 1.89) |
⊕⊝⊝⊝ VERY LOW h | 37 per 1000 | 33 per 1000 | 4 fewer per 1000 (22 fewer to 33 more) |
||
| Vaginal birth: 30 per 1000 | Vaginal birth: 27 per 1000 | Vaginal birth: 3 fewer per 1000 (18 fewer to 27 more) | |||||||||
| Caesarean birth: 133 per 1000 | Caesarean birth: 118 per 1000 | Caesarean birth: 15 fewer per 1000 | |||||||||
| Ergometrine | 1.30 (0.52 to 3.27) |
⊕⊝⊝⊝ VERY LOW f | 0.61 (0.22 to 1.67) |
⊕⊕⊝⊝ LOW i | 0.94 (0.48 to 1.84) |
⊕⊕⊝⊝ LOW j | 37 per 1000 | 35 per 1000 | 2 fewer per 1000 (19 fewer to 31 more) | ||
| Vaginal birth: 30 per 1000 | Vaginal birth: 28 fewer per 1000 | Vaginal birth: 2 fewer per 1000 (16 fewer to 25 more) | |||||||||
| Caesarean birth: 133 per 1000 | Caesarean birth: 122 per 1000 | Caesarean birth: 8 fewer per 1000 (69 fewer to 112 more) | |||||||||
| Ergometrine plus oxytocin | 0.73 (0.57 to 0.93) |
⊕⊕⊕⊕ HIGH | 1.07 (0.75 to 1.54) |
⊕⊕⊕⊝ MODERATE k | 0.83 (0.66 to 1.03) |
⊕⊕⊕⊕ HIGH e | 37 per 1000 | 31 per 1000 | 6 fewer per 1000 (13 fewer to 1 more) | ||
| Vaginal birth: 30 per 1000 | Vaginal birth: 25 per 1000 | Vaginal birth: 5 fewer per 1000 (10 fewer to 1 more) | |||||||||
| Caesarean birth: 133 per 1000 | Caesarean birth: 124 per 1000 | Caesarean birth: 9 fewer per 1000 (45 fewer to 4 more) | |||||||||
| Misoprostol plus oxytocin | 0.87 (0.69 to 1.09) |
⊕⊕⊕⊝ MODERATE l | 1.17 (0.47 to 2.86) |
⊕⊕⊕⊕ HIGH | 0.88 (0.70 to 1.11) |
⊕⊕⊕⊕ HIGH e | 37 per 1000 | 31 per 1000 | 6 fewer per 1000 (13 fewer to 1 more) | ||
| Vaginal birth: 30 per 1000 | Vaginal birth: 25 per 1000 | Vaginal birth: 5 fewer per 1000 (10 fewer to 1 more) | |||||||||
| Caesarean birth: 133 per 1000 | Caesarean birth: 124 per 1000 | Caesarean birth: 9 fewer per 1000 (45 fewer to 4 more) | |||||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. * No included studies or there are no event in included studies to estimate the baseline risk ** Absolute risk with uterotonic cannot be estimated in the absence of absolute risk with oxytocin ***Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin CI: Confidence interval; RR: Risk ratio. | |||||||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||||||
a Direct evidence downgraded ‐2 due to serious imprecision and strong suspicion of publication bias
b Indirect evidence downgraded ‐2 due to very serious imprecision
c Network evidence downgraded ‐2 due to low certainty direct and indirect evidence, and ‐1 due to incoherence between the direct and indirect estimates (no intransitivity, network estimate remains imprecise)
d Indirect evidence downgraded ‐1 due to serious imprecision
e Network evidence not downgraded due to high certainty indirect evidence (no intransitivity, incoherence, or imprecision)
f Direct evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
g Indirect evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
h Network evidence downgraded ‐3 due to very low certainty direct and indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
i Indirect evidence downgraded ‐2 due to multiple limitations in study design and serious imprecision
j Network evidence downgraded ‐2 due to low certainty indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
k Indirect evidence downgraded ‐1 due to multiple limitations in study design
l Direct evidence downgraded ‐1 due to serious imprecision
Summary of findings 3. Additional uterotonics.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: use of additional uterotonics Setting: hospital or community setting | |||||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |||
| Carbetocin | 0.48 (0.34 to 0.68) |
⊕⊕⊝⊝ LOW a | 0.35 (0.22 to 0.57) |
⊕⊕⊝⊝ LOW b | 0.45 (0.34 to 0.59) |
⊕⊕⊝⊝ LOW c | 135 per 1000 | 61 per 1000 | 74 fewer per 1000 (89 fewer to 55 fewer) | ||
| Vaginal birth: 116 per 1000 | Vaginal birth: 52 per 1000 | Vaginal birth: 64 fewer per 1000 (77 fewer to 48 fewer) | |||||||||
| Caesarean birth: 304 per 1000 | Caesarean birth: 137 per 1000 | Caesarean birth: 167 fewer per 1000 (201 fewer to 125 fewer) | |||||||||
| Misoprostol | 1.01 (0.85 to 1.20) |
⊕⊕⊝⊝ LOW a | 1.18 (0.81 to 1.73) |
⊕⊕⊝⊝ LOW b | 1.04 (0.88 to 1.24) |
⊕⊕⊝⊝ LOW c | 135 per 1000 | 140 per 1000 | 5 more per 1000 (16 fewer to 32 more) | ||
| Vaginal birth: 116 per 1000 | Vaginal birth: 121 per 1000 | Vaginal birth: 5 more per 1000 (14 fewer to 28 more) | |||||||||
| Caesarean birth: 304 per 1000 | Caesarean birth: 316 per 1000 | Caesarean birth: 12 more per 1000 (36 fewer to 73 more | |||||||||
| Injectable prostaglandins | 0.29 (0.09 to 0.94) |
⊕⊕⊝⊝ LOW d | 0.78 (0.38 to 1.59) |
⊕⊕⊝⊝ LOW e | 0.55 (0.31 to 0.96) |
⊕⊕⊝⊝ LOW c | 135 per 1000 | 74 per 1000 | 61 fewer per 1000 (93 fewer to 5 fewer) | ||
| Vaginal birth: 116 per 1000 | Vaginal birth: 64 per 1000 | Vaginal birth: 52 fewer per 1000 (80 fewer to 5 fewer) | |||||||||
| Caesarean birth: 304 per 1000 | Caesarean birth: 167 per 1000 | Caesarean birth: 137 fewer per 1000 (210 fewer to 12 fewer) | |||||||||
| Ergometrine | 1.46 (0.61 to 3.48) |
⊕⊝⊝⊝ VERY LOW f | 0.83 (0.55 to 1.26) |
⊕⊝⊝⊝ VERY LOW g | 0.97 (0.69 to 1.36) |
⊕⊝⊝⊝ VERY LOW h | 135 per 1000 | 131 per 1000 | 4 fewer per 1000 (42 fewer to 49 more) | ||
| Vaginal birth: 116 per 1000 | Vaginal birth: 113 per 1000 | Vaginal birth: 3 fewer per 1000 (36 fewer to 42 more) | |||||||||
| Caesarean birth: 304 per 1000 | Caesarean birth: 295 per 1000 | Caesarean birth: 9 fewer per 1000 (94 fewer to 109 more) | |||||||||
| Ergometrine plus oxytocin | 0.79 (0.59 to 1.07) |
⊕⊝⊝⊝ VERY LOW f | 0.57 (0.40 to 0.81) |
⊕⊕⊝⊝ LOW b | 0.65 (0.50 to 0.85) |
⊕⊕⊝⊝ LOW c | 135 per 1000 | 89 per 1000 | 46 fewer per 1000 (66 fewer to 20 fewer) | ||
| Vaginal birth: 116 per 1000 | Vaginal birth: 77 per 1000 | Vaginal birth: 39 fewer per 1000 (57 fewer to 17 fewer) | |||||||||
| Caesarean birth: 304 per 1000 | Caesarean birth: 201 per 1000 | 1Caesarean birth: 03 fewer per 1000 (149 fewer to 46 fewer) | |||||||||
| Misoprostol plus oxytocin | 0.54 (0.44 to 0.67) |
⊕⊕⊕⊕ HIGH | 0.68 (0.31 to 1.51) |
⊕⊕⊝⊝ LOW b | 0.56 (0.42 to 0.73) |
⊕⊕⊕⊕ HIGH i | 135 per 1000 | 77 per 1000 | 58 fewer per 1000 (76 fewer to 35 fewer) | ||
| Vaginal birth: 116 per 1000 | Vaginal birth: 66 per 1000 | Vaginal birth: 50 fewer per 1000 (65 fewer to 30 fewer) | |||||||||
| Caesarean birth: 304 per 1000 | Caesarean birth: 173 per 1000 | Caesarean birth: 131 fewer per 1000 (170 fewer to 79 fewer) | |||||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin groups in the network meta‐analysis.The corresponding risks in the Carbetocin, Misoprostol, Injectable prostaglandins, Ergometrine, Ergometrine plus oxytocin (Syntometrine ®), Misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. * No included studies or there are no event in included studies to estimate the baseline risk ** Absolute risk with uterotonic cannot be estimated in the absence of absolute risk with oxytocin ***Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin CI: Confidence interval; RR: Risk ratio. | |||||||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||||||
a Direct evidence downgraded ‐2 due to multiple limitations in study design and severe unexplained statistical heterogeneity
b Indirect evidence downgraded ‐2 due to multiple limitations in study design and severe unexplained statistical heterogeneity
c Network evidence downgraded ‐2 due to low certainty direct and indirect evidence (no intransitivity, incoherence or serious imprecision)
dDirect evidence downgraded ‐2 due to multiple crucial limitations in study design
e Indirect evidence downgraded ‐2 due to multiple limitations in study design, severe unexplained statistical heterogeneity and serious imprecision
f Direct evidence downgraded ‐3 due to multiple limitations in study design, severe unexplained statistical heterogeneity and serious imprecision
g Indirect evidence downgraded ‐3 due to multiple limitations in study design, severe unexplained statistical heterogeneity and serious imprecision
h Network evidence downgraded ‐3 due to very low certainty direct and indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
i Network evidence not downgraded due to high certainty direct evidence (no intransitivity, incoherence, or imprecision)
Summary of findings 4. Blood transfusion.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: blood transfusion Setting: hospital or community setting | |||||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |||
| Carbetocin | 0.68 (0.38 to 1.22) | ⊕⊕⊕⊝ MODERATE a | 0.62 (0.21 to 1.85) | ⊕⊕⊝⊝ LOW b | 0.81 (0.49 to 1.32) | ⊕⊕⊕⊝ MODERATE c | 22 per 1000 | 18 per 1000 | 4 fewer per 1000 (11 fewer to 7 more) | ||
| Vaginal birth: 15 per 1000 |
Vaginal birth: 12 per 1000 | Vaginal birth: 3 fewer per 1000 (5 fewer to 4 more) | |||||||||
| Caesarean birth: 81 per 1000 |
Caesarean birth:66 per 1000 | Caesarean birth:15 fewer per 1000 (41 fewer to 26 more) | |||||||||
| Misoprostol | 0.81 (0.65 to 1.00) |
⊕⊕⊕⊝ MODERATE a | 1.02 (0.59 to 1.77) | ⊕⊕⊝⊝ LOW d | 0.88 (0.68 to 1.13) | ⊕⊕⊕⊝ MODERATE c | 22 per 1000 | 19 per 1000 | 3 fewer per 1000 (7 fewer to 3 more) | ||
| Vaginal birth: 15 per 1000 | Vaginal birth: 13 per 1000 | Vaginal birth: 2 fewer per 1000 (5 fewer to 2 more) | |||||||||
| Caesarean birth: 81 per 1000 | Caesarean birth: 71 per 1000 | Caesarean birth: 10 fewer per 1000 (26 fewer to 11 more) | |||||||||
| Injectable prostaglandins | 1.01 (0.04 to 23.65) | ⊕⊝⊝⊝ VERY LOW e | 0.49 (0.16 to 1.52) | ⊕⊝⊝⊝ VERY LOW f | 0.66 (0.25 to 1.72) | ⊕⊝⊝⊝ VERY LOW g | 22 per 1000 | 15 per 1000 | 7 fewer per 1000 (17 fewer to 16 more) | ||
| Vaginal birth: 15 per 1000 | Vaginal birth: 10 per 1000 | Vaginal birth: 5 fewer per 1000 (11 fewer to 11 more) | |||||||||
| Caesarean birth: 81 per 1000 | Caesarean birth: 56 per 1000 | Caesarean birth: 28 fewer per 1000 (61 fewer to 58 more) | |||||||||
| Ergometrine | 1.44 (0.20 to 10.23) | ⊕⊝⊝⊝ VERY LOW h | 1.01 (0.38 to 2.68) | ⊕⊕⊝⊝ LOW i | 1.11 (0.54 to 2.28) | ⊕⊕⊝⊝ LOW j | 22 per 1000 | 24 per 1000 | 2 more per 1000 (10 fewer to 28 more) | ||
| Vaginal birth: 15 per 1000 | Vaginal birth: 17 per 1000 | Vaginal birth: 2 more per 1000 (7 fewer to 19 more) | |||||||||
| Caesarean birth: 81 per 1000 | Caesarean birth: 90 per 1000 | Caesarean birth: 9 more per 1000 (37 fewer to 104 more) | |||||||||
| Ergometrine plus oxytocin | 0.88 (0.53 to 1.44) | ⊕⊕⊝⊝ LOW k | 0.64 (0.41 to 1.00) | ⊕⊕⊝⊝ LOW i | 0.77 (0.58 to 1.03) | ⊕⊕⊝⊝ LOW j | 22 per 1000 | 17 per 1000 | 5 fewer per 1000 (9 fewer to 1 more) | ||
| Vaginal birth: 15 per 1000 | Vaginal birth: 12 per 1000 | Vaginal birth: 3 fewer per 1000 (6 fewer to 0 fewer) | |||||||||
| Caesarean birth: 81 per 1000 | Caesarean birth: 63 per 1000 | Caesarean birth: 18 fewer per 1000 (33 fewer to 2 more) | |||||||||
| Misoprostol plus oxytocin | 0.50 (0.37 to 0.67) | ⊕⊕⊝⊝ LOW l | 0.77 (0.27 to 2.26) | ⊕⊕⊕⊝ MODERATE m | 0.51 (0.37 to 0.70) | ⊕⊕⊕⊝ MODERATE c | 22 per 1000 | 11 per 1000 | 11 fewer per 1000 (14 fewer to 7 fewer) | ||
| Vaginal birth: 15 per 1000 | Vaginal birth: 8 per 1000 | Vaginal birth: 7 fewer per 1000 (9 fewer to 5 fewer) | |||||||||
| Caesarean birth: 81 per 1000 | Caesarean birth: 42 per 1000 | Caesarean birth: 39 fewer per 1000 (50 fewer to 24 fewer) | |||||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin groups in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. * No included studies or there are no event in included studies to estimate the baseline risk ** Absolute risk with uterotonic cannot be estimated in the absence of absolute risk with oxytocin ***Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin CI: Confidence interval; RR: Risk ratio. | |||||||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||||||
a Direct evidence downgraded ‐1 due to serious imprecision
b Indirect evidence downgraded ‐2 due to very serious imprecision
c Network evidence downgraded ‐1 due to moderate certainty direct evidence (no intransitivity or incoherence, network estimate remains imprecise)
d Indirect evidence downgraded ‐2 due to multiple limitations in study design and strong suspicion of publication bias
e Direct evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
f Indirect evidence downgraded ‐3 due to multiple crucial limitations in study design and very serious imprecision
g Network evidence downgraded ‐3 due to very low certainty direct and indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
h Direct evidence downgraded ‐3 due to multiple limitations in study design, severe unexplained statistical heterogeneity and very serious imprecision
i Indirect evidence downgraded ‐2 due to multiple limitations in study design and serious imprecision
j Network evidence downgraded ‐2 due to low certainty indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
k Direct evidence downgraded ‐2 due to multiple limitations in study design and serious imprecision
l Direct evidence downgraded ‐2 due to multiple limitations in study design and strong suspicion of publication bias
m Indirect evidence downgraded ‐1 due to multiple limitations in study design and serious imprecision
Summary of findings 5. Vomiting.
|
Patient or population: women in the third stage of labour
Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin
Comparison (reference): oxytocin
Outcome: vomiting Setting: hospital or community setting | |||||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |||
| Carbetocin | 0.90 (0.53 to 1.50) | ⊕⊕⊕⊝ MODERATE a | 1.00 (0.51 to 1.95) | ⊕⊕⊝⊝ LOW b | 0.93 (0.64 to 1.35) | ⊕⊕⊕⊝ MODERATE c | 28 per 1000 | 26 per 1000 | 2 fewer per 1000 (10 fewer to 10 more) |
||
| Vaginal birth: 13 per 1000 | Vaginal birth: 12 per 1000 | Vaginal birth: 1 fewer per 1000 (5 fewer to 5 more) | |||||||||
| Caesarean birth: 97 per 1000 | Caesarean birth: 91 per 1000 | Caesarean birth: 6 fewer per 1000 (34 fewer to 35 more) | |||||||||
| Misoprostol | 1.51 (1.19 to 1.91) | ⊕⊕⊕⊕ HIGH |
2.73 (1.66 to 4.50) | ⊕⊕⊝⊝ LOW d | 1.63 (1.25 to 2.14) | ⊕⊕⊕⊝ MODERATE e | 28 per 1000 | 46 per 1000 | 18 more per 1000 (7 more to 32 more) | ||
| Vaginal birth: 13 per 1000 | Vaginal birth: 21 per 1000 | Vaginal birth: 8 more per 1000 (3 more to 15 more) | |||||||||
| Caesarean birth: 97 per 1000 | Caesarean birth: 158 per 1000 | Caesarean birth: 61 more per 1000 (24 more to 111 more) | |||||||||
| Injectable prostaglandins | 2.48 (0.57 to 10.73) | ⊕⊝⊝⊝ VERY LOW f | 4.07 (1.93 to 8.60) | ⊕⊝⊝⊝ VERY LOW g | 3.76 (1.90 to 7.42) | ⊕⊕⊝⊝ LOW h | 28 per 1000 | 105 per 1000 | 77 more per 1000 (25 more to 180 more) | ||
| Vaginal birth: 13 per 1000 | Vaginal birth: 49 per 1000 | Vaginal birth: 36 more per 1000 (12 more to 83 more) | |||||||||
| Caesarean birth: 97 per 1000 | Caesarean birth: 365 per 1000 | Caesarean birth: 268 more per 1000 (87 more to 623 more) | |||||||||
| Ergometrine | 3.83 (1.10 to 13.28) | ⊕⊕⊝⊝ LOW i | 1.83 (1.18 to 2.84) | ⊕⊕⊝⊝ LOW j | 2.36 (1.56 to 3.55) | ⊕⊕⊕⊝ MODERATE k | 28 per 1000 | 66 per 1000 | 38 more per 1000 (16 more to 71 more) |
||
| Vaginal birth: 13 per 1000 | Vaginal birth: 31 per 1000 | Vaginal birth: 18 more per 1000 (7 more to 33 more) | |||||||||
| Caesarean birth: 97 per 1000 | Caesarean birth: 229 per 1000 | Caesarean birth: 132 more per 1000 (54 more to 247 more) | |||||||||
| Ergometrine plus oxytocin | 3.05 (1.76 to 5.29) | ⊕⊕⊕⊝ MODERATE l | 2.77 (1.75 to 4.38) | ⊕⊕⊝⊝ LOW d | 2.93 (2.08 to 4.13) | ⊕⊕⊕⊝ MODERATE m | 28 per 1000 | 82 per 1000 | 54 more per 1000 (30 more to 88 more) | ||
| Vaginal birth: 13 per 1000 | Vaginal birth: 38 per 1000 | Vaginal birth: 25 more per 1000 (14 more to 41 more) | |||||||||
| Caesarean birth: 97 per 1000 | Caesarean birth: 284 per 1000 | Caesarean birth: 187 more per 1000 (105 more to 304 more) | |||||||||
| Misoprostol plus oxytocin | 2.24 (1.52 to 3.31) | ⊕⊕⊕⊕ HIGH | 1.48 (0.52 to 4.27) | ⊕⊝⊝⊝ VERY LOW g | 2.11 (1.39 to 3.18) | ⊕⊕⊕⊕ HIGH n | 28 per 1000 | 59 per 1000 | 31 more per 1000 (11 more to 61 more) | ||
| Vaginal birth: 13 per 1000 | Vaginal birth: 27 per 1000 | Vaginal birth: 14 more per 1000 (5 more to 28 more) | |||||||||
| Caesarean birth: 97 per 1000 | Caesarean birth: 205 per 1000 | Caesarean birth: 108 more per 1000 (38 more to 211 more) | |||||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin groups in the network meta‐analysis.The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. * No included studies or there are no event in included studies to estimate the baseline risk ** Absolute risk with uterotonic cannot be estimated in the absence of absolute risk with oxytocin ***Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin CI: Confidence interval; RR: Risk ratio. | |||||||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||||||
a Direct evidence downgraded ‐1 due to serious imprecision
b Indirect evidence downgraded ‐2 due to very serious imprecision
c Network evidence downgraded ‐1 due to moderate certainty direct evidence (no intransitivity or incoherence, network estimate remains imprecise)
d Indirect evidence downgraded ‐2 due to multiple crucial limitations in study design and serious imprecision
e Network evidence not initially downgraded given high certainty direct evidence; however, downgraded ‐1 due to incoherence between the direct and indirect estimates (no intransitivity or imprecision)
f Direct evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
g Indirect evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
h Network evidence initially downgraded ‐3 due to very low certainty direct and indirect evidence, however upgraded +1 due to precision of network estimate (when direct and indirect were both imprecise; no intransitivity or incoherence)
i Direct evidence downgraded ‐2 due to multiple crucial limitations in study design
j Indirect evidence downgraded ‐2 due to multiple limitations in study design and serious imprecision
k Network evidence initially downgraded ‐2 due to low certainty direct and indirect evidence, however upgraded +1 due to precision of network estimate (when direct and indirect were both imprecise; no intransitivity or incoherence)
l Direct evidence downgraded ‐1 due to multiple limitations in study design
m Network evidence downgraded ‐1 due to moderate certainty direct evidence (no intransitivity, incoherence, or serious imprecision)
n Network evidence not downgraded due to high certainty direct evidence (no intransitivity, incoherence, or serious imprecision)
Summary of findings 6. Hypertension.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: hypertension Setting: hospital or community setting | |||||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |||
| Carbetocin | Not reported by included studies | _ | 1.24 (0.28 to 5.56) |
⊕⊝⊝⊝ VERY LOW a | 1.24 (0.28 to 5.56) |
⊕⊝⊝⊝ VERY LOW b | 82 per 1000 | 102 per 1000 | 20 more per 1000 (59 fewer to 374 more) |
||
| Vaginal birth: 76 per 1000 | Vaginal birth: 94 per 1000 | Vaginal birth: 18 more per 1000 (55 fewer to 347 more) | |||||||||
| Caesarean birth: 167 per 1000 | Caesarean birth: 207 per 1000 | Caesarean birth: 40 more per 1000 (120 fewer to 762 more) | |||||||||
| Misoprostol | 3.64 (0.60 to 22.27) |
⊕⊝⊝⊝ VERY LOW c | 1.01 (0.28 to 3.65) |
⊕⊕⊝⊝ LOW d | 1.50 (0.49 to 4.61) |
⊕⊕⊝⊝ LOW e | 82 per 1000 | 123 per 1000 | 41 more per 1000 (42 fewer to 296 more) | ||
| Vaginal birth: 76 per 1000 | Vaginal birth: 114 per 1000 | Vaginal birth: 38 more per 1000 (39 fewer to 274 more) | |||||||||
| Caesarean birth: 167 per 1000 | Caesarean birth: 250 per 1000 | Caesarean birth: 83 more per 1000 (85 fewer to 603 more) | |||||||||
| Injectable prostaglandins | Not reported by included studies | _ | 1.40 (0.09 to 20.66) |
⊕⊝⊝⊝ VERY LOW a | 1.40 (0.09 to 20.66) |
⊕⊝⊝⊝ VERY LOW b | 82 per 1000 | 115 per 1000 | 33 more per 1000 (75 fewer to 1000 more) |
||
| Vaginal birth: 76 per 1000 | Vaginal birth: 106 per 1000 | Vaginal birth: 30 more per 1000 (69 fewer to 1000 more) |
|||||||||
| Caesarean birth: 167 per 1000 | Caesarean birth: 234 per 1000 | Caesarean birth: 67 more per 1000 (152 fewer to 1000 more) | |||||||||
| Ergometrine | 13.39 (2.01 to 89.44) |
⊕⊕⊝⊝ LOW f | 12.42 (0.91 to 168.67) |
⊕⊝⊝⊝ VERY LOW g | 8.54 (2.12 to 34.48) |
⊕⊕⊝⊝ LOW h | 82 per 1000 | 700 per 1000 | 618 more per 1000 (92 more to 2745 more) |
||
| Vaginal birth: 76 per 1000 | Vaginal birth: 649 per 1000 | Vaginal birth: 573 more per 1000 (85 more to 1000 more) |
|||||||||
| Caesarean birth: 167 per 1000 | Caesarean birth: 1000 per 1000 | Caesarean birth: 1000 more per 1000 (187 more to 1000 more) | |||||||||
| Ergometrine plus oxytocin | 2.00 (0.29 to 13.97) |
⊕⊕⊝⊝ LOW i | 5.16 (0.63 to 42.13) |
⊕⊝⊝⊝ VERY LOW j | 2.48 (0.89 to 6.88) |
⊕⊕⊝⊝ LOW k | 82 per 1000 | 203 per 1000 | 121 more per 1000 (9 fewer to 482 more) | ||
| Vaginal birth: 76 per 1000 | Vaginal birth: 188 per 1000 | Vaginal birth: 112 more per 1000 (8 fewer to 447 more) | |||||||||
| Caesarean birth: 167 per 1000 | Caesarean birth: 414 per 1000 | Caesarean birth: 247 more per 1000 (18 fewer to 982 more) | |||||||||
| Misoprostol plus oxytocin | Not reported by included studies | _ | Not reported by included studies | _ | Not reported by included studies | _ | see comment* | see comment** | see comment*** | ||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin groups in the network meta‐analysis.The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. * No included studies or there are no event in included studies to estimate the baseline risk ** Absolute risk with uterotonic cannot be estimated in the absence of absolute risk with oxytocin ***Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin CI: Confidence interval; RR: Risk ratio. | |||||||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||||||
a Indirect evidence downgraded ‐3 due to multiple limitations in study design, very serious imprecision and severe unexplained statistical heterogeneity
b Network evidence downgraded ‐3 due to very low certainty indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
c Direct evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
d Indirect evidence downgraded ‐2 due to multiple limitations in study design, serious imprecision and severe unexplained statistical heterogeneity
e Network evidence downgraded ‐2 due to low certainty indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
f Direct evidence downgraded ‐2 due to multiple limitations in study design and severe unexplained statistical heterogeneity
g Indirect evidence downgraded ‐3 due to multiple crucial limitations in study design and severe unexplained statistical heterogeneity
h Network evidence downgraded ‐3 due to very low certainty indirect evidence (no intransitivity, incoherence or imprecision; although CI is wide there is a clear increase in this outcome for ergometrine)
i Direct evidence downgraded ‐2 due to severe unexplained statistical heterogeneity and serious imprecision
j Indirect evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
k Network evidence downgraded ‐2 due to low certainty direct evidence (no intransitivity or incoherence, network estimate remains imprecise)
Summary of findings 7. Fever.
| Patient or population: women in the third stage of labour Interventions: carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin Comparison (reference): oxytocin Outcome: fever Setting: hospital or community setting | |||||||||||
| Uterotonic agent(s) | Direct evidence | Indirect evidence | NMA evidence | Anticipated absolute effects for NMA estimate | |||||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with oxytocin | Risk with intervention (other uterotonics) | Risk difference with intervention | |||
| Carbetocin | 1.58 (0.27 to 9.35) | ⊕⊕⊕⊝ MODERATE a | 0.77 (0.18 to 3.42) |
⊕⊕⊝⊝ LOWb | 1.07 (0.43 to 2.69) |
⊕⊕⊕⊝ MODERATE c | 29 per 1000 | 31 per 1000 | 2 more per 1000 (17 fewer to 49 more) |
||
| Vaginal birth: 24 per 1000 | Vaginal birth: 26 per 1000 | Vaginal birth: 2 more per 1000 (14 fewer to 41 more) |
|||||||||
| Caesarean birth: 55 per 1000 | Caesarean birth: 59 per 1000 | Caesarean birth: 4 more per 1000 (31 fewer to 93 more) | |||||||||
| Misoprostol | 3.75 (2.73 to 5.15) |
⊕⊕⊝⊝ LOW d | 6.49 (2.24 to 18.76) |
⊕⊕⊕⊝ MODERATE e | 3.87 (2.90 to 5.16) |
⊕⊕⊕⊝ MODERATE c | 29 per 1000 | 112 per 1000 | 83 more per 1000 (55 more to 121 more) | ||
| Vaginal birth: 24 per 1000 | Vaginal birth: 93 per 1000 | Vaginal birth: 69 more per 1000 (46 more to 100 more) | |||||||||
| Caesarean birth: 55 per 1000 | Caesarean birth: 213 per 1000 | Caesarean birth: 158 more per 1000 (105 more to 229 more) | |||||||||
| Injectable prostaglandins | 2.00 (0.18 to 21.71) | ⊕⊝⊝⊝ VERY LOW f | 0.96 (0.24 to 3.87) |
⊕⊕⊝⊝ LOW b | 1.12 (0.33 to 3.86) |
⊕⊕⊝⊝ LOWg | 29 per 1000 | 32 per 1000 | 3 more per 1000 (19 fewer to 83 more) |
||
| Vaginal birth: 24 per 1000 | Vaginal birth: 27 per 1000 | Vaginal birth: 3 more per 1000 (16 fewer to 69 more) |
|||||||||
| Caesarean birth: 55 per 1000 (for caesarean birth) | Caesarean birth: 61 per 1000 (for caesarean birth) | Caesarean birth: 6 more per 1000 (37 fewer to 153 more) | |||||||||
| Ergometrine | 2.97 (0.97 to 9.05) |
⊕⊝⊝⊝ VERY LOW f | 0.63 (0.35 to 1.16) |
⊕⊕⊝⊝ LOW h | 0.77 (0.44 to 1.35) |
⊕⊝⊝⊝ VERY LOW i | 29 per 1000 | 22 per 1000 | 7 fewer per 1000 (16 fewer to 10 more) |
||
| Vaginal birth: 24 per 1000 | Vaginal birth: 18 per 1000 | Vaginal birth: 6 fewer per 1000 (13 fewer to 8 more) |
|||||||||
| Caesarean birth: 55 per 1000 | Caesarean birth: 42 per 1000 | Caesarean birth: 13 fewer per 1000 (31 fewer to 18 more) | |||||||||
| Ergometrine plus oxytocin | 1.08 (0.48 to 2.43) |
⊕⊕⊝⊝ LOW j | 0.54 (0.22 to 1.32) |
⊕⊕⊝⊝ LOW k | 0.70 (0.35 to 1.42) |
⊕⊕⊝⊝ LOW l | 29 per 1000 | 20 per 1000 | 9 fewer per 1000 (19 fewer to 12 more) |
||
| Vaginal birth: 24 per 1000 | Vaginal birth: 17 per 1000 | Vaginal birth: 7 fewer per 1000 (16 fewer to 10 more) |
|||||||||
| Caesarean birth: 55 per 1000 | Caesarean birth: 42 per 1000 | Caesarean birth: 13 fewer per 1000 (31 fewer to 19 more) | |||||||||
| Misoprostol plus oxytocin | 2.99 (2.00 to 4.45) |
⊕⊕⊕⊝ MODERATE m | 5.43 (1.48 to 19.95) |
⊕⊕⊝⊝ LOW n | 3.14 (2.20 to 4.49) |
⊕⊕⊕⊝ MODERATE o | 29 per 1000 | 91 per 1000 | 62 more per 1000 (35 more to 101 more) | ||
| Vaginal birth: 24 per 1000 | Vaginal birth: 75 per 1000 | Vaginal birth: 51 more per 1000 (29 more to 84 more) | |||||||||
| Caesarean birth: 55 per 1000 | Caesarean birth: 173 per 1000 | Caesarean birth: 118 more per 1000 (66 more to 192 more) | |||||||||
|
The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin groups in the network meta‐analysis.The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effect of individual uterotonic when compared with oxytocin (and its 95% CI) derived from the network meta‐analysis. * No included studies or there are no event in included studies to estimate the baseline risk ** Absolute risk with uterotonic cannot be estimated in the absence of absolute risk with oxytocin ***Risk difference cannot be estimated in the absence of absolute risks with intervention and oxytocin CI: Confidence interval; RR: Risk ratio. | |||||||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||||||
a Direct evidence downgraded ‐1 due to serious imprecision
b Indirect evidence downgraded ‐2 due to multiple limitations in study design, severe unexplained statistical heterogeneity and serious imprecision
c Network evidence downgraded ‐1 due to moderate certainty direct evidence (no intransitivity or incoherence, network estimate remains imprecise)
d Direct evidence downgraded ‐2 due to multiple limitations in study design and severe unexplained statistical heterogeneity
e Indirect evidence downgraded ‐1 due to multiple limitations in study design and serious imprecision
f Direct evidence downgraded ‐3 due to multiple limitations in study design and very serious imprecision
g Network evidence downgraded ‐2 due to low certainty indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
h Indirect evidence downgraded ‐2 due to multiple limitations in study design, severe unexplained statistical heterogeneity and strong suspicion of publication bias. The indirect estimate is imprecise, however the effect estimates for the two head‐to‐head comparisons in the dominant first‐order loop were not imprecise, so we have not downgraded for imprecision
i Network evidence initially downgraded ‐2 due to low certainty indirect evidence; however, downgraded further ‐1 due to incoherence between the direct and indirect estimates (no intransitivity. Network estimate is imprecise, unlike indirect evidence, however no further downgrade considered because certainty already very low)
j Direct evidence downgraded ‐2 due to very serious imprecision
k Indirect evidence downgraded ‐2 due to multiple limitations in study design and severe unexplained statistical heterogeneity. The indirect estimate is imprecise, however the effect estimates for the two head‐to‐head comparisons in the dominant first‐order loop were not imprecise, so we have not downgraded for imprecision
l Network evidence downgraded ‐2 due to low certainty direct and indirect evidence (no intransitivity or incoherence, network estimate remains imprecise)
m Direct evidence downgraded ‐1 due to multiple limitations in study design
n Indirect evidence downgraded ‐2 due to multiple limitations in study design and severe unexplained statistical heterogeneity
o Network evidence downgraded ‐1 due to moderate certainty direct evidence (no intransitivity, incoherence or imprecision)
Background
Description of the condition
An estimated 303,000 women died during childbirth in 2015 (Alkema 2016). Postpartum haemorrhage (PPH) accounted for up to a third of all these maternal deaths (Say 2014). Almost all deaths occurred in low‐ or middle‐income countries. Even when death from PPH is avoided, the need for blood transfusion, hysterectomy and additional intervention place a huge burden on women's health and health services (Penney 2007; Souza 2013).
The third stage of labour, defined as the period of time from birth until the delivery of the placenta, and the immediate postpartum period are the most hazardous periods of childbirth due to the risk of PPH. The World Health Organization (WHO) defines PPH as when the blood loss after birth equals or exceeds 500 mL in the first 24 hours (WHO 2012). The most common cause of PPH is uterine atony (failure of the uterus to contract after birth). Even though risk factors for adverse maternal outcomes from severe haemorrhage have been identified (Souza 2013), often PPH is unpredictable as it occurs in the absence of identifiable clinical or historical risk factors (Combs 1991). Therefore, effective prevention of PPH is advocated for all women during childbirth (WHO 2012). The administration of uterotonic agents routinely in the third stage of labour is the key intervention that prevents PPH, although there is uncertainty about which agent may be the most effective.
Description of the intervention
The administration of uterotonic agents to prevent PPH is part of the active management of the third stage of labour (Begley 2015). The active management of the third stage of labour refers to the administration of a uterotonic agent, early cord clamping, and controlled cord traction until delivery of the placenta. In 2012, a WHO guideline panel revisited the evidence underpinning each component of active management of the third stage of labour and considered the use of uterotonics as the main intervention within this package (WHO 2012).
How the intervention might work
Several different uterotonic agents have been used for preventing PPH. These agents include ergometrine, misoprostol, carbetocin, oxytocin, injectable prostaglandins (such as carboprost and sulprostone) and the combinations of agents such as misoprostol plus oxytocin and ergometrine plus oxytocin.
Oxytocin
Oxytocin (Syntocinon®) is the most widely used uterotonic agent. At low doses, it produces rhythmic uterine contractions that are indistinguishable in frequency, force and duration from those observed during spontaneous labour, but at higher dosages, it causes sustained uterine contractions (MEDICINES.ORG.UK). It has a short half‐life, approximately three to five minutes, and can be used as an infusion to maintain uterine contraction. When used intramuscularly, the latent phase lasts three to seven minutes, but produces a longer‐lasting clinical effect of up to one hour (MEDICINES.ORG.UK). However, oxytocin cannot be used orally. It is unstable in ambient temperatures and it requires a cold chain through storage and transport. It should also not be given intravenously as a large bolus, because it can cause severe hypotension (Thomas 2007). Because of its anti‐diuretic effect, water intoxication can occur with prolonged infusion of oxytocin (MEDICINES.ORG.UK).
Ergometrine
Ergometrine and methylergometrine are ergot alkaloids that increase the uterine muscle tone by causing sustained uterine contractions. They have a latent phase of two to five minutes after intramuscular injection and the plasma half‐life is 30 to 120 minutes (de Groot 1998). After intravenous administration, the onset of action is one minute or less and the duration of action is 45 minutes (although rhythmic contractions may persist for up to three hours). However, ergometrine and methylergometrine have an unpredictable bioavailability, which prevents oral use of the agent and requires protection from light, and storage at a temperature between 2° and 8°C to prolong shelf life (de Groot 1996a). They are vasoconstrictive and are contraindicated in women with hypertensive or cardiovascular disorders (MEDICINES.ORG.UK).
Misoprostol
Misoprostol is a prostaglandin E1 analogue, which is licensed for the prevention and treatment of gastric ulcers. It is well known for its off‐label use as a uterotonic agent (Tuncalp 2012). It is water‐soluble and heat stable (Davies 2001). It is absorbed nine to 15 minutes after sublingual, oral, vaginal, and rectal use. The half‐life is about 20 to 40 minutes. Oral and sublingual routes have the advantage of rapid onset of action, while the vaginal and rectal routes result in prolonged activity and greater bioavailability (Schaff 2005).
Injectable prostaglandins
Prostaglandin preparations are available in injectable forms and the most commonly used agents are carboprost tromethamine (Hemabate), an analogue of 15‐methyl‐prostaglandin F2a, and sulprostone, which is a PGE2 analogue. After intramuscular administration, the time to peak plasma concentration is between 15 and 60 minutes. The half‐life is about eight minutes. They require storage at a temperature between 2° and 8°C to prolong shelf life (MEDICINES.ORG.UK). They both enhance uterine contractility and cause vasoconstriction in postpartum women (MEDICINES.ORG.UK). However, they are not contraindicated in hypertensive women (MEDICINES.ORG.UK). In the management of the third stage of labour, injectable prostaglandins have been mainly used for intractable PPH as a last resort when other measures fail. Important disadvantages of injectable prostaglandins have been their cost and availability.
Carbetocin
Carbetocin is a newer long‐acting synthetic analogue of oxytocin with agonist properties. After intravenous injection, it produces sustained uterine contractions within two minutes, lasting for approximately six minutes followed by rhythmic contractions for 60 minutes (Hunter 1992). When carbetocin is administered by an intramuscular injection, the sustained uterine contractions last for approximately 11 minutes and the rhythmic contractions for 120 minutes (Hunter 1992). A heat stable carbetocin is now available and has been evaluated against oxytocin in a large randomised trial (Widmer 2018). Carbetocin also appears to have a favourable side‐effect profile (Su 2012).
Combination agents
The use of combinations of uterotonic agents is also popular and the most commonly used agent is ergometrine plus oxytocin (Syntometrine ®). This is a fixed‐combination agent containing 5 international units (IU) of oxytocin and 500 mcg of ergometrine. Intramuscular injection is the recommended route (MEDICINES.ORG.UK). When used intramuscularly, the latent period for the occurrence of the uterine response is about 2.5 minutes and the uterotonic effects last for around three hours. Another combination that has been investigated is misoprostol plus oxytocin. This combination is not in synthetic (fixed‐drug) or naturally occurring forms.
The WHO recommends that all women giving birth should be offered uterotonics during the third stage of labour for the prevention of PPH; oxytocin (intramuscular/intravenous, 10 IU is the uterotonic agent of choice (WHO 2012). Other injectable uterotonics and misoprostol are recommended as alternatives for the prevention of PPH in settings where oxytocin is not available.
Why it is important to do this review
The individual uterotonics described above have been compared in existing Cochrane Reviews and all comparisons are based on trials that directly compared one uterotonic against another uterotonic agent in head‐to‐head trials (Begley 2015; Liabsuetrakul 2018; McDonald 2004; Su 2012; Tuncalp 2012; Westhoff 2013). The existing Cochrane Reviews have variable eligibility criteria for study inclusion, uterotonic agent comparisons and outcomes. In the absence of a single randomised controlled trial comparing all available uterotonic agents, uncertainty remains over their relative effectiveness and ranking. When multiple interventions are available, a network meta‐analysis is better placed for synthesising and interpreting the wider picture of the evidence and to understand the relative effects of all available interventions. Network meta‐analysis has advantages over conventional pairwise meta‐analysis, as the technique uses both direct and indirect evidence in a single coherent analysis to improve certainty about all possible treatment comparisons. Indirect evidence is obtained when the relative effectiveness of two competing interventions is inferred through a common comparator, even though this pair may not have been compared directly (Caldwell 2005; Lumley 2002).
This is an update of a review first published in April 2018. It has been updated to incorporate results from a large WHO trial (Widmer 2018) and a number of other large recently published trials.
Objectives
To identify the most effective uterotonic agent(s) to prevent postpartum haemorrhage (PPH) with the least side effects, and generate a ranking according to their effectiveness and side‐effect profile.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials or cluster‐randomised trials comparing the effectiveness and side effects of uterotonic agents with other uterotonic agents, placebo or no treatment for preventing postpartum haemorrhage (PPH) were eligible for inclusion. Quasi‐randomised trials were excluded. Randomised trials published only as abstracts were eligible if sufficient information could be retrieved.
Types of participants
The review included studies of women in the third stage of labour following a vaginal or caesarean birth in hospital or community settings.
Types of interventions
Trials were eligible if they administered uterotonic agents of any dosage, route or regimen systemically at birth for preventing PPH, and compared them with other uterotonic agents, placebo or no treatment. Trials evaluating uterotonic agents not administered systemically, such as intrauterine administration, or not immediately after birth, or exclusively comparing different dosages, routes or regimens of the same uterotonic agent were excluded. We included trials in which non‐pharmacologic co‐interventions such as controlled cord traction, cord clamping, or uterine massage was performed as a randomised intervention in all arms of the trial and the effects of such co‐interventions were tested through a sensitivity analysis.
We classified agents into single agents including oxytocin, carbetocin, injectable prostaglandins (carboprost tromethamine, sulprostone), misoprostol, ergometrine (included also ergonovine, methylergonovine), and combination agents including ergometrine plus oxytocin (Syntometrine ® as a fixed‐combination agent containing 5 international units (IU) of oxytocin and 500 mcg of ergometrine, any oxytocin dose and route when combined with any dose and route of ergometrine, ergonovine, or methylergonovine), and misoprostol plus oxytocin (any oxytocin dose and route when combined with any dose and route of misoprostol).
For this review, we assumed that any woman who meets the inclusion criteria is, in principle, equally likely to be randomised to any of the eligible uterotonic agents.
Types of outcome measures
We estimated the relative effects and rankings of the competing interventions according to the following outcomes.
Primary outcomes
The primary outcomes of the review were:
PPH ≥ 500 mL; and
PPH ≥ 1000 mL.
Secondary outcomes
The secondary outcomes of the review were:
maternal deaths;
severe maternal morbidity: intensive care admissions;
severe maternal morbidity: shock (as defined by the trialists);
additional uterotonics;
blood transfusion;
mean volumes of blood loss (mL);
change in haemoglobin measurements before versus after birth (g/L);
breastfeeding at hospital discharge;
nausea;
vomiting;
hypertension;
headache;
fever (>= 38°C);
shivering;
abdominal pain;
diarrhoea;
maternal sense of well‐being (as defined by the trialists);
maternal satisfaction (as defined by the trialists).
Search methods for identification of studies
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (24 May 2018).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports using the terms given in Appendix 1 (24 May 2018).
Searching other resources
We retrieved additional relevant references cited in papers identified through the above search strategy and we did search for the full texts of trials initially identified as abstracts. For randomised trials published only as abstracts, we sought information from primary authors to investigate whether these studies met our eligibility criteria before including them. Trials that compared at least two of the agents were eligible and we searched for all possible comparisons. We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Three review authors retrieved and independently assessed for inclusion all the potential studies we identified (IDG, AP, NA). We resolved any disagreements through discussion or, if required, in consultation with a third person (AC). We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).
1.
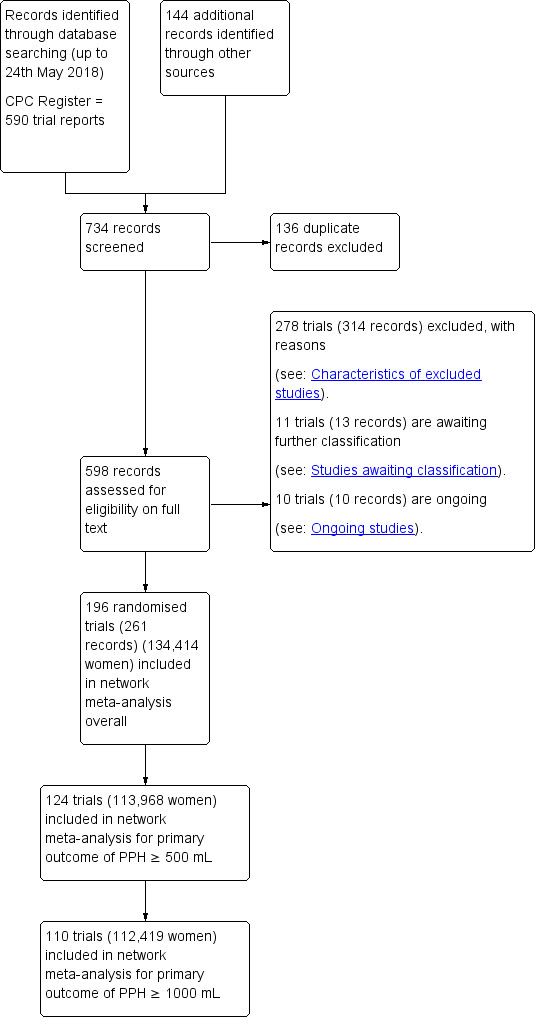
Study flow diagram.
Data extraction and management
We designed an electronic form on ©Microsoft Access to extract data. For eligible studies, at least three review authors independently extracted the data using a blank electronic form (IDG, AP, NA, RM, OT). We resolved discrepancies through discussion or, if required, we consulted another person (AC). We entered data into STATA and Review Manager software (RevMan 2014) and checked for accuracy. When information was unclear, we attempted to contact authors of the original reports to provide further details. The following data were extracted.
Outcome data
From each included study we extracted: the number of participants, the gestational age and the parity of participants, and any exclusion criteria. We also extracted: the interventions being compared, and their respective primary and secondary outcomes. All relevant arm level data were extracted (e.g. number of events and number of patients for binary outcomes and means and standard deviations per study arm for continuous outcomes).
Data on potential effect modifiers
From each included study we extracted the following study, intervention and population characteristics that may act as effect modifiers:
mode of delivery (vaginal or caesarean birth);
prior risk of PPH (as defined by trialists and categorised as low, high, mixed or not stated);
dosage, regimen, and route of administration (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion); and
setting of the study (community or hospital).
Other data
From each included study we extracted the following additional information:
country or countries in which the study was performed;
date of publication and dates of recruitment;
type of publication (full‐text publication, abstract publication, unpublished data); and
trial registration reference.
Assessment of risk of bias in included studies
At least three (IDG, AP, NA, RM) review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving another assessor (AC).
(1) Random sequence generation (checking for possible selection bias)
Studies were excluded if found to be at high risk for bias for random sequence generation (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number). We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to have affected the results.
We assessed the methods as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses. We assessed methods to handle incomplete outcome data as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups and less than 10% of missing outcome data);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation or more than 10% of missing outcome data); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns about other possible sources of bias, such as the source of funding and potential conflicts of interest.
We assessed these interests as:
low risk of other bias (public funding or no funding and no significant conflicts of interest identified);
high risk of other bias (industry funding or significant conflicts of interest identified); or
unclear risk of other bias.
Another source of bias was generated by the method of measuring blood loss. We assessed the method described in each study and classified it as at:
low risk of other bias (objective measurements such as weighing sponges, measurements in drapes, volumetric assessment, tagged red cells, etc);
high risk of other bias (subjective measurement such as clinical or visual estimates); or
unclear risk of other bias (unspecified methods of measurement).
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For our primary outcomes, we combined quality items and judged trials as “low risk of bias” if they were double‐blinded, had allocation concealment and with little loss to follow‐up (less than 10%). Trials were judged as “intermediate risk of bias” if they demonstrated adequate allocation concealment, with assessor blinding and little loss to follow‐up (less than 10%). Alternatively, trials were considered to be at “high risk of bias”. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis for information about how the risk of bias was incorporated in the sensitivity analysis.
Summary of findings
The 'Summary of findings' tables present evidence comparing all other uterotonic agents with a reference comparator, oxytocin. Each table describes key features of the evidence relating to a single outcome, and there is one table for each of our seven most important outcomes in accordance with the GRADE approach. These include PPH ≥ 500 mL, PPH ≥ 1000 mL, blood transfusion, additional uterotonics, vomiting, hypertension and fever.
We used the GRADE working group’s approach (Brignardello‐Petersen 2018; Puhan 2014) for rating the certainty of the network meta‐analysis effect estimates for all the comparisons and all outcomes. We appraised the certainty of the direct, indirect, and network evidence sequentially (in this order). First, we assessed the certainty of the direct evidence (where available) for a given outcome, and rated the evidence using the standard GRADE approach based on consideration of: study design limitations (risk of bias); inconsistency; imprecision; indirectness and publication bias (Higgins 2011). On the network diagram for all the comparisons and all outcomes we display the certainty of the direct evidence. Then we rated the certainty of the indirect evidence for the same outcome, and this was determined based on the lower of the certainty ratings of the two arms forming the dominant ‘first‐order’ loop in the network diagram for this outcome. Our final step was to determine the quality of network evidence based on: (i) the higher certainty rating of the direct and indirect evidence, (ii) whether the relevant network diagram exhibited ‘intransitivity’, i.e. whether all the comparisons contributing data to the estimate were directly consistent with the PICO question, (iii) consideration of coherence between direct and indirect effect estimates, and (iv) precision of the network effect estimate. Where the network estimate was precise, and the direct and/or indirect evidence contributing to the certainty ratings were not, the certainty of the network evidence was upgraded by one level for precision. At each of these stages, two review authors (MJW, VD, JP, MC) independently appraised the certainty ratings for the direct, indirect and network evidence. Disagreements between authors were resolved through discussion and consultation with a third review author (OTO, JPV) where necessary.
The quality of network evidence for each outcome was rated as ‘high’, ‘moderate’, ‘low’ or ‘very low’ in accordance with the GRADE approach:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect;
Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect; and
Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
For ease of comparison when interpreting the relative effects of all uterotonic agents, the 'Summary of findings' tables include the effect estimate and certainty judgements for each of the direct evidence, indirect evidence and the network meta‐analysis, describing all the findings for a single outcome in each table. The anticipated absolute effects are also included, based on the network effect estimate for each agent/agent combination in comparison with oxytocin. The assumed risks in the oxytocin group are based on weighted means of baseline risks from the studies with oxytocin arms in the network meta‐analysis. The corresponding risks in the carbetocin, misoprostol, injectable prostaglandins, ergometrine, ergometrine plus oxytocin (Syntometrine ®), misoprostol plus oxytocin groups (and their 95% confidence interval (CI)) are based on the assumed risk in the oxytocin group and the relative effect of the individual uterotonic when compared with oxytocin (and its 95% CI) as derived from the network meta‐analysis. The baseline risks differed significantly by the mode of birth subgroups, so the anticipated absolute effects are presented separately for vaginal and caesarean births based on the weighted means of baseline risks according to these modes of birth.
Measures of treatment effect
Relative treatment effects
We summarised relative treatment effects for dichotomous outcomes as risk ratios (RR) and for continuous outcomes as mean difference (MD) with 95% CIs (Dias 2013). These are summarised in forest plots displaying the results from pairwise, indirect and network (combining direct and indirect) analyses for the comparisons of uterotonic agents versus placebo or no treatment and the comparisons of uterotonic agents versus oxytocin. All other comparisons are available from Appendix 2.
Relative treatment ranking
We estimated the cumulative probabilities for each uterotonic agent being at each possible rank and obtained a treatment hierarchy using the surface under the cumulative ranking curve (SUCRA); the larger the SUCRA the higher its rank among all available agents (Salanti 2011). The probabilities to rank the treatments are estimated under a Bayesian model with flat priors, assuming that the posterior distribution of the parameter estimates is approximated by a normal distribution with mean and variance equal to the frequentist estimates and variance–covariance matrix. Rankings are constructed drawing 1000 samples from their approximate posterior density. For each draw, the linear predictor is evaluated for each study, and the largest linear predictor is noted (White 2011).
Unit of analysis issues
Cluster‐randomised trials
For a cluster‐randomised trial included in this review (Stanton 2013), we used the unadjusted standard errors as the clusters and the Intracluster Correlation Co‐efficient (ICC) was small (ICC = 0.012). Another cluster‐randomised trial (Chandhiok 2006) did not report the ICC and the ICC from Stanton 2013 was used. Chandhiok 2006 was reduced it to its effective sample size taking into account the design effects as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered it reasonable to combine the results from the cluster‐randomised and the individually‐randomised trials as there was little heterogeneity between the study designs and any interaction between the relative effects of agents and the choice of randomisation unit was considered to be unlikely. The effect of the unit of randomisation was also assessed in sensitivity analysis (Higgins 2011).
Multi‐arm trials
Multi‐arm trials were included and we accounted for the correlation between the effect estimates in the network meta‐analysis. We treated multi‐arm studies as multiple independent comparisons in pairwise meta‐analyses and these were not combined in any analysis.
Dealing with missing data
For included studies, we noted the levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. We used the number randomised minus any participants whose outcomes were known to be missing as the denominator for each outcome in each trial.
Assessment of clinical and methodological heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, we described the study population characteristics across all included trials. We assessed the presence of clinical heterogeneity by comparing these characteristics.
Assessment of intransitivity across treatment comparisons
In this context we expect that the intransitivity assumption holds assuming the following: 1) the common treatment used to compare different uterotonics indirectly is similar when it appears in different trials (e.g. oxytocin is administered in a similar way in oxytocin versus misoprostol trials and in oxytocin versus oxytocin plus ergometrine trials); 2) all pairwise comparisons do not differ with respect to the distribution of effect modifiers (e.g. the design and study characteristics of oxytocin versus misoprostol trials are similar to oxytocin versus oxytocin plus ergometrine trials). The assumption of intransitivity was evaluated epidemiologically by comparing the clinical and methodological characteristics of sets of studies from the various treatment comparisons.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. The funnel plots were assessed visually for asymmetry. We also assessed potential reporting bias for the primary outcomes by assessing the sensitivity of results to exclusion of studies with fewer than 400 participants.
Data synthesis
Methods for direct treatment comparisons
Initially, we performed pairwise meta‐analyses using a random‐effects model in Stata and Review Manager software (RevMan 2014) for every treatment comparison with at least two studies (DerSimonian 1986).
Methods for indirect and network comparisons
We initially generated and assessed the network diagrams to determine if a network meta‐analysis is feasible. Then we performed the network meta‐analysis within a frequentist framework using multivariate meta‐analysis estimated by restricted maximum likelihood. All analyses were done using Stata statistical software, release 15 (StataCorp, College Station, TX). We used the network suite of Stata commands designed for this purpose (White 2012; White 2015).
Assessment of statistical heterogeneity
Assumptions when estimating the heterogeneity
In pairwise meta‐analyses, we estimated the heterogeneity for each comparison. In network meta‐analysis we assumed a common estimate for the heterogeneity variance across all of the different comparisons.
Measures and tests for heterogeneity
We assessed statistically the presence of heterogeneity within each pairwise comparison for the primary outcomes using the I2 statistic that measures the percentage of variability that cannot be attributed to random error (Higgins 2002). The certainty of the evidence was downgraded for inconsistency where I2 ≥ 60%. The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter estimated from the multivariate meta‐analysis.
Assessment of statistical inconsistency
To check the assumption of consistency in the entire network we used the “design‐by‐ treatment” interaction model as described by Higgins (Higgins 2012). This method accounts for a different source of inconsistency that can occur when studies with different designs (two‐arm trials versus three‐arm trials) give different results as well as disagreement between direct and indirect evidence. Using this approach we inferred about the presence of inconsistency from any source in the entire network based on a Chi2 test.
Investigation of heterogeneity and inconsistency
Where we found important heterogeneity and/or inconsistency, we explored the possible sources for primary outcomes. Where sufficient studies were available, we performed multivariate meta‐analyses for subgroups and sensitivity analyses by using potential effect modifiers as possible sources of inconsistency and/or heterogeneity.
Subgroup analysis
For the primary outcomes we carried out the following pre‐specified subgroup analyses.
Population: prior risk of PPH (high versus low), mode of delivery (vaginal versus caesarean birth), setting (hospital versus community).
Intervention: dose of misoprostol (≥ 600 mcg versus < 600 mcg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
We assessed subgroup differences by firstly comparing the network diagram for each subgroup. Next, we performed a pairwise and network meta‐analysis for each subgroup and we compared their relative treatment effects and their relative treatment ranking. We examined the subgroups for qualitative interactions where the direction of effect could be reversed, that is if an intervention was beneficial in one subgroup but harmful in another.
Sensitivity analysis
For the primary outcomes we performed sensitivity analysis for the following.
Risk of bias (restricted to low risk of bias studies only): studies are ranked as 'low risk of bias' if they are double‐blinded, and have allocation concealment with little loss to follow‐up (less than 10%). The concealed studies with assessor blinding and little loss to follow‐up (less than 10%) are ranked as 'intermediate risk of bias' and the rest as 'high risk of bias'. We considered that assessor blinding was likely to be very important, in order to eliminate any risk of bias in subjective measurements or estimates of blood loss (not all studies measure this outcome objectively). We considered protocol publication in advance of the results to be an unsuitable criterion for sensitivity analyses, because protocol publication only became widespread in recent years.
Funding source (restricted to studies with funding source at low risk of bias (public or no funding)).
Whether an objective method of outcome assessment was employed (restricted to studies with an objective method of measuring blood loss). Objective methods of blood loss measurement were considered to be all methods that employed a measurement of the blood loss. This is in contrast to subjective methods where a healthcare professional is estimating the blood loss, usually visually.
Trial size (restricted to large studies (> 400 participants), in recognition of the greater likelihood for small studies than large or multi‐centre studies to suffer publication bias). In terms of trial size, there is evidence that smaller studies can exaggerate estimated benefits (Nüesch 2010). However, the cut‐off for deciding the definition of a small study can vary between research topics. For this topic, it appears that trials with more than 400 participants are more likely to be of higher quality, prospectively registered and overall at low risk of bias.
Removing trials that also randomised participants to co‐interventions such as uterine massage or controlled cord traction.
Removing trials with more than 10% missing data.
Removing trials published before 1990.
Randomisation unit (restricted to individually‐randomised trials and removing cluster‐randomised trials).
Choice of relative effect measure (risk ratio (RR) versus odds ratio (OR)).
Use of fixed‐effect versus random‐effects model.
Differences were assessed by evaluating the relative effects and assessment of model fit.
Results
Description of studies
Results of the search
The results of the search are summarised in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Figure 1).
The search of Cochrane Pregnancy and Childbirth's (CPC) Trials Register on 24 May 2018 retrieved in total 590 records. We retrieved a further 144 records from additional author searches and manual searching of reference lists for a total of 734 available records. From these, we excluded 136 records as duplicates. We examined the full text of 598 records and included in the network meta‐analysis 196 randomised trials (reported in 261 publications).
We contacted the authors from 98 randomised trials for additional data or clarifications. We were able to obtain additional data from trial authors for 39 randomised trials (Characteristics of included studies and Appendix 2). We excluded 278 trials (reported in 314 publications) (Characteristics of excluded studies), 11 trials (reported in 13 publications) could not be classified (Studies awaiting classification) and 10 trials were still ongoing (Ongoing studies).
Included studies
The network meta‐analysis includes 196 randomised trials involving 135,559 women. Most studies were reported in English; 11 translations were obtained (six Spanish, two French, two Turkish and one Chinese). The studies were conducted across 53 countries (including high‐, middle‐ and low‐income countries) and often involved more than one country. A number of multi‐arm trials were identified: two five‐arm trials, eight four‐arm trials and 22 three‐arm trials. The median size of the trials was around 213 participants (interquartile range (IQR) 123 to 529).
Most trials (95.4%, 187/196) were performed in a hospital setting, seven were performed in a community setting (3.6%), one (0.5%) in a mixed setting and one (0.5%) of unspecified setting. The majority of the trials included women undergoing a vaginal birth (71.5%, 140/196), and 53 trials (27%) involved women undergoing elective or emergency caesareans. Only two (1%) trials included women undergoing either a vaginal birth or caesarean and in one trial (0.5%) the mode of birth was not specified. Women included in the trials were judged to be at high risk for postpartum haemorrhage (PPH) in 66 of 196 trials (33.7%), low risk in 52 trials (26.5%) and 68 trials (34.7%) included women both at high or low risk for PPH. The risk for PPH was not specified in 10 trials (5.1%).
Women with a singleton pregnancy only were recruited in 124 trials (63.3%), 36 trials (18.4%) included women with either singleton or multiple pregnancies and only one trial (0.5%) included women with twin pregnancies only. Thirty‐five trials (17.8%) did not specify. Six trials (3.1%) included only nulliparous or primigravida women, one trial included only multiparous women (0.5%), 108 trials (55.1%) included both nulliparous and multiparous women of all parities, and 81 trials (41.3%) did not specify the parity of the women included in the trials. Exclusion criteria varied significantly and usually encompassed women with significant medical comorbidities.
Across all 196 trials (412 trial arms) in the network meta‐analysis, the following agents were used either as intervention or comparison:
137 trial arms (33.3%) used oxytocin;
96 trial arms (23.3%) used misoprostol;
39 trial arms (9.5%) used ergometrine;
35 trial arms (8.5%) used ergometrine plus oxytocin;
33 trial arms (8%) used carbetocin;
29 trial arms (7%) used placebo or no treatment;
26 trial arms (6.3%) used misoprostol plus oxytocin;
17 trial arms (4.1%) used injectable prostaglandins.
See Characteristics of included studies for details.
Excluded studies
We excluded 278 trials (for details seeCharacteristics of excluded studies). The most common reasons for exclusion were because trials were comparing exclusively doses or routes of the same uterotonic agents, trials that were quasi randomised or trials investigating ineligible interventions such as tranexamic acid.
Risk of bias in included studies
We present summaries of the methodological quality of the included studies for each of the domains we assessed across all studies (Figure 2) and for each included study (Figure 3).
2.
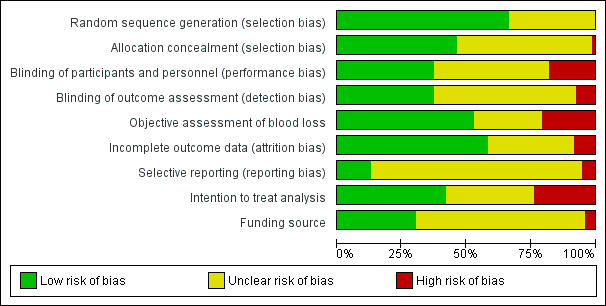
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Trials with evidence of inadequate random sequence generation were excluded from this review. As a result 130 of 196 included trials (66.3%) were found to have used an adequate method generating the random sequence and were at low risk of bias. However, 66 trials (33.7%) did not report the method used in sufficient detail and the risk of bias was judged to be unclear. Ninety of 196 trials (45.9%) reported adequate methods for allocation concealment and were judged to be at low risk of bias. Only three trials (1.5%) showed evidence of inadequate allocation concealment and 103 trials (52.6%), did not provide enough information to assess allocation concealment and the risk of bias was judged to be unclear.
Blinding
In total, 73 of 196 trials (37.2%) reported adequate methods for blinding both participants and personnel to treatment allocation. Thirty‐five trials (17.9%) were judged to be at high risk of bias for blinding of participants and personnel. Eighty‐eight trials (44.9%) did not provide enough information to assess the blinding of participants and personnel and the risk of bias was judged to be unclear. Seventy‐three of 196 trials (37.2%) reported adequate methods for blinding the assessment of the primary outcomes. Fifteen trials (7.7%) were judged to be at high risk of bias for blinding the assessment of the primary outcomes. There were 108 trials (55.1%) that did not provide enough information for blinding the assessment of the primary outcomes and the risk of bias was judged to be unclear.
Incomplete outcome data
There were 114 of 196 trials (58.1%) that were judged to be at a low risk of bias. In these trials, missing outcome data were less than 10% for the primary outcomes of the review and balanced in numbers across intervention groups with similar reasons for missing data across groups. In 16 trials (8.2%), more than 10% of patients dropped out or were not analysed as per the “intention‐to‐treat” principles following randomisation, indicating a high risk of bias. Sixty‐six trials (33.7%) did not provide enough information to assess so that it was uncertain whether or not the handling of incomplete data was appropriate and the risk of bias was judged to be unclear in these trials.
Selective reporting
Only 25 of 196 trials (12.8%) pre‐specified all outcomes in publicly available study protocols and were judged to be at low risk of bias. Ten trials (5.1%) did not report all pre‐specified outcomes as reported in their published protocols or methodology within the main report and were judged to be at high risk of bias for selective reporting. For most trials (161 trials; 82.1%), we were unable to identify a published protocol and the risk of bias was judged to be unclear.
Other potential sources of bias
Eighty‐two of 196 trials (41.8%) analysed data by the intention‐to‐treat principle and were judged to be at low risk of bias. Forty‐seven trials (24%) did not analyse data by the intention‐to‐treat principle and were judged to be at high risk of bias. For 67 trials (34.2%), we were unable to identify whether data were analysed by the intention‐to‐treat principle and the risk of bias was judged to be unclear.
We found that 59 of 196 trials (30.1%) were either conducted with public or no funding, and declared that they had no potential conflicts of interest. Eight trials (4.1%) were judged to be at high risk of bias as they were funded directly by the manufacturer of the drug under investigation. There were 129 trials (65.8%) that did not provide enough information to assess the source of funding or potential conflicts of interest and the risk of bias was judged to be unclear.
Among all the studies, 103 of 196 trials (52.6%) reported relatively objective methods for measuring blood loss such as weighing sponges, measurements in drapes or volumetric assessment and were judged to be at low risk of bias. The studies that did not measure blood loss as this was not an outcome of interest were also considered at low risk of bias. Forty‐one trials (20.9%) were judged to be at high risk of bias for measuring blood loss as they used subjective measurement such as clinical or visual estimates. Fifty‐two trials (26.5%) did not provide enough information to assess the method for measuring blood loss, and the risk of bias was judged to be unclear.
For the purpose of sensitivity analysis we analysed how many trials were judged to be at low, intermediate or high overall risk of bias. For PPH ≥ 500 mL, 38 of 124 trials (30.6%) were found to be at low overall risk of bias. Eighty‐six of 124 trials (69.4%) were judged to be at high risk of bias as they were judged to be either at high risk or unclear risk of bias for at least one of the domains mentioned above. There were no trials judged as intermediate risk of bias ‐ see Sensitivity analysis for information about how this risk of bias has impacted the results.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Please note that all of the analyses presented in the Data and analyses section relate to the 'direct evidence' and were used as per our methods to grade the evidence. The results from Data and analyses were also used to check the direction of effect in the subgroups and not to formally check for subgroup effects using the interaction test. These results are not described.
The following section presents the results as reported in all of the figures (Figure 4 to Figure 5). The figures present the results from the network diagrams, the forest plots with the pairwise, indirect and network (combining direct and indirect) effect estimates and the cumulative rankograms for all the outcomes with available data. The figures present the results for different uterotonics in comparison to placebo or no treatment and different uterotonics in comparison to the reference uterotonic agent oxytocin. All other comparisons are available from Appendix 2.
4.
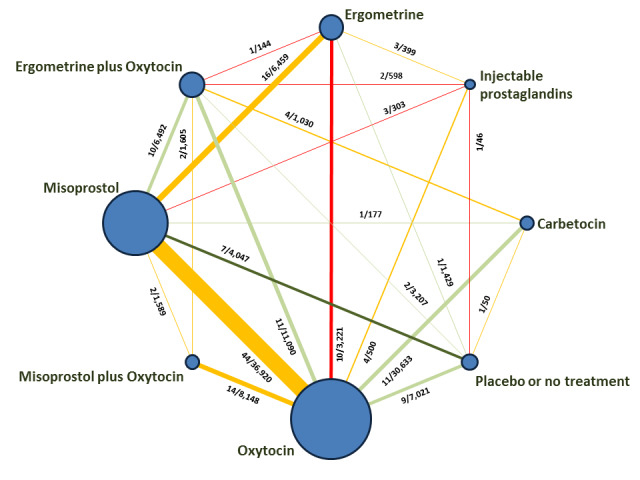
Network Diagram for PPH ≥ 500 mL. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
5.
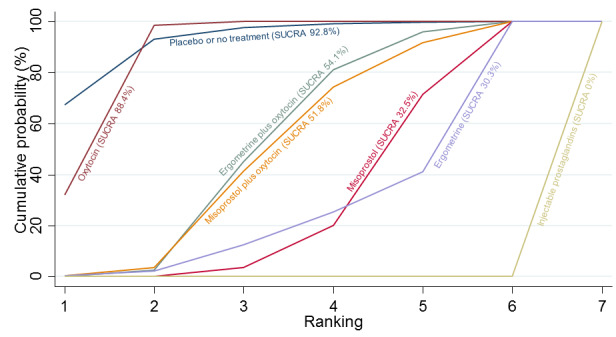
Cumulative rankograms comparing each of the uterotonic agents for diarrhoea. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Primary outcomes
Postpartum haemorrhage (PPH) ≥ 500 mL
The network diagram for PPH ≥ 500 mL is presented in Figure 4. Oxytocin was the most frequently investigated uterotonic agent (88 of 124 trials, 71%) for this outcome (Figure 4).
Relative effects from the network meta‐analysis of 124 trials suggested that all agents were effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 6). When compared with oxytocin, ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination were more effective in preventing PPH ≥ 500 mL. When compared with oxytocin, moderate‐certainty evidence suggests that carbetocin (risk ratio (RR) 0.72, 95% confidence interval (CI) 0.56 to 0.93) and ergometrine plus oxytocin (RR 0.70, 95% CI 0.59 to 0.84) probably reduce PPH ≥ 500 mL, while low‐certainty evidence suggests that misoprostol plus oxytocin (RR 0.70, 95% CI 0.58 to 0.86) may reduce PPH ≥ 500 mL. Low‐certainty evidence suggests that misoprostol, injectable prostaglandins, and ergometrine may make little or no difference to this outcome compared with oxytocin (Table 1). Based on these results, about 122 per 1000 women given oxytocin for a vaginal birth would experience a PPH of ≥ 500 mL compared with 85 given ergometrine plus oxytocin combination, 87 given carbetocin, and 85 given misoprostol plus oxytocin (Table 1).
6.
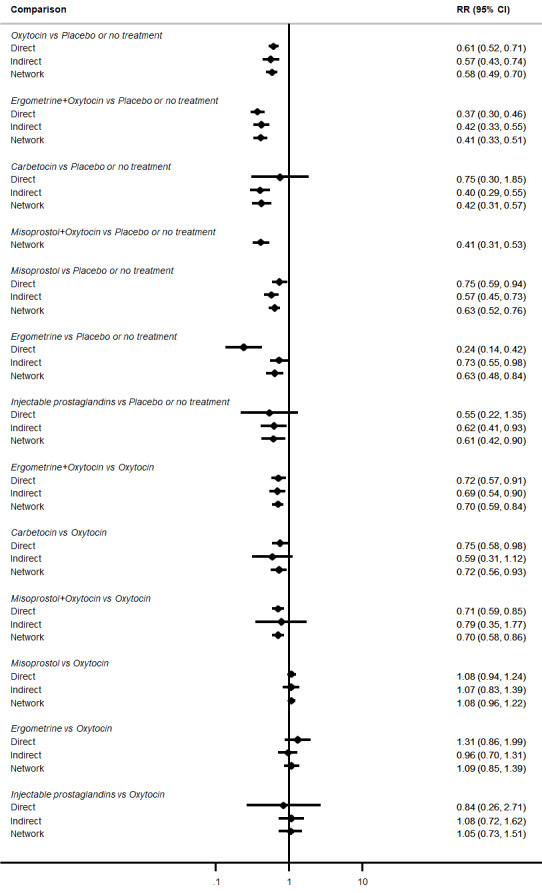
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of PPH ≥ 500 mL.
The cumulative probabilities for each agent being at each possible rank for preventing PPH ≥ 500 mL are shown in Figure 7. Treatment hierarchies are presented with the surface under the cumulative ranking curve (SUCRA); the larger the SUCRA the higher its rank among all available agents. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. A SUCRA of 100% means the uterotonic agent is the best and a SUCRA of 0% means the agent is the worst. The highest ranked agents were ergometrine plus oxytocin combination (SUCRA 86.8%), misoprostol plus oxytocin combination (SUCRA 85.7%) and carbetocin (SUCRA 83.1%). Oxytocin ranked fourth (47%) followed by injectable prostaglandins (SUCRA 37.6%), ergometrine (SUCRA 30.8%), misoprostol (SUCRA 28.7%) and placebo or no treatment (SUCRA 0.2%).
7.
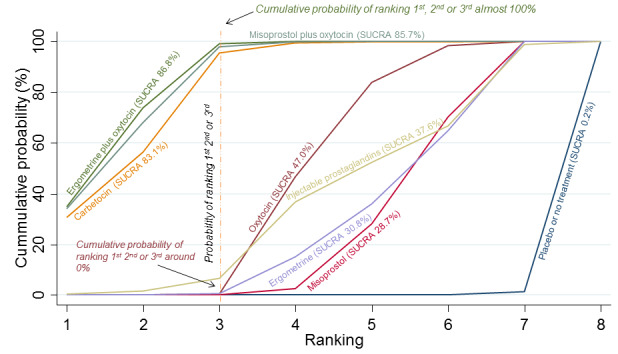
Cumulative rankograms comparing each of the uterotonic agents for prevention of PPH ≥ 500 mL. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Postpartum haemorrhage (PPH) ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL is presented in Figure 8. Oxytocin was the most frequently investigated uterotonic agent (72.7%, 80 of 110 trials) for this outcome (Figure 8).
8.
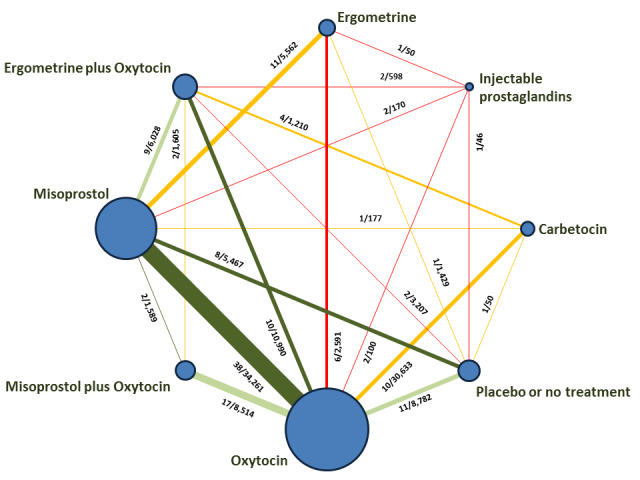
Network Diagram for PPH ≥ 1000 mL. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
Relative effects from the network meta‐analysis of 110 trials suggested that all agents except ergometrine and injectable prostaglandins were effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment (Figure 9). No differences were observed in the effects of uterotonic agents compared with the reference uterotonic agent oxytocin for PPH ≥ 1000 mL. High‐certainty evidence suggests that misoprostol plus oxytocin (RR 0.88, 95% CI 0.70 to 1.11) and ergometrine plus oxytocin (RR 0.83, 95% CI 0.66 to 1.03) make little or no difference to PPH ≥ 1000 mL when compared with oxytocin. In absolute terms, these results suggest that about 30 per 1000 women given oxytocin for a vaginal birth would experience PPH ≥ 1000 mL, compared with 26 given misoprostol plus oxytocin and 25 given ergometrine plus oxytocin. Low‐certainty evidence suggests that ergometrine (RR 0.94, 95% CI 0.48 to 1.84) may make little or no difference to this outcome when compared with oxytocin. The evidence for carbetocin and injectable prostaglandins was uncertain. High‐certainty evidence suggests that misoprostol is less protective against PPH ≥ 1000 mL when compared with oxytocin (RR 1.19, 95% CI 1.01 to 1.42) (Table 2).
9.
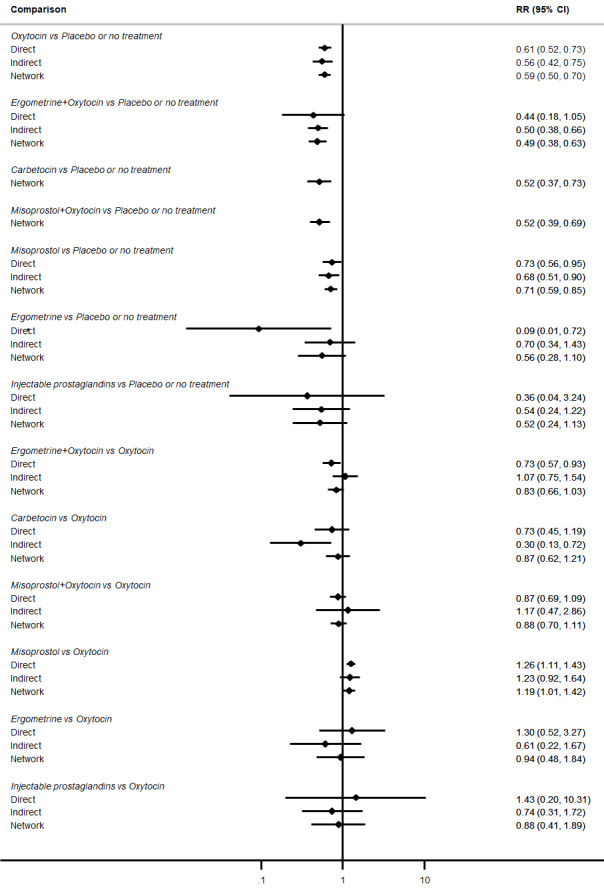
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of PPH ≥ 1000 mL.
Despite the comparable relative treatment effects between all uterotonics (except misoprostol) and oxytocin, cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL are shown in Figure 10. Ergometrine plus oxytocin (SUCRA 77.7%), misoprostol plus oxytocin (SUCRA 68.7%) combinations and carbetocin (SUCRA 68.4%) were the highest ranked agents. Oxytocin ranked sixth (45.6%) after injectable prostaglandins (SUCRA 61.3%) and ergometrine (SUCRA 55.5%). Misoprostol was seventh (SUCRA 21.5%) ranking higher than placebo or no treatment (1.4%).
10.
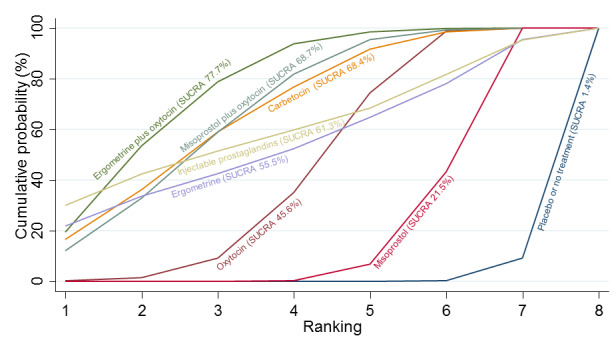
Cumulative rankograms comparing each of the uterotonic agents for prevention of PPH ≥ 1000 mL. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Secondary outcomes
Maternal death
The network diagram for maternal death is presented in Figure 11. Relative effects from the network meta‐analysis of 59 trials suggested that no meaningful differences could be detected between all uterotonic agents for maternal deaths as this outcome was rare (14 deaths across all trials were reported) (Figure 12). When compared with oxytocin, carbetocin (RR 2.00, 95% CI 0.37 to 10.92) and misoprostol (RR 0.62, 95% CI 0.14 to 2.74) probably make little or no difference to maternal death. Network relative effects were not estimable for the comparisons of other uterotonics with oxytocin (Figure 12).
11.
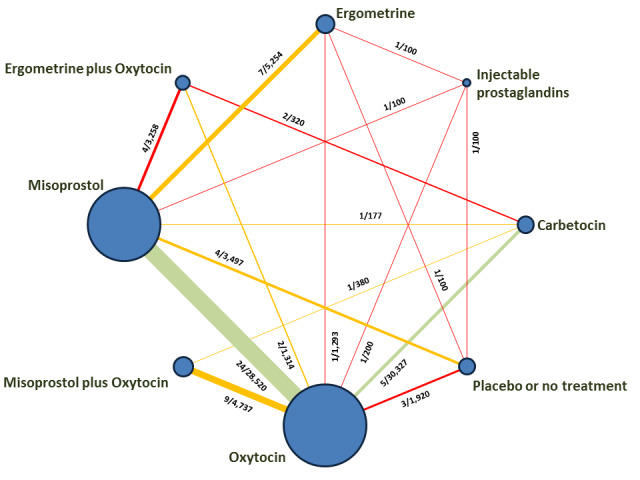
Network Diagram for maternal death. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
12.
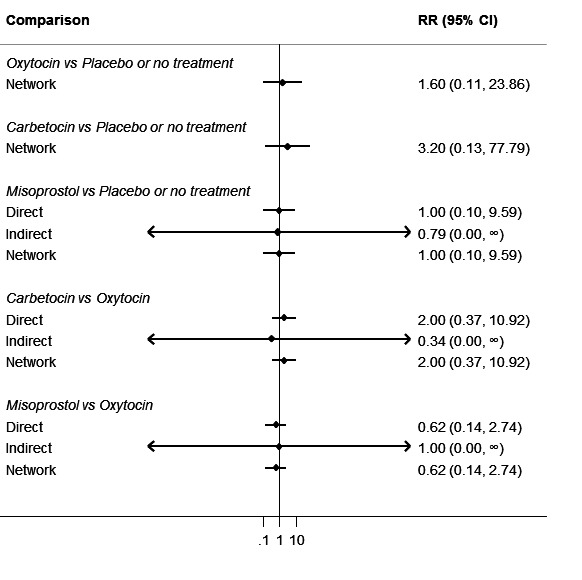
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of maternal death.
Figure 13 shows the cumulative probabilities for each agent being at each possible rank for maternal death. No reliable ranking could be derived for this outcome because of the rarity of maternal deaths.
13.
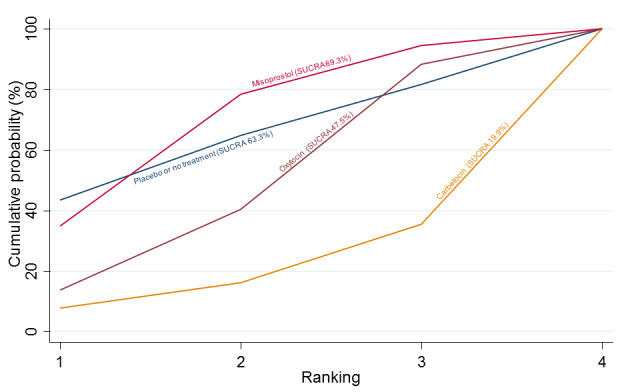
Cumulative rankograms comparing each of the uterotonic agents for prevention of maternal death. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Severe maternal morbidity: intensive care admissions
The network diagram for intensive care admissions as an outcome of severe morbidity is presented in Figure 14. Relative effects from the network meta‐analysis of 21 trials for the various comparisons suggested that there were no detectable differences among uterotonic agents for intensive care admissions as this outcome was rare. This outcome was not reported for any trial involving injectable prostaglandins (Figure 15).
14.
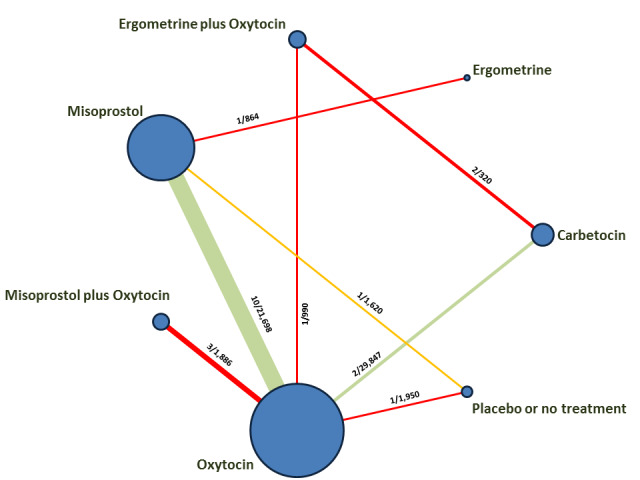
Network Diagram for severe maternal morbidity: intensive care admissions. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
15.
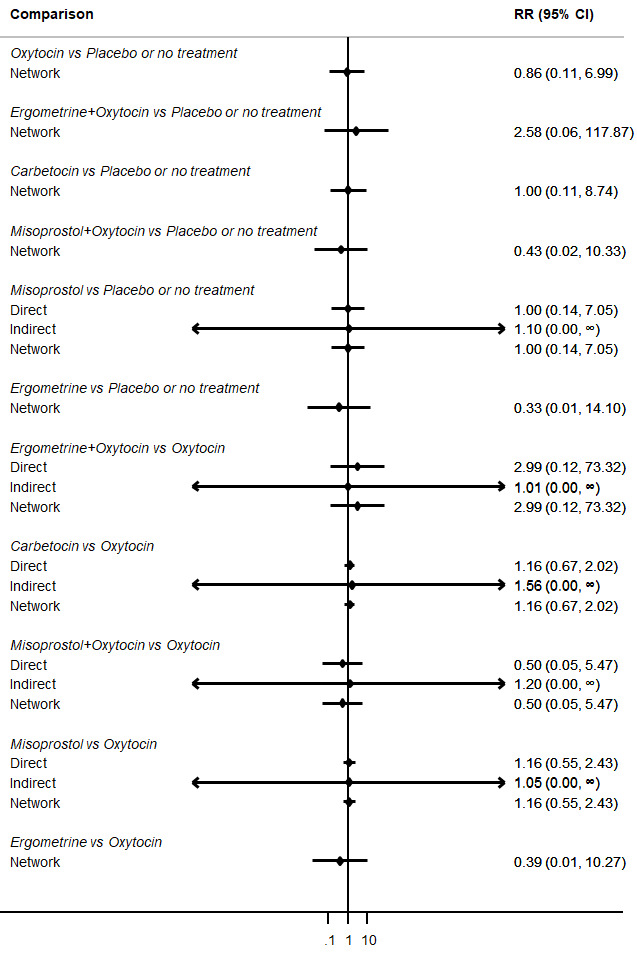
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for prevention of severe maternal morbidity: intensive care admissions.
Figure 16 shows the cumulative probabilities for each agent being at each possible rank for intensive care admissions. The ranking for all agents was not clear for this outcome due to limited data.
16.
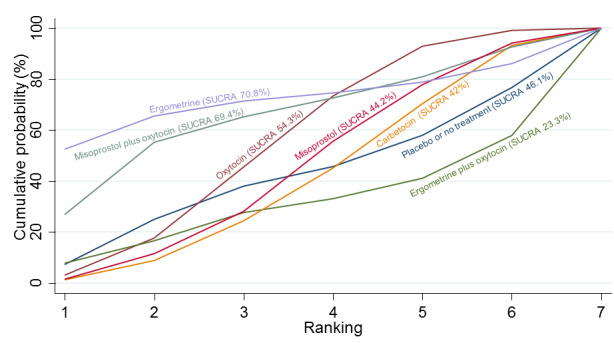
Cumulative rankograms comparing each of the uterotonic agents for prevention of severe maternal morbidity: intensive care admissions. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking. We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Severe maternal morbidity: shock
There were no trials reporting shock as an outcome of severe maternal morbidity.
Additional uterotonics
The network diagram for the use of additional uterotonics is presented in Figure 17. Relative effects from the network meta‐analysis of 142 trials suggested that all agents were effective at reducing the use of additional uterotonics when compared with placebo or no treatment (Figure 18). High‐certainty evidence suggests that misoprostol plus oxytocin (RR 0.56, 95% CI 0.42 to 0.73) reduces the use of additional uterotonics when compared with oxytocin (Table 3). Based on these results, about 116 per 1000 women given oxytocin for a vaginal birth would require the administration of additional uterotonic agents, compared with 66 given misoprostol plus oxytocin. There is low‐certainty evidence that carbetocin (RR 0.45, 95% CI 0.34 to 0.59), injectable prostaglandins (RR 0.55, 95% CI 0.31 to 0.96) and ergometrine plus oxytocin (RR 0.65, 95% CI 0.50 to 0.85) may also reduce the use of additional uterotonics compared with oxytocin. It is uncertain whether ergometrine reduces use of additional uterotonics because the certainty of this evidence is very low (Table 3).
17.
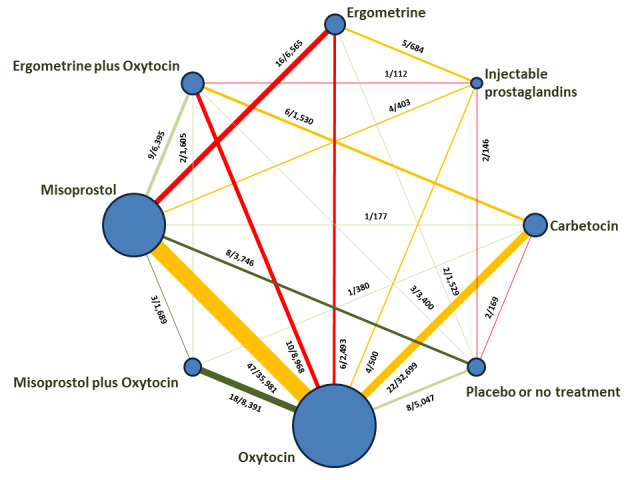
Network Diagram for additional uterotonics. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
18.
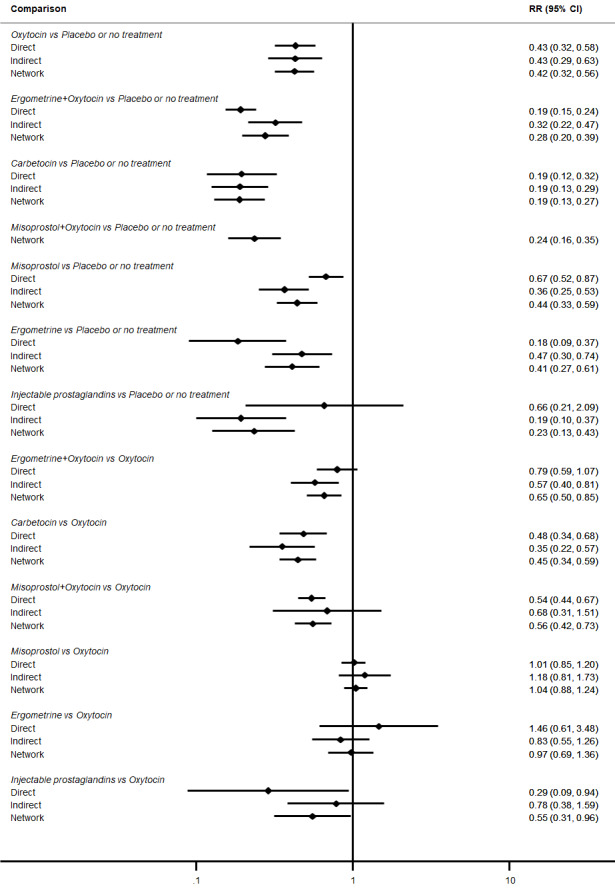
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for additional uterotonics.
Figure 19 shows the cumulative probabilities for each agent being at each possible rank for the use of additional uterotonics. The highest ranked agents were carbetocin (SUCRA 94.5%), injectable prostaglandins (77.5%), misoprostol plus oxytocin (SUCRA 77.2%) and ergometrine plus oxytocin (SUCRA 64.2%). Oxytocin was ranked sixth (SUCRA 30.7%) behind ergometrine (SUCRA 32.4%). The lowest ranked agents were misoprostol (SUCRA 23.4%), and placebo or no treatment (SUCRA 0%).
19.
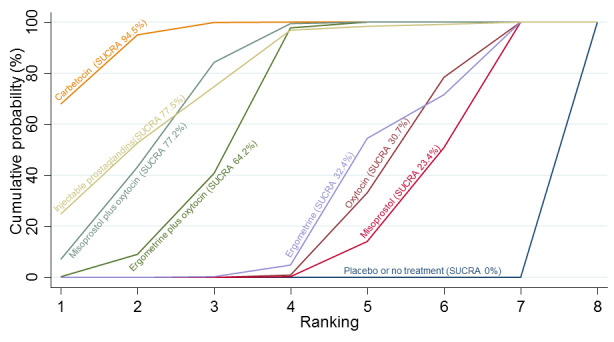
Cumulative rankograms comparing each of the uterotonic agents for additional uterotonics. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Blood transfusion
The network diagram for blood transfusion is presented in Figure 20. Relative effects from the network meta‐analysis of 124 trials suggested that all agents except ergometrine and injectable prostaglandins were effective for preventing blood transfusion when compared with placebo or no treatment (Figure 21). Moderate‐certainty evidence suggests that misoprostol plus oxytocin probably prevents the need for blood transfusion when compared with oxytocin (RR 0.51, 95% CI 0.37 to 0.70). This suggests that whilst around 15 per 1000 women would require a blood transfusion when given oxytocin for a vaginal birth, about 8 per 1000 women would need a transfusion with misoprostol plus oxytocin. Moderate‐certainty evidence suggests that carbetocin (RR 0.81, 95% CI 0.49 to 1.32) and misoprostol (RR 0.88, 95% CI 0.68 to 1.13) make little or no difference to the need for blood transfusion when compared with oxytocin. Low‐certainty evidence suggests that ergometrine (RR 1.11, 95% CI 0.54 to 2.28) and ergometrine plus oxytocin (RR 0.77, 95% CI 0.58 to 1.03) may make little or no difference to this outcome when compared with oxytocin. The evidence for injectable prostaglandins is uncertain (Table 4).
20.
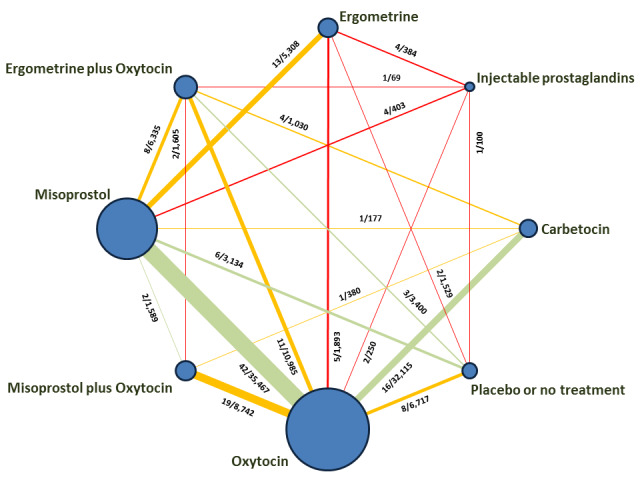
Network Diagram for blood transfusion. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
21.
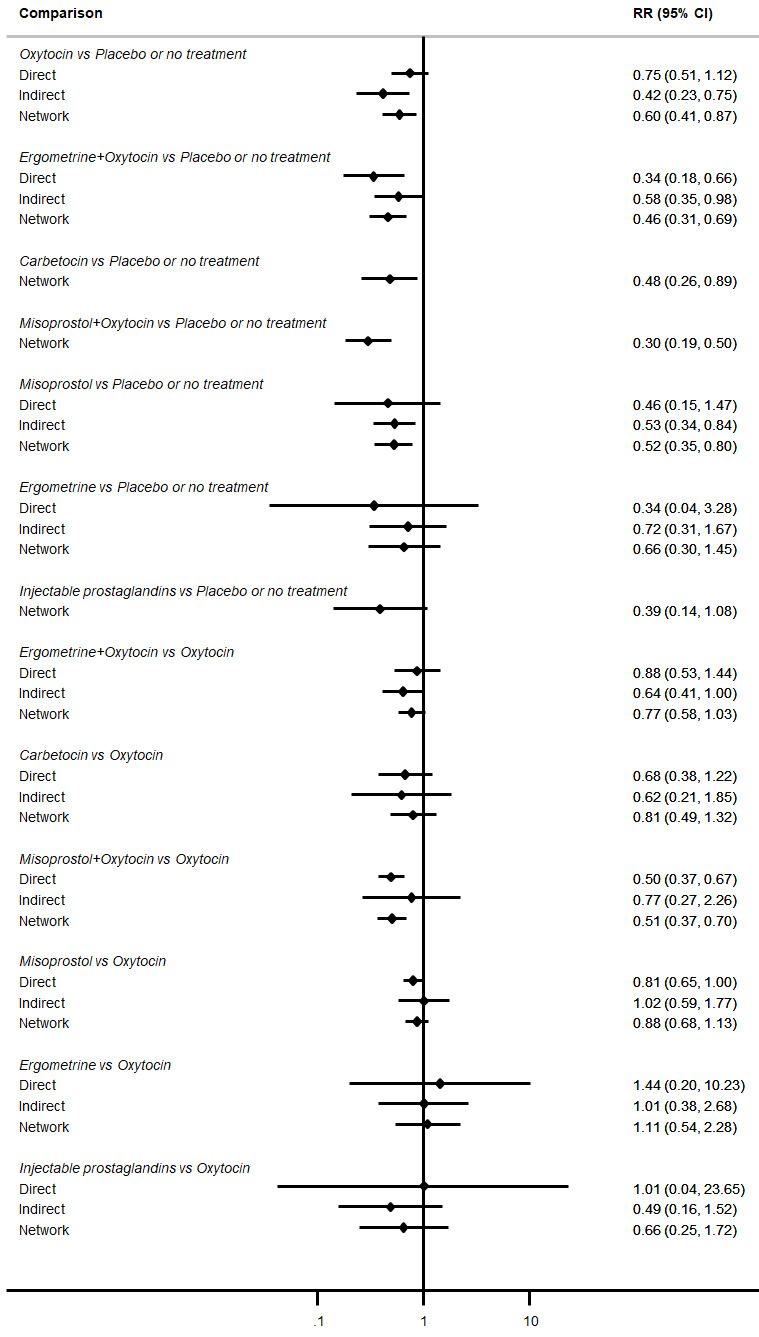
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for blood transfusion.
Figure 22 shows the cumulative probabilities for each agent being at each possible rank for preventing blood transfusion. The highest ranked agents were misoprostol plus oxytocin (SUCRA 94.1%), injectable prostaglandins (SUCRA 69.5%) and ergometrine plus oxytocin (SUCRA 64.5%). Oxytocin was ranked sixth (SUCRA 31.8%) behind carbetocin (SUCRA 57.6%) and misoprostol (SUCRA 50.7%), but higher than ergometrine (SUCRA 28.8%) and placebo or no treatment (SUCRA 3%).
22.
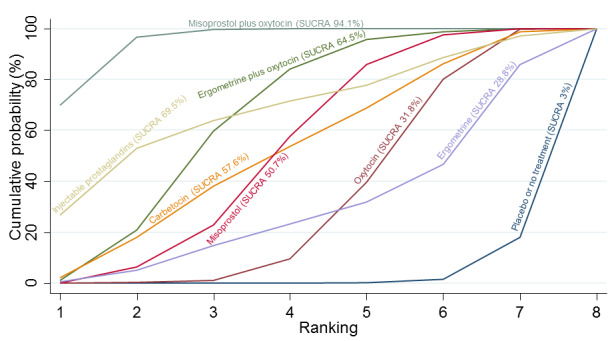
Cumulative rankograms comparing each of the uterotonic agents for blood transfusion. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Mean volumes of blood loss
The network diagram for blood loss (mL) as a continuous outcome is presented in Figure 23. Relative effects from the network meta‐analysis of 136 trials suggested that all agents are effective for reducing blood loss as a continuous outcome when compared with placebo or no treatment (Figure 24). When compared with oxytocin, moderate‐certainty evidence suggests that blood loss is probably on average reduced among women receiving misoprostol plus oxytocin (mean difference (MD) 88.31 mL lower, 95% CI 127.08 mL lower to 49.54 mL lower), and low‐certainty evidence suggests that it may be reduced among women receiving carbetocin (MD 81.39 mL lower, 95% CI 119.91mL lower to 42.87 mL lower). Low‐certainty evidence suggests that there may be little or no difference between ergometrine (MD 4.82 mL higher, 95% CI 28.00 mL lower to 37.64 mL higher) and oxytocin for this outcome. The effects of misoprostol, injectable prostaglandins and ergometrine plus oxytocin were unclear because the certainty of the evidence was very low (Figure 24).
23.
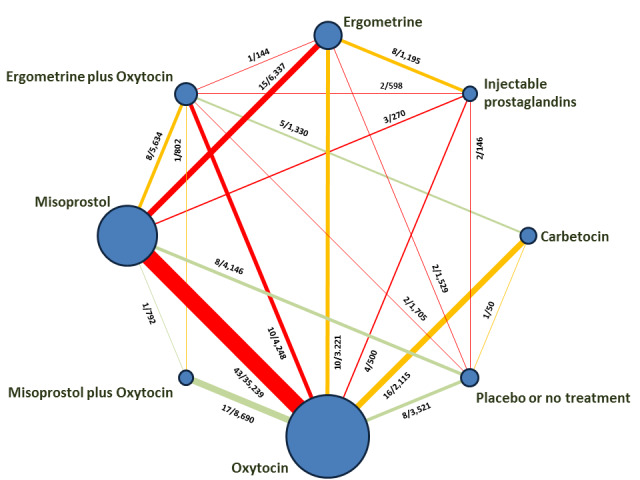
Network Diagram for mean blood loss (mL). The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
24.
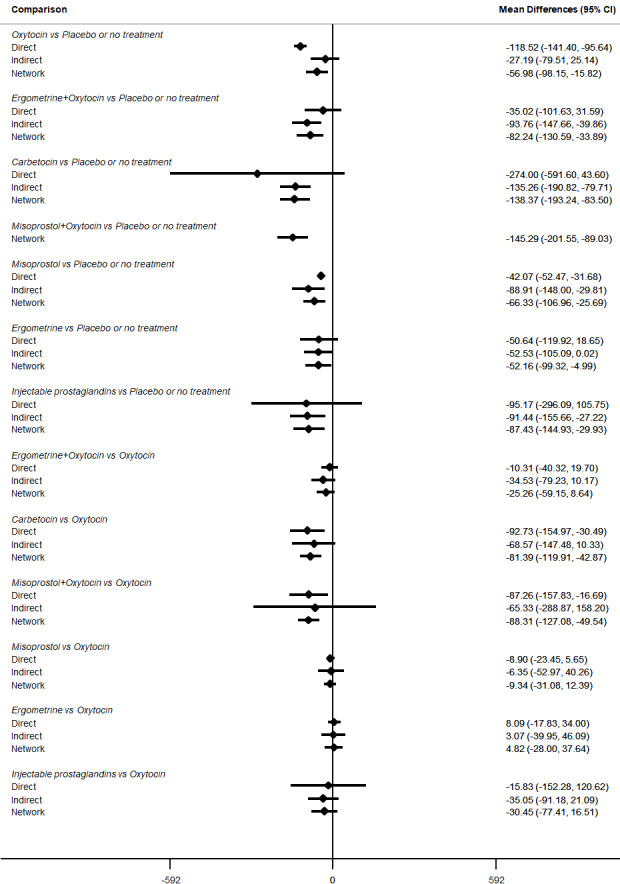
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for mean blood loss (mL).
Figure 25 shows the cumulative probabilities for each agent being at each possible rank for preventing blood loss (mL) as a continuous outcome. The highest ranked agents were misoprostol plus oxytocin (SUCRA 93.7%), carbetocin (SUCRA 90.9%), injectable prostaglandins (SUCRA 60.8%) and ergometrine plus oxytocin (SUCRA 58.5%). Oxytocin was ranked sixth (SUCRA 28.3%) behind misoprostol (SUCRA 42.8%). The lowest ranked agents were ergometrine (SUCRA 24.6%) and placebo or no treatment (SUCRA 0.4%).
25.
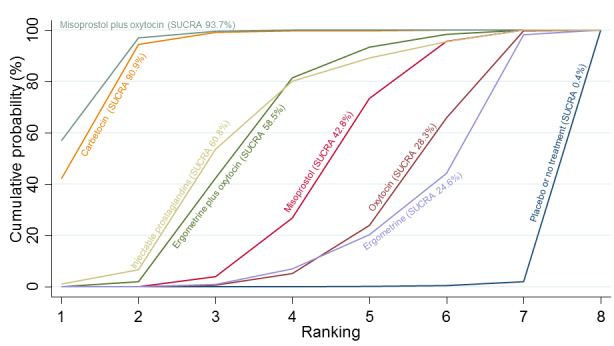
Cumulative rankograms comparing each of the uterotonic agents for mean blood loss (mL). Ranking indicates the cumulative probability of being the best, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Change in haemoglobin
The network diagram for the change in haemoglobin measurements before versus after birth (g/L) is presented in Figure 26. Relative effects from the network meta‐analysis of 86 trials suggested that all agents except ergometrine and the injectable prostaglandins were effective for reducing the change in haemoglobin measurements when compared with placebo or no treatment (Figure 27). There is low‐certainty evidence to suggest that the mean change in haemoglobin level before versus after birth may be lower among women receiving misoprostol plus oxytocin (MD 2.53 g/L lower, 95% CI 3.80 g/L lower to 1.26 g/L lower) and carbetocin (MD 2.18 g/L lower, 95% CI from 3.57 g/L lower to 0.79 g/L lower) compared with those receiving oxytocin. Low‐certainty evidence suggests that there may be little or no difference between ergometrine (MD 0.98 g/L higher, 95% CI from 0.74 g/L lower to 2.69 g/L higher); or ergometrine plus oxytocin (MD 1.07 g/L lower, 95% CI 2.38 g/L lower to 0.25 g/L higher) and oxytocin for this outcome. The effects of misoprostol and injectable prostaglandins were unclear because the certainty of the evidence was very low (Figure 27).
26.
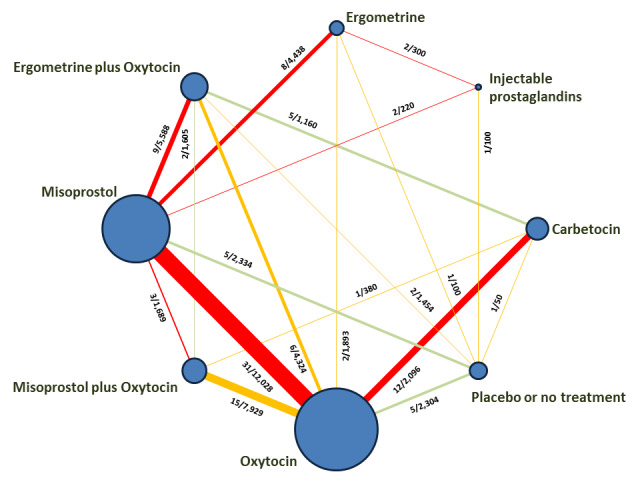
Network Diagram for change in haemoglobin measurements before and after birth (g/L). The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
27.
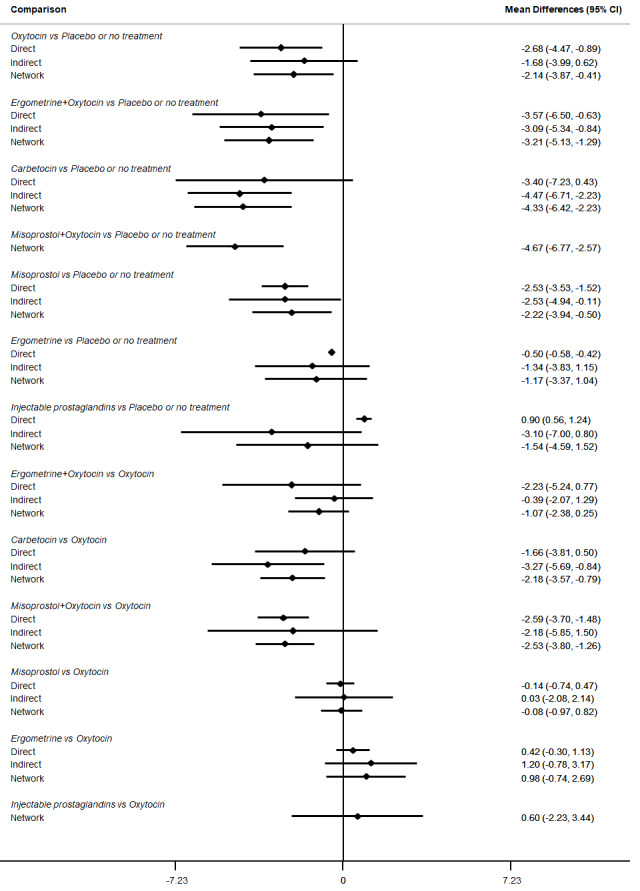
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for change in haemoglobin measurements before and after birth (g/L).
Figure 28 shows the cumulative probabilities for each agent being at each possible rank for change in haemoglobin measurements before versus after birth (g/L). The highest ranked agents were misoprostol plus oxytocin (93.9%), carbetocin (88.7%), and ergometrine plus oxytocin (69.1%). Oxytocin ranked fifth (SUCRA 42.3%) behind misoprostol (SUCRA 46.4%), but ranked better than injectable prostaglandins (SUCRA 33.7%), ergometrine (SUCRA 21.3%) and placebo or no treatment (SUCRA 4.6%).
28.
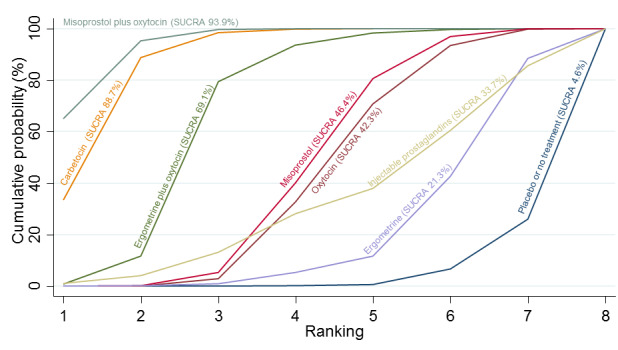
Cumulative rankograms comparing each of the uterotonic s for change in haemoglobin measurements before and after birth (g/L). Ranking indicates the cumulative probability of being the best, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Breastfeeding at hospital discharge
The network diagram for breastfeeding at hospital discharge is presented in Figure 29. Relative effects from the network meta‐analysis of six trials suggested that there were no detectable differences among oxytocin, carbetocin, ergometrine plus oxytocin for breastfeeding at hospital discharge when compared with placebo or no treatment. HIgh‐certainty evidence suggests that ergometrine plus oxytocin (RR 0.99, 95% CI 0.96 to 1.03) makes little or no difference to the proportion of women who are breastfeeding at the time of discharge from hospital when compared with oxytocin. In absolute terms, these results suggest that about 849 per 1000 women given oxytocin for vaginal birth would be breastfeeding at discharge, compared to 841 per 1000 women with ergometrine plus oxytocin. The findings for carbetocin were unclear, because we found the evidence to be of very low certainty. There were no clear findings relating to any other uterotonics as the outcome was not reported in any of the included trials involving misoprostol, injectable prostaglandins, ergometrine and misoprostol plus oxytocin (Figure 30).
29.
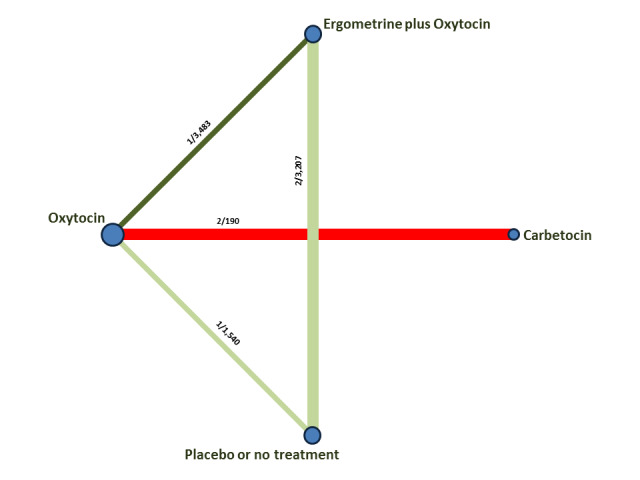
Network Diagram for breastfeeding at discharge. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
30.
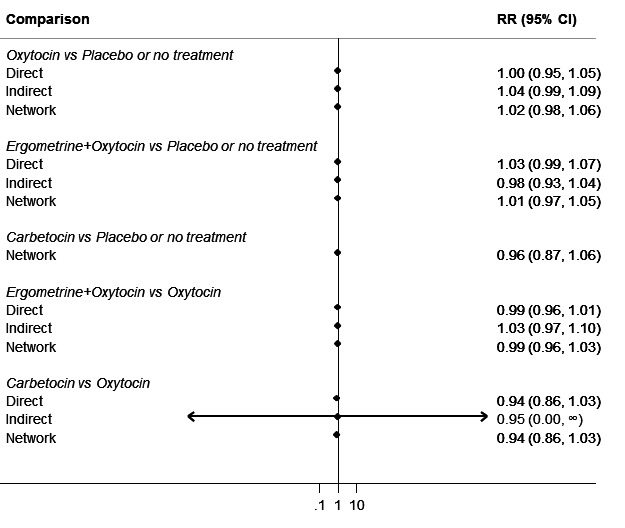
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for breastfeeding at discharge.
Figure 31 shows the cumulative probabilities for each agent being at each possible rank for breastfeeding at hospital discharge. The ranking for all agents was not clear for this outcome due to limited data.
31.
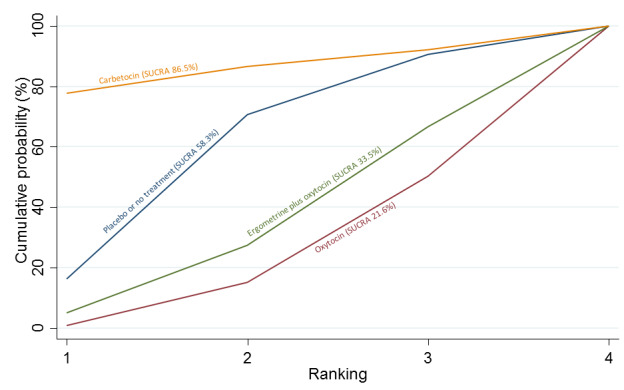
Cumulative rankograms comparing each of the uterotonic agents for breastfeeding at discharge. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Side effects
Nausea
The network diagram for nausea is presented in Figure 32. Relative effects from the network meta‐analysis of 100 trials suggest that ergometrine and ergometrine plus oxytocin are worse than placebo or no treatment in causing nausea (Figure 33). When compared with oxytocin, there is high‐certainty evidence to suggest that women receiving ergometrine plus oxytocin (RR 2.03, 95% CI 1.47 to 2.80) and misoprostol plus oxytocin (RR 1.88, 95% CI 1.14 to 3.09) are more likely to experience nausea and moderate‐certainty evidence that women receiving misoprostol (RR 1.41, 95% CI 1.10 to 1.81), ergometrine (RR 2.40, 95% CI 1.65 to 3.49) or injectable prostaglandins (RR 2.25, 95% CI 1.16 to 4.39) are more likely to experience nausea than women receiving oxytocin alone (Figure 33). Based on these results, about 86 per 1000 women given oxytocin for a vaginal birth would experience nausea, compared with 175 given ergometrine plus oxytocin, 162 given misoprostol plus oxytocin, 121 given misoprostol, 206 given ergometrine, and 193 given injectable prostaglandins. Low‐certainty evidence suggests that carbetocin may make little or no difference to experience of nausea among women when compared with oxytocin (RR 1.00, 95% CI 0.71 to 1.41). With carbetocin, the anticipated absolute effect is the same as oxytocin, with 86 per 1000 women experiencing nausea.
32.
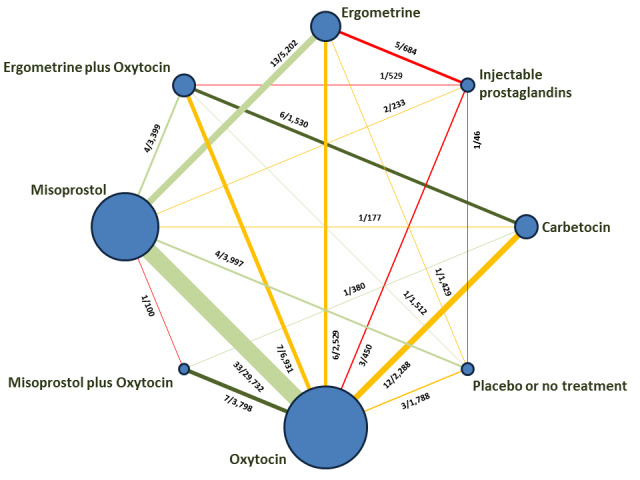
Network Diagram for nausea. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
33.
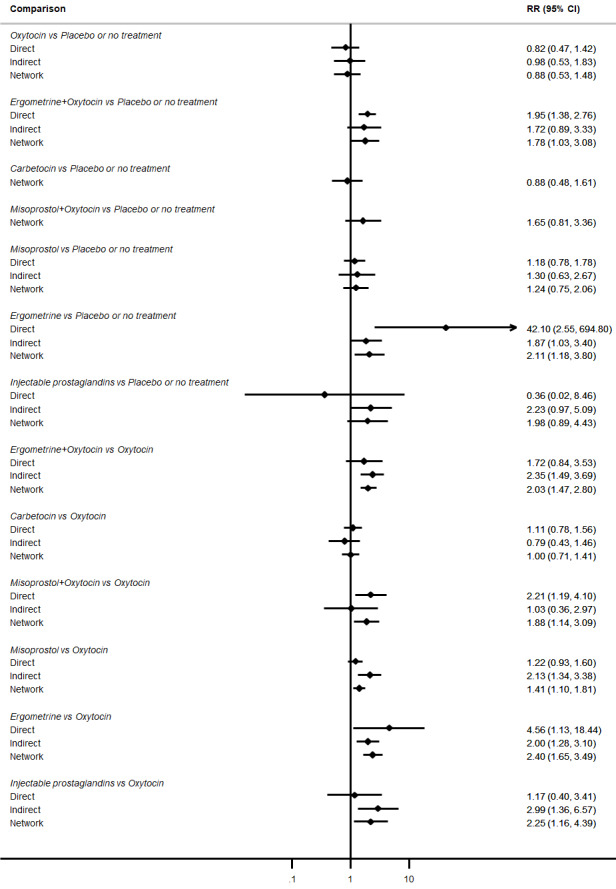
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for nausea.
Figure 34 shows the cumulative probabilities for each agent being at each possible rank for causing nausea. The highest ranked agents with which women are less likely to experience nausea are oxytocin (SUCRA 88.5%), carbetocin (SUCRA 87.3%) and placebo or no treatment (SUCRA 75.5%). These are followed by misoprostol (SUCRA 57%) and misoprostol plus oxytocin (SUCRA 32.9%). The lowest ranked agents are ergometrine plus oxytocin (SUCRA 26.7%), injectable prostaglandins (SUCRA 19.4%) and ergometrine (SUCRA 12.7%).
34.
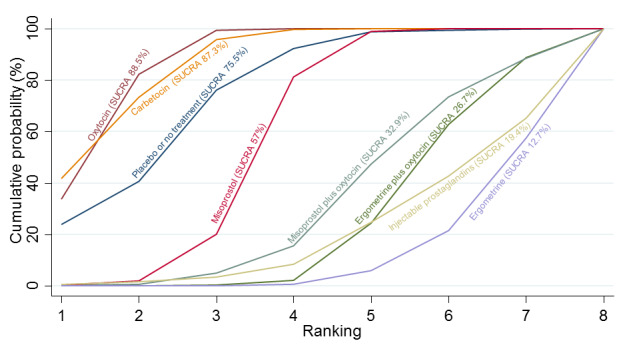
Cumulative rankograms comparing each of the uterotonic agents for nausea. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Vomiting
The network diagram for vomiting is presented in Figure 35. Relative effects from the network meta‐analysis of 110 trials suggested that ergometrine, injectable prostaglandins, misoprostol plus oxytocin and ergometrine plus oxytocin are worse than placebo or no treatment in causing vomiting (Figure 36). When compared with oxytocin, there is evidence that all agents besides carbetocin increase the incidence of vomiting. High‐certainty evidence suggests misoprostol plus oxytocin combination (RR 2.11, 95% CI 1.39 to 3.18) increases the likelihood of vomiting, while moderate‐certainty evidence suggests that ergometrine plus oxytocin (RR 2.93, 95% CI 2.08 to 4.13), misoprostol (RR 1.63, 95% CI 1.25 to 2.14), and ergometrine (RR 2.36, 95% CI 1.56 to 3.55) probably increase the likelihood of vomiting. These results suggest that 13 per 1000 women given oxytocin experience vomiting, compared to 12 per 1000 with carbetocin, 27 with misoprostol plus oxytocin, 38 with ergometrine plus oxytocin, 21 with misoprostol, and 31 with ergometrine. Low‐certainty evidence also suggests that injectable prostaglandins (RR 3.76, 95% CI 1.90 to 7.42) may increase women’s experience of vomiting. Moderate‐certainty evidence suggests that carbetocin probably makes little or no difference to women’s experience of vomiting compared with oxytocin (RR 0.93, 95% CI 0.64 to 1.35) (Table 5).
35.
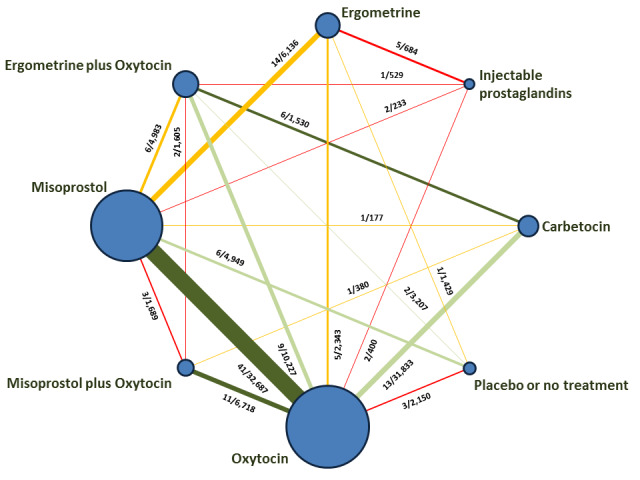
Network Diagram for vomiting. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
36.
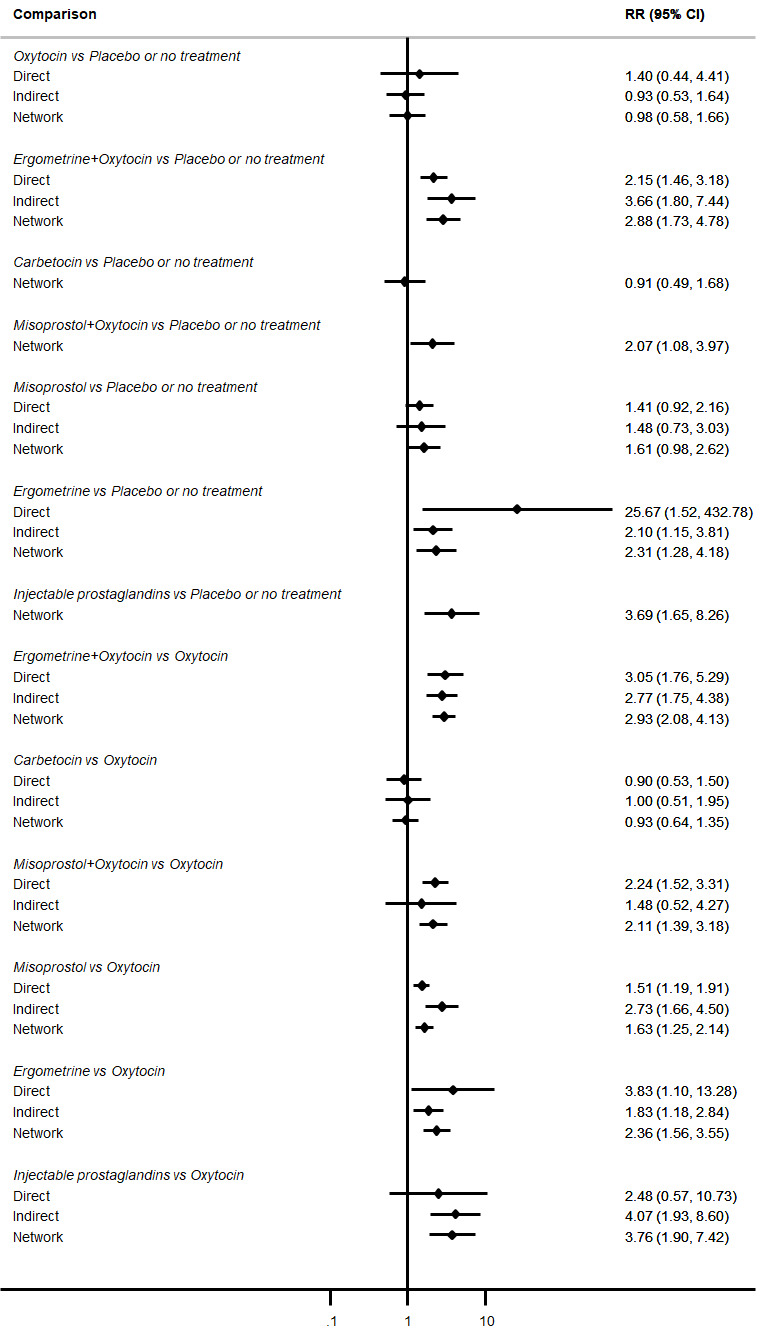
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for vomiting.
Figure 37 shows the cumulative probabilities for each agent being at each possible rank for causing vomiting. The highest ranked agents were carbetocin (SUCRA 90%), oxytocin (SUCRA 83.7%) and placebo or no treatment (SUCRA 82.6%). These are followed by misoprostol (SUCRA 55.2%) and misoprostol plus oxytocin (SUCRA 37.9%). The lowest ranked agents were ergometrine (SUCRA 30%), ergometrine plus oxytocin (SUCRA 15.1%) and injectable prostaglandins (SUCRA 5.6%).
37.
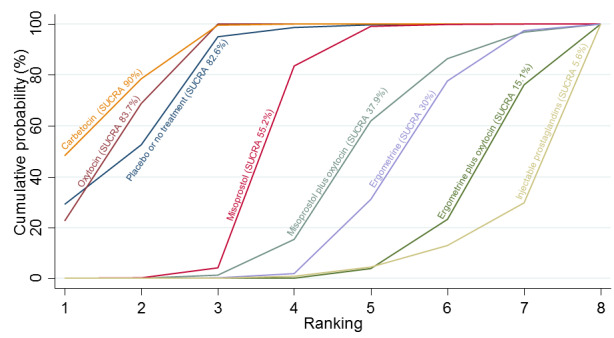
Cumulative rankograms comparing each of the uterotonic agents for vomiting. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Hypertension
The network diagram for hypertension is presented in Figure 38. Relative effects from the network meta‐analysis of 20 trials suggest that ergometrine is worse than placebo or no treatment in causing hypertension (Figure 39). Low‐certainty evidence suggests that ergometrine (RR 8.54, 95% CI 2.12 to 34.48) may increase the risk of hypertension when compared with oxytocin, whereas misoprostol (RR 1.50, 95% 0.49 to 4.61) and ergometrine plus oxytocin (RR 2.48, 95% CI 0.89 to 6.88) may make little or no difference to this outcome. The baseline risk of hypertension for women receiving oxytocin is 76 per 1000 women. Taking into account the very wide 95% CIs, this suggests that the range of possible true effects varies substantially for each agent. It is uncertain whether carbetocin or injectable prostaglandins increase hypertension because the certainty of evidence was very low (Table 6).
38.
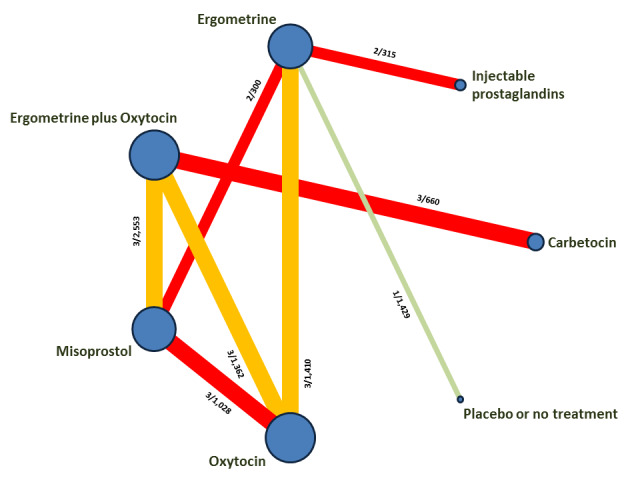
Network Diagram for hypertension. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
39.
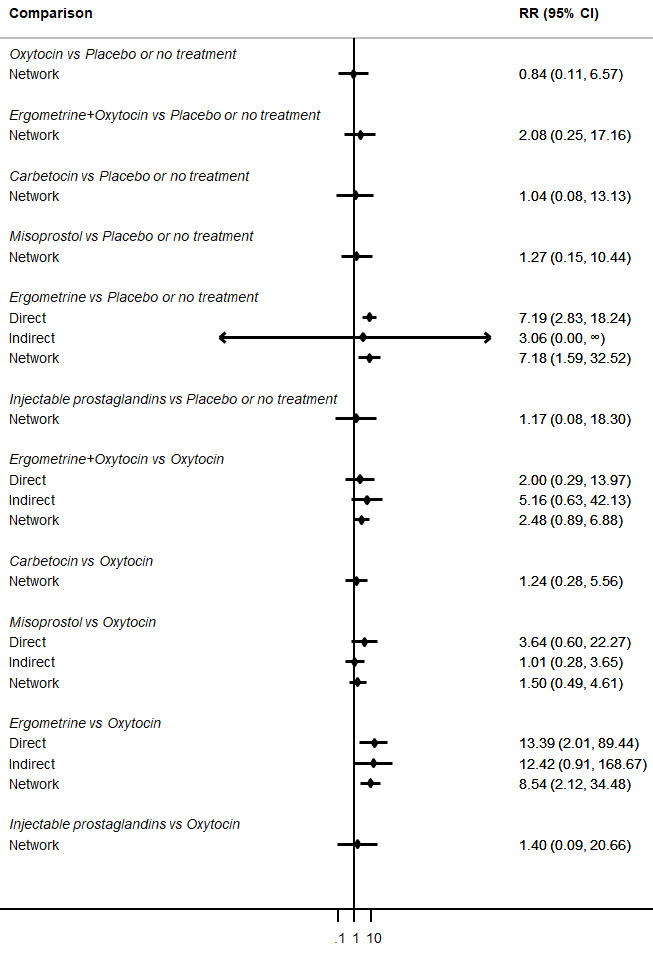
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for hypertension.
Figure 40 shows the cumulative probabilities for each agent being at each possible rank for causing hypertension. The lowest ranked agents were ergometrine (SUCRA 2.6%) and ergometrine plus oxytocin (31.1%). The rest of the agents were of comparable ranking. There were no trials involving misoprostol plus oxytocin so this agent could not be ranked.
40.

Cumulative rankograms comparing each of the uterotonic agents for hypertension. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Headache
The network diagram for headache is presented in Figure 41. Relative effects from the network meta‐analysis of 57 trials suggested that ergometrine is worse than placebo or no treatment in causing headache (Figure 42). When compared with oxytocin, there is low‐certainty evidence to suggest that women receiving ergometrine (RR 1.89, 95% CI 1.02 to 3.50) may be more likely to experience headache (Figure 42), with 167 per 1000 women given oxytocin experiencing headache compared to 316 with ergometrine. Low‐certainty evidence also suggests that carbetocin, misoprostol, and misoprostol plus oxytocin may make little or no difference to experience of headache when compared with oxytocin. It is uncertain whether injectable prostaglandins impact on women’s experience of headache because the certainty of evidence was very low.
41.
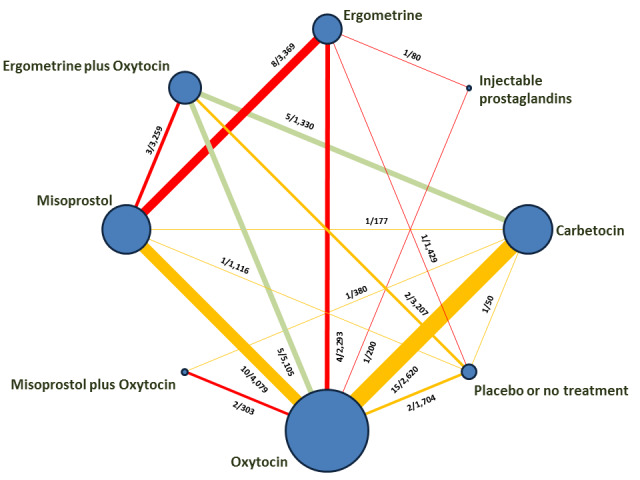
Network Diagram for headache. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
42.
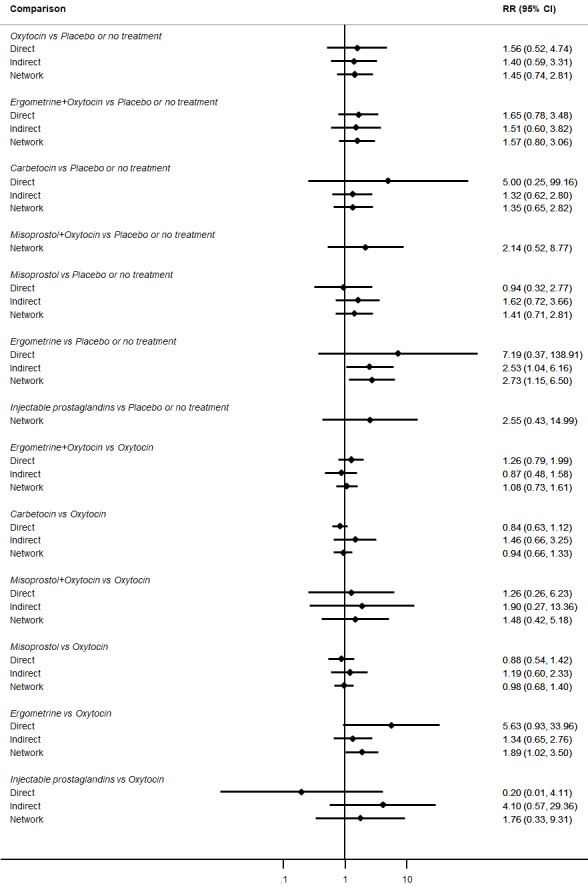
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for headache.
Figure 43 shows the cumulative probabilities for each agent being at each possible rank for causing headache. The highest ranked intervention was placebo or no treatment (SUCRA 87.9%), carbetocin (SUCRA 66.2%), misoprostol (SUCRA 60.5%) and oxytocin (SUCRA 57.6%). The lowest ranked agents were ergometrine plus oxytocin (SUCRA 47.2%), misoprostol plus oxytocin (SUCRA 35.1%), injectable prostaglandins (SUCRA 32.2%) and ergometrine (SUCRA 13.3%).
43.
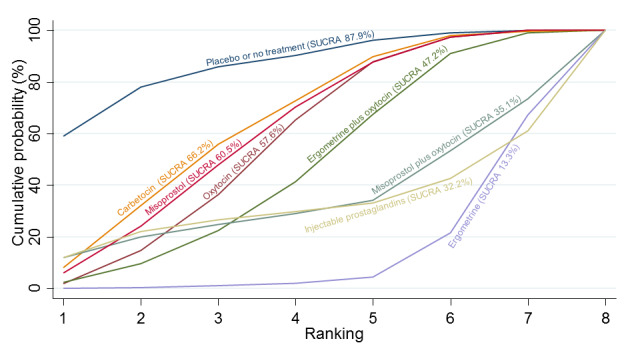
Cumulative rankograms comparing each of the uterotonic agents for headache. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Fever
The network diagram for fever is presented in Figure 44. Relative effects from the network meta‐analysis of 83 trials suggested that misoprostol and misoprostol plus oxytocin are worse than placebo or no treatment in causing fever (Figure 45). Moderate‐certainty evidence suggests that misoprostol (RR 3.87, 95% CI 2.90 to 5.16) and misoprostol plus oxytocin (RR 3.14, 95% CI 2.20 to 4.49) probably increase the occurrence of fever when compared with oxytocin. Moderate‐certainty evidence suggests that carbetocin (RR 1.07, 95% CI 0.43 to 2.69) probably makes little or no difference to women’s experience of fever. These results suggests that 24 per 1000 women given oxytocin would experience fever, compared to 93 with misoprostol, 75 with misoprostol plus oxytocin and 26 with carbetocin. Low‐certainty evidence suggests that injectable prostaglandins (RR 1.12, 95% CI 0.33 to 3.86) and ergometrine plus oxytocin (RR 0.70, 95% CI 0.35 to 1.42) may make little or no difference to this outcome, when compared with oxytocin. Evidence regarding the comparative effect of ergometrine on this outcome is uncertain because the certainty of the evidence was very low (Table 7).
44.
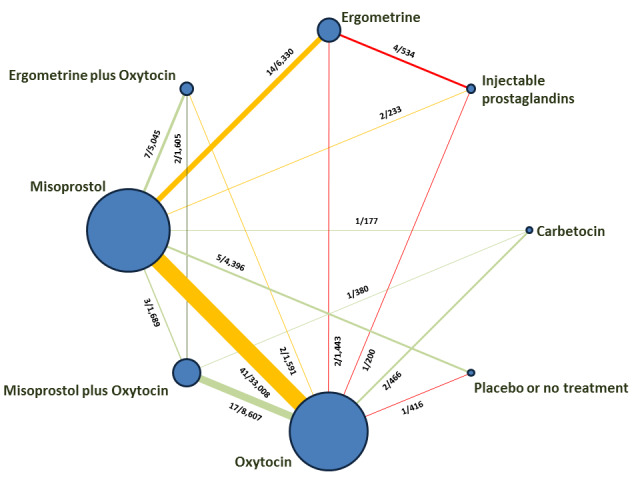
Network Diagram for fever. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
45.
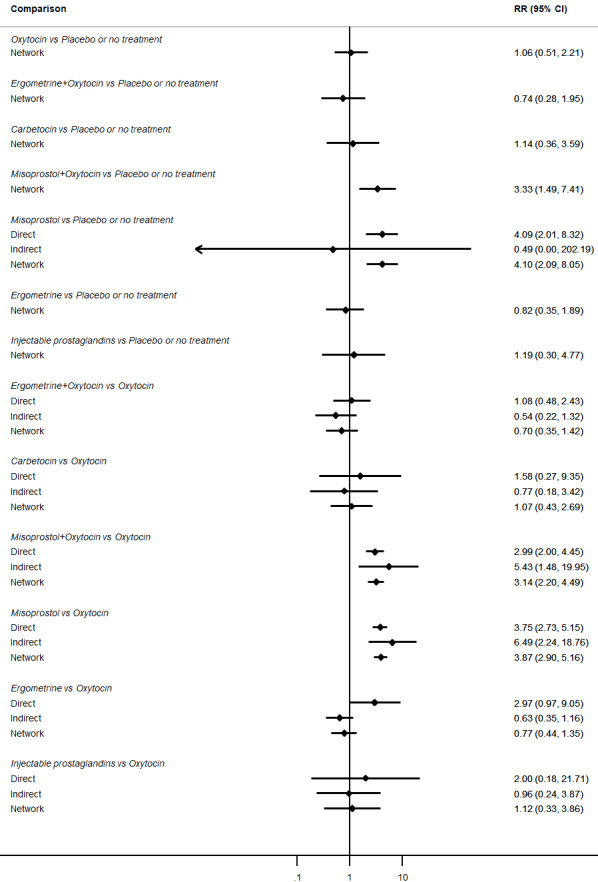
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for fever.
Figure 46 shows the cumulative probabilities for each agent being at each possible rank for causing fever. The lowest ranked agents were misoprostol (SUCRA 2.7%) and misoprostol plus oxytocin (SUCRA 12.9%). The rest of the agents were similar in ranking to the placebo or no treatment group.
46.
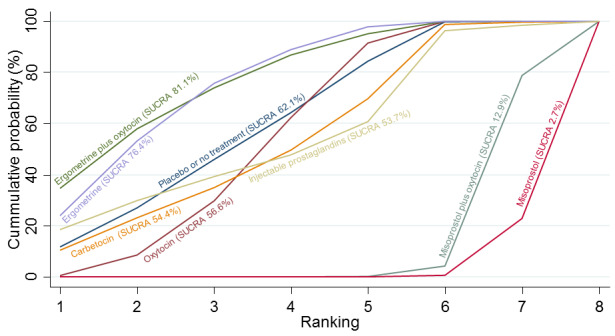
Cumulative rankograms comparing each of the uterotonic agents for fever. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Shivering
The network diagram for shivering is presented in Figure 47. Relative effects from the network meta‐analysis of 109 trials suggested that misoprostol and misoprostol plus oxytocin are worse than placebo or no treatment in causing shivering (Figure 48). When compared with oxytocin, there is moderate‐certainty evidence to suggest that women receiving misoprostol plus oxytocin (RR 3.62, 95% CI 2.59 to 5.05) are probably more likely to experience shivering (Figure 48). In absolute terms, whereas 89 per 1000 women given oxytocin would experience shivering with oxytocin, 322 would experience shivering with misoprostol plus oxytocin. Low‐certainty evidence suggests that misoprostol (RR 4.18, 95% CI 3.34 to 5.23) may also increase the experience of shivering. Moderate‐certainty evidence suggests that ergometrine plus oxytocin (RR 1.38, 95% CI 0.86 to 2.22) probably makes little or no difference to shivering when compared with oxytocin. Likewise, low‐certainty evidence suggests that carbetocin and injectable prostaglandins may make little or no difference to this outcome when compared with oxytocin (Figure 48).
47.
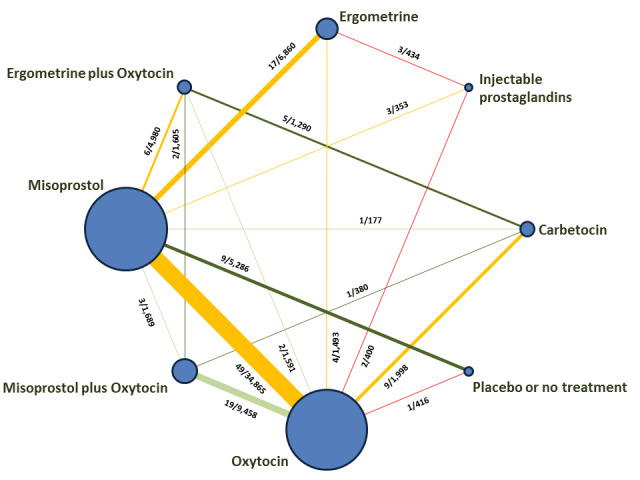
Network Diagram for shivering. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
48.
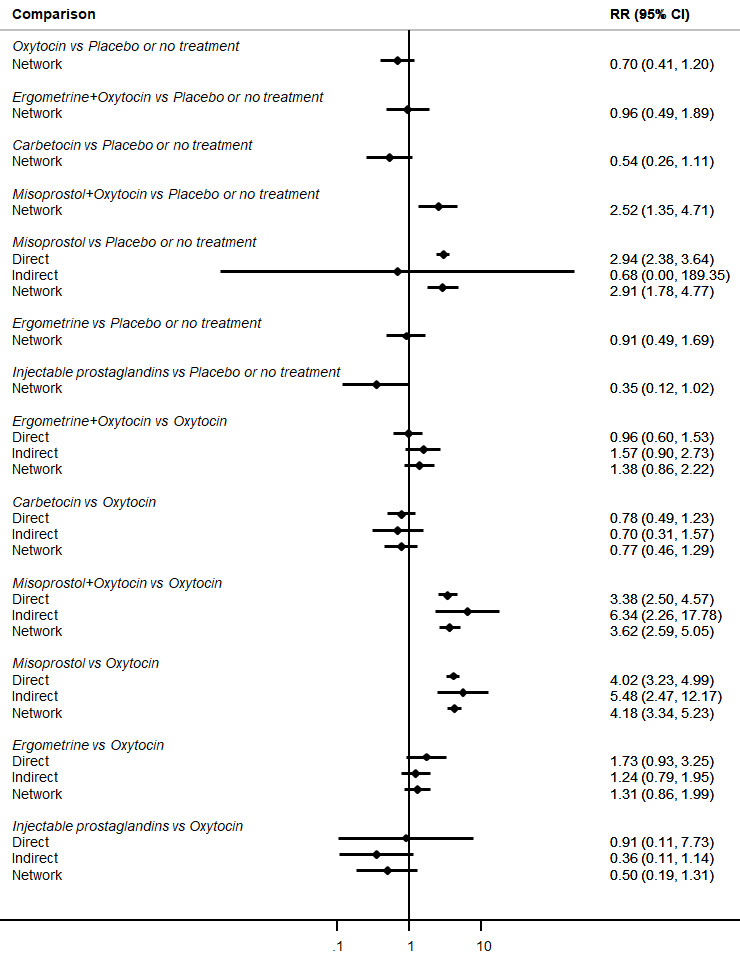
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for shivering.
Figure 49 shows the cumulative probabilities for each agent being at each possible rank for causing shivering. The highest ranked agents are injectable prostaglandins (SUCRA 94.4%), carbetocin (SUCRA 84.3%) and oxytocin (SUCRA 71.1%). These are followed by ergometrine (SUCRA 48.3%), ergometrine plus oxytocin and placebo or no treatment (SUCRA 42.7%). The lowest ranked agents were misoprostol plus oxytocin (SUCRA 11.1%) and misoprostol (SUCRA 3.2%).
49.
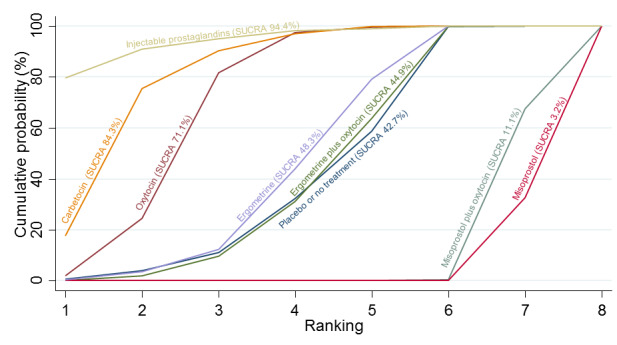
Cumulative rankograms comparing each of the uterotonic agents for shivering. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Abdominal pain
The network diagram for abdominal pain is presented in Figure 50. Relative effects from the network meta‐analysis of 32 trials suggested that ergometrine is worse than placebo or no treatment in causing abdominal pain (Figure 51). High‐certainty evidence suggests that misoprostol and misoprostol plus oxytocin make little or no difference to women’s experience of abdominal pain, when compared with oxytocin. Low‐certainty evidence suggests that ergometrine plus oxytocin probably make little or no difference to women’s experience of abdominal pain compared with oxytocin. The effects of injectable prostaglandins and ergometrine were uncertain as the certainty of evidence was very low (Figure 51).
50.
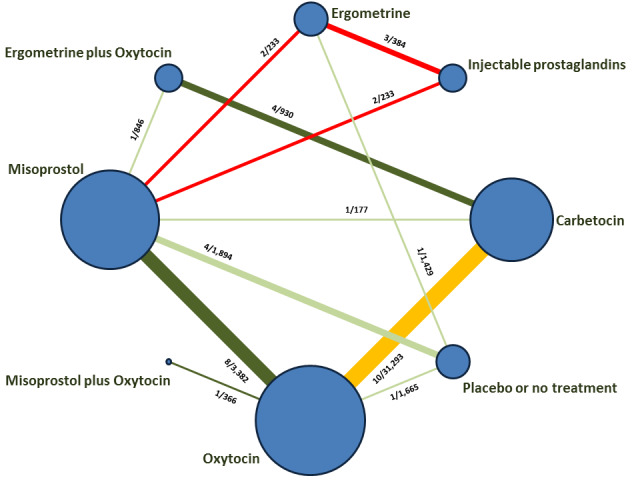
Network Diagram for abdominal pain. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
51.
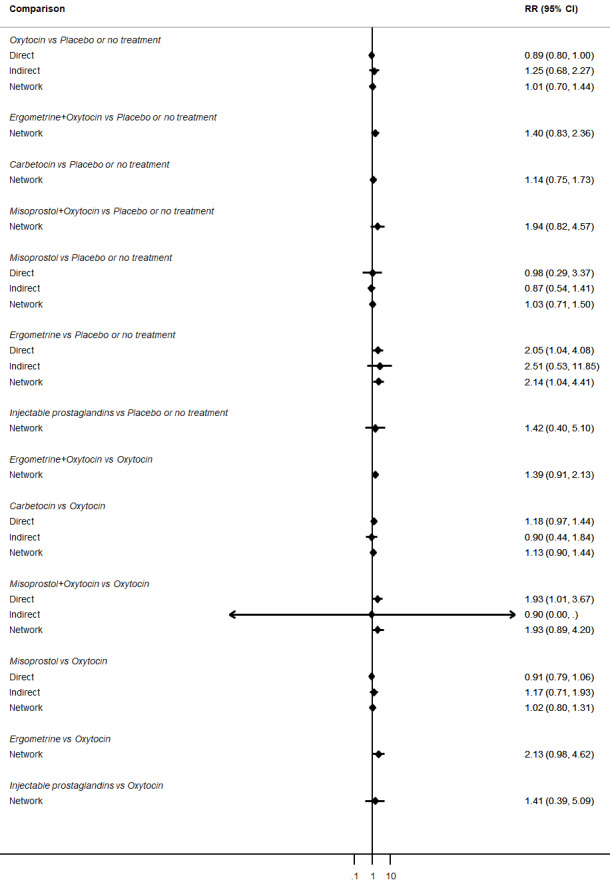
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for abdominal pain.
Figure 52 shows the cumulative probabilities for each agent being at each possible rank for causing abdominal pain. The highest ranked agent was oxytocin (SUCRA 77.9%), misoprostol (SUCRA 72.8%) and placebo or no treatment (SUCRA 75.7%). These were followed by carbetocin (SUCRA 56.3%), injectable prostaglandins (SUCRA 47.4%) and ergometrine plus oxytocin (SUCRA 35.9%). The lowest ranked agents are misoprostol plus oxytocin (SUCRA 20%) and ergometrine (SUCRA 13.9%).
52.
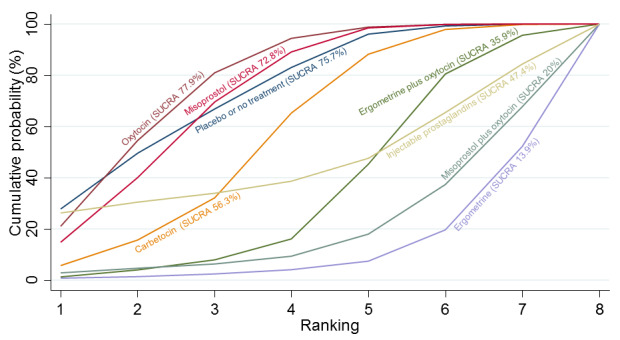
Cumulative rankograms comparing each of the uterotonic agents for abdominal pain. Ranking indicates the cumulative probability of being the best agent, the second best, the third best, etc. The x axis shows the relative ranking and the y‐axis the cumulative probability of each ranking.We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available agents.
Diarrhoea
The network diagram for diarrhoea is presented in Figure 53. Relative effects from the network meta‐analysis of 55 trials suggested that misoprostol, ergometrine and injectable prostaglandins are worse than placebo or no treatment in causing diarrhoea (Figure 54). High‐certainty evidence shows that misoprostol (RR 2.24, 95% CI 1.64 to 3.05) and misoprostol plus oxytocin (RR 1.82, 95% CI 1.12 to 2.98) increase the likelihood of diarrhoea when compared with oxytocin (Figure 54), Moderate‐certainty evidence suggests that ergometrine plus oxytocin (RR 1.80, 95% CI 1.18 to 2.75) and injectable prostaglandins (RR 23.41, 95% CI 11.03 to 49.70) probably increase the likelihood of diarrhoea, when compared with oxytocin (Figure 54). These results suggest that 11 women per 1000 given oxytocin for vaginal birth would experience diarrhoea, compared to 25 per 1000 women with misoprostol, 23 with misoprostol plus oxytocin, 20 with ergometrine plus oxytocin and 254 with injectable prostaglandins. There is also low‐certainty evidence that ergometrine (RR 2.51, 95% CI 1.20 to 5.26) may increase diarrhoea (Figure 54).
53.
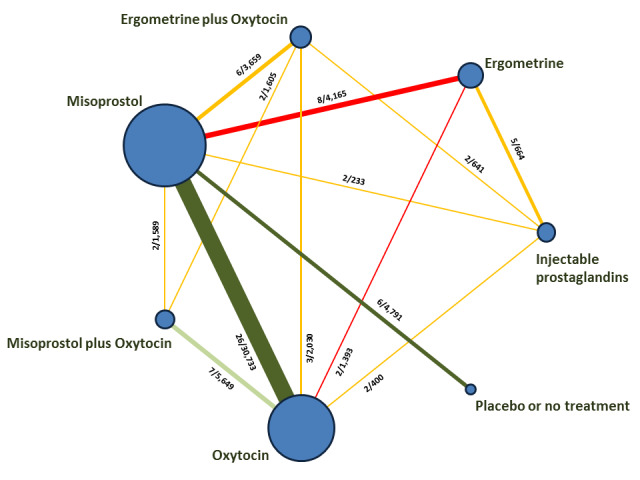
Network Diagram for diarrhoea. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green for high‐certainty evidence; light green for moderate‐certainty evidence; orange for low‐certainty evidence and red for very low‐certainty evidence. Multi‐arm trials contribute to more than one comparison.
54.
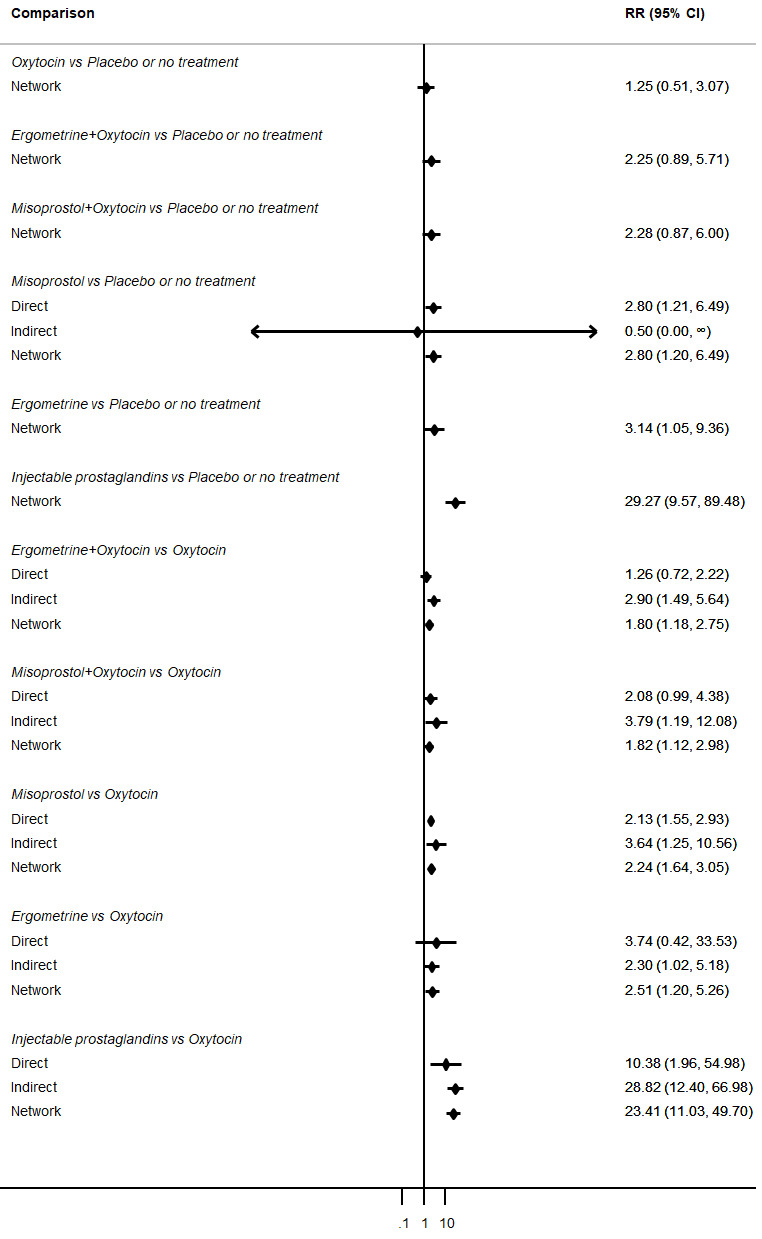
Forest plot with relative risk ratios and 95% CIs from pairwise, indirect and network (combining direct and indirect) analyses for diarrhoea.
Figure 5 shows the cumulative probabilities for each agent being at each possible rank for causing diarrhoea. The highest ranked agents were placebo or no treatment (SUCRA 92.8%) and oxytocin (SUCRA 88.4%). These were followed by ergometrine plus oxytocin (SUCRA 54.1%), misoprostol plus oxytocin (SUCRA 51.8%). The lowest ranked agents are misoprostol (SUCRA 32.5%), ergometrine (SUCRA 30.3%) and injectable prostaglandins (SUCRA 0%).
Maternal sense of well‐being
In total there were two trials reporting outcomes relevant to maternal sense of well‐being. However, because of the heterogeneous ways maternal sense of well‐being was defined in these trials a decision was made not to perform a meta‐analysis.
This outcome was reported in two different ways by one trial comparing oxytocin with no treatment (Jans 2017). Low‐certainty evidence suggests that the use of prophylactic oxytocin may make little or no difference to women’s experience of less energy than before birth at three months postpartum (RR 1.02, 95% CI 0.93 to 1.13), or to experience of fatigue at three months postpartum (RR 0.99, 95% CI 0.95 to 1.04), Analysis 1.19.
1.19. Analysis.
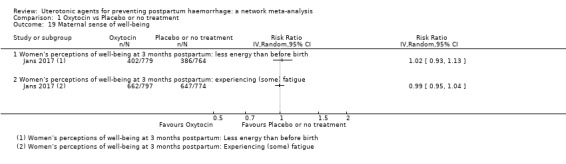
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 19 Maternal sense of well‐being.
This outcome was reported in eight different ways by another trial comparing ergometrine plus oxytocin with no treatment (Rogers 1998), Analysis 6.19. Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women’s general health at six weeks postpartum when compared to no treatment (RR 0.99, 95% CI 0.71 to 1.37). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to women's exhaustion since birth when compared to no treatment (RR 0.95, 95% CI 0.79 to 1.15). Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women's exhaustion at six weeks postpartum when compared to no treatment (RR 0.95, 95% CI 0.74 to 1.21). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to women's blues at six weeks postpartum when compared to no treatment (RR 0.93, 95% CI 0.83 to 1.04). Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women experiencing depression at six weeks postpartum when compared to no treatment (RR 1.22, 95% CI 0.84 to 1.78). Low‐certainty evidence suggests that prophylactic ergometrine plus oxytocin may make little or no difference to women looking for help for depression at six weeks postpartum when compared to no treatment (RR 1.05, 95% CI from 0.82 to 1.35). It is uncertain whether prophylactic ergometrine plus oxytocin reduces admissions to hospital for depression at six weeks postpartum when compared to no treatment because the certainty of this evidence is very low (RR 3.06, 95% CI 0.12 to 75.06). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to women reporting health problems at six weeks postpartum when compared to no treatment (RR 0.95, 95% CI 0.90 to 1.01).
6.19. Analysis.
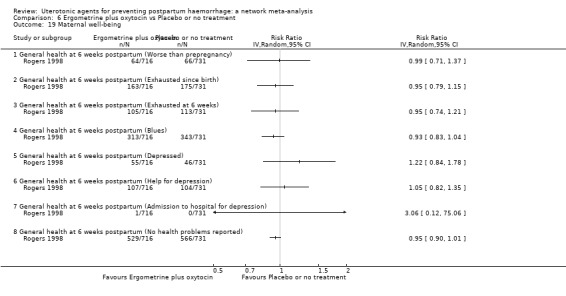
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 19 Maternal well‐being.
Maternal satisfaction
In total, there were five trials reporting outcomes relevant to maternal satisfaction. However, because of the heterogeneous ways maternal satisfaction was defined in these trials a decision was made not to perform a meta‐analysis.
This outcome was reported in three different ways by one trial comparing oxytocin with no treatment (Jangsten 2011). Moderate‐certainty evidence suggests that the use of prophylactic oxytocin may make little or no difference to women’s perception of whether management of the birth positively influenced the childbirth experience for mothers (RR 1.01, 95% CI 0.89 to 1.15); or made little or no difference to the mother’s childbirth experience (RR 1.02, 95% CI 0.90 to 1.15). Low‐certainty evidence suggests that the use of prophylactic oxytocin may make little or no difference to the extent to which women perceive that management of the birth negatively influenced the childbirth experience for mothers (RR 0.73, 95% CI 0.47 to 1.13), Analysis 1.20.
1.20. Analysis.
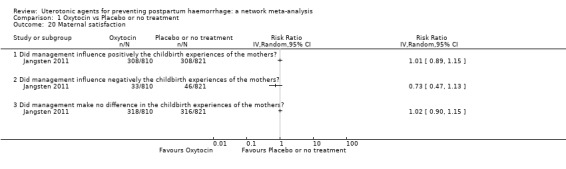
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 20 Maternal satisfaction.
This outcome was reported in two different ways by one trial comparing ergometrine plus oxytocin with no treatment (Rogers 1998). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably makes little or no difference to satisfaction with third‐stage management when compared to no treatment (RR 1.03, 95% CI from 1.00 to 1.05). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin probably decreased women feeling in control during third stage when compared to no treatment (RR 0.95, 95% CI 0.91 to 0.99), Analysis 6.20.
6.20. Analysis.
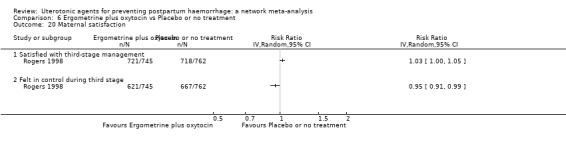
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 20 Maternal satisfaction.
This outcome was reported in four different ways by one trial comparing misoprostol with oxytocin (Diop 2016). Moderate‐certainty evidence suggests that prophylactic misoprostol, when compared to oxytocin, probably makes little or no difference to women being satisfied or very satisfied with the uterotonic agent they received (RR 1.01, 95% CI 1.00 to 1.02), or that women would make a complaint about, or have problems with, the uterotonic agent they received (RR 0.36, 95% CI 0.20 to 0.64), or that women would take the specific uterotonic agent again after subsequent deliveries (RR 1.01, 95% CI 1.00 to 1.02), or that women would recommend the specific uterotonic agent to a friend (RR 1.01, 95% CI 1.00 to 1.02), Analysis 8.20.
8.20. Analysis.
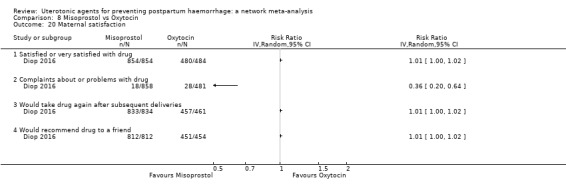
Comparison 8 Misoprostol vs Oxytocin, Outcome 20 Maternal satisfaction.
This outcome was reported by one trial comparing ergometrine plus oxytocin with misoprostol using an eight‐item Client Satisfaction Questionnaire (Ng 2007). Moderate‐certainty evidence suggests that prophylactic ergometrine plus oxytocin, when compared to misoprostol, probably makes little or no difference to women being satisfied with the uterotonic agent they received (MD 0.6 lower, 95% CI 1.22 lower to 0.02 higher).
Subgroup analyses
We carried out subgroup analyses for PPH ≥ 500 mL and PPH ≥ 1000 mL by mode of birth (caesarean versus vaginal birth), setting (hospital versus community), risk of PPH (high versus low risk for PPH), dose of misoprostol (≥ 600 mcg versus < 600 mcg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only). The network diagrams for all subgroups are available from Appendix 2. Relative effects and cumulative probabilities for each agent being at each possible rank from the network meta‐analysis for each subgroup are also available from Appendix 2. Subgroup analyses did not reveal important subgroup differences for any of the subgroups.
Sensitivity analyses
We carried out pre‐specified sensitivity analyses by restricting our analyses to studies at low risk of bias, studies at low risk of bias in terms of funding sources, to studies that used an objective method of measuring blood loss and large trials with more than 400 participants. Details of these analyses are available from Appendix 2. Sensitivity analyses were also performed according to the choice of relative effect measure (risk ratio (RR) versus odds ratio (OR)) and the statistical model (fixed‐effect versus random‐effects model). Further sensitivity analyses identified during the review process were performed by removing trials published earlier than 1990, cluster trials, removing trials with a high level of missing data and removing trials where participants were also randomised to co‐agents such as uterine massage or early controlled cord traction, or both. The sensitivity analyses show that the overall results are not affected by the abovementioned criteria or decisions.
Discussion
Summary of main results
This network meta‐analysis of 196 randomised trials (135,559 women) shows that all uterotonic agents are effective in preventing postpartum haemorrhage (PPH) ≥ 500 mL when compared with placebo or no treatment. The three highest ranked uterotonic agents were ergometrine plus oxytocin combination, carbetocin and misoprostol plus oxytocin combination. Ergometrine plus oxytocin combination and carbetocin are probably more effective uterotonic agents for preventing PPH ≥ 500 mL than oxytocin. Misoprostol plus oxytocin may also be more effective but the certainty of the evidence is low. Misoprostol, injectable prostaglandins, and ergometrine have comparable relative effects to oxytocin for preventing PPH ≥ 500 mL but again the certainty of the evidence is low.
This network meta‐analysis shows all agents except ergometrine and injectable prostaglandins were effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment. Misoprostol plus oxytocin and ergometrine plus oxytocin combinations make little or no difference to PPH ≥ 1000 mL when compared with oxytocin. Ergometrine also may make little or no difference to this outcome when compared with oxytocin but the evidence was of low certainty. The evidence for carbetocin and injectable prostaglandins was of very low quality. Misoprostol is less effective against PPH ≥ 1000 mL when compared with oxytocin. Despite the comparable relative treatment effects between all uterotonics (except misoprostol) and oxytocin, ergometrine plus oxytocin, misoprostol plus oxytocin combinations and carbetocin were the highest ranked agents for PPH ≥ 1000 mL.
Misoprostol plus oxytocin reduces the use of additional uterotonics and probably also reduces the risk of blood transfusion when compared with oxytocin. Carbetocin, injectable prostaglandins and ergometrine plus oxytocin may also reduce the use of additional uterotonics but the certainty of the evidence is low. No meaningful differences could be detected between all agents for maternal deaths or severe morbidity as these outcomes were so rare in the included randomised trials where they were reported.
The two combination regimens were associated with important side effects. When compared with oxytocin, misoprostol plus oxytocin combination increases the likelihood of vomiting and fever. No included studies reported on hypertension for misoprostol plus oxytocin versus oxytocin. Ergometrine plus oxytocin probably increases the likelihood of vomiting and may make little or no difference to the risk of hypertension, however absolute effects varied considerably and the certainty of the evidence was low for this outcome.
Subgroup analyses did not reveal important subgroup differences by mode of birth (caesarean versus vaginal birth), setting (hospital versus community), risk of PPH (high versus low risk for PPH), dose of misoprostol (≥ 600 mcg versus < 600 mcg) and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
Overall completeness and applicability of evidence
This network meta‐analysis provides the relative effectiveness of all agents used for the prevention of PPH in a coherent and methodologically robust way across important clinical outcomes by combining both direct and indirect evidence, thus increasing the statistical power and confidence in the results. We found that most of the included trials reported our primary outcomes and most of the secondary outcomes. This increased the power across most of our analyses and contributed to the consistency in the ranking across all blood loss outcomes. We were thorough in our evaluation of the important potential treatment effect modifiers (mode of birth, prior risk of PPH, healthcare setting, dose, route and regimen of the agents). We did not encounter important differences in the distribution of the effect modifiers between the different comparisons. In addition, the ranking of the agents in each of the subgroups was comparable with the overall ranking. The results of the network meta‐analyses were mostly consistent and where there was significant inconsistency this was likely due to unstable estimates from single studies.
Many trials excluded women with significant comorbidities and at very high risk for PPH. Women recruited to the included studies were predominantly delivered at more than 37 weeks of gestation. Most of the trials were carried out in hospital settings and with women having a vaginal birth. For women having a vaginal birth, uterotonic agent administration used to be a component of the active management of the third stage of labour, alongside controlled cord traction and early cord clamping. The most up‐to‐date guidelines from the WHO (WHO 2012), place emphasis on the administration of a uterotonic agent as the main aspect within this package for prevention of PPH. These guidelines state that early cord clamping is generally not advised, whilst controlled cord traction is optional where skilled birth attendants are present (WHO 2012). Rankings of the available agents were similar in subgroups where trials included either only women having a vaginal birth or only women undergoing a caesarean section. Evidently, uterine tone plays a major role in PPH at caesarean section, with a relative reduction of PPH ≥500 mL similar to the reduction seen in women undergoing vaginal births when more effective agents are used. The ranking is relevant to women at either high or low risk for PPH in hospital settings. There were not enough trials to be able to recommend a ranking in community settings, even though a similar ranking in terms of effectiveness can be expected.
The dosages, regimens and routes of administration for the most effective uterotonic agents varied. In most of the studies investigating this agent, carbetocin was administered as a single intravenous bolus of 100 mcg or intramuscularly. The combination of ergometrine plus oxytocin was usually administered intramuscularly combining 500 mcg of ergometrine plus 5 IU (international units) of oxytocin. Misoprostol plus oxytocin combinations varied greatly, with some studies administering an intravenous infusion of 20 IU of oxytocin and 400 mcg of misoprostol sublingually, or 200 mcg of misoprostol sublingually, others administering an intravenous bolus of oxytocin of 10 IU plus 400 mcg misoprostol sublingually, while others administered an intravenous infusion of 10 IU of oxytocin and 400 mcg of misoprostol rectally. There were also several other ways of administering the misoprostol plus oxytocin combination described (see Characteristics of included studies).
Quality of the evidence
We recognise that there is no single established approach for assessing the certainty of the effect estimates generated by the network meta‐analysis. We applied the rigorous method for appraising quality of network evidence as proposed by the GRADE Working group. Overall, the evidence presented varied widely in quality, and our confidence in the effect estimates ranged from very low to high certainty. When we compared oxytocin with all the other uterotonic agents and agent combinations, most individual outcomes included a range in quality of evidence across the different interventions, and this was equally true for our most important outcomes. Our reasons for downgrading the evidence also varied across comparisons and outcomes.
Summarising the quality of the evidence for the seven most important outcomes (also described in the summary of findings), for PPH ≥ 500 mL, moderate‐certainty evidence pointed to the probable superiority of both carbetocin and ergometrine plus oxytocin when compared with oxytocin alone; in both cases this evidence was downgraded due to some concerns regarding risk of bias and unexplained statistical heterogeneity in both the direct and indirect comparisons contributing most weight to the network estimate, however the direct and indirect effect estimates were coherent with one another.
For PPH ≥ 1000 mL, we had high‐certainty evidence that there is little difference between oxytocin and the uterotonic combinations of ergometrine plus oxytocin and misoprostol plus oxytocin, whereas misoprostol is slightly worse. For carbetocin and injectable prostaglandins, very low‐certainty evidence suggested unclear effects due to imprecision, strong suspicion of publication bias for the direct evidence, and incoherence between the direct and indirect effect estimates.
We had high‐certainty evidence that misoprostol plus oxytocin reduces the need for additional uterotonic agents compared to oxytocin alone, and low‐certainty evidence suggested that carbetocin, injectable prostaglandins and ergometrine plus oxytocin may all also reduce the use of additional uterotonics. The low‐certainty findings were all downgraded due to risk of bias and unexplained statistical heterogeneity, with findings on injectable prostaglandins and ergometrine plus oxytocin also being downgraded for imprecision. Moderate‐certainty evidence suggested misoprostol plus oxytocin may reduce the need for blood transfusion compared to oxytocin, with the evidence being downgraded due to imprecision.
The quality of the evidence on side effects was also somewhat varied, although with less variation within the evidence for each individual outcome. For vomiting, most of the findings were high or moderate certainty, with the main reasons for downgrading being concerns about risk of bias and imprecision. For hypertension, the evidence was all low or very low quality due to wide‐ranging concerns about risk of bias, imprecision and unexplained statistical heterogeneity. The evidence on fever ranged from moderate to very low certainty, and we had most confidence in the finding that misoprostol alone or in combination with oxytocin led to a considerable increase in this outcome for women. We downgraded these findings due to concerns about risk of bias, severe unexplained statistical heterogeneity and imprecision.
Potential biases in the review process
Several authors have been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. They did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review – these tasks were carried out by other members of the team who were not directly involved in the trials.
The quality of the evidence was assessed by a team of authors based in different countries. Before we could GRADE the network meta‐analysis evidence, we had to determine the methodology for this process because there is no well‐established approach or accompanying tools such as software. All GRADE assessments were undertaken by one individual (MJW, VD, MC or JP) and then re‐assessed independently by another of those four authors, in consultation with OTO and JPV where additional decision‐making was required.
The earliest included trial was conducted in 1976 (Moodie 1976), and in the decades since then, the clinical care and the clinical response to PPH may have improved. These temporal changes could have contributed to heterogeneity and increased the uncertainty of findings. However, we carried out a sensitivity analysis by removing trials published before 1990 and this did not vary the ranking of the agents. As objective methods of measuring blood loss became increasingly available this could perhaps have also led to apparent changes in reported blood loss. The trials included in the review recruited women with varied clinical characteristics, and it is important to consider this when interpreting results. The inclusion criteria were not always reported in detail and, when they were, these varied across trials. Further heterogeneity may also be present in the overall analysis related to the dose, route or regimen of the uterotonic agents. Even though we did not observe subgroup effects when we examined the dose of misoprostol or regimen of oxytocin administration, we were not able to perform subgroup analyses for every single increment in dosage or route of administration. Lastly, not all trials reported data on side effects, hence these analyses were often underpowered.
Agreements and disagreements with other studies or reviews
In this update of the review first published in April 2018, we have incorporated results from a large WHO trial (Widmer 2018) and overall, 56 new trials involving 46,612 women. The conclusions remain largely the same. The results for the primary outcome of PPH ≥ 500 mL were similar to the previously published review (Gallos 2018), although the quality of the evidence for carbetocin has changed from ‘very low‐’ to ‘moderate‐certainty evidence’ for this outcome, due to the addition of data from three studies including approximately 30,000 women. For the primary outcome of PPH ≥ 1000 mL, none of the agents is significantly more effective when compared with the reference uterotonic agent oxytocin. In the previous version of the review, high‐quality evidence suggested that ergometrine plus oxytocin was more effective in reducing PPH ≥ 1000 mL in comparison to oxytocin. For all other outcomes (blood transfusion; additional uterotonics; and side effects), the results are largely the same.
Our results agree with existing Cochrane Reviews (Begley 2015; Liabsuetrakul 2018; McDonald 2004; Su 2012; Tuncalp 2012; Westhoff 2013) that focus on the comparison of a uterotonic agent versus another (direct comparisons). However, this network meta‐analysis has several more studies than included in the previous reviews because of its nature of comparing all available uterotonic agents in one single analysis and because it is the most up‐to‐date including recently published trials. Hence, some estimates differ slightly, as expected.
Authors' conclusions
Implications for practice.
The current WHO recommendation on the choice of uterotonics for preventing postpartum haemorrhage (PPH) is 10 IU of intramuscular or intravenous oxytocin (WHO 2012). We found that oxytocin has substantial desirable effects compared with placebo or no treatment and trivial side effects. As a result, the balance of effects is expected to favour oxytocin. A problem with oxytocin, though, is that it needs to be kept refrigerated (2 °C to 8 °C) to maintain its potency. Several studies have demonstrated that oxytocin loses potency if stored at room temperature for too long or at higher temperatures, making its use difficult in low‐resource settings (Hogerzeil 1993; WHO 1993).
We found that ergometrine plus oxytocin combination (Syntometrine ®), misoprostol plus oxytocin combination and carbetocin have additional desirable effects compared with oxytocin, whereas misoprostol, injectable prostaglandins and ergometrine have no additional benefits compared with oxytocin. However, these uterotonic agents with the exception of carbetocin also have substantial undesirable effects as they increase the likelihood of side effects compared with oxytocin.
While the combination of ergometrine plus oxytocin may be more effective than oxytocin alone for some desirable outcomes, this combination also increases important side effects for the woman. Notably, caution should be exercised when using ergot derivatives for PPH prevention as these drugs have clear contraindications in women with underlying hypertensive or cardiovascular disorders. Thus, it is probably safer to avoid the use of ergot derivatives containing uterotonics in unscreened populations.
It is important to note that although the combination of misoprostol plus oxytocin may be more effective than oxytocin alone for some desirable outcomes, this combination also increases important side effects for the woman. In addition, as misoprostol and oxytocin are not available as a fixed drug combination (like Syntometrine®), and the two agents have to be administered through separate routes (parenteral and oral/rectal), the application of this combination may be less feasible in routine clinical settings compared with using either oxytocin or misoprostol as a single uterotonic agent. Therefore, the care provider and the parturient woman may need to carefully balance the additional benefits of a combination of misoprostol and oxytocin (over either of these agents alone) with the drawbacks (including side effects, and the challenges and inconvenience) of using two drugs through separate routes before using this combination.
There is evidence that carbetocin may be more effective than oxytocin for some desirable outcomes but with a comparable side‐effect profile when compared with oxytocin. While this risk‐benefit balance appears to favour carbetocin, carbetocin is more expensive and currently not widely available. A room temperature stable formulation of carbetocin is also now available, which could make it an attractive option for settings where maintaining the cold chain for storage and transport of oxytocin is problematic, if the cost limitations can be addressed. Nonetheless, despite the unit cost of carbetocin being higher than oxytocin it may still be cost‐effective in high‐income settings such as the UK where the cost of caring for PPH and its complication is substantial (Gallos 2018).
Before making decisions, policymakers would need to balance the desirable and undesirable effects of the range of effective uterotonics presented with their available resources and other contextual issues. An economic assessment would need to assess the consequences of various single or combination uterotonic agents compared with their current standard, with consideration of differences between their effects (benefits and harms), supply costs, and other resource requirements (staffing and training, equipment and infrastructure, staff time, supplies, supervision and monitoring). Other important considerations for decision‐making include the potential impact of introducing or scaling up the uterotonic on health equity, acceptability to key stakeholders and feasibility of using these uterotonics in routine clinical practice.
Implications for research.
There is still uncertainty around the best doses and routes of administration for each of the uterotonic agents. For oxytocin for example, there are uncertainties around the optimal dose at caesarean section, whether it should be administered intravenously or intramuscularly and whether it should be administered as an intravenous bolus or infusion. The current network meta‐analysis analyses the effectiveness and side effects for the various agents grouping together all doses and routes of the agents analysing them at an aggregate level only. The current network meta‐analysis cannot answer if a specific dose or route for any of the agents is preferred as it excludes trials that have compared different doses or routes of the same agents. We propose to update our existing network meta‐analysis by adding evidence from all the trials comparing the various doses and routes for all available agents. We wish to analyse each agent by disaggregating the various doses and routes available and then analyse in the context of the network. In this way we plan to make use of both existing direct evidence and indirect evidence from the whole network. This approach potentially can give us answers about preferred doses and routes for each of the agents and identify research gaps in the evidence base.
Consultation with our consumer group demonstrated the need for more research into outcomes identified as priorities for women and their families, such as women’s views regarding the agents used, severe maternal morbidity such as shock, and breastfeeding at discharge. To date, trials have rarely investigated these outcomes. Consumers also considered the side effects of uterotonic agents to be important but these were often not reported.
What's new
| Date | Event | Description |
|---|---|---|
| 24 May 2018 | New search has been performed | Search updated. We have included 56 new trials in this update involving 46,612 women. We have updated the methods ‐ all changes are summarised in detail in 'Differences between protocol and review'. Six authors have stepped down from the team and 10 new authors have joined the review team. |
| 24 May 2018 | New citation required but conclusions have not changed | With the addition of 56 new trials (46,612 women), the update now includes a total of 196 trials (135,559 women). The conclusions remain largely the same. The results for the primary outcome of postpartum haemorrhage (PPH) ≥ 500 mL were similar to the previously published review (Gallos 2018), although the quality of the evidence for carbetocin has changed from ‘very low‐certainly’ to ‘moderate‐certainty evidence’ for this outcome, due to the addition of data from three studies including approximately 30,000 women. For the primary outcome of PPH ≥ 1000 mL, none of the agents is significantly more effective when compared with the reference uterotonic agent oxytocin. In the previous version of the review, high‐quality evidence suggested that ergometrine plus oxytocin was more effective in reducing PPH ≥ 1000 mL in comparison to oxytocin. For all other outcomes (blood transfusion; additional uterotonics; and side effects), the results are largely the same. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peer referees (an editor and one referee who is external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
The first published version of this review (Gallos 2018) was supported by Ammalife (www.ammalife.org), and was undertaken as part of independent research funded by the UK National Institute for Health Research (Health Technology Assessment Project 14/139/17 Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis). The views expressed in this publication are those of the review authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
The World Health Organization (WHO) and Ioannis D Gallos, Argyro Papadopoulou, Rebecca Man, Nikos Athanasopoulos, Aurelio Tobias, Malcolm J Price, Myfanwy Williams, Virginia Diaz, Julia Pasquale, Monica Chamillard, Justus Hofmeyr and Arri Coomarasamy retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
We wish to thank Helen Williams, David Lissauer, Abi Merriel, Vidhya Moorthy and Harold Gee for their help with screening trials and data extraction for the previous version of this review. We thank Edgardo Abalos and Leanne Jones for their help with grading of the evidence for this 2018 update.
We are grateful for the advice by the investigators of the Postpartum Haemorrhage Core Outcome Sets Project and particularly to Shireen Meher, Anna Cuthbert, Zarko Alfirevic, Jamie Kirkham and Paula Williamson.
Appendices
Appendix 1. Search terms
ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP)
Third stage AND labo(u)r AND oxytocin
Third stage AND labo(u)r AND misoprostol
Third stage AND labo(u)r AND carbetocin
Third stage AND labo(u)r AND ergometrine
uterotonic* AND oxytocin
uterotonic* AND misoprostol
uterotonic* AND carbetocin
uterotonic* AND ergometrine
uterotonic* AND labo(u)r
uterotonic* AND h(a)emorrhage
h(a)emorrhage AND postpartum AND ergometrine
h(a)emorrhage AND postpartum AND oxytocin
h(a)emorrhage AND postpartum AND carbetocin
h(a)emorrhage AND postpartum AND misoprostol
Appendix 2. Supplementary Appendix
For all subgroup and sensitivity analyses please see Appendix 1 https://edata.bham.ac.uk/284/
For all forest plots for all outcomes and interventions see Appendix 2 https://edata.bham.ac.uk/284/
For additional data from trialists see Appendix 3 https://edata.bham.ac.uk/284/
Data and analyses
Comparison 1. Oxytocin vs Placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | 1920 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 11 | 8782 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.52, 0.73] |
| 3 Blood transfusion | 8 | 6717 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.51, 1.12] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 1950 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 9 | 7021 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.52, 0.71] |
| 7 Additional uterotonics | 8 | 5047 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.32, 0.58] |
| 8 Blood loss | 8 | 3521 | Mean Difference (IV, Random, 95% CI) | ‐118.52 [‐141.40, ‐95.64] |
| 9 Change in haemoglobin | 5 | 2304 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐4.47, ‐0.89] |
| 10 Breastfeeding | 1 | 1540 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 11 Nausea | 3 | 1788 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.47, 1.42] |
| 12 Vomiting | 3 | 2150 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.44, 4.41] |
| 13 Headache | 2 | 1704 | Risk Ratio (IV, Random, 95% CI) | 1.56 [0.52, 4.74] |
| 14 Abdominal pain | 1 | 1665 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal sense of well‐being | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Women’s perceptions of well‐being at 3 months postpartum: less energy than before birth | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Women’s perceptions of well‐being at 3 months postpartum: experiencing (some) fatigue | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Did management influence positively the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Did management influence negatively the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Did management make no difference in the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 1 Death.
1.2. Analysis.
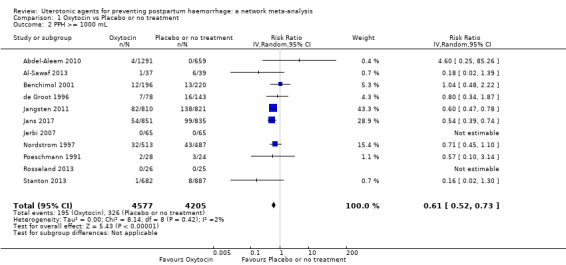
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 2 PPH >= 1000 mL.
1.3. Analysis.
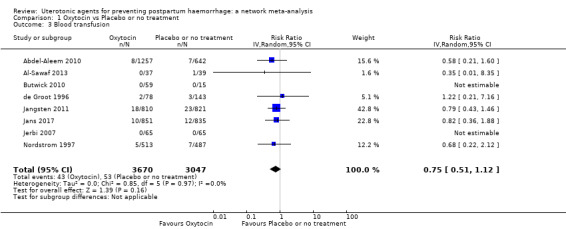
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 3 Blood transfusion.
1.4. Analysis.
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 4 Severe maternal morbidity: intensive care admissions.
1.6. Analysis.
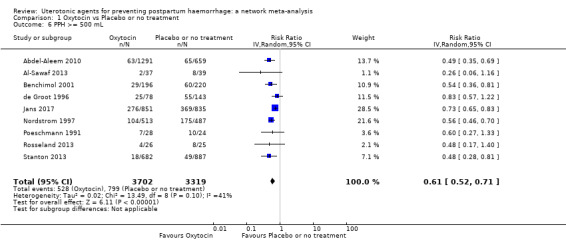
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 6 PPH >= 500 mL.
1.7. Analysis.
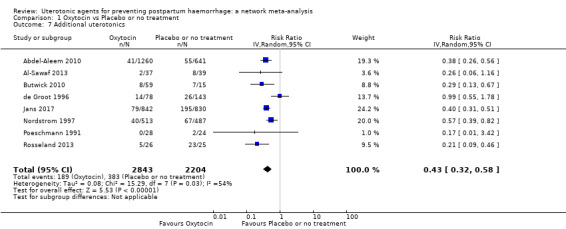
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 7 Additional uterotonics.
1.8. Analysis.
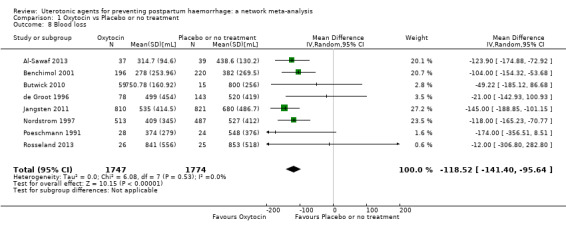
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 8 Blood loss.
1.9. Analysis.
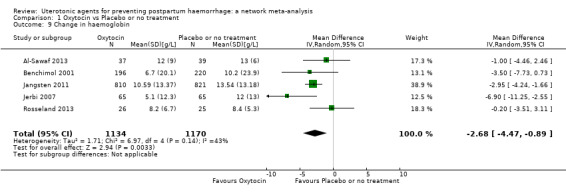
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 9 Change in haemoglobin.
1.10. Analysis.
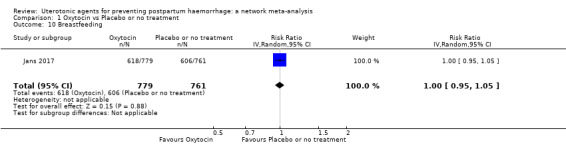
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 10 Breastfeeding.
1.11. Analysis.
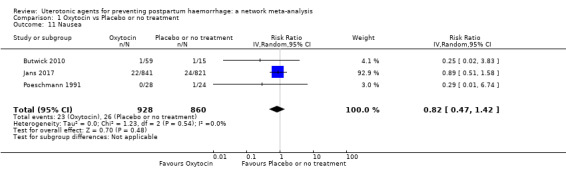
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 11 Nausea.
1.12. Analysis.
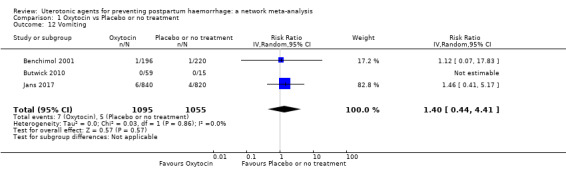
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 12 Vomiting.
1.13. Analysis.
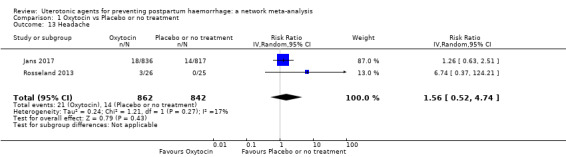
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 13 Headache.
1.14. Analysis.
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 14 Abdominal pain.
1.16. Analysis.
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 16 Shivering.
1.17. Analysis.
Comparison 1 Oxytocin vs Placebo or no treatment, Outcome 17 Fever.
Comparison 2. Carbetocin vs Placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.30, 1.85] |
| 7 Additional uterotonics | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.12, 0.32] |
| 8 Blood loss | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐274.0 [‐591.60, 43.60] |
| 9 Change in haemoglobin | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.23, 0.43] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
2.2. Analysis.
Comparison 2 Carbetocin vs Placebo or no treatment, Outcome 2 PPH >= 1000 mL.
2.6. Analysis.
Comparison 2 Carbetocin vs Placebo or no treatment, Outcome 6 PPH >= 500 mL.
2.7. Analysis.
Comparison 2 Carbetocin vs Placebo or no treatment, Outcome 7 Additional uterotonics.
2.8. Analysis.
Comparison 2 Carbetocin vs Placebo or no treatment, Outcome 8 Blood loss.
2.9. Analysis.
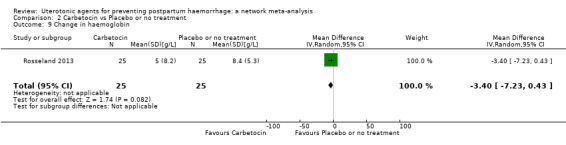
Comparison 2 Carbetocin vs Placebo or no treatment, Outcome 9 Change in haemoglobin.
2.13. Analysis.
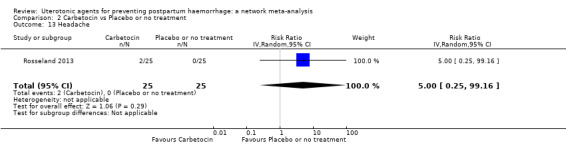
Comparison 2 Carbetocin vs Placebo or no treatment, Outcome 13 Headache.
Comparison 3. Misoprostol vs Placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 3497 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.10, 9.59] |
| 2 PPH >= 1000 mL | 8 | 5467 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.56, 0.95] |
| 3 Blood transfusion | 6 | 3134 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.15, 1.47] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 1620 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.14, 7.05] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 7 | 4047 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 7 Additional uterotonics | 8 | 3746 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.52, 0.87] |
| 8 Blood loss | 8 | 4146 | Mean Difference (IV, Random, 95% CI) | ‐42.07 [‐52.47, ‐31.68] |
| 9 Change in haemoglobin | 5 | 2334 | Mean Difference (IV, Random, 95% CI) | ‐2.53 [‐3.53, ‐1.52] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 4 | 3997 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.78, 1.78] |
| 12 Vomiting | 6 | 4949 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.92, 2.16] |
| 13 Headache | 1 | 1116 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.32, 2.77] |
| 14 Abdominal pain | 4 | 1894 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.29, 3.37] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 9 | 5286 | Risk Ratio (IV, Random, 95% CI) | 2.94 [2.38, 3.64] |
| 17 Fever | 5 | 4396 | Risk Ratio (IV, Random, 95% CI) | 4.09 [2.01, 8.32] |
| 18 Diarrhoea | 6 | 4791 | Risk Ratio (IV, Random, 95% CI) | 2.80 [1.21, 6.49] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
3.1. Analysis.
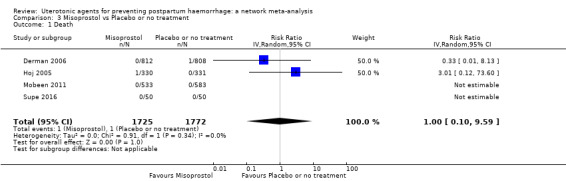
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 1 Death.
3.2. Analysis.
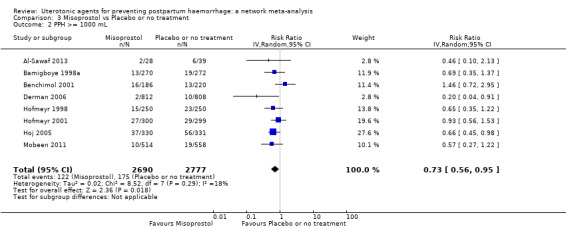
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 2 PPH >= 1000 mL.
3.3. Analysis.
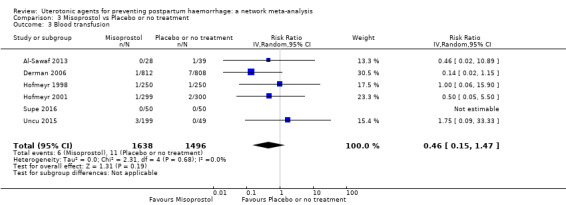
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 3 Blood transfusion.
3.4. Analysis.
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 4 Severe maternal morbidity: intensive care admissions.
3.6. Analysis.
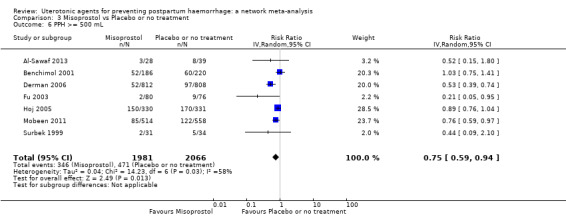
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 6 PPH >= 500 mL.
3.7. Analysis.
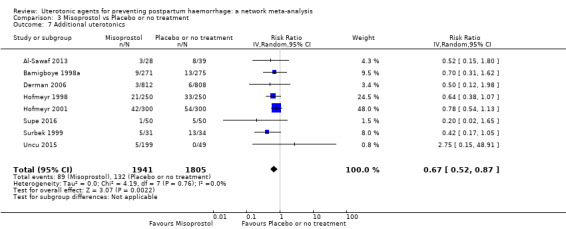
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 7 Additional uterotonics.
3.8. Analysis.
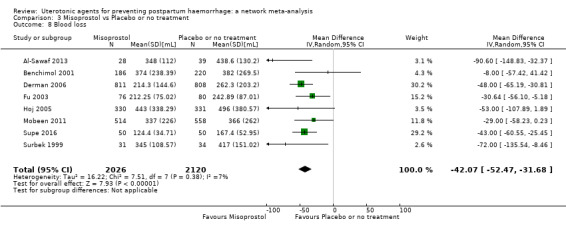
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 8 Blood loss.
3.9. Analysis.
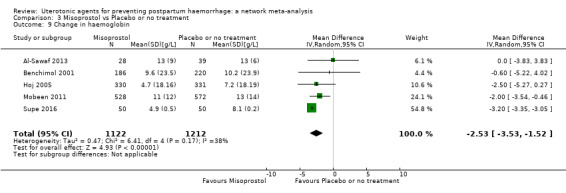
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 9 Change in haemoglobin.
3.11. Analysis.
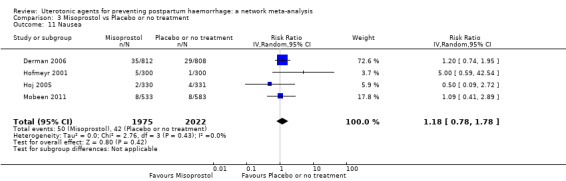
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 11 Nausea.
3.12. Analysis.
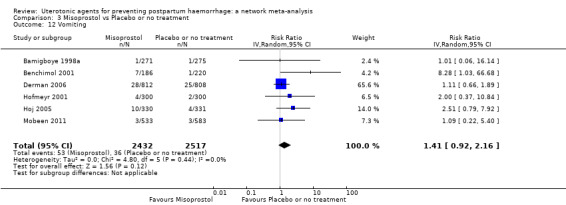
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 12 Vomiting.
3.13. Analysis.
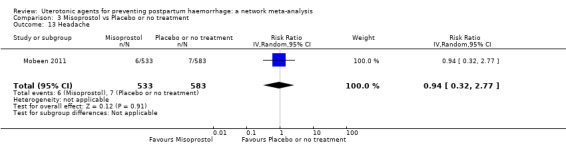
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 13 Headache.
3.14. Analysis.
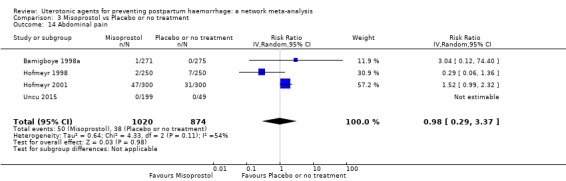
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 14 Abdominal pain.
3.16. Analysis.
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 16 Shivering.
3.17. Analysis.
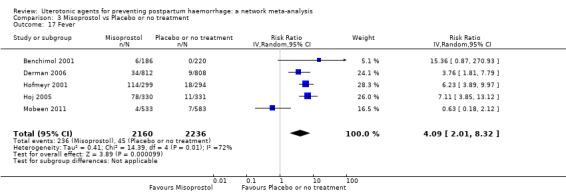
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 17 Fever.
3.18. Analysis.
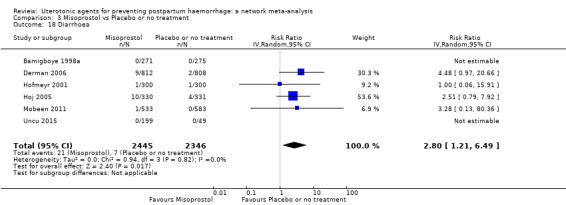
Comparison 3 Misoprostol vs Placebo or no treatment, Outcome 18 Diarrhoea.
Comparison 4. Injectable prostaglandins vs Placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.04, 3.24] |
| 3 Blood transfusion | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.22, 1.35] |
| 7 Additional uterotonics | 2 | 146 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.21, 2.09] |
| 8 Blood loss | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐95.17 [‐296.09, 105.75] |
| 9 Change in haemoglobin | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.56, 1.24] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.02, 8.46] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
4.1. Analysis.
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 1 Death.
4.2. Analysis.
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 2 PPH >= 1000 mL.
4.3. Analysis.
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 3 Blood transfusion.
4.6. Analysis.
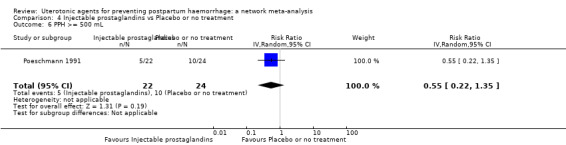
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 6 PPH >= 500 mL.
4.7. Analysis.
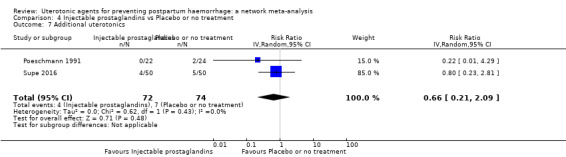
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 7 Additional uterotonics.
4.8. Analysis.
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 8 Blood loss.
4.9. Analysis.
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 9 Change in haemoglobin.
4.11. Analysis.
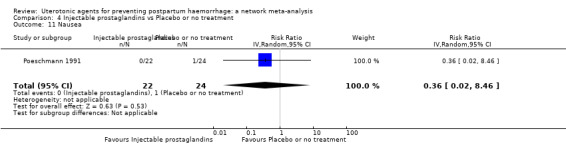
Comparison 4 Injectable prostaglandins vs Placebo or no treatment, Outcome 11 Nausea.
Comparison 5. Ergometrine vs Placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| 3 Blood transfusion | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.04, 3.28] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.14, 0.42] |
| 7 Additional uterotonics | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.37] |
| 8 Blood loss | 2 | 1529 | Mean Difference (IV, Random, 95% CI) | ‐50.64 [‐119.92, 18.65] |
| 9 Change in haemoglobin | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 42.10 [2.55, 694.80] |
| 12 Vomiting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 25.67 [1.52, 432.78] |
| 13 Headache | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [0.37, 138.91] |
| 14 Abdominal pain | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.04, 4.08] |
| 15 Hypertension | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [2.83, 18.24] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
5.1. Analysis.
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 1 Death.
5.2. Analysis.
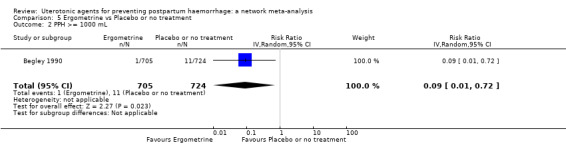
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 2 PPH >= 1000 mL.
5.3. Analysis.
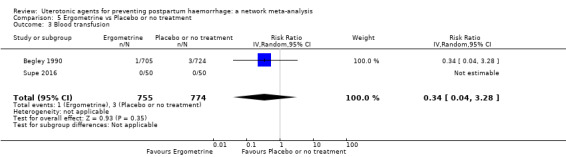
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 3 Blood transfusion.
5.6. Analysis.
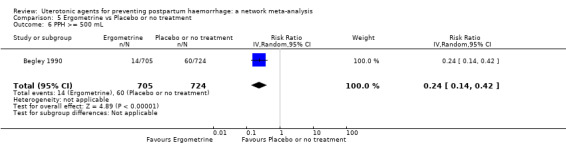
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 6 PPH >= 500 mL.
5.7. Analysis.
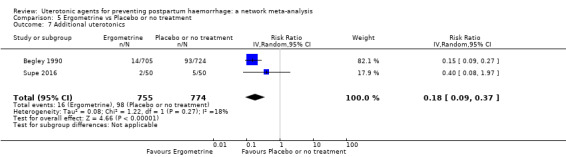
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 7 Additional uterotonics.
5.8. Analysis.
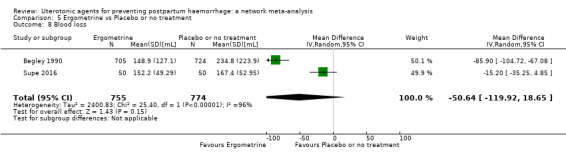
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 8 Blood loss.
5.9. Analysis.
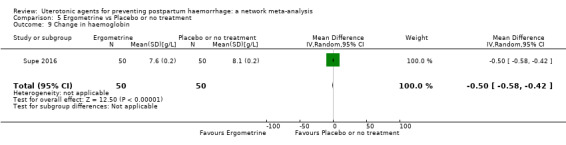
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 9 Change in haemoglobin.
5.11. Analysis.
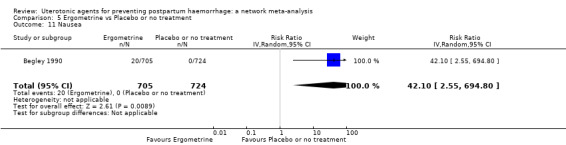
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 11 Nausea.
5.12. Analysis.
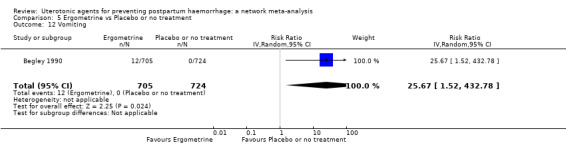
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 12 Vomiting.
5.13. Analysis.
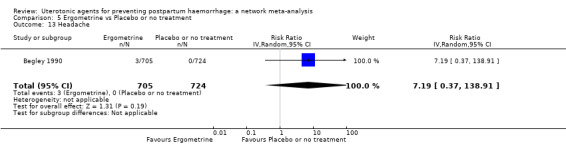
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 13 Headache.
5.14. Analysis.
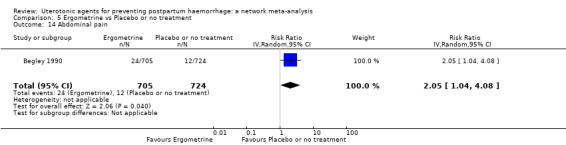
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 14 Abdominal pain.
5.15. Analysis.
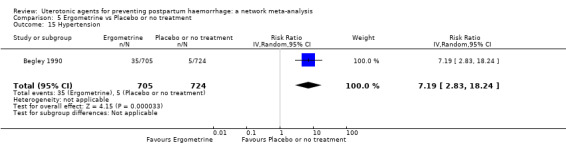
Comparison 5 Ergometrine vs Placebo or no treatment, Outcome 15 Hypertension.
Comparison 6. Ergometrine plus oxytocin vs Placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.18, 1.05] |
| 3 Blood transfusion | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.18, 0.66] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.30, 0.46] |
| 7 Additional uterotonics | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.15, 0.24] |
| 8 Blood loss | 2 | 1705 | Mean Difference (IV, Random, 95% CI) | ‐35.02 [‐101.63, 31.59] |
| 9 Change in haemoglobin | 2 | 1454 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐6.50, ‐0.63] |
| 10 Breastfeeding | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 11 Nausea | 1 | 1512 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.38, 2.76] |
| 12 Vomiting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 2.15 [1.46, 3.18] |
| 13 Headache | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.78, 3.48] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 General health at 6 weeks postpartum (Worse than prepregnancy) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 General health at 6 weeks postpartum (Exhausted since birth) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 General health at 6 weeks postpartum (Exhausted at 6 weeks) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.4 General health at 6 weeks postpartum (Blues) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.5 General health at 6 weeks postpartum (Depressed) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.6 General health at 6 weeks postpartum (Help for depression) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.7 General health at 6 weeks postpartum (Admission to hospital for depression) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.8 General health at 6 weeks postpartum (No health problems reported) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Satisfied with third‐stage management | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Felt in control during third stage | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.2. Analysis.

Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 2 PPH >= 1000 mL.
6.3. Analysis.
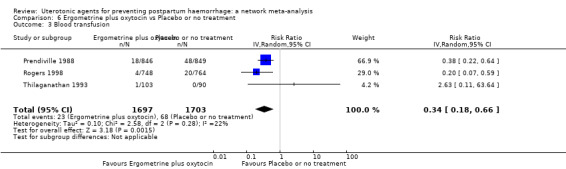
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 3 Blood transfusion.
6.6. Analysis.
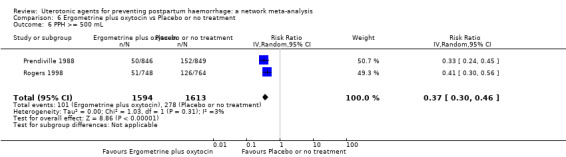
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 6 PPH >= 500 mL.
6.7. Analysis.
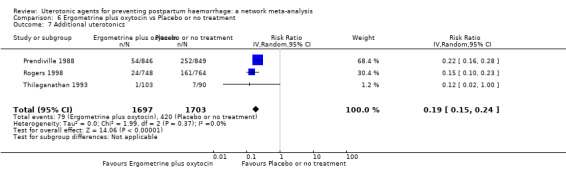
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 7 Additional uterotonics.
6.8. Analysis.
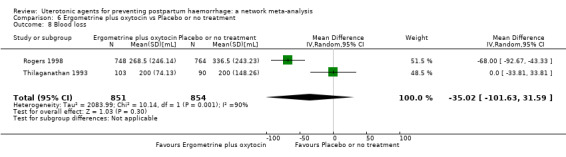
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 8 Blood loss.
6.9. Analysis.
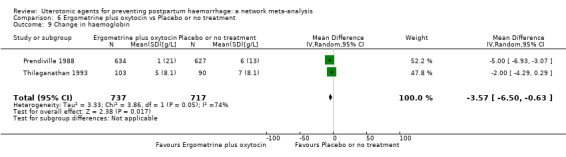
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 9 Change in haemoglobin.
6.10. Analysis.
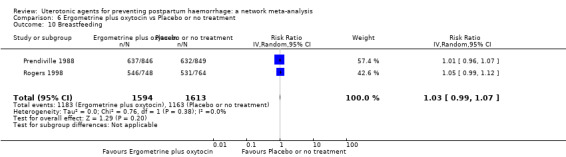
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 10 Breastfeeding.
6.11. Analysis.
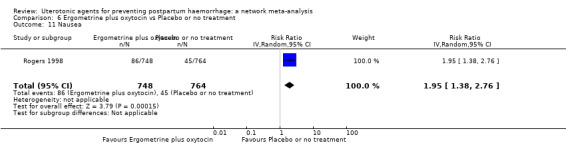
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 11 Nausea.
6.12. Analysis.
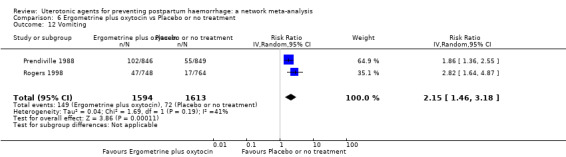
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 12 Vomiting.
6.13. Analysis.
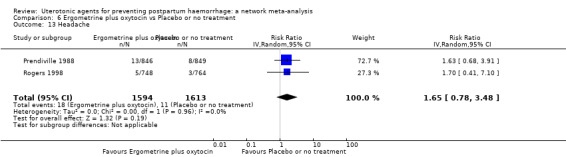
Comparison 6 Ergometrine plus oxytocin vs Placebo or no treatment, Outcome 13 Headache.
Comparison 7. Misoprostol plus oxytocin vs Placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Misoprostol vs Oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 24 | 28520 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.14, 2.74] |
| 2 PPH >= 1000 mL | 38 | 34261 | Risk Ratio (IV, Random, 95% CI) | 1.26 [1.11, 1.43] |
| 3 Blood transfusion | 42 | 35467 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 4 Severe maternal morbidity: intensive care admissions | 10 | 21698 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.55, 2.43] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 44 | 36920 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 7 Additional uterotonics | 47 | 35981 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 8 Blood loss | 43 | 35239 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐23.45, 5.65] |
| 9 Change in haemoglobin | 31 | 12028 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.74, 0.47] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 33 | 29732 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.93, 1.60] |
| 12 Vomiting | 41 | 32687 | Risk Ratio (IV, Random, 95% CI) | 1.51 [1.19, 1.91] |
| 13 Headache | 10 | 4079 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.54, 1.42] |
| 14 Abdominal pain | 8 | 3382 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 15 Hypertension | 3 | 1028 | Risk Ratio (IV, Random, 95% CI) | 3.64 [0.60, 22.27] |
| 16 Shivering | 49 | 34865 | Risk Ratio (IV, Random, 95% CI) | 4.02 [3.23, 4.99] |
| 17 Fever | 41 | 33008 | Risk Ratio (IV, Random, 95% CI) | 3.75 [2.73, 5.15] |
| 18 Diarrhoea | 26 | 30733 | Risk Ratio (IV, Random, 95% CI) | 2.13 [1.55, 2.93] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Satisfied or very satisfied with drug | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Complaints about or problems with drug | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Would take drug again after subsequent deliveries | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Would recommend drug to a friend | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
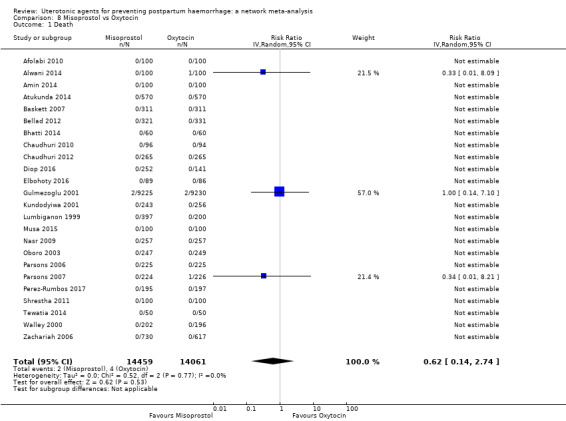
Comparison 8 Misoprostol vs Oxytocin, Outcome 1 Death.
8.2. Analysis.
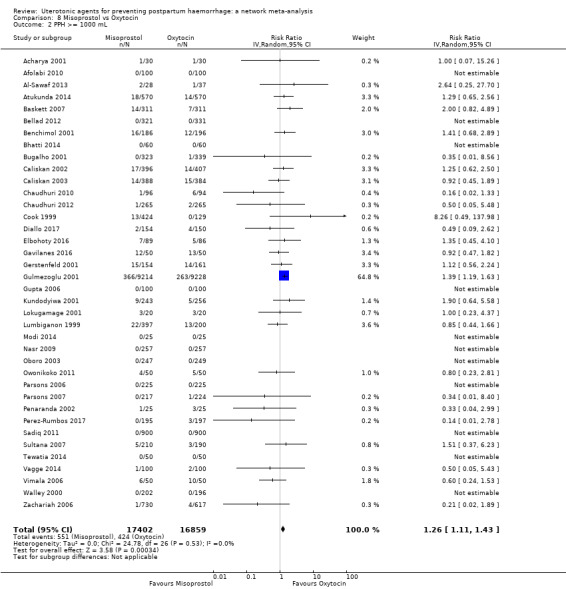
Comparison 8 Misoprostol vs Oxytocin, Outcome 2 PPH >= 1000 mL.
8.3. Analysis.
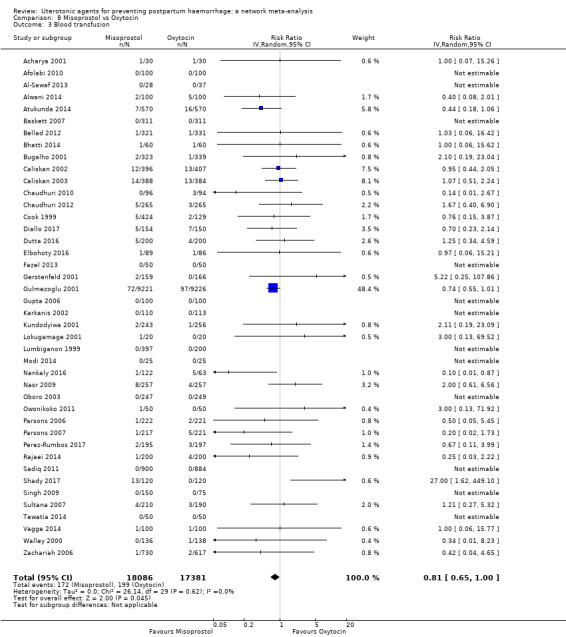
Comparison 8 Misoprostol vs Oxytocin, Outcome 3 Blood transfusion.
8.4. Analysis.
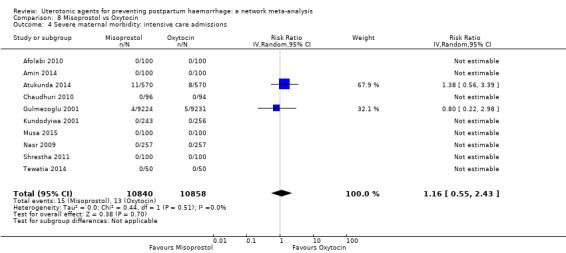
Comparison 8 Misoprostol vs Oxytocin, Outcome 4 Severe maternal morbidity: intensive care admissions.
8.6. Analysis.
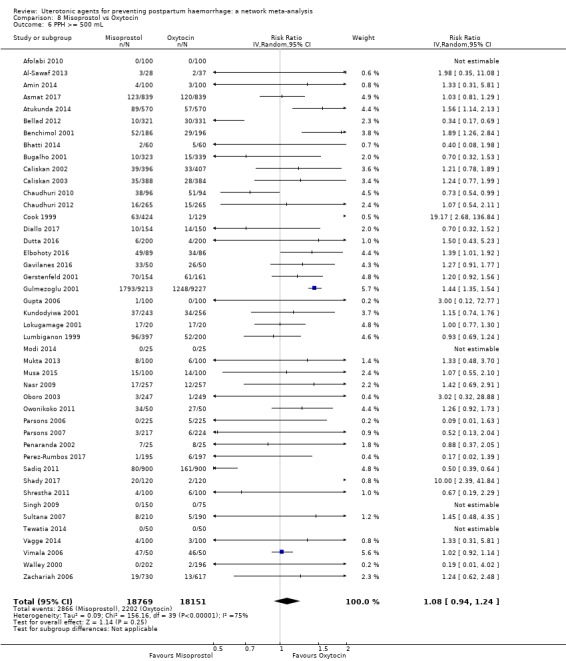
Comparison 8 Misoprostol vs Oxytocin, Outcome 6 PPH >= 500 mL.
8.7. Analysis.
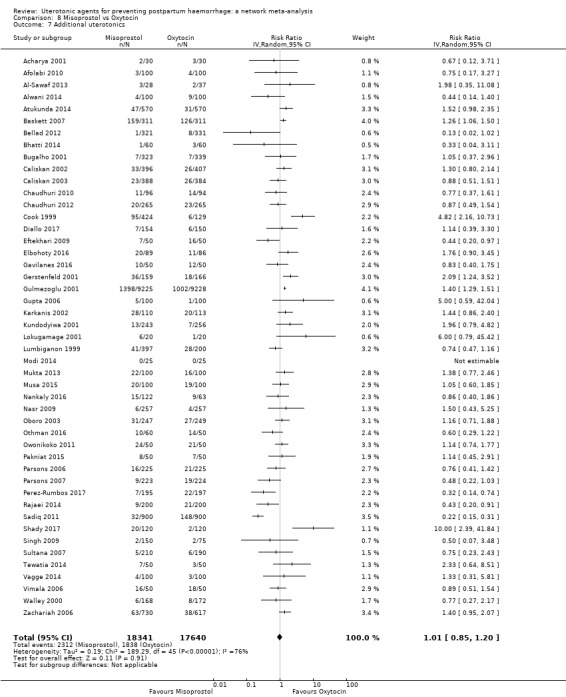
Comparison 8 Misoprostol vs Oxytocin, Outcome 7 Additional uterotonics.
8.8. Analysis.
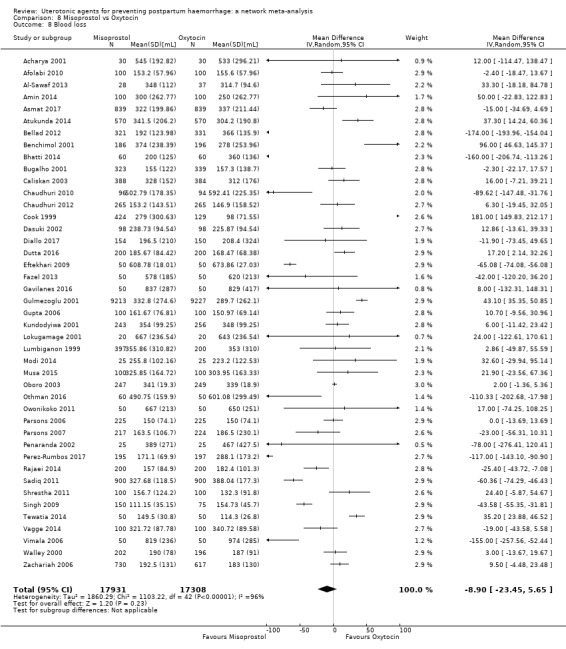
Comparison 8 Misoprostol vs Oxytocin, Outcome 8 Blood loss.
8.9. Analysis.
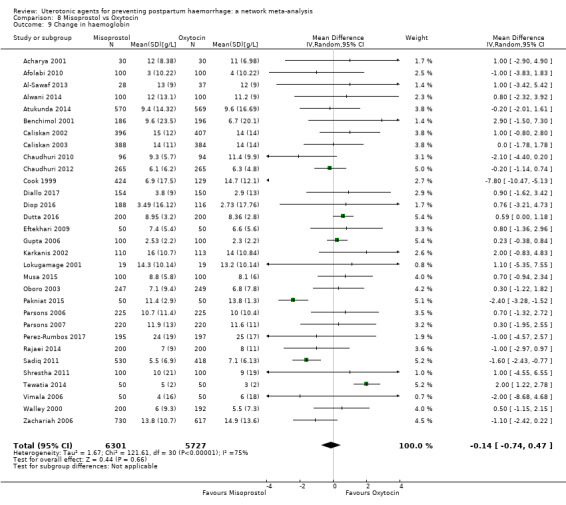
Comparison 8 Misoprostol vs Oxytocin, Outcome 9 Change in haemoglobin.
8.11. Analysis.
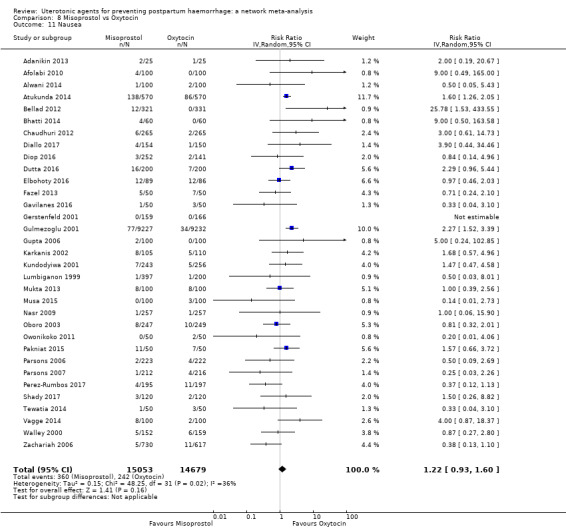
Comparison 8 Misoprostol vs Oxytocin, Outcome 11 Nausea.
8.12. Analysis.
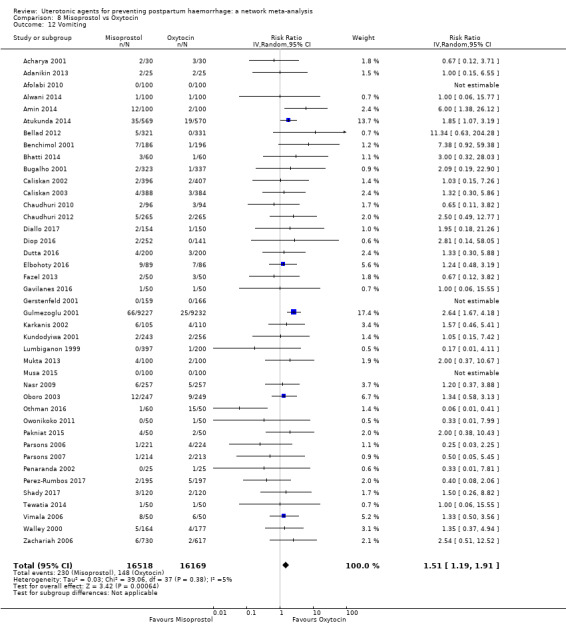
Comparison 8 Misoprostol vs Oxytocin, Outcome 12 Vomiting.
8.13. Analysis.
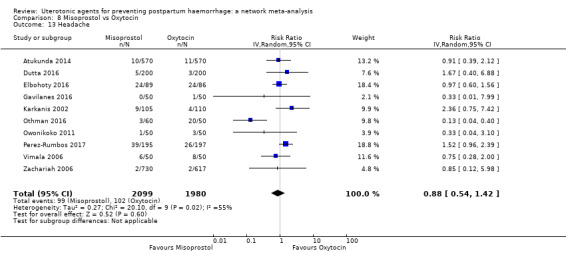
Comparison 8 Misoprostol vs Oxytocin, Outcome 13 Headache.
8.14. Analysis.
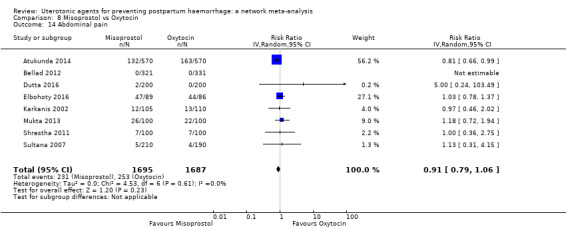
Comparison 8 Misoprostol vs Oxytocin, Outcome 14 Abdominal pain.
8.15. Analysis.
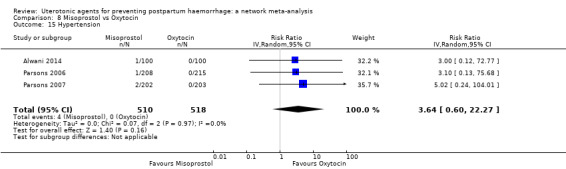
Comparison 8 Misoprostol vs Oxytocin, Outcome 15 Hypertension.
8.16. Analysis.
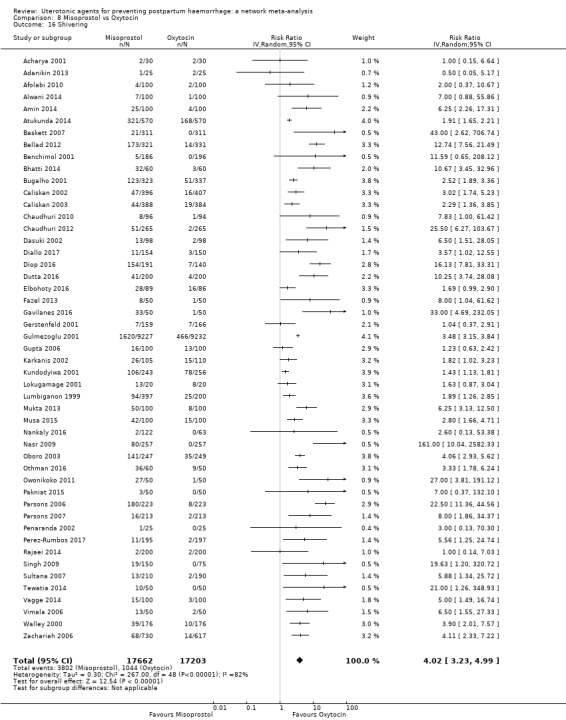
Comparison 8 Misoprostol vs Oxytocin, Outcome 16 Shivering.
8.17. Analysis.
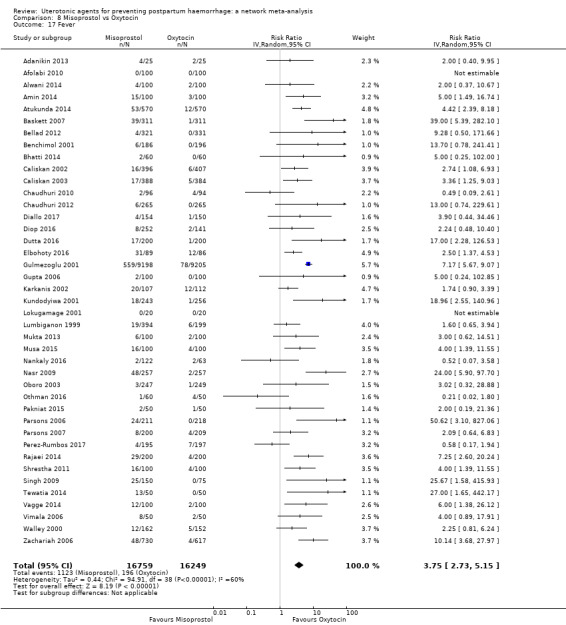
Comparison 8 Misoprostol vs Oxytocin, Outcome 17 Fever.
8.18. Analysis.
Comparison 8 Misoprostol vs Oxytocin, Outcome 18 Diarrhoea.
Comparison 9. Injectable prostaglandins vs Oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.20, 10.31] |
| 3 Blood transfusion | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.65] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.26, 2.71] |
| 7 Additional uterotonics | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.09, 0.94] |
| 8 Blood loss | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐15.83 [‐152.28, 120.62] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 3 | 450 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.40, 3.41] |
| 12 Vomiting | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 2.48 [0.57, 10.73] |
| 13 Headache | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.01, 4.11] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.11, 7.73] |
| 17 Fever | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.18, 21.71] |
| 18 Diarrhoea | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 10.38 [1.96, 54.98] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
9.1. Analysis.
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 1 Death.
9.2. Analysis.
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 2 PPH >= 1000 mL.
9.3. Analysis.
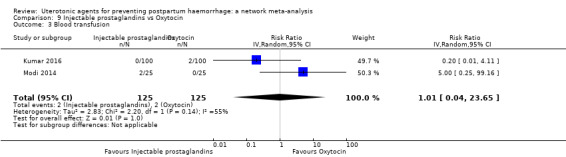
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 3 Blood transfusion.
9.6. Analysis.
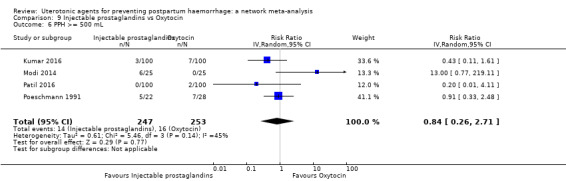
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 6 PPH >= 500 mL.
9.7. Analysis.
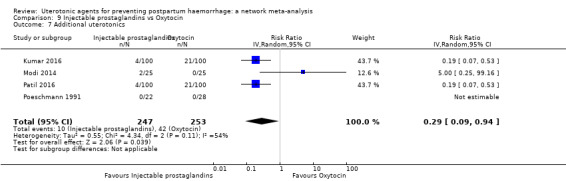
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 7 Additional uterotonics.
9.8. Analysis.

Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 8 Blood loss.
9.11. Analysis.
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 11 Nausea.
9.12. Analysis.
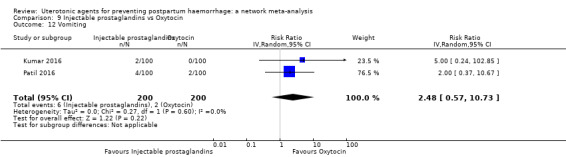
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 12 Vomiting.
9.13. Analysis.
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 13 Headache.
9.16. Analysis.
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 16 Shivering.
9.17. Analysis.
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 17 Fever.
9.18. Analysis.
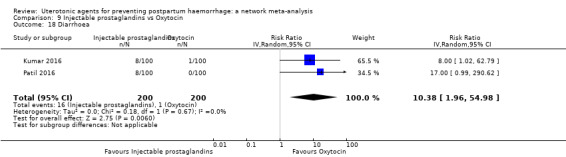
Comparison 9 Injectable prostaglandins vs Oxytocin, Outcome 18 Diarrhoea.
Comparison 10. Carbetocin vs Oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 5 | 30327 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.37, 10.92] |
| 2 PPH >= 1000 mL | 10 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.45, 1.19] |
| 3 Blood transfusion | 16 | 32115 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.38, 1.22] |
| 4 Severe maternal morbidity: intensive care admissions | 2 | 29847 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.67, 2.02] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 11 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.58, 0.98] |
| 7 Additional uterotonics | 22 | 32699 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.34, 0.68] |
| 8 Blood loss | 16 | 2115 | Mean Difference (IV, Random, 95% CI) | ‐92.73 [‐154.97, ‐30.49] |
| 9 Change in haemoglobin | 12 | 2096 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐3.81, 0.50] |
| 10 Breastfeeding | 2 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.86, 1.03] |
| 11 Nausea | 12 | 2288 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.78, 1.56] |
| 12 Vomiting | 13 | 31833 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.53, 1.50] |
| 13 Headache | 15 | 2620 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.63, 1.12] |
| 14 Abdominal pain | 10 | 31293 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 9 | 1998 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.49, 1.23] |
| 17 Fever | 4 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.27, 9.35] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
10.1. Analysis.
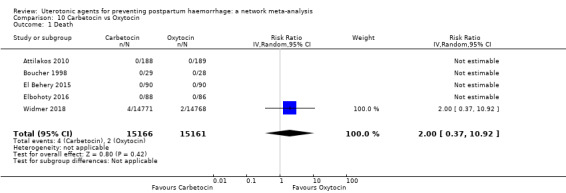
Comparison 10 Carbetocin vs Oxytocin, Outcome 1 Death.
10.2. Analysis.
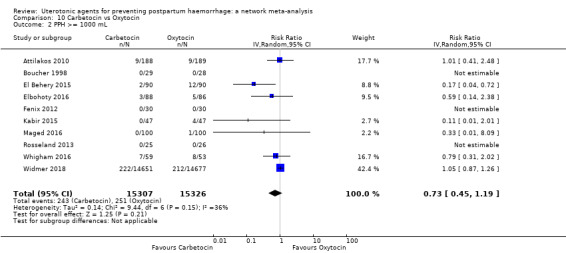
Comparison 10 Carbetocin vs Oxytocin, Outcome 2 PPH >= 1000 mL.
10.3. Analysis.
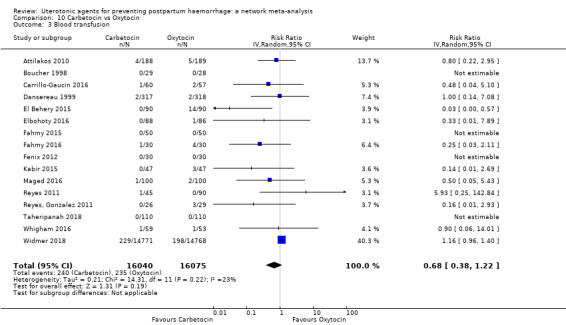
Comparison 10 Carbetocin vs Oxytocin, Outcome 3 Blood transfusion.
10.4. Analysis.
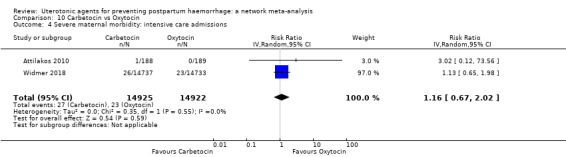
Comparison 10 Carbetocin vs Oxytocin, Outcome 4 Severe maternal morbidity: intensive care admissions.
10.6. Analysis.
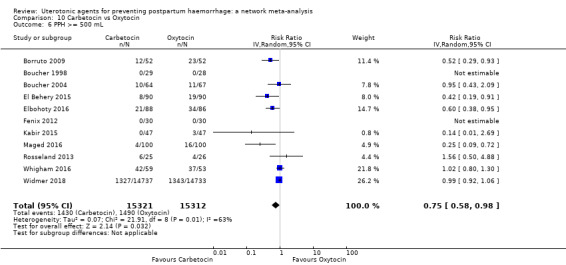
Comparison 10 Carbetocin vs Oxytocin, Outcome 6 PPH >= 500 mL.
10.7. Analysis.
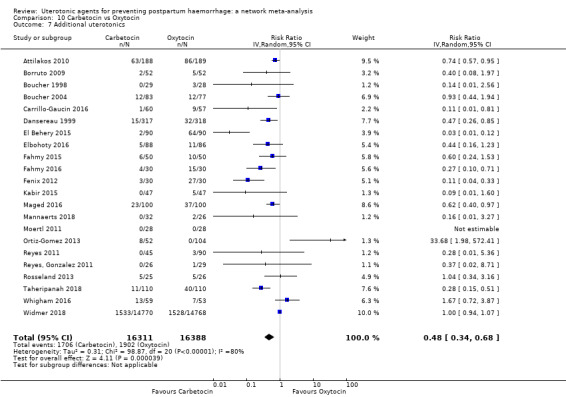
Comparison 10 Carbetocin vs Oxytocin, Outcome 7 Additional uterotonics.
10.8. Analysis.
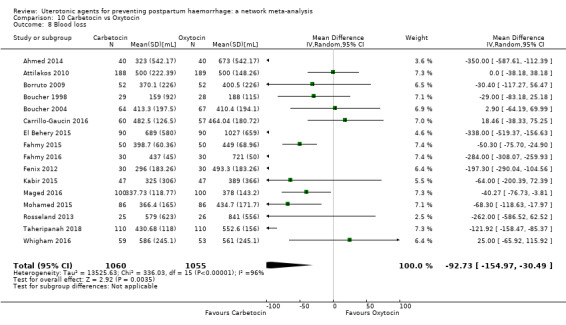
Comparison 10 Carbetocin vs Oxytocin, Outcome 8 Blood loss.
10.9. Analysis.
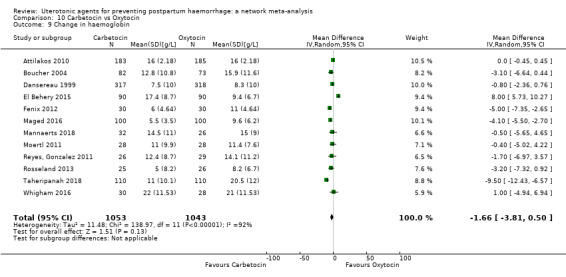
Comparison 10 Carbetocin vs Oxytocin, Outcome 9 Change in haemoglobin.
10.10. Analysis.
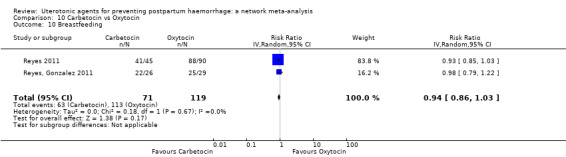
Comparison 10 Carbetocin vs Oxytocin, Outcome 10 Breastfeeding.
10.11. Analysis.
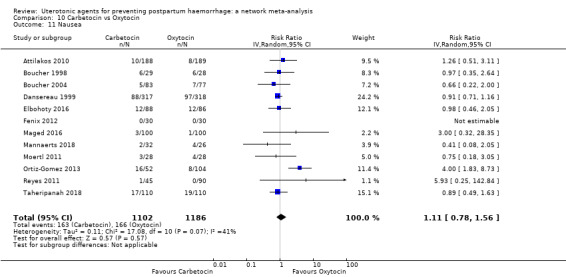
Comparison 10 Carbetocin vs Oxytocin, Outcome 11 Nausea.
10.12. Analysis.
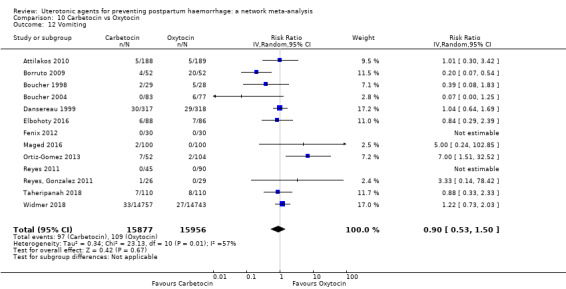
Comparison 10 Carbetocin vs Oxytocin, Outcome 12 Vomiting.
10.13. Analysis.
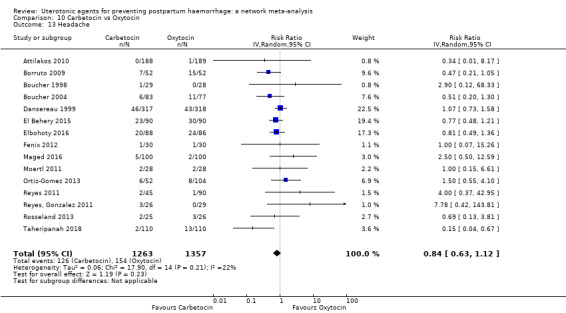
Comparison 10 Carbetocin vs Oxytocin, Outcome 13 Headache.
10.14. Analysis.
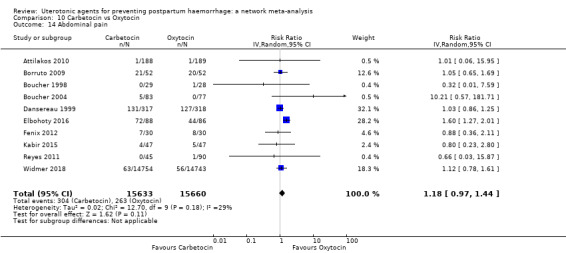
Comparison 10 Carbetocin vs Oxytocin, Outcome 14 Abdominal pain.
10.16. Analysis.
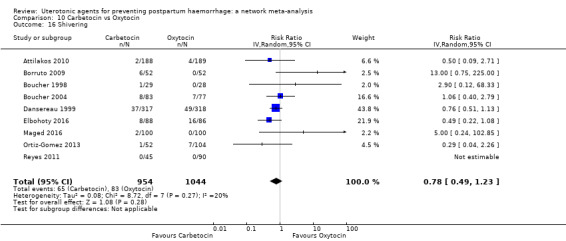
Comparison 10 Carbetocin vs Oxytocin, Outcome 16 Shivering.
10.17. Analysis.
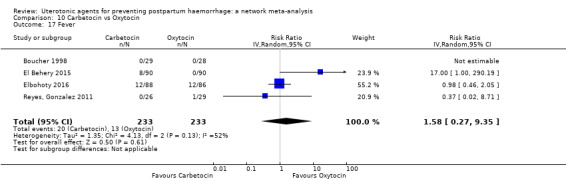
Comparison 10 Carbetocin vs Oxytocin, Outcome 17 Fever.
Comparison 11. Ergometrine vs Oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 1293 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 6 | 2591 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.52, 3.27] |
| 3 Blood transfusion | 5 | 1893 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.20, 10.23] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 10 | 3221 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.86, 1.99] |
| 7 Additional uterotonics | 6 | 2493 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.61, 3.48] |
| 8 Blood loss | 10 | 3221 | Mean Difference (IV, Random, 95% CI) | 8.09 [‐17.83, 34.00] |
| 9 Change in haemoglobin | 2 | 1893 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.30, 1.13] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 6 | 2529 | Risk Ratio (IV, Random, 95% CI) | 4.56 [1.13, 18.44] |
| 12 Vomiting | 5 | 2343 | Risk Ratio (IV, Random, 95% CI) | 3.83 [1.10, 13.28] |
| 13 Headache | 4 | 2293 | Risk Ratio (IV, Random, 95% CI) | 5.63 [0.93, 33.96] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 1410 | Risk Ratio (IV, Random, 95% CI) | 13.39 [2.01, 89.44] |
| 16 Shivering | 3 | 1493 | Risk Ratio (IV, Random, 95% CI) | 1.73 [0.93, 3.25] |
| 17 Fever | 2 | 1443 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.97, 9.05] |
| 18 Diarrhoea | 2 | 1393 | Risk Ratio (IV, Random, 95% CI) | 3.74 [0.42, 33.53] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
11.1. Analysis.
Comparison 11 Ergometrine vs Oxytocin, Outcome 1 Death.
11.2. Analysis.
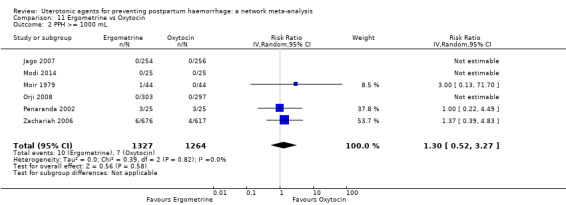
Comparison 11 Ergometrine vs Oxytocin, Outcome 2 PPH >= 1000 mL.
11.3. Analysis.
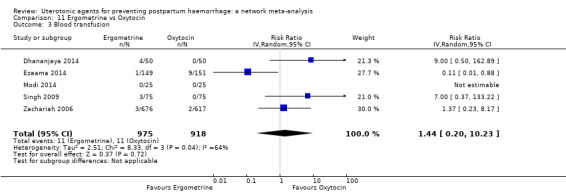
Comparison 11 Ergometrine vs Oxytocin, Outcome 3 Blood transfusion.
11.6. Analysis.
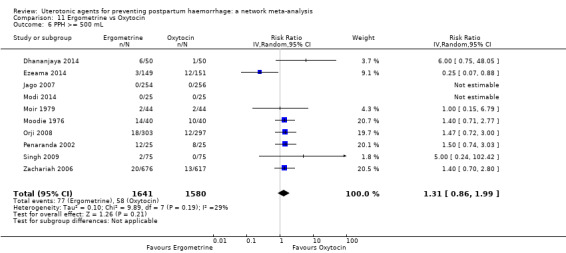
Comparison 11 Ergometrine vs Oxytocin, Outcome 6 PPH >= 500 mL.
11.7. Analysis.
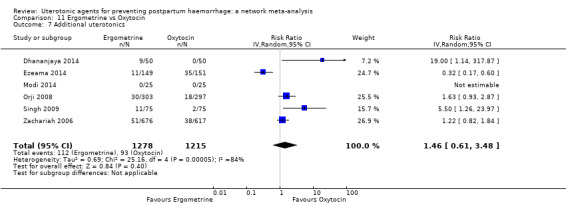
Comparison 11 Ergometrine vs Oxytocin, Outcome 7 Additional uterotonics.
11.8. Analysis.
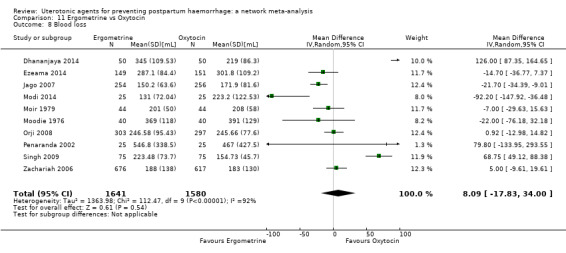
Comparison 11 Ergometrine vs Oxytocin, Outcome 8 Blood loss.
11.9. Analysis.
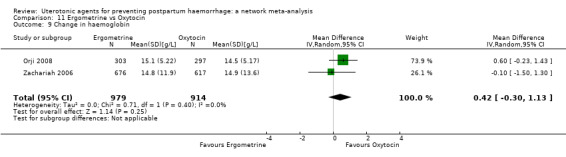
Comparison 11 Ergometrine vs Oxytocin, Outcome 9 Change in haemoglobin.
11.11. Analysis.
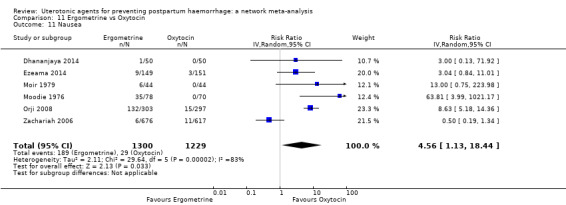
Comparison 11 Ergometrine vs Oxytocin, Outcome 11 Nausea.
11.12. Analysis.
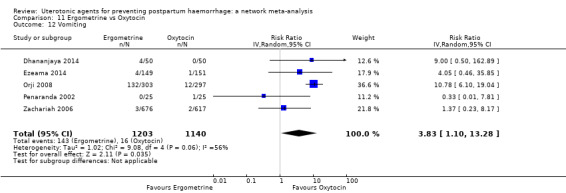
Comparison 11 Ergometrine vs Oxytocin, Outcome 12 Vomiting.
11.13. Analysis.
Comparison 11 Ergometrine vs Oxytocin, Outcome 13 Headache.
11.15. Analysis.
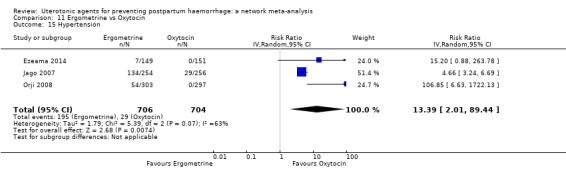
Comparison 11 Ergometrine vs Oxytocin, Outcome 15 Hypertension.
11.16. Analysis.
Comparison 11 Ergometrine vs Oxytocin, Outcome 16 Shivering.
11.17. Analysis.
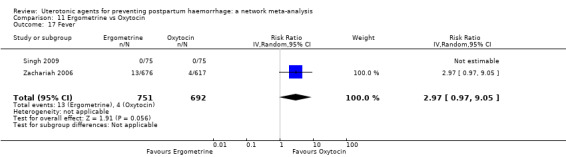
Comparison 11 Ergometrine vs Oxytocin, Outcome 17 Fever.
11.18. Analysis.
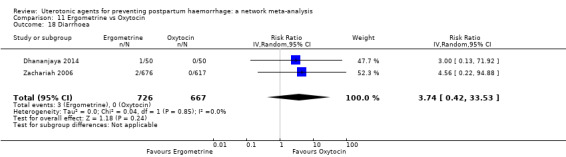
Comparison 11 Ergometrine vs Oxytocin, Outcome 18 Diarrhoea.
Comparison 12. Ergometine plus oxytocin vs Oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 1314 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 10 | 10990 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.57, 0.93] |
| 3 Blood transfusion | 11 | 10985 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.53, 1.44] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 991 | Risk Ratio (IV, Random, 95% CI) | 2.99 [0.12, 73.32] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 11 | 11090 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.57, 0.91] |
| 7 Additional uterotonics | 10 | 8968 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.59, 1.07] |
| 8 Blood loss | 10 | 4248 | Mean Difference (IV, Random, 95% CI) | ‐10.31 [‐40.32, 19.70] |
| 9 Change in haemoglobin | 6 | 4324 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐5.24, 0.77] |
| 10 Breastfeeding | 1 | 3483 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 11 Nausea | 7 | 6931 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.84, 3.53] |
| 12 Vomiting | 9 | 10227 | Risk Ratio (IV, Random, 95% CI) | 3.05 [1.76, 5.29] |
| 13 Headache | 5 | 5105 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.79, 1.99] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 1362 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.29, 13.97] |
| 16 Shivering | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.60, 1.53] |
| 17 Fever | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.48, 2.43] |
| 18 Diarrhoea | 3 | 2030 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.72, 2.22] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
12.1. Analysis.
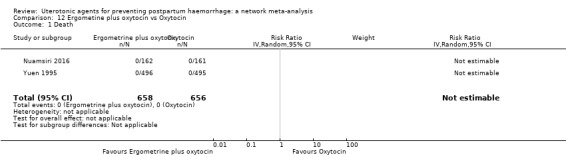
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 1 Death.
12.2. Analysis.
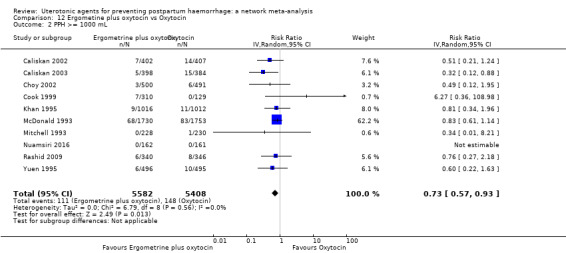
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 2 PPH >= 1000 mL.
12.3. Analysis.
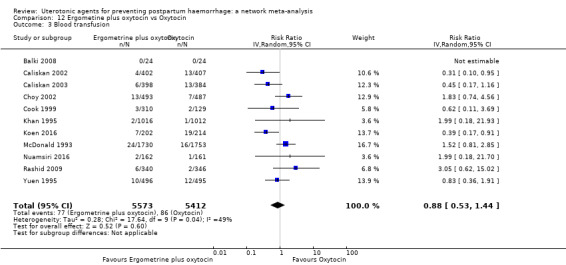
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 3 Blood transfusion.
12.4. Analysis.
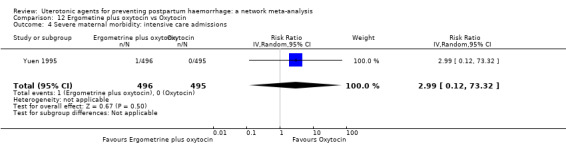
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 4 Severe maternal morbidity: intensive care admissions.
12.6. Analysis.
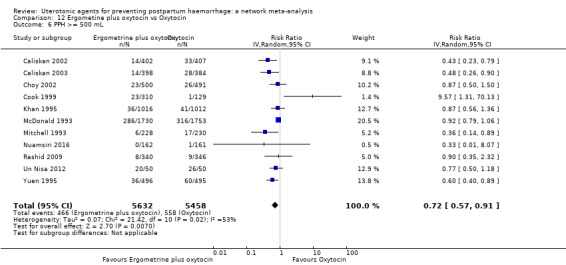
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 6 PPH >= 500 mL.
12.7. Analysis.
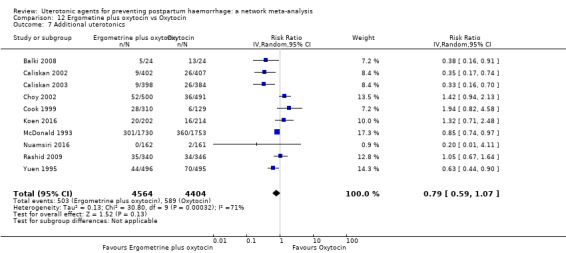
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 7 Additional uterotonics.
12.8. Analysis.
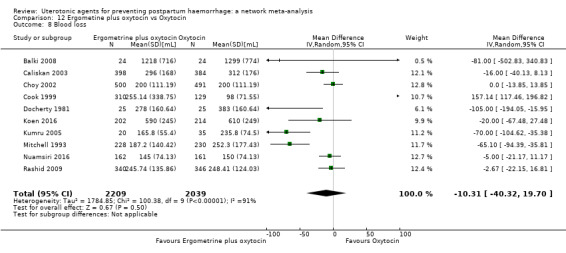
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 8 Blood loss.
12.9. Analysis.

Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 9 Change in haemoglobin.
12.10. Analysis.
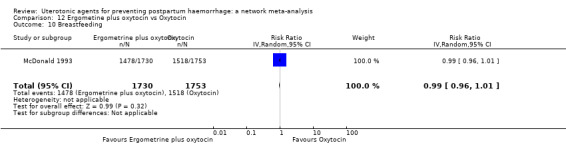
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 10 Breastfeeding.
12.11. Analysis.
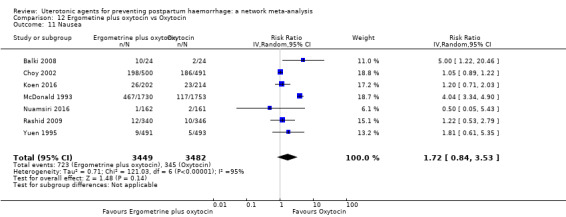
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 11 Nausea.
12.12. Analysis.
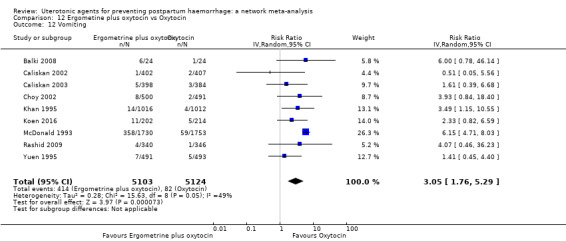
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 12 Vomiting.
12.13. Analysis.
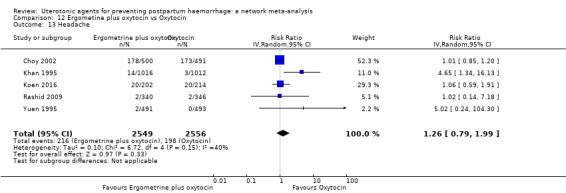
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 13 Headache.
12.15. Analysis.
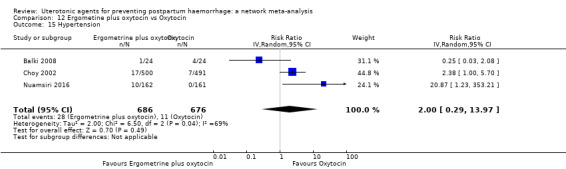
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 15 Hypertension.
12.16. Analysis.
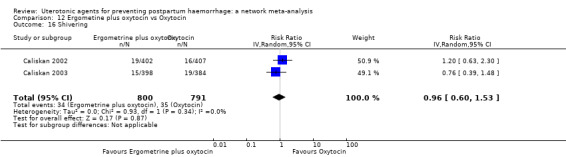
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 16 Shivering.
12.17. Analysis.
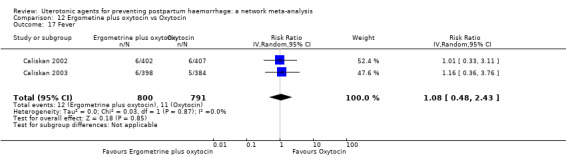
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 17 Fever.
12.18. Analysis.
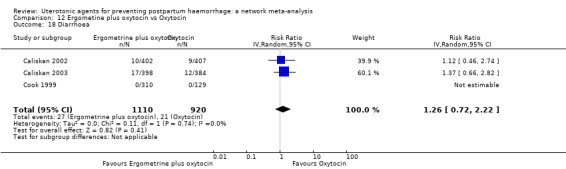
Comparison 12 Ergometine plus oxytocin vs Oxytocin, Outcome 18 Diarrhoea.
Comparison 13. Misoprostol plus oxytocin vs Oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 9 | 4737 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 17 | 8514 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 3 Blood transfusion | 19 | 8742 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.37, 0.67] |
| 4 Severe maternal morbidity: intensive care admissions | 3 | 1886 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.47] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 14 | 8148 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 7 Additional uterotonics | 18 | 8391 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.44, 0.67] |
| 8 Blood loss | 17 | 8690 | Mean Difference (IV, Random, 95% CI) | ‐87.26 [‐157.83, ‐16.69] |
| 9 Change in haemoglobin | 15 | 7929 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐3.70, ‐1.48] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 7 | 3798 | Risk Ratio (IV, Random, 95% CI) | 2.21 [1.19, 4.10] |
| 12 Vomiting | 11 | 6718 | Risk Ratio (IV, Random, 95% CI) | 2.24 [1.52, 3.31] |
| 13 Headache | 2 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.26, 6.23] |
| 14 Abdominal pain | 1 | 366 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.01, 3.67] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 19 | 9458 | Risk Ratio (IV, Random, 95% CI) | 3.38 [2.50, 4.57] |
| 17 Fever | 17 | 8607 | Risk Ratio (IV, Random, 95% CI) | 2.99 [2.00, 4.45] |
| 18 Diarrhoea | 7 | 5649 | Risk Ratio (IV, Random, 95% CI) | 2.08 [0.99, 4.38] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
13.1. Analysis.
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 1 Death.
13.2. Analysis.
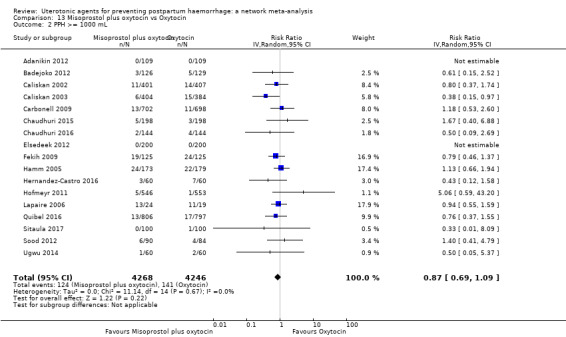
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 2 PPH >= 1000 mL.
13.3. Analysis.
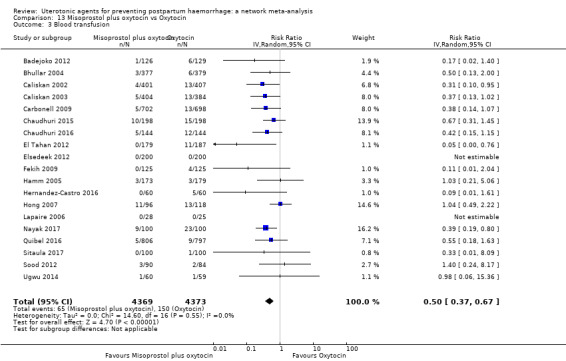
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 3 Blood transfusion.
13.4. Analysis.
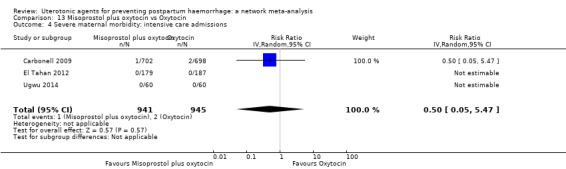
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 4 Severe maternal morbidity: intensive care admissions.
13.6. Analysis.
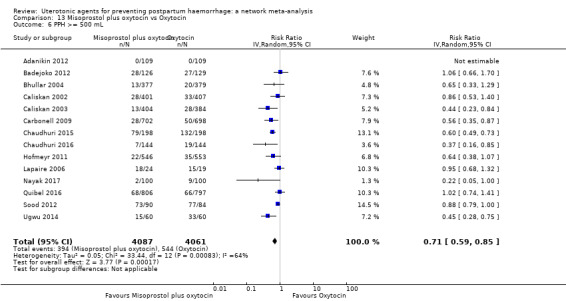
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 6 PPH >= 500 mL.
13.7. Analysis.
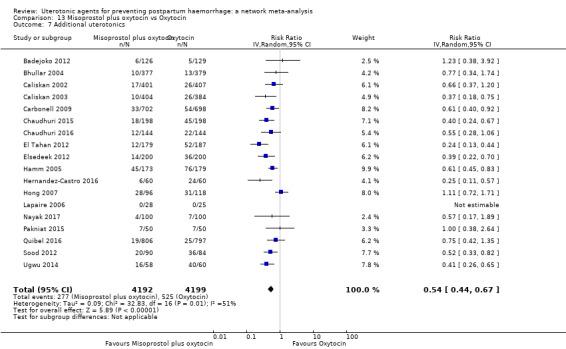
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 7 Additional uterotonics.
13.8. Analysis.
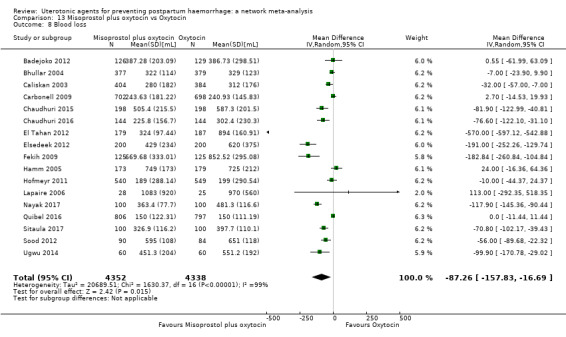
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 8 Blood loss.
13.9. Analysis.
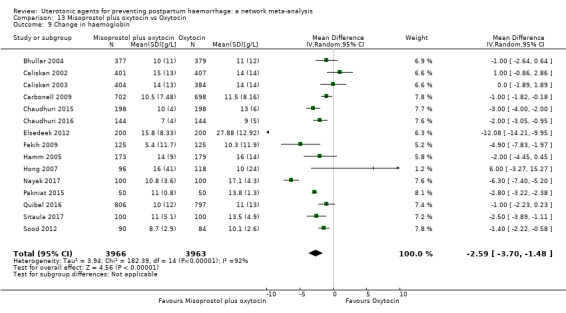
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 9 Change in haemoglobin.
13.11. Analysis.
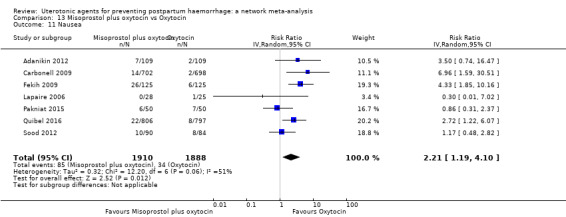
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 11 Nausea.
13.12. Analysis.
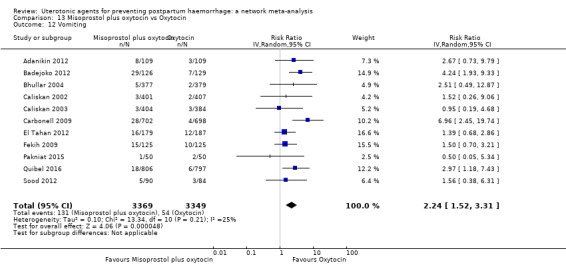
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 12 Vomiting.
13.13. Analysis.
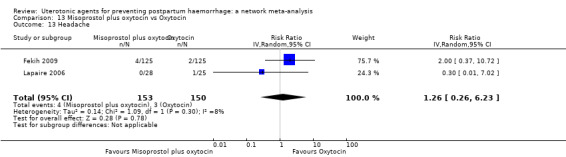
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 13 Headache.
13.14. Analysis.
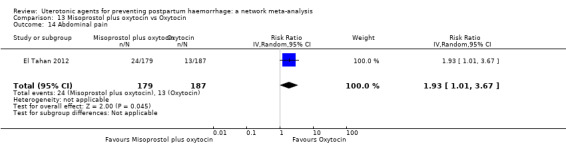
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 14 Abdominal pain.
13.16. Analysis.
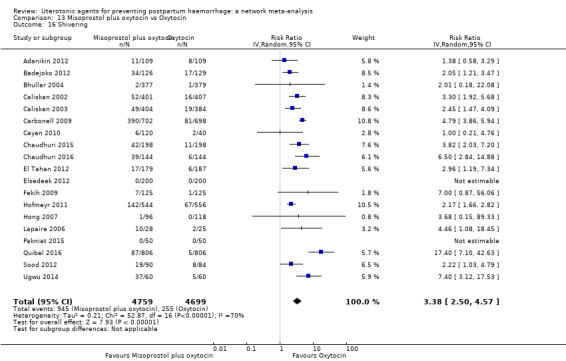
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 16 Shivering.
13.17. Analysis.
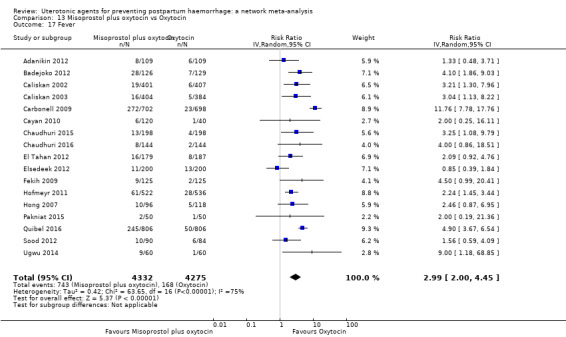
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 17 Fever.
13.18. Analysis.
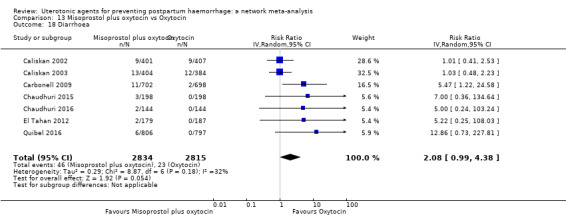
Comparison 13 Misoprostol plus oxytocin vs Oxytocin, Outcome 18 Diarrhoea.
Comparison 14. Injectable prostaglandins vs Misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 170 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 3 Blood transfusion | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.14, 5.39] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 3 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.60 [0.57, 4.52] |
| 7 Additional uterotonics | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.28, 3.69] |
| 8 Blood loss | 3 | 270 | Mean Difference (IV, Random, 95% CI) | 52.99 [‐39.74, 145.71] |
| 9 Change in haemoglobin | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐1.35, 5.90] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.06, 0.92] |
| 12 Vomiting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.01, 163.15] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.20, 4.80] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 353 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.01, 0.21] |
| 17 Fever | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.01, 0.39] |
| 18 Diarrhoea | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 9.03 [1.70, 48.00] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
14.1. Analysis.
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 1 Death.
14.2. Analysis.
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 2 PPH >= 1000 mL.
14.3. Analysis.
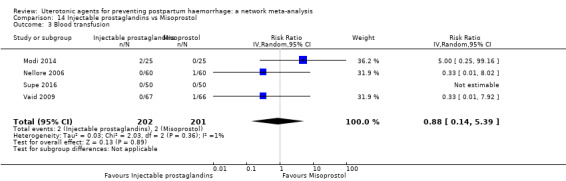
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 3 Blood transfusion.
14.6. Analysis.
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 6 PPH >= 500 mL.
14.7. Analysis.
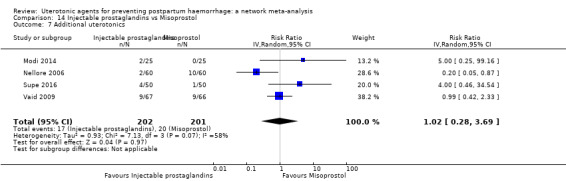
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 7 Additional uterotonics.
14.8. Analysis.
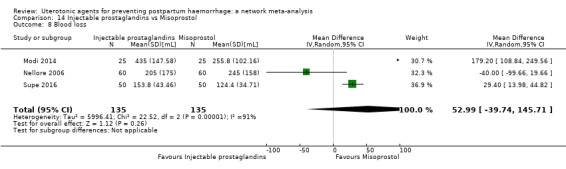
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 8 Blood loss.
14.9. Analysis.
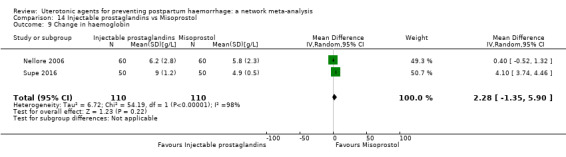
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 9 Change in haemoglobin.
14.11. Analysis.
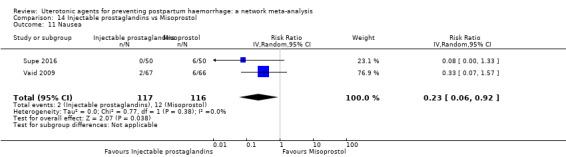
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 11 Nausea.
14.12. Analysis.
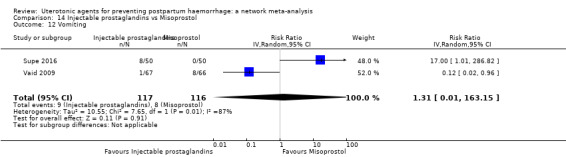
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 12 Vomiting.
14.14. Analysis.
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 14 Abdominal pain.
14.16. Analysis.
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 16 Shivering.
14.17. Analysis.
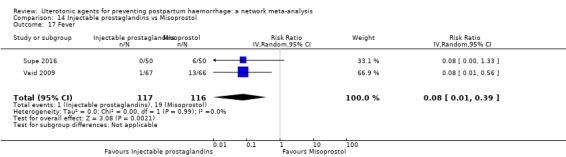
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 17 Fever.
14.18. Analysis.
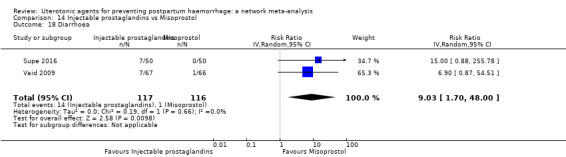
Comparison 14 Injectable prostaglandins vs Misoprostol, Outcome 18 Diarrhoea.
Comparison 15. Misoprostol vs Carbetocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [0.62, 8.64] |
| 3 Blood transfusion | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 71.85] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [1.52, 3.50] |
| 7 Additional uterotonics | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.96 [1.55, 10.07] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.47, 2.08] |
| 12 Vomiting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.55, 3.99] |
| 13 Headache | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.71, 1.99] |
| 14 Abdominal pain | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.46 [1.67, 7.17] |
| 17 Fever | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.55 [1.41, 4.64] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
15.1. Analysis.
Comparison 15 Misoprostol vs Carbetocin, Outcome 1 Death.
15.2. Analysis.
Comparison 15 Misoprostol vs Carbetocin, Outcome 2 PPH >= 1000 mL.
15.3. Analysis.
Comparison 15 Misoprostol vs Carbetocin, Outcome 3 Blood transfusion.
15.6. Analysis.
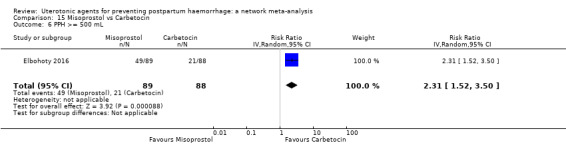
Comparison 15 Misoprostol vs Carbetocin, Outcome 6 PPH >= 500 mL.
15.7. Analysis.
Comparison 15 Misoprostol vs Carbetocin, Outcome 7 Additional uterotonics.
15.11. Analysis.
Comparison 15 Misoprostol vs Carbetocin, Outcome 11 Nausea.
15.12. Analysis.
Comparison 15 Misoprostol vs Carbetocin, Outcome 12 Vomiting.
15.13. Analysis.
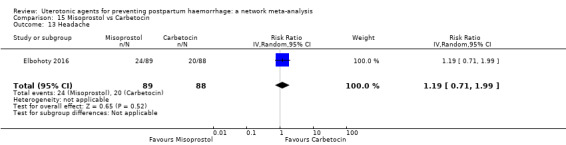
Comparison 15 Misoprostol vs Carbetocin, Outcome 13 Headache.
15.14. Analysis.
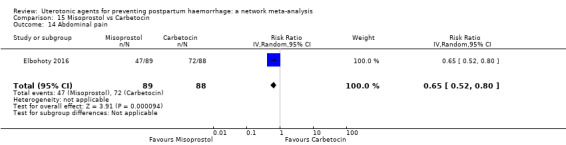
Comparison 15 Misoprostol vs Carbetocin, Outcome 14 Abdominal pain.
15.16. Analysis.
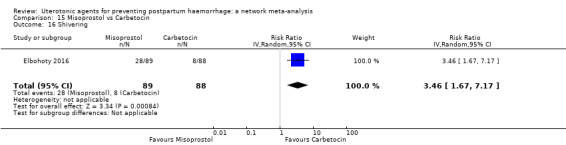
Comparison 15 Misoprostol vs Carbetocin, Outcome 16 Shivering.
15.17. Analysis.
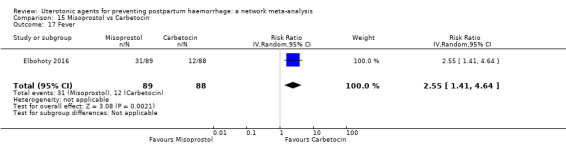
Comparison 15 Misoprostol vs Carbetocin, Outcome 17 Fever.
Comparison 16. Ergometrine vs Misoprostol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 7 | 5254 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 11 | 5562 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.45, 3.48] |
| 3 Blood transfusion | 13 | 5308 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.65, 4.50] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 864 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.16] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 16 | 6459 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.79, 1.75] |
| 7 Additional uterotonics | 16 | 6565 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.60] |
| 8 Blood loss | 15 | 6337 | Mean Difference (IV, Random, 95% CI) | 11.66 [‐9.85, 33.17] |
| 9 Change in haemoglobin | 8 | 4438 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.43, 2.26] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 13 | 5202 | Risk Ratio (IV, Random, 95% CI) | 1.53 [1.15, 2.04] |
| 12 Vomiting | 14 | 6136 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.81, 1.83] |
| 13 Headache | 8 | 3369 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.70, 4.12] |
| 14 Abdominal pain | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.04, 65.80] |
| 15 Hypertension | 2 | 300 | Risk Ratio (IV, Random, 95% CI) | 7.55 [0.94, 60.53] |
| 16 Shivering | 17 | 6860 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.26, 0.44] |
| 17 Fever | 14 | 6330 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.33] |
| 18 Diarrhoea | 8 | 4165 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.46, 2.34] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
16.1. Analysis.
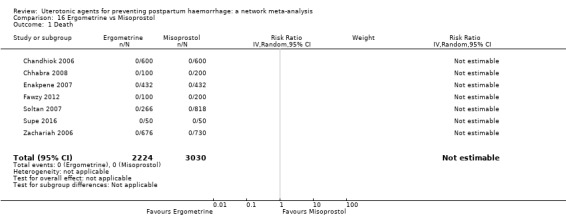
Comparison 16 Ergometrine vs Misoprostol, Outcome 1 Death.
16.2. Analysis.
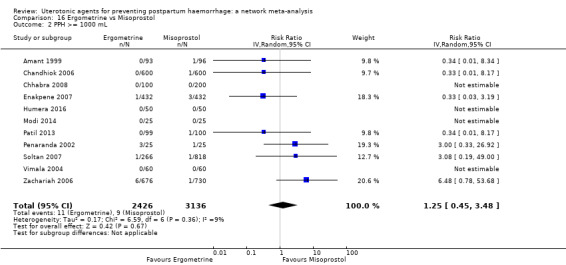
Comparison 16 Ergometrine vs Misoprostol, Outcome 2 PPH >= 1000 mL.
16.3. Analysis.
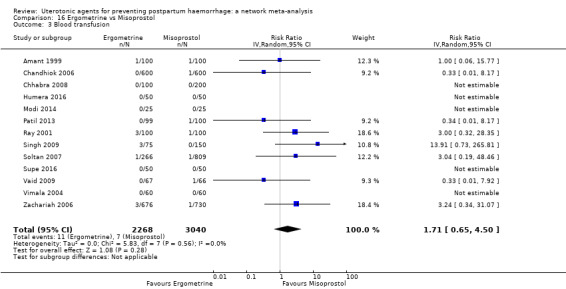
Comparison 16 Ergometrine vs Misoprostol, Outcome 3 Blood transfusion.
16.4. Analysis.
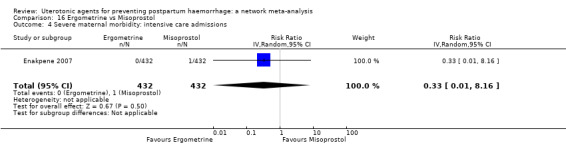
Comparison 16 Ergometrine vs Misoprostol, Outcome 4 Severe maternal morbidity: intensive care admissions.
16.6. Analysis.
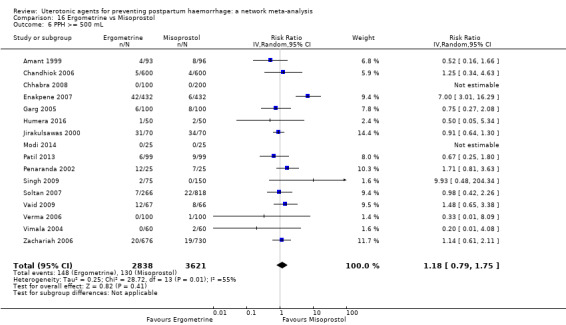
Comparison 16 Ergometrine vs Misoprostol, Outcome 6 PPH >= 500 mL.
16.7. Analysis.
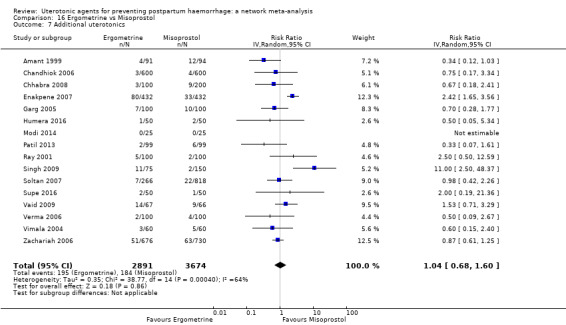
Comparison 16 Ergometrine vs Misoprostol, Outcome 7 Additional uterotonics.
16.8. Analysis.
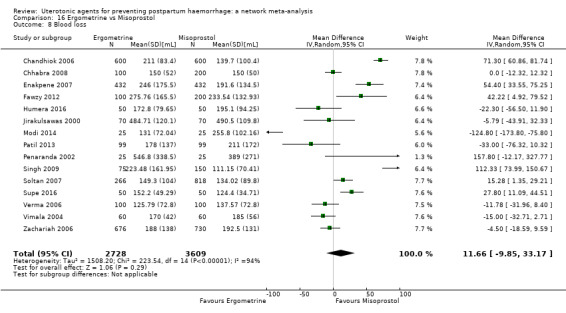
Comparison 16 Ergometrine vs Misoprostol, Outcome 8 Blood loss.
16.9. Analysis.
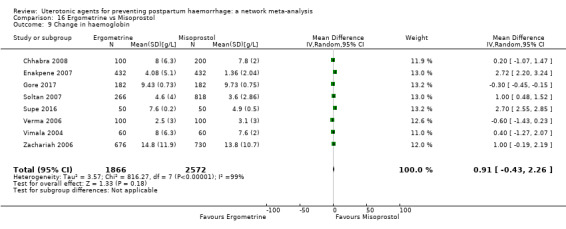
Comparison 16 Ergometrine vs Misoprostol, Outcome 9 Change in haemoglobin.
16.11. Analysis.

Comparison 16 Ergometrine vs Misoprostol, Outcome 11 Nausea.
16.12. Analysis.
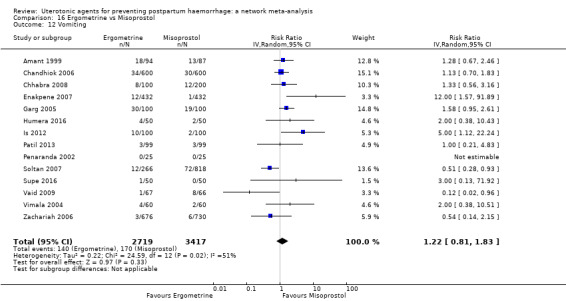
Comparison 16 Ergometrine vs Misoprostol, Outcome 12 Vomiting.
16.13. Analysis.
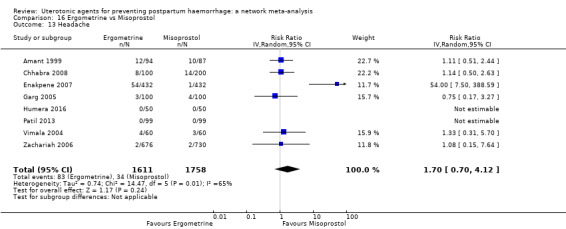
Comparison 16 Ergometrine vs Misoprostol, Outcome 13 Headache.
16.14. Analysis.
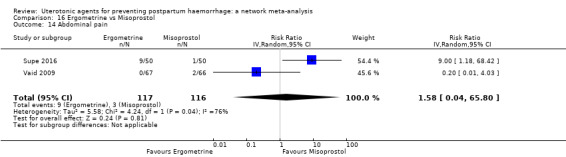
Comparison 16 Ergometrine vs Misoprostol, Outcome 14 Abdominal pain.
16.15. Analysis.
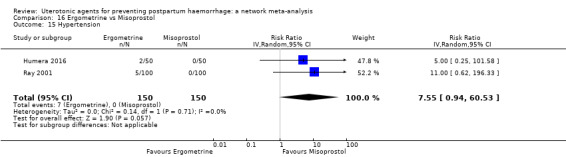
Comparison 16 Ergometrine vs Misoprostol, Outcome 15 Hypertension.
16.16. Analysis.
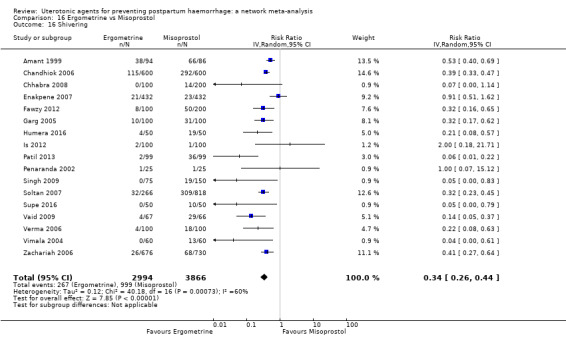
Comparison 16 Ergometrine vs Misoprostol, Outcome 16 Shivering.
16.17. Analysis.
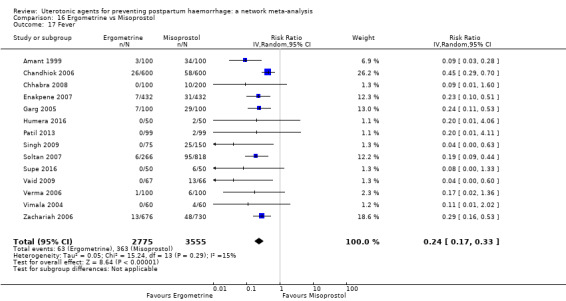
Comparison 16 Ergometrine vs Misoprostol, Outcome 17 Fever.
16.18. Analysis.
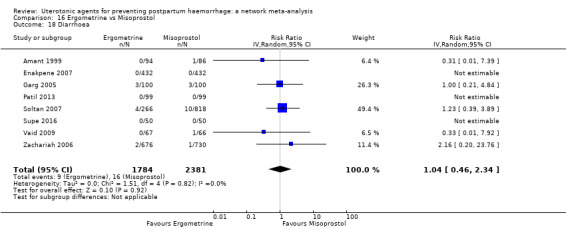
Comparison 16 Ergometrine vs Misoprostol, Outcome 18 Diarrhoea.
Comparison 17. Misoprostol vs Ergometrine plus oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 3258 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 9 | 6028 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.08, 2.40] |
| 3 Blood transfusion | 8 | 6335 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.91, 2.21] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 10 | 6492 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.35, 2.26] |
| 7 Additional uterotonics | 9 | 6395 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.49, 2.36] |
| 8 Blood loss | 8 | 5634 | Mean Difference (IV, Random, 95% CI) | 22.86 [4.50, 41.22] |
| 9 Change in haemoglobin | 9 | 5588 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐0.49, 2.67] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 4 | 3399 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.61, 0.84] |
| 12 Vomiting | 6 | 4983 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.50, 1.04] |
| 13 Headache | 3 | 3259 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.47, 1.76] |
| 14 Abdominal pain | 1 | 846 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 15 Hypertension | 3 | 2553 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.18, 1.41] |
| 16 Shivering | 6 | 4980 | Risk Ratio (IV, Random, 95% CI) | 2.71 [1.95, 3.76] |
| 17 Fever | 7 | 5045 | Risk Ratio (IV, Random, 95% CI) | 5.33 [2.87, 9.88] |
| 18 Diarrhoea | 6 | 3659 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.57] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Woman's satisfaction using an eight item Client Satisfaction Questionnaire | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
17.1. Analysis.
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 1 Death.
17.2. Analysis.
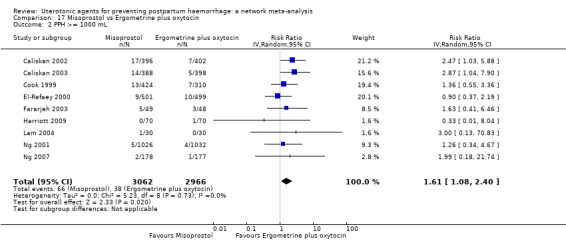
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 2 PPH >= 1000 mL.
17.3. Analysis.
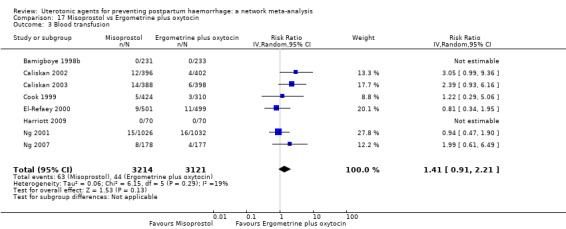
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 3 Blood transfusion.
17.6. Analysis.
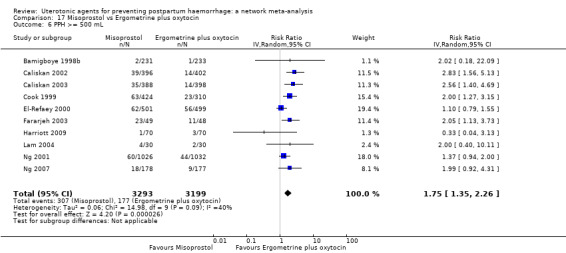
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 6 PPH >= 500 mL.
17.7. Analysis.
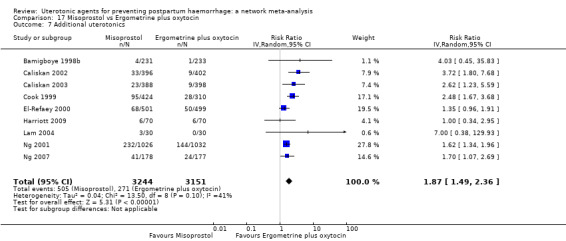
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 7 Additional uterotonics.
17.8. Analysis.
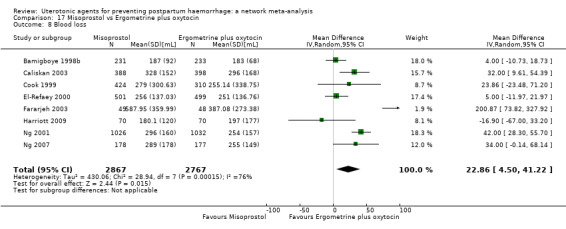
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 8 Blood loss.
17.9. Analysis.
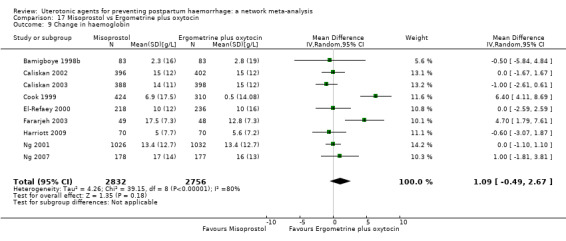
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 9 Change in haemoglobin.
17.11. Analysis.
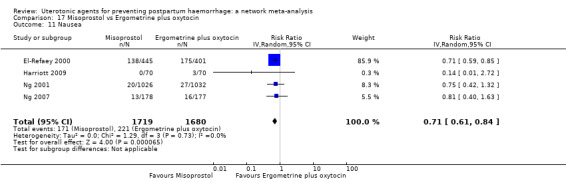
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 11 Nausea.
17.12. Analysis.
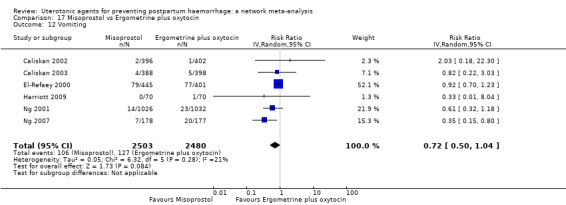
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 12 Vomiting.
17.13. Analysis.
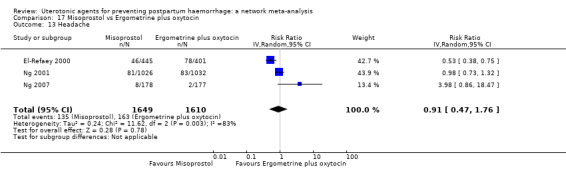
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 13 Headache.
17.14. Analysis.

Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 14 Abdominal pain.
17.15. Analysis.
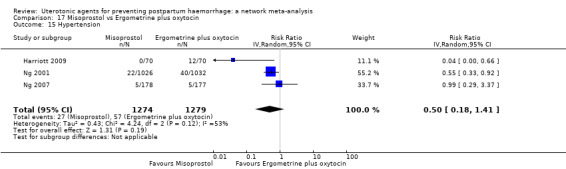
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 15 Hypertension.
17.16. Analysis.
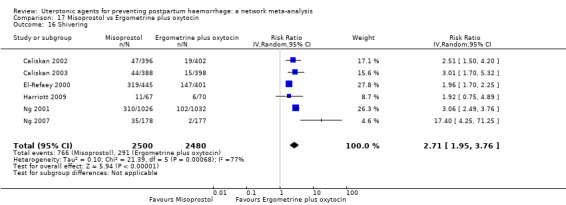
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 16 Shivering.
17.17. Analysis.
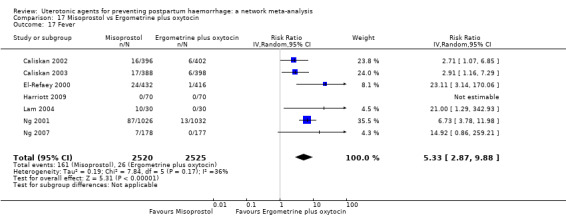
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 17 Fever.
17.18. Analysis.
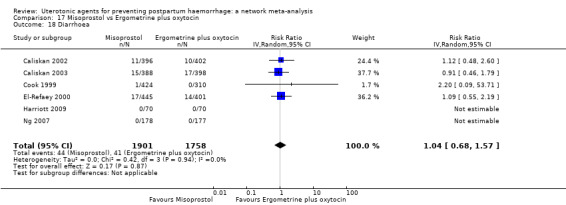
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 18 Diarrhoea.
17.20. Analysis.
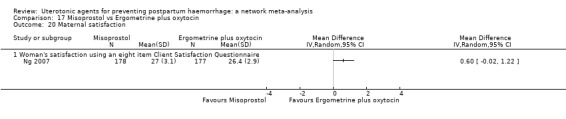
Comparison 17 Misoprostol vs Ergometrine plus oxytocin, Outcome 20 Maternal satisfaction.
Comparison 18. Misoprostol vs Misoprostol plus oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.85 [1.03, 3.33] |
| 3 Blood transfusion | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 2.97 [1.40, 6.30] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.93 [0.99, 3.76] |
| 7 Additional uterotonics | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.89 [1.26, 2.83] |
| 8 Blood loss | 1 | 792 | Mean Difference (IV, Random, 95% CI) | 48.0 [24.68, 71.32] |
| 9 Change in haemoglobin | 3 | 1689 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.42, 0.96] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.83 [0.73, 4.57] |
| 12 Vomiting | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.51, 3.82] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 17 Fever | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.62, 1.53] |
| 18 Diarrhoea | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.70, 2.13] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
18.2. Analysis.

Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 2 PPH >= 1000 mL.
18.3. Analysis.
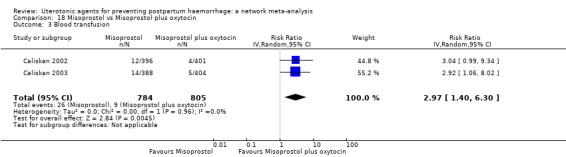
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 3 Blood transfusion.
18.6. Analysis.
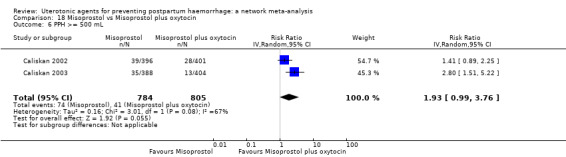
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 6 PPH >= 500 mL.
18.7. Analysis.
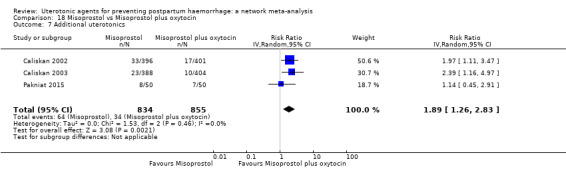
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 7 Additional uterotonics.
18.8. Analysis.

Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 8 Blood loss.
18.9. Analysis.
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 9 Change in haemoglobin.
18.11. Analysis.
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 11 Nausea.
18.12. Analysis.
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 12 Vomiting.
18.16. Analysis.
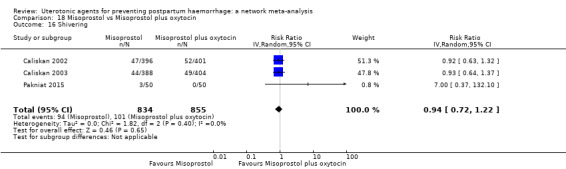
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 16 Shivering.
18.17. Analysis.
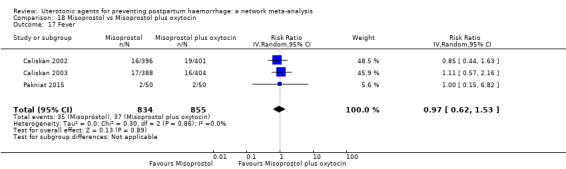
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 17 Fever.
18.18. Analysis.
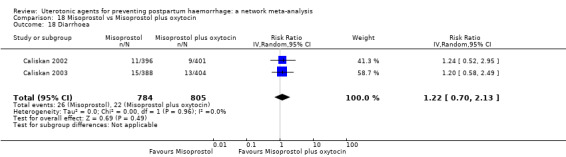
Comparison 18 Misoprostol vs Misoprostol plus oxytocin, Outcome 18 Diarrhoea.
Comparison 19. Carbetocin vs Injectable prostaglandins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 20. Injectable prostaglandins vs Ergometrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 3 Blood transfusion | 4 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.58] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 3 | 399 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.64, 3.17] |
| 7 Additional uterotonics | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.40, 1.51] |
| 8 Blood loss | 8 | 1195 | Mean Difference (IV, Random, 95% CI) | ‐16.08 [‐57.17, 25.00] |
| 9 Change in haemoglobin | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐6.58, 4.01] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.98, 2.94] |
| 12 Vomiting | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 2.70 [0.97, 7.55] |
| 13 Headache | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 14 Abdominal pain | 3 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.07, 40.44] |
| 15 Hypertension | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.02, 1.37] |
| 16 Shivering | 3 | 434 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.16, 0.91] |
| 17 Fever | 4 | 534 | Risk Ratio (IV, Random, 95% CI) | 4.52 [0.76, 26.80] |
| 18 Diarrhoea | 5 | 664 | Risk Ratio (IV, Random, 95% CI) | 10.09 [2.75, 37.00] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
20.1. Analysis.
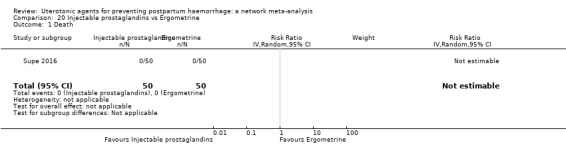
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 1 Death.
20.2. Analysis.
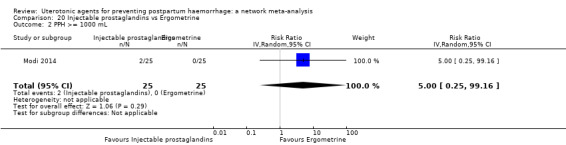
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 2 PPH >= 1000 mL.
20.3. Analysis.
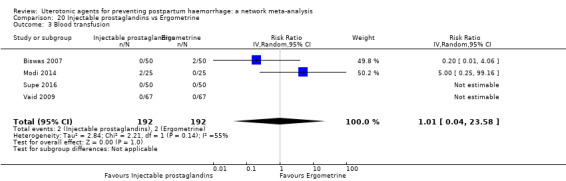
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 3 Blood transfusion.
20.6. Analysis.
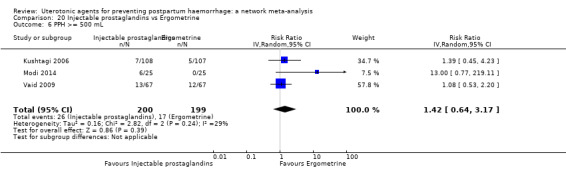
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 6 PPH >= 500 mL.
20.7. Analysis.
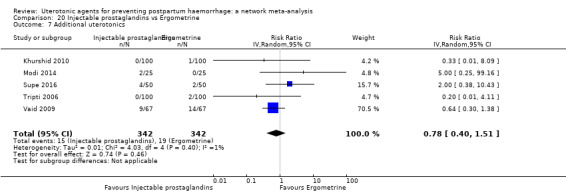
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 7 Additional uterotonics.
20.8. Analysis.
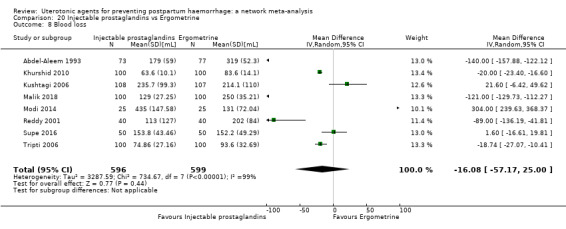
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 8 Blood loss.
20.9. Analysis.
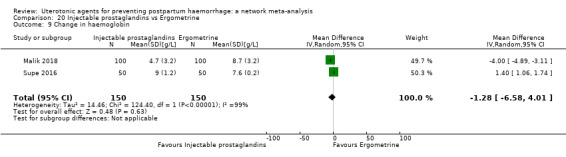
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 9 Change in haemoglobin.
20.11. Analysis.
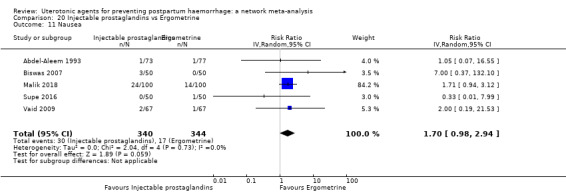
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 11 Nausea.
20.12. Analysis.
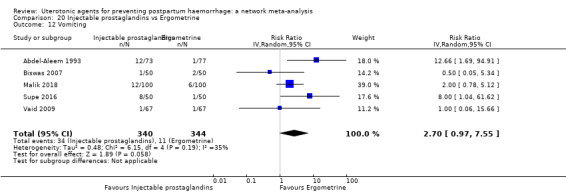
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 12 Vomiting.
20.13. Analysis.
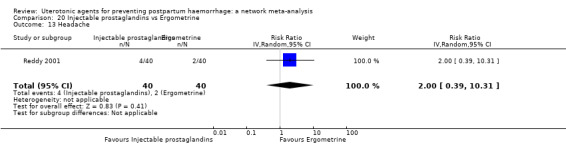
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 13 Headache.
20.14. Analysis.

Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 14 Abdominal pain.
20.15. Analysis.
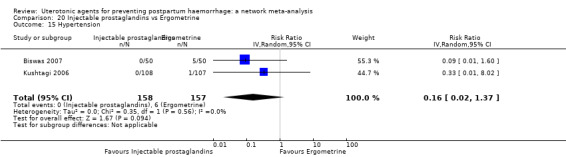
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 15 Hypertension.
20.16. Analysis.
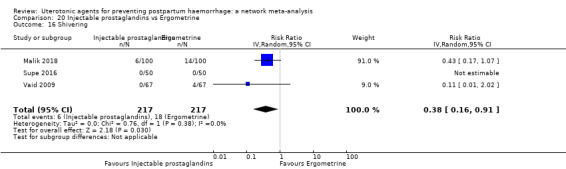
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 16 Shivering.
20.17. Analysis.
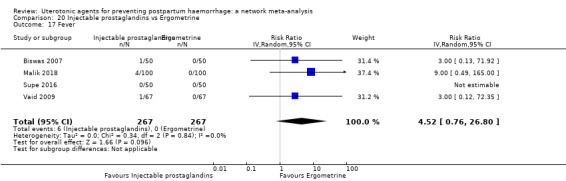
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 17 Fever.
20.18. Analysis.
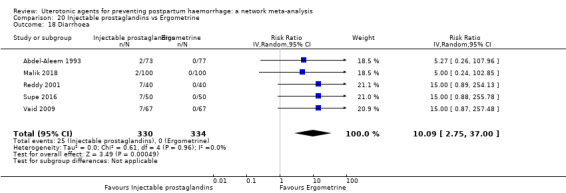
Comparison 20 Injectable prostaglandins vs Ergometrine, Outcome 18 Diarrhoea.
Comparison 21. Injectable prostaglandins vs Ergometrine plus oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.17, 3.78] |
| 3 Blood transfusion | 1 | 69 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.17, 2.53] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.92, 1.79] |
| 7 Additional uterotonics | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.16, 7.36] |
| 8 Blood loss | 2 | 598 | Mean Difference (IV, Random, 95% CI) | ‐26.18 [‐104.63, 52.27] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 5.06 [1.12, 22.86] |
| 12 Vomiting | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.40, 2.13] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 641 | Risk Ratio (IV, Random, 95% CI) | 23.70 [7.55, 74.45] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
21.2. Analysis.
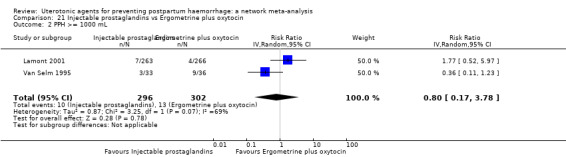
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 2 PPH >= 1000 mL.
21.3. Analysis.
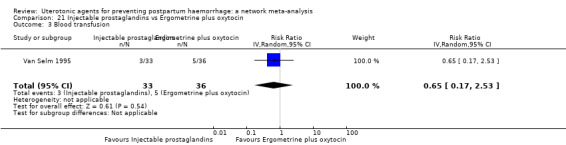
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 3 Blood transfusion.
21.6. Analysis.
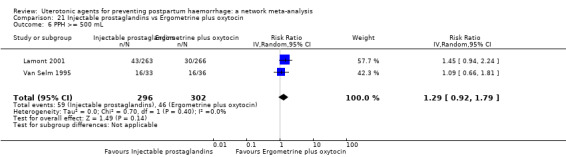
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 6 PPH >= 500 mL.
21.7. Analysis.
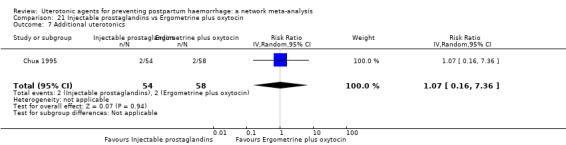
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 7 Additional uterotonics.
21.8. Analysis.
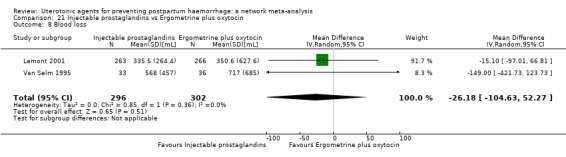
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 8 Blood loss.
21.11. Analysis.
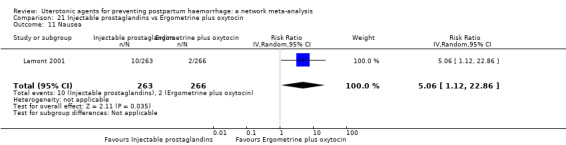
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 11 Nausea.
21.12. Analysis.
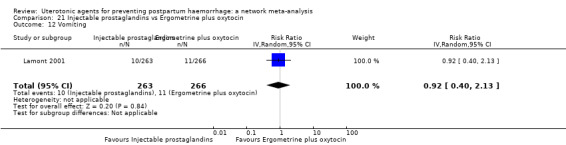
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 12 Vomiting.
21.18. Analysis.
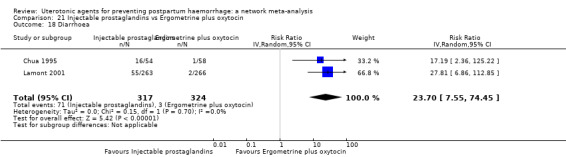
Comparison 21 Injectable prostaglandins vs Ergometrine plus oxytocin, Outcome 18 Diarrhoea.
Comparison 22. Misoprostol plus oxytocin vs Injectable prostaglandins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 23. Ergometrine vs Carbetocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 24. Carbetocin vs Ergometrine plus oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 4 | 1210 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.13, 0.86] |
| 3 Blood transfusion | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.42, 4.82] |
| 4 Severe maternal morbidity: intensive care admissions | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.44, 2.09] |
| 7 Additional uterotonics | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 8 Blood loss | 5 | 1330 | Mean Difference (IV, Random, 95% CI) | ‐44.08 [‐82.41, ‐5.75] |
| 9 Change in haemoglobin | 5 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐4.32, ‐0.82] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.19, 0.45] |
| 12 Vomiting | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.16, 0.46] |
| 13 Headache | 5 | 1330 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.45, 4.38] |
| 14 Abdominal pain | 4 | 930 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 15 Hypertension | 3 | 660 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.22, 1.39] |
| 16 Shivering | 5 | 1290 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.26, 0.80] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
24.1. Analysis.
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 1 Death.
24.2. Analysis.
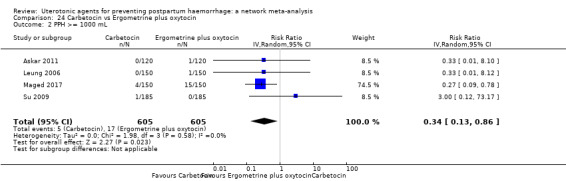
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 2 PPH >= 1000 mL.
24.3. Analysis.
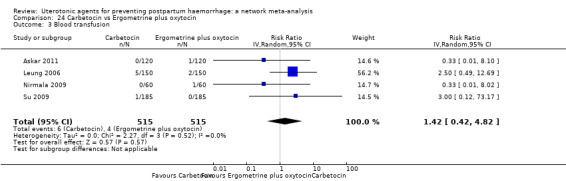
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 3 Blood transfusion.
24.4. Analysis.
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 4 Severe maternal morbidity: intensive care admissions.
24.6. Analysis.
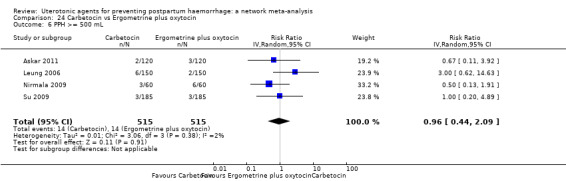
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 6 PPH >= 500 mL.
24.7. Analysis.
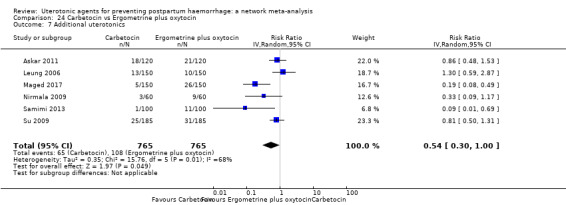
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 7 Additional uterotonics.
24.8. Analysis.
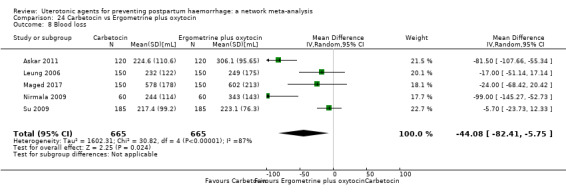
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 8 Blood loss.
24.9. Analysis.
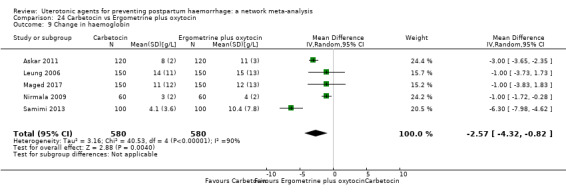
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 9 Change in haemoglobin.
24.11. Analysis.
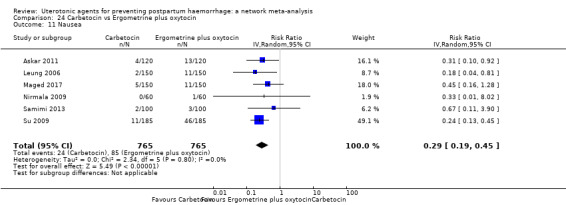
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 11 Nausea.
24.12. Analysis.
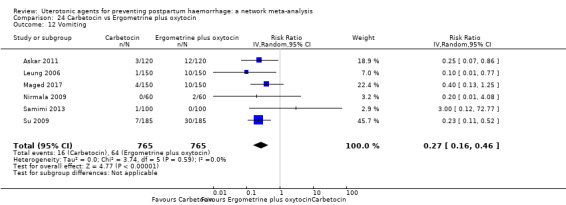
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 12 Vomiting.
24.13. Analysis.
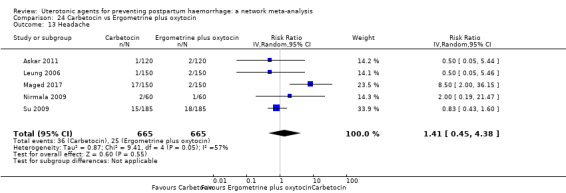
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 13 Headache.
24.14. Analysis.
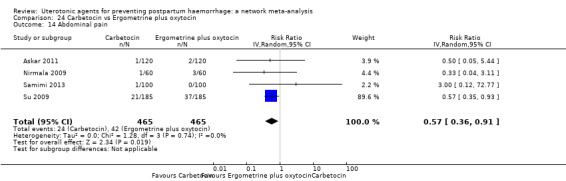
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 14 Abdominal pain.
24.15. Analysis.
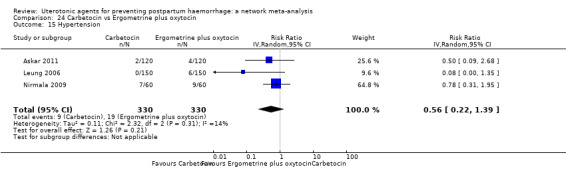
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 15 Hypertension.
24.16. Analysis.
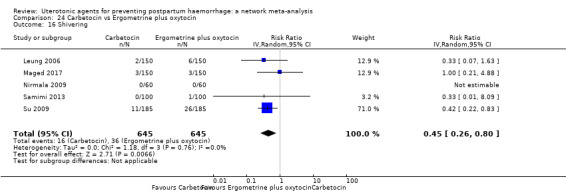
Comparison 24 Carbetocin vs Ergometrine plus oxytocin, Outcome 16 Shivering.
Comparison 25. Misoprostol plus oxytocin vs Carbetocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.45, 35.46] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.74, 1.93] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 380 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.72, 0.32] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.62, 2.39] |
| 12 Vomiting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.58, 3.09] |
| 13 Headache | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.37, 10.79] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 7.83 [3.43, 17.88] |
| 17 Fever | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 8.5 [1.99, 36.28] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
25.1. Analysis.
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 1 Death.
25.3. Analysis.
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 3 Blood transfusion.
25.7. Analysis.
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 7 Additional uterotonics.
25.9. Analysis.
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 9 Change in haemoglobin.
25.11. Analysis.
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 11 Nausea.
25.12. Analysis.
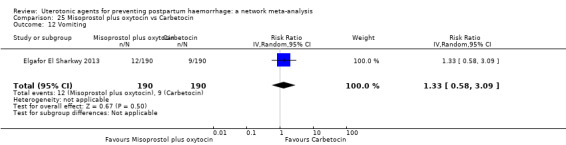
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 12 Vomiting.
25.13. Analysis.
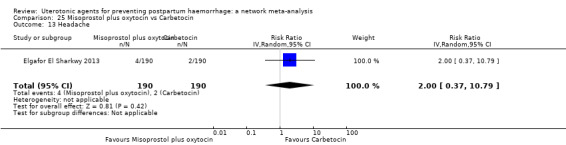
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 13 Headache.
25.16. Analysis.
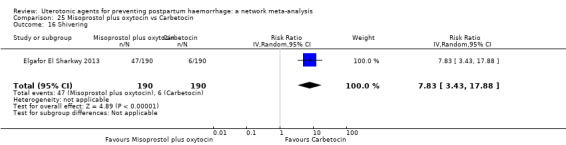
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 16 Shivering.
25.17. Analysis.
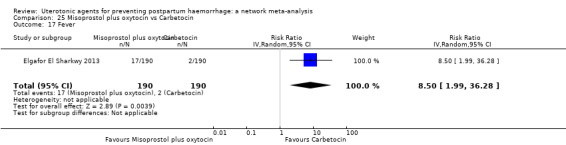
Comparison 25 Misoprostol plus oxytocin vs Carbetocin, Outcome 17 Fever.
Comparison 26. Ergometrine vs Ergometrine plus oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 144 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 20.10 [5.76, 34.44] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
26.6. Analysis.
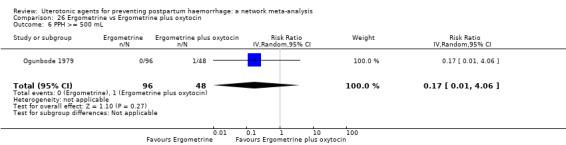
Comparison 26 Ergometrine vs Ergometrine plus oxytocin, Outcome 6 PPH >= 500 mL.
26.8. Analysis.

Comparison 26 Ergometrine vs Ergometrine plus oxytocin, Outcome 8 Blood loss.
Comparison 27. Misoprostol plus oxytocin vs Ergometrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 28. Misoprostol plus oxytocin vs Ergometrine plus oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.68, 2.94] |
| 3 Blood transfusion | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.36, 2.19] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.65, 2.99] |
| 7 Additional uterotonics | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.82, 2.69] |
| 8 Blood loss | 1 | 802 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐40.24, 8.24] |
| 9 Change in haemoglobin | 2 | 1605 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.72, 0.72] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.23, 4.77] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.95 [2.02, 4.29] |
| 17 Fever | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.89 [1.51, 5.54] |
| 18 Diarrhoea | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.46, 1.41] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
28.2. Analysis.
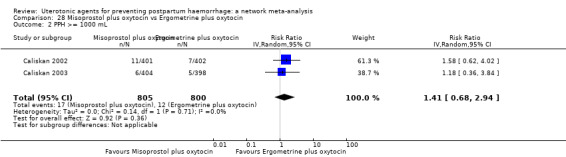
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 2 PPH >= 1000 mL.
28.3. Analysis.
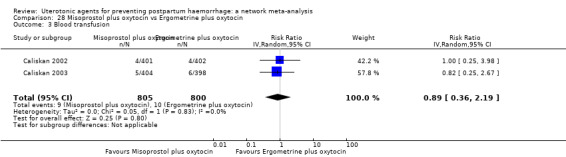
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 3 Blood transfusion.
28.6. Analysis.
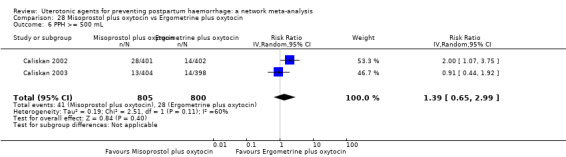
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 6 PPH >= 500 mL.
28.7. Analysis.
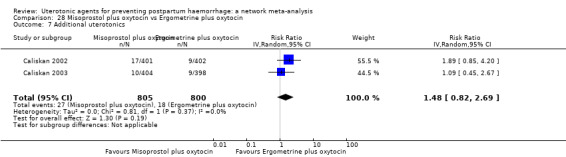
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 7 Additional uterotonics.
28.8. Analysis.
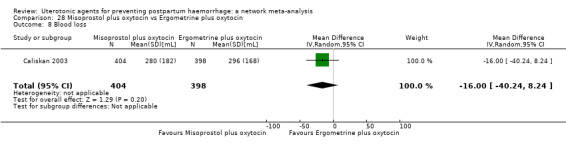
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 8 Blood loss.
28.9. Analysis.
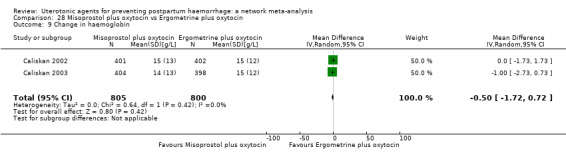
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 9 Change in haemoglobin.
28.12. Analysis.
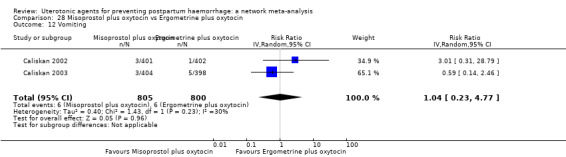
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 12 Vomiting.
28.16. Analysis.
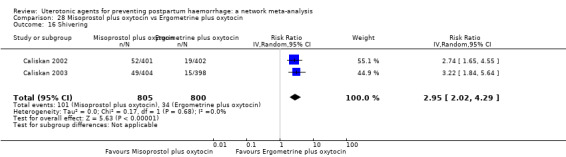
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 16 Shivering.
28.17. Analysis.
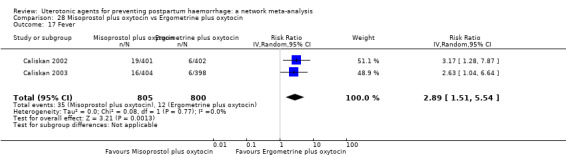
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 17 Fever.
28.18. Analysis.
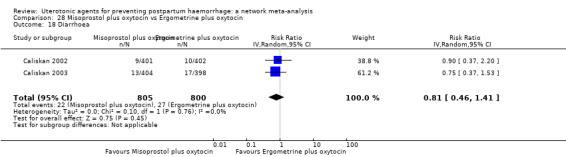
Comparison 28 Misoprostol plus oxytocin vs Ergometrine plus oxytocin, Outcome 18 Diarrhoea.
Comparison 29. Oxytocin vs Placebo or no treatment (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | 1920 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 3 | 1920 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 11 | 8782 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.51, 0.72] |
| 2.1 Vaginal birth | 10 | 8731 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.51, 0.72] |
| 2.2 Caesarean | 1 | 51 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 8 | 6717 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.51, 1.12] |
| 3.1 Vaginal birth | 7 | 6643 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.51, 1.12] |
| 3.2 Caesarean | 1 | 74 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 1950 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 1 | 1950 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 9 | 7021 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.52, 0.71] |
| 6.1 Vaginal birth | 8 | 6970 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.52, 0.72] |
| 6.2 Caesarean | 1 | 51 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.17, 1.40] |
| 7 Additional uterotonics | 8 | 5047 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.32, 0.58] |
| 7.1 Vaginal birth | 6 | 4922 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.35, 0.66] |
| 7.2 Caesarean | 2 | 125 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.14, 0.44] |
| 8 Blood loss | 8 | 3521 | Mean Difference (IV, Random, 95% CI) | ‐118.52 [‐141.40, ‐95.64] |
| 8.1 Vaginal birth | 6 | 3396 | Mean Difference (IV, Random, 95% CI) | ‐121.22 [‐144.50, ‐97.94] |
| 8.2 Caesarean | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐42.70 [‐166.12, 80.72] |
| 9 Change in haemoglobin | 5 | 2304 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐4.47, ‐0.89] |
| 9.1 Vaginal birth | 4 | 2253 | Mean Difference (IV, Random, 95% CI) | ‐3.19 [‐4.98, ‐1.40] |
| 9.2 Caesarean | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐3.51, 3.11] |
| 10 Breastfeeding | 1 | 1540 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 10.1 Vaginal birth | 1 | 1540 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 3 | 1788 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.47, 1.42] |
| 11.1 Vaginal birth | 2 | 1714 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.49, 1.51] |
| 11.2 Caesarean | 1 | 74 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.02, 3.83] |
| 12 Vomiting | 3 | 2150 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.44, 4.41] |
| 12.1 Vaginal birth | 2 | 2076 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.44, 4.41] |
| 12.2 Caesarean | 1 | 74 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 2 | 1704 | Risk Ratio (IV, Random, 95% CI) | 1.56 [0.52, 4.74] |
| 13.1 Vaginal birth | 1 | 1653 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.63, 2.51] |
| 13.2 Caesarean | 1 | 51 | Risk Ratio (IV, Random, 95% CI) | 6.74 [0.37, 124.21] |
| 14 Abdominal pain | 1 | 1665 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 14.1 Vaginal birth | 1 | 1665 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Ceasarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal sense of well‐being | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth ‐ Women’s perceptions of well‐being at 3 months postpartum: Less energy than before birth | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Vaginal birth ‐ Women’s perceptions of well‐being at 3 months postpartum: Experiencing (some) fatigue | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth ‐ Did management influence positively the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Vaginal birth ‐ Did management influence negatively the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Vaginal birth ‐ Did management make no difference in the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
29.1. Analysis.
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 1 Death.
29.2. Analysis.
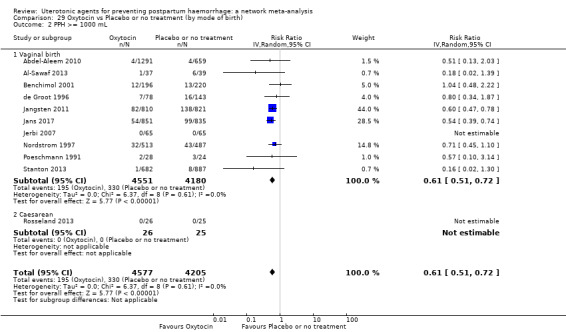
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 2 PPH >= 1000 mL.
29.3. Analysis.
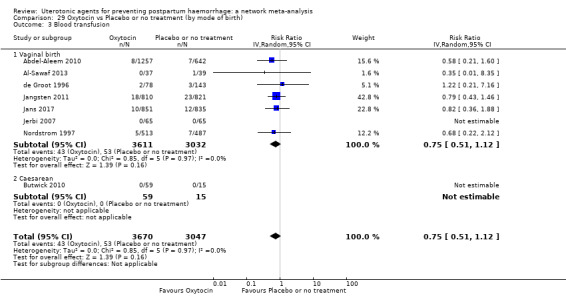
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 3 Blood transfusion.
29.4. Analysis.
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
29.6. Analysis.
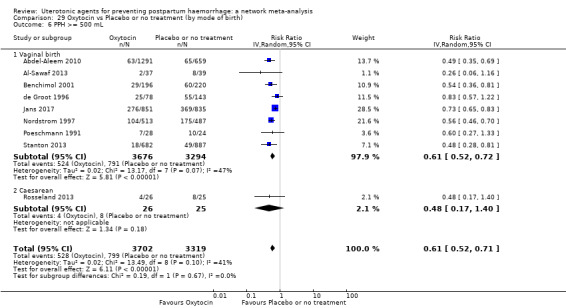
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 6 PPH >= 500 mL.
29.7. Analysis.
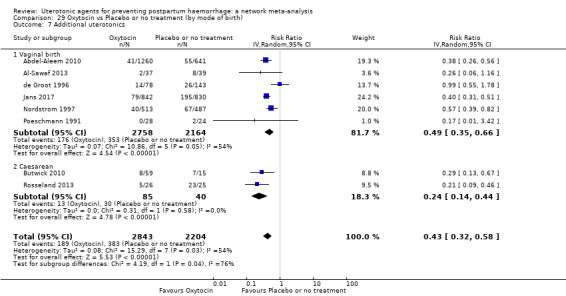
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 7 Additional uterotonics.
29.8. Analysis.
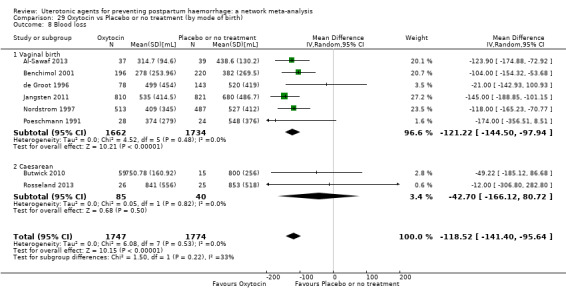
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 8 Blood loss.
29.9. Analysis.
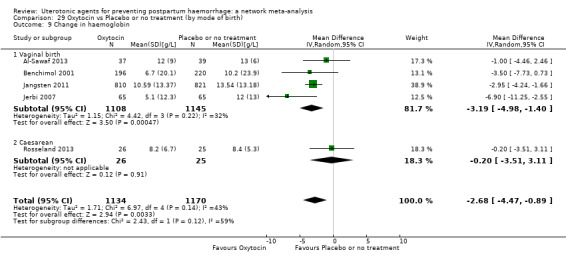
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 9 Change in haemoglobin.
29.10. Analysis.
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 10 Breastfeeding.
29.11. Analysis.
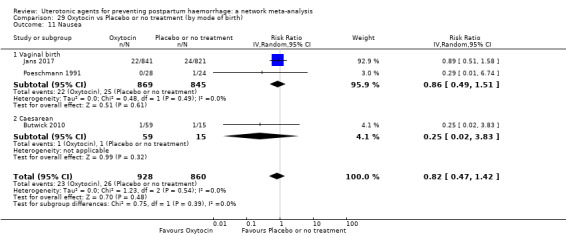
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 11 Nausea.
29.12. Analysis.
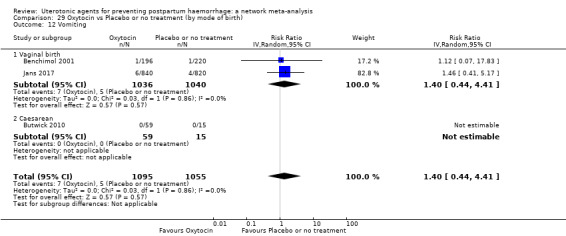
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 12 Vomiting.
29.13. Analysis.

Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 13 Headache.
29.14. Analysis.
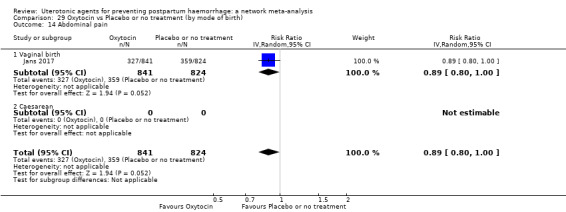
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 14 Abdominal pain.
29.16. Analysis.
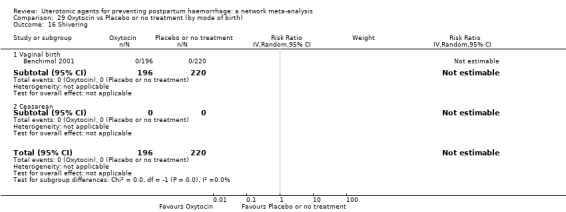
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 16 Shivering.
29.17. Analysis.
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 17 Fever.
29.19. Analysis.
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 19 Maternal sense of well‐being.
29.20. Analysis.
Comparison 29 Oxytocin vs Placebo or no treatment (by mode of birth), Outcome 20 Maternal satisfaction.
Comparison 30. Carbetocin vs Placebo or no treatment (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.30, 1.85] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.30, 1.85] |
| 7 Additional uterotonics | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.12, 0.32] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.12, 0.32] |
| 8 Blood loss | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐274.0 [‐591.60, 43.60] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐274.0 [‐591.60, 43.60] |
| 9 Change in haemoglobin | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.23, 0.43] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.23, 0.43] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
30.2. Analysis.
Comparison 30 Carbetocin vs Placebo or no treatment (by mode of birth), Outcome 2 PPH >= 1000 mL.
30.6. Analysis.

Comparison 30 Carbetocin vs Placebo or no treatment (by mode of birth), Outcome 6 PPH >= 500 mL.
30.7. Analysis.
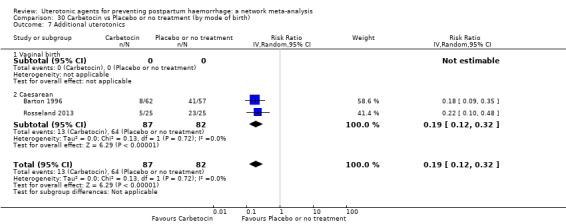
Comparison 30 Carbetocin vs Placebo or no treatment (by mode of birth), Outcome 7 Additional uterotonics.
30.8. Analysis.
Comparison 30 Carbetocin vs Placebo or no treatment (by mode of birth), Outcome 8 Blood loss.
30.9. Analysis.
Comparison 30 Carbetocin vs Placebo or no treatment (by mode of birth), Outcome 9 Change in haemoglobin.
30.13. Analysis.
Comparison 30 Carbetocin vs Placebo or no treatment (by mode of birth), Outcome 13 Headache.
Comparison 31. Misoprostol vs Placebo or no treatment (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 3497 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.10, 9.59] |
| 1.1 Vaginal birth | 4 | 3497 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.10, 9.59] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 8 | 5467 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.56, 0.95] |
| 2.1 Vaginal birth | 8 | 5467 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.56, 0.95] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 6 | 3134 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.15, 1.47] |
| 3.1 Vaginal birth | 6 | 3134 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.15, 1.47] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 1620 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.14, 7.05] |
| 4.1 Vaginal birth | 1 | 1620 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.14, 7.05] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 7 | 4047 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 6.1 Vaginal birth | 7 | 4047 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 8 | 3746 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.52, 0.87] |
| 7.1 Vaginal birth | 8 | 3746 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.52, 0.87] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 8 | 4146 | Mean Difference (IV, Random, 95% CI) | ‐42.07 [‐52.47, ‐31.68] |
| 8.1 Vaginal birth | 8 | 4146 | Mean Difference (IV, Random, 95% CI) | ‐42.07 [‐52.47, ‐31.68] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 5 | 2334 | Mean Difference (IV, Random, 95% CI) | ‐2.53 [‐3.53, ‐1.52] |
| 9.1 Vaginal birth | 5 | 2334 | Mean Difference (IV, Random, 95% CI) | ‐2.53 [‐3.53, ‐1.52] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 4 | 3997 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.78, 1.78] |
| 11.1 Vaginal birth | 4 | 3997 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.78, 1.78] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 6 | 4949 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.92, 2.16] |
| 12.1 Vaginal birth | 6 | 4949 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.92, 2.16] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 1116 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.32, 2.77] |
| 13.1 Vaginal birth | 1 | 1116 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.32, 2.77] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 4 | 1894 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.29, 3.37] |
| 14.1 Vaginal birth | 4 | 1894 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.29, 3.37] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 9 | 5286 | Risk Ratio (IV, Random, 95% CI) | 2.94 [2.38, 3.64] |
| 16.1 Vaginal birth | 9 | 5286 | Risk Ratio (IV, Random, 95% CI) | 2.94 [2.38, 3.64] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 5 | 4396 | Risk Ratio (IV, Random, 95% CI) | 4.09 [2.01, 8.32] |
| 17.1 Vaginal birth | 5 | 4396 | Risk Ratio (IV, Random, 95% CI) | 4.09 [2.01, 8.32] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 6 | 4791 | Risk Ratio (IV, Random, 95% CI) | 2.80 [1.21, 6.49] |
| 18.1 Vaginal birth | 6 | 4791 | Risk Ratio (IV, Random, 95% CI) | 2.80 [1.21, 6.49] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
31.1. Analysis.
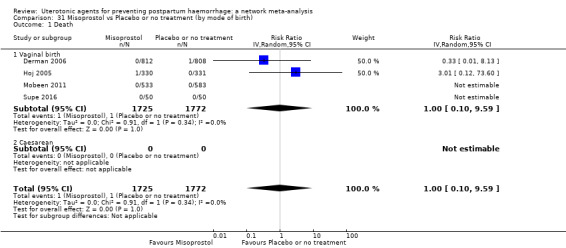
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 1 Death.
31.2. Analysis.
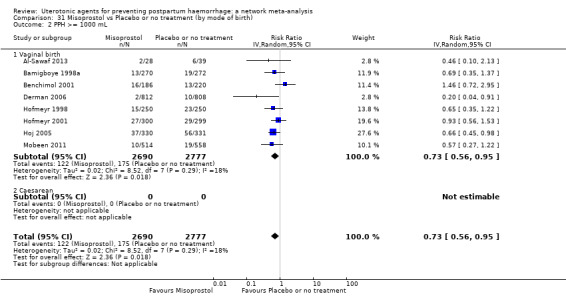
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 2 PPH >= 1000 mL.
31.3. Analysis.
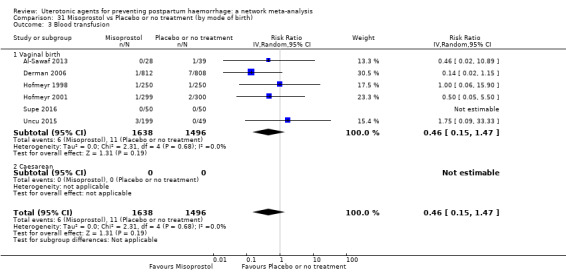
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 3 Blood transfusion.
31.4. Analysis.
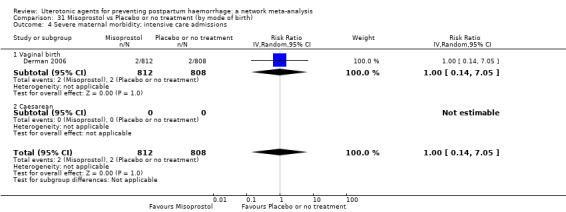
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
31.6. Analysis.
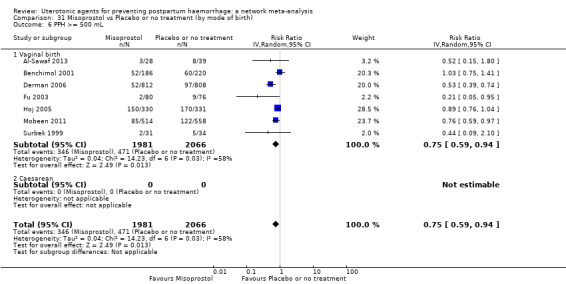
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 6 PPH >= 500 mL.
31.7. Analysis.
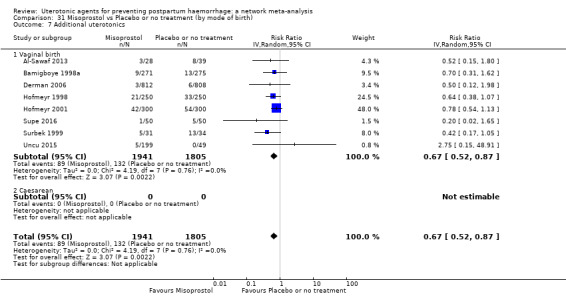
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 7 Additional uterotonics.
31.8. Analysis.
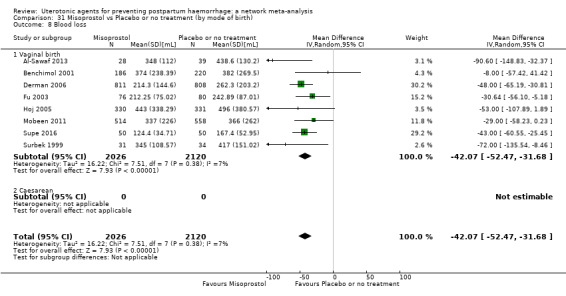
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 8 Blood loss.
31.9. Analysis.
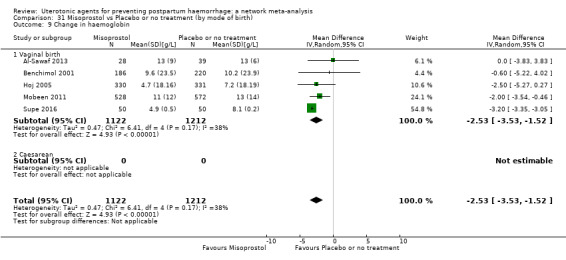
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 9 Change in haemoglobin.
31.11. Analysis.
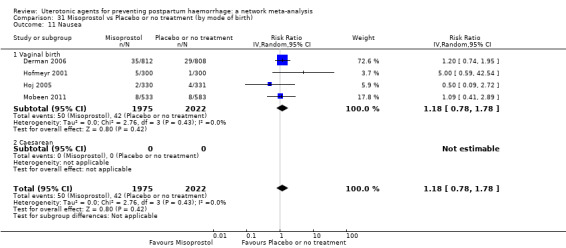
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 11 Nausea.
31.12. Analysis.
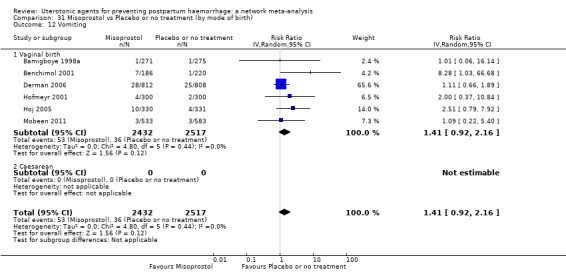
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 12 Vomiting.
31.13. Analysis.
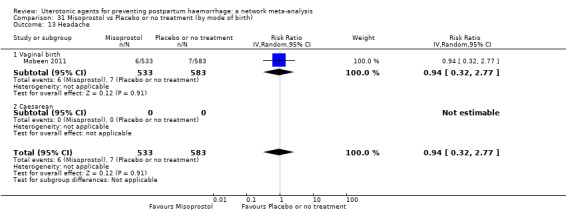
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 13 Headache.
31.14. Analysis.
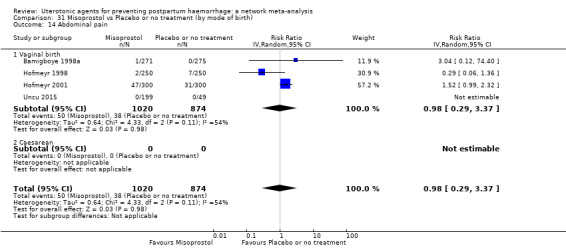
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 14 Abdominal pain.
31.16. Analysis.
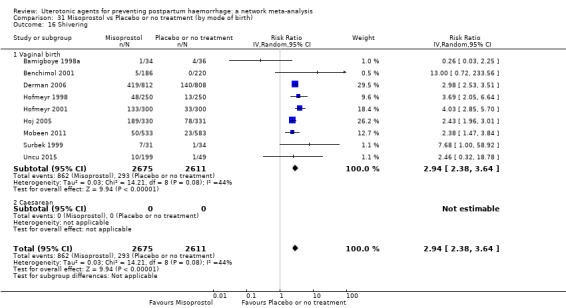
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 16 Shivering.
31.17. Analysis.
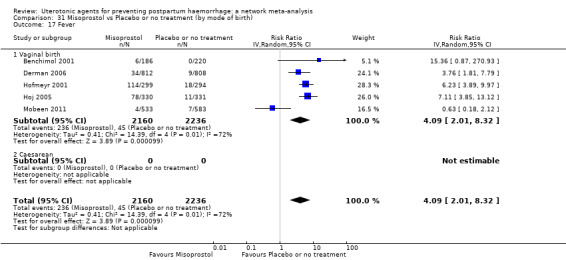
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 17 Fever.
31.18. Analysis.
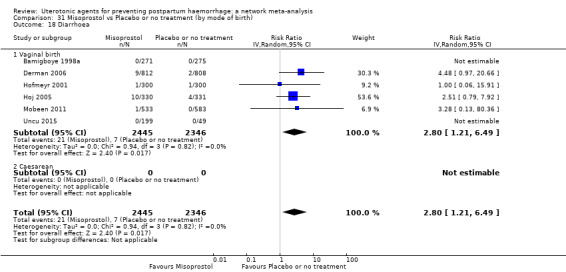
Comparison 31 Misoprostol vs Placebo or no treatment (by mode of birth), Outcome 18 Diarrhoea.
Comparison 32. Injectable prostaglandins vs Placebo or no treatment (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.04, 3.24] |
| 2.1 Vaginal birth | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.04, 3.24] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 vaginal birth | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.22, 1.35] |
| 6.1 Vaginal birth | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.22, 1.35] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 2 | 146 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.21, 2.09] |
| 7.1 Vaginal birth | 2 | 146 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.21, 2.09] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐95.17 [‐296.09, 105.75] |
| 8.1 Vaginal birth | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐95.17 [‐296.09, 105.75] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.56, 1.24] |
| 9.1 Vaginal birth | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.56, 1.24] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.02, 8.46] |
| 11.1 Vaginal birth | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.02, 8.46] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
32.1. Analysis.
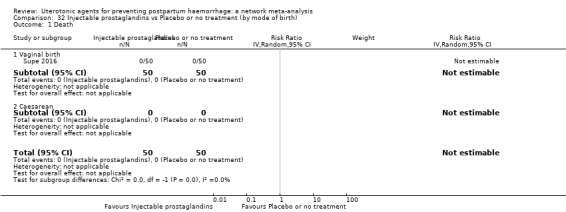
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 1 Death.
32.2. Analysis.
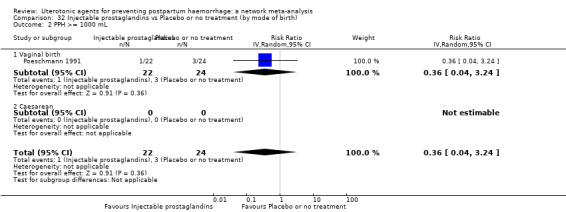
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 2 PPH >= 1000 mL.
32.3. Analysis.
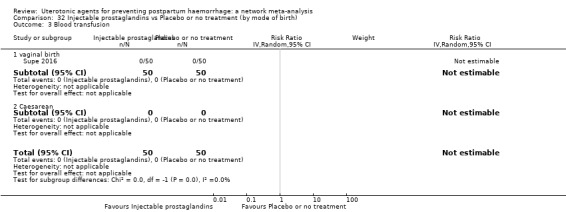
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 3 Blood transfusion.
32.6. Analysis.
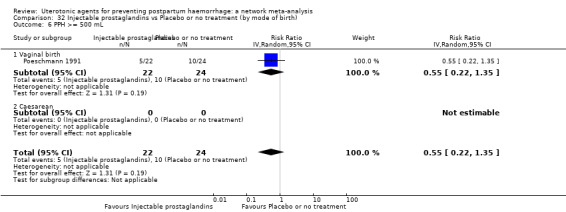
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 6 PPH >= 500 mL.
32.7. Analysis.
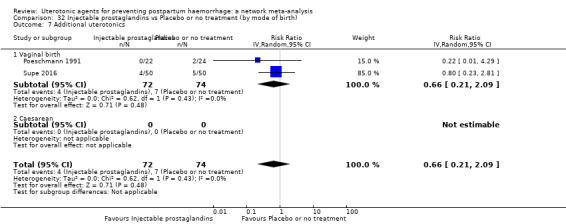
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 7 Additional uterotonics.
32.8. Analysis.
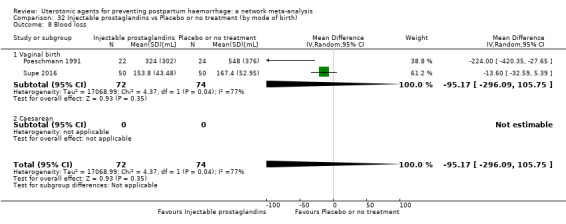
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 8 Blood loss.
32.9. Analysis.
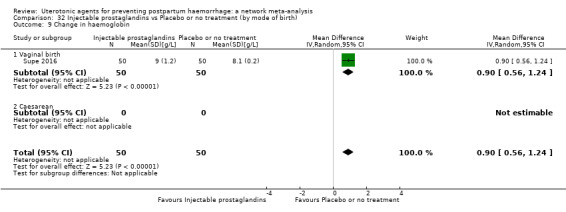
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 9 Change in haemoglobin.
32.11. Analysis.
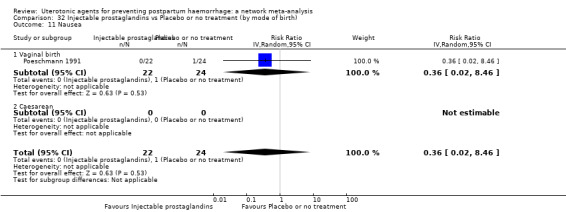
Comparison 32 Injectable prostaglandins vs Placebo or no treatment (by mode of birth), Outcome 11 Nausea.
Comparison 33. Ergometrine vs Placebo or no treatment (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| 2.1 Vaginal birth | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.04, 3.28] |
| 3.1 Vaginal birth | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.04, 3.28] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.14, 0.42] |
| 6.1 Vaginal birth | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.14, 0.42] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.37] |
| 7.1 Vaginal birth | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.37] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 1529 | Mean Difference (IV, Random, 95% CI) | ‐50.64 [‐119.92, 18.65] |
| 8.1 Vaginal birth | 2 | 1529 | Mean Difference (IV, Random, 95% CI) | ‐50.64 [‐119.92, 18.65] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 9.1 Vaginal birth | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 42.10 [2.55, 694.80] |
| 11.1 Vaginal birth | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 42.10 [2.55, 694.80] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 25.67 [1.52, 432.78] |
| 12.1 Vaginal birth | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 25.67 [1.52, 432.78] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [0.37, 138.91] |
| 13.1 Vaginal birth | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [0.37, 138.91] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.04, 4.08] |
| 14.1 Vaginal birth | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.04, 4.08] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [2.83, 18.24] |
| 15.1 Vaginal birth | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [2.83, 18.24] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Casarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
33.1. Analysis.
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 1 Death.
33.2. Analysis.
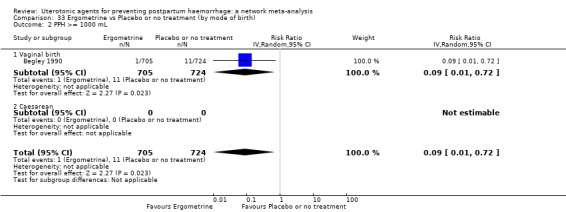
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 2 PPH >= 1000 mL.
33.3. Analysis.
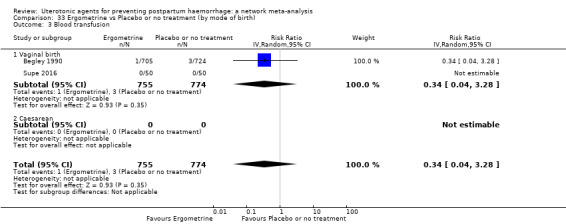
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 3 Blood transfusion.
33.6. Analysis.
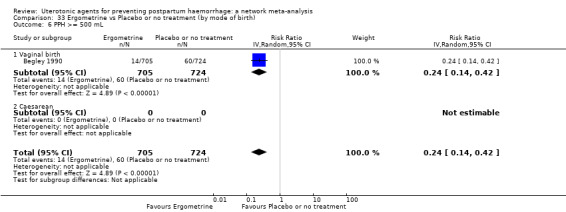
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 6 PPH >= 500 mL.
33.7. Analysis.
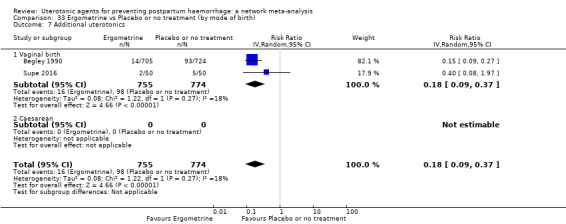
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 7 Additional uterotonics.
33.8. Analysis.
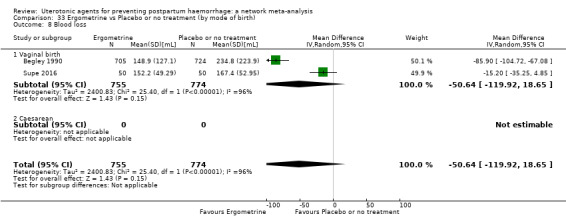
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 8 Blood loss.
33.9. Analysis.
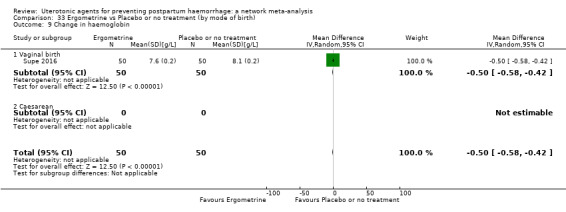
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 9 Change in haemoglobin.
33.11. Analysis.
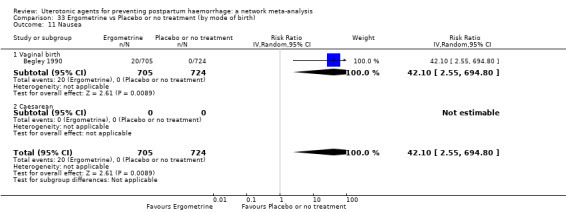
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 11 Nausea.
33.12. Analysis.
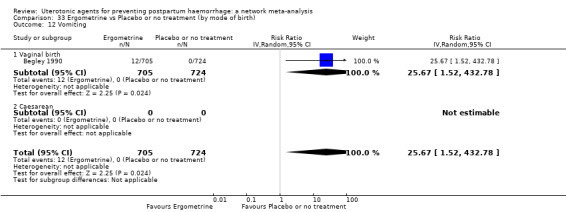
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 12 Vomiting.
33.13. Analysis.
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 13 Headache.
33.14. Analysis.

Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 14 Abdominal pain.
33.15. Analysis.
Comparison 33 Ergometrine vs Placebo or no treatment (by mode of birth), Outcome 15 Hypertension.
Comparison 34. Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.18, 1.05] |
| 2.1 Vaginal birth | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.18, 1.05] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.18, 0.66] |
| 3.1 Vaginal birth | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.18, 0.66] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.30, 0.46] |
| 6.1 Vaginal birth | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.30, 0.46] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.15, 0.24] |
| 7.1 Vaginal birth | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.15, 0.24] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 1705 | Mean Difference (IV, Random, 95% CI) | ‐35.02 [‐101.63, 31.59] |
| 8.1 Vaginal birth | 2 | 1705 | Mean Difference (IV, Random, 95% CI) | ‐35.02 [‐101.63, 31.59] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 1454 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐6.50, ‐0.63] |
| 9.1 Vaginal birth | 2 | 1454 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐6.50, ‐0.63] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 10.1 Vaginal birth | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 1512 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.38, 2.76] |
| 11.1 Vaginal birth | 1 | 1512 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.38, 2.76] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 2.15 [1.46, 3.18] |
| 12.1 Vaginal delivery | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 2.15 [1.46, 3.18] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.78, 3.48] |
| 13.1 Vaginal birth | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.78, 3.48] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth ‐ General health at 6 weeks postpartum (Worse than prepregnancy) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Vaginal birth ‐ General health at 6 weeks postpartum (Exhausted since birth) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Vaginal birth ‐ General health at 6 weeks postpartum (Exhausted at 6 weeks) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.4 Vaginal birth ‐ General health at 6 weeks postpartum (Blues) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.5 Vaginal birth ‐ General health at 6 weeks postpartum (Depressed) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.6 Vaginal birth ‐ General health at 6 weeks postpartum (Help for depression) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.7 Vaginal birth ‐ General health at 6 weeks postpartum (Admission to hospital for depression) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.8 Vaginal birth ‐ General health at 6 weeks postpartum (No health problems reported) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.9 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth ‐ Satisfied with third‐stage management | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Vaginal birth ‐ Felt in control during third stage | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
34.2. Analysis.
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 2 PPH >= 1000 mL.
34.3. Analysis.
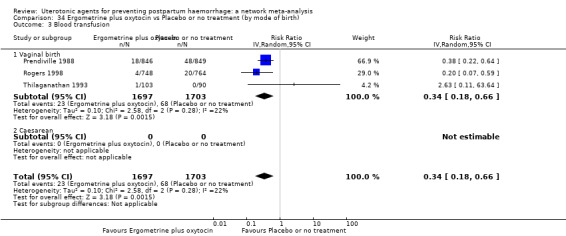
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 3 Blood transfusion.
34.6. Analysis.
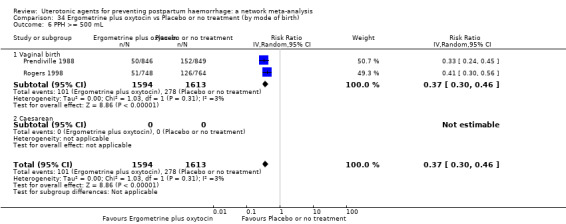
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 6 PPH >= 500 mL.
34.7. Analysis.
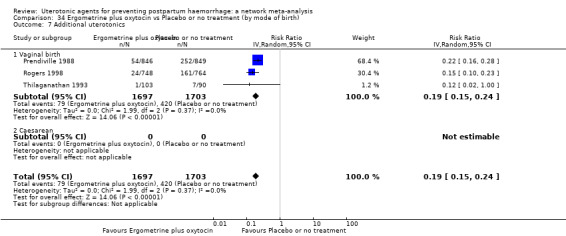
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 7 Additional uterotonics.
34.8. Analysis.
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 8 Blood loss.
34.9. Analysis.
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 9 Change in haemoglobin.
34.10. Analysis.
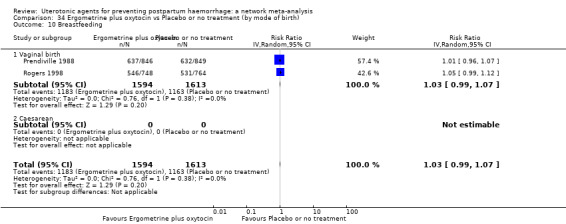
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 10 Breastfeeding.
34.11. Analysis.
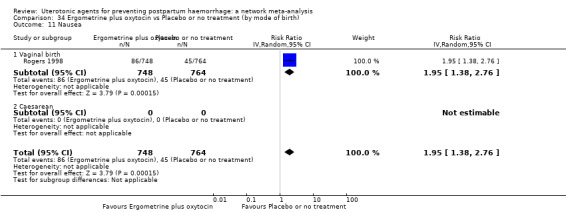
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 11 Nausea.
34.12. Analysis.
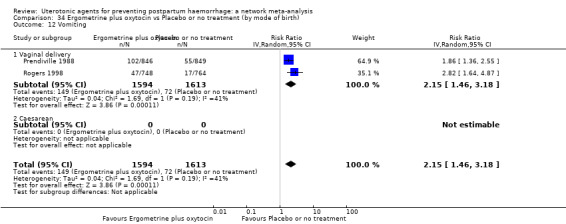
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 12 Vomiting.
34.13. Analysis.
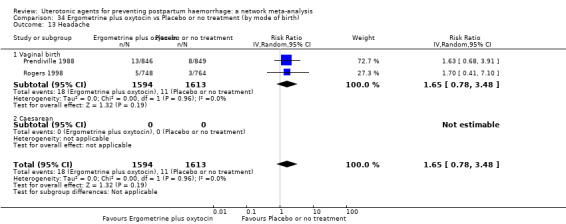
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 13 Headache.
34.19. Analysis.
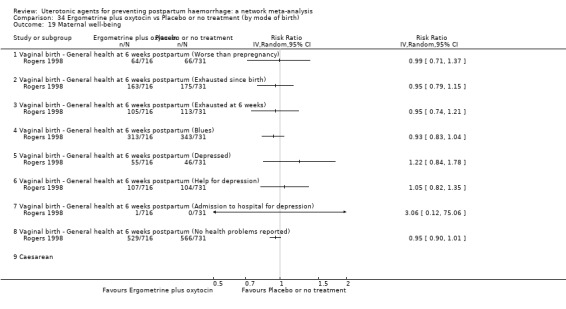
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 19 Maternal well‐being.
34.20. Analysis.
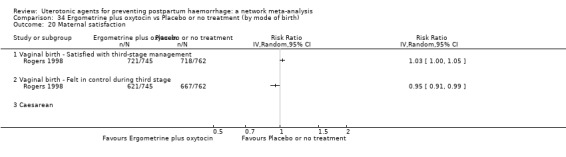
Comparison 34 Ergometrine plus oxytocin vs Placebo or no treatment (by mode of birth), Outcome 20 Maternal satisfaction.
Comparison 35. Misoprostol plus oxytocin vs Placebo or no treatment (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginl birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 36. Misoprostol vs Oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 24 | 28520 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.14, 2.74] |
| 1.1 Vaginal birth | 21 | 27955 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.14, 3.95] |
| 1.2 Caesarean | 3 | 565 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.09] |
| 2 PPH >= 1000 mL | 38 | 34261 | Risk Ratio (IV, Random, 95% CI) | 1.26 [1.11, 1.43] |
| 2.1 Vaginal birth | 31 | 33496 | Risk Ratio (IV, Random, 95% CI) | 1.31 [1.15, 1.49] |
| 2.2 Caesarean | 7 | 765 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.54, 1.26] |
| 3 Blood transfusion | 42 | 35467 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 3.1 Vaginal birth | 34 | 34417 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.67, 1.03] |
| 3.2 Caesarean | 8 | 1050 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.19, 1.21] |
| 4 Severe maternal morbidity: intensive care admissions | 10 | 21698 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.55, 2.43] |
| 4.1 Vaginal birth | 9 | 21508 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.55, 2.43] |
| 4.2 Caesarean | 1 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 44 | 36920 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 6.1 Vaginal birth | 38 | 36215 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.90, 1.31] |
| 6.2 Caesarean | 6 | 705 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.92, 1.25] |
| 7 Additional uterotonics | 47 | 35981 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 7.1 Vaginal birth | 35 | 34521 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.86, 1.30] |
| 7.2 Caesarean | 12 | 1460 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.69, 1.16] |
| 8 Blood loss | 43 | 35239 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐23.45, 5.65] |
| 8.1 Vaginal birth | 34 | 34339 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐16.92, 12.77] |
| 8.2 Caesarean | 9 | 900 | Mean Difference (IV, Random, 95% CI) | ‐59.79 [‐89.04, ‐30.54] |
| 9 Change in haemoglobin | 31 | 12028 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.74, 0.47] |
| 9.1 Vaginal birth | 24 | 11240 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.62, 0.62] |
| 9.2 Caesarean | 7 | 788 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.26, 0.73] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 33 | 29732 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.93, 1.60] |
| 11.1 Vaginal birth | 26 | 28907 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.94, 1.79] |
| 11.2 Caesarean | 7 | 825 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.61, 1.54] |
| 12 Vomiting | 41 | 32687 | Risk Ratio (IV, Random, 95% CI) | 1.51 [1.19, 1.91] |
| 12.1 Vaginal birth | 30 | 31402 | Risk Ratio (IV, Random, 95% CI) | 1.82 [1.43, 2.33] |
| 12.2 Caesarean | 11 | 1285 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.55, 1.45] |
| 13 Headache | 10 | 4079 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.54, 1.42] |
| 13.1 Vaginal birth | 5 | 3494 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.99, 2.04] |
| 13.2 Caesarean | 5 | 585 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.19, 1.15] |
| 14 Abdominal pain | 8 | 3382 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 14.1 Vaginal birth | 7 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.73, 1.04] |
| 14.2 Caesarean | 1 | 175 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.78, 1.37] |
| 15 Hypertension | 3 | 1028 | Risk Ratio (IV, Random, 95% CI) | 3.64 [0.60, 22.27] |
| 15.1 Vaginal birth | 2 | 828 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.44, 36.03] |
| 15.2 Caesarean | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.12, 72.77] |
| 16 Shivering | 49 | 34865 | Risk Ratio (IV, Random, 95% CI) | 4.02 [3.23, 4.99] |
| 16.1 Vaginal birth | 36 | 33355 | Risk Ratio (IV, Random, 95% CI) | 4.16 [3.27, 5.29] |
| 16.2 Caesarean | 13 | 1510 | Risk Ratio (IV, Random, 95% CI) | 3.58 [2.06, 6.21] |
| 17 Fever | 41 | 33008 | Risk Ratio (IV, Random, 95% CI) | 3.75 [2.73, 5.15] |
| 17.1 Vaginal birth | 32 | 31858 | Risk Ratio (IV, Random, 95% CI) | 4.62 [3.33, 6.42] |
| 17.2 Caesarean | 9 | 1150 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.80, 2.87] |
| 18 Diarrhoea | 26 | 30733 | Risk Ratio (IV, Random, 95% CI) | 2.13 [1.55, 2.93] |
| 18.1 Vaginal birth | 26 | 30733 | Risk Ratio (IV, Random, 95% CI) | 2.13 [1.55, 2.93] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Satisfied or very satisfied with drug | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Complaints about or problems with drug | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Would take drug again after subsequent deliveries | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Would recommend drug to a friend | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
36.1. Analysis.
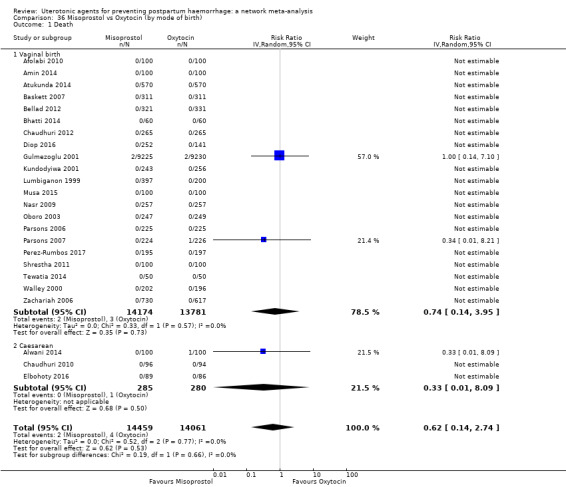
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 1 Death.
36.2. Analysis.
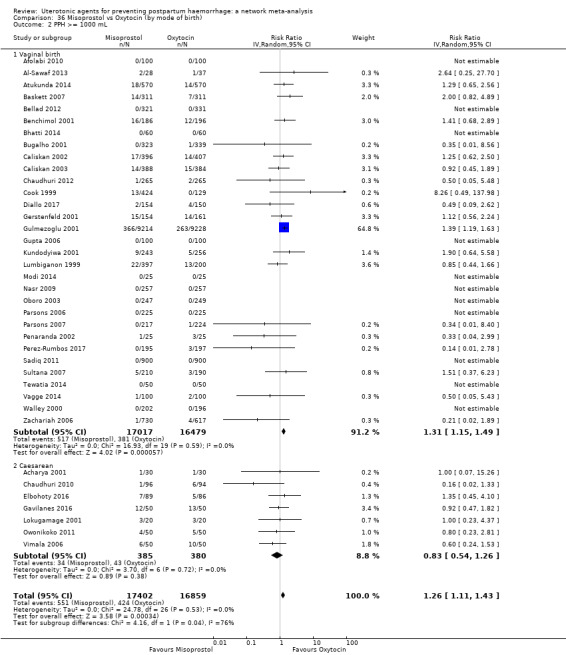
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
36.3. Analysis.
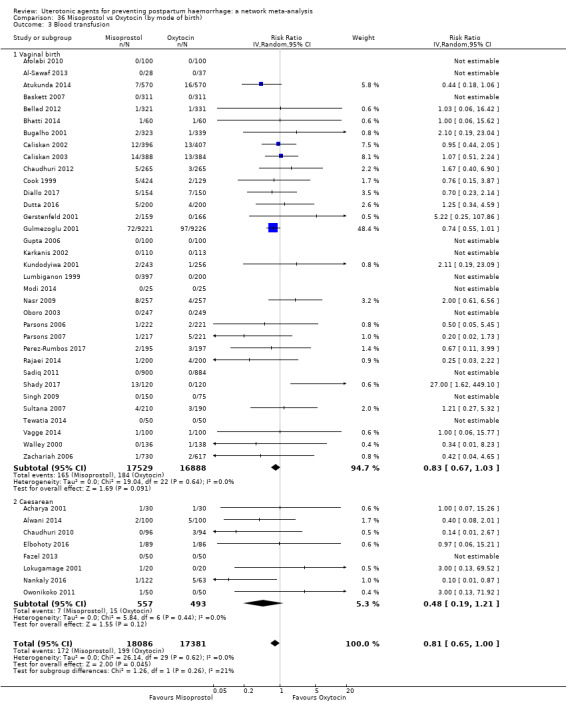
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 3 Blood transfusion.
36.4. Analysis.
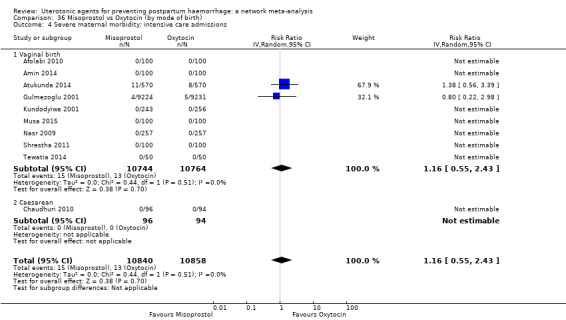
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
36.6. Analysis.
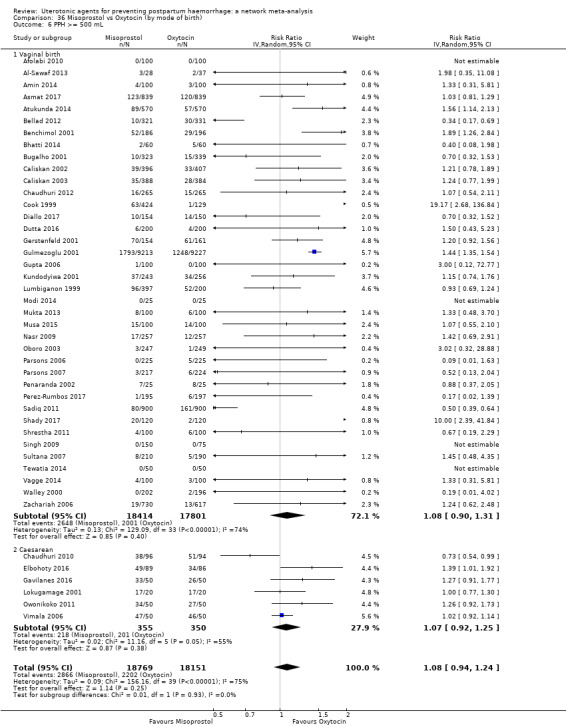
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
36.7. Analysis.
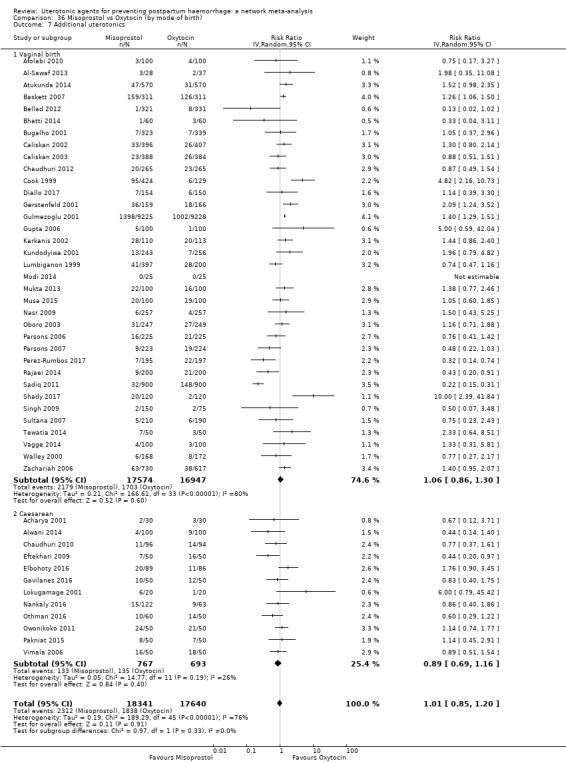
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
36.8. Analysis.
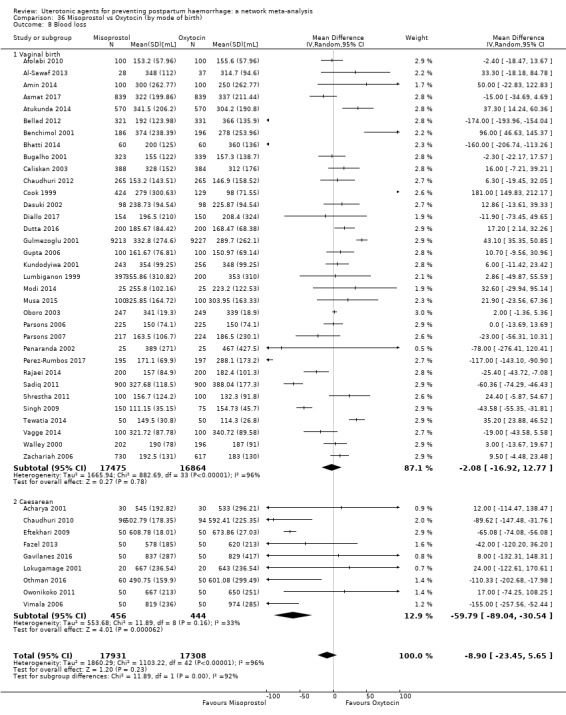
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 8 Blood loss.
36.9. Analysis.
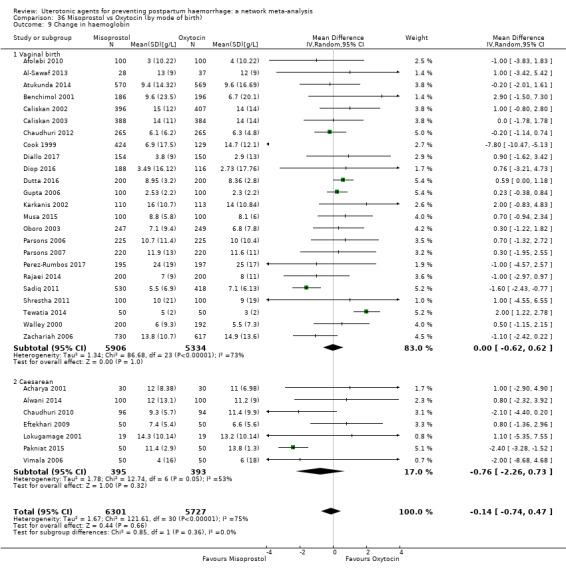
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
36.11. Analysis.
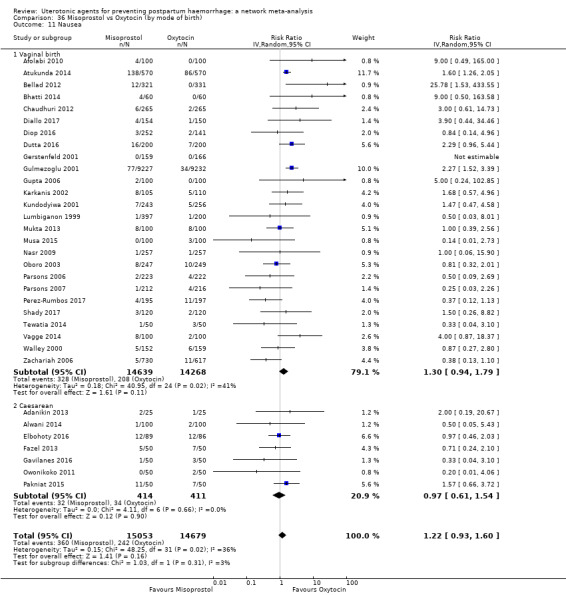
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 11 Nausea.
36.12. Analysis.
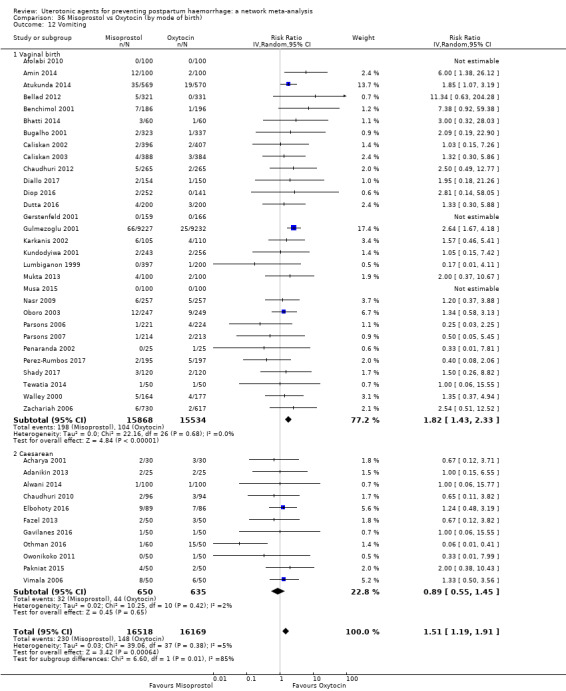
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 12 Vomiting.
36.13. Analysis.
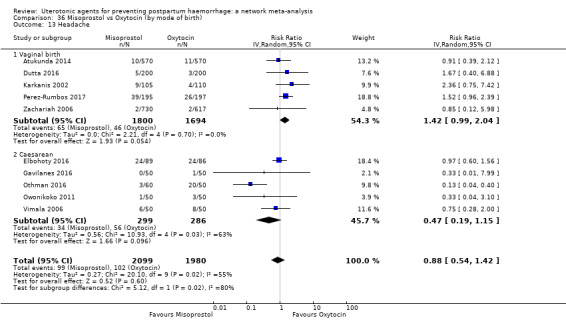
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 13 Headache.
36.14. Analysis.
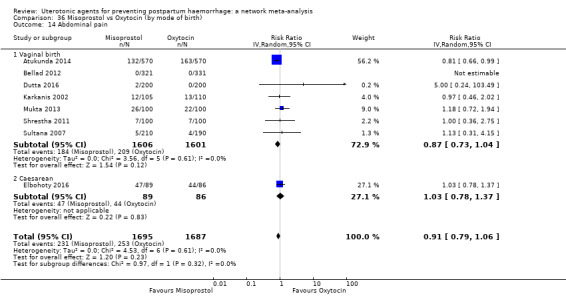
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 14 Abdominal pain.
36.15. Analysis.
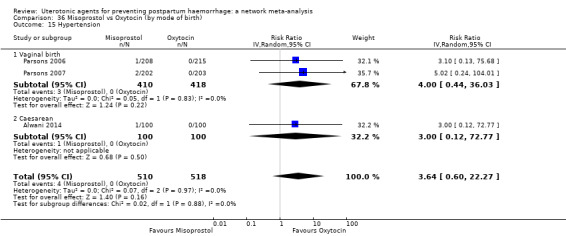
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 15 Hypertension.
36.16. Analysis.
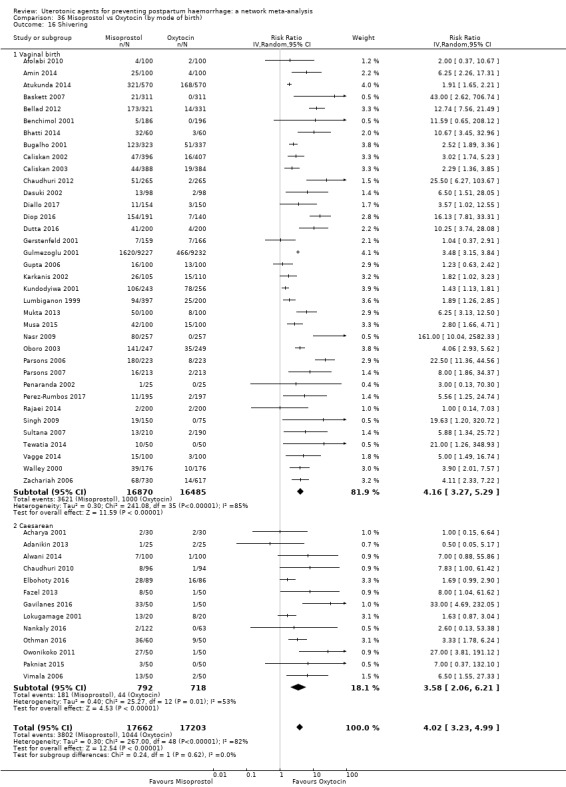
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 16 Shivering.
36.17. Analysis.
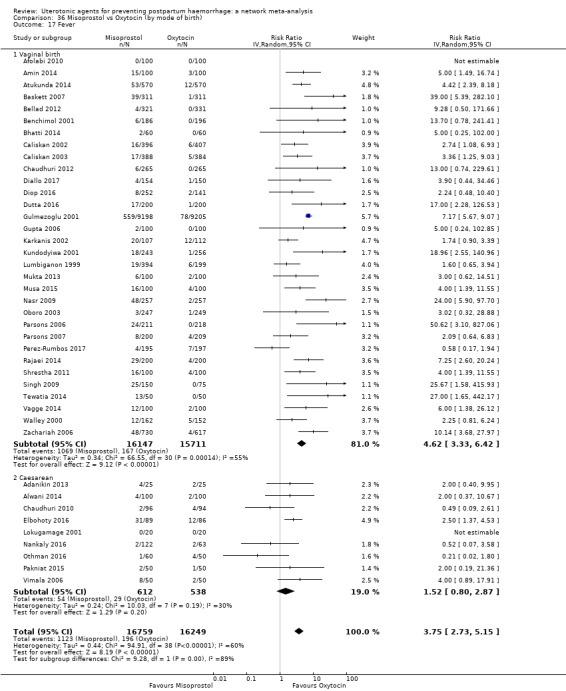
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 17 Fever.
36.18. Analysis.
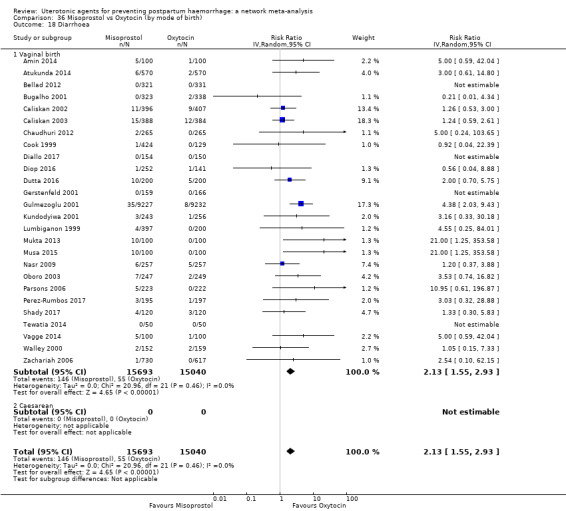
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 18 Diarrhoea.
36.20. Analysis.
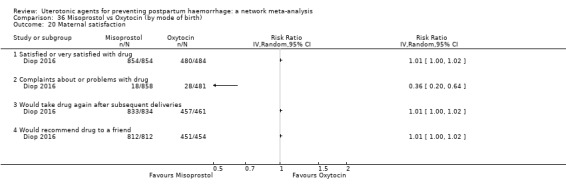
Comparison 36 Misoprostol vs Oxytocin (by mode of birth), Outcome 20 Maternal satisfaction.
Comparison 37. Injectable prostaglandins vs Oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.20, 10.31] |
| 2.1 Vaginal birth | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.20, 10.31] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.65] |
| 3.1 Vaginal birth | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.65] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.26, 2.71] |
| 6.1 Vaginal birth | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.26, 2.71] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.09, 0.94] |
| 7.1 Vaginal birth | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.09, 0.94] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐15.83 [‐152.28, 120.62] |
| 8.1 Vaginal birth | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐15.83 [‐152.28, 120.62] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 3 | 450 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.40, 3.41] |
| 11.1 Vaginal birth | 3 | 450 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.40, 3.41] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 2.48 [0.57, 10.73] |
| 12.1 Vaginal birth | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 2.48 [0.57, 10.73] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.01, 4.11] |
| 13.1 Vaginal birth | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.01, 4.11] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.11, 7.73] |
| 16.1 Vaginal birth | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.11, 7.73] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.18, 21.71] |
| 17.1 Vaginal birth | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.18, 21.71] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 10.38 [1.96, 54.98] |
| 18.1 Vaginal birth | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 10.38 [1.96, 54.98] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
37.1. Analysis.
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 1 Death.
37.2. Analysis.
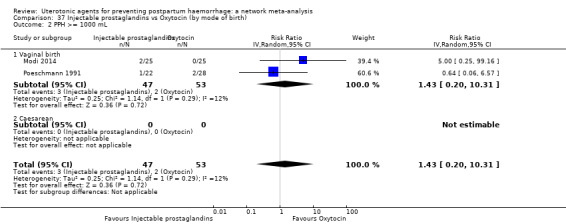
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
37.3. Analysis.
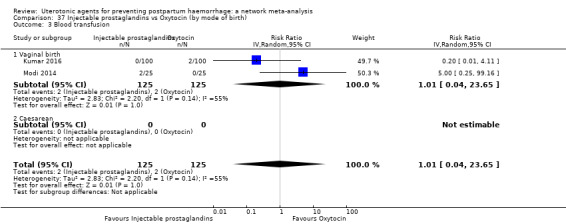
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 3 Blood transfusion.
37.6. Analysis.
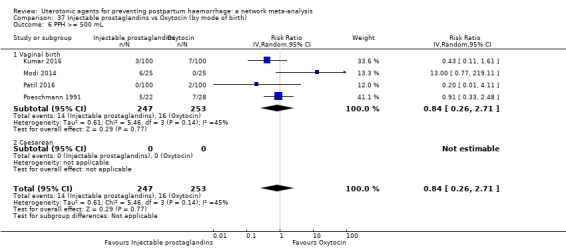
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
37.7. Analysis.
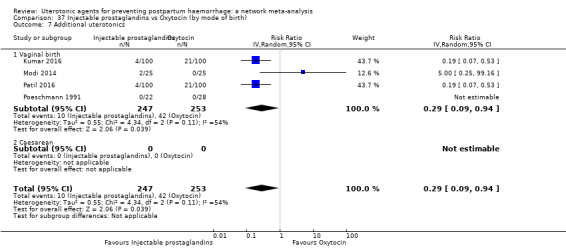
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
37.8. Analysis.
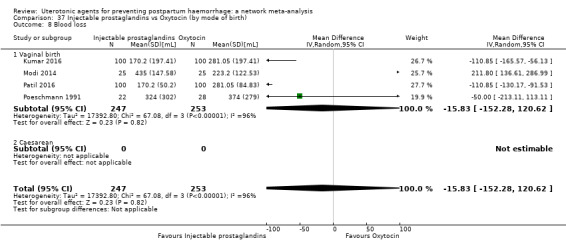
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 8 Blood loss.
37.11. Analysis.

Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 11 Nausea.
37.12. Analysis.
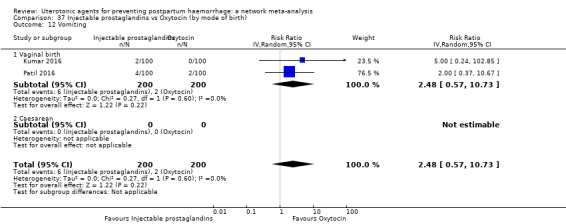
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 12 Vomiting.
37.13. Analysis.
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 13 Headache.
37.16. Analysis.
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 16 Shivering.
37.17. Analysis.
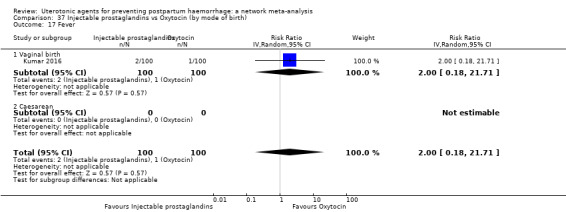
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 17 Fever.
37.18. Analysis.
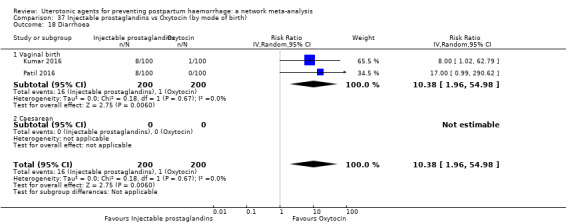
Comparison 37 Injectable prostaglandins vs Oxytocin (by mode of birth), Outcome 18 Diarrhoea.
Comparison 38. Carbetocin vs Oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 5 | 30327 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.37, 10.92] |
| 1.1 Vaginal birth | 1 | 29539 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.37, 10.92] |
| 1.2 Caesarean | 4 | 788 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 10 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.45, 1.19] |
| 2.1 Vaginal birth | 4 | 29682 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.21, 2.20] |
| 2.2 Caesarean | 6 | 951 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.31, 1.23] |
| 3 Blood transfusion | 16 | 32115 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.38, 1.22] |
| 3.1 Vaginal birth | 5 | 30028 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.52, 2.10] |
| 3.2 Caesarean | 10 | 2032 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.23, 1.10] |
| 3.3 Both caesarean and vaginal birth | 1 | 55 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.01, 2.93] |
| 4 Severe maternal morbidity: intensive care admissions | 2 | 29847 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.67, 2.02] |
| 4.1 Vaginal birth | 1 | 29470 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.65, 1.98] |
| 4.2 Caesarean | 1 | 377 | Risk Ratio (IV, Random, 95% CI) | 3.02 [0.12, 73.56] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 11 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.58, 0.98] |
| 6.1 Vaginal birth | 5 | 29955 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.34, 1.30] |
| 6.2 Caesarean | 6 | 678 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.47, 1.07] |
| 7 Additional uterotonics | 22 | 32699 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.34, 0.68] |
| 7.1 Vaginal birth | 6 | 30187 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.30, 0.99] |
| 7.2 Caesarean | 15 | 2457 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.27, 0.74] |
| 7.3 Both caesarean and vaginal birth | 1 | 55 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.02, 8.71] |
| 8 Blood loss | 16 | 2115 | Mean Difference (IV, Random, 95% CI) | ‐92.73 [‐154.97, ‐30.49] |
| 8.1 Vaginal birth | 4 | 485 | Mean Difference (IV, Random, 95% CI) | ‐68.42 [‐143.52, 6.68] |
| 8.2 Caesarean | 12 | 1630 | Mean Difference (IV, Random, 95% CI) | ‐101.54 [‐178.53, ‐24.55] |
| 9 Change in haemoglobin | 12 | 2096 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐3.81, 0.50] |
| 9.1 Vaginal birth | 3 | 415 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐5.34, ‐3.07] |
| 9.2 Caesarean | 8 | 1626 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐3.48, 2.22] |
| 9.3 Both caesarean and vaginal birth | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐6.97, 3.57] |
| 10 Breastfeeding | 2 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.86, 1.03] |
| 10.1 Vaginal birth | 1 | 135 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.85, 1.03] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Both caesarean and vaginal birth | 1 | 55 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 11 Nausea | 12 | 2288 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.78, 1.56] |
| 11.1 Vaginal birth | 4 | 555 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.37, 4.60] |
| 11.2 Caesarean | 8 | 1733 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.75, 1.61] |
| 12 Vomiting | 13 | 31833 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.53, 1.50] |
| 12.1 Vaginal birth | 5 | 30055 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.14, 5.25] |
| 12.2 Caesarean | 7 | 1723 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.44, 1.60] |
| 12.3 Both caesarean and vaginal birth | 1 | 55 | Risk Ratio (IV, Random, 95% CI) | 3.33 [0.14, 78.42] |
| 13 Headache | 15 | 2620 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.63, 1.12] |
| 13.1 Vaginal birth | 4 | 555 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.41, 3.26] |
| 13.2 Caesarean | 10 | 2010 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.61, 1.08] |
| 13.3 Both caesarean and vaginal birth | 1 | 55 | Risk Ratio (IV, Random, 95% CI) | 7.78 [0.42, 143.81] |
| 14 Abdominal pain | 10 | 31293 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 14.1 Vaginal birth | 5 | 29946 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.79, 1.49] |
| 14.2 Caesarean | 5 | 1347 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.90, 1.62] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 9 | 1998 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.49, 1.23] |
| 16.1 Vaginal birth | 3 | 495 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.49, 3.07] |
| 16.2 Caesarean | 6 | 1503 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.40, 1.20] |
| 17 Fever | 4 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.27, 9.35] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 3 | 411 | Risk Ratio (IV, Random, 95% CI) | 2.90 [0.19, 43.87] |
| 17.3 Both caesarean and vaginal birth | 1 | 55 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.02, 8.71] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
38.1. Analysis.
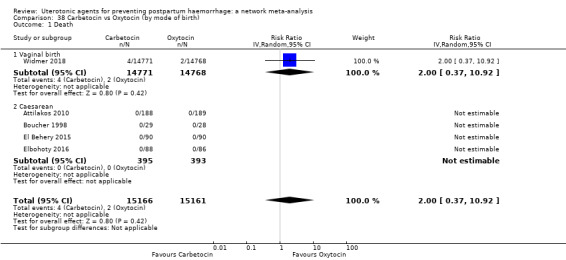
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 1 Death.
38.2. Analysis.
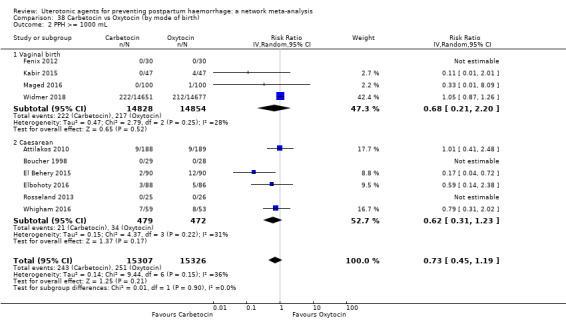
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
38.3. Analysis.

Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 3 Blood transfusion.
38.4. Analysis.
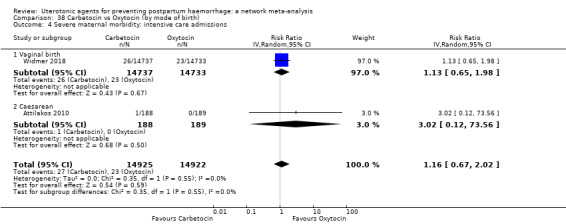
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
38.6. Analysis.
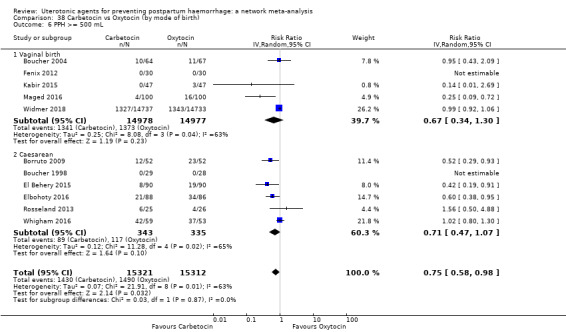
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
38.7. Analysis.
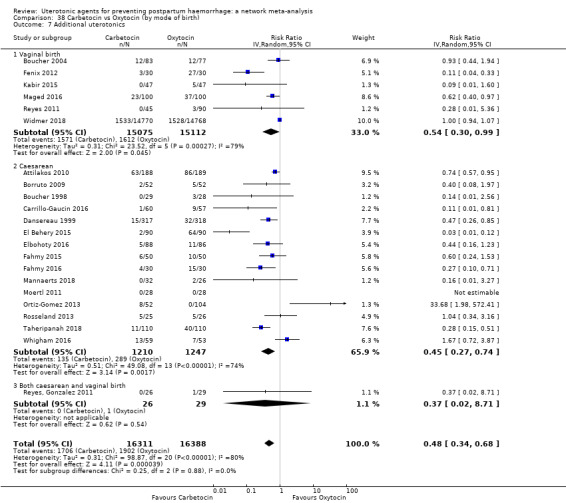
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
38.8. Analysis.
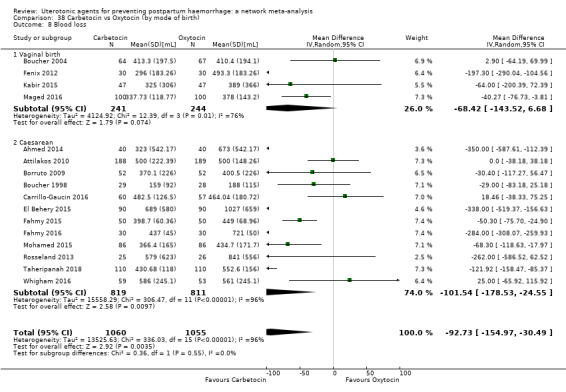
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 8 Blood loss.
38.9. Analysis.
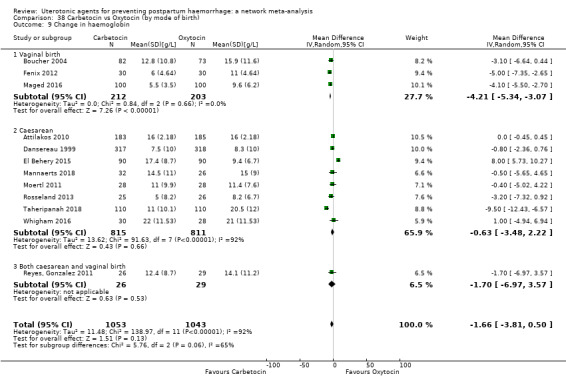
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
38.10. Analysis.
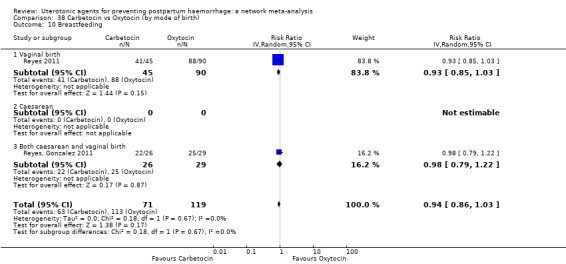
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 10 Breastfeeding.
38.11. Analysis.
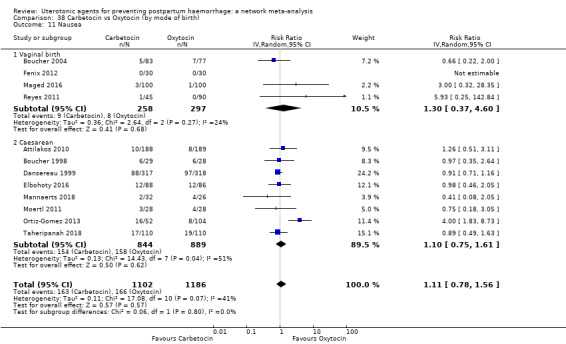
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 11 Nausea.
38.12. Analysis.
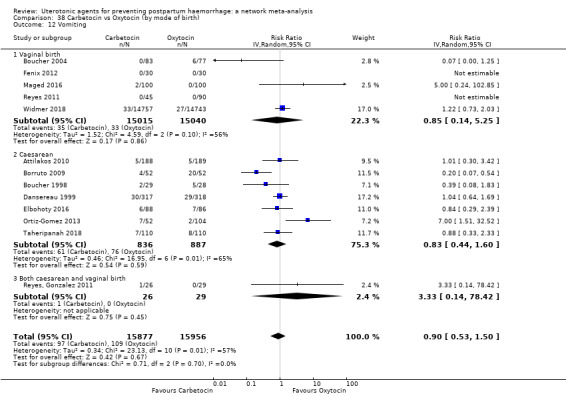
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 12 Vomiting.
38.13. Analysis.
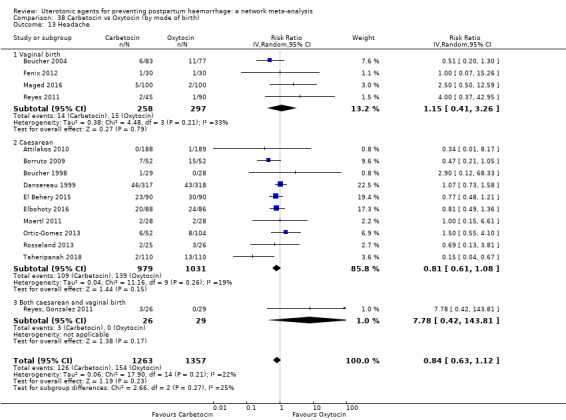
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 13 Headache.
38.14. Analysis.
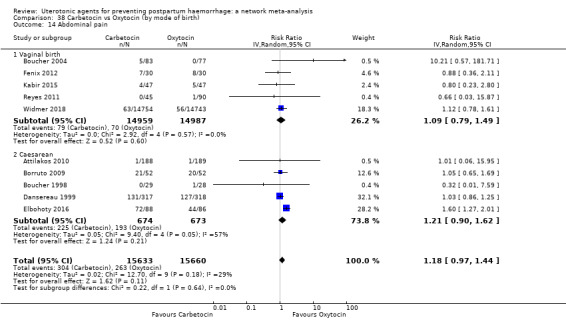
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 14 Abdominal pain.
38.16. Analysis.
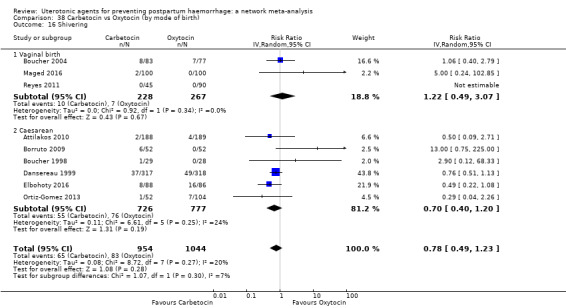
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 16 Shivering.
38.17. Analysis.
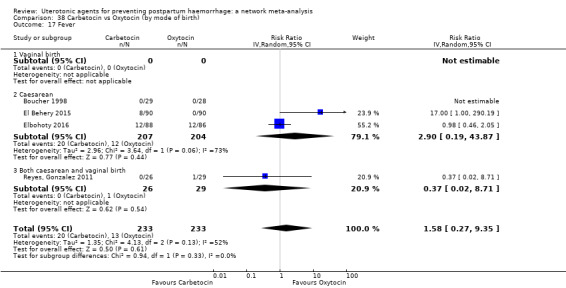
Comparison 38 Carbetocin vs Oxytocin (by mode of birth), Outcome 17 Fever.
Comparison 39. Ergometrine vs Oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 1293 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 1 | 1293 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 6 | 2591 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.52, 3.27] |
| 2.1 Vaginal birth | 6 | 2591 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.52, 3.27] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 5 | 1893 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.20, 10.23] |
| 3.1 Vaginal birth | 5 | 1893 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.20, 10.23] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 10 | 3221 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.86, 1.99] |
| 6.1 Vaginal birth | 10 | 3221 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.86, 1.99] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 6 | 2493 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.61, 3.48] |
| 7.1 Vaginal birth | 6 | 2493 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.61, 3.48] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 10 | 3221 | Mean Difference (IV, Random, 95% CI) | 8.09 [‐17.83, 34.00] |
| 8.1 Vaginal birth | 10 | 3221 | Mean Difference (IV, Random, 95% CI) | 8.09 [‐17.83, 34.00] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 1893 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.30, 1.13] |
| 9.1 Vaginal birth | 2 | 1893 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.30, 1.13] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 6 | 2529 | Risk Ratio (IV, Random, 95% CI) | 4.56 [1.13, 18.44] |
| 11.1 Vaginal birth | 6 | 2529 | Risk Ratio (IV, Random, 95% CI) | 4.56 [1.13, 18.44] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 5 | 2343 | Risk Ratio (IV, Random, 95% CI) | 3.83 [1.10, 13.28] |
| 12.1 Vaginal birth | 5 | 2343 | Risk Ratio (IV, Random, 95% CI) | 3.83 [1.10, 13.28] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 4 | 2293 | Risk Ratio (IV, Random, 95% CI) | 5.63 [0.93, 33.96] |
| 13.1 Vaginal birth | 4 | 2293 | Risk Ratio (IV, Random, 95% CI) | 5.63 [0.93, 33.96] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 1410 | Risk Ratio (IV, Random, 95% CI) | 13.39 [2.01, 89.44] |
| 15.1 Vaginal birth | 3 | 1410 | Risk Ratio (IV, Random, 95% CI) | 13.39 [2.01, 89.44] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 1493 | Risk Ratio (IV, Random, 95% CI) | 1.73 [0.93, 3.25] |
| 16.1 Vaginal birth | 3 | 1493 | Risk Ratio (IV, Random, 95% CI) | 1.73 [0.93, 3.25] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 1443 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.97, 9.05] |
| 17.1 Vaginal birth | 2 | 1443 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.97, 9.05] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 1393 | Risk Ratio (IV, Random, 95% CI) | 3.74 [0.42, 33.53] |
| 18.1 Vaginal birth | 2 | 1393 | Risk Ratio (IV, Random, 95% CI) | 3.74 [0.42, 33.53] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
39.1. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 1 Death.
39.2. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
39.3. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 3 Blood transfusion.
39.6. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
39.7. Analysis.
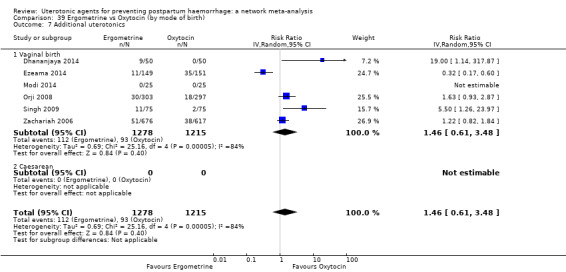
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
39.8. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 8 Blood loss.
39.9. Analysis.

Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
39.11. Analysis.

Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 11 Nausea.
39.12. Analysis.
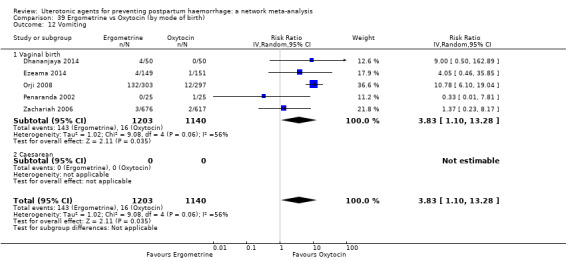
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 12 Vomiting.
39.13. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 13 Headache.
39.15. Analysis.
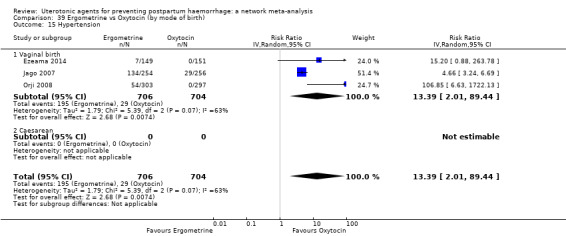
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 15 Hypertension.
39.16. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 16 Shivering.
39.17. Analysis.
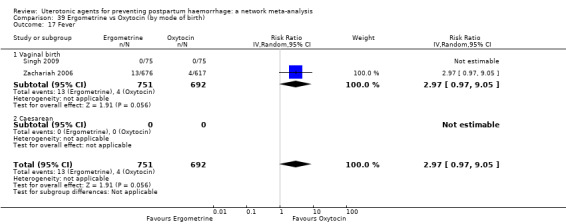
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 17 Fever.
39.18. Analysis.
Comparison 39 Ergometrine vs Oxytocin (by mode of birth), Outcome 18 Diarrhoea.
Comparison 40. Ergometine plus oxytocin vs Oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 1314 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 2 | 1314 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 10 | 10990 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.57, 0.93] |
| 2.1 Vaginal birth | 10 | 10990 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.57, 0.93] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 11 | 10985 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.53, 1.44] |
| 3.1 Vaginal birth | 9 | 10521 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.61, 1.64] |
| 3.2 Caesarean | 2 | 464 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.17, 0.91] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 991 | Risk Ratio (IV, Random, 95% CI) | 2.99 [0.12, 73.32] |
| 4.1 Vaginal birth | 1 | 991 | Risk Ratio (IV, Random, 95% CI) | 2.99 [0.12, 73.32] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 11 | 11090 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.57, 0.91] |
| 6.1 Vaginal birth | 11 | 11090 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.57, 0.91] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 10 | 8968 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.59, 1.07] |
| 7.1 Vaginal birth | 8 | 8504 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.58, 1.10] |
| 7.2 Caesarean | 2 | 464 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.22, 2.48] |
| 8 Blood loss | 10 | 4248 | Mean Difference (IV, Random, 95% CI) | ‐10.31 [‐40.32, 19.70] |
| 8.1 Vaginal birth | 7 | 3729 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐33.85, 33.60] |
| 8.2 Caesarean | 3 | 519 | Mean Difference (IV, Random, 95% CI) | ‐49.54 [‐88.07, ‐11.01] |
| 9 Change in haemoglobin | 6 | 4324 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐5.24, 0.77] |
| 9.1 Vaginal birth | 6 | 4324 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐5.24, 0.77] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 1 | 3483 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 10.1 Vaginal birth | 1 | 3483 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 7 | 6931 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.84, 3.53] |
| 11.1 Vaginal birth | 5 | 6467 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.65, 3.86] |
| 11.2 Caesarean | 2 | 464 | Risk Ratio (IV, Random, 95% CI) | 2.09 [0.53, 8.22] |
| 12 Vomiting | 9 | 10227 | Risk Ratio (IV, Random, 95% CI) | 3.05 [1.76, 5.29] |
| 12.1 Vaginal birth | 7 | 9763 | Risk Ratio (IV, Random, 95% CI) | 2.93 [1.50, 5.71] |
| 12.2 Caesarean | 2 | 464 | Risk Ratio (IV, Random, 95% CI) | 2.83 [1.12, 7.15] |
| 13 Headache | 5 | 5105 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.79, 1.99] |
| 13.1 Vaginal birth | 4 | 4689 | Risk Ratio (IV, Random, 95% CI) | 1.74 [0.67, 4.55] |
| 13.2 Caesarean | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.59, 1.91] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 1362 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.29, 13.97] |
| 15.1 Vaginal birth | 2 | 1314 | Risk Ratio (IV, Random, 95% CI) | 4.57 [0.65, 32.04] |
| 15.2 Caesarean | 1 | 48 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.03, 2.08] |
| 16 Shivering | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.60, 1.53] |
| 16.1 Vaginal birth | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.60, 1.53] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.48, 2.43] |
| 17.1 Vaginal birth | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.48, 2.43] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 3 | 2030 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.72, 2.22] |
| 18.1 Vaginal birth | 3 | 2030 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.72, 2.22] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
40.1. Analysis.
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 1 Death.
40.2. Analysis.
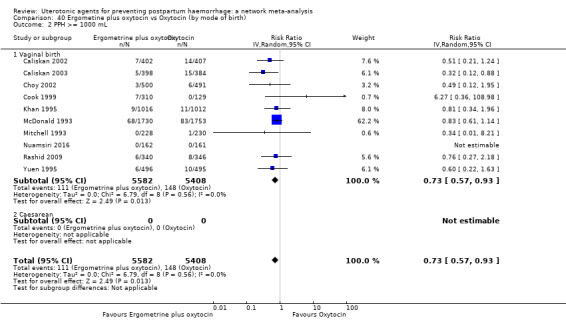
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
40.3. Analysis.
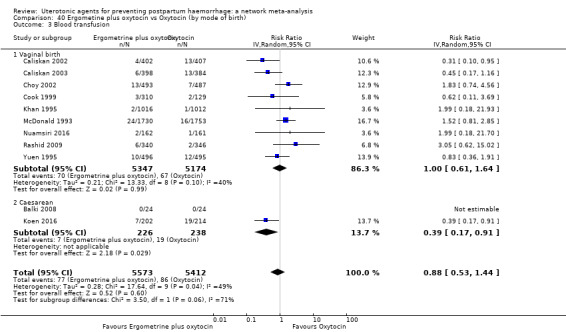
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 3 Blood transfusion.
40.4. Analysis.
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
40.6. Analysis.
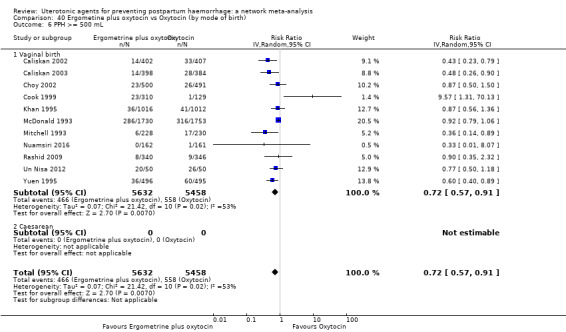
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
40.7. Analysis.
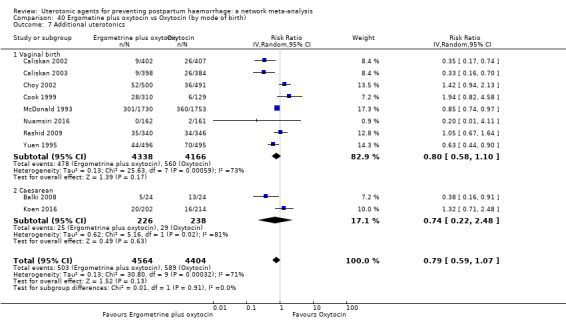
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
40.8. Analysis.
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 8 Blood loss.
40.9. Analysis.
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
40.10. Analysis.
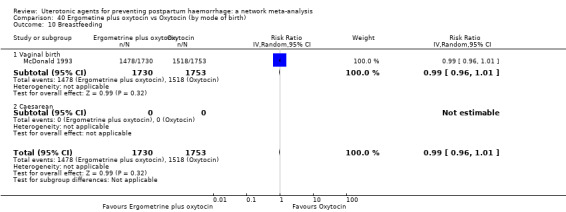
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 10 Breastfeeding.
40.11. Analysis.
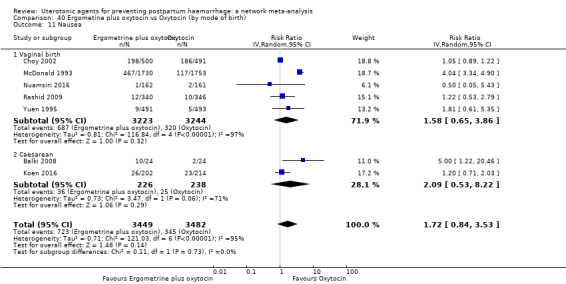
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 11 Nausea.
40.12. Analysis.
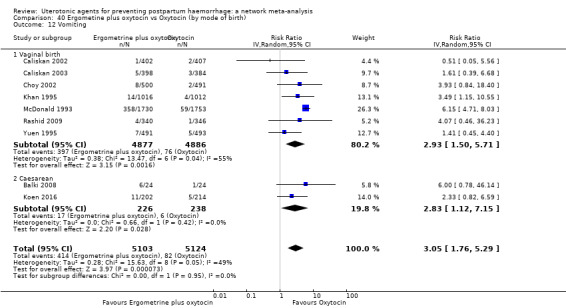
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 12 Vomiting.
40.13. Analysis.
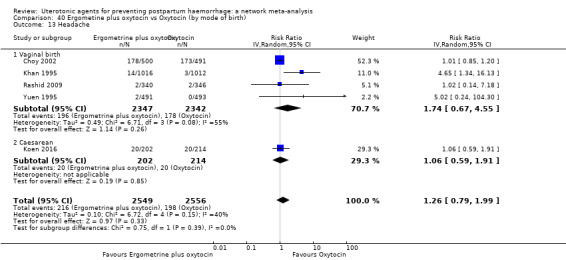
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 13 Headache.
40.15. Analysis.
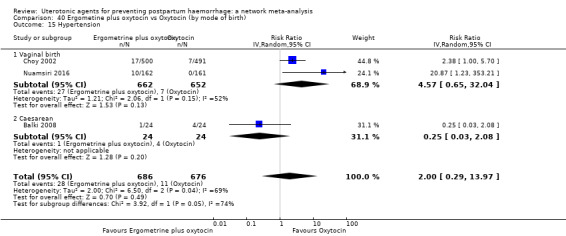
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 15 Hypertension.
40.16. Analysis.
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 16 Shivering.
40.17. Analysis.
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 17 Fever.
40.18. Analysis.
Comparison 40 Ergometine plus oxytocin vs Oxytocin (by mode of birth), Outcome 18 Diarrhoea.
Comparison 41. Misoprostol plus oxytocin vs Oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 9 | 4737 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 5 | 3802 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 4 | 935 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 17 | 8514 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 2.1 Vaginal birth | 7 | 6241 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.52, 1.14] |
| 2.2 Caesarean | 10 | 2273 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.70, 1.24] |
| 3 Blood transfusion | 19 | 8742 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.37, 0.67] |
| 3.1 Vaginal birth | 7 | 5898 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.25, 0.61] |
| 3.2 Caesarean | 12 | 2844 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.36, 0.96] |
| 4 Severe maternal morbidity: intensive care admissions | 3 | 1886 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.47] |
| 4.1 Vaginal birth | 1 | 1400 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.47] |
| 4.2 Caesarean | 2 | 486 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 14 | 8148 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 6.1 Vaginal birth | 8 | 6997 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.55, 0.92] |
| 6.2 Caesarean | 6 | 1151 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.51, 0.92] |
| 7 Additional uterotonics | 18 | 8391 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.44, 0.67] |
| 7.1 Vaginal birth | 7 | 5898 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.49, 0.79] |
| 7.2 Caesarean | 11 | 2493 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.36, 0.66] |
| 8 Blood loss | 17 | 8690 | Mean Difference (IV, Random, 95% CI) | ‐87.26 [‐157.83, ‐16.69] |
| 8.1 Vaginal birth | 7 | 6179 | Mean Difference (IV, Random, 95% CI) | ‐12.23 [‐26.51, 2.06] |
| 8.2 Caesarean | 10 | 2511 | Mean Difference (IV, Random, 95% CI) | ‐134.79 [‐276.45, 6.88] |
| 9 Change in haemoglobin | 15 | 7929 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐3.70, ‐1.48] |
| 9.1 Vaginal birth | 6 | 5643 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.61, ‐0.17] |
| 9.2 Caesarean | 9 | 2286 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐5.60, ‐2.39] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 7 | 3798 | Risk Ratio (IV, Random, 95% CI) | 2.21 [1.19, 4.10] |
| 11.1 Vaginal birth | 2 | 3003 | Risk Ratio (IV, Random, 95% CI) | 3.52 [1.55, 7.99] |
| 11.2 Caesarean | 5 | 795 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.76, 3.84] |
| 12 Vomiting | 11 | 6718 | Risk Ratio (IV, Random, 95% CI) | 2.24 [1.52, 3.31] |
| 12.1 Vaginal birth | 6 | 5610 | Risk Ratio (IV, Random, 95% CI) | 3.32 [2.03, 5.44] |
| 12.2 Caesarean | 5 | 1108 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.96, 2.36] |
| 13 Headache | 2 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.26, 6.23] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 2 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.26, 6.23] |
| 14 Abdominal pain | 1 | 366 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.01, 3.67] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 1 | 366 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.01, 3.67] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 19 | 9458 | Risk Ratio (IV, Random, 95% CI) | 3.38 [2.50, 4.57] |
| 16.1 Vaginal birth | 8 | 7007 | Risk Ratio (IV, Random, 95% CI) | 3.68 [2.41, 5.60] |
| 16.2 Caesarean | 11 | 2451 | Risk Ratio (IV, Random, 95% CI) | 3.04 [2.00, 4.61] |
| 17 Fever | 17 | 8607 | Risk Ratio (IV, Random, 95% CI) | 2.99 [2.00, 4.45] |
| 17.1 Vaginal birth | 7 | 6209 | Risk Ratio (IV, Random, 95% CI) | 4.30 [2.57, 7.21] |
| 17.2 Caesarean | 10 | 2398 | Risk Ratio (IV, Random, 95% CI) | 1.85 [1.28, 2.67] |
| 18 Diarrhoea | 7 | 5649 | Risk Ratio (IV, Random, 95% CI) | 2.08 [0.99, 4.38] |
| 18.1 Vaginal birth | 5 | 4887 | Risk Ratio (IV, Random, 95% CI) | 1.89 [0.82, 4.36] |
| 18.2 Caesarean | 2 | 762 | Risk Ratio (IV, Random, 95% CI) | 6.07 [0.73, 50.35] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
41.1. Analysis.
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 1 Death.
41.2. Analysis.
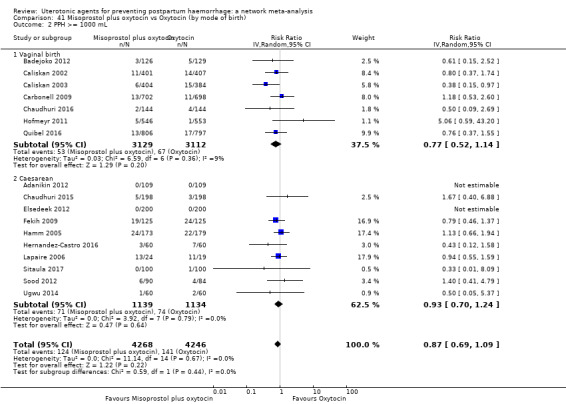
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
41.3. Analysis.
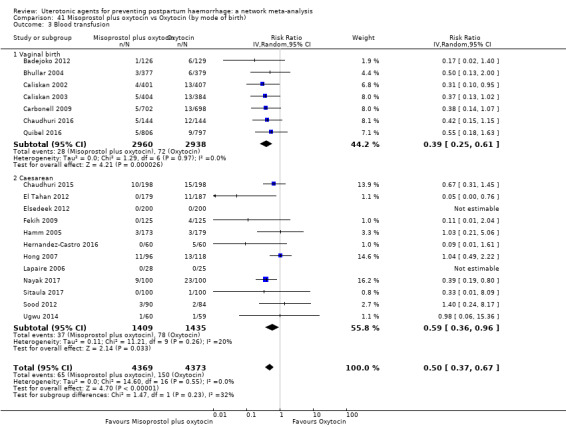
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 3 Blood transfusion.
41.4. Analysis.
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
41.6. Analysis.
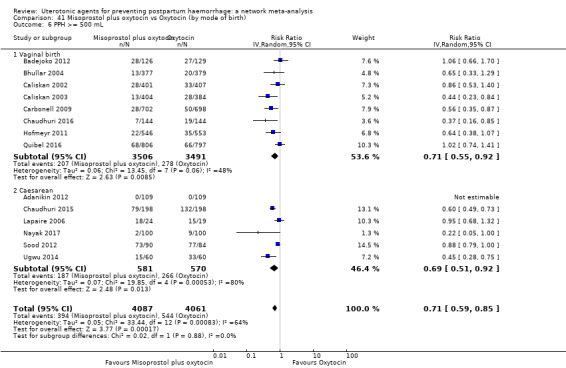
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
41.7. Analysis.
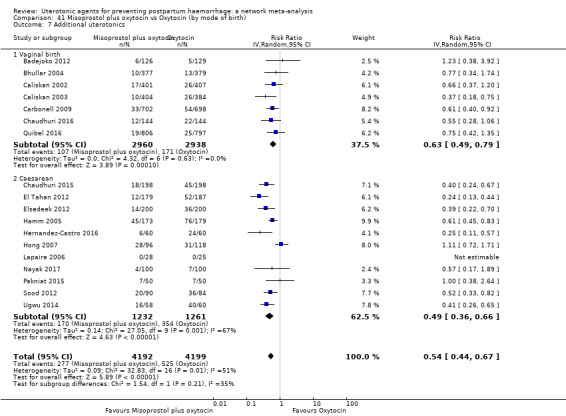
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
41.8. Analysis.
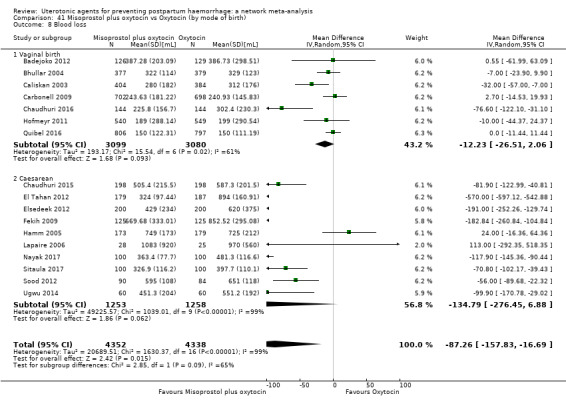
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 8 Blood loss.
41.9. Analysis.
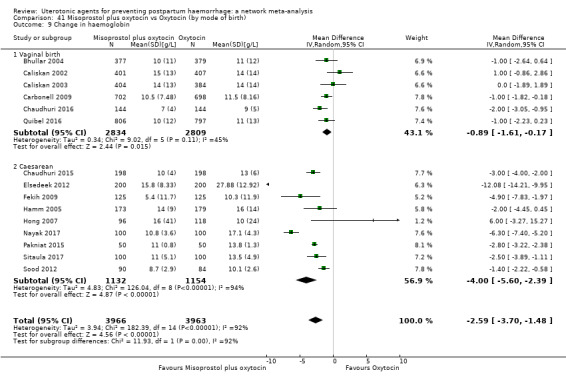
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
41.11. Analysis.
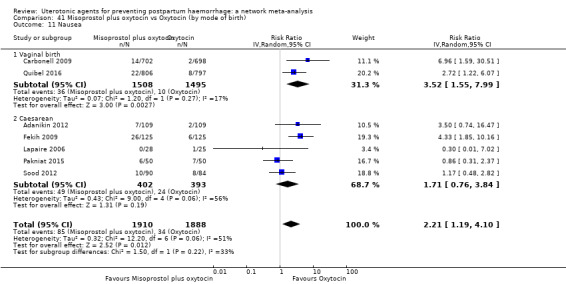
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 11 Nausea.
41.12. Analysis.
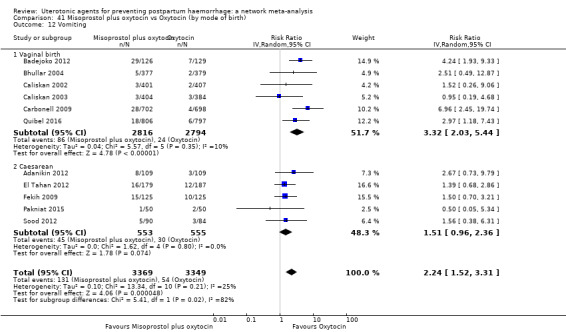
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 12 Vomiting.
41.13. Analysis.
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 13 Headache.
41.14. Analysis.
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 14 Abdominal pain.
41.16. Analysis.
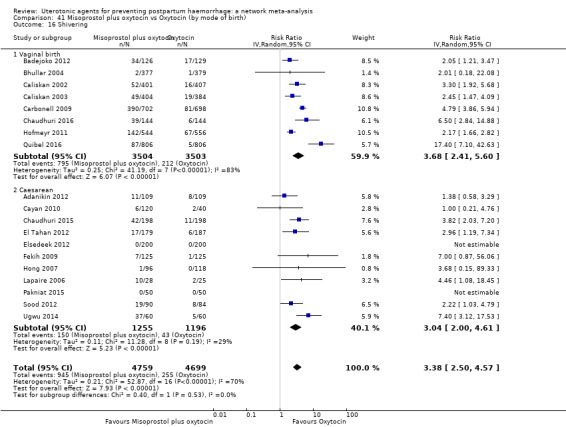
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 16 Shivering.
41.17. Analysis.
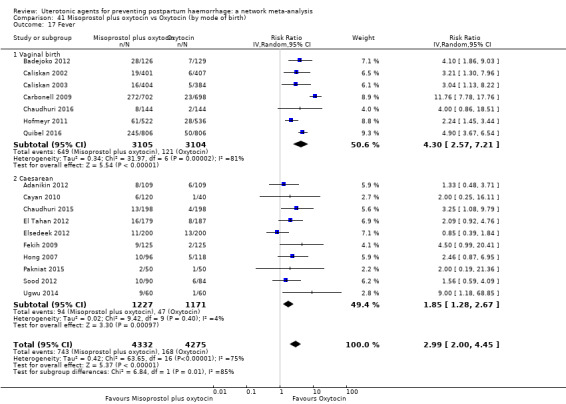
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 17 Fever.
41.18. Analysis.
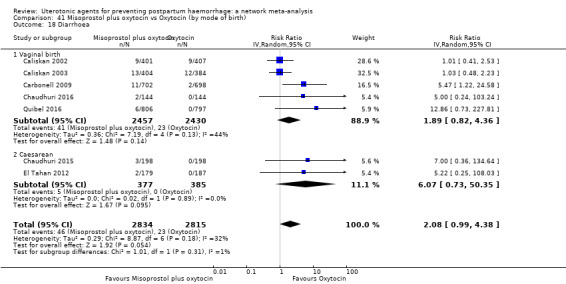
Comparison 41 Misoprostol plus oxytocin vs Oxytocin (by mode of birth), Outcome 18 Diarrhoea.
Comparison 42. Injectable prostaglandins vs Misoprostol (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 170 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.1 Vaginal birth | 2 | 170 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.14, 5.39] |
| 3.1 Vaginal birth | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.14, 5.39] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 3 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.60 [0.57, 4.52] |
| 6.1 Vaginal birth | 3 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.60 [0.57, 4.52] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.28, 3.69] |
| 7.1 Vaginal birth | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.28, 3.69] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 3 | 270 | Mean Difference (IV, Random, 95% CI) | 52.99 [‐39.74, 145.71] |
| 8.1 Vaginal birth | 3 | 270 | Mean Difference (IV, Random, 95% CI) | 52.99 [‐39.74, 145.71] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐1.35, 5.90] |
| 9.1 Vaginal birth | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐1.35, 5.90] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.06, 0.92] |
| 11.1 Vaginal birth | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.06, 0.92] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.01, 163.15] |
| 12.1 Vaginal birth | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.01, 163.15] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.20, 4.80] |
| 14.1 Vaginal birth | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.20, 4.80] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 353 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.01, 0.21] |
| 16.1 Vaginal birth | 3 | 353 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.01, 0.21] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.01, 0.39] |
| 17.1 Vaginal birth | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.01, 0.39] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 9.03 [1.70, 48.00] |
| 18.1 Vaginal birth | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 9.03 [1.70, 48.00] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
42.1. Analysis.
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 1 Death.
42.2. Analysis.
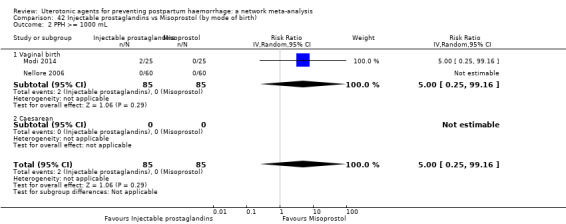
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 2 PPH >= 1000 mL.
42.3. Analysis.
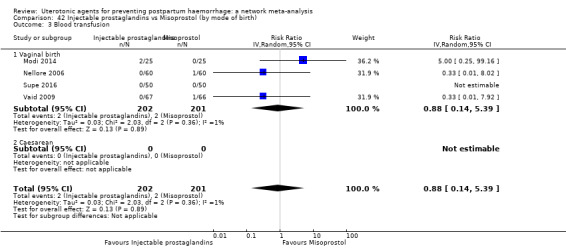
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 3 Blood transfusion.
42.6. Analysis.
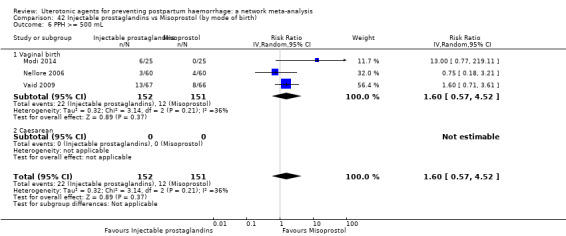
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 6 PPH >= 500 mL.
42.7. Analysis.
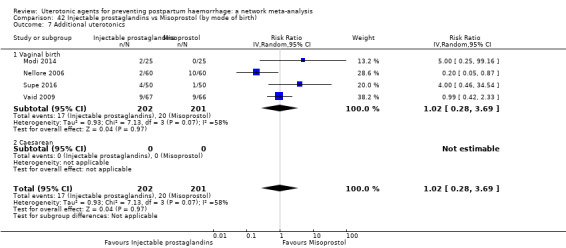
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 7 Additional uterotonics.
42.8. Analysis.
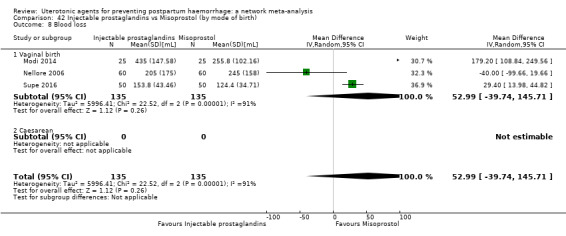
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 8 Blood loss.
42.9. Analysis.
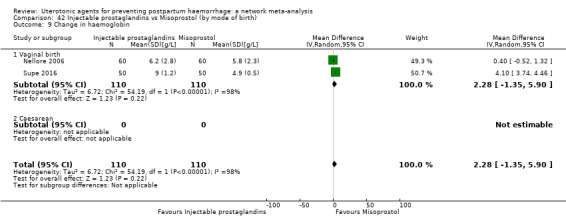
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 9 Change in haemoglobin.
42.11. Analysis.
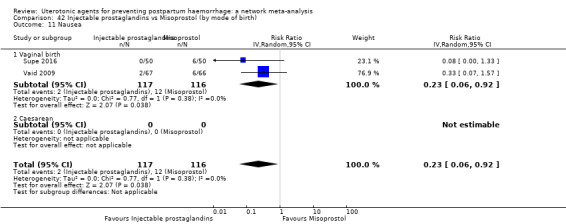
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 11 Nausea.
42.12. Analysis.
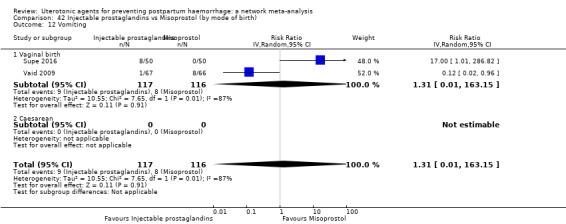
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 12 Vomiting.
42.14. Analysis.
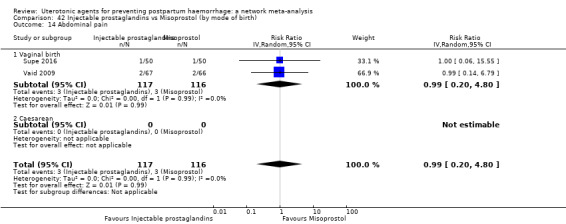
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 14 Abdominal pain.
42.16. Analysis.
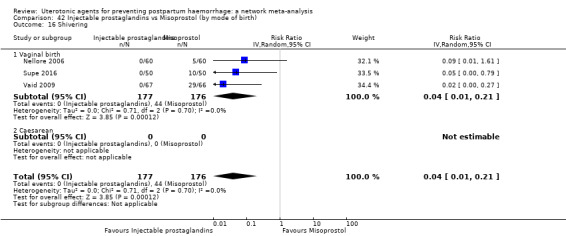
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 16 Shivering.
42.17. Analysis.
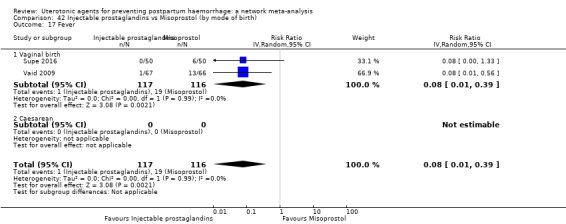
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 17 Fever.
42.18. Analysis.
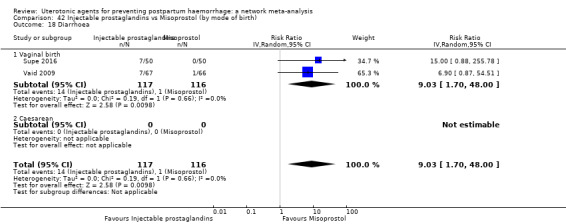
Comparison 42 Injectable prostaglandins vs Misoprostol (by mode of birth), Outcome 18 Diarrhoea.
Comparison 43. Misoprostol vs Carbetocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [0.62, 8.64] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [0.62, 8.64] |
| 3 Blood transfusion | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 71.85] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 71.85] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [1.52, 3.50] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [1.52, 3.50] |
| 7 Additional uterotonics | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.96 [1.55, 10.07] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.96 [1.55, 10.07] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.47, 2.08] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.47, 2.08] |
| 12 Vomiting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.55, 3.99] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.55, 3.99] |
| 13 Headache | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.71, 1.99] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.71, 1.99] |
| 14 Abdominal pain | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.46 [1.67, 7.17] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.46 [1.67, 7.17] |
| 17 Fever | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.55 [1.41, 4.64] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.55 [1.41, 4.64] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
43.1. Analysis.
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 1 Death.
43.2. Analysis.
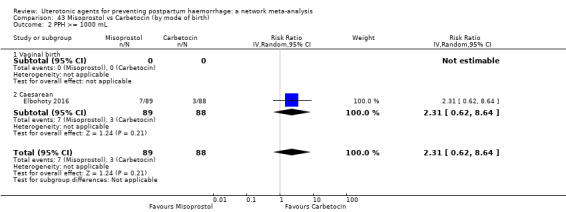
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
43.3. Analysis.
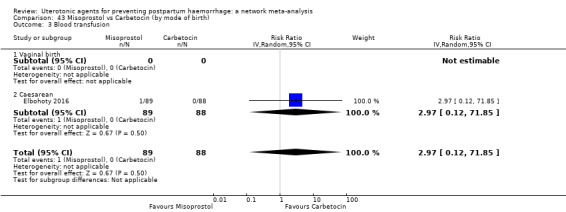
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 3 Blood transfusion.
43.6. Analysis.
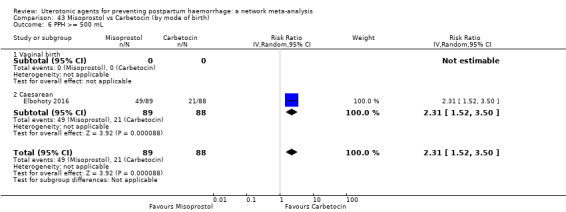
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 6 PPH >= 500 mL.
43.7. Analysis.
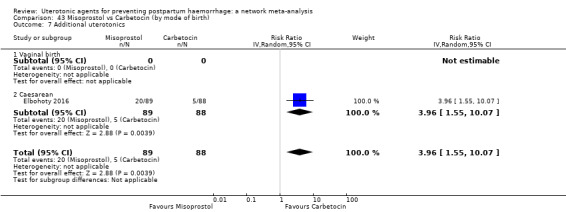
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 7 Additional uterotonics.
43.11. Analysis.
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 11 Nausea.
43.12. Analysis.
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 12 Vomiting.
43.13. Analysis.
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 13 Headache.
43.14. Analysis.
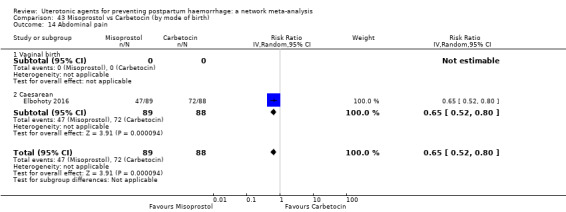
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 14 Abdominal pain.
43.16. Analysis.
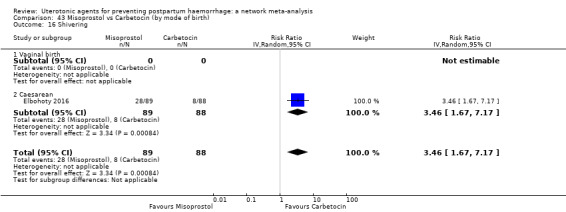
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 16 Shivering.
43.17. Analysis.
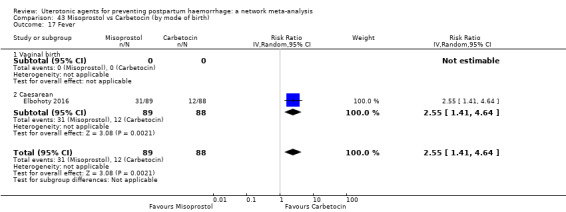
Comparison 43 Misoprostol vs Carbetocin (by mode of birth), Outcome 17 Fever.
Comparison 44. Ergometrine vs Misoprostol (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 7 | 5254 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 7 | 5254 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 11 | 5562 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.45, 3.48] |
| 2.1 Vaginal birth | 11 | 5562 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.45, 3.48] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 13 | 5308 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.65, 4.50] |
| 3.1 Vaginal birth | 13 | 5308 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.65, 4.50] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 864 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.16] |
| 4.1 Vaginal birth | 1 | 864 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.16] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 16 | 6459 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.79, 1.75] |
| 6.1 Vaginal birth | 16 | 6459 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.79, 1.75] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 16 | 6565 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.60] |
| 7.1 Vaginal birth | 16 | 6565 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.60] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 15 | 6337 | Mean Difference (IV, Random, 95% CI) | 11.66 [‐9.85, 33.17] |
| 8.1 Vaginal birth | 15 | 6337 | Mean Difference (IV, Random, 95% CI) | 11.66 [‐9.85, 33.17] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 8 | 4438 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.43, 2.26] |
| 9.1 Vaginal birth | 8 | 4438 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.43, 2.26] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 13 | 5202 | Risk Ratio (IV, Random, 95% CI) | 1.53 [1.15, 2.04] |
| 11.1 Vaginal birth | 13 | 5202 | Risk Ratio (IV, Random, 95% CI) | 1.53 [1.15, 2.04] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 14 | 6136 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.81, 1.83] |
| 12.1 Vaginal birth | 14 | 6136 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.81, 1.83] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 8 | 3369 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.70, 4.12] |
| 13.1 Vaginal birth | 8 | 3369 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.70, 4.12] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.04, 65.80] |
| 14.1 Vaginal birth | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.04, 65.80] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 2 | 300 | Risk Ratio (IV, Random, 95% CI) | 7.55 [0.94, 60.53] |
| 15.1 Vaginal birth | 2 | 300 | Risk Ratio (IV, Random, 95% CI) | 7.55 [0.94, 60.53] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 17 | 6860 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.26, 0.44] |
| 16.1 Vaginal birth | 17 | 6860 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.26, 0.44] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 14 | 6330 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.33] |
| 17.1 Vaginal birth | 14 | 6330 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.33] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 8 | 4165 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.46, 2.34] |
| 18.1 Vaginal birth | 8 | 4165 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.46, 2.34] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
44.1. Analysis.
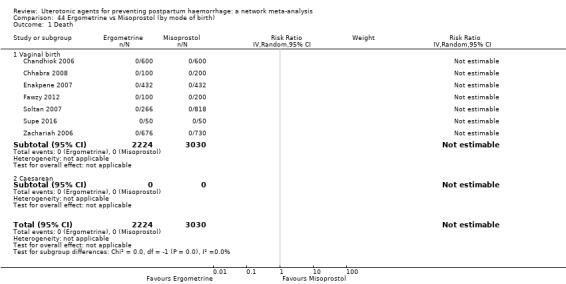
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 1 Death.
44.2. Analysis.
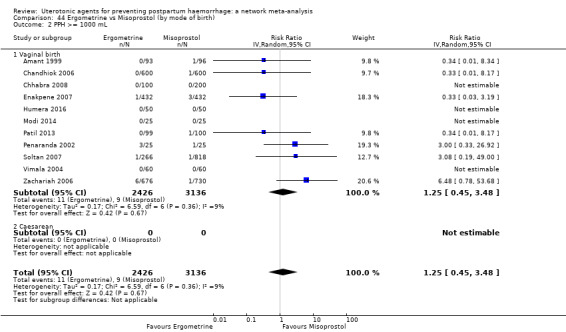
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 2 PPH >= 1000 mL.
44.3. Analysis.
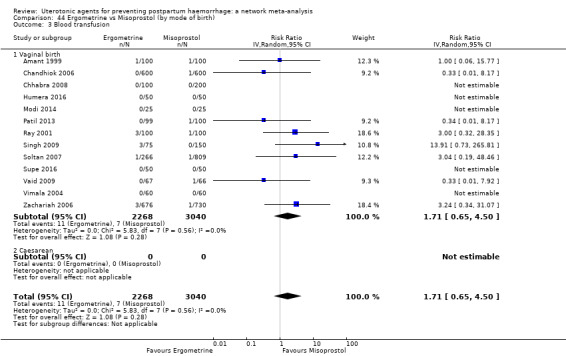
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 3 Blood transfusion.
44.4. Analysis.
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
44.6. Analysis.
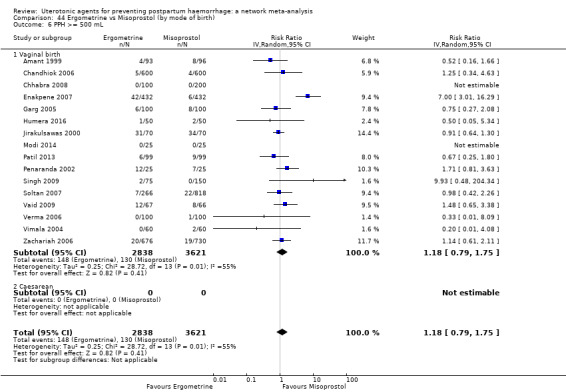
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 6 PPH >= 500 mL.
44.7. Analysis.
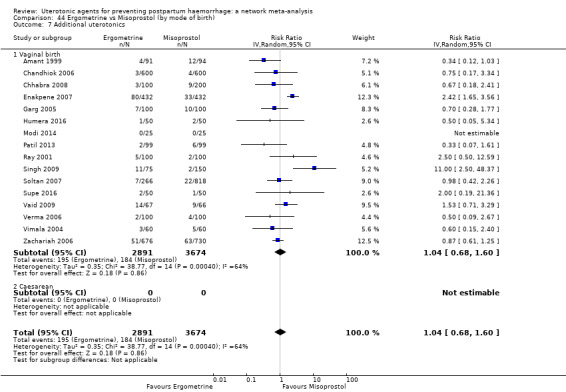
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 7 Additional uterotonics.
44.8. Analysis.
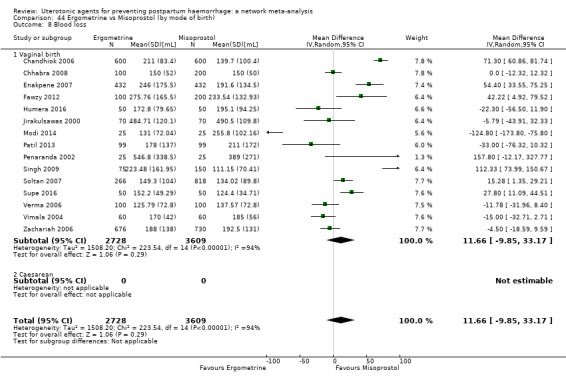
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 8 Blood loss.
44.9. Analysis.
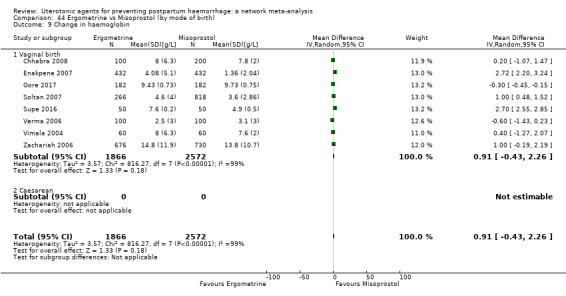
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 9 Change in haemoglobin.
44.11. Analysis.
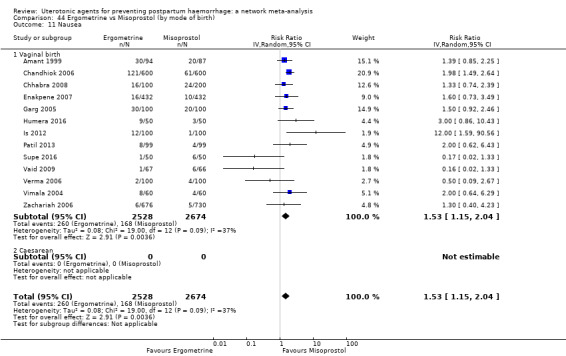
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 11 Nausea.
44.12. Analysis.
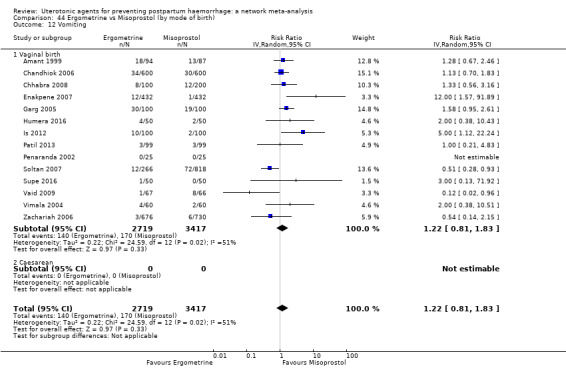
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 12 Vomiting.
44.13. Analysis.
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 13 Headache.
44.14. Analysis.
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 14 Abdominal pain.
44.15. Analysis.
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 15 Hypertension.
44.16. Analysis.
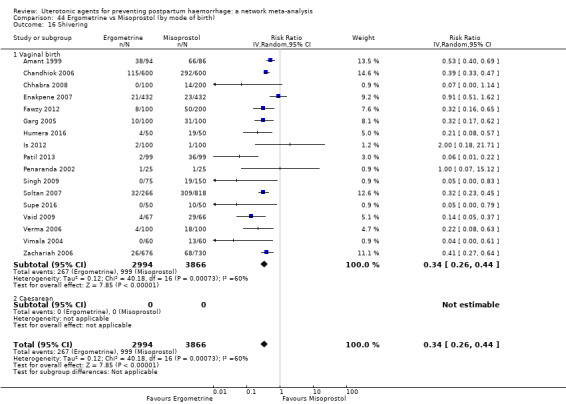
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 16 Shivering.
44.17. Analysis.
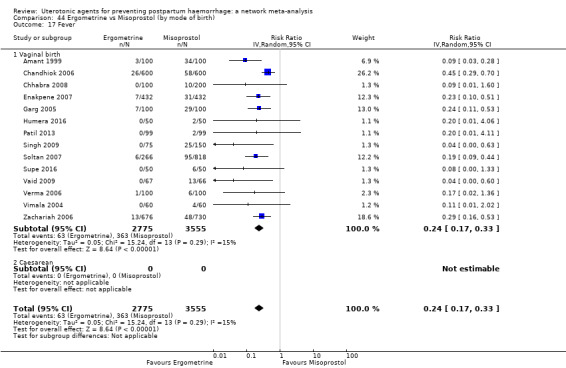
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 17 Fever.
44.18. Analysis.
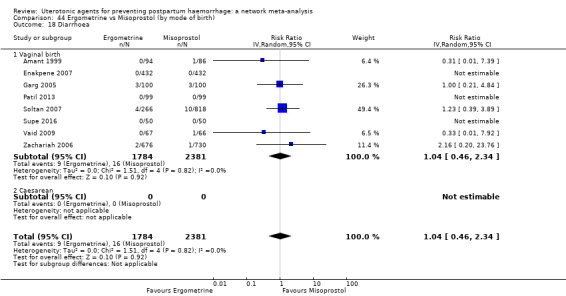
Comparison 44 Ergometrine vs Misoprostol (by mode of birth), Outcome 18 Diarrhoea.
Comparison 45. Misoprostol vs Ergometrine plus oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 3258 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 4 | 3258 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 9 | 6028 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.08, 2.40] |
| 2.1 Vaginal birth | 9 | 6028 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.08, 2.40] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 8 | 6335 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.91, 2.21] |
| 3.1 Vaginal birth | 8 | 6335 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.91, 2.21] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 10 | 6492 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.35, 2.26] |
| 6.1 Vaginal birth | 10 | 6492 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.35, 2.26] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 9 | 6395 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.49, 2.36] |
| 7.1 Vaginal birth | 9 | 6395 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.49, 2.36] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 8 | 5634 | Mean Difference (IV, Random, 95% CI) | 22.86 [4.50, 41.22] |
| 8.1 Vaginal birth | 8 | 5634 | Mean Difference (IV, Random, 95% CI) | 22.86 [4.50, 41.22] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 9 | 5588 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐0.49, 2.67] |
| 9.1 Vaginal birth | 9 | 5588 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐0.49, 2.67] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 4 | 3399 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.61, 0.84] |
| 11.1 Vaginal birth | 4 | 3399 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.61, 0.84] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 6 | 4983 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.50, 1.04] |
| 12.1 Vaginal birth | 6 | 4983 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.50, 1.04] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 3 | 3259 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.47, 1.76] |
| 13.1 Vaginal birth | 3 | 3259 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.47, 1.76] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 1 | 846 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 14.1 Vaginal birth | 1 | 846 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 2553 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.18, 1.41] |
| 15.1 Vaginal birth | 3 | 2553 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.18, 1.41] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 6 | 4980 | Risk Ratio (IV, Random, 95% CI) | 2.71 [1.95, 3.76] |
| 16.1 Vaginal birth | 6 | 4980 | Risk Ratio (IV, Random, 95% CI) | 2.71 [1.95, 3.76] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 7 | 5045 | Risk Ratio (IV, Random, 95% CI) | 5.33 [2.87, 9.88] |
| 17.1 Vaginal birth | 7 | 5045 | Risk Ratio (IV, Random, 95% CI) | 5.33 [2.87, 9.88] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 6 | 3659 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.57] |
| 18.1 Vaginal birth | 6 | 3659 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.57] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
45.1. Analysis.
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 1 Death.
45.2. Analysis.
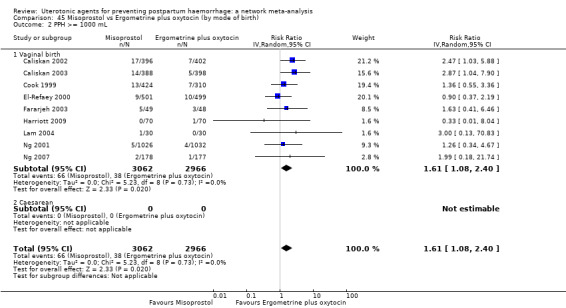
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
45.3. Analysis.
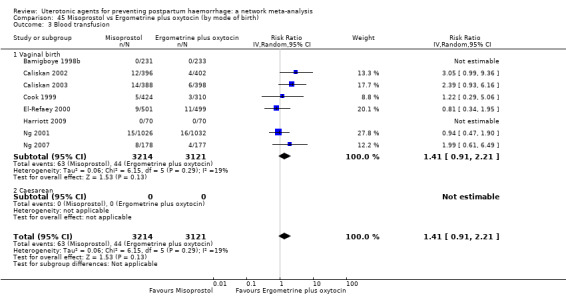
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 3 Blood transfusion.
45.6. Analysis.
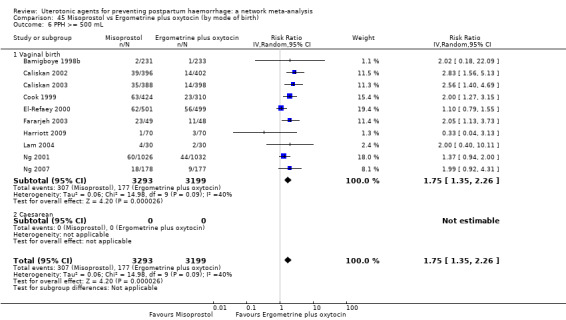
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
45.7. Analysis.
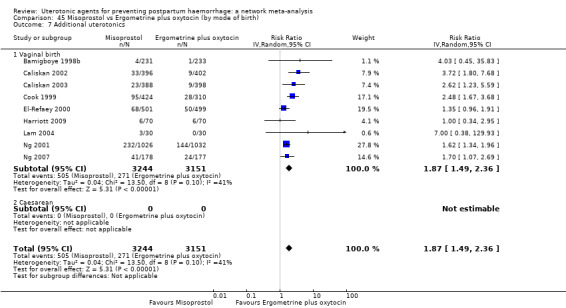
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
45.8. Analysis.
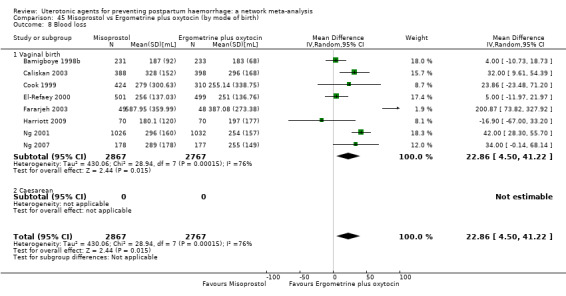
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 8 Blood loss.
45.9. Analysis.
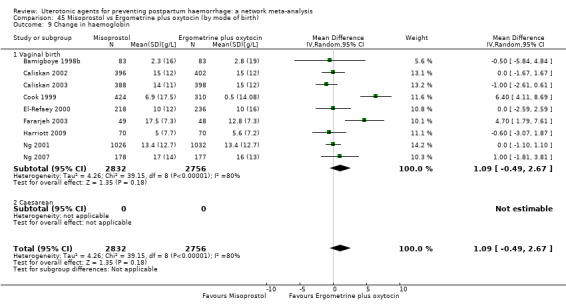
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
45.11. Analysis.
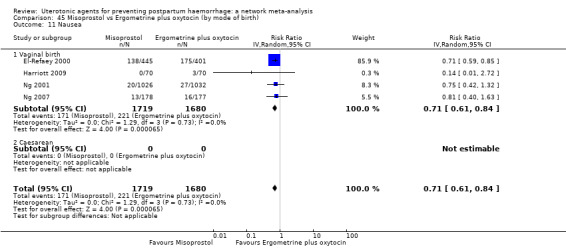
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 11 Nausea.
45.12. Analysis.
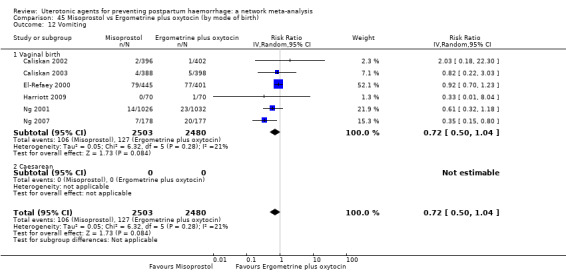
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 12 Vomiting.
45.13. Analysis.
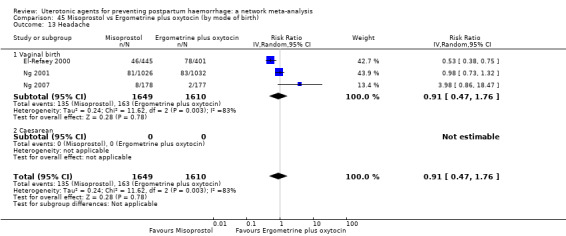
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 13 Headache.
45.14. Analysis.
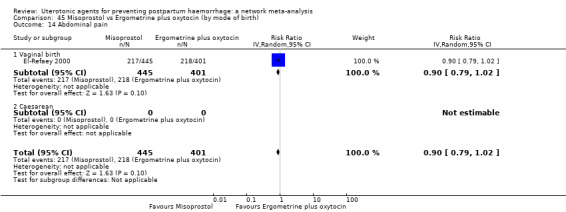
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 14 Abdominal pain.
45.15. Analysis.
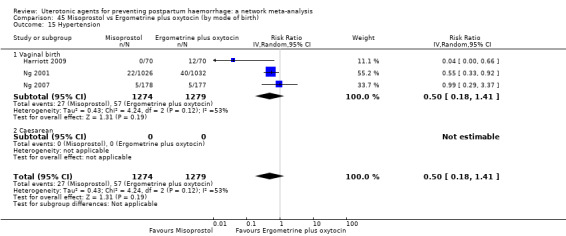
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 15 Hypertension.
45.16. Analysis.
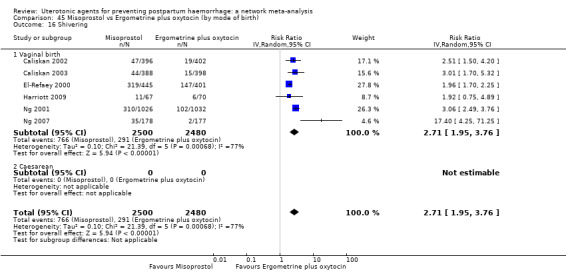
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 16 Shivering.
45.17. Analysis.
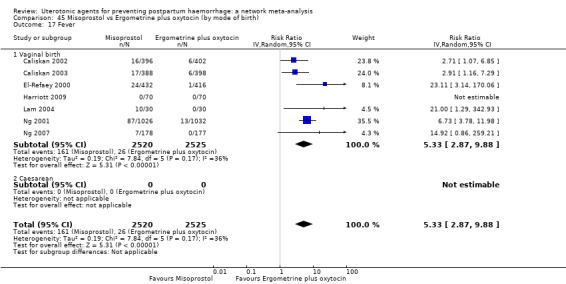
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 17 Fever.
45.18. Analysis.
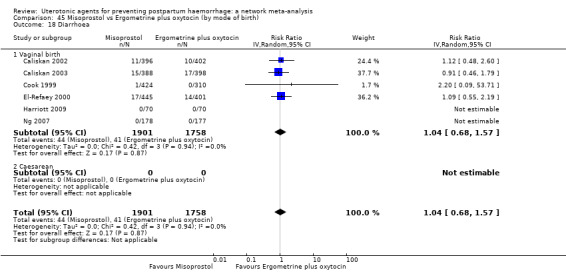
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 18 Diarrhoea.
45.20. Analysis.
Comparison 45 Misoprostol vs Ergometrine plus oxytocin (by mode of birth), Outcome 20 Maternal satisfaction.
Comparison 46. Misoprostol vs Misoprostol plus oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.85 [1.03, 3.33] |
| 2.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.85 [1.03, 3.33] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 2.97 [1.40, 6.30] |
| 3.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 2.97 [1.40, 6.30] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity:shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.93 [0.99, 3.76] |
| 6.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.93 [0.99, 3.76] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.89 [1.26, 2.83] |
| 7.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 2.12 [1.35, 3.32] |
| 7.2 Caesarean | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.45, 2.91] |
| 8 Blood loss | 1 | 792 | Mean Difference (IV, Random, 95% CI) | 48.0 [24.68, 71.32] |
| 8.1 Vaginal birth | 1 | 792 | Mean Difference (IV, Random, 95% CI) | 48.0 [24.68, 71.32] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 3 | 1689 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.42, 0.96] |
| 9.1 Vaginal birth | 2 | 1589 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.21, 1.21] |
| 9.2 Caesarean | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.43, 1.23] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.83 [0.73, 4.57] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.83 [0.73, 4.57] |
| 12 Vomiting | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.51, 3.82] |
| 12.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.33, 3.24] |
| 12.2 Caesarean | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 4.0 [0.46, 34.54] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 16.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.71, 1.21] |
| 16.2 Caesarean | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 7.0 [0.37, 132.10] |
| 17 Fever | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.62, 1.53] |
| 17.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.61, 1.54] |
| 17.2 Caesarean | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.0 [0.15, 6.82] |
| 18 Diarrhoea | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.70, 2.13] |
| 18.1 Vaginal birth | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.70, 2.13] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
46.2. Analysis.
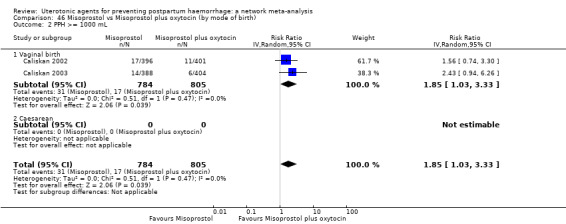
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
46.3. Analysis.
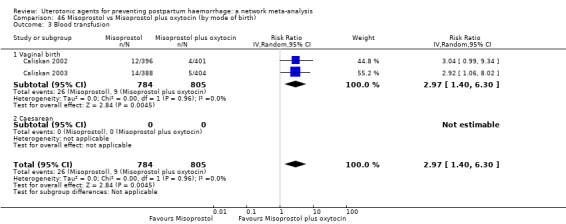
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 3 Blood transfusion.
46.6. Analysis.
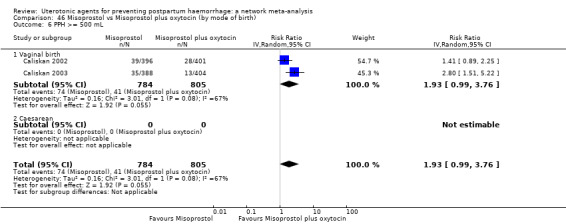
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
46.7. Analysis.
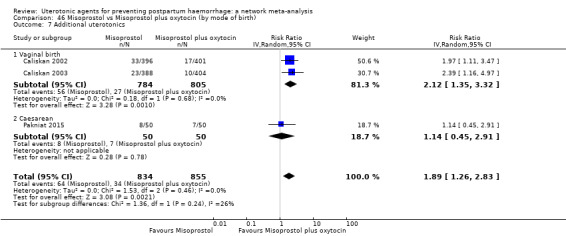
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
46.8. Analysis.
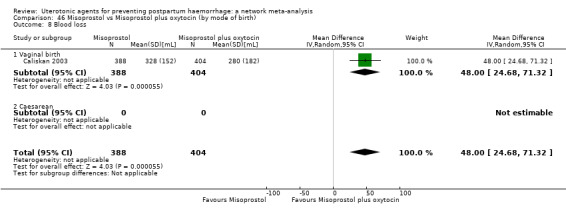
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 8 Blood loss.
46.9. Analysis.
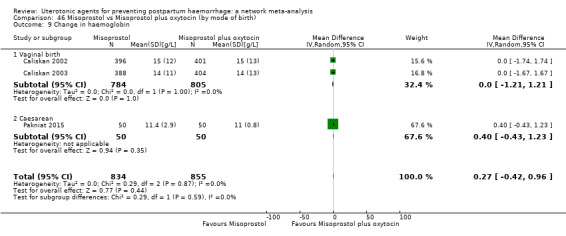
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
46.11. Analysis.
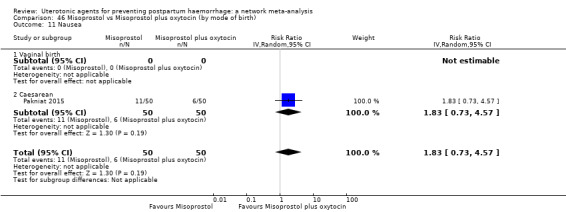
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 11 Nausea.
46.12. Analysis.
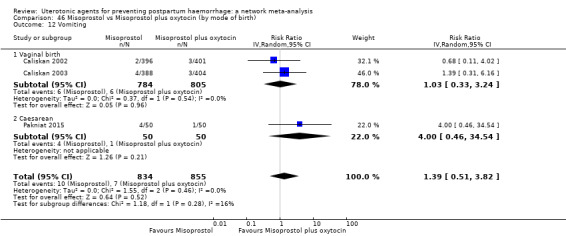
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 12 Vomiting.
46.16. Analysis.
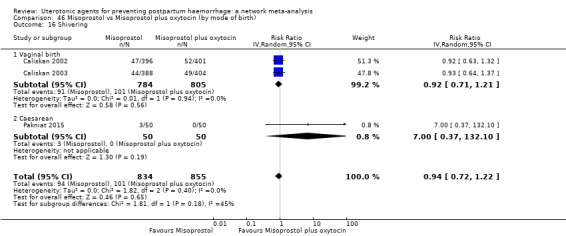
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 16 Shivering.
46.17. Analysis.
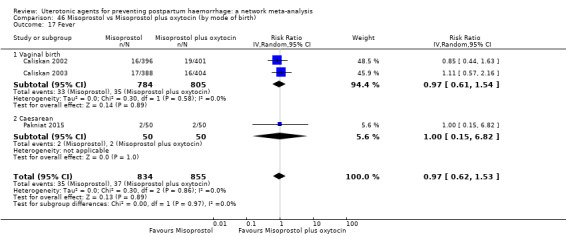
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 17 Fever.
46.18. Analysis.
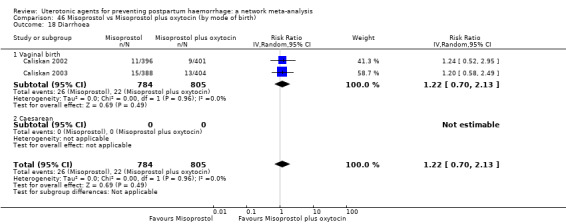
Comparison 46 Misoprostol vs Misoprostol plus oxytocin (by mode of birth), Outcome 18 Diarrhoea.
Comparison 47. Carbetocin vs Injectable prostaglandins (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 48. Injectable prostaglandins vs Ergometrine (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.1 Vaginal birth | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 4 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.58] |
| 3.1 Vaginal birth | 4 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.58] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 3 | 399 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.64, 3.17] |
| 6.1 Vaginal birth | 3 | 399 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.64, 3.17] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.40, 1.51] |
| 7.1 Vaginal birth | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.40, 1.51] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 8 | 1195 | Mean Difference (IV, Random, 95% CI) | ‐16.08 [‐57.17, 25.00] |
| 8.1 Vaginal birth | 8 | 1195 | Mean Difference (IV, Random, 95% CI) | ‐16.08 [‐57.17, 25.00] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐6.58, 4.01] |
| 9.1 Vaginal birth | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐6.58, 4.01] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.98, 2.94] |
| 11.1 Vaginal birth | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.98, 2.94] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 2.70 [0.97, 7.55] |
| 12.1 Vaginal birth | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 2.70 [0.97, 7.55] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 13.1 Vaginal birth | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 3 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.07, 40.44] |
| 14.1 Vaginal birth | 3 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.07, 40.44] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.02, 1.37] |
| 15.1 Vaginal birth | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.02, 1.37] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 434 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.16, 0.91] |
| 16.1 Vaginal birth | 3 | 434 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.16, 0.91] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 4 | 534 | Risk Ratio (IV, Random, 95% CI) | 4.52 [0.76, 26.80] |
| 17.1 Vaginal birth | 4 | 534 | Risk Ratio (IV, Random, 95% CI) | 4.52 [0.76, 26.80] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 5 | 664 | Risk Ratio (IV, Random, 95% CI) | 10.09 [2.75, 37.00] |
| 18.1 Vaginal birth | 5 | 664 | Risk Ratio (IV, Random, 95% CI) | 10.09 [2.75, 37.00] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
48.1. Analysis.
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 1 Death.
48.2. Analysis.
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 2 PPH >= 1000 mL.
48.3. Analysis.
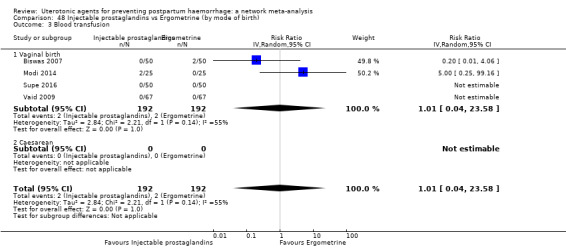
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 3 Blood transfusion.
48.6. Analysis.
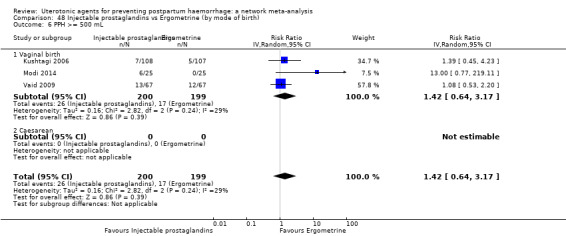
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 6 PPH >= 500 mL.
48.7. Analysis.
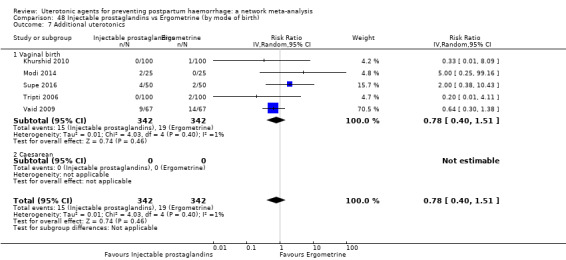
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 7 Additional uterotonics.
48.8. Analysis.
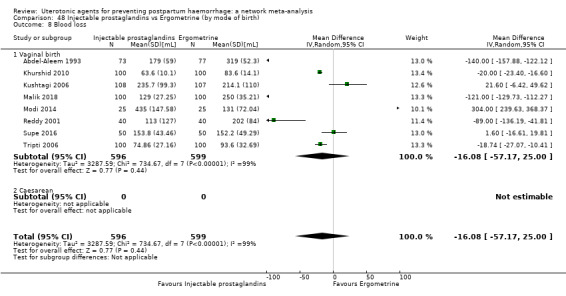
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 8 Blood loss.
48.9. Analysis.
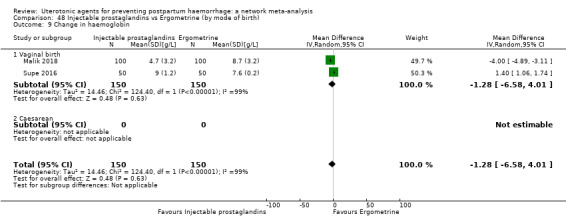
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 9 Change in haemoglobin.
48.11. Analysis.
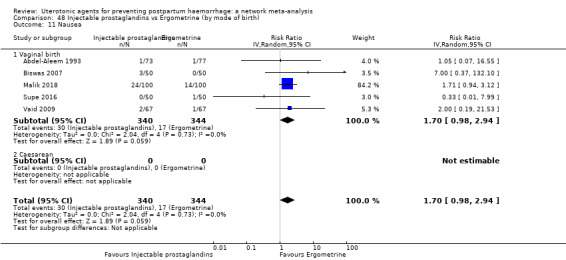
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 11 Nausea.
48.12. Analysis.
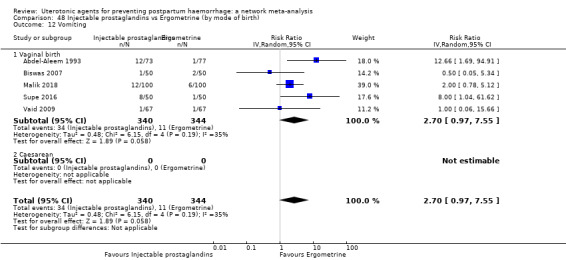
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 12 Vomiting.
48.13. Analysis.
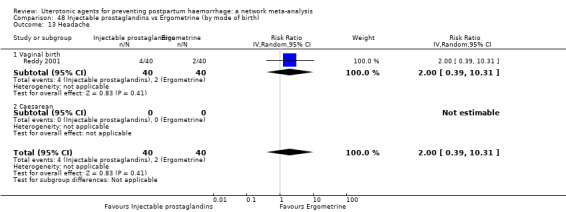
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 13 Headache.
48.14. Analysis.
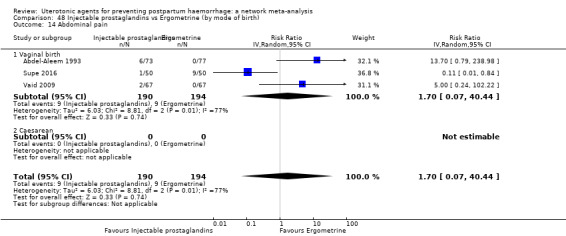
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 14 Abdominal pain.
48.15. Analysis.
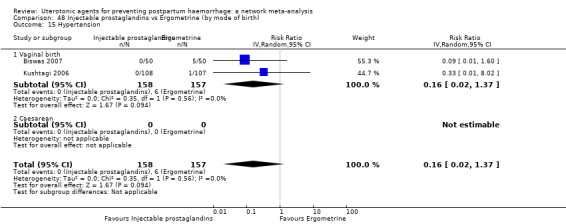
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 15 Hypertension.
48.16. Analysis.
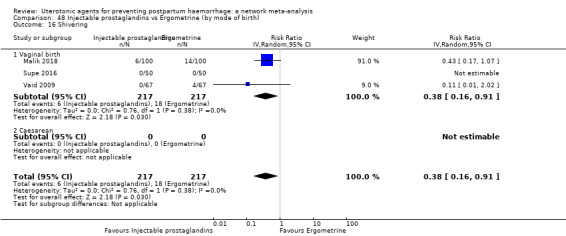
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 16 Shivering.
48.17. Analysis.
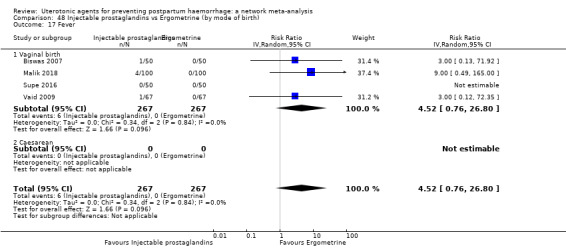
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 17 Fever.
48.18. Analysis.
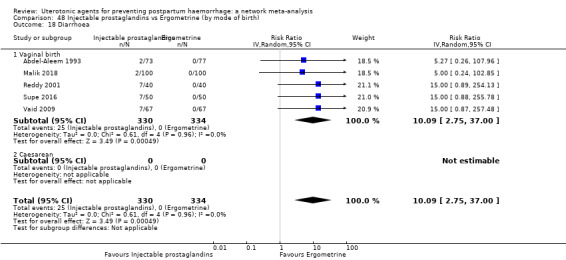
Comparison 48 Injectable prostaglandins vs Ergometrine (by mode of birth), Outcome 18 Diarrhoea.
Comparison 49. Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.17, 3.78] |
| 2.1 Vaginal birth | 1 | 69 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.11, 1.23] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Both caesarean and vaginal birth | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.52, 5.97] |
| 3 Blood transfusion | 1 | 69 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.17, 2.53] |
| 3.1 Vaginal birth | 1 | 69 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.17, 2.53] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.92, 1.79] |
| 6.1 Vaginal birth | 1 | 69 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.66, 1.81] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Both caesarean and vaginal birth | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 1.45 [0.94, 2.24] |
| 7 Additional uterotonics | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.16, 7.36] |
| 7.1 Vaginal birth | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.16, 7.36] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 598 | Mean Difference (IV, Random, 95% CI) | ‐26.18 [‐104.63, 52.27] |
| 8.1 Vaginal birth | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐149.0 [‐421.73, 123.73] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Both caesarean and vaginal birth | 1 | 529 | Mean Difference (IV, Random, 95% CI) | ‐15.10 [‐97.01, 66.81] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 5.06 [1.12, 22.86] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Both caesarean and vaginal birth | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 5.06 [1.12, 22.86] |
| 12 Vomiting | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.40, 2.13] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Both caesarean and vaginal birth | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.40, 2.13] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 641 | Risk Ratio (IV, Random, 95% CI) | 23.70 [7.55, 74.45] |
| 18.1 Vaginal birth | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 17.19 [2.36, 125.22] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Both caesarean and vaginal birth | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 27.81 [6.86, 112.85] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
49.2. Analysis.
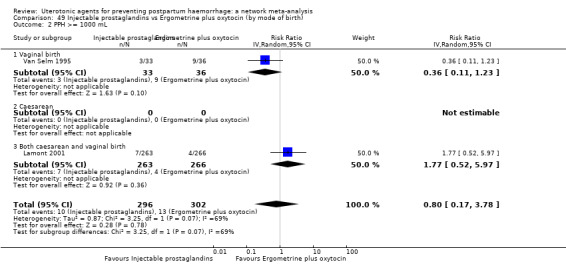
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
49.3. Analysis.
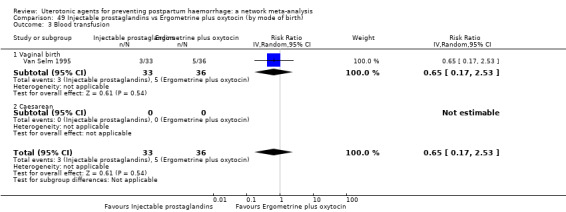
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 3 Blood transfusion.
49.6. Analysis.
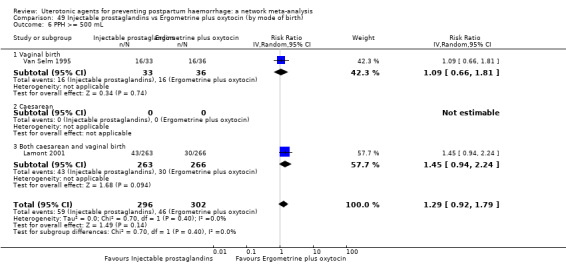
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
49.7. Analysis.
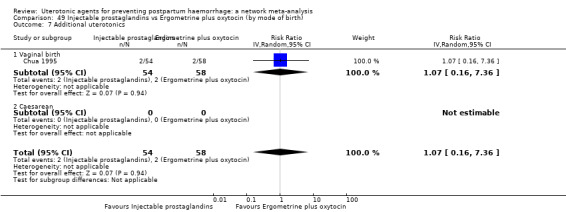
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
49.8. Analysis.
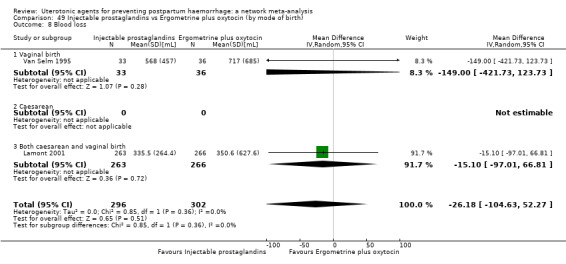
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 8 Blood loss.
49.11. Analysis.
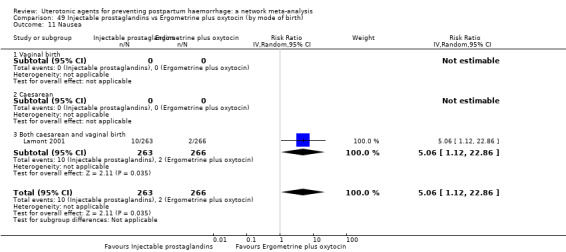
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 11 Nausea.
49.12. Analysis.
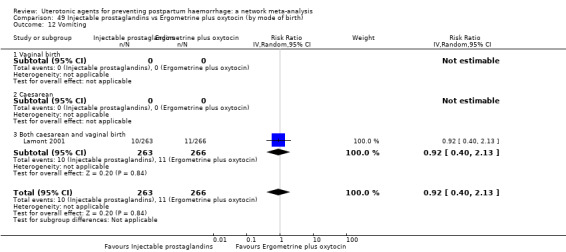
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 12 Vomiting.
49.18. Analysis.
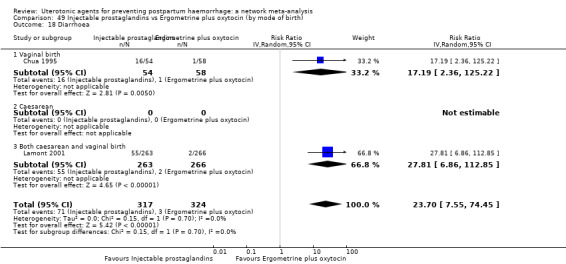
Comparison 49 Injectable prostaglandins vs Ergometrine plus oxytocin (by mode of birth), Outcome 18 Diarrhoea.
Comparison 50. Misoprostol plus oxytocin vs Injectable prostaglandins (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 51. Ergometrine vs Carbetocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 52. Carbetocin vs Ergometrine plus oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 4 | 1210 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.13, 0.86] |
| 2.1 Vaginal birth | 3 | 910 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.11, 4.38] |
| 2.2 Caesarean | 1 | 300 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.09, 0.78] |
| 3 Blood transfusion | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.42, 4.82] |
| 3.1 Vaginal birth | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.42, 4.82] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.44, 2.09] |
| 6.1 Vaginal birth | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.44, 2.09] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 7.1 Vaginal birth | 5 | 1230 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.44, 1.21] |
| 7.2 Caesarean | 1 | 300 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.08, 0.49] |
| 8 Blood loss | 5 | 1330 | Mean Difference (IV, Random, 95% CI) | ‐44.08 [‐82.41, ‐5.75] |
| 8.1 Vaginal birth | 4 | 1030 | Mean Difference (IV, Random, 95% CI) | ‐48.84 [‐94.82, ‐2.85] |
| 8.2 Caesarean | 1 | 300 | Mean Difference (IV, Random, 95% CI) | ‐24.0 [‐68.42, 20.42] |
| 9 Change in haemoglobin | 5 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐4.32, ‐0.82] |
| 9.1 Vaginal birth | 4 | 860 | Mean Difference (IV, Random, 95% CI) | ‐2.86 [‐4.81, ‐0.90] |
| 9.2 Caesarean | 1 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.83, 1.83] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.19, 0.45] |
| 11.1 Vaginal birth | 5 | 1230 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.16, 0.43] |
| 11.2 Caesarean | 1 | 300 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.16, 1.28] |
| 12 Vomiting | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.16, 0.46] |
| 12.1 Vaginal birth | 5 | 1230 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.13, 0.44] |
| 12.2 Caesarean | 1 | 300 | Risk Ratio (IV, Random, 95% CI) | 0.4 [0.13, 1.25] |
| 13 Headache | 5 | 1330 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.45, 4.38] |
| 13.1 Vaginal birth | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.46, 1.49] |
| 13.2 Caesarean | 1 | 300 | Risk Ratio (IV, Random, 95% CI) | 8.5 [2.00, 36.15] |
| 14 Abdominal pain | 4 | 930 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 14.1 Vaginal birth | 4 | 930 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 660 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.22, 1.39] |
| 15.1 Vaginal birth | 3 | 660 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.22, 1.39] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 5 | 1290 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.26, 0.80] |
| 16.1 Vaginal birth | 4 | 990 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.22, 0.74] |
| 16.2 Caesarean | 1 | 300 | Risk Ratio (IV, Random, 95% CI) | 1.0 [0.21, 4.88] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
52.1. Analysis.
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 1 Death.
52.2. Analysis.
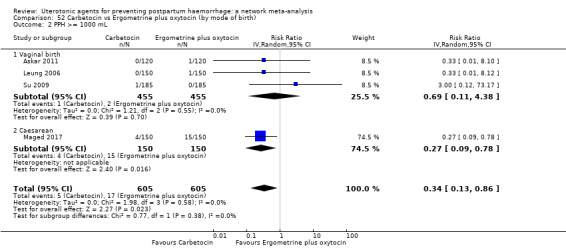
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
52.3. Analysis.
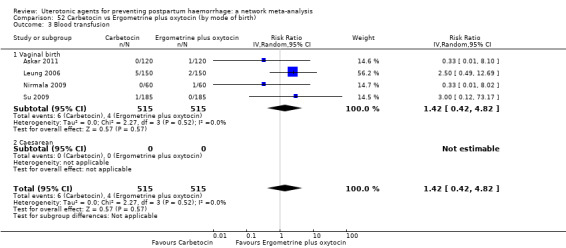
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 3 Blood transfusion.
52.4. Analysis.
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 4 Severe maternal morbidity: intensive care admissions.
52.6. Analysis.
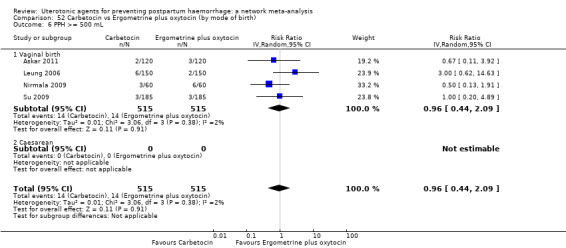
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
52.7. Analysis.
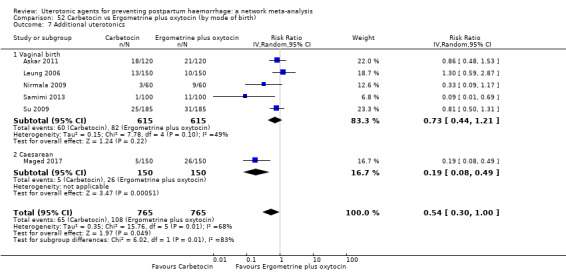
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
52.8. Analysis.
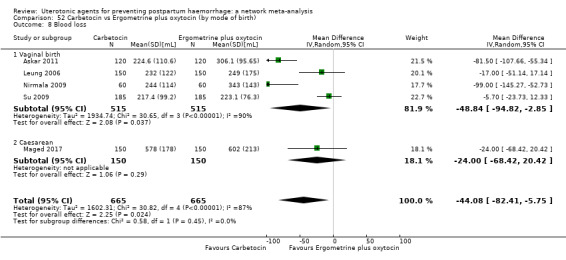
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 8 Blood loss.
52.9. Analysis.
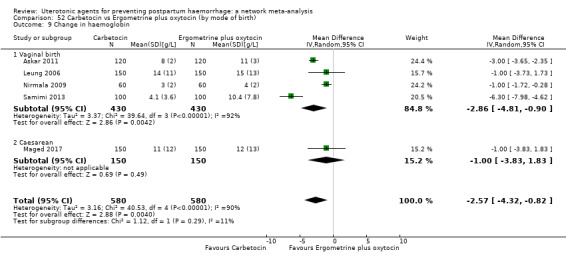
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
52.11. Analysis.
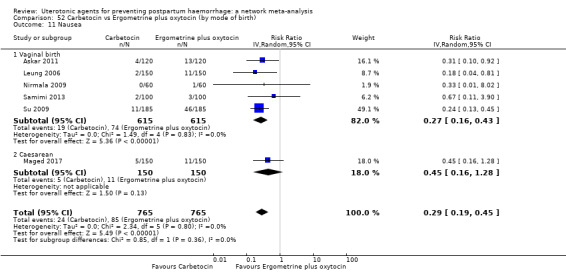
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 11 Nausea.
52.12. Analysis.
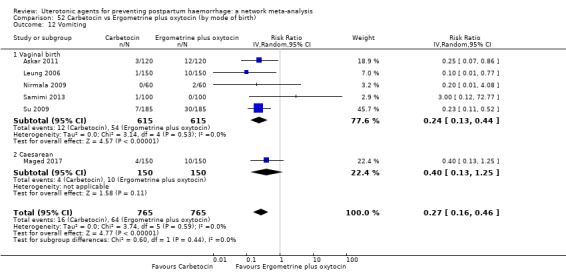
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 12 Vomiting.
52.13. Analysis.
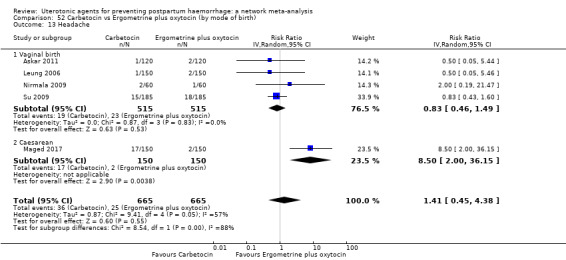
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 13 Headache.
52.14. Analysis.

Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 14 Abdominal pain.
52.15. Analysis.
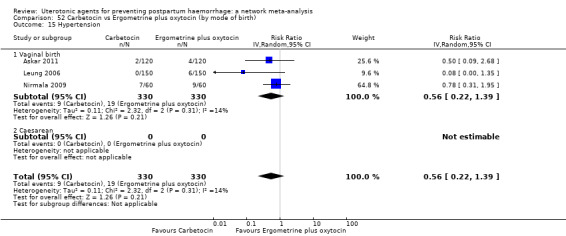
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 15 Hypertension.
52.16. Analysis.
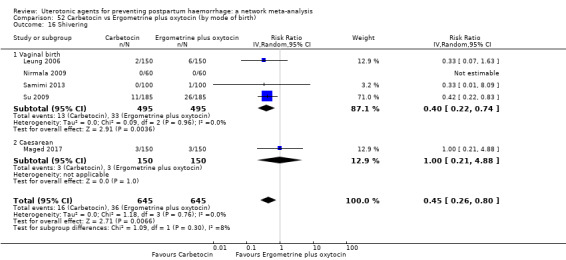
Comparison 52 Carbetocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 16 Shivering.
Comparison 53. Misoprostol plus oxytocin vs Carbetocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.45, 35.46] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.45, 35.46] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.74, 1.93] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.74, 1.93] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 380 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.72, 0.32] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 1 | 380 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.72, 0.32] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.62, 2.39] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.62, 2.39] |
| 12 Vomiting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.58, 3.09] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.58, 3.09] |
| 13 Headache | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.37, 10.79] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.37, 10.79] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 7.83 [3.43, 17.88] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 7.83 [3.43, 17.88] |
| 17 Fever | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 8.5 [1.99, 36.28] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 8.5 [1.99, 36.28] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
53.1. Analysis.
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 1 Death.
53.3. Analysis.

Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 3 Blood transfusion.
53.7. Analysis.
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 7 Additional uterotonics.
53.9. Analysis.
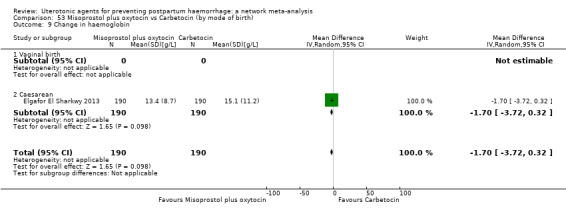
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 9 Change in haemoglobin.
53.11. Analysis.
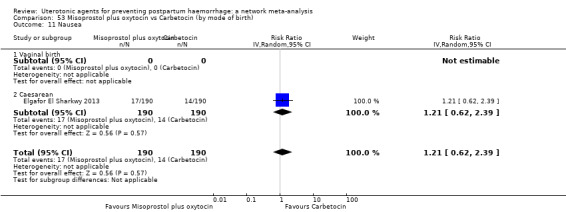
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 11 Nausea.
53.12. Analysis.
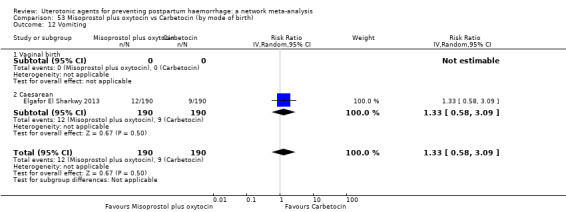
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 12 Vomiting.
53.13. Analysis.
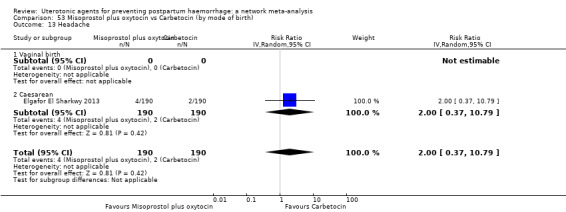
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 13 Headache.
53.16. Analysis.
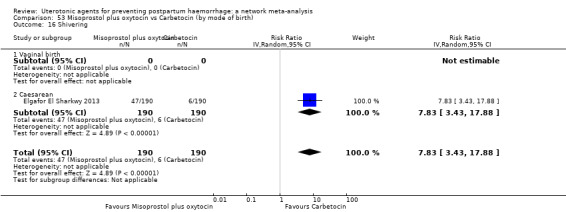
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 16 Shivering.
53.17. Analysis.
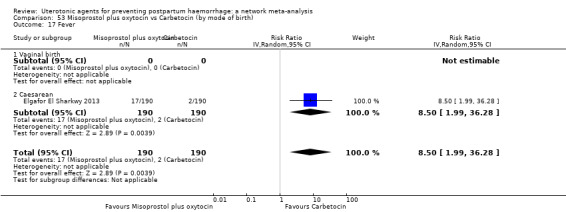
Comparison 53 Misoprostol plus oxytocin vs Carbetocin (by mode of birth), Outcome 17 Fever.
Comparison 54. Ergometrine vs Ergometrine plus oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 144 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 6.1 Vaginal birth | 1 | 144 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 20.10 [5.76, 34.44] |
| 8.1 Vaginal birth | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 20.10 [5.76, 34.44] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
54.6. Analysis.
Comparison 54 Ergometrine vs Ergometrine plus oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
54.8. Analysis.
Comparison 54 Ergometrine vs Ergometrine plus oxytocin (by mode of birth), Outcome 8 Blood loss.
Comparison 55. Misoprostol plus oxytocin vs Ergometrine (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Vaginal birth | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 56. Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.68, 2.94] |
| 2.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.68, 2.94] |
| 2.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.36, 2.19] |
| 3.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.36, 2.19] |
| 3.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.65, 2.99] |
| 6.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.65, 2.99] |
| 6.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.82, 2.69] |
| 7.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.82, 2.69] |
| 7.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 1 | 802 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐40.24, 8.24] |
| 8.1 Vaginal birth | 1 | 802 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐40.24, 8.24] |
| 8.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 1605 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.72, 0.72] |
| 9.1 Vaginal birth | 2 | 1605 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.72, 0.72] |
| 9.2 Caesarean | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.23, 4.77] |
| 12.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.23, 4.77] |
| 12.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Vaginal birth | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.95 [2.02, 4.29] |
| 16.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.95 [2.02, 4.29] |
| 16.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.89 [1.51, 5.54] |
| 17.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.89 [1.51, 5.54] |
| 17.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.46, 1.41] |
| 18.1 Vaginal birth | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.46, 1.41] |
| 18.2 Caesarean | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Vaginal birth | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Caesarean | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
56.2. Analysis.
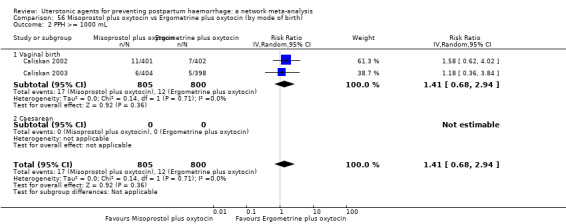
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 2 PPH >= 1000 mL.
56.3. Analysis.
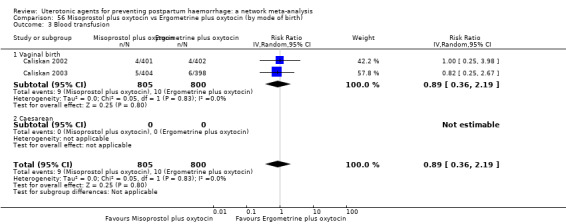
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 3 Blood transfusion.
56.6. Analysis.
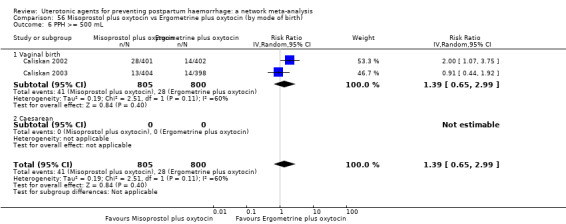
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 6 PPH >= 500 mL.
56.7. Analysis.
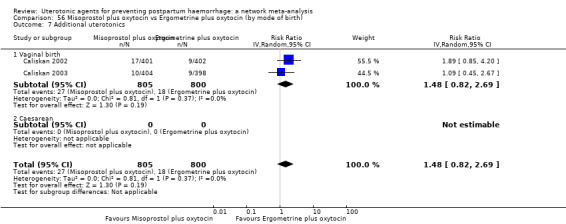
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 7 Additional uterotonics.
56.8. Analysis.
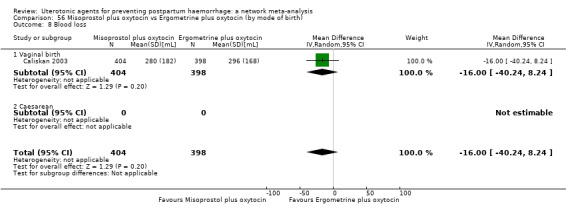
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 8 Blood loss.
56.9. Analysis.
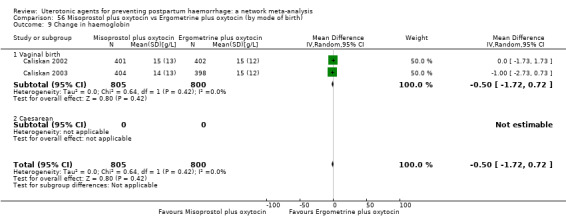
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 9 Change in haemoglobin.
56.12. Analysis.
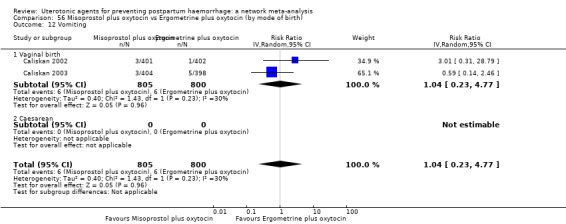
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 12 Vomiting.
56.16. Analysis.
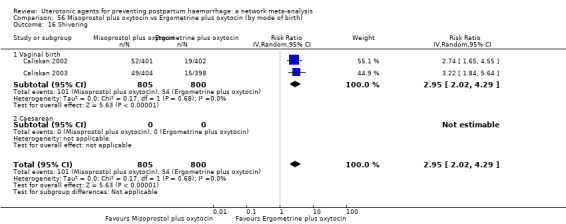
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 16 Shivering.
56.17. Analysis.
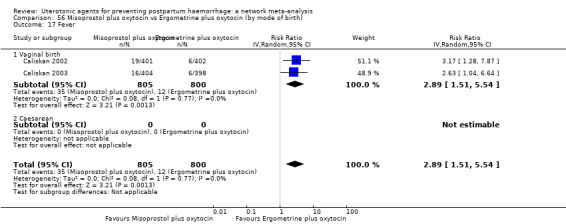
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 17 Fever.
56.18. Analysis.
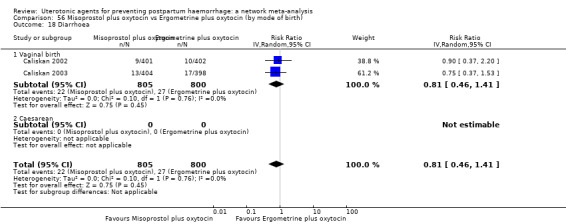
Comparison 56 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by mode of birth), Outcome 18 Diarrhoea.
Comparison 57. Oxytocin vs Placebo or no treatment (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | 1920 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 130 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 1 | 1569 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Both community and hospital setting | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 11 | 8782 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.51, 0.72] |
| 2.1 Hospital setting | 8 | 5306 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.52, 0.79] |
| 2.2 Community setting | 2 | 3255 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.22, 0.98] |
| 2.3 Both community and hospital setting | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.34, 1.87] |
| 3 Blood transfusion | 8 | 6717 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.51, 1.12] |
| 3.1 Hospital setting | 6 | 4810 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.44, 1.14] |
| 3.2 Community setting | 1 | 1686 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.36, 1.88] |
| 3.3 Both community and hospital setting | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.21, 7.16] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 1950 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 1 | 1950 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 9 | 7021 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.52, 0.71] |
| 6.1 Hospital setting | 6 | 3545 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.46, 0.63] |
| 6.2 Community setting | 2 | 3255 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.43, 0.95] |
| 6.3 Both community and hospital setting | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.57, 1.22] |
| 7 Additional uterotonics | 8 | 5047 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.32, 0.58] |
| 7.1 Hospital setting | 6 | 3154 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.27, 0.53] |
| 7.2 Community setting | 1 | 1672 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.31, 0.51] |
| 7.3 Both community and hospital setting | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.55, 1.78] |
| 8 Blood loss | 8 | 3521 | Mean Difference (IV, Random, 95% CI) | ‐118.52 [‐141.40, ‐95.64] |
| 8.1 Hospital setting | 7 | 3300 | Mean Difference (IV, Random, 95% CI) | ‐122.08 [‐145.37, ‐98.79] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Both community and hospital setting | 1 | 221 | Mean Difference (IV, Random, 95% CI) | ‐21.0 [‐142.93, 100.93] |
| 9 Change in haemoglobin | 5 | 2304 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐4.47, ‐0.89] |
| 9.1 Hospital setting | 5 | 2304 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐4.47, ‐0.89] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Both community and hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 1 | 1540 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 1 | 1540 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 10.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 3 | 1788 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.47, 1.42] |
| 11.1 Hospital setting | 2 | 126 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.03, 2.10] |
| 11.2 Community setting | 1 | 1662 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.51, 1.58] |
| 11.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 3 | 2150 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.44, 4.41] |
| 12.1 Hospital setting | 2 | 490 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.07, 17.83] |
| 12.2 Community setting | 1 | 1660 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.41, 5.17] |
| 12.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 2 | 1704 | Risk Ratio (IV, Random, 95% CI) | 1.56 [0.52, 4.74] |
| 13.1 Hospital setting | 1 | 51 | Risk Ratio (IV, Random, 95% CI) | 6.74 [0.37, 124.21] |
| 13.2 Community setting | 1 | 1653 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.63, 2.51] |
| 13.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 1 | 1665 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 1 | 1665 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 14.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 1 | 416 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Both community and hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal sense of well‐being | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting ‐ Women’s perceptions of well‐being at 3 months postpartum: Less energy than before birth | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Community setting ‐ Women’s perceptions of well‐being at 3 months postpartum: Experiencing (some) fatigue | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.4 Both community and hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting ‐ Did management influence positively the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Hospital setting ‐ Did management influence negatively the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Hospital setting ‐ Did management make no difference in the childbirth experiences of the mothers? | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 Both community and hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
57.1. Analysis.
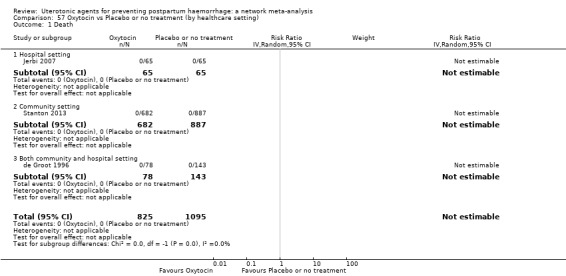
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 1 Death.
57.2. Analysis.
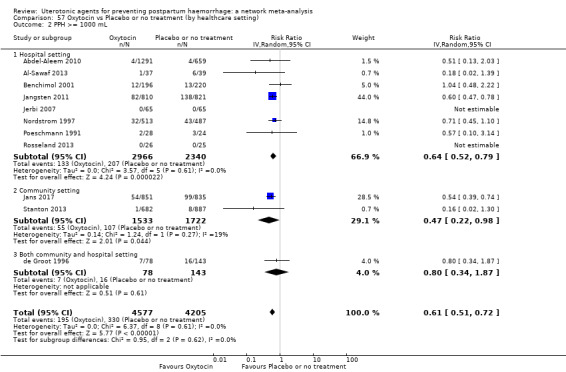
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 2 PPH >= 1000 mL.
57.3. Analysis.
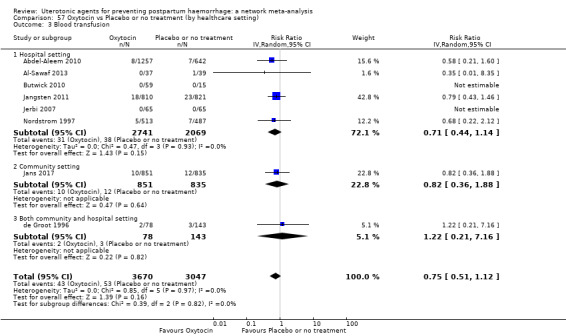
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 3 Blood transfusion.
57.4. Analysis.
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
57.6. Analysis.
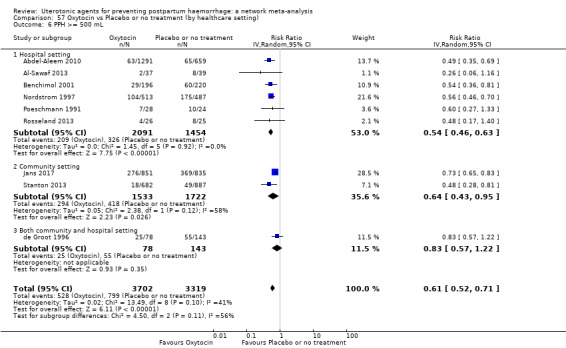
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 6 PPH >= 500 mL.
57.7. Analysis.
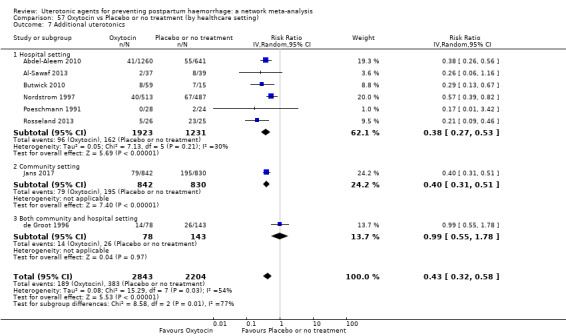
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 7 Additional uterotonics.
57.8. Analysis.
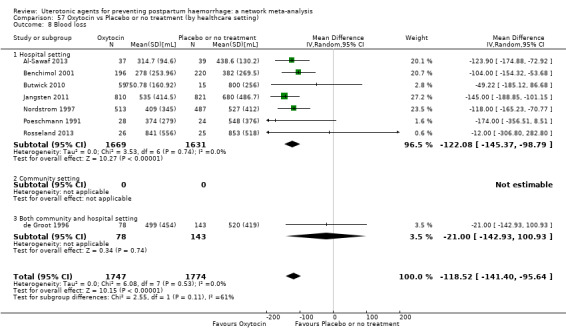
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 8 Blood loss.
57.9. Analysis.

Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 9 Change in haemoglobin.
57.10. Analysis.
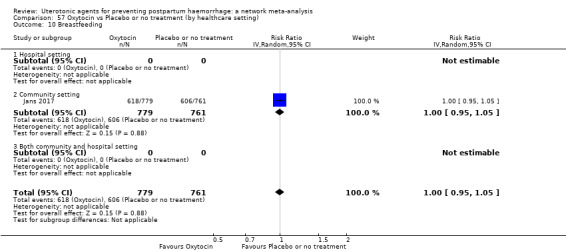
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 10 Breastfeeding.
57.11. Analysis.
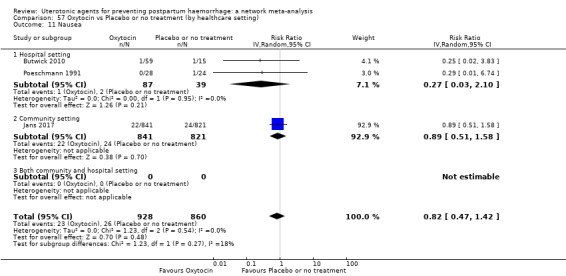
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 11 Nausea.
57.12. Analysis.
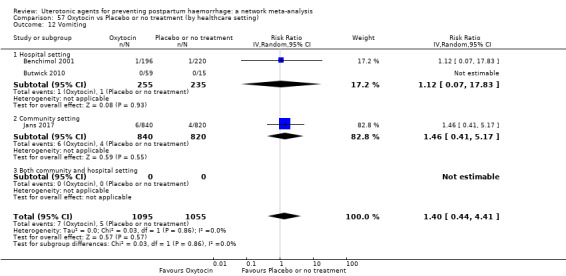
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 12 Vomiting.
57.13. Analysis.
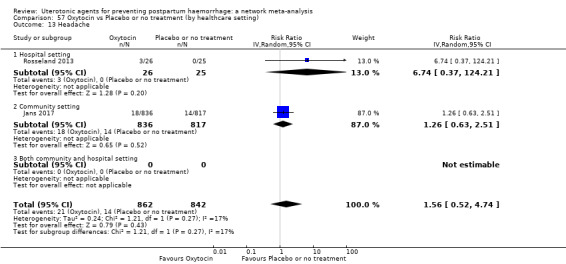
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 13 Headache.
57.14. Analysis.
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 14 Abdominal pain.
57.16. Analysis.
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 16 Shivering.
57.17. Analysis.
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 17 Fever.
57.19. Analysis.
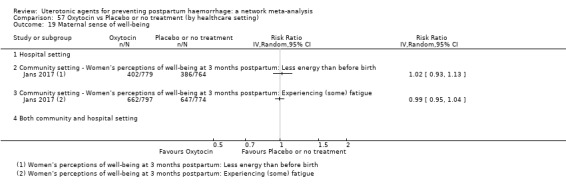
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 19 Maternal sense of well‐being.
57.20. Analysis.
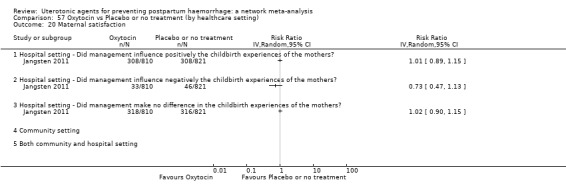
Comparison 57 Oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 20 Maternal satisfaction.
Comparison 58. Carbetocin vs Placebo or no treatment (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.30, 1.85] |
| 6.1 Hospital setting | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.30, 1.85] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.12, 0.32] |
| 7.1 Hospital setting | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.12, 0.32] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐274.0 [‐591.60, 43.60] |
| 8.1 Hospital setting | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐274.0 [‐591.60, 43.60] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.23, 0.43] |
| 9.1 Hospital setting | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐7.23, 0.43] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 13.1 Hospital setting | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
58.2. Analysis.
Comparison 58 Carbetocin vs Placebo or no treatment (by healthcare setting), Outcome 2 PPH >= 1000 mL.
58.6. Analysis.

Comparison 58 Carbetocin vs Placebo or no treatment (by healthcare setting), Outcome 6 PPH >= 500 mL.
58.7. Analysis.
Comparison 58 Carbetocin vs Placebo or no treatment (by healthcare setting), Outcome 7 Additional uterotonics.
58.8. Analysis.
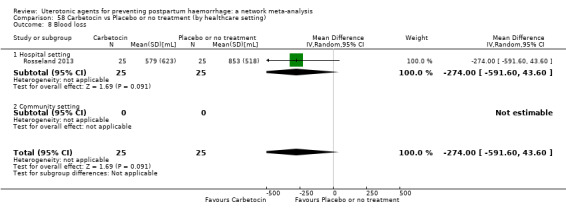
Comparison 58 Carbetocin vs Placebo or no treatment (by healthcare setting), Outcome 8 Blood loss.
58.9. Analysis.
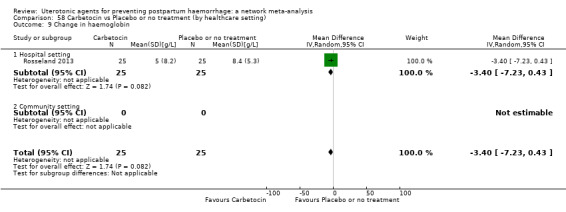
Comparison 58 Carbetocin vs Placebo or no treatment (by healthcare setting), Outcome 9 Change in haemoglobin.
58.13. Analysis.
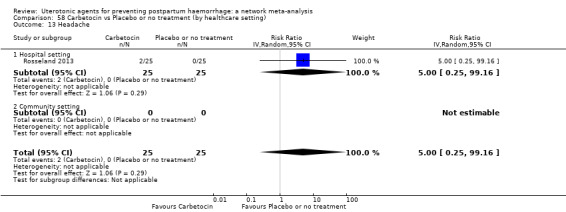
Comparison 58 Carbetocin vs Placebo or no treatment (by healthcare setting), Outcome 13 Headache.
Comparison 59. Misoprostol vs Placebo or no treatment (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 3497 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.10, 9.59] |
| 1.1 Hospital setting | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 3 | 3397 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.10, 9.59] |
| 2 PPH >= 1000 mL | 8 | 5467 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.56, 0.95] |
| 2.1 Hospital setting | 5 | 2114 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.63, 1.15] |
| 2.2 Community setting | 3 | 3353 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.39, 0.88] |
| 3 Blood transfusion | 6 | 3134 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.15, 1.47] |
| 3.1 Hospital setting | 5 | 1514 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.19, 3.10] |
| 3.2 Community setting | 1 | 1620 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.02, 1.15] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 1620 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.14, 7.05] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 1 | 1620 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.14, 7.05] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 7 | 4047 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.59, 0.94] |
| 6.1 Hospital setting | 4 | 694 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.29, 1.25] |
| 6.2 Community setting | 3 | 3353 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.56, 0.96] |
| 7 Additional uterotonics | 8 | 3746 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.52, 0.87] |
| 7.1 Hospital setting | 7 | 2126 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.52, 0.88] |
| 7.2 Community setting | 1 | 1620 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.12, 1.98] |
| 8 Blood loss | 8 | 4146 | Mean Difference (IV, Random, 95% CI) | ‐42.07 [‐52.47, ‐31.68] |
| 8.1 Hospital setting | 5 | 794 | Mean Difference (IV, Random, 95% CI) | ‐41.82 [‐61.16, ‐22.49] |
| 8.2 Community setting | 3 | 3352 | Mean Difference (IV, Random, 95% CI) | ‐43.79 [‐58.09, ‐29.49] |
| 9 Change in haemoglobin | 5 | 2334 | Mean Difference (IV, Random, 95% CI) | ‐2.53 [‐3.53, ‐1.52] |
| 9.1 Hospital setting | 3 | 573 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐4.30, 0.19] |
| 9.2 Community setting | 2 | 1761 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐3.46, ‐0.77] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 4 | 3997 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.78, 1.78] |
| 11.1 Hospital setting | 1 | 600 | Risk Ratio (IV, Random, 95% CI) | 5.00 [0.59, 42.54] |
| 11.2 Community setting | 3 | 3397 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.74, 1.70] |
| 12 Vomiting | 6 | 4949 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.92, 2.16] |
| 12.1 Hospital setting | 3 | 1552 | Risk Ratio (IV, Random, 95% CI) | 2.79 [0.85, 9.15] |
| 12.2 Community setting | 3 | 3397 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.80, 2.01] |
| 13 Headache | 1 | 1116 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.32, 2.77] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 1 | 1116 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.32, 2.77] |
| 14 Abdominal pain | 4 | 1894 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.29, 3.37] |
| 14.1 Hospital setting | 4 | 1894 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.29, 3.37] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 9 | 5286 | Risk Ratio (IV, Random, 95% CI) | 2.94 [2.38, 3.64] |
| 16.1 Hospital setting | 6 | 1889 | Risk Ratio (IV, Random, 95% CI) | 3.55 [2.13, 5.90] |
| 16.2 Community setting | 3 | 3397 | Risk Ratio (IV, Random, 95% CI) | 2.71 [2.33, 3.15] |
| 17 Fever | 5 | 4396 | Risk Ratio (IV, Random, 95% CI) | 4.09 [2.01, 8.32] |
| 17.1 Hospital setting | 2 | 999 | Risk Ratio (IV, Random, 95% CI) | 6.38 [4.01, 10.14] |
| 17.2 Community setting | 3 | 3397 | Risk Ratio (IV, Random, 95% CI) | 2.87 [0.90, 9.18] |
| 18 Diarrhoea | 6 | 4791 | Risk Ratio (IV, Random, 95% CI) | 2.80 [1.21, 6.49] |
| 18.1 Hospital setting | 3 | 1394 | Risk Ratio (IV, Random, 95% CI) | 1.0 [0.06, 15.91] |
| 18.2 Community setting | 3 | 3397 | Risk Ratio (IV, Random, 95% CI) | 3.11 [1.28, 7.51] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
59.1. Analysis.
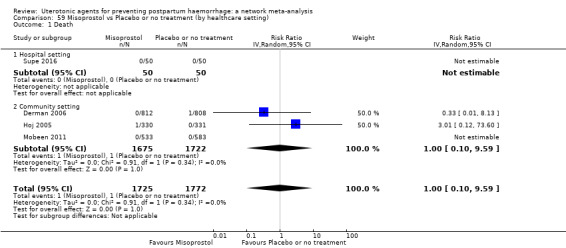
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 1 Death.
59.2. Analysis.
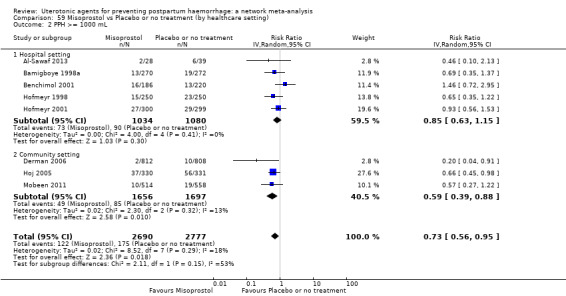
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 2 PPH >= 1000 mL.
59.3. Analysis.
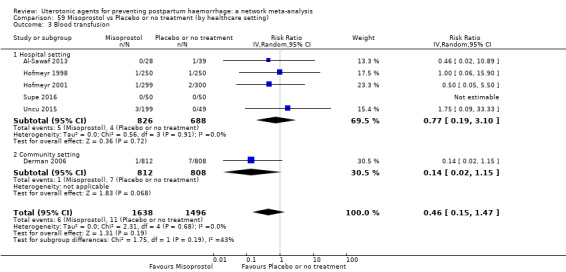
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 3 Blood transfusion.
59.4. Analysis.
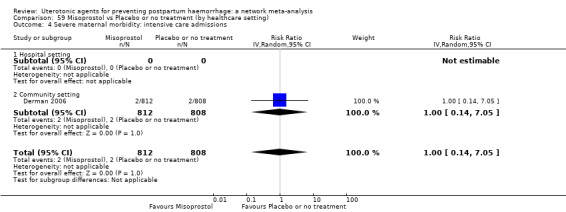
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
59.6. Analysis.
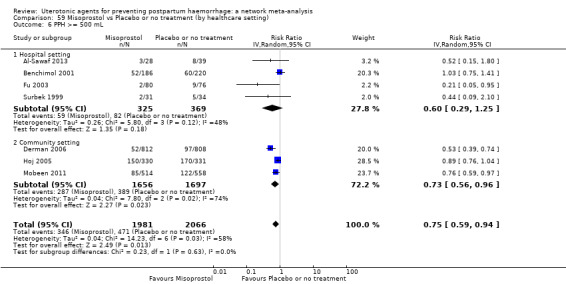
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 6 PPH >= 500 mL.
59.7. Analysis.
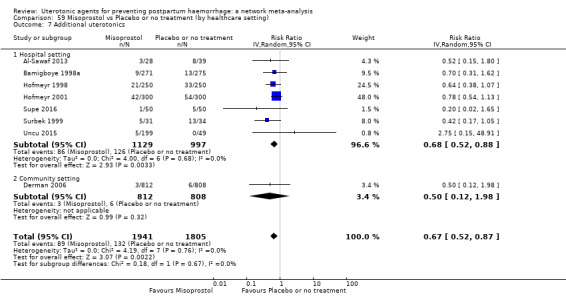
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 7 Additional uterotonics.
59.8. Analysis.
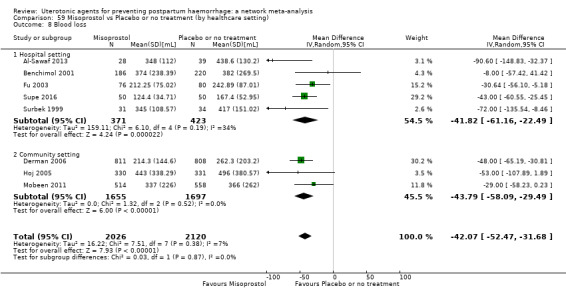
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 8 Blood loss.
59.9. Analysis.
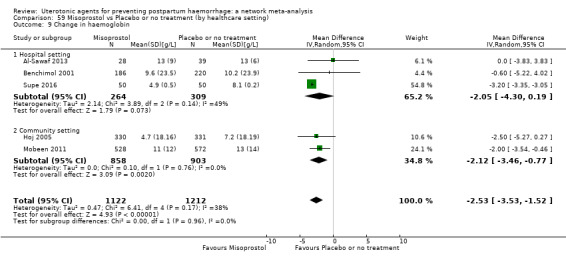
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 9 Change in haemoglobin.
59.11. Analysis.
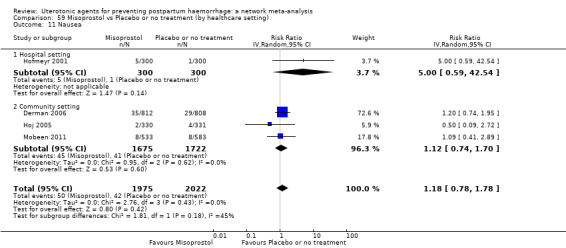
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 11 Nausea.
59.12. Analysis.
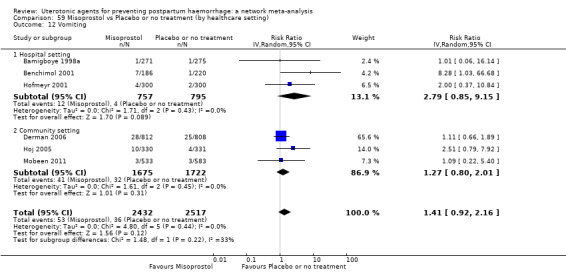
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 12 Vomiting.
59.13. Analysis.
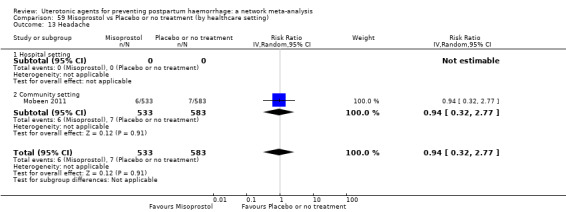
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 13 Headache.
59.14. Analysis.
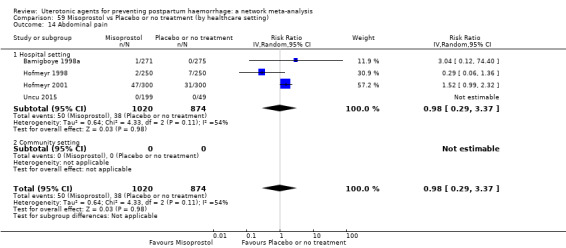
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 14 Abdominal pain.
59.16. Analysis.
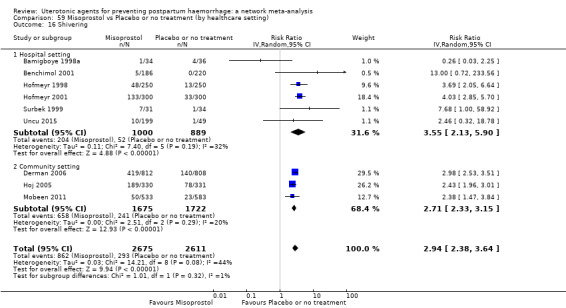
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 16 Shivering.
59.17. Analysis.
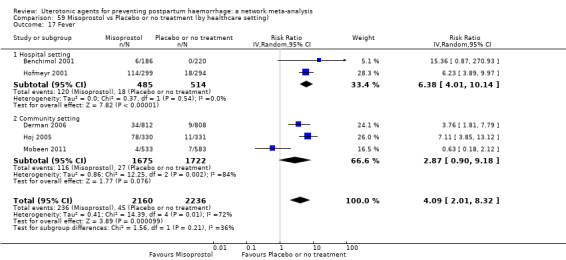
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 17 Fever.
59.18. Analysis.
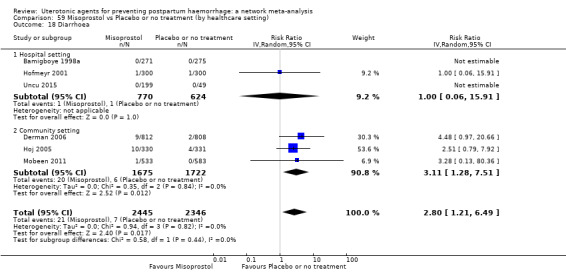
Comparison 59 Misoprostol vs Placebo or no treatment (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 60. Injectable prostaglandins vs Placebo or no treatment (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.04, 3.24] |
| 2.1 Hospital setting | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.04, 3.24] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.22, 1.35] |
| 6.1 Hospital setting | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.22, 1.35] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 2 | 146 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.21, 2.09] |
| 7.1 Hospital setting | 2 | 146 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.21, 2.09] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐95.17 [‐296.09, 105.75] |
| 8.1 Hospital setting | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐95.17 [‐296.09, 105.75] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.56, 1.24] |
| 9.1 Hospital setting | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.56, 1.24] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.02, 8.46] |
| 11.1 Hospital setting | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.02, 8.46] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
60.1. Analysis.
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 1 Death.
60.2. Analysis.
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 2 PPH >= 1000 mL.
60.3. Analysis.
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 3 Blood transfusion.
60.6. Analysis.
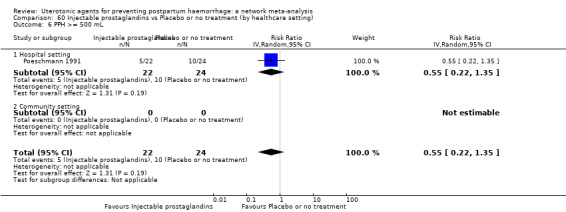
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 6 PPH >= 500 mL.
60.7. Analysis.
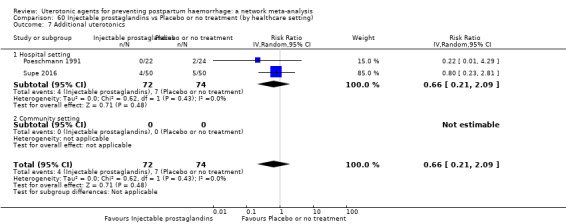
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 7 Additional uterotonics.
60.8. Analysis.
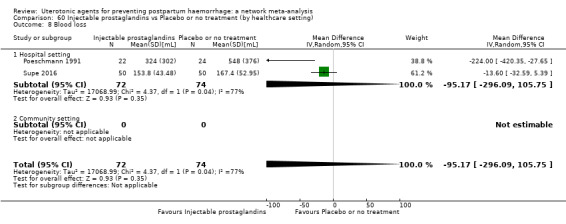
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 8 Blood loss.
60.9. Analysis.
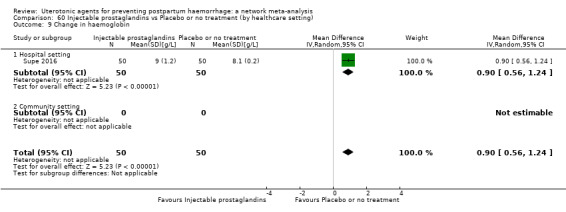
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 9 Change in haemoglobin.
60.11. Analysis.
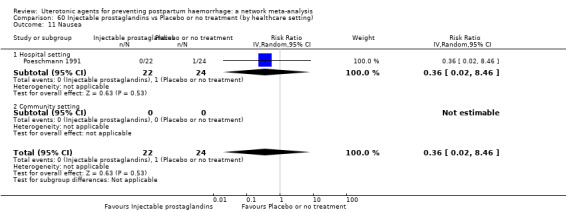
Comparison 60 Injectable prostaglandins vs Placebo or no treatment (by healthcare setting), Outcome 11 Nausea.
Comparison 61. Ergometrine vs Placebo or no treatment (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| 2.1 Hospital setting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.04, 3.28] |
| 3.1 Hospital setting | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.04, 3.28] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.14, 0.42] |
| 6.1 Hospital setting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.14, 0.42] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.37] |
| 7.1 Hospital setting | 2 | 1529 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.09, 0.37] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 1529 | Mean Difference (IV, Random, 95% CI) | ‐50.64 [‐119.92, 18.65] |
| 8.1 Hospital setting | 2 | 1529 | Mean Difference (IV, Random, 95% CI) | ‐50.64 [‐119.92, 18.65] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 9.1 Hospital setting | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.58, ‐0.42] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 42.10 [2.55, 694.80] |
| 11.1 Hospital setting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 42.10 [2.55, 694.80] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 25.67 [1.52, 432.78] |
| 12.1 Hospital setting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 25.67 [1.52, 432.78] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [0.37, 138.91] |
| 13.1 Hospital setting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [0.37, 138.91] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.04, 4.08] |
| 14.1 Hospital setting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.04, 4.08] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [2.83, 18.24] |
| 15.1 Hospital setting | 1 | 1429 | Risk Ratio (IV, Random, 95% CI) | 7.19 [2.83, 18.24] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
61.1. Analysis.
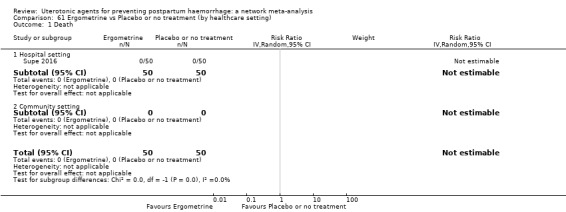
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 1 Death.
61.2. Analysis.
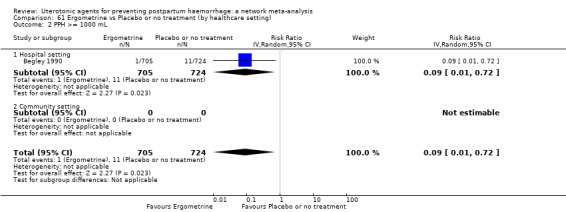
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 2 PPH >= 1000 mL.
61.3. Analysis.
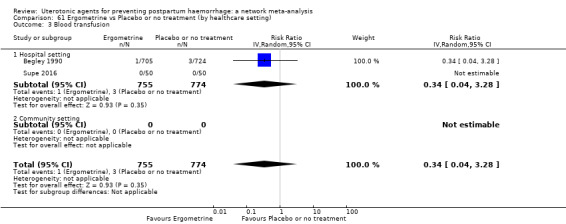
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 3 Blood transfusion.
61.6. Analysis.
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 6 PPH >= 500 mL.
61.7. Analysis.
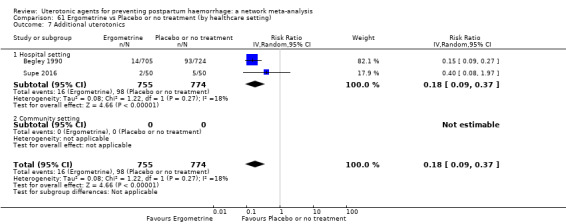
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 7 Additional uterotonics.
61.8. Analysis.
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 8 Blood loss.
61.9. Analysis.
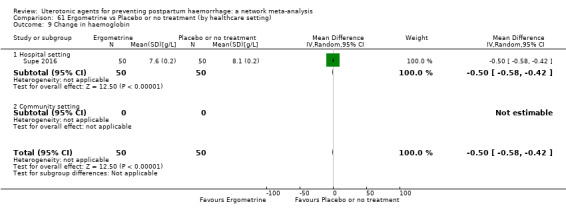
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 9 Change in haemoglobin.
61.11. Analysis.
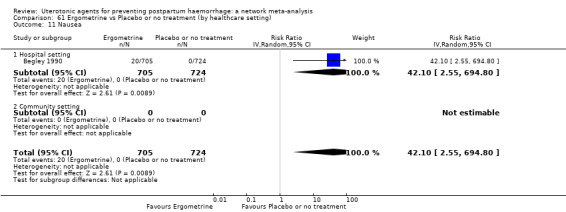
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 11 Nausea.
61.12. Analysis.
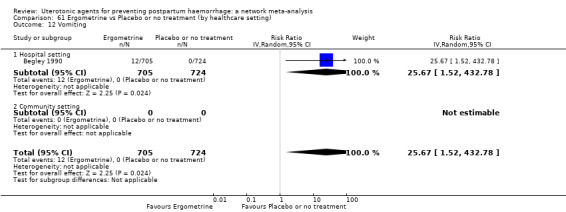
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 12 Vomiting.
61.13. Analysis.
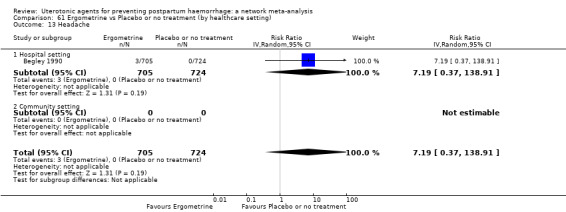
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 13 Headache.
61.14. Analysis.
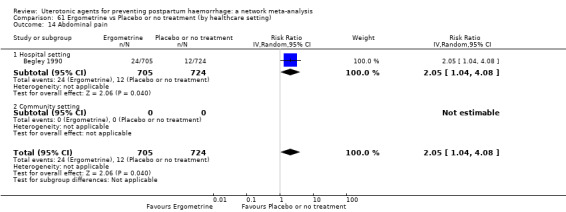
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 14 Abdominal pain.
61.15. Analysis.
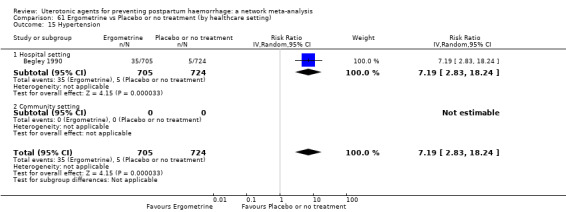
Comparison 61 Ergometrine vs Placebo or no treatment (by healthcare setting), Outcome 15 Hypertension.
Comparison 62. Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.18, 1.05] |
| 2.1 Hospital setting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.18, 1.05] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.18, 0.66] |
| 3.1 Hospital setting | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.18, 0.66] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.30, 0.46] |
| 6.1 Hospital setting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.30, 0.46] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.15, 0.24] |
| 7.1 Hospital setting | 3 | 3400 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.15, 0.24] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 1705 | Mean Difference (IV, Random, 95% CI) | ‐35.02 [‐101.63, 31.59] |
| 8.1 Hospital setting | 2 | 1705 | Mean Difference (IV, Random, 95% CI) | ‐35.02 [‐101.63, 31.59] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 1454 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐6.50, ‐0.63] |
| 9.1 Hospital setting | 2 | 1454 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐6.50, ‐0.63] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 10.1 Hospital setting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.99, 1.07] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 1512 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.38, 2.76] |
| 11.1 Hospital setting | 1 | 1512 | Risk Ratio (IV, Random, 95% CI) | 1.95 [1.38, 2.76] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 2.15 [1.46, 3.18] |
| 12.1 Hospital setting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 2.15 [1.46, 3.18] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.78, 3.48] |
| 13.1 Hospital setting | 2 | 3207 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.78, 3.48] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting ‐ General health at 6 weeks postpartum (Worse than prepregnancy) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Hospital setting ‐ General health at 6 weeks postpartum (Exhausted since birth) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Hospital setting ‐ General health at 6 weeks postpartum (Exhausted at 6 weeks) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.4 Hospital setting ‐ General health at 6 weeks postpartum (Blues) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.5 Hospital setting ‐ General health at 6 weeks postpartum (Depressed) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.6 Hospital setting ‐ General health at 6 weeks postpartum (Help for depression) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.7 Hospital setting ‐ General health at 6 weeks postpartum (Admission to hospital for depression) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.8 Hospital setting ‐ General health at 6 weeks postpartum (No health problems reported) | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.9 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting ‐ Satisfied with third‐stage management | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Hospital setting ‐ Felt in control during third stage | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
62.2. Analysis.
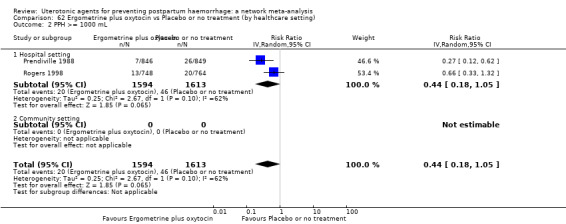
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 2 PPH >= 1000 mL.
62.3. Analysis.
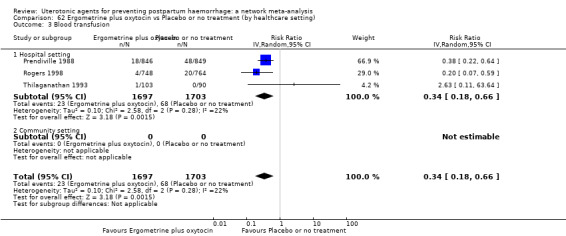
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 3 Blood transfusion.
62.6. Analysis.
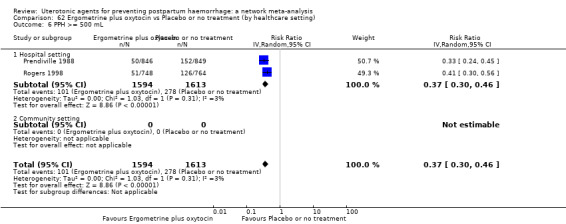
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 6 PPH >= 500 mL.
62.7. Analysis.
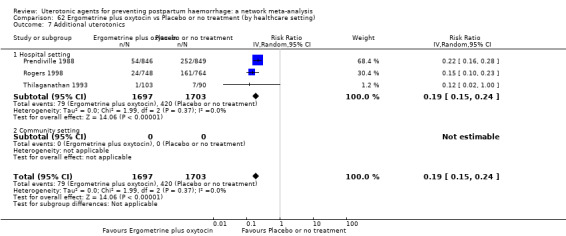
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 7 Additional uterotonics.
62.8. Analysis.
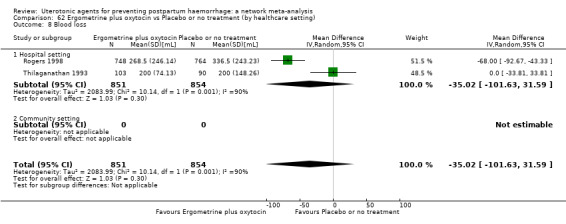
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 8 Blood loss.
62.9. Analysis.
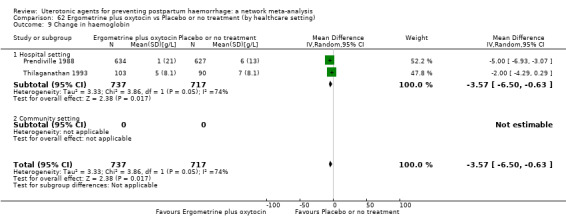
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 9 Change in haemoglobin.
62.10. Analysis.
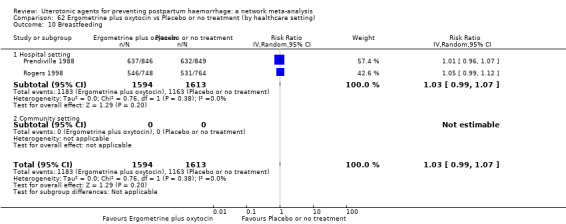
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 10 Breastfeeding.
62.11. Analysis.
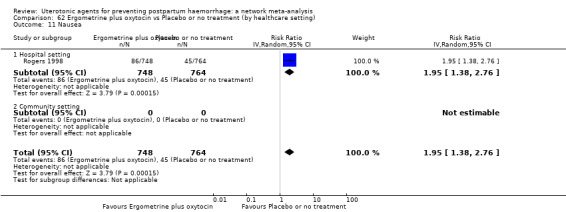
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 11 Nausea.
62.12. Analysis.
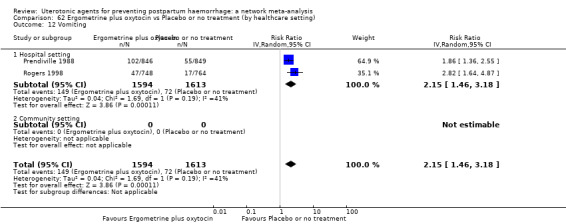
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 12 Vomiting.
62.13. Analysis.
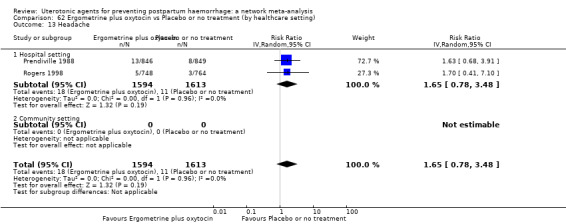
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 13 Headache.
62.19. Analysis.
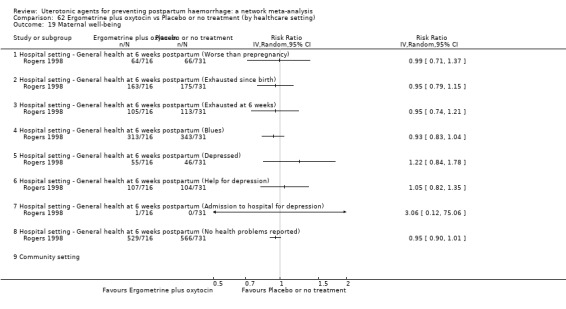
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 19 Maternal well‐being.
62.20. Analysis.
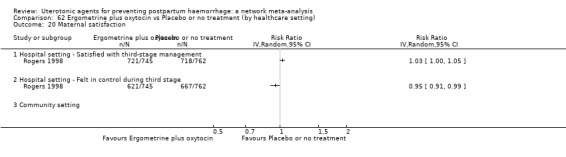
Comparison 62 Ergometrine plus oxytocin vs Placebo or no treatment (by healthcare setting), Outcome 20 Maternal satisfaction.
Comparison 63. Misoprostol plus oxytocin vs Placebo or no treatment (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 64. Misoprostol vs Oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 24 | 28520 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.14, 2.74] |
| 1.1 Hospital setting | 23 | 28127 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.14, 2.74] |
| 1.2 Community setting | 1 | 393 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 38 | 34261 | Risk Ratio (IV, Random, 95% CI) | 1.26 [1.11, 1.43] |
| 2.1 Hospital setting | 38 | 34261 | Risk Ratio (IV, Random, 95% CI) | 1.26 [1.11, 1.43] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 42 | 35467 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 3.1 Hospital setting | 42 | 35467 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.00] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 10 | 21698 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.55, 2.43] |
| 4.1 Hospital setting | 10 | 21698 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.55, 2.43] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 44 | 36920 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 6.1 Hospital setting | 44 | 36920 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 47 | 35981 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 7.1 Hospital setting | 47 | 35981 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 43 | 35239 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐23.45, 5.65] |
| 8.1 Hospital setting | 43 | 35239 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐23.45, 5.65] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 31 | 12028 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.74, 0.47] |
| 9.1 Hospital setting | 30 | 11724 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.77, 0.46] |
| 9.2 Community setting | 1 | 304 | Mean Difference (IV, Random, 95% CI) | 0.76 [‐3.21, 4.73] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 33 | 29732 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.93, 1.60] |
| 11.1 Hospital setting | 32 | 29339 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.93, 1.61] |
| 11.2 Community setting | 1 | 393 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.14, 4.96] |
| 12 Vomiting | 41 | 32687 | Risk Ratio (IV, Random, 95% CI) | 1.51 [1.19, 1.91] |
| 12.1 Hospital setting | 40 | 32294 | Risk Ratio (IV, Random, 95% CI) | 1.48 [1.16, 1.89] |
| 12.2 Community setting | 1 | 393 | Risk Ratio (IV, Random, 95% CI) | 2.81 [0.14, 58.05] |
| 13 Headache | 10 | 4079 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.54, 1.42] |
| 13.1 Hospital setting | 10 | 4079 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.54, 1.42] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 8 | 3382 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 14.1 Hospital setting | 8 | 3382 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 1028 | Risk Ratio (IV, Random, 95% CI) | 3.64 [0.60, 22.27] |
| 15.1 Hospital setting | 3 | 1028 | Risk Ratio (IV, Random, 95% CI) | 3.64 [0.60, 22.27] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 49 | 34865 | Risk Ratio (IV, Random, 95% CI) | 4.02 [3.23, 4.99] |
| 16.1 Hospital setting | 48 | 34534 | Risk Ratio (IV, Random, 95% CI) | 3.82 [3.09, 4.74] |
| 16.2 Community setting | 1 | 331 | Risk Ratio (IV, Random, 95% CI) | 16.13 [7.81, 33.31] |
| 17 Fever | 41 | 33008 | Risk Ratio (IV, Random, 95% CI) | 3.75 [2.73, 5.15] |
| 17.1 Hospital setting | 40 | 32615 | Risk Ratio (IV, Random, 95% CI) | 3.80 [2.76, 5.25] |
| 17.2 Community setting | 1 | 393 | Risk Ratio (IV, Random, 95% CI) | 2.24 [0.48, 10.40] |
| 18 Diarrhoea | 26 | 30733 | Risk Ratio (IV, Random, 95% CI) | 2.13 [1.55, 2.93] |
| 18.1 Hospital setting | 25 | 30340 | Risk Ratio (IV, Random, 95% CI) | 2.17 [1.57, 2.99] |
| 18.2 Community setting | 1 | 393 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.04, 8.88] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting ‐ Complaints about or problems with drug | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Community setting ‐ Satisfied or very satisfied with drug | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Community setting ‐ Would take drug again after subsequent deliveries | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 Community setting ‐ Would recommend drug to a friend | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
64.1. Analysis.
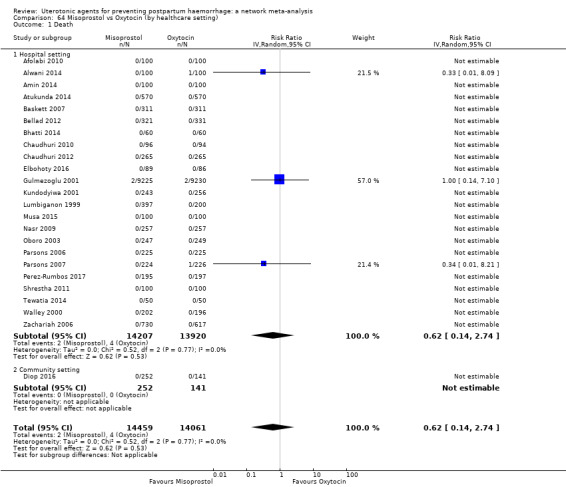
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 1 Death.
64.2. Analysis.
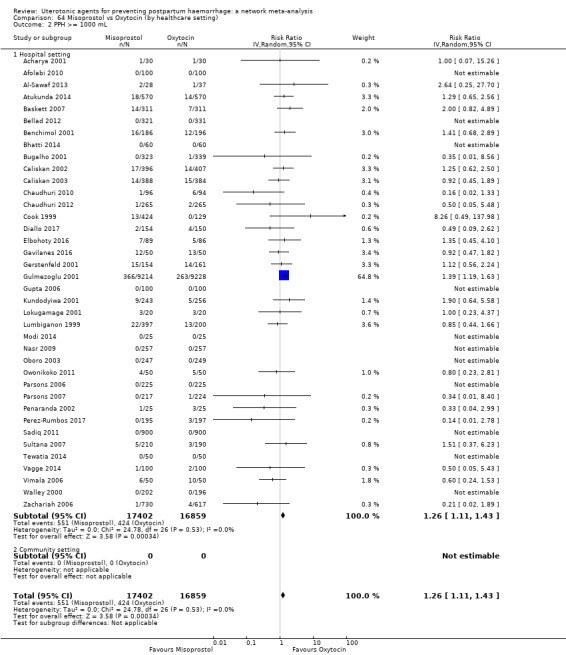
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
64.3. Analysis.
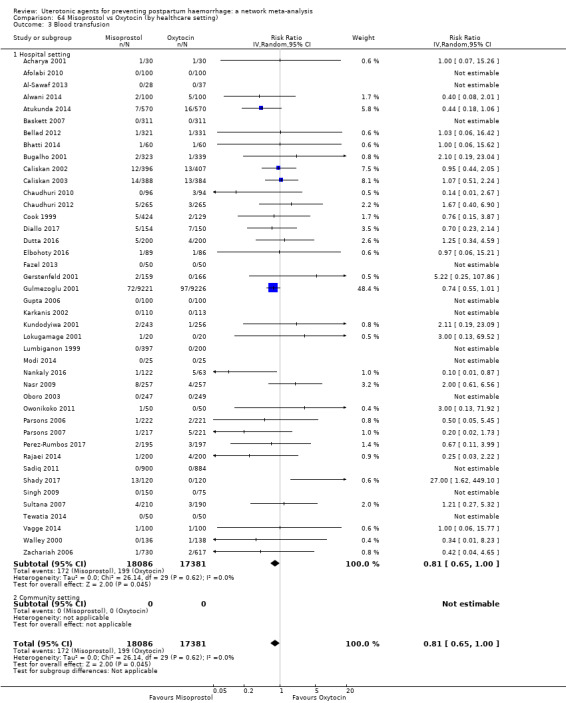
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
64.4. Analysis.
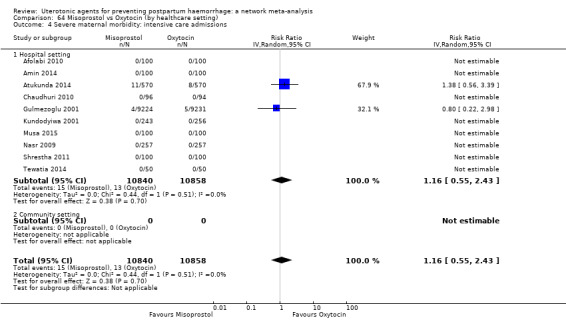
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
64.6. Analysis.
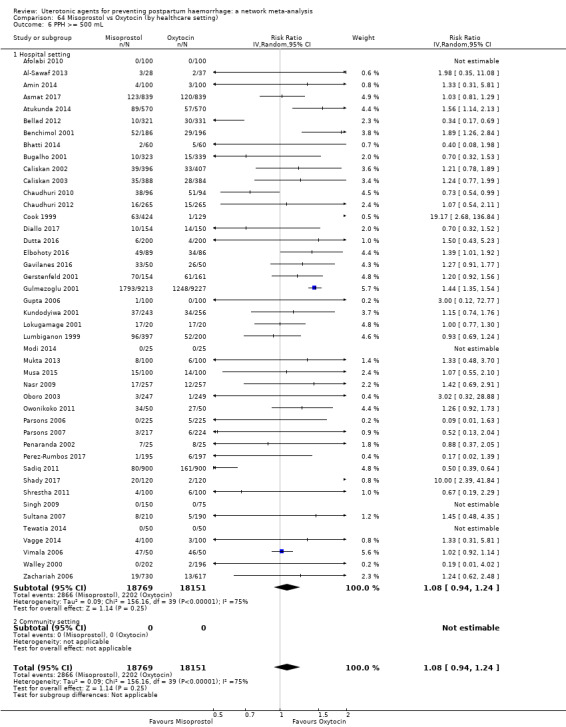
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
64.7. Analysis.
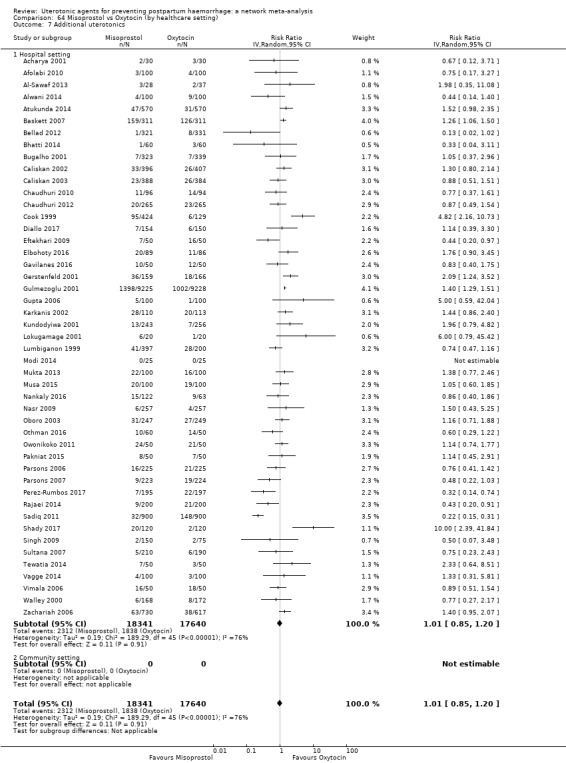
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
64.8. Analysis.
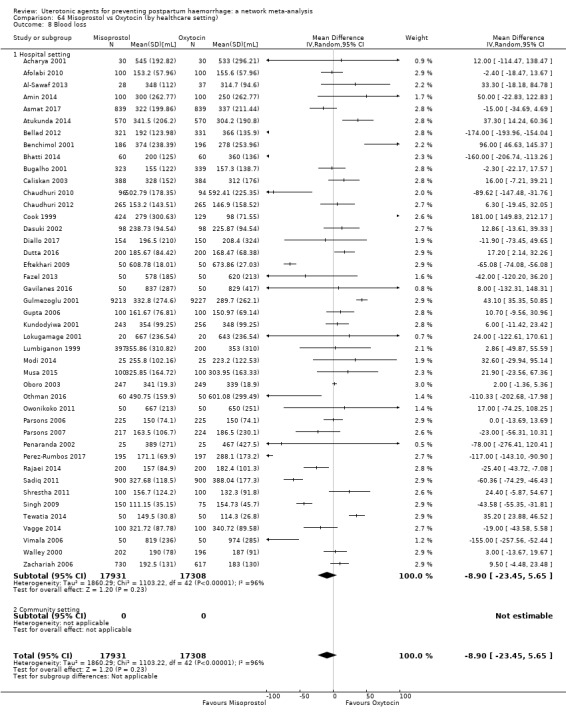
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 8 Blood loss.
64.9. Analysis.
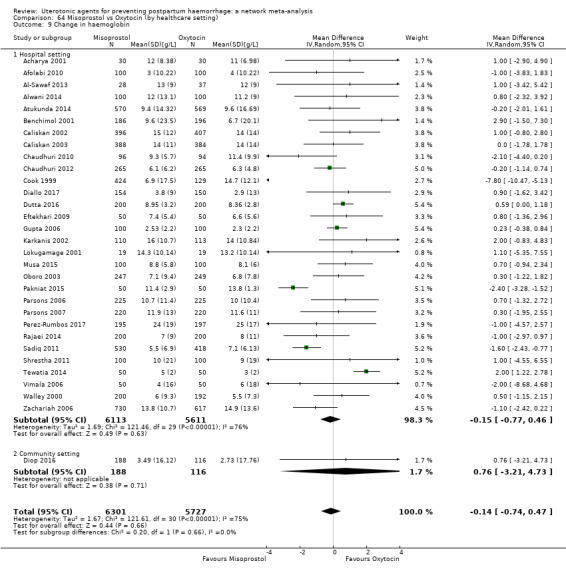
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
64.11. Analysis.
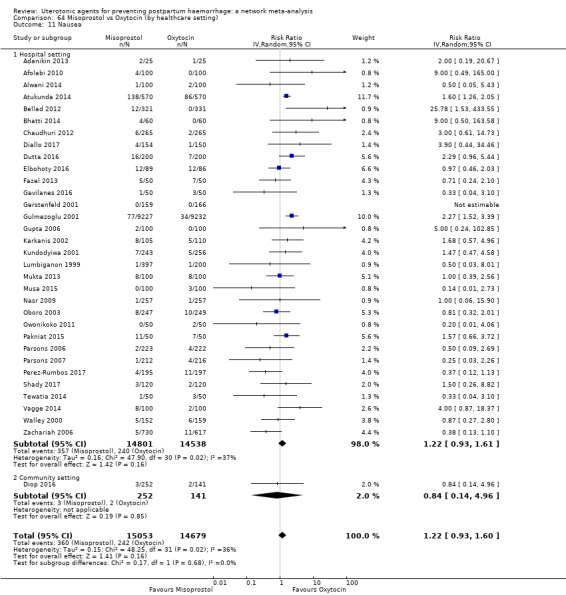
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 11 Nausea.
64.12. Analysis.
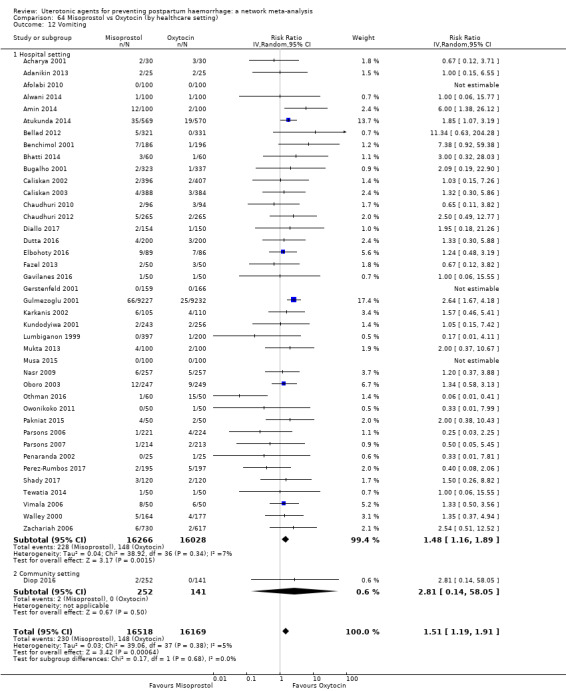
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 12 Vomiting.
64.13. Analysis.
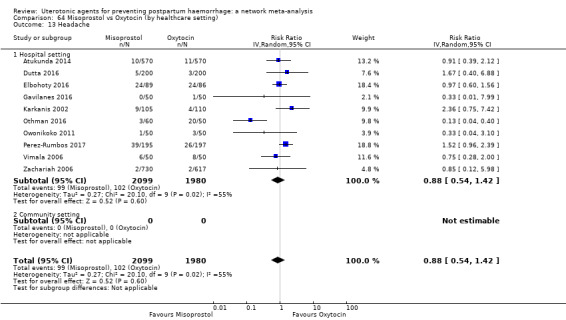
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 13 Headache.
64.14. Analysis.
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 14 Abdominal pain.
64.15. Analysis.
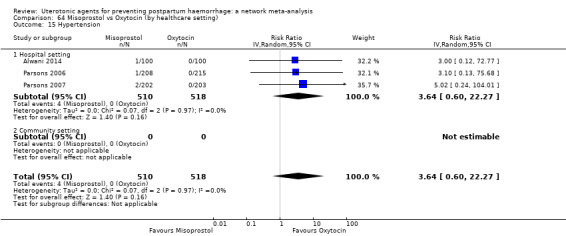
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 15 Hypertension.
64.16. Analysis.
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 16 Shivering.
64.17. Analysis.
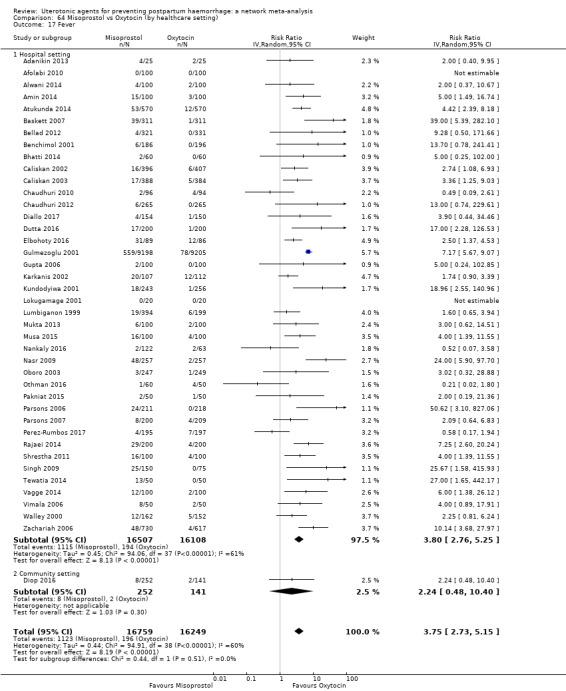
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 17 Fever.
64.18. Analysis.
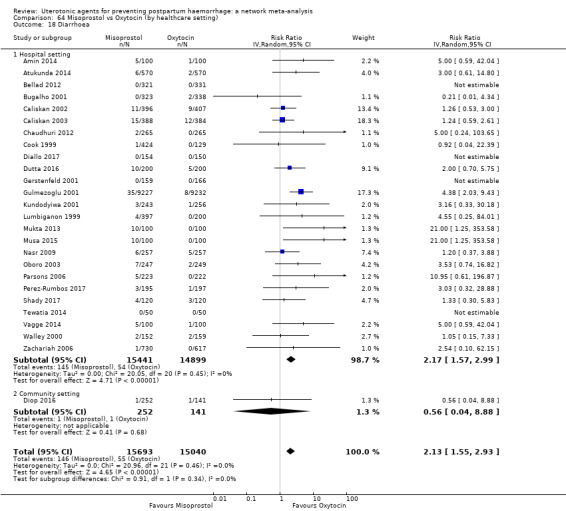
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
64.20. Analysis.
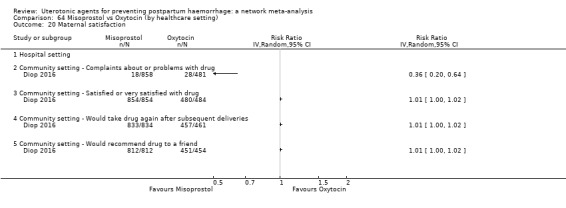
Comparison 64 Misoprostol vs Oxytocin (by healthcare setting), Outcome 20 Maternal satisfaction.
Comparison 65. Injectable prostaglandins vs Oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.20, 10.31] |
| 2.1 Hospital setting | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.20, 10.31] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.65] |
| 3.1 Hospital setting | 2 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.65] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.26, 2.71] |
| 6.1 Hospital setting | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.26, 2.71] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.09, 0.94] |
| 7.1 Hospital setting | 4 | 500 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.09, 0.94] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐15.83 [‐152.28, 120.62] |
| 8.1 Hospital setting | 4 | 500 | Mean Difference (IV, Random, 95% CI) | ‐15.83 [‐152.28, 120.62] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 3 | 450 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.40, 3.41] |
| 11.1 Hospital setting | 3 | 450 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.40, 3.41] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 2.48 [0.57, 10.73] |
| 12.1 Hospital setting | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 2.48 [0.57, 10.73] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.01, 4.11] |
| 13.1 Hospital setting | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.01, 4.11] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.11, 7.73] |
| 16.1 Hospital setting | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.11, 7.73] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.18, 21.71] |
| 17.1 Hospital setting | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.18, 21.71] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 10.38 [1.96, 54.98] |
| 18.1 Hospital setting | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 10.38 [1.96, 54.98] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
65.1. Analysis.
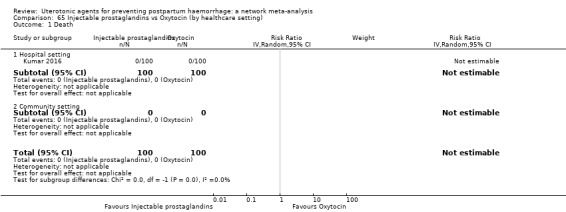
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 1 Death.
65.2. Analysis.
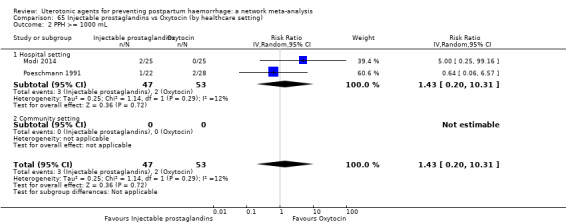
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
65.3. Analysis.
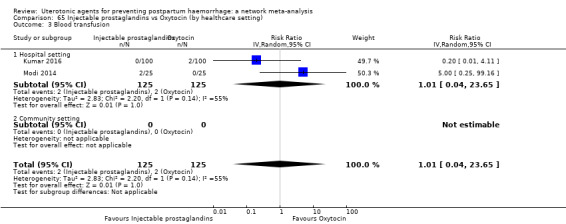
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
65.6. Analysis.
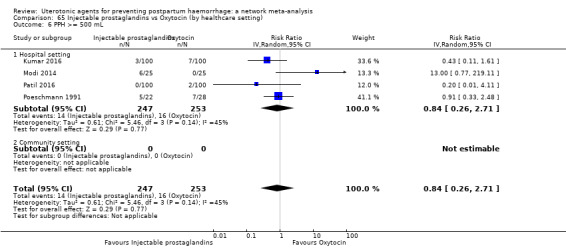
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
65.7. Analysis.
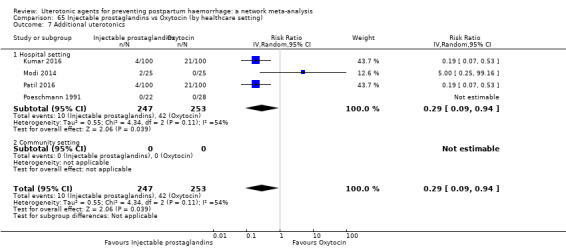
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
65.8. Analysis.
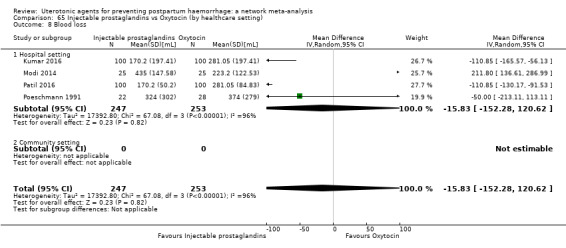
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 8 Blood loss.
65.11. Analysis.
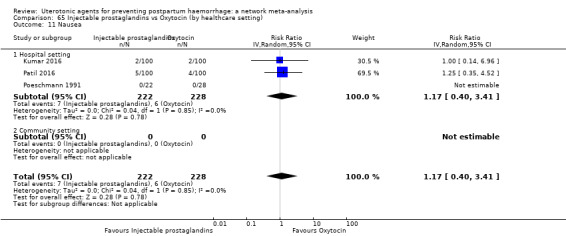
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 11 Nausea.
65.12. Analysis.
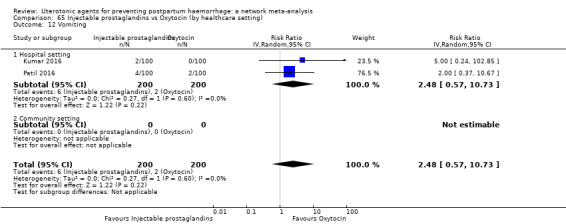
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 12 Vomiting.
65.13. Analysis.
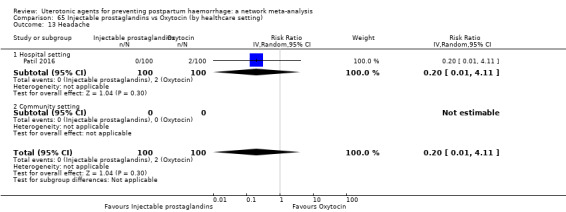
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 13 Headache.
65.16. Analysis.
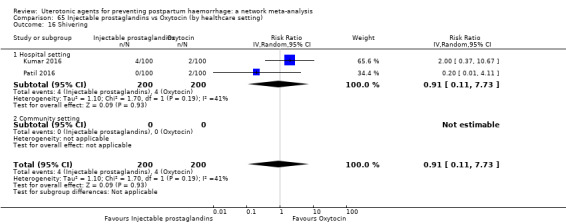
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 16 Shivering.
65.17. Analysis.
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 17 Fever.
65.18. Analysis.
Comparison 65 Injectable prostaglandins vs Oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 66. Carbetocin vs Oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 5 | 30327 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.37, 10.92] |
| 1.1 Hospital setting | 5 | 30327 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.37, 10.92] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 10 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.45, 1.19] |
| 2.1 Hospital setting | 10 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.45, 1.19] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 16 | 32115 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.38, 1.22] |
| 3.1 Hospital setting | 16 | 32115 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.38, 1.22] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 2 | 29847 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.67, 2.02] |
| 4.1 Hospital setting | 2 | 29847 | Risk Ratio (IV, Random, 95% CI) | 1.16 [0.67, 2.02] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 11 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.58, 0.98] |
| 6.1 Hospital setting | 11 | 30633 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.58, 0.98] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 22 | 32699 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.34, 0.68] |
| 7.1 Hospital setting | 22 | 32699 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.34, 0.68] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 16 | 2115 | Mean Difference (IV, Random, 95% CI) | ‐92.73 [‐154.97, ‐30.49] |
| 8.1 Hospital setting | 16 | 2115 | Mean Difference (IV, Random, 95% CI) | ‐92.73 [‐154.97, ‐30.49] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 12 | 2096 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐3.81, 0.50] |
| 9.1 Hospital setting | 12 | 2096 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐3.81, 0.50] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 2 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.86, 1.03] |
| 10.1 Hospital setting | 2 | 190 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.86, 1.03] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 12 | 2288 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.78, 1.56] |
| 11.1 Hospital setting | 12 | 2288 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.78, 1.56] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 13 | 31833 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.53, 1.50] |
| 12.1 Hospital setting | 13 | 31833 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.53, 1.50] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 15 | 2620 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.63, 1.12] |
| 13.1 Hospital setting | 15 | 2620 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.63, 1.12] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 10 | 31293 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 14.1 Hospital setting | 10 | 31293 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.97, 1.44] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 9 | 1998 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.49, 1.23] |
| 16.1 Hospital setting | 9 | 1998 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.49, 1.23] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 4 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.27, 9.35] |
| 17.1 Hospital setting | 4 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.27, 9.35] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
66.1. Analysis.
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 1 Death.
66.2. Analysis.
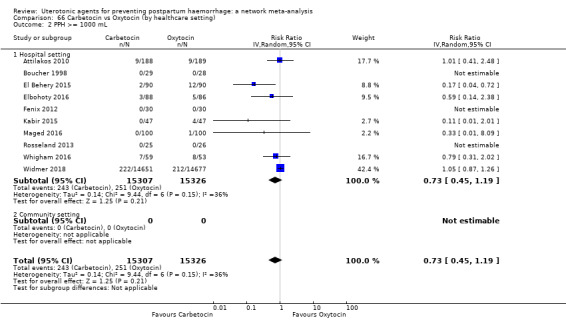
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
66.3. Analysis.
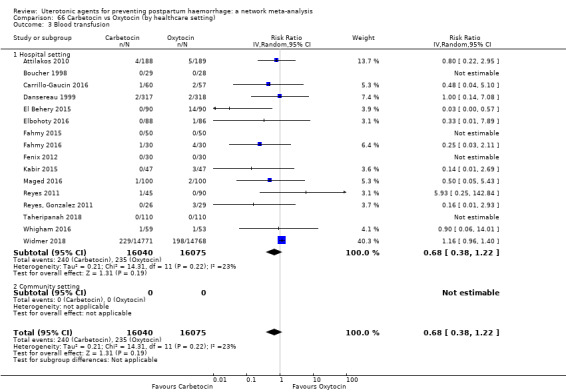
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
66.4. Analysis.
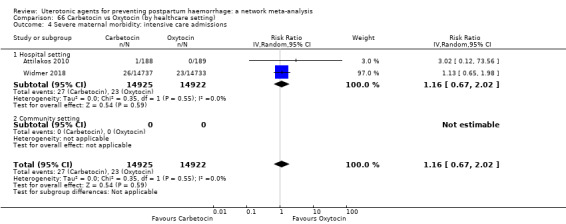
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
66.6. Analysis.
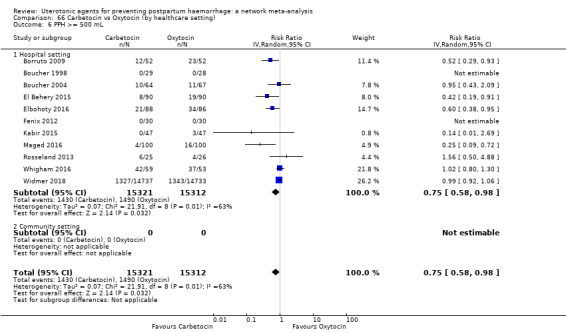
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
66.7. Analysis.
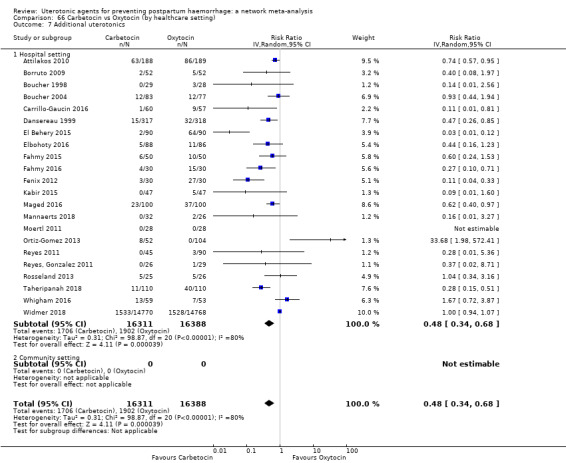
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
66.8. Analysis.
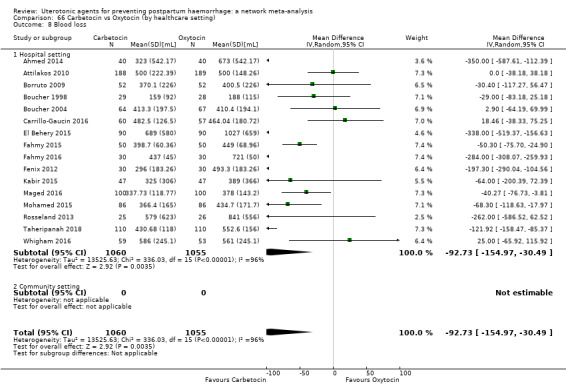
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 8 Blood loss.
66.9. Analysis.
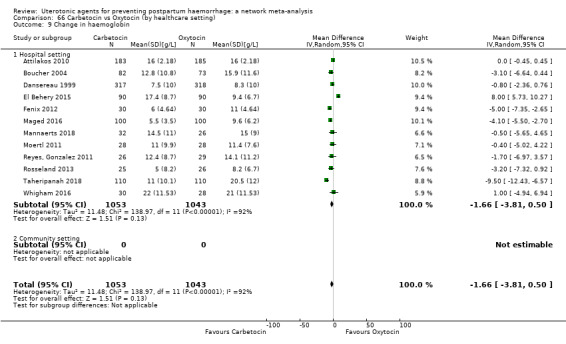
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
66.10. Analysis.
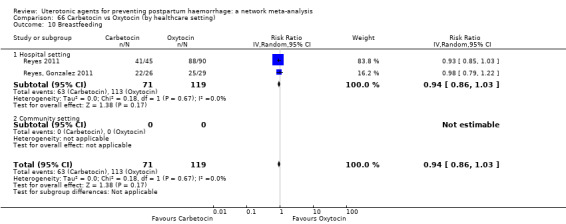
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 10 Breastfeeding.
66.11. Analysis.
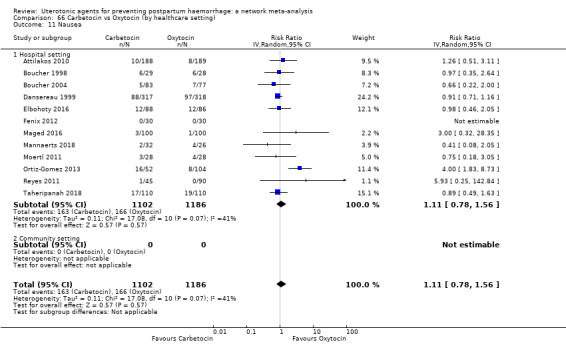
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 11 Nausea.
66.12. Analysis.
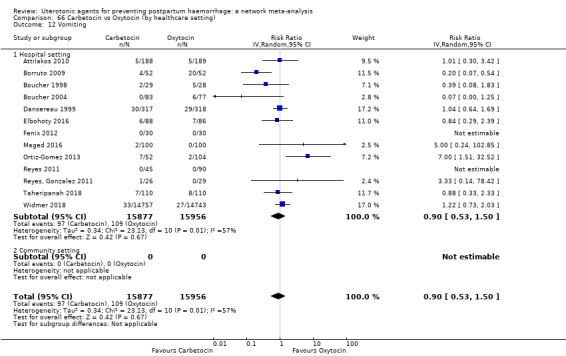
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 12 Vomiting.
66.13. Analysis.
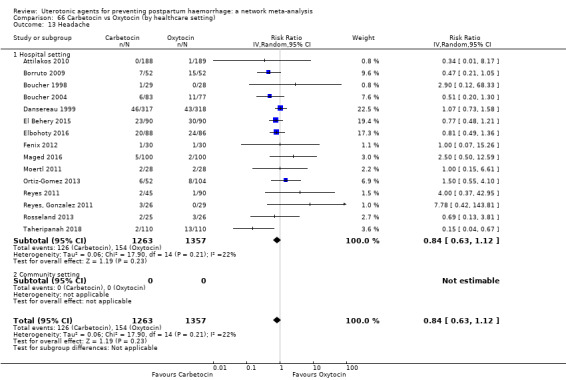
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 13 Headache.
66.14. Analysis.
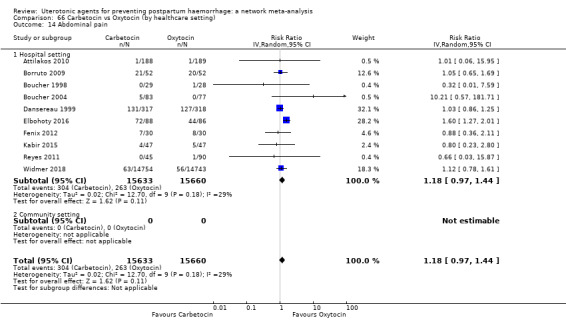
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 14 Abdominal pain.
66.16. Analysis.
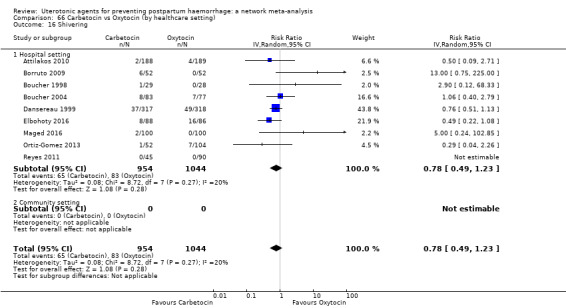
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 16 Shivering.
66.17. Analysis.
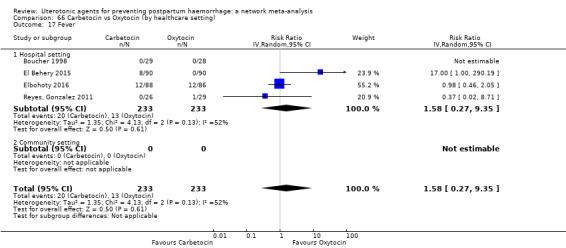
Comparison 66 Carbetocin vs Oxytocin (by healthcare setting), Outcome 17 Fever.
Comparison 67. Ergometrine vs Oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 1293 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 1293 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 6 | 2591 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.52, 3.27] |
| 2.1 Hospital setting | 6 | 2591 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.52, 3.27] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 5 | 1893 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.20, 10.23] |
| 3.1 Hospital setting | 5 | 1893 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.20, 10.23] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 10 | 3221 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.86, 1.99] |
| 6.1 Hospital setting | 10 | 3221 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.86, 1.99] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 6 | 2493 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.61, 3.48] |
| 7.1 Hospital setting | 6 | 2493 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.61, 3.48] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 10 | 3221 | Mean Difference (IV, Random, 95% CI) | 8.09 [‐17.83, 34.00] |
| 8.1 Hospital setting | 10 | 3221 | Mean Difference (IV, Random, 95% CI) | 8.09 [‐17.83, 34.00] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 1893 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.30, 1.13] |
| 9.1 Hospital setting | 2 | 1893 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.30, 1.13] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 6 | 2529 | Risk Ratio (IV, Random, 95% CI) | 4.56 [1.13, 18.44] |
| 11.1 Hospital setting | 6 | 2529 | Risk Ratio (IV, Random, 95% CI) | 4.56 [1.13, 18.44] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 5 | 2343 | Risk Ratio (IV, Random, 95% CI) | 3.83 [1.10, 13.28] |
| 12.1 Hospital setting | 5 | 2343 | Risk Ratio (IV, Random, 95% CI) | 3.83 [1.10, 13.28] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 4 | 2293 | Risk Ratio (IV, Random, 95% CI) | 5.63 [0.93, 33.96] |
| 13.1 Hospital setting | 4 | 2293 | Risk Ratio (IV, Random, 95% CI) | 5.63 [0.93, 33.96] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 1410 | Risk Ratio (IV, Random, 95% CI) | 13.39 [2.01, 89.44] |
| 15.1 Hospital setting | 3 | 1410 | Risk Ratio (IV, Random, 95% CI) | 13.39 [2.01, 89.44] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 1493 | Risk Ratio (IV, Random, 95% CI) | 1.73 [0.93, 3.25] |
| 16.1 Hospital setting | 3 | 1493 | Risk Ratio (IV, Random, 95% CI) | 1.73 [0.93, 3.25] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 1443 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.97, 9.05] |
| 17.1 Hospital setting | 2 | 1443 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.97, 9.05] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 1393 | Risk Ratio (IV, Random, 95% CI) | 3.74 [0.42, 33.53] |
| 18.1 Hospital setting | 2 | 1393 | Risk Ratio (IV, Random, 95% CI) | 3.74 [0.42, 33.53] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
67.1. Analysis.
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 1 Death.
67.2. Analysis.
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
67.3. Analysis.
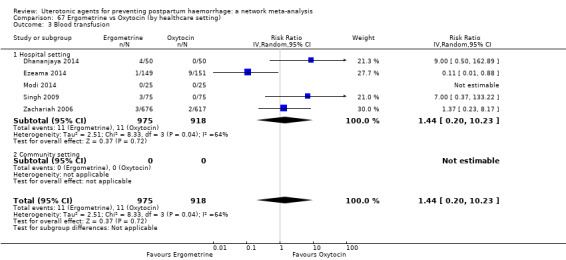
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
67.6. Analysis.
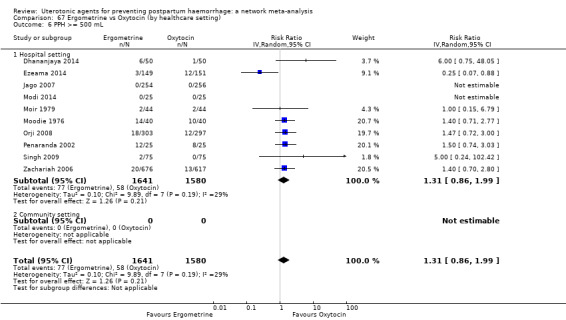
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
67.7. Analysis.
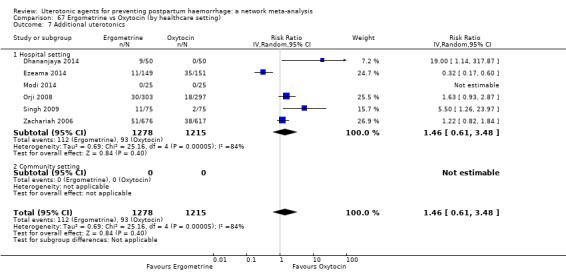
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
67.8. Analysis.
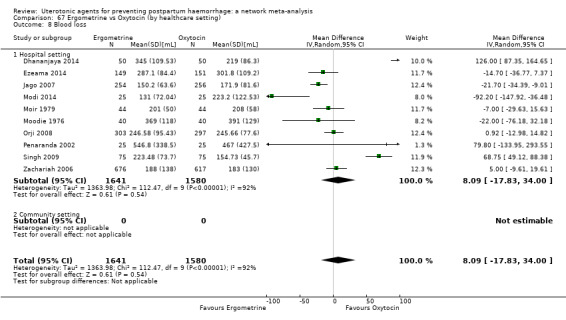
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 8 Blood loss.
67.9. Analysis.
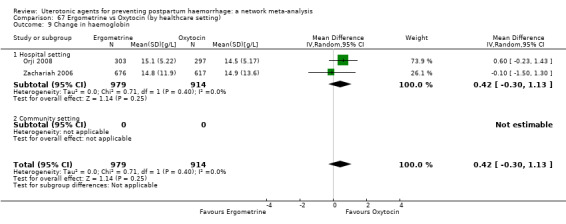
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
67.11. Analysis.
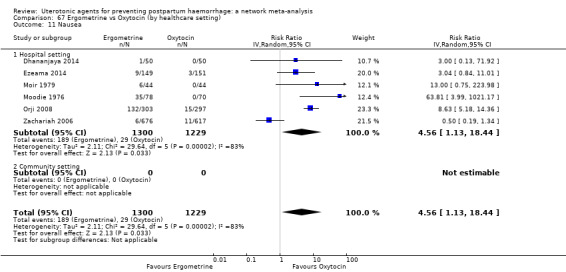
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 11 Nausea.
67.12. Analysis.
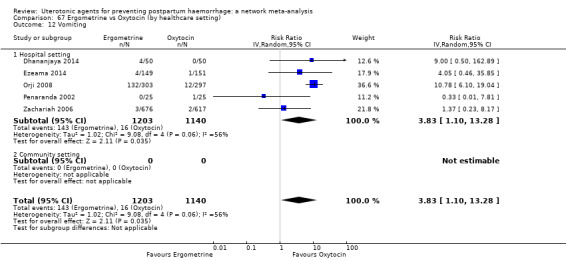
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 12 Vomiting.
67.13. Analysis.
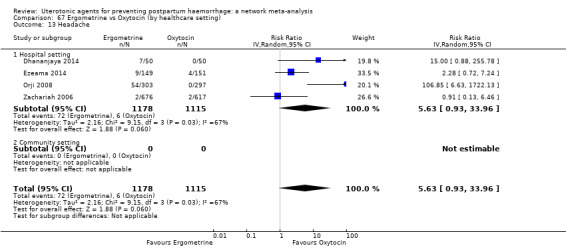
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 13 Headache.
67.15. Analysis.
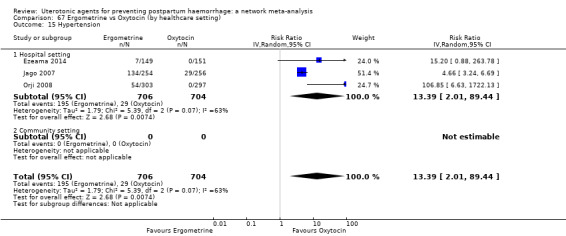
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 15 Hypertension.
67.16. Analysis.
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 16 Shivering.
67.17. Analysis.
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 17 Fever.
67.18. Analysis.
Comparison 67 Ergometrine vs Oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 68. Ergometine plus oxytocin vs Oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 1314 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 2 | 1314 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 10 | 10990 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.57, 0.93] |
| 2.1 Hospital setting | 10 | 10990 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.57, 0.93] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 11 | 10985 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.53, 1.44] |
| 3.1 Hospital setting | 11 | 10985 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.53, 1.44] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 991 | Risk Ratio (IV, Random, 95% CI) | 2.99 [0.12, 73.32] |
| 4.1 Hospital setting | 1 | 991 | Risk Ratio (IV, Random, 95% CI) | 2.99 [0.12, 73.32] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 11 | 11090 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.57, 0.91] |
| 6.1 Hospital setting | 11 | 11090 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.57, 0.91] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 10 | 8968 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.59, 1.07] |
| 7.1 Hospital setting | 10 | 8968 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.59, 1.07] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 10 | 4248 | Mean Difference (IV, Random, 95% CI) | ‐10.31 [‐40.32, 19.70] |
| 8.1 Hospital setting | 10 | 4248 | Mean Difference (IV, Random, 95% CI) | ‐10.31 [‐40.32, 19.70] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 6 | 4324 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐5.24, 0.77] |
| 9.1 Hospital setting | 6 | 4324 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐5.24, 0.77] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 1 | 3483 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 10.1 Hospital setting | 1 | 3483 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 7 | 6931 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.84, 3.53] |
| 11.1 Hospital setting | 7 | 6931 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.84, 3.53] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 9 | 10227 | Risk Ratio (IV, Random, 95% CI) | 3.05 [1.76, 5.29] |
| 12.1 Hospital setting | 9 | 10227 | Risk Ratio (IV, Random, 95% CI) | 3.05 [1.76, 5.29] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 5 | 5105 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.79, 1.99] |
| 13.1 Hospital setting | 5 | 5105 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.79, 1.99] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 1362 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.29, 13.97] |
| 15.1 Hospital setting | 3 | 1362 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.29, 13.97] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.60, 1.53] |
| 16.1 Hospital setting | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.60, 1.53] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.48, 2.43] |
| 17.1 Hospital setting | 2 | 1591 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.48, 2.43] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 3 | 2030 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.72, 2.22] |
| 18.1 Hospital setting | 3 | 2030 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.72, 2.22] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
68.1. Analysis.
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 1 Death.
68.2. Analysis.
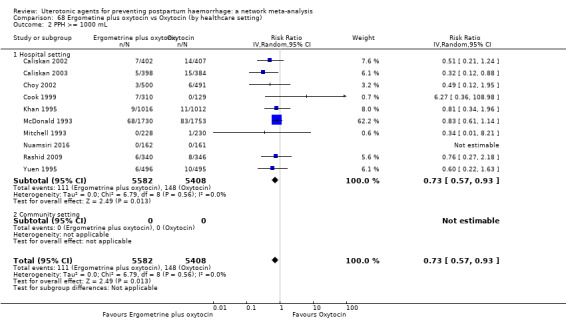
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
68.3. Analysis.
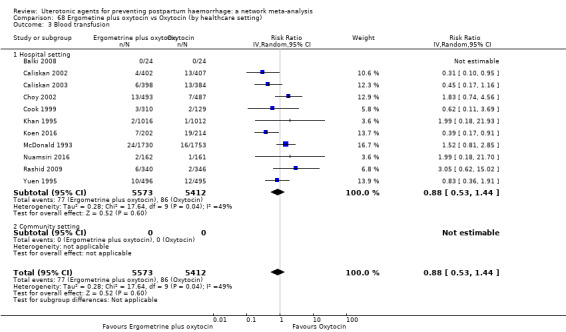
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
68.4. Analysis.
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
68.6. Analysis.
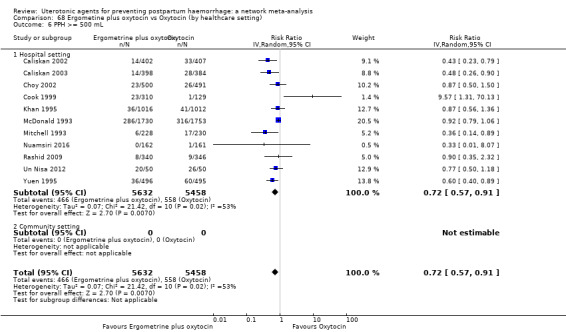
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
68.7. Analysis.
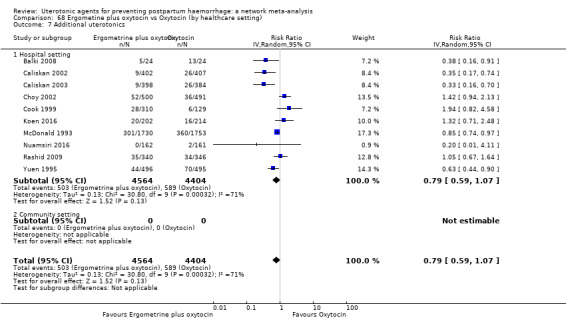
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
68.8. Analysis.
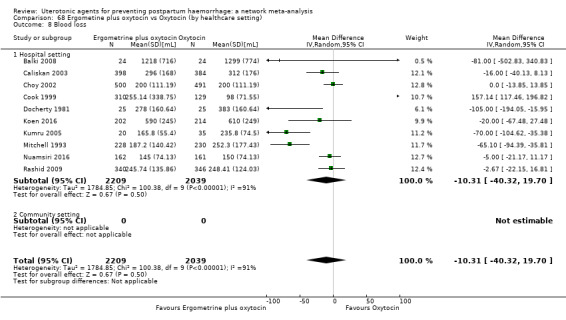
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 8 Blood loss.
68.9. Analysis.
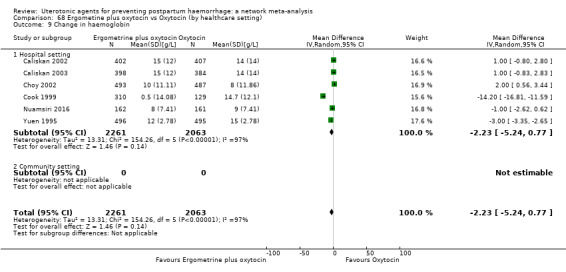
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
68.10. Analysis.
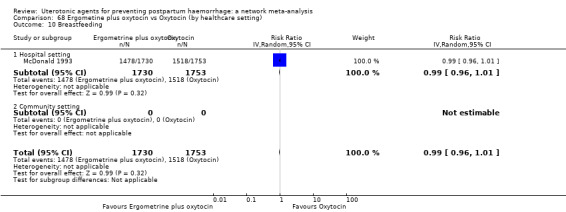
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 10 Breastfeeding.
68.11. Analysis.
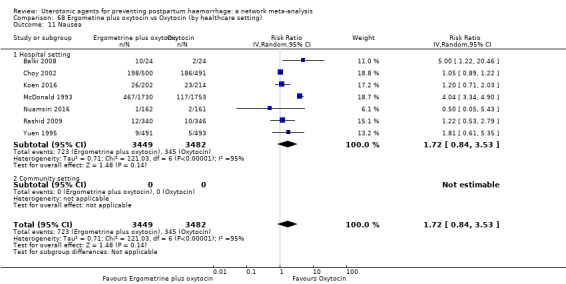
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 11 Nausea.
68.12. Analysis.
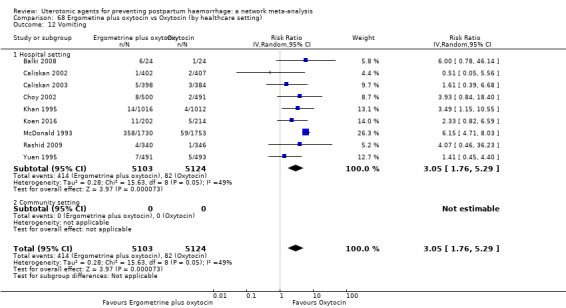
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 12 Vomiting.
68.13. Analysis.
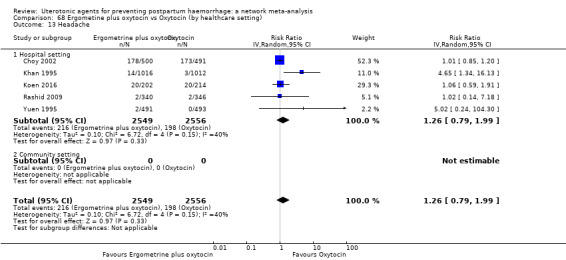
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 13 Headache.
68.15. Analysis.
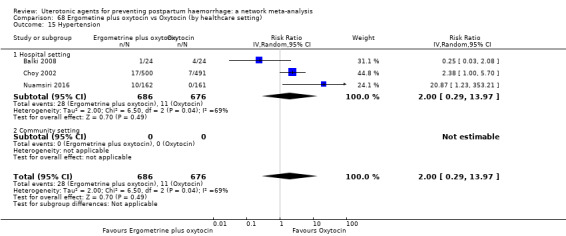
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 15 Hypertension.
68.16. Analysis.
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 16 Shivering.
68.17. Analysis.
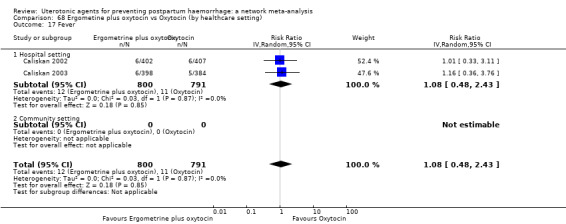
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 17 Fever.
68.18. Analysis.
Comparison 68 Ergometine plus oxytocin vs Oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 69. Misoprostol plus oxytocin vs Oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 9 | 4737 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 9 | 4737 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 17 | 8514 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 2.1 Hospital setting | 17 | 8514 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 19 | 8742 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.37, 0.67] |
| 3.1 Hospital setting | 19 | 8742 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.37, 0.67] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 3 | 1886 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.47] |
| 4.1 Hospital setting | 3 | 1886 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.47] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 14 | 8148 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 6.1 Hospital setting | 14 | 8148 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 18 | 8391 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.44, 0.67] |
| 7.1 Hospital setting | 18 | 8391 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.44, 0.67] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 17 | 8690 | Mean Difference (IV, Random, 95% CI) | ‐87.26 [‐157.83, ‐16.69] |
| 8.1 Hospital setting | 17 | 8690 | Mean Difference (IV, Random, 95% CI) | ‐87.26 [‐157.83, ‐16.69] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 15 | 7929 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐3.70, ‐1.48] |
| 9.1 Hospital setting | 15 | 7929 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐3.70, ‐1.48] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 7 | 3798 | Risk Ratio (IV, Random, 95% CI) | 2.21 [1.19, 4.10] |
| 11.1 Hospital setting | 7 | 3798 | Risk Ratio (IV, Random, 95% CI) | 2.21 [1.19, 4.10] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 11 | 6718 | Risk Ratio (IV, Random, 95% CI) | 2.24 [1.52, 3.31] |
| 12.1 Hospital setting | 11 | 6718 | Risk Ratio (IV, Random, 95% CI) | 2.24 [1.52, 3.31] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 2 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.26, 6.23] |
| 13.1 Hospital setting | 2 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.26, 6.23] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 1 | 366 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.01, 3.67] |
| 14.1 Hospital setting | 1 | 366 | Risk Ratio (IV, Random, 95% CI) | 1.93 [1.01, 3.67] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 19 | 9458 | Risk Ratio (IV, Random, 95% CI) | 3.38 [2.50, 4.57] |
| 16.1 Hospital setting | 19 | 9458 | Risk Ratio (IV, Random, 95% CI) | 3.38 [2.50, 4.57] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 17 | 8607 | Risk Ratio (IV, Random, 95% CI) | 2.99 [2.00, 4.45] |
| 17.1 Hospital setting | 17 | 8607 | Risk Ratio (IV, Random, 95% CI) | 2.99 [2.00, 4.45] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 7 | 5649 | Risk Ratio (IV, Random, 95% CI) | 2.08 [0.99, 4.38] |
| 18.1 Hospital setting | 7 | 5649 | Risk Ratio (IV, Random, 95% CI) | 2.08 [0.99, 4.38] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
69.1. Analysis.
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 1 Death.
69.2. Analysis.
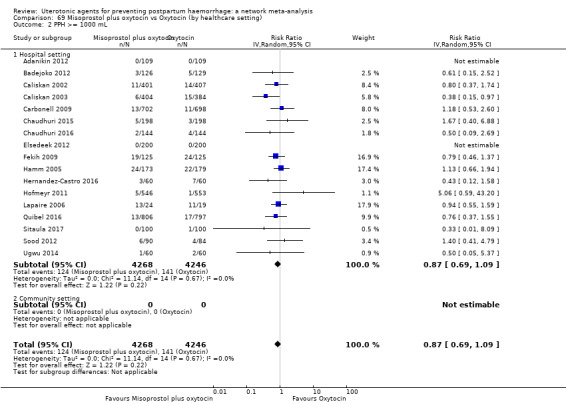
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
69.3. Analysis.
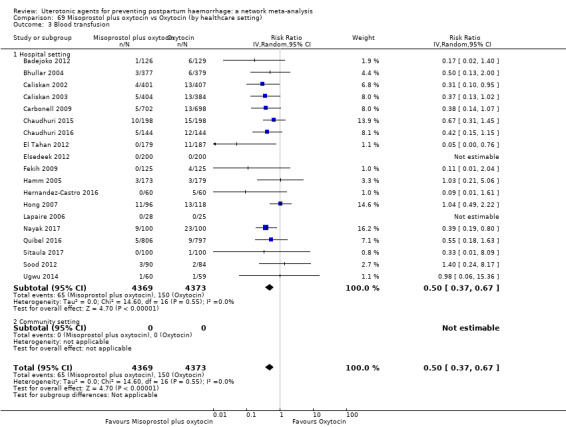
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
69.4. Analysis.
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
69.6. Analysis.
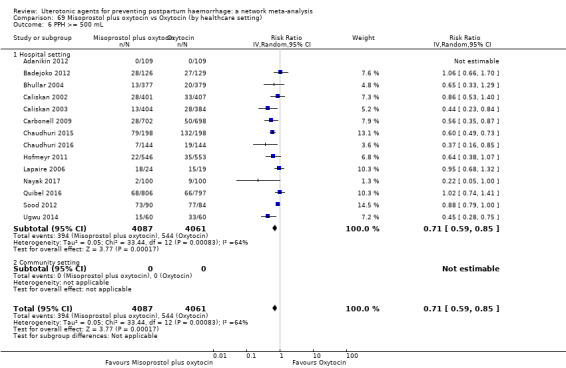
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
69.7. Analysis.
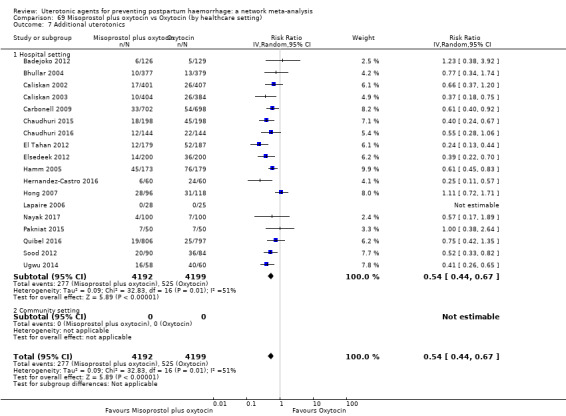
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
69.8. Analysis.

Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 8 Blood loss.
69.9. Analysis.
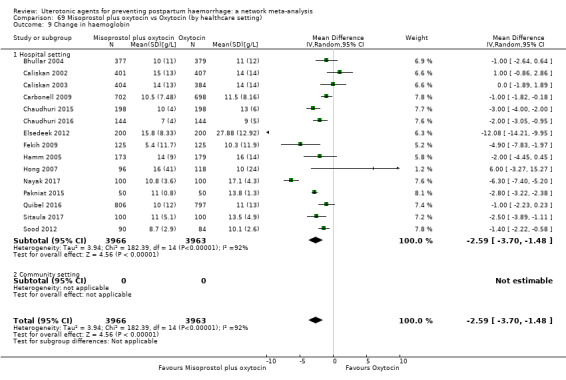
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
69.11. Analysis.
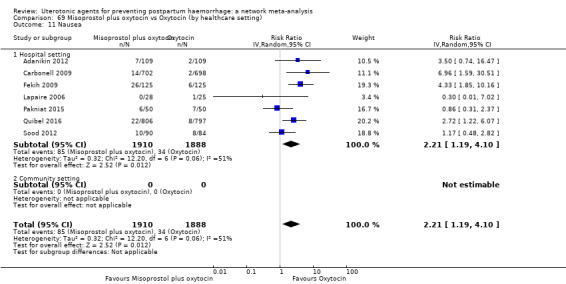
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 11 Nausea.
69.12. Analysis.

Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 12 Vomiting.
69.13. Analysis.
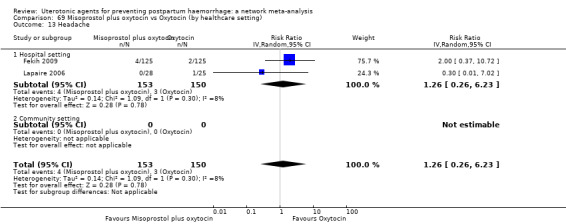
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 13 Headache.
69.14. Analysis.
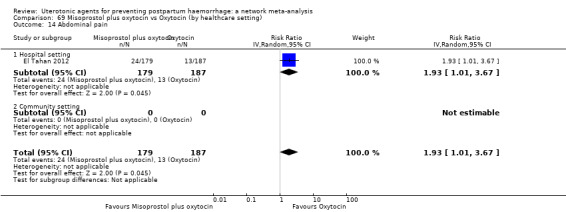
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 14 Abdominal pain.
69.16. Analysis.
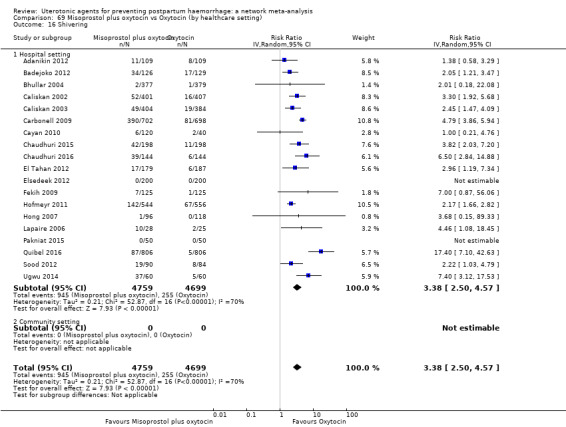
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 16 Shivering.
69.17. Analysis.
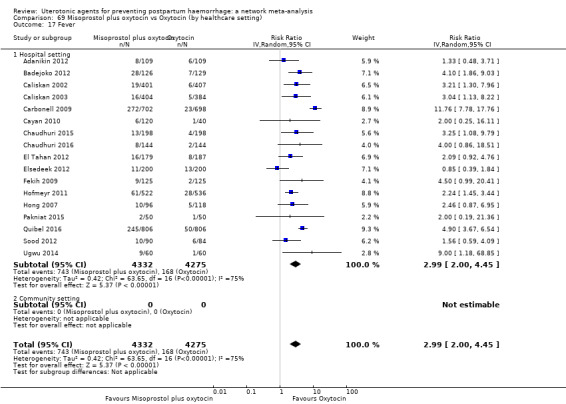
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 17 Fever.
69.18. Analysis.
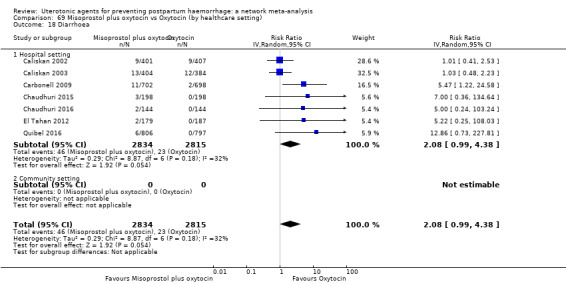
Comparison 69 Misoprostol plus oxytocin vs Oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 70. Injectable prostaglandins vs Misoprostol (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 170 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.1 Hospital setting | 2 | 170 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.14, 5.39] |
| 3.1 Hospital setting | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.14, 5.39] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 3 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.60 [0.57, 4.52] |
| 6.1 Hospital setting | 3 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.60 [0.57, 4.52] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.28, 3.69] |
| 7.1 Hospital setting | 4 | 403 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.28, 3.69] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 3 | 270 | Mean Difference (IV, Random, 95% CI) | 52.99 [‐39.74, 145.71] |
| 8.1 Hospital setting | 3 | 270 | Mean Difference (IV, Random, 95% CI) | 52.99 [‐39.74, 145.71] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐1.35, 5.90] |
| 9.1 Hospital setting | 2 | 220 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐1.35, 5.90] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.06, 0.92] |
| 11.1 Hospital setting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.06, 0.92] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.01, 163.15] |
| 12.1 Hospital setting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.01, 163.15] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.20, 4.80] |
| 14.1 Hospital setting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.20, 4.80] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 353 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.01, 0.21] |
| 16.1 Hospital setting | 3 | 353 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.01, 0.21] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.01, 0.39] |
| 17.1 Hospital setting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.01, 0.39] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 9.03 [1.70, 48.00] |
| 18.1 Hospital setting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 9.03 [1.70, 48.00] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
70.1. Analysis.
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 1 Death.
70.2. Analysis.
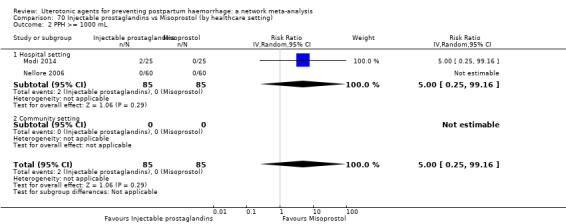
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 2 PPH >= 1000 mL.
70.3. Analysis.
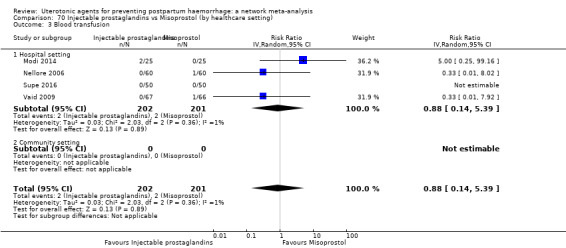
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 3 Blood transfusion.
70.6. Analysis.
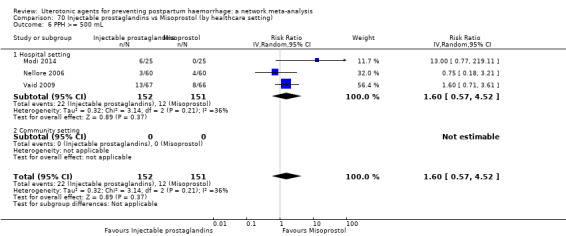
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 6 PPH >= 500 mL.
70.7. Analysis.
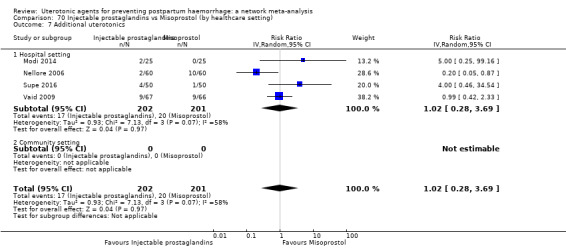
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 7 Additional uterotonics.
70.8. Analysis.
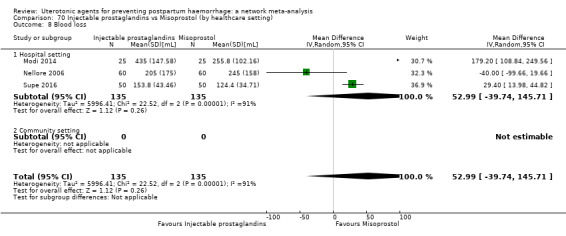
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 8 Blood loss.
70.9. Analysis.
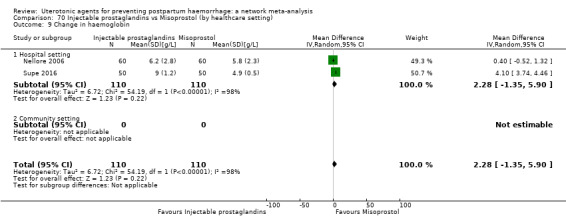
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 9 Change in haemoglobin.
70.11. Analysis.
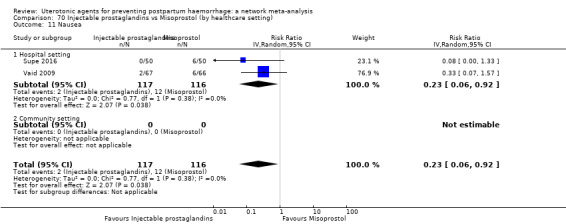
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 11 Nausea.
70.12. Analysis.
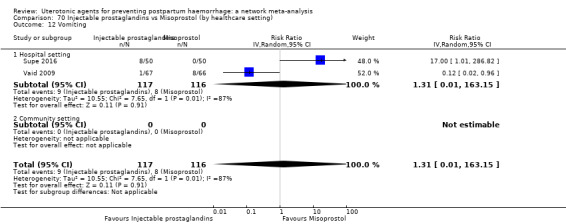
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 12 Vomiting.
70.14. Analysis.
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 14 Abdominal pain.
70.16. Analysis.
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 16 Shivering.
70.17. Analysis.
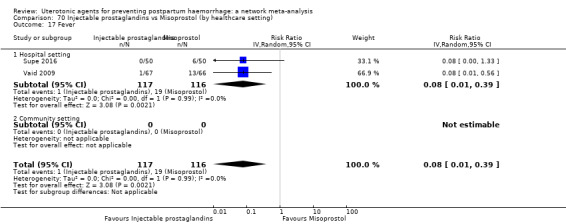
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 17 Fever.
70.18. Analysis.
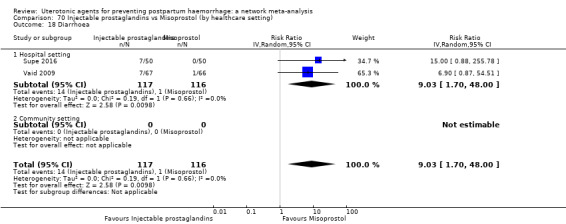
Comparison 70 Injectable prostaglandins vs Misoprostol (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 71. Misoprostol vs Carbetocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [0.62, 8.64] |
| 2.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [0.62, 8.64] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 71.85] |
| 3.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 71.85] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [1.52, 3.50] |
| 6.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.31 [1.52, 3.50] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.96 [1.55, 10.07] |
| 7.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.96 [1.55, 10.07] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.47, 2.08] |
| 11.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.47, 2.08] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.55, 3.99] |
| 12.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.55, 3.99] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.71, 1.99] |
| 13.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.71, 1.99] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 14.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.52, 0.80] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.46 [1.67, 7.17] |
| 16.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 3.46 [1.67, 7.17] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.55 [1.41, 4.64] |
| 17.1 Hospital setting | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 2.55 [1.41, 4.64] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
71.1. Analysis.
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 1 Death.
71.2. Analysis.
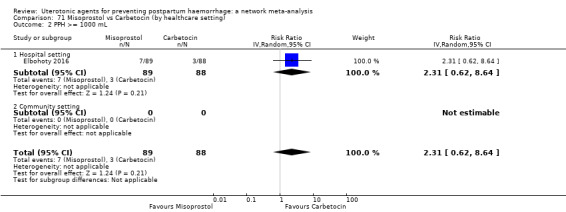
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
71.3. Analysis.
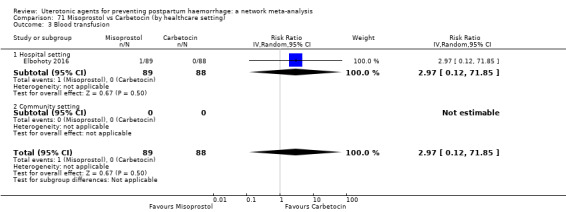
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 3 Blood transfusion.
71.6. Analysis.
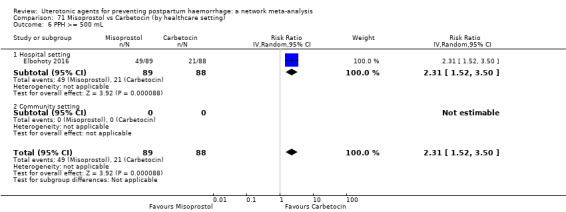
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
71.7. Analysis.
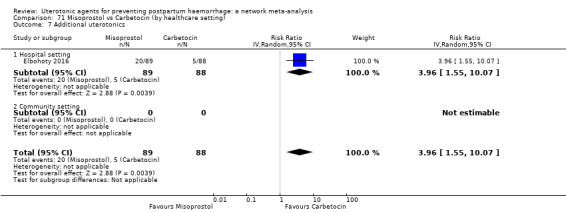
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 7 Additional uterotonics.
71.11. Analysis.
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 11 Nausea.
71.12. Analysis.
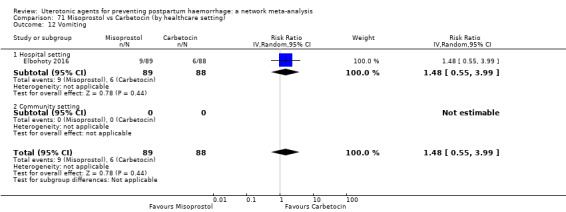
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 12 Vomiting.
71.13. Analysis.
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 13 Headache.
71.14. Analysis.
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 14 Abdominal pain.
71.16. Analysis.
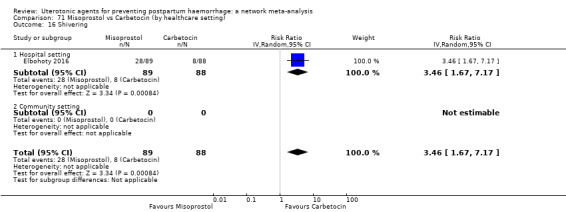
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 16 Shivering.
71.17. Analysis.
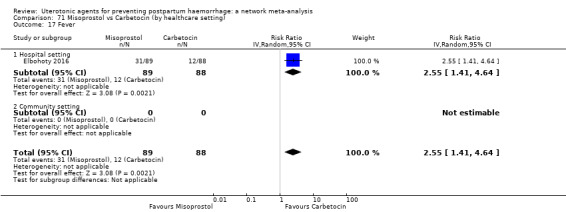
Comparison 71 Misoprostol vs Carbetocin (by healthcare setting), Outcome 17 Fever.
Comparison 72. Ergometrine vs Misoprostol (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 7 | 5254 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 6 | 4054 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 11 | 5562 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.45, 3.48] |
| 2.1 Hospital setting | 10 | 4362 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.47, 4.34] |
| 2.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.17] |
| 3 Blood transfusion | 13 | 5308 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.65, 4.50] |
| 3.1 Hospital setting | 12 | 4108 | Risk Ratio (IV, Random, 95% CI) | 2.02 [0.73, 5.57] |
| 3.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.17] |
| 4 Severe maternal morbidity: intensive care admissions | 1 | 864 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.16] |
| 4.1 Hospital setting | 1 | 864 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.16] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 16 | 6459 | Risk Ratio (IV, Random, 95% CI) | 1.18 [0.79, 1.75] |
| 6.1 Hospital setting | 15 | 5259 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.77, 1.79] |
| 6.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.34, 4.63] |
| 7 Additional uterotonics | 16 | 6565 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.60] |
| 7.1 Hospital setting | 15 | 5365 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.68, 1.65] |
| 7.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.17, 3.34] |
| 8 Blood loss | 15 | 6337 | Mean Difference (IV, Random, 95% CI) | 11.66 [‐9.85, 33.17] |
| 8.1 Hospital setting | 14 | 5137 | Mean Difference (IV, Random, 95% CI) | 6.55 [‐11.42, 24.51] |
| 8.2 Community setting | 1 | 1200 | Mean Difference (IV, Random, 95% CI) | 71.30 [60.86, 81.74] |
| 9 Change in haemoglobin | 8 | 4438 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.43, 2.26] |
| 9.1 Hospital setting | 8 | 4438 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.43, 2.26] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 13 | 5202 | Risk Ratio (IV, Random, 95% CI) | 1.53 [1.15, 2.04] |
| 11.1 Hospital setting | 12 | 4002 | Risk Ratio (IV, Random, 95% CI) | 1.43 [1.02, 2.00] |
| 11.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 1.98 [1.49, 2.64] |
| 12 Vomiting | 14 | 6136 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.81, 1.83] |
| 12.1 Hospital setting | 13 | 4936 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.77, 2.05] |
| 12.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.70, 1.83] |
| 13 Headache | 8 | 3369 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.70, 4.12] |
| 13.1 Hospital setting | 8 | 3369 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.70, 4.12] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.04, 65.80] |
| 14.1 Hospital setting | 2 | 233 | Risk Ratio (IV, Random, 95% CI) | 1.58 [0.04, 65.80] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 2 | 300 | Risk Ratio (IV, Random, 95% CI) | 7.55 [0.94, 60.53] |
| 15.1 Hospital setting | 2 | 300 | Risk Ratio (IV, Random, 95% CI) | 7.55 [0.94, 60.53] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 17 | 6860 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.26, 0.44] |
| 16.1 Hospital setting | 16 | 5660 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.22, 0.44] |
| 16.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.33, 0.47] |
| 17 Fever | 14 | 6330 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.33] |
| 17.1 Hospital setting | 13 | 5130 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.15, 0.28] |
| 17.2 Community setting | 1 | 1200 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.29, 0.70] |
| 18 Diarrhoea | 8 | 4165 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.46, 2.34] |
| 18.1 Hospital setting | 8 | 4165 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.46, 2.34] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
72.1. Analysis.
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 1 Death.
72.2. Analysis.
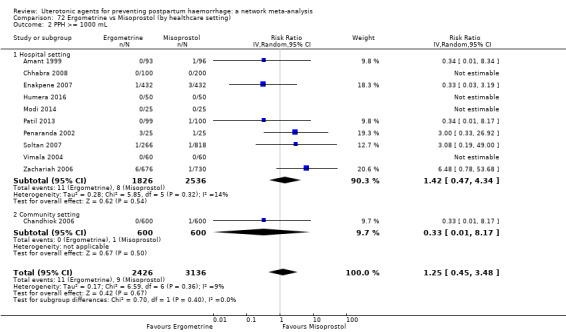
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 2 PPH >= 1000 mL.
72.3. Analysis.
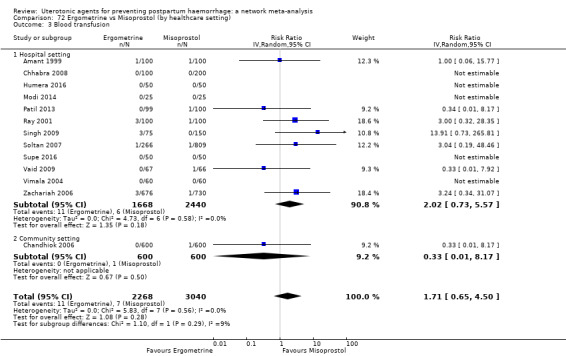
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 3 Blood transfusion.
72.4. Analysis.
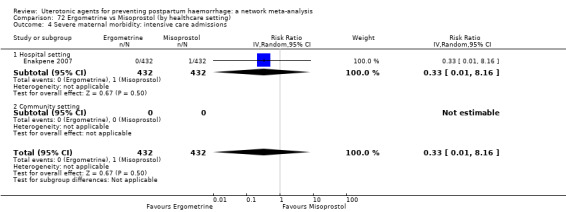
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
72.6. Analysis.
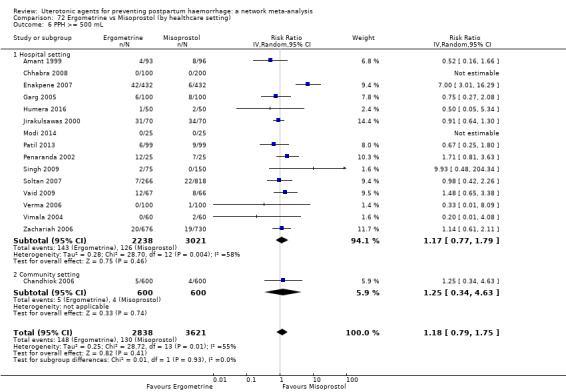
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 6 PPH >= 500 mL.
72.7. Analysis.
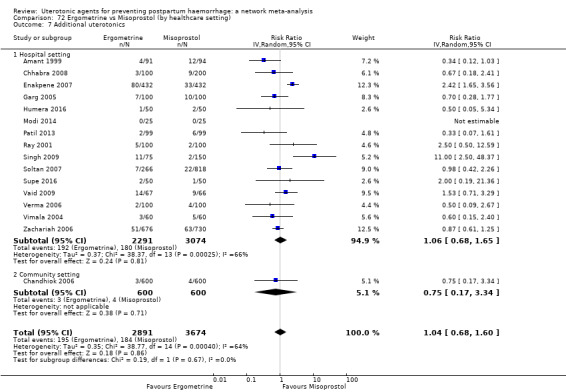
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 7 Additional uterotonics.
72.8. Analysis.
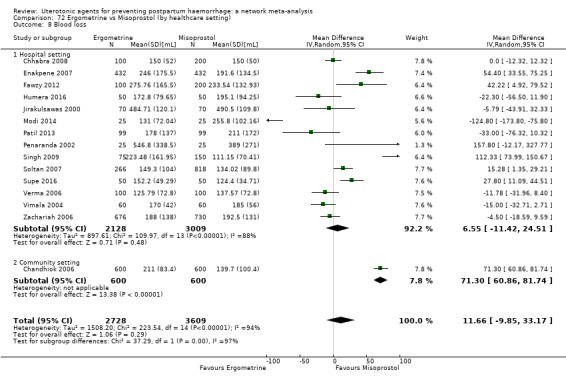
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 8 Blood loss.
72.9. Analysis.
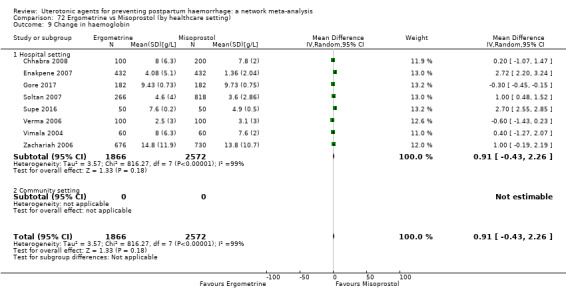
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 9 Change in haemoglobin.
72.11. Analysis.
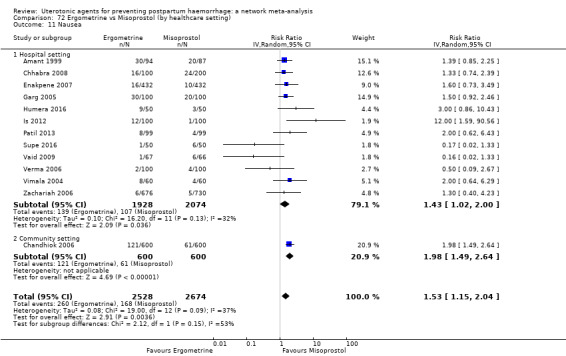
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 11 Nausea.
72.12. Analysis.
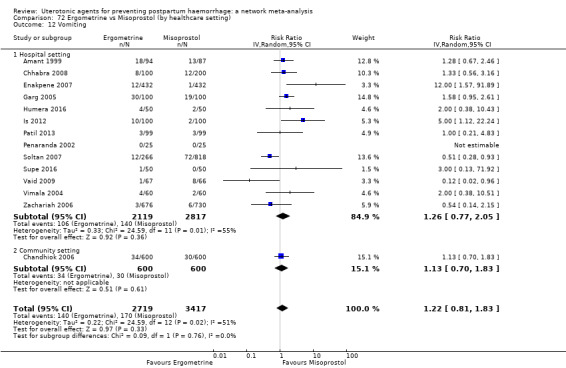
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 12 Vomiting.
72.13. Analysis.
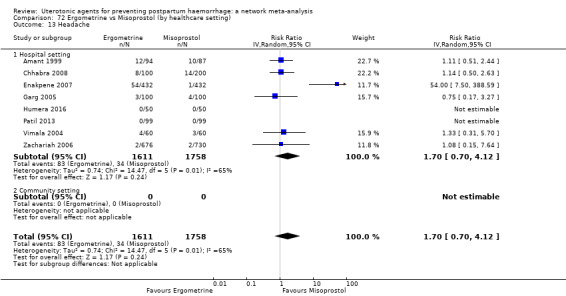
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 13 Headache.
72.14. Analysis.
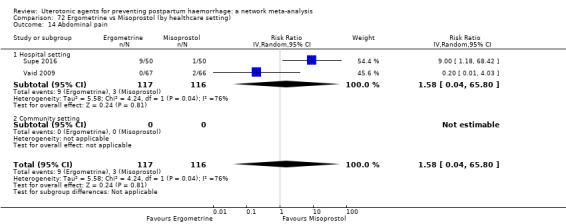
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 14 Abdominal pain.
72.15. Analysis.
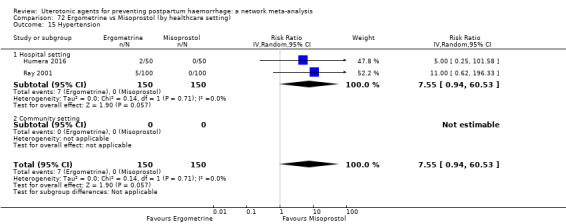
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 15 Hypertension.
72.16. Analysis.
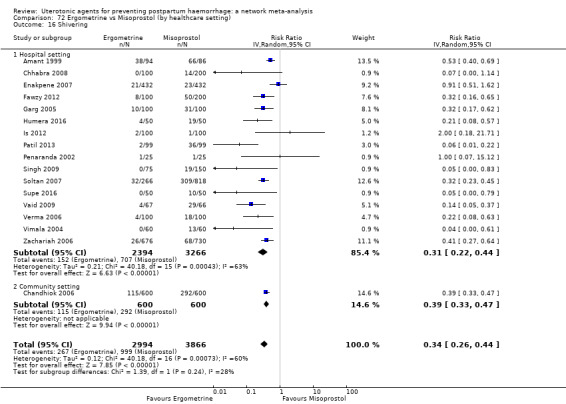
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 16 Shivering.
72.17. Analysis.
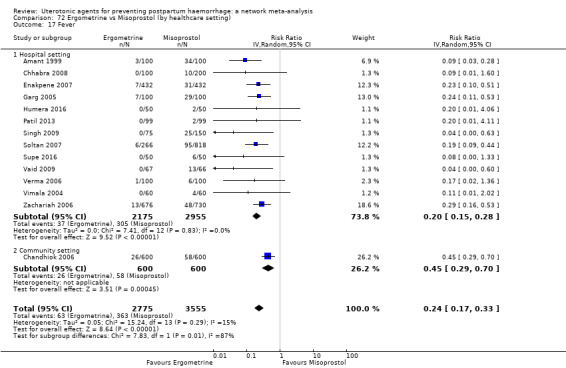
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 17 Fever.
72.18. Analysis.
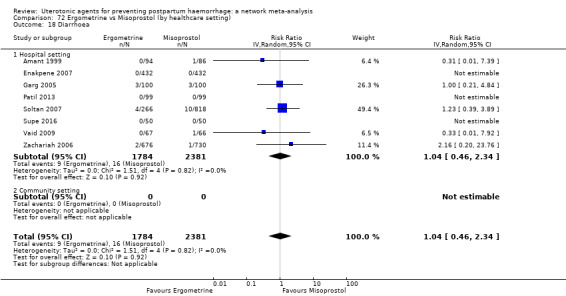
Comparison 72 Ergometrine vs Misoprostol (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 73. Misoprostol vs Ergometrine plus oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 3258 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 4 | 3258 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 9 | 6028 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.08, 2.40] |
| 2.1 Hospital setting | 9 | 6028 | Risk Ratio (IV, Random, 95% CI) | 1.61 [1.08, 2.40] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 8 | 6335 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.91, 2.21] |
| 3.1 Hospital setting | 8 | 6335 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.91, 2.21] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 10 | 6492 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.35, 2.26] |
| 6.1 Hospital setting | 10 | 6492 | Risk Ratio (IV, Random, 95% CI) | 1.75 [1.35, 2.26] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 9 | 6395 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.49, 2.36] |
| 7.1 Hospital setting | 9 | 6395 | Risk Ratio (IV, Random, 95% CI) | 1.87 [1.49, 2.36] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 8 | 5634 | Mean Difference (IV, Random, 95% CI) | 22.86 [4.50, 41.22] |
| 8.1 Hospital setting | 8 | 5634 | Mean Difference (IV, Random, 95% CI) | 22.86 [4.50, 41.22] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 9 | 5588 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐0.49, 2.67] |
| 9.1 Hospital setting | 9 | 5588 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐0.49, 2.67] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 4 | 3399 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.61, 0.84] |
| 11.1 Hospital setting | 4 | 3399 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.61, 0.84] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 6 | 4983 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.50, 1.04] |
| 12.1 Hospital setting | 6 | 4983 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.50, 1.04] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 3 | 3259 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.47, 1.76] |
| 13.1 Hospital setting | 3 | 3259 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.47, 1.76] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 1 | 846 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 14.1 Hospital setting | 1 | 846 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 2553 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.18, 1.41] |
| 15.1 Hospital setting | 3 | 2553 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.18, 1.41] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 6 | 4980 | Risk Ratio (IV, Random, 95% CI) | 2.71 [1.95, 3.76] |
| 16.1 Hospital setting | 6 | 4980 | Risk Ratio (IV, Random, 95% CI) | 2.71 [1.95, 3.76] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 7 | 5045 | Risk Ratio (IV, Random, 95% CI) | 5.33 [2.87, 9.88] |
| 17.1 Hospital setting | 7 | 5045 | Risk Ratio (IV, Random, 95% CI) | 5.33 [2.87, 9.88] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 6 | 3659 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.57] |
| 18.1 Hospital setting | 6 | 3659 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.68, 1.57] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
73.1. Analysis.
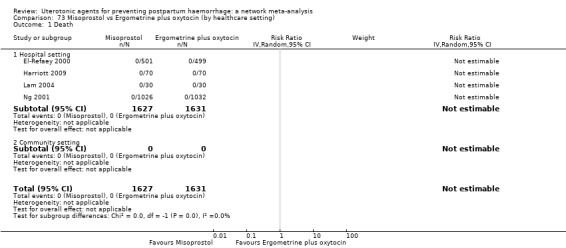
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 1 Death.
73.2. Analysis.
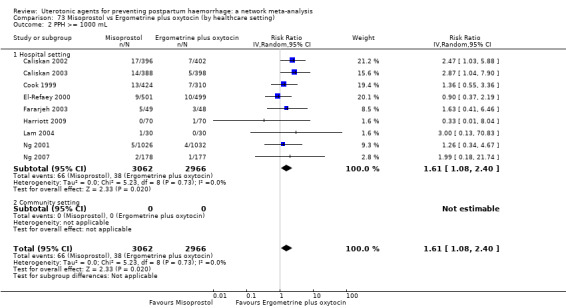
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
73.3. Analysis.
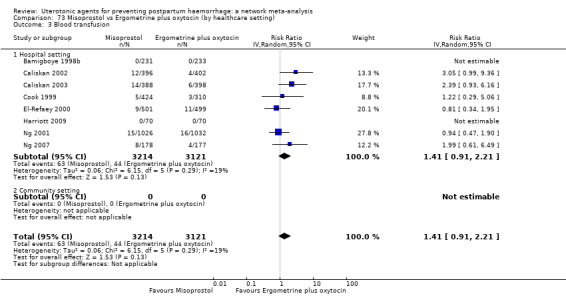
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
73.6. Analysis.
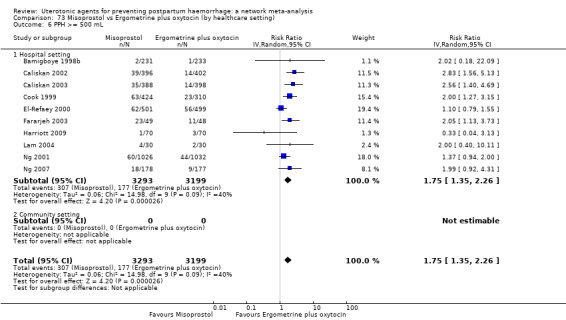
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
73.7. Analysis.
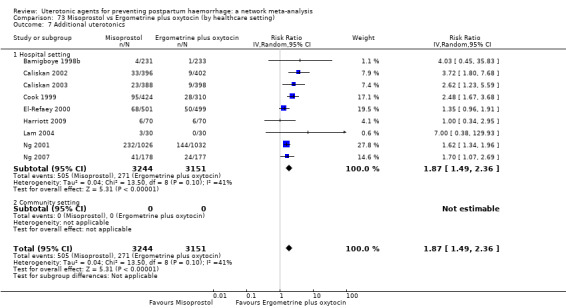
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
73.8. Analysis.
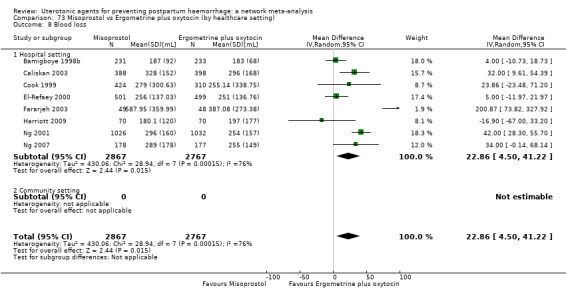
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 8 Blood loss.
73.9. Analysis.
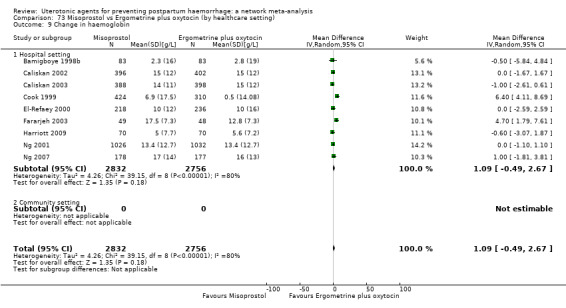
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
73.11. Analysis.
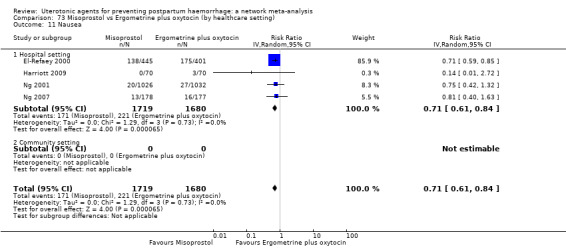
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 11 Nausea.
73.12. Analysis.
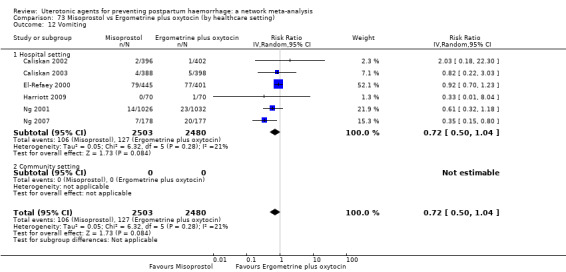
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 12 Vomiting.
73.13. Analysis.
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 13 Headache.
73.14. Analysis.
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 14 Abdominal pain.
73.15. Analysis.
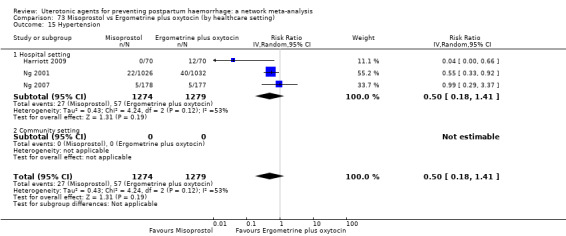
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 15 Hypertension.
73.16. Analysis.
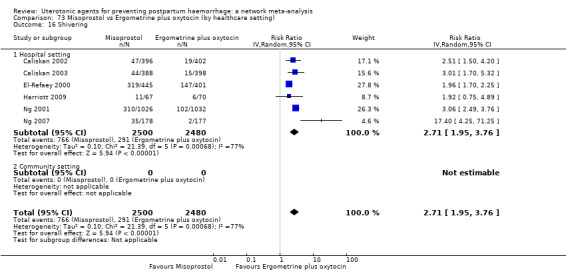
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 16 Shivering.
73.17. Analysis.
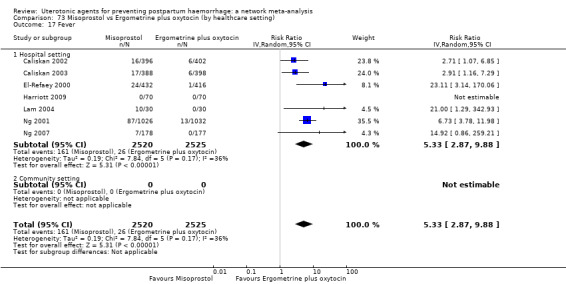
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 17 Fever.
73.18. Analysis.
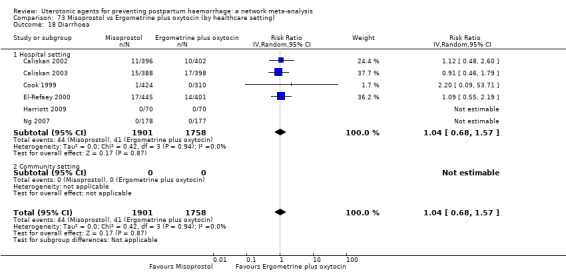
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
73.20. Analysis.
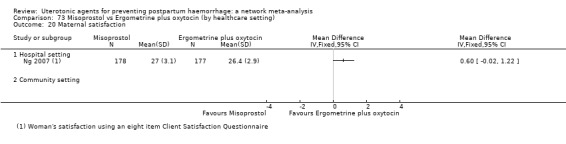
Comparison 73 Misoprostol vs Ergometrine plus oxytocin (by healthcare setting), Outcome 20 Maternal satisfaction.
Comparison 74. Misoprostol vs Misoprostol plus oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.85 [1.03, 3.33] |
| 2.1 Hospital setting | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.85 [1.03, 3.33] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 2.97 [1.40, 6.30] |
| 3.1 Hospital setting | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 2.97 [1.40, 6.30] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.93 [0.99, 3.76] |
| 6.1 Hospital setting | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.93 [0.99, 3.76] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.89 [1.26, 2.83] |
| 7.1 Hospital setting | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.89 [1.26, 2.83] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 1 | 792 | Mean Difference (IV, Random, 95% CI) | 48.0 [24.68, 71.32] |
| 8.1 Hospital setting | 1 | 792 | Mean Difference (IV, Random, 95% CI) | 48.0 [24.68, 71.32] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 3 | 1689 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.42, 0.96] |
| 9.1 Hospital setting | 3 | 1689 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.42, 0.96] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.83 [0.73, 4.57] |
| 11.1 Hospital setting | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.83 [0.73, 4.57] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.51, 3.82] |
| 12.1 Hospital setting | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.51, 3.82] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 16.1 Hospital setting | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.62, 1.53] |
| 17.1 Hospital setting | 3 | 1689 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.62, 1.53] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.70, 2.13] |
| 18.1 Hospital setting | 2 | 1589 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.70, 2.13] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
74.2. Analysis.
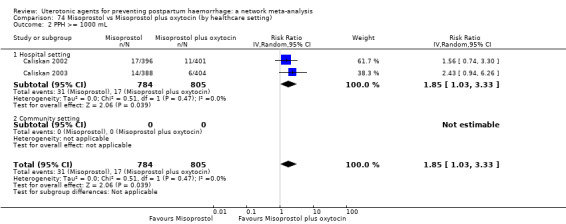
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
74.3. Analysis.
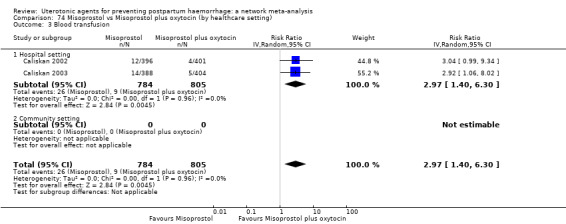
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
74.6. Analysis.

Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
74.7. Analysis.
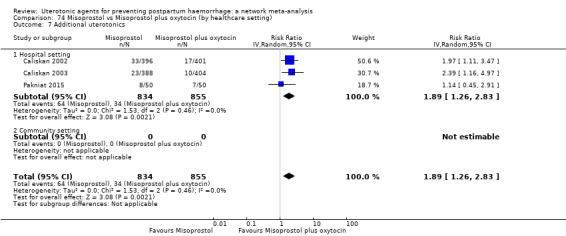
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
74.8. Analysis.
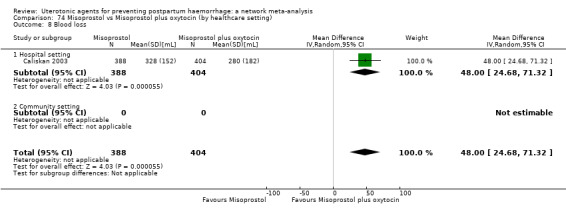
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 8 Blood loss.
74.9. Analysis.
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
74.11. Analysis.
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 11 Nausea.
74.12. Analysis.
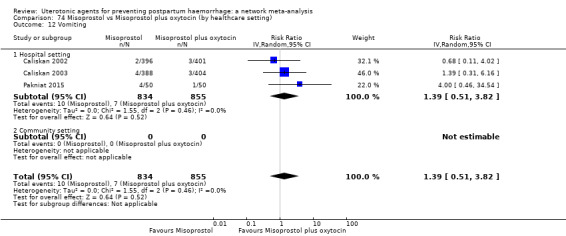
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 12 Vomiting.
74.16. Analysis.
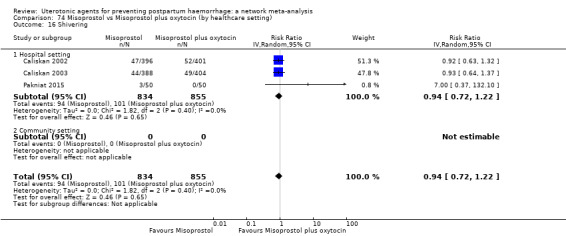
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 16 Shivering.
74.17. Analysis.
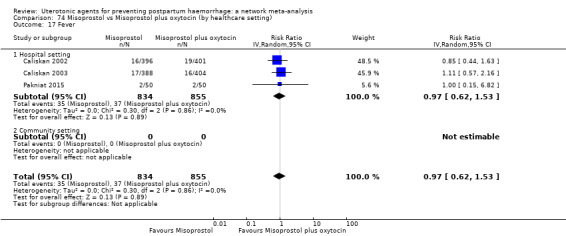
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 17 Fever.
74.18. Analysis.
Comparison 74 Misoprostol vs Misoprostol plus oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 75. Carbetocin vs Injectable prostaglandins (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 76. Injectable prostaglandins vs Ergometrine (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.1 Hospital setting | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 4 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.58] |
| 3.1 Hospital setting | 4 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.04, 23.58] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 3 | 399 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.64, 3.17] |
| 6.1 Hospital setting | 3 | 399 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.64, 3.17] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.40, 1.51] |
| 7.1 Hospital setting | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.40, 1.51] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 8 | 1195 | Mean Difference (IV, Random, 95% CI) | ‐16.08 [‐57.17, 25.00] |
| 8.1 Hospital setting | 8 | 1195 | Mean Difference (IV, Random, 95% CI) | ‐16.08 [‐57.17, 25.00] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐6.58, 4.01] |
| 9.1 Hospital setting | 2 | 300 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐6.58, 4.01] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.98, 2.94] |
| 11.1 Hospital setting | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.98, 2.94] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 2.70 [0.97, 7.55] |
| 12.1 Hospital setting | 5 | 684 | Risk Ratio (IV, Random, 95% CI) | 2.70 [0.97, 7.55] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 13.1 Hospital setting | 1 | 80 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.39, 10.31] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 3 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.07, 40.44] |
| 14.1 Hospital setting | 3 | 384 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.07, 40.44] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.02, 1.37] |
| 15.1 Hospital setting | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.02, 1.37] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 3 | 434 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.16, 0.91] |
| 16.1 Hospital setting | 3 | 434 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.16, 0.91] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 4 | 534 | Risk Ratio (IV, Random, 95% CI) | 4.52 [0.76, 26.80] |
| 17.1 Hospital setting | 4 | 534 | Risk Ratio (IV, Random, 95% CI) | 4.52 [0.76, 26.80] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 5 | 664 | Risk Ratio (IV, Random, 95% CI) | 10.09 [2.75, 37.00] |
| 18.1 Hospital setting | 5 | 664 | Risk Ratio (IV, Random, 95% CI) | 10.09 [2.75, 37.00] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
76.1. Analysis.
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 1 Death.
76.2. Analysis.
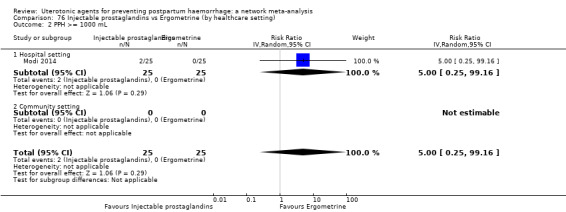
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 2 PPH >= 1000 mL.
76.3. Analysis.
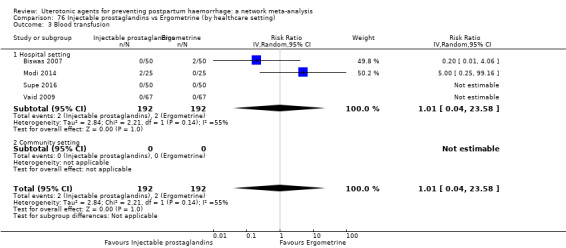
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 3 Blood transfusion.
76.6. Analysis.
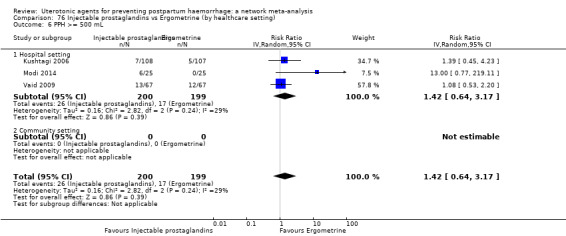
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 6 PPH >= 500 mL.
76.7. Analysis.
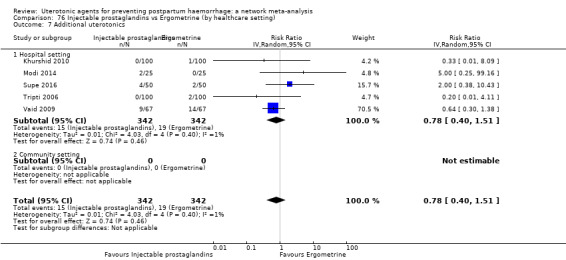
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 7 Additional uterotonics.
76.8. Analysis.
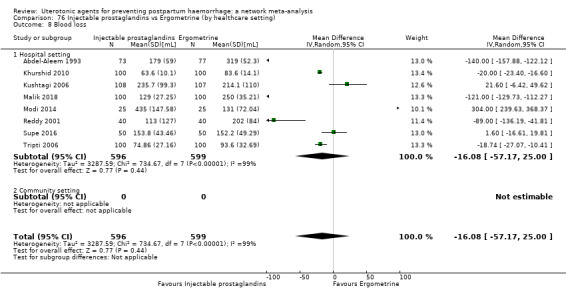
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 8 Blood loss.
76.9. Analysis.
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 9 Change in haemoglobin.
76.11. Analysis.
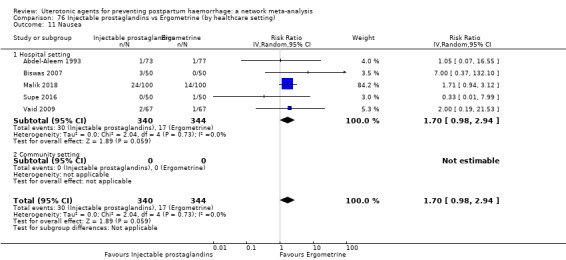
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 11 Nausea.
76.12. Analysis.
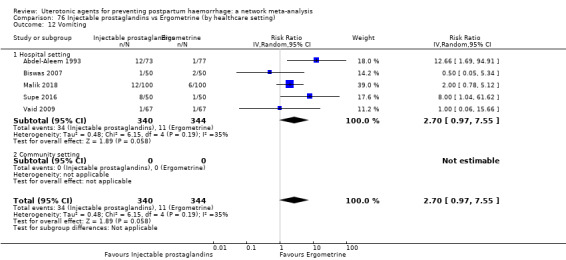
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 12 Vomiting.
76.13. Analysis.
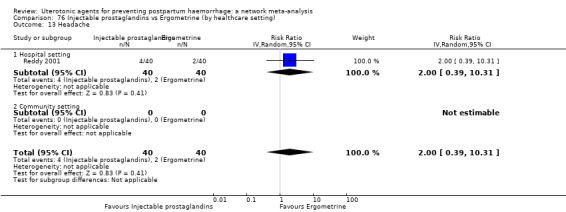
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 13 Headache.
76.14. Analysis.
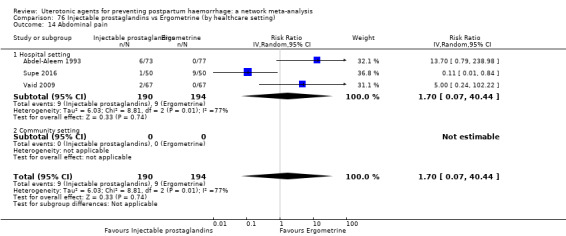
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 14 Abdominal pain.
76.15. Analysis.
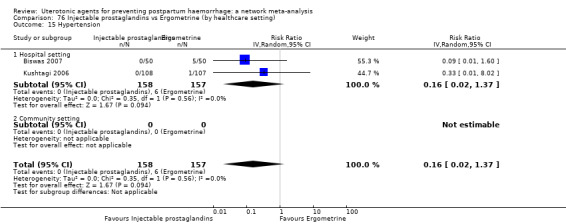
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 15 Hypertension.
76.16. Analysis.
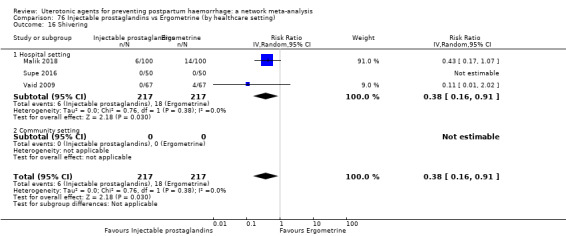
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 16 Shivering.
76.17. Analysis.
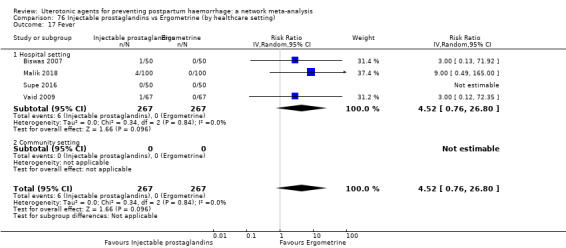
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 17 Fever.
76.18. Analysis.
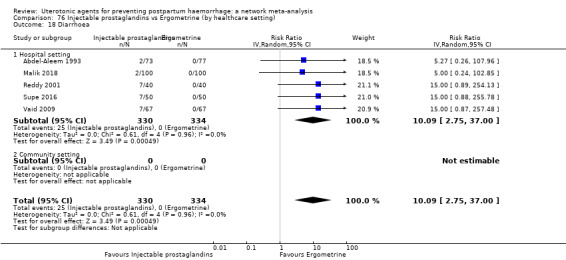
Comparison 76 Injectable prostaglandins vs Ergometrine (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 77. Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.17, 3.78] |
| 2.1 Hospital setting | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.17, 3.78] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 1 | 69 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.17, 2.53] |
| 3.1 Hospital setting | 1 | 69 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.17, 2.53] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.92, 1.79] |
| 6.1 Hospital setting | 2 | 598 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.92, 1.79] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.16, 7.36] |
| 7.1 Hospital setting | 1 | 112 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.16, 7.36] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 2 | 598 | Mean Difference (IV, Random, 95% CI) | ‐26.18 [‐104.63, 52.27] |
| 8.1 Hospital setting | 2 | 598 | Mean Difference (IV, Random, 95% CI) | ‐26.18 [‐104.63, 52.27] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 5.06 [1.12, 22.86] |
| 11.1 Hospital setting | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 5.06 [1.12, 22.86] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.40, 2.13] |
| 12.1 Hospital setting | 1 | 529 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.40, 2.13] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 641 | Risk Ratio (IV, Random, 95% CI) | 23.70 [7.55, 74.45] |
| 18.1 Hospital setting | 2 | 641 | Risk Ratio (IV, Random, 95% CI) | 23.70 [7.55, 74.45] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
77.2. Analysis.
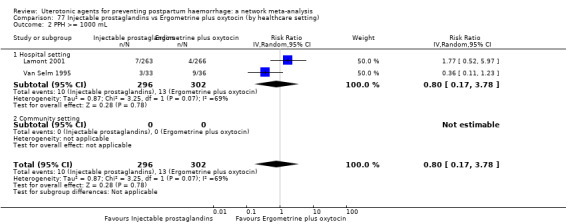
Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
77.3. Analysis.
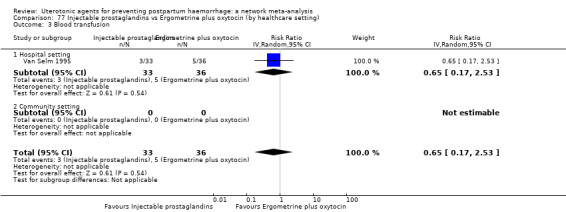
Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
77.6. Analysis.

Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
77.7. Analysis.
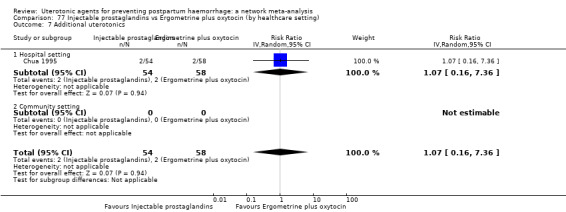
Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
77.8. Analysis.
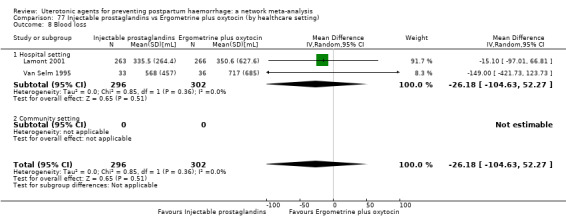
Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 8 Blood loss.
77.11. Analysis.
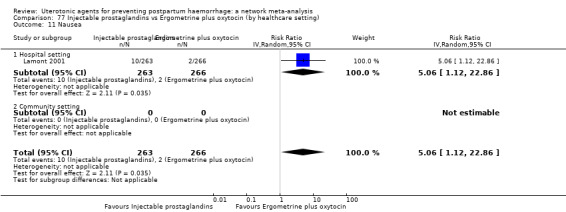
Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 11 Nausea.
77.12. Analysis.
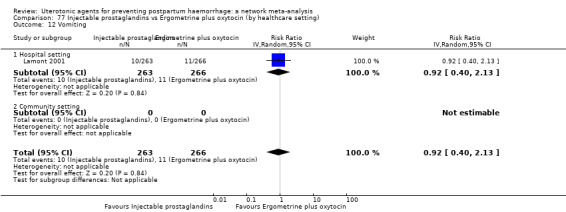
Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 12 Vomiting.
77.18. Analysis.
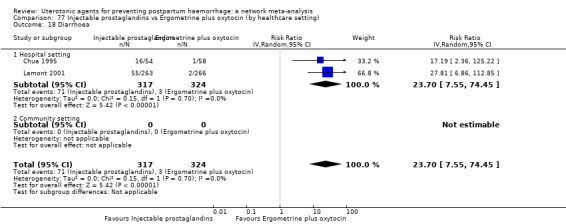
Comparison 77 Injectable prostaglandins vs Ergometrine plus oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
Comparison 78. Misoprostol plus oxytocin vs Injectable prostaglandins (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 79. Ergometrine vs Carbetocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 80. Carbetocin vs Ergometrine plus oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 4 | 1210 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.13, 0.86] |
| 2.1 Hospital setting | 4 | 1210 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.13, 0.86] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.42, 4.82] |
| 3.1 Hospital setting | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.42, 4.82] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 2 | 320 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.44, 2.09] |
| 6.1 Hospital setting | 4 | 1030 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.44, 2.09] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 7.1 Hospital setting | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.30, 1.00] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 5 | 1330 | Mean Difference (IV, Random, 95% CI) | ‐44.08 [‐82.41, ‐5.75] |
| 8.1 Hospital setting | 5 | 1330 | Mean Difference (IV, Random, 95% CI) | ‐44.08 [‐82.41, ‐5.75] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 5 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐4.32, ‐0.82] |
| 9.1 Hospital setting | 5 | 1160 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐4.32, ‐0.82] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.19, 0.45] |
| 11.1 Hospital setting | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.29 [0.19, 0.45] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.16, 0.46] |
| 12.1 Hospital setting | 6 | 1530 | Risk Ratio (IV, Random, 95% CI) | 0.27 [0.16, 0.46] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 5 | 1330 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.45, 4.38] |
| 13.1 Hospital setting | 5 | 1330 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.45, 4.38] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 4 | 930 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 14.1 Hospital setting | 4 | 930 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.36, 0.91] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 3 | 660 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.22, 1.39] |
| 15.1 Hospital setting | 3 | 660 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.22, 1.39] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 5 | 1290 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.26, 0.80] |
| 16.1 Hospital setting | 5 | 1290 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.26, 0.80] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
80.1. Analysis.
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 1 Death.
80.2. Analysis.
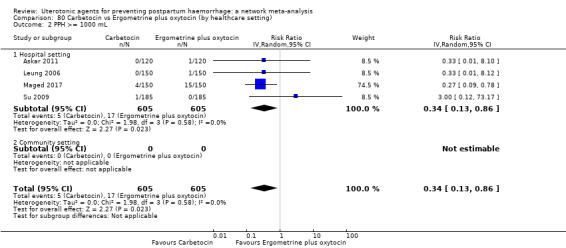
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
80.3. Analysis.

Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
80.4. Analysis.
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 4 Severe maternal morbidity: intensive care admissions.
80.6. Analysis.
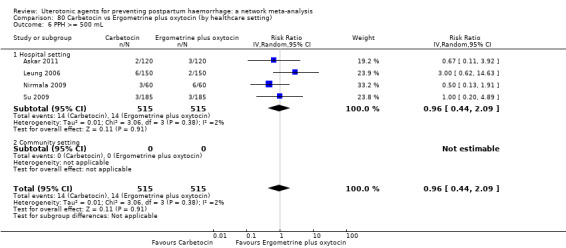
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
80.7. Analysis.
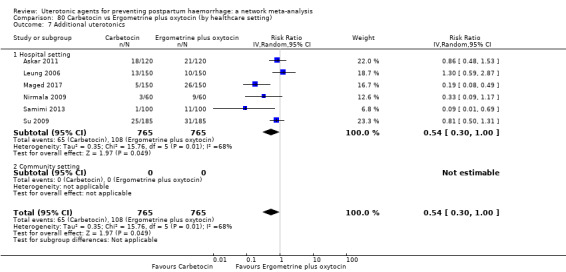
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
80.8. Analysis.
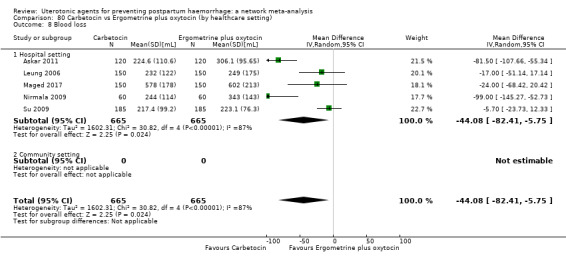
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 8 Blood loss.
80.9. Analysis.
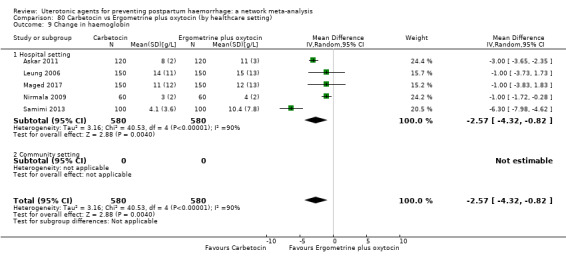
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
80.11. Analysis.
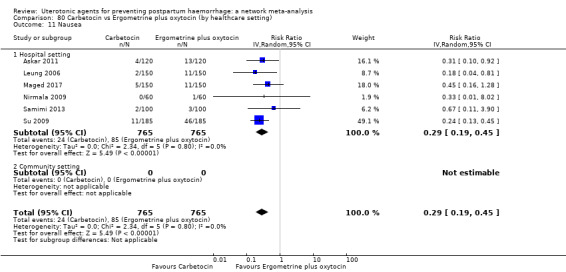
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 11 Nausea.
80.12. Analysis.
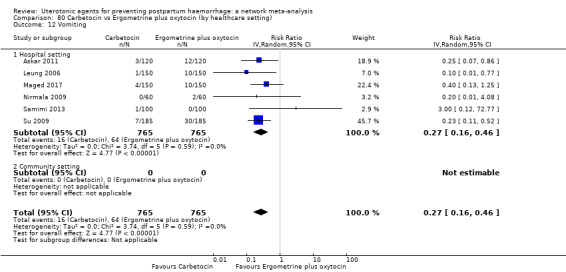
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 12 Vomiting.
80.13. Analysis.
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 13 Headache.
80.14. Analysis.
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 14 Abdominal pain.
80.15. Analysis.
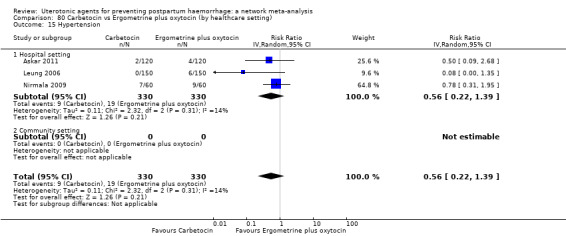
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 15 Hypertension.
80.16. Analysis.
Comparison 80 Carbetocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 16 Shivering.
Comparison 81. Misoprostol plus oxytocin vs Carbetocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.45, 35.46] |
| 3.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.45, 35.46] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.74, 1.93] |
| 7.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.74, 1.93] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 1 | 380 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.72, 0.32] |
| 9.1 Hospital setting | 1 | 380 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐3.72, 0.32] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.62, 2.39] |
| 11.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.62, 2.39] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.58, 3.09] |
| 12.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.58, 3.09] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.37, 10.79] |
| 13.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.37, 10.79] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 7.83 [3.43, 17.88] |
| 16.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 7.83 [3.43, 17.88] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 8.5 [1.99, 36.28] |
| 17.1 Hospital setting | 1 | 380 | Risk Ratio (IV, Random, 95% CI) | 8.5 [1.99, 36.28] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
81.1. Analysis.
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 1 Death.
81.3. Analysis.
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 3 Blood transfusion.
81.7. Analysis.
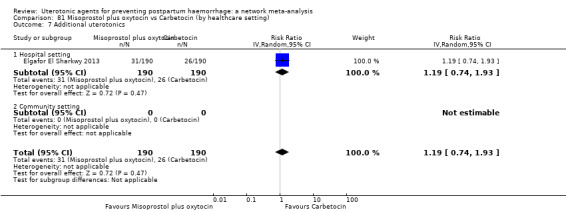
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 7 Additional uterotonics.
81.9. Analysis.
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 9 Change in haemoglobin.
81.11. Analysis.
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 11 Nausea.
81.12. Analysis.
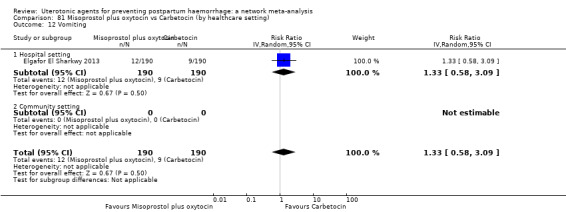
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 12 Vomiting.
81.13. Analysis.
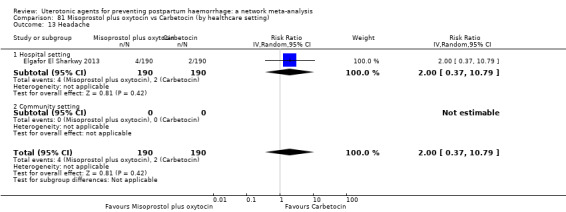
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 13 Headache.
81.16. Analysis.
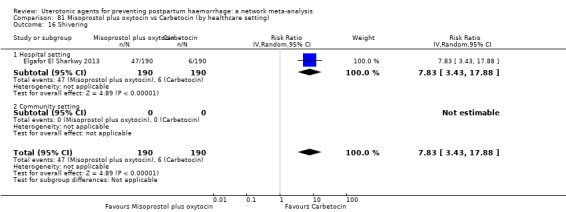
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 16 Shivering.
81.17. Analysis.
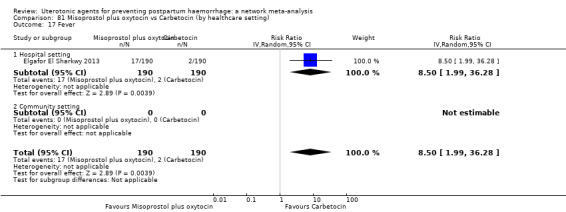
Comparison 81 Misoprostol plus oxytocin vs Carbetocin (by healthcare setting), Outcome 17 Fever.
Comparison 82. Ergometrine vs Ergometrine plus oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 1 | 144 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 6.1 Hospital setting | 1 | 144 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.01, 4.06] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 20.10 [5.76, 34.44] |
| 8.1 Hospital setting | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 20.10 [5.76, 34.44] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
82.6. Analysis.
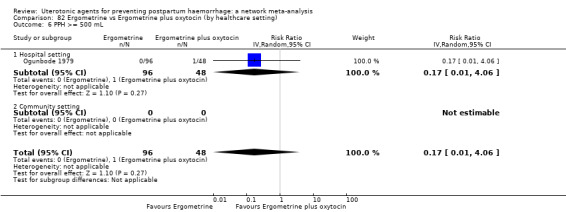
Comparison 82 Ergometrine vs Ergometrine plus oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
82.8. Analysis.
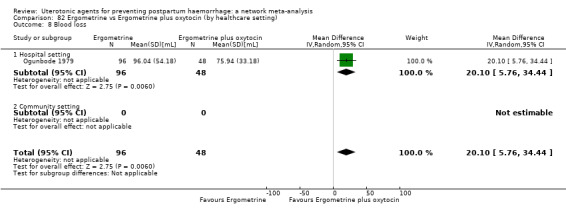
Comparison 82 Ergometrine vs Ergometrine plus oxytocin (by healthcare setting), Outcome 8 Blood loss.
Comparison 83. Misoprostol plus oxytocin vs Ergometrine (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Hospital setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 84. Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPH >= 1000 mL | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.68, 2.94] |
| 2.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.68, 2.94] |
| 2.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Blood transfusion | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.36, 2.19] |
| 3.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.36, 2.19] |
| 3.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Severe maternal morbidity: intensive care admissions | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Severe maternal morbidity: shock | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 PPH >= 500 mL | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.65, 2.99] |
| 6.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.65, 2.99] |
| 6.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Additional uterotonics | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.82, 2.69] |
| 7.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.82, 2.69] |
| 7.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Blood loss | 1 | 802 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐40.24, 8.24] |
| 8.1 Hospital setting | 1 | 802 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐40.24, 8.24] |
| 8.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in haemoglobin | 2 | 1605 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.72, 0.72] |
| 9.1 Hospital setting | 2 | 1605 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.72, 0.72] |
| 9.2 Community setting | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Breastfeeding | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Nausea | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Vomiting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.23, 4.77] |
| 12.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.23, 4.77] |
| 12.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Headache | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Abdominal pain | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Hypertension | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Hospital setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Shivering | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.95 [2.02, 4.29] |
| 16.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.95 [2.02, 4.29] |
| 16.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Fever | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.89 [1.51, 5.54] |
| 17.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 2.89 [1.51, 5.54] |
| 17.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Diarrhoea | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.46, 1.41] |
| 18.1 Hospital setting | 2 | 1605 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.46, 1.41] |
| 18.2 Community setting | 0 | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal well‐being | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Maternal satisfaction | 0 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Hospital setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Community setting | 0 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
84.2. Analysis.
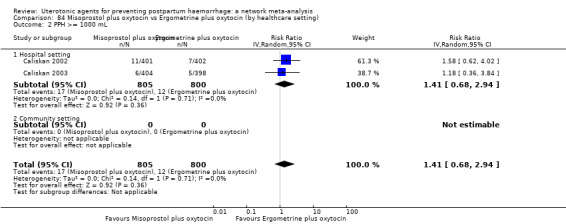
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 2 PPH >= 1000 mL.
84.3. Analysis.
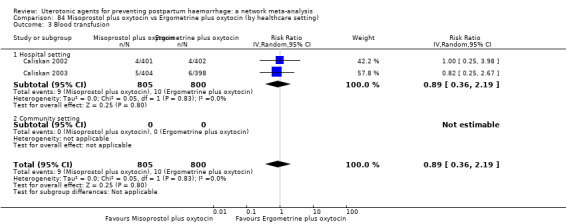
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 3 Blood transfusion.
84.6. Analysis.
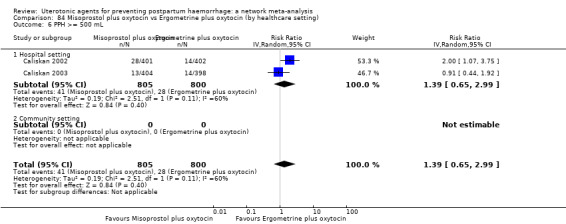
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 6 PPH >= 500 mL.
84.7. Analysis.
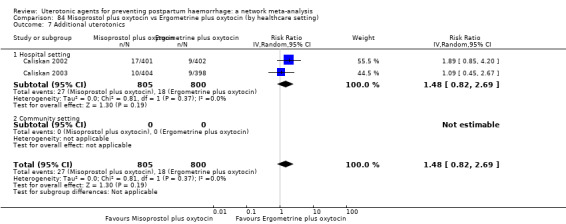
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 7 Additional uterotonics.
84.8. Analysis.
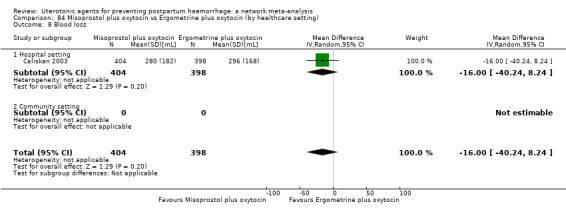
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 8 Blood loss.
84.9. Analysis.
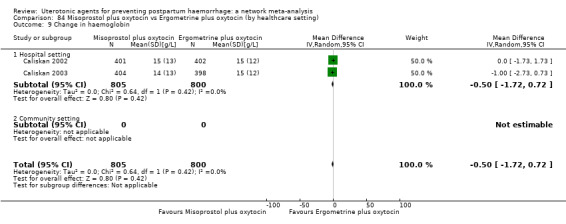
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 9 Change in haemoglobin.
84.12. Analysis.
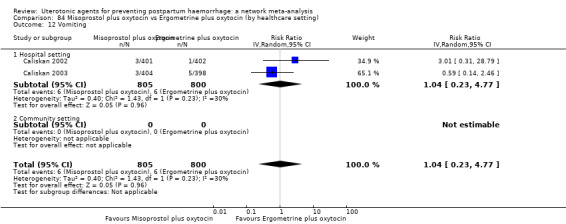
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 12 Vomiting.
84.16. Analysis.
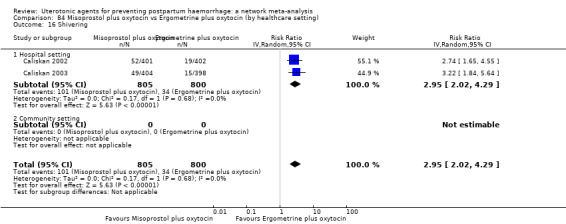
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 16 Shivering.
84.17. Analysis.
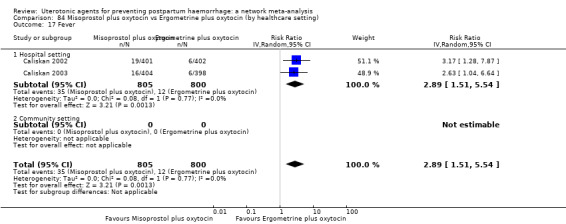
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 17 Fever.
84.18. Analysis.
Comparison 84 Misoprostol plus oxytocin vs Ergometrine plus oxytocin (by healthcare setting), Outcome 18 Diarrhoea.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Aleem 1993.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 150 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with risk factors for PPH: duration of labour less than 2 hours or prolonged labour more than 24 hours, MgSO4 for pre‐eclampsia, chorioamnionitis, multiple pregnancy, previous PPH, APH and episiotomy. | |
| Interventions | 200 mcg of ergometrine administered by an IV bolus versus 250 mcg of carboprost administered IM | |
| Outcomes | The study recorded the following outcomes: blood loss (mL); third stage duration (minutes); diarrhoea; nausea; vomiting; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers was used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Blood was collected in a tray and measured. Sterile pads were placed over the vulva before and after use for a period of 4 hours. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Carboprost kindly supplied by Prof. S. Bergstrom, Sweden but source(s) of funding for the study were not reported. |
Abdel‐Aleem 2010.
| Methods | 3‐arm controlled randomised trial | |
| Participants | 1964 women were randomised in a hospital setting in Egypt and South Africa. The population comprised women of any parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with medical complications such as hypertension and diabetes, previous caesarean section, or an abdominal wall that was not thin enough to allow easy palpation of the uterus after delivery. | |
| Interventions | 10 IU of oxytocin administered IM versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to 1 of 3 groups by selecting the next number in a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | The allocated group was noted inside opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | In Assiut, investigators appraised blood loss by collection with a calibrated plastic drape placed under the mother within 30 minutes of delivery. At the East London Hospital Complex, investigators appraised blood loss by collection with a low profile plastic “fracture” bedpan placed under the mother. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Investigators were unable to collect outcome data from 14 randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ACTRN: 12609000372280). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors, or conducted without external funding. |
Acharya 2001.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 60 women were randomised in a hospital setting in UK. The population comprised women of both nulliparous and multiparous, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus 400 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; transfusion; blood loss (mL; change in Hb; vomiting; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised intra‐operative blood loss by the estimation of attending physicians, and by measurement of preoperative and postoperative Hb concentration and hematocrit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Adanikin 2012.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 218 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with altered serum electrolytes, peritonitis, sepsis, previous bowel surgery, thyroid disease, inflammatory bowel disease, or chronic constipation. | |
| Interventions | 25 IU of oxytocin administered by an IV bolus + infusion versus 600 mcg plus 5 IU of misoprostol plus oxytocin administered rectally plus by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence developed by 1 researcher (O.O.) using a computer‐generated table of random numbers with varied permutated blocks. |
| Allocation concealment (selection bias) | Low risk | Used sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The same researcher administered the drugs intra‐operation and set up the infusions in the operating room; he was the only person who was not blind to the drug allocation and he did not take any further part in the active running of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Adanikin 2013.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 50 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with asthma or with hypersensitivity to prostaglandins. | |
| Interventions | 600 mcg of misoprostol administered rectally versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | The pharmacy department provided the study drugs and placebos in unidentifiable form but the resident doctor was responsible for the patient’s allocation according to the randomisation table. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Objective assessment of blood loss | Low risk | Investigators weighed the pads 4 hours postpartum for assessment of blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Afolabi 2010.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction of labour or caesarean section, or those with hematocrit of less than 30%, pre‐eclampsia/eclampsia, grand multiparity (5 or more), multiple pregnancy, coagulopathy, or medical disorders. | |
| Interventions | 10 IU of oxytocin administered IM versus 400 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised into 2 groups, A and B, by blocked (restrictive) double‐blind randomisation using random table generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss at delivery by collection with a large kidney dish, for measurement in a graduated measuring jar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ahmed 2014.
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | 80 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with risk factors for excessive blood loss e.g. those with placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 10 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was "single‐blind" but the identity of those blinded and the method of blinding were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Al‐Sawaf 2013.
| Methods | 3‐arm controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in Egypt. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction of labour or instrumental delivery, or those with previous caesarean section, extensive perineal, vaginal or cervical lacerations, bleeding disorders, HB less than 100 g/L, uterine malformations, grand multiparity, multiple pregnancy, polyhydramnios, intrauterine fetal death, medical problems such as pre‐eclampsia, diabetes, cardiopulmonary problems, bowel disease, or allergy to prostaglandins. | |
| Interventions | No treatment versus 200 mcg of misoprostol administered sublingually versus 5 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Used closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with sterile packs weighed beforehand and afterwards. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Following randomisation, 16 study participants were excluded from our analysis. Of these, 14 patients received intrapartum oxytocin, 1 patient experienced extensive vaginal laceration and another experienced a cervical laceration". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Alwani 2014.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of parity 3 or less, either singleton or multiple pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; death; change in Hb; nausea; vomiting; hypertension; fever;.shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomised in 2 groups using random number table generated online (http://www.graphpad.com/quickcalcs/randomize1/). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | No funding was sought for this study. |
Amant 1999.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 213 women were randomised in a hospital setting in Belgium. The population comprised women of both nulliparous and multiparous, either singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with hypertensive disorders, gestational age less than 32 weeks, intrauterine fetal death, uterine malformations, inflammatory bowel disease, obliterative vascular or coronary disease, sepsis, allergy to prostaglandins or alkaloids. | |
| Interventions | 600 mcg of misoprostol administered orally versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; diarrhoea; nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by a computer‐generated list and randomisation in blocks. |
| Allocation concealment (selection bias) | Low risk | The study box contained either 2 capsules of misoprostol and an ampoule containing placebo, or 2 capsules with placebo and an ampoule containing methylergometrine. The study boxes and capsules were indistinguishable in the 2 groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "213 women were enrolled in the study, but the data for 13 were excluded because a caesarean section was performed after randomisation (n = 3), or because no predelivery (n = 3) or postpartum (n = 7, short hospital stay) blood sample was taken". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Amin 2014.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in Pakistan. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with traumatic PPH, bleeding disorders, prolonged labour, placenta praevia, placental abruption, multiple pregnancy, BMI more than 30, or previous PPH. | |
| Interventions | 5 IU of oxytocin administered by an IV bolus versus 800 mcg of misoprostol administered rectally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; severe maternal morbidity: intensive care admissions; manual removal of placenta; death; blood loss (mL); third stage duration (minutes); diarrhoea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with special drapes placed under the mother until 1 hour postpartum, and weighed beforehand and afterwards. Blood was also collected in graduated plastic bags. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Askar 2011.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 240 women were randomised in a hospital setting in Kuwait. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women less than 18 years old and those with known or suspected coagulopathy, grand multiparity (5 or more), uterine fibroids, polyhydramnios, multiple pregnancy, fetal macrosomia, severe anaemia, cervical tears or who required prophylactic oxytocin infusion. The presence of contraindications for the use of either syntometrine or carbetocin that include pre‐existing hypertension, pre‐eclampsia, asthma, cardiac, renal or liver diseases, epilepsy, or history of hypersensitivity to syntometrine or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; nausea; vomiting; hypertension; headache; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by a computer‐generated code prepared before the recruitment. |
| Allocation concealment (selection bias) | Low risk | Used sealed, consecutively‐numbered, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a new plastic sheet placed under the mother following delivery of the placenta, and weighed (together with any gauzes, tampons and pads applied during the delivery) beforehand and 2 hours afterwards. A digital scale was used for weight measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Asmat 2017.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 1678 women were randomised in a hospital setting in Pakistan. The population comprised women of any parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with malpresentations such as breech, compound or transverse presentation, multiple pregnancy, placenta praevia type III, IV, placenta accreta, placental abruption, uterine rupture, myomectomy (uterine cavity opened), coagulation disorders, DIC, cardiac diseases, diabetes, and anaemia. | |
| Interventions | 800 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A lottery method was used. |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported but unlikely to have been implemented with a lottery method of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Quote: "Pads soaked were used to asses the amount of blood loss." Methods of evaluating blood loss were not reported in sufficient detail. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Attilakos 2010.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 377 women were randomised in a hospital setting in UK. The population comprised women of both nulliparous and multiparous, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women undergoing caesarean section with general anaesthesia, gestational age less than 37 weeks performed for fetal or maternal distress where, due to time constraints, it was not possible to recruit or randomise, or those with multiple pregnancy, placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 5 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; death; blood loss (mL); change in Hb; nausea; vomiting; headache; tachycardia; hypotension; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence (1:1 ratio—blocks of ten, no stratification) was generated by computer. |
| Allocation concealment (selection bias) | Low risk | The preparation of the ampoules was undertaken by DHP Ltd. (Powys, UK) which provided sequentially numbered and labelled boxes each containing a 1‐mL ampoule of the study drug. All boxes and ampoules were identically labelled, with the study number being the only differentiating feature between different drug packs. the random allocation sequence was not known to the investigators until the study had finished and the analysis was started. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Blood loss was estimated by the attending surgeon quote: "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (EudraCT 2005‐002812‐94). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | Ferring Pharmaceuticals funded the cost of preparation of blinded medication ampoules. No other external funding was required for the study. |
Atukunda 2014.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 1140 women were randomised in a hospital setting in Uganda. The population comprised women of both nulliparous and multiparous, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour or elective caesarean section, or those with intrauterine fetal death, heart disease, severe malaria or acute bacterial infection, multiple pregnancy, antepartum haemorrhage, altered cognitive status or reported hypersensitivity to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered IM versus 600 mcg of misoprostol administered sublingually | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; headache; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A study biostatistician generated a randomisation list with a block size of 10. |
| Allocation concealment (selection bias) | Low risk | The study clinical pharmacist prepared the study drugs and placebos. The midwife research assistants received opaque envelopes with affixed study codes, containing both an injection (1 mL of oxytocin 10 IU or its placebo) and 3 pills (misoprostol 600 mg or its placebo). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To achieve blinding of the participants and assessors, both inactive agents were manufactured and packaged to resemble actual study medicines in terms of shape, size, and colour". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a clean plastic sheet placed under the mother during and after the third stage of labour. The sheet was specifically designed and piloted for the purpose. Blood was then drained into a calibrated container to improve accuracy in blood loss measurement. Furthermore, quote: "mothers were given pre‐weighed standard sanitary pads to place in the perineum at all times. These pads were changed and weighed hourly for the first 6 hours, and then every 6 hours until 24 hours postpartum. Blood loss was estimated as 1 mL per g of weight of the pad after subtracting the dry pad weight". Investigators added the estimated blood loss in pads, to the volume of blood already collected with the plastic sheet. To improve consistency in the estimation of blood loss, standardised electronic scales were used to weigh soiled sanitary pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT01866241). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by scholarship funding from the Father Bash Foundation (public funding). |
Badejoko 2012.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 264 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women in the second or third stage of labour, or those with cervical lacerations or coagulopathy. | |
| Interventions | 30 IU of oxytocin administered by an IV bolus + infusion versus 600 mcg plus 20 IU of misoprostol plus oxytocin administered rectally plus by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code produced by an independent statistician using a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered sealed packets made of identical opaque brown‐paper envelopes prepared by the hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a BRASS‐V calibrated drape, quote: "which is a sterile intrapartum blood collection mat with a calibrated receptacle" placed under the mother after the delivery of the baby and immediate clamping of the umbilical cord. The drape included ribbons tied around the abdomen of the mother to optimise blood collection." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "6 women from the misoprostol group and 3 from the oxytocin group were excluded from statistical analysis. 5 of these women in the misoprostol group and all 3 in the oxytocin group were excluded because of the occurrence of cervical lacerations in them. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was conducted without external funding. |
Balki 2008.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 48 women were randomised in a hospital setting in Canada. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised women requiring general anaesthesia, or those with cardiac disease, hypertension or any condition predisposing to uterine atony and PPH, such as placenta praevia, multiple pregnancy, pre‐eclampsia, macrosomia, polyhydramnios, uterine fibroids, bleeding disorders, chorioamnionitis, previous uterine atony, previous PPH or allergy/hypersensitivity to oxytocin or ergot derivatives. | |
| Interventions | 250 mcg plus 20 IU of ergometrine plus oxytocin administered by an IV bolus versus 20 IU of oxytocin administered by an IV bolus + infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL); nausea; vomiting; hypertension; tachycardia; hypotension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of numbers. |
| Allocation concealment (selection bias) | Low risk | Used consecutively‐numbered opaque sealed packets or envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by measurement of hematocrit preoperatively and 48 hours postoperatively. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors. |
Bamigboye 1998a.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 550 women were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; manual removal of placenta; third stage duration (minutes); diarrhoea; vomiting; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by means of sealed, opaque containers containing 400 mg misoprostol or placebo tablets. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. Blinding of the midwife administering the tablets was therefore not possible". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with an absorbent plastic‐backed linen saver and a low‐profile plastic “fracture” bedpan placed under the mother. Blood collection in the plastic bedpan continued until 1 hour after delivery of the baby. At 1 hour after delivery, all the blood on the linen saver was scooped into the bedpan with the blood already collected there, and quote: "the total blood was carefully measured". All the used linen savers and vaginal pads were weighed, and the known dry weights of these materials were subtracted from the measured total weight. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Records of 4 of the 550 allocations (all from the placebo group) could not be traced". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Bamigboye 1998b.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 491 women were randomised in a hospital setting in South Africa. The population comprised women of any parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally versus 500 mcg and 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by means of sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "About halfway through enrolment it was discovered that a small number of women had been excluded from the syntometrine [ergometrine plus oxytocin] group because of hypertension detected after enrolment (thus contraindicating the use of syntometrine [ergo |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding). |
Barton 1996.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 119 women were randomised in a hospital setting in USA. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria were not specified. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus placebo | |
| Outcomes | The study recorded the following outcomes: additional uterotonics. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Baskett 2007.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 622 women were randomised in a hospital setting in Canada. The population comprised women of any parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with placenta praevia, placental abruption, coagulopathy or unstable asthma. | |
| Interventions | 5 IU of oxytocin administered by an IV bolus versus 400 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation cards. |
| Allocation concealment (selection bias) | Low risk | Used sealed, opaque, sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The packages were prepared by the hospital pharmacy and their active drug unknown to the physicians and nurses". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by a combination of the visual estimation of attending physicians and measurement of blood volume in a kidney dish placed under the mother during the third stage of labour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Nova Scotia Health Research Foundation (public funding). |
Begley 1990.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 1429 women were randomised in a hospital setting in Ireland. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, vaginal breech or instrumental delivery, or those with hypertension, epidural anaesthesia, antepartum haemorrhage, placenta praevia, placental abruption, first stage of labour more than 15 hours, "quick" delivery or needing resuscitation. | |
| Interventions | 500 mcg of ergometrine administered IV bolus versus No treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); third stage duration (minutes); nausea; vomiting. Hypertension. Headache. Abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used. The first number was selected from the table and the numbers were then allocated in blocks of 100, following in sequence. |
| Allocation concealment (selection bias) | Low risk | Used numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | A sterile receiver was placed against the perineum to collect the blood lost and was measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses but dropouts for change in Hb. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by public funding, or conducted without external funding. |
Begum 2015.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in Bangladesh. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by caesarean. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 20 IU of oxytocin administered by an IVinfusion | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Bellad 2012.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 652 women were randomised in a hospital setting in India. The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section or instrumental delivery, or those with medical disorders, in active labour with more than 4 cm dilatation or stillbirths. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death blood loss (mL); third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paticipants were assigned to treatment with a 1 : 1 ratio using computer‐generated simple randomisation |
| Allocation concealment (selection bias) | Low risk | The study medications and placebos were packaged in appropriately coded envelopes by administrative staff from the department of clinical pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a BRASS‐V calibrated drape placed under the mother before delivery of the baby. Quote: "The calibrated blood collection receptacle was opened after delivery and drainage of amniotic fluid. The blood collected in the drape was transferred to a measuring jar with 10‐mL calibrations for accuracy. Blood‐soaked swabs were weighed in g, and the known dry weight of the swabs was subtracted; this volume was added to the measured blood volume from the drape (assuming an equivalence of 1 g and 1 mL)". Blood loss was measured at 1 and 2 hours after delivery of the baby. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ClinicalTrials.gov NCT01373359). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Jawaharlal Nehru Medical College (the institution of the authors). Study medications were donated by Cipla (misoprostol) and AstraZeneca (oxytocin). |
Benchimol 2001.
| Methods | 3‐arm controlled randomised trial | |
| Participants | 602 women were randomised in a hospital setting in France. The population comprised women of any parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with gestational age less than 32 weeks, previous PPH, intrauterine fetal death, previous uterine scar, multiple pregnancy or pre‐eclampsia. | |
| Interventions | No treatment versus 2.5 IU of oxytocin administered by an IV bolus versus 600 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; blood loss (mL); change in Hb; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Slips with the words “control,” “Syntocinon,” and “Cytotec” were placed into envelopes which were then drawn at random upon admission into the delivery room to determine to which group the woman would belong. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by weighing (methods of collecting blood were not reported). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Bhatti 2014.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in Pakistan. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with medical disorders, multiple pregnancy, instrumental births, stillbirths and over 42 weeks. | |
| Interventions | 400 mcg of Misoprostol administered sublingually versus 10 IU of Oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 1:1 simple randomisation but the sequence generation was not reported in sufficient detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Visual assessment of blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Bhullar 2004.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 756 women were randomised in a hospital setting in USA. The population comprised women of any parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with a bleeding disorder. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IV infusion versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes; vomiting; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Agent vials were coded with a number, which had been assigned using a random number table. |
| Allocation concealment (selection bias) | Low risk | Used opaque vials containing either a 200 mcg misoprostol tablet or a placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The placebo tablets were similar in size and colour, but not identical in shape to the misoprostol tablet". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote:"The placebo tablets were similar in size and colour, but not identical in shape to the misoprostol tablet". |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Biswas 2007.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women of gravida 3 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with heart, renal or liver disease, previous caesarean and severe hypertension. | |
| Interventions | 125 mcg of carboprost administered IM versus 200 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes:transfusion; manual removal of placenta; nausea; vomiting; hypertension; fever. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Weighed blood clots and vaginal pads before and after use. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Borruto 2009.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 104 women were randomised in a hospital setting in Italy. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with toxemia, eclampsia or epilepsy. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; blood loss (mL); vomiting; headache; hypotension; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients were divided in two groups with blinding to the study medication". Blinding of caregivers was not confirmed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by quote:" a sensitive colorimetric method". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The authors, quote: "do not have a financial relationship with the organisation that sponsored the research". No other source(s) of funding for the study were reported. |
Boucher 1998.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 60 women were randomised in a hospital setting in Canada. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with heart disease or cardiac arrhythmia, hypertension or liver/renal/endocrine disease. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 32.5 IU of oxytocin administered by an IV bolus + infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); nausea; vomiting; headache; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by a sensitive colorimetric measurement of the Hb concentration of blood loss collected, quote: "by means of aspiration from the operative field [that] began immediately after administration of the study drug and ceased at the time of skin closure. All gauzes used during this timeframe were placed in 15% Lyse solution. All aspirated blood, gauzes, and the reference blood sample were sent to the laboratory for quantification of total blood volume. Blood on gauzes was extracted with Lyse solution, and haemoglobin content was determined with a sensitive colorimetric method adapted to the Cobas FARA analyser. Haemoglobin concentration is proportional to the absorbance of a hydrogen peroxide‐activated aminophenazone‐phenol mixture measured at a wavelength of 500 nm. The inter‐assay coefficient of variation averaged 3.3%, and the limit of detection of the assay was 14 mg/dL. The amount of blood collected in gauzes was calculated with the following formula: blood loss in dL = amount of haemoglobin in surgical gauzes in mg/haemoglobin concentration in mg/dL before caesarean section. Total blood loss was calculated by means of summing the volumes of blood aspirated and collected with gauzes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "3 patients who received general instead of epidural anaesthesia were excluded from the study and did not receive the study medication" but the study report did not specify whether these exclusions occurred before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
Boucher 2004.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 164 women were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women younger than 18 years old, or those without known PPH risk, known or suspected coagulopathy, heart disease or cardiac arrhythmia, chronic liver/renal/endocrine disease or hypersensitivity to study drugs. | |
| Interventions | 100 mcg of carbetocin administered IM versus 10 IU of oxytocin administered IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; blood loss (mL); change in Hb; nausea; vomiting; headache; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generaterd randomisation codes using a block size of 4. |
| Allocation concealment (selection bias) | Unclear risk | Used consecutively‐numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was 'double‐blind': Quote: "for each study subject, kits containing both the study medication and a placebo were prepared in the hospital pharmacy according to the randomisation schedule, to assure blinding of the clinical staff". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 164 women were randomised in the study, but 4 were excluded because they did not receive the study medication (3 oxytocin and 1 carbetocin) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
Bugalho 2001.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 700 women were randomised in a hospital setting in Mozambique. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour. | |
| Interventions | 400 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); third stage duration (minutes); diarrhoea; vomiting; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the investigators nor the nurses participating in the study had access to the codes until the completion of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss with a metallic collector placed under the mother, from immediately after delivery of the baby until the mother was removed from the delivery room. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A few subjects were excluded after randomisation for emergency caesarean section or incomplete data collection". |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of retained placenta were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | This study was financed by the Maputo Central Hospital (the institution of the authors) and the Special Program on Research and Research Training in Human Reproduction of the WHO (public funding). |
Butwick 2010.
| Methods | 5‐arm placebo‐controlled randomised trial | |
| Participants | 75 women were randomised in a hospital setting in the USA. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with active labour, ruptured membranes, drug allergy, multiple pregnancy, significant obstetric disease, risk factors for PPH (abnormal placentation, fibroids, previous PPH, previous classical uterine incision), coagulopathy or thrombocytopenia. | |
| Interventions | Placebo versus 5, 3, 1, or 0.5 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL); nausea; vomiting; tachycardia; hypotension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using Microsoft Excel‐generated random number allocations |
| Allocation concealment (selection bias) | Unclear risk | Used opaque envelopes containing group assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The obstetrician and anaesthetist involved in each case were blinded to the oxytocin dose assignments". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss quote: "by estimating blood collected by suction and by calculating the weight of blood on surgical swabs". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "75 patients were enrolled, and 74 patients completed the study; 1 patient was excluded due to protocol violation (obstetrician request for supplemental oxytocin despite adequate uterine tone)". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the Department of Anesthesia of the Stanford University School of Medicine (the institution of the authors). |
Caliskan 2002.
| Methods | 4‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 1633 women were randomised in a hospital setting in Turkey. The population comprised women of any parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with gestational age less than 32 weeks or hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus by an IV infusion versus 400 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered by an IV infusion versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered IM plus by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; change in Hb; third stage duration (minutes); diarrhoea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was based on a table of computer‐generated blocks of random numbers. |
| Allocation concealment (selection bias) | Low risk | Used sealed consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To overcome the limitation of the shape of the placebo, all medications were applied by midwives, but residents who treat the birth and the third stage of labour were blinded to the identity of medication. Only the midwife who applied the medication opened the envelope once to read the code and then transferred the randomisation code into another identical envelope. The identities of the placebo and active medication were also concealed from caregivers and residents who followed up the patient for the next 24 hours. The randomisation code was not broken until study completion." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To overcome the limitation of the shape of the placebo, all medications were applied by midwives, but residents who treat the birth and the third stage of labour were blinded to the identity of medication. Only the midwife who applied the medication opened the envelope once to read the code and then transferred the randomisation code into another identical envelope. The identities of the placebo and active medication were also concealed from caregivers and residents who followed the patient for the next 24 hours. The randomisation code was not broken until study completion." |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a sterile steel bedpan and plastic bed linen. Gauzes and pads were also collected and weighed until 1 hour after delivery of the placenta. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The study enrolled 1633 women, but the data for 27 women were excluded because of lack of predelivery (n = 13) or postpartum (n = 14, short hospital stay) haemoglobin concentrations". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Caliskan 2003.
| Methods | 4‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 1800 women were randomised in a hospital setting in Turkey. The population comprised women of any parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with gestational age less than 32 weeks or hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered orally plus by an IV infusion versus 400 mcg of misoprostol administered orally versus 10 IU of oxytocin administered by an IV versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered IM plus by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated without any blocking or stratification. |
| Allocation concealment (selection bias) | Low risk | Used sealed, consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. To minimise this limitation, the preparation and administration of the medication were carried out by a midwife who had not been involved in the management of the patient except for drug administration." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. To minimise this limitation, the preparation and administration of the medication were carried out by a midwife who had not been involved in the management of the patient except for drug administration." |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a sterile steel bedpan and plastic bed linen from immediately after delivery. Gauzes and pads were also collected 1 hour after delivery of the placenta and weighed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The data for 226 patients were excluded because of caesarean deliveries performed after randomisation (n = 206) and the lack of predelivery (n = 6) or postpartum (n = 14, short hospital stay) haemoglobin concentrations." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Carbonell 2009.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 1410 women were randomised in a hospital setting in Spain. The population comprised women of parity 4 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section or instrumental delivery, or those with gestational age less than 32 weeks, coagulopathy, Hb less than 80 g/L, liver or kidney disorder, grand multiparity (5 or more), hypersensitivity or any contraindication for use of prostaglandins. | |
| Interventions | 400 mcg and 200 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually and rectally plus IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity; intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); NNU admissions; diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments generated by computer. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes prepared by people not related to the study. This process was supervised by an analyst. Every morning a secretary received the sealed envelopes for distribution and this process was monitored by someone working on the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After delivery of the baby, investigators appraised blood loss by collection with a sterile waterproof cloth placed under the mother, to channel blood into a bottle with capacity of 2 L: the volume reading was collected once beyond the third stage of labour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1410 women were randomised in the study, but 10 were excluded because they did not receive the allocated agents (3 in the misoprostol plus oxytocin group and 7 in the oxytocin group) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by the Science and Ethics Committee of the Hospital Eusebio Hernandez in Habana, Cuba in conjunction with the Clinica Mediterranea Medica in Valencia, Spain (the institutions of the authors). |
Carrillo‐Gaucin 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in Mexico. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised women with allergies to oxytocin or carbetocin or previous coagulation disorder. | |
| Interventions | unspecified dose of carbetocin administered by an unspecified route versus unspecified dose of oxytocin administered by an unspecified route | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Simple randomisation but sequence generation was not reported in sufficient detail. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is mentioned that the study was double blinded but blinding methods (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 3 losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Cayan 2010.
| Methods | 4‐arm controlled randomised trial | |
| Participants | 160 women were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with thyroid disorder, inflammatory bowel disease or other bowel diseases, previous bariatric surgery or hypersensitivity to prostaglandins. | |
| Interventions | 200, 400, or 600 mcg of misoprostol plus oxytocin administered rectally plus by an IV infusion versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Chalermpolprapa 2010.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in Thailand. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by caesareans. Exclusion criteria were not specified. | |
| Interventions | Unspecified dose of misoprostol plus oxytocin administered by an unspecified route versus unspecified dose of oxytocin administered by an unspecified route | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Chandhiok 2006.
| Methods | 2‐arm cluster‐controlled randomised trial | |
| Participants | 1200 women were randomised in a community setting in India. The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with multiple pregnancy, known systemic disease or previous uterine surgery, or who were designated as high risk and scheduled for transfer to an advanced care facility at the time of labour. | |
| Interventions | 600 mcg of misoprostol administered orally versus 200 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); third stage duration (minutes; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not explained in sufficient detail. |
| Allocation concealment (selection bias) | Low risk | Randomisation process not explained in sufficient detail but lack of allocation concealment usually not an issue in cluster trials. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not applicable. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not applicable. |
| Objective assessment of blood loss | Low risk | Immediately after the cord was clamped and cut, the paramedical worker in both groups placed a calibrated blood collection drape (BRASS‐V drape) under the women’s buttocks for quantification of blood loss. This consists of a plastic sheet to which a funnelled pouch is attached. The volume of blood collected in the first hour was recorded. In the event of persistent bleeding, another measurement was made at the end of 2 hours. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | This ICMR Task Force study was funded in part by the WHO Country Office, New Delhi; Cipla Pharmaceuticals provided the misoprostol tablets. |
Chaudhuri 2010.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women undergoing caesarean section for cord prolapse or bradycardia, or those with cardiovascular, respiratory, liver or haematological disorders or known hypersensitivity to prostaglandins. | |
| Interventions | 800 mcg of misoprostol administered rectally versus 40 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity; intensive care admissions; additional uterotonics; transfusion; death; blood loss (mL); change in Hb; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer‐generated random numbers in a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | The packets containing the 2 drugs were sealed and opaque, and could not be identified by the surgeons and anaesthetists |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The packets containing the 2 types of drug were sealed and opaque, and could not be identified by the surgeons and anaesthetist". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised intraoperative blood loss by collection with a suction bottle for volumetric measurement, combined with linen savers and mops weighed before and after delivery. They added the approximate volume of the contents of the suction bottle (a) to the difference in weight between dry (b) and soaked (c) linen savers and mops (1 g equivalent to 1 mL). Amniotic fluid volume (d) was calculated by multiplying amniotic fluid index by 30 mL. Finally, intraoperative blood loss was determined by subtracting amniotic fluid volume from approximate blood loss ((a + (c ‐ b)) ‐ d). Furthermore, investigators appraised postoperative bleeding over the next 8 hours by weighing soaked pads and subtracting the dry weight. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "4 women in group 1 [misoprostol] and 6 women in group 2 [oxytocin] were excluded from the analysis: 4 women required conversion to general anaesthesia, 5 women had traumatic intraoperative bleeding (extension of lower segment incision or broad ligament" |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2009/091/000075). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Chaudhuri 2012.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 530 women were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing augmentation of labour, caesarean section or instrumental delivery, or those with risk factors for PPH, including BMI more than 30, grand multiparity (5 or more), polyhydramnios, fetal macrosomia, antepartum haemorrhage, prolonged labour, previous PPH, Hb less than 80 g/L, severe pre‐eclampsia, asthma or coagulopathy. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death. Blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Used pre‐prepared sealed and opaque packet. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The misoprostol and placebo tablets were similar in size, shape, and colour. The ampoules of oxytocin and placebo were also similar. Selection, enrolment, and randomisation were done by the resident doctors, whereas preparation of packets and confidential record maintenance was done by the labour room nursing staff in charge." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Quote: "Investigators appraised blood loss by collection with specially designed, pre‐weighed absorbent thick cotton pads with plastic lining, placed under the mother. Blood clots, if any, were expressed from the vagina into a polythene bag. Any episiotomy wound was repaired immediately, and the swabs used for the purpose of episiotomy were not included in blood loss assessment. If necessary, pads were replaced during the observational hour after delivery. Then the soaked pad(s) and the blood clots were weighed. "The specific gravity of blood being 1.08, the amount of blood lost in mL was approximately equal to the weight in g". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 women in the study group and 1 woman in the control group refused sublingual administration of the drug". |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2009/091/000672). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Chaudhuri 2015.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 396 women were randomised in a hospital setting in India. The population comprised women of parity 5 or less, either singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised women requiring conversion to general anaesthesia, or those with cardiovascular, hepatic, or haematologic disorders or any contraindication for the use of misoprostol or oxytocin. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IM bolus and IV infusion versus 20 IU of oxytocin administered IM bolus plus an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); change in Hb; diarrhoea; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated random number sequence and blocks of size 8. |
| Allocation concealment (selection bias) | Low risk | Assignments were contained in sealed, opaque and sequentially‐numbered packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Randomisation and confidential record maintenance were performed by residents who were not involved in the trial, and the operation theatre midwife prepared the sealed packets and allocated and administered the drugs. Thus, clinicians, investigators, data analysts, and participants were masked to the treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised intraoperative blood loss from after delivery of the placenta. Blood was collected with a suction bottle, linen savers and mops: the dry weights of these materials were subtracted from the soaked weights, and the total volume of intraoperative blood loss calculated on the basis that 1 g is equivalent to 1 mL. Investigators also appraised postoperative blood loss by weighing soaked pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2013/05/003645). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Chaudhuri 2016.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 288 women were randomised in a hospital setting in India. The population comprised women of parity 5 or less, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women who had caesareans or instrumental birth, known hypersensitivity to misoprostol and/or oxytocin, major cardiovascular, hepatic, or haematological disorders or intrauterine fetal death or stillbirth. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; diarrhoea; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated random number sequence and block randomisation (blocks of 6–8). |
| Allocation concealment (selection bias) | Low risk | Used sealed, opaque, and sequentially numbered packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, and data analysts were masked to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, and data analysts were masked to group assignment. |
| Objective assessment of blood loss | Low risk | Linens soaked with amniotic fluid were removed soon after delivery of the newborn, and a pre‐weighed thick cotton pad with plastic lining was placed under the buttocks. All blood clots were removed from the vagina and kept in a plastic bag. The pad was replaced if completely soaked during the 1‐hour observation period. Episiotomies were repaired immediately after complete delivery of the placenta, and cotton swabs used during this procedure were not included in the blood loss assessment. The difference in weight between the soaked and dry pad was added to the weight of blood clots to calculate the total blood loss (1mL was considered equal to 1 g given the specific gravity of blood of 1.08). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | Registered with Clinical Trial Registry India (Registration No. CTRI/2014/03/004491). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Chhabra 2008.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 300 women were randomised in a hospital setting in India. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing augmentation of labour, caesarean section or instrumental delivery, or those with grand multiparity (more than 5), multiple pregnancy, pregnancy‐induced hypertension, antepartum haemorrhage, previous caesarean, Hb less than 80 g/L, other obstetric problems or known hypersensitivity to prostaglandins. | |
| Interventions | 100 or 200 mcg of misoprostol administered sublingually versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); change in Hb; third stage duration (minutes); nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss by quote: "measuring blood and blood clots collected in sponges". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Choy 2002.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 991 women were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with medical conditions that precluded the use of ergometrine, such as pre‐eclampsia, cardiac disease or conditions that required prophylactic oxytocin infusion after delivery such as grand multiparity (4 or more) or presence of uterine fibroids. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 10 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; nausea; vomiting; hypertension;headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | Low risk | Used sealed consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The preparation and administration of the medication was carried out by a second midwife who was not involved in the management of the patient except for the drug administration. The medical attendant who delivered the baby was not informed of the type of oxytocics used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss quote: "by measuring the amount of blood clots and weighing the towels and swabs used". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Chua 1995.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 115 women were randomised in a hospital setting in Singapore. The population comprised women of unspecified parity, a singleton pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 125 mcg of carboprost administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; manual removal of placenta; diarrhoea. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | All blood and blood clots lost in the first 2 hours after delivery were collected by mopping the blood and clots with absorbent paper, and collect the paper in a plastic bag. The bags were sent to the laboratory for processing within 2 hours of completion of blood collection. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 115 women were randomised in the study, but 3 were excluded because they gave birth precipitously before preparing the bed for accurate collection of blood after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Cook 1999.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 930 women were randomised in a hospital setting in Australia, Papua and China. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective caesarean section, or those with coagulopathy, asthma, heart disease, severe renal disease, epilepsy or hypertension. | |
| Interventions | 400 mcg of misoprostol administered orally versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by random number list in blocks of 20 with a separate randomisation for each centre. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered sealed security (opaque) envelopes containing the appropriate drug label for each centre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss by combining "estimated" and "measured" values according to the standard clinical practice of each study centre. The "estimated" blood loss was judged by the attending senior midwives and/or clinicians. The "measured" blood loss was calculated as the actual volume of blood collected in a calibrated measuring jug, combined with the difference in weight between dry and blood‐stained undersheets and sanitary pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were not collected completely from 67 study participants: quote: "the main reasons for exclusion prior to randomisation, and following randomisation but before treatment, were the need for caesarean section and development of hypertension, either before or during labour." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Dabbaghi Gale 2012.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 269 women were randomised in a hospital setting in Iran. The population comprised women of parity less than 3, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with previous PPH, asthma, clotting disorders, placental abruption, PPH due to lacerations, or those requiring instrumental delivery or caesarean section. | |
| Interventions | 400 mcg of misoprostol administered orally versus 10 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Dansereau 1999.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 694 women were randomised in a hospital setting in Canada. The population comprised women of parity 5 or less, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women undergoing general anaesthesia or requiring a classical uterine incision, or those with heart disease, chronic hypertension requiring treatment, liver, renal, or endocrine disorders, coagulopathy, placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 25 IU of oxytocin administered by an IV bolus + infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; change in Hb; nausea; vomiting; headache; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code, stratified by centre and with use of random blocks of 2. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All physicians and nurses involved, all investigators and their staff, and all sponsor representatives were kept blinded to the treatment codes at all times". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 694 women were enrolled in the study, but 59 were excluded because of withdrawals (n = 5) or protocol violations (n = 54) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
Dasuki 2002.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 196 women were randomised in a hospital setting in Indonesia. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: blood loss (mL); third stage duration (minutes); shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
de Groot 1996.
| Methods | 3‐arm placebo‐controlled randomised trial | |
| Participants | 371 women were randomised in a hospital and community setting in the Netherlands. The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour or instrumental delivery, requiring tocolysis or those who refuse to take part or with cardiac disease, multiple pregnancy, non‐cephalic presentation, polyhydramnios, coagulopathy, stillbirth, antepartum haemorrhage, Hb less than 4.8 mmol/L or previous complication in third stage. | |
| Interventions | Placebo versus 5 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death;blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Used identical study boxes. Care was taken that no difference could be seen or heard between the packages of the ergometrine/placebo tablets and the oxytocin ampoules. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study made use of placebo tablets to minimise detection bias between the placebo and the oral ergometrine arm but also included an unblinded oxytocin arm and the comparison of oxytocin versus placebo was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a "fresh" perineal pad placed under the mother from immediately after birth until 1 hour after the delivery of the placenta. The difference in the weight of the pad before and after delivery was calculated on the basis that 1 g is equivalent to 1 mL of blood. "During delivery some blood was usually spattered on the drapes and gowns of the attendants, although attempts were made to minimise such losses. This gave a constant error of approximately 10%. In addition, the placental interstices contain maternal blood (about 9% of placental weight). As systematic overestimations (amniotic fluid) and underestimations (blood loss) are likely to be equally distributed among the groups, no corrections have been made for them". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "4 women with exclusion criteria were entered erroneously (3 forceps, 1 augmentation). They are considered as non‐participants". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Del Angel‐Garcia 2006.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 152 women were randomised in a hospital setting in Mexico. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by an unspecified method. Exclusion criteria were not specified. | |
| Interventions | unspecified dose of oxytocin administered by an unspecified route versus unspecified dose of carbetocin administered by an unspecified route | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no (Abstract only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Derman 2006.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 1620 women were randomised in a community setting in India. The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women at high risk and inappropriate for home or community births according to India’s ministry of health guidelines including those undergoing elective caesarean section or breech vaginal delivery, or those previous caesarean section, Hb less than 80 g/L, antepartum haemorrhage, hypertension, multiple pregnancy, history of previous antepartum or PPH, retained placenta, uterine inversion, diabetes, heart disease, seizures, placenta praevia, asthma or contraindications to misoprostol. | |
| Interventions | 600 mcg of misoprostol administered orally versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated randomisation list with a random block size by the data co‐ordinating centre and was stratified by the midwife. |
| Allocation concealment (selection bias) | Low risk | The envelopes were numbered and each envelope had a 5‐digit code number assigned to it. The first 2 digits were the auxiliary nurse midwife number, followed by a sequence number beginning with 001 and ending with 100, assigned to the individual participant. Non‐distinguishable envelopes in batches of 100 were distributed to each of the midwifes affiliated with the 4 selected primary‐health centres. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The identical placebo was specifically manufactured for the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a polyurethane blood collection drape placed under the mother from immediately after birth until 1 hour after delivery of the baby. The blood collection drape included a calibrated receptacle specifically developed for the study. In the event of persistent bleeding beyond 1 hour, the drape was removed at 1 hour, blood loss measured, and a new drape used with a second measurement made at 2 hours. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00097123). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Institute of Child Health and Human Development (public funding) and the Bill and Melinda Gates Foundation (public funding). |
Dhananjaya 2014.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women of parity 4 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with grand multiparity (not defined), rhesus negative blood group, cardiac disease, diabetes, bleeding disorder, precipitated labour, overdistended uterus, traumatic PPH, PROM/chorioamnionitis, intrauterine death, previous caesarean section/scar on uterus or inability to obtain the informed consent. | |
| Interventions | 10 IU of oxytocin administered IM versus 200 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; blood loss (mL); third stage duration (minutes); diarrhoea; nausea; vomiting; headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Systematic random sampling method. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with drapes that were weighed together with mops and clots, and by measurement of Hb concentration and haematocrit of a sample of venous blood before delivery and 24 hours after birth. A sample of venous blood before delivery and 24 hours after the birth was also collected, for Hb and haematocrit measurement quote: "as an objective index of blood loss". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Diallo 2017.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 304 women were randomised in a hospital setting in Senegal. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women who could not give their consent, those requiring a caesarean delivery and those with asthma allergy to misoprostol, pregnancies of less than 36 weeks, temperature above 38°C, chorioamnionitis, multiple pregnancy, severe cardiopathy, severe anaemia, clotting disorders, or complex perineal tear. | |
| Interventions | 400 mcg of misoprostol administered orally versus 5 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion' blood loss (mL); change in Hb; diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomised sequence. |
| Allocation concealment (selection bias) | Low risk | Cards assigning patients into groups were placed in envelopes which were then sealed and numbered as and when patients were included. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | If an oxytocin drip was used during labour, it was continued for patients in the “oxytocin” group and replaced by a bottle of 5% glucose solution in the “misoprostol” group. The patient was then attended by the midwife who was not informed of the type of uterotonic administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient was then attended by the midwife who was not informed of the type of uterotonic administered." |
| Objective assessment of blood loss | Low risk | The blood lost was collected in a basin placed after the clamping of the umbilical cord and the removal of the amniotic fluid. Episiotomies were repaired immediately after delivery. Blood loss was collected for up to 2 hours after delivery. This blood was transferred into a graduated jar to measure its exact volume. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | No funding sought for this study. |
Diop 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 1820 women were randomised in a community setting in Senegal. The population comprised women of any parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with known allergies to prostaglandins or pregnancy complications. | |
| Interventions | 600 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: death; change in Hb; diarrhoea; nausea; vomiting; fever; shivering; maternal satisfaction.; | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The computer‐generated random allocation was overseen by Gynuity Health Projects, which also assigned clusters. Maternity huts with auxiliary midwives located 3–21 km from the closest referral centre were randomly assigned (1:1) by staff at Gynuity Health Projects to either oral misoprostol or oxytocin in Uniject, stratified by reported previous year clinic volume (deliveries) and geographical location (inland or coastal). |
| Allocation concealment (selection bias) | Low risk | Study drugs were packed into individually numbered single‐dose envelopes by staff at Gynuity Health Projects and supplied to maternity huts by ChildFund Senegal. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Objective assessment of blood loss | High risk | The perceived amount of blood loss was documented as “normal”, “moderate”, or “significant”. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 1820 recruited initially through the clusters but 1412 were included in the analysis and 1049 had data available for the study's primary outcome. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (ClinicalTrials.gov, number NCT01713153). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | This study was funded by the Bill & Melinda Gates Foundation. |
Docherty 1981.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 50 women were randomised in a hospital setting in UK. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Dutta 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 400 women were randomised in a hospital setting in India. The population comprised women of parity 2 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women requiring caesarean section or instrumental delivery, Hb less than 8 g/dL, APH, severe pregnancy‐induced hypertension, pre‐eclampsia or eclampsia, prolonged labour or precipitate labour, fetal weight > 3.5 kg, polyhydramnios, and medical disorders (cardiovascular disease, diabetes mellitus, thyroid disorders and other coagulation abnormalities. | |
| Interventions | 600 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; transfusion; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; headache; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study is stated to be double‐blinded but blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Any blood clot which expressed from the uterus was measured in the calibrated glass container. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Eftekhari 2009.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in Iran. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with multiple pregnancy, prolonged labour more than 12 hours, 2 or more previous caesarean sections, previous uterine rupture, Hb less than 80 g/L,who had a history of heart, renal or liver disorders or had a coagulopathy did not enter the study. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; blood loss (mL); change in Hb | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | By a simple randomisation method, patients were allocated into 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection in a suction bottle, and with drapes and pads beneath the mother. Amniotic fluid was suctioned and measured, and then subtracted from the total volume of the suction bottle. Meanwhile the known dry weight(s) of drapes and pads were subtracted from the soaked weights of these materials. Measurements of blood collected in the suction bottle and on drapes and pads were added together. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion were omitted). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
El Behery 2015.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 180 women were randomised in a hospital setting in Egypt. The population comprised women of nulliparous, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised women undergoing elective caesarean section, vaginal delivery or general anaesthesia, or those who are multigravida, or with malpresentation, fetal anomalies, placenta praevia, diabetes, hypertension, pre‐eclampsia or cardiac disease. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL;. change in Hb; headache; fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code. |
| Allocation concealment (selection bias) | Low risk | Used sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was quote: "double‐blinded": "a double dummy system for administration was used". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss quote: "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 180 women were included in the study, but 100 were excluded because 4 had congenital fetal anomalies, 7 cases had placenta praevia, 5 cases were diabetic, 8 had hypertension, 9 had pre‐eclampsia, 3 cases were cardiac, 28 cases needs general anaesthesia, 17 cases delivered vaginally and 19 cases delivered by elective caesarean section). It was unclear if these were excluded before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
El Tahan 2012.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 382 women were randomised in a hospital setting in Egypt. The population comprised women of parity 3 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with asthma, anaemia, bleeding disorders, cardiac disease, inflammatory disease, bowel disease, multiple pregnancy, pre‐eclampsia, placenta praevia, placental abruption, previous APH, previous PPH, grand multiparity (not defined), fibroids, growth restriction, fetal malformations or allergy to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus by an IV bolus versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; death; blood loss (mL); diarrhoea; vomiting; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated randomisation code. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and misoprostol tables quote: "looked identical in size, colour, and packing". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised intraoperative blood loss by collection in a suction bottle minus sonographically estimated amniotic fluid volume, together with visual estimates of the volume of blood on the floor and the weight differences between dry and used towels, linens, and swabs. Visual estimates were performed by obstetricians blinded to treatment allocation. Towels, linen and swabs were weighed with an electronic scale. Weights were added to volumetric values on the basis that 1 g is equivalent to 1 mL. Investigators appraised postoperative blood loss by weighing bed linen, gowns and perineal pads. Furthermore, blinded investigators estimated blood loss by multiplying maternal blood volume in mL by the difference between preoperative and postoperative hematocrit measurements, all divided by preoperative haematocrit measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "4 patients in the placebo group and 12 patients in the misoprostol group were excluded from the study due to loss to follow‐up or missed preoperative haematocrit data". |
| Selective reporting (reporting bias) | Unclear risk | The study report matches the study protocol that was registered retrospectively (ClinicalTrials.gov NCT01466530). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by funding from Mansoura University (the institution of the authors). |
El‐Refaey 2000.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 1000 women were randomised in a hospital setting in UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section or water birth, or those with severe asthma. | |
| Interventions | 500 mcg of misoprostol administered orally versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes). Diarrhoea. Nausea. Vomiting. Headache. Fever. Shivering. Abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician using computer‐generated block randomisation with varying block size |
| Allocation concealment (selection bias) | Low risk | Used opaque, sequentially‐numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Elbohoty 2016.
| Methods | 3‐arm active‐controlled triple‐dummy randomised trial | |
| Participants | 270 women were randomised in a hospital setting in Egypt. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with hypersensitivity to oxytocin, carbetocin, or prostaglandins; contraindication to treatment with prostaglandins (e.g. glaucoma); history of significant heart disease; severe asthma; epilepsy; history or evidence of liver, renal, or vascular disease; history of coagulopathy, thrombocytopenia, or anticoagulant therapy; HELLP syndrome or eclampsia; placental abruption; or contraindication to spinal anaesthesia. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 400 mcg of misoprostol administered sublingually versus 30 IU of oxytocin administered by an IV bolus + infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; nausea; vomiting; headache; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in a 1:1:1 ratio using a computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes were prepared, with each envelope containing 1 of the 3 study drugs and placebos for the other 2 drugs. The randomisation protocol was concealed from the research team and the primary investigator contacted a central co‐ordinating investigator to identify the envelope to be distributed to each patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tablet placebos, containing hydrogenated castor oil, hypromellose, microcrystalline cellulose, and sodium starch glycolate were prepared to be identical in size, colour, shape, and packing to the tablet study drug. Intravenous placebo ampoules containing normal saline were prepared and were identical in shape and packing to the IV study drugs used. All envelopes were prepared by Sigma Pharmaceuticals and were already sealed when received by the research team. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Consequently, patients, investigators, and data analysts were masked to group assignments and unmasking only occurred after data analysis was completed. |
| Objective assessment of blood loss | Low risk | Surgical towels were weighed with their wrapping before and after delivery using a highly accurate digital balance. The difference in mass between the dry and soaked towels was calculated. Operative blood loss was calculated using 3 parameters: (A) the volume of the suction bottle contents (mL), (B) the difference in towel mass (g), and © the amniotic fluid volume (mL). Intraoperative blood loss (mL) was calculated as: Intraoperative blood loss = (A + B) −C. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 270 women were randomised in the study, but 7 were excluded because they had general anaesthesia (n = 4) or the drug ampoules were damaged after randomisation. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov: NCT02053922). |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Elgafor El Sharkwy 2013.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 380 women were randomised in a hospital setting in Egypt. The population comprised women of any parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women undergoing general anaesthesia, or those with coagulopathy, coronary artery disease, hypertension, PPH due to causes other than uterine atony or hypersensitivity to carbetocin. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IV infusion versus 100 mcg of carbetocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: severe maternal morbidity: additional uterotonics; transfusion; death; change in Hb; nausea; vomiting; headache; hypotension; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Drugs were in pre‐prepared sealed and opaque packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Caesarean delivery was performed by 4 senior obstetricians who were blinded to the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss quote: "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Elsedeek 2012.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 400 women were randomised in a hospital setting in Egypt. The population comprised women of parity 4 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women undergoing their first elective caesarean section, or those unsure of gestation or with hypertension, diabetes, oligohydramnios, abnormal placenta or abnormal laboratory investigations. | |
| Interventions | 400 mcg plus 10 IU of Misoprostol plus Oxytocin administered rectally plus by an intravenous infusion versus 10 IU of Oxytocin administered by an intravenous infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb. NNU admissions; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was placed in sealed envelopes until the time of operation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Attending obstetricians and other caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss from after uterine incision, by collection in 2 separate suction sets administered by a nurse, and by weighing surgical towels before and after each operation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ACTRN 12611000638932). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors, or conducted without external funding. |
Enakpene 2007.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 864 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at Low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with pre‐eclampsia, hypertension, cardiac disease, severe anaemia, asthma, renal/hepatic disorders, grand multiparity (not defined), multiple pregnancy, polyhydramnios, previous PPH, fibroids or contraindications to misoprostol or ergometrine. | |
| Interventions | 400 mcg of misoprostol administered orally versus 500 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by simple random selection. An independent statistician generated sets of 4 random letters, which were in boxes, and each box contained 4 separate random allocations which was equivalent to an opaque sealed envelope stratified in a block of 4. |
| Allocation concealment (selection bias) | Low risk | Used opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was "single‐blinded". The identity of those blinded was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by a combination of careful collection in a receptacle after the delivery of the baby, by visual estimation of blood loss, and by extrapolation of blood loss using the weight difference of the total perineal pad used up to 24 hours postpartum. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion, chest pain and abdominal pain were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Postgraduate Medical College and Faculty of Obstetrics and Gynecology of the University College Hospital in Ibadan, Nigeria (the institution of the authors). |
Ezeama 2014.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 300 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with premature labour (less than 28 weeks), multiple pregnancy, APH, hypertension in pregnancy, severe anaemia or haemoglobinopathy. | |
| Interventions | 10 IU of oxytocin administered IM versus 500 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); third stage duration (minutes); nausea; vomiting; hypertension; headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation numbers. |
| Allocation concealment (selection bias) | Low risk | A person uninvolved with the study prepared the study drugs. The labels on the ampoules (which were similar in size and colour) were removed and the ampoules were placed in opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A person uninvolved with the study prepared the study drugs: 1‐mL ampoules containing either 10 IU of oxytocin (Labtocin; Laborate Pharmaceutical India, Panipat, India) or 0.5 mg of ergometrine (Ergosav; Savorite Pharmaceuticals, Vadodara, India). The labels on the ampoules (which were similar in size and colour) were removed and the ampoules were placed in opaque sealed envelopes, such that only the computer generated randomisation numbers on the envelopes were available to identify the study drug. Both drugs were purchased from a public pharmacy." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with "a fresh large perineal pad with plastic backing". They placed all the gauzes and perineal pads used to absorb the blood into a polythene bag, and subtracted the dry weight from the wet weight. Volume of blood loss was calculated on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered (PACTR 201105000292708). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors. |
Fahmy 2015.
| Methods | 4‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 200 women were randomised in a hospital setting in Egypt. The population comprised women of parity 4 or less, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with coagulopathy, thrombocytopenia, fibroids, placenta praevia, history of previous obstetric haemorrhage more than 1 litre, and women who received anticoagulant and antiplatelets therapy. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus 100 mcg of carbetocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An online randomisation program (http://ww.randomizer.org) was used to generate random list and to allocate patients into the 4 study groups. |
| Allocation concealment (selection bias) | Low risk | Random allocation numbers were concealed in opaque closed envelops but there is no mention of the envelopes being sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear as a placebo saline infusion is mentioned but no sufficient details of how blinding was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | The calculated estimated blood loss = Estimated blood volume X (preoperative PCV – postoperative PCV)/preoperative PCV. (Where estimated blood volume = Booking weight (kg) X 85 mL) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fahmy 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 60 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a twin pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with hypertension, pre‐eclampsia, cardiac, respiratory, renal or liver disease, pre‐existing bleeding disorder such as haemophilia and women taking therapeutic anticoagulants, hypersensitivity to carbetocin or oxytocin. Patients with Hb less than 9.5 g% and those who are pregnant with more than 2 babies. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 20 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by using computer‐generated program. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both drugs were prepared preoperatively and coded so that the working investigator and the obstetrician were blinded to the type of drug injected. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fakour 2013.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 200 women were randomised in a hospital setting in Iran. The population comprised women of nulliparous, unspecified whether singleton or multiple pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 20 IU of oxytocin administered IV | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study used double‐dummy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study used double‐dummy. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fararjeh 2003.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 97 women were randomised in a hospital setting in Turkey. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective caesarean section or instrumental delivery, or those with premature labour (less than 37 weeks), postmaturity (more than 43 weeks), grand multiparity (more than 4), twin pregnancy, growth restriction, macrosomia, Hb less than 100 g/L, systemic disorder, prolonged third stage, manual removal of placenta or additional lacerations due to episiotomy or where it took longer than 30 minutes to repair lacerations after episiotomy. | |
| Interventions | 400 mcg of misoprostol administered rectally versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; blood loss (mL); change in Hb. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used urn block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with scale vessels, and by subtraction of the dry weight(s) of cloths and pads from the soaked weight(s) of these items. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fawole 2011.
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | 1345 parturients were randomised in a hospital setting in Nigeria. The population comprised multiparous women, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered vaginally. Exclusion criteria comprised severe allergic conditions or asthma, age below 18 years, pyrexia above 38°C, or abortion of the pregnancy. | |
| Interventions | 400 mcg of misoprostol administered sublingually plus 10 IU of oxytocin or 250 mcg to 500 mcg of ergometrine administered IM or by an intravenous bolus (n = 658) or IV bolus versus 10 IU of oxytocin or 250 mcg to 500 mcg of ergometrine administered IM or intravenously (n = 660). | |
| Outcomes | Could not include in the analysis as could not separate out the patients who received oxytocin from those who received ergometrine. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes, but data not provided separate for each drug used and could not be included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated in blocks of 6‐8 women by the research nurse, who used a computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | The trial drugs were concealed in sealed, sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was identical in shape, colour, size, and design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Blood collection was initiated as soon as possible after administration of the trial medication. A low‐profile plastic fracture bedpan was placed below the woman’s perineum to collect all subsequent blood loss for a period of 1 hour. Blood collected in the bedpan and all blood‐soaked small gauze swabs were emptied into a plastic measuring jar and the volume was measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses stated by authors but 27 women randomised were not included in the analysis for the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | No available protocol. |
| Intention to treat analysis | Unclear risk | 27 women randomised were not included in the analysis for the primary outcome. |
| Funding source | Low risk | The trial was funded by theMedical Research Council of South Africa. |
Fawzy 2012.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 300 women were randomised in a hospital setting in Egypt, Libya. The population comprised women of nulliparous, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women at high risk for PPH such as multiple pregnancy, polyhydramnios, placenta praevia, diabetes mellitus, renal disorders. | |
| Interventions | 500 mcg of ergometrine administered by an IV bolus versus 200 mcg of misoprostol administered sublingually or rectally | |
| Outcomes | The study recorded the following outcomes: death; blood loss (mL); third stage duration (minutes); shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated but no further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Objective assessment of blood loss | High risk | All patients were closely observed for time of placental delivery, amount of blood loss by Hb and haematocrit value pre and immediately post delivery (within 1 hour), {then calculation of estimated blood loss using the following equation EBL = (BV)X(HCTO‐HCTf)/HCT where: EBL = estimated blood loss, BV: blood volume= body weight X600 cc KG&HCTO = initial haematocrit HCTf = final haematocrit HCTave = (HCTO + HCTF)/2} |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fazel 2013.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in Iran. The population comprised women of parity 3 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with twin pregnancy, fetal distress, pregnancy‐induced hypertension, oligohydramnios, polyhydramnios, macrosomia, grand multiparity (4 or more), HELLP syndrome, coagulopathy, asthma, heart/lung/liver disease, previous more than 1 caesarean section, previous myomectomy, previous other abdominal operations, febrile diseases or sensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: transfusion; blood loss (mL); nausea; vomiting; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised intraoperative blood loss by collection with an isolated suction. The volume of blood collected in suction was combined with the volume of blood collected in gauzes and gowns: every small gauze soaked with blood was considered to contain 20 mL, and every large gauze soaked with blood 50 mL, and every g increase in the weight of a gown was considered as equivalent to 1 mL of blood. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Kashan University of Medical Sciences (the institution of the authors). |
Fekih 2009.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 250 women were randomised in a hospital setting in Tunisia. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women undergoing caesarean section with general anaesthesia, or those with placenta praevia, retroplacental clot, multiple pregnancy, premature labour (less than 32 weeks), intrauterine death, Hb less than 80 g/L, coagulopathy, HELLP syndrome, antepartum haemorrhage, ruptured uterus, previous more than 2 caesareans or other uterine scar, prolonged labour (more than 12 hours) or pyrexia. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IV bolus and infusion versus 20 IU of oxytocin administered by an IV bolus + infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; transfusion; blood loss (mL); change in Hb; nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | A slip of paper was placed inside an opaque, sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised perioperative blood loss as a combination of the volume of liquid in the suction collection jar, and the weight of swabs and pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fenix 2012.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 75 women were randomised in a hospital setting in Phillipines. The population comprised women of any parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with pre‐existing hypertension, pre‐eclampsia, diabetes, asthma, cardiac/renal diseases, coagulopathy, abnormal laboratory tests or allergy to the study medication. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb; nausea; vomiting; headache; tachycardia; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code. |
| Allocation concealment (selection bias) | Unclear risk | Used sealed, consecutively‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patient and the principal investigator attending the delivery were blinded to the type of medication administered" [additional information from the authors]. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient and the principal investigator attending the delivery were blinded to the type of medication administered" [additional information from the authors]. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by visual estimation, not including blood loss considered to result from repair of lacerations. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "9 women in the carbetocin group and 6 women in the oxytocin group failed to have a paired haemoglobin test to measure the change in haemoglobin 24 hours after delivery because they refused further blood extraction. These 15 women were excluded". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fu 2003.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 156 women were randomised in a hospital setting in China. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered orally versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 500; blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss in the 2 hours after delivery and after all amniotic fluids had been drained, by collection in a small tray and absorption into disposable, sterile, water‐resistant gauze. The contents were weighed and volume was determined on the basis that 1.05 g is equivalent to 1 mL of blood. A measuring cup was used to estimate the blood in the tray; blood that soaked into the gauze was measured on the basis that material measuring 10 cm by 10 cm holds 10 mL of blood. These 3 measurements were combined to ascertain total blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Fuks 2014.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 143 women were randomised in a hospital setting in Jamaica. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with multiple pregnancy, grand multiparous, intrauterine fetal demise, pre‐eclampsia, polyhydramnios, third‐ or fourth‐degree laceration, and caesarean delivery. | |
| Interventions | 600 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Garg 2005.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of nulliparous, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; manual removal of placenta; third stage duration (minutes); diarrhoea; nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in 1:1 ratio by random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Gavilanes 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in Equador. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with Hb less than 80 g/L, multiple pregnancy, polyhydramnios, previous uterine rupture, bleeding disorders, intrauterine death or hyperthermia (more than 38.5C). | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; blood loss (mL); nausea; vomiting; headache; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised postoperative blood loss by collection with quote: "suction apparatus and sterile drapes before irrigation" and by weighing the blood collected in abdominal swabs and gauzes with a calibrated scale (Zhongshan Camry Electronic Co Ltd, model EK 4052‐E, Guangdong, China). Investigators estimated the volume of blood loss quote: "by subtraction of amniotic fluid at 30 cc per each centimetre reported by amniotic fluid index". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Gerstenfeld 2001.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 400 women were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with multiple pregnancy, coagulopathy, Hb less than 70 g/L, indication for caesarean section or contraindication to prostaglandin or oxytocin use. | |
| Interventions | 400 mcg of misoprostol administered rectally versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; diarrhoea; nausea; vomiting; shivering. | |
| Notes | Contact with study authors for additional information: Yes. Additional data from authors: No | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was carried out by an uninvolved party and was determined by a random number sequence. |
| Allocation concealment (selection bias) | Low risk | The random number sequence was prepared by a third party and was concealed until the patient was enrolled. Packets were prepared in advance of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The random number sequence was quote: "concealed until the patient was enrolled" and "packets were prepared in advance of randomisation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss (a) by collection with drapes placed under the mother. Each drape included a plastic pouch and measured volume in mL. Meanwhile the dry weights of delivery linen and sponges were subtracted from bloodied weights to determine the volume of blood collected with these materials, on the basis that 1 g is equivalent to 1 mL. The volumes of blood in drapes and linen were added together. Furthermore quote: "if amniotic fluid loss [after placement of the drape] was significant... the approximate percentage was recorded on the data sheet and blood loss was adjusted accordingly". Investigators appraised blood loss (b) by estimation of the delivery attendant(s). Investigators appraised blood loss (c) by measurement of Hb and haematocrit values were obtained on admission and on postpartum day 1. The differences between these 2 values were recorded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 75 women who were excluded from analysis, 73 underwent cesarean deliveries, one woman was discharged to home before delivery, and one had an initial haemoglobin of 6.8 mg/dL". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Gore 2017.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 364 women were randomised in a hospital setting in India. The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women of gestational age less than 37 years, polyhydramnios, APH, pre‐eclampsia, multiple pregnancy, intrauterine fetal distress, coagulation disorders, asthma, epilepsy, heart disease, kidney disease, severe anaemia with Hb less than 7 g/dL, complicated or eventful first and second stage of labour. | |
| Interventions | 400 mcg of misoprostol administered orally versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: change in Hb; third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | The evaluation of blood loss was assessed by placing cotton pads under the buttocks prior to the delivery of baby. After the delivery of the placenta the total pads and linen used were weighed in grams. The weight of 1 g of cotton pad or linen was equal to 1 mL (Langford 2000). From this the known dry weight subtracted and the calculated volume added. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The authors report no funding sources. |
Gulmezoglu 2001.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 18,530 women were randomised in a hospital setting in Argentina, China, Egypt, Ireland, Nigeria, South Africa, Switzerland, Thailand and Vietnam Nigeria, South Africa, Switzerland, Thailand, and Vietnam. The population comprised women of both nulliparous and multiparous, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective or emergency caesarean section after randomisation, or those with asthma, severe chronic allergic conditions, abortion, pyrexia (more than 38°C) or inability to give consent. | |
| Interventions | 600 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM or by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000. Severe maternal morbidity; intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation schedule was generated centrally at WHO, Geneva, Switzerland, by computer‐generated random numbers and was stratified by country. Within the strata, women were individually randomised into 1 of 2 intervention groups with randomly varying block sizes of 4–6 women. |
| Allocation concealment (selection bias) | Low risk | The treatment packs were sealed, numbered sequentially, and could only be taken from the dispenser consecutively. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The treatment packs and their contents were identical in shape, colour, weight, and feel." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss from the time of delivery of the baby until the third stage of the labour was completed, when the mother was transferred to postnatal care (usually up to 1 hour postpartum). Immediately after the cord was clamped and cut, they passed a flat bedpan or an unsoiled receiver under the mother. The collected blood was poured into a standard measuring jar provided by WHO for volumetric measurement. Quote: "To simplify the procedure... small gauze swabs soaked with blood were put into the measuring jar and included in the measurement together with the blood and clots". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Investigators excluded quote: "37 and 34 women with emergency caesarean section, and 13 and 4 women lost to follow‐up in misoprostol and oxytocin groups, respectively, for blood loss ≥ 1000 mL, and 2 and 4 women without information on the need for additional uterotonics". |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was published in advance. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the UNDP/UNFPA/WHO/World Bank (public funding). Special Programme of Research, Development and Research Training Human Reproduction of WHO. Searle (Skokie, IL, USA) and Novartis (Basel, Switzerland) donated the active and placebo medications used in the trial. |
Gupta 2006.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes); nausea; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random tables. |
| Allocation concealment (selection bias) | Unclear risk | A sealed envelope with a code number was opened when vaginal delivery was imminent. The code was not broken till the end of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind". "Each envelope contained either 3 tablets of 200 mcg misoprostol and an ampoule of normal saline or 3 identical looking placebo tablets and an ampoule of 10 IU oxytocin". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a BRASS‐V calibrated drape placed under the mother. Pre‐weighed gauzes were used to clean any perineal tears or episiotomy. After 1 hour the dry weight of the sponges was subtracted from the soiled weight, and added to the volume of blood collected in the drape on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Hamm 2005.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 352 women were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria were not specified. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IV infusion versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "The group assignments were available only to the pharmacy. The nurse selected an opaque vial from the drug cabinet that contained either a 200‐mg misoprostol tablet or placebo. The vial number (which had been assigned in the pharmacy) and patient identification were sent to the pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
Harriott 2009.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 140 women were randomised in a hospital setting in the West Indies. The population comprised women of both nulliparous and multiparous, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with previous PPH, hypertension, previous caesarean, intrauterine death, sepsis/pyrexia (more than 38°C), APH or Hb less than 80 g/L. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 400 mcg of misoprostol administered rectally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb.;third stage duration (minutes); diarrhoea; nausea; vomiting; hypertension; fever; shivering; maternal satisfaction. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation was used to randomly assign participants. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Both the patient and the midwife conducting the delivery were aware of the drug administered". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a modified plastic drape placed under the mother from the commencement of the third stage of labour, until 1 hour after delivery. The collection drape measured 168 cm by 84 cm, and contained folded over side‐wings (to act as a chute) and a 34‐cm collection pouch made by folding the distal end of the drape. Standard sterile drapes were placed above the blood collection drape. Every effort was made to avoid soiling the sterile drapes before delivery of the baby, because they were not weighed. After delivery, overlying sterile drapes were removed to facilitate the use of the collection drape. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Mona Campus and Research Publication Committee of the University of the West Indies (the institution of the authors). |
Hernandez‐Castro 2016.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 123 women were randomised in a hospital setting in Mexico. The population comprised women of any parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean delivery. Exclusion criteria comprised women with hypersensitivity to prostaglandins, hyperthermia, coagulation defects, or history of vaginal bleeding (placental abruption or placenta praevia) and those who required general anaesthesia. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IV infusion versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; transfusion. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated sequence in blocks of 6. |
| Allocation concealment (selection bias) | Low risk | The drugs were kept in opaque containers, prepared by the hospital’s pharmacy department, marked with the number assigned to the patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients, clinicians, investigators, and data analysts were masked to group assignment is stated but the placebo used was folic acid tablets which are different shape than misoprostol. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Patients, clinicians, investigators, and data analysts were masked to group assignment is stated but the placebo used was folic acid tablets which are different shape than misoprostol. |
| Objective assessment of blood loss | High risk | Visual estimation of blood loss was performed by the anaesthesiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 123 women were randomised in the study, but 3 were excluded because of inadequate drug administration (n = 1), uterine artery injury (n = 1) and incorrect fetal weight calculation (n = 1) after randomisation. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (ClinicalTrials.gov:NCT01733329). |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Hofmeyr 1998.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 500 women were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing augmentation of labour, or those with hypertension, diabetes or previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered orally versus placebo | |
| Outcomes | The study recorded the following outcomes:PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; shivering; abdominal pain | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence, in balanced blocks of 8. |
| Allocation concealment (selection bias) | Low risk | The containers were ordered according to a computer‐generated random sequence, in balanced blocks of 8. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The tablets were either misoprostol 2 x 200 mcg or 2 placebo tablets similar in size and colour but not shape. Efforts to obtain identical placebo tablets were unsuccessful. This method of blinding proved to be effective. In only 1 case did the attending midwife inadvertently catch sight of the tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Within a minute of delivery, investigators removed any linen soiled with amniotic fluid, and placed a fresh, disposable absorbent linen‐saver sheet with plastic backing, and a low wedge‐shaped plastic "fracture" bedpan under the mother. Quote: "This was found to be a comfortable and efficient way of collecting the great majority of blood lost after delivery, and could be left in place without discomfort even during perineal suturing. When active bleeding had stopped, any blood clots were expressed from the uterus, the bedpan was removed and a sanitary towel was applied. The [volume of] blood in the bedpan was measured in a measuring jug. An hour after delivery, any bloodstained linen‐savers and sanitary towels were placed in a plastic bag and weighed in g". After subtracting the known dry weights of these materials, the bloodstained weights were added to the volume of blood collected in the bedpan to ascertain the total blood loss in the first hour after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding). |
Hofmeyr 2001.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 600 women were randomised in a hospital setting in South Africa. The population comprised women of any parity, unspecified whether singleton or multiple pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally versus placebo | |
| Outcomes | The study recorded the following outcomes:PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; diarrhoea; nausea; vomiting; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments generated by computer in blocks of 18. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque test tubes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Misoprostol and placebo were similar in size and colour but not shape. Efforts to obtain identical placebo tablets were unsuccessful. This method of blinding proved to be effective. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Within a minute of delivery, investigators removed any linen soiled with amniotic fluid, and placed a fresh, disposable absorbent linen‐saver sheet with plastic backing, and a low wedge‐shaped plastic "fracture" bedpan under the mother. Quote: "This was found to be a comfortable and efficient way of collecting the great majority of blood lost after delivery, and could be left in place without discomfort even during perineal suturing. When active bleeding had stopped, any blood clots were expressed from the uterus, the bedpan was removed and a sanitary towel was applied. The [volume of] blood in the bedpan was measured in a measuring jug. An hour after delivery, any bloodstained linen‐savers and sanitary towels were placed in a plastic bag and weighed in g". After subtracting the known dry weights of these materials, the bloodstained weights were added to the volume of blood collected in the bedpan to ascertain the total blood loss in the first hour after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no withdrawals after randomisation and all outcomes were analysed in the allocated group". However the primary outcome data of 1 study participant in the placebo group were unavailable. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding) and University of the Witwatersrand (the institution of the authors). |
Hofmeyr 2011.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 1103 women were randomised in a hospital setting in South Africa, Uganda, and Nigeria. The population comprised women of any parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section or instrumental delivery, or those who declined participation or were unable to consent, were too ill or distressed to participate or with a not viable pregnancy. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes :PPH at 500; PPH at 1000; manual removal of placenta; death; blood loss (mL); fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers and was stratified by country in blocks of 6–8. |
| Allocation concealment (selection bias) | Low risk | Quote: "The trial medication was provided, and the study drug packs were prepared, by Gynuity Health Projects. When a participant enrolled, the researcher took the next study drug pack from the dispenser and immediately wrote the woman's name both on the pack and in the participant number list, which was kept separate from the case record forms. Enrolment took place when the pack was removed from the pack dispenser. The pack could not be used for another woman or returned to the dispenser." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind". Quote: "The packs were identical in shape, colour, weight, and feel, and contained either 2 tablets of 200 mcg of misoprostol (HRA Pharma, Paris, France) or 2 matching placebo tablets". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Similarly to the study team of Gulmezoglu 2001, investigators appraised blood loss by collection with a fresh non‐absorbent sheet and low plastic “fracture” bedpan placed under the mother from as soon as possible after delivery until 1 hour postpartum. Investigators considered that quote: "longer‐term blood loss measurement is more difficult to standardise". They transferred the blood collected in the sheet and the bedpan (together with any soaked small gauze swabs) to a measuring jar to ascertain the volume. Alternatively, they collected blood with a plastic sheet placed under the mother immediately after delivery. If bleeding continued beyond 1 hour, investigators restarted collection and measurement until bleeding subsided. Attempts were made to minimise any losses on the drapes and gowns of delivery attendants. In addition, quote: "the placental interstices also contain maternal blood (about 9% of placental weight)." Because overestimations (amniotic fluid) and underestimations (blood loss) were likely to be distributed equally between the 2 study groups, and most would have occurred before the onset of measurement, the data were not corrected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data for the primary outcome were not available for 4 of the 1103 women". |
| Selective reporting (reporting bias) | High risk | The prospectively registered protocol of the study (ClinicalTrials.gov NCT 00124540) lists some secondary outcomes different to those included the study report (≥ 1000 mL within the first hour only, transfusion, Hb < 8 g/dL 24 hours after delivery). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Gynuity Health Projects through a grant from the Bill and Melinda Gates Foundation (public funding). |
Hoj 2005.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 661 women were randomised in a community setting in Guinea‐Bissau. The population comprised women of parity 3 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered sublingually versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Used opaque envelopes that were consecutively‐numbered and filled with the study drugs. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Misoprostol and placebo tablets of identical form, size, colour, and packing were produced". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | After delivery of the baby and drainage of the amniotic fluid, investigators placed a clean plastic‐lined absorbent drape under the mother. They changed the drape as many times as needed. The mother stayed on the drape or was asked to wear a pad over the next 60 minutes. All drapes and pads were weighed with an electronic scale and the known dry weights were subtracted in order to ascertain the volume of blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Danish Society of Obstetrics and Gynaecology, the Illum Foundation, and the Danish International Development Agency (public funding). |
Hong 2007.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 214 women were randomised in a hospital setting in Korea. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by caesarean (unspecified whether elective or emergency). Exclusion criteria were not specified. | |
| Interventions | 20 IU of oxytocin administered by an IV infusion versus 400 mcg plus 20 IU of misoprostol plus oxytocin administered rectally plus by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; change in HB; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo is mentioned but insufficient detail is reported to decide on blinding (of study participants and caregivers). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Placebo is mentioned but insufficient detail is reported to decide on blinding of outcome assessors. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Humera 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women of any parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with pre‐eclampsia or eclampsia, previous caesarean, previous retained placenta, APH, coagulation disorder, cardiac diseases, diabetes, hypertension and epilepsy. | |
| Interventions | 600 mcg of misoprostol administered orally versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); third stage duration (minutes); nausea.; vomiting; hypertension; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After delivery of the baby amniotic fluid was allowed to drain away (if present) and amniotic fluid soaked bed linen covered with dry disposable linen saver, corrugated rubber sheet placed under buttocks, sterile kidney tray placed at the vulva was used to collect blood loss over next 1 hour. Collected blood was measured using a measuring jar, blood clots weighed separately (1 g = 1 mL). Blood soaked swabs were weighed, the known dry weight subtracted and the calculated volume added to that of the blood volume of measuring jar. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | No funding sought for this study. |
Is 2012.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally versus unspecified of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: third stage duration (minutes); nausea; vomiting; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Jago 2007.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 510 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour or instrumental delivery, or those requiring epidural analgesia or with hypertension in pregnancy, existing hypertension, chronic renal disease, diabetes, vascular diseases, cardiac disease, anticoagulation therapy or allergy to ergometrine or oxytocin. | |
| Interventions | 500 mcg of ergometrine administered IM versus 10 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; blood loss (mL); hypertension. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Used numbers that were labelled on envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Jangsten 2011.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 1802 women were randomised in a hospital setting in Sweden. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective caesarean section, or those who were non‐Swedish speaking or with previous PPH, pre‐eclampsia, grand multiparity (more than 4) or intrauterine death. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; transfusion; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes); maternal satisfaction. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes containing the randomisation group prepared in consecutive order and kept in another unit. At randomisation, midwives phoned the staff at the other unit who opened the envelopes and disclosed the assigned intervention and trial number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Because of the nature of the study, blinding was not possible for the midwives, but the women were not informed of which management was to be used for them". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by removing pads soaked with amniotic fluid and placing a dry sanitary pad under the mother, immediately after the birth of the baby. They weighed all sanitary towels and pads before and after use. Blood loss was recorded (a) between the birth of the baby and the expulsion of the placenta, and (b) from expulsion of the placenta up to 2 hours postpartum. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 171 randomised women were not included in the study analysis. Among those randomised to receive oxytocin, 4 withdrew consent, 75 had caesareans, and 14 were lost to follow up. In the control group, 2 withdrew consent, 56 had caesareans, and 20 were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | The authors excluded 131 randomised study participants from the analysis because they experienced caesarean deliveries. |
| Funding source | Low risk | The study was supported by funding from the Research and Development Board in Göteborg and Bohuslän, Baby Bag and the SU Foundation in Sweden (public funding). |
Jans 2017.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 1704 women were randomised in a community setting in the Netherlands. The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with indications for a prophylactic approach to the third stage management in primary midwifery care and women with poor command of the Dutch language. | |
| Interventions | 5 IU of oxytocin administered IM versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000.;additional uterotonics; transfusion; third stage duration (minutes); breastfeeding; nausea; vomiting; headache; abdominal pain; maternal well‐being. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by a lottery method Quote: "Randomization was achieved using two numbered and sealed opaque envelopes. Each envelope contained a sticker indicating one of the allotted treatments. When the midwife was confident that the birth would be completed in her care (defined for primigravid women when a large part of the baby’s head was presenting and for multiparous women at the beginning of the second stage of labor), the woman herself or someone else designated by her would choose one of the two envelopes." |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported but unlikely to have been implemented with a lottery method of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Objective assessment of blood loss | Low risk | Used digital scales, 10 disposable pre‐weighed incontinence pads (a small impermeable multilayered sheet with high absorbency) and graduated measuring cups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1704 women were randomised in the study, but 18 were excluded because of referral to hospital (n = 16) and were lost to follow‐up or withdrew from the study (n = 2) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The trial was funded by the Prevention Fund of the Netherlands. |
Jerbi 2007.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 130 women were randomised in a hospital setting in Tunisia. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with placenta praevia, APH, non‐cephalic presentation, intrauterine death, grand multiparity, (more than 5), fibroids, anticoagulation therapy, previous PPH or previous caesarean. | |
| Interventions | 5 IU of oxytocin administered by an IV bolus versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; transfusion; manual removal of placenta; death; change in Hb; third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
Jirakulsawas 2000.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 140 women were randomised in a hospital setting in Thailand. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally versus 200 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Kabir 2015.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 110 women were randomised in a hospital setting in Bangladesh. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with placenta praevia, multiple pregnancy, placental abruption, hypertensive disorders, pre‐eclampsia, cardiac/renal/liver disorders, epilepsy, moderate anaemia (Hb < 9 g/dLl), intrauterine fetal death and unwilling to participate in the study. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Used pre‐weighted standardised delivery mat (Quaiyum’s mat) and pre‐weighted sanitary pads for blood collection after delivery to each of the pregnant woman to measure blood loss and measured the amount of blood loss in g by digital postal scale. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 110 women were randomised in the study, but 16 were excluded because of pre‐eclampsia (n = 5), eclampsia (n = 5), placenta praevia (n = 2), placental abruption (n = 2) and multiple pregnancy (n = 2) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Karkanis 2002.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 238 women were randomised in a hospital setting in Canada. The population comprised women of parity 5 or less, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with coagulopathy, anticoagulation therapy, previous PPH or previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered rectally versus 5 IU of oxytocin administered by an IV bolus or IM | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; manual removal of placenta; change in Hb; third stage duration (minutes); nausea; vomiting; headache; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician developed blocked randomisation tables for each centre. |
| Allocation concealment (selection bias) | Low risk | Pharmacy assembled consecutively‐numbered opaque, sealed packets that contained the group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "13 women randomised subsequently delivered by caesarean and were excluded from analysis. 2 women were lost to follow‐up early in the trial when their packets were opened but the manoeuvre was not completed and no data were recorded". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the physicians of Ontario, through the Physician Services Incorporated Foundation (public funding). |
Kerekes 1979.
| Methods | 3‐arm controlled randomised trial | |
| Participants | 140 women were randomised in a hospital setting in Hungary. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 200 mcg of ergometrine administered IV bolus versus no treatment | |
| Outcomes | The study recorded the following outcomes: third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss by collection in a container placed under the mother during the third stage of labour until 2 hours postpartum. The contents of the container were transferred to a measuring cylinder. However, blood loss data were not reported in a format that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Khan 1995.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 2040 women were randomised in a hospital setting in United Arab Emirates. The population comprised women of any parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour, caesarean section or instrumental delivery, or requiring general anaesthesia, epidural or diazepam, or those with antenatal hypertension (160/100 mmHg or more), hypertension on antihypertensive drugs, multiple pregnancy, cardiac disease or Hb of 90 g/L or less. | |
| Interventions | 10 IU of oxytocin administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; transfusion; manual removal of placenta; vomiting; headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Number code by the hospital pharmacist who alone was aware of the content of the ampoules. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned an opaque sealed envelope. Each envelope carried the instruction to use a numbered vial of the study drug. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss "in the standard way" by measurement of blood and clots in a graduated jug, and by weighing swabs and linen. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "12 patients had to be excluded from the trial (oxytocin 5; ergometrine plus oxytocin 7) after randomisation because they no longer fulfilled the inclusion criteria (2 who required caesarean section and 10 who were delivered by forceps or ventouse (oxytocin, 4; Ergometrine plus oxytocin 6)." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Khurshid 2010.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of any parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with hypertension, cardiac disease, renal disease, gastro‐intestinal disorders, respiratory disease, endocrinal problems, coagulation disorder and sensitivity to prostaglandin or methergin. | |
| Interventions | 125 mcg of carboprost administered IM versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; manual removal of placenta; blood loss (mL;.third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using random tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Blood loss was estimated by collecting blood and blood clots in the kidney tray and adding the difference in the weight of the drapes before use and after birth. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Koen 2016.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 540 women were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women not willing or not able to provide consent, previous classic CS, < 18 years of age, pre‐eclampsia, eclampsia, uncontrolled hypertension, cardiac/liver/renal disorders, hypersensitivity to oxytocin or oxytocin + ergometrine, occlusive vascular disease, autoimmune vasculitis. | |
| Interventions | 12.5 IU of oxytocin administered by an IV bolus + infusion versus 500 mcg plus 15 IU of ergometrine plus oxytocin administered IM plus by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL); nausea; vomiting; headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out by a lottery method "Randomisation was by means of sealed not transparent envelopes. Each had a label inside with a letter A (oxytocin group) or B (oxytocin + ergometrine), which corresponded to a pair of pre‐packed colour‐coded ampoules that were used for the two different groups." |
| Allocation concealment (selection bias) | High risk | Quote: "Randomisation was carried out by a lottery method "Randomisation was by means of sealed not transparent envelopes. Each had a label inside with a letter A (oxytocin group) or B (oxytocin + ergometrine), which corresponded to a pair of pre‐packed colour‐coded ampoules that were used for the two different groups." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Calculation of blood loss was done using calculated pregnancy preoperative blood volume (0.75 × [{height inches × 50} + {weight pounds × 25}) × percentage of blood volume lost ([pre‐delivery haematocrits – post‐delivery haematocrits]/pre‐delivery haematocrits). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 540 women were randomised in the study, but 124 were excluded because of giving birth vaginally (n = 80), incomplete data or protocol violations (n = 44) after randomisation. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (ClinicalTrials.gov NCT02046499). |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Kumar 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 201 women were randomised in a hospital setting in India. The population comprised women of any parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesareans, with hypersensitivity to drugs, asthma, cardiac diseases, epilepsy, psychiatric disorders, liver and renal diseases. | |
| Interventions | 125 mcg of carboprost administered IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; death; blood loss (mL); third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Perineal drapes were replaced by calibrated Brasss V obstetric drape after the delivery of the baby. The average time taken for episiotomy suturing was around 10 minutes in both the groups and did not have any significant impact on the blood loss and duration of bleeding. Brasss V drape was removed 10 minutes after the episiotomy suturing in all patients unless the patient continued to have significant PPH. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 women was excluded because of a fourth degree tear after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Kumru 2005.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 55 women were randomised in a hospital setting in Turkey. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with multiple pregnancy, hypertension or vascular diseases. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus + infusion versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered by an IV bolus plus by IV bolus plus infusion | |
| Outcomes | The study recorded the following outcomes: blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised intraoperative blood loss by weighing compresses and rolls before and after the birth of the baby, and calculating the difference between these measurements. Pre‐weighted pads were distributed in advance to each mother, and collected at intervals of 3‐6 hours hour intervals after the aspiration of amniotic fluid. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Kundodyiwa 2001.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 500 women were randomised in a hospital setting in Zimbabwe. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing instrumental delivery, or those with previous PPH, antepartum haemorrhage, coagulopathy, multiple pregnancy, asthma or allergies to prostaglandins or oxytocin. | |
| Interventions | 400 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated using a random sequence. |
| Allocation concealment (selection bias) | Low risk | The participant was asked to randomly pick a numbered sealed opaque envelope from the study cooler‐box. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical placebo tablets could not be obtained from the manufacturers. The tablets were similar in size and colour but not in shape. However, most reviewed trials on misoprostol had this similar problem although this method of blinding proved to be effective." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The data sheet was completed by the midwife supervising the delivery and collected and checked by the research assistant". |
| Objective assessment of blood loss | Low risk | After delivery, investigators appraised blood loss by removing linen soiled with amniotic fluid, and then placing a fresh disposable incontinence pad with a plastic backing under the mother. Blood expressed from the uterus was measured with a calibrated measuring jug. The volume of blood soiling linen savers and sanitary pads was determined as the difference between dry weights and soiled weights: these measurements were added to the volume recorded by the calibrated jug. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data for 1 woman were excluded because she delivered undiagnosed twins after randomisation". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Kushtagi 2006.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 215 women were randomised in a hospital setting in India. The population comprised women of any parity, unspecified whether singleton or multiple pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 200 mcg of ergometrine administered by an IV bolus versus 125 mcg of carboprost administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; blood loss (mL); third stage duration (minutes); hypertension. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Amount of blood loss was quantified by noting the increment in weight of standardised tampons which were placed high up in the vagina immediately after placental delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Lam 2004.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 60 women were randomised in a hospital setting in China (Hong Kong SAR). The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour, or those with antepartum haemorrhage, anaemia, 2 or more surgical terminations, previous manual removal of placenta, previous PPH or previous third stage complications. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered by an IV bolus versus 600 mcg of misoprostol administered sublingually | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; manual removal of placenta; death; fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated using a random number‐generated table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss during the third stage by visual estimation, and by objective measurement on the basis of a method previously described by Newton and colleagues. Whilst any blood clots were collected and measured with a jug, white linen was placed under the mother during delivery and subsequently processed for 15 minutes with sodium hydroxide solution in an automatic stomacher (laboratory blender), to achieve the formation of alkaline hematin. Quote: "The optical density at 550 nm of the alkaline hematin was measured by spectrophotometry and compared with that of a known volume of a sample of the patient’s venous blood" to calculate the volume of blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | It was unclear from the study report whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Lamont 2001.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 529 women were randomised in a hospital setting in the UK. The population comprised women of both nulliparous and multiparous, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by both caesarean and vaginal delivery. Exclusion criteria comprised women with known sensitivity to either prostaglandins, ergometrine or oxytocin, had a history of asthma, glaucoma, raised intraocular pressure or were known to have cardiac, pulmonary, renal or hepatic disease, hypertension, sepsis or obliterative vascular disorders. Women were excluded if they were currently taking anticoagulant treatment or participating in other clinical trials. | |
| Interventions | 250 mcg of carboprost administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; manual removal of placenta; blood loss (mL); diarrhoea; nausea; vomiting. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The randomisation slips were contained in envelopes which were opened by a person not involved in the postpartum assessments who resealed the envelope and drew 1 mL of the appropriate medication into a syringe. The nature of the medication was not revealed and the resealed envelope was retained in the woman’s notes. The medication was administered by a competent person other than the one who had opened the envelope and filled the syringe. |
| Objective assessment of blood loss | Unclear risk | Blood loss was measured as accurately as possible, taking into consideration the liquor amnii and soiling of the surgical drapes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 530 women were randomised in the study, but 1 was excluded because did not receive the allocated agent (carboprost) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Lapaire 2006.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 56 women were randomised in a hospital setting in Switzerland. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women undergoing emergency caesarean section, or those with fetal distress, fetal malformations, pre‐eclampsia, HELLP syndrome, coagulopathy, severe systemic disorders, an American Society of Anesthesiologists physical status of 3 or greater, severe asthma, previous myomectomy, pyrexia (more than 38.5C) or hypersensitivity to prostaglandins. | |
| Interventions | 25 IU of oxytocin administered by an IV bolus + infusion versus 800 mcg plus 5 IU of misoprostol plus oxytocin administered orally plus by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); nausea; headache; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The hospital pharmacy performed the 1:1 computer‐generated randomisation that assigned the participants to their group. |
| Allocation concealment (selection bias) | Low risk | Used identical study boxes from pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": Quote: "the study drugs and placebos [were provided by the pharmacy] in unidentifiable form". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | When the membranes ruptured before delivery, investigators appraised intraoperative and postoperative blood loss by determining the difference in weight of cloths and pads used to absorb blood during surgery and in the intermediate care unit. When membranes did not rupture preoperatively, investigators appraised blood loss by collection in suction bottles and subtracting estimated amniotic fluid volume. Investigators considered that 1 g is equivalent to 1 mL of blood or amniotic fluid. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "3 patients in the oxytocin group were excluded from statistical analysis because of errors in drug administration". Moreover calculated blood loss data were unavailable in 13 cases and for these women the primary outcome was estimated clinically." |
| Selective reporting (reporting bias) | High risk | The study protocol that was registered retrospectively (ClinicalTrials.gov) lists PPH as the primary outcome of the study, but the study report lists the primary outcomes as intraoperative and postoperative blood loss and drug‐related adverse effects (these items are listed only as secondary outcomes in the registration file). The study does not report the incidence of PPH ≥ 500 mL, nor PPH ≥ 1000 mL. |
| Intention to treat analysis | High risk | The authors excluded 3 study participants in the oxytocin group from the analysis because they incurred errors in drug administration. |
| Funding source | Low risk | The study was supported by funding from the Scientific Pool of Basel University Hospital (the institution of the authors). |
Leung 2006.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 329 women were randomised in a hospital setting in Hong Kong. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women requiring prophylactic oxytocin infusion, or those with pre‐existing hypertension, pre‐eclampsia, asthma, cardiac/renal/liver diseases, grand multiparity or fibroids. | |
| Interventions | 100 mcg of carbetocin administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hbl third stage duration (minutes); nausea; vomiting; hypertension; headache; tachycardia; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code before the recruitment. |
| Allocation concealment (selection bias) | Low risk | This was performed by opening a sealed, consecutively‐numbered, opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by visual estimation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "15 women in the carbetocin group and 14 women in the ergometrine plus oxytocin group failed to have a paired haemoglobin test to measure the change in haemoglobin 48 hours after delivery either because they had requested early home or refused further blood taking. These 29 women were excluded." |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of fever were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
Lokugamage 2001.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 40 women were randomised in a hospital setting in UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with two or more previous caesarean sections or previous uterine rupture. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus 500 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb; fever;.shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was undertaken by means of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Used sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The obstetrician, surgical assistant, scrub nurse and recovery midwife were blinded to the treatment. The anaesthetist and the anaesthetic assistant were not blinded as it was important for patient safety that a record was kept of all drugs administered." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised intraoperative and postoperative (up to 1 hour) blood loss by visual estimation, quote: "in a standard manner (volume of blood in suction bottle plus soiling of swabs and bed sheets)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by "assistance" from the Department of Anaesthesia at University College London Hospitals NHS Trust (the institution of the authors). |
Lumbiganon 1999.
| Methods | 3‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 597 women were randomised in a hospital setting in South Africa and Thailand. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective caesarean section or abortion, or those with asthma, other severe chronic allergic conditions a contraindication to use of misoprostol or if they were not willing or able to give informed consent. | |
| Interventions | 600 mcg or 400 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence, generated centrally. |
| Allocation concealment (selection bias) | Low risk | The treatment packs were consecutively‐numbered and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The packs were identical in shape, colour, weight and feel. Each woman received an injection and 3 tablets. Thus, the trial was double‐blinded using double placebos". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss from the delivery of the baby until the mother was transferred to postnatal care. The collected blood was poured into a standard measuring jar provided by WHO for the purpose of volumetric measurement. Linen was not weighed but clots and small gauze swabs soaked with blood were included in the measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion after randomisation: 8 women in the oxytocin group did not comply with treatment (6 had an emergency caesarean section, 1 was HIV positive and mistakenly excluded, 1 whose ampoule was not located). 1 woman in the 600 mcg group was excluded. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the WHO (public funding). Active and placebo medications, syringes and swabs were donated by Searle, Novartis Pharma AG and Becton Dickinson International. |
Maged 2016.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 200 women were randomised in a hospital setting in Egypt. The population comprised women of any parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with placenta praevia, coagulopathy, pre‐eclampsia, cardiac/renal/liver disorders, epilepsy or known hypersensitivity to oxytocin or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered IM versus 5 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion.; blood loss (mL); change in Hb; third stage duration (minutes); nausea; vomiting; headache; tachycardia; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were equally randomised using automated web‐based randomisation system. |
| Allocation concealment (selection bias) | Unclear risk | Only states that ensured allocation concealment with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported in sufficient detail even though the authors state it was double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by weighing swabs and using pictorial charts. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Maged 2017.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 300 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with placenta previa, coagulopathy, pre‐eclamptic or known sensitivity to oxytocin or methergine. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 200 mcg plus 5 IU of ergometrine plus oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; blood loss (mL; change in Hb; nausea; vomiting; headache; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using automated web based randomisation system. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported in sufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors state the study was double‐blinded but blinding (of study participants and caregivers) was not described in sufficient detail. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Calculated estimated blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Malik 2018.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with anaemia, pregnancy‐induced hypertension, placental abruption/placenta praevia, multiple pregnancy, grand multiparous, malpresentation, polyhydramnios, previous uterine scar, chorioamnionitis, prolonged labour, intrauterine fetal death, coagulation disorder, asthma/epilepsy/heart/renal disorder. | |
| Interventions | 125 mcg of carboprost administered IM versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Amount of blood loss was calculated by weighing the gauzes/sponges before delivery followed by again weighing them after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Mannaerts 2018.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 68 women were randomised in a hospital setting in Belgium. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with medical conditions potentially influencing outcome measures (nausea, vomitus, and hypotension): diabetes, preexisting hypertension, pre‐eclampsia, gestational hypertension, and known gastrointestinal diseases. | |
| Interventions | 15 IU of oxytocin administered by an IV bolus + infusion versus 100 mcg of carbetocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; change in Hb; nausea. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants are randomly assigned following simple randomisation procedure in 1 : 1 ratio to 1 of the 2 treatment groups. A computer‐generated randomisation list was generated using SPSS21. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication was prepared by a midwife not treating the patient to make sure that patient, gynaecologist, anaesthesiologist, and midwife clinically in charge of the patient are blinded for the medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medication was prepared by a midwife not treating the patient to make sure that patient, gynaecologist, anaesthesiologist, and midwife clinically in charge of the patient are blinded for the medication. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 68 women were randomised in the study, but 10 were excluded because of incomplete data after randomisation. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (ISRCTN 95504420). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
McDonald 1993.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 3497 women were randomised in a hospital setting in Australia. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing emergency or elective caesarean section, or requiring general anaesthetic for instrumental delivery, or those with hypertension in labour (more than 150/100 mm Hg), antenatal hypertension, maternal distress, advanced stage in labour, language barrier, fetal abnormality, intrauterine death or medical disorder. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; NNU admissions; breastfeeding; nausea; vomiting. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The ampoules were numbered by Sandoz by using simple randomisation. There was no blocking or prognostic stratification. |
| Allocation concealment (selection bias) | Low risk | The ampoules were numbered by third party (Sandoz). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Delivery attendants were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending obstetricians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All women allocated to receive a drug were included in that group, excluding only the 14 women for whom drug allocation was not recorded". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Sandoz. |
Mitchell 1993.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 461 women were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective caesarean section, or those with significant hypertension or cardiac disease. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 5 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; manual removal of placenta; blood loss (mL); third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear sequence: described as without any blocking or stratification. |
| Allocation concealment (selection bias) | Low risk | Used identical study boxes prepared by third party (Sandoz). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss quote: "in the standard way by graduated jug measurement plus an allowance for spillage". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Perinatal Trials Service (public funding), for the Department of Health for England and Wales, and for Birthright (the charitable arm of the RCOG). Coded medication ampoules were provided by Sandoz. |
Mobeen 2011.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 1119 women were randomised in a community setting in Pakistan. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with hypertension, non‐cephalic presentation, polyhydramnios, previous caesarean, multiple pregnancy, intrauterine death, antepartum haemorrhage or Hb less than 80 g/L. | |
| Interventions | 600 mcg of misoprostol administered orally versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random code in blocks of 6 was maintained by Gynuity Health Projects in New York and not revealed until data collection and cleaning were completed. |
| Allocation concealment (selection bias) | Low risk | Study medication was packed in numbered colour‐coded boxes by Gynuity Health Projects in New York. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both women and TBAs were blinded to study assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | To appraise postpartum blood loss, blood was collected with a perineal sheet and bedpan placed under the mother for a minimum of 1 hour or until active bleeding stopped (whichever occurred last). Quote: "Blood collected in the bedpan was transferred to a measuring jar, which was then closed, and the perineal sheet and cotton roll were placed in a sealed plastic bag. The closed measuring jar and sealed plastic bag were then placed inside a plastic cooler which was tightly closed and stored in a secure place in the woman’s home until the local health visitor or community health nurse arrived for weighing, 1–2 days after delivery". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Invalid blood loss measures, which mainly occurred when monitoring visits were not possible because of poor weather conditions, were excluded from our analysis". |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00120237). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Bill and Melinda Gates Foundation (public funding). |
Modi 2014.
| Methods | 4‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women of parity 3 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with gestations less than 37 or more than 42 weeks, intrauterine death, fetal growth restriction, hypertensive or cardiac or renal disorders, multiple pregnancies, placenta praevia, placenta abruption, grand multiparous, coagulation disorders, anaemia (< 8 g/dL), tachycardia or hypotension, malpresentations, chorioamnionitis, or known allergy to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered IM versus 200 mcg of ergometrine administered by an IV bolus versus 125 mcg of carboprost administered IM versus 600 mcg of misoprostol administered rectally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; Blood loss (mL); third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Used BRASS‐V drapes to measure the blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | No funding sought for this study. |
Moertl 2011.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 84 women were randomised in a hospital setting in Austria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women requiring general anaesthesia, or those with placenta praevia, placental abruption, multiple pregnancy, pre‐eclampsia, gestational diabetes, pre‐existing insulin‐dependent diabetes, cardiovascular/renal disorders, hypo‐/hyperthyroidism or women on cardiovascular system medications. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 5 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: additional uterotonics change in Hb; nausea; headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer‐generated randomisation sequence 1:1 ratio—blocks of 10 without stratification. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medication was double‐blinded to the clinical staff (obstetricians as well as anaesthesiologists) and the technicians performing the measurements". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators did not appraise blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After randomisation, investigators excluded 28 women from analysis for technical problems (n = 15), change to general anaesthesia (n = 9), recording artefacts (n = 3) and patient withdrawal (n = 1). |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (EudraCT 2007‐005498‐78). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | CNSystems Medizintechnik AG in Graz, Austria provided the Task Force® Monitor 3040i system used to measure haemodynamic parameters. No other external funding was required for the study. |
Mohamed 2015.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 172 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with medical disorder as hypertension, diabetes or on an anticoagulant, severe polyhydramnios, multiple pregnancy, placenta praevia or placental abruption, previous uterine scar other than lower segment caesarean section or who had more than 1 previous section. | |
| Interventions | 5 IU of oxytocin administered by an IV bolus versus 100 mcg of carbetocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by computer generated randomisation system. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After delivery of the placenta, the volume of blood loss was assessed by weight or saturation assessment techniques by subtracting the dry weight of absorbing materials (pads, sponges, etc) from the weight of blood‐containing materials and using the conversion 1 g weight = 1 mL to quantify the blood volume contained in the materials. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Moir 1979.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 88 women were randomised in a hospital setting in the UK. The population comprised women of primigravidas, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 500 mcg of ergometrine administered by an IV bolus versus 10 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; blood loss (mL); nausea. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by quote: "the haemoglobin extraction‐dilution technique, which is acceptably accurate (Roe, Gardiner and Dudley, 1962; Thornton et al, 1963) and particularly suited to obstetric use (Moir and Wallace, 1967; Wallace, 1967). The perdometer apparatus was used and all blood and blood‐stained linen were collected". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Moodie 1976.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 148 women were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 500 mcg of ergometrine administered by an IV bolus versus 5 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; blood loss (mL); nausea. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with the placenta bowl and soiled linen and swabs. Quote: "The principles of the haemoglobin extraction‐dilution technique employed have been discussed by Roe, Gardiner and Dudley (1962) and Thornton and colleagues (1963). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 148 study participants but blood loss data were available in only 80 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Mukta 2013.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of any parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing emergency or elective caesarean section, or those with eclampsia, asthma, epilepsy, cardiac/kidney disorder or coagulopathy. | |
| Interventions | 600 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; diarrhoea; nausea; vomiting; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly divided into 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss in mL, by collection with a calibrated plastic drape, after the drainage of amniotic fluid and delivery of the baby until the third stage of labour was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Musa 2015.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 235 women were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing planned instrumental, or those who received oxytocin and/or misoprostol other than in the third stage of labour, or those with grand multiparity (more than 4), multiple pregnancy, fibroids, polyhydramnios, pre‐eclampsia, eclampsia, hypertension, cardiac disorder, asthma,APH, previous PPH, prolonged rupture of membranes or Hb less than 100 g/L). | |
| Interventions | 600 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; severe maternal morbidity: intensive care admissions; additional uterotonics; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done by blocked (restrictive), using computer‐generated random numbers prepared by an independent statistician. |
| Allocation concealment (selection bias) | Unclear risk | Used opaque envelopes but no other details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, caregivers, and outcome assessors (researchers or research assistants) were masked to group allocation. Investigators were not masked for data analysis". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Participants, caregivers, and outcome assessors (researchers or research assistants) were masked to group allocation. Investigators were not masked for data analysis". |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by quote: "the gravimetric method" (Ambardekar 2009) until 1 hour after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 235 study participants were randomised but only 200 were analysed due to protocol deviations and missing data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (PACTR 201407000825227). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the University of Ilorin Teaching Hospital (the institution of the authors). |
Nankaly 2016.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 185 women were randomised in a hospital setting in Iran. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with anaemia, multiple pregnancy, polyhydramnios, prolonged labour, PROM, placenta praevia, placental abruption, vaginal bleeding, diabetes, blood pressure, kidney disease, cardiovascular disease and coagulation disorders or other underlying disease. | |
| Interventions | 20 IU of oxytocin administered by an IV infusion versus 400 mcg or 200 mcg of misoprostol administered sublingually | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported in Quote: "The randomisation was done via block randomisation and in the form of four blocks". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Objective assessment of blood loss | Unclear risk | Lost blood volume gained from calculating the total collected blood in suction container and counting the number of blood gases. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Nasr 2009.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 514 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with antepartum haemorrhage, coagulopathy, hypertension in pregnancy or the need for anticoagulants. | |
| Interventions | 800 mcg of misoprostol administered rectally versus 5 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated by a computer‐generated random allocation system created at the Statistics Unit of Assiut University Hospital. |
| Allocation concealment (selection bias) | Low risk | Allocation codes were placed in sealed, opaque, consecutively‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was quote: "double‐blind": active treatments and placebo treatments were "identical‐looking". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
Nayak 2017.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women having severe medical and surgical complications including the heart, liver, kidney, brain disease and blood disorders, any contraindication to misoprostol including mitral stenosis, glaucoma and diastolic blood pressure over 100 mmHg and known allergic to prostaglandins, history of thromboembolic disorders, abnormal placentation such as placenta praevia, placental abruption and placental adhesions caused by repeated artificial abortions, pregnancy complications such as severe pre‐eclampsia, multiple pregnancies, macrosomia and polyhydramnios, complication with myoma and with any blood dyscrasia. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus by an IV infusion versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; blood loss (mL); change in Hb. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | The quantity of blood (mL) = (weight of (used material + unused material) after surgery‐weight of all materials prior to surgery)/1.05 plus the volume included in the suction container after placental delivery. In addition, pads used after completion of caesarean section to 2 hours postpartum weighed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Nellore 2006.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women requiring oxytocin induction or augmentation of labor, caesarean delivery, or those with gestational age less than 37 weeks, multiple pregnancy, Hb concentration less than 8 g/dL, and known allergy to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered rectally versus 125 mcg of carboprost administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes); shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ng 2001.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 2058 women were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, asthma, grand multiparity (more than 3), fibroids or contraindications for the use of either misoprostol or syntometrine. | |
| Interventions | 600 mcg of misoprostol administered orally versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death. Blood loss (mL); change in Hb; nausea; vomiting; hypertension; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation based on a table of computer‐generated blocks of random numbers. |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This was not a double‐blinded study". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ng 2004.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 298 women were randomised in an unspecified setting in Hong Kong. The population comprised women of unspecified parity, a singleton pregnancy, at unspecified risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with multiple pregnancy or non‐vaginal delivery. | |
| Interventions | 400 mcg of misoprostol administered orally versus 1 mL of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinding of personnel and participants (placebo use) but insufficient details from abstract only. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ng 2007.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 360 women were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, asthma, grand multiparity (more than 3), fibroids or contraindications for the use of either misoprostol or syntometrine. | |
| Interventions | 400 mcg of misoprostol administered orally versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; diarrhoea; nausea; vomiting; hypertension; headache; fever; shivering; maternal satisfaction. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was based on a table of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Used consecutively‐numbered and sealed opaque packages. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo was identical in size and colour but had a different shape to the misoprostol tablet. All women were asked to swallow the tablets directly from the opaque cup without looking at them. The identity of the active medication and placebo were concealed from the caregivers and the parturient." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "5 women were excluded from the analysis because of missing post‐delivery haemoglobin level". |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of tachycardia and dizziness were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Nirmala 2009.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in Malaysia. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women younger than 18 years old, or those with cardiac disorder, hypertension requiring treatment, liver/renal/vascular/endocrine disorder (excluding gestational diabetes) or hypersensitivity to oxytocin or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; nausea; vomiting; hypertension; headache; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Used sealed, sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The preparation and administration of the medication was carried out by midwives who were not involved in the management of the patient except for the drug administration". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by "the gravimetric method" from immediately after drug administration. They used a digital scale (Soehnle, Venezia) for weight measurement. In order to minimise confounding by fluid absorbed into drapes, they collected blood with a new plastic sheet placed under the mother after delivery of the baby. They also weighed any gauzes, tampons and pads used in the first hour after delivery of the placenta, and subtracted the dry weights of these materials to calculate blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Nordstrom 1997.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 1000 women were randomised in a hospital setting in Sweden. The population comprised women of any parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Ampoules were prepared at the hospital pharmacy and consecutively‐numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The content of the ampoules was unknown to mothers, midwives and doctors until the study was completed". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss quote: "by measuring collected blood and adding what was estimated to have been absorbed by surgical cloths and tissues". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the County Council and County Health Authority Research and Development Foundation in the County of Jämtland, Sweden (public funding). |
Nuamsiri 2016.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 323 women were randomised in a hospital setting in Thailand. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with multiple pregnancy, polyhydramnios, uterine fibroids, previous PPH, APH, parity greater than 4, previous caesarean section, severe anaemia (Hb level of ≤ 8 g/dL), coagulopathy, contraindications to the use of ergometrine, estimated fetal birthweight > 4000 g. and inability to obtain written informed consent. Women who ended up having a caesarean section or instrumental delivery were also excluded from this study. | |
| Interventions | 200 mcg plus 20 IU of ergometrine plus oxytocin administered by an IV bolus + infusion versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); change in Hb; third stage duration (minutes); nausea; hypertension. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation scheme using a computer‐generated list of numbers. |
| Allocation concealment (selection bias) | Low risk | Used sealed and consecutively numbered opaque envelopes were prepared by a research assistant not involved in the study. The women were randomly allocated to 1 of the 2 study groups by opening the next available envelope just before delivery. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study drug and placebo were prepared by the research assistant not involved in the study. The obstetrician and nursing staff were all blinded to the type of injectable substance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study drug and placebo were prepared by the research assistant not involved in the study. The obstetrician and nursing staff were all blinded to the type of injectable substance. |
| Objective assessment of blood loss | Low risk | Used the blood collection drape, which was placed under the buttocks after placental delivery. Blood‐soaked swabs were weighed in grams, and the known dry weight of the swabs was subtracted, this volume was added to the measured blood volume from the drape (assuming an equivalence of 1 g to 1 mL). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study report matches the study protocol that was registered retrospectively (TCTR20150820001). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding were not reported. |
Oboro 2003.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 496 women were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour, or those with previous caesarean, Hb less than 80 g/L, previous PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, fibroids or precipitate labour. | |
| Interventions | 10 IU of oxytocin administered IM versus 600 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by using random tables. |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared opaque sealed sequentially‐numbered packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The identity of the active medication and placebo were concealed from the caregivers and women". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending obstetricians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ogunbode 1979.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 144 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing instrumental delivery, or those with previous PPH, multiple pregnancy, polyhydramnios or vaginal lacerations. | |
| Interventions | 200 mcg or 500 mcg of ergometrine administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; manual removal of placenta; blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Restricted random allocation. |
| Allocation concealment (selection bias) | Unclear risk | Used sealed sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The identity of the various drugs was not known to the investigators until after completion of the trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by collection in a dish pressed against the vulva for 3 minutes: the contents were carefully measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Sandoz. |
Orji 2008.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 600 women were randomised in a hospital setting in Nigeria. The population comprised women of parity 6 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with hypertension in pregnancy, packed cell volume less than 30%, previous PPH, haemoglobinopathy or cardiac disorder. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus 250 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; manual removal of placenta. Blood loss (mL); change in Hb; third stage duration (minutes); nausea; vomiting; hypertension; headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done by sealed sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss by "using a pre‐weighed gauze that was weighed again after delivery". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion and PPH ≥ 1000 mL were omitted). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ortiz‐Gomez 2013.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 156 women were randomised in a hospital setting in Spain. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with comorbidities, refractory hypotension due to neuraxial blockage, vasoactive drugs needed to control haemodynamic issues or multiple pregnancy. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 61 IU of oxytocin administered by an IV bolus + infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; nausea; vomiting; headache; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss by the estimation of delivery attendants, but blood loss data were not reported in a format that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Othman 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with anaemia (Hb < 8 g/dL), multiple pregnancy, placental abnormality (e.g. placenta praevia, placenta abruption), polyhydramnios, 2 or more previous caesarean deliveries, current or previous history of heart disease, liver, renal disorders or known coagulopathy. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; blood loss (mL); vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated random table. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was done using serially numbered closed opaque envelopes. Each envelope was labelled with a serial number and had a card noting the intervention type inside. Allocation was never changed after opening the envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Quote: "The volume of blood loss during caesarean delivery and 2 hours postoperatively was assessed. Total blood loss during caesarean delivery was measured by adding the volume of the suction bottle with the blood soaked sponges (know dry weight). Blood loss 2 hours after caesarean delivery was measured by using blood collection drape. The whole blood loss was estimated by adding the blood in the suction bottle, blood soaked sponges and blood collection drape." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 120 women were randomised in the study, but 10 were excluded from the analysis from the oxytocin group after randomisation. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (NCT02562300). |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Owonikoko 2011.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, antepartum haemorrhage, cardiac/renal/liver disorders, coagulopathy, asthma, glaucoma, pre‐eclampsia, eclampsia, prolonged labour or contraindications to administration of prostaglandins. | |
| Interventions | 20 IU of oxytocin administered by an IV infusion versus 400 mcg of misoprostol administered sublingually | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); nausea; vomiting; headache; hypotension; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was developed by a statistician who was not otherwise involved with the study using computer‐generated table of random numbers and varied permutated blocks. |
| Allocation concealment (selection bias) | Low risk | Used sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The anaesthetist was blind to the allocation until he opened each participant’s envelope at surgery. The obstetricians were unaware of what oxytocic was given as the faces of the patients were screened off during the surgery". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The obstetricians were unaware of what oxytocic was given as the faces of the patients were screened off during the surgery". |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection in a suction bottle, and by weighing delivery drapes and gauzes on the basis that 1 g is equivalent to 1 mL of blood. Quote: "Both the surgeon and anaesthetist estimated blood loss independently. The scrub nurse weighed the drapes and gauze before and after the operation, noted the amount of blood in the suction bottle, and recorded these. The postoperative care nurse also recorded the blood loss during the first 4 hours after surgery". Finally a research assistant (not part of the medical team) calculated the mean estimated blood loss from all these values. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Pakniat 2015.
| Methods | 3‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 150 women were randomised in a hospital setting in Iran. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with any risk factor of postpartum haemorrhage i.e. anaemia (Hb < 8 g/dL), multiple pregnancy, antepartum haemorrhage, polyhydramnios, 2 or more previous caesarean sections and/or a history of previous uterine rupture, cardiac/liver/renal disorders, or known coagulopathy. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 200 mcg plus 5 IU of misoprostol plus oxytocin administered sublingually plus by an IV bolus versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; change in Hb; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study is stated to be double‐blinded but blinding (of study participants and caregivers) was unclear. The study used dummy infusion and tablets but there was no mention of a dummy for the IV bolus that 1 of the groups received. There is insufficient detail reported to decide on the adequacy of the blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | The volume of blood in the suction bottle and blood‐soaked sponges was measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (NCT01571323). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Parsons 2006.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 450 women were randomised in a hospital setting in Ghana. The population comprised women of both nulliparous and multiparous, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with asthma, epilepsy or contraindications to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered IM versus 800 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; hypertension; fever;.shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "We acknowledge that unblinding for some participants was possible because the envelopes for women who were initially randomised but who subsequently underwent caesarean section were returned and used for the next women enrolled". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Matercare International and the Society of Obstetricians and Gynaecologists of Canada (public funding). |
Parsons 2007.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 450 women were randomised in a hospital setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with asthma, epilepsy or contraindications to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered IM versus 800 mcg of misoprostol administered rectally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); nausea; vomiting; hypertension; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Unblinding for some participants was possible because the envelopes for women who were initially randomised but who subsequently underwent caesarean section were returned and used for the next women enrolled". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Estimated blood loss data were unavailable in 9 cases (misoprostol 7; oxytocin 2) and haemoglobin measurements (misoprostol 4; oxytocin 6) were unavailable in 10 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Matercare International and the Society of Obstetricians and Gynaecologists of Canada (public funding). |
Patil 2013.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with Hb level less than 7 g/dL, APH, multiple pregnancy, non‐cephalic presentations, pregnancy induced hypertension, previous LSCS, induced labour, instrumental delivery, cervical tear and third‐degree perineal tear, body temperature > 38o C on admission, cardiac disease, hepatic disorders and known hypersensitivity to prostaglandins. | |
| Interventions | 600 mcg of misoprostol administered orally versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); diarrhoea; nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation table was used to randomise participants. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Once the active bleeding stopped, collected blood was weighed. Swabs and pads used during 3rd stage were not counted for blood loss, but were kept to minimum of < 3. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 200 women were randomised in the study, but 2 were excluded because of third degree perineal tear (n = 1) and adherent placenta (n = 1) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Patil 2016.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with hypersensitivity to drugs, respiratory diseases, cardiac disease, renal, liver disorder, epilepsy, psychiatric disorders, pre‐eclampsia, severe anaemia, multiple pregnancy, poly/oligohydramnios, previous PPH, grand multiparous. | |
| Interventions | 10 IU of oxytocin administered IM versus 125 mcg of carboprost administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; blood loss (mL); third stage duration (minutes); diarrhoea; nausea; vomiting; headache; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | The blood loss during third stage of labour and the immediate postpartum period (1 hour after delivery) was estimated quantitatively using Brasss V Drape. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Penaranda 2002.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 78 women were randomised in a hospital setting in Colombia. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with asthma, multiple pregnancy, intrauterine death, coagulopathy, cervical tear or water in the blood collector. | |
| Interventions | 50 mcg of misoprostol administered sublingually versus 16 mIU/min of oxytocin administered by an IV versus 200 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; blood loss (mL; third stage duration (minutes); vomiting; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss from cord clamping until 1 hour after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women were excluded from the analysis after entering the study because of liquor contamination during blood collection. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Perez‐Rumbos 2017.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 500 women were randomised in a hospital setting in Venezuela. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesareans, grand multiparous (>= 5), multiple pregnancy, previous caesareans, precipitate labour, anaemia (< 6 g/dL), chorioamnionitis, previous PPH, polyhydramnios, intrauterine fetal death, APH, asthma and hypersensitivity in any of the agents, clotting disorders, renal/liver disorders, epilepsy, hypertension, or those who did not consent to the study. | |
| Interventions | 600 mcg of misoprostol administered rectally versus 20 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The numbers for the assignment to each treatment group were generated with a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | A sealed system was used that contained the location of each patient to the treatment groups. The envelopes were opened at the beginning of each treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | The blood lost was collected in a calibrated and all the gauzes used were weighed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 500 women were randomised in the study, but 108 were excluded because of missing data after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Poeschmann 1991.
| Methods | 3‐arm controlled randomised trial | |
| Participants | 77 women were randomised in a hospital setting in the Netherlands. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women if they had a Hobel Score of more than 10. | |
| Interventions | 5 IU of oxytocin administered IM versus 500 mcg of sulprostone administered IM versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; manual removal of placenta; blood loss (mL); third stage duration (minutes); nausea. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was within blocks of 10 but the sequence generation method was not reported. |
| Allocation concealment (selection bias) | Low risk | Allocated identical numbered boxes containing trial medications. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A nurse not involved with the delivery room prepared the injections. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Blood loss was calculated by measuring the amount of blood and clots collected in the bedpan and by weighing the blood‐stained swabs and linen obtained for 1 hour postpartum. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77 women were randomised in the study, but 3 were excluded because of induction of labour (n = 2) and instrumental delivery (n = 1) after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Sulprostone was supplied by Schering without charge but no other funding sources are reported. |
Prendiville 1988.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 1695 women were randomised in a hospital setting in the UK. The population comprised women of both nulliparous and multiparous, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with cardiac disorder, antepartum haemorrhage, non‐cephalic presentation, multiple pregnancy, intrauterine death but after change in the protocol multiple other exclusion criteria were introduced. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; change in Hb; NNU admissions; breastfeeding; vomiting; headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South Western Regional Health Authority of the UK (public funding). |
Quibel 2016.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 1721 women were randomised in a hospital setting in France. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with multiple pregnancies, known hypersensitivity to prostaglandins, caesarean delivery, or participation in any other treatment trial. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered orally plus by an IV bolus versus 10 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL;.change in Hb; diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent, centralised, computer‐generated randomisation sequence (Clean‐Web; Télémedecine Technologies, Boulogne, France) was used for this allocation based on a randomisation list established by an independent statistician according to a permuted block method balanced and stratified by centre. |
| Allocation concealment (selection bias) | Low risk | To conceal allocation, treatment boxes were sealed and numbered sequentially according to the randomisation sequence and were stored in the predelivery unit of each maternity ward. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo tablets were provided by the pharmacy of the Assistance Publique‐Hôpitaux de Paris. They were identical to misoprostol tablets in colour but their shape was slightly different. Therefore, the treatment was administered by a research midwife who did not otherwise participate in this trial, to maintain the treatment blind of patients and staff, before or after randomisation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The placebo tablets were provided by the pharmacy of the Assistance Publique‐Hôpitaux de Paris. They were identical to misoprostol tablets in colour but their shape was slightly different. Therefore, the treatment was administered by a research midwife who did not otherwise participate in this trial, to maintain the treatment blind of patients and staff, before or after randomisation." |
| Objective assessment of blood loss | Low risk | Quote: "Blood loss was collected into a calibrated plastic bag placed under the mother’s pelvis. The transparent, graduated bag allowed continuous monitoring of blood loss and was maintained in place for at least 2 hours after the neonate’s delivery. It did not require sterilization and could be used in a dorsal, lateral, or lithotomy position. Blood from blood‐soaked gauze swabs was also transferred into the plastic bag." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1721 women were randomised in the study, but 118 were excluded because of caesarean during labour (n = 113) and withdrawals from the study (n = 5) after randomisation. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (NCT01113229). |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | Supported by a grant from Programme Hospitalier de Recherche Clinique Clinique—PHRC 2009 (Ministère de la Santé N° AOR 09010). |
Rajaei 2014.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 400 women were randomised in a hospital setting in Iran. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with placenta praevia, placental abruption, coagulopathy, previous caesarean, macrosomia (more than 4 kg), polyhydramnios or uncontrolled asthma. | |
| Interventions | 20 IU of oxytocin administered by an IV infusion versus 400 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL); change in Hb; hypotension; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation using simple randomisation with computer‐generated numbers in 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was quote: "double‐blind": "for blinding the study, identical‐appearing solutions and tablets corresponding to the 2 pharmacological groups were prepared by the pharmacy and kept in the fridge until required". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss during the first hour after delivery, by collection with pads weighed before and after absorbance of blood. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | The study protocol was registered (ClinicalTrials.gov NCT01863706) but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of diarrhoea, nausea and vomiting were not completely reported). Moreover, the study publication reports outcomes (hypotension, nausea, transfusion) not listed in the registered protocol. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Hormozgan University of Medical Sciences (the institution of the authors). |
Ramirez 2001.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | An unspecified number of parturients were randomised in a hospital setting in Spain. The population comprised women of nulliparous, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised multiparous women, severe anaemia, hypertensive disorders. | |
| Interventions | 5 IU of oxytocin administered by an IV bolus versus 200 mcg of ergometrine administered by an IV bolus versus no treatment | |
| Outcomes | The study recorded the following outcomes: (No outcome data found) | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Rashid 2009.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 686 women were randomised in a hospital setting in Saudi Arabia. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section or requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, hypertension on treatment, APH, pre‐term labour (less than 37 weeks), postmaturity (more than 42 weeks) or Hb less or equal to 90 g/L. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); third stage duration (minutes); nausea; vomiting; headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence of numbers. |
| Allocation concealment (selection bias) | Unclear risk | Used sequentially‐numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss quote: "clinically in a standard way" by collection with a plastic sheet that was subsequently drained (with clots) into a graduated measuring jug, and by weighing swabs and towels. Quote "Any delayed haemorrhage within 24 hours after delivery was calculated". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of requirement for additional syntometrine [ergometrine plus oxytocin] were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ray 2001.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective caesarean section, or those with pre‐term labour (less than 32 weeks), prolonged labour, antepartum haemorrhage, pre‐eclampsia, intrauterine death, multiple pregnancy, epilepsy, asthma, cardiac/kidney disorder, coagulopathy or anaemia. | |
| Interventions | 400 mcg of misoprostol administered orally versus unspecified dose of ergometrine administered by an unspecified route | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; manual removal of placenta; hypertension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss in the first 2 hours after delivery of the placenta, by "clinical estimation". However, blood loss data were not reported in a format that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Reddy 2001.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in India. The population comprised women of any parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with heart, liver or renal disease, asthma, epilepsy, Rhesus‐negative, traumatic PPH, severe anaemia (< 6 g/dL) or hypertension. | |
| Interventions | 200 mcg of ergometrine administered by an IV bolus versus 250 mcg of carboprost administered IM | |
| Outcomes | The study recorded the following outcomes: blood loss (mL); third stage duration (minutes); diarrhoea; headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Reyes 2011.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 144 women were randomised in a hospital setting in Panama. The population comprised women of parity 5 or more, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing emergency caesarean section, or those with coagulopathy, unknown parity or known allergy to carbetocin. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; manual removal of placenta; breastfeeding; nausea; vomiting; headache; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of PPH were omitted). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | Ferring Pharmaceuticals donated carbetocin. No other external funding was required for the study. |
Reyes, Gonzalez 2011.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 57 women were randomised in a hospital setting in Panama. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both caesarean and vaginal delivery. Exclusion criteria comprised women with HELLP syndrome, blood dyscrasia or multiple pregnancy. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 10 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; change in Hb; third stage duration (minutes); breastfeeding; vomiting; headache; fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code. |
| Allocation concealment (selection bias) | Low risk | Used opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was quote "double‐blind": "because the two drugs are administered differently, a double dummy system for administration was used". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women were excluded from the study analysis after randomisation (quote "1 given drug before expulsion of placenta; 1 ampoule of the drug broken before use"). |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Rogers 1998.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 1512 women were randomised in a hospital setting in the UK. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing augmentation of labour or instrumental delivery or requiring epidural analgesia, or those with placenta praevia, previous PPH, antepartum haemorrhage, Hb less than 100 g/L or mean corpuscular volume less than 75 fL, non‐cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than 5), fibroids, anticoagulation therapy, pre‐term labour (less than 32 weeks) or contraindications to any of the drugs. | |
| Interventions | Unspecified of ergometrine plus oxytocin administered IM versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); third stage duration (minute); NNU admissions; breastfeeding; nausea; vomiting; headache; maternal satisfaction. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule used variably sized balanced blocks, and the randomisation envelopes were prepared in advance in the National Perinatal Epidemiology Unit (NEPU). |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Blood loss data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Public Health and Operational Research Committee of the Anglia and Oxford Regional Health Authority, UK (public funding). |
Rosseland 2013.
| Methods | 3‐arm placebo‐controlled randomised trial | |
| Participants | 76 women were randomised in a hospital setting in Norway. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with pre‐eclampsia, placenta praevia, placenta accreta, von Willebrand disease or other bleeding disorder or preoperative systolic arterial pressure less than 90 mmHg. | |
| Interventions | 5 IU of oxytocin administered by an IV bolus versus 100 mcg of carbetocin administered by an IV bolus versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; blood loss (mL); change in Hb; headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated list of random numbers was used. The block size varied between 6 and 9. Stratified randomisation with 2 strata, BMI less than 30 and BMI of 30 or more. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was quote "double‐blinded": "to maintain blinding of the participants and investigators, the test medicine was delivered to the Department of Anaesthesiology in 10 mL syringes containing 5 mL of solution marked only with trial identification and randomisation numbers. The 10‐mL syringes with the test medicines were prepared by a staff anaesthesiologist, who was otherwise uninvolved in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss with the following formula: (0.75 x height in inches x 50) + (weight in pounds x 50) x ((predelivery haematocrit measurement ‐ postdelivery haematocrit measurement)/predelivery haematocrit measurement). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00977769). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
Sadiq 2011.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 1865 women were randomised in a hospital setting in Nigeria. The population comprised women of parity 6 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing instrumental delivery, or those with diabetes, non‐cephalic presentation, anaemia, APH, multiple pregnancy, grand multiparity (more than 6) or known allergy. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus 600 mcg of misoprostol administered orally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments generated by dice‐box. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss at delivery by collection with pre‐calibrated kidney dishes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "46 of the administered questionnaires were invalidated leaving a total of 1819 valid questionnaires (912 for oxytocin and 907 for misoprostol)." The data were further reduced through a process of computer randomisation so as to have equal study populations. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the University of Maiduguri Teaching Hospital. Study medications were donated by Emzor Pharmaceutical Industries. |
Samimi 2013.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 216 women were randomised in a hospital setting in Iran. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with hypertension, pre‐eclampsia, uterine rupture, cervical tear, asthma, cardiovascular/renal/liver disorders, grand multiparity (not defined), fibroids or previous PPH. | |
| Interventions | 100 mcg of carbetocin administered IM versus 200 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: severe maternal morbidity: intensive care admissions; additional uterotonics; death; change in Hb; nausea; vomiting; tachycardia; hypotension; shivering abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "Patients and medical personnel were blinded to the type of drug". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Blood loss was not measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 24 hours postpartum, blood samples could not be collected from 16 women (9 in the carbetocin group and 7 in the ergometrine plus oxytocin group). |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (Iranian registry of clinical trials number 138810212854N2). |
| Intention to treat analysis | High risk | The authors excluded 16 study participants from the analysis because postpartum haemoglobin measurements were not available. |
| Funding source | Unclear risk | The study was supported by funding from the Kashan University of Medical Sciences (the institution of the authors). |
Shady 2017.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 360 women were randomised in a hospital setting in Egypt. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with medical disorders as cardiac, hepatic, renal, neurologic disorders, thromboembolic disease, blood disorders, diabetes, gestational hypertension and pre‐eclampsia, grand multiparous (> 5), multiple pregnancy, polyhydramnios, macrosomia, APH, prolonged and obstructed labour, scarred uterus or previous instrumental delivery and those suffering from hypersensitivity to tranexamic acid. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus 600 mcg of misoprostol administered sublingually | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; diarrhoea; nausea; vomiting. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician prepared computer‐generated randomisation tables. |
| Allocation concealment (selection bias) | Low risk | Investigators placed the allocation data in serially numbered closed opaque envelopes. Each envelope had a card noting the intervention type inside. The envelopes were opened only by the principal investigator administering the study medications according to the order of attendance of women. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Immediately after delivery of the baby, and after liquor drainage, the patient was placed over a blood drape of known weight and a graduated container was placed under the delivery bed to collect blood. The amount of blood collected in the blood drape was measured. Then the patient was given pre‐weighed pads, which were weighed 4 hours postpartum. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Shrestha 2011.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in Nepal. The population comprised women of any parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with polyhydramnios, chorioamnionitis, preterm labour, previous caesarean, asthma, cardiac disorder or contraindication/hypersensitivity to the use of prostaglandin and uterotonics. | |
| Interventions | 1000 mcg of misoprostol administered rectally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; severe maternal morbidity: intensive care admissions; death; blood loss (mL); change in Hb; third stage duration (minutes); fever; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated as per the lottery technique. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss in the 48 hours postpartum, by collection with pre‐weighed sterile pads and a calibrated bucket. All the soaked drapes and pads were weighed and the dry weights of these materials were subtracted to calculate blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Singh 2009.
| Methods | 4‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 300 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing augmentation of labour, or those with intrauterine death, antepartum haemorrhage, multiple pregnancy, malpresentation, cardiac disorder, Rhesus‐negative mother, hypertension, Hb less than 70 g/L or hypersensitivity/contraindication to prostaglandins. | |
| Interventions | 400 or 600 mcg of misoprostol administered sublingually versus 5 IU of oxytocin administered by an IV bolus versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); third stage duration (minutes); fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The drug packets were sealed and coded using a computer‐generated random number chart by the same individual. |
| Allocation concealment (selection bias) | Unclear risk | Used sealed drug packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was quote "double‐blind": active treatments and placebo treatments were "identical" and investigators were "thus blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators removed any linen soiled with amniotic fluid, and placed a disposable and absorbent pre‐weighed linen saver sheet with a pre‐weighed polythene bag under the mother to collect blood from the uterine cavity. Any blood clots were expressed from the vagina into the polythene bag, which was then removed and weighed. A fresh pre‐weighed sanitary napkin was applied. Separate swabs were not included in the final calculation (addition of the various gravimetric measurements), that was performed 1 hour after delivery. Quote "The specific gravity of blood being 1.08, the amount of blood lost in mL was equal to the weight in grams". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (changes in Hb measurements were unspecified beyond textual summary that quote "all groups showed a slight decrease in mean haemoglobin concentration 24 hours postpartum [maximum decrease of 0.6 g/dL]; however, the difference was not significant [ANOVA, P > 0.05]"). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Sitaula 2017.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in Nepal. The population comprised women of parity 4 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised women with polyhydramnios, uncontrolled diabetes mellitus, previous 2 or more caesarean deliveries, severe pre‐eclampsia, multiple pregnancy, grand multipara, known coagulation disorder, caesarean delivery under GA, previous myomectomy, previous uterine rupture, abnormal placentation, sensitivity to misoprostol. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered rectally plus by an IV infusion versus 20 IU of oxytocin administered by an Quote infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; transfusion; blood loss (mL); change in Hb. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were objective involved weighing the swabs but also visual estimation quote "fist full of clot was 500 ml". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Soltan 2007.
| Methods | 4‐arm active‐controlled randomised trial | |
| Participants | 1228 women were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with traumatic PPH, blood disorders, chorioamnionitis, placenta praevia or placental abruption. | |
| Interventions | 200 mcg of ergometrine administered IM versus 600‐1000 mcg of misoprostol administered sublingually | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Used opaque, closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by collection with a graduated plastic bag, and by weighing towels, linen and gauzes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote "144 women were excluded from analysis because they were exposed to trauma to the perineum, vagina or cervix during labour and had traumatic excessive bleeding". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Sood 2012.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 174 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria were not specified. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IV infusion versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes made at pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised intraoperative blood loss by collection with suction apparatus and sterile drapes before irrigation, and by evaluating the blood in abdominal swabs and gauzes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Stanton 2013.
| Methods | 2‐arm cluster‐controlled randomised trial | |
| Participants | 1586 women were randomised in a community setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered IM versus no treatment | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; death. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 52 CHOs were randomly allocated equally to either the intervention or the control group; this allocation was stratified by both district and distance (#10 km or .10 km) to emergency obstetric care. The randomisation sequence was determined using Stata (version 12) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was not reported but less of an issue in cluster‐randomised trials. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote "The random allocation was not masked". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised postpartum blood loss by collection with a BRASS‐V calibrated plastic drape placed under the mother, who was asked to remain recumbent for 1 hour following delivery of the baby, or for 2 hours if active bleeding persisted. Quote "Fluids, urine, and faeces were excluded from the blood loss measure by sweeping them to the side and into a receptacle". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "7 and 9 enrolled women in the oxytocin and control arms, respectively, lacked a blood‐loss measure". |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT01108289). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Bill and Melinda Gates Foundation (public funding). |
Su 2009.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 370 women were randomised in a hospital setting in Singapore. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing elective caesarean section, or those with multiple pregnancy, previous PPH, coagulopathy, coronary artery disease, hypertension or hypersensitivity/contraindications for the use of syntometrine or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered IM versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); third stage duration (minutes); nausea; vomiting; headache; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was blocked and stratified by parity. The randomisation list with the allocation of the mode of intervention was forwarded from the Biostatistics Unit to the Department of Pharmacy at National University Hospital, where the purchased medications were kept. |
| Allocation concealment (selection bias) | Low risk | Used opaque packages made at pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "The identities of the medications were not known to the midwives, obstetricians and the participants. The medication codes were only broken following completion of the trial". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the visual estimation of attending obstetricians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered 2 years after beginning recruitment (ClinicalTrial.gov NCT00499005). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Healthcare Group of Singapore (public funding). |
Sultana 2007.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 400 women were randomised in a hospital setting in Bangladesh. The population comprised women of both nulliparous and multiparous, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500. PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians after collection in a plastic bowl. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Supe 2016.
| Methods | 4‐arm controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of any parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with medical disorders like pregnancy‐induced hypertension, cardiac disease, sensitivity to prostaglandins, and history of previous caesarean section. | |
| Interventions | 800 mcg of misoprostol administered rectally versus 200 mcg of ergometrine administered IM versus 125 mcg of carboprost administered IM versus no treatment | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by using a randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | The blood and blood clots in the kidney tray were weighed. A plastic pouch was placed under the buttocks prior to the delivery. The blood lost was collected in this pouch. After the delivery of the placenta, the content of the pouch was transferred to a graduated jar. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | Funding was not required. |
Surbek 1999.
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 65 women were randomised in a Hospital setting in Switzerland. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with multiple pregnancy, pre‐eclampsia, previous PPH or antepartum haemorrhage. | |
| Interventions | 600 mcg of misoprostol administered orally versus placebo | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; blood loss (mL); third stage duration (minute); NNU admissions; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random tables. |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was quote "double‐masked": "for proper masking, the study drugs were prepared by the hospital pharmacy as three identical gelatine capsules". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Taheripanah 2018.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 220 women were randomised in a hospital setting in Iran. The population comprised women of nulliparous and multiparous, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised women refusing to co‐operate, major therapeutic side effects, history of cardiac and renal diseases, pre‐eclampsia, and twin pregnancy. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 30 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; blood loss (mL); change in Hb; nausea; vomiting; headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as block randomisation. |
| Allocation concealment (selection bias) | Low risk | Selection and randomisation of the patients were performed by a coordinating nurse, using a series of sequentially numbered sealed envelopes; therefore, the sequence of allocation was hidden. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The authors state Quote "The women and practitioners were not aware of the type of intervention" but blinding (of study participants and caregivers) was unclear as it is not described in sufficient detail. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was registered retrospectively (NCT02079558). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Tewatia 2014.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with grand multiparity (more than 4), anaemia, malpresentation, polyhydramnios, antepartum haemorrhage, liver/renal disorder, previous caesarean, previous PPH, uterine anomaly, traumatic PPH or contraindications to use misoprostol or oxytocin. | |
| Interventions | 10 IU of oxytocin administered by an IV infusion versus 600 mcg of misoprostol administered sublingually | |
| Outcomes | The study recorded the following outcomes: PPH at 500. PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote "Due to [the] nature of administration of the drugs, [the] patient or clinical care team could not be blinded. However, [the] statistician was unaware of the group allocation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators removed any linen soiled with amniotic fluid, and placed a calibrated plastic bag under the mother to collect blood from the uterine cavity. After delivery of the placenta, a pre‐weighed pad was placed high up in vagina until 1 hour afterwards. In cases of episiotomy, a separate pad was applied to the episiotomy site, and the fluid collected by this pad was not included in blood loss measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Thilaganathan 1993.
| Methods | 2‐arm controlled randomised trial | |
| Participants | 193 women were randomised in a hospital setting in UK. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour or instrumental delivery, or those with grand multiparity (not defined), malpresentation, multiple pregnancy, previous caesarean, previous PPH, APH, hypertension in pregnancy, intrauterine death, PROM, cervical lacerations or third degree perineal tears. | |
| Interventions | No treatment versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated using standard randomisation tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was conducted without external funding. |
Tripti 2006.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of nulliparous and multiparous, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with hypertension, cardiac disease, renal disease, gastrointestinal disorders, respiratory disease, endocrinal problems, coagulation disorder, and sensitivity to prostaglandin or methergin. | |
| Interventions | 125 mcg of carboprost administered IM versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; manual removal of placenta; blood loss (mL); third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Blood loss was estimated by blood and blood clots collected in the kidney tray and adding the difference in the weight of the drapes before use and after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Ugwu 2014.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in Nigeria. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, pre‐eclampsia, eclampsia, undiagnosed vaginal bleeding, prolonged labour, prolonged obstructed labour, cardiac/renal/liver disorders or fever. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an IV infusion versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additionaluUterotonics; transfusion; death; blood loss (mL); fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random tables. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote There were no look‐alike placebo tablets for women who had oxytocin alone but the fact that no member of the obstetric team had knowledge of which agent the patient received, is expected to ensure allocation concealment. In addition, there was incomplete blinding of the anaesthetist, although this was not likely to affect the study outcome, since the anaesthetist's estimated blood loss was not used." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "There were no look‐alike placebo tablets for women who had oxytocin alone but the fact that no member of the obstetric team had knowledge of which agent the patient received, is expected to ensure allocation concealment. In addition, there was incomplete blinding of the anaesthetist, although this was not likely to affect the study outcome, since the anaesthetist's estimated blood loss was not used." |
| Objective assessment of blood loss | Low risk | Investigators appraised intraoperative and postoperative blood loss by collection in a suction bottle. Furthermore, soiled drapes, abdominal packs and pieces of gauze were weighed and the known dry weights subtracted. Finally, vulva pads applied during the 4 hours post‐operation, were also weighed and the known dry weights subtracted. Measurements obtained by these 3 methods were added together. Weight measurements were performed with a weighing scale made in China, of total weighing capacity of 5 kg and graduations of 0.25 g. Investigators considered that 1 g is equivalent to 1 mL of blood. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of nausea, vomiting, diarrhoea, headaches, fatigue, dizziness, chills, flatulence and abdominal pain were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Un Nisa 2012.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women of parity 2 to 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with previous PPH, multiple pregnancy, previous caesarean, macrosomia, pre‐eclampsia, diabetes, cardiac/lung/bleeding/clotting disorders or taking anticoagulants. | |
| Interventions | 10 IU of oxytocin administered by an IV bolus versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study participants (patients) were divided by lottery system in the 2 groups, each group comprising of 50 patients. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss after the delivery of baby quote "by squeezing the soaked pads and quantifying the amount of blood clots in a kidney tray of standard size to be equal to 500 mL". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Uncu 2015.
| Methods | 5‐arm controlled randomised trial | |
| Participants | 248 women were randomised in a hospital setting in Turkey. The population comprised women of parity 5 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with placenta praevia, previous PPH, APH, non‐cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than 5), fibroids, pre‐eclampsia or anticoagulation therapy. | |
| Interventions | No treatment versus 400 mcg to 800 mcg of misoprostol administered orally, vaginally or rectally | |
| Outcomes | The study recorded the following outcomes: additional uterotonics; transfusion; third stage duration (minutes); diarrhoea; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Vagge 2014.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with cardiac disorder in pregnancy, uterine tumour in pregnancy, secondary PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, anaemia, coagulopathy, antepartum haemorrhage, previous PPH, prolonged labour, precipitate labour or known allergic or hypersensitivity reaction to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered by an IV infusion versus 800 mcg of misoprostol administered rectally | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; blood loss (mL); diarrhoea; nausea; fever; shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used simple random sampling. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of appraising blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Vaid 2009.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with grand multiparity (more than 4), multiple pregnancy, preterm labour (less than 32 weeks), HELLP syndrome, polyhydramnios, coagulopathy, asthma, cardiac/renal disorder, epilepsy, hypertension, Hb less than 80 g/L or known drug allergy. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 200 mcg of ergometrine administered IM versus 125 mcg of carboprost administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; transfusion; manual removal of placenta; diarrhoea; nausea; vomiting; fever; shivering; abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by a computer‐generated random number. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After the drainage of amniotic fluid, investigators appraised blood loss by collection with a sterile calibrated BRASS‐V drape placed under the mother. The drape remained in placed for 1 hour. Furthermore, quote "blood loss in gauze pieces was calculated by subtracting the weight of dry gauze from the weight of blood‐soaked gauze pieces". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Van Selm 1995.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 81 women were randomised in a hospital setting in the Netherlands. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women with coagulation disorder, anticoagulant medication, multiple pregnancy, fibroids, hypertension, induction of labour. | |
| Interventions | 200 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 500 mcg of sulprostone administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; transfusion;manual removal of placenta; blood loss (mL); third stage duration (minutes). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Assignment to pharmacy coded boxes occurred, after informed consent, in first stage labour. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding of personnel and participants (placebo use). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinding of personnel and participants (placebo use). |
| Objective assessment of blood loss | Low risk | Measured the blood and clots by collecting and weighing the blood stained linen and pads. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 81 women were randomised in the study, but 12 were excluded because of exclusion criteria all in the ergometrine plus oxytocin group after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Verma 2006.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 200 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 200 mcg of ergometrine administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; additional uterotonics; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes); nausea; fever; Shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": active treatments and placebo treatments were "identical‐looking". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss quote: "accurately with a specially designed calibrated blood collection drape (BRASS‐V drape)". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | It was unclear from the study report whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
Vimala 2004.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 120 women were randomised in a hospital setting in India. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour or caesarean section, or those with preterm labour (less than 37 weeks), grand multiparity (more than 5), multiple pregnancy, hypertension in pregnancy, Hb less than 80 g/L or known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; blood loss (mL); change in Hb; third stage duration (minutes); nausea; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by random tables. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatments were administered via different routes and the authors did not report any double dummy. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss by the estimation of attending nurses and obstetricians. After delivery of the baby, amniotic fluid was allowed to drain away, and amniotic fluid‐soaked bed linens were covered with dry disposable ‘linen‐savers’. A wedge‐shaped plastic bedpan was placed under the mother for 1 hour. Blood and clots from the bedpan were decanted into a measuring cylinder and measured. Blood‐soaked swabs and linen‐savers were weighed; the known dry weights were subtracted, for the weight of blood contained within them to be added to the value indicated by the measuring cylinder. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Vimala 2006.
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both elective or emergency caesarean. Exclusion criteria comprised women with multiple pregnancy, APH, polyhydramnios, prolonged labour (more than 12 hours), previous more than 1 caesarean, previous uterine rupture, cardiac/liver/renal disorder, coagulopathy or Hb less than 80 g/L. | |
| Interventions | 400 mcg of misoprostol administered sublingually versus 20 IU of oxytocin administered by an IV infusion | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; blood loss (mL); change in Hb; vomiting; headache; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | Low risk | Used opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators appraised blood loss intraoperatively and in the first hour postoperatively "in a standard manner". They measured the volume of blood in the suction bottle, and weighed blood‐soaked sponges and linen savers. Then they added the difference between dry and blood‐soaked weights of sponges and linen savers, to the volume measured in the suction bottle. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Division of Reproductive Health and Nutrition, Indian Council of Medical Research (public funding). |
Walley 2000.
| Methods | 2‐arm active‐controlled double‐dummy randomised trial | |
| Participants | 401 women were randomised in a hospital setting in Ghana. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing induction or augmentation of labour or caesarean section, or those with grand multiparity (more than 5), multiple pregnancy, preterm labour (less than 32 weeks), hypertension in pregnancy, HELLP syndrome, polyhydramnios, previous PPH, coagulopathy, precipitate labour, chorioamnionitis, Hb less than 80 g/L or a known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes); diarrhoea; nausea; vomiting; fever; shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Used sequentially‐numbered, opaque packets made by administrative staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The identity of the placebo and active medications were concealed from caregivers and participants". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators appraised blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of those women randomised, blood loss measurements were unavailable in 3 cases, and postpartum Hb samples were unavailable in 9 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from MaterCare International and the Canadian International Development Agency (public funding). |
Whigham 2016.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 122 women were randomised in a hospital setting in Australia. The population comprised women of any parity, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised women undergoing elective caesarean section or requiring general anaesthesia, or those with vascular/liver/renal disorders, preterm labour (less than 37 weeks), multiple pregnancy, placenta praevia, placental abruption, previous more than 2 caesareans or an adverse reaction to carbetocin/oxytocin. | |
| Interventions | 100 mcg of carbetocin administered by an IV bolus versus 5 IU of oxytocin administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 1000; additional uterotonics; transfusion; blood loss (mL); change in Hb. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation at pharmacy level and none of the operating or anaesthetic doctors will have access to this. |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pharmacy used a study label, which included study title, number and expiry date to cover the trade label. Patients, anaesthetists and operating obstetricians were blinded to the intervention drug. These ampoules were stocked in the emergency theatre fridge in boxes labelled only with the matching study label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators appraised intra‐operative blood loss by the estimation of attending physicians. Excess blood was collected in measuring container by suction, and weighed together with any swabs soaked in blood. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 114 women were randomised in the study, but 10 were excluded because they had a general anaesthetic (n = 2) or ampoules discarded (n = 8) after randomisation. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (ACTRN 12612000466842). |
| Intention to treat analysis | High risk | Those who were excluded from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | This project was awarded the Peninsula Health Grant for Health Research. |
Widmer 2018.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 29645 women were randomised in a hospital setting in Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda and the UK. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women in an advanced stage of labour (cervical dilatation > 6 cm) or who were too distressed to give informed consent, who had known allergies to carbetocin, oxytocin homologues or excipients, who had serious cardiovascular disorders, serious hepatic or renal disease, or who had epilepsy. | |
| Interventions | 100 mcg of carbetocin administered IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; vomiting; abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation sequence was generated at WHO using computer‐generated random numbers. Randomisation was stratified by country using permuted blocks of size ten, with an allocation ratio 1:1. |
| Allocation concealment (selection bias) | Low risk | Both HS carbetocin and oxytocin were in 1 mL ampoules in consecutively numbered treatment packs arranged in dispensers. Allocation was by opening the consecutively numbered treatment pack in the dispenser. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The ampoules, trial packs and dispensers were identical in shape, size and weight ensuring that investigators were blinded to individual treatment allocation. Although carbetocin was heat stable and did not require cold storage we kept the dispensers in cold storage (2⁰C to 8⁰C) to give oxytocin maximum efficacy and maintain double‐blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Once the cord was clamped and cut, a blood collection plastic drape (BRASSS‐V™) was placed under the woman's buttocks. The blood was collected for 1 hour, or 2 hours if the bleeding continued beyond 1 hour. The drape with the blood was then weighed by a digital scale, the weight recorded in grams and then converted to volume (mL) at the analysis stage. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (Trial registration: HRP Trial A65870; UTN U1111‐1162‐8519; ACTRN12614000870651; CTRI/2016/05/006969, EUDRACT 2014‐004445‐26). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The research in this publication was supported by funding from MSD, through its MSD for Mothers Program. MSD for Mothers is an initiative of Merck & Co., Inc., Kenilworth, N.J., USA.. The funder had no commercial interest in the investigational drug, no influence on the protocol, the statistical analysis plan and the final manuscript; the funder could provide comments, but there was no obligation on the trial team to accept any. The HS carbetocin was provided by Ferring International Center S.A. (Saint Prex, Switzerland) and oxytocin by Novartis (Basel, Switzerland) free of charge. Neither company had any influence on any of the trial documents or processes. |
Yuen 1995.
| Methods | 2‐arm active‐controlled double‐blinded randomised trial | |
| Participants | 1000 women were randomised in a hospital setting in Hong Kong. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women requiring oxytocin infusion in the third stage, or those with pre‐eclampsia or cardiac disorder. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered IM versus 10 IU of oxytocin administered IM | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; severe maternal morbidity: intensive care admissions; additional uterotonics; transfusion; manual removal of placenta; death; change in Hb; nausea; vomiting; headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "When a patient entered the study, a nursing officer who was not involved in the management of the patient drew up the indicated medication and handed this to the patient’s attendants". Study participants and caregivers were thus blinded to treatment allocations until the codes were revealed after all data were collected in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators appraised blood loss during delivery quote: "by measuring the amount of blood clots and weighing the towels used". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "9 [randomised participants] were excluded: 3 had a twin pregnancy, 1 had blood transfusion during labour, and the other 5 had unavailable records". |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
Zachariah 2006.
| Methods | 3‐arm active‐controlled randomised trial | |
| Participants | 2023 women were randomised in a hospital setting in India. The population comprised women of both nulliparous and multiparous, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised women undergoing caesarean section, or those with asthma, cardiac disorder, rhesus factor incompatibility or hypertension. | |
| Interventions | 400 mcg of misoprostol administered orally versus 10 IU of oxytocin administered IM versus 200 mcg of ergometrine administered by an IV bolus | |
| Outcomes | The study recorded the following outcomes: PPH at 500; PPH at 1000; additional uterotonics; transfusion; manual removal of placenta; death; blood loss (mL); change in Hb; third stage duration (minutes). Diarrhea. Nausea. Vomiting. Headache. Fever. Shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After the drainage of amniotic fluid, investigators appraised blood loss by collection with a large sterile plastic bag placed under the mother until she was transferred to the postnatal department. The blood collected in the plastic bag was then transferred to a measuring jar. Mops were not used in the labour room, and gauze pieces were counted. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
ACTRN: Australian Clinical Trials Registration Number; ANOVA: one‐way Analysis of variance; APH: antepartum haemorrhage; ASA I or II: ASA Physical Status Classification System: ASA I represents a normal healthy patient, ASA II represents a patient with mild systemic disease; BMI: Body Mass Index; cc: cubic centimetres; CHOs: community health officers; cm: centimetres; CS: caesarean section; CTRI: Clinical Trials Registry of India; DIC: disseminated intravascular coagulopathy; dL: decilitres; EudraCT: European Clinical Trials database; fL: femtolitres (measurement of mean corpuscular volume); g: gram; Hb: haemoglobin; HELLP syndrome: Hemolysis (destruction of red blood cells), Elevated Liver enzymes (which indicate liver damage), and Low Platelet count; HIV: human Immunodeficiency virus; Hong Kong SAR: Hong Kong Special Adminstrative Region; IM: intramuscularly; IU: International Units; IV: intravenous; kg: kilograms; km: kilometres; L, litres; mcg: micrograms; MCV: mean cell volume; mg: milligrams; mgSO4: magnesium sulphate; min: minutes; mL: millilitres; mmHG: millimetres of mercury (unit of pressure); mmol: millimoles; NCT: National Clinical Trial (number); NEPU: National Perinatal Epidemiology Unit; NHS: National Health Service; nm: nanometres; NNU: Neonatal Unit; PACTR: Pan African Clinical Trials Registry; PCV: packed cell volume; PPH: postpartum haemorrhage; PROM: premature rupture of membranes; RCOG: Royal College of Obstetricians and Gynaecologists; UK: United Kingdom; UNDP/UNFPA: United Nations Development Programme/United Nations Population Fund; USA: United States of America; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Aleem 2013 | Not eligible intervention |
| Abdel‐Aleem 2018 | Same drug intervention both arms and only different timing of oxytocin administration |
| Abdollahy 2000 | Not eligible intervention |
| Adhikari 2007 | Quasi‐randomised |
| Adnan 2017 | Same drug intervention both arms only different route of oxytocin administration |
| Ahmed 2015 | Not eligible intervention |
| Akinaga 2016 | Not eligible intervention |
| Al‐Harazi 2009 | Same drug intervention both arms and only different route of misoprostol administration |
| Alam 2017 | Not randomised |
| Ali 2012 | Quasi‐randomised |
| Ali 2018 | Not randomised |
| Anandakrishnan 2013 | Same drug intervention both arms and only different dose of carbetocin administration |
| Anjaneyulu 1988 | Not eligible intervention |
| Anvaripour 2013 | Intervention given after the third stage of labour |
| Ashwal 2016 | Same drug intervention both arms only different regimen of oxytocin administration |
| Athavale 1991 | Not eligible intervention |
| Ayedi 2011 | Same drug intervention both arms and only different dose of oxytocin administration |
| Ayedi 2011b | Not eligible intervention |
| Ayedi 2012 | Not eligible intervention |
| Aziz 2014 | Quasi‐randomised |
| Bader 2000 | Not eligible intervention |
| Badhwar 1991 | Not eligible intervention |
| Bai 2014 | Not eligible intervention |
| Baig 2015 | Not eligible intervention |
| Balki 2006 | Same drug intervention both arms and only different dose of oxytocin administration |
| Banovska 2013 | Not eligible intervention |
| Barbaro 1961 | Not eligible intervention |
| Baumgarten 1983 | Intervention given after the third stage of labour |
| Bhattacharya 1988 | Not eligible intervention |
| Bhavana 2013 | Not eligible intervention |
| Bider 1991 | Not eligible intervention |
| Bider 1992 | Not eligible intervention |
| Bisri 2011 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Bivins 1993 | Not eligible intervention |
| Blum 2010 | Intervention for treatment of PPH |
| Bonham 1963 | Quasi‐randomised |
| Bonis 2012 | Quasi‐randomised |
| Boopathi 2014 | Quasi‐randomised |
| Bose 2017 | Not eligible intervention |
| Bulusu 2017 | Same drug intervention both arms only different route of misoprostol administration |
| Cappiello 2006 | Not eligible intervention |
| Carvalho 2004 | Same drug intervention both arms and only different dose of oxytocin administration |
| Catanzarite 1990 | Not eligible intervention |
| Chaplin 2009 | Same drug intervention both arms and only different dose of oxytocin administration |
| Chatterjee 2016 | Intervention given after the third stage of labour |
| Chaudhuri 2014 | Intervention given after the third stage of labour |
| Chestnut 1987 | Not eligible intervention |
| Chou 1994 | Not eligible intervention |
| Chou 2015 | Same drug intervention both arms only different dose of oxytocin administration |
| Chukudebelu 1963 | Quasi‐randomised |
| Cooper 2004 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Cordovani 2011 | Same drug intervention all arms only different dose of carbetocin administration |
| Cordovani 2012 | Same drug intervention both arms and only different dose of carbetocin administration |
| Dagdeviren 2016 | Same drug intervention both arms only different route of oxytocin administration |
| Dahiya 1995 | Not eligible intervention |
| Daley 1951 | Quasi‐randomised |
| Daly 1999 | Inappropriate population |
| Dao 2009 | Intervention for treatment of PPH |
| Davies 2005 | Same drug intervention both arms and only different regimen of oxytocin administration |
| De bonis 2012 | Quasi‐randomised |
| Dell‐Kuster 2017 | Same drug intervention both arms only different infusion rate of carbetocin administration |
| Dennehy 1998 | Not eligible intervention |
| Deshpande 2016 | Not eligible intervention |
| Diab 1999 | Quasi‐randomised |
| Dickinson 2009 | Not eligible population (terminations 2nd trimester) |
| Diop 2011 | Study withdrawn |
| Dommisse 1980 | Not randomised |
| Dong 2011 | Not eligible intervention |
| Dumoulin 1981 | Not randomised |
| Durocher 2012 | Not randomised |
| Dutta 2000 | Quasi‐randomised |
| Dweck 2000 | Not eligible intervention |
| Dzuba 2012 | Same drug intervention both arms and only different route of oxytocin administration |
| Elati 2011 | Same drug intervention both arms and only different dose of misoprostol administration |
| Erkkola 1984 | Not eligible intervention |
| Farber 2013 | Not eligible intervention |
| Farber 2015 | Not eligible intervention |
| Fatemeh 2011 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Forster 1957 | Quasi‐randomised |
| Francis 1965 | Quasi‐randomised |
| Friedman 1957 | Quasi‐randomised |
| Fugo 1958 | Quasi‐randomised |
| Gai 2004 | Not eligible intervention |
| Gavhane 2017 | Not randomised |
| George 2010 | Same drug intervention both arms and only different dose of oxytocin administration |
| Ghulmiyyah 2007 | Not eligible intervention |
| Ghulmiyyah 2017 | Same drug intervention all arms only different dose of oxytocin administration |
| Gobbur 2011 | Not eligible intervention |
| Gohel 2007 | Not eligible intervention |
| Goswami 2013 | Not eligible intervention |
| Groeber 1960 | Quasi‐randomised |
| Gungorduk 2010 | Not eligible intervention |
| Gungorduk 2010b | Same drug intervention both arms and only different regimen of oxytocin administration |
| Gungorduk 2011 | Not eligible intervention |
| Gungorduk 2013 | Not eligible intervention |
| Gupta 2014 | Not eligible intervention |
| Habek 2007 | Not eligible intervention |
| Hacker 1979 | Not randomised |
| Halder 2013 | Not eligible intervention |
| Hoffman 2006 | Same drug intervention both arms and only different timing of oxytocin administration |
| Hofmeyr 2004 | Intervention for treating PPH |
| Howard 1964 | Not eligible intervention |
| Huh 2004 | Same drug intervention both arms and only different timing of oxytocin administration |
| Hunt 2013 | Not eligible intervention |
| Häivä 1994 | Quasi‐randomised |
| Ilancheran 1990 | Not randomised |
| Irons 1994 | Inappropriate population (excluded women who had PPH) |
| Islam 2008 | Not randomised |
| Jackson 2001 | Same drug intervention both arms and only different timing of oxytocin administration |
| Jagielska 2015 | Not randomised |
| Javadi 2015 | Not eligible intervention |
| Jiang 2001 | Same drug intervention both arms and only different dose of oxytocin administration |
| Jin 2000 | Not eligible intervention |
| Jolivet 1978 | Not eligible intervention |
| Jonsson 2010 | Same drug intervention both arms and only different dose of oxytocin administration |
| Kashanian 2010 | Intervention administered after the third stage of labour |
| Kemp 1963 | Quasi‐randomised |
| Khan 1997 | Not eligible intervention |
| Khan 2003 | Same drug intervention both arms and only different route of misoprostol administration |
| Khan 2012 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Khan 2013 | Same drug intervention all arms only different dose of carbetocin administration |
| Khanun 2011 | Same drug intervention both arms and only different route of misoprostol administration |
| Kikutani 2003a | Innapropriate population |
| Kikutani 2003b | Innapropriate population |
| Kikutani 2006 | Not randomised |
| King 2010 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Kintu 2012 | Same drug intervention both arms and only different dose of oxytocin administration |
| Kiran 2012 | Same drug intervention both arms and only different dose of oxytocin administration |
| Kore 2000 | Not eligible intervention |
| Kovacheva 2015 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Kovavisarach 1998 | Not eligible intervention |
| Le 2000 | Not randomised |
| Leader 2002 | Not eligible population (2nd trimester) |
| Li 2002 | Not eligible intervention |
| Li 2003 | Not eligible intervention |
| Li 2011 | Not eligible intervention |
| Lin 2009 | Not eligible intervention |
| Liu 1997 | Not eligible intervention |
| Liu 2002 | Not eligible intervention |
| Liu 2015 | Not eligible intervention |
| Liu 2016 | Not eligible intervention |
| Luamprapas 1994 | Not eligible intervention |
| Maged 2015 | Not eligible intervention |
| Makvandi 2013 | Not eligible intervention |
| Mangla 2012 | Not eligible intervention |
| Mankuta 2006 | Not eligible intervention |
| Mansouri 2011 | Same drug intervention both arms and only different route of misoprostol administration |
| Martinez 2006 | Not eligible intervention |
| McGinty 1956 | Quasi‐randomised |
| Miller 2009 | Not eligible intervention |
| Mirghafourvand 2015 | Not eligible intervention |
| Mirteimouri 2013 | Not randomised |
| Mockler 2015 | Same drug intervention both arms only different route of oxytocin administration |
| Mohamadian 2013 | Same drug intervention both arms only different timing of oxytocin administration |
| Mollitt 2009 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Moore 1956 | Same drug intervention both arms and only different type of the same drug |
| Movafegh 2011 | Not eligible intervention |
| Munishankarappa 2009 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Munn 2001 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Murphy 2009 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Murphy 2015 | Same drug intervention both arms only different route of oxytocin administration |
| Nankali 2013 | Not eligible intervention |
| Narenji 2012 | Not randomised |
| Nelson 1983 | Not eligible intervention |
| Neri‐Mejia 2016 | Same drug intervention all arms only different route and regimen of oxytocin administration |
| Newton 1961 | Quasi‐randomised |
| Nguyen‐Lu 2015 | Same drug intervention all arms only different dose of carbetocin administration |
| Nieminen 1964 | Not randomised |
| Oberbaum 2005 | Not eligible intervention |
| Oguz 2014 | Same drug intervention both arms and only different route and timing of oxytocin administration |
| Ononge 2015 | Self‐administration of uterotonic agent |
| Ozalp 2010 | Not eligible intervention |
| Ozcan 1996 | Not eligible intervention |
| Ozkaya 2005 | Inappropriate population (excluded women who had PPH) |
| Padhy 2006 | Not eligible intervention |
| Palacio 2011 | Same drug intervention both arms and only different dose of oxytocin administration |
| Paull 1977 | Same drug intervention both arms and only different doses of drug administration |
| Pei 1996 | Not randomised |
| Perdiou 2009 | Not eligible intervention |
| Phromboot 2010 | Not eligible intervention |
| Pierre 1992 | Quasi‐randomised |
| Pinder 2002 | Same drug intervention both arms and only different doses of drug administration |
| Pisani 2012 | Quasi‐randomised |
| Porter 1991 | Not eligible intervention |
| Priya 2015 | Inappropriate population (measured blood loss after the delivery of the placenta) |
| Puri 2012 | Not eligible intervention |
| Qiu 1999 | Not eligible population (second stage of labour) |
| Quiroga 2009 | Not eligible intervention |
| Ragab 2016 | Same drug intervention both arms only different timing of misoprostol administration |
| Raghavan 2016 | Intervention given for treatment of PPH |
| Rahbar 2018 | Quasi‐randomised |
| Rajwani 2000 | Not eligible intervention |
| Ray 2012 | Same drug intervention both arms only different regimen of oxytocin administration |
| Razali 2016 | Quasi‐randomised |
| Reddy 1989 | Not eligible intervention |
| Rezk 2018 | Same drug intervention both arms only different route of misoprostol administration |
| Rooney 1985 | Quasi‐randomised |
| Rosales‐Ortiz 2014 | Quasi‐randomised |
| Rouse 2011 | Same drug intervention both arms and only different doses of drug administration |
| Sadeghipour 2013 | Not eligible intervention |
| Saito 2007 | Quasi‐randomised |
| Sallam 2018 | Intervention administered for treatment of PPH |
| Samuels 2005 | Not eligible intervention |
| Sangkhomkhamhang 2012 | Same drug intervention both arms only different route of oxytocin administration |
| Sariganont 1999 | Not randomised |
| Sarna 1997 | Same drug intervention both arms and only different doses of drug administration |
| Sartain 2008 | Same drug intervention both arms and only different doses of drug administration |
| Savitha 2017 | Quasi‐randomised |
| Schaefer 2004 | Same drug intervention both arms and only different timings of drug administration |
| Schemmer 2001 | Same drug intervention both arms and only different timings of drug administration |
| Sekhavat 2009 | Not eligible intervention |
| Sentilhes 2015 | Not eligible intervention |
| Senturk 2013 | Not eligible intervention |
| Senturk 2016 | Not randomised |
| Shahid 2013 | Not eligible intervention |
| Sharma 2014 | Not randomised |
| Sheehan 2011 | Same drug intervention both arms and only different doses of drug administration |
| Shirazi 2013 | Not eligible intervention |
| Shrestha 2007 | Not eligible intervention |
| Shrestha 2008 | Not eligible intervention |
| Singh 2005 | Quasi‐randomised |
| Siriwarakul 1991 | Not eligible intervention |
| Soiva 1964 | Not randomised |
| Soleimani 2014 | Quasi‐randomised |
| Sorbe 1978 | Quasi‐randomised |
| Soriano 1995 | Quasi‐randomised |
| Sreelatha 2017 | Same drug intervention all arms only different route of misoprostol administration |
| Stearn 1963 | Not randomised |
| Svanstrom 2008 | Innapropriate population |
| Swapnika 2018 | Not randomised |
| Symes 1984 | Inapropriate population |
| Taj 2014 | Not eligible intervention |
| Takagi 1976 | Not eligible intervention |
| Tali 2016 | Not eligible intervention |
| Tanir 2009 | Not eligible intervention |
| Tarabrin 2012 | Not eligible intervention |
| Tariq 2015b | Administered for treatment of PPH |
| Tehseen 2008 | Not eligible intervention |
| Terry 1970 | Not eligible intervention |
| Tessier 2000 | Same drug intervention both arms and only different regimen of drug administration |
| Tharakan 2008 | Same drug intervention both arms and only different timings of drug administration |
| Thomas 2007 | Same drug intervention both arms and only different regimen of drug administration |
| Thornton 1988 | Quasi‐randomised |
| Tita 2012 | Same drug intervention both arms and only different doses of drug administration |
| Tripti 2009 | Not randomised |
| Tudor 2006 | Not eligible intervention |
| Ugwu 2016 | Same drug intervention both arms and only different doses of drug administration |
| Van den Enden 2009 | Same drug intervention both arms and only different regimen of drug administration |
| Vasegh 2005 | Quasi‐randomised |
| Vaughan Williams 1974 | Innapropriate population |
| Ventoskovskiy 1990 | Not eligible intervention |
| Vogel 2004 | Not eligible outcomes |
| Wallace 2007 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Walraven 2005 | Not eligible uterotonic (oral ergometrine) |
| Wang 2000 | Not eligible intervention |
| Wang 2018 | Not randomised |
| Weeks 2015 | Self‐administration of uterotonic agent |
| Weihong 1998 | Same drug intervention both arms and only different routes of drug administration |
| Weiss 1975 | Not eligible outcomes |
| Wellmann 2016 | Intervention administered before the third stage of labour |
| Wetta 2013 | Same drug intervention both arms and only different doses of drug administration |
| Winikoff 2012 | Same drug intervention both arms and only different routes of drug administration |
| Winikoff 2016 | Same drug intervention all arms only different route of oxytocin administration |
| Wong 2005a | Same drug intervention both arms and only different doses of drug administration |
| Wong 2005b | Study withdrawn |
| Wright 2005 | Not eligible intervention |
| Wu 2007 | Not eligible intervention |
| Xu 2003 | Not eligible intervention |
| Xu 2013 | Not eligible intervention |
| Yamaguchi 2011 | Same drug intervention both arms and only different regimens of drug administration |
| Yan 2000 | Not eligible intervention |
| Yang 2001 | Not eligible intervention |
| Young 1988 | Not eligible intervention |
| Zamora 1999 | Same drug intervention both arms and only different timings of drug administration |
| Zaporozhan 2013 | Not eligible intervention |
| Zhao 1998 | Not eligible intervention |
| Zhao 2003 | Not eligible intervention |
| Zhou 1994 | Same drug intervention both arms and only different routes of drug administration |
PPH: postpartum haemorrhage
Characteristics of studies awaiting assessment [ordered by study ID]
Abdel‐Aleem 1997.
| Methods | Randomised trial |
| Participants | High‐risk women after vaginal delivery in Assiut, Egypt. |
| Interventions | Carboprost 250 mcg IM versus methylergonovine maleate 0.4 mg IV versus oxytocin 10 IU IV |
| Outcomes | Blood loss |
| Notes | Abstract only and awaiting reply from authors for additional information or full text |
Alli 2013.
| Methods | Randomised double‐blinded trial. |
| Participants | Women undergoing caesareans. |
| Interventions | Sublingual misoprostol 600 mcg or 10 IU bolus intravenous oxytocin |
| Outcomes | Blood loss, need for additional uterotonics, and side effects. |
| Notes | Abstract only and unable to contact authors for additional information or full text |
Amornpetchakul 2017.
| Methods | Randomised controlled trial |
| Participants | Women undergoing vaginal delivery in high‐risk singleton pregnancies. |
| Interventions | 5 IU of oxytocin or 100 mcg of carbetocin intravenously. |
| Outcomes | Blood loss, PPH, additional uterotonics. |
| Notes | Abstract only and awaiting reply from authors for additional information or full text |
Beigi 2009.
| Methods | Randomised trial. |
| Participants | 542 nulliparous pregnant women |
| Interventions | 20 IU of oxytocin administered intravenously or 400 mcg of misoprostol administered sublingually |
| Outcomes | PPH (not defined), third‐stage duration (minutes), headache, shivering. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Written in Persian and awaiting translation. |
Muller 1996.
| Methods | Randomised trial |
| Participants | Women with singleton pregnancies in hospital setting |
| Interventions | Oxytocin 5 IU IV versus no treatment |
| Outcomes | Change in Hb level |
| Notes | Abstract only and unable to contact authors for additional information or full text |
Norchi 1988.
| Methods | Controlled clinical trial |
| Participants | No details available |
| Interventions | Sulprostone versus methylergometrine |
| Outcomes | No details available |
| Notes | Abstract only and unable to contact authors for additional information or full text |
Rabow 2017.
| Methods | Randomised trial |
| Participants | Healthy, singleton pregnant women undergoing elective caesarean section in spinal anaesthesia |
| Interventions | Carbetocin 100 mcg IV versus oxytocin 5 IU IV |
| Outcomes | Cardiovascular parameters and need for additional uterotonics |
| Notes | Abstract only and awaiting reply from authors for additional information or full text |
Roy 2017.
| Methods | Randomised trial |
| Participants | Women in the third stage of labour |
| Interventions | Misoprostol 400 mcg PO versus oxytocin 10 IU IM |
| Outcomes | Blood loss, postpartum Hb and side effects |
| Notes | Abstract only and unable to contact authors for additional information or full text |
Said 2017.
| Methods | Randomised trial |
| Participants | Women undergoing elective caesareans |
| Interventions | Misoprostol 600 mcg PR versus oxytocin unspecified dose IV |
| Outcomes | Blood loss and postpartum Hb |
| Notes | Abstract only and unable to contact authors for additional information or full text |
Shrivasatava 2012.
| Methods | Randomised trial. |
| Participants | Not known how many women randomised |
| Interventions | 200 mcg of methylergometrine of unknown route or 400 mcg of misoprostol administered sublingually. |
| Outcomes | PPH (not defined), additional uterotonics, change in Hb level, third‐stage duration (minutes), blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
Sunil 2016.
| Methods | Randomised trial. |
| Participants | Women at term with spontaneous onset of labour |
| Interventions | Oxytocin 10 IU IM versus carboprost tromethamine 125 mcg IM |
| Outcomes | Blood loss, PPH, diarrhoea |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
Hb: haemoglobin; IM: intramuscular; IU: international unit;IV: intravascular; mcg: microgram; mL:: millilitre; PO: by mouth; PPH: postpartum haemorrhage; PR: rectally.
Characteristics of ongoing studies [ordered by study ID]
Balki 2017.
| Trial name or title | Carbetocin versus oxytocin at elective cesarean section: a double‐blind, randomized controlled non‐inferiority trial of high and low dose regimens |
| Methods | Randomised double‐blinded |
| Participants | Women undergoing elective caesareans. |
| Interventions | Carbetocin 20 mcg IV versus carbetocin 100 mcg IV versus oxytocin 0.5 IU IV versus oxytocin 5 IU IV |
| Outcomes | Additional uterotonics, side effects |
| Starting date | May 25, 2017 |
| Contact information | Mrinalini Balki, Samuel Lunenfeld Research Institute, Mount Sinai Hospital |
| Notes | Recruiting |
Draycott 2014.
| Trial name or title | Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and syntometrine for the third stage of labour following vaginal birth (IMox) |
| Methods | Randomised trial |
| Participants | Women delivering vaginally, singleton pregnancy |
| Interventions | 1 dose of 100 mcg intramuscular carbetocin given for active management of the third stage of labour, immediately after the birth of the baby. 1 dose of 10 IU intramuscular syntocinon given for active management of the third stage of labour, immediately after the birth of the baby. 1 dose of 500 mcg/5 IU intramuscular Ssyntometrine® given for active management of the third stage of labour, immediately after the birth of the baby. |
| Outcomes | Use of additional uterotonic agents |
| Starting date | February 2015 |
| Contact information | Tim Draycott, North Bristol NHS Trust/University of Bristol |
| Notes | Study Chair: |
Gomez 2011.
| Trial name or title | Efficiency of carbetocin in the prevention of the postpartum haemorrhage: a clinical double‐blinded randomised study |
| Methods | Open‐label randomised trial. |
| Participants | Women undergoing a vaginal birth at home with a trained study provider. |
| Interventions | 600 mcg of misoprostol administered orally or 10 IU of oxytocin administered intramuscularly. |
| Outcomes | PPH at 1000, additional uterotonics, transfusion, nausea, headache, abdominal pain. |
| Starting date | 15/07/2010 |
| Contact information | Milton Cesar Gomez Gomez |
| Notes | This study is shown as not yet recruiting. |
Goudar 2016.
| Trial name or title | Room temperature stable carbetocin for preventing blood loss after delivery |
| Methods | Randomised, parallel group, active controlled trial |
| Participants | Pregnant women and women who have had a vaginal birth |
| Interventions | Carbetocin RTS 100 mcg IM versus oxytocin 10 IU IM |
| Outcomes | Primary: post‐delivery (48‐72 hours) Hb level adjusted for pre‐delivery haemoglobin. Secondary: 1 Blood loss of 500 mL or more within 1 hour 2 Blood loss of 1000 mL or more within 1 hour 3 Additional uterotonics 4 Blood transfusion 5 Manual removal of placenta 6 Additional surgical procedures 7 Maternal death 8 Composite outcome of maternal death or severe morbidity up to time of discharge. 9 Incidence and severity of adverse or serious adverse events up to time of discharge. |
| Starting date | 01/07/2016 |
| Contact information | Dr Shivaprasad S Goudar Womens and Childrens Health Research Unit KLE Universitys J N Medical College Nehru Nagar Belgaum KARNATAKA 590010 India |
| Notes | Completed but results not available to date |
Kalahroudi 2010a.
| Trial name or title | Comparison of the effect of rectal misoprostol and syntometrin in prevention of postpartum hemorrhage |
| Methods | Double‐blinded randomised trial. |
| Participants | 200 women with a singleton pregnancy undergoing a vaginal birth. |
| Interventions | 500 mcg of ergometrine plus 5 IU of oxytocin administered intramuscularly or 600 mcg of misoprostol administered rectally. |
| Outcomes | Additional uterotonics, change in Hb level. |
| Starting date | 21/4/2010 |
| Contact information | Dr Mansoureh Samimi |
| Notes | This study is shown as recruitment complete. |
Kalahroudi 2010b.
| Trial name or title | Comparison effect of carbetocine and syntometrin in prevention of postpartum hemorrhage |
| Methods | Double‐blinded randomised trial. |
| Participants | 200 women with a singleton pregnancy undergoing a vaginal birth. |
| Interventions | 500 mcg of ergometrine plus 5 IU of oxytocin administered intramuscularly or 100 mcg of carbetocin administered intramuscularly. |
| Outcomes | Additional uterotonics, change in Hb level. |
| Starting date | 21/1/2010 |
| Contact information | Dr Mansoureh Samimi |
| Notes | This study is shown as recruitment complete. |
Maged 2018.
| Trial name or title | Carbetocin versus rectal misoprostol for management of third stage of labor in women at low risk of postpartum hemorrhage |
| Methods | Interventional (clinical trial) |
| Participants | Women admitted for spontaneous, induced or augmented vaginal delivery and categorized as low risk for PPH |
| Interventions | Carbetocin 100 mcg IV versus misoprostol 800 mcg PR |
| Outcomes | Prevention of PPH after vaginal delivery and side effects |
| Starting date | July 2, 2017 |
| Contact information | Ahmed Maged, Cairo University |
| Notes | Completed but no results posted |
Moradi 2010.
| Trial name or title | Comparison of misoprostol and oxytocin in reduction of postpartum hemorrhage |
| Methods | Randomised trial. |
| Participants | 300 women with singleton, term pregnancies. |
| Interventions | 10 IU of oxytocin administered intravenously or 400 mcg of misoprostol administered orally. |
| Outcomes | Change in Hb. |
| Starting date | 22/12/2009 |
| Contact information | Simindokht Moradi |
| Notes | This study is shown as recruitment complete. |
Sweed 2014.
| Trial name or title | Comparison between rectal and sublingual misoprostol before caesarian section to reduce intra and post‐operative blood loss |
| Methods | Placebo‐controlled randomised trial. |
| Participants | 635 women undergoing elective caesarean with a singleton term pregnancy and only 1 previous caesarean. |
| Interventions | 400 mcg of misoprostol administered rectally or 400 mcg of misoprostol administered sublingually or placebo. |
| Outcomes | Change in Hb level, blood loss. |
| Starting date | February 2013 |
| Contact information | Mohamed S Sweed |
| Notes | Completed but no report available. |
Thakur 2015.
| Trial name or title | A clinical trial to compare the effects of 4 drugs, oxytocin, misoprostol, 15‐methylprostaglandinF2alpha and methylergometrine in active management of third stage of labor. |
| Methods | Randomised, parallel group, multiple‐arm trial |
| Participants | All non high‐risk women at term pregnancy (37 to 40 weeks of gestation) who delivered vaginally |
| Interventions | Oxytocin 10 IU IM versus misoprostol 600 mcg PR versus 15‐methyl prostaglandin F2alpha 125 mcg IM versus methylergometrine 200 mcg IM |
| Outcomes | Blood loss, PPH, blood transfusion, need for additional uterotonics, side effects |
| Starting date | 01/01/2013 |
| Contact information | Dr Priyanka Thakur |
| Notes | Completed but no report available |
Hb: haemoglobin; IM: intramuscular; IU: international unit;IV: intravenous; mcg: microgram; PPH: postpartum haemorrhage; PR: rectally; RTS: room temperature stable
Differences between protocol and review
There are some differences between the published protocol for this review (Gallos 2015) and the full review, these are listed below.
Objectives
We have clarified the objectives of this review.
In our protocol the stated objectives were: "We aim to assess the clinical effectiveness and side‐effect profile of uterotonic agents to prevent PPH, and to generate a clinically useful ranking of available uterotonics according to their effectiveness and side effects. We will explore the effects according to various key prognostic and treatment factors. The population of interest is women following a vaginal birth or a caesarean section in the hospital or the community setting. All uterotonic agents considered by the WHO are eligible and the outcomes include blood loss‐related outcomes and side effects."
In the review, our objectives are listed as:
To identify the most effective uterotonic agent(s) to prevent PPH with the least side effects, and generate a ranking according to their effectiveness and side‐effect profile.
Methods/types of interventions
The text in this section has been edited to add sensitivity analyses that became necessary during the review and explain how we grouped the agents for analysis.
In the protocol, this section stated:
"We will consider trials of uterotonics described by WHO (WHO 2012) (oxytocin, ergometrine, misoprostol, carbetocin, or combinations of uterotonics) administered prophylactically by healthcare professionals for preventing PPH via any systemic route (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion) compared with another uterotonic or with placebo or no treatment. If we identify in the included studies interventions that we are not aware of, we will consider them as eligible and include them in the network after assessing their comparability with those named above. We will include trials in which non‐pharmacologic co‐interventions such as controlled cord traction, cord clamping, or uterine massage was performed as a randomised intervention in all arms of the trial. We will stratify all agents according to mode of birth, prior risk of PPH, healthcare setting, specific dosage, regimen and route, to detect inequalities in subgroups that could affect comparative effectiveness."
Figure 1 (in the published protocol) shows the overall network of eligible comparisons in the review at the agent level.
"Multi‐arm trials that compare different dosages, regimens or routes of one uterotonic agent, but also compare those versus another uterotonic agent, will be included. Intervention arms of different dosages, regimens or routes of the same uterotonic agent will be merged together for the global analysis of all outcomes and treated as separate independent comparisons only for the relevant subgroup analysis according to dosage, regimen and route of administration, while taking into account the correlation between the comparisons. We will exclude trials comparing exclusively different dosages, regimens or routes of administration of the same uterotonic agent. The review will be restricted to studies evaluating uterotonic agents administered systemically at the birth of the baby for preventing PPH. Studies considering non‐uterotonic agents, uterotonic agents administered locally (for example, via intraumbilical or intrauterine routes) or at a later stage of delivery (for example, for the treatment of PPH or for retained placenta) will be excluded."
In our review this section now states:
Trials were eligible if they administered uterotonic agents of any dosage, route or regimen systemically at birth for preventing postpartum haemorrhage (PPH), and compared them with other uterotonic agents, placebo or no treatment. Trials evaluating uterotonic agents not administered systemically, such as intrauterine administration, or not immediately after birth, or exclusively comparing different dosages, routes or regimens of the same uterotonic agent were excluded. We included trials in which non‐pharmacologic co‐interventions such as controlled cord traction, cord clamping, or uterine massage was performed as a randomised intervention in all arms of the trial and the effects of such co‐interventions were tested through a sensitivity analysis.
We classified agents into single agents including oxytocin, carbetocin, injectable prostaglandins (carboprost tromethamine, sulprostone), misoprostol, ergometrine (included also ergonovine, methylergonovine), and combination agents including ergometrine plus oxytocin (Syntometrine ® as a fixed‐combination agent containing 5 international units (IU) of oxytocin and 500 mcg of ergometrine, any oxytocin dose and route when combined with any dose and route of ergometrine, ergonovine, or methylergonovine), and misoprostol plus oxytocin (any oxytocin dose and route when combined with any dose and route of misoprostol).
Methods/search methods
The search methods have been updated in line with the current standard search methods text of Cochrane Pregnancy and Childbirth.
Methods/types of outcomes/secondary outcomes
We have edited our outcome list based on the core outcome set for prevention of PPH. We have edited the composite outcome 'maternal deaths or severe morbidity events' and split that into 'severe maternal morbidity: 'intensive care admissions' and 'severe maternal morbidity: shock (as defined by the trialists)'. We have removed the outcomes 'manual removal of placenta'; 'mean durations of the third stage of labour (minutes)'; 'neonatal unit admission requirement'; 'tachycardia'; and 'hypotension'. We have added outcomes 'diarrhoea'; 'maternal sense of well‐being (as defined by the trialists)'; 'maternal satisfaction (as defined by the trialists)'.
Assessment of reporting biases
We have edited the assessment of reporting biases. In the protocol, these sections stated:
We assessed potential reporting bias for the primary outcomes by assessing the sensitivity of results to exclusion of studies with fewer than 400 participants.
In our review these sections now state:
If there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. The funnel plots were assessed visually for asymmetry. We also assessed potential reporting bias for the primary outcomes by assessing the sensitivity of results to exclusion of studies with fewer than 400 participants.
Methods/investigation of heterogeneity and inconsistency and also subgroup analysis
We have edited the intervention subgroups for exploring heterogeneity and inconsistency and also subgroup analysis. In the protocol, these sections stated:
Intervention: dose, regimen or route.
We assessed subgroup differences by firstly comparing the network diagram for each subgroup. Next, we performed a network meta‐analysis for each subgroup and we compared their relative treatment effects and their relative treatment ranking.
In our review these sections now state:
Intervention: Dose of misoprostol (≥ 600 mcg versus < 600 mcg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
We assessed subgroup differences by firstly comparing the network diagram for each subgroup. Next, we performed a pairwise and network meta‐analysis for each subgroup and we compared their relative treatment effects and their relative treatment ranking. We examined the subgroups for qualitative interactions where the direction of effect could be reversed, that is if an intervention was beneficial in one subgroup but harmful in another.
Methods/investigation of heterogeneity and inconsistency and also subgroup analysis
We have carried out additional sensitivity analyses that became necessary during the conduct of the review. These are listed below.
Trials that also randomised participants to co‐interventions such as uterine massage or controlled cord traction.
Trials with more than 10% missing data.
Trials published before 1990.
Analysis
Since publication of the protocol for this review, further methods became available to perform the analysis with a frequentist approach in STATA. We changed our analysis for this reason to STATA rather than WinBUGS and a Bayesian environment.
'Summary of findings' table
We have modified our approach to assessing confidence in the evidence generated by this network meta‐analysis, in line with recent guidance published by the GRADE working group (see Brignardello‐Petersen 2018; Puhan 2014). This was not planned at the protocol stage, because we were not aware then of the most up‐to‐date guidance.
Contributions of authors
Ioannis D Gallos (IDG) and Arri Coomarasamy (AC) conceived the idea for this study. IDG, Malcolm J Price (MJP), Aurelio Tobias (AT), Olufemi T Oladapo (OTO) and AC designed the meta‐analysis. IDG designed all electronic data collection forms. IDG, Argyro Papadopoulou (AP), Rebecca Man (RM), Nikos Athanasopoulos (NA) screened trials and extracted data. MJP and AT performed the statistical analysis. Myfanwy Williams (MJW), Virginia Diaz (VD), Julia Pasquale (JP), Monica Chamillard (MC), Josh Vogel (JPV) and OTO graded the evidence and created the 'summary of findings' tables. IDG drafted the protocol and all versions of the review. AP, RM, NA, MP, AT, MP, OT, MJW, VD, JP, MC, Mariana Widmer (MW), Özge Tuncalp (OT), G Justus Hofmeyr (GJH), Fernando Althabe (FA), A Metin Gulmezoglu (AMG), JPV, OTO and AC edited and revised the review.
Sources of support
Internal sources
-
University of Birmingham, UK.
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
-
World Health Organization, Switzerland.
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
-
University of the Witwatersrand, South Africa.
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
External sources
-
National Institute for Health Research, UK.
This review is undertaken as part of independent research funded by the UK National Institute for Health Research (Health Technology Assessment Project 14/139/17 Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis).
-
Birmingham Women’s NHS Foundation Trust, UK.
Additional financial support to meet the employment costs of Helen M Willliams (HMW) and Abi Merriel (AM) is provided by Birmingham Women’s NHS Foundation Trust.
-
Ammalife, UK.
Additional financial support to meet the employment costs of Abi Merriel (AM) is provided by Ammalife (UK Registered Charity 1120236).
Declarations of interest
Ioannis D Gallos (IDG): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis. He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that were eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis supplied carbetocin and oxytocin for these studies. IDG did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review (and will not for future updates) – these tasks were carried out by other members of the team who were not directly involved in the trials.
Argyro Papadopoulou (AP): none known.
Rebecca Man (RM): none known.
Nikolaos Athanasopoulos (NA): none known.
Malcolm J Price (MJP): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis.
Aurelio Tobias: none known.
Myfanwy Williams (MJW): is employed by the University of Liverpool as a Research Associate at Cochrane Pregnancy and Childbirth. Her role is supported by the World Health Organization.
Virginia Diaz (VD): none known.
Julia Pasquale (JP): none known.
Monica Chamillard (MC): none known.
Mariana Widmer (MW): has been involved in a trial related to the use of uterotonics for the prevention of PPH that is included in this review. Ferring Pharmaceuticals and Novartis supplied carbetocin and oxytocin for the trial and the study is supported by WHO/Merck for Mothers. MW did not participate in any decisions regarding this trial (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates – these tasks were carried out by other members of the team who were not directly involved in the trial.
Özge Tunçalp (OT): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis.
G Justus Hofmeyr (GJH): has been and continues to be involved in a number of studies that may be eligible for inclusion in this review, but has not been (and will not participate in) data extraction or quality assessment of the studies in which he was involved. GJH is a co‐investigator on the UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis. Neither he nor his institution receives funding from this grant.
Fernando Althabe: none known.
A Metin Gulmezoglu (AMG): was part of the central coordination unit of the large World Health Organization multicentre trial comparing carbetocin with oxytocin included in the review. He is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis.
Joshua Vogel (JPV): led the updating of WHO recommendations on uterotonics for the prevention of postpartum haemorrhage based on this review.
Olufemi T Oladapo (OTO): led the updating of WHO recommendations on uterotonics for the prevention of postpartum haemorrhage based on the findings of this review update.
Arri Coomarasamy (AC): is the Chief Investigator of UK National Institute for Health Research HTA Project Award 14/139/17 entitled Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis. He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that were eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis supplied carbetocin and oxytocin for these studies and another study is supported by WHO/Merck for Mothers. AC did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates – these tasks have been carried out by other members of the team who were not directly involved in the trials. AC is a member of the Executive Board of Ammalife (UK registered charity 1120236). He does not receive any payment for this relationship.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdel‐Aleem 1993 {published data only}
- Abdel‐Aleem H, Abol‐Oyoun EM, Moustafa SA, Kamel HS, Abdel‐Wahab HA. Carboprost trometamol in the management of the third stage of labor. International Journal of Gynecology & Obstetrics 1993;42:247‐50. [DOI] [PubMed] [Google Scholar]
Abdel‐Aleem 2010 {published data only}
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32‐6. [DOI] [PubMed] [Google Scholar]
Acharya 2001 {published data only}
- Acharya G, Al‐Sammarai MT, Patel N, Al‐Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra‐operative blood loss during cesarean section. Acta Obstetricia et Gynecologica Scandinavica 2001;80(3):245‐50. [DOI] [PubMed] [Google Scholar]
Adanikin 2012 {published data only}
- Adanikin AI, Orji EO, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage post‐caesarean section. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S825. [Google Scholar]
- Adanikin AI, Orji EO, Fasubaa OB, Onwudiegwu U, Ijarotimi OA, Olaniyan O. The effect of post‐cesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(2):159‐62. [DOI] [PubMed] [Google Scholar]
- Orji EO, Adanikin AI. Prospective randomised double blind study on the effect of post‐caesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S446‐S447. [DOI] [PubMed] [Google Scholar]
- Orji EO, Adanikin AO. The effect of post‐caesarean rectal misoprostol on intestinal motility. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):21. [DOI] [PubMed] [Google Scholar]
Adanikin 2013 {published data only}
- Adanikin AI, Orji E, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage after caesarean section. Nepal Journal of Obstetrics and Gynaecology 2013;8(2):34‐7. [Google Scholar]
Afolabi 2010 {published data only}
- Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Medical Journal 2010;51(3):207‐11. [PubMed] [Google Scholar]
Ahmed 2014 {published data only}
- Ahmed WA, Ibrahim ZM, Mostafa I, Kishk EA, Elbahie MA. Safety and efficacy of carbetocin in hypertensive pregnant women undergoing cesarean delivery. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):49. [Google Scholar]
Al‐Sawaf 2013 {published data only}
- Al‐Sawaf A, El‐Mazny A, Shohayeb A. A randomised controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2013;33(3):277‐9. [DOI] [PubMed] [Google Scholar]
Alwani 2014 {published data only}
- Alwani M, Singh S, Thakur R, Mishra S. A randomized study comparing rectally administered misoprostol after spinal anesthesia versus intramuscular oxytocin for prevention of postpartum hemorrhage in caesarean section. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2014;3(3):512‐5. [Google Scholar]
Amant 1999 {published data only}
- Amant F, Spitz B, Timmerman D, Corresmans A, Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double‐blind randomised trial. British Journal of Obstetrics and Gynaecology 1999;106:1066‐70. [DOI] [PubMed] [Google Scholar]
Amin 2014 {published data only}
- Amin N. Prophylactic use of misoprostol in management of third stage of labour and prevention of atonic uterus. Journal of Postgraduate Medical Institute 2014;28(2):196‐200. [Google Scholar]
Askar 2011 {published data only}
- Askar AA, Ismail MT, El‐Ezz AA, Rabie NH. Carbetocin versus syntometrine in the management of third stage of labor following vaginal delivery. Archives of Gynecology and Obstetrics 2011;284(6):1359‐65. [DOI] [PubMed] [Google Scholar]
Asmat 2017 {published data only}
- Asmat R, Ashraf T, Asmat F, Asmat S, Asmat N. Effectiveness of per rectal misoprostol versus intramuscular oxytocin for prevention of primary postpartum haemorrhage. Journal of the College of Physicians and Surgeons Pakistan 2017;27(1):13‐7. [PubMed] [Google Scholar]
Attilakos 2010 {published data only}
- Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Can a new oxytocin analogue reduce the need for additional oxytocics after caesarean section? The results of a double‐blind randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa51. [Google Scholar]
- Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double‐blind randomised trial. BJOG: an international journal of obstetrics and gynaecology 2010;117(8):929‐36. [DOI] [PubMed] [Google Scholar]
- Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. The haemodynamic effects of oxytocin and carbetocin following caesarean section: the results of a double‐blind randomised study. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):140‐1. [DOI] [PubMed] [Google Scholar]
- Attilakos G, Psaroudakis D, Ash J, Buchanen R, Winter C, Draycott T. Low recruitment rate for a drug trial in obstetrics: an effect of the publicity following the TGN1412 clinical trial at the PAREXEL Research Unit in Northwick Park Hospital? [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4‐6; London, UK. 2007:110.
Atukunda 2014 {published data only}
- Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of post‐partum haemorrhage in Uganda: a randomised, controlled, non‐inferiority trial. Lancet 2014;384(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double‐blind randomized non‐inferiority trial. PLOS Medicine 2014;11(11):e1001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badejoko 2012 {published data only}
- Badejoko OO, Ijarotimi AO, Awowole IO, Loto OM, Badejoko BO, Olaiya DS, et al. Adjunctive rectal misoprostol versus oxytocin infusion for prevention of postpartum hemorrhage in women at risk: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2012;38(11):1294‐301. [DOI] [PubMed] [Google Scholar]
Balki 2008 {published data only}
- Balki M, Dhumne S, Kasodekar S, Kingdom J, Windrim R, Carvalho JC. Oxytocin‐ergometrine co‐administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG: an international journal of obstetrics and gynaecology 2008;115(5):579‐84. [DOI] [PubMed] [Google Scholar]
Bamigboye 1998a {published data only}
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum haemorrhage: a placebo controlled trial. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:49‐52. [DOI] [PubMed]
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1998;179(4):1043‐6. [DOI] [PubMed] [Google Scholar]
Bamigboye 1998b {published data only}
- Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. Randomized comparison of rectal misoprostol with syntometrine for management of third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 1998;77:178‐81. [PubMed] [Google Scholar]
Barton 1996 {published data only}
- Barton SR, Jackson A. The safety and efficiency of carbetocin to control uterine bleeding following caesarean section. Prenatal and Neonatal Medicine 1996;1(Suppl 1):185. [Google Scholar]
Baskett 2007 {published data only}
- Baskett TF, Persad V, Clough H, Young D. Prophylactic use of misoprostol in the third stage of labor [abstract]. Obstetrics & Gynecology 2005;105(4 Suppl):39S. [Google Scholar]
- Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. International Journal of Gynecology & Obstetrics 2007;97(1):2‐5. [DOI] [PubMed] [Google Scholar]
- Chandra S, Persad V, Young D, Baskett T. A preliminary study of cutaneous blood flow associated with postpartum use of oral misoprostol. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(12):1073‐6. [DOI] [PubMed] [Google Scholar]
Begley 1990 {published data only}
- Begley CM. Comparative studies in the third stage of labour [MSc thesis]. Dublin: Trinity College, University of Dublin, 1990. [Google Scholar]
- Begley CM. A comparison of 'active' and 'physiological' management of the third stage of labour. Midwifery 1990;6:3‐17. [DOI] [PubMed] [Google Scholar]
- Begley CM. The effect of ergometrine on breast feeding. Midwifery 1990;6:60‐72. [DOI] [PubMed] [Google Scholar]
Begum 2015 {published data only}
- Begum T, Yeasmin S, Chakma S. Sublingual misoprostol versus oxitocin infusion to reduce blood loss in caesarean section. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):258. [Google Scholar]
Bellad 2012 {published data only}
- Bellad M, Ganachari TD, Mallapur M. Sublingual (SL) powdered misoprostol (400 mcg) vs IM oxytocin (10 IU) for prevention of postpartum blood loss ‐ a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S124‐5. [Google Scholar]
- Bellad MB, Tara D, Ganachari MS, Mallapur MD, Goudar SS, Kodkany BS, et al. Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double‐blind randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2012;119(8):975‐86. [DOI] [PubMed] [Google Scholar]
Benchimol 2001 {published data only}
- Benchimol M, Gondry J, Mention JE, Gagneur O, Boulanger JC. Role of misoprostol in the delivery outcome. [French] [Place du misoprostol dans la direction de la delivrance.]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(6):576‐83. [PubMed] [Google Scholar]
Bhatti 2014 {published data only}
- Bhatti K, Mahar T, Hafeez R, Shoaib‐u‐Nisa. A randomized controlled trial on prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin. Medical Forum Monthly 2014;25(1):10‐2. [Google Scholar]
Bhullar 2004 {published data only}
- Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstetrics & Gynecology 2004;104(6):1282‐8. [DOI] [PubMed] [Google Scholar]
Biswas 2007 {published data only}
- Biswas A, Bal R, Kundu MK, Kyal A, Halder M. A study of prophylactic use of 15‐methyl prostaglandin f2alpha in the active management of third stage of labour. Journal of the Indian Medical Association 2007;105(9):506‐9. [PubMed] [Google Scholar]
Borruto 2009 {published data only}
- Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum hemorrhage after cesarean section: a randomized clinical trial. Archives of Gynecology and Obstetrics 2009;280(5):707‐12. [DOI] [PubMed] [Google Scholar]
Boucher 1998 {published data only}
- Boucher M, Horbay GL, Griffin P, Deschamps Y, Desjardins C, Schulz M, et al. Double‐blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. Journal of Perinatology 1998;18(3):202‐7. [PubMed] [Google Scholar]
Boucher 2004 {published data only}
- Boucher M, Nimrod C, Tawagi G. Carbetocin IM injection vs oxytocin IV infusion for prevention of postpartum hemorrhage in women at risk following vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185(6 Pt 2):Abstract no: 494. [Google Scholar]
- Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery:a double‐blind randomized trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(5):481‐8. [DOI] [PubMed] [Google Scholar]
Bugalho 2001 {published data only}
- Bugalho A, Daniel A, Foundes A, Cunha M. Misoprostol for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2001;73:1‐6. [DOI] [PubMed] [Google Scholar]
Butwick 2010 {published data only}
- Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. A study of the minimum effective dose of oxytocin in patients undergoing elective cesarean delivery. American Society of Anaesthesiologists Annual Meeting; 2009 Oct 17‐21; New Orleans, USA. 2009.
- Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective caesarean delivery. British Journal of Anaesthesia 2010;104(3):338‐43. [DOI] [PubMed] [Google Scholar]
Caliskan 2002 {published data only}
- Caliskan E, Meydanli M, Dilbaz B, Aykan B, Sonmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2002;187:1038‐45. [DOI] [PubMed] [Google Scholar]
Caliskan 2003 {published data only}
- Caliskan E, Dilbaz B, Meydanli MM, Ozturk N, Narin MA, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2003;101(5 Pt 1):921‐8. [DOI] [PubMed] [Google Scholar]
Carbonell 2009 {published data only}
- Carbonell i Esteve JL, Hernandez JM, Piloto M, Setien SA, Texido CS, Tomasi G, et al. Active management of the third phase of labour plus 400 mug of sublingual misoprostol and 200 mug of rectal misoprostol versus active management only in the prevention of post‐partum haemorrhage. A randomised clinical trial [Manejo activo de la tercera fase del parto mas 400 mug de misoprostol sublingual y 200 mug de misoprostol rectal frente a manejo activo solo en la prevencion de la hemorragia posparto. Ensayo clinico aleatorizado]. Progresos de Obstetricia y Ginecologia 2009;52(10):543‐51. [Google Scholar]
Carrillo‐Gaucin 2016 {published data only}
- Carrillo‐Gaucin S, Torres‐Gomez LG. Carbetocin and oxytocin: prevention of postpartum hemorrhage in patients with risk factors for uterine atony [Carbetocina y oxitocina: prevención de hemorragia posparto en pacientes con factores de riesgo para atonía uterina]. Revista Médica del Instituto Mexicano del Seguro Social 2016;54 Suppl 3:S284‐90. [PubMed] [Google Scholar]
Cayan 2010 {published data only}
- Cayan F, Doruk A, Sungur MA, Dilek S. Comparison of the different dosages of rectal misoprostol on I=intestinal motility and pain score in high risk cesarean delivery. Turkiye Klinikleri Journal of Medical Sciences 2010;30(4):1154‐9. [Google Scholar]
Chalermpolprapa 2010 {published data only}
- Chalermpolprapa V. Efficacy of sublingual misoprostol in prevention of postpartum hemorrhage in cesarean section: A randomized double‐blinded, placebo‐controlled trial. Region 4‐5 Medical Journal 2010;29(3):325‐35. [Google Scholar]
Chandhiok 2006 {published data only}
- Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. International Journal of Gynecology & Obstetrics 2006;92(2):170‐5. [DOI] [PubMed] [Google Scholar]
Chaudhuri 2010 {published data only}
- Chaudhuri P, Banerjee GB, Mandal A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. International Journal of Gynecology & Obstetrics 2010;109(1):25‐9. [DOI] [PubMed] [Google Scholar]
Chaudhuri 2012 {published data only}
- Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low‐risk women. International Journal of Gynecology and Obstetrics 2012;116(2):138‐42. [DOI] [PubMed] [Google Scholar]
Chaudhuri 2015 {published data only}
- Chaudhuri P, Majumdar A. Sublingual misoprostol as an adjunct to oxytocin during cesarean delivery in women at risk of postpartum hemorrhage. International Journal of Gynaecology & Obstetrics 2015;128:48‐52. [DOI] [PubMed] [Google Scholar]
Chaudhuri 2016 {published data only}
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third‐stage management among women at risk of postpartum hemorrhage. International Journal of Gynecology and Obstetrics 2016;132:191‐5. [DOI] [PubMed] [Google Scholar]
Chhabra 2008 {published data only}
- Chhabra S, Tickoo C. Low‐dose sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 2008;34(5):820‐3. [DOI] [PubMed] [Google Scholar]
Choy 2002 {published data only}
- Choy CM, Lau WC, Tam WH, Yuen PM. A randomised controlled trial of intramuscular syntometrine and intravenous oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:173‐7. [DOI] [PubMed] [Google Scholar]
Chua 1995 {published data only}
- Chua S, Chew SL, Yeoh CL, Roy AC, Ho LM, Selamat N, et al. A randomized controlled study of prostaglandin 15‐Methyl F2 alpha compared with syntometrine for prophylactic use in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1995;35:413‐6. [DOI] [PubMed] [Google Scholar]
Cook 1999 {published data only}
- Cook CM, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or syntometrine in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(4):414‐9. [DOI] [PubMed] [Google Scholar]
Dabbaghi Gale 2012 {published data only}
- Dabbaghi Gale T, Elmizadeh KH, Moradi SD, Rashvand Melli E. Comparison of intravenous oxytocin and oral misoprostol in reduction of postpartum hemorrhage. Journal of Zanjan University of Medical Sciences and Health Services 2012;20(81):1‐8. [Google Scholar]
Dansereau 1999 {published data only}
- Dansereau J. Comparison of carbetocin vs. oxytocin in prevention of uterine atony post cesarean section. Prenatal and Neonatal Medicine 1996;1(Suppl 1):80. [Google Scholar]
- Dansereau J, Gambling D, Joshi A, Helewa M, Doran T, Lange I, et al. Double‐blind comparison of carbetocin vs oxytocin in preventing uterine atony post cesarean section. International Journal of Gynecology & Obstetrics 1994;46 Suppl:77. [Google Scholar]
- Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. American Journal of Obstetrics and Gynecology 1999;18(3 Pt 1):670‐6. [DOI] [PubMed] [Google Scholar]
- Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double‐blind comparison of carbetocin vs oxytocin in preventing uterine atony post cesarean section. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69:37. [DOI] [PubMed] [Google Scholar]
- Gambling D, Dansereau J, Schulz M, Horbay GL, Waasenaar W. Double‐blind, randomized comparison of a single dose of carbetocin vs 8 hours oxytocin infusion after cesarean delivery: safety data. A Canadian multi‐center trial [abstract]. International Journal of Obstetric Anesthesia 1994;3:113‐4. [Google Scholar]
- Gambling DR, Dansereau J, Wassenaar W, Schulz M, Horbay GLA. Double‐blind randomized comparison of a single dose of carbetocin versus 8 hours oxytocin infusion after cesarean delivery: safety data. Anesthesia & Analgesia 1994;78 Suppl:S127. [Google Scholar]
Dasuki 2002 {published data only}
- Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum hemorrhage [abstract]. Journal of Obstetrics and Gynaecology Research 2002;28(1):46. [Google Scholar]
de Groot 1996 {published data only}
- Groot AN, Roosmalen J, Dongen PWJ, Borm GF. A placebo‐controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1996;75:464‐8. [DOI] [PubMed] [Google Scholar]
Del Angel‐Garcia 2006 {published data only}
- Angel‐Garcia G, Garcia‐Contreras F, Constantino‐Casas P, Nevarez‐Sida A, Lopez‐Gonzalez N, Garcia‐Constantino M, et al. Economic evaluation of carbetocin for the prevention of uterine atony in patients with risk factors in Mexico. Value in Health 2006;9(6):A254. [Google Scholar]
Derman 2006 {published data only}
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource‐poor communities: a randomised controlled trial. Lancet 2006;368(9543):1248‐53. [DOI] [PubMed] [Google Scholar]
- Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, Bellad MB, et al. Factors associated with acute postpartum hemorrhage in low‐risk women delivering in rural India. International Journal of Gynecology & Obstetrics 2008;101(1):94‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, et al. Conducting international collaborative research in developing nations. International Journal of Gynecology & Obstetrics 2004;87(3):267‐71. [DOI] [PubMed] [Google Scholar]
- Goudar SS, Chakraborty H, Edlavitch SA, Naik VA, Bellad MB, Patted SS, et al. Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. Journal of Maternal‐Fetal & Neonatal Medicine 2008;21(8):559‐64. [DOI] [PubMed] [Google Scholar]
- Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al. Initiating a novel therapy in preventing postpartum hemorrhage in rural India: a joint collaboration between the United States and India. International Journal of Fertility & Womens Medicine 2004;49(2):91‐6. [PubMed] [Google Scholar]
- Kodkany BS, Goudar SS, Derman RJ. The efficacy of oral misoprostol in preventing postpartum hemorrhage in a community setting: a randomized double‐blind placebo‐controlled trial. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S141‐S142. [DOI] [PubMed] [Google Scholar]
- NCT00097123. RCT of misoprostol for postpartum hemorrhage in India. clinicaltrials.gov/ct2/show/NCT00097123 (first received 18 November 2004).
- Patted SS, Goudar SS, Naik VA, Bellad MB, Edlavitch SA, Kodkany BS, et al. Side effects of oral misoprostol for the prevention of postpartum hemorrhage: results of a community‐based randomised controlled trial in rural India. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(1):24‐8. [DOI] [PubMed] [Google Scholar]
Dhananjaya 2014 {published data only}
- Dhananjaya BS, Charishma S. Comparative study of efficacy and safety of intramuscular oxytocin with intramuscular methylergometrine in the active management of third stage of labour. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(3):734‐9. [Google Scholar]
Diallo 2017 {published data only}
- Diallo M, Sylla T, Diouf AA, Moreira PM, Gassama O, Gueye MD, et al. Active management of third stage of labour with low doses of oral misoprostol and oxytocin on low: risk parturient in a Sub‐Saharan hospital, Dakar, Sénégal. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2017;6(2):516‐22. [Google Scholar]
- Vlassoff M, Diallo A, Philbin J, Kost K, Bankole A. Cost‐effectiveness of two interventions for the prevention of postpartum hemorrhage in Senegal. International Journal of Gynecology and Obstetrics 2016;133(3):307‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diop 2016 {published data only}
- Diop A, Daff B, Sow M, Blum J, Diagne M, Sloan NL, et al. Oxytocin via Uniject (a prefilled single‐use injection) versus oral misoprostol for prevention of postpartum haemorrhage at the community level: a cluster‐randomised controlled trial. Lancet. Global Health 2016;4(1):e37‐44. [DOI] [PubMed] [Google Scholar]
- NCT01713153. Comparing misoprostol and oxytocin in uniject for postpartum hemorrhage (PPH) prevention in Senegal. clinicaltrials.gov/ct2/show/NCT01713153 (first received: 22 October 2012).
Docherty 1981 {published data only}
- Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60. [Google Scholar]
Dutta 2016 {published data only}
- Dutta BK, Gupta KR. A comparative study on rectal misoprostol versus intramuscular oxytocin to prevent postpartum haemorrhage. New Indian Journal of OBGYN 2016;2(2):98‐103. [Google Scholar]
Eftekhari 2009 {published data only}
- Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. Journal of Obstetrics and Gynaecology 2009;29(7):633‐6. [DOI] [PubMed] [Google Scholar]
El Behery 2015 {published data only}
Elbohoty 2016 {published data only}
- Elbohoty AE, Mohammed WE, Sweed M, Eldin AM, Nabhan A, Abd‐El‐Maeboud KH. Randomized controlled trial comparing carbetocin, misoprostol, and oxytocin for the prevention of postpartum hemorrhage following an elective cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;134(3):324‐8. [DOI] [PubMed] [Google Scholar]
Elgafor El Sharkwy 2013 {published data only}
- Elgafor El Sharkwy IA. Carbetocin versus sublingual misoprostol plus oxytocin infusion for prevention of postpartum hemorrhage at cesarean section in patients with risk factors: a randomized, open trail study. Archives of Gynecology and Obstetrics 2013;288(6):1231‐6. [DOI] [PubMed] [Google Scholar]
El‐Refaey 2000 {published data only}
- El‐Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, et al. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG: an international journal of obstetrics and gynaecology 2000;107:1104‐10. [DOI] [PubMed] [Google Scholar]
Elsedeek 2012 {published data only}
- Elsedeek MS. Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery. International Journal of Gynecology & Obstetrics 2012;118(2):149‐52. [DOI] [PubMed] [Google Scholar]
El Tahan 2012 {published data only}
- Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, et al. Effects of preoperative sublingual misoprostol on uterine tone during isoflurane anesthesia for cesarean section. Revista Brasileira de Anestesiologia 2012;62(5):625‐35. [DOI] [PubMed] [Google Scholar]
Enakpene 2007 {published data only}
- Enakpene CA, Morhason‐Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post‐partum hemorrhage during third stage of labor. Journal of Obstetrics and Gynaecology Research 2007;33(6):810‐7. [DOI] [PubMed] [Google Scholar]
Ezeama 2014 {published data only}
- Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, et al. A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. International Journal of Gynecology & Obstetrics 2014;124(1):67‐71. [DOI] [PubMed] [Google Scholar]
Fahmy 2015 {published data only}
- Fahmy AA, Fawzy M. Oxytocin infusion after oxytocin bolus and carbetocin bolus to reduce blood loss during and after cesarean section ‐ a randomized clinical trial. Medical Journal of Cairo University 2015;83(1):79‐83. [Google Scholar]
Fahmy 2016 {published data only}
- Fahmy NG, Yousef HM, Zaki HV. Comparative study between effect of carbetocin and oxytocin on isoflurane‐induced uterine hypotonia in twin pregnancy patients undergoing cesarean section. Egyptian Journal of Anaesthesia 2016;32(1):117‐21. [Google Scholar]
Fakour 2013 {published data only}
- Fakour F, Mirzayi M, Reza Naghipour M, Ebrahimi H, Mahdavi M. Comparison between sublingual misoprostol and intravenous oxytocin in management of third stage of labor. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;15(34):7‐14. [Google Scholar]
Fararjeh 2003 {published data only}
- Fararjeh C, Gezer A, Cepni I, Benian A, Ocal P, Kosebay D. The efficacy of misoprostol in preventing postpartum bleeding [Postpartum Kanama Profilaksisinde Rektal Mizoprostol Kulanımının Etkinliği.]. Jinekoloji ve Obstetrik Dergisi 2003;17(4):218‐23. [Google Scholar]
Fawole 2011 {published data only}
- Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al. A double‐blind, randomized, placebo‐controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2011;112(2):107‐11. [DOI] [PubMed] [Google Scholar]
Fawzy 2012 {published data only}
- Fawzy AE, Swelem M, Abdelrehim AI, Titeli S, Elghazal ZS, El‐Gahwagi MM, et al. Active management of third stage of labor by intravenous ergometrine and rectal versus sublingual misoprostol (a double‐center study). Alexandria Journal of Medicine 2012;48(4):381‐5. [Google Scholar]
Fazel 2013 {published data only}
- Fazel MR, Mansoure‐Samimi, Esmaeil‐Fakharian. A comparison of rectal misoprostol and intravenous oxytocin on hemorrhage and homeostatic changes during cesarean section. Middle East Journal of Anesthesiology 2013;22(1):41‐6. [PubMed] [Google Scholar]
Fekih 2009 {published data only}
- Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al. Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2009;38(7):588‐93. [DOI] [PubMed] [Google Scholar]
Fenix 2012 {published data only}
- Fenix AM. Double‐blind randomized controlled trial comparing the effect of carbetocin with oxytocin for the prevention of postpartum hemorrhage among high risk women following vaginal delivery. International Journal of Gynaecology & Obstetrics 2012;119(Suppl 3):S347‐S348. [Google Scholar]
Fu 2003 {published data only}
- Fu YX, Ran KQ, Wang M. Prevention of early postpartum hemorrhage by way of oral misoprostol. Journal of Nursing Science 2003;18(12):910‐1. [Google Scholar]
Fuks 2014 {published data only}
- Fuks AM, Khanna P, Yusaf T, Aslian A, Kowalska D, Salafia CM. Use of prophylactic misoprostol in reduction of blood loss at vaginal delivery. Obstetrics and Gynecology 2014;123(5 Suppl):144S‐5S. [Google Scholar]
Garg 2005 {published data only}
- Garg P, Batra S, Gandhi G. Oral misoprostol versus injectable methylergometrine in management of the third stage of labor. International Journal of Gynecology & Obstetrics 2005;91(2):160‐1. [DOI] [PubMed] [Google Scholar]
Gavilanes 2016 {published data only}
- Gavilanes P, Morales MF, Velasco S, Teran E. Sublingual misoprostol is as effective as intravenous oxytocin to reduce intra‐operative blood loss during cesarean delivery in women living at high altitude. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(4):559‐61. [DOI] [PubMed] [Google Scholar]
Gerstenfeld 2001 {published data only}
- Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185:878‐82. [DOI] [PubMed] [Google Scholar]
Gore 2017 {published data only}
- Gore S, Padmawar A, Pathan SK. A prospective randomized controlled trial for comparison of oral misoprostol with methyl ergometrine in the third stage of labour for prevention of post‐partum haemorrhage. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2017;6(7):2825‐8. [Google Scholar]
Gulmezoglu 2001 {published data only}
- Gulmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358:689‐95. [DOI] [PubMed] [Google Scholar]
- Lumbiganon P, Hofmeyr J, Gulmezoglu AM, Pinol A, Villar J. Misoprostol dose‐related shivering and pyrexia in the third stage of labour Who collaborative trial of misoprostol in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106(4):304‐8. [DOI] [PubMed] [Google Scholar]
- Lumbiganon P, Villar J, Piaggio G, Gulmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:1222‐6. [DOI] [PubMed] [Google Scholar]
Gupta 2006 {published data only}
- Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage ‐ a pilot study. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S139‐S140. [DOI] [PubMed] [Google Scholar]
Hamm 2005 {published data only}
- Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. American Journal of Obstetrics and Gynecology 2005;192:1404‐6. [DOI] [PubMed] [Google Scholar]
Harriott 2009 {published data only}
- Harriott J, Christie L, Wynter S, DaCosta V, Fletcher H, Reid M. A randomized comparison of rectal misoprostol with syntometrine on blood loss in the third stage of labour. West Indian Medical Journal 2009;58(3):201‐6. [PubMed] [Google Scholar]
Hernandez‐Castro 2016 {published data only}
- Hernandez‐Castro F, Lopez‐Serna N, Trevino‐Salinas EM, Soria‐Lopez JA, Sordia‐Hernandez LH, Cardenas‐Estrada E. Randomized double‐blind placebo‐controlled trial of buccal misoprostol to reduce the need for additional uterotonic drugs during cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;132(2):184‐7. [DOI] [PubMed] [Google Scholar]
- NCT01733329. Buccal misoprostol during cesarean section for preventing postpartum hemorrhage in women with risk factors for uterine atony. clinicaltrials.gov/ct2/show/NCT01733329 (first received 16 November 2012).
Hofmeyr 1998 {published data only}
- Hofmeyr GJ, Nikodem C, Jager M, Drakely A, Gilbart B. Oral misoprostol for labour third stage management: randomised assessment of side effects. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:53‐4.
- Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105(9):971‐5. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Jager M, Rose L, Nikodem VC, Lawrie T. Misoprostol for third stage of labour management: a double blind, placebo controlled clinical trial. Proceedings of the 16th Conference on Priorities in Perinatal Care; 1997; South Africa. 1997:29‐31.
Hofmeyr 2001 {published data only}
- Hofmeyr GJ, Nikodem VC, Jager M, Drakely AJ. Side effects of oral misoprostol in the third stage of labour: a random allocation placebo controlled trial. Journal of Obstetrics & Gynaecology 2000;20(Suppl 1):S40‐1. [Google Scholar]
- Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Side‐effects of oral misoprostol in the third stage of labour‐‐a randomised placebo‐controlled trial. South African Medical Journal 2001;91(5):432‐5. [PubMed] [Google Scholar]
Hofmeyr 2011 {published data only}
- Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, et al. Administration of 400mug of misoprostol to augment routine active management of the third stage of labor. International Journal of Gynecology and Obstetrics 2011;112(2):98‐102. [DOI] [PubMed] [Google Scholar]
- NCT00124540. Misoprostol for preventing postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT00124540 (first received: 26 July 2005).
Hoj 2005 {published data only}
- Hoj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea‐Bissau: randomised double blind clinical trial. BMJ 2005;331:723‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen BB, Hoj L, Hvidman LE, Nielsen J, Cardoso P, Aaby P. Reduced post‐partum bleeding after treatment with sublingual misoprostol: a randomized double‐blind clinical study in a developing country ‐ secondary publication [Reduceret post partum‐blodning efter sublingval misoprostol: et randomiseret dobbeltblindt klinisk studie i et udviklingsland ‐ sekundaerpublikation]. Ugeskrift for Laeger 2006;168(13):1341‐3. [PubMed] [Google Scholar]
Hong 2007 {published data only}
- Hong SC, Kim JW, Park HT, Seol HJ, Kim HJ, Kim SH, et al. Additional rectal misoprostol plus intravenous oxytocin versus intravenous oxytocin for the prevention of postpartum hemorrhage after cesarean section. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S99, Abstract no: 321. [Google Scholar]
Humera 2016 {published data only}
- Humera A, Kerure SB. Randomized comparative study of oral misoprostol with intravenous methyl ergometrine in prevention of postpartum haemorrhage in cases high risk for postpartum haemorrhage. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2016;5(3):798‐802. [Google Scholar]
Is 2012 {published data only}
- Is S, Gr V, Keranahalli S. Comparison of intramuscular ergometrine and per rectal misoprostol for prophylaxis against atonic post partum haemorrhage. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S797‐S798. [Google Scholar]
Jago 2007 {published data only}
- Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. Journal of Maternal‐Fetal & Neonatal Medicine 2007;20(9):703‐5. [DOI] [PubMed] [Google Scholar]
Jangsten 2011 {published data only}
- Jangsten E, Mattsson L, Lyckestam I, Hellstrom A, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:362–9. [DOI] [PubMed] [Google Scholar]
Jans 2017 {published data only}
- Jans S, Herschderfer KC, Diem MT, Aitink M, Rijnders M, Pal‐Bruin K, et al. LENTE study: effectiveness of prophylactic intramuscular oxytocin during third stage of labour amongst low risk women. A randomized controlled trial. 31st International Confederation of Midwives Triennial Congress. Midwives ‐ Making a Difference in the World; 2017 June 18‐22; Toronto, Canada. 2017:Abstract no: P1.063.
Jerbi 2007 {published data only}
- Jerbi M, Hidar S, Elmoueddeb S, Chaieb A, Khairi H. Oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2007;96(3):198‐9. [DOI] [PubMed] [Google Scholar]
Jirakulsawas 2000 {published data only}
- Jirakulsawas J, Khooarmompattana S. Comparison of oral misoprostol and intramuscular methylergonovine for prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 2000;12(4):332. [Google Scholar]
Kabir 2015 {published data only}
- Kabir N, Akter D, Daisy TA, Jesmin S, Razzak M, Tasnim S, et al. Efficacy and safety of carbetocin in comparison to oxytocin in the active management of third stage of labour following vaginal delivery: an open label randomized control trial. Bangladesh Journal of Obstetrics and Gynecology 2015;30(1):3‐9. [Google Scholar]
Karkanis 2002 {published data only}
- Karkanis SG, Caloia D, Salenieks ME, Kingdom J, Walker M, Meffe F, et al. Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. Journal of Obstetrics and Gynaecology Canada: JOGC 2002;24(2):149‐54. [DOI] [PubMed] [Google Scholar]
Kerekes 1979 {published data only}
- Kerekes L, Domokos N. The effect of prostaglandin F2alpha on third stage labour. Prostaglandins 1979;18:161‐6. [DOI] [PubMed] [Google Scholar]
Khan 1995 {published data only}
- Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin vs syntometrine in the active management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:147‐51. [DOI] [PubMed] [Google Scholar]
- Khan GQ, Susheela JI, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin versus syntometrine in the active management of the third stage of labour. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:Abstract no: 212. [DOI] [PubMed]
Khurshid 2010 {published data only}
- Khurshid R, Fatima K, Parveen S, Ul Shamas I, Salman R. A comparison between intramuscular PGF2 a125 mG and intravenous methyl ergometrine 0.2 Mg in the active management of third stage labor. Internet Journal of Gynecology and Obstetrics 2010; Vol. 14, issue 1.
Koen 2016 {published data only}
- Koen S, Snyman LC, Pattinson RC, Makin JA. A randomised controlled trial comparing oxytocin and oxytocin + ergometrine for prevention of postpartum haemorrhage at caesarean section. South African Medical Journal = Suid‐Afrikaanse Tydskrif Vir Geneeskunde 2016;106(4):399‐402. [DOI] [PubMed] [Google Scholar]
Kumar 2016 {published data only}
- Kumar SK, Shyam S, Batakurki P. Carboprost versus oxytocin for active management of third stage of labor: a prospective randomized control study. Journal of Obstetrics and Gynecology of India 2016;66(S1):S229‐S234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumru 2005 {published data only}
- Kumru S, Gurates B, Parmaksiz C. Investigation of the usefulness of methyl ergonovine application in cesarean section cases [Sezaryen olgularinda metil ergonovin uygulamasinin yararliliginin arastirilmasi]. Journal of the Turkish German Gynecology Association Artemis 2005;6(1):42‐5. [Google Scholar]
Kundodyiwa 2001 {published data only}
- Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2001;75:235‐41. [DOI] [PubMed] [Google Scholar]
Kushtagi 2006 {published data only}
- Kushtagi P, Verghese LM. Evaluation of two uterotonic medications for the management of the third stage of labor. International Journal of Gynecology & Obstetrics 2006;94(1):47‐8. [DOI] [PubMed] [Google Scholar]
- Verghese L, Kushtagi P. Evaluation of carboprost as a prophylactic oxytocic in the management of third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):74. [Google Scholar]
Lam 2004 {published data only}
- Lam H, Tang OS, Lee CP, Ho PC. A pilot‐randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:647‐50. [DOI] [PubMed] [Google Scholar]
Lamont 2001 {published data only}
- Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins & Other Lipid Mediators 2001;66(3):203‐10. [DOI] [PubMed] [Google Scholar]
Lapaire 2006 {published data only}
- Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. International Journal of Gynecology & Obstetrics 2006;95(1):2‐7. [DOI] [PubMed] [Google Scholar]
Leung 2006 {published data only}
- Leung SW, Ng PS, Wong WY, Cheung TH. A randomised trial of carbetocin versus syntometrine in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2006;113:1459‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lokugamage 2001 {published data only}
- Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durban primary postpartum haemorrhage study. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. RCOG, 1999:77‐8.
- Lokugamage AU, Paine M, Bassau‐Balroop H, El‐Refaey K, Sullivan K, Rodek C. Active management of the third stage at caesarean section: misoprostol vs syntocinon. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000; Vol. Book 2:54.
- Lokugamage AU, Paine M, Bassaw‐Balroop K, Sullivan KR, El‐Refaey H, Rodeck CH. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(4):411‐4. [DOI] [PubMed] [Google Scholar]
Lumbiganon 1999 {published data only}
- Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose‐related shivering and pyrexia in the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106:304‐8. [DOI] [PubMed] [Google Scholar]
Maged 2016 {published data only}
- Maged AM, Hassan AM, Shehata NA. Carbetocin versus oxytocin for prevention of postpartum hemorrhage after vaginal delivery in high risk women. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(4):532‐6. [DOI] [PubMed] [Google Scholar]
Maged 2017 {published data only}
- Maged AM, Ragab AS, Elnassery N, Al Mostafa W, Dahab S, Kotb A. Carbetocin versus syntometrine for prevention of postpartum hemorrhage after cesarean section. Journal of Maternal‐Fetal & Neonatal Medicine 2017;30(8):962‐6. [DOI] [PubMed] [Google Scholar]
Malik 2018 {published data only}
- Sabha M Cimona LS Fida M. A comparative study between prostaglandin 2ɑ and methyl ergometrine in the active management of third stage of labour. Modern Approaches in Drug Designing 2018;1(5):MADD.000522. [Google Scholar]
Mannaerts 2018 {published data only}
- Mannaerts D, Veeken L, Coppejans H, Jacquemyn Y. Adverse effects of carbetocin versus oxytocin in the prevention of postpartum haemorrhage after caesarean section: a randomized controlled trial. Journal of Pregnancy 2018;2018:1374150. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 1993 {published data only}
- McDonald S. The Perth third stage oxytocic trial. Proceedings of National Conference on Research in Midwifery; 1992; Reading, UK. 1992.
- McDonald S, Prendiville WJ. A randomized controlled trial of syntocinon vs syntometrine as part of the active management of the third stage of labour. Journal of Perinatal Medicine 1992;20(Suppl 1):97. [Google Scholar]
- McDonald S, Prendiville WJ, Blair E. A randomised controlled trial of syntocinon vs syntometrine as part of the active management of the third stage of labour. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:87.
- McDonald SJ. Management in the Third Stage of Labour [thesis]. University of Western Australia, 1996. [Google Scholar]
- McDonald SJ, Prendiville WJ, Blair E. Randomised controlled trial of oxytocin alone vs oxytocin and ergometrine in active management of third stage of labour. BMJ 1993;307:1167‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1993 {published data only}
- Mitchell GG, Elbourne DR. The Salford third stage trial: oxytocin plus ergometrine vs oxytocin alone in the active management of the third stage of labor. Online Journal of Current Clinical Trials 1993;2:Doc 83. [PubMed] [Google Scholar]
Mobeen 2011 {published data only}
- Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Use of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage during home deliveries in Pakistan: a randomised placebo‐controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00120237. Misoprostol for the prevention of postpartum hemorrhage in rural Pakistan. clinicaltrials.gov/ct2/show/NCT00120237 (first received: 7 July 2005).
Modi 2014 {published data only}
- Modi V, Goel JK, Kashyap A, Arya SB, Kar J, Goel R. Active management of third stage of labor: A comparison of various uterotonic. Journal of South Asian Federation of Obstetrics and Gynaecology ‐ SAFOG 2014;6(3):151‐5. [Google Scholar]
Moertl 2011 {published data only}
- Moertl M, Kraschl J, Friedrich S, Pickel K, Ulrich D, Eder M, et al. Hemodynamic changes of carbetocin and oxytocin in women undergoing cesarean section. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S112. [Google Scholar]
- Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2011;118(11):1349‐56. [DOI] [PubMed] [Google Scholar]
- Mortl M, Pickel K, Friedrich S, Ulrich D, Lang U, Schlembach D. Hemodynamic changes of carbetocin and oxytocin given as i.v. bolus on women undergoing cesarean section. Geburtshilfe und Frauenheilkunde 2008;68:S46. [Google Scholar]
Mohamed 2015 {published data only}
- Mohamed HF, Mustafa GF, Ibrahim MA, Stefanos GE. Comparative study between intravenous bolus dose of carbetocin versus oxytocin during cesarean delivery in healthy parturients on blood loss: a randomized control trial. Medical Journal of Cairo University 2015;83(2):167‐72. [Google Scholar]
Moir 1979 {published data only}
- Moir DD, Amoa AB. Ergometrine or oxytocin? Blood loss and side‐effects at spontaneous vertex delivery. British Journal of Anaesthesia 1979;51(2):113‐7. [DOI] [PubMed] [Google Scholar]
Moodie 1976 {published data only}
- Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. British Journal of Anaesthesia 1976;48:571‐4. [DOI] [PubMed] [Google Scholar]
Mukta 2013 {published data only}
- Mukta M, Sahay PB. Role of misoprostol 600 mcg oral in active management of third stage of labor: a comparative study with oxytocin 10 IU i.m. Journal of Obstetrics and Gynecology of India 2013;6:325‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musa 2015 {published data only}
- Musa AO, Ijaiya MA, Saidu R, Aboyeji AP, Jimoh AA, Adesina KT, et al. Double‐blind randomized controlled trial comparing misoprostol and oxytocin for management of the third stage of labor in a Nigerian hospital. International Journal of Gynecology & Obstetrics 2015; Vol. 129, issue 3:227‐30. [DOI] [PubMed]
Nankaly 2016 {published data only}
- Nankaly A, Jalilian N, Eshghiali S, Rezaei M. The effects of sublingual misoprostol and intravenous oxytocin in reducing bleeding among cesarean deliveries. Acta Medica Mediterranea 2016;32:953‐7. [Google Scholar]
Nasr 2009 {published data only}
- Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;105(3):244‐7. [DOI] [PubMed] [Google Scholar]
Nayak 2017 {published data only}
- Nayak L Pradhan K Mishra S. Role of 400 mcg intraoperative sublingual misoprostol for reduction of caesarean blood loss. Journal of Evidence Based Medicine and Healthcare 2017;4(10):573‐7. [Google Scholar]
Nellore 2006 {published data only}
- Nellore V, Mittal S, Dadhwal V. Rectal misoprostol vs. 15‐methyl prostaglandin F2alpha for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94(1):45‐6. [DOI] [PubMed] [Google Scholar]
Ng 2001 {published data only}
- Ng PS, Chan AS, Sin WK, Tang LC, Cheung KB, Yuen PM. Comparison of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor ‐ a multicenter randomised controlled trial. XVI FIGO World Congress of Obstetrics & Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29.
- Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and im syntometrine in the management of the third stage of labour. Human Reproduction 2001;16(1):31‐5. [DOI] [PubMed] [Google Scholar]
Ng 2004 {published data only}
- Ng PS, Yuen PM, Sahota DS. Comparison of oral misoprostol and intravascular syntocinon in the management of the third stage of labour ‐ a double‐blind randomised controlled trial. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK. 2004:69.
Ng 2007 {published data only}
- Ng PS, Lai CY, Sahota DS, Yuen PM. A double‐blind randomized controlled trial of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor. Gynecologic and Obstetric Investigation 2007;63(1):55‐60. [DOI] [PubMed] [Google Scholar]
- Yuen PM, Ng PS, Sahota DS. A double‐blind randomised controlled trial of oral misoprostol in addition to intra‐muscular syntometrine in the management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK. 2004:62.
Nirmala 2009 {published data only}
- Nirmala K, Zainuddin AA, Ghani NA, Zulkifli S, Jamil MA. Carbetocin versus syntometrine in prevention of post‐partum hemorrhage following vaginal delivery. Journal of Obstetrics and Gynaecology Research 2009;35(1):48‐54. [DOI] [PubMed] [Google Scholar]
Nordstrom 1997 {published data only}
- Nordstrom L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. British Journal of Obstetrics and Gynaecology 1997;104(7):781‐6. [DOI] [PubMed] [Google Scholar]
Nuamsiri 2016 {published data only}
- Nuamsiri T Kaewkiattikun K. Prevention of postpartum hemorrhage with oxytocin versus ergometrine plus oxytocin in the third stage of labor. Thai Journal of Obstetrics and Gynaecology 2016;24:97‐103. [Google Scholar]
Oboro 2003 {published data only}
- Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Obstetrics & Gynaecology 2003;23(1):13‐6. [DOI] [PubMed] [Google Scholar]
Ogunbode 1979 {published data only}
- Ogunbode O, Obisesan K, Ayeni O. Methergin in the management of the third stage of labor: a comparative clinical trial with syntometrine and ergometrine. Current Therapeutic Research, Clinical and Experimental 1979;26:460‐5. [Google Scholar]
Orji 2008 {published data only}
- Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. International Journal of Gynecology & Obstetrics 2008;101(2):129‐32. [DOI] [PubMed] [Google Scholar]
Ortiz‐Gomez 2013 {published data only}
- Ortiz‐Gomez JR, Morillas‐Ramirez F, Fornet‐Ruiz I, Palacio‐Abizanda FJ, Bermejo‐Albares L. [Clinical and pharmacological study of the efficacy of carbetocin in elective caesareans compared to low and usual doses of oxytocin]. [Spanish]. Revista Espanola de Anestesiologia y Reanimacion 2013;60(1):7‐15. [DOI] [PubMed] [Google Scholar]
Othman 2016 {published data only}
- Othman ER, Fayez MF, Aal DEMA, El‐Dine Mohamed HS, Abbas AM, Ali MK. Sublingual misoprostol versus intravenous oxytocin in reducing bleeding during and after cesarean delivery: a randomized clinical trial. Taiwanese Journal of Obstetrics and Gynecology 2016;55(6):791‐5. [DOI] [PubMed] [Google Scholar]
Owonikoko 2011 {published data only}
- Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: A randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(7):715‐21. [DOI] [PubMed] [Google Scholar]
Pakniat 2015 {published data only}
- Pakniat H, Khezri MB. The effect of combined oxytocin‐misoprostol versus oxytocin and misoprostol alone in reducing blood loss at cesarean delivery: a prospective randomized double‐blind study. Journal of Obstetrics and Gynaecology of India 2015;65(6):376‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parsons 2006 {published data only}
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2006;28(1):20‐6. [DOI] [PubMed] [Google Scholar]
Parsons 2007 {published data only}
- Parsons S, Ntumy YM, Walley RL, Wilson JB, Crane JM, Matthews K, et al. Rectal misoprostol vs intramuscular oxytocin in the routine management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK. 2004:18.
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2007;29(9):711‐8. [DOI] [PubMed] [Google Scholar]
Patil 2013 {published data only}
- Patil NB, Patted SS. A randomised controlled trial of oral misoprostol vs injection methylergometrine for prevention of post partum hemorrhage. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(3):296‐303. [Google Scholar]
Patil 2016 {published data only}
- Patil AS, Dadavate V, Thobbi VA. Carboprost versus oxytocin for active management of third stage of labour. Al Ameen Journal of Medical Sciences 2016;9(3):196‐201. [Google Scholar]
Penaranda 2002 {published data only}
- Penaranda WA, Arrieta OB, Yances BR. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en al hospital de maternidad rafael calvo de cartagena]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87‐92. [Google Scholar]
Perez‐Rumbos 2017 {published data only}
- Perez‐Rumbos A, Reyna‐Villasmil E Rondon‐Tapia M, Reyna‐Villasmil N. Rectal misoprostol or intramuscular oxytocin in the management of the third phase of labour. Perinatología y Reproducción Humana 2017;31(2):78‐84. [Google Scholar]
Poeschmann 1991 {published data only}
- Poeschmann RP, Doesburg WH, Eskes TK. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1991;98:528‐30. [DOI] [PubMed] [Google Scholar]
- Poeschmann RP, Eskes TK, Doesburg WH. Oxytocin and sulprostone reduce post partum blood loss and shorten the third stage in low risk term women. International Journal of Gynecology & Obstetrics 1991;36 Suppl:312. [Google Scholar]
- Poeschmann RP, Eskes TK, Doesburg WH, Lemmens WA, Benneker JC. Oxytocin and sulprostone reduce postpartum blood loss in low risk term women compared to saline. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:176.
Prendiville 1988 {published data only}
- Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ 1988;297(6659):1295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quibel 2016 {published data only}
- Quibel T, Ghout I, Goffinet F, Salomon LJ, Fort J, Javoise S, et al. Active management of the third stage of labor with a combination of oxytocin and misoprostol to prevent postpartum hemorrhage: a randomized controlled trial. Obstetrics and Gynecology 2016;128(4):805‐11. [DOI] [PubMed] [Google Scholar]
- Rozenberg P, Quibel T, Ghout I, Salomon L, Bussiere L, Goffinet F. Active management of the third stage of labor with routine oxytocin and misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S18. [DOI] [PubMed] [Google Scholar]
Rajaei 2014 {published data only}
- NCT01863706. Misoprostol versus oxytocin for prevention of post partum hemorrhage. clinicaltrials.gov/ct2/show/NCT01863706 (first received: 23 May 2013).
- Rajaei M, Karimi S, Shahboodaghi Z, Mahboobi H, Khorgoei T, Rajaei F. Safety and efficacy of misoprostol versus oxytocin for the prevention of postpartum hemorrhage. Journal of Pregnancy 2014;2014:713879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez 2001 {published data only}
- Ramirez O, Benito V, Jimenez R, Valido C, Hernandez C, Garcia JA. Third stage of labour: active or expectant management? preliminary results [abstract]. Journal of Perinatal Medicine 2001;Suppl 1(Pt 2):364. [Google Scholar]
Rashid 2009 {published data only}
- Rashid M, Clark A, Rashid MH. A randomised controlled trial comparing the efficacy of intramuscular syntometrine and intravenous syntocinon, in preventing postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2009;29(5):396‐401. [DOI] [PubMed] [Google Scholar]
Ray 2001 {published data only}
- Chatterjee A. Misoprostol and the 3rd stage. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000; Vol. Book 4:29.
- Ray A, Mukherjee P, Basu G, Chatterjee A. Misoprostol and third stage of labour. Journal of Obstetrics and Gynecology of India 2001;51(6):53‐4. [Google Scholar]
Reddy 2001 {published data only}
- Reddy R, Shenoy JV. Active management of third stage of labour. A comparative study in high risk patients for atonic postpartum haemorrhage. Journal of Obstetrics and Gynecology of India 2001;51(2):44‐7. [Google Scholar]
Reyes, Gonzalez 2011 {published data only}
- Reyes OA, Gonzalez GM. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in patients with severe preeclampsia: a double‐blind randomized controlled trial. Journal of Obstetrics and Gynaecology Canada: JOGC 2011;33(11):1099‐104. [DOI] [PubMed] [Google Scholar]
Reyes 2011 {published data only}
- Reyes OA. Carbetocin vs oxytocin for the prevention of postpartum hemorrhage in grand multiparous patients: A randomized controlled trial. [Spanish]. Clinica e investigacion en ginecologia y obstetricia 2011; Vol. 38, issue 1:2‐7.
Rogers 1998 {published data only}
- Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet 1998;351(9104):693‐9. [DOI] [PubMed] [Google Scholar]
Rosseland 2013 {published data only}
- Gawecka E, Rosseland LA. A secondary analysis of a randomized placebo‐controlled trial comparing the analgesic effects of oxytocin with carbetocin: postcesarean delivery morphine equivalents. Anesthesia and Analgesia 2014;119(4):1004. [DOI] [PubMed] [Google Scholar]
- Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology 2013;119(3):541‐51. [DOI] [PubMed] [Google Scholar]
Sadiq 2011 {published data only}
- Sadiq UG, Kwanashie O, Mairiga G, Gamaniel S, Isa H, Abdu A, et al. A randomised clinical trial comparing the efficacy of oxytocin injection and oral misoprostol tablet in the prevention of postpartum haemorrhage in Maiduguri Nigeria. International Research Journal of Pharmacy 2011;2(8):76‐81. [Google Scholar]
Samimi 2013 {published data only}
- Samimi M, Imani‐Harsini A, Abedzadeh‐Kalahroudi M. Carbetocin vs. syntometrine in prevention of postpartum hemorrhage: a double blind randomized control trial. Iranian Red Crescent Medical Journal 2013;15(9):817‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shady 2017 {published data only}
- Shady NW, Sallam HF, Elsayed AH, Abdelkader AM, Ali SS, Alanwar A, et al. The effect of prophylactic oral tranexamic acid plus buccal misoprostol on blood loss after vaginal delivery: a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2017;27:1‐7. [DOI] [PubMed] [Google Scholar]
Shrestha 2011 {published data only}
- Shrestha A, Dongol A, Chawla CD, Adhikari RK. Rectal misoprostol versus intramuscular oxytocin for prevention of post partum hemorrhage. Kathmandu University Medical Journal 2011;33(1):8‐12. [DOI] [PubMed] [Google Scholar]
Singh 2009 {published data only}
- Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2009;107(2):130‐4. [DOI] [PubMed] [Google Scholar]
Sitaula 2017 {published data only}
- Sitaula S Uprety DK Thakur A Pradhan T. Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery: a randomized controlled trial. Nepal Journal of Obstetrics and Gynaecology 2017;11(2):37‐41. [DOI] [PubMed] [Google Scholar]
Soltan 2007 {published data only}
- Soltan MH, El‐Gendi E, Imam HH, Fathi O. Different doses of sublingual misoprostol versus methylergometrine for the prevention of atonic postpartum haemorrhage. International Journal of Health Sciences 2007;1(2):229‐36. [PMC free article] [PubMed] [Google Scholar]
Sood 2012 {published data only}
- Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. Journal of Obstetrics and Gynaecology of India 2012;62(2):162‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 2013 {published data only}
- NCT01108302. Effectiveness, safety and feasibility of auxiliary nurse midwives' (ANM) use of oxytocin in uniject™ to prevent postpartum hemorrhage in India. clinicaltrials.gov/ct2/show/NCT01108302 (first received: 2 April 2010).
- Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: Design of a community‐based cluster‐randomized trial. BMC Pregnancy and Childbirth 2012;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CK, Newton S, Mullany LC, Cofie P, Tawiah Agyemang C, Adiibokah E, et al. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community‐based, cluster‐randomized trial. PLOS Medicine. NCT01108289 2013; Vol. 10, issue 10:e1001524. [DOI] [PMC free article] [PubMed]
Su 2009 {published data only}
- Su LL, Rauff M, Chan YH, Mohamad Suphan N, Lau TP, Biswas A, et al. Carbetocin versus syntometrine for the third stage of labour following vaginal delivery‐‐a double‐blind randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2009;116(11):1461‐6. [DOI] [PubMed] [Google Scholar]
Sultana 2007 {published data only}
- Sultana N, Khatun M. Misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Bangladesh College of Physicians and Surgeons 2007;25(2):73‐6. [Google Scholar]
Supe 2016 {published data only}
- Supe PA, Kore SJ, Nandanwar YS. A comparative study of efficacy of misoprostol with methyl ergometrine and carboprost in active management of third stage of labour. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2016;5(5):1525‐31. [Google Scholar]
Surbek 1999 {published data only}
- Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Misoprostol for prevention of postpartum hemorrhage: a randomized controlled trial [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA 2000;Book 1:33. [Google Scholar]
- Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Oral misoprostol vs placebo for third stage of labour [Orales misoprostol reduziert den postpartalen blutverlust]. Gynakologisch Geburtshilfliche Rundschau 1999;39:144. [Google Scholar]
Taheripanah 2018 {published data only}
- Taheripanah R, Shoman A, Ali Karimzadeh M, Zamaniyan M, Malih N. Efficacy of oxytocin vs. Carbetocin in prevention of postpartum hemorrhage after cesarean section under general anesthesia: a prospective randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2018;31(21):2807‐12. [DOI] [PubMed] [Google Scholar]
Tewatia 2014 {published data only}
- Tewatia R, Rani S, Srivastav U, Makhija B. Sublingual misoprostol versus intravenous oxytocin in prevention of post‐partum hemorrhage. Archives of Gynecology and Obstetrics 2014;289:739‐42. [DOI] [PubMed] [Google Scholar]
Thilaganathan 1993 {published data only}
- Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:19‐22. [DOI] [PubMed] [Google Scholar]
Tripti 2006 {published data only}
- Tripti N, Manju E. Intramuscular PGF2 alpha 125 microg versus intravenous methyl ergometrine 0.2 mg in the active management of third stage of labor. Journal of Obstetrics and Gynecology of India 2006;56(5):396‐8. [Google Scholar]
Ugwu 2014 {published data only}
- Ugwu IA, Enabor OO, Adeyemi AB, Lawal OO, Oladokun A, Olayemi O. Sublingual misoprostol to decrease blood loss after caesarean delivery: a randomised controlled trial. Journal of Obstetrics and Gynaecology 2014;34(5):407‐11. [DOI] [PubMed] [Google Scholar]
Uncu 2015 {published data only}
- Uncu Y, Karahasan M, Uyaniklar O, Uncu G. Prophylactic misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. European Review for Medical and Pharmacological Sciences 2015;19(1):15‐22. [PubMed] [Google Scholar]
Un Nisa 2012 {published data only}
- Un Nisa S, Usmani SY. Role of intravenous syntocinon in prevention of primary postpartum haemorrhage. Pakistan Journal of Medical and Health Sciences 2012;6(4):1020‐4. [Google Scholar]
Vagge 2014 {published data only}
- Vagge DS, Mamatha KR, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol versus intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. Indian Journal of Pharmacology 2013;45 Suppl:S45. [Google Scholar]
- Vagge DS, Mamatha KR, Shivamurthy G, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol and intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. Journal of Chemical and Pharmaceutical Research 2014;6(3):1134‐40. [Google Scholar]
Vaid 2009 {published data only}
- Vaid A, Dadhwal V, Mittal S, Deka D, Misra R, Sharma JB, et al. A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl‐ergometrine versus intramuscular 15‐methyl PGF2alpha in active management of third stage of labor. Archives of Gynecology and Obstetrics 2009;280(6):893‐7. [DOI] [PubMed] [Google Scholar]
Van Selm 1995 {published data only}
- Selm M, Kanhai HH, Keirse MJ. Preventing the recurrence of atonic postpartum hemorrhage: a double‐blind trial. Acta Obstetricia et Gynecologica Scandinavica 1995;74:270‐4. [DOI] [PubMed] [Google Scholar]
Verma 2006 {published data only}
- Verma P, Aggarwal N, Jain V, Suri V. A double‐blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S137‐S138. [DOI] [PubMed] [Google Scholar]
Vimala 2004 {published data only}
- Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methylergometrine for active management of third stage of labor. International Journal of Gynecology & Obstetrics 2004;87:1‐5. [DOI] [PubMed] [Google Scholar]
Vimala 2006 {published data only}
- Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. International Journal of Gynecology & Obstetrics 2006;92(2):106‐10. [DOI] [PubMed] [Google Scholar]
Walley 2000 {published data only}
- Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double‐blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1111‐5. [DOI] [PubMed] [Google Scholar]
Whigham 2016 {published data only}
- Whigham CA, Gorelik A, Loughnan T, Trivedi A. Carbetocin versus oxytocin in active labour. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):88. [Google Scholar]
- Whigham CA, Gorelik A, Loughnan TE, Trivedi A. Carbetocin versus oxytocin to reduce additional uterotonic use at non‐elective caesarean section: a double‐blind, randomised trial. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(23):3866‐9. [DOI] [PubMed] [Google Scholar]
Widmer 2018 {published data only}
- Gulmezoglu M. The WHO Champion Trial. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E29‐30. [Google Scholar]
- Widmer M, Piaggio G, Abdel‐Aleem H, Carroli G, Chong Y, Coomarasamy A, et al. Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally: study protocol for a randomized controlled trial. Trials 2016;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer M, Piaggio G, Nguyen TM, et al. Heat‐stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. New England Journal of Medicine 2018;379(8):743‐52. [DOI] [PubMed] [Google Scholar]
Yuen 1995 {published data only}
- Yuen PM, Chan NS, Yim SF, Chang AM. A randomised double blind comparison of syntometrine and syntocinon in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1995;102:377‐80. [DOI] [PubMed] [Google Scholar]
Zachariah 2006 {published data only}
- Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labor. International Journal of Gynecology & Obstetrics 2006;92(1):23‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Aleem 2013 {published data only}
- Abdel‐Aleem H, Alhusaini TK, Abdel‐Aleem MA, Menoufy M, Gulmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(17):1705‐9. [DOI] [PubMed] [Google Scholar]
Abdel‐Aleem 2018 {published data only}
- Abdel‐Aleem AA, Abbas AM, Thabet AL, Badran E, El‐Nashar IH. The effect of initiating intravenous oxytocin infusion before uterine incision on the blood loss during elective cesarean section: a randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2018 [Epub ahead of print]. [DOI] [PubMed]
Abdollahy 2000 {published data only}
- Abdollahy F. Comparison effect of oxytocin and normal salin injection intra umbelical venuse [abstract]. Gynecological Endocrinology 2000;14(Suppl 2):49. [Google Scholar]
Adhikari 2007 {published data only}
- Adhikari S, Rana A, Bista KD. Active management of third stage of labour: comparison between prophylactic intramuscular methylergometrine and intramuscular oxytocin. Nepal Journal of Obstetrics and Gynaecology 2007;2(2):24‐8. [Google Scholar]
Adnan 2017 {published data only}
- Adnan N, Boland F, Murphy DJ. Intramuscular oxytocin versus intravenous oxytocin to prevent postpartum haemorrhage at vaginal delivery (LabOR trial): study protocol for a randomised controlled trial. Trials 2017;18(1):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2015 {published data only}
- Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(9):1014‐8. [DOI] [PubMed] [Google Scholar]
Akinaga 2016 {published data only}
- Akinaga C, Uchizaki S, Kurita T, Taniguchi M, Makino H, Suzuki A, et al. Randomized double‐blind comparison of the effects of intramyometrial and intravenous oxytocin during elective cesarean section. Journal of Obstetrics and Gynaecology Research 2016;42(4):404‐9. [DOI] [PubMed] [Google Scholar]
Alam 2017 {published data only}
- Alam A, Shyam P, Goswami S. A comparative study of efficacy of oxytocin, methylergometrine and misoprostol in prevention of post‐partum haemorrhage. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2017;6(5):1960‐4. [Google Scholar]
Al‐Harazi 2009 {published data only}
- Al‐Harazi AH, Frass KA. Sublingual misoprostol for the prevention of postpartum hemorrhage. Saudi Medical Journal 2009;30(7):912‐6. [PubMed] [Google Scholar]
Ali 2012 {published data only}
- Ali R, Hina F. Postpartum hemorrhage; comparison of efficacy of ergometrine with misoprostol in prophylaxis in cesarean section. Professional Medical Journal 2012;19(3):360‐4. [Google Scholar]
Ali 2018 {published data only}
- Ali AA, Nasr AA, Ahmed HH, El‐ Rasheedy MI, Badawy M. Carbetocin versus oxytocin and misoprostol in prevention of atonic post‐partum hemorrhage in high risk patients planed for cesarean delivery. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2018;7(1):10‐4. [Google Scholar]
Anandakrishnan 2013 {published data only}
- Anandakrishnan S, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose, part 2. Canadian Journal of Anaesthesia 2013;60(11):1054‐60. [DOI] [PubMed] [Google Scholar]
Anjaneyulu 1988 {published data only}
- Anjaneyulu R, Devi PK, Kanthamani CR, Vijaya R, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route ‐ a controlled clinical trial. Acta Obstetricia et Gynecologica Scandinavica Supplementa 1988;145:9‐11. [DOI] [PubMed] [Google Scholar]
Anvaripour 2013 {published data only}
- Anvaripour A, Shahryari H, Ahmadi S, Ghasemi S, Mirzaei K. Comparison the effects of oxytocin and methylergonovine in elective caesarean section under spinal anesthesia. Archives of Gynecology and Obstetrics 2013;287(5):979‐83. [DOI] [PubMed] [Google Scholar]
Ashwal 2016 {published data only}
- Ashwal E, Hiersch L, Wertheimer A, Krispin E, Aviram A, Dayan DB, et al. The effect of post‐partum oxytocin regimen on hemoglobin decline–a randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S197‐S198, Abstract no: 351. [Google Scholar]
Athavale 1991 {published data only}
- Athavale RD, Nerurkar NM, Dalvi SA, Bhattacharya MS. Umbilical vein oxytocin in the management of third stage of labour. Journal of Postgraduate Medicine 1991;37(4):219‐20. [PubMed] [Google Scholar]
Ayedi 2011 {published data only}
- Ayedi M, Zouche I, Smaoui L, Bouaziz I, Smaoui M, Kolsi K. Comparison of 2 versus 5 units of oxytocin in caesarean section. European Journal of Anaesthesiology 2011;28 Suppl:159‐60. [Google Scholar]
Ayedi 2011b {published data only}
- Ayedi M, Jarraya A, Smaoui M, Zouari J, Smaoui L, Kolsi K. Effect of tranexamic acid on post partum hemorrhage by uterine atony: a preliminary result of a randomized, placebo controlled trial. European Journal of Anaesthesiology 2011;28 Suppl 1:165. [Google Scholar]
Ayedi 2012 {published data only}
- Ayedi M, NCT01599468. Effects of tranexamic acid on post partum hemorrhage by uterine atony after cesarean section delivery: a randomized, placebo controlled trial. clinicaltrials.gov/ct2/show/NCT01599468 (first received 14 May 2012).
Aziz 2014 {published data only}
- Aziz S, Kazi S, Haq G, Soomro N. Oral misoprostol versus oxytocin in the management of third stage of labour. JPMA ‐ Journal of the Pakistan Medical Association 2014;64(4):428‐32. [PubMed] [Google Scholar]
Bader 2000 {published data only}
- Bader W, Ast S, Hatzmann W. The significance of acupuncture in the third stage of labour [Dit Bedeutung der Akupunktur in der Plazentarperiode]. Deutsche Zeitschrift fur Akupunktur 2000;43:264‐8. [Google Scholar]
- Bader W, Ast S, Reinehr J, Hackmann J, Hatzmann W. Oxytocin versus acupuncture in the third stage of labour ‐ a prospective randomized study [Oxytocin versus Akupunktur in der Plazentarperiode ‐ eine prospektiv randomisierte Studie]. Geburtshilfe und Frauenheilkunde 2000;60 Suppl 1:S73. [Google Scholar]
Badhwar 1991 {published data only}
- Badhwar L, Singh K, Sethi N, Gupta I, Aggarwal N. The value of nipple stimulation in the management of third stage of labour. International Journal of Gynecology & Obstetrics 1991;36 Suppl:16. [Google Scholar]
Bai 2014 {published data only}
- Bai J, Sun Q, Zhai H. A comparison of oxytocin and carboprost tromethamine in the prevention of postpartum hemorrhage in high‐risk patients undergoing cesarean delivery. Experimental and Therapeutic Medicine 2014;7(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baig 2015 {published data only}
- Baig FS, Shahzad N, Khurshid HN, Malik A. Postpartum haemorrhage; comparison of intra umbilical and intra venous injection of oxytocin on blood loss in third stage of labour. Professional Medical Journal 2015;22(6):793‐7. [Google Scholar]
Balki 2006 {published data only}
- Balki M, Ronayne M, Davies S, Fallah S, Kingdom J, Windrim R, et al. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstetrics & Gynecology 2006;107(1):45‐50. [DOI] [PubMed] [Google Scholar]
- Balki M, Ronayne M, Davies S, Kingdom J, Windrim R, Carvalho J. Oxytocin requirements at cesarean section for failure to progress in labor: a dose‐finding study [abstract]. Anesthesiology 2005;102(Suppl 1):10. [Google Scholar]
Banovska 2013 {published data only}
- Banovska J, Goffard P, Suball M, Origer P, Delatte P, Kapessidou P. Efficiency of temporary balloon occlusion of iliac arteries in patients at high hemorrhagic risk undergoing cesarean section. European Journal of Anaesthesiology 2013;30:176. [Google Scholar]
Barbaro 1961 {published data only}
- Barbaro CA, Smith GO. Clinical trial of SE505 ‐ a new oxytocic mixture. Australian and New Zealand Journal of Obstetrics and Gynaecology 1961;1:147‐50. [Google Scholar]
Baumgarten 1983 {published data only}
- Baumgarten K, Schmidt J, Horvat A, Neumann M, Cerwenka R, Gruber W, et al. Uterine motility after post‐partum application of sulprostone and other oxytocics. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;16:181‐92. [DOI] [PubMed] [Google Scholar]
Bhattacharya 1988 {published data only}
- Bhattacharya P, Devi PK, Jain S, Kanthamani CR, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route for control of postpartum bleeding ‐ a comparative trial with methylergometrine. Acta Obstetricia et Gynecologica Scandinavica Supplement 1988;145:13‐5. [DOI] [PubMed] [Google Scholar]
- Devi PK, Sutaria UD, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha for control of postpartum bleeding. Acta Obstetricia et Gynecologica Scandinavica Supplementa 1988;145:7‐8. [DOI] [PubMed] [Google Scholar]
Bhavana 2013 {published data only}
- Bhavana G, Mittal S. Evaluation of efficacy of prophylactic injection tranexamic acid in decreasing blood loss before and after caesarean section. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):32. [Google Scholar]
Bider 1991 {published data only}
- Bider D, Menashe Y, Dulitzky M, Mashiach S, Ben‐Rafael Z. Oxytocin or saline injected intra‐umbilically did not influence the third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 1991;70:321‐3. [DOI] [PubMed] [Google Scholar]
Bider 1992 {published data only}
- Bider D, Ben‐Rafael Z, Dulitzky M, Menashe Y, Mashiach S, Barkai G. Effect of intraumbilical prostaglandin F2alpha injection on the third stage of labor. Journal of Reproductive Medicine 1992;37(4):317‐9. [PubMed] [Google Scholar]
Bisri 2011 {published data only}
- Bisri Y, Redjeki IS, Himendra A. The comparative of effect of bolus‐infusion oxytocine with infusion oxytocine on blood pressure, heart rate, and uterine contraction of women undergoing elective caesarean section with general anesthesia N2O‐sevoflurane. European Journal of Anaesthesiology 2011;28 Suppl:159. [Google Scholar]
Bivins 1993 {published data only}
- Bivins HA, Cope DA, Newman RB, Eller DP. Randomized trial of intraumbilical vein oxytocin. American Journal of Obstetrics and Gynecology 1993;168:435. [DOI] [PubMed] [Google Scholar]
- Bivins HA, Cope DA, Newman RB, Eller DP. Randomized trial of intraumbilical vein oxytocin in midtrimester pregnancy losses. American Journal of Obstetrics and Gynecology 1993;169(4):1070‐3. [DOI] [PubMed] [Google Scholar]
Blum 2010 {published data only}
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of post‐partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double‐blind, randomised, non‐inferiority trial. Lancet 2010;375(9710):217‐23. [DOI] [PubMed] [Google Scholar]
Bonham 1963 {published data only}
- Bonham DG. Intramuscular oxytocics and cord traction in third stage of labour. BMJ 1963;2:1620‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonis 2012 {published data only}
- Bonis M, Torricelli M, Leoni L, Berti P, Ciani V, Puzzutiello R, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(6):732‐5. [DOI] [PubMed] [Google Scholar]
Boopathi 2014 {published data only}
- Boopathi A, Nayak SR, Rao A, Rao B. Oxytocin versus methylergometrine in the active management of third stage of labour. Open Journal of Obstetrics and Gynecology 2014;4:666‐71. [Google Scholar]
Bose 2017 {published data only}
- Bose D, Beegum R. Sublingual misoprostol vs intravenous tranexamic acid in reducing blood loss during cesarean section: a prospective randomized study. Journal of South Asian Federation of Obstetrics and Gynaecology ‐ SAFOG 2017;9(1):9‐13. [Google Scholar]
Bulusu 2017 {published data only}
- Bulusu R, Ray P, Rani A, Handa P. Comparison of side effects of misoprostol by oral and rectal routes in active management of third stage of labour. Journal of Evidence Based Medicine and Healthcare 2017;4(3):146‐9. [Google Scholar]
Cappiello 2006 {published data only}
- Cappiello E, Lugo L, Kodali B, Hepner D, Harnett M, Tsen LC. A double‐blinded, randomized, placebo‐controlled trial of calcium chloride for the augmentation of uterine tone following cesarean delivery [abstract]. Anesthesiology 2006;104(Suppl 1):32. [Google Scholar]
Carvalho 2004 {published data only}
- Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: A dose finding study. Obstetrics & Gynecology 2004;104:1005‐10. [DOI] [PubMed] [Google Scholar]
Catanzarite 1990 {published data only}
- Catanzarite VA. Prophylactic intramyometrial carboprost tromethamine does not substantially reduce blood loss relative to intramyometrial oxytocin at routine cesarean section. American Journal of Perinatology 1990;7:39‐42. [DOI] [PubMed] [Google Scholar]
Chaplin 2009 {published data only}
- Chaplin AC, George RB, McKeen D, McLeod LC. Up‐down determination of the ED90 of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing an elective caesarean delivery. Canadian Journal of Anaesthesia 2009;56(Suppl 1):S62. [DOI] [PubMed] [Google Scholar]
Chatterjee 2016 {published data only}
- Chatterjee S, Sarkar A, Rao KD. Using misoprostol for primary versus secondary prevention of postpartum haemorrhage ‐ Do costs matter?. PLOS One 2016;11(10):e0164718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhuri 2014 {published data only}
- Chaudhuri P, Mandi S, Mazumdar A. Rectally administrated misoprostol as an alternative to intravenous oxytocin infusion for preventing post‐partum hemorrhage after cesarean delivery. Journal of Obstetrics and Gynaecology Research 2014;40(9):2023‐30. [DOI] [PubMed] [Google Scholar]
Chestnut 1987 {published data only}
- Chestnut DH, Wilcox LL. Influence of umbilical vein administration of oxytocin on the third stage of labor. Proceedings of 19th Annual Meeting of Society for Obstetric Anesthesia and Perinatology; 1987 May 20‐23; Halifax, Nova Scotia, Canada. 1987:49.
- Chestnut DH, Wilcox LL. Influence of umbilical vein administration of oxytocin on the third stage of labor: a randomized, double‐blind, placebo‐controlled study. American Journal of Obstetrics and Gynecology 1987;157:160‐2. [DOI] [PubMed] [Google Scholar]
Chou 1994 {published data only}
- Chou MM, MacKenzie IZ. A prospective, double‐blind, randomized comparison of prophylactic intramyometrial 15‐methyl prostaglandin F2, 125 micrograms, and intravenous oxytocin, 20 units, for the control of blood loss at elective cesarean section. American Journal of Obstetrics and Gynecology 1994;171:1356‐60. [DOI] [PubMed] [Google Scholar]
Chou 2015 {published data only}
- Chou LT, Da AW, Murizah MZ, Rushdan M, Rashid Z. A randomised controlled trial on low dose versus high dose oxytocin infusion in prevention of uterine atony at caesarean delivery. Journal of Obstetrics and Gynaecology Research 2015;41(Suppl S1):44‐5, Abstract no: FC 8.11. [Google Scholar]
Chukudebelu 1963 {published data only}
- Chukudebelu WO, Marshall AT, Chalmers JA. Use of 'syntometrine' in the third stage of labour. BMJ 1963;1:1390‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2004 {published data only}
- ISRCTN07452238. A study to determine the cardiovascular effects of different methods of administering the oxytocic drug syntocinon. isrctn.com/ISRCTN07452238 (first received: 12 September 2003).
Cordovani 2011 {published data only}
- Cordovani D, Farine D, Balki M, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: A dose‐finding study. Canadian Journal of Anesthesia 2011;58(Suppl 1):S90. [Google Scholar]
Cordovani 2012 {published data only}
- Cordovani D, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose. Canadian Journal of Anesthesia 2012;59(8):751‐7. [DOI] [PubMed] [Google Scholar]
Dagdeviren 2016 {published data only}
- Dagdeviren H, Cengiz H, Heydarova U, Caypinar SS, Kanawati A, Guven E, et al. Intramuscular versus intravenous prophylactic oxytocin for postpartum hemorrhage after vaginal delivery: a randomized controlled study. Archives of Gynecology and Obstetrics 2016;294(5):911‐6. [DOI] [PubMed] [Google Scholar]
- NCT02080104. Intramuscular versus intravenous prophylactic oxytocin for hemorrhage after vaginal delivery (oxytocin). clinicaltrials.gov/ct2/show/NCT02080104 (first received: 1 March 2014).
Dahiya 1995 {published data only}
- Dahiya P, Puri M, Rathee S. Influence of intraumbilical oxytocin on the third stage of labour. Indian Journal of Medical Science 1995;49:23‐7. [PubMed] [Google Scholar]
Daley 1951 {published data only}
- Daley D. The use of intramuscular ergometrine at the end of the second stage of normal labour. Journal of Obstetrics and Gynaecology of the British Empire 1951;57:388‐97. [DOI] [PubMed] [Google Scholar]
Daly 1999 {published data only}
- Daly S, Andolina K, Tolosa JE, Roberts N, Wapner R. A randomized controlled trial of misoprostol versus oxytocin in preventing postpartum blood loss. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S68. [Google Scholar]
Dao 2009 {published data only}
- Dao B, Blum J, Barrera G, Cherine Ramadan M, Dabash R, Darwish E, et al. Side effect profiles for misoprostol and oxytocin in the treatment of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S150. [Google Scholar]
Davies 2005 {published data only}
- Davies GA, Tessier JL, Woodman MC, Lipson A, Hahn PM. Maternal hemodynamics after oxytocin bolus compared with infusion in the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2005;105:294‐9. [DOI] [PubMed] [Google Scholar]
De bonis 2012 {published data only}
- Bonis M, Torricelli M, Leoni L, Berti P, Ciani V, Puzzutiello R, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(6):732‐5. [DOI] [PubMed] [Google Scholar]
- Voltolini C, Bonis M, Vellucci F, Regini C, Orlandini C, Vannuccini S, et al. Carbetocin versus oxytocin after caesarean section: Similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. International Journal of Gynecology and Obstetrics 2012;119:S806‐7. [DOI] [PubMed] [Google Scholar]
Dell‐Kuster 2017 {published data only}
- Dell‐Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of carbetocin applied as an intravenous bolus compared to as a short‐infusion for caesarean section: study protocol for a randomised controlled trial. Trials 2016;17(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell‐Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of carbetocin given as an intravenous bolus compared with short infusion for caesarean section ‐ double‐blind, double‐dummy, randomized controlled non‐inferiority trial. British Journal of Anaesthesia 2017;118(5):772‐80. [DOI] [PubMed] [Google Scholar]
- Dell‐Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of intravenous carbetocin as a bolus compared to a short infusion for caesarean section. Journal of Obstetric Anesthesia 2016;26(Suppl 1):S7. [Google Scholar]
Dennehy 1998 {published data only}
- Dennehy KC, Rosaeg OP, Cicutti NJ, Krepski B, Sylvain JP. Oxytocin injection after caesarean delivery: intravenous or intramyometrial?. Canadian Journal of Anaesthesia 1998;45(7):635‐9. [DOI] [PubMed] [Google Scholar]
Deshpande 2016 {published data only}
- Deshpande HG, Madkar CS, Patel KK. Comparative study between intravenous and intraumbilical oxytocin as active management of third stage in elective and emergency caesarean section. Indian Journal of Obstetrics and Gynaecology Research 2016;3(1):55‐8. [Google Scholar]
Diab 1999 {published data only}
- Diab KM, Ramy AR, Yehia MA. The use of rectal misoprostol as active pharmacological management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 1999;25(5):327‐32. [DOI] [PubMed] [Google Scholar]
Dickinson 2009 {published data only}
- Dickinson JE, Doherty DA. Optimization of third‐stage management after second‐trimester medical pregnancy termination. American Journal of Obstetrics & Gynecology 2009;201(3):303.e1‐303.e7. [DOI] [PubMed] [Google Scholar]
Diop 2011 {published data only}
- NCT01487278. Comparing misoprostol and oxytocin in UnijectTM postpartum hemorrhage (PPH) prevention in Mali. clinicaltrials.gov/ct2/show/NCT01487278 Date first received: 5 December 2011.
Dommisse 1980 {published data only}
- Dommisse J. The routine use of oxytocic drugs in the third stage of labour [letter]. South African Medical Journal 1980;46:549. [PubMed] [Google Scholar]
Dong 2011 {published data only}
- Dong Y. Effects of carboprost on prevention of hemorrhage after induced labor with scarred uterus. Journal of Shanghai Jiaotong University (Medical Science) 2011;31(8):1212‐5. [Google Scholar]
Dumoulin 1981 {published data only}
- Dumoulin JG. A reappraisal of the use of ergometrine. Journal of Obstetrics and Gynaecology 1981;1:178‐81. [Google Scholar]
Durocher 2012 {published data only}
- Durocher J, Blum J, Sheldon WR, Trussell J, Winikoff B. Does the effect of oxytocin prophylaxis on post‐partum blood loss depend on route of administration?. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S332. [Google Scholar]
Dutta 2000 {published data only}
- Dutta DK, Saha KK. Comparative study on role of syntometrine and prostaglandin in the prevention of PPH. XVI FIGO World Congress of Obstetrics & Gynecology (Book 4); 2000 Sept 3‐8; Washington DC, USA. 2000:29.
Dweck 2000 {published data only}
- Dweck MF, Lynch CM, Spellacy WN. Use of methergine for the prevention of postoperative endometritis in non‐elective cesarean section patients. Infectious Diseases in Obstetrics & Gynecology 2000;8:151‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dzuba 2012 {published data only}
- Dzuba I, Durocher J, Dilbaz B, Gelisen O, Ngoc NT, Montesinos R, et al. Route of administration of oxytocin in prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S333. [Google Scholar]
Elati 2011 {published data only}
- Elati A, Elmahaishi MS, Elmahaishi MO, Elsraiti OA, Weeks AD. The effect of misoprostol on postpartum contractions: a randomised comparison of three sublingual doses. BJOG: an international journal of obstetrics and gynaecology 2011;118(4):466‐73. [DOI] [PubMed] [Google Scholar]
Erkkola 1984 {published data only}
- Erkkola R, Kero P, Kanto J, Korvenranta H, Nanto V, Peltonen T. Delayed cord clamping in cesarean section with general anesthesia. American Journal of Perinatology 1984;1(2):165‐9. [DOI] [PubMed] [Google Scholar]
Farber 2013 {published data only}
- NCT02026297. Tranexamic acid and thromboelastography during cesarean delivery (TA TEG). clinicaltrials.gov/ct2/show/NCT02026297 (first received: 22 December 2013).
Farber 2015 {published data only}
- Farber MK, Schultz R, Lugo L, Liu X, Huang C, Tsen LC. The effect of co‐administration of intravenous calcium chloride and oxytocin on maternal hemodynamics and uterine tone following cesarean delivery: a double‐blinded, randomized, placebo‐controlled trial. International Journal of Obstetric Anesthesia 2015;24(3):217‐24. [DOI] [PubMed] [Google Scholar]
Fatemeh 2011 {published data only}
- Fatemeh F, Zohreh S, Abbas MG, Layla H. Maternal haemodynamic effects of oxytocin bolus or infusion in the third stage of labour. Pakistan Journal of Medical Sciences 2011;27(3):656‐9. [Google Scholar]
Forster 1957 {published data only}
- Forster FM. A comparative study of ergometrine and 'methergin' used in the management of the third stage of labour. Medical Journal of Australia 1957;2:155‐6. [Google Scholar]
Francis 1965 {published data only}
- Francis HH, Miller JM, Porteous CR. Clinical trial of an oxytocin‐ergometrine mixture (first of two trials). Australian and New Zealand Journal of Obstetrics and Gynaecology 1965;5:47‐51. [DOI] [PubMed] [Google Scholar]
Friedman 1957 {published data only}
- Friedman EA. Comparative clinical evaluation of postpartum oxytocics. American Journal of Obstetrics and Gynecology 1957;73:1306‐13. [DOI] [PubMed] [Google Scholar]
Fugo 1958 {published data only}
- Fugo NW, Dieckmann WJ. A comparison of oxytocic drugs in the management of the placental stage. American Journal of Obstetrics and Gynecology 1958;76:141‐6. [DOI] [PubMed] [Google Scholar]
Gai 2004 {published data only}
- Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi‐center, randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;112:154‐7. [DOI] [PubMed] [Google Scholar]
Gavhane 2017 {published data only}
- Gavhane Satyajit P, Harshad T, Bangal VB, Verma P, Bhavsar Dhruval K. Comparative outcome of active management of third stage of labour with prophylactic use of oxytocin, methyl ergometrine and misoprostol. Pravara Medical Review 2017;9(4):4‐9. [Google Scholar]
George 2010 {published data only}
- George RB, McKeen D, Chaplin AC, McLeod L. Up‐down determination of the ED(90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Canadian Journal of Anaesthesia 2010;57(6):578‐82. [DOI] [PubMed] [Google Scholar]
Ghulmiyyah 2007 {published data only}
- Ghulmiyyah LM, Wehbe SA, Saltzman SL, Ehleban C, Sibai BM. Effects of intraumbilical vein injection of saline versus oxytocin plus saline on duration of the third stage of labor: a randomized double‐blind placebo trial [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S18. [Google Scholar]
- Ghulmiyyah LM, Wehbe SA, Saltzman SL, Ehleben C, Sibai BM. Intraumbilical vein injection of oxytocin and the third stage of labor: randomized double‐blind placebo trial. American Journal of Perinatology 2007;24(6):347‐52. [DOI] [PubMed] [Google Scholar]
Ghulmiyyah 2017 {published data only}
- Ghulmiyyah LM, Usta IM, Ghazeeri G, Taher N, Abu‐Ghannam G, Nassar AH, et al. Intravenous oxytocin use to decrease blood loss during scheduled cesarean delivery: a randomized double‐blinded controlled trial (oxytrial). American Journal of Perinatology 2017;34(4):379‐87. [DOI] [PubMed] [Google Scholar]
Gobbur 2011 {published data only}
- Gobbur VR, Reddy SV, Bijapur UJ. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:92.
Gohel 2007 {published data only}
- Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. Journal of Obstetrics and Gynaecology of India 2007;57(3):228‐30. [Google Scholar]
Goswami 2013 {published data only}
- Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment cesarean section: a double‐blind randomized case control prospective trial. Saudi Journal of Anaesthesia 2013;7(4):427‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groeber 1960 {published data only}
- Groeber WR, Bishop EH. Methergine and ergonovine in the third stage of labor. Obstetrics & Gynecology 1960;15:85‐8. [PubMed] [Google Scholar]
Gungorduk 2010 {published data only}
- Gungorduk K, Asicioglu O, Besimoglu B, Gungorduk OC, Yildirm G, Ark C, et al. Using intraumbilical vein injection of oxytocin in routine practice with active management of the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2010;116(3):619‐24. [DOI] [PubMed] [Google Scholar]
Gungorduk 2010b {published data only}
- Gungorduk K, Asicioglu O, Celikkol O, Olgac Y, Ark C. Use of additional oxytocin to reduce blood loss at elective caesarean section: a randomised control trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2010;50(1):36‐9. [DOI] [PubMed] [Google Scholar]
Gungorduk 2011 {published data only}
- Gungorduk K, Yildirim G, Asicioglu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double‐blind, placebo‐controlled study. American Journal of Perinatology 2011;28(3):233‐40. [DOI] [PubMed] [Google Scholar]
Gungorduk 2013 {published data only}
- Gungorduk K, Asicioglu O, Yildirim G, Ark C, Tekirdag AI, Besimoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. American Journal of Perinatology 2013;30(5):407‐13. [DOI] [PubMed] [Google Scholar]
Gupta 2014 {published data only}
- Gupta M, Bhosale U. Comparative study of methylergometrine and low dose carboprost (PGF2‐x) in active management of 3rd stage labor. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):139. [Google Scholar]
Habek 2007 {published data only}
- Habek D, Franicevic D. Intraumbilical injection of uterotonics for retained placenta. International Journal of Gynecology & Obstetrics 2007;99(2):105‐9. [DOI] [PubMed] [Google Scholar]
Hacker 1979 {published data only}
- Hacker NF, Biggs JS. Blood pressure changes when uterine stimulants are used after normal delivery. British Journal of Obstetrics and Gynaecology 1979;86:633‐6. [DOI] [PubMed] [Google Scholar]
Häivä 1994 {published data only}
- Häivä L, Hartikainen A. Pharmacological management of third stage of labor in primiparae and multiparae [Kohtua supistavan lääkkeen valinta ensi‐ ja uudelleensynnyttäjille]. Suomen Lääkärilehti 1994;49(33):3442‐4. [Google Scholar]
Halder 2013 {published data only}
- Halder S, Samanta B, Sardar R, Chattopadhyay S. Tranexamic acid used before caesarean section reduces blood loss based on pre‐ and postoperative hemoglobin level: A case‐control study. Journal of the Indian Medical Association 2013;111(3):184‐6. [PubMed] [Google Scholar]
Hoffman 2006 {published data only}
- Hoffman M, Castagnola D, Naqvi F. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S107. [Google Scholar]
- Hoffman M, Naqvi F, Sciscione A. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S82. [Google Scholar]
Hofmeyr 2004 {published data only}
- Hofmeyr GJ, Ferreira S, Nikodem VC, Mangesi L, Singata M, Jafta Z, et al. Misoprostol for treating postpartum haemorrhage: a randomized controlled trial. BMC Pregnancy and Childbirth 2004;4(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 1964 {published data only}
- Howard WF, McFadden PR, Keettel WC. Oxytocic drugs in fourth stage of labor. Journal of the American Medical Association 1964;189:411‐3. [DOI] [PubMed] [Google Scholar]
Huh 2004 {published data only}
- Huh W, Chelmow D, Malone FD. A randomized, double‐blinded, placebo controlled trial of oxytocin at the beginning versus the end of the third stage of labor for prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S130. [DOI] [PubMed] [Google Scholar]
- Huh WK, Chelmow D, Malone FD. A double‐blinded, randomized, controlled trial of oxytocin at the beginning versus the end of the third stage of labour for prevention of postpartum hemorrhage. Gynecologic and Obstetric Investigation 2004;58(2):72‐6. [DOI] [PubMed] [Google Scholar]
Hunt 2013 {published data only}
- Hunt BJ. Tranexamic acid for the treatment of postpartum haemorrhage‐preliminary results of the woman trial. Transfusion Medicine 2013;23(Suppl 1):7. [Google Scholar]
Ilancheran 1990 {published data only}
- Ilancheran A, Ratnam SS. Effect of oxytocics on prostaglandin levels in the third stage of labour. Gynecologic and Obstetric Investigation 1990;29:177‐80. [DOI] [PubMed] [Google Scholar]
Irons 1994 {published data only}
- Irons DW, Sriskandabalan P, Bullough CH. A simple alternative to parenteral oxytocics for the third stage of labor. International Journal of Gynecology & Obstetrics 1994;46:15‐8. [DOI] [PubMed] [Google Scholar]
Islam 2008 {published data only}
- Islam A, Siraj A, Arif N. Post partum hemorrhage prophylaxis; comparison of the efficacy of misoprostol and ergometrine in cesarean delivery. Professional Medical Journal 2008;15(3):323‐7. [Google Scholar]
Jackson 2001 {published data only}
- Jackson KW, Allbert JR, Schemmer GK, Elliot M, Humphrey A, Taylor J. A randomized controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2001;185:873‐7. [DOI] [PubMed] [Google Scholar]
Jagielska 2015 {published data only}
- Jagielska I, Kazdepka‐Zieminska A, Kaczorowska A, Madej A, Kolossa T, Grabiec M. [Evaluation of carbetocin and oxytocin efficacy in prevention of postpartum hemorrhage in women after cesarean section]. [Polish]. Ginekologia Polska 2015;86(9):689‐93. [PubMed] [Google Scholar]
Javadi 2015 {published data only}
- Javadi EH, Sadeghipour Z, Barikani A, Javadi M. Tranexamic acid in the control of uterine atony during labor. Biotechnology and Health Sciences 2015;2(2):e26898. [Google Scholar]
Jiang 2001 {published data only}
- Jiang Q, Wang P, Cao W. Effect on different doses of misoprostol to prevent postpartum hemorrhage. Chinese Nursing Research 2001;15(6):313‐4. [Google Scholar]
Jin 2000 {published data only}
- Jin LJ, Zhou L. Application of anus misoprostol to decrease the volume of post partum hemorrhage. Journal of Practical Nursing 2000;16(2):9‐10. [Google Scholar]
Jolivet 1978 {published data only}
- Jolivet A, Robyn C, Huraux‐Rendu C, Gautray JP. Effect of ergot alkaloid derivatives on milk secretion in the immediate postpartum period. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1978;7:129‐34. [PubMed] [Google Scholar]
Jonsson 2010 {published data only}
- Jonsson M, Hanson U, Lidell C, Norden‐Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2010;117(1):76‐83. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Norden Lindeberg S, Hanson U. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S214. [DOI] [PubMed] [Google Scholar]
Kashanian 2010 {published data only}
- Kashanian M, Fekrat M, Masoomi Z, Sheikh Ansari N. Comparison of active and expectant management on the duration of the third stage of labour and the amount of blood loss during the third and fourth stages of labour:a randomised controlled trial. Midwifery 2010;26:241‐5. [DOI] [PubMed] [Google Scholar]
Kemp 1963 {published data only}
- Kemp J. Clinical trial of "syntometrine" in the third stage of labour. British Medical Journal 1963;1(5342):1391‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 1997 {published data only}
- Khan GQ, John IS, Wani S, Doherty T, Sibai BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. American Journal of Obstetrics and Gynecology 1997;177(4):770‐4. [DOI] [PubMed] [Google Scholar]
Khan 2003 {published data only}
- Khan RU, El‐Refaey H. Pharmacokinetics and adverse‐effect profile of rectally administered misoprostol in the third stage of labor. Obstetrics & Gynecology 2003;101(5 Pt 1):968‐74. [DOI] [PubMed] [Google Scholar]
Khan 2012 {published data only}
- Khan MS, Sinha SK, Sultana T, Singhal S. Comparison of two oxytocin infusions in patients undergoing emergency cesarean sections: A double blind study. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S389. [Google Scholar]
Khan 2013 {published data only}
- Khan M, Balki M, Ahmed I, Farine D, Searward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose, part 3 final. Society for Obstetric Anesthesia and Perinatology (SOAP) 45th Annual Meeting; 2013 April 24‐28; San Juan, Puerto Rico. 2013:Abstract no: GM 3.
Khanun 2011 {published data only}
- Khanun A, Khanum S. Oral versus rectal misoprostol in the prevention of primary postpartum hemorrage. Pakistan Journal of Medical and Health Sciences 2011;5(3):587‐8. [Google Scholar]
Kikutani 2003a {published data only}
- Kikutani T, Shimada Y. Effects of methylergometrine and oxytocin on thoracic epidural pressure during cesarean section. Journal of Obstetrics and Gynaecology Research 2003;29(3):180‐5. [DOI] [PubMed] [Google Scholar]
Kikutani 2003b {published data only}
- Kikutani T, Oshima M, Sugimoto K, Shimada Y. Effects of intravenous infusion rate of oxytocin on thoracic epidural pressure in parturients undergoing elective cesarean section. Journal of Nippon Medical School = Nihon Ika Daigahu Zasshi 2003;70(6):475‐9. [DOI] [PubMed] [Google Scholar]
Kikutani 2006 {published data only}
- Kikutani T, Kikutani M, Oshima M, Sugimoto K, Shimada Y. Effects of methylergometrine and oxytocin on blood loss and uterine contraction during cesarean section. Masui ‐ Japanese Journal of Anesthesiology 2006;55(5):590‐4. [PubMed] [Google Scholar]
King 2010 {published data only}
- King KJ, Douglas J, Unger W, Wong AB. A randomized double‐blind comparison of a 5 unit intravenous oxytocin bolus versus placebo as a strategy to prevent uterine atony at cesarean section in women who are at increased risk of post‐partum hemorrhage [abstract]. Anesthesiology 2006;104(Suppl 1):41. [Google Scholar]
- King KJ, Douglas J, Unger W, Wong AB, Espinosa V, King RA. 5U bolus oxytocin at cesarean section in women at risk of atony [abstract]. Anesthesiology 2007;106(Suppl 1):14. [Google Scholar]
- King KJ, Douglas MJ, Unger W, Wong A, King RA. Five unit bolus oxytocin at cesarean delivery in women at risk of atony: a randomized, double‐blind, controlled trial. Anesthesia & Analgesia 2010;111(6):1460‐6. [DOI] [PubMed] [Google Scholar]
Kintu 2012 {published data only}
- Kintu A, Nakubulwa S, Mijumbi C, Kwizera A, Tindimwebwa J. Uterotonic efficacy of oxytocin 2.5 versus 10 units during caesarean section at mulago hospital: a double blinded placebo controlled randomised clinical trial. British Journal of Anaesthesia 2012;108:ii197‐8. [Google Scholar]
Kiran 2012 {published data only}
- Kiran S, Anand A, Singh T, Gupta N. Effective dose of oxytocin in caesarean delivery. British Journal of Anaesthesia 2012;108:ii195. [Google Scholar]
Kore 2000 {published data only}
- Kore S, Srikrishna S, Hegde A, Ambiye VR, Vaidya PR. Active management of third stage of labour with intraumbilical oxytocin injection. Journal of Obstetrics and Gynecology of India 2000;50(3):54‐5. [Google Scholar]
Kovacheva 2015 {published data only}
- Kovacheva VP, Soens MA, Tsen LC. A randomized, double‐blinded trial of a "rule of threes" algorithm versus continuous infusion of oxytocin during elective cesarean delivery. Anesthesiology 2015;123(1):92‐100. [DOI] [PubMed] [Google Scholar]
Kovavisarach 1998 {published data only}
- Kovavisarach E, Rojsangruang S. Effect of umbilical vein oxytocin injection on the third stage of labor : a randomized controlled study. 9th Congress of the Federation of the Asia and Oceania Perinatal Societies; 1996 November 10‐14; Singapore. 1996:59. [PubMed]
- Kovavisarach E, Rojsangruang S. Effect of umbilical vein oxytocin injection on the third stage of labor: a randomized controlled study. Journal of the Medical Association of Thailand 1998;81:693‐7. [PubMed] [Google Scholar]
Le 2000 {published data only}
- Le J. Prevention of postpartum hemorrhage by carboprost and oxytocin in 90 cases analysis. Acta Medicinae Sinica 2000;13(2):140‐1. [Google Scholar]
Leader 2002 {published data only}
- Leader J, Bujnovsky M, Carlan SJ, Triana T, Richichi K. Effect of oral misoprostol after second‐trimester delivery: a randomized, blinded study. Obstetrics & Gynecology 2002;100(4):689‐94. [DOI] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li X, Wang H, Wang J, Cao X L, Ma Y. Prophylactic and therapeutic effect of misoprofil plus oxytocin on postpartum hemorrhage in patients with pregnancy‐induced hypertension syndrome. Journal of Postgraduates of Medicine 2002;25(7):34‐5. [Google Scholar]
Li 2003 {published data only}
- Li DP, Bei HZ. Clinical study on reduction of postpartum bleeding in the risk factors by misoprostol. Hainan Medical Journal 2003;14(11):11‐2. [Google Scholar]
Li 2011 {published data only}
- Li H, Afzal A, Lian Q, Kramer GC, Svenson C, Prough D. Restricted fluid therapy decreases surgical blood loss ‐ a clinical study of two fluid regimens during cesarean section under spinal anesthesia. Anesthesia & Analgesia 2011;112:S‐291. [Google Scholar]
- Li H, Simon M, Lian Q, Afzal A, Christer Svenson C, Prough D. Restricted fluid therapy decreases surgical blood loss ‐ A clinical study of two fluid regimens during cesarean section under spinal anesthesia. American Society of Anesthesiologists Annual Meeting; 2011, October 15‐19; Chicago, Illinois. 2011.
Lin 2009 {published data only}
- Lin JH, Lin QD, Liu XH, Yan JY, He J, Li L, et al. [Multi‐center study of motherwort injection to prevent postpartum hemorrhage after caesarian section]. [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2009;44(3):175‐8. [PubMed] [Google Scholar]
Liu 1997 {published data only}
- Liu C, Wang D, Li X. Clinical study on reduction of postpartum bleeding by methyl carprost suppository. Chung‐Hua Fu Chan Ko Tsa Chih/Chinese Journal of Obstetrics and Gynaecology 1997;32(1):22‐4. [PubMed] [Google Scholar]
Liu 2002 {published data only}
- Liu DY, Fan L, Huang XH. Clinical observation on treatment of postpartum hemorrhage by xuesaitong soft capsule. Chinese Journal of Integrated Traditional and Western Medicine 2002;22(3):182‐4. [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu Y, Chen HX, Kang DL, Kuang XH, Liu WX, Ni J. Influence of dexmedetomidine on incidence of adverse reactions introduced by hemabate in postpartum hemorrhage during cesarean section. International Journal of Clinical and Experimental Medicine 2015;8(8):13776‐82. [PMC free article] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu W, Ma S, Pan W, Tan W. Combination of motherwort injection and oxytocin for the prevention of postpartum hemorrhage after cesarean section. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(51):2489‐92. [DOI] [PubMed] [Google Scholar]
Luamprapas 1994 {published data only}
- Luamprapas A. A study of umbilical vein administration of oxytocin to shorten the third stage of labor. Chon Buri Hospital Journal 1994;19(2):14‐25. [Google Scholar]
Maged 2015 {published data only}
- Maged AM, Helal OM, Elsherbini MM, Eid MM, Elkomy RO, Dahab S, et al. A randomized placebo‐controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. International Journal of Gynecology and Obstetrics 2015;131:265‐8. [DOI] [PubMed] [Google Scholar]
Makvandi 2013 {published data only}
- Makvandi S, Shoushtari SZ, Hosseini VZ. Management of third stage of labour: A comparison of intraumbilical oxytocin and placental cord drainage. Shiraz E Medical Journal 2013;14(2):83‐90. [Google Scholar]
Mangla 2012 {published data only}
- Mangla D, Goel JK, Goel R. Prophylactic intramyometrial oxytocin before placenta delivery during cesarean section prevents postpartum hemorrhage: a prospective randomized study of 150 women. Journal of South Asian Federation of Obstetrics and Gynaecology 2012;4(2):93‐6. [Google Scholar]
Mankuta 2006 {published data only}
- NCT00405626. Double blind placebo controlled bellis perenis and arnica montana as a drug for PPH. clinicaltrials.gov/ct2/show/NCT00405626 (first received: 29 November 2006).
Mansouri 2011 {published data only}
- Mansouri HA, Alsahly N. Rectal versus oral misoprostol for active management of third stage of labor: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283(5):935‐9. [DOI] [PubMed] [Google Scholar]
Martinez 2006 {published data only}
- Martinez MM, Lopez Farfan JA, Ramos Alvarez G, Lopez Colombo A. Oxitocin trough umbilical vein to shorten the third stage of labor [Oxitocina transvena umbilical para acortar el tercer periodo de trabajo de parto]. Ginecologia y Obstetricia de Mexico 2006;74(2):89‐94. [PubMed] [Google Scholar]
McGinty 1956 {published data only}
- McGinty LB. A study of the vasopressor effects of oxytocics when used intravenously in the third stage of labour. Western Journal of Surgery 1956;64:22‐8. [PubMed] [Google Scholar]
Miller 2009 {published data only}
- Miller S, Tudor C, Thorsten V, Nyima, Kalyang, Sonam, et al. Randomized double masked trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum hemorrhage in Lhasa, Tibet. Journal of Midwifery & Women's Health 2009;54(2):133‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirghafourvand 2015 {published data only}
- Mirghafourvand M, Alizadeh SM, Abasalizadeh F, Shirdel M. The effect of intravenous tranexamic acid on hemoglobin and hematocrit levels after vaginal delivery: a randomized controlled trial. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;16(60):1‐8. [Google Scholar]
- Mirghafourvand M, Mohammad‐Alizadeh S, Abbasalizadeh F, Shirdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: a double‐blind randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2015;55(1):53‐8. [DOI] [PubMed] [Google Scholar]
Mirteimouri 2013 {published data only}
- Mirteimouri M, Tara F, Teimouri B, Sakhavar N, Vaezi A. Efficacy of rectal misoprostol for prevention of postpartum hemorrhage. Iranian Journal of Pharmaceutical Research 2013; Vol. 12, issue 2:469‐74. [PMC free article] [PubMed]
Mockler 2015 {published data only}
- Mockler JC, Malkoutzis V, Davis‐Tuck M, Wallace EM. Oxytocin infusion at elective caesarean section: a double blind, randomised controlled trial. Journal of Paediatrics and Child Health 2015;51(Suppl 1):54. [Google Scholar]
Mohamadian 2013 {published data only}
- Mohamadian S, Shorab NJ, Mirzakhani K. The effect of the timing of intramuscular oxytocin injection on maternal bleeding during the third stage of labour. Journal of Midwifery & Reproductive Health 2013;1(2):66‐70. [Google Scholar]
Mollitt 2009 {published data only}
- Mollitt C, Ssenoga A, Grassman C, Barclay PM. Randomised controlled trial comparing the effects of oxytocin i.v. bolus vs. oxytocin i.v. infusion on cardiac output during caesarean section. International Journal of Obstetric Anesthesia 2009;18(Suppl 1):S11. [Google Scholar]
Moore 1956 {published data only}
- Moore JH. Is methylergonovine tartrate superior to ergonovine maleate. American Journal of Obstetrics and Gynecology 1956;71:908‐11. [DOI] [PubMed] [Google Scholar]
Movafegh 2011 {published data only}
- Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. International Journal of Gynecology & Obstetrics 2011;115(3):224‐6. [DOI] [PubMed] [Google Scholar]
Munishankarappa 2009 {published data only}
- Munishankarappa B, McLeod GA, MacGregor H, Murphy D. Maternal haemodynamic at elective caesarean section following oxytocin 5‐unit bolus and placebo infusion compared to oxytocin 5‐unit bolus and 30‐unit infusion. International Journal of Obstetric Anesthesia 2009;18(Suppl 1):S48. [DOI] [PubMed] [Google Scholar]
Munn 2001 {published data only}
- Munn MB, Owen J, Hauth J. Oxytocin regimens for the prevention of uterine atony at cesarean delivery [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S14. [DOI] [PubMed] [Google Scholar]
- Munn MB, Owen J, Vincent R, Wakefield M, Chestnut DH, Hauth JC. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstetrics & Gynecology 2001;98(3):386‐90. [DOI] [PubMed] [Google Scholar]
Murphy 2009 {published data only}
- ISRCTN17813715. A randomised controlled trial of oxytocin bolus versus oxytocin bolus and infusion for the control of blood loss at elective caesarean section. isrctn.com/ISRCTN17813715 (first received: 25 March 2008).
- Murphy DJ, Carey M, Montgomery AA, Sheehan SR, the ECSSITSG. Study protocol. ECSSIT ‐ Elective Caesarean Section Syntocinon Infusion Trial. A multi‐centre randomised controlled trial of oxytocin (Syntocinon) 5 IU bolus and placebo infusion versus oxytocin 5 IU bolus and 40 IU infusion for the control of blood loss at elective caesarean section. BMC Pregnancy and Childbirth 2009;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, MacGregor H, Munishankar B, McLeod G. A randomised controlled trial of oxytocin 5IU and placebo infusion versus oxytocin 5IU and 30IU infusion for the control of blood loss at elective caesarean section‐‐pilot study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2009;142(1):30‐3. [DOI] [PubMed] [Google Scholar]
Murphy 2015 {published data only}
- Murphy D, ISRCTN14718882. A study to compare the effectiveness of intravenous oxytocin with intramuscular oxytocin given at the third stage of labour at preventing bleeding at vaginal birth. isrctn.com/ISRCTN14718882 (first received 14 December 2015).
Nankali 2013 {published data only}
- Nankali A, Keshavarzi F, Fakheri T, Zare S, Rezaei M, Daeichin S. Effect of intraumbilical vein oxytocin injection on third stage of labor. Taiwanese Journal of Obstetrics & Gynecology 2013;52(1):57‐60. [DOI] [PubMed] [Google Scholar]
Narenji 2012 {published data only}
- IRCT201206099981N1. Comparison the effect of intramuscular injection of oxytocin and nipple stimulation on the third stage of delivery length and bleeding. en.irct.ir/trial/10487?revision=10487 (first received 25 June 2012).
Nelson 1983 {published data only}
- Nelson GH. Use of 15‐methyl prostaglandin F2alpha postpartum to contract the uterus in normal pregnant women. Journal of the Medical Association of Georgia 1983;72:703‐6. [PubMed] [Google Scholar]
Neri‐Mejia 2016 {published data only}
- Neri‐Mejia M, Pedraza‐Aviles AG. Active management of the third stage of labor: three schemes of oxytocin: randomised clinical trial. Ginecologia y Obstetricia De Mexico 2016;84(5):306‐13. [PubMed] [Google Scholar]
Newton 1961 {published data only}
- Newton M, Mosey LM, Egli GE, Gifford WB, Hull CT. Blood loss during and immediately after delivery. Obstetrics & Gynecology 1961;17:9‐18. [PubMed] [Google Scholar]
Nguyen‐Lu 2015 {published data only}
- Nguyen‐Lu N, Carvalho J, Farine D, Seaward G, Downey K, Balki M. Carbetocin at cesarean delivery for labor arrest: A randomised controlled trial to determine ED90. Canadian Journal of Anesthesia 2013;60(Suppl 1):S112. [Google Scholar]
- Nguyen‐Lu N, Carvalho JC, Farine D, Seaward G, Ye XY, Balki M. Carbetocin at cesarean delivery for labour arrest: a sequential allocation trial to determine the effective dose. Canadian Journal of Anaesthesia//Journal Canadien D'anesthesie 2015;62(8):866‐74. [DOI] [PubMed] [Google Scholar]
Nieminen 1964 {published data only}
- Nieminen U, Jaervinen PA. A comparative study of different medical treatments of the third stage of labour. Annales Chirurgiae et Gynaecologiae Fenniae 1964;53:424‐9. [PubMed] [Google Scholar]
Oberbaum 2005 {published data only}
- NCT01156194. Effect of a homeopathic remedy on the third stage of delivery: a prospective, randomized, double‐blind study. clinicaltrials.gov/show/NCT01156194 (first received: 1 July 2010).
- Oberbaum M, Galoyan N, Lerner‐Geva L, Singer SR, Grisaru S, Shashar D, et al. The effect of the homeopathic remedies arnica montana and bellis perennis on mild postpartum bleeding‐‐a randomized, double‐blind, placebo‐controlled study‐‐preliminary results. Complementary Therapies in Medicine 2005;13(2):87‐90. [DOI] [PubMed] [Google Scholar]
Oguz 2014 {published data only}
- Oguz Orhan E, Dilbaz B, Aksakal SE, Altinbas S, Erkaya S. Prospective randomized trial of oxytocin administration for active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2014;127(2):175‐9. [DOI] [PubMed] [Google Scholar]
Ononge 2015 {published data only}
- Ononge S, Campbell OM, Kaharuza F, Lewis JJ, Fielding K, Mirembe F. Effectiveness and safety of misoprostol distributed to antenatal women to prevent postpartum haemorrhage after child‐births: a stepped‐wedge cluster‐randomized trial. BMC Pregnancy and Childbirth 2015;15:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozalp 2010 {published data only}
- Ozalp E, Tanir HM, Sener T. Dinoprostone vaginal insert versus intravenous oxytocin to reduce postpartum blood loss following vaginal or cesarean delivery. Clinical and Experimental Obstetrics and Gynecology 2010;37(1):53‐5. [PubMed] [Google Scholar]
Ozcan 1996 {published data only}
- Ozcan T, Sahin G, Senoz S. The effect of intraumbilical oxytocin on the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1996;36:9‐11. [DOI] [PubMed] [Google Scholar]
Ozkaya 2005 {published data only}
- Ozkaya O, Sezik M, Kaya H, Desdicioglu R, Dittrich R. Placebo‐controlled randomized comparison of vaginal with rectal misoprostol in the prevention of postpartum hemorrhage. Journal of Obstetrics & Gynaecology Research 2005;31(5):389‐93. [DOI] [PubMed] [Google Scholar]
Padhy 2006 {published data only}
- Padhy AK, Panigrahi R, Mohapatra KR. Alternative method of active management of 3rd stage of labour with 10 units of intraumbilical oxytocin injection [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:76.
Palacio 2011 {published data only}
- Palacio FJ, Morillas F, Ortiz‐Gomez JR, Fornet I, Bermejo L, Cantalejo F. [Efficacy of low‐dose oxytocin during elective cesarean section]. [Spanish]. Revista Espanola de Anestesiologia y Reanimacion 2011;58(1):6‐10. [DOI] [PubMed] [Google Scholar]
Paull 1977 {published data only}
- Paull JD, Ratten GJ. Ergometrine and third stage blood loss. Medical Journal of Australia 1977;1:178‐9. [Google Scholar]
Pei 1996 {published data only}
- Pei JL, Zhao DF. Study of the effects of using uterine stimulants on milk secretion during delivery. Zhonghua Hu Li Za Zhi 1996;31(7):384‐5. [PubMed] [Google Scholar]
Perdiou 2009 {published data only}
- Perdiou A. The effect of 3rd generation colloids on primary haemostasis in pregnant women. European Hematology Association 14th Annual Congress; 2009 June 4‐7; Berlin, Germany. 2009.
- Perdiou A, Kousoulakou A, Papadopoulou G, Leveta G, Trigka A, Andromida M, et al. The effect of 3rd generation colloids on primary haemostasis in pregnant women. Haematologica 2009;94(s2):527, Abstract no. 1334. [Google Scholar]
Phromboot 2010 {published data only}
- Phromboot T. Efficacy of intraumbilical vein methylergonovine maleate on duration of third stage of labor. Thai Journal of Obstetrics and Gynaecology 2010;20:29‐33. [Google Scholar]
Pierre 1992 {published data only}
- Pierre F, Mesnard L, Body G. For a systematic policy of iv oxytocin inducted placenta deliveries in a unit where a fairly active management of third stage of labour is yet applied: results of a controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1992;43:131‐5. [DOI] [PubMed] [Google Scholar]
Pinder 2002 {published data only}
- Pinder AJ, Dresner M, Calow C, ORiordan J, Johnson R. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. International Journal of Obstetric Anesthesia 2002;11:156‐9. [DOI] [PubMed] [Google Scholar]
Pisani 2012 {published data only}
- Pisani I, Tiralongo GM, Gagliardi G, Scala RL, Todde C, Frigo MG, et al. The maternal cardiovascular effect of carbetocin compared to oxytocin in women undergoing caesarean section. Pregnancy Hypertension 2012;2(2):139‐42. [DOI] [PubMed] [Google Scholar]
Porter 1991 {published data only}
- Porter KB, O'Brien WF, Bruskivage L, Collins MK, Knuppel RA, Givens P. Prospective randomized study on the effects of umbilical vein oxytocin on puerperal blood loss, length of the third stage of labor and on alpha‐fetoprotein levels. American Journal of Obstetrics and Gynecology 1991;164:326. [Google Scholar]
- Porter KB, O'Brien WF, Collins MK, Givens P, Knuppel R, Bruskivage L. A randomized comparison of umbilical vein and intravenous oxytocin during the puerperium. Obstetrics & Gynecology 1991;78:254‐6. [PubMed] [Google Scholar]
Priya 2015 {published data only}
- Priya GP, Veena P, Chaturvedula L, Subitha L. A randomized controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum hemorrhage. Archives of Gynecology and Obstetrics 2015;292(6):1231‐7. [DOI] [PubMed] [Google Scholar]
Puri 2012 {published data only}
- Puri M, Taneja P, Gami N, Rehan HS. Effects of different doses of intraumbilical oxytocin on the third stage of labor. International Journal of Gynecology & Obstetrics 2012;118(3):210‐2. [DOI] [PubMed] [Google Scholar]
Qiu 1999 {published data only}
- Qiu H, Zhu H, Ouyang W. Clinical study on chanlibao in accelerating second stage of labor [Chinese]. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1998;18:214‐6. [PubMed] [Google Scholar]
- Qiu H, Zhu H, Ouyang W, Wang Z, Sun H. Clinical effects and mechanism of chanlibao in accelerating second stage of labor. Journal of Tongji Medical University 1999;19(2):141‐4. [DOI] [PubMed] [Google Scholar]
Quiroga 2009 {published data only}
- Quiroga Diaz R, Cantu Mata R, Tello Gutierrez HE, Puente Villalobos M, Montemayor Garza R, Martinez Mendoza A. Intrauterine misoprostol for the prevention of bleeding cesarean. Ginecologia y Obstetricia de Mexico 2009;77(10):469‐74. [PubMed] [Google Scholar]
Ragab 2016 {published data only}
- Ragab A, Barakat R, Alsammani MA. A randomized clinical trial of preoperative versus postoperative misoprostol in elective cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;132(1):82‐4. [DOI] [PubMed] [Google Scholar]
Raghavan 2016 {published data only}
- Raghavan S, Geller S, Miller S, Goudar SS, Anger H, Yadavannavar MC, et al. Misoprostol for primary versus secondary prevention of postpartum haemorrhage: a cluster‐randomised non‐inferiority community trial. BJOG: an international journal of obstetrics and gynaecology 2016;123:120‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahbar 2018 {published data only}
- Rahbar N, Mirjan N, Ghorbani R. Comparison of sublingual misoprostol and intravenous oxytocin in the management of hemorrhage after cesarean section. Middle East Journal of Rehabilitation and Health Studies 2018;5(1):e62025. [Google Scholar]
Rajwani 2000 {published data only}
- Rajwani J, Survana K. Active management of third stage of labor ‐ a comparative study [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 3); 2000 Sept 3‐8; Washington DC, USA. 2000:54.
Ray 2012 {published data only}
- Ray D, Ghosh S, Bhattacharya S, Mandal RD, Basak A. Oxytocin administration during caesarian delivery: comparison between bolus versus infusion. Society for Obstetric Anesthesia and Perinatology (SOAP) 44th Annual Meeting; 2015 May 2‐5; Monterey, USA. 2012:T‐26.
Razali 2016 {published data only}
- Razali N, Md Latar IL, Chan YK, Omar SZ, Tan PC. Carbetocin compared to oxytocin in emergency cesarean section: a randomized trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;198:35‐9. [DOI] [PubMed] [Google Scholar]
Reddy 1989 {published data only}
- Reddy VV, Carey JC. Effect of umbilical vein oxytocin on puerperal blood loss and length of the third stage of labor. American Journal of Obstetrics and Gynecology 1989;160(1):206‐8. [DOI] [PubMed] [Google Scholar]
Rezk 2018 {published data only}
- Rezk M. A randomized clinical trial of sublingual versus rectal misoprostol for the prevention of postpartum hemorrhage in low resource settings. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201802003154375 (first received 27 February 2018).
Rooney 1985 {published data only}
- Rooney I, Hughes P, Calder AA. Is routine administration of syntometrine still justified in the management of the third stage of labour?. Health Bulletin 1985;43:99‐101. [PubMed] [Google Scholar]
Rosales‐Ortiz 2014 {published data only}
- Rosales‐Ortiz S. Prophylaxis of obstetric haemorrhage: Experience using carbetocin vs. oxytocin in patients with risk factors for postpartum haemorrhage. Journal of Perinatal Medicine 2013;41(Suppl 1):140. [Google Scholar]
- Rosales‐Ortiz S, Aguado RP, Hernandez RS, Castorena M, Cristobal FL, Gonzalez MC, et al. Carbetocin versus oxytocin for prevention of postpartum haemorrhage: A randomised controlled trial. Lancet 2014;383:S51. [DOI] [PubMed] [Google Scholar]
Rouse 2011 {published data only}
- Rouse D, Abramovici A, Szychowski J, Seals S, Andrews W, Hauth J, et al. Oxytocin dose‐regimens to prevent uterine atony after vaginal delivery: does treatment efficacy vary by risk status?. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S50‐S51. [Google Scholar]
Sadeghipour 2013 {published data only}
- IRCT2013052613473N1. The role of tranexamic acid in management of uterine atony during delivery. en.search.irct.ir/view/13710 (first received: 14 July 2013).
Saito 2007 {published data only}
- Saito K, Haruki A, Ishikawa H, Takahashi T, Nagase H, Koyama M, et al. Prospective study of intramuscular ergometrine compared with intramuscular oxytocin for prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2007;33(3):254‐8. [DOI] [PubMed] [Google Scholar]
Sallam 2018 {published data only}
- Sallam HF Shady NW. Adjunctive sublingual misoprostol for secondary prevention of post‐partum hemorrhage during cesarean delivery: double blind placebo randomized controlled trial. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2018;7(2):495‐502. [Google Scholar]
Samuels 2005 {published data only}
- Samuels N, Oberbaum M. The effect of the homoeopathic remedies arnica montana and bellis perennis on postpartum bleeding ‐ a randomised, double‐blind, placebo‐controlled study [abstract]. Focus on Alternative and Complementary Therapies 2005;10 Suppl 1:47. [DOI] [PubMed] [Google Scholar]
Sangkhomkhamhang 2012 {published data only}
- Sangkhomkhamhang U, ACTRN12612000624886. A randomised controlled trial of intravenous versus intramuscular oxytocin in the management of third stage of labor. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000624886 (first received 12 June 2012).
Sariganont 1999 {published data only}
- Sariganont J. Comparative study between syntocinon and methergin in prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 1999;11(4):248. [Google Scholar]
Sarna 1997 {published data only}
- Sarna MC, Soni AK, Gomez M, Oriol NE. Intravenous oxytocin in patients undergoing elective cesarean section [see comments]. Anesthesia & Analgesia 1997;84(4):753‐6. [DOI] [PubMed] [Google Scholar]
Sartain 2008 {published data only}
- Sartain JB, Barry JJ, Howat PW, McCormack DI, Bryant M. Intravenous oxytocin bolus of 2 units is superior to 5 units during elective caesarean section. British Journal of Anaesthesia 2008;101(6):822‐6. [DOI] [PubMed] [Google Scholar]
Savitha 2017 {published data only}
- Savitha A Sarita H Kashinath G. Randomized controlled trial of rectal misoprostol and intramuscular oxytocin in the prevention of PPH. International Journal of Basic & Clinical Pharmacology 2017;6(5):1101‐3. [Google Scholar]
Schaefer 2004 {published data only}
- Schaefer A, Klein L, Wolfe P, Heindricks G, Downs L, Guinn D. Double blind rct of early versus traditional oxytocin management in the third stage to prevent blood loss [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S69. [Google Scholar]
Schemmer 2001 {published data only}
- Schemmer G. A randomized controlled trial comparing prophylactic administration of oxytocin before and after placental delivery in the prevention of postpartum hemorrhage [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S20. [DOI] [PubMed] [Google Scholar]
Sekhavat 2009 {published data only}
- Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(1):72‐5. [DOI] [PubMed] [Google Scholar]
Sentilhes 2015 {published data only}
- NCT02302456. Tranexamic acid for preventing postpartum haemorrhage following a vaginal delivery (TRAAP). clinicaltrials.gov/ct2/show/NCT02302456 (first received: 17 November 2014).
- Sentilhes L, Daniel V, Darsonval A, Deruelle P, Vardon D, Perrotin F, et al. Study protocol. TRAAP ‐ TRAnexamic Acid for Preventing postpartum hemorrhage after vaginal delivery: a multicenter randomized, double‐blind, placebo‐controlled trial. BMC Pregnancy and Childbirth 2015;15:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senturk 2013 {published data only}
- Senturk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double‐blind, placebo‐controlled, randomized clinical trial. Archives of Gynecology and Obstetrics 2013;287(4):641‐5. [DOI] [PubMed] [Google Scholar]
Senturk 2016 {published data only}
- Senturk S, Kagitci M, Balik G, Arslan H, Kir Sahin F. The effect of the combined use of methylergonovine and oxytocin during caesarean section in the prevention of post‐partum haemorrhage. Basic & Clinical Pharmacology & Toxicology 2016;118(5):338‐43. [DOI] [PubMed] [Google Scholar]
Shahid 2013 {published data only}
- Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. Journal of the College of Physicians and Surgeons‐‐Pakistan : JCPSP 2013; Vol. 23, issue 7:459‐62. [PubMed]
Sharma 2014 {published data only}
- Sharma M, Kaur P, Kaur K, Kaur A, Kaur PK, Kaur MM. A comparative study of oxytocin/misoprostol/methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynecology of India 2014;64(3):175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheehan 2011 {published data only}
- Sheehan S, Carey M, Murphy D. A cohort study of 500 patients recruited to ECSSIT ‐ Elective Caesarean Section Syntocinon Infusion Trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S492. [Google Scholar]
- Sheehan S, Montgomery AA, Carey M, McAuliffe F, Eogan M, Gleeson R, et al. Ecssit‐elective caesarean section Syntocinon infusion trial a multicentre randomized controlled trial of oxytocin (Syntocinon) 5 IU bolus and placebo infusion versus oxytocin 5 IU bolus and 40 IU infusion for the control of blood loss at elective caesarean section. Irish Journal of Medical Science 2011;180(Suppl 4):S119. [Google Scholar]
- Sheehan SR, Montgomery AA, Carey M, McAuliffe FM, Eogan M, Gleeson R, et al. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective caesarean section: Double blind, placebo controlled, randomised trial. BMJ 2011;343(7819):d4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirazi 2013 {published data only}
- IRCT201204079399N1. A placebo‐controlled clinical trial to assess efficacy of tranexamic acid in reducing hemorrhage after vaginal delivery. en.search.irct.ir/view/9264 (first received: 15 November 2012).
Shrestha 2007 {published data only}
- Shrestha P, Babu CS. Influence of umbilical vein oxytocin on blood loss and length of third stage of labour. Nepal Medical College Journal 2007;9(3):176‐8. [PubMed] [Google Scholar]
Shrestha 2008 {published data only}
- Shrestha A, Urala MS, Upreti D, Niraula S. Comparison of intramyometrial and intramuscular 15 methyl PGF2x against traditional prophylactic intramuscular methergin for the active management of third stage of labor. Nepal Journal of Obstetrics and Gynaecology 2008;3(2):35‐9. [Google Scholar]
Singh 2005 {published data only}
- Singh N, Singh U. Methylergometrine and carboprost tromethamine prophylaxis for postpartum hemorrhage. Journal of Obstetrics and Gynaecology of India 2005;55(4):325‐8. [Google Scholar]
Siriwarakul 1991 {published data only}
- Siriwarakul W. A study of umbilical vein administration of oxytocin to shorten the third stage of labor. Chon Buri Hospital Journal 1991;16(1):40‐51. [Google Scholar]
Soiva 1964 {published data only}
- Soiva K, Koistinen O. Clinical experience with simultaneous intramuscular injection of oxytocin and methylergometrine. Annales Chirurgiae et Gynaecologiae Fenniae 1964;53:173‐8. [PubMed] [Google Scholar]
Soleimani 2014 {published data only}
- Soleimani Z, Naini AA. The effectiveness of sublingual misoprostol in prevention of bleeding during cesarean delivery. [Persian]. Iranian Journal of Obstetrics, Gynecology and Infertility 2014; Vol. 17, issue 125:1‐7.
Sorbe 1978 {published data only}
- Sorbe B. Active pharmacologic management of the third stage of labor. A comparison of oxytocin and ergometrine. Obstetrics & Gynecology 1978;52:694‐7. [PubMed] [Google Scholar]
Soriano 1995 {published data only}
- Soriano D, Dulitzki M, Schiff E, Barkai G, Seidman DS. A randomized prospective trial of oxytocin plus ergometrin versus oxytocin alone for prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 2):361. [Google Scholar]
Sreelatha 2017 {published data only}
- Sreelatha S, Nethra HS, Nadagoudar S, Ambastha V, Rajeshwari. A comparative study of different route of administration of misoprostol in the management of third stage of labour. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2017;6(9):3865‐71. [Google Scholar]
Stearn 1963 {published data only}
- Stearn RH. Syntometrine in the management of the third stage of labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1963;70:593‐6. [DOI] [PubMed] [Google Scholar]
Svanstrom 2008 {published data only}
- Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double‐blind comparison of oxytocin and methylergometrine during caesarean section. British Journal of Anaesthesia 2008;100(5):683‐9. [DOI] [PubMed] [Google Scholar]
Swapnika 2018 {published data only}
- Swapnika D, Prema Priya G, Senthil Priya S, Allirathinam AS. A comparative study between intramuscular oxytocin and intramuscular methyl ergometrine in the active management of third stage of labour. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2018;7(5):1943‐8. [Google Scholar]
Symes 1984 {published data only}
- Symes JB. A study on the effect of ergometrine on serum prolactin levels following delivery. Journal of Obstetrics and Gynaecology 1984;5:36‐8. [Google Scholar]
Taj 2014 {published data only}
- Taj N, Firdous A, Akhtar N, Chaudhary MH, Sarah, Bajwa Z, et al. Efficacy of tranexamic acid in reducing blood loss during and after cesarean section [Nergis Taj, Ahsan Firdaus, Nadeem Akhtar, Muhammad Hamid Chaudhary, Sarah, Zunaira Bajwa, Ehsan Ullah.]. Rawal Medical Journal 2014;39(3):311‐3. [Google Scholar]
Takagi 1976 {published data only}
- Takagi S, Yoshida T, Togo Y, Tochigi H, Abe M, Sakata H, et al. The effects of intramyometrial injection of prostaglandin F2alpha on severe post‐partum hemorrhage. Prostaglandins 1976;12(4):565‐79. [DOI] [PubMed] [Google Scholar]
Tali 2016 {published data only}
- Tali K, Ignacio Alensuela A. The effect of prophylactic intravenous tranexamic acid in reducing blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: A prospective, randomised, double‐blind, placebo‐controlled study. Australian and New Zealand Journal of Obstetrics and Gynaecology 2016;56(Suppl 1):61. [Google Scholar]
Tanir 2009 {published data only}
- Tanir H, Sener T, Ozalp E. Dinoprostone vaginal insert versus intravenous oxytocin to reduce the postpartum blood loss following vaginal or cesarean delivery. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S506‐7. [PubMed] [Google Scholar]
Tarabrin 2012 {published data only}
- Tarabrin O, Kaminskiy V, Galich S, Tkachenko R, Gulyaev A, Shcherbakov S, et al. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2012;16 Suppl 1:S157. [Google Scholar]
Tariq 2015b {published data only}
- Tariq N, Khakwani M, Parveen R. Effectiveness of misoprostol in the prevention of postpartum hemorrhage. Pakistan Journal of Medical and Health Sciences 2015;9(1):268‐70. [Google Scholar]
Tehseen 2008 {published data only}
- Tehseen F, Anwar A, Arfat Y. Intraumbilical veinous injection oxytocin in the active management of third stage of labour. Journal of the College of Physicians and Surgeons‐‐Pakistan 2008;18(9):551‐4. [PubMed] [Google Scholar]
Terry 1970 {published data only}
- Terry MF. A management of the third stage to reduce feto‐maternal transfusion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1970;77:129‐32. [DOI] [PubMed] [Google Scholar]
Tessier 2000 {published data only}
- Tessier JL, Davies GA, Woodman MC, Lipson A. Maternal hemodynamics after oxytocin bolus versus infusion in the third stage of labor. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S128. [DOI] [PubMed] [Google Scholar]
Tharakan 2008 {published data only}
- Tharakan T, Jha J. Randomized double blind prospective trial of active management of the third stage of labor. Archives of Medical Science 2008;4(1):79‐82. [Google Scholar]
- Tharakan T, Jha J. Randomized double‐blind prospective trial of active management of the third stage of labor [abstract]. Obstetrics & Gynecology 2007;109(4 Suppl):1S. [Google Scholar]
Thomas 2007 {published data only}
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of intravenous bolus or infusion of oxytocin in women undergoing caesarean section [abstract]. International Journal of Obstetric Anesthesia 2006;15 Suppl 1:S13. [Google Scholar]
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. British Journal of Anaesthesia 2007;98(1):116‐9. [DOI] [PubMed] [Google Scholar]
Thornton 1988 {published data only}
- Thornton S, Davison JM, Baylis PH. Plasma oxytocin during third stage of labour: comparison of natural and active management. BMJ 1988;297:167‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S, Davison JM, Baylis PH. Plasma oxytocin in the third stage of human labour with and without syntometrine [abstract]. Clinical Science 1987;73 Suppl 17:2P. [Google Scholar]
Tita 2012 {published data only}
- Tita AT, Szychowski JM, Rouse DJ, Bean CM, Chapman V, Nothern A, et al. Higher‐dose oxytocin and hemorrhage after vaginal delivery: A randomized controlled trial. Obstetrics and Gynecology 2012;119(2 Pt 1):293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripti 2009 {published data only}
- Tripti N, Balram S. 400 ug oral misoprostol versus 0.2mg intravenous methyl ergometrine for the active management of third stage of labor. Journal of Obstetrics and Gynecology of India 2009;59(3):228‐34. [Google Scholar]
Tudor 2006 {published data only}
- Tudor C, Miller S, Nyima, Sonam, Droyoung, Varner M. Preliminary progress report: randomized double‐blind trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum hemorrhage in Lhasa, Tibet. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S145‐6. [DOI] [PubMed] [Google Scholar]
Ugwu 2016 {published data only}
- Ugwu IA, Oluwasola TA, Enabor OO, Anayochukwu‐Ugwu NN, Adeyemi AB, Olayemi OO. Randomized controlled trial comparing 200mug and 400mug sublingual misoprostol for prevention of primary postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2016;133:173‐7. [DOI] [PubMed] [Google Scholar]
Van den Enden 2009 {published data only}
- Enden E, Lahousse J, Devlieger R, Vandermeersch E, Velde M. Haemodynamic effects of a bolus or infusion of oxytocin: a randomised double‐blind trial. International Journal of Obstetric Anesthesia 2009;18(Suppl 1):S45. [DOI] [PubMed] [Google Scholar]
Vasegh 2005 {published data only}
- Vasegh FR, Bahiraie A, Mahmoudi M, Salehi L. Comparison of active and physiologic management of third stage of labor. HAYAT: The Journal of Tehran Faculty of Nursing & Midwifery 2005;10(23):102. [Google Scholar]
Vaughan Williams 1974 {published data only}
- Vaughan Williams CA, Johnson A, Ledward R. A comparison of central venous pressure changes in the third stage of labour following oxytocic drugs and diazepam. Journal of Obstetrics and Gynaecology of the British Commonwealth 1974;81:596‐9. [DOI] [PubMed] [Google Scholar]
Ventoskovskiy 1990 {published data only}
- Ventoskovskiy BM, Popov AV. Homoeopathy as a practical alternative to traditional obstetrics methods. British Homoeopathic Journal 1990;79:201‐5. [Google Scholar]
Vogel 2004 {published data only}
- Vogel D, Burkhardt T, Rentsch K, Schweer H, Watzer B, Zimmerman R, et al. Misoprostol versus methylergometrine: pharmacokinetics in human milk. American Journal of Obstetrics and Gynecology 2004;191:2168‐73. [DOI] [PubMed] [Google Scholar]
Wallace 2007 {published data only}
- Wallace EM. A double‐blind randomised controlled trial of oxytocin bolus plus placebo infusion versus oxytocin bolus plus oxytocin infusion at elective caesarean section. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12607000631404 (first received 12 December 2007).
Walraven 2005 {published data only}
- Walraven G, Blum J, Dampha Y, Sowe M, Morison L, Winikoff B, et al. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112:1277‐83. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang BI, Du JM. Clinical study on reduction of postpartum bleeding using carprost suppository. Henan Medical Research 2000;9(2):155‐6. [Google Scholar]
Wang 2018 {published data only}
- Wang Y. Therapeutic efficacy and safety of carbetocin on postpartum hemorrhage. chictr.org.cn/showproj.aspx?proj=26431 (first received 11 April 2018).
Weeks 2015 {published data only}
- Frye L, Durocher J, Weeks A, Ditai J, Ononge S, Faragher B, et al. On the trail of misoprostol in the community: A secondary analysis of self‐administered misoprostol for the prevention of postpartum hemorrhage in Uganda. International Journal of Gynaecology and Obstetrics 2015;131(Suppl 5):E354‐5. [Google Scholar]
- Weeks A, Ditai J, Ononge S, Faragher B, Mirembe F, Byamugisha J, et al. Self‐administered misoprostol to prevent bleeding after homebirths in Uganda: a placebo‐controlled randomised trial. BJOG: an international journal of obstetrics and gynaecology 2013;120:76. [Google Scholar]
- Weeks AD, Ditai J, Ononge S, Faragher B, Frye LJ, Durocher J, et al. The MamaMiso study of self‐administered misoprostol to prevent bleeding after childbirth in rural Uganda: a community‐based, placebo‐controlled randomised trial. BMC Pregnancy and Childbirth 2015;15(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weihong 1998 {published data only}
- Weihong H, Hanrong C, Hong L, Linan C. Preventing of postpartum haemorrhage by carboprost methylate suppository administered through vagina or sublingually. Acta Academiae Medicinae Shanghai 1998;25:137‐9. [Google Scholar]
Weiss 1975 {published data only}
- Weiss G, Klein S, Shenkman L, Kataoka K, Hollander CS. Effect of methylergonovine on puerperal prolactin secretion. Obstetrics & Gynecology 1975;46:209‐10. [PubMed] [Google Scholar]
Wellmann 2016 {published data only}
- Wellmann S, Koslowski A, Spanaus K, Zimmermann R, Burkhardt T. Fetal release of copeptin in response to maternal oxytocin administration: a randomized controlled trial. Obstetrics and Gynecology 2016;128(4):699‐703. [DOI] [PubMed] [Google Scholar]
Wetta 2013 {published data only}
- Wetta L, Szychowski J, Seals S, Mancuso M, Hauth J, Tita A. Risk factors for uterine atony at vaginal delivery: a comprehensive evaluation. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S71‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. American Journal of Obstetrics and Gynecology 2013;209(1):51e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winikoff 2012 {published data only}
- NCT01608958. Intravenous and intramuscular administration of oxytocin in the third stage of labor for prevention of postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT01608958 (first received: 29 May 2012).
Winikoff 2016 {published data only}
- Winikoff B, Dzuba I, Carroli G, NCT02954068. Intravenous versus intramuscular administration of oxytocin and its relationship with postpartum bleeding and other clinical signs: a randomized placebo‐controlled study. clinicaltrials.gov/show/NCT02954068 (first received 28 October 2016).
Wong 2005a {published data only}
- NCT00257803. Does the rapid intravenous administration of oxytocin after delivery of the baby decrease the bleeding during cesarean section in women at risk of bleeding during cesarean section?. clinicaltrials.gov/ct2/show/NCT00257803 (first received: 21 November 2005).
Wong 2005b {published data only}
- NCT01710566. Misoprostol and oxytocin in Uniject® for postpartum hemorrhage prevention in communities. clinicaltrials.gov/ct2/show/NCT01710566 (first received: 8 May 2012).
Wright 2005 {published data only}
- NCT00147420. RCT of Zhi Byed 11 (ZB11) Versus Misoprostol in Tibet. clinicaltrials.gov/ct2/show/NCT00147420 (first received 7 September 2005).
Wu 2007 {published data only}
- Wu LF, Liu Y, Ruan Y. Clinical study on prevention of postpartum hemorrhage of cesarean section using hemabat in high risk pregnant women. Chinese Journal of Obstetrics & Gynecology 2007;42(9):577‐81. [PubMed] [Google Scholar]
Xu 2003 {published data only}
- Xu H. Misoprostol on preventing postpartum bleeding in cesarean. Hebei Medicine 2003;9(9):806‐7. [Google Scholar]
Xu 2013 {published data only}
- Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double‐blind randomization trial. Archives of Gynecology and Obstetrics 2013;287(3):463‐8. [DOI] [PubMed] [Google Scholar]
Yamaguchi 2011 {published data only}
- Yamaguchi ET, Cardoso MM, Torres ML, Nascimento RC, Ribeiro MC, Frerichs E, et al. Serum oxytocin concentrations in elective caesarean delivery: a randomized comparison of three infusion regimens. International Journal of Obstetric Anesthesia 2011;20(3):224‐8. [DOI] [PubMed] [Google Scholar]
Yan 2000 {published data only}
- Yan WG, Ling MX, Mao HY. Clinical study on reduction of postpartum bleeding in cesarean operation by misoprostol. Journal of Zhenjiang Medical College 2000;10(3):440‐1. [Google Scholar]
Yang 2001 {published data only}
- Yang H, Zheng S, Shi C. [Clinical study on the efficacy of tranexamic acid in reducing postpartum blood lose: a randomized comparative, multicenter trial] [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynaecology] 2001;36(10):590‐2. [PubMed] [Google Scholar]
Young 1988 {published data only}
- Young SB, Martelly PD, Greb L, Considine G, Coustan DR. The effect of intraumbilical oxytocin on the third stage of labor. Obstetrics & Gynecology 1988;71:736‐8. [PubMed] [Google Scholar]
Zamora 1999 {published data only}
- Zamora LA. A randomized controlled trial of oxytocin administered at the end of the second stage of labor versus oxytocin administered at the end of the third stage of labor in the prevention of postpartum hemorrhage. Philippine Journal of Obstetrics and Gynecology 1999;23(4):125‐33. [PubMed] [Google Scholar]
Zaporozhan 2013 {published data only}
- Zaporozhan V, Tarabrin O, Gavrychenko D, Mazurenko G, Saleh O, Lyoshenko I. Effcacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2013;17(Suppl 2):S135‐6. [Google Scholar]
Zhao 1998 {published data only}
- Zhao Y, Li X, Peng Y. Clinical study on reduction of postpartum bleeding in cesarean section by misoprostol. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 1998;33:403‐5. [PubMed] [Google Scholar]
Zhao 2003 {published data only}
- Zhao SF, Sun XF. Clinical study on preventing and curing postpartum hemorrhage in the third stage of labor. Journal of Practical Obstetrics and Gynecology 2003;19(5):278‐80. [Google Scholar]
Zhou 1994 {published data only}
- Zhou HL, Zhang L. Study on the effect of third stage of labor through different channels of injection of oxytocin. Chinese Journal of Nursing 1994;29(8):453‐5. [Google Scholar]
References to studies awaiting assessment
Abdel‐Aleem 1997 {published data only}
- Abdel‐Aleem H. [Personal communication] Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum haemorrhage. Letter to: Cochrane Pregnancy and Childbirth, Liverpool, UK 1997.
- Abdel‐Aleem H, Mostafa SA, Makarem MH, Abol‐Oyoun EM, Makhlouf A, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum hemorrhage. Research Activities on Reproductive Health: Annual Report of Assiut University Department of Obstetrics and Gynecology November 1997. Assiut University, Faculty of Medicine, 1997:75. [Google Scholar]
Alli 2013 {published data only}
- Alli QO. Comparing effectiveness of sublingual misoprostol with oxytocin infusion to reduce blood loss at caesarean section: double blind, randomised study. BJOG: an international journal of obstetrics and gynaecology 2013;120:77‐8. [Google Scholar]
Amornpetchakul 2017 {published data only}
- Amornpetchakul P, Lertbunnaphong T, Boriboonhiransarn D, Leetheeragul J, Sirisomboon R, Jiraprasertwong R. Efficacy of intravenous 100 mcg carbetocin versus intravenous 5 units oxytocin for prevention of immediate postpartum hemorrhage after normal vagina delivery among high risk pregnancies; a triple‐blinded randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2017;43:24‐5. [Google Scholar]
- Lertbunnaphong T. Efficacy of intravenous 100 mcg carbetocin versus intravenous 5 units oxytocin for prevention of immediate postpartum hemorrhage after normal vagina delivery among high risk pregnancies; a triple‐blinded randomized controlled trial. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1973 (first received 15 July 2016).
Beigi 2009 {published data only}
- Beigi A, Tabarestani H, Moini A, Zarrinkoub F, Kazempour M, Hadian Amree A. [Sublingual misoprostol versus intravenous oxytocin in the management of postpartum hemorrhage]. Tehran University Medical Journal 2009;67(8):556‐61. [Google Scholar]
Muller 1996 {published data only}
- Muller R, Beck G. Active management of the third stage of labour. 19th Swiss Congress of the Swiss Society of Gynecology and Obstetrics; 1996 June; Interlaken, Switzerland. 1996.
Norchi 1988 {published data only}
- Norchi S, Beretta E, Zanini A, Bottino S. Prevention of primary post‐partum haemorrhage (PPH). Controlled clinical trial: Sulprostone vs Metilergometrina. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988.
Rabow 2017 {published data only}
- Rabow S, Jonsson E, Jonsson H, Olofsson P. Cardiovascular effects of oxytocin and carbetocin at caesarean section, a prospective double‐blind randomised study using non‐invasive pulse wave analysis. Acta Anaesthesiologica Scandinavica 2017; Vol. 61, issue 8:1053.
Roy 2017 {published data only}
- Roy R Vernekar M. Comparative study on effect of misoprostol and oxytocin in the active management of third stage of labor in a tertiary hospital in Manipur, India. Indian Journal of Public Health Research and Development 2017;8(4):376‐81. [Google Scholar]
Said 2017 {published data only}
- Said SK. A comparative study of rectal misoprostol to oxytocin infusion during cesarean delivery to reduce intra operative & postoperative blood loss. Al‐Kufa Journal for Biology 2017;9(3):55‐62. [Google Scholar]
Shrivasatava 2012 {published data only}
- Shrivasatava DD, Khamsara D. Critical evaluation of sublingual misoprostol and methyl ergometrine in active management of third stage of labour. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S484. [Google Scholar]
Sunil 2016 {published data only}
- Sunil Kumar KS, Shyam S, Batakurki P. Carboprost versus oxytocin for active management of third stage of labor: a prospective randomized control study. Journal of Obstetrics and Gynaecology of India 2016;66(Suppl 1):S229‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Balki 2017 {published data only}
- NCT03168698. Carbetocin vs. oxytocin at elective cesarean section: a double‐blind, randomized controlled non‐inferiority trial of high and low dose regimens. https://clinicaltrials.gov/ct2/show/NCT03168698 (first received 30 May 2017).
Draycott 2014 {published data only}
- NCT02216383. Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and syntometrine for the third stage of labour following vaginal birth (IMox). clinicaltrials.gov/ct2/show/NCT02216383 (first received 15 August 2014).
Gomez 2011 {published data only}
- ACTRN12610000550000. Comparison of the effectiveness of carbetocin vs oxytocin in managing the third stage of labor in a group of women with risk factors for postpartum hemorrhage. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335628 (first received 25 June 2010).
Goudar 2016 {published data only}
- CTRI/2016/06/006996. Effect of carbetocin RTS vs oxytocin for preventing postpartum hemorrhage on post‐delivery hemoglobin: a randomized controlled trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10619 (first received 6 June 2016).
Kalahroudi 2010a {published data only}
- IRCT138810212854N2. Comparison effect of carbetocine and syntometrin in prevention of post partum hemorrhage. en.search.irct.ir/view/2059 (first received 15 March 2010).
Kalahroudi 2010b {published data only}
- IRCT201008212854N5. Comparison of the effect of rectal misoprostol and syntometrin in prevention of post partum hemorrhage. en.search.irct.ir/view/3932 (first received 9 September 2010).
Maged 2018 {published data only}
- NCT03556852. Carbetocin versus rectal misoprostol for management of third stage of labor in women at low risk of postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT03556852 (first received 14 June 2018).
Moradi 2010 {published data only}
- IRCT138812223548N1. Comparison of misoprostol and oxytocin in reduction of postpartum hemorrhage. en.search.irct.ir/view/2616 (first received 29 May 2010).
Sweed 2014 {published data only}
- NCT02083107. Comparison between rectal and sublingual misoprostol before caesarian section to reduce intra & post‐operative blood loss. clinicaltrials.gov/ct2/show/NCT02083107 (first received 7 March 2014).
Thakur 2015 {published data only}
- CTRI/2015/06/005918. Efficacy of oxytocin, misoprostol, 15‐methylprostaglandinF2alpha and methylergometrine in active management of third stage of labor. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10133 (first received 16 June 2015).
Additional references
Alkema 2016
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario‐based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter‐Agency Group. Lancet 2016;387:462‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begley 2015
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
Brignardello‐Petersen 2018
- Brignardello‐Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta‐analysis. Journal of Clinical Epidemiology 2018;93:36‐44. [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Combs 1991
- Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstetrics and Gynecology 1991;77:69‐76. [PubMed] [Google Scholar]
Davies 2001
- Davies NM, Longstreth J, Jamali F. Misoprostol therapeutics revisited. Pharmacotherapy 2001;21:60‐73. [DOI] [PubMed] [Google Scholar]
de Groot 1996a
- Groot AN. The role of oral (methyl) ergometrine in the prevention of postpartum haemorrhage. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;69:31‐6. [DOI] [PubMed] [Google Scholar]
de Groot 1998
- Groot AN, Dongen PW, Vree TB, Hekster YA, Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998;56:523‐35. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dias 2013
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Medical Decision Making 2013; Vol. 33, issue 5:607‐17. [DOI] [PMC free article] [PubMed]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency innetwork meta‐analysis: Concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogerzeil 1993
- Hogerzeil HV, Walker GJ, Goeje MJ. Stability of Injectable Oxytocics in Tropical Climates. Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin. Geneva: World Health Organization (WHO), 1993. [Google Scholar]
Hunter 1992
- Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long‐acting oxytocin analog on the postpartum uterus. Clinical Pharmacology and Therapeutics 1992;52:60‐7. [DOI] [PubMed] [Google Scholar]
Liabsuetrakul 2018
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD005456.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumley 2002
- Lumley T. Network meta‐analysis for indirect treatment comparisons. Statistics in Medicine 2002;21(16):2313‐24. [DOI] [PubMed] [Google Scholar]
McDonald 2004
- McDonald SJ, Abbott JM, Higgins SP. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MEDICINES.ORG.UK
- Medicines.org.uk. Syntocinon Ampoules 5 IU/ml ‐ Summary of Product Characteristics (SPC) ‐ (eMC). Available from: http://www.medicines.org.uk/emc/medicine/16423/SPC 2014; Vol. [cited 10 October 2014].
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penney 2007
- Penney G, Brace V. Near miss audit in obstetrics. Current Opinion in Obstetrics & Gynecology 2007;19:145‐50. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: An overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Say 2014
- Say L, Chou D, Gemmill A, Tunçalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2(6):e323‐e333. [DOI] [PubMed] [Google Scholar]
Schaff 2005
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception 2005;71(1):22‐5. [DOI] [PubMed] [Google Scholar]
Souza 2013
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross‐sectional study. Lancet 2013;381:1747‐55. [DOI] [PubMed] [Google Scholar]
Su 2012
- Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD005457.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuncalp 2012
- Tuncalp O, Hofmeyr GJ, Gulmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westhoff 2013
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]
White 2011
- White I. Multivariate random‐effects meta‐regression:Updates to mvmeta. The Stata Journal 2011;11(2):255‐270. [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network metaanalysis:Model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2015
- White IR. Network meta‐analysis. Stata Journal 2015;15(4):951‐85. [Google Scholar]
WHO 1993
- WHO. Stability of Injectable Oxytocics in Tropical Climates: Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine, and Oxytocin. Geneva: World Health Organization, 1993. [Google Scholar]
WHO 2012
- WHO. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: Department of Reproductive Health, World Health Organization, 2012. [PubMed] [Google Scholar]
References to other published versions of this review
Gallos 2015
- Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallos 2018
- Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD011689.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


