Abstract
Purpose: The purpose of this study was to evaluate the effect of different cardioplegic solutions on endothelial integrity and oxidative stress in cardiovascular surgery.
Methods: In this randomized prospective study, after ethics approval and informed consent, 60 surgical patients were included. Patients undergoing coronary bypass surgery were randomized into two groups as warm blood cardioplegia (n = 30) and cold crystalloid cardioplegia (n = 30) following the cross-clamping. Measurements were performed at three time points: before induction of anesthesia (T1), at admission to intensive care unit (ICU) (T2) and at the 24th postoperative hour (T3). Besides biochemical routine hemodynamic monitoring, patients were assessed for the sialic acid (SA), ischemic-modified albumin (IMA), advanced oxide protein products (AOPPs), total thiol (SH), and free hemoglobin (fHb) level.
Results: Neither crystalloid nor blood cardioplegia led to significant changes in the AOPPs, T-SH, and SA level (p >0.05). Crystalloid cardioplegia, however, increased IMA level compared to both baseline (p <0.01) and blood cardioplegia group (p <0.05). fHb levels were transiently increased in both groups at the second-time point (p <0.001). fHb level was lower in the crystalloid group compared to that in the other group (p <0.05) at T2.
Conclusion: Cardioplegia type creates similar effects on glycocalyx integrity. However, myocardial protection could be provided with warm blood cardioplegia.
Keywords: cardiovascular surgery, cardioplegic solutions, endothelial glycocalyx, sialic acid
Introduction
Myocardial damage is one of the significant causes of mortality and morbidity in cardiovascular surgery. Thus, myocardial protection is a critical approach to prevent or to reduce the myocardial complications that occur during and after cardiac surgery. The cardioplegic arrest is commonly used as myocardial protection strategies.1) A wide variety of cardioplegic solutions are routinely used. Cardioplegic solutions concerning standardization in the world do not exist. There is still ongoing discussion on the relative effectiveness of these solutions considering myocardial protection. Current cardioplegic solutions contain various chemical agents that immediately stop the heart in diastole and provide secure protection against myocardial ischemia/reperfusion (I/R) damage. Overall, there are two types of cardioplegic solutions as crystalloid and blood cardioplegia and are often supplied as hypothermic. Blood cardioplegia is made by adding potassium and other substances (Mg++, HCO3−, glucose, etc.) to oxygenated blood from the patient.2)
In cardiac surgery, ischemic episodes result to produce oxidative mediators leading to disrupt cellular integrity.3) Like all components of the cell, the endothelial glycocalyx, which is the inner cover of the vessel, is also adversely affected by oxidative mediators.4) Glycocalyx fragments broken off by oxidative mediators during this process can be collected from plasma. Sialic acid (SA) in the structure of sialoproteins is one of the carbohydrate components of the glycocalyx and previously shown that of high level in plasma after cardiac surgery.5)
Besides glycocalyx, oxidative stress negatively affects both erythrocytes and intravascular compartments. Reactive reagents can break apart from the membranes of erythrocytes and hemoglobin can come out of the erythrocytes that is defective in membrane integrity.6) In this hemolytic processes, the increment in free hemoglobin (fHb) level is correlated with morbidity.7) Regarding intravascular events, albumin is the most commonly found protein in plasma. Likewise, the maintenance of plasma oncotic pressure and drug delivery, albumin has a potent antioxidant property with thiol (SH−) bounds in its structure.8) The three-dimensional structure could be changed during oxidative stress and the resulting new albumin is called ischemia-modified albumin (IMA). IMA is an increasing biomarker especially in cardiac ischemia such as creatine kinase-MB (CK-MB), troponin-I.9) In this ischemic-oxidative process, SH groups in albumin work to neutralize the highly reactive oxidative species (ROS) and subsequently, plasma total SH level is found to be decreased.8) In this protein oxidation process, advanced oxidation protein products (AOPPs), another mediator, are further increased acting as an inflammatory mediator. Moreover, previously shown that AOPPs are risen even in acute injuries in different organs.10)
The aim of the current study was to evaluate the hypothesis that blood cardioplegia could be better on maintaining redox homeostasis than crystalloid cardioplegia in patients undergoing cardiovascular surgery. To assess the effects of cardiac surgery and cardioplegia type on the multifactorial relation between glycocalyx integrity and reactive oxygen species, we measured plasma levels of total SH and AOPPs (oxidative stress markers). Furthermore, glycocalyx degradation was evaluated by measuring plasma levels of SA (glycocalyx compartment) and plasma levels of IMA, CK-MB, troponin-I was determined as a marker of cardiac ischemia and oxidative stress and plasma fHb level was measured for evaluation of erythrocyte membrane integrity preservation against to oxidative stress.
Subjects and Methods
Ethical approval, informed consent, and patients: The current study design was approved by the medical ethics committee of Acibadem University (ATADEK 2014-652). Written informed consent was obtained from all participating patients. The current study was designed as prospectively. In all, 60 patients undergoing elective bypass surgery were included in the present study. Patients with systemic disorders other than hypertension and ejection fraction (EF) less than 40% were excluded from the study. Patients were randomized by the sealed envelope method and were divided into two groups; in the first group, antegrade warm (32–34°C) blood cardioplegia (10 mL/kg; sodium 10 mmol/L, potassium 20 mmol/L, magnesium 12 mmol/L) was administered (n = 30); in the second group, antegrade cold crystalloid cardioplegia (Plegisol) was administered (n = 30) following the cross-clamping. Topical cooling was not performed. All operations were performed by the same surgeon.
Anesthesia and surgical procedure: Alprazolam 0.5 mg (Xanax) was given by oral route to all patients at the preoperative night. Midazolam was administered by intramuscular route 125 μg/kg 30 minutes before the operation. Venous vascular 16G cannulas were used in all patients in the operating room and all patients received saline at a rate of 100 mL/h. Two-channel ECG (DII,V5), pulse oximetry, invasive blood pressure (by 18G pressure cannula), and central venous pressure (CVP) monitoring (8F intraducer is inserted via right internal jugular vein for this purpose under local anesthesia) were provided. Anesthesia was induced with midazolam (50 μg/kg i.v.), followed by pancuronium (2 mg/kg i.v.) and fentanyl (25–35 μg/kg i.v.) and maintenance was achieved by both pancuronium infusion (0.1 mg/kg/h) and 50% oxygen, 50% N2O and 0.7%–1% sevoflurane inhalation. Midazolam and vecuronium infusion were initiated at a rate of 80 μg/kg/hr in all patients. Furosemide was given at a dose of 0.5 mg/kg i.v. Following harvesting of the left internal thoracic artery, activated clotting time (ACT) was increased up to 450–600 seconds by administration of heparin. Cardiopulmonary bypass (CPB) procedure was initiated using 1200 mL Ringer Lactate as priming solution. After CPB initiation, all patients were randomly divided into two groups according to the description above.
Extracorporeal circulation: During extracorporeal circulation (ECC) hematocrit (Hct) was kept at above 20%, and mean arterial blood pressure (MAP) was kept between 50 and 80 mmHg, the pump flow rate was maintained higher than 2/m2 level. Tissue perfusion was maintained through systemic blood pressure, carbon dioxide partial differential of arterial-venous (Pv-aCO2), lactate level, urinary flow rate, and blood gases analysis during ECC. Moderate hypothermia (32°C) was performed in all patients and midazolam and vecuronium doses were decreased at a rate of 60 mg/kg/h following hypothermia. Midazolam and vecuronium were decreased to a rate of 50 μg/kg/h after ECC and then midazolam and vecuronium infusions were stopped after the skin is closed.
Postoperative follow-up: Body temperature was measured with an axillary temperature probe and maintained at 37.0°C with a heating blanket in intensive care unit (ICU). To prevent the patients’ uncontrolled shaking, meperidine (0.4 mg/kg iv) was administered. Postoperative analgesia was achieved with diclofenac sodium at a dose of 1.25 μg/kg. To prevent the postoperative hypertension, beta-blockers (metoprolol: Beloc-Astra) were used according to the patients’ heart rate (HR) and myocardial contractility (EF > 40%). Patients were mechanically ventilated in SIMV + PS mode after the surgery. The respiratory rate at 12/min, tidal volume as 8 mL/kg, FiO2 as 0.5, PEEP as 0–5 cmH2O, pressure support (PS) as 10 cmH2O, trigger sensitivity as 2 cm H2O were set in all patients. Respiratory rate was diminished to 8/min and then 4/min with the beginning of spontaneous breathing of the patient. PS value was reduced step by step to 4 cm H2O according to patient’s respiratory effort and tidal volumes. Conscious patients with PaCO2 <48 mmHg, pH >7.30 and arterial PaO2/FiO2 >250 dopamine dose lower than 5 μg/kg/min, hemodynamically stable and no drainage were extubated. Blood gases, glucose and electrolytes levels were periodically measured after extubation.
Blood sampling and data collection: Measurements were performed at three-time points: Before induction of anesthesia (T1), at the time at ICU admission (T2) and the 24th postoperative hour (T3). At these time points, hemodynamic parameters (HR, MAP), level of hemoglobin, glucose and lactate levels were recorded. Blood samples were collected at tubes without anticoagulant.
Manual biochemical measurements: Advanced oxidative protein products (AOPPs) measurement: AOPPs were determined using the modified method of Hanasand et al.11) Measurement of IMA levels: A modification of the Bar-Or et al. method was used to estimate IMA levels.12) Total thiols (T-SH) determination: The assay of T-SH determination is based on the method of Sedlak and Lindsay was used.13) SA determination: SA concentration was determined by a method of Sydow.14) fHb measurement: All serum samples were diluted. After absorbance measurement, following formula was used to calculate the fHb concentration as g/L
cHb (g/L) = 1.65 A415 − 0.93 A380 − 0.73 A450.15)
Statistical analysis: Normal distribution of all data was tested using Kolmogorov–Smirnov and Shapiro–Wilk’s test. All data are presented as mean ± SEM. The statistical analysis was performed using GraphPad Prism v5.0 (GraphPad Software, La Jolla, CA, USA). A comparative analysis of two groups at same time point was performed using unpaired-t test and one-way ANOVABonferroni post-hoc test was used for repeated measurements. Differences between values were considered statistically significant at p <0.05.
Results
The demographic data for 60 patients who underwent cardiac surgery are presented in Table 1. There were no significant differences between two groups concerning age, height, weight, duration of anesthesia, operation time, cross-clamping, and CPB time (p >0.05). Similarly, there were no significant differences between the hemodynamic parameter such as HR, MAP at all time points (T1, T2, and T3). Hemodynamic parameters are given in Table 2. When two groups were compared for hemoglobin, glucose and lactate levels at all time points, there were no significant differences (p >0.05). These results are given in Table 3.
Table 1. Demographic data.
| Blood | Crystalloid | p level | |
|---|---|---|---|
| Age (Years) | 66 ± 8 | 64 ± 9 | ns |
| Gender (M:F, 1:0) | 14:16 | 15:15 | ns |
| Weight (kg) | 76 ± 14 | 77 ± 13 | ns |
| Height (cm) | 165 ± 9 | 163 ± 8 | ns |
| Operation period (min) | 208 ± 35 | 210 ± 27 | ns |
| Cross-clamping period (min) | 53 ± 13 | 55 ± 14 | ns |
Table 2. Hemodynamic parameters.
| Parameters | T1 | T2 | T3 |
|---|---|---|---|
| Heart rate (min-) | |||
| Blood | 76 ± 3 | 78 ± 2 | 84 ± 2 |
| Crystalloid | 72 ± 3 | 74 ± 3 | 85 ± 3 |
| Mean arterial pressure (mmHg) | |||
| Blood | 76 ± 3 | 78 ± 2 | 84 ± 2 |
| Crystalloid | 72 ± 3 | 74 ± 3 | 85 ± 3 |
Table 3. Blood gases parameters.
| Hb (g/dL) | |||
| Blood | 12.7 ± 0.3 | 10.7 ± 0.2 | 10.2 ± 0.3 |
| Crystalloid | 13.6 ± 0.3 | 10.1 ± 0.2 | 9.6 ± 03 |
| Glucose (mg/dL) | |||
| Blood | 128 ± 7 | 166 ± 10 | 171 ± 8 |
| Crystalloid | 128 ± 9 | 151 ± 9 | 171 ± 12 |
| Lactate (mmol/L) | |||
| Blood | 1.2 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 |
| Crystalloid | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.2 |
All oxidative stress parameters are given in Table 4. When the basal values of all parameters were evaluated (T1), there were no significant differences (p >0.05). In T1 time point, SA levels (mg/g protein) were measured as 3.9 ± 2 (T1), 3.5 ± 1.7 (T2), 2.4 ± 1.5 (T3) in blood cardioplegia group and 6.2 ± 2.4 (T1), 7.0 ± 2.8 (T2), 4.9 ± 1.9 (T3) in crystalloid cardioplegia group. When these results were compared between two groups, there were no significant differences (p >0.05). AOPPs levels (mmol/g protein) were measured as 3.9 ± 0.6 (T1), 2.9 ± 0.5 (T2), 3.1 ± 0.5 (T3) in blood cardioplegia group and 6.2 ± 2.4 (T1), 7.0 ± 2.8 (T2), 4.9 ± 1.9 (T3) in crystalloid cardioplegia group. T-SH levels (umol/gr protein) were measured as 36.4 ± 5.3 (T1), 37.9 ± 6.2 (T2), 55.8 ± 9.7 (T3) in blood cardioplegia group and 34.7 ± 8.6 (T1), 44.0 ± 9.0 (T2), 46.1 ± 12.0 (T3) in crystalloid cardioplegia group. fHb (g/dL) levels were measured as 0.09 ± 0.04 (T1), 0.25 ± 0.02 (T2), 0.04 ± 0.01 (T3) in blood cardioplegia group and 0.05 ± 0.01 (T1), 0.17 ± 0.02 (T2), 0.04 ± 0.01 (T3) in crystalloid cardioplegia group. AOPPs, T-SH, and fHb levels were compared in two groups. There were also no significant differences between two groups and all time points (p > 0.05). But when fHb was compared in two groups and three-time points, fHb level significantly increased in T2 according to T1 and then decreased to first level again in T3 in two group. When T2 fHb levels were compared between two group, significantly higher levels were detected in blood cardioplegia group.
Table 4. Oxidative stress and glycocalyx degradation parameters.
| Parameters | T1 | T2 | T3 |
|---|---|---|---|
| Sialic acid (mg/g protein) | |||
| Blood | 3.9 ± 2.0 | 3.5 ± 1.7 | 2.4 ± 1.5 |
| Crystalloid | 6.2 ± 2.4 | 7.0 ± 2.8 | 4.9 ± 1.9 |
| Advanced oxidation protein products (mmoL/g protein) | |||
| Blood | 3.9 ± 0.6 | 2.9 ± 0.5 | 3.1 ± 0.5 |
| Crystalloid | 6.2 ± 2.4 | 7.0 ± 2.8 | 4.9 ± 1.9 |
| Total thiol (umoL/g protein) | |||
| Blood | 36.4 ± 5.3 | 37.9 ± 6.2 | 55.8 ± 9.7 |
| Crystalloid | 34.7 ± 8.6 | 44.0 ± 9.0 | 46.1 ± 12.0 |
| Free Hb (g/L) | |||
| Blood | 0.09 ± 0.04 | 0.25 ± 0.02aaa | 0.04 ± 0.01bbb |
| Crystalloid | 0.05 ± 0.01 | 0.17 ± 0.02aaa* | 0.04 ± 0.01bbb |
| Ischemia-modified albumin (AbsUnit) | |||
| Blood | 0.49 ± 0.04 | 0.48 ± 0.03 | 0.45 ± 0.06 |
| Crystalloid | 0.45 ± 0.06 | 0.51 ± 0.06 | 0.62 ± 0.05*aa |
| Creatine kinase-MB (IU) | |||
| Blood | 15.4 ± 4.6 | 51.4 ± 15cc** | 29.4 ± 8cc |
| Crystalloid | 13.4 ± 5.3 | 60.8 ± 26.2cc** | 32.3 ± 17.1cc |
| Troponin-I (pg/mL) | |||
| Blood | 0.1 ± 0.4 | 260 ± 118** | 294 ± 189 |
| Crystalloid | 0.2 ± 0.4 | 552 ± 799** | 390 ± 231 |
*p <0.05; vs blood cardioplegia group at the same point. aap <0.01; aaap <0.001; vs T1 time point. bbbp <0.001; vs T2 time point. ccp <0.05; vs T1 point. **p <0.05; vs blood cardioplegia group at the same point.
IMA levels were measured as 0.49 ± 0.04 (T1), 0.48 ± 0.03 (T2), 0.45 ± 0.06 (T3) in blood cardioplegia group and 0.45 ± 0.06 (T1), 0.51 ± 0.06 (T2), 0.62 ± 0.05 (T3) in crystalloid cardioplegia group. There were significant differences between two groups in T3 time point (p <0.05).
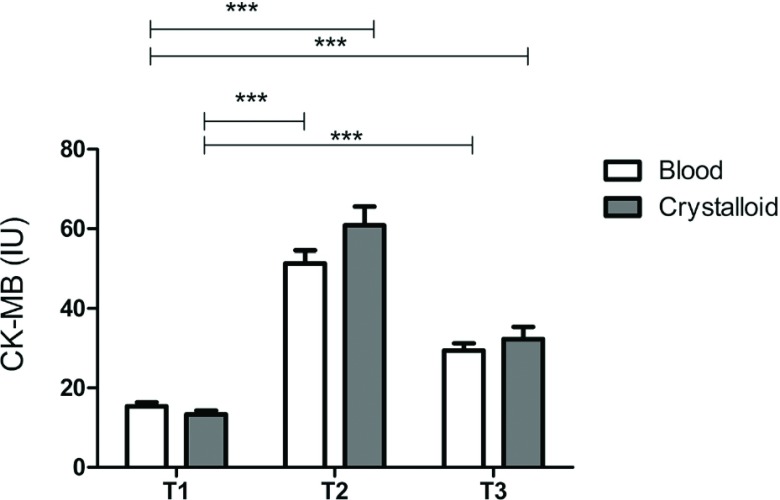
CK-MB and troponin levels are given in Table 4. At the T2 and T3 time points, CK-MB and troponin levels in plasma were significantly increased compared to respective baseline time points (p <0.05). In T2 time point, both CK-MB and troponin-I levels were found to be insignificantly higher in crystalloid group. CK-MB levels are also given in Fig. 1.
Fig. 1. Creatine kinase-MB levels. ***p <0.001.
Discussion
In our study, we evaluated the effects of crystalloid and warm blood cardioplegia on glycocalyx integrity and oxidative stress status in patients undergoing bypass surgery. The main results of our study showed that type of cardioplegia did not effect on SA shedding which could be attributed to preserved glycocalyx integrity. Moreover, we are aware that other glycocalyx substances must be measured to clarify this situation.
SA, one of the components of glycocalyx, increases in plasma when glycocalyx is damaged. In this case, the barrier function of vascular endothelial cells is lost and interstitial edema occurs as fluid extravagates from intravascular to extravascular space. Thus, a positive fluid balance is seen when intravascular volume is maintained. However, there is no study in the literature showing the type of cardioplegia effect on the integrity of the glycocalyx and fluid balance in open-heart surgery. Hence, our study is the first one which points out the relation between cardioplegia solution and glycocalyx degradation.
It has been known for 10 years that high blood SA levels pose a cardiovascular risk and may cause myocardial damage. Lindenberg et al. have stated that the serum level of SA is the independent predictor of cardiovascular mortality.16) However, contrary results have also been published.17) Subsequently, SA has been used to compare various surgical procedures or medical treatments.18) In our study, crystalloid and blood cardioplegia were found to have similar effects on serum SA level. Berkan et al. have investigated whether SA is a marker of myocardial injury, like troponin-T and CK-MB. Samples were collected from coronary sinus, arterial and venous blood. SA levels were found to correlate with troponin-T and CK-MB levels after cross-clamping. According to these results, the authors have stated that SA could be a biomarker for myocardial damage.19) We believe that these results may provide us to choose liberally the cardioplegic solution.
The shedding of SA to plasma following glycocalyx damage occurs after I/R injury in cardiovascular surgery. The first study showing the effect of I/R on glycocalyx integrity was published by Rehm et al.20) They found significantly increased serum levels of glycocalyx component after I/R in aortic surgery. Bruegger et al. found the same results in pediatric patients.21) Although the two studies used the same cardioplegic solutions, we compared a different cardioplegic solution. Thus, we were able to assess the effect type of cardioplegia on glycocalyx integrity.
Glycocalyx damage is described only by I/R in patients undergoing open-heart surgery. However, Bruegger et al. have compared the on-pump and off-pump surgery, unlike us.22) They found the plasma increment of glycocalyx components in both groups at the end of operation. The results of the on-pump group were similar in our study. As a conclusive of the study, the researchers stated there could be a different mechanism of glycocalyx damage than I/R in open-heart surgery, such as protease activation, increased proinflammatory cytokines, atrial natriuretic peptide or increasing mechanical stress.
Other factors that affect the barrier function of the glycocalyx include the applied fluids in surgery. Many studies have investigated the effect of fluids on glycocalyx integrity in both cardiac surgery and non-cardiac surgery. In our research, our comparison of crystalloid cardioplegia and colloid cardioplegia is the first in the literature. Mathru et al. compared pregnant women who received and did not receive crystalloid bolus to prevent hypotension before spinal anesthesia.23) Glycocalyx degradation products were significantly higher in plasma with patients given a crystalloid bolus.
We also assessed IMA, CK-MB and troponin-I level to determine oxidative stress and the status of cardiac ischemia. Recently, IMA levels have been interpreted as a marker that can detect the earliest phase of myocardial ischemia. Kanko et al. found that the level of IMA increased in the early period of the ischemic phase in a patient who received coronary artery bypass grafting (CABG).24) Similarly, in the literature, the levels of IMA in both groups in our study were found to be higher after reperfusion than before. Otherwise, we found a significantly lesser increase in IMA used blood cardioplegia than used crystalloid cardioplegic group in our study, which compared two different cardioplegic solutions. This result is essential in terms of showing the myocardial protection of blood cardioplegia during surgery. Karahan et al. investigated the effectiveness of cold blood with added anti-oxidant (N-acetyl cysteine, NAC) cardioplegia on myocardial protection by measuring serum IMA level.25) Similar to our study, they noticed that serum IMA level was significantly lesser increase in the NAC than the other group, could show myocardial damage, and could be used in follow-up to affect cardioplegia on myocardial protection. Unlike their study, there was a comparison on colloid cardioplegia with crystalloid cardioplegia instead of two colloid cardioplegia. As a result, serum IMA levels were also significantly lower in the blood cardioplegia group. Furthermore, in T2 time point, both CK-MB and troponin-I levels were found to be insignificantly higher in crystalloid group. This findings were consistent with higher level of IMA as attributed to cardiac ischemia and oxidative stress.
Serum T-SH level as an oxidative stress parameter was further evaluated in our study. Plasma proteins (mainly albumin) are susceptible to oxidation due to the free thiol (sulfhydryl) moieties in their molecular structures. The amount of free thiol is reduced when encountering oxidative stress.8) Hu et al. found that defense status against oxidative mediators can be determined by measuring the thiol groups.26) A study by Pepper and colleagues found that higher levels of total serum thiol in patients used blood cardioplegia as in crystalloid cardioplegia.27) These authors emphasized that use of blood cardioplegia may be protective against oxidative stress, thanks to higher thiol levels. It is known that oxidative stress occurs for various reasons during bypass surgery. We also have found that total thiol insignificantly increased in the group that used blood cardioplegia at the end of the surgery, compared to the beginning of the surgery in our study. We believe this increase might have been due to the thiols in the donor’s blood have passed to the patient. Hence, blood cardioplegia can make patients more resistant to surgical stress.
The AOPPs levels were measured as another oxidative stress parameter in our study. Free oxygen radicals during inflammatory and oxidative processes cause the formation of AOPPs with structural malformation by affecting protein–carbonyl compounds in plasma. WitkoSarsat et al. stated that AOPPs might be a biomarker of oxidative stress and inflammatory process.28) Limited numbers of publications are available on the relationship of AOPPs to bypass surgery in the literature. The relationship between acute kidney injury (AKI) and AOPPs in patients receiving CABG was established.29) Both studies, which applied standard anesthesia protocols in all patients, detected a significant correlation between the increase in AOPPs and postoperative AKI incidence. AOPPs levels were similar between the groups in our study and we used different cardioplegic solutions. Within the group, there were not significantly different AOPPs levels before induction and postoperative 24 hours. Therefore, we believe that our patients may have had exposure to minimal and recycled oxide-inflammatory stress and none of the patients developed AKI in our study. In another study conducted in our clinic, we assessed the effects of dilutional anemia or blood transfusion on the development of AKI by neutrophil gelatinase-associated lipocalin (NGAL) and AOPPs level. Unlike the above two studies, we determined that AOPPs levels were similar in patients who received and did not receive the blood.29)
Finally, we measured fHb levels to evaluate the fluid homeostasis and hemolytic process. ECC, transfusion of banked blood and aspiration systems are the sources of fHb in open-heart surgery. A study done by Rinne compared crystalloid cardioplegia with blood cardioplegia in their study.30) Contrary to popular belief, it was pointed out that the use of blood cardioplegia did not increase the amount of fHb more than expected. Additionally, fHb level increased similarly in both groups with the onset of ECC and reached initial values at 24th postoperative hour. The absence of any significant increment at the end of surgery may be due to the nature of the surgery in both groups. Although the increase in fHb has not continued, unknown biochemical processes may have begun in the growing period and hence, it could be an upturning investment for later. Additionally, it is noteworthy that the blood–cardioplegia lead to increase in plasma level of fHb. The fHb increment may be related to the occurrence of storage lesions in the blood bank. In recent years, the incidence of increased in hemolysis and fHb level has reduced with technological advances in the ECC systems, showing the relationship between AKI with erythrocyte transfusion after bypass surgery and lowering the transfusion threshold in open-heart surgery.30)
Blood samples were taken from the systemic circulation in our study because the cannulation of the sinus coronarius, which allows taking blood samples from cardiac circulation, is not routinely applied in our clinic, due to possible complications. Although this situation can be considered a limitation of our study, we believe that the results were not affected. Although cardioplegia type does not appear to be a decisive factor for glycocalyx damage, crystalloid cardioplegia could cause stress on the heart. Therefore, blood storing at the blood bank should be used with caution. However, the effects of each scenario and strategy on endothelial glycocalyx need to be studied in more extensive prospective randomized series in open-heart surgery.
Acknowledgment
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University. Project number: 44902.
Disclosure Statement
None of the authors have any conflicts of interest to disclose.
References
- 1).Liakopoulos OJ, Kuhn EW, Choi YH, et al. Myocardial protection in cardiac surgery patients requiring prolonged aortic cross-clamp times: a single-center evaluation of clinical outcomes comparing two blood cardioplegic strategies. Thorac Cardiovasc Surg 2010; 51: 895-905. [PubMed] [Google Scholar]
- 2).Siddiqi S, Blackstone EH, Bakaeen FG. Bretschneider and del Nido solutions: Are they safe for coronary artery bypass grafting? If so, how should we use them? J Card Surg 2018; 33: 229-34. [DOI] [PubMed] [Google Scholar]
- 3).Zakkar M, Ascione R, James AF, et al. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol Ther 2015; 154: 13-20. [DOI] [PubMed] [Google Scholar]
- 4).Song JW, Goligorsky MS. Perioperative implication of the endothelial glycocalyx. Korean J Anesthesiol 2018; 71: 92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Berkan O, Sagban M. Sialic acid or troponin T to detect perioperative myocardial damage in patients undergoing elective coronary artery bypass grafting. Circ J 2002; 66: 1019-23. [DOI] [PubMed] [Google Scholar]
- 6).Caimi G, Montana M, Canino B, et al. Erythrocyte deformability, plasma lipid peroxidation and plasma protein oxidation in a group of OSAS subjects. Clin Hemorheol Microcirc 2016; 64: 7-14. [DOI] [PubMed] [Google Scholar]
- 7).Plewes K, Kingston HWF, Ghose A, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis 2017; 17: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Taverna M, Marie AL, Mira JP, et al. Specific antioxidant properties of human serum albumin. Ann Intensive Care 2013; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Voudris KV, Chanin J, Feldman DN, et al. Novel inflammatory biomarkers in coronary artery disease: potential therapeutic approaches. Curr Med Chem 2015; 22: 2680-9. [DOI] [PubMed] [Google Scholar]
- 10).Du SL, Zeng XZ, Tian JW, et al. Advanced oxidation protein products in predicting acute kidney injury following cardiac surgery. Biomarkers 2015; 20: 206-11. [DOI] [PubMed] [Google Scholar]
- 11).Hanasand M, Omdal R, Norheim KB, et al. Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta 2012; 413: 901-6. [DOI] [PubMed] [Google Scholar]
- 12).Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med 2000; 19: 311-5. [DOI] [PubMed] [Google Scholar]
- 13).Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with ellman’s reagent. Anal Biochem 1968; 25: 192-205. [DOI] [PubMed] [Google Scholar]
- 14).Sydow G. A simplified quick method for determination of sialic acid in serum. Biomed Biochim Acta 1985; 44: 1721-3. [PubMed] [Google Scholar]
- 15).Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand J Clin Lab Invest 1959; 11: 66-70. [DOI] [PubMed] [Google Scholar]
- 16).Lindberg G, Eklund GA, Gullberg B, et al. Serum sialic acid concentration and cardiovascular mortality. BMJ 1991; 302: 143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Wu EB, Lumb P, Chambers JB, et al. Plasma sialic acid and coronary artery atheromatous load in patients with stable chest pain. Atherosclerosis 1999; 145: 261-6. [DOI] [PubMed] [Google Scholar]
- 18).Aytekin B, Ünal EU, Demir A, et al. Unilateral antegrade cerebral perfusion and moderate hypothermia: assessing safety with novel biomarkers. Heart Lung Circ 2017; 26: 495-503. [DOI] [PubMed] [Google Scholar]
- 19).Berkan O, Göl MK, Günay L, et al. Sialic acid is an indicator of inflammation due to cardiopulmonary bypass but not myocardial damage. J Cardiovasc Surg 2002; 43: 489-93. [PubMed] [Google Scholar]
- 20).Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007; 116: 1896-906. [DOI] [PubMed] [Google Scholar]
- 21).Bruegger D, Brettner F, Rossberg I, et al. Acute degradation of the endothelial glycocalyx in infants undergoing cardiac surgical procedures. Ann Thorac Surg 2015; 99: 926-31. [DOI] [PubMed] [Google Scholar]
- 22).Bruegger D, Rehm M, Abicht J, et al. Shedding of the endothelial glycocalyx during cardiac surgery: on-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2009; 138: 1445-7. [DOI] [PubMed] [Google Scholar]
- 23).Powell MF, Mathru M, Brandon A, et al. Assessment of endothelial glycocalyx disruption in term parturients receiving a fluid bolus before spinal anesthesia: a prospective observational study. Int J Obstet Anesth 2014; 23: 330-4. [DOI] [PubMed] [Google Scholar]
- 24).Kanko M, Yavuz S, Duman C, et al. Ischemia-modified albumin use as a prognostic factor in coronary bypass surgery. J Cardiothorac Surg 2012; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Karahan SC, Koramaz I, Altun G, et al. Ischemia-modified albumin reduction after coronary bypass surgery is associated with the cardioprotective efficacy of cold-blood cardioplegia enriched with N-acetylcysteine: a preliminary study. Eur Surg Res 2010; 44: 30-6. [DOI] [PubMed] [Google Scholar]
- 26).Pepper JR, Mumby S, Gutteridge JM. Blood cardioplegia increases plasma iron overload and thiol levels during cardiopulmonary bypass. Ann Thorac Surg 1995; 60: 1735-40. [DOI] [PubMed] [Google Scholar]
- 27).Witko-Sarsat V, Nguyen Khoa T, Jungers P, et al. Advanced oxidation protein products: oxidative stress markers and mediators of inflammation in uremia. Adv Nephrol Necker Hosp 1998; 28: 321-41. [PubMed] [Google Scholar]
- 28).Liang X, Chen Y, Zhuang J, et al. Advanced oxidation protein products as prognostic biomarkers for recovery from acute kidney injury after coronary artery bypass grafting. Biomarkers 2012; 17: 507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Arıtürk C, Ozgen ZS, Kilercik M, et al. Comparative effects of hemodilutional anemia and transfusion during cardiopulmonary bypass on acute kidney injury: a prospective randomized study. Heart Surg Forum 2015; 18: E154-60. [DOI] [PubMed] [Google Scholar]
- 30).Rinne TT. Blood cardioplegia does not increase haemolysis. A comparison between crystalloid and blood cardioplegia in coronary artery bypass grafting. Scand J Thorac Cardiovasc Surg 1996; 30: 65-9. [DOI] [PubMed] [Google Scholar]