Abstract
Background:
Dasatinib is a potent BCR-ABL1 and Src family tyrosine kinase inhibitor (TKI). It is approved at a dose of 100 mg orally daily for the treatment of chronic myeloid leukemia in chronic phase (CML-CP). This dose schedule is associated with myelosuppression and pleural effusions. Anecdotal data suggests that lower doses may be as effective and less toxic. The aim of this study was to assess the efficacy and safety of lower dose of dasatinib 50 mg daily in patients with newly diagnosed CML-CP.
Methods:
Seventy-five patients with newly diagnosed CML-CP received dasatinib 50 mg daily. Eligibility and response criteria were standards as used in previous protocols.
Results:
At a median follow-up of 9 months, 60 patients were evaluable for response at 3 months. Patients achieving BCR-ABL1 transcript levels by International Standard (IS) ≤10% and ≤1% at 3 months were 93% and 72% respectively. The rates of complete cytogenetic response (CCyR) by conventional cytogenetics or fluorescence-in-situ hybridization (FISH) at 6 and 12 months were 86% and 88%, respectively. At 12 months, 79%, 71% and 46% patients achieved MMR, MR4.0 and MR4.5, respectively. Nine patients had dose interruption for ≤14 days. Only 1 patient developed pleural effusion requiring dose reduction to 20mg. All patients remain alive and with no transformation so far.
Conclusion:
Dasatinib 50 mg daily is active and well tolerated in newly diagnosed CML-CP. It should be further explored as a new potential standard of care option in CML. (ClinicalTrials.gov number, NCT02689440).
Keywords: chronic myeloid leukemia, dasatinib, complete cytogenetic response, major molecular response
Precis:
1. Dasatinib at lower dose of 50mg daily is active and well tolerated in the treatment of newly diagnosed chronic phase chronic myeloid leukemia.
2. Dasatinib at lower dose is also likely to be more cost effective.
Introduction
The therapeutic landscape of chronic myeloid leukemia (CML) changed dramatically with the development of small molecule tyrosine kinase inhibitors (TKIs) targeting BCR-ABL1. The 10-year survival rate in CML in chronic phase (CML-CP) improved from approximately 20% to 80–90%.1 Four TKIs, imatinib, nilotinib, dasatinib and most recently bosutinib are approved for frontline therapy of patients with newly diagnosed CML-CP.1–3 Therapy with second generation TKIs reported significantly deeper and faster responses with no impact on long-term survival.8
Dasatinib is an oral second generation TKI that is 325 times more potent than imatinib in vitro.4–6 It also inhibits the Src family of kinases, which may be important in blunting critical cell signaling pathways.7 Dasatinib was initially evaluated in patients in the salvage setting, and later compared to imatinib and approved for frontline CML therapy.2 A five-year follow-up showed that dasatinib induced more rapid and deeper responses at early time points compared with imatinib.8 At 3 months, a higher proportion of patients treated with dasatinib achieved BCR-ABL1 transcripts (IS) ≤10% (84% vs 64%, P < 0.0001).9 Dasatinib induced higher rates of 12-month confirmed complete cytogenetic response (CCyR) (77% vs 66%, p = 0.007), and higher 5-year cumulative major molecular response (MMR) rates (76% vs 64%, p < 0.0022) and molecular response with 4.5 log reduction (MR4.5) (42% vs 33%, P = 0.025). Pleural effusions occurred more frequently with dasatinib therapy (28% vs <1%).8 Additional side effects encountered with dasatinib included myelosuppression (20%), and rare pulmonary hypertension (5%).8, 9
In early clinical trials evaluating dasatinib, the drug was noted to be active at lower doses with better safety profile.10 In a randomized trial of four dose schedules of dasatinib (100mg vs 140mg daily; single dose vs twice daily schedule), dasatinib 100 mg single daily dose was found as effective as 140 mg daily with a better safety profile.11 Furthermore investigators from the DASISION trial have reported the ability to maintain the efficacy of dasatinib among patients who had their dose reduced, while improving its safety profile.12
The aim of the study was to evaluate a lower dose of dasatinib, 50 mg daily, in patients with newly diagnosed CML. Lower doses may improve efficacy by improving drug exposure and minimizing drug interruptions due to adverse events. Additionally, this strategy may prove to be more cost-effective, particularly with the availability of generic formulations of imatinib.
Patients and Methods
Patients with a new diagnosis of Philadelphia chromosome (Ph)-positive CML-CP (diagnosis within 12 months from the start of study treatment) were eligible. Patients should have received no prior CML therapy other than hydroxyurea or a maximum of 1 month of therapy with a TKI. Prior therapy with 1 to 2 doses of cytarabine was allowed. Other eligibility criteria included age ≥ 18 years, performance status of 0 to 2, and adequate organ function. Patients with clonal evolution were eligible provided they met all other criteria for chronic phase disease (blasts < 15%, blasts plus promyelocytes < 30%, basophils < 20%, and platelets > 100 × 109/L unless related to therapy). Patients with uncontrolled angina within 3 months, diagnosed or suspected with congenital long QT syndrome, with any history of clinically significant ventricular arrhythmias (such as ventricular tachycardia, ventricular fibrillation, or torsade de pointe) with prolonged QTc interval on pre-entry electrocardiogram (> 460 msec), or with a history of a significant bleeding disorder unrelated to CML were not eligible. The study was approved by the institutional review board (IRB); all patients signed an IRB–approved informed consent.
Patients received dasatinib 50 mg once daily. Treatment was continued until disease progression or unacceptable toxicity. Patients with grade 3 to 4 non-hematologic toxicity had treatment transiently interrupted, and dasatinib was restarted (after resolution to grade ≤1) at one dose level reduction to 40 mg once daily. For hematologic toxicity, treatment was interrupted for grade 4 neutropenia (neutrophils <0.5 × 109/L) or thrombocytopenia (platelets <40 × 109/L). Treatment was restarted at the same dose if recovery to above these levels occurred within 2 weeks. Treatment was restarted with a reduction of one dose level if recovery time was >2 weeks.
Patients who did not achieve BCR-ABL1 transcripts (IS) ≤10% by 3 months, a complete cytogenetic response after 6 months (Ph FISH positivity ≤2%; BCR-ABL1 transcripts (IS) ≤1%) or MMR after 12 months of therapy (BCR-ABL1 transcripts (IS) ≤0.1%) and have experienced no grade ≥3 toxicity may have the dasatinib dose escalated to 100 mg daily. These patients will be counted as failures for the purpose of statistical monitoring described below.
Patients were monitored by routine blood counts with differential and blood chemistry every 1 to 2 weeks for the first month, then every 4 to 8 weeks thereafter. Bone marrow aspiration with cytogenetic analysis and peripheral-blood quantitative reverse transcription polymerase chain reaction (PCR) for BCR-ABL1 transcripts were performed at baseline, then peripheral blood FISH and PCR for BCR-ABL1 transcripts every 3 months for 1 year, and every 6 months thereafter. From patient 19 and onwards, a confirmatory conventional cytogenetic analysis was performed at 6 months. For FISH testing, 200 cells were routinely counted. Response criteria were as previously defined.13 Complete cytogenetic response was defined by 0% Ph-positive metaphases based on G-banding with at least 20 metaphases counted, or by FISH ≤2%, or by BCR-ABL1 transcripts (IS) ≤1%. As the data matured showing discrepant results between FISH testing and PCR at 3 months, and based on the published literature, BCR-ABL1 transcripts (IS) ≤1% was used to define CCyR. Molecular responses were categorized as major molecular response (MMR) defined as BCR-ABL1 transcripts (IS) ≤0.1% and MR4.5 defined as BCR-ABL1 transcripts (IS) ≤0.0032% with detection sensitivity of ≥1 in 105 cells.13 The (IS) conversion factor at our institution is × 0.35. Therefore a patient reported to have BCR-ABL1 transcripts <0.01% has a converted value of BCR-ABL1 transcripts (IS) of <0.01% × 0.35 that equals to <0.0035%. This is interpreted as equivalent to ≤0.0032%, or equal to MR4.5. Patients with reported BCR-ABL1 transcripts 0.01% has a converted value of BCR-ABL1 transcripts (IS) of 0.01% × 0.35 that equals to 0.0035% and still considered MR4.0 for the purpose of this analysis.
This was a pilot study (NCT02689440), initially designed to enroll 50 patients. Additional 25 patients were enrolled to assess and confirm the efficacy. The primary objective was to meet a target of MMR rate of 50% at 12 months. This was based on historical experience with dasatinib.14 Two end points were monitored, the MMR probability at 12 months and the toxicity rate. The secondary endpoint was to meet a target of 75% rate of CCyR at 6 months. The goal was to determine whether lower dose of dasatinib would at least maintain the same efficacy as standard-dose dasatinib 100mg daily with a better safety profile. Seventy-five patients were to be treated, with early stopping rules for response and toxicity. The study would stop if, at any time during the study, it was determined that there is more than 5% chance that the MMR probability at 12 months is less than 50%, and more than 5% chance that the CCyR probability at 6 months is less than 75%. For toxicity monitoring, the study would stop if, at any time during the study, it was determined that there is more than 95% chance that the toxicity of grade 3 or higher, is more than 20%. This evaluation would be done at 6 months. These stopping rules for responses and toxicity would be applied starting from the first patient enrolled and in cohorts of 10.
Results
Patient and treatment characteristics
Between March 2016 and November 2017, 75 patients were consecutively treated. Baseline characteristics are summarized in Table 1. The median age was 47 years (range, 19 to 84 years). The median time from diagnosis to treatment was 6 days (range, 0–40); 15 (20%) patients had received TKI for less than 28 days prior to enrollment: 8 with dasatinib, 5 with imatinib, and 2 with nilotinib, with median time on prior TKI therapy of 19 days (range, 1–29). By Sokal risk score, 49 (65%) patients had low-risk, 20 (27%) had intermediate-risk, and 6 (8%) had high-risk. The median follow-up time for all patients is 9 months (range, 0.7 – 21.2). The data cutoff was 11/2017.
Table 1.
Baseline patient characteristics, N=75
| Parameter | No (%); or median [range] |
|---|---|
| Median age, yr | 47 [19–84] |
| Male gender | 42 (56) |
| Median WBC, ×109/L | 44.4 [2–508] |
| Median hemoglobin, g/dl | 12.4 [8–17] |
| Median platelets, × 109/L | 353 [42–1399] |
| Median peripheral-blood basophils, % | 3 [0–17] |
| Median peripheral-blood blasts, % | 0 [0–7] |
| Median bone marrow blasts, % | 1 [0–9] |
| Sokal risk | |
| Low | 49 (65) |
| Intermediate | 20 (27) |
| High | 6 (8) |
| Previous CML therapy (≤30 days) | 15 |
| Dasatinib | 8 (53) |
| Imatinib | 5 (33) |
| Nilotinib | 2 (13) |
Response and Outcome
Overall, 2 patients discontinued therapy; one patient after 5 days of treatment initiation due to a traumatic brain bleed and the second patient due to failure to achieve CCyR and MMR at 12 months into therapy. Fifteen patients have been observed for less than 3 months. Of the 75 patients who started therapy on low-dose dasatinib, 60 patients have been observed for ≥3 months and are thus evaluable for cytogenetic and molecular response.
Table 2 lists the rates of responses at 3, 6, 9 and 12 months respectively. For this analysis, all 60 evaluable patients are kept in the denominator up to the time of their last follow-up, with patients who discontinued therapy included up to the follow-up time that would correspond to the time of this report had they stayed on the study. Therefore all rates are calculated at the time point, rather than as cumulative rates (by the time point). This is a more conservative form of reporting responses compared with previous trials (reporting cumulative rates). Sixty patients reached the 3-month follow-up, 50 patients reached the 6-month follow-up, 34 patients reached the 9-month follow-up and 24 patients reached the 12-month follow-up time-point respectively. Responses occurred early on the study. At 3 months, 56/60 (93%) patients achieved a BCR-ABL1 transcripts (IS) ≤10%, and 43/60 (72%) patients achieved BCR-ABL1 transcripts (IS) ≤1%, equivalent to CCyR. At 6 months, 43/50 (86%) patients achieved CCyR.
Table 2.
Response rates – at 3, 6, 9 and 12 months of dasatinib at 50mg daily*
| No. Response/Total (%) | 3 months | 6 months | 9 months | 12 months |
|---|---|---|---|---|
| BCR-ABL1 transcripts (IS) ≤10% | 56/60 (93) | 48/50 (96) | 32/34 (94) | 23/24 (96) |
| PCR ≤1% | 43/60 (72) | 42/50 (84) | 30/34 (88) | 22/24 (92) |
| CCyR | 26/60 (43) | 43/50 (86) | 29/34 (85) | 21/24 (88) |
| MMR | 17/60 (28) | 33/50 (66) | 28/34 (82) | 19/24 (79) |
| MR4.0 | 2/60 (3) | 16/50 (32) | 19/34 (56) | 17/24 (71) |
| MR4.5 | 0/60 (0) | 9/50 (18) | 9/34 (26) | 11/24 (46) |
CCyR=complete cytogenetic response; MMR=major molecular response (BCR-ABL1 transcripts [IS] ≤0.1%); MR4.0 = BCR-ABL transcripts (IS) ≤0.01%; MR4.5 = BCR-ABL transcripts (IS) ≤0.0032%
As the data matured showing discrepancy between PCR and FISH testing at 3 months, BCR-ABL1 transcripts (IS) ≤1% was used define CCyR. In addition cytogenetic assessment by conventional cytogenetics was mandated at 6 months, starting patient 19 and onwards.
The rates of CCyR with 95% confidence intervals (CI) at 6, 9 and 12 months after the initiation of dasatinib treatment were 86% (CI 73–94), 85% (CI 69–95) and 88% (CI 68–97) respectively. At 12 months, 19/24 (79%) patients achieved MMR. The rates of MMR at 3, 6, 9, and 12 months after the initiation of dasatinib were 28% (CI 17–41), 66% (CI 51–79), 82% (CI 65–93) and 79% (CI 58–93) respectively. At 12 months, 17/24 (71%) patients and 11/23 (46%) patients achieved MR4.0 and MR4.5 respectively. There was a rapid reduction in transcript levels, with median BCR-ABL1 transcripts (IS) of 0.34% (0.007–12.9) at 3 months, 0.02% (0–11.2) at 6 months, and 0.0035% (0–9.5) at 12 months. At 12 months, CCyR had been achieved in 88% of patients, MMR in 79%, MR4.0 in 71% of patients and MR4.5 in 46%. The median time to CCyR was 3.2 months (range, 2.7–6.3), the median time to MMR was 5.8 months (range, 2.7–9.0), and the median time to MR4.5 was 7.6 months (range, 5.7–12.6).
Two patients did not meet the milestones per protocol specifications (failure to achieve a cytogenetic response at 6 months) and had the dasatinib dose increased to 100mg daily. These patients were considered as treatment failure. Neither of the 2 patients tested positive for ABL kinase domain mutations. The first patient was noted to have a decline in hemoglobin to 6 g/dl and platelets of 60×109/L 8 weeks into treatment with dasatinib 100mg daily dose. Work-up for anemia was negative. Dasatinib was held for 3 weeks and resumed with the recovery of blood counts, at a dose reduction to 70mg daily dose. At the 12 month follow-up, repeat bone marrow exam showed persistent disease without evidence of cytogenetic or molecular response. No ABL kinase domain mutations were detected. The patient was subsequently taken off study due to lack response to dasatinib therapy. The second patient did not achieve a cytogenetic response at 6 months. Dasatinib was increased to 100mg daily. Two patients were taken off study: In addition to the first patient described above (taken off study due to lack of response), a second patient was taken off study because of a traumatic brain bleed 4 days into therapy (withholding dasatinib judged by the treating physician to be in the patient’s best interest).
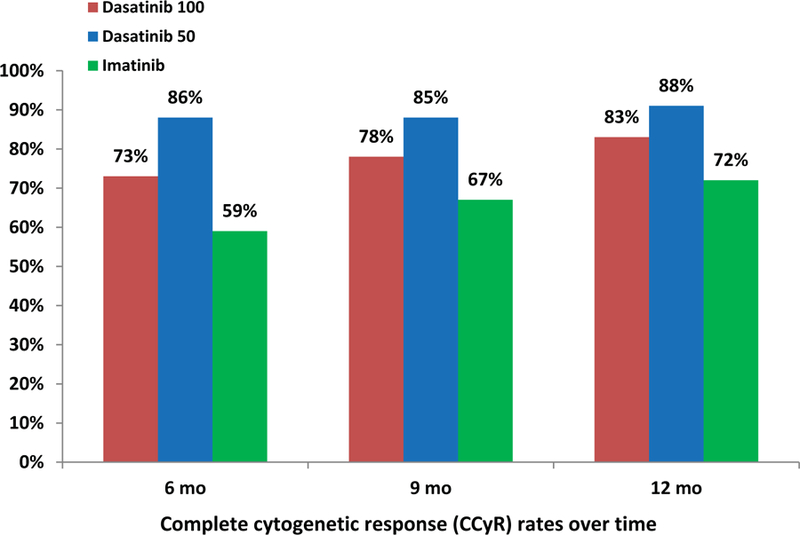
So far, the responses have been durable: none of the patients who achieved CCyR and MMR on the study have lost their response to therapy. All patients are alive, and none had disease transformation to accelerated or blast phase. When compared with historical data (DASISION trial) in patients treated with imatinib (n=260), dasatinib 100mg daily (n=259), the results obtained with lower doses of dasatinib were more favorable.8 Higher rates of CCyR and MMR were noted with dasatinib 50mg daily compared to dasatinib 100mg daily and imatinib (Figure 1).2,8 Early response to therapy with BCR-ABL1 transcripts (IS) ≤10% and ≤1% at 3 months was also noted to be higher with lower dose dasatinib compared to historical data with dasatinib 100mg and imatinib (Figure 2).9
Figure 1.
A. CCyR rates compared to historical data (DASISION trial) 2,8
B. MMR rates compared to historical data (DASISION trial) 2,8
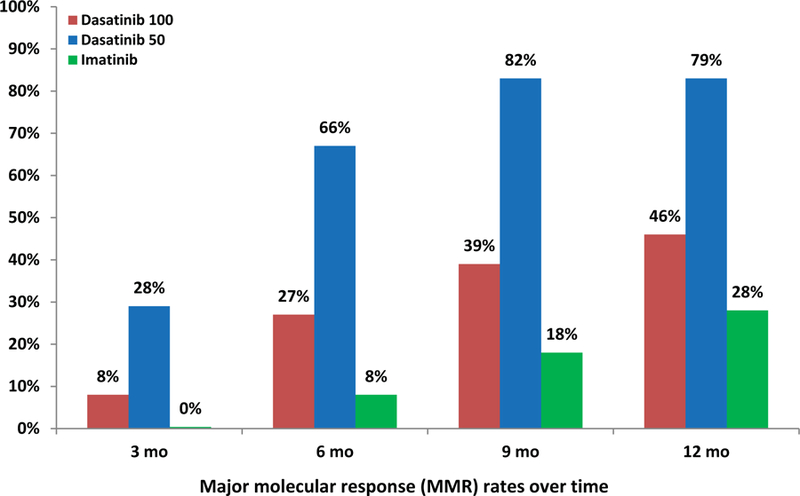
Figure 2.
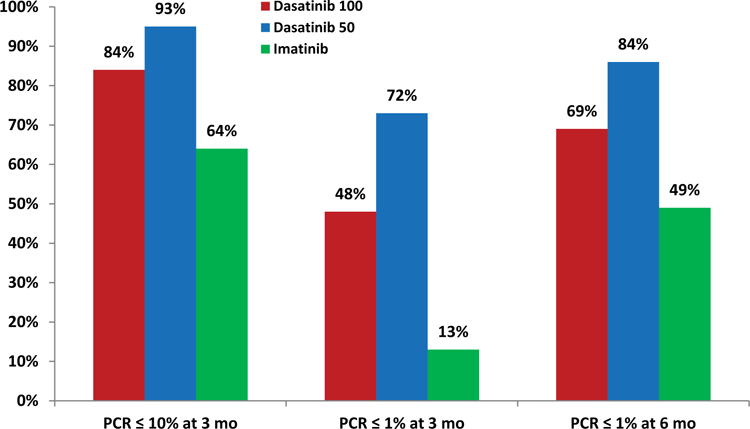
Responses according to BCR-ABL1 transcript levels at 3 and 6 months – a comparison with historical data (DASISION trial) 9
An interesting observation was the discrepant results between the PCR and FISH studies at 3 months. FISH ≤2% (equivalent to CCyR) at 3 months was observed in 26 of 60 (43%) patients (3 patients had FISH ≤2% at 3 months, and BCR-ABL1 transcripts (IS) >1%). BCR-ABL1 transcripts (IS) ≤1% (also equivalent to CCyR) at 3 months was observed in 43 of 60 (72%) patients. However, 20 of these 43 patients who achieved a BCR-ABL1 transcripts (IS) ≤1% at 3 months had FISH positivity >2% (Table 3). In fact, 7 of these patients had achieved MMR despite testing positive by FISH. This highlights the limitation of peripheral blood FISH testing at 3 months, possibly giving rise to false results. Due to this finding, we subsequently amended the study to have bone marrow cytogenetic evaluation instead of FISH testing at 6 months, for assessment of cytogenetic response. Among 12 of the 20 patients who reached the 6-month evaluation, 11 had achieved CCyR: 9 by conventional cytogenetic analysis (4 patients had both conventional cytogenetics and FISH and tested negative; 5 had conventional cytogenetics alone and tested negative) and 2 by FISH alone. One patient remains in partial cytogenetic response. The remaining 8 of the 20 patients are receiving dasatinib and have not yet reached the 6-month evaluation.
Table 3.
Discrepancies of BCR-ABL1 transcripts ≤1% and FISH positivity (>2%) at 3 months among 20 patients
| Patient | BCR1-ABL1 transcripts (IS) | FISH-positivity (%) |
|---|---|---|
| 1 | 0.01 | 3.0 |
| 2 | 0.03 | 2.5 |
| 3 | 0.04 | 3.0 |
| 4 | 0.06 | 3.0 |
| 5 | 0.08 | 3.5 |
| 6 | 0.09 | 5.5 |
| 7 | 0.09 | 2.5 |
| 8 | 0.13 | 4.5 |
| 9 | 0.15 | 5.0 |
| 10 | 0.17 | 3.0 |
| 11 | 0.25 | 4.5 |
| 12 | 0.34 | 4.0 |
| 13 | 0.39 | 4.0 |
| 14 | 0.47 | 6.5 |
| 15 | 0.49 | 3.0 |
| 16 | 0.59 | 3.0 |
| 17 | 0.61 | 7.0 |
| 18 | 0.64 | 4.0 |
| 19 | 0.69 | 9.0 |
| 20 | 0.69 | 8.5 |
The first 7 patients highlighted, achieved a MMR (BCR-ABL1 transcripts ≤0.1%) despite testing positive by FISH (>2%) at 3 months
Safety
Treatment was well-tolerated overall. Nine patients (12%) had dose interruption for ≤14 days in the first 3 months into therapy: two for lower gastrointestinal bleed, two for renal dysfunction, two for transaminitis, and three for thrombocytopenia. Treatment was resumed (after resolution to grade ≤1) without any dose modification. Only one patient with prior dose interruption for renal dysfunction (secondary to dehydration) at the time of the 3-month follow-up visit developed pleural effusion and required dose reduction to 20mg daily. The patient remains on 20mg daily without any recurrence of the pleural effusion. One patient, 75-year-old, had traumatic subdural bleeding on Day 4 from the start of therapy. This was unlikely related to dasatinib, but the patient was taken off study on Day 5 of dasatinib and was not evaluable for response.
Discussion
In this study, dasatinib 50 mg daily in patients with early CML-CP was effective and safe. Eighty-six percent of patients achieved CCyR at 6 months from the start of therapy. The 12-month MMR rate was 79%; the 12-month MR4.5 rate was 46%. Treatment was well tolerated with only 9 patients (12%) requiring treatment interruption for a median of 14 days (range, 7–14). Pleural effusion was encountered in only one patient (1% so far) requiring dose reduction to 20mg. These results compare favorably with the historical experience at our institution in similar patients treated with standard-dose dasatinib as well as the DASISION study.8,14–15 In the 5 year follow-up of the DASISION study, 28% patients developed dasatinib-related pleural effusion requiring drug interruption in 62%, and/or dose reduction in 41% of the cases.8 Responses with low-dose dasatinib occurred considerably faster and ultimately at higher rates compared with responses reported with standard-dose dasatinib. For example, the 6-month CCyR rate with dasatinib 50mg daily was 86%, higher than that reported with standard-dose dasatinib (73% at 6 months) and with imatinib (59% at 6 months).
Discordance was noted between BCR-ABL-ABL1 transcripts (IS) ≤1% and FISH testing at 3 months. Similar discordances were reported in the DASISION trial when assessing responses at 3 and 6 months.9 One possible explanation of this discordance could be related to the fact our PCR analysis may not accurately reflect the amount of disease in patients at early milestones (e.g. at 3 months) with transcript levels <1%. With further follow-up at 6 months, a higher level of concordance was observed. To limit this discordance, the protocol was amended to include cytogenetic analysis by G-banding technique at 6 months to confirm the CCyR.
We have previously reported on the favorable impact of the achievement of 3- and 6-month CCyR and BCR-ABL-ABL1 transcripts (IS) ≤1% on long-term event-free and progression-free survival in patients treated with second generation TKI in the frontline setting.16,9 In the current study, the 3- and 6-month rates of BCR-ABL1 transcripts (IS) ≤1% were 72% and 84%, compared with 48% and 69% with dasatinib 100mg, and 13% and 49% with imatinib 400mg, respectively, in historical series.9 Furthermore, the 12-month MMR and MR4.5 rates (79% and 46%) were significantly higher than the rates obtained with standard-dose dasatinib (46% and 5%, respectively).8 Although, a higher percentage of patients in the current study were low risk by Sokal, the distribution was similar to what has been reported in our previous studies with dasatinib and nilotinib.14,17 The high efficacy of this strategy may therefore reside in the safety profile of low-dose dasatinib with minimal treatment interruptions and constant drug exposure. Previous studies have shown that treatment interruptions correlated with worse outcomes.
These extrapolations should be considered with caution because the comparisons refer to historical controls. Moreover the follow-up is still limited. The inclusion criteria for all these studies (imatinib and standard-dose dasatinib) were identical, and there were no obvious differences in the patient characteristics.14 The long-term impact of low-dose dasatinib as initial therapy in CML will require a longer follow-up. But the results reported here appear to be encouraging when compared to those achieved with other second generation TKIs.8,18–19
The high rate of MR4.5 may play a crucial role in treatment discontinuation. Several studies have evaluated whether TKIs can be safely discontinued in patients who have achieved long-term deep molecular responses. Higher treatment-free remission rates can be achieved with low-dose dasatinib due to the relatively deeper and more sustained molecular responses achieved with this strategy, when compared to imatinib and standard-dose second generation TKIs. In the EURO-SKI trial, the largest study to date of TKI discontinuation in CML, among 821 patients with CML treated with frontline imatinib, nilotinib or dasatinib, who had achieved at least MR4.0, and who subsequently stopped TKI therapy, the molecular recurrence-free survival at 2 years was 52%.20 In a multivariate analysis, duration of MR4.0 for 3+ years was the only significant factor for persistent deep molecular response after stopping therapy. Additional factors include the duration of TKI therapy for 6+ years.21 The low-dose dasatinib strategy induced the highest rates of deep molecular responses so far, and may be preferred as initial therapy for patients in whom the eventual TKI discontinuation may be particularly valued (e.g. younger patients expected to live for 2+ decades and who may benefit from TKI discontinuation).
Lower dose dasatinib constitutes a cost-effective strategy. It may allow the achievement of an optimal response at a lower cost than standard-dose dasatinib. Recently, generic formulations of imatinib became available, although at a higher cost than expected.22–23 Despite the availability of 4 imatinib generics in the United States in 2017, the average wholesale price (AWP) of a year of any generic imatinib is $110,000, compared with $140,000+ for patented imatinib. The price of generic imatinib in Canada is $3,000-$8,000 per year, and in India is $400 per year. With proper market forces in the U.S., historically, the price of a generic drug was expected to decrease to ≤10% of the patented drug once 4 or more generics were available. Thus, the current experience with generic imatinib is unusual or raises the perception of potentially unusual market forces. The AWP for a year of dasatinib 100mg is $170,000+, compared with $96,000 for dasatinib 50mg. Therefore, lower dose dasatinib therefore offers an optimal solution with at least equivalent efficacy compared to second generation TKIs but at a significant lower cost, a price currently even lower than generic formulations of imatinib in the US.
Today, most patients with CML-CP are functionally cured with long-term TKI therapy. Strategies should be explored in order to increase the proportion of patients who achieve treatment-free remissions. Carter and colleagues reported on emerging data suggesting that the combination of Bcl-2 inhibitors (venetoclax) with TKIs enhanced cytotoxicity, depleted the CML stem cells, and prolonged survival in a murine CML model.24–25 Such a strategy may lead to a potentially higher percent of patients who achieve long-term complete molecular remissions and a treatment-free remission status. A study combining dasatinib 50 mg daily with venetoclax in frontline CML-CP is ongoing.
In summary, dasatinib 50 mg daily was effective and safe as initial therapy for CML-CP. High rates of responses and rapid achievement of CCyR were observed in nearly all patients after 6 months from the start of therapy. The 12-month MMR and MR4.5 rates of 79% and 46%, respectively, were encouraging. The results were achieved with very favorable toxicity profiles. Dasatinib 50mg daily may be considered a new cost-effective option for frontline therapy in CML-CP. A confirmation of these findings is warranted. A randomized study comparing 50mg of dasatinib with the standard dose could be considered.
Acknowledgments
This study was supported by the MD Anderson Cancer Center Leukemia SPORE CA100632 and the Charif Souki Cancer Research Fund.
Footnotes
Conflict-of-interest: The authors declare no competing financial interests.
References
- 1.Hochhaus A, Larson RA, Guilhot F, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med 2017;376(10):917–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362:2260–2270 [DOI] [PubMed] [Google Scholar]
- 3.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362(24):2251–2259 [DOI] [PubMed] [Google Scholar]
- 4.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. Journal of medicinal chemistry 2004;47(27):6658–61 [DOI] [PubMed] [Google Scholar]
- 5.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer research 2005;65(11):4500–5 [DOI] [PubMed] [Google Scholar]
- 6.Tokarski JS, Newitt JA, Chang CY, et al. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Research 2006;66(11):5790–7 [DOI] [PubMed] [Google Scholar]
- 7.Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004;305(5682):399–401 [DOI] [PubMed] [Google Scholar]
- 8.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol 2016;34(20):2333–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014;123(4):494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006;354(24):2531–41 [DOI] [PubMed] [Google Scholar]
- 11.Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or - intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180–034. Am J Hematol 2016;91(9):869–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes J, Hochhaus A, Kantarjian H, et al. Impact of dose reductions on 5-year efficacy in newly diagnosed patients with chronic myeloid leukemia in chronic phase (CML-CP) from DASISION. ASCO 2017; abstract 3036
- 13.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013;122:872–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol 2010;28(3):398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain P, Kantarjian H, Alattar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400mg, imatinib 800mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. Lancet Haematol, 2 (2015), pp. e118–e128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabbour E, Kantarjian HM, O’Brien S, et al. Front-line therapy with second-generation tyrosine kinase inhibitors in patients with early chronic phase chronic myeloid leukemia: what is the optimal response? J Clin Oncol 2011;29(32):4260–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes JE, Jones D, O’brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol 2010;28(3):392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016;30(5):1044–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus Iimatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE Trial. J Clin Oncol 2017. November 1FF [DOI] [PMC free article] [PubMed]
- 20.Mahon FX, Richter J, Guilhot J, et al. Cessation of Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia Patients with Deep Molecular Response: Results of the Euro-Ski Trial. Blood 2016;128(22):787–787 [Google Scholar]
- 21.Duration of deep molecular response has most impact on the success of cessation of tyrosine kinase inhibitor treatment in chronic myeloid leukemia - results from the EURO-SKI trial. Blood 2017;130: abstract 313 [Google Scholar]
- 22.Sacha T, Góra-Tybor J, Szarejko M, et al. A multicenter prospective study on efficacy and safety of imatinib generics: A report from Polish Adult Leukemia Group imatinib generics registry. Am J Hematol 2017;92(7):E125–E128 [DOI] [PubMed] [Google Scholar]
- 23.Madhav D India Generic imatinib in chronic myeloid leukemia: survival of the cheapest. Blood 2016;128: abstract 630 [DOI] [PubMed]
- 24.Carter BZ, Mak PY, Mu H, et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med 2016;8(355):355ra117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko TK, Chuah CT, Huang JW, Ng KP, Ong ST. The BCL2 inhibitor ABT-199 significantly enhances imatinib-induced cell death in chronic myeloid leukemia progenitors. Oncotarget 2014;5(19):9033–9038 [DOI] [PMC free article] [PubMed] [Google Scholar]


