Abstract
Mitochondria are an essential component of multicellular life – from primitive organisms, to highly complex entities like mammals. The importance of mitochondria is underlined by their plethora of well-characterized essential functions such as energy production through oxidative phosphorylation (OX-PHOS), calcium and reactive oxygen species (ROS) signaling, and regulation of apoptosis. In addition, novel roles and attributes of mitochondria are coming into focus through the recent years of mitochondrial research. In particular, over the past decade the study of mitochondrial shape and dynamics has achieved special significance, as they are found to impact mitochondrial function. Recent advances indicate that mitochondrial function and dynamics are inter-connected, and maintain the balance between health and disease at a cellular and an organismal level. For example, excessive mitochondrial division (fission) is associated with functional defects, and is implicated in multiple human diseases from neurodegenerative diseases to cancer. In this chapter we examine the recent literature on the mitochondrial dynamics–function relationship, and explore how it impacts on the development and progression of human diseases. We will also highlight the implications of therapeutic manipulation of mitochondrial dynamics in treating various human pathologies.
Keywords: Cancer, DRP1, DRP1S616, Fis1, Fission, Fusion, Mff, Mfn1, Mfn2, MiD49/51, MiD49/51OPA1, Mitochondrial dynamics, Neurodegenerative diseases
1. Introduction
Since their first inclusion into cells through endosymbiosis millions of years ago, mitochondria are an essential component of multicellular life – from primitive organisms to highly complex entities like mammals. The importance of mitochondria is underlined by their plethora of well-characterized essential functions such as energy production through oxidative phosphorylation (OX-PHOS), calcium and reactive oxygen species (ROS) signaling, and apoptosis. In addition, novel roles and attributes of mitochondria are coming into focus through the recent years of mitochondrial research. In particular, over the past decade the study of mitochondrial shape and dynamics has achieved special significance, as they are found to impact mitochondrial function. Recent advances indicate that mitochondrial function and dynamics are inter-connected, and maintain the balance between health and disease at a cellular and an organismal level. For example, excessive mitochondrial division (fission) is associated with functional defects, and is implicated in multiple human diseases from neurodegenerative diseases to cancer. The purpose of this chapter is to examine the recent literature on the mitochondrial dynamics–function relationship, and explore how it impacts on the development and progression of human diseases. We will also highlight how therapeutic manipulation of mitochondrial dynamics may be utilized to modulate mitochondrial health with potential in treating various human pathologies.
1.1. Mitochondrial Biology: Functions
Mitochondria are highly versatile organelles with a multitude of vital functions within the cell. Primarily, mitochondria act as the main source of cellular energy production, by hosting the TCA cycle and the OX-PHOS pathway machinery to utilize fuels (e.g., pyruvate and NADH produced via glycolysis, acetyl co-a produced by lipid β-oxidation, and glutamine) to generate energy in the form of ATP (Ward and Thompson 2012b). The OX-PHOS pathway is also known as aerobic respiration as it requires the presence of oxygen. The mitochondrial respiratory function is particularly essential in organs and tissues with high energy demand, such as the heart and tissues of the central nervous system (Ikeda et al. 2014). Mitochondria also play an important role in the biosynthesis of cellular building blocks such as nucleotides, amino acids, and lipids required for cellular growth and proliferation (Ward and Thompson 2012b). Another important function of mitochondria is calcium buffering and modulating calcium signaling through controlled uptake and release of calcium from cellular stores (Walsh et al. 2009). Ca2+ buffering is achieved in part via a close contact between mitochondria and the endoplasmic reticulum (ER) network, creating calcium micro-domains that facilitate Ca2+ uptake by mitochondria to maintain cellular calcium homeostasis (de Brito and Scorrano 2008; Walsh et al. 2009). Mitochondrial ATP, NADH, pyruvate, and ROS also modulate Ca2+ signaling machinery, while the uptake and release of Ca2+ by mitochondria directly influence cytosolic calcium concentrations and Ca2+ signaling (Walsh et al. 2009). As the primary site for ROS generation in the cell, mitochondria are also responsible for cellular redox signaling, as well as maintaining oxidative homeostasis through a series of antioxidants (Collins et al. 2012; Hamanaka and Chandel 2010). Signaling pathways that utilize ROS include cellular response to hypoxia, growth factor stimulation for cell proliferation and survival, and generation of inflammatory responses (Hamanaka and Chandel 2010; Schieber and Chandel 2014). Last, but not least, mitochondria play a central role in the apoptotic pathway of cell death. Multiple upstream signaling pathways converge on mitochondria in a complex network of pro- and anti-apoptotic proteins to signal for mitochondrial outer membrane permeabilization (MOMP) and the release of cytochrome c (Chipuk et al. 2010). Mitochondria play a pivotal role in apoptosis, by compartmentalizing the cell death inducing cytochrome c under normal healthy conditions, and orchestrating its release upon stress conditions to undergo apoptosis (Elkholi et al. 2014).
1.2. Mitochondrial Biology: Dynamics
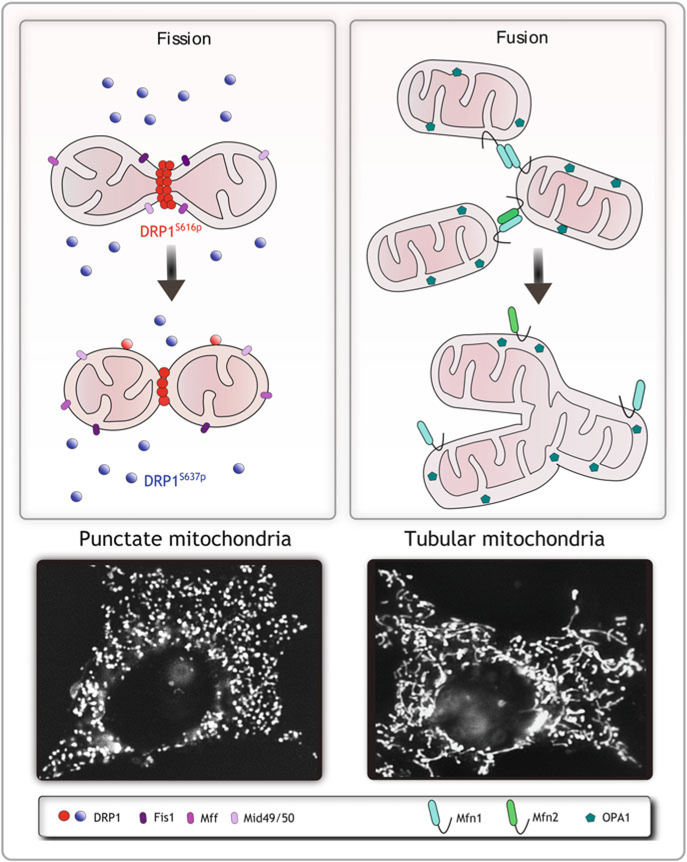
Over the past few decades, an important characteristic feature of mitochondria emerged in the field of mitochondrial research. In place of the original “text book” idea of a static bean-shaped mitochondrion, it is now appreciated that mitochondria exist as a network of highly dynamic organelles and may be present in multiple shapes and sizes within the cell, ranging from small puncta to short and long tubules (Fig. 2). These different shapes are the result of the ability of mitochondria to divide, fuse together, and move through the cell. These processes are collectively termed mitochondrial dynamics, and involve two specific, highly regulated opposing processes known as fission (division) and fusion (Fig. 1). In healthy cells in most tissues, mitochondria continuously undergo these fission and fusion events, changing their shape and size and moving within short distances to do so.
Fig. 2.
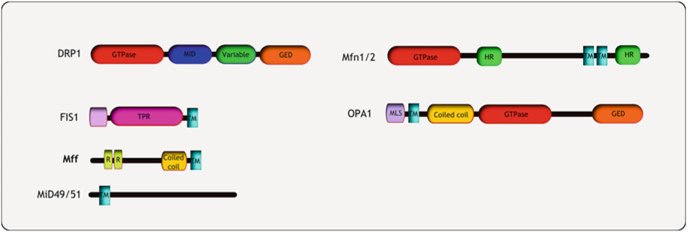
Regulators of mitochondrial dynamics. Several large GTPases govern mitochondrial dynamics. DRP1 is a cytosolic protein that is recruited to the OMM by membrane anchored adaptor proteins such as Fis1, Mff and Mid49/50. Post translational modifications of DRP1 include phosphorylation, SUMOylation, ubiquitinylation and s-nitrosylation. The outer membrane fusion proteins Mfn1 and Mfn2 are anchored on the OMM via two transmembrane domains. Mfn1 is regulated by phosphorylation as well as ubiquitinylation. The inner membrane fusion GTPase OPA1 is found in the IM space attached to the IMM, and is regulated through proteolytic cleavage to produce fusion competent shorter isoforms. Abbreviations: GED GTPase effector domain, HR heptad repeat, TPR tetra-tricopeptide repeat, R conserved repeats, MLS mitochondrial localization signal, TM trans-membrane
Fig. 1.
Dynamic mitochondria. Mitochondrial morphology is maintained through a balance of fission and fusion events and involve the activity of several large GTPases and adapter proteins. Mitochondrial fission is mediated by DRP1 and the OMM anchored adapter proteins Fis1, Mff and MID49/51. Fusion is regulated by the mitofusins Mfn1 and 2 on the outer membrane, and OPA1 in the inner membrane. If increased fission is favored, mitochondria form short punctate structures (fragmented mitochondria). If fusion is favored mitochondria form tubular networks (fused mitochondria)
Mitochondrial dynamics are considered important for maintaining healthy functional mitochondria in the cell. Through fusion and fission events, mitochondria are able to mix and exchange their DNA and protein content, or allow for the segregation and elimination of unhealthy components through an autophagic process termed mitophagy. Mitochondrial fission is also required for distribution of mitochondria to daughter cells during cell division, as well as distribution of the organelle to distal, energy demanding regions of the cell such as neuronal axons, and lamellipodia during cell motility. More recent developments in mitochondrial research indicate that mitochondrial dynamics are also intricately involved with the functions of mitochondria, and there is emerging evidence that connects mitochondrial dynamics directly to the manifestation of certain pathologies, such as neuro-degenerative diseases, cancer, cardiomyopathies, and metabolic disorders. In this section of the chapter we will introduce the major players in mitochondrial dynamics and how cellular signaling controls these processes. We will also discuss how aberrant fission and fusion of mitochondria arise, and how these aberrations result in mitochondrial dysfunction and human diseases.
1.2.1. Mitochondrial Fission: Executors and Regulators
Mitochondrial fission is the process by which a mitochondrion divides into two smaller units. The major executor of fission is the dynamin related protein 1 (DRP1). DRP1 is mainly cytosolic, but translocates to the mitochondrial surface in order to mediate fission of the organelle. DRP1 assembles on the surface of mitochondria, forms helical oligomers, and undergoes self-interaction mediated GTP hydrolysis and subsequent membrane constriction resulting in the scission of both the inner and outer mitochondrial membranes (OMM). In a series of elegant in vitro experiments, both DRP1 and its yeast homolog Dnm1 were shown to self-assemble into helices and tubulate liposomes (Ingerman et al. 2005). These DRP1 helices have a diameter of ~120 nm (Ingerman et al. 2005; Mears et al. 2011), and since the diameter of an average mitochondrion is approximately fivefold to sevenfold larger, it is speculated that already existing membrane constrictions may provide the sites for the self-assembly of DRP1 oligomers. Accordingly, it has been shown that ER–mitochondria contact sites constrict mitochondria to a diameter that is permissive for DRP1 oligomer formation, and therefore may mark future fission sites (Friedman et al. 2011).
As DRP1 is mostly cytosolic, there may be a requirement for a mitochondrial membrane anchored receptor to recruit DRP1 to the mitochondria for fission. Several such receptor proteins have been described, including mitochondrial fission 1 (Fis1), mitochondrial fission factor (Mff), Mid 49/51 (MIEF1/2), and ganglioside-induced differentiation-associated protein 1 (GDAP) (Gandre-Babbe and van der Bliek 2008; Niemann et al. 2005; Palmer et al. 2011; Serasinghe and Yoon 2008; Yoon et al. 2003). Fis1 is the first receptor identified to recruit DRP1, and is thought to interact with DRP1 via its two tetra-tricopeptide repeat (TPR)-like motifs. Fis1 forms oligomers on the OMM which may provide the scaffold for DRP1 recruitment (Serasinghe and Yoon 2008; Yoon et al. 2003). More recently Mff was identified as an adaptor for DRP1 (Gandre-Babbe and van der Bliek 2008). Mff is also an OMM anchored protein which recruits DRP1 via its cytosolic domain. Mid49 and Mid50 were also recently identified as mitochondrial outer membrane anchored adapters of DRP1; however, their exact role in mitochondrial fission remains to be clarified (Liu et al. 2013; Palmer et al. 2011; Zhao et al. 2011). GDAP1 is an OMM anchored protein that regulates mitochondrial fission in neuronal cells by cooperating with DRP1 and Fis1 (Niemann et al. 2005, 2009). The presence of multiple adaptor proteins for DRP1 suggests that there may be several pathways regulating mitochondrial fission. It also indicates that these proteins may be functionally redundant and have cell type specific or context dependent activity (Loson et al. 2013; Niemann et al. 2005, 2009), further suggesting additional points of control in the fission process.
Transcriptional Regulation of DRP1
Both transcriptional and post-translational modifications of DRP1 regulate mitochondrial fission. Several reports indicate that DRP1 is transcriptionally upregulated in cancer (Choudhary et al. 2011; Serasinghe et al. 2015). While the precise transcriptional regulators of Drp1 are currently not known, several putative transcriptional activators have been reported in the recent literature. For example, the androgen receptor was shown to directly activate Drp1 transcription by binding to the promoter region of the DRP1 gene in prostate cancer, suggesting hormone responsive regulation of Drp1 (Choudhary et al. 2011). Drp1 has also been proposed as a p53 transcriptional target indicating a potential intersection between cellular stress pathways and mitochondrial fission (Li et al. 2010). More recently it was shown that oncogenic RAS-MAPK signaling upregulates Drp1 mRNA levels via ERK, and that blocking RAS-MAPK signaling with targeted inhibitors drastically reduces Drp1 mRNA levels (Serasinghe et al. 2015). It is likely that a transcription factor downstream of ERK is responsible for this upregulation. It is intriguing that certain disease conditions, cancer in particular, are associated with transcriptional upregulation of Drp1, which suggests an important role/function of the protein in disease pathology. Identification of Drp1 specific transcription factors and upstream signaling networks will yield important insights into the function of Drp1 and mitochondrial fission in these diseases.
Post-Translational Regulation of DRP1
Mechanisms of post-translational regulation of DRP1 include phosphorylation, SUMOylation, nitrosylation, and ubiquitinylation. One of the best characterized post-translational modifications of DRP1 leading to the modulation of its function is phosphorylation. Two main phosphorylation sites in DRP1 have been identified, both on serine residues residing on the GTPase effector domain (GED) of DRP1 (Fig. 2). DRP1 serine 616 phosphorylation is an activating event resulting in the OMM localization of DRP1 and subsequent mitochondrial fragmentation. It has been reported that DRP1S616 phosphorylation by calcium/calmodulin-dependent protein kinase-1 (CAMK-1) facilitates DRP1 interaction with Fis1 on the OMM (Han et al. 2008). DRP1S616 is phosphorylated by protein kinase C (PKC) under oxidative stress conditions (Qi et al. 2011), by CDK1/cyclin B during cell cycle progression (Taguchi et al. 2007), and by ERK 1/2 under pathological conditions leading to the activation of fission (Kashatus et al. 2015; Serasinghe et al. 2015; Yu et al. 2011). The specific phosphatases responsible for dephosphorylation on this site are currently not known. However, it has been reported that dephosphorylation at S616 leads to mitochondrial fusion and protects from autophagy in nutrient starvation conditions (Rambold et al. 2011). Indeed it is interesting to speculate that there may exist a series of thus far unidentified phosphatases having equal significance to kinases, regulating DRP1 with potential implications on mitochondrial shape and function.
While the putative DRP1S616 phosphatases are not known, it has been demonstrated that DRP1 S637 phosphorylation acts as a countermeasure to inactivate DRP1 mediated fission, thereby establishing a balance between active and inactive forms of DRP1. DRP1 S637 when phosphorylated by the protein kinase A (PKA) acts to prevent mitochondrial fission by translocating DRP1 away from mitochondria (Chang and Blackstone 2007). This site is dephosphorylated by calcineurin. On the other hand, DRP1 phosphorylation at the same site by CAMK-1 and rho-associated coiled-coil containing protein kinase 1 (ROCK 1) has been shown to induce mitochondrial fission by activating DRP1 function in cultured hippocampal neurons and induction of fission, leading to diabetic neuropathies, respectively (Han et al. 2008; Wang et al. 2012). These contrasting observations may be reconciled with the argument that the regulatory phosphorylation events of DRP1 may be highly tissue specific (e.g., nervous system), and may depend on the availability and function of DRP1 receptors in these cells (e.g., hippocampal neurons), and dependent on the mitochondrial requirement of the tissue. It also may depend on the cellular context such as specific stress conditions and associated signaling pathways (e.g., calcium signaling). We will discuss these tissue and disease specific regulation of mitochondrial dynamics further in the context of human pathologies in a subsequent section of this chapter. It is important however to stress that further investigations into these unknowns would enable a better dissection of the function of DRP1 in mitochondrial fission.
A third phosphorylation site with functional consequence has been recently identified at DRP1 serine 693 residue, which resides in the GED (Fig. 2). This site is phosphorylated by glycogen synthase kinase 3 beta (GSK3β), and inhibits DRP1 oligomerization dependent GTP hydrolysis to block mitochondrial fission (Chou et al. 2012). This phosphorylation event, in particular, has been observed in association with apoptosis (Chou et al. 2012).
Another type of post-translational modification that impacts DRP1 function is ubiquitinylation. Membrane associated ring finger 5 (MARCH 5, also known as MITOL) is a mitochondrial outer membrane localized E3 ligase that ubiquitinates DRP1 on the OMM. MARCH 5 mediated ubiquitinylation of DRP1 was originally reported to mark DRP1 for proteolytic degradation leading to reduced fission and elongated mitochondrial networks (Nakamura et al. 2006; Yonashiro et al. 2006). In more recent reports however, MARCH 5 mediated DRP1 ubiquitinylation was shown to increase DRP1 mitochondrial localization and fission (Karbowski et al. 2007; Park et al. 2010) suggesting that this protein modification may be required for mitochondrial fission. These contradictory reports warrant further study to clarify the role of MARCH 5 mediated ubiquitinylation of DRP1. Parkin is another E3 ubiquitin ligase that was shown to ubiquitinylate DRP1 and Fis1 leading to the proteasomal degradation of these proteins (Cui et al. 2010; Wang et al. 2011). These reports indicate that ubiquitinylation by Parkin acts as an inhibitory event for DRP1 mediated mitochondrial fission. The relevance of this DRP1 regulatory pathway in Parkinson’s disease will be discussed later in this chapter.
The SUMO-conjugating enzyme Ubc9 and the mitochondrial SUMO E3 ligase MAPL SUMOylate DRP1, resulting in increased DRP1 protein stability on the OMM, leading to increased fission (Braschi et al. 2009; Harder et al. 2004; Prudent et al. 2015; Wasiak et al. 2007) (Fig. 3). Furthermore, it was shown that during apoptosis, the BCL2 associated X (BAX) protein and Bcl2 homologous antagonist killer (BAK) protein oligomerization stabilizes DRP1 on the OMM in a SUMOylation dependent manner, suggesting that this may be a mechanism of apoptotic fragmentation of mitochondria (Wasiak et al. 2007). Conversely, Sentrin/SUMO specific protease 5 (SENP5) was identified as a SUMO-protease that removes the SUMO groups from DRP1 resulting in decreased mitochondrial fragmentation, and knock down of SENP5 was found to fragment mitochondria by stabilizing DRP1 SUMOylation (Zunino et al. 2007). Similar activity was reported recently from SENP3, another member of the SEN protease family, in an in vitro model of ischemia (Guo et al. 2013). Under ischemic conditions, SENP3 is downregulated leading to extended SUMOylation and resulting fragmentation of mitochondria, which is reversed upon re-oxygenation (Guo et al. 2013). However, the depletion of SENP3 in this case, which led to DRP1-sumo stabilization, was shown to suppress cytochrome c release and caspase mediated cell death, thereby acting in a cell protective manner.
Fig. 3.
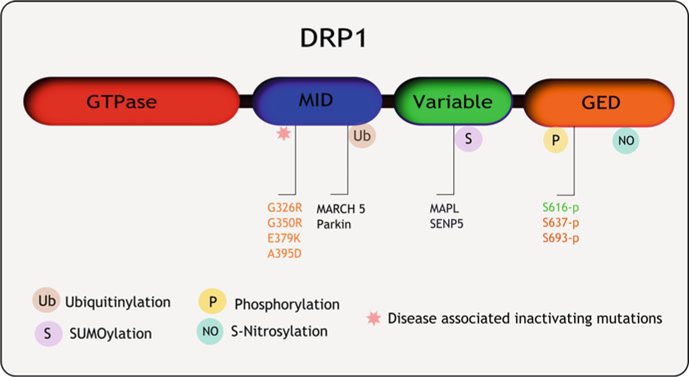
DRP1 modifications and mutations associated with human diseases. DRP1 activity is regulated by post-translational modifications such as (a) Ubiquitinylation: MARCH 5, Parkin (b) SUMOylation: MAPL, SENP4 (deubiquitinase) (c) Phosphorylation; S616: CAMK-1,CDK1/Cyclin B, ERK; S637: PKA1, CAMK-1, ROCK1, Calcinuerin (phosphatase); S693: GSK3b. De-regulation of these processes affects DRP1 function and manifests in several human pathologies. Additionally, de novo mutations in the DRP1 middle domain which resulted DRP1 loss of function resulting in neuropathological and birth defects are indicated here
It is interesting that the same DRP1 modification can give opposing results under different stimuli, as seen with DRP1 phosphorylation at S637 and ubiquitinylation by MARCH 5 discussed earlier. These differences however could be addressed by taking into consideration the tissue specific context; for example, neuronal cells may have specific requirement for mitochondrial dynamics compared to other tissues, normal vs stress conditions – such as de-SUMOylation under normoxic and ischemic conditions, and involvement of complex signaling networks in regulating multiple fission/fusion factors – e.g., MARCH mediated ubiquitination of DRP1, Fis1 as well as the mitochondrial fusion protein, mitofusin 1 (Mfn1 – to be discussed in the next section). The final phenotypic outcome may be the cumulative effect of several factors, including multiple protein modifications on DRP1 at a given time. It is worth noting that some of these modifications are under normal physiological conditions and others are under disease or stress conditions. This alludes to a highly sophisticated regulatory framework that maintains the fine balance of mitochondrial dynamics in a cell, and can be offset in pathologies. We will discuss this in more detail in Sect. 1.4 of the chapter.
In addition to the modifications occurring in normal cellular conditions, specific disease related post-translational modifications of DRP1 have also been identified. These are S-nitrosylation and O-glucNAcylation, and both were shown to activate DRP1 and increase mitochondrial fragmentation (Bossy et al. 2010; Cho et al. 2009; Gawlowski et al. 2012). Amyloid beta mediated Nitric oxide (NO) production leading to DRP1 S-nitrosylation was shown to fragment mitochondria and induce neurotoxicity in Alzheimer’s disease and Parkinson’s disease (Bossy et al. 2010; Cho et al. 2009). O-glucNAcylation was shown to induce mitochondrial fragmentation in rat neonatal cardiomyocytes, with implications in diabetes induced mitochondrial dysfunction and cardiovascular complications (Gawlowski et al. 2012).
1.2.2. Opposing Fission: Mitochondrial Fusion
Unlike fission, mitochondrial fusion has two distinct systems in place for the fusion of inner and outer membranes. Even though these processes are separate, the inner and outer membrane fusion is highly coordinated. OMM fusion is achieved by Mfn1 and 2, which are anchored on the OMM via two transmembrane domains which span the membrane twice (Hoppins et al. 2007) (Fig. 2). Mfn1 and 2 form homo- and heterotypic interactions when two apposing mitochondria come together at a permissive proximity. This initial tethering of mitochondria is followed by the outer membrane fusion through mixing of the lipid bilayers (Koshiba et al. 2004). Mfn1 and 2 perform overlapping functions; however, they are not functionally redundant (Chen and Chan 2004; Chen et al. 2003). Both proteins are generally expressed in cells, and the abundance of each may be cell type dependent.
Regulation of Mitochondrial Fusion Proteins
Both Mfn1 and 2 show regulation at both transcriptional and post-transcriptional levels (Santel et al. 2003). Mfn1 is regulated by peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α) during postnatal cardiac growth (Martin et al. 2014). Other reports indicate tissue specific Mfn1 transcriptional repression by the corticosteroid dexamethasone in liver and in hepatoma cells (Hernandez-Alvarez et al. 2013) and by microRNA 140 in cardiomyocytes (Li et al. 2014). Mfn2 transcription is driven by the PGC-1α and PGC-1β, key factors in mitochondrial biogenesis, as well as the transcription factor Sp1 in skeletal and smooth muscle cells (Sorianello et al. 2012). Mfn2 has also shown to have hormone responsive transcriptional regulation as Estrogen-Related Receptor-alpha (ERRα) binds to the human Mfn2 promoter and stimulates transcription (Scarpulla et al. 2012; Soriano et al. 2006). Mfn1 and 2 are also regulated at a post-translational level. One of the best characterized post-translational modifications of these proteins is ubiquitinylation. Mfn1 and 2 are both ubiquitinylated by MARCH-5 and Parkin (Gegg et al. 2010; Park and Cho 2012; Park et al. 2010; Tanaka et al. 2010). Mfn1 is also activated by deacetylation mediated by HDAC6 (Lee et al. 2014). Mfn2, on the other hand, is ubiquitinylated by Parkin and marked for proteasomal degradation (Gegg et al. 2010; Poole et al. 2010; Tanaka et al. 2010; Ziviani and Whitworth 2010). Additional Mfn2 E3 ligases include HUWE1 (regulated by phosphorylation of JNK), and by the ubiquitin ligase Mul1 (Leboucher et al. 2012; Lokireddy et al. 2012) leading to proteasomal degradation. However, MARCH 5 mediated ubiquitinylation of Mfn1 and 2 did not drive proteasomal degradation of these proteins (Sugiura et al. 2013). Mfn2 levels are also regulated by activation of the deubiquitinase USP30 (Yue et al. 2014).
Inner mitochondrial membrane fusion is mediated by OPA1, a GTPase anchored on the inner mitochondrial membrane (Fig. 2). Apart from its function in inner membrane fusion OPA1 has a distinct function in cristae remodeling and cytochrome c release during apoptosis (Frezza et al. 2006). Eight splice variants of OPA1 have been identified, and categorized as long (L) and short (S) isoforms. Both isoforms localize to the intermembrane space of mitochondria. The L-isoform is membrane anchored and the S-isoform is found peripherally attached to the inner membrane lipids (Olichon et al. 2002; Satoh et al. 2003). The OPA1-L isoform is considered the fusion competent form of OPA1, and further cleavage of the protein at protease sites S1 and S2 generates short forms of the protein that regulate inner membrane fusion (Griparic et al. 2007; Ishihara et al. 2006). Therefore, proteolytic cleavage is the major regulatory mechanism for OPA1 function in membrane fusion (Ishihara et al. 2006).
The intermembrane space rhomboid protease, PARL (presenilin-associated rhomboid-like), cleaves OPA1 at the S1 site to short soluble isoforms, and PRRL knockout resulted in mitochondrial fragmentation and sensitization to apoptosis, suggesting that proteolytic cleavage of OPA1 by PARL is a regulatory mechanism during cell death (Cipolat et al. 2006). However, PARL mediated cleavage of OPA1 does not affect mitochondrial fusion (Cipolat et al. 2006). YME1L another intermembrane space AAA protease was found to cleave OPA1 at the S2 site, resulting in inner membrane fusion. Yme1l is regulated by the OX-PHOS activity of the inner membrane (Anand et al. 2014; Ishihara et al. 2006; Mishra et al. 2014; Song et al. 2007). Under high OX-PHOS conditions, Yme1L cleaves Opa1 with higher efficiency to induce inner mitochondrial membrane fusion, suggesting that mitochondrial inner membrane fusion supports increased OX-PHOS activity, and is required for maintaining this function of mitochondria. Paraplegin is another IMM AAA metalloprotease that participates in OPA1 cleavage to produce a short fusion incompetent form (Duvezin-Caubet et al. 2006; Ishihara et al. 2006). These opposing cleavage events indicate that OPA1 processing is a coordinated event that determines fusion and fission under different physiological conditions.
The requirement of mitochondrial dynamics for cellular function is highlighted by studies from genetic models of fission and fusion including yeast, Drosophila, and mouse models. Since the focus of this chapter is on human disease, we will discuss findings from mouse models of genetic knockdown and disease, and correlate these findings with human pathologies presenting aberrant mitochondrial dynamics and resulting functional defects.
1.3. Insights from Genetic Models of Fission and Fusion
1.3.1. DRP1, Mff, Mfn1, and Mfn2 Genetic Knockout Mice
Over the past decade knockout mouse models for the fission protein DRP1 and the fusion proteins Mfn1 and 2 were developed and characterized (Chen et al. 2003; Ishihara et al. 2009; Kageyama et al. 2012; Wakabayashi et al. 2009). The most interesting parallel between all three mouse models is the embryonic lethality that is observed during early mid gestation in the null homozygotes, indicating an essential role of mitochondrial dynamics in embryonic development. In the DRP1 knockout mouse the embryonic lethality was observed at approximately day 11.5, and Mfn1 and Mfn2 knockout mice display a high level of lethality by day 11.5 and 12.5, respectively (Chen et al. 2003; Wakabayashi et al. 2009). Mfn1/2 double knockout mice die sooner, indicating that these two proteins have non-redundant function in the early stages of embryo development (Chen et al. 2003). As expected, all three knockout phenotypes show changes in mitochondrial morphology, dynamics, and function. All three knockouts produced embryos that are smaller than wild type, indicating a requirement of mitochondrial dynamics in cell proliferation, growth, and differentiation (Wakabayashi et al. 2009), while the Mfn1 and 2 knockout embryos showed developmental delays and deformations (Chen et al. 2003). It is noteworthy however that the defects of Mfn1 and 2 knockout phenotypes can be corrected by expressing the dominant negative form of DRP1 (K38A), indicating a possible therapeutic implication for blocking DRP1 function in these conditions where mitochondrial fusion is dysregulated (Chen et al. 2003). More recently an Mff homozygous knockout mouse model was generated and studied (Chen et al. 2015). While the mice were viable at birth, they succumbed prematurely at postnatal 13 weeks to dilated cardiomyopathy leading to heart failure. This phenotype was reversed by deletion of the Mfn1 which restored the fission–fusion balance, demonstrating that re-fusing mitochondria may be a viable therapeutic strategy in diseases with excessive mitochondrial fission (Chen et al. 2015).
1.3.2. Conditional Knockout Mouse Models
DRP1 knockout has the most deleterious effects on the brain, CNS, and heart development. The neonatal brain and heart in particular are organs that express high levels of DRP1, and possibly depend on the protein for their development and function. Accordingly, the most striking phenotypes of DRP1 loss manifest in these organs, suggesting tissue specific requirement of DRP1. In particular, the DRP1 KO embryonic cardiomyocytes show reduced beating rates, and this may be responsible for the death of the embryo (Wakabayashi et al. 2009). To further understand the requirement of DRP1 in the function of these organs in the embryonic and postnatal development, conditional knockout mice have been established (Ishihara et al. 2009; Kageyama et al. 2012; Wakabayashi et al. 2009). The heart-specific knockout of DRP1 in particular showed neonatal lethality as a result of dilated heart, and thin heart walls, displaying clear features of cardiac dysfunction at the time of death at p7–10 after birth (Ishihara et al. 2009) suggesting that Drp1 is essential for neonatal heart development. The brain-specific knockout of DRP1 generated using brain-specific En1-Cre resulted in severe decrease in cerebellum development and neonatal death within 24 h of birth (Wakabayashi et al. 2009). Proliferation of the cerebellar cells, and in particular the number of Purkinje cells, showed dramatic reduction in this conditional knockout model (Wakabayashi et al. 2009). DRP1 deletion in a larger region of the brain using Nes-Cre recombinase resulted in apoptotic cell death in the premature superficial layer neurons and deep cortical layers (Ishihara et al. 2009). These phenotypes are characterized by increased size and reduced number of mitochondria, loss of mitochondria from the synapse, and defective synapse formation in culture (Ishihara et al. 2009). The requirement of DRP1 in synapse formation may be due at least in part to the loss of mitochondrial fission, but also due to the direct role of DRP1 in the clathrin coated endocytic vesicle formation in response to synaptic stimulation (Li et al. 2013). DRP1 mediated apoptosis in neuronal cells depends on the cell type and physiological context. Cerebellar Purkinje cells and neurons of the forebrain are among specific cell types that are affected severely by the loss of DRP1. In both cases cells undergo initial mitochondrial elongation followed by oxidative damage, resulting in fragmented and swollen mitochondria (Kageyama et al. 2012). In neural tube closure, developmentally regulated apoptosis is decreased by the loss of DRP1 (Wakabayashi et al. 2009), while increased in the neuroepithelium of the brain (Ishihara et al. 2009). Finally, DRP1 was shown to ensure survival of post-mitotic neurons in mice (Kageyama et al. 2012; Zhang et al. 2012). DRP1 null post-mitotic Purkinje cells became elongated and accumulated oxidative damage, became defective in respiration, and gradually degenerated over a period of 6 months. These aberrations manifested in defective motor and coordination behavior in these mice (Kageyama et al. 2012), suggesting that oxidative damage downstream of blocking mitochondrial fission is responsible for the neurodegeneration.
Similarly, Mfn1/2 conditional knockout mice have been developed to study the role of these proteins in the heart and brain function. Cardiomyocyte specific deletion of Mfn2 resulted in enlarged cardiomyocytes, and displayed mild mitochondrial dysfunction and modest cardiac hypertrophy and functional deterioration (Papanicolaou et al. 2011). The absence of Mfn2 prevented ROS mediated mitochondrial permeability transition (MPT), and protected adult cardiomyocytes from a number of cell death inducing stimuli, and better recovery from reperfusion injury. However, an Mfn1/2 double knockout in cardiomyocytes resulted in a much more severe phenotype (Chen et al. 2011; Papanicolaou et al. 2012). The cells displayed abnormally expanded or fragmented mitochondria, cardiomyocyte and respiratory dysfunction and rapidly progressive cardiomyopathies at postnatal day 7. These conditions led to heart failure, resulting in rapid decline in survival and death by 16 days post-birth (Chen et al. 2011; Papanicolaou et al. 2012). These studies established that Mfn1 and 2 are essential for the mitochondrial remodeling and metabolic reprogramming of the heart during neonatal heart development (Papanicolaou et al. 2011, 2012).
Tissue specific knockout mice have also been generated to study the role of Mfn1 and 2 in the nervous system. In particular, Mfn2 knockout and mutant studies were conducted focusing on modeling the Charcot–Marie–Tooth type 2A (CMT2A) disease. Mfn1 and 2 deficiency in the placental giant cell layer is responsible for the embryonic lethality of the Mfn1 and Mfn2 knockout mice (Chen et al. 2003), therefore mice were developed to specifically knockdown these proteins avoiding cells of placental lineage (Chen et al. 2007). These mice were viable for both Mfn1 and 2 knockdown; however, the Mfn2 deficiency showed more dramatic abnormalities. A percentage of Mfn2 knockout pups died post-birth, and the surviving mice showed severe defects in movement and balance, and succumbed at day 17 (Chen et al. 2007). Mfn1 knockout in the same system showed much less severity in phenotype, and the mice survived to adulthood with no obvious defects, indicating lesser significance of this mitofusin in the nervous system. Cerebellum specific knockout of Mfn2 showed severe defects in postnatal cerebellar growth. With the Mfn2 knockout, the cerebellar Purkinje cells, in particular, showed aberrant mitochondrial distribution, ultrastructure, and ETC activity, and the study showed that Mfn2, but not Mfn1 is required for dendritic outgrowth, spine formation, and cell survival (Chen et al. 2007). Mitochondrial transport also seems to be affected in Mfn2 specific knockout neurons, suggesting a novel function for Mfn2 distinct from its function in OMM fusion (Misko et al. 2010; Pham et al. 2012). A study using the expression of Mfn2 mutant forms in cultured dorsal ganglion neurons to recapitulate the neuronal pathology of the CMT2A disease reported abnormal clustering of small fragmented mitochondria in the cell body as well as axons, along with impaired transport of mitochondria (Baloh et al. 2007). Similar results were observed from a dopaminergic neuron specific Mfn2 knockout mouse model (Pham et al. 2012). Mitochondrial fragmentation in the cell body and neuronal processes as well as markedly reduced mitochondrial mass and transport were observed, and these presumably led to the loss of neurons and movement defects characteristic of the CMT2A disease in these mice (Pham et al. 2012).
Similar to DRP1, Mfn1, and Mfn2 knockout mice, OPA1 knockout mice were also found to be embryonic lethal; death occurring in this case around day 13.5 is indicative of the requirement of this protein in embryonic development. OPA1+/‒ heterozygotes are viable, and several models were developed to characterize the requirement of OPA1 (Davies et al. 2007; Williams et al. 2010). Many of these studies were developed to model the DOA disease in mice and focus on the effect of OPA1 loss on optic neuronal function. While these studies do not recapitulate effect of the complete loss of OPA1, they represent moderate effects of OPA1 reduction and recapitulate DOA phenotypes. These studies reported that the reduction of OPA1 levels led to Retinal ganglion cell dendritic pruning, increased autophagy, and may contribute to RGC loss and atrophy, a marked reduction in retinal ganglion cell synaptic connectivity (White et al. 2009; Williams et al. 2011, 2012). Study of the heart in this heterozygous mouse model showed alterations in mitochondrial morphology, but not changes to the electron transport chain activity. Delay in Ca2+ dependent PTP opening, and associated myocardial hypertrophy induced by pressure overload were also observed (Piquereau et al. 2012). OPA1 loss was examined in the context of embryonic development, and these studies indicate that OPA1 is crucial for the survival of RGCs and essential for early embryonic survival.
1.4. From Mouse Models to Human Diseases
As we discussed in the previous section, genetic disruption of mitochondrial dynamics impacts most severely the tissues and processes that are most energy demanding and dependent on mitochondrial function. For example, the brain and heart are among the organs that are most severely impacted by the loss of mitochondrial dynamics proteins. Of similar importance is embryonic development. It is therefore not surprising that most of the mitochondrial dynamics associated diseases are related to developmental, neurodegenerative, and metabolic disorders, and cardiomyopathies. These diseases manifest in aberrant mitochondrial function, cell growth, proliferation, and apoptosis. Considering the role of mitochondrial dynamics in cell proliferation in particular, recent advances illuminating these processes in cancer are particularly insightful. It is interesting to note that most of the human diseases are related to increased mitochondrial fragmentation, and in this section we will discuss the role of mitochondrial fission in human diseases.
1.4.1. Cancer: A Novel Role for Mitochondrial Fission
One of the more recent developments on the disease relevance of mitochondrial fission comes from the study of cancer. Increased mitochondrial fission was reported as a pathogenicity factor in several distinct cancer models. It is interesting to note that these reports come from different cancers, originating in distinct organs, and may have entirely different oncogenic signaling circuitry yet result in the upregulation of DRP1 activity leading to mitochondrial fission. Also while some reports indicate a crucial role for DRP1 in cancer initiation and transformation, others show an important role for DRP1 in the later metastatic stages suggesting a role for DRP1 in different stages of the disease progression (Zhao et al. 2013). These observations indicate a fundamental role of DRP1 in cancer.
Mitochondrial fragmentation mediated by the upregulation of DRP1 is implicated in cellular transformation by oncogenic RAS in a mouse embryonic fibroblast (MEF) model (Serasinghe et al. 2015). Excessive mitochondrial fragmentation by oncogenic RAS is crucial for the development of cancer metabolism, and transformation of MEFs. Genetic ablation and pharmacological inhibition of DRP1 was shown to abolish the transformation, suggesting a specific role for this protein in cancer development. The fission reported here is mainly mediated by DRP1S616 phosphorylation by ERK. DRP1S616 is a mitotic phosphorylation site and is responsible for fragmenting mitochondria during mitosis. Extrapolating from this observation, it is possible to envision that RAS mediated phosphorylation of DRP1S616 mimics this mitotic phosphorylation event and may facilitate or drive excessive cell proliferation in these RAS-MAPK driven cancers. Additionally, mitochondrial fission results in cancer metabolism, by preferentially utilizing the glycolytic pathway for energy production and increased biosynthesis of macromolecules. This metabolic switch could also support the development of cancer by supporting growth and proliferation of cancer cells. ERK was found to directly phosphorylate DRP1S616 in BRAFV600E positive melanoma as well as pancreatic cancer (Kashatus et al. 2015; Serasinghe et al. 2015). In melanoma patient samples, DRP1S616 phosphorylation shows a clear correlation with the presence of BRAFV600E, and a strong correlation with increasing DRP1S616 phosphorylation levels with cancer progression (Serasinghe et al. 2015; Wieder et al. 2015).
DRP1 is dysregulated in breast, lung, pancreatic, and thyroid carcinomas (Kashatus et al. 2015; Serasinghe et al. 2015). Recently DRP was found to play a crucial role in the development of glioblastoma (Xie et al. 2015). Brain tumor initiating cells (BTIC) and non-BTIC cells were found to harbor differential post-translational modification patterns of DRP1 enhancing or blocking the development of tumors. BTIC cells had a predominant phosphorylation-activated form of DRP1S616, while non-BTIC cells showed a predominantly inactive 637 phosphorylation of DRP1. While total DRP1 levels remained unchanged, the presence of DRP1S616 phosphorylation was elevated selectively in a panel of glioblastoma tissue samples compared to non-tumor tissues. The activation of DRP1S616 was found to be regulated by the kinase CDK5. This study was further supported by patient data which indicated an inverse correlation between patient survival and the presence of DRP1S616. This comprehensive study points to the differential regulation of DRP1 post-translational modification as the switch between tumor growth and differentiation of cells, suggesting that DRP1 phosphorylation may be an important point of control in tumorigenesis.
A similar report from the study of lung cancer (Rehman et al. 2012) showed that DRP1 expression is upregulated and present in its activated form (S616p) in cancer cell lines, as well as tissue microarrays. This is associated with a parallel downregulation of the fusion protein Mfn2. Blocking fission using Mdivi-1, a small molecule inhibitor to DRP1, or overexpressing Mfn2 in lung cancer cells blocked cell cycle progression at G2/M stage. This study showed increased expression of DRP1, along with increased serine 616 phosphorylation, and decreased serine 637 phosphorylation in lung cancer cell lines, again drawing attention to the differential regulation of DRP1 post-translational modifications in cancer.
The underlying observation from these cases is that DRP1 expression or activation seems to be the predominant cause for the fragmentation (deferential expression of MFN2 has been noted less frequently) (Ferreira-da-Silva et al. 2015; Rehman et al. 2012), even though the genetic and etiologic factors for these cancers are distinct. Also, the signaling pathways responsible for the activation of DRP1 are distinct between the cancers. Some are directly under the regulation of cell cycle, while others are activated by oncogenic signaling pathways such as the RAS-MAPK pathway. The genotypes of these cancers are not reported except for in a few cases including BRAFV600E in melanoma, so it will be worthwhile to genotype these cancers and cancer cell lines to interrogate whether there exist common components between the signaling circuitry among different cancers. This would enable us to determine if there is indeed a shared component in the upstream DRP1 regulation in cancer or if it is the result of many different factors. In either case it will be interesting to see how this novel, and thus far under explored aspect of mitochondrial dynamics is implicated in cancer.
The fact that different signaling pathways lead to DRP1 activation and mitochondrial fragmentation suggests a fundamental role for mitochondrial fission mediated by DRP1 in cancer. It is possible that excessive mitochondrial fission augments the uncontrolled cell division phenotype seen in cancer. Indeed mitochondrial fission leads to cancer-like metabolism which may be a factor that triggers transformation (Serasinghe et al. 2015), or mitochondrial fragmentation may promote cell cycle progression (Qian et al. 2012; Rehman et al. 2012). Indeed, the end result may be a combination of both these processes which facilitates cells to synthesize the cellular building blocks via a predominant glycolytic metabolic pathway (Ward and Thompson 2012a) and subsequently utilize those components for cellular growth and divide and multiply rapidly as characteristic of cancer.
Another intriguing possibility with mitochondrial dynamics in cancer is the ability of mitochondrial shape to determine the susceptibility to apoptosis. It has been shown that DRP1 depletion sensitizes cancer cells to apoptosis (Inoue-Yamauchi and Oda 2012; Rehman et al. 2012). This suggests that not only does the increased fission lead to enhanced proliferation, but it also enable the cells to evade apoptosis. A key piece of evidence for the ability of mitochondrial shape to determine apoptosis susceptibility come from a recent report, that showed with mechanistic evidence that small mitochondria are more resistant to MOMP compared to intermediate and long mitochondria (Renault et al. 2015). This suggests that the excessive mitochondrial fission may also be a cellular adaptation to avoid apoptosis, and this characteristic maybe selected for in cancer cell survival. This observation, along with the earlier discussed DRP1 involvement in cancer development, reiterates a potential therapeutic intervention point in modulating mitochondrial fission in cancer.
1.4.2. Familial Disorders Arising from Defects in Mitochondrial Dynamics Proteins
It is interesting to note that familial defects of the fusion proteins Mfn1 and OPA1 both lead to disease conditions arising from neuropathies, highlighting the importance of mitochondrial dynamics on the correct functioning of the nervous system. Mutations of OPA1 leading to its loss of function cause Autosomal Dominant Optic Atrophy (ADOA). Mutations in its GTPase domain and C-terminal end result in autosomal dominancy and haplo-insufficiency leading to the loss of function of OPA1 (Olichon et al. 2007). The disease is characterized by progressive loss of vision leading to blindness in the second decade of life (Olichon et al. 2006). Loss of OPA1 and unopposed fission leads to apoptosis of retinal ganglion cells and degradation of the optic nerve. Interestingly rare Mfn2 mutations leading to similar phenotypes and ADOA have been identified, suggesting that aberrant OMM mitochondrial fusion may also contribute to the disease phenotype (Zuchner et al. 2006). On the other hand, autosomal dominant missense mutations in Mfn2 GTPase and coiled-coil domains lead to the development of the CMT2A. This leads to metabolic defects, reduced energy production, and limited mitochondrial transport to synapses, and usually manifests as muscle atrophy of the legs and feet (Klein et al. 2011; Zuchner et al. 2006). While mutations in DRP1 are not affiliated with any familial disease conditions, a DRP1 mutation in the Alanine 395 residue to Aspartic acid was reported to cause death in a new born infant as a result of multiple developmental disorders (Waterham et al. 2007). The infant manifested microcephaly, abnormal brain development, optic atrophy and hypoplasia, persistent lactic acidemia, and a mildly elevated plasma concentration of very-long-chain fatty acids (Waterham et al. 2007). Fibroblasts from this patient showed hyperfused mitochondria due to defective DRP1 function; however, the OX-PHOS and the respiratory complex activity were normal in these cells. This isolated case was due to a random point mutation of the Drp1 gene, but provides insight into the importance of proper functioning of the mitochondrial fission machinery in human health.
1.4.3. Neurodegenerative Disorders
Parkinson’s Disease
Parkinson’s disease (PD) is characterized by resting tremor, rigidity, and bradykinesia resulting from death of dopaminergic neurons in the substantia nigra. While most cases of PD are sporadic, there is a minority of rare heritable juvenile cases resulting from autosomal recessive mutations in PINK1 and Parkin genes (Pickrell and Youle 2015; Valente et al. 2004). Mitochondrial dysfunction is strongly implicated in PD. As we discussed at the beginning of this review PINK1 and Parkin regulate mitochondrial quality control through mitophagy. Knockdown of PINK1 leads to mitochondrial fragmentation and autophagy in neurons through oxidative stress, and PINK1 expression restored tubular mitochondria and suppressed toxin induced autophagy/mitophagy (Dagda et al. 2009). Expression of the dominant negative DRP1 mutant K38A inhibited both fission and mitophagy in PINK1-deficient cells, prevented stress induced mitochondrial depolarization, and apoptosis by reducing oxidative stress and mitochondrial fission (Dagda et al. 2009). Additionally, Pink1 phosphorylates and targets Parkin to depolarize mitochondria and enhances mitophagy (Kim et al. 2008). PINK1/Parkin dysfunction leading to increased DRP1 mediated mitochondrial fission is an important factor contributing to neuronal death in PD (Deng et al. 2008; Wang et al. 2011). For example, loss of PINK1 increases DRP1 levels and results in increased mitochondrial fission, increased oxidative stress, and reduced ATP generation (Dagda et al. 2009; Lutz et al. 2009). These phenotypes were rescued by expression of Mfn1 and 2 and OPA1 and treatment with the DRP1 inhibitor Mdivi-1 (Cui et al. 2010). Mitochondrial fission due to the loss of PINK1 was observed in skin fibroblasts of PD patients (Exner et al. 2007). It has been shown that pesticides such as rotenone and paraquat (inhibitors of mitochondrial respiratory complex I) that promote PD induce mitochondrial fission (Barsoum et al. 2006; Gomez-Lazaro et al. 2008). Reduced complex I activity in the substantia nigra of the brain is characteristic of PD, suggesting a mitochondrial fission-function axis on the disease onset and progression of Parkinson’s disease (Schapira 2006).
Alzheimer’s Disease
Alzheimer’s disease is characterized by progressive senile or pre-senile dementia with associated biochemical features such as selective neuronal loss, synaptic alterations leading to loss of connectivity between neurons, neurofibrillary degeneration, and extracellular deposits of αβ plaques (Baloyannis 2006). Alzheimer’s disease (AD) displays signs of overall mitochondrial dysfunction such as altered lipid metabolism, calcium homeostasis, decreased energy metabolism, and increased oxidative damage and is considered an early event in disease pathology (Ferreira et al. 2010; Hirai 2000; Supnet and Bezprozvanny 2010). Intriguingly, abnormal mitochondrial shape and dynamics have also been reported from AD studies, and the majority of these studies show a clear association between the form and function of mitochondria in this disease. For example, abnormal small mitochondria with defective cristae structure were observed in an electron microscopy (EM) study of human AD neurons (Baloyannis 2006). Abnormal expression levels of mitochondrial dynamics proteins, including increased expression of DRP1 and Fis 1, were reported from postmortem brains of Alzheimer patients, along with reduced expression of Mfn1, 2, and OPA1 (Reddy et al. 2012; Wang et al. 2009b). This and other studies in mouse models and APP cell lines indicate that aberrantly fragmented mitochondria in AD are at least in part due to the increased expression of fission factors and decreased expression of fusion proteins, resulting an imbalance in mitochondrial dynamics favoring mitochondrial fission (Barsoum et al. 2006; Reddy et al. 2011; Rui et al. 2006; Wang et al. 2008b). Additionally, many reports implicate post-translational modifications of DRP1 as causative of mitochondrial fragmentation in AD. Accumulation of β amyloid protein leads to the production of nitric oxide (NO), which activates DRP1 function by s-nitrosylating DRP1, resulting in mitochondrial fission, synaptic loss, and neuronal damage (Cho et al. 2009). Mutation of DRP1 to prevent nitrosylation protected neurons from these effects, suggesting a direct role of DRP1 (Cho et al. 2009). This is supported by the observation that exposure to NO rapidly fragments mitochondria in primary cortical neuronal cells, with an associated decline in ATP generation and increase in free radicals (Barsoum et al. 2006). This fission event is thought to precede the neuronal injury and cell death, and is modulated by the regular mitochondrial dynamics machinery (Barsoum et al. 2006). However, in the same system mitochondrial fission was shown to support BAX mediated apoptosis when challenged with nitrosative stress, indicating a direct role of fission in the subsequent neuronal cell death. DRP1 interaction with amyloid precursor protein was reported from AD patient samples and studies done using ABPP (amyloid beta precursor protein) transgenic mice with increased interactions correlating with increased disease pathology (Manczak et al. 2011). Similarly, a study using an AD cybrid cell system showed increased mitochondrial fission mediated by DRP1 and associated mitochondrial dysfunction. ERK signaling facilitated the DRP1 mitochondrial localization and fission, and blocking ERK signaling or DRP1 with Mdivi-1 rescued the morphology and function (Gan et al. 2014). This observation in particular draws a parallel with the ERK-mediated phosphorylation and activation of DRP1 at S616 resulting in increased mitochondrial fission in cancer, described in the previous section, suggesting a similar mode of activation in this system. This also implies an underlying common signaling network governing mitochondrial fission, between these unrelated pathologies. The early onset of mitochondrial dysfunction in AD may be a consequence of altered mitochondrial shape and dynamics, suggesting that interrogation of the mitochondrial dynamics machinery in Alzheimer’s disease may be useful in understanding and treating the disease.
Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant disease characterized by choreoathetosis, dementia, and premature death. The disease arises due to a mutation in the huntingtin gene (Htt) that results in amplification of a stretch of “CAG” trinucleotide sequences encoding a tract of polyglutamine in the protein (Wang et al. 2009a). This polyglutamine results in mis-folding of the Htt protein and aberrant protein interactions. Mitochondrial dysfunction is one of the prominent features of the pathophysiology of this neurodegenerative disease (Brouillet et al. 1995; Panov et al. 2002; Schapira and Patel 2014; Tabrizi et al. 1999). Interestingly, increased mitochondrial fission is a characteristic of the disease. Overexpression of mutant Htt was shown to cause mitochondrial fragmentation, and the severity of the fragmentation depended on the length of the polyglutamine repeat (Wang et al. 2008a, 2009a). Upregulation of DRP1 and Fis1 and downregulation of Mfn1, Mfn2, and OPA1 was reported from the frontal cortex of HD patients (Costa et al. 2010; Shirendeb et al. 2011) suggesting that the aberrant phenotype is due to shifting the mitochondrial dynamics to favor fission. In addition, mutant Htt was shown to interact with DRP1, increase its GTPase activity, and promote mitochondrial fission in BAC-HD transgenic mouse neurons (Shirendeb et al. 2012). This resulted in defective anterograde movement of mitochondria on the axons and synaptic defects (Shirendeb et al. 2012). The Htt–DRP1 interaction and mitochondrial fission was associated with increased sensitivity of cells to apoptosis, in both rat neurons and Htt patient derived fibroblasts (Song et al. 2011). Inhibition of DRP1, and increasing Mfn1 and 2 expression restored mitochondrial fusion and prevented cell death. S-Nitrosylation, and activation of DRP1, was reported as a consequence of NO production by the mutant Htt protein in Htt transgenic mice and postmortem brains of HD patients. This draws an interesting parallel with the disease pathology of AD where NO plays an important role in DRP1 activation mediated mitochondrial fission and mitochondrial dysfunction (Cho et al. 2009), suggesting that common disease pathways are shared between these neurodegenerative disorders.
1.4.4. Cardiovascular Diseases
As the major energy demanding organ in the body, the heart relies on mitochondrial function to maintain ATP production to fuel cardiomyocyte metabolism and contractile function. Therefore it is logical to anticipate a role for mitochondrial dynamics in cardiomyocyte function. In support of this notion, increased mitochondrial fission associated with the loss of OPA1 has been reported in failing rat and human hearts (Chen et al. 2009). More recently it was shown that stress induced processing of OPA1 by the protease OMA1 leads to mitochondrial fragmentation, dilated cardiomyopathy, and heart failure in mice (Wai et al. 2015). While defects in mitochondrial fission and fusion factors have not been identified thus far as direct causative factors in cardiac diseases in humans, studies done in mouse models indicate that excessive mitochondrial fission is associated with programmed cardiomyocyte death during heart failure and ischemia–reperfusion injury (Disatnik et al. 2013; Sharp et al. 2014). This provides a therapeutic opportunity for manipulation of mitochondrial dynamics in cardiac diseases to delay or even reverse the cell death occurring during cardiac hypertrophy or heart failure, providing a window of opportunity for the management of the disease. Accordingly, pharmacological inhibition of DRP1 using the small molecule inhibitor Mdivi-1 or siRNA mediated knockdown of DRP1 was shown to rescue mouse hearts from ischemia and reperfusion injury (Ong et al. 2010, 2015; Sharp et al. 2014). DRP1 is activated by dephosphorylation at serine 637 by calcineurin in a cardiac arrest mouse model (Sharp et al. 2014), and blocking calcineurin function reversed the mitochondrial fission and maintained mitochondrial network connectivity. Similarly, acute inhibition of DRP1–Fis1 interaction by the peptide P110 at the onset of reperfusion blocked mitochondrial fission and resulted in improved bioenergetics leading to long-term benefits postmyocardial infarction (Disatnik et al. 2013). Taken together these observations suggest a therapeutic utility for pharmacologically modulating mitochondrial dynamics in cardiovascular diseases.
1.4.5. Metabolic Disorders and Obesity
Recent evidence suggest that aberrant mitochondria dynamics contribute to metabolic disorders either directly or indirectly. For example, diabetic cardiomyopathy and non-alcoholic liver disease both show gross alterations in mitochondrial morphology with functional consequences such as swollen or misshapen mitochondria in patients (Galloway and Yoon 2013). In DCM patients these defects are associated with reduced Mfn1 levels and correspondingly shorter mitochondria. The mitochondrial network remodeling and associated dysfunction seen in these defects is hyperglycemia driven (Croston et al. 2014).
While a link between obesity and mitochondrial dynamics has not been reported in humans, studies show that this may be the case in mouse models. For example, in the hypothalamic axis of feeding and satiety, neuropeptide Y and agouti-related protein (NPY-AgRP) neurons showed increased mitochondrial fusion via Mfn1 and Mfn2 with feeding and overfeeding, in turn leading to elevated levels of leptin and glucose (Dietrich et al. 2013). Mitochondrial fusion was also shown to regulate neuronal activity via modulation of cellular ATP levels in this mouse model of dietinduced obesity. In proopiomelanocortin (POMC) neurons diet-induced obesity reduced Mfn2 levels impacting on ER–mitochondrial tethering and increased ER stress leading to leptin resistance in these mice (Schneeberger et al. 2013). In mouse and rat models of type I and type II diabetes excessive mitochondrial fragmentation has been reported, with associated detrimental effects such as increased ROS production, reduced mitochondrial function, and increased susceptibility to apoptosis (Makino et al. 2010; Trudeau et al. 2010; Yu et al. 2006). ROS generation in this context was found to be a consequence of mitochondrial fragmentation, providing a direct link to this pathology (Yu et al. 2006).
1.5. Perspectives: Targeting Mitochondrial Fission in Disease Management and Therapy
Considering the role of excessive mitochondrial fission in the development and progression of multiple pathologies as we described in this chapter, it is possible to envision the utility of mitochondrial fission and fusion factors in predicting disease etiology, progression, and maintenance. For example, increased expression of DRP1 is reported in melanoma, lung, breast, and thyroid cancers and linked to the pathology (Ferreira-da-Silva et al. 2015; Serasinghe et al. 2015; Zhao et al. 2013). In addition, DRP1S616 phosphorylation status has been clearly linked to disease progression in melanoma as well as glioblastoma (Serasinghe et al. 2015; Wieder et al. 2015; Xie et al. 2015). In melanoma, nevus to melanoma progression was correlated with increased DRP1S616 phosphorylation; while in glioblastoma, the differential phosphorylation at S616 vs S637 sites determined the ability of a cell to initiate brain tumors. ERK-mediated DRP1S616 phosphorylation was also reported from human pancreatic cancer (Kashatus et al. 2015). These novel findings foreshadow potential clinical utility of DRP1 as well as DRP1S616/S637 phosphorylation as potential biomarkers for cancer initiation and progression, and may allow novel opportunities for early detection and prevention of the these cancers.
As we discussed throughout this chapter, there is also a large body of evidence to date that show how disease phenotypes resulting from excessive fission can be reversed by blocking fission through pharmacological inhibition of DRP1. Therefore, it is reasonable to predict that future therapies targeting mitochondrial fission may enter into the clinic. Close to a decade ago Mdivi-1, a small molecule inhibitor to Dnm1 – the yeast isoform of DRP1, was identified through a chemical screen (Cassidy-Stone et al. 2008). Mdivi-1 was also found to inhibit mammalian DRP1 function by blocking its GTPase activity and inhibit mitochondrial fission, and has since become a valuable research tool in interrogating mitochondrial dynamics under various contexts. Of note, pharmacological inhibition of DRP1 mediated mitochondrial fission using Mdivi-1 as well as short peptide inhibitors such as p110 has highlighted the potential of blocking mitochondrial fission in multiple disease models, ranging from cancer and neurodegenerative diseases to cardiovascular and metabolic diseases, as discussed earlier in the chapter. While compounds like Mdivi-1 has pioneered the proof-of-principle that DRP1 mediated mitochondrial fission is indeed a viable therapeutic target, it is necessary to develop drugs with higher affinity, specificity, and selectivity in order to achieve clinical benefit. Additionally, depending on the cellular context other fission proteins may serve as viable candidates for blocking mitochondrial fission (Serasinghe et al. 2010). While multiple avenues remain to be explored in terms of effectively blocking excessive mitochondrial fission in the treatment of human diseases, it is evident that mitochondrial fission is a promising target that may reveal exciting clinical outcomes in the near future.
References
- Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T (2014) The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204: 919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J (2007) Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci 27: 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloyannis SJ (2006) Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis 9: 119–126 [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25:3900–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy B, Petrilli A, Klinglmayr E, Chen J, Lutz-Meindl U, Knott AB, Masliah E, Schwarzenbacher R, Bossy-Wetzel E (2010) S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J Alzheimers Dis 20(Suppl 2): S513–S526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM (2009) MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 10:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall NW, Beal MF (1995) Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci U S A 92:7105–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14:193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CR, Blackstone C (2007) Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282:21583–21587 [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC (2004) Mitochondrial dynamics in mammals. Curr Top Dev Biol 59:119–144 [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130:548–562 [DOI] [PubMed] [Google Scholar]
- Chen L, Gong Q, Stice JP, Knowlton AA (2009) Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu Y, Dorn GW 2nd (2011) Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109:1327–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ren S, Clish C, Jain M, Mootha V, McCaffery JM, Chan DC (2015) Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J Cell Biol 211:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR (2010) The BCL-2 family reunion. Mol Cell 37:299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA (2009) S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324: 102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CH, Lin CC, Yang MC, Wei CC, Liao HD, Lin RC, Tu WY, Kao TC, Hsu CM, Cheng JT, Chou AK, Lee CI, Loh JK, Howng SL, Hong YR (2012) GSK3beta-mediated Drp1 phosphorylation induced elongated mitochondrial morphology against oxidative stress. PLoS One 7, e49112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V, Kaddour-Djebbar I, Lakshmikanthan V, Ghazaly T, Thangjam GS, Sreekumar A, Lewis RW, Mills IG, Bollag WB, Kumar MV (2011) Novel role of androgens in mitochondrial fission and apoptosis. Mol Cancer Res 9:1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, De Strooper B (2006) Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126:163–175 [DOI] [PubMed] [Google Scholar]
- Collins Y, Chouchani ET, James AM, Menger KE, Cocheme HM, Murphy MP (2012) Mitochondrial redox signalling at a glance. J Cell Sci 125:801–806 [DOI] [PubMed] [Google Scholar]
- Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, Malorni W, Davies KJ, Carafoli E, Scorrano L (2010) Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Mol Med 2: 490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croston TL, Thapa D, Holden AA, Tveter KJ, Lewis SE, Shepherd DL, Nichols CE, Long DM, Olfert IM, Jagannathan R, Hollander JM (2014) Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am J Physiol Heart Circ Physiol 307: H54–H65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K (2010) Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem 285:11740–11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ 3rd, Kulich SM, Tandon A, Park D, Chu CT (2009) Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem 284:13843–13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, Votruba M (2007) Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet 16:1307–1318 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2008) Mitofusin 2: a mitochondria-shaping protein with signaling roles beyond fusion. Antioxid Redox Signal 10:621–633 [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M (2008) The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A 105:14503–14508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Liu ZW, Horvath TL (2013) Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D (2013) Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc 2, e000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS (2006) Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem 281:37972–37979 [DOI] [PubMed] [Google Scholar]
- Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T (2009) Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol 187:1023–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkholi R, Renault TT, Serasinghe MN, Chipuk JE (2014) Putting the pieces together: How is the mitochondrial pathway of apoptosis regulated in cancer and chemotherapy? Cancer Metab 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C (2007) Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27:12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira IL, Resende R, Ferreiro E, Rego AC, Pereira CF (2010) Multiple defects in energy metabolism in Alzheimer’s disease. Curr Drug Targets 11:1193–1206 [DOI] [PubMed] [Google Scholar]
- Ferreira-da-Silva A, Valacca C, Rios E, Populo H, Soares P, Sobrinho-Simoes M, Scorrano L, Maximo V, Campello S (2015) Mitochondrial dynamics protein Drp1 is overexpressed in oncocytic thyroid tumors and regulates cancer cell migration. PLoS One 10, e0122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126:177–189 [DOI] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334:358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway CA, Yoon Y (2013) Mitochondrial morphology in metabolic diseases. Antioxid Redox Signal 19:415–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Huang S, Wu L, Wang Y, Hu G, Li G, Zhang H, Yu H, Swerdlow RH, Chen JX, Yan SS (2014) Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochim Biophys Acta 1842:220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM (2008) The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell 19: 2402–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR 3rd, Hoshijima M, Dillmann W (2012) Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem 287:30024–30034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19:4861–4870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Bonekamp NA, Galindo MF, Jordan J, Schrader M (2008) 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic Biol Med 44:1960–1969 [DOI] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM (2007) Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol 178:757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Hildick KL, Luo J, Dearden L, Wilkinson KA, Henley JM (2013) SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J 32:1514–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Chandel NS (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M (2008) CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol 182:573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H (2004) Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 14:340–345 [DOI] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM (2009) Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187: 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Alvarez MI, Paz JC, Sebastian D, Munoz JP, Liesa M, Segales J, Palacin M, Zorzano A (2013) Glucocorticoid modulation of mitochondrial function in hepatoma cells requires the mitochondrial fission protein Drp1. Antioxid Redox Signal 19:366–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S (2000) Alzheimer disease: current therapy and future therapeutic strategies. Alzheimer Dis Assoc Disord 14(Suppl 1):S11–S17 [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J (2007) The machines that divide and fuse mitochondria. Annu Rev Biochem 76:751–780 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Sciarretta S, Nagarajan N, Rubattu S, Volpe M, Frati G, Sadoshima J (2014) New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxid Med Cell Longev 2014:210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J (2005) Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol 170:1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Yamauchi A, Oda H (2012) Depletion of mitochondrial fission factor DRP1 causes increased apoptosis in human colon cancer cells. Biochem Biophys Res Commun 421:81–85 [DOI] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 25:2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11:958–966 [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Zhang Z, Roda R, Fukaya M, Wakabayashi J, Wakabayashi N, Kensler TW, Reddy PH, Iijima M, Sesaki H (2012) Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J Cell Biol 197:535–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Neutzner A, Youle RJ (2007) The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol 178:71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, Counter CM, Kashatus DF (2015) Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol Cell 57:537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J (2008) PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun 377:975–980 [DOI] [PubMed] [Google Scholar]
- Klein CJ, Shi Y, Fecto F, Donaghy M, Nicholson G, McEntagart ME, Crosby AH, Wu Y, Lou H, McEvoy KM, Siddique T, Deng HX, Dyck PJ (2011) TRPV4 mutations and cytotoxic hypercalcemia in axonal Charcot-Marie-Tooth neuropathies. Neurology 76:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305:858–862 [DOI] [PubMed] [Google Scholar]
- Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TD, Glickman MH, Weissman AM (2012) Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell 47:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kapur M, Li M, Choi MC, Choi S, Kim HJ, Kim I, Lee E, Taylor JP, Yao TP (2014) MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria. J Cell Sci 127:4954–4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P (2010) miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet 6:e1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Alavian KN, Lazrove E, Mehta N, Jones A, Zhang P, Licznerski P, Graham M, Uo T, Guo J, Rahner C, Duman RS, Morrison RS, Jonas EA (2013) A Bcl-xL-Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat Cell Biol 15:773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li Y, Jiao J, Wang J, Li Y, Qin D, Li P (2014) Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis. Mol Cell Biol 34:1788–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Yu R, Jin SB, Han L, Lendahl U, Zhao J, Nister M (2013) The mitochondrial elongation factors MIEF1 and MIEF2 exert partially distinct functions in mitochondrial dynamics. Exp Cell Res 319:2893–2904 [DOI] [PubMed] [Google Scholar]
- Lokireddy S, Wijesoma IW, Teng S, Bonala S, Gluckman PD, McFarlane C, Sharma M, Kambadur R (2012) The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metab 16:613–624 [DOI] [PubMed] [Google Scholar]
- Loson OC, Song Z, Chen H, Chan DC (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, Brunner B, Kurz-Drexler A, Vogel F, Reichert AS, Bouman L, Vogt-Weisenhorn D, Wurst W, Tatzelt J, Haass C, Winklhofer KF (2009) Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem 284:22938–22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Scott BT, Dillmann WH (2010) Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53:1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet 20:2495–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP (2014) A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res 114:626–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE (2011) Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol 18:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Carelli V, Manfredi G, Chan DC (2014) Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab 19:630–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30:4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S (2006) MARCH-V is a novel mitofusin 2and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep 7:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann A, Ruegg M, La Padula V, Schenone A, Suter U (2005) Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol 170:1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann A, Wagner KM, Ruegg M, Suter U (2009) GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis 36:509–520 [DOI] [PubMed] [Google Scholar]
- Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, Ducommun B, Lenaers G, Belenguer P (2002) The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the intermembrane space. FEBS Lett 523:171–176 [DOI] [PubMed] [Google Scholar]
- Olichon A, Guillou E, Delettre C, Landes T, Arnaune-Pelloquin L, Emorine LJ, Mils V, Daloyau M, Hamel C, Amati-Bonneau P, Bonneau D, Reynier P, Lenaers G, Belenguer P (2006) Mitochondrial dynamics and disease, OPA1. Biochim Biophys Acta 1763:500–509 [DOI] [PubMed] [Google Scholar]
- Olichon A, Elachouri G, Baricault L, Delettre C, Belenguer P, Lenaers G (2007) OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ 14:682–692 [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022 [DOI] [PubMed] [Google Scholar]
- Ong SB, Kalkhoran SB, Cabrera-Fuentes HA, Hausenloy DJ (2015) Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur J Pharmacol 763:104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT (2011) MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep 12:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT (2002) Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci 5:731–736 [DOI] [PubMed] [Google Scholar]
- Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K (2011) Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31:1309–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Ngoh GA, Dabkowski ER, O’Connell KA, Ribeiro RF Jr, Stanley WC, Walsh K (2012) Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol 302:H167–H179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YY, Cho H (2012) Mitofusin 1 is degraded at G2/M phase through ubiquitylation by MARCH5. Cell Div 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H (2010) Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci 123:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham AH, Meng S, Chu QN, Chan DC (2012) Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit. Hum Mol Genet 21: 4817–4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85:257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, Fortin D, le Huynh H, Nicolas V, Alavi MV, Brenner C, Ventura-Clapier R, Veksler V, Joubert F (2012) Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res 94:408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L (2010) The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One 5:e10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent J, Zunino R, Sugiura A, Mattie S, Shore GC, McBride HM (2015) MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol Cell 59:941–955 [DOI] [PubMed] [Google Scholar]
- Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D (2011) Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell 22:256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Choi S, Gibson GA, Watkins SC, Bakkenist CJ, Van Houten B (2012) Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J Cell Sci 125:5745–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A 108:10190–10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67: 103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M (2012) Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta 1822:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C, Archer SL (2012) Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J 26:2175–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault TT, Floros KV, Elkholi R, Corrigan KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla JJ, Buettner C, Newmeyer DD, Chipuk JE (2015) Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol Cell 57:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Tiwari P, Xie Z, Zheng JQ (2006) Acute impairment of mitochondrial trafficking by betaamyloid peptides in hippocampal neurons. J Neurosci 26:10480–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT (2003) Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 116: 2763–2774 [DOI] [PubMed] [Google Scholar]
- Satoh M, Hamamoto T, Seo N, Kagawa Y, Endo H (2003) Differential sublocalization of the dynamin-related protein OPA1 isoforms in mitochondria. Biochem Biophys Res Commun 300:482–493 [DOI] [PubMed] [Google Scholar]
- Scarpulla RC, Vega RB, Kelly DP (2012) Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH (2006) Etiology of Parkinson’s disease. Neurology 66:S10–S23 [DOI] [PubMed] [Google Scholar]
- Schapira AH, Patel S (2014) Targeting mitochondria for neuroprotection in Parkinson disease. JAMA Neurol 71:537–538 [DOI] [PubMed] [Google Scholar]
- Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M (2013) Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155:172–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serasinghe MN, Yoon Y (2008) The mitochondrial outer membrane protein hFis1 regulates mitochondrial morphology and fission through self-interaction. Exp Cell Res 314:3494–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serasinghe MN, Seneviratne AM, Smrcka AV, Yoon Y (2010) Identification and characterization of unique proline-rich peptides binding to the mitochondrial fission protein hFis1. J Biol Chem 285:620–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serasinghe MN, Wieder SY, Renault TT, Elkholi R, Asciolla JJ, Yao JL, Jabado O, Hoehn K, Kageyama Y, Sesaki H, Chipuk JE (2015) Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol Cell 57:521–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL (2014) Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28:316–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH (2011) Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum Mol Genet 20:1438–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, Mao P, Reddy PH (2012) Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum Mol Genet 21:406–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC (2007) OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 178:749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman M, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G, Bossy-Wetzel E (2011) Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med 17:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorianello E, Soriano FX, Fernandez-Pascual S, Sancho A, Naon D, Vila-Caballer M, Gonzalez-Navarro H, Portugal J, Andres V, Palacin M, Zorzano A (2012) The promoter activity of human Mfn2 depends on Sp1 in vascular smooth muscle cells. Cardiovasc Res 94:38–47 [DOI] [PubMed] [Google Scholar]
- Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A (2006) Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes 55:1783–1791 [DOI] [PubMed] [Google Scholar]
- Sugiura A, Nagashima S, Tokuyama T, Amo T, Matsuki Y, Ishido S, Kudo Y, McBride HM, Fukuda T, Matsushita N, Inatome R, Yanagi S (2013) MITOL regulates endoplasmic reticulum-mitochondria contacts via Mitofusin2. Mol Cell 51:20–34 [DOI] [PubMed] [Google Scholar]
- Supnet C, Bezprozvanny I (2010) Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J Alzheimers Dis 20(Suppl 2):S487–S498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH (1999) Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol 45:25–32 [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282:11521–11529 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Molina AJ, Guo W, Roy S (2010) High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol 177:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–1160 [DOI] [PubMed] [Google Scholar]
- Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Ruperez FJ, Barbas C, Ibanez B, Langer T (2015) Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350, aad0116. [DOI] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H (2009) The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 186:805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A (2009) Modulation of calcium signalling by mitochondria. Biochim Biophys Acta 1787:1374–1382 [DOI] [PubMed] [Google Scholar]
- Wang CE, Tydlacka S, Orr AL, Yang SH, Graham RK, Hayden MR, Li S, Chan AW, Li XJ (2008a) Accumulation of N-terminal mutant huntingtin in mouse and monkey models implicated as a pathogenic mechanism in Huntington’s disease. Hum Mol Genet 17:2738–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X (2008b) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105:19318–19323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lim PJ, Karbowski M, Monteiro MJ (2009a) Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet 18:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009b) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29:9090–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Song P, Du L, Tian W, Yue W, Liu M, Li D, Wang B, Zhu Y, Cao C, Zhou J, Chen Q (2011) Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem 286:11649–11658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W,Wang Y,Long J,Wang J,Haudek SB,Overbeek P,Chang BH,Schumacker PT,Danesh FR (2012) Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15:186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB (2012a) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB (2012b) Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol 4:a006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM (2007) Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177:439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV (2007) A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med 356:1736–1741 [DOI] [PubMed] [Google Scholar]
- White KE, Davies VJ, Hogan VE, Piechota MJ, Nichols PP, Turnbull DM, Votruba M (2009) OPA1 deficiency associated with increased autophagy in retinal ganglion cells in a murine model of dominant optic atrophy. Invest Ophthalmol Vis Sci 50:2567–2571 [DOI] [PubMed] [Google Scholar]
- Wieder SY, Serasinghe MN, Sung JC, Choi DC, Birge MB, Yao JL, Bernstein E, Celebi JT, Chipuk JE (2015) Activation of the mitochondrial fragmentation protein DRP1 correlates with BRAF(V600E) melanoma. J Invest Dermatol 135:2544–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Morgan JE, Votruba M (2010) Opa1 deficiency in a mouse model of dominant optic atrophy leads to retinal ganglion cell dendropathy. Brain 133:2942–2951 [DOI] [PubMed] [Google Scholar]
- Williams PA, Morgan JE, Votruba M (2011) Mouse models of dominant optic atrophy: what do they tell us about the pathophysiology of visual loss? Vision Res 51:229–234 [DOI] [PubMed] [Google Scholar]
- Williams PA, Piechota M, von Ruhland C, Taylor E, Morgan JE, Votruba M (2012) Opa1 is essential for retinal ganglion cell synaptic architecture and connectivity. Brain 135:493–505 [DOI] [PubMed] [Google Scholar]
- Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, Dombrowski SM, Huang Z, Fang X, Shi Y, Ferguson AN, Kashatus DF, Bao S, Rich JN (2015) Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci 18:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, Inatome R, Yanagi S (2006) A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J 25:3618–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA (2003) The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23:5409–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y (2006) Increased production of reactive oxygen species in hyper-glycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103:2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Jhun BS, Yoon Y (2011) High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal 14:425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Chen Z, Liu H, Yan C, Chen M, Feng D, Yan C, Wu H, Du L, Wang Y, Liu J, Huang X, Xia L, Liu L, Wang X, Jin H, Wang J, Song Z, Hao X, Chen Q (2014) A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res 24: 482–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kageyama Y, Sesaki H (2012) Mitochondrial division prevents neurodegeneration. Autophagy 8:1531–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Liu T, Jin S, Wang X, Qu M, Uhlen P, Tomilin N, Shupliakov O, Lendahl U, Nister M (2011) Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J 30:2762–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y (2013) Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32:4814–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziviani E, Whitworth AJ (2010) How could Parkin-mediated ubiquitination of mitofusin promote mitophagy? Autophagy 6:660–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S,DeJonghe P,Jordanova A,Claeys KG,Guergueltcheva V,Cherninkova S,Hamilton SR, Van Stavern G, Krajewski KM, Stajich J, Tournev I, Verhoeven K, Langerhorst CT, de Visser M, Baas F, Bird T, Timmerman V, Shy M, Vance JM (2006) Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol 59:276–281 [DOI] [PubMed] [Google Scholar]
- Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM (2007) The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci 120: 1178–1188 [DOI] [PubMed] [Google Scholar]