Abstract
Background:
Because HIV viral suppression is essential for optimal outcomes and prevention efforts, understanding trends and predictors is imperative to inform public health policy.
Objective:
To evaluate viral suppression trends in people living with HIV (PLWH), including the relationship of associated factors, such as demographic characteristics and integrase strand transfer inhibitor (ISTI) use.
Design:
Longitudinal observational cohort study.
Setting:
8 HIV clinics across the United States.
Participants:
PLWH receiving clinical care.
Measurements:
To understand trends in viral suppression (≤400 copies/mL), annual viral suppression rates from 1997 to 2015 were determined. Analyses were repeated with tests limited to 1 random test per person per year and using inverse probability of censoring weights to address loss to follow-up. Joint longitudinal and survival models and linear mixed models of PLWH receiving antiretroviral therapy (ART) were used to examine associations between viral suppression or continuous viral load (VL) levels and demographic factors, substance use, adherence, and ISTI use.
Results:
Viral suppression increased from 32% in 1997 to 86% in 2015 on the basis of all tests among 31 930 PLWH. In adjusted analyses, being older (odds ratio [OR], 0.76 per decade [95% CI, 0.74 to 0.78]) and using an ISTI-based regimen (OR, 0.54 [CI, 0.51 to 0.57]) were associated with lower odds of having a detectable VL, and black race was associated with higher odds (OR, 1.68 [CI, 1.57 to 1.80]) (P < 0.001 for each). Similar patterns were seen with continuous VL levels; when analyses were limited to 2010 to 2015; and with adjustment for adherence, substance use, or depression.
Limitation:
Results are limited to PLWH receiving clinical care.
Conclusion:
HIV viral suppression rates have improved dramatically across the United States, which is likely partially attributable to improved ART, including ISTI-based regimens. However, disparities among younger and black PLWH merit attention.
Approximately 1.2 million adults are living with HIV in the United States, with a disproportionate burden among men who have sex with men and among African Americans (1). Since 1996, the availability of potent antiretroviral therapy (ART) has led to large decreases in HIV-related mortality and morbidity. Antiretroviral therapy has transformed HIV from a fatal disease into a manageable chronic illness for people living with HIV (PLWH) who are aware of their infection and can access and adhere to ART. Furthermore, ART regimens have become increasingly better tolerated and easier to take (2).
Achieving and maintaining HIV viral suppression with ART can optimize health outcomes among PLWH and limit transmission to others (3, 4). Monitoring viral suppression is thus an important aspect of HIV care. Identifying predictors of nonsuppression and groups at increased risk for it can enhance intervention efforts.
Reports from individual clinics suggest high levels of viral suppression (5), but current large-scale estimates from across the United States are limited. One of the best assessments was from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), which found peak viral suppression of 72%; however, key potential predictors, such as adherence and substance use, were not examined (6). Furthermore, this analysis only included data through 2008 and did not examine potential improvements in viral suppression due to recent changes in ART regimens, such as increased use of integrase strand transfer inhibitors (ISTIs). These drugs may have superior tolerability, reduced pill burden, and improved virologic and immune outcomes (7–15). Up-to-date evaluations are needed to assess improvements in HIV care, specifically viral suppression, over the past decade.
The Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) is a cohort collaboration that integrates data from a large and diverse population of PLWH across the United States (16). CNICS captures a broad range of information associated with the rapidly changing course of HIV disease management. Our objective was to examine changes in viral suppression over time and identify associated factors. We were particularly interested in the association of viral suppression with changing demographic and clinical characteristics, such as substance use patterns, medication adherence, and ISTI use.
METHODS
Data Source
CNICS is a dynamic cohort of more than 32 000 PLWH who have had 2 or more HIV clinical care visits at 8 sites across the United States (Appendix Figure 1, available at Annals.org). Each CNICS clinic is at a Centers for AIDS Research site. The CNICS data repository integrates comprehensive clinical data, including laboratory test results; ART and other medications; diagnoses; demographic data; and historical information, including prior ART use, collected at initial clinic visits via standardized intake processes (16). In addition, in recent years, sites have initiated the CNICS clinical assessment of patient-reported measures and outcomes, including illicit drug use, alcohol use, adherence, and depression (17–20). During routine clinical care visits, PLWH use touchscreen tablets equipped with Web-based survey software to complete the CNICS clinical assessments (17, 21). Those who appear intoxicated; have cognitive impairment; or do not speak English, Spanish, or Amharic are not asked to complete the assessment.
Institutional review boards at each site approved CNICS protocols.
Study Participants
The overall study sample included all CNICS participants aged 18 years or older who had a viral load (VL) measure as part of clinical care between 1997 and 2015. We also examined key subgroups, including those who were receiving ART. We included a subgroup of PLWH receiving ART in or after 2010 to minimize the effect of changes in treatment initiation guidelines. Additional subgroup sensitivity analyses were done among PLWH who had completed the CNICS clinical assessment of patient-reported measures, such as adherence and illicit drug use, and among those known to be ART-naive at initiation of CNICS care.
Outcomes
The primary outcome was viral suppression versus detection, which we defined as a VL of no more than 400 versus greater than 400 copies/mL to exclude clinically insignificant VL blips (22) and account for changing thresholds of detection over time. In addition, we examined a secondary outcome of VL (in copies/mL) at each time point as a continuous variable, which we log-transformed (in base10) due to skew. We then back-transformed VL coefficients by raising them to a power of 10. We calculated relative VL values, which we defined as the ratio of VL for PLWH with and without each characteristic of interest (such as ISTI use).
Predictors
We examined age, race/ethnicity, sex, HIV transmission risk factors, and CD4 cell count at ART initiation. Self-reported adherence to ART (23, 24), illicit drug use (including and excluding marijuana) (25–29), and depression (30, 31) were collected as part of the CNICS clinical assessment (Appendix Table 1, available at Annals.org), which was integrated into clinical care between 2005 and 2011 at 7 CNICS sites.
Appendix Table 1.
Categories and Instruments Used for Key Covariates
| Covariate | Category |
|---|---|
| Race/ethnicity | Non-Hispanic white |
| Non-Hispanic black | |
| Hispanic | |
| Other | |
| HIV transmission risk factor | Men who have sex with men |
| Injection drug use or injection drug use and men who have sex with men | |
| Heterosexual | |
| Other | |
| Adherence to ART | 30-day visual analogue scale (continuous variable) |
| Illicit drug use | ASSIST instrument categorized 2 ways: |
| Binary variable indicating any illicit drug use (not including marijuana) | |
| Categories of individual drug use: cocaine/crack, methamphetamine/crystal methamphetamine, opiate/heroin, or marijuana use in the past 3 months | |
| Hazardous alcohol use | AUDIT-C instrument with a score ≥5 for men and ≥4 for women to define hazardous use |
| Depression symptom severity | PHQ-9, categorized as depressed or not based on a score ≥10 |
ART = antiretroviral therapy; ASSIST = Alcohol, Smoking and Substance Involvement Screening Test; AUDIT-C = Alcohol Use Disorders Identification Test–Consumption; PHQ-9 = 9-item Patient Health Questionnaire.
Statistical Analysis
We used χ2 tests for categorical variables and t tests for continuous variables to assess differences in demographic and clinical characteristics among persons with and without viral suppression based on VL at the end of follow-up. To understand VL trends in unadjusted ecological analyses (1997 to 2015), we determined the percentage of VL tests showing no more than 400 copies/mL each year for all patients receiving care that year (overall). We then examined VL trends among PLWH categorized by various factors, including age, sex, race/ethnicity, and HIV transmission risk factor. We examined time to viral suppression for the majority (>50%) of VL tests by year of ART initiation. People living with HIV typically had multiple measures in all years since CNICS enrollment. Because we were concerned that those who were lost to follow-up may have been less likely to have viral suppression, we repeated trend analyses limiting tests to 1 random VL result per calendar year per person and accounting for possible differential loss to follow-up using inverse probability of censoring weights based on prior VL values and demographic characteristics (age, sex, and race/ethnicity) to rebalance the sample on the basis of these parameters (32). To operationalize this approach, separate logistic models (with age, sex, and race as covariates) were fit to each time point to estimate the weights. This limited potential selection bias due to loss to follow-up that differed on the basis of characteristics of participants who were lost to follow-up.
Our primary analysis was a joint longitudinal and survival model that examined associations between demographic and clinical characteristics and having a detectable VL among persons receiving ART from 1997 to 2015 while accounting for loss to follow-up (33). In this model, we specified the longitudinal process as a mixed logistic model with detectable VL as the outcome. We specified the time-to-event process as loss to follow-up (due to death or leaving the cohort) using a Weibull distribution. This approach was selected because of known limitations with less complex models (34). In particular, there was concern that the loss-tofollow-up process was related to the detectable VL process, and so the models for these 2 processes needed to be estimated jointly. Using this model allowed us to account for potential informative dropout due to loss to follow-up that was related to both the covariates included in the model and the detectable VL measures.
We repeated the analyses and limited them to patients known to be ART-naive at enrollment. We also conducted sensitivity analyses that added adherence, illicit drug use (opiates or heroin, cocaine or crack, and methamphetamine or crystal methamphetamine), and depression among the subset who completed the CNICS clinical assessment and included current substance use categorized by class (opiates or heroin, cocaine or crack, methamphetamine or crystal methamphetamine, marijuana, and hazardous alcohol use) with adherence. To minimize the effect of changes in treatment initiation guidelines, we conducted a subgroup analysis for 2010 to 2015, during which time the guidelines changed little after having been expanded to include initiation of treatment independent of CD4 cell count. Additional sensitivity analyses were conducted that included CD4 cell count at ART initiation, added a quadratic term for calendar time (year of cohort entry, centered around 2010), excluded site, and used a different VL cut point (50 copies/mL). Finally, we repeated the analyses using linear mixed models with VL (log10) as a continuous outcome because these models inherently handle unbalanced numbers and times of observations between patients and clustering by participant (35).
All models were adjusted for age, sex, race/ethnicity, site, ISTI use, calendar time, and years of follow-up. Statistical models were fit using Stata, version 14 (Stata-Corp), with the GSEM package used to fit the joint longitudinal and survival models.
RESULTS
We included 31 930 PLWH from 8 CNICS sites (Appendix Figure 2, available at Annals.org) who had at least 1 HIV VL measurement after 1 January 1997 (mean number of measurements per person, 17 [SD, 16]), of whom 47% were alive and receiving care at the end of the study period. Eighty-two percent were men, 55% were nonwhite, and the mean number of HIV care visits was 22. Table 1 shows demographic and clinical characteristics by viral suppression status based on the most recent VL measurement. Patients with viral suppression were older (46 vs. 41 years [P < 0.001]) and were more likely to have a current CD4 count of 0.500 × 109 cells/L or greater (58% vs. 22% [P < 0.001]) than those without viral suppression.
Table 1.
Patient Demographic and Clinical Characteristics, by Most Recent Viral Suppression Status*
| Characteristic at Most Recent VL Test | Viral Suppression (n = 22 594[71%])† | No Viral Suppression(n = 9336[29%])‡ | Overall(n = 31 930[100%]) | P Value |
|---|---|---|---|---|
| Mean age (SD), y | 46(11) | 41 (10) | 44(11) | <0.001 |
| Female, % | 17 | 21 | 18 | <0.001 |
| Race/ethnicity, % | <0.001 | |||
| White | 47 | 40 | 45 | |
| Black | 35 | 46 | 38 | |
| Hispanic | 13 | 9 | 12 | |
| Other | 5 | 4 | 5 | |
| HIV transmission risk factor, % | <0.001 | |||
| MSM | 56 | 44 | 53 | |
| Heterosexual | 24 | 25 | 24 | |
| IDU | 15 | 23 | 18 | |
| Other | 5 | 7 | 5 | |
| CD4 cell count at cohort entry, % | <0.001 | |||
| ≥0.500 × 109 cells/L | 33 | 28 | 31 | |
| 0.250–0.499 × 109 cells/L | 33 | 31 | 32 | |
| <0.250 × 109 cells/L | 35 | 40 | 36 | |
| Most recent CD4 cell count, % | <0.001 | |||
| ≥0.500 × 109 cells/L | 58 | 22 | 47 | |
| 0.250–0.499 × 109 cells/L | 29 | 30 | 29 | |
| <0.250 × 109 cells/L | 13 | 49 | 23 | |
| Mean HIV care visits (SD), n | 25 (25) | 15(18) | 22 (23) | <0.001 |
| Mean VL tests (SD), n | 20 (17) | 11 (12) | 17 (16) | <0.001 |
| Year of cohort entry, % | <0.001 | |||
| 2003 or earlier | 38 | 57 | 44 | |
| 2004–2009 | 33 | 28 | 32 | |
| 2010 or later | 28 | 15 | 24 |
IDU = injection drug use; MSM = men who have sex with men; VL = viral load.
Percentages may not sum to 100 due to rounding.
VL ≤400 copies/mL.
VL >400 copies/mL.
Unadjusted Viral Suppression Trends
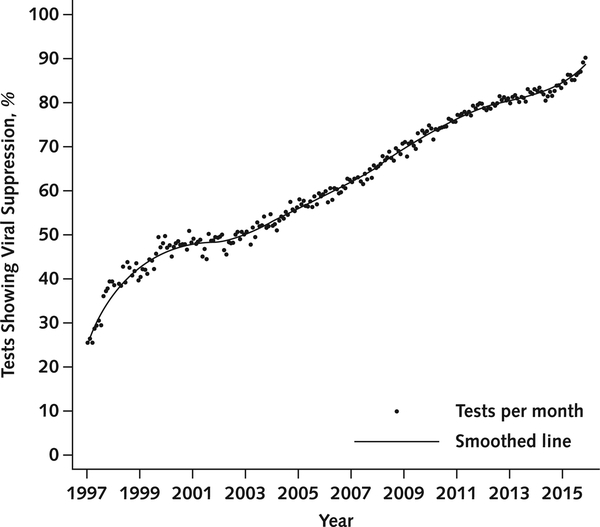
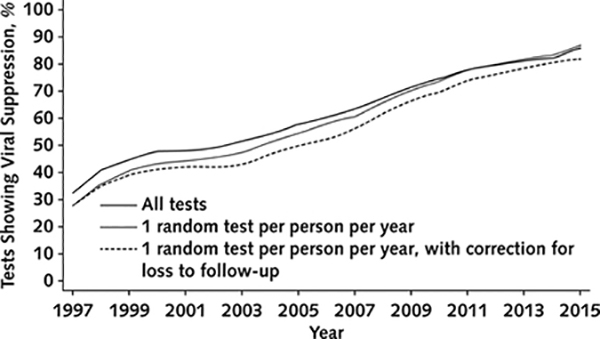
We examined viral suppression trends from 1997 to 2015 using 553 737 VL results among both PLWH who were using ART and those not using it. The number of PLWH with VL tests ranged from 3004 to 14 659 per year, and the number of tests ranged from 9193 to 39 715 per year. Viral suppression increased from 32% in 1997 to 86% in 2015 based on all VL values (Figure 1). Results were similar when we limited random VL tests to 1 per person per year and when we corrected for loss to follow-up using inverse probability weights (Appendix Figure 3, available at Annals.org).
Figure 1.
Percentage of tests showing viral suppression over time among all patients.
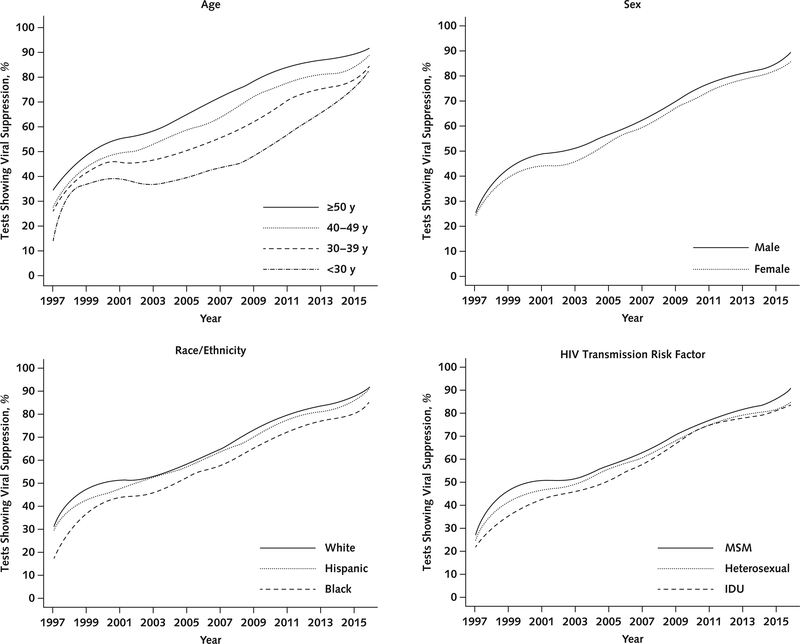
The percentages of tests showing viral suppression by demographic group are shown in Figure 2. The percentage of tests showing viral suppression increased by about 5% with each decade of age, was about 3% higher for men versus women, was about 8% lower in black versus white PLWH, and was about 6% lower in those whose HIV transmission risk factor was injection drug use compared with men who have sex with men.
Figure 2.
Percentage of tests showing viral suppression over time among all patients, by demographic and clinical characteristics. IDU = injection drug use; MSM = men who have sex with men.
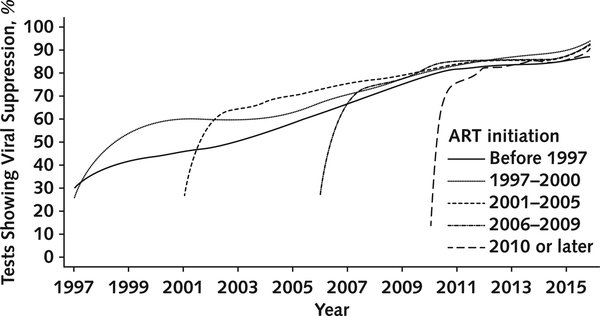
We examined viral suppression after ART initiation by calendar period. For example, among the cohort commencing ART between 1997 and 2000, most VL tests showed suppression in 9 months. In contrast, in the cohort initiating ART after 2010, most tests showed suppression in 2 months (Figure 3). Differences in testing frequency were probably not the primary driver of these results given that the median time to the initial test was 77 days for the cohort initiating ART in 1997 to 2000 versus 75 days for the cohort initiating ART after 2010.
Trends Among PLWH Receiving ART in 2010 to 2015
Among the subgroup of 20 281 PLWH receiving ART in the current treatment era (2010 to 2015), the percentage receiving ART increased each year, from 86% in 2010 to 93% in 2015. The percentage for whom all results in a given year were no more than 400 copies/mL also increased each year, from 75% in 2010 to 86% in 2015. Mean adherence did not increase over this period, with about 71% reporting greater than 95% adherence and about 82% reporting greater than 90% adherence. Similarly, about 18% of PLWH reported current illicit drug use each year from 2010 to 2015.
Factors Associated With VL in Adjusted Analyses
In joint longitudinal and survival models of patients receiving ART from 1997 to 2015, black race (odds ratio [OR], 1.68 [95% CI, 1.57 to 1.80]; P < 0.001) was associated with increased odds of having a detectable VL in adjusted analyses (Table 2). Older age (OR, 0.76 per decade [CI, 0.74 to 0.78]), Hispanic ethnicity (OR, 0.81 [CI, 0.74 to 0.90]), calendar time (OR, 0.83 [CI, 0.83 to 0.84]), years of follow-up (OR, 0.79 [CI, 0.79 to 0.80]), and use of ISTI-based regimens (OR, 0.54 [CI, 0.51 to 0.57]) were associated with lower odds of having a detectable VL (P < 0.001 for each). The shape parameter of the Weibull distribution was less than 1 (k = 0.59 [CI, 0.58 to 0.60]), indicating that attrition tends to occur early in care and PLWH are less likely to censor the longer they are in care.
Table 2.
Factors Associated With Detectable VL or Amount of VL in Adjusted Models Among Patients Receiving ART*
| Covariate | Joint Longitudinal and Survival Model† |
Linear Mixed Model‡ |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | Relative VL (95% CI) | P Value | |
| Integrase strand transfer inhibitor use | 0.54 (0.51–0.57) | <0.001 | 0.71 (0.69–0.74) | <0.001 |
| Female | 1.01 (0.93–1.08) | 0.89 | 0.98 (0.92–1.05) | 0.64 |
| Age (per decade, centered at 40 y) | 0.76 (0.74–0.78) | <0.001 | 0.83 (0.81–0.85) | <0.001 |
| Race/ethnicity (reference is white race) | ||||
| Black | 1.68 (1.57–1.80) | <0.001 | 1.60 (1.51–1.70) | <0.001 |
| Hispanic | 0.81 (0.74–0.90) | <0.001 | 0.89 (0.82–0.96) | 0.003 |
| Other | 0.83 (0.73–0.96) | 0.009 | 0.90 (0.80–1.00) | 0.053 |
| Calendar time (year of cohort entry, centered around 2010) | 0.83 (0.83–0.84) | <0.001 | 0.84 (0.83–0.84) | <0.001 |
| Years of follow-up | 0.79 (0.79–0.80) | <0.001 | 0.74 (0.74–0.75) | <0.001 |
ART = antiretroviral therapy; OR = odds ratio; VL = viral load.
Also adjusted for site.
Outcome is detectable VL (>400 copies/mL). The survival model component is included in Appendix Table 7 (available at Annals.org).
Outcome is VL in log10 copies/mL.
Examining VL as a continuous outcome led to similar findings, such as black race and younger age being associated with higher relative VL and Hispanic ethnicity and ISTI use being associated with lower relative VL (Table 2).
We repeated the analyses among the subgroup of patients receiving ART who were known to be ART-naive when joining CNICS (n = 16 019) (Appendix Table 2, available at Annals.org). The findings in this subpopulation were similar to the overall findings, with black race being associated with higher odds of a detectable VL (OR, 1.69 [CI, 1.55 to 1.84]) and greater relative VL (1.62 [CI, 1.51 to 1.74]) in adjusted analyses. Older age and Hispanic ethnicity were associated with lower odds of a detectable VL (ORs, 0.83 [CI, 0.80 to 0.86] and 0.84 [CI, 0.75 to 0.94], respectively) and lower relative VL (0.88 [CI, 0.86 to 0.91] and 0.89 [CI, 0.81 to 0.98], respectively). Finally, ISTI use also was associated with lower odds of a detectable VL (OR, 0.66 [CI, 0.62 to 0.71]) and lower relative VL (0.79 [CI, 0.76 to 0.83]).
In sensitivity analyses among the subgroup of patients receiving ART who had completed the CNICS clinical assessment, older age and ISTI use were consistently associated with lower relative VL. Higher adherence also was associated with lower relative VL (0.84 [CI, 0.83 to 0.84]), whereas illicit drug use (1.48 [CI, 1.40 to 1.57]) and depression (1.29 [CI, 1.23 to 1.35]) were associated with higher relative VL (Appendix Table 3, available at Annals.org). Similar patterns also were found in joint longitudinal and survival models with both adherence and category of substance use included in the same model (Appendix Table 4, available at Annals.org). Inclusion of these covariates led to a minimal attenuation in the association of calendar time with viral suppression, with an OR of 0.90 (CI, 0.87 to 0.93) compared with 0.83 (CI, 0.83 to 0.84) in models not adjusted for these confounding factors.
Findings were similar in sensitivity analyses that were limited to PLWH receiving ART between 2010 and 2015 to minimize the effect of changes in ART treatment guidelines (Appendix Table 5, available at Annals.org). Findings were also similar in sensitivity analyses that also included CD4 cell count at ART initiation, with black race associated with higher relative VL and older age, Hispanic ethnicity, and ISTI use associated with lower relative VL (Appendix Table 6, available at Annals.org). In addition, higher CD4 count at ART initiation was associated with lower relative VL (0.83 [CI, 0.82 to 0.84] per 0.100 × 109 cells/L). Findings were also similar in sensitivity analyses that included models that added a quadratic term for calendar time (Appendix Table 6), used a lower limit of VL quantitation, and excluded site as a covariate (data not shown).
DISCUSSION
We examined patterns of viral suppression among PLWH over time across the United States and showed a nearly 3-fold improvement in the rate of viral suppression, from 32% in 1997 to 86% in 2015. In unadjusted analyses, we found that the percentage of VL tests showing suppression differed among key demographic groups, such as black versus white persons, young versus old persons, and women versus men. Overall, time from ART initiation to viral suppression decreased and viral suppression increased over time. In adjusted analyses of patients receiving ART, factors associated with viral suppression and lower relative VL included demographic and clinical characteristics, such as older age and ISTI use. These findings persisted in analyses limited to 2010 and after (when almost everyone met criteria for ART initiation) and in sensitivity analyses that included substance use and ART adherence.
The large improvements in viral suppression at sites across the United States corroborate findings from prior studies that have shown increased suppression over time (6). For example, NA-ACCORD demonstrated a 26% increase in the percentage of PLWH who had viral suppression between 2000 and 2008, but this period did not capture use of newer regimens that are potentially more potent and tolerable.
Of note, when we focused on the current treatment era (for which published data are sparse), we showed increased viral suppression over time among patients receiving ART, which suggests that factors beyond wider ART use accounted for the improved outcomes. Although adherence and substance use are important factors in viral suppression rates at an individual level (36, 37), average adherence did not improve and substance use did not decrease over time between 2010 and 2015. Statistical models that were further adjusted for these 2 factors showed only small levels of attenuation in the association between calendar time and viral suppression. These results do not support the idea that population-level changes in adherence and substance use were driving improved suppression rates over time in the most recent era.
More widespread ART use, including initiation of ART, was the most important driver of viral suppression among PLWH. However, among those receiving ART, we found that ISTI use increased and was likely an important contributing factor to viral suppression. This was true in all sensitivity models, including those limited to patients who were known to be ART-naive when they initiated care. Sensitivity analyses that included CD4 cell count at ART initiation were done to address the possibility that patients using ISTIs or initiating ART more recently were doing so at a higher CD4 cell count, but results were similar even after adjustment for baseline CD4 cell count. Several factors may contribute to improved suppression in PLWH using ISTIs compared with those using other ART regimens, including greater tolerability, enhanced potency, single-tablet regimens containing ISTIs, or a higher bar to the development of drug resistance during periods of less-than-perfect adherence.
Despite the clinically and statistically significant achievements in viral suppression, disparities persist. For example, we found that PLWH who were younger or black were more likely to have a detectable VL, whereas those who were Hispanic were less likely to have a detectable VL in most but not all models. These findings are consistent with a study demonstrating differences in HIV care cascade steps in 2009, including viral suppression by demographic characteristics (38). Although National HIV Surveillance System data showed lower overall viral suppression rates in 2014 than we observed, they also showed lower rates of viral suppression among black compared with white or Hispanic men who had sex with men (39). Other studies demonstrated that suppression rates improved during 2009 to 2013 but still remained lower among black versus white persons (40), possibly due to differences in adherence (41). Whether due to differences in substance use, adherence, or other factors (such as medical mistrust) (42), taken together, these findings suggest the need for culturally tailored interventions to encourage ART use, enhance medication adherence, and improve viral suppression and other outcomes.
Strengths of our study include the large number of PLWH from multiple sites across the United States who are engaged in care, thus providing geographic, racial/ ethnic, and clinical diversity. This enhances generalizability compared with trials and interval cohorts that are restricted to PLWH who are willing to return for scheduled visits instead of clinical care. CNICS contains comprehensive clinical data, including factors that are often not available, such as measures of adherence and substance use.
Observational data have limitations, particularly with regard to causal inference, although the prospective data collection and comprehensive clinical data of CNICS minimize some of these concerns. Temporal trends in increased ISTI use overlapped with increased viral suppression rates, but these rates were improving before ISTIs were introduced. However, models of the association of ISTI use with viral suppression were adjusted for calendar time and both factors were statistically significant, so both are probably independent associations. Our adherence measure was self-reported, and its accuracy may have varied across demographic groups; however, we do not expect the accuracy to have changed over time. Self-reported adherence may have lacked sensitivity to detect changes in adherence. In 1997, 11% of VL tests had a lower limit of detection of less than 500 copies/mL (rather than <400 copies/mL); therefore, values of 400 to 500 copies/mL from these tests would have been misclassified as viral suppression. People living with HIV may have previously received care elsewhere, although results of sensitivity analyses among the subset of PLWH who were known to be ART-naive at initiation of CNICS care were similar to the main analyses. Differences in the number of clinic visits and VL measures among those with viral suppression versus those without could be concerning; however, trend findings were the same in analyses using 1 random VL test per person per year rather than all VL tests. We used inverse probability weights to provide reassurance that loss to follow-up was likely not driving trends, and we also used joint longitudinal and survival models, which account for censoring due to loss from care. Although we used statistical techniques to account for the possibility of bias due to loss to follow-up, there may be residual bias due to an unmeasured or unknown driver of this loss to follow-up. Finally, CNICS sites may differ from lower-resource clinical settings, and PLWH in CNICS differ from those from other data sources, such as the National HIV Surveillance System (39), in that CNICS includes only adult PLWH receiving clinical care, which may limit generalizability. Similar analyses that include different clinical settings could broaden understanding of viral suppression trends in the United States.
In conclusion, because viral suppression is essential for optimal HIV outcomes as well as HIV prevention efforts, understanding current trends and predictors is imperative to target public health policy. We must strive to decrease disparities for black PLWH and younger patient groups who may have poorer access to treatment. However, marked improvement in levels of viral suppression over time among PLWH across the United States bodes well for the long-term health outcomes of the current generation of PLWH and the possibility of limiting HIV transmission to others. Changes in ART treatment guidelines, with earlier ART initiation, are clearly a major contributor to higher viral suppression rates; however, even among patients receiving ART in the recent treatment era, suppression rates continue to improve. More potent, better-tolerated regimens, including ISTIs, may play a role in this success.
Figure 3.
Percentage of tests showing viral suppression over time among patients receiving ART, by time of ART initiation. ART = antiretroviral therapy.
Acknowledgment:
The authors thank the providers, the staff, and particularly the patients across all of the CNICS sites for their ongoing support.
Grant Support: This work was supported by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (U24AA020801, U01AA020793, and U01AA020802). Additional support came from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (CNICS R24 AI067039, UW CFAR NIAID Grant P30 AI027757, UNC CFAR grant P30 AI50410, JHU CFAR grant P30 AI094189, Providence/Boston CFAR grant P30 AI042853, and UAB CFAR grant P30 AI027767), the National Institute of General Medical Sciences (U54 GM115677), and the National Institute on Drug Abuse (DA U01036935).
Role of the Funding Source
CNICS is funded by grants from the National Institutes of Health. The funders had no role in the design, conduct, or analysis or the decision to publish this manuscript.
Primary Funding Source: National Institutes of Health.
Appendix
Appendix Figure 1.
Map of CNICS sites. CNICS = Centers for AIDS Research Network of Integrated Clinical Systems.
Appendix Figure 2.
CNICS inclusion and exclusion criteria. Table 1 and Figures 1 and 2 include all participants with ≥1 VL measurement (n = 31 930) (middle box), whereas Table 2 is limited to those receiving ART (n = 28 520) (bottom box). ART = antiretroviral therapy; CNICS = Centers for AIDS Research Network of Integrated Clinical Systems; VL = viral load.
Appendix Figure 3.
Percentage of tests showing viral suppression, with all tests included; limited to 1 random test per person per year; and limited to 1 random test per person per year, with correction for loss to follow-up with inverse probability of censoring weights based on prior viral load values and demographic characteristics.
Appendix Table 2.
Factors Associated With Detectable VL or Amount of VL in Adjusted Models Among Patients Receiving ART Who Were Known to be ART-Naive at CNICS Enrollment (n = 16 019)*
| Covariate | Joint Longitudinal and Survival Model† |
Linear Mixed Model‡ |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | Relative VL | 95% CI | P Value | |
| Integrase strand transfer inhibitor use | 0.66 | 0.62–0.71 | <0.001 | 0.79 | 0.76–0.83 | <0.001 |
| Female | 1.10 | 1.00–1.20 | 0.041 | 1.06 | 0.98–1.15 | 0.133 |
| Age (per decade, centered at 40 y) | 0.83 | 0.80–0.86 | <0.001 | 0.88 | 0.86–0.91 | <0.001 |
| Race/ethnicity (reference is white race) | ||||||
| Black | 1.69 | 1.55–1.84 | <0.001 | 1.62 | 1.51–1.74 | <0.001 |
| Hispanic | 0.84 | 0.75–0.94 | 0.002 | 0.89 | 0.81–0.98 | 0.020 |
| Other | 0.76 | 0.65–0.89 | 0.001 | 0.85 | 0.75–0.96 | 0.012 |
| Calendar time (year of cohort entry, centered around 2010) | 0.88 | 0.87–0.89 | <0.001 | 0.87 | 0.87–0.88 | <0.001 |
| Years of follow-up | 0.83 | 0.82–0.84 | <0.001 | 0.75 | 0.74–0.76 | <0.001 |
ART = antiretroviral therapy; CNICS = Centers for AIDS Research Network of Integrated Clinical Systems; OR = odds ratio; VL = viral load.
Also adjusted for site.
Outcome is detectable VL (>400 copies/mL).
Outcome is VL in log10 copies/mL.
Appendix Table 3.
Factors Associated With Amount of VL in Adjusted Models That Also Included Adherence, Substance Use, and Depression Among Patients Receiving ART*
| Covariate | Linear Mixed Models† |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Relative VL | 95% CI | PValue | Relative VL 95% CI | PValue | Relative VL | 95% CI | PValue | ||
| Integrase strand transfer inhibitor use | 0.92 | 0.88–0.97 | 0.001 | 0.90 | 0.86–0.95 | <0.001 | 0.91 | 0.87–0.96 | <0.001 |
| Female | 1.05 | 0.96–1.15 | 0.29 | 1.17 | 1.06–1.28 | 0.002 | 1.11 | 1.01–1.22 | 0.039 |
| Age (per decade, centered at 40 y) | 0.92 | 0.89–0.95 | <0.001 | 0.87 | 0.84–0.90 | <0.001 | 0.86 | 0.84–0.89 | <0.001 |
| Race/ethnicity (reference is white race) | |||||||||
| Black | 1.35 | 1.25–1.46 | <0.001 | 1.46 | 1.35–1.59 | <0.001 | 1.48 | 1.36–1.61 | <0.001 |
| Hispanic | 0.95 | 0.86–1.04 | 0.24 | 0.92 | 0.83–1.01 | 0.091 | 0.92 | 0.83–1.01 | 0.082 |
| Other | 0.99 | 0.86–1.14 | 0.89 | 1.03 | 0.88–1.20 | 0.73 | 1.02 | 0.87–1.18 | 0.82 |
| Calendar time (year of cohort entry, centered around 2010) | 0.92 | 0.90–0.94 | <0.001 | 0.92 | 0.90–0.95 | <0.001 | 0.93 | 0.91–0.95 | <0.001 |
| Years of follow-up | 0.89 | 0.88–0.90 | <0.001 | 0.84 | 0.83–0.85 | <0.001 | 0.84 | 0.83–0.85 | <0.001 |
| Adherence (per 10%)‡ | 0.84 | 0.83–0.84 | <0.001 | - | - | - | - | - | - |
| Current illicit drug use§ | - | - | - | 1.48 | 1.40–1.57 | <0.001 | - | - | - |
| Depression∥ | - | - | - | - | - | - | 1.29 | 1.23–1.35 | <0.001 |
ART = antiretroviral therapy; VL = viral load.
Also adjusted for site.
Outcome is VL in log10 copies/mL.
Measured using a 30-day visual analogue scale.
Includes cocaine/crack, methamphetamine/crystal methamphetamine use, and heroin/opiates.
Based on 9-item Patient Health Questionnaire score ≥10.
Appendix Table 4.
Factors Associated With Detectable VL in Adjusted Models That Also Included Adherence and Substance Use Among Patients Receiving ART*
| Undetectable VL | OR | 95% CI | P Value |
|---|---|---|---|
| Integrase strand transfer inhibitor use | 0.81 | 0.73–0.90 | <0.001 |
| Female | 1.14 | 0.95–1.37 | 0.167 |
| Age (per decade, centered at 40 y) | 0.77 | 0.72–0.82 | <0.001 |
| Race/ethnicity (reference is white race) | |||
| Black | 1.97 | 1.69–2.31 | <0.001 |
| Hispanic | 0.93 | 0.76–1.14 | 0.49 |
| Other | 1.02 | 0.74–1.40 | 0.92 |
| Calendar time (year of cohort entry, centered around 2010) | 0.90 | 0.87–0.93 | <0.001 |
| Years of follow-up | 0.89 | 0.87–0.91 | <0.001 |
| Adherence | 0.97 | 0.97–0.97 | <0.001 |
| Cocaine/crack use | 1.16 | 0.99–1.36 | 0.069 |
| Methamphetamine/crystal methamphetamine use | 1.90 | 1.63–2.22 | <0.001 |
| Opiate/heroin use | 1.25 | 0.96–1.61 | 0.093 |
| Marijuana use | 1.10 | 0.99–1.23 | 0.069 |
| Hazardous alcohol use | 0.90 | 0.81–1.01 | 0.070 |
ART = antiretroviral therapy; OR = odds ratio; VL = viral load.
Survival model component included in Appendix Table 8.
Appendix Table 5.
Factors Associated With Detectable VL in Adjusted Models Among Patients Receiving ART in 2010 to 2015
| Covariate | Joint Longitudinal Survival Model* |
Linear Mixed Model† |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | Relative VL | 95% CI | P Value | |
| Integrase strand transfer inhibitor use | 0.67 | 0.63–0.72 | <0.001 | 0.78 | 0.76–0.81 | <0.001 |
| Female | 1.23 | 1.11–1.36 | <0.001 | 1.14 | 1.07–1.21 | <0.001 |
| Age (per decade, centered at 40 y) | 0.69 | 0.67–0.72 | <0.001 | 0.85 | 0.83–0.87 | <0.001 |
| Race/ethnicity (reference is white race) | ||||||
| Black | 2.09 | 1.89–2.30 | <0.001 | 1.54 | 1.46–1.65 | <0.001 |
| Hispanic | 0.84 | 0.74–0.96 | 0.01 | 0.91 | 0.84–0.98 | 0.014 |
| Other | 0.88 | 0.73–1.06 | 0.2 | 0.93 | 0.83–1.04 | 0.197 |
| Calendar time (year of cohort entry, centered around 2010) | 1.14 | 1.10–1.17 | <0.001 | 0.98 | 0.96–1.00 | 0.016 |
| Years of follow-up | 0.79 | 0.78–0.81 | <0.001 | 0.78 | 0.77–0.79 | <0.001 |
ART = antiretroviral therapy; OR = odds ratio; VL = viral load.
Outcome is detectable VL (>400 copies/mL).
Outcome is VL in log10 copies/mL.
Appendix Table 6.
Factors Associated With Amount of VL in Adjusted Models That Also Included CD4 Cell Count at ART Initiation and Cohort Year Squared Among Patients Receiving ART*
| Covariate | Linear Mixed Models† |
|||||
|---|---|---|---|---|---|---|
| Relative VL | 95% CI | P Value | Relative VL | 95% CI | P Value | |
| Integrase strand transfer inhibitor use | 0.71 | 0.68–0.73 | <0.001 | 0.70 | 0.67–0.72 | <0.001 |
| Female | 1.05 | 0.98–1.12 | 0.141 | 0.99 | 0.93–1.06 | 0.86 |
| Age (per decade, centered at 40 y) | 0.82 | 0.80–0.84 | <0.001 | 0.83 | 0.81–0.85 | <0.001 |
| Race/ethnicity (reference is white race) | ||||||
| Black | 1.41 | 1.33–1.50 | <0.001 | 1.60 | 1.51–1.70 | <0.001 |
| Hispanic | 0.77 | 0.71–0.83 | <0.001 | 0.90 | 0.83–0.97 | 0.006 |
| Other | 0.81 | 0.73–0.90 | <0.001 | 0.89 | 0.80–0.99 | 0.039 |
| Calendar time (year of cohort entry, centered around 2010) | 0.85 | 0.85–0.86 | <0.001 | 0.88 | 0.87–0.89 | <0.001 |
| Years of follow-up | 0.75 | 0.74–0.75 | <0.001 | 0.75 | 0.74–0.75 | <0.001 |
| CD4 count at ART initiation (per 0.100 × 109 cells/L) | 0.83 | 0.82–0.84 | <0.001 | - | - | - |
| Cohort entry year squared (from 2010) | - | - | - | 1.01 | 1.00–1.01 | <0.001 |
ART = antiretroviral therapy; VL = viral load.
Also adjusted for site.
Outcome is VL in log10 copies/mL.
Appendix Table 7.
Survival Model for the Joint Longitudinal and Survival Model (Using a Weibull Distribution With Shape Parameter k = 0.59) for Loss to Follow-up Among Patients Receiving ART
| Covariate | HR | 95% CI | P Value |
|---|---|---|---|
| Integrase strand transfer inhibitor use | 0.35 | 0.33–0.38 | <0.001 |
| Female | 0.96 | 0.91–1.01 | 0.08 |
| Age (per decade, centered at 40 y) | 0.85 | 0.83–0.86 | <0.001 |
| Race/ethnicity (reference is white race) | |||
| Black | 1.08 | 1.04–1.13 | <0.001 |
| Hispanic | 0.67 | 0.63–0.71 | <0.001 |
| Other | 1.06 | 0.97–1.15 | 0.192 |
| Calendar time (year of cohort entry, centered around 2010) | 1.05 | 1.05–1.06 | <0.001 |
ART = antiretroviral therapy; HR = hazard ratio.
Appendix Table 8.
Survival Model for the Joint Longitudinal and Survival Model (Using a Weibull Distribution with Shape Parameter k = 0.59) With Adherence and Substance Use Classes Included
| Time to Loss to Follow-up | HR | 95% CI | P Value |
|---|---|---|---|
| Integrase strand transfer inhibitor use | 0.58 | 0.52–0.64 | <0.001 |
| Female | 0.90 | 0.80–1.02 | 0.113 |
| Age (per decade, centered at 40 y) | 0.87 | 0.83–0.90 | <0.001 |
| Race/ethnicity (reference is white race) | |||
| Black | 0.87 | 0.78–0.96 | 0.008 |
| Hispanic | 0.69 | 0.62–0.78 | <0.001 |
| Other | 0.93 | 0.77–1.12 | 0.45 |
| Calendar time (year of cohort entry, centered around 2010) | 1.15 | 1.12–1.18 | <0.001 |
| Adherence | 1.00 | 1.00–1.00 | 0.61 |
| Cocaine/crack use | 1.00 | 0.85–1.17 | 0.99 |
| Methamphetamine/crystal methamphetamine use | 1.01 | 0.88–1.16 | 0.87 |
| Opiate/heroin use | 1.10 | 0.85–1.42 | 0.46 |
| Marijuana use | 1.08 | 0.99–1.18 | 0.074 |
| Hazardous alcohol use | 1.46 | 1.33–1.60 | <0.001 |
HR = hazard ratio.
Footnotes
Disclosures: Dr. Wilson reports consulting fees from Pfizer outside the submitted work. Dr. Mayer reports grants from Gilead Sciences and ViiV Healthcare outside the submitted work. Dr. Mugavero reports personal fees from the Gilead Foundation and a grant from Bristol-Myers Squibb outside the submitted work. Dr. Christopoulos reports grants and personal fees from Gilead and personal fees from Roche outside the submitted work. Dr. Eron reports personal fees from Merck and grants and personal fees from ViiV Healthcare, Janssen Pharmaceutical, Gilead Sciences, and Bristol-Myers Squibb outside the submitted work. Dr. Moore reports personal fees from Medscape outside the submitted work. Dr. Rodriguez reports personal fees from Gilead during the conduct of the study. Dr. Saag reports grants from Merck, Bristol-Myers Squibb, Gilead, ViiV Healthcare, AbbVie, and Proteus and personal fees from Merck, Bristol-Myers Squibb, and Gilead outside the submitted work. Dr. Crane reports a grant from ViiV Healthcare outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17–2242.
Reproducible Research Statement: Study protocol: Not applicable. Statistical code: Investigators interested in the statistical code used to generate the joint longitudinal and survival or other models may contact Ms. Nance (e-mail, rmnance@u.washington.edu). Data set: The CNICS cohort strives to have an open-access approach to data. Investigators who are interested in replicating this study or conducting other work within CNICS should visit the CNICS Web site (www.uab.edu/cnics) for information on how to submit a concept proposal to receive data and conduct studies with the data.
Presented in part at the 11th International Conference on HIV Treatment and Prevention Adherence, Fort Lauderdale, Florida, 9–11 May 2016, and at the 23rd Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 22–25 February 2016.
Current author addresses and author contributions are available at Annals.org.
Contributor Information
Robin M. Nance, From University of Washington, Seattle, Washington.
J.A. Chris Delaney, From University of Washington, Seattle, Washington.
Jane M. Simoni, From University of Washington, Seattle, Washington.
Ira B. Wilson, Brown University, Providence, Rhode Island.
Kenneth H. Mayer, Harvard Medical School and Fenway Institute, Boston, Massachusetts.
Bridget M. Whitney, From University of Washington, Seattle, Washington.
Frances M. Aunon, From University of Washington, Seattle, Washington.
Steven A. Safren, University of Miami, Miami, Florida, and Fenway Institute, Boston, Massachusetts.
Michael J. Mugavero, University of Alabama at Birmingham, Birmingham, Alabama.
W. Christopher Mathews, University of California, San Diego, San Diego, California.
Katerina A. Christopoulos, University of California, San Francisco, San Francisco, California.
Joseph J. Eron, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Sonia Napravnik, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Richard D. Moore, Johns Hopkins University, Baltimore, Maryland.
Benigno Rodriguez, Case Western Reserve University, Cleveland, Ohio.
Bryan Lau, Johns Hopkins University, Baltimore, Maryland.
Rob J. Fredericksen, From University of Washington, Seattle, Washington.
Michael S. Saag, University of Alabama at Birmingham, Birmingham, Alabama.
Mari M. Kitahata, From University of Washington, Seattle, Washington.
Heidi M. Crane, From University of Washington, Seattle, Washington.
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report. Vol. 27. Diagnoses of HIV Infection in the United States and Dependent Areas, 2015. Atlanta: Centers for Disease Control and Prevention; 2016. Accessed at www.cdc.gov/hiv/library/reports/hiv-surveillance.html on 20 July 2018.
- 2.Looney D, Ma A, Johns S. HIV therapy—the state of art. Curr Top Microbiol Immunol 2015;389:1–29. [PMID: ] doi: 10.1007/82_2015_440 [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. ; HPTN 052 Study Team. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375: 830–9. [PMID: ] doi: 10.1056/NEJMoa1600693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. ; PARTNER Study Group. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316:171–81. [PMID: ] doi: 10.1001/jama.2016.5148 [DOI] [PubMed] [Google Scholar]
- 5.Gale HB, Rodriguez MD, Hoffman HJ, Benator DA, Gordin FM, Labriola AM, et al. Progress realized: trends in HIV-1 viral load and CD4 cell count in a tertiary-care center from 1999 through 2011. PLoS One 2013;8:e56845 [PMID: ] doi: 10.1371/journal.pone.0056845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. ; North American AIDS Cohort Collaboration on Research and Design. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med 2012;157:325–35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, et al. ; GS-US-236–0102 Study Team. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013;63:96–100. [PMID: ] doi: 10.1097/QAI.0b013e318289545c [DOI] [PubMed] [Google Scholar]
- 8.DeJesus E, Rockstroh JK, Lennox JL, Saag MS, Lazzarin A, Zhao J, et al. ; STARTMRK Investigators. Efficacy of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1infected patients: week-192 overall and subgroup analyses from STARTMRK. HIV Clin Trials 2012;13:228–32. [PMID: ] doi: 10.1310/hct1304-228 [DOI] [PubMed] [Google Scholar]
- 9.Rockstroh JK, DeJesus E, Lennox JL, Yazdanpanah Y, Saag MS, Wan H, et al. ; STARTMRK Investigators. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr 2013;63:77–85. [PMID: ] doi: 10.1097/QAI.0b013e31828ace69 [DOI] [PubMed] [Google Scholar]
- 10.Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, et al. ; ACTG A5257 Team. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014;161: 461–71. [PMID: ] doi: 10.7326/M14-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zolopa AR, Berger DS, Lampiris H, Zhong L, Chuck SL, Enejosa JV, et al. Activity of elvitegravir, a once-daily integrase inhibitor, against resistant HIV type 1: results of a phase 2, randomized, controlled, dose-ranging clinical trial. J Infect Dis 2010;201:814–22. [PMID: ] doi: 10.1086/650698 [DOI] [PubMed] [Google Scholar]
- 12.Wohl DA, Cohen C, Gallant JE, Mills A, Sax PE, Dejesus E, et al. ; GS-US-236–0102 Study Team. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/ tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/ tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results [Letter]. J Acquir Immune Defic Syndr 2014;65:e118–20. [PMID: ] doi: 10.1097/QAI.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 13.Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. ; SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013;381:735–43. [PMID: ] doi: 10.1016/S0140-6736(12)61853-4 [DOI] [PubMed] [Google Scholar]
- 14.Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al. ; ING114915 Study Team. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014;383:2222–31. [PMID: ] doi: 10.1016/S0140-6736(14)60084-2 [DOI] [PubMed] [Google Scholar]
- 15.D’Abbraccio M, Busto A, De Marco M, Figoni M, Maddaloni A, Abrescia N. Efficacy and tolerability of integrase inhibitors in antiretroviral-naive patients. AIDS Rev 2015;17:171–85. [PMID: ] [PubMed] [Google Scholar]
- 16.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol 2008;37: 948–55. [PMID: ] doi: 10.1093/ije/dym231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crane HM, Lober W, Webster E, Harrington RD, Crane PK, Davis TE, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res 2007;5:109–18. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 18.Fredericksen R, Crane PK, Tufano J, Ralston J, Schmidt S, Brown T, et al. Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. J AIDS HIV Res 2012;4:47–55. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mimiaga MJ, Reisner SL, Grasso C, Crane HM, Safren SA, Kitahata MM, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health 2013;103:1457–67. [PMID: ] doi: 10.2105/AJPH.2012.301162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman BJ, Fredericksen RJ, Crane PK, Safren SA, Mugavero MJ, Willig JH, et al. Evaluation of the single-item self-rating adherence scale for use in routine clinical care of people living with HIV. AIDS Behav 2013;17:307–18. [PMID: ] doi: 10.1007/s10461-012-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence ST, Willig JH, Crane HM, Ye J, Aban I, Lober W, et al. Routine, self-administered, touch-screen, computer-based suicidal ideation assessment linked to automated response team notification in an HIV primary care setting. Clin Infect Dis 2010;50:1165–73. [PMID: ] doi: 10.1086/651420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Sighem A, Zhang S, Reiss P, Gras L, van der Ende M, Kroon F, et al. Immunologic, virologic, and clinical consequences of episodes of transient viremia during suppressive combination antiretroviral therapy. J Acquir Immune Defic Syndr 2008;48:104–8. [PMID: ] doi: 10.1097/QAI.0b013e31816a1d4f [DOI] [PubMed] [Google Scholar]
- 23.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006;10:227–45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amico KR, Fisher WA, Cornman DH, Shuper PA, Redding CG, Konkle-Parker DJ, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr 2006;42:455–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 25.Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev 2005;24:217–26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 26.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 2002;97:1183–94. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 27.Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med 2003;163:821–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 28.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-c): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789–95. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 29.Gual A, Segura L, Contel M, Heather N, Colom J. Audit-3 and Audit-4: effectiveness of two short forms of the Alcohol Use Disorders Identification Test. Alcohol Alcohol 2002;37:591–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 30.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000;56: 779–88. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 33.Lawrence Gould A, Boye ME, Crowther MJ, Ibrahim JG, Quartey G, Micallef S, et al. Joint modeling of survival and longitudinal nonsurvival data: current methods and issues. Report of the DIA Bayesian Joint Modeling Working Group. Stat Med 2015;34:2181–95. [PMID: ] doi: 10.1002/sim.6141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeting MJ, Thompson SG. Joint modelling of longitudinal and time-to-event data with application to predicting abdominal aortic aneurysm growth and rupture. Biom J 2011;53:750–63. [PMID: ] doi: 10.1002/bimj.201100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCulloch C, Searle S. Generalized, Linear, and Mixed Models. New York: J Wiley; 2001. [Google Scholar]
- 36.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 37.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med 2002;17:377–81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015; 175:588–96. [PMID: ] doi: 10.1001/jamainternmed.2014.8180 [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Mitsch A, Wu B. HIV care outcomes among men who have sex with men with diagnosed HIV infection—United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:969–74. [PMID: ] doi: 10.15585/mmwr.mm6637a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL; Medical Monitoring Project. Trends in racial and ethnic disparities in antiretroviral therapy prescription and viral suppression in the United States, 2009–2013. J Acquir Immune Defic Syndr 2016;73:446–53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simoni JM, Huh D, Wilson IB, Shen J, Goggin K, Reynolds NR, et al. Racial/ethnic disparities in ART adherence in the United States: findings from the MACH14 study. J Acquir Immune Defic Syndr 2012;60:466–72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dale SK, Bogart LM, Wagner GJ, Galvan FH, Klein DJ. Medical mistrust is related to lower longitudinal medication adherence among African-American males with HIV. J Health Psychol 2016;21: 1311–21. [PMID: ] doi: 10.1177/1359105314551950 [DOI] [PMC free article] [PubMed] [Google Scholar]