Abstract
TEMPO (2, 2, 6, 6-tetramethylphiperidine-1-oxyl) and its derivatives are stable free radical nitroxides widely used in the field of chemistry, biology, and pharmacology. TEMPO was previously found to be mutagenic and to induce micronuclei in mammalian cells. In this study, we investigated and quantified the genotoxicity of 4 structurally similar nitroxides, TEMPO and 3 of its derivatives (4-hydroxy-TEMPO, 4-oxo-TEMPO, and 4-methoxy-TEMPO), using the mouse lymphoma assay (MLA) and Comet assay in L5178Y Tk+/− cells. The results showed that all tested nitroxides were cytotoxic and mutagenic in the MLA, both in the presence and absence of S9, with metabolic activation significantly enhancing the cytotoxicity and/or utagenicity. In addition, the 4 nitroxides caused DNA-strand breakage. The mutagenicity and DNA damaging dose-responses of the test articles were compared using the PROAST benchmark dose software package. The potency ranking of the 4 nitroxides for mutagenicity was different from the ranking of the DNA damaging effects. The mode of action analysis by a multi-endpoint DNA damage pathway assay classified all 4 nitroxides as clastogens. In addition, the majority of the induced Tk mutants showed loss of heterozygosity at the Tk and D11Mit42 loci (ie, chromosome damage <31 Mbp). These results suggest that TEMPO and its 3 derivatives are cytotoxic and mutagenic in mouse lymphoma cells through a mechanism that involves strand breakage and large alterations to DNA. The potency rankings indicate that the different TEMPO derivatives vary in their mutagenic and DNA damaging potential.
Keywords: nitroxide, mouse lymphoma assay, loss of heterozygosity, DNA damage, benchmark dose
Piperidine nitroxides, a group of low-molecular weight, stable and membrane permeable free radicals, have been employed in the fields of chemistry, biochemistry, biology, and pharmacology. They can be used as spin probes in electron paramagnetic resonance studies, and there is now an accumulating body of evidence indicating that they also can be used as radiation protective agents and radical scavengers to mitigate oxidative stress in biological systems (Czepas et al., 2008; Matsumoto et al., 2004; Yamasaki et al., 2011). Nitroxides possess superoxide dismutase-like activity and easily cross cell membranes, thus providing antioxidant effects by scavenging intracellular oxygen radicals (Soule et al., 2007). Recently, nitroxides have been proposed for therapeutic and clinical applications, such as preventing radiation-or ethanol-induced liver injury, for treating hypertension, neurodegenerative diseases, and glaucoma, and for chemoprevention and cancer treatment (Dikalova et al., 2010; Gariboldi et al., 2003; Liang et al., 2005; Perez and Cederbaum, 2001). Considering their significance as potential pharmaceutical agents and probes for various biological applications, it is of paramount importance to understand their possible toxic effects.
TEMPO (2, 2, 6, 6-tetramethylphiperidine-1-oxyl), a classic nitroxide radical, is a nitroxyl (N–O•)-based 6-membered-ring piperidine radical. Previously we demonstrated that TEMPO was mutagenic in the mouse lymphoma assay (MLA) and induced micronuclei in TK6 human lymphoblastoid cells (Guo et al., 2013). Various derivatives of TEMPO, such as 4-hydroxy-TEMPO (also known as TEMPOL), 4-oxo-TEMPO, 4-methoxy-TEMPO, can be obtained by the addition of a substituent group on the 4-position of the piperidine ring (Supplementary Figure 1). Although these derivatives have interesting and perhaps useful biological properties, it remains unclear whether or not they induce similar mutagenic and cytotoxic effects as does TEMPO (Zhang et al., 2018). In fact, paradoxical results on these compounds have been reported in the literature. For example, 4-hydroxy-TEMPO inhibited cell growth in a panel of human and rodent cultured cell lines, possibly by triggering an apoptotic mechanism with a consistent preference for neoplastic cells (Gariboldi et al., 1998). Whereas another study reported that 4-hydroxy-TEMPO itself was not mutagenic or toxic to Chinese hamster ovary AS52 cells, but that it was actually protective against the cytotoxic and mutagenic effects induced by hydrogen peroxide and hypoxanthine/xanthine oxidase (DeGraff et al., 1992).
In addition, the biological activities of nitroxides depend upon their structure (Gallez et al., 1992); that is, the addition of different substituent groups may result in modified biological effects. TEMPO, 4-hydroxy-TEMPO, 4-oxo-TEMPO, and 4-amino-TEMPO produced different toxicity in human HaCaT keratinocytes (Kroll et al., 1999). Following a 24-h treatment, TEMPO was the most cytotoxic nitroxide, with an IC50 (the half maximal inhibitory concentration) value of 2.66 mM, while 4-hydroxy-TEMPO showed the least cytotoxicity with an IC50 value of 11.4 mM. Besides cytotoxicity, the structure of the nitroxides also affected their mutagenic activity (Gallez et al., 1992). Radicals that were more sensitive to reduction were more mutagenic in Salmonella typhimurium tester strain TA 100.
This study investigated the cytotoxicity induced by TEMPO and 3 of its derivatives and compared their genotoxic potential in order to augment the limited information on these important compounds. Two widely accepted genotoxicity assays were employed, the MLA and the Comet assay. The MLA, using the thymidine kinase (Tk) gene of the L5178Y/Tk+/− −3.7.2 C mouse lymphoma cell line, is capable of detecting a broad spectrum of genetic damage, including both point mutations and chromosomal breakage events. The Comet assay is a gel electrophoresis-based method that measures DNA damage in individual cells. The mutagenicity and DNA damaging dose-response data that we generated were analyzed quantitatively in order to rank the genotoxic potency of the test articles. The mode of action (MoA) of the genotoxic effects of the test compounds were investigated using a multiplexed assay for determining the pathways for DNA damage and the Tk mutants from the MLA were evaluated for loss of heterozygocity (LOH) by genotyping the mutants using 5 microsatellite loci spanning the entire chromosome 11 of mouse lymphoma cells.
MATERIALS AND METHODS
Materials.
TEMPO (molecular formula C9H18NO, CAS no. 2564–83-2, 4-hydroxy-TEMPO (C9H18NO2, CAS no. 2226–96-2), 4-oxo-TEMPO (C9H16NO2, CAS no. 2896–70-0), 4-methoxy-TEMPO (C10H20NO2, CAS no. 95407–69-5), dimethyl sulfoxide (DMSO), and trifluorothymidine (TFT) were purchased from Sigma-Aldrich (St Louis, Missouri). Fischer’s medium and fetal bovine serum (FBS) was obtained from Quality Bio (Gaithersburg, Maryland) and Atlanta Bio (Flowery Branch, Georgia), respectively. RPMI 1640 medium and all other cell culture supplies were acquired from Invitrogen Life Technologies (Carlsbad, California). Aroclor 1254-induced male Sprague-Dawley rats liver postmitochondrial fraction (S9) was purchased from Molecular Toxicology (Boone, North Carolina).
PCR Master Mix, proteinase K, and kits for performing the CellTiter-Blue cell viability, cell proliferation (MTS), and ATP assays were obtained from Promega (Madison, Wisconsin). The primers used for detection of LOH at the Tk locus and the D11Mit42, D11Mit36, D11Mit20, and D11Mit74 loci were synthesized by Integrated DNA Technologies (Coralville, Iowa). In vitro Multiflow High Content Assays kit was purchased from Litron Laboratories (Rochester, New York).
Cells culture and treatments.
The L5178Y/Tk+/− −3.7.2C mouse lymphoma cell line was used for conducting all the assays except the Multiflow assay which was performed with TK6 human lymphoblastoid cells. Mouse lymphoma cells were grown in growth medium containing 10% horse serum and cleansed of pre-existing Tk−/− and Tk−/0 mutants periodically according to the methods described previously (Mei et al., 2014). TK6 cells were cultured in RPMI 1640 medium containing 10% FBS. All 4 chemicals were tested both in the absence and presence of S9 metabolic activation in the MLA. The S9 cofactor mixture was prepared by mixing 180 mg/ml of glucose-6-phosphate, 25 mg/ml of NADP, 150 mM KCl, and rat liver S9 in the ratio 1:1:1:2. The final S9 concentration in the treatment medium was 1% (about 0.38 mg protein/ml).
The chemical working solutions (100 ×) were prepared just prior to use. In the absence or presence of 1% S9 mix, 6 × 106 mouse lymphoma cells were exposed to various concentrations of the chemicals or vehicle (DMSO) in a total volume of 10 ml treatment medium that contained 5% horse serum for 4 h at 37°C. The final concentration of DMSO in the medium was 1%. Cells were harvested immediately following exposure and the following assays performed.
Cell viability and cell proliferation assays.
Cell viability and cell proliferation were measured following the treatment using CellTiter-Blue and MTS [3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 H-tetrazolium, inner salt] assay kits, respectively, according to the manufacturer’s manual. The CellTiter-Blue assay uses the ability of living cells to convert resazurin (a redox dye) into fluorescent resorufin. The MTS assay is based on the conversion of MTS tetrazolium compound into a colored formazon product by NADPH or NADH in metabolically active cells. Briefly, following the 4-h exposure, 100 μl of treated cells were transferred into the wells of 96-microwell plates in quadruplicate, and 10 μl of CellTiter-Blue or MTS reagent were added into each well. Following a 1-h incubation at 37°C in a humidified incubator with 5% CO2 in air, fluorescence (CellTiter-Blue assay) or absorbance (MTS assay) was measured in a Synergy H4 Hybrid multimode microplate reader (BioTek, Winooski, Vermont).
Measurement of cellular ATP levels.
Cellular ATP levels were measured following the treatments as described previously in Guo et al. (2015). Following the 4-h treatment, cells were mixed with reaction reagent at a ratio of 1:1 and were dispensed into the wells of a white 96-well flat-bottomed plate in quadruplicate. Luminescence was read immediately with a Synergy H4 Hybrid microplate reader.
Mouse lymphoma assay.
The Tk mutants were selected using the microwell version of the MLA as described by OECD Test Guideline 490 (Guo et al., 2011; OECD, 2015). Positive controls (0.3 μg/ml of benzo[a]pyrene +S9 or 0.1 μg/ml of 4-nitroquinoline-1-oxide) were included in each assay. Following the 4-h treatment, cells were washed twice with fresh medium by centrifugation, and then were resuspended in 20 ml of growth medium. The cultures were placed on a roller drum (15 rpm) in a 37°C incubator and grown 2 days for phenotypic expression. For mutant enumeration, 200 μl cells in the cloning medium containing 20% horse serum and 3 μg/ml of TFT were seeded into each well of four 96-well flat-bottomed microtiter plates at a final density of 2000 cells/well. For determination of the plating efficiency, approximately 1.6 cells were aliquoted in 200 μl per well into two 96-well plates. All plates were incubated at 37°C in a humidified incubator with 5% CO2 in air for 11 days and mutant colonies were counted and categorized as small colonies or large colonies using criteria described previously (Mei et al., 2014). Cytotoxicity and mutagenicity were expressed as relative total growth (RTG) and mutant frequency (MF), respectively.
Alkaline comet assay.
The alkaline Comet assay was performed using the method described previously in Zhang et al. (2015). Briefly, mouse lymphoma cells were exposed to various concentrations of TEMPO and its derivatives for 4 h. Following exposure, the cells were washed with cold phosphate-buffered saline (PBS) and Comet assays were performed using single cell gel electrophoresis kit according to the manufacturer’s protocol (Trevigen, Gaithersburg, Maryland). Three independent experiments were performed and the percentage of DNA in tails from more than 300 cells for each concentration was scored using Trevigen Comet Analysis Software.
Multiflow DNA damage assay.
The genotoxic MoA of TEMPO and its 3 derivatives was investigated by a method for evaluating multiple endpoints that are associated with DNA damage response pathways (MultiFlow DNA Damage Kit—p53, γ-H2AX, Phospho-Histone H3) according to the manufacturer’s manual (Litron Laboratories, Rochester, New York). Briefly, 4 × 104 TK6 cells were seeded into each well of a 96-well plate and exposed to 14 concentrations of each chemical. After 4- and 24-h of incubation, 25 μl of cells were removed from each well and added to a 96-well U-bottomed plate preloaded with 50 μl of working MultiFlow Kit Labeling solution. Following 30 min incubation at room temperature, flow cytometric analysis was performed using a FACSCanto II flow cytometer equipped with a High Throughput Sampler (BD Biosciences, San Jose, California). Ten thousand total events were acquired or the entire 30 μl volume was exhausted, whichever came first. A total of 5 endpoints were obtained. More specifically, chemical-induced cytotoxicity was calculated using nuclei to counting bead ratios; phosphorylated γ-H2AX and p53 responses were evaluated based on median fluorescence intensity; and the percentages of phosphorylated histone H3 (p-H3) and polyploidy were quantified as the proportion of p-H3-positive and 8n-positive events relative to the total events with 2n and greater DNA content.
LOH evaluation of the Tk mutants.
Forty-eight large and 48 small Tk mutant colonies resulting from treatment with each of the test agents were directly taken from TFT-selection plates following colony counting. The mutant cells were washed once with 200 μl of PBS by centrifugation. The genomic DNA was extracted, and PCR and agarose gel electrophoresis were performed as described previously (Guo et al., 2011). A total of 5 loci (Tk, D11Mit42, D11Mit36, D11Mit20, and D11Mit74 loci) covering the whole of chromosome 11 were analyzed. The presence of only 1 band was scored as LOH and nonLOH was scored as retention of 2 bands at a given locus.
Data analysis.
The MLA data were evaluated using criteria developed by the MLA Expert Workgroup of the International Workgroup for Genotoxicity Testing (Moore et al., 2003). Positive responses were defined as those where the induced MF in 1 or more treated cultures exceeded the global evaluation factor (GEF, 126 mutants per million cells). Given that differences were shown in the proportion of large and small colony mutants between treatment groups and control, weighted sums of the number of large and small Tk mutant colonies were used in the comparison of mutation spectra between different groups. LOH patterns of mutants were compared using the computer program written by Cariello et al. (1994) for the Monte Carlo analysis developed by Adams and Skopek (Adams and Skopek, 1987).
Both the MLA and Comet assay data were quantitatively evaluated by benchmark dose (BMD) analysis using PROAST Software (version 38.9, developed by the Dutch National Institute for Public Health and the Environment, RIVM) following the technical guidance (RIVM, 2013). Briefly, the Hill model combined with covariate approach of the software was used for modeling the continuous concentration-dependent data (Wills et al., 2016a). The BMD5, BMD10, BMD50, BMD100, and BMD200 values (a 5%, 10%, 50%, 100%, or 200%, respectively, increase over the vehicle control response) and the BMDU and BMDL values (the upper and lower 95% CIs of the BMD) were calculated for each data set.
The MoA of the 4 compounds were evaluated using data generated from the MultiFlow DNA damage kit and evaluated by the 2 approaches described previously. The first compares each biomarker response against GEFs expressed as fold-increase over concurrent solvent controls (Bryce et al., 2017). GEFs were generated from pooled fold-increase response data for a total of 84 chemicals generated by 7 laboratories. A clastogen or aneugen call requires that 2 successive concentrations meet or exceed the GEF for at least 2 out of 4 clastogen- or aneugen-sensitive biomarkers listed in Table 1. The second approach used an ensemble of several machine learning algorithms described previously in Bryce et al. (2018). In this case, data were applied to multinomial logistic regression, artificial neural network learners, and random forest models that were built with JMP Pro software for Macintosh (v13, SAS Institute, Cary, North Carolina). The majority vote ensemble required 2 or more models to show a clastogenic or aneugenic call with 80% probability over 2 successive concentrations, or 90% probability at any 1 concentration. For both the GEF and machine learning approaches, only concentrations that were below a predefined cytotoxicity limit (≤80% relative nuclei count at 24 h) were considered.
Table 1.
Criteria for Classification of Chemical’s MoAa
| GEFsb |
|||
|---|---|---|---|
| Treatments | Biomarkers | Clastogen | Aneugen |
| 4-h | γ-H2AX | 1.51-fold | |
| p53 | 1.40-fold | ||
| p-H3 | 1.71-fold | ||
| 24-h | γ-H2AX | 2.11-fold | |
| p53 | 1.45-fold | 1.45-fold | |
| p-H3 | 1.52-fold | ||
| polyploidy | 5.86-fold | ||
From Bryce et al. (2017). All assay data were restricted to ≤80% cytotoxicity following a 24-h treatment, which was evaluated by relative nuclei counts (≥20%) at the highest concentration.
GEFs, expressed as fold-increase over concurrent solvent controls, were generated from pooled fold-increase response data for a total of 84 chemicals studied across 7 laboratories in the world.
The ATP assay, cell viability assay, and Comet assay data are presented as the mean ± SD from at least 3 independent experiments. Statistical significance was determined by 1-way analysis of variance followed by the Dunnett’s test for pairwise-comparisons using Sigmaplot 13.0 (Systat Software, San Jose, California). A p-value < .05 was considered to be statistically significant. IC20 values of the nitroxides were calculated with data from 3 independent cytotoxicity assays by Nonlinear Regression (Curve fit) using Graphpad Prism 6 (La Jolla, California).
RESULTS
TEMPO and Its Derivatives Induce Distinct Patterns of Cytotoxicity in the Various Assays
The cytotoxicity of TEMPO and 3 of its derivatives was assessed in L5178Y cells by quantifying cell proliferation (MTS assay), cell viability (CellTiter-Blue assay), and metabolically active cells (ATP assay). Following a 4-h treatment, all 4 chemicals significantly decreased cell viability as measured by all 3 assays. However, the endpoints differed in their sensitivity to the toxicity produced by the compounds (Figure 1). IC20 values for the 4 nitroxides were calculated as some nitroxides produced cytotoxic effects of <50% in 1 or more assays. As shown in Table 2, the MTS assay exhibited the greatest sensitivity to the nitroxides, especially for TEMPO, 4-hydroxy-TEMPO, and 4-methoxy-TEMPO, which produced reductions in cell viability of approximately 50% at the lowest concentrations tested in this study (Figure 1). Interestingly, the cytotoxicity dose-responses for 4-oxo-TEMPO were very similar in the 3 assays (Figure 1C), while TEMPO displayed obvious differences in cytotoxicity in the 3 assays. 4-Hydroxy-TEMPO and 4-methoxy-TEMPO exhibited very similar cytotoxicity dose responses in the 3 assays, with the MTS assay being considerably more sensitive to their cytotoxic effects than either the ATP or CellTiter-Blue assay.
Figure 1.
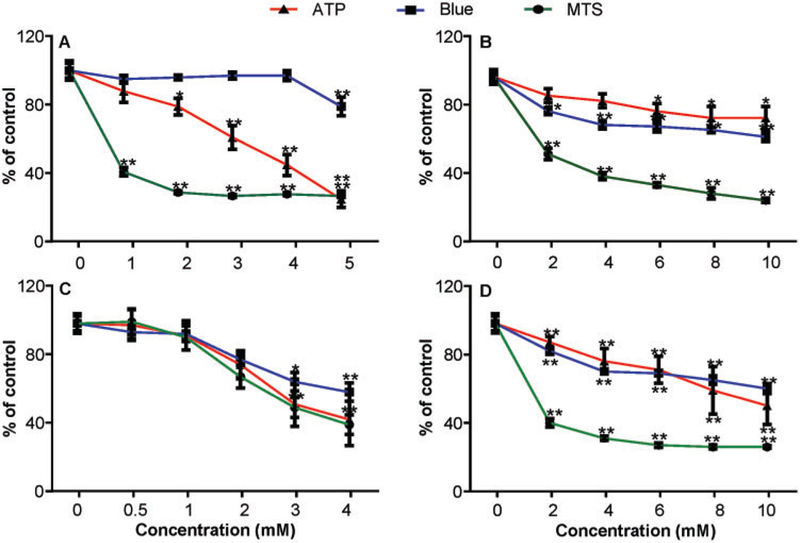
Relative viability of mouse lymphoma cells exposed to TEMPO and its derivatives. Cells were exposed to various concentrations (0.5–10 mM) of 4 piperidine nitroxides for 4h and cell viability was measured using the ATP, Titer Blue, and MTS assay. (A) TEMPO; (B) 4-hydroxy-TEMPO; (C) 4-oxo-TEMPO; (D) 4-methoxy-TEMPO. The data points represent the mean ± SD from 3 independent experiments with 4 parallel replicate wells per concentration in each experiment. * p < .05 and ** p < .01 compared with the vehicle control.
Table 2.
IC20 Values of TEMPO and 3 oflts Derivativesa
| Nitroxide | MTS assay (mM) |
Blue assay (mM) |
ATP assay (mM) |
|---|---|---|---|
| TEMPO | 0.011 | 4.968 | 1.923 |
| 4-hydroxy-TEMPO | 0.356 | 1.781 | 6.389 |
| 4-oxo-TEMPO | 1.553 | 1.950 | 1.730 |
| 4-methoxy-TEMPO | 0.030 | 2.577 | 3.874 |
L5178Y mouse lymphoma cells were treated with the 4 nitroxides at various concentrations and cell viabilities were measured. IC20 values were calculated by Nonlinear Regression (Curve fit) using Graphpad Prism 6 software.
TEMPO and Its Derivatives Are Mutagenic in the MLA, Both With and Without S9
Our previous study showed that TEMPO-induced cytotoxicity and mutagenicity in the MLA both with and without 1% S9 (Guo et al., 2013). In this study, we evaluated the mutagenicity of TEMPO’s 3 derivatives in the MLA both with and without metabolic activation. Although all tested nitroxides produced concentration-dependent cytotoxicity and mutagenicity effects under both conditions, metabolic activation had distinct effects on these endpoints (Figure 2). When S9 was employed in the culture, lower concentrations of TEMPO, 4-oxo-TEMPO, and 4-methoxy-TEMPO were required to produce the highest acceptable cytotoxicity, ie, RTG values of 10%–20% in treated cells, than treatments without S9. In addition, metabolic activation also increased the MFs in TEMPO- and 4-methoxy-TEMPO-treated cells as compared with those produced in treatments without S9, 502 versus 208 and 961 versus 508 per million cells, respectively, at concentrations that produced 10%–20% RTG (Figs. 2A and 2D). In contrast, treatments with 2–10 mM 4-hydroxy-TEMPO resulted in very similar concentration-related increases in cytotoxicity and mutagenicity with and without metabolic activation (Figure 2B).
Figure 2.
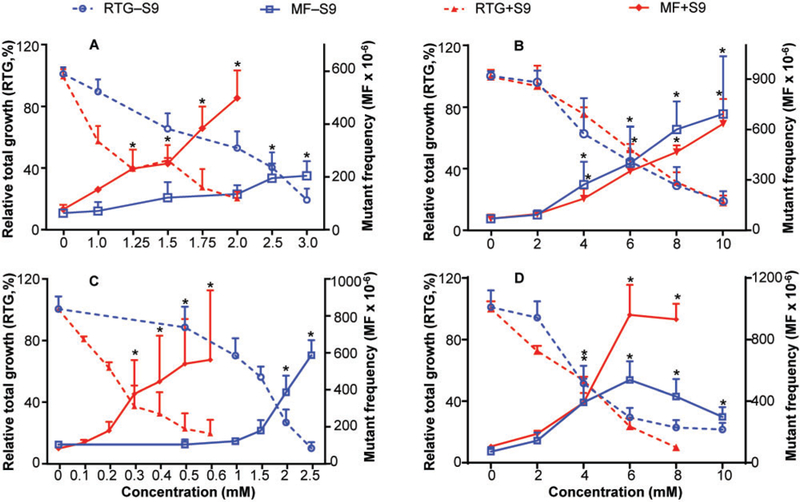
Cytotoxicity and genotoxicity induced by TEMPO and its derivatives in MLA. Cells were treated with various concentrations (0.1–10 mM) of 4 nitroxides for 4 h in the presence or absence of metabolic activation S9. (A) TEMPO (data from Guo et al., 2013); (B) 4-hydroxy-TEMPO; (C) 4-oxo-TEMPO; (D) 4-methoxy-TEMPO. The data points represent the mean ± SD from 3 independent experiments. * indicates positive response in the MLA, exceeding the GEF of 126 × 10−6.
TEMPO and Its Derivatives Cause DNA Damage in L5178Y Cells
The alkaline Comet assay was used to evaluate the extent of DNA damage induced by TEMPO and its derivatives in the absence of S9 activation. The maximum concentration of each compound used for these assays produced approximately 70% cell viability in the CellTiter-Blue assay. Treatments with the 4 nitroxides all resulted in concentration-dependent increases in the percentage of tail DNA (Figure 3). The lowest concentration that induced significant DNA damage was 2, 4, 3, and 6 mM for TEMPO, 4-hydroxy-TEMPO, 4-oxo-TEMPO, and 4-methoxy-TEMPO, respectively. The highest percentage of DNA in tail, 28% (vs a background of approximately 5%), was produced by treatment with TEMPO (Figure 3A), followed by 4-hydroxy-TEMPO, 4-oxo-TEMPO, and 4-methoxy-TEMPO, which produced maximum levels of 21%, 14%, and 13% DNA in the tail, respectively (Figs. 3B–D).
Figure 3.
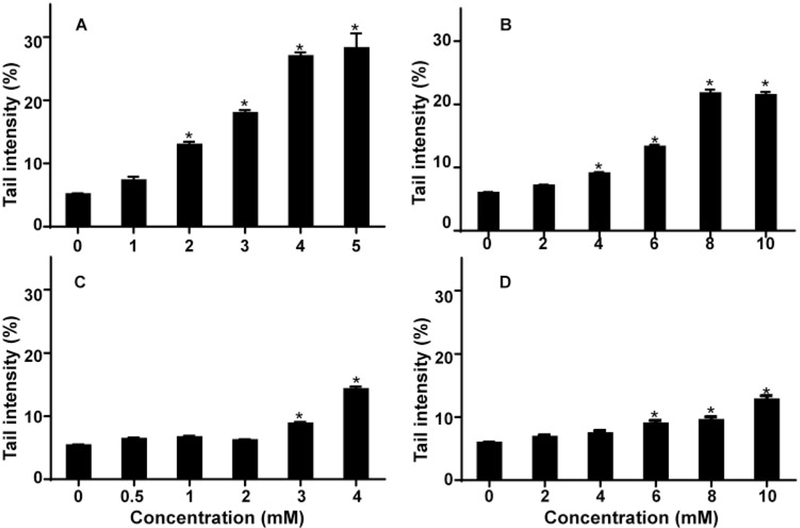
TEMPO and its derivatives cause DNA-strand breaks in mouse lymphoma cells. Following a 4-h treatment, DNA single- and double-strand breaks induced by 4 nitroxides were evaluated by Comet assay. (A) TEMPO; (B) 4-hydroxy-TEMPO; (C) 4-oxo-TEMPO; (D) 4-methoxy-TEMPO. The data points represent the mean ± SD from 3 independent experiments. * p < .05 compared with the vehicle control.
Tk Mutants Induced by TEMPO and Its Derivatives Possess Different LOH Patterns Than Those of the Untreated Control
DNA extracted from 48 large and 48 small Tk mutant colonies collected from cultures treated with the highest concentration of each nitroxide, both with and without S9, were analyzed for LOH at 5 microsatellite loci (Tk locus and D11Mit42, D11Mit36, D11Mit20, and D11Mit74). These 5 polymorphic markers were almost evenly distributed along the full length of chromosome 11. The percentages of the different types of mutations in large and small colony mutants combined are displayed in Figure 4. The most common type of mutation for both the control and nitroxide treatments was LOH, with the deletion limited to Tk through the D11Mit42 locus, which comprises approximately 4.5 Mbp of chromosome 11. Statistical analysis revealed significantly different mutational spectra between the vehicle control and all treatment groups, both with and without S9 (p < .05), except for 4-hydroxy-TEMPO with S9. In addition, treatments with metabolic activation significantly altered the mutational spectra of TEMPO and 4-hydroxy-TEMPO (p < .05), but not the other nitroxides. It is worth noting that similar mutational spectra were produced by all 4 nitroxides in the presence of S9 (Supplementary Table 1).
Figure 4.
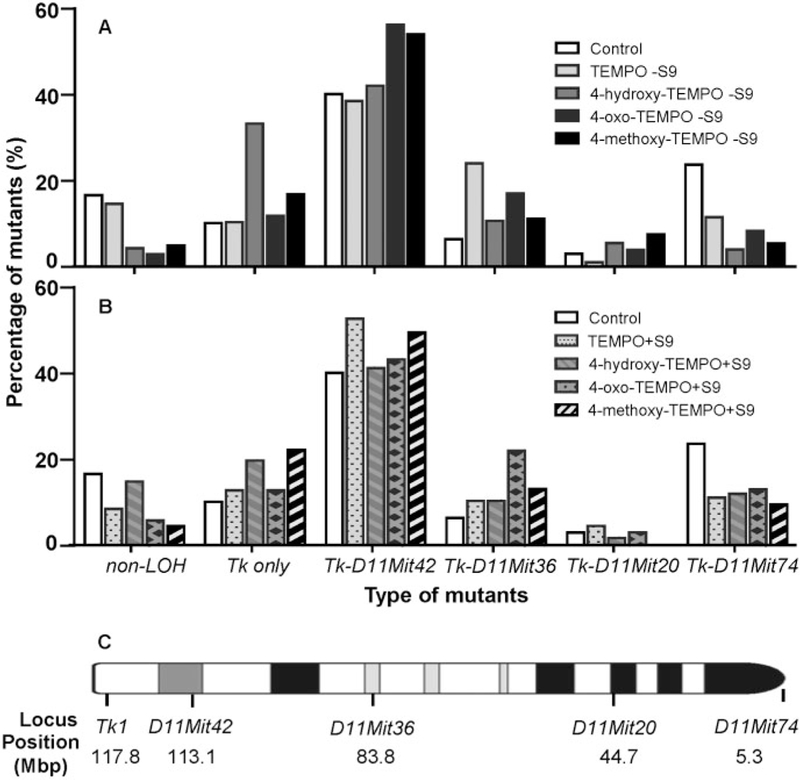
Mutational spectra evaluated by LOH analysis in induced Tk mutants at 5 heterozygous loci along mouse chromosome 11. Following 11 days incubation, the mutants were collected from TFT-selective plates in the MLA. The data were the weighted sum of mutation percentages from large and small colony mutants. The “–” indicates the LOH extends from the Tk locus to either D11Mit42, D11Mit36, D11Mit20, or D11Mit74. (A) Treatment without S9; (B) Treatment with S9; (C) The loci that were analyzed for LOH are marked on the chromosome 11.
TEMPO and Its Derivatives Display a Clastogenic MoA in Treated Cells
Using the GEF criteria (Table 1) recommended by Bryce et al. (2017), TEMPO and its 3 derivatives were classified as clastogens, as the 4-h γ-H2AX, 24-h γ-H2AX, and 24-h p53 showed 1.1- to 2.8-, 2.8- to 3.5-, and 2.1- to 2.8-fold increases, respectively, in treated cells as compared with the concurrent control (Figure 5 and Supplementary Table 2). In addition to evaluating GEFs, we also evaluated the data using an ensemble approach that included 3 machine learning algorithms, ie, multinomial logistic regression, random forest, and artificial neural network learners. As shown in Figure 6, all 3 models demonstrated a high probability of clastogenic MoA, as 4 nitroxides had multiple successive concentrations exhibiting clastogen probability scores ≥80%. Specifically, both logistic regression and neural network models showed clastogenic probability scores ≥90% for most of the concentrations tested.
Figure 5.
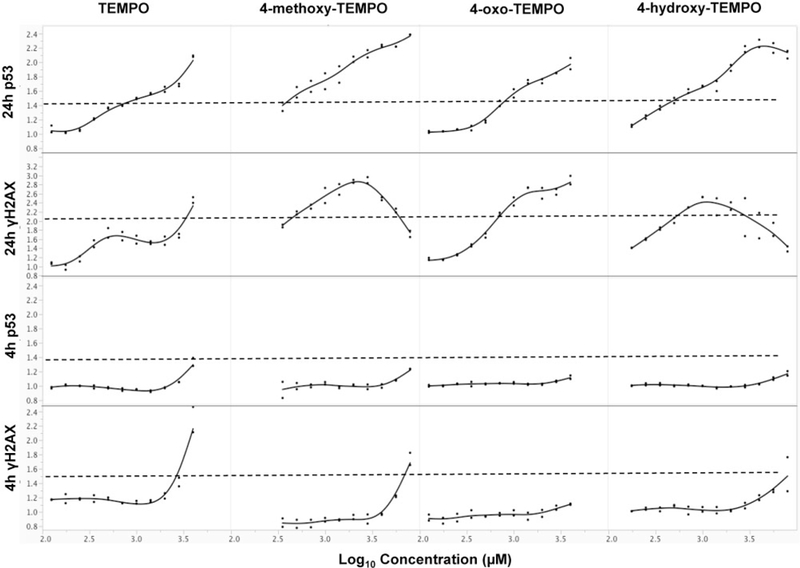
MoA of TEMPO and its derivatives evaluated by GEF for multiflow DNA damage assay. Data are expressed as fold-increase over concurrent solvent control and plotted for each chemical. Each dot represents a different concentration of the chemical. Dash line represents the GEF for each endpoint.
Figure 6.
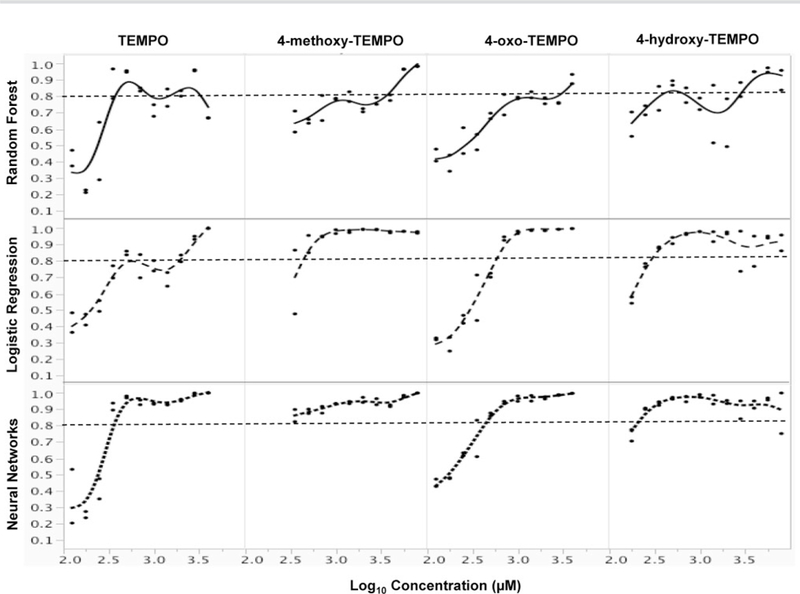
Clastogenic probabilities of TEMPO and its derivatives. The probabilities for an clastogen classification were calculated using an ensemble approach containing 3 machine learning algorithms, multinomial logistic regression, random forest, and artificial neural network learners. Each dot represents a different concentration of the chemical.
Quantification of the Mutagenic and DNA Damage Potencies of TEMPO and Its Derivatives
Both the MLA and Comet assay data were analyzed using BMD quantitative approaches. We first calculated the BMD10 and BMDL10 (ie, 10% increase above the background response and the lower bound of the 95% CI) using the covariant approach of the PROAST Software package. The use of the covariant approach was justified by the 4 nitroxides sharing a similar 6-membered ring structure and a clastogenic MoA for their genotoxicity. In this analysis, the BMDL10 was used for potency ranking the nitroxides in terms of their mutagenicity and ability to induce DNA damage (Table 3). For example, when L5178Y cells were treated with the 4 chemicals without S9, 4-oxo-TEMPO was the most potent nitroxide in the MLA, followed by 4-methoxy-TEMPO > TEMPO > 4-hydroxy-TEMPO.
Table 3.
Relative Genotoxic Potential of 4 Nitroxides
| MLA Without S9 |
MLA With S9 |
Comet Assay |
||||
|---|---|---|---|---|---|---|
| Nitroxide | BMDL10a | Ranking | BMDL10a | Ranking | BMDL10a | Ranking |
| TEMPO | 0.981 | 3 | 0.257 | 2 | 0.454 | 1 |
| 4-hydroxy-TEMPO | 1.300 | 4 | 1.024 | 4 | 1.612 | 3 |
| 4-oxo-TEMPO | 0.661 | 1 | 0.058 | 1 | 1.114 | 2 |
| 4-methoxy-TEMPO | 0.756 | 2 | 0.770 | 3 | 3.358 | 4 |
BMDL10, the lower limit of the 10% BMD, is calculated from Hill model of the PROAST Software using chemical as a covariate for both the MLA MF data and the %DNA in tail data from the Comet assay.
Similar BMD potency rankings were obtained when the MLA dose-response data were analyzed using different BMDs, eg, 4-oxo-TEMPO had the lowest BMD values for BMD10, BMD50, BMD100, and BMD200 (Figure 7, top panels). However, the BMDU and BMDL values for the 4 –S9 responses overlapped each other, regardless of the BMD that was used. In contrast, the 4 nitroxides exhibited clear differences in mutagenic potency with metabolic activation. As shown in Figure 7 (middle panel), the BMD10 values for the 4 dose-responses formed 3 groups without overlapping 95% CIs, while the BMD50, BMD100, and BMD200 values were able to separate the +S9 mutagenic potencies completely.
Figure 7.
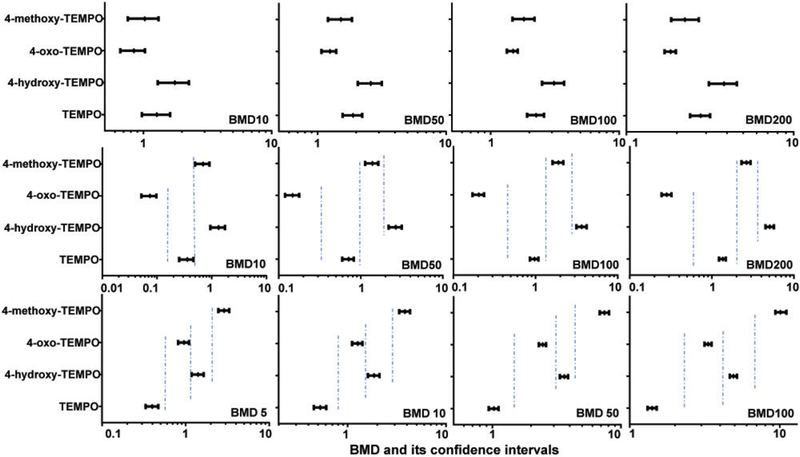
Comparison of BMD values for the mutagenicity and DNA damaging effects induced by TEMPO and its derivatives. The BMD (BMD5, BMD10, BMD50, BMD100, and BMD200) estimates producing a 5%, 10%, 50%, 100%, or200% increase in the background responses were calculated using Hill model of PROAST with covariate analysis approach. Top panel, treatments without S9 in the MLA; middle panel, treatments with S9 in the MLA; bottom panel, data from the Comet assay. The bars represent the calculated lower and upper 95% CI of each value.
The BMD5, BMD10, BMD50, and BMD100 were calculated for DNA damage responses in Comet assays run in the absence of S9 activation. BMD200 values were not calculated for the responses, since they would have produced values much higher than the highest concentrations tested in this study. The BMDL and BMDU metrics for the BMD5s, BMD10s, BMD50s, and BMD100s all had non-overlapping 95% CIs (Figure 7, bottom panel). TEMPO was the most potent inducer of DNA damage, followed by 4-oxo-TEMPO and 4-hydroxy-TEMPO; and 4-methoxy-TEMPO was the weakest (Table 3).
DISCUSSION
Nitroxides are sometimes used in vivo at very high concentrations and can achieve high levels of tissue accumulation. For example, C3H mice injected with 5 ml/kg body weight of 150 mM 4-hydroxy-TEMPO in the tail vein had maximum blood and tissue concentrations of 6.1–8.1 mM (Davis et al., 2011); the progression of multiple sclerosis was alleviated in mice fed 10 g/kg powdered 4-hydroxy-TEMPO (equivalent to 58 mM in drinking water) (Neil et al., 2017); incubation of Chinese hamster cells with 10 and 100 mM 4-hydroxy-TEMPO for 10 min prior to X-irradiation resulted in radiation protection factors of 1.3 and 2.5, respectively (Mitchell et al., 1991). As there is a positive correlation between the level of radioprotection and the concentration of the nitroxide, the balance between beneficial and potential adverse effects should be considered. In addition, there are very little data on the toxic effects of TEMPO and its derivatives (Zhang et al., 2018). This study evaluated the cytotoxic and genotoxic effects of TEMPO and 3 TEMPO derivatives using the MLA and Comet assay. The resulting genotoxicity dose-response data were used to rank the genotoxic potencies of the 4 compounds.
We first compared the cytotoxic effects of the 4 nitroxides in mouse lymphoma cells using 3 assays, the MTS, CellTiter-Blue, and ATP assays. Overall, the results showed that TEMPO and 4-oxo-TEMPO were more cytotoxic than 4-hydroxy-TEMPO or 4-methoxy-TEMPO, a finding that was consistent with a previous study using the Amido black assay and reporting that TEMPO and 4-oxo-TEMPO had lower IC50 values than 4-hydroxy-TEMPO in HaCaT cells (Kroll et al., 1999). Cytotoxicity is closely related to the lipid/water partition coefficients of the nitroxides (Kroll et al., 1999). Lipophilic compounds such as TEMPO are expected to accumulate in the cell membrane to a greater extent than hydrophilic compounds such as 4-hydroxy-TEMPO, which would facilitate the cellular toxicity of TEMPO. The different nitroxide concentrations in the cell membrane and subcellular compartments may result in the activation of different cellular signaling pathways (Suy et al., 1998).
Another observation was that all 4 nitroxides exhibited the highest sensitivity to the MTS assay (Figure 1 and Table 2). As the MTS assay measures cytotoxicity by increases in NAD(P)H oxidoreductase-mediated reduction of the MTS tetrazolium compound in metabolically active cells, this suggests that the nitroxides produced a high NAD(P)H flux in treated cells. We assume that the increase in cellular NAD(P)H results from nitroxide-induced mitochondrial impairment (Aleshin et al., 2015) based on the fact that TEMPO caused concentration-dependent ATP depletion and decreases in mitochondrial membrane potential in L5178Y cells (Guo et al., 2015). Although sharing the same basic chemical structure, the 4 nitroxides exhibited distinct patterns of cytotoxicity in the 3 viability assays, indicating that the compounds were cytotoxic by some-what different biological pathways. This observation suggests the importance of careful method selection for the in vitro assessment of cell viability.
All 4 nitroxides significantly increased Tk gene mutation frequencies and the number of DNA-strand breaks in L5178Y cells, but with different efficiencies (Figs. 2 and 3). Metabolic activation increased the cytotoxicity and genotoxicity of all the compounds except 4-hydroxy-TEMPO (Figure 2B), indicating that these nitroxides were direct genotoxicants and that the metabolites of most nitroxides were more cytotoxic and mutagenic than their parent compounds. Under biological conditions, most nitroxides are reduced to stable hydroxylamines (Gallez et al., 1992). Nitroxides, however, may also undergo 1-electron oxidation to yield the respective oxoammonium derivatives, which can chemically modify macromolecules, oxidize organic and inorganic molecules found in biological systems, and initiate cell signaling events related to oxidative stress (Dragutan and Mehlhorn, 2007). In addition, genotoxicity may result from adduct formation by the nitroxides with DNA or other radicals (Sies and Mehlhorn, 1986).
Another important finding of this study was that the mutagenic potential of the 4 nitroxides was not clearly related to their potential for causing DNA damage. At the cytotoxicity limit for performing the MLA, TEMPO induced the lowest MF (208 × 10−6) of the 4 nitroxides when tested in the absence of S9 activation. TEMPO, however, at the limit concentration in the Comet assay, produced the most DNA damage of the 4 nitroxides (28% DNA in tail) (Figs. 2A and3A). In contrast, 4-oxo-TEMPO and 4-methoxy-TEMPO, which had the highest mutant frequencies in the MLA at the limit cytotoxicity (584 and 537 × 10−6, respectively), produced the lowest increases in % DNA in tail at the cytotoxicity limit for the Comet assay (13%–14% vs a control frequency of approximately 5%). These results provide additional evidence indicating that structurally similar nitroxides may damage DNA and induce mutations and cytotoxicity via different genotoxicity and stress signaling pathways (Suy et al., 1998).
The MoA for the genotoxicity of the nitroxides was investigated using both a multiplexed DNA damage response pathway assay and by LOH analysis of the Tk mutants induced by the nitroxides in the MLA. The multiplexed assay is an add-and-read type test combining measurement of 4 biomarkers relevant to DNA damage response pathways. These biomarkers include phosphorylated p-H3 and γ-H2AX (indicators of mitotic cells and DNA double-strand breaks, respectively), nuclear p53 content (a measure of DNA damage response) and the frequency of 8n cells (an indicator of polyploidization). The nitroxides had little effect on p-H3 expression but produced significant increases in γ-H2AX and nuclear p53 content, clearly suggesting a clastogenic rather than aneugenic genotoxic MoA. The increased DNA-strand breakage measured by the Comet assay for each of the 4 nitroxides is consistent with a clastogenic MoA.
L5178Y cells are heterozygous at the thymidine kinase locus (Tk+/−), which facilitates detecting single mutagenic events by the MLA. One common way of mutating the Tk+ allele is through allelic loss, usually referred to as LOH (Honma et al., 2001). The extent of allele loss can be used to construct mutation spectra, as was done in this study for mutants induced by the 4 nitroxides, both with and without S9. Almost all the treatment groups had significantly different allele loss mutational spectra from the concurrent vehicle control. The most common mutational type found in the nitroxide-induced mutants had LOH involving Tk and the D11Mit42 locus, indicating chromosomal damage that spanned <31 Mbp. The DNA-strand breakage that accompanied this loss of DNA sequence lends further support to a clastogenic MoA. Another interesting observation from this analysis was that, in the absence of S9, the mutational spectrum of TEMPO-induced Tk mutants was significantly different from the spectra of other 3 nitroxides. In addition, the 4-methoxy-TEMPO-induced Tk mutants shared similar mutational spectra with 4-hydroxy-TEMPO and 4-oxo-TEMPO mutants. In the presence of S9, however, the mutational spectra of all the 4 nitroxides were similar (Supplementary Table 1). 4-Hydroxy-TEMPO, along with the diamagnetic 1, 4-dihydroxy-TEMPO and its hydroxylamine, are the major metabolites of 4-oxo-TEMPO (Kroll and Borchert, 1999). Thus, these studies suggest that somewhat different mechanisms might exist for mutation induction by the 4 nitroxides in the absence of metabolic activation. However, with S9 activation, the mutational responses produced by the nitroxides seem, at least qualitatively, to be quite similar. Thus, their metabolites appear to cause mutagenic effects through similar mechanisms, possibly because TEMPO and its derivatives may have 1 or more metabolic end-products in common.
Quantitative approaches have been used recently to evaluate genotoxicity dose-responses as a means for supporting risk assessment and regulatory decision-making (Wills et al., 2016a,b). Quantitative dose-response analysis has been used to analyze data generated from various in vitro and in vivo genotoxicity assays, ie, the Ames mutagenicity test (Tang et al., 2014), micronucleus assay (Bemis et al., 2016), transgenic rodent mutation assay (Wills et al., 2016b), and the Pig-a and Comet assays (Guerard et al., 2017). We previously demonstrated that quantitative analysis of MLA data could be used for discriminating between the mutagenic potencies of structurally diverse chemicals (Guo et al., 2016) and whole cigarette smoke solutions generated from different types of cigarettes using different smoking regimens (Guo et al., 2018). The present study demonstrated that BMD estimates was also able to rank order the mutagenic and DNA-damaging potency of these structurally similar nitroxides (Table 3). We would like to mention that the BMD approach takes all of the dose-response data into account for curve-fitting and produces BMD5–200 values that focus on multiple points along the dose-responses, ie, from the lower end to the middle or upper end of the dose-response curve.
One of the advantages of BMD potency ranking is that responses can be differentiated statistically on the basis of non-overlapping upper and lower CIs for the BMD estimates. It has been suggested that increasing the number of chemical doses is able to improve the BMD precision. BMDs calculated from 10 doses of B(a)P had narrower upper and lower CIs compared with those calculated from 3 doses of dibenz(a, h)anthracene (Wills et al., 2016b). Our data used 5 treatment concentrations for each compound in both assays. The BMD potency approach separated the 4 nitroxide dose responses for data generated from the Comet assay without S9 and the MLA with S9. However, when the MLA without S9 data were evaluated, the BMDs and their upper and lower CIs overlapped each other due to relatively wide CIs. This suggests that testing additional concentrations of the test articles may be required in order to improve the power of the quantitative approaches for potency ranking.
In summary, we found that TEMPO and 3 TEMPO derivatives caused concentration-dependent increases in MF and DNA damage as measured by the MLA and Comet assay, respectively; cytotoxicity and genotoxicity were generally greater in the presence of exogenous metabolic activation. A molecular mechanism study indicated that all the 4 nitroxides had a clastogenic MoA for genotoxicity. Quantitative dose-response modeling (ie, using the BMD approach) of in vitro MLA and Comet data were useful for rank ordering the genotoxic potencies of the test compounds, with the derivatives generally being less potent genotoxins than the parent compound, TEMPO.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Vasily Dobrovolsky and Dr Volodymyr Tryndyak for their critical review of this article.
FUNDING J.E.S. and J.T. were supported by appointments to the Postgraduate Research Program and Summer Student Research Program, respectively, at the National Center for Toxicological Research (NCTR) administered by the Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration (FDA).
Footnotes
Disclaimer: The information in this paper is not a formal dissemination of information by the U.S. Food and Drug Administration (FDA) and does not represent the agency position or policy.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
REFERENCES
- Adams WT, and Skopek TR (1987). Statistical test for the comparison of samples from mutational spectra. J. Mol. Biol. 194, 391–396. [DOI] [PubMed] [Google Scholar]
- Aleshin VA, Artiukhov AV, Oppermann H, Kazantsev AV, Lukashev NV, and Bunik VI (2015). Mitochondrial impairment may increase cellular NAD(P)H: Resazurin oxidoreductase activity, perturbing the NAD(P)H-based viability assays. Cells 4, 427–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Wills JW, Bryce SM, Torous DK, Dertinger SD, and Slob W (2016). Comparison of in vitro and in vivo clastogenic potency based on benchmark dose analysis of flow cytometric micronucleus data. Mutagenesis 31, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce SM, Bernacki DT, Bemis JC, Spellman RA, Engel ME, Schuler M, Lorge E, Heikkinen PT, Hemmann U, Thybaud V, et al. (2017). Interlaboratory evaluation of a multiplexed high information content in vitro genotoxicity assay. Environ. Mol. Mutagen. 58,146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce SM, Bernacki DT, Smith-Roe SL, Witt KL, Bemis JC, and Dertinger SD (2018). Investigating the generalizability of the multiflow(R) DNA damage assay and several companion machine learning models with a set of 103 diverse test chemicals. Toxicol. Sci. 162,146–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariello NF, Piegorsch WW, Adams WT, and Skopek TR (1994). Computer program for the analysis of mutational spectra: Application to p53 mutations. Carcinogenesis 15, 2281–2285. [DOI] [PubMed] [Google Scholar]
- Czepas J, Koceva-Chyła A, Gwoździński K, and Jóźwiak Z (2008). Different effectiveness of piperidine nitroxides against oxidative stress induced by doxorubicin and hydrogen peroxide. Cell Biol. Toxicol. 24,101–112. [DOI] [PubMed] [Google Scholar]
- Davis RM, Matsumoto S, Bernardo M, Sowers A, Matsumoto K, Krishna MC, and Mitchell JB (2011). Magnetic resonance imaging of organic contrast agents in mice: Capturing the whole-body redox landscape. Free Radic. Biol. Med. 50,459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraff WG, Krishna MC, Russo A, and Mitchell JB (1992). Antimutagenicity of a low molecular weight superoxide dismutase mimic against oxidative mutagens. Environ. Mol. Mutagen. 19, 21–26. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, and Dikalov SI (2010). Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 107,106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragutan I, and Mehlhorn RJ (2007). Modulation of oxidative damage by nitroxide free radicals. Free Radic. Res. 41, 303–315. [DOI] [PubMed] [Google Scholar]
- Gallez B, De Meester C, Debuyst R, Dejehet F, and Dumont P (1992). Mutagenicity of nitroxyl compounds: Structure-activity relationships. Toxicol. Lett. 63, 35–45. [DOI] [PubMed] [Google Scholar]
- Gariboldi MB, Lucchi S, Caserini C, Supino R, Oliva C, and Monti E (1998). Antiproliferative effect of the piperidine nitroxide TEMPOL on neoplastic and nonneoplastic mammalian cell lines. Free Radic. Biol. Med. 24, 913–923. [DOI] [PubMed] [Google Scholar]
- Gariboldi MB, Ravizza R, Petterino C, Castagnaro M, Finocchiaro G, and Monti E (2003). Study of in vitro and in vivo effects of the piperidine nitroxide Tempol-a potential new therapeutic agent for gliomas. Eur. J. Cancer 39, 829–837. [DOI] [PubMed] [Google Scholar]
- Guerard M, Johnson G, Dertinger S, Duran-Pacheco G, Funk J, and Zeller A (2017). Dose-response relationship of temozolomide, determined by the Pig-a, comet, and micronucleus assay. Arch. Toxicol. 91, 2443–2453. [DOI] [PubMed] [Google Scholar]
- Guo X, Chen S, Zhang Z, Dobrovolsky VN, Dial SL, Guo L, and Mei N (2015). Reactive oxygen species and c-Jun N-terminal kinases contribute to TEMPO-induced apoptosis in L5178Y cells. Chem. Biol. Interact. 235, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Heflich RH, Dial SL, De M, Richter PA, and Mei N (2018). Quantitative differentiation of whole smoke solution-induced mutagenicity in the mouse lymphoma assay. Environ. Mol. Mutagen 59, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Heflich RH, Dial SL, Richter PA, Moore MM, and Mei N (2016). Quantitative analysis of the relative mutagenicity of five chemical constituents of tobacco smoke in the mouse lymphoma assay. Mutagenesis 31, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Mittelstaedt RA, Guo L, Shaddock JG, Heflich RH, Bigger AH, Moore MM, and Mei N (2013). Nitroxide TEMPO: A genotoxic and oxidative stress inducer in cultured cells. Toxicol. In Vitro 27,1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M, Momose M, Sakamoto H, Sofuni T, and Hayashi M (2001). Spindle poisons induce allelic loss in mouse lymphoma cells through mitotic non-disjunction. Mutat. Res. 493,101–114. [DOI] [PubMed] [Google Scholar]
- Kroll C, and Borchert HH (1999). Metabolism of the stable nitroxyl radical 4-oxo-2, 2, 6, 6-tetramethylpiperidine-N-oxyl(TEMPONE). Eur.J. Pharm. Sci. 8, 5–9. [DOI] [PubMed] [Google Scholar]
- Kroll C, Langner A, and Borchert HH (1999). Nitroxide metabolism in the human keratinocyte cell line HaCaT. Free Radic. Biol. Med. 26,850–857. [DOI] [PubMed] [Google Scholar]
- Liang Q, Smith AD, Pan S, Tyurin VA, Kagan VE, Hastings TG, and Schor NF (2005). Neuroprotective effects of TEMPOL in central and peripheral nervous system models of Parkinson’s disease. Biochem. Pharmacol. 70, 1371–1381. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Krishna MC, and Mitchell JB (2004). Novel pharmacokinetic measurement using electron paramagnetic resonance spectroscopy and simulation of in vivo decay of various nitroxyl spin probes in mouse blood. J. Pharmacol. Exp. Ther. 310, 1076–1083. [DOI] [PubMed] [Google Scholar]
- Mei N, Guo X, and Moore MM (2014). Methods for using the mouse lymphoma assay to screen for chemical mutagenicity and photo-mutagenicity In Optimization in Drug Discovery: In-Vitro Methods (Caldwell GW, Eds.), Humana Press, Totowa, New Jersey: pp. 561–592. [Google Scholar]
- Mitchell JB, DeGraff W, Kaufman D, Krishna MC, Samuni A, Finkelstein E, Ahn MS, Hahn SM, Gamson J, and Russo A (1991). Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch. Biochem. Biophys. 289,62–70. [DOI] [PubMed] [Google Scholar]
- Moore MM, Honma M, Clements J, Bolcsfoldi G, Cifone M, Delongchamp R, Fellows M, Gollapudi B, Jenkinson P, Kirby P, et al. (2003). Mouse lymphoma thymidine kinase gene mutation assay: International Workshop on Genotoxicity Tests Workgroup report–Plymouth, UK 2002. Mutat. Res. 540,127–140. [DOI] [PubMed] [Google Scholar]
- Neil S, Huh J, Baronas V, Li X, McFarland HF, Cherukuri M, Mitchell JB, and Quandt JA (2017). Oral administration of the nitroxide radical TEMPOL exhibits immunomodulatory and therapeutic properties in multiple sclerosis models. Brain Behau. Immun. 62, 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. (2015). Test No. 490: In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene. Available at: http://www.oecd-ilibrary.org/docserver/download/9716211e.pdf?expires=1508177736&id=id&accname=guest&checksum= 1C88770EB8BA2B05FB05F3FBA098FE4D. Accessed February 2, 2018.
- Perez MJ, and Cederbaum AI (2001). Spin trapping agents (Tempol and POBN) protect HepG2 cells overexpressing CYP2E1 against arachidonic acid toxicity. Free Radic. Biol. Med. 30, 734–746. [DOI] [PubMed] [Google Scholar]
- RIVM. (2013). PROAST. Available at: http://www.rivm.nl/en/Documents_and_publications/Scientific/Models/PROAST. Accessed February 2, 2018.
- Sies H, and Mehlhorn R (1986). Mutagenicity of nitroxide-free radicals. Arch. Biochem. Biophys. 251, 393–396. [DOI] [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, and Mitchell JB (2007). Therapeutic and clinical applications of nitroxide compounds. Antioxid. Redox. Signal. 9,1731–1743. [DOI] [PubMed] [Google Scholar]
- Suy S, Mitchell JB, Ehleiter D, Haimovitz-Friedman A, and Kasid U (1998). Nitroxides tempol and tempo induce divergent signal transduction pathways in MDA-MB 231 breast cancer cells. J. Biol. Chem. 273,17871–17878. [DOI] [PubMed] [Google Scholar]
- Tang L, Guerard M, and Zeller A (2014). Quantitative assessment of the dose-response of alkylating agents in DNA repair proficient and deficient ames tester strains. Environ. Mol. Mutagen. 55,15–23. [DOI] [PubMed] [Google Scholar]
- Wills JW, Johnson GE, Doak SH, Soeteman-Hernandez LG, Slob W, and White PA (2016a). Empirical analysis of BMD metrics in genetic toxicology part I: In vitro analyses to provide robust potency rankings and support MOA determinations. Mutagenesis 31, 255–263. [DOI] [PubMed] [Google Scholar]
- Wills JW, Long AS, Johnson GE, Bemis JC, Dertinger SD, Slob W, and White PA (2016b). Empirical analysis of BMD metrics in genetic toxicology part II: In vivo potency comparisons to promote reductions in the use of experimental animals for genetic toxicity assessment. Mutagenesis 31, 265–275. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Mito F, Ito Y, Pandian S, Kinoshita Y, Nakano K, Murugesan R, Sakai K, Utsumi H, and Yamada K (2011). Structure-reactivity relationship of piperidine nitroxide: Electrochemical, ESR and computational studies. J. Org. Chem. 76,435–440. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen S, Mei H, Xuan J, Guo X, Couch L, Dobrovolsky VN, Guo L, and Mei N (2015). Ginkgo biloba leaf extract induces DNA damage by inhibiting topoisomerase II activity in human hepatic cells. Sci. Rep. 5,14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ren Z, Chen S, Guo X, Liu F, Guo L, and Mei N (2018). ROS generation and JNK activation contribute to 4-methoxy-TEMPO-induced cytotoxicity, autophagy, and DNA damage in HepG2 cells. Archives of toxicology. 92, 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


