Abstract
Introduction:
Alzheimer’s Disease (AD) is a progressive neurodegenerative condition in which individuals exhibit memory loss, dementia, and impaired metabolism. Nearly all previous sin-gle-treatment studies to treat AD have failed, likely because it is a complex disease with multiple un-derlying drivers contributing to risk, onset, and progression. Here, we explored the efficacy of a mul-ti-therapy approach based on the disease risk factor status specific to individuals with AD diagnosis or concern.
Methods:
Novel software from uMETHOD Health was designed to execute a precision-medicine-based approach to de¬velop personalized treatment recommendations with the goal of slowing or re-versing biologic drivers of AD. AD-associated inputs encompassed genomic data, bio-specimen measurements, imaging data (such as MRIs or PET scans), medical histories, medications, allergies, co-morbidities, relevant lifestyle factors, and results of neuropsychological testing. Algorithms were then employed to prioritize physiologic and lifestyle states with the highest probability of contributing to disease status, and these priorities were incorporated into a personalized care plan, which was de-livered to physicians and supported by health coaches to increase adherence. The sample included 40 subjects with Subjective Cognitive Decline patients (SCD), and Mild Cognitive Impairment Patients (MCI).
Results:
Software analysis was completed for 40 individuals. They remained on their treatment plan for an average of 8.4 months, equal to 2.8 iterations of care plans. 80% of individuals overall showed improved memory function scores or held steady, as meas¬ured by standardized cognitive evaluations. Cognitive assessments showed significant improvement in the SCD group (Composite P value .002, Executive P value .01), and the CNS-VS Executive domain showed significant results in the com-bined group as well (P value .01). There was also biomarker improvement over time observed from the blood panels. 8 out of 12 selected biomarkers showed slight, though statistically non-significant, improvement overall for symptomatic individuals, and 6 out of 12 for the overall population. Only one biomarker, homocysteine, showed significant improvement, though (P values .03, .04, .002).
Conclusions:
Our analysis of these individuals lead to several interesting observations to¬gether sug-gesting that AD risk factors comprise a network of interlocking feedback loops that may be modifia-ble. Our findings indicate previously unidentified connectivity between AD risk factors, suggesting that treatment regimens should be tailored to the individual and multi-modal to simultaneously return sev¬eral risk factors to a normative state. If successfully performed, the possibility to slow progression of AD and possibly reverse aspects of cognitive decline may become achievable.
Keywords: Alzheimer’s disease, mild cognitive impairment, precision medicine, combination therapy, software, treatment
1. Introduction
Alzheimer’s Disease (AD) is a progressive neurodegenerative condition in which individuals exhibit memory loss, dementia, and impaired metabolism. It is commonly a late-onset disease, with symptoms developing around the age of 65. AD is one of the most common forms of dementia, accounting for 50 to 80 percent of all dementia cases [1] and is a growing economic and social burden [2-4]. Early symptoms include difficulty in recalling recent events, personality changes, trouble with problem-solving, and confusion. As the disease progresses, symptoms include mood swings, irritability, aggression, trouble with language, and long-term memory loss. In the late stages of AD, bodily functions are lost, leading to death. Life expectancy after diagnosis is seven years [1].
Nearly all previous single-domain studies to treat AD have failed, likely because it is a complex disease with multiple underlying drivers contributing to risk, onset, and progression. Often potential pathologic drivers of AD (e.g., high homocysteine, genetic biases, insulin resistance, poor diet, poor sleep, lack of exercise, chronic inflammation, toxicity) are active simultaneously and need to be addressed accordingly [5]. It is now recognized that many of these underlying drivers are modifiable, allowing persons to reduce their risk and potentially delay disease onset. Even without any personal optimization, as many of one-third of cases could be attributed to modifiable risk factors [6, 7].
It is also increasingly being recognized that early intervention is key to developing an effective therapy. The neuropathological changes of AD evolve many years before clinical onset of the disease. If the diagnosis and treatment are left until a patient has progressed to dementia, their “cerebral compensatory reserves have been exhausted because of extensive neurodegeneration” [8]. Therefore, it is believed that targeting patients who present with Mild Cognitive Impairment (MCI) due to AD and Subjective Cognitive Decline (SCD) are good targets for early treatment. MCI due to AD, also known as amnestic MCI, is when a patient’s cognitive capacity is below the level appropriate to their age, gender, and education level, but the cognitive decline has not reached dementia level. SCD is a cognitively-normal individual with self-reported cognitive impairment, suggestive of a prodromal AD phase [8, 9].
Clinical informatics platforms can improve the treatment of those with chronic, complex diseases such as AD (and its early phases) by optimizing a personalized multi-factorial approach. With a multitude of underlying drivers of AD, genetic factors, comorbidities, medications, and optimizations for each person, the amount of data used in generating a care plan quickly accumulates, making a repeatable and reliable process beyond the scope of what a physician can do by hand, or do well quickly. But where manual methods fail, clinical informatics platforms excel. These platforms can provide a personalized treatment method for each individual in a repeatable, predictable, and timely manner.
2. Materials and methods
uMETHOD Health has developed a precision-medicine platform that follows the multi-modal principles of the FINGER trial [7], the Weill-Cornell Alzheimer’s Prevention Clinic [10], the AHRQ Report [11], and others [12-16]. Large datasets about each person are analyzed to generate personalized treatment plans for those at risk (SCD) or in early stages (MCI). The platform identifies and addresses active issues, and creates repeatable and practical treatment plans for use in doctors’ practices. The algorithms identify more than 50 drivers of AD [17-41].
2.1. Overview of Population
All individuals presented here self-selected to follow the care plan. The program was recommended by their local physician or they found uMH’s website through their own searches and chose to purchase the evaluation and follow the care plan under the guidance of their physician. As this was not a controlled trial and the care plan was available to all patients through the physician, only those who wanted to continue for multiple iterations did so. Some did not continue due to cost, others due to complexity, and others were not yet due to renew for a second care plan at the time of this publication.
Patients often had a family history of AD and, out of concern for their own health, sought out our program. Others were recommended for the program by their physician, based on the physician’s office analysis of the patient’s cognitive state. uMH was not involved in these initial analyses, but physicians were expected to have followed standard of care for these initial assessments.
In order to enroll a patient in the program, physicians were instructed to follow a set of inclusion and exclusion criteria for interested patients before enrolling them (see Table 1). All participants were initially assessed with the Self-Administered Gerocognitive Examination (SAGE) to determine their cognitive status and the amount of cognitive impairment present. SCD vs. MCI was defined following the University of Ohio’s validity and normality data [42], with SCD patients scoring 19 or higher, and MCI patients scoring from 12-18. Participants who scored below 12 on SAGE were excluded, as the care plan is best suited for those with mild symptoms, and not those who are have progressed into the dementia stages.
Table 1.
Acceptance and exclusion criteria.
| Acceptance Criteria |
| Diagnosis of mild cognitive impairment or subjective cognitive impairment |
| Progressive memory loss |
| SAGE score of 12 or higher |
| Exclusion Criteria |
| Alcoholic (patient must be sober for at least 3 months) |
| Diagnosed with any other neurological disease outside of MCI or Alzheimer’s disease |
| BMI above 35 |
| Cancer, both current and recurring |
| Current Lyme disease |
| Currently in an Alzheimer’s trial |
| History of major stroke, repeated strokes, recurring TIA’s, or speech impediment due to stroke |
| Smoking |
| Stage 4 or 5 of chronic kidney disease (using K/DOQI guidelines) |
| Uncontrolled blood pressure greater than 140/90 (must be medically controlled to enroll) |
| Uncontrolled seizures or on multiple medications to treat seizures |
| Major depression; as defined by 3 or more medications needed to treat, counseling more than once a month, currently having suicidal thoughts/ideas |
Of the 40 individuals who continued for multiple iterations of their care plan, it was observed that they were of an appropriate age to be registering cognitive concerns due to AD. The MCI individuals (n = 20) were an average of 5 years older than SCD (n = 20) ones. These self-selected individuals have a higher percentage of APOE ε4, a lower occurrence of diabetes, metabolic syndrome, and obesity, and are well-educated when compared to the general public (Table 2). In other words, this population is not representative of the general public.
Table 2.
Overview of baseline: Population and cognitive testing results.
| - | MCI n = 20 mean (SD) | SCD n = 20 mean (SD) |
|---|---|---|
| Demographic Characteristics | ||
| Age | 67.20 (9.45) | 61.09 (9.47) |
| ApoE4 | 80.00% | 65.00% |
| Education (yrs.) | 16.85 (2.58) | 18.89 (2.40) |
| Number of medications | 15.75 (11.84) | 12.50 (9.32) |
| BMI | 24.07 (3.36) | 23.01 (3.46) |
| Depression | 35.00% | 10.00% |
| Gender: Male Female |
7 13 |
6 14 |
| Cognitive tests | ||
| SAGE (best = 22) | 16.77 (3.02) | 21.35 (1.34) |
| CNS-VS Composite Memory (%tile) | 6.83 (8.45) | 51.61 (28.78) |
| CNS-VS Executive Function (%tile) | 5.17 (6.68) | 44.50 (25.80) |
| SF-36 mental health | 63.56 (31.33) | 72.32 (17.89) |
| SF-36 physical health | 80.44 (15.93) | 79.00 (19.63) |
Unless otherwise indicated, data reported as mean (±SD).
SAGE = Self-Administered Gerocognitive Exam, CNS-VS = CNS Vital Signs. APOE4 = Apolipoprotein E ε4 allele.
2.2. Input: Diverse Medical Data
Data collected from multiple health domains contribute to individuals’ risk profile, helps identify underlying drivers of decline, and leads to their treatment recommendations.
Raw data files of genomic information are collected from consumer-focused companies such as 23andMe or Ancestry.com. Alternatively, a full-exome VCF file of genomic data can be read. More than 2,000 Single Nucleotide Polymorphism (SNPs) were analyzed per individual.
Personal medical history is gathered from forms completed by the individual or their caretaker. This information includes current medications, nutraceuticals, over the counter drugs, along with comorbidities, past procedures and surgeries, allergies, imaging such as MRI, EEG, or PET scans, immunization history, and family history of dementia or cardiovascular conditions.
Lifestyle data, vitals, and biometrics are also supplied. Information on sleep, diet, stress, educational attainment, physical activity, quality of life, and activities of daily living are all collected for input into the precision-medicine engine.
All forms were completed by the participants and/or their caregiver via an online portal, or using printed forms depending on computer skills and access. See Table 3 for a full list of questions provided to the patient.
Table 3.
Participant forms and questions.
| Medications |
| Current medications |
| Current supplements |
| Dosage |
| When started |
| Who prescribed |
| Allergies |
| Food |
| Medication |
| Environmental |
| Past Surgeries and Procedures |
| Date |
| Reason |
| Successful or not |
| Family History |
| Cognitive impairment |
| Cardiovascular conditions |
| Lifestyle |
| Alcohol intake per week |
| Average exercise intensity (1-10) |
| Average time spent exercising |
| Caffeine intake per day |
| Daily stress level (1-10) |
| Days of exercise per week |
| Hours of sleep per night |
| SF-36 |
| Tobacco use |
| Female Only |
| Have you entered menopause? When? |
| Have you had a hysterectomy? |
| Medical History |
| Past head trauma |
| Past medical conditions |
A panel of more than 100 blood tests was requested. These include complete blood count, comprehensive metabolic panel, homocysteine level, lipid panel, endocrine panel, essential and toxic metals, and vitamin levels. Other bio-specimen results, such as metabolomics, urine, saliva, and cerebrospinal fluid, are also incorporated if available.
Cognitive testing is performed before each care plan generation. Care plans are updated every 3 months. The battery includes the Montreal Cognitive Assessment (MoCA) [43], the Self-Administered Gerocognitive Exam (SAGE) [44], and CNS Vital Signs (CNS-VS) [45]. CNS-VS employs a normative dataset comprising the scores from 1,069 cognitively-normal people. Each test-taker’s results are organized by age-group and compared to the appropriate normative data set for percentile scoring [45]. Alternate forms of all cognitive tests were used at subsequent administrations to prevent learning curves.
General list of questions asked to each participant. Answers are used in creating their care plan.
The blood labs panel, a subset of the lifestyle and diet information, and cognitive tests are updated with each iteration of the care plan.
All granular data that is received is retained not only for the purpose of the algorithm processing, but also so that it is available to the physicians to help them in their decision process when reviewing the recommended treatment plan.
2.3. Output: Recommended Interventions
Each participant’s information is analyzed by the informatics platform and compared to standardized databases, peer-reviewed publications, and reference tables to generate treatment recommendations. Databases and reference tables used were sourced from the Centers for Disease Control, Food and Drug Administration, National Institutes of Health, DrugBank, and OMIM. The information in these databases and tables relates to SNPs, drug-drug interactions, drug-gene interactions, drug indications, and diagnostics.
The care plan recommendations consist of prescription medications, nutraceuticals, lifestyle changes, as well as specific additional diagnostics to be pursued. Lifestyle changes focus on nutrition, beginning with the MIND diet (17), exercise, sleep, autophagy, stress reduction, and brain stimulation. Each recommendation is listed by priority from highest to lowest, so the care plan may be more easily tailored to the person’s capabilities. Every recommendation is personalized to the individual, including the prioritization and dosage components. Medication recommendations may involve dosage changes, changes in formulation, or deprescribing.
Each care plan is updated after three months of implementation, allowing individual’s blood levels and medication response to be closely monitored and recommendations adjusted accordingly.
Separate care plan reports generated by the software are optimized for the physician, the person under treatment, and their coaching team – with the goal of suggesting what can best be achieved in a clinical setting in the long term, taken in three-month segments.
Coaches meet with individuals once a week for about one hour. They focus on explaining which areas of lifestyle changes to make in the coming week, so that improvements may be readily observable (an encouragement factor). They design personalized goals around what’s achievable over a week or month and are a resource for the individual and caregiver for 1) Scheduling, 2) Explaining the complexities of terminology, 3) Understanding what others have observed, and 4) interfacing with the medical team.
2.4. Informatics Platform
This informatics platform reads medical data about a person and generates reports that describe recommended therapeutics given their current state. Internal software is written in the Python language. Interfaces to an external portal, used by the medical, care, and coaching teams to gather input and return reports, is written in PHP and Java. External medical databases support internal rules-processing algorithms. These are sourced from bodies such as the NIH, FDA, pharmaceutical trade groups, and consortia focused on topics such as genetics or allergies.
The initial input for a person is often on the order of one million data points. This count can vary (generally upwards), depending on several input categories: 1) The completeness of the genetic exome data, 2) The resolution and number of images and image files, 3) The number of historical biospecimen lab results and cognitive assessments, 4) The granularity and history of wearable data samples, and 5) Any attached photos, scans, and faxes. Some of this input data is well-structured, such as genome data files or raw image data; other data is less well-structured, such as personal medical histories, medication lists, and image processing summaries. Natural language processing (NLP) techniques are used throughout the input steps, particularly for precise identification of lab tests, medications, drug indications, and comorbidities. A range of NLP techniques is employed to normalize input data.
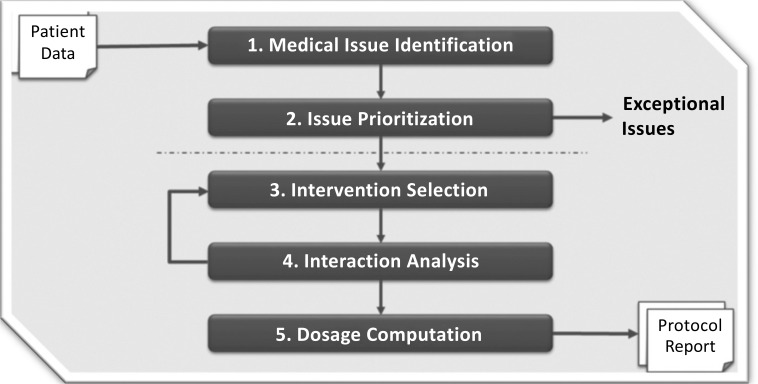
The algorithms that implement this information platform go through a consistent set of steps each time they load a person’s input to generate a new set of reports. These steps are rules-based, so the logic and evidence sources can be tracked (and evaluated). First, the person’s current medical issues are identified, based on their available input data. There are frequently dozens of issues, so decision theory techniques are used to assign weights, priorities, and strategies to the issues. This prioritization is drawn from a wide array of medical knowledge and is done in such a way that they are self-consistent for a person’s current state. Exceptional conditions can be encountered, where data values are far out of range or the need to address an issue is urgent. Examples include untreated kidney or liver disease, or a serum value, such as creatine kinase, that is at an alarm level. When exceptional issues are determined, the generation of a regular care plan is stopped, the issues are described in detail, and the physician and care team are alerted: these exceptional issues are beyond the scope of recommending a protocol for addressing cognitive decline in the next few months.
Next, interventions are selected to address the issues prioritized high enough to be the focus for the care plan period of three months. For each issue, interventions may range from those that work slowly and have few side effects to those that work quickly but may have undesirable effects for the person in their current state. Interventions also have a wide range of costs, including financial costs, pain, an effort by the person being treated, and so on. Currently, no invasive interventions are recommended in the generated care plans. The selected interventions are fitted together as a group, in an iterative manner. Many interactions may be observed, such as drug-to-drug, drug-to-genome, drug-to-diet, and diet-to-existing-comorbidity. Algorithms determine an appropriate path forward, given the many potential conflicts.
Finally, a recommended dosage or amount for each intervention is calculated, and again, the interactions among their current medications (and their dosages) and the recommended interventions are compared (Fig. 1). The generated care plan contains recommendations for prescription and supplemental medications to be added, increased, decreased, changed, or discontinued. Specific changes to lifestyle and diet are recommended. Additional diagnostics, such as lab tests and imaging, may be recommended.
Fig. (1).
Informatics platform process.
Every word in every report is generated by the informatics algorithms. Natural Language Generation (NLG) techniques assure all text, tables, and images are human-readable, in high-quality natural language (such as American English). Multiple versions of the reports are generated suited to the presumed education (e.g., physician), reading ability (e.g., those under treatment and their caregivers), and vocabulary (e.g., dietitian, coach, physical therapist) of the readers.
3. Statistical Analysis
The rate of change between data values was established by calculating the delta of the final and baseline visit and controlling for the baseline score (x2 – x1)/x1 for each biomarker reported, SAGE, and two CNS-VS subdomains.
To test for significance, normal distribution was established with the Anderson-Darling test. Samples that were found to be normally distributed were further analyzed with a paired t-test to assess if results were significant. Welch’s t-test was used for any non-normally distributed samples.
Power calculations for the paired t-test were done using the following:
4. Results
4.1. Memory Function & Biomarkers Improved
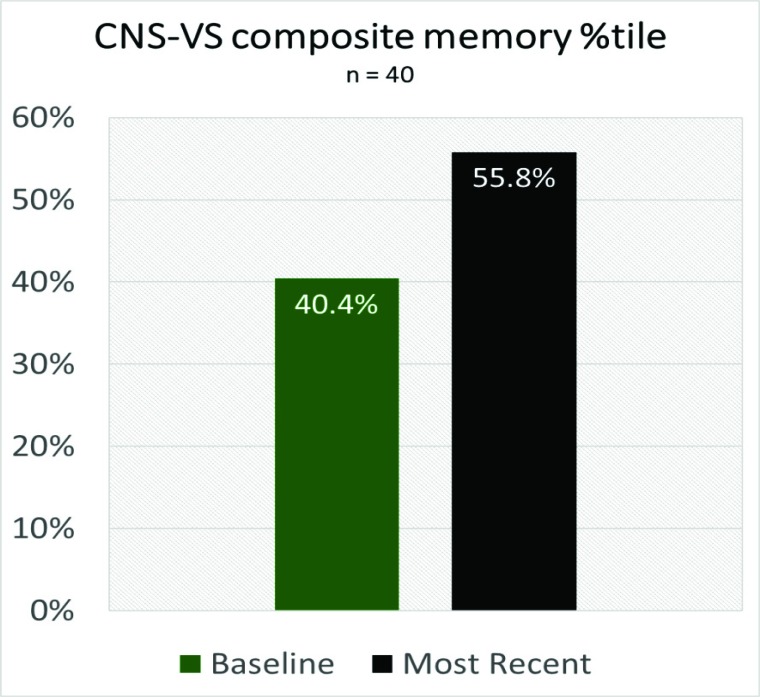
Of the 40 individuals who followed the care plan for an average of 8.4 months, 80% of the overall population (SCD + MCI) improved or held steady on their CNS-VS composite memory scores (Table 4), with overall percentile scores improving from 40.4 (below average), to 55.87 (slightly above average) (Fig. 2). For the MCI subset of that population, 57% showed improvement or held steady in their CNS-VS composite memory percentile scores (Table 4). This data is promising since the improvement in memory scores is rarely observed, especially in the context of MCI, and suggests that adherence to the treatment regime may have long-lasting benefits.
Table 4.
Percent of individuals with steady or improved cognitive status.
| Cognitive Tests | MCI n=20 | SCD n=20 | Combined n=40 |
|---|---|---|---|
| SAGE | 71% | 78% | 76% |
| CNS-VS Composite Memory | 57% | 88% | 80% |
| CNS-VS Executive Function | 43% | 94% | 76% |
SAGE = Self-Administered Gerocognitive Exam, CNS-VS = CNS Vital Signs.
Fig. (2).
Overall Memory Improvement.
76% of all individuals also showed improvement or held steady in their SAGE scores, with 71% of the MCI portion of that population showing improvement or no decline (Table 4), indicating the consistency of program results across multiple testing measures.
Of the cognitive assessments given, both CNS-VS domains showed significant improvement in the SCD group (Composite P value .002, Executive P value .01), and the CNS-VS Executive domain showed significant results in the overall population (SCD+MCI) as well (P value .01) (Table 5).
Table 5.
Changes for Individuals with Multiple Care Plans.
| MCI n=20 | SCD n=20 | Combined n=40 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarkers | xStart | xEnd | P(T<=t) two-tail | Cohen's D | xStart | xEnd | P(T<=t) two-tail | Cohen's D | xStart | xEnd | P(T<=t) two-tail | Cohen's D |
| Homocysteine | 9.65 (2.45) | 8.36 (1.64) | 0.03 | -0.63 | 8.85 (2.27) | 7.49 (1.62) | 0.04 | -0.70 | 9.25 (2.37) | 7.925 (1.67) | 0.002 | -0.66 |
| Fasting insulin | 7.63 (8.14) | 6.945 (8.1) | 0.79 | -0.08 | 6.53 (6.29) | 6.28 (7.16) | 0.91 | -0.04 | 7.11 (7.25) | 6.63 (7.57) | 0.80 | -0.06 |
| Glucose | 90.75 (11.99) | 93.2 (9.51) | 0.47 | 0.23 | 89.75 (8.19) | 88.3 (11) | 0.63 | -0.15 | 90.25 (10.15) | 90.75 (10.44) | 0.79 | 0.05 |
| Total cholesterol | 212.21 (40.19) | 190.26 (43.22) | 0.12 | -0.53 | 202.74 (32.52) | 205.79 (28.13 | 0.80 | 0.09 | 207.47 (38.36) | 198.03 (36.81) | 0.30 | -0.25 |
| TSH | 2.05 (1.16) | 1.28 (1.2) | 0.05 | -0.65 | 1.91 (1.29) | 1.73 (1.17) | 0.61 | -0.15 | 1.98 (1.21) | 1.5 (1.2) | 0.09 | -0.40 |
| rT3 | 5.39 (5.89) | 17.24 (7.57) | 0.39 | 0.27 | 15.93 (3.92) | 15.96 (4.66) | 0.99 | 0.01 | 15.63 (5.05) | 16.67 (6.39) | 0.45 | 0.18 |
| AM cortisol | 16.62 (4.82) | 16.94 (4.49) | 0.83 | 0.07 | 14.24 (4.98) | 14.29 (4.8) | 0.99 | 0.01 | 15.46 (4.98) | 15.65 (4.77) | 0.84 | 0.04 |
| hs-CRP | 1.05 (1.13) | 1.07 (1.55) | 0.96 | 0.02 | 0.73 (1.03) | 0.75 (1.07) | 0.96 | 0.01 | 0.89 (1.08) | 0.91 (1.33) | 0.96 | 0.02 |
| A/G ratio | 2.03 (0.2) | 2.03 (0.27) | 0.95 | 0.02 | 1.87 (0.37) | 1.83 (0.4) | 0.79 | -0.11 | 1.96 (0.3) | 1.94 (0.35) | 0.84 | -0.05 |
| Zinc | 92.1 (17.72) | 92.39 (13.87) | 0.96 | 0.02 | 96.31 (26.05) | 92.06 (20.11) | 0.60 | -0.18 | 94.12 (21.9) | 92.23 (16.9) | 0.70 | -0.10 |
| 25(OH)D | 45.55 (18.54) | 54.28 (27.92) | 0.25 | 0.38 | 51.26 (24.25) | 48.17 (13.7) | 0.71 | -0.13 | 48.4 (27.67) | 51.22 (21.93) | 0.61 | 0.11 |
| Creatinine clearance | 68.17 (17.1) | 68.8 (16.73) | 0.91 | 0.04 | 74.6 (17.4) | 79.16 (19.53) | 0.47 | 0.25 | 71.38 (17.33) | 73.98 (18.7) | 0.54 | 0.14 |
| Cognitive tests | ||||||||||||
| SAGE (best =22) | 17.07 (3.83) | 16.29 (5.31) | 0.43 | -0.17 | 21.5 (1.14) | 21.27 (2.39) | 0.69 | -0.13 | 19.78 (3.32) | 19.33 (4.47) | 0.63 | -0.11 |
| CNS-VS Composite Memory (%tile) | 6.83 (8.02) | 3.67 (3.07) | 0.42 | -0.55 | 51.6 (27.93) | 73.28 (20.71) | 0.002 | 0.89 | 40.42 (31.71) | 55.87 (35.6) | 0.15 | 0.46 |
| CNS-VS Executive Function (%tile) | 5.17 (6.68) | 32 (32.27) | 0.10 | 1.38 | 44.5 (25.17) | 64.33 (19.77) | 0.01 | 0.88 | 34.67 (27.94) | 56.25 (26.83) | 0.01 | 0.79 |
Unless otherwise indicated, data reported as mean (±SD).
*One outlier with an unexplained high hs-CRP of 37.5 was removed from mean and SD.
Improvements are highlighted in green.
TSH = Thyroid-stimulating hormone, rT3= reverse triiodothyronine, hs-CRP = high-sensitivity C-reactive protein, A/G ratio = albumin to globulin ratio, 25(OH)D = 25-hydroxy vitamin D, SAGE = Self-Administered Gerocognitive Exam, CNS-VS = CNS Vital Signs.
Although trends were noted, no other cognitive test showed significant improvement or decline in scores. Longer adherence to the program and/or more enrolled subjects is likely necessary to determine whether these cognitive outcomes are significantly impacted.
There was also biomarker improvement over time observed from the blood panels. Eight out of 12 selected biomarkers showed slight improvement overall for symptomatic individuals and six out of 12 for the overall sample population (Table 5). Only one biomarker, homocysteine, showed significant improvement though (P values .03, .04, .002). Although not currently feasible due to the limited number of subjects, it will be interesting to compare the behavior of different variables relative to each other. For instance, cognitive performance may be more impacted by improvements in some biomarkers relative to others. This analysis may provide further prioritization and simplification as to the key parameters that treated and identify those subjects most likely to respond to the program.
5. Discussion
The results of this work show that not only are individuals able to follow a multi-modal treatment, but this approach also produced measurable improvements in both cognitive testing and biomarkers (Tables 4 and 5). Longer-term studies are needed to show that addressing multiple disease drivers simultaneously can be an effective path to improve cognitive function and to delay or prevent AD for patients in the preclinical and prodromal stages.
Precision medicine is quickly becoming more realistic with advances in genetics, proteomics, lipidomics, metabolomics, and many other fields of scientific and medical study. But implementing it in the clinic by hand is not simple. Algorithmic platforms are necessary to combine and analyze the wide range of information necessary to create individualized, detailed reports. The need for these platforms is increasingly being recognized in peer-reviewed publications as well as in industry settings [46, 47]. The United States government has recognized the need for precision medicine with the 2015 Precision Medicine Initiative [48, 49].
As Galvin points out, a well-balanced diet and healthy lifestyle may be paramount to continued overall and brain health, but every disease risk factor has the potential to act both independently and to augment the effect of other risk factors [46]. Alzheimer’s, and potentially other complex
diseases, must be approached as a disease that results from a system of risk factors with a multi-modal care plan that targets the system as a whole.
A precision-medicine platform enables an actionable combination therapy for AD. Well-established, big-data analytics techniques are utilized, including prioritizing, weightings, probabilities, partial differential equations, and tools from artificial intelligence. Diagnosis, treatment recommendations and ongoing tracking is extensible. Health coaches are considered essential to adherence, providing guidance and influencing individuals' compliance. Individuals comply with the recommendations and can achieve goals leading to measurable cognitive improvements sustained over an average of 8.4 months.
80% of the population, and, of those, 57% of the MCI individuals, improved or held steady in their cognitive status as measured by CNS-VS composite memory. Even though MCI SAGE scores decreased by 0.6 points over 8.4 months (Table 5), this is still less than the average decrease of 1.91 points per year observed in previous studies for the untreated AD [50].
No cognitive test score showed a significant change for MCI individuals. This finding does show that while individuals did not improve their scores, they also did not decline.
The individuals here have a high rate of APOE ε4, are well-educated, average in their 60s, and can sense their cognitive decline. We saw a range of natural biological variability in the population data, although the population was not representative of the general public.
Conclusion
As such, further research on a larger population is warranted to conclude if these results remain consistent, and perhaps show more significant trends for a larger and more generalized population.
Additionally, future research could focus on finding ways to bring the cost of care down even further for this type of multi-modal approach. Every recommendation is given a priority and weight, as well as a monetary cost. The current algorithm has the ability to filter on those requirements for each person and setting.
This multi-modal approach also has the potential for use with other dementias and disease states. uMH’s software platform was developed to specifically address the issues and contributors to AD, but parts such as improved diet, exercise, and sleep can be applied to large populations. Software-enabled, personalized combination treatment plans could be developed for many other disease states backed by similar research. Our research has shown that patients are able to follow these more complex treatment plans, and applying them to a broader audience through the development of other disease state-specific algorithms could increase the quality of life for many in the aging population, and subsequently reduce costs associated with many aging-related disease states.
Acknowledgements
Partial funding for this work and article processing charges were funded by uMETHOD Health located in Raleigh, NC, USA.
All authors had full access to all of the data in this work and take complete responsibility for the integrity of the data and accuracy of the data analysis.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No animals were used in this study. All humans research procedures followed were in accordance with the guidelines from National Institutes of Health approved by the Human Research Protections.
Consent for Publication
Informed consent was obtained from all participants starting the care plan.
Conflict of Interest
Please note the following authors are employed by and/or hold stock in uMETHOD Health: Dorothy Keine, MS, John Q. Walker, PhD, Marwan N. Sabbagh, MD, FAAN. The following author is employed by AFFIRMATIVhealth which has a financial relationship with uMETHOD Health: Brian K. Kennedy, PhD.
REFERENCES
- 1.Alzheimer’s Association http://www.alz.org/
- 2.2012 http://www.who.int/mental_health/publications/dementia_ report_2012/en/
- 3. World Health Organization. First WHO ministerial conference on global action against dementia: Meeting report. WHO Headquarters Geneva, Switzerland; ; 2015. [Google Scholar]
- 4.Winblad B., Amouyel P., Andrieu S., et al. Defeating alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016;15(5):455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 5.Doraiswamy P.M., Steffens D.C. Combination therapy for early Alzheimer’s disease: What are we waiting for? J. Am. Geriatr. Soc. 1998;46(10):1322–1324. doi: 10.1111/j.1532-5415.1998.tb04556.x. [DOI] [PubMed] [Google Scholar]
- 6.Livingston G., Sommerlad A., Orgeta V., et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 7.Ngandu T., Lehtisalo J., Solomon A., et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomized controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 8.Bachurin S., Gavrilova S., Samsonova A., et al. Mild cognitive impairment due to alzheimer disease: Contemporary approaches to diagnostics and pharmacological intervention. Pharmacol. Res. 2017;129:216–226. doi: 10.1016/j.phrs.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Grau S., Rodriguez-Gomez O., Sanabria A., et al. Exploring APOE genotype effects on Alzheimer’s disease risk and amyloid β burden in individuals with subjective cognitive decline: The FundacioACE Healthy Brain Initiative (FACEHBI) study baseline results. Alzheimers Dementia J Alzheimers Assoc. 2017;14(5):634–643. doi: 10.1016/j.jalz.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Seifan A., Isaacson R. The alzheimer’s prevention clinic at Weill Cornell medical college/ New-York Presbyterian hospital: Risk stratification and personalized early intervention. J. Prev. Alzheimers Dis. 2015;2(4):254–266. doi: 10.14283/jpad.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane R.L., Butler M., Fink H.A., et al. Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia Rockville, MD. US: Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 12.Cummings J.L., Isaacson R.S., Schmitt F.A., et al. A practical algorithm for managing Alzheimer’s disease: What, when, and why? Ann. Clin. Transl. Neurol. 2015;2(3):307–323. doi: 10.1002/acn3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Østergaard S.D., Mukherjee S., Sharp S.J., et al. Associations between potentially modifiable risk factors and Alzheimer disease: A Mendelian randomization study. PLoS Med. 2015;12(6):e1001841. doi: 10.1371/journal.pmed.1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson D., Perry D., Bens C., et al. Charting a path toward combination therapy for Alzheimer’s disease. Expert Rev. Neurother. 2015;15(1):107–113. doi: 10.1586/14737175.2015.995168. [DOI] [PubMed] [Google Scholar]
- 15.Xu W., Tan L., Wang H.F., et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2015;86:1284–1285. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 16.Norton S., Matthews F.E., Barnes D.E., et al. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 17.Morris M.C., Tangney C.C., Wang Y., et al. MIND diet associated with reduced incidence of Alzheimer’s disease. J Alzheimers Dement. 2015;11(9):1007–1014. doi: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paddock C.P. Concussion linked to brain changes in people at genetic risk for Alzheimer’s. Medical News Today; 2017. [Google Scholar]
- 19.Joubert L.M., Manore M. Exercise, nutrition, and homocysteine. Int. J. Sport Nutr. Exerc. Metab. 2006;16(4):341–361. doi: 10.1123/ijsnem.16.4.341. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigue K.M., Rieck J.R., Kennedy K.M., et al. Risk factors for β-Amyloid deposition in healthy aging: Vascular and genetic effects. JAMA Neurol. 2013;70(5):600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong H., Kim B.S., Im H. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int Neurol J. 2016;20(Suppl. 1):S2–S7. doi: 10.5213/inj.1632604.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaker L., Wolters F., Korevaar T.I., et al. Thyroid function and the risk of dementia: The rotterdam study. Neurology. 2016;87(16):1688–1695. doi: 10.1212/WNL.0000000000003227. [DOI] [PubMed] [Google Scholar]
- 23.Rosales-Corral S., Tan D.X., Manchester L., et al. Diabetes and alzheimer’s disease, two overlapping pathologies with the same background: Oxidative stress. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/985845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee S., Peters S.A.E., Woodward M., et al. Type 2 Diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bucossi S., Ventriglia M., Panetta V., et al. Copper in alzheimer’s disease: A Meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimers Dis. 2011;24(1):175–185. doi: 10.3233/JAD-2010-101473. [DOI] [PubMed] [Google Scholar]
- 26.Singh I., Sagare A.P., Coma M., et al. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. USA. 2013 doi: 10.1073/pnas.1302212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lövheim H., Gilthorpe J., Adolfsson R., et al. Reactivated herpes simplex infection increases the risk of Alzheimer’s disease. Alzheimers Dement. 2015;11(6):593–599. doi: 10.1016/j.jalz.2014.04.522. [DOI] [PubMed] [Google Scholar]
- 28.Lovheim H., Gilthorpe J., Johansson A., et al. Herpes simplex infection and the risk of Alzheimer’s disease- A nested case-control study. Alzheimers Dement. 2015;11(6):587–592. doi: 10.1016/j.jalz.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 29.Itzhaki R.F., Lathe R., Balin B.J., et al. Microbes and Alzheimer’s disease. J. Alzheimers Dis. 2016;51(4):979–984. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31(19):454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell N., Boustani M., Limbil T., et al. The cognitive impact of anticholinergics: A clinical review. Clin. Interv. Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han L., Agostini J.V., Allore H.G. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J. Am. Geriatr. Soc. 2008;56(12):2203–2210. doi: 10.1111/j.1532-5415.2008.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirai K., Aliev G., Ninomura A., et al. Mitochondrial abnormalities in alzheimer’s disease. J. Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Escudero V., Martín-Maestro P., Perry G., et al. Deconstructing mitochondrial dysfunction in alzheimer disease. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W., Tan L., Wang H., et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. Cognitive Neurol. 2015;86(12):1299–1306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabarti S., Khemka V., Banerjee A., et al. Metabolic risk factors of sporadic alzheimer’s disease: Implications in the Pathology, Pathogenesis and Treatment. Aging Dis. 2015;6(4):282–299. doi: 10.14336/AD.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerutti-Kopplin D., Feine J., Padilha D.M., et al. Tooth Loss Increases the Risk of Diminished Cognitive Function: A Systematic Review and Meta-analysis. JDR Clin. Trans. Res. 2016:10–19. doi: 10.1177/2380084416633102. [DOI] [PubMed] [Google Scholar]
- 38.Last W. Health Science. [Online]. 2016 [cited 2017]. Available from: www.health¬science-spirit.com\pyroluria.htm. 2016
- 39.Xie L., Kang H., Xu Q., et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–37. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira L.K., Tamashiro-Duran J.H., Squarzoni P., et al. The link between cardiovascular risk, Alzheimer’s disease, and mild cognitive impairment: Support from recent functional neuroimaging studies. Br. J. Psychiatry. 2014;36(4):344–357. doi: 10.1590/1516-4446-2013-1275. [DOI] [PubMed] [Google Scholar]
- 41.Kim H.A., Miller A.A., Drummond G.R., et al. Vascular cognitive impairment and Alzheimer’s disease: Role of cerebral hypoperfusion and oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2012;385(10):953–959. doi: 10.1007/s00210-012-0790-7. [DOI] [PubMed] [Google Scholar]
- 42.Ohio State University 2017 https://wexnermedical.osu.edu/brain-spine-neuro/memory-disorders/sage
- 43.Nasreddine Z.S., Phillips N.A., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2015;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 44.Scharre D.W., Chang S.I., Murden R.A., et al. Self-administered Gerocognitive Examination (SAGE): A brief cognitive assessment Instrument for Mild Cognitive Impairment (MCI) and early dementia. Alzheimer Dis. Assoc. Disord. 2010;24(1):64–71. doi: 10.1097/WAD.0b013e3181b03277. [DOI] [PubMed] [Google Scholar]
- 45.Gualtieri C.T., Johnson L.G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch. Clin. Neuropsychol. 2006;21(7):623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Galvin J. The prevention of alzheimer’s disease: Lessons learned and applied. J. Am. Geriatr. Soc. 2017;65(10):2128–2133. doi: 10.1111/jgs.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isaacson R. Is alzheimer’s prevention possible? J. Am. Geriatr. Soc. 2017;65(10):2153–2154. doi: 10.1111/jgs.15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.2015 https://obamawhitehouse.archives.gov/node/333101
- 49.National Institutes of Health 2017 https://allofus.nih.gov/
- 50.Scharre D., Chang S., Nagaraja H., et al. Longitudinal Changes in Self-Administered Gerocognitive Examination (SAGE) and Mini-Mental State Exam (MMSE) Score for Subjective Cognitive Impairment (SCI), Mild Cognitive Impairment (MCI), Dementia Converters, and Alzheimer’s Disease (AD) Patients. Alzheimers Dement J Alzheimers Assoc. 2015;11(7):570. [Google Scholar]