Abstract
Objective
A correlation between physical exercise and cognitive improvement has been found in Alzheimer's disease (AD). This study aimed to investigate the effects of aerobic and resistance exercise on the recognition memory and acetylcholinesterase (AChE) activity in beta amyloid (Aβ) model of AD in rat.
Materials and Methods
Fifty male 8-week-old Wistar rats (250–280 g) were divided into 5 groups (n = 10 each) of control, sham surgery, Aβ-received sedentary, Aβ-received with aerobic exercise and Aβ-received with resistance exercise. AD was induced by intracerebroventricular injection of Aβ25–35 peptide. The sham surgery group received normal saline using the same route and condition. Two groups of Aβ-received animals were trained by treadmill for aerobic exercise and by ladder for strength exercise for 8 weeks (4 days/week). Novel object recognition (NOR) task was used to assess recognitional memory in groups. AChE activity in the brain tissue was assessed using the Spectrophotometry method.
Results
There was no significant difference in memory index and AChE activity between the sham surgery and control groups (p > 0.05). Also, impairment of NOR indices was seen in the Aβ-injected sedentary rats (p < 0.05). However, both aerobic and strength training improved the exploration index in this test (p < 0.05). Further, AChE activity increased in the Aβ-injected sedentary group but declined in the aerobic and resistance exercise groups (p < 0.01).
Conclusion
Aerobic and resistance exercise could improve recognition memory and decrease AChE activity in Aβ-induced AD in rats. The decrease in AChE activity may be one of the mechanisms by which exercise improves cognition and memory in AD.
Key Words: Alzheimer's disease, Beta amyloid, Acetylcholinesterase, Resistance exercise, Aerobic exercise
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in elderly adults. Memory and cognitive impairment due to the progressive loss of neurons is considered the hallmark of AD-type dementia [1, 2, 3, 4]. From the histopathological point of view, AD progression is mostly associated with the extracellular deposition of beta amyloid (Aβ) peptides [5]. Evidence supports that Aβ plays the main role in the cholinergic dysfunction during AD [6].
According to the literature, physical activities or exercise may improve memory decline and cognitive impairment and also delay the onset of dementias including AD [7, 8, 9, 10]. Similarly, investigations on the animal models of AD have provided compelling evidence for a preventive role of physical activity in AD [11, 12, 13, 14]. There is evidence that following a strict exercise regimen during the middle age of an individual is associated with the reduction of dementia risk, improved cognitive scores, larger hippocampal volumes, and lower rate of gray matter volume loss [15]. However, the exact mechanisms by which physical activity improves cognitive performance remain unclear.
Physical activity may attenuate cognitive impairment through Aβ-dependent or independent mechanisms [10]. Cholinergic modulation of central nervous system in mild cognitive impairment or AD patients is one of the most frequently used methods for prevention of disease progression [16] and it seems that aerobic or resistance exercises could affect this neurotransmission.
The aim of this study was to examine the effects of aerobic and resistance exercise on the recognitional memory and acetylcholinesterase (AChE) activity in Aβ-induced AD in rat.
Materials and Methods
Study Design
Fifty male Wistar rats (8 weeks old, weighing 250–280 g) were randomly assigned to 5 groups of control, sham surgery, Aβ-received sedentary, Aβ-received with aerobic exercise, and Aβ-received with resistance exercise (n = 10 in each). Control animals did not receive any treatment or exercise. For modeling of AD, rats received aggregated Aβ25–35 via the intracerebroventricular route; the sham surgery group received normal saline using the same route. Separate groups of Aβ-received animals were divided into aerobic exercise, resistance exercise, and sedentary groups. All procedures were approved by the regional Ethics Committee of Tabriz University of Medical Sciences.
AD Induction
Aggregated Aβ Preparation
Aβ25–35 peptide (Sigma Aldrich, USA) was dissolved in 200 µL of distilled water at the concentration of 5 μg/μL and the solution was incubated at 37°C for 1 week before use.
Surgical Procedure
The rats were anesthetized intra-peritoneally using the mixture of ketamine (70 mg/kg) and xylazine (10 mg/kg) then were placed in a stereotaxic instrument. Using a micro-injection pump, 50 μg of the aggregated Aβ peptide was administrated into each of the ventricles over 3 min. The coordinates were chosen based on the Paxinos and Watson rat brain atlas (antero-posterior −0.8 mm, lateral ±1.6 mm and ventro-dorsal −4.5 mm). For prevention of reflux, the needle was left in place for 5 min before it was withdrawn. Sham surgery group rats were injected with normal saline using the same procedure. Resistance training and aerobic training were started 1 week after the surgery.
Resistance Training
Resistance training of the Aβ-received rats comprised of climbing a ladder (100 cm length, 2 cm grid, 85° incline) with weights attached to their tails.
Three days before training, the rats were familiarized with the apparatus by climbing it twice with and without weight to reach a cage at the top of the ladder. When the rats reached the top of the ladder, they were allowed to recover in the resting area. After familiarization, the rats began resistance training with weights attached to the base of their tail with tape and strap. The rats were positioned at the bottom of the ladder and motivated to climb the ladder by striking or touching their tail. The initial weight attached to the tail was 10% of the rat body weight and was escalated progressively until 100% throughout the 8 weeks of the training period. The weight increase was at the beginning of each week and was not altered throughout that week. The resistance training consisted of 1 set of 10 repetitions with a 10–20 s rest interval between the repeats. The rats in the training groups were trained once a day (9–12 am) every 2 day for 8 weeks.
Aerobic Training Protocol
For aerobic exercise, rats were trained in a motorized treadmill. Animals were first subjected to a 1-week familiarity course in order to reduce handling and environment-related stimulants. Initially, rats were forced to run on a treadmill at a speed of 10 m/min for 20 min. The speed and duration of running were gradually escalated and at the end of the fourth week reached 20 m/min for 40 min and the condition continued up to the end of the eighth week. A gentle 0.04 mA electric shock was sufficient to make the rats run and the entire training process was carried out without any further tail shock.
Behavioral Testing
Novel object recognition (NOR) task was used to assess learning and memory in the rats. This procedure consists of 3 phases: habituation, familiarization, and test phases. In the habituation phase, rats were habituated to the testing apparatus for 10 min. Animals were allowed to freely explore and manipulate the open field arena in the absence of objects. One day after the first phase and during the familiarization phase, animals were separately placed in the open field arena containing 2 identical objects (A and A') for 10 min. Then during the test phase (24 h after familiarization phase), animals were made to return to the open field arena with 2 objects one of which was identical and another one was novel (A and B). The time of objects exploration was measured up to 20 s and novel object exploration time was used as an index of memory.
Tissue Collection and Processing
One day after behavioral test, the rats were euthanized by decapitation under ketamine (70 mg/kg) and xylazine (10 mg/kg) anesthesia and brains were rapidly removed on ice and stored at −80°C until use.
Measurement of AChE Activity
The AChE activity assay was performed based on the method described by Ellman et al. [17]. This method measures the thiocoline production rate by spectrophotometry, while AChE catalyzes the hydrolysis of acetylcholine. Rats' brain tissues were homogenized in 0.1 M of phosphate buffer (pH 8.0) and centrifuged at 14,000 rpm, 4°C for 5 min. Then 0.2 mL of obtained supernatant was added to the cuvette containing 2.8 mL of 0.1 M phosphate buffer and 100 µL of Ellman's reagent (0.01 M; 5,5′-dithiobis-2-nitrobenzoic acid). Accordingly, absorption was measured at 412 nm. Then 20 µL of substrate (acetylthiocholine iodide) was added. After 2 min of incubation at 30°C, the product of thiocholine reaction with 5,5′-dithiobis-2-nitrobenzoic acid was determined at 412 nm for a period of 10 min at 2 min intervals for the absorbance per minute.
Statistical Analysis
Mean values and SEM were used for descriptive data. Also, the analysis of other data was performed using a one-way analysis of variance, and a Tukey post-hoc test. p < 0.05 was considered statistically significant. SPSS 17.0 software was used for all the statistical analyses.
Results
Comparison of Memory Index within the Groups
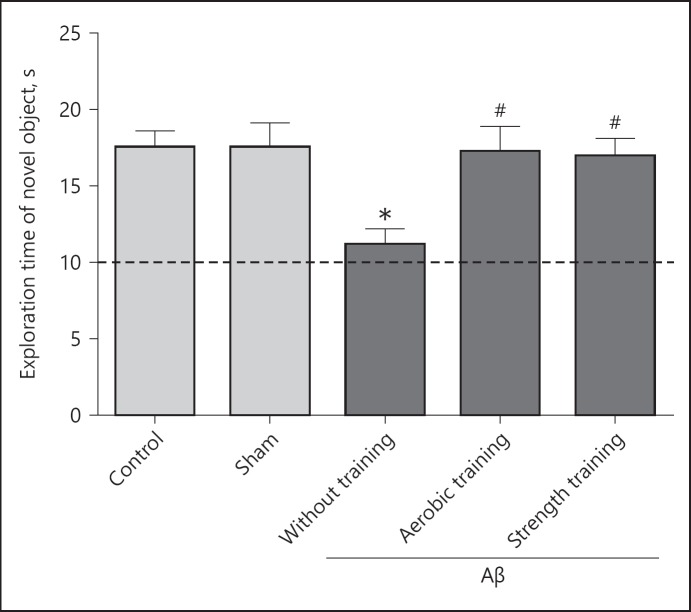
Control and sham surgery groups showed no significant difference in the memory index (p > 0.05). The Aβ-injected sedentary group showed significantly lower novel object exploring time compared to the control and sham surgery groups (p < 0.05). Novel object exploring time significantly increased in the Aβ-injected strength exercise or aerobic exercise groups compared with the Aβ-injected sedentary group (p < 0.05; Fig. 1).
Fig. 1.
Exploration time of the novel object during the test phase of novel object recognition (NOR) task in different groups. Each bar represents the mean ± SEM (n = 10). * p < 0.05 compared to the control group and # p < 0.05 compared to the beta amyloid (Aβ)-received sedentary group respectively. All values were different from the chance exploration (10 s) illustrated by the dashed line (p < 0.05).
Comparison of AChE Activity within the Groups
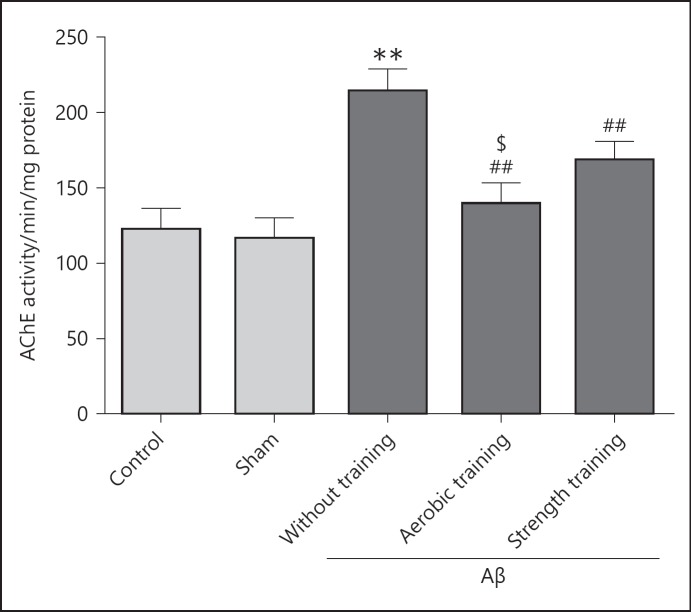
The brain AChE activities are presented in Figure 2. The highest AChE activity was observed in the Aβ injected sedentary group, and the lowest activity was observed in the sham surgery group. No significant difference in AChE activity was observed between control and sham surgery groups (p > 0.05). Strength and aerobic exercises reduced AChE activity in Aβ-injected groups compared to the Aβ-injected sedentary group (p < 0.001). The Aβ-injected sedentary group showed significantly higher AChE activity in comparison with control and sham surgery groups (p < 0.001). The aerobic exercise group revealed significant decrease in AChE activity (p < 0.05) compared to the Aβ-injected strength exercise group.
Fig. 2.
AChE activity in different groups. Each bar represents the mean ± SEM, (n = 10). ** p < 0.01 compared to the control group and ## p < 0.01 compared to the Aβ-received without training group, respectively. $ p < 0.05 compared to the Aβ-received strength training group.
Discussion
According to the cholinergic hypothesis of AD, the acetylcholine level significantly decreases in AD patients' brain [18, 19, 20]. AChE is an important component of all cholinergic synapses in the brain, where it rapidly hydrolyzes the acetylcholine; so AChE inhibitors that reverse the activation of this enzyme are now used for the symptomatic treatment of AD [21, 22, 23]. Moreover, the progressive loss of cholinergic neurons and synapses is influenced by the cholinergic enzymes activity.
There is evidence that physical activity and exercise could improve cognitive function and memory impairment in AD [24, 25, 26, 27]. Animal studies have shown that exercise improves decreased neurotrophic factors levels and enhances neurogenesis, synaptic plasticity, antioxidant capacity, and angiogenesis in AD models [28, 29, 30]. However, the exact mechanism is still unclear.
According to the Cho et al. [31] study, treadmill exercise reversed cognitive impairment in AD animals. Ke et al. [32] evaluated the effects of treadmill exercise on the transgenic AD mice. They found that exercise improves learning and memory via an increase in the cholinergic neurons in the medial septum and vertical diagonal band [32]. Cassilhas et al. [33] showed that aerobic and resistance exercise improves spatial memory through divergent molecular mechanisms. The Souza et al. [34] study demonstrated that 8 weeks of swimming training prevents recognition memory impairment in the Aβ-received animals. Also, according to the Yuede et al. [35] study, voluntarily running AD animals had better performance in the recognition memory tests compared with the sedentary group. In our study, NOR test results showed significant improvement in memory following both resistant and strength exercises in AD animals, which is in line with other similar studies [34, 35]. Based on the cholinergic hypothesis of AD, any reduction in the acetylcholine level is the probable cause of AD [36]. Also, the degeneration of cholinergic neurons in basal forebrain plays a key role in AD-induced memory loss and cognitive impairment [37]. Aβ peptides deposition induces cholinergic denervation in AD [38]. Moreover, studies have shown that AChE could precipitate Aβ plaques deposition in the AD patients' brain [39, 40, 41]. Somani et al. [42] showed that chemical and physical stressors decrease choline acetyltransferase and AChE enzymes in the brain. Kim et al. [43] showed a decreased hippocampal AChE activity after 21 days of treadmill training in the rat model of stroke. Our data show that the reduction of AChE activity in rats is parallel to the improvement in NOR task, which was used in this study for recognitional memory assessment.
In summary, the results of our study indicate that aerobic exercise reduces the AChE activity more effectively than resistance exercise. Also, exercise improves AD-induced memory impairment. This is partly due to the change in the cholinergic function resulting from decline in the AChE activity.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
Funding Sources
This work was supported by Neurosciences Research Center (NSRC), Tabriz University of medical sciences.
Author Contribution
All the authors contributed to conceptualization, investigation, data analysis, validation, literature search, laboratory studies, data acquisition, manuscript preparation, manuscript editing, and manuscript review.
References
- 1.Dickson DW. Neuropathology of Alzheimer's disease and other dementias. Clin Geriatr Med. 2001;17:209–228. doi: 10.1016/s0749-0690(05)70066-5. [DOI] [PubMed] [Google Scholar]
- 2.Kar S, Quirion R. Amyloid beta peptides and central cholinergic neurons: functional interrelationship and relevance to Alzheimer's disease pathology. Prog Brain Res. 2004;145:261–274. doi: 10.1016/S0079-6123(03)45018-8. [DOI] [PubMed] [Google Scholar]
- 3.Mott RT, Hulette CM. Neuropathology of Alzheimer's disease. Neuroimaging Clin N Am. 2005;15:755–765. doi: 10.1016/j.nic.2005.09.003. ix. [DOI] [PubMed] [Google Scholar]
- 4.Souder E. Neuropathology in Alzheimer's disease: target of pharmacotherapy. J Am Acad Nurse Pract. 2005;(Suppl):3–5. [PubMed] [Google Scholar]
- 5.Sadigh-Eteghad S, Talebi M, Farhoudi M, Golzari SE, Sabermarouf B, Mahmoudi J. Beta-amyloid exhibits antagonistic effects on alpha 7 nicotinic acetylcholine receptors in orchestrated manner. J Med Hypotheses Ideas. 2014;8:49–52. [Google Scholar]
- 6.Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J. Amyloid-beta: a crucial factor in Alzheimer's disease. Med Princ Pract. 2015;24:1–10. doi: 10.1159/000369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton LE, Mitnitski A, Fallah N, Kirkland SA, Rockwood K. Changes in cognition and mortality in relation to exercise in late life: a population based study. PLoS One. 2008;3:e3124. doi: 10.1371/journal.pone.0003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K. Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008;56:1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Souto Barreto P, Denormandie P, Lepage B, Armaingaud D, Rapp T, Chauvin P, et al. Effects of a long-term exercise programme on functional ability in people with dementia living in nursing homes: Research protocol of the LEDEN study, a cluster randomised controlled trial. Contemp Clin Trials. 2016;47:289–295. doi: 10.1016/j.cct.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimi K, Majdi A, Baghaiee B, Hosseini SH, Sadigh-Eteghad S. Physical activity and beta-amyloid pathology in Alzheimer's disease: a sound mind in a sound body. EXCLI J. 2017;16:959–972. doi: 10.17179/excli2017-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]
- 12.Sim YJ. Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer's disease rats. J Exerc Rehabil. 2014;10:81–88. doi: 10.12965/jer.140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pareja-Galeano H, Brioche T, Sanchis-Gomar F, Escriva C, Dromant M, Gomez-Cabrera MC, et al. [Effects of physical exercise on cognitive alterations and oxidative stress in an APP/PSN1 transgenic model of Alzheimer's disease] Rev Esp Geriatr Gerontol. 2012;47:198–204. doi: 10.1016/j.regg.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Lee JM, Shin MS, Ji ES, Kim TW, Cho HS, Kim CJ, et al. Treadmill exercise improves motor coordination through ameliorating Purkinje cell loss in amyloid beta23–35-induced Alzheimer's disease rats. J Exerc Rehabil. 2014;10:258–264. doi: 10.12965/jer.140163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadigh-Eteghad S, Majdi A, Mahmoudi J, Golzari SE, Talebi M. Astrocytic and microglial nicotinic acetylcholine receptors: An overlooked issue in Alzheimer's disease. J Neural Transm. 2016;123:1359–1367. doi: 10.1007/s00702-016-1580-z. [DOI] [PubMed] [Google Scholar]
- 17.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 18.Pomara N, Stanley M. The cholinergic hypothesis of memory dysfunction in Alzheimer's disease–revisited. Psychopharmacol Bull. 1986;22:110–118. [PubMed] [Google Scholar]
- 19.Adams PR. Cholinergic hypothesis of Alzheimer's disease: biophysical aspects. Res Publ Assoc Res Nerv Ment Dis. 1987;65:169–185. [PubMed] [Google Scholar]
- 20.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Recanatini M, Valenti P. Acetylcholinesterase inhibitors as a starting point towards improved Alzheimer's disease therapeutics. Curr Pharm Des. 2004;10:3157–3166. doi: 10.2174/1381612043383313. [DOI] [PubMed] [Google Scholar]
- 22.Raschetti R, Maggini M, Sorrentino GC, Martini N, Caffari B, Vanacore N. A cohort study of effectiveness of acetylcholinesterase inhibitors in Alzheimer's disease. Eur J Clin Pharmacol. 2005;61:361–368. doi: 10.1007/s00228-005-0946-1. [DOI] [PubMed] [Google Scholar]
- 23.Racchi M, Porrello E, Lanni C, Lenzken SC, Mazzucchelli M, Govoni S. Role of acetylcholinesterase inhibitors in pharmacological regulation of amyloid precursor protein processing. Aging Clin Exp Res. 2006;18:149–152. doi: 10.1007/BF03327431. [DOI] [PubMed] [Google Scholar]
- 24.Brodaty H. Regular exercise and behavioural management by caregivers improves physical and mental health of people with Alzheimer's disease. Evid Based Ment Health. 2004;7:43. doi: 10.1136/ebmh.7.2.43. [DOI] [PubMed] [Google Scholar]
- 25.Wang PN, Yang CL, Lin KN, Chen WT, Chwang LC, Liu HC. Weight loss, nutritional status and physical activity in patients with Alzheimer's disease. A controlled study. J Neurol. 2004;251:314–320. doi: 10.1007/s00415-004-0316-4. [DOI] [PubMed] [Google Scholar]
- 26.Foldi NS, Schaefer LA, White RE, Johnson R, Jr, Berger JT, Carney MT, et al. Effects of graded levels of physical similarity and density on visual selective attention in patients with Alzheimer's disease. Neuropsychology. 2005;19:5–17. doi: 10.1037/0894-4105.19.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Scherder E, Eggermont L, Sergeant J, Boersma F. Physical activity and cognition in Alzheimer's disease: relationship to vascular risk factors, executive functions and gait. Rev Neurosci. 2007;18:149–158. doi: 10.1515/revneuro.2007.18.2.149. [DOI] [PubMed] [Google Scholar]
- 28.Lange-Asschenfeldt C, Kojda G. Alzheimer's disease, cerebrovascular dysfunction and the benefits of exercise: from vessels to neurons. Exp Gerontol. 2008;43:499–504. doi: 10.1016/j.exger.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, et al. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Dao AT, Zagaar MA, Levine AT, Salim S, Eriksen JL, Alkadhi KA. Treadmill exercise prevents learning and memory impairment in Alzheimer's disease-like pathology. Curr Alzheimer Res. 2013;10:507–515. doi: 10.2174/1567205011310050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho J, Shin MK, Kim D, Lee I, Kim S, Kang H. Treadmill running reverses cognitive declines due to Alzheimer's disease. Med Sci Sports Exerc. 2015;47:1814–1824. doi: 10.1249/MSS.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 32.Ke HC, Huang HJ, Liang KC, Hsieh-Li HM. Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise. Brain Res. 2011;1403:1–11. doi: 10.1016/j.brainres.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 33.Cassilhas RC, Lee KS, Fernandes J, Oliveira MG, Tufik S, Meeusen R, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Souza LC, Jesse CR, Del Fabbro L, de Gomes MG, Goes ATR, Filho CB, et al. Swimming exercise prevents behavioural disturbances induced by an intracerebroventricular injection of amyloid-beta1–42 peptide through modulation of cytokine/NF-kappaB pathway and indoleamine-2,3-dioxygenase in mouse brain. Behav Brain Res. 2017;331:1–13. doi: 10.1016/j.bbr.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanabria-Castro A, Alvarado-Echeverria I, Monge-Bonilla C. Molecular pathogenesis of Alzheimer's disease: an update. Ann Neurosci. 2017;24:46–54. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 38.Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer's disease. J Neural Transm (Vienna) 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- 39.Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer's disease. Drugs Today (Barc) 2003;39:75–83. doi: 10.1358/dot.2003.39.1.740206. [DOI] [PubMed] [Google Scholar]
- 40.Rees T, Hammond PI, Soreq H, Younkin S, Brimijoin S. Acetylcholinesterase promotes beta-amyloid plaques in cerebral cortex. Neurobiol Aging. 2003;24:777–787. doi: 10.1016/s0197-4580(02)00230-0. [DOI] [PubMed] [Google Scholar]
- 41.Reyes AE, Chacon MA, Dinamarca MC, Cerpa W, Morgan C, Inestrosa NC. Acetylcholinesterase-Abeta complexes are more toxic than Abeta fibrils in rat hippocampus: effect on rat beta-amyloid aggregation, laminin expression, reactive astrocytosis, and neuronal cell loss. Am J Pathol. 2004;164:2163–2174. doi: 10.1016/s0002-9440(10)63774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somani SM, Babu SR, Arneric SP, Dube SN. Effect of cholinesterase inhibitor and exercise on choline acetyltransferase and acetylcholinesterase activities in rat brain regions. Pharmacol Biochem Behav. 1991;39:337–343. doi: 10.1016/0091-3057(91)90189-9. [DOI] [PubMed] [Google Scholar]
- 43.Kim G, Kim E. Effects of treadmill training on limb motor function and acetylcholinesterase activity in rats with stroke. J Phys Ther Science. 2013;25:1227–1230. doi: 10.1589/jpts.25.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]