Abstract
Background
Although presepsin (P-SEP) is an early sepsis biomarker, sepsis is often suspected after starting hemodialysis (HD). To enhance the utility of P-SEP, we investigated whether pre-HD P-SEP levels could be predicted using the P-SEP levels from blood samples collected after starting HD.
Methods
We observed P-SEP level changes due to HD and dialyzer passage in HD patients using a dialysis membrane with a β2-microglobulin (β2-MG) clearance of either ≥50 mL/min (high-flux) or < 30 mL/min (intermediate-flux). We calculated the removal ratios for the elimination of P-SEP or the predicted pre-HD P-SEP levels based on the correction of hemoconcentration.
Results
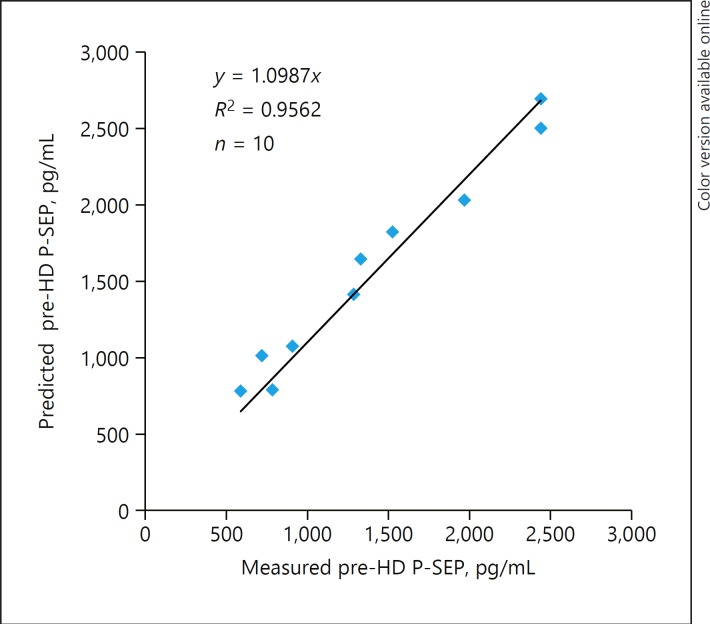
The P-SEP levels significantly decreased at 4 h after starting HD (n = 8) using membranes with a β2-MG clearance ≥50 mL/min; the removal ratios at 2 and 4 h were 42.8 ± 7.9% and 58.8 ± 18.4%, respectively. In contrast, the P-SEP levels did not decrease during the passage of dialyzer in 2 patients with a β2-MG clearance < 30 mL/min, and the P-SEP levels increased during HD in all patients (n = 10, including the abovementioned 2 patients) with a β2-MG clearance < 30 mL/min. The predicted pre-HD P-SEP levels (y) were strongly correlated with the actually measured pre-HD P-SEP levels (x) (R2 = 0.9562) using the regression equation: y = 1.0987x.
Conclusion
The levels of P-SEP with a molecular weight near that of β2-MG decreased similarly to those of β2-MG during HD using membranes with a β2-MG clearance ≥50 mL/min. On the contrary, the levels of P-SEP rather increased during HD with a β2-MG clearance < 30 mL/min, suggesting that P-SEP appeared not to be eliminated. Furthermore, the pre-HD P-SEP levels might be predictable by the correction of hemoconcentration using even blood samples collected after starting HD with a β2-MG clearance < 30 mL/min.
Keywords: Biomarker, CD14, Dialysis membrane, Hemodialysis, Presepsin
Introduction
Sepsis is the leading cause of death in hemodialysis (HD) patients; therefore, highly sensitive biomarkers are required. The 2008 Guidelines of the Surviving Sepsis Campaign recommended that the infection focus should be established as rapidly as possible within the first 6 h of presentation [1]. Although many biomarkers have been investigated for diagnosing sepsis, no reliable biomarkers have been established in the clinical settings; only procalcitonin (PCT) and presepsin (P-SEP) are weakly recommended in “The Japanese Clinical Guidelines for Management of Sepsis and Septic Shock 2016” [2].
P-SEP is an N-terminal fragment of CD14 on monocytes with a molecular weight of 13,000 Da that was discovered in Japan in 2002 as a biomarker for diagnosing sepsis early. The novel production mechanism of P-SEP has been reported to be closely related to phagocytosis [3]. PCT is also a conventionally used biomarker for diagnosing sepsis. Although the superiority between P-SEP and PCT is still controversial [4], we previously reported the usefulness of P-SEP over PCT for predicting bacterial proliferation in skin wounds in HD patients, where no marked differences were found in the PCT levels between patients with skin wound infections and those without infection foci [5]. Although P-SEP might be a promising candidate for early sepsis biomarker, we have a long way to go in utilizing P-SEP for HD patients, because the P-SEP levels increase considerably in HD patients [6, 7], and the P-SEP levels in early sepsis have not been reported to date. Therefore, we must accumulate as much data as possible on P-SEP levels when early sepsis is suspected.
In clinical practice, early sepsis is suspected from signs such as a high fever when HD is being performed, as the peripheral blood mononuclear cells of HD patients are “primed” to produce IL-1β and TNF and easily stimulated by the HD procedure itself to cause a fever [8]. The “primed” response of dendritic cells to lipopolysaccharide (intracellular TNFα production) in HD patients has also been observed in vitro [9]. In such cases, we cannot take advantage of early sepsis biomarkers, since blood collection is conventionally performed at the next HD session (inevitably 48–72 h later). If the pre-HD biomarker level could be predicted to a certain extent, even using the blood samples obtained after starting HD, the utility of the biomarker for detecting early sepsis might be remarkably enhanced. In the present study, to determine the P-SEP levels in cases of early sepsis as accurately as possible, we investigated whether or not the pre-HD levels could be predicted using even the blood samples obtained after starting HD as a preliminary study in HD patients without infection.
In Japan, dialysis membranes have been developed to ensure good removal of β2-microglobulin (β2-MG). Through March 2016, commercial dialyzers were classified into 5 categories based on β2-MG clearance (mL/min) under the Japanese reimbursement system: class I (< 10; low-flux dialyzers), class II (10–< 30; “classic” high-flux dialyzers), class III (30–< 50; “modern” high-flux dialyzers), class IV (50–< 70; super-flux dialyzers), and class V (≥70; also super-flux dialyzers). In clinical practice, class IV and V membranes are used for > 90% of Japanese HD patients [10, 11]. Since class II is referred to as high-flux dialyzers worldwide, the standard definition of high-performance membrane dialyzers is membranes providing β2-MG clearance ≥10 mL/min in actual clinical use [12]. Therefore, dialysis membranes with a β2-MG clearance < 30 mL/min are intermediate-flux dialyzers, falling between low-flux and high-flux. Indeed, class IV and V membranes are designed to eliminate β2-MG, but we investigated whether P-SEP might be eliminated or not, because the molecular weight of P-SEP (13,000 Da) is slightly greater than β2-MG (11,800 Da). Consequently, although P-SEP was remarkably eliminated as expected during HD with a β2-MG clearance ≥50 mL/min, it appeared not to have been eliminated during HD with a β2-MG clearance < 30 mL/min. We therefore explored the utility of P-SEP for detecting sepsis early using even the blood samples obtained after starting HD with a β2-MG clearance < 30 mL/min.
Patients and Methods
Study Patients and Design
We enrolled HD patients using either various dialysis membranes with β2-MG clearance ≥50 mL/min (n = 8) or those with β2-MG clearance < 30 mL/min (n = 10). The following are the exclusion criteria: active inflammation (high-sensitivity C-reactive protein [hsCRP] exceeding 0.3 mg/dL), infection foci, and active malignancy. We compared changes in the P-SEP levels during HD between the group with β2-MG clearance ≥50 mL/min using class IV and V dialyzers and the group with β2-MG clearance < 30 mL/min using class I and II dialyzers. The dialyzer with a β2-MG clearance < 30 mL/min was mainly selected for patients who had recently started HD and/or those with hypoalbuminemia. The membranes used in the group with β2-MG clearance ≥50 mL/min were a Hollow fiber dialyzer FD series (FDY-180GW; polyester polymer alloy [PEPA®]; class IV; NIKKISO Co., Ltd., Tokyo, Japan) (n = 2), a Triacetate hollow fiber dialyzer FB-eco series (FB-190Pβeco; cellulose triacetate [CTA]; class IV; Nipro Co., Ltd., Osaka, Japan) (n = 2), a Toraylight® NV (NV-21X; polysulfone [PS]; class V; TORAY Industries, Inc., Tokyo, Japan) (n = 2), and a PEPA dialyzer FDW (FDW-18; [PEPA®]; class V; NIKKISO Co., Ltd.) (n = 2). The membrane used in the group with β2-MG clearance of < 30 mL/min was a Nipro polyethersulfone dialyzer (PES-15Mαeco and PES-21Mαeco; polyethersulfone [PES]; classes I and II; Nipro Co., Ltd.) (n = 10). In addition, we investigated the permeability of dialysis membranes with both a β2-MG clearance < 30 mL/min and a high protein adsorption capacity for β2-MG and P-SEP. The membrane used was a Hollow fiber dialyzer KF-201 (KF-15C; ethylene vinylalcohol [EVAL®]; class II; Kawasumi Laboratories, Inc., Tokyo, Japan) (n = 2).
Laboratory Measurements
Hematocrit (Ht) was measured using an automated analyzer, DxH (Beckman Coulter, Tokyo, Japan). Serum albumin, hsCRP, and β2-MG were measured using an automated analyzer BM-8060G (JEOL, Tokyo, Japan) based on an improved bromocresol purple method (Aqua-auto Kainos ALB Test Kit; KAINOS Laboratories, Inc., Tokyo, Japan), a latex turbidimeteric immunoassay (CRP-LATEX X2 “SEIKEN”; DENKA SEIKEN Co., Ltd., Tokyo, Japan), and a latex turbidimeteric immunoassay (LZ test “Eiken” β2-M; Eiken Chemical Co., Ltd., Tokyo, Japan), respectively. Plasma P-SEP was measured using an automated immunoanalyzer, STACIA® (LSI Medience Corporation, Tokyo, Japan), based on a chemiluminescence enzyme immunoassay [13].
Calculation of the Removal Ratio during HD Using Dialysis Membranes with a β2-MG Clearance ≥50 mL/min
We calculated the removal ratio (%) of P-SEP and β2-MG during HD as follows: [1 − {pre Ht (1 − post Ht/100) post C}/{post Ht (1 − pre Ht/100) pre C}] × 100, where pre C is the P-SEP or β2-MG level before HD, post C is the P-SEP or β2-MG level at 2 or 4 h after starting HD, pre Ht is the Ht before HD, and post Ht is the Ht at 2 or 4 h after starting HD [14].
Prediction of the Pre-HD P-SEP Levels Based on the Correction of the Hemoconcentration Using the Post-HD P-SEP Levels
The Pre-HD P-SEP levels were calculated as follows:
Pre-HD P-SEP level = post-HD P-SEP level/(1 + [BWpre – BWpost] / [0.2 × BWpost]), where BWpre and BWpost indicate the body weight (kg) pre- and post-HD, respectively [15].
Statistical Analysis
Continuous variables were described using the median (interquartile range [IQR]) or the mean ± standard deviation. Comparisons of group differences for continuous variables were made by the Mann-Whitney U test, the Wilcoxon signed-rank test, or the Kruskal-Wallis test with Steel-Dwass correction as appropriate. The regression equation between predicted and measured pre-HD P-SEP levels was calculated using the least-square method. Significance was set at p < 0.05 (two-tailed).
Results
Patient Characteristics and Dialysis Condition
Table 1 shows the comparison between the two groups (β2-MG clearance ≥50 mL/min and < 30 mL/min). There were no differences except in dialysis vintage, which was significantly shorter in the group with β2-MG clearance < 30 mL/min than in the ≥50 mL/min group (p < 0.05).
Table 1.
Comparison of the characteristics and the factors determining clearance during HD between enrolled patients dialyzed using high-flux and intermediate-flux membranes
| ≥50 mL/min | <30 mL/min | p value | |
|---|---|---|---|
| Patients, n | 8 | 10 | |
| Age, years | 63.0±13.7 | 67.0±12.5 | 0.5276 |
| Male, % | 83 | 80 | |
| Dialysis vintage, years | 5.0±5.1 | 1.0±0.5* | 0.0263 |
| Diabetes mellitus, % | 50 | 50 | |
| BMI | 21.9±3.6 | 24.6±4.0 | 0.1505 |
| DW, kg | 62.5±12.8 | 62.8±13.4 | 0.9574 |
| Serum albumin, g/dL | 3.6±0.3 | 3.4±0.2 | 0.1011 |
| White blood cells, /μL | 5,838±1,408 | 7,310±1,626 | 0.0602 |
| hsCRP, mg/dL | 0.06 (0.01–0.30) | 0.23 (0.17–0.84) | 0.1416 |
| Blood flow rate, mL/min | 200 | 200 | |
| Duration of HD, h | 4.0 | 4.0 | |
| UF, kg | 2.8±0.4 | 3.3±0.8 | 0.1634 |
| UF/DW, % | 4.6±1.0 | 5.3±1.0 | 0.2144 |
| KT/Vsp | 1.42±0.28 | 1.44±0.24 | 0.8834 |
High-flux membranes: β2-MG clearance ≥50 mL/min; intermediate-flux membranes: β2-MG clearance <30 mL/min. HD, hemodialysis; β2-MG, β2-microglobulin; BMI, body mass index; DW, dry weight; hsCRP, high-sensitivity C-reactive protein; UF, ultrafiltration; KT/Vsp, KT/V single pool.
p < 0.05, Student's t test.
Elimination of P-SEP during HD Similar to β2-MG in the Group with β2-MG Clearance ≥50 mL/min
Table 2 indicates the changes in the P-SEP and β2-MG levels during HD in the group with β2-MG clearance ≥50 mL/min. Although both P-SEP and β2-MG significantly decreased after 4 h of HD (p < 0.05 and p < 0.01, respectively), β2-MG significantly decreased also after 2 h after starting HD (p < 0.01). The removal ratios of P-SEP and β2-MG were 42.8 ± 7.9% and 55.7 ± 7.4% at 2 h after starting HD and 58.8 ± 18.4% and 69.1 ± 7.1% at 4 h after starting HD, respectively.
Table 2.
Changes in β2-MG and P-SEP levels during HD using high-flux dialysis membranes with a β2-MG clearance ≥50 mL/min
| 0 h | 2 h | 4 h | R (2 h) | R (4 h) | |
|---|---|---|---|---|---|
| β2-MG, mg/L | 26.3 (24.6–28.4) | 12.6 (10.5–15.2)** | 9.1 (7.7–11.5)** | 55.7±7.4 | 69.1±7.1 |
| P-SEP, pg/mL | 1,295 (988–1,928) | 801 (684–1,108) | 667 (463–750)* | 42.8±7.9 | 58.8±18.4 |
| Ht, % | 36.1±3.5 | 38.3±3.5 | 39.9±3.2 |
Blood samples were collected before HD (0 h) and at 2 or 4 h after starting HD. The membranes used in the present study were a Hollow fiber dialyzer FD series (FDY-180GW; polyester polymer alloy [PEPA®]; class IV; NIKKISO Co., Ltd., Tokyo, Japan) (n = 2), a Triacetate hollow fiber dialyzer FB-eco series (FB-190Pβeco; cellulose triacetate [CTA]; class IV; Nipro Co., Ltd, Osaka, Japan) (n = 2), a Toraylight® NV (NV-21X; polysulfone [PS]; class V; TORAY Industries, Inc., Tokyo, Japan) (n = 2), and a PEPA dialyzer FDW (FDW-18; [PEPA®]; class V; NIKKISO Co., Ltd.) (n = 2). β2-MG, β2-microglobulin; P-SEP, presepsin; HD, hemodialysis; R, removal ratio; Ht, hematocrit.
p < 0.05 versus 0 h
p < 0.01 versus 0 h, Kruskal-Wallis test with Steel-Dwass correction.
The Retaining of P-SEP Levels in the Blood during HD Using a Dialysis Membrane with a β2-MG Clearance < 30 mL/min
Table 3 shows the β2-MG and P-SEP levels in the blood samples obtained from the inflow and outflow side of the dialyzer at 0.5 h after starting HD, and at 2 and 4 h after starting HD with a β2-MG clearance ≥50 mL/min (NV-15X and NV-21X) and < 30 mL/min (PES-15Mαeco and PES-21Mαeco), with patients 4 and 9 as the individual patients. The levels of β2-MG and P-SEP decreased during the passage of the dialyzer and subsequently decreased at 2 and 4 h after starting HD with a β2-MG clearance ≥50 mL/min. In contrast, the levels of β2-MG and P-SEP did not decrease during dialyzer passage and subsequently increased at 2 and 4 h after starting HD with a β2-MG clearance < 30 mL/min.
Table 3.
Comparison of the behavior of β2-MG and P-SEP levels during HD using dialysis membranes with a β2-MG clearance ≥50 mL/min and <30 mL/min
| Patient | Dialysis membrane | Substance | 0.5 h |
2 h | 4 h | |
|---|---|---|---|---|---|---|
| IN | OUT | |||||
| 4 | NV-21X | β2-MG | 19.3 | 5.7 | 14.4 | 11.1 |
| P-SEP | 881 | 483 | 781 | 762 | ||
| 4 | PES-21Mαeco | β2-MG | 22.3 | 24.3 | 24.3 | 27.6 |
| P-SEP | 1,040 | 1,170 | 1,150 | 1,330 | ||
| 9 | NV-15X | β2-MG | 21.0 | 10.0 | 14.1 | 11.1 |
| P-SEP | 2,030 | 1,390 | 1,450 | 1,300 | ||
| 9 | PES-15Mαeco | β2-MG | 29.2 | 31.0 | 32.5 | 36.0 |
| P-SEP | 2,640 | 2,710 | 2,960 | 3,190 | ||
β2-MG values are mg/L; P-SEP values are pg/mL. Individual data of HD patients 4 and 9 are included. Blood samples were collected at 0.5, 2, and 4 h after starting HD. Blood samples were collected from either the inflow side (IN) or the outflow side (OUT) of the dialyzer at 0.5 h after starting HD. NV-15 and NV-21X are dialysis membranes with a β2-MG clearance ≥50 mL/min. PES-15Mαeco and PES-21Mαeco are dialysis membranes with a β2-MG clearance <30 mL/min. β2-MG, β2-microglobulin; P-SEP, presepsin; HD, hemodialysis.
Adsorption of P-SEP by the Dialysis Membrane Made of EVAL with a β2-MG Clearance < 30 mL/min
Table 4 shows the β2-MG and P-SEP levels in the blood samples obtained from the inflow and outflow side of the dialyzer at 0.5 h after starting HD, and at 2 and 4 h after starting HD using a dialysis membrane, KF-15C (ethylene vinylalcohol [EVAL]), with both a β2-MG clearance < 30 mL/min and a high protein adsorption capacity. The levels of β2-MG and P-SEP slightly decreased or increased during the passage of the dialyzer, and subsequently slightly decreased at 2 and 4 h after starting HD.
Table 4.
Behavior of β2-MG and P-SEP levels during HD using dialysis membranes with both a β2-MG clearance <30 mL/min and a high protein adsorption capacity in 2 patients
| Dialysis membrane | Substance | 0.5 h |
2 h | 4 h | |
|---|---|---|---|---|---|
| IN | OUT | ||||
| KF-15C | β2-MG | 35.9 | 34.6 | 34.4 | 33.6 |
| P-SEP | 1,460 | 1,510 | 1,450 | 1,380 | |
| KF-15C | β2-MG | 16.0 | 14.3 | 14.7 | 14.1 |
| P-SEP | 933 | 807 | 925 | 868 | |
β2-MG values are mg/L; P-SEP values are pg/mL. Blood samples were collected at 0.5, 2, and 4 h after starting HD. Blood samples were collected from either the inflow side (IN) or the outflow side (OUT) of the dialyzer at 0.5 h after starting HD. The membrane used in the present study was a Hollow fiber dialyzer KF-201 (KF-15C; ethylene vinylalcohol [EVAL®]; class II; Kawasumi Laboratories, Inc.). β2-MG, β2-microglobulin; P-SEP, presepsin; HD, hemodialysis.
Prediction of the Pre-HD P-SEP Levels Based on the Correction of Hemoconcentration Using the Post-HD P-SEP Levels
Table 5 shows the individual data of HD patients with a β2-MG clearance < 30 mL/min (patients 4 and 9 were also indicated in Table 3). Post-HD P-SEP levels (2,010 [1,225–2,415] pg/mL) were higher than pre-HD P-SEP levels (1,305 [814–1,858] pg/mL) (p < 0.01). Body weight decreased from 66.9 ± 13.7 kg to 63.6 ± 13.1 kg (p < 0.01), resulting in a decrease of 5.5 ± 0.8% compared to dry weight. The predicted pre-HD P-SEP levels (1,522 [1,024–1,972] pg/mL), which were obtained based on the correction of hemoconcentration, remained slightly higher than the measured pre-HD levels (p < 0.01). The predicted pre-HD P-SEP levels were positively correlated with the measured pre-HD levels (R2 = 0.9562) using the regression equation: y = 1.0987x. The predicted pre-HD P-SEP levels were 10% higher than the measured pre-HD levels (Fig. 1).
Table 5.
Levels of P-SEP obtained with pre-HD and post-HD blood samples, and the predicted pre-HD P-SEP levels using the correction of hemoconcentration
| Patient | Measured pre-HD | Predicted pre-HD | Measured post-HD | BWpre | BWpost | ([BWpre - BWpost]/DW) × 100, % |
|---|---|---|---|---|---|---|
| 1 | 586 | 774 | 987 | 50.0 | 47.4 | 5.5 |
| 2 | 715 | 1008 | 1,190 | 59.4 | 56.1 | 5.9 |
| 3 | 783 | 787 | 1,000 | 60.5 | 57.4 | 5.7 |
| 4 | 906 | 1,073 | 1,330 | 101.3 | 96.7 | 4.7 |
| 5 | 1,280 | 1,408 | 1,910 | 73.6 | 68.7 | 7.4 |
| 6 | 1,330 | 1,636 | 2,110 | 59.4 | 56.1 | 5.9 |
| 7 | 1,520 | 1,817 | 2,280 | 66.0 | 62.8 | 5.2 |
| 8 | 1,970 | 2,024 | 2,460 | 68.0 | 65.2 | 4.3 |
| 9 | 2,440 | 2,690 | 3,390 | 64.7 | 61.5 | 5.2 |
| 10 | 2,440 | 2,496 | 3,120 | 59.0 | 56.2 | 5.0 |
| Total | 1,305 (814–1,858) | 1,522 (1,024–1,972)** | 2,010 (1,225–2,415)** | 66.9±13.7 | 63.6±13.1*** | 5.5±0.8 |
P-SEP values are pg/mL; BW and DW values are kg. HD was performed using a membrane with a β2-MG clearance <30 mL/min (PES-15Mαeco or PES-21Mαeco). The pre-HD P-SEP level was predicted based on the correction of the post-HD value using the following formula: predicted pre-HD P-SEP = measured post-HD P-SEP/(1 + [BWpre – BWpost]/[0.2 × BWpost]). P-SEP, presepsin; HD, hemodialysis; BWpre, body weight before HD; BWpost, BW after HD; DW, dry weight.
p < 0.01 versus measured pre-HD, Wilcoxon signed-rank test
p < 0.01 versus BWpre, Student's t test.
Fig. 1.
Scatter plot with the best-fit line between the predicted pre-HD P-SEP levels and the pre-HD P-SEP levels. The pre-HD P-SEP levels appeared to be fairly predictable based on the correction of hemoconcentration using the post-HD P-SEP levels. HD, hemodialysis; P-SEP: presepsin.
Discussion
In the group with β2-MG clearance ≥50 mL/min, a significant decrease in P-SEP was only noted between pre-HD and 4 h after starting HD, while that of β2-MG was verified between pre-HD and 2 and 4 h after starting HD. These findings indicate that the elimination of β2-MG appears to be more aggressive than that of P-SEP, based on their molecular weights (β2-MG: 11,800 Da; P-SEP: 13,000 Da). Despite P-SEP having a greater molecular weight than β2-MG, a considerable amount of P-SEP appeared to be eliminated through high-flux dialysis membranes. The removal ratio (between pre-HD and 4 h after starting HD) of P-SEP (58.8 ± 18.4%) using class IV and V dialyzers was greater than that (37.7 ± 13.1%) between pre-HD and 4 h after starting HD using only class IV dialyzers [16], suggesting the molecular weight dependence of P-SEP elimination. Therefore, the P-SEP levels during HD using high-flux dialysis membranes could not substitute for pre-HD P-SEP levels. On the contrary, the P-SEP level increased during HD in all patients with a β2-MG clearance < 30 mL/min (Table 5). Because P-SEP and β2-MG were removed by high-flux membranes that had been developed to eliminate β2-MG (Table 2), we suspected that the protein binding of P-SEP was negligible. Therefore, the increase in the P-SEP levels when using dialysis membranes with a β2-MG clearance < 30 mL/min appears to be dependent on the molecular weight (13,000 Da). Herget-Rosenthal et al. [17] also reported that the levels of PCT (molecular weight, 13,000 Da) did not decrease during HD using a low-flux membrane.
It has previously been reported that both P-SEP and PCT were superior to hsCRP for diagnosing sepsis in patients who underwent surgery for acute abdomen [1]; thus P-SEP might also be useful for detecting an exacerbating localized infection [5]. Therefore, P-SEP is expected to be useful for diagnosing early sepsis. In order to emphasize the merits of P-SEP, we should make the best use of not only the blood samples collected at pre-HD but also those obtained when early sepsis is suspected. In doing so, we will be able to start the administration of antibiotics and/or to consider surgical treatment such as drainage, debridement, or surgical operation without waiting for the next HD session (48–72 h later).
Due to our suspicions that the hemoconcentration influenced the increase in P-SEP during HD with a β2-MG clearance < 30 mL/min, we corrected the post-HD P-SEP levels for predicting the pre-HD P-SEP levels using a method based on changes in the ultrafiltration (obtained by the change of body weight) [15]. In clinical practice in HD outpatients, early sepsis is often suspected when HD is being performed. We must therefore estimate the body weight at that time in another way, but it is not necessarily difficult for skilled dialysis room staff to estimate the body weight based on the ultrafiltration rate and time course.
The predicted pre-HD P-SEP levels still increased even after correction for the hemoconcentration using the post-HD P-SEP levels (1,522 [1,024–1,972] pg/mL) compared to pre-HD P-SEP levels (1,305 [814–1,858] pg/mL), suggesting the potential for P-SEP load during HD procedure. Masuda and colleagues reported at “The 57th Annual Meeting of Japanese Society of Laboratory Medicine, Kobe, Japan” that the P-SEP level using plasma prepared by allowing a sample to stand for 5 min followed by centrifugation for 5 min and the P-SEP level using plasma prepared by mixing thoroughly with gentle inversion for 5 min followed by centrifugation for 5 min were 111.5 and 729.7 pg/mL, respectively, in 3 healthy individuals. Therefore, we must consider the possibility of stimulated P-SEP release from monocytes in the blood circuit compressed by the roller pump during HD, which may resemble a blood mixing procedure.
In the present study, we investigated the PES membrane (class I and II) in the group with β2-MG clearance < 30 mL/min. Because the dialysis membranes of classes IV and V are also included in Nipro's PES membrane lineup, the increase in P-SEP level in class I and II membranes might not necessarily be due to the material (PES) itself but rather to the chemical composition, morphology, and geometry [18]. Since April 2017 in Japan, the dialysis membranes (EVAL and polymethylmethacrylate [PMMA]) with a high protein adsorption capacity were newly classified as class S [19]. In the present study, the levels of β2-MG and P-SEP slightly decreased during HD using a dialysis membrane made of EVAL with a β2-MG clearance < 30 mL/min despite the absence of apparent decrease of the β2-MG and P-SEP levels during dialyzer passage as is the case with a β2-MG clearance ≥50 mL/min (Tables 3, 4), suggesting that the decrease of β2-MG and P-SEP level is mainly due to protein adsorption and the decrease is offset by the hemoconcentration. Therefore, P-SEP appears again not to be eliminated during HD with a β2-MG clearance < 30 mL/min.
The predicted pre-HD P-SEP levels were estimated to be 10% higher than the measured pre-HD levels in the present study. On the other hand, the pre-HD P-SEP levels in the HD patients using membrane with β2-MG clearance < 30 mL/min were expected to be higher than those using membrane with β2-MG clearance ≥50 mL/min. These aspects were expected to result in an increase of sensitivity and a decrease of false-negative rate for detecting sepsis early. We therefore purposely applied dialysis membranes with β2-MG clearance < 30 mL/min (such as PES-15Mαeco or PES-21Mαeco) so as not to “rule out” but to “rule in” early sepsis. The target HD patients are those with advanced age, aspiration pneumonia, pyogenic spondylitis, critical limb illness, infectious endocarditis, and immunosuppressive agent administration.
Our study has several limitations. First, the number of patients was small. Second, the patients did not have infection or inflammation. A fundamental issue at hand is that the threshold values of P-SEP levels of HD patients have not yet been established not only in sepsis but also for noninfectious conditions [6]. To enhance the value of P-SEP as an early sepsis biomarker, we must diligently accumulate more data on P-SEP values not only in cases of sepsis but also in cases of focal infections that progress to sepsis. Thereby, we hope to be able to predict the pre-HD P-SEP levels without waiting for the next HD session when early sepsis is suspected, so a dialysis membrane with a β2-MG clearance < 30 mL/min might be useful for increasing our opportunities to obtain data on an infection in HD patients. In addition, we must collect data on both pre- and mid-HD P-SEP levels in order to establish a more reliable regression equation between predicted pre-HD P-SEP levels and measured pre-HD P-SEP levels.
In conclusion, the levels of P-SEP with a molecular weight near that of β2-MG decreased similarly to those of β2-MG during HD using membranes with a β2-MG clearance ≥50 mL/min. On the contrary, the levels of P-SEP rather increased during HD with a β2-MG clearance < 30 mL/min, suggesting that P-SEP appeared not to be eliminated. Furthermore, the pre-HD P-SEP levels might be predictable by the correction of hemoconcentration using even the blood samples collected after starting HD with a β2-MG clearance < 30 mL/min (PES 15Mαeco and PES-21Mαeco), although the predicted pre-HD P-SEP levels were nearly 10% higher than the measured pre-HD values.
Statement of Ethics
The study was approved by our institution's Institutional Review Board. Written informed consent was obtained from all patients.
Disclosure Statement
The author declares no conflict of interest. There was no funding for the present study.
Acknowledgments
The author thanks all the stuff members working at Tsunashima Kidney Clinic. The author also thanks Japan Medical Communication (Fukuoka, Japan) for editing the manuscript for its English content.
References
- 1.Vodnik T, Kaljevic G, Tadic T, Majkic-Singh N. Presepsin (sCD14-ST) in preoperative diagnosis of abdominal sepsis. Clin Chem Lab Med. 2013;51:2053–2062. doi: 10.1515/cclm-2013-0061. [DOI] [PubMed] [Google Scholar]
- 2.Nishida O, Ogura H, Egi M, Fujishima S, Hayashi Y, Iba T, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock (J-SSCG 2016) J Intensive Care. 2018;6:7–77. doi: 10.1186/s40560-017-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother. 2015;21:564–569. doi: 10.1016/j.jiac.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Hayashida K, Kondo Y, Hara Y, Aihara M. Head-to-head comparison of procalcitonin and presepsin for the diagnosis of sepsis in critically ill adult patients: a protocol for a systematic review and meta-analysis. BMJ Open. 2017;7:e014305. doi: 10.1136/bmjopen-2016-014305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiota J, Tagawa H, Ohura N, Kasahara H. Presepsin is a potent biomarker for diagnosing skin wound infection in hemodialysis patients compared to white blood cell count, high-sensitivity C-reactive protein, procalcitonin, and soluble CD14. Ren Replace Ther. 2017;3:31. [Google Scholar]
- 6.Nagata T, Yasuda Y, Ando M, Abe T, Katsuno T, Kato S, Tsuboi N, Matsuo S, Maruyama S. Clinical impact of kidney function on presepsin levels. PLoS One. 2015;10:e0129159. doi: 10.1371/journal.pone.0129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y, Ishikura H, Nishida T, Kawano Y, Yuge R, Ichiki R. Usefulness of presepsin in the diagnosis of sepsis in patients with or without acute kidney injury. BMC Anesthesiology. 2014;14:88. doi: 10.1186/1471-2253-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horl WH. Hemodialysis membranes: Interleukins, biocompatibility, and middle molecules. J Am Soc Nephrol. 2002;13:S62–S71. [PubMed] [Google Scholar]
- 9.Agrawal S, Gollapudi P, Elahimehr R, Pahl MV, Vaziri ND. Effect of end-stage renal disease and haemodialysis on dendritic cell subset and basal and LPS-stimulated cytokine production. Nephrol Dial Transplant. 2010;25:746. doi: 10.1093/ndt/gfp580. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita AC. Mass transfer mechanisms in high-performance membrane dialyzers. Contrib Nephrol. 2011;173:95–102. doi: 10.1159/000328946. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe Y, Kawanishi H, Suzuki K, Nakai S, Tsuchida K, Tabei K, et al. “Maintenance Hemodialysis Hemodialysis Prescriptions” Guideline Working Group, Japanese Society for Dialysis Therapy: Japanese society for dialysis therapy clinical guideline for “Maintenance hemodialysis: hemodialysis prescriptions”. Ther Apher Dial. 2015;19((suppl 1)):67–92. doi: 10.1111/1744-9987.12294. [DOI] [PubMed] [Google Scholar]
- 12.Abe M, Hamano T, Wada A, Nakai S, Masakane I, Renal Data Registry Committee Japanease Society for Dialysis Therapy Effect of dialyzer membrane materials on survival in chronic hemodialysis patients: results from the annual survey of the Japanese Nationwide Dialysis Registry. PLoS One. 2017;12:e0184424. doi: 10.1371/journal.pone.0184424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamura Y, Yokoi H. Development of a point-of care assay system for measurement of presepsin (sCD14-ST) Clin Chim Acta. 2011;412:2157–2161. doi: 10.1016/j.cca.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Kawanishi H, Mineshima M, Hirakata H, Akizawa T. Performance evaluation method for blood purifier (in Japanese) J Jpn Soc Dial Ther. 2012;45:435–445. [Google Scholar]
- 15.Bergstrom J, Wehle B. No change in corrected beta 2-microglobulin concentration after cuprophane haemodialysis. Lancet. 1987;1:628–629. doi: 10.1016/s0140-6736(87)90266-2. [DOI] [PubMed] [Google Scholar]
- 16.Imagawa A, Uozumi E, Shiota Y, Shiraishi R, Ikezawa A, Morita S. Presepsin level in renal dysfunction and hemodialysis patients (in Japanese) Med Technol. 2015;64:169–172. [Google Scholar]
- 17.Herget-Rosenthal S, Marggraf G, Pietruck F, Husing J, Strupat M, Philipp T, Kribben A. Procalcitonin for accurate detection of infection in haemodialysis. Nephrol Dial Transplant. 2001;16:975–979. doi: 10.1093/ndt/16.5.975. [DOI] [PubMed] [Google Scholar]
- 18.Ouseph R, Hutchison CA, Ward RA. Differences in solute removal by two high-flux membranes of nominally similar synthetic polymers. Nephrol Dial Transplant. 2008;23:1704–1712. doi: 10.1093/ndt/gfm916. [DOI] [PubMed] [Google Scholar]
- 19.Kawanishi H, Mineshima M, Tomo M, Minakuchi J. Functional classification of blood purifier (in Japanese) J Jpn Soc Dial Ther. 2013;46:501–506. [Google Scholar]