Abstract
Reinforcement learning has achieved tremendous success in recent years, notably in complex games such as Atari, Go, and chess. In large part, this success has been made possible by powerful function approximation methods in the form of deep neural networks. The objective of this paper is to introduce the basic concepts of reinforcement learning, explain how reinforcement learning can be effectively combined with deep learning, and explore how deep reinforcement learning could be useful in a medical context.
Keywords: Artificial intelligence, Reinforcement learning, Deep learning
Introduction
Reinforcement learning (RL) algorithms have experienced unprecedented success in the last few years, reaching human-level performance in several domains, including Atari video games [1] or the ancient games of Go [2] and chess [3]. This success has been largely enabled by the use of advanced function approximation techniques in combination with large-scale data generation from self-play games. The aim of this paper is to introduce basic RL algorithms and describe state-of-the-art extensions of these algorithms to deep learning. We also discuss the potential of RL in medicine and review the literature to study existing practical applications of RL. Even though RL offers several benefits in comparison to other artificial intelligence (AI) techniques, such as the ability to optimize long-term benefit to patients rather than immediate benefit, there are a number of obstacles that have to be overcome in order to apply RL on a large scale.
Reinforcement Learning
RL is an area of machine learning concerned with sequential decision problems [4]. Concretely, a learner or agent interacts with an environment by taking actions, and the aim of the agent is to maximize its expected cumulative reward. Each action affects the next, and the agent cannot simply maximize the immediate reward that it will get, but rather has to plan ahead and select actions that will maximize reward in the long run.
Notation: Given a finite set X, a probability distribution on X is a vector µ ∈ RX whose elements are non-negative (i.e., µ(x) ≥ 0 for each x ∈ X) and whose sum equals 1 (i.e., Σxµ(x) = 1). We use Δ(X) = {µ ∈ RX: Σxµ(x) = 1, µ(x) ≥ 0 (∀x)} to denote the set of all such probability distributions.
Markov Decision Processes
Sequential decision problems are usually modelled mathematically as Markov decision processes, or MDPs. An MDP is a tuple M = (S, A, P, r), where
S is the finite state space,
A is the finite action space,
P: S × A → Δ(S) is the transition function, with P (s′ ∣ s, a) denoting the probability of moving to state s′ when taking action a in state s,
r: S × A → R is the reward function, with r (s, a) denoting the expected reward obtained when taking action a in state s.
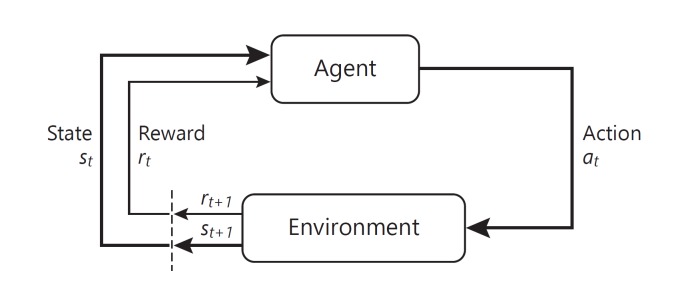
Intuitively, the agent controls which action to select, while the environment controls the outcome of each action. In each round t, the agent observes a state st ∈ S and selects an action at ∈ A. As a result, the environment returns a new state st + 1 ∼ P (• ∣ st, at) and reward rt+ 1 ∼ r (st, at). This process is illustrated in Figure 1. By repeating the procedure for t = 0, 1, 2, ... the result is a trajectory
The aim of the agent is to select actions as to maximize some measure of expected cumulative reward. The most common reward criterion is the discounted cumulative reward
where γ ∈ (0, 1] is a discount factor which ensures that the sum remains bounded.
Fig. 1.
Illustration of the agent-environment interface [4].
The decision strategy of the agent is represented by a policy π: S → Δ (A), with π (a | s) denoting the probability that the agent selects action a in state s. For each policy π, we can define an associated value function Vπ, which measures how much expected reward the agent will accumulate from a given state when acting according to π. Specifically, the value in state s is defined as
The values of consecutive states satisfy a recursive relationship called the Bellman equations:
As an alternative to Vπ, we can instead define an action-value function Qπ, which measures how much expected reward the agent will accumulate from a given state when taking a specific action and then acting according to π. The action-value for state s and action a is defined as
There is a straightforward relationship between the value function Vπ and action-value function Qπ:
Consequently, the Bellman equations can be stated either for Vπ or for Qπ.
We can also define the optimal value function V* as the maximum amount of expected reward that an agent can accumulate from a given state. The optimal value function in state s is given by V* (s) = maxπVπ (s), i.e., the maximum value among the individual policies. The optimal policy π* is the policy that attains the maximum value in each state s, i.e., π* (• ∣ s) = arg maxπ Vπ (s). The optimal values of consecutive states satisfy the optimal Bellman equations
Just as before, we can instead define the optimal action-value function Q*, which is related to V* as follows:
RL Algorithms
Most RL algorithms work by maintaining an estimate πˆ of the optimal policy, an estimate Vˆ of the optimal value function, and/or an estimate Qˆ of the optimal action-value function. If the transition function P and reward function r are known, πˆ and Vˆ can be estimated directly. Specifically, from the Bellman equations we can derive a Bellman operator Tπ which can be applied to a value function Vˆ to produce a new value function TπVˆ defined as
Likewise, from the optimal Bellman equations we can derive an optimal Bellman operator T* that, when applied to a value function Vˆ, is defined as
Value iteration works by repeatedly applying the optimal Bellman operator T* to an initial value function estimate Vˆ0:
If the value of each state is stored in a table, value iteration is guaranteed to eventually converge to the optimal value function V*.
Policy iteration instead starts with an initial policy estimate πˆ0 and alternates between a policy estimation step and a policy improvement step. In the policy estimation step, we simply estimate the value function Vˆπˆk associated with the current policy πˆk. To do so, we can repeatedly apply the Bellman operator Tπˆk to an initial value function estimate Vˆ0:
In the policy improvement step, we update the policy such that it is greedy with respect to the value function estimate Vˆπˆk:
If the value of each state is stored in a table, policy iteration is also guaranteed to eventually converge to the optimal value function V*.
If the transition function P and reward function r are unknown, we have to resort to different techniques. In this case, πˆ, Vˆ, and Qˆ have to be estimated from transitions on the form st, at, rt+ 1, st+ 1. Unlike value iteration and policy iteration, which update the values of all states in each iteration, temporal difference (TD) methods update the value of a single state for a given transition. The most popular TD method is Q-learning [5], which maintains an estimate Qˆt of the optimal action-value function, which is updated after each transition (st, at, rt+ 1, st+ 1). The new estimate Qˆt+ 1 is identical to Qˆt for each state-action pair different from (st, at), while Qˆt+ 1 (st, at) is given by
Here, αt is a learning rate. If the value of each state-action pair is stored in a table and αt is appropriately tuned, Q-learning is guaranteed to eventually converge to the optimal action-value function Q*, even if the transition function P and reward function r are unknown.
Deep RL
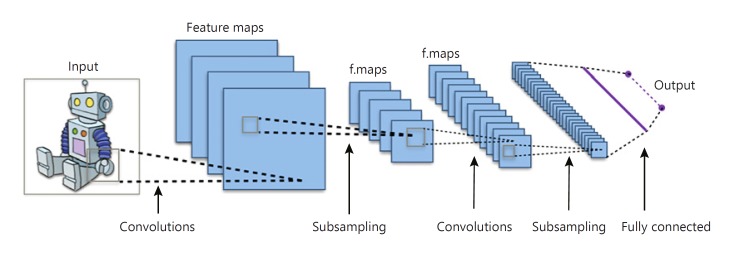
In most realistic domains, the state space S is too large to store the estimated value function Vˆ in a table. In this case, it is common to parameterize Vˆθ (or πˆθ, Qˆθ) on some parameter vector θ. The value in a state is completely determined by the current parameters in θ, and the update rules for RL algorithms are modified such that they no longer update the values of states directly, but rather the parameters in θ. In deep RL, Vˆθ (or πˆθ, Qˆθ) is represented using a deep neural network, with θ being the parameters of the network. When the input is an image, a convolutional neural network is typically deployed, such as the one in Figure 2.
Fig. 2.
Convolutional neural network. By Aphex 34 (CC BY-SA 4.0 [https://creativecommons.org/licences/by-sa/4.0]), from Wikimedia Commons.
A deep Q network, or DQN [1], is a deep neural network that estimates the action-value function Qˆθ. Given a transition st, at, rt+ 1, st+ 1, the parameters θ of the neural network are updated as to minimize the Bellman error
In order to prevent overfitting, the algorithm performs a technique called experience replay in which many transitions are stored in a database. In each iteration, a number of transitions are sampled at random from the database in order to update the network parameters θ.
Asynchronous advantage actor-critic, or A3C [6], maintains both an estimate πˆθ of the policy (the actor) and an estimate Vˆϕ of the value function (the critic). Given a transition (st, at, rt+ 1, st+ 1), the parameter vector θ of πˆθ is updated using the regularized policy gradient rule
where Aˆϕ (st, at) is an estimate of the advantage function
H(πˆθ (• ∣ st)) is the entropy of the policy πˆθ in state st, and the parameter β controls the amount of regularization. The stability of the algorithm increases by using n-step returns, i.e., reward accumulated during n consecutive transitions. Moreover, the vectors θ and ϕ often share parameters, e.g., in a neural network setup all non-output layers are shared, and only the output layers differ for πˆθ and Vˆϕ.
AlphaZero [3, 7] also maintains both a policy estimate πˆθ and a value estimate Vˆϕ. The parameters are not updated using the policy gradient rule; rather, the algorithm performs Monte-Carlo tree search (MCTS) to estimate a target action distribution p(•) given by the empirical visitation count of each branch of the search tree (MCTS also determines which action at to perform next). The parameters are then updated using the loss function
where Rt is the observed return from state st, and β again controls the amount of regularization.
RL in Medicine
Many decision problems in medicine are by nature sequential. When a patient visits a doctor, the doctor has to decide which treatment to administer to the patient. Later, when the patient returns, the treatment previously administered affects their current state, and consequently the next decision regarding future treatment. This type of decision problem can be effectively modelled as an MDP and solved using RL algorithms.
In most AI systems implemented in medicine, the sequential nature of decisions is ignored, and the systems instead base their decisions exclusively on the current state of patients. RL offers an attractive alternative to such systems, taking into account not only the immediate effect of treatment, but also the long-term benefit to the patient.
In spite of the potential of RL in medicine, there are a number of obstacles that have to be overcome in order to apply RL algorithms in the hospital. RL algorithms typically learn by trial-and-error, but submitting patients to exploratory treatment strategies is of course not an option in practice. Instead, RL algorithms would have to learn from existing data collected using fixed treatment strategies. This process is called off-policy learning and will play an important role in practical RL algorithms, especially in a medical setting.
Another important issue is to establish what the reward should be, which in turn determines the behavior of the optimal policy. Defining an appropriate reward function involves weighing different factors against each other, such as the monetary cost of a given treatment versus the life expectancy of a patient. This dilemma is not exclusive to RL, however, and is already being discussed on a large scale in different countries.
There exist several examples of medical RL applications in the literature. RL has been used to develop treatment strategies for epilepsy [8] and lung cancer [9]. An approach based on deep RL was recently proposed for developing treatment strategies based on medical registry data [10]. Deep RL has also been used to learn treatment policies for sepsis [11].
In nephrology, the problem of anemia treatment in hemodialysis patients is particularly well-suited to model as a sequential decision-making problem. A common treatment for patients with chronic kidney disease are erythropoiesis-stimulating agents (ESAs), but the effects of ESAs are unpredictable, making it necessary to closely monitor the patient's condition. At regular time intervals, the medical team has to decide what action to take, and in turn, this action will affect the patient's condition in the future. Several authors have proposed using RL to control the administration of ESAs [12, 13].
Statement of Ethics
The author has no ethical conflicts to disclose.
Disclosure Statement
The author has no conflicts of interest to declare.
Acknowledgements
This work is partially funded by the grant TIN2015-67959 of the Spanish Ministry of Science.
References
- 1.Mnih V, Kavukcuoglu K, Silver D, Rusu AA, Veness J, Bellemare MG, et al. Human-level control through deep reinforcement learning. Nature. 2015 Feb;518((7540)):529–33. doi: 10.1038/nature14236. [DOI] [PubMed] [Google Scholar]
- 2.Silver D, Huang A, Maddison CJ, Guez A, Sifre L, van den Driessche G, et al. Mastering the game of Go with deep neural networks and tree search. Nature. 2016 Jan;529((7587)):484–9. doi: 10.1038/nature16961. [DOI] [PubMed] [Google Scholar]
- 3.Silver David, Hubert Thomas, Schrittwieser Julian, Antonoglou Ioannis, Lai Matthew, Guez Arthur, Lanctot Marc, Sifre Laurent, Kumaran Dharshan, Graepel Thore, Lillicrap Timothy, Simonyan Karen, Hassabis Demis. Mastering Chess and Shogi by Self-Play with a General Reinforcement Learning Algorithm. 12. 2017 doi: 10.1126/science.aar6404. [DOI] [PubMed] [Google Scholar]
- 4.Sutton RS, Barto AG. Introduction to Reinforcement Learning. 1st ed. Cambridge (MA): MIT Press; 1998. [Google Scholar]
- 5.Watkins CJ, Dayan P, Q-learning In Machine Learning. 1992:pages 279–292. [Google Scholar]
- 6.Mnih Volodymyr, Badia Adri Puigdomnech, Mirza Mehdi, Graves Alex, Lillicrap Timothy P, Harley Tim, Silver David, Kavukcuoglu Koray. Asynchronous Methods for Deep Reinforcement Learning. arXiv. 2016;48:1–28. [Google Scholar]
- 7.Silver D, Schrittwieser J, Simonyan K, Antonoglou I, Huang A, Guez A, et al. Mastering the game of Go without human knowledge. Nature. 2017 Oct;550((7676)):354–9. doi: 10.1038/nature24270. [DOI] [PubMed] [Google Scholar]
- 8.Pineau J, Guez A, Vincent R, Panuccio G, Avoli M. Treating epilepsy via adaptive neurostimulation: a reinforcement learning approach. Int J Neural Syst. 2009 Aug;19((4)):227–40. doi: 10.1142/S0129065709001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Zeng D, Socinski MA, Kosorok MR. Reinforcement learning strategies for clinical trials in nonsmall cell lung cancer. Biometrics. 2011 Dec;67((4)):1422–33. doi: 10.1111/j.1541-0420.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Logan B, Liu N, Xu Z, Tang J, Wang Y. Deep reinforcement learning for dynamic treatment regimes on medical registry data. In 2017 IEEE International Conference on Healthcare Informatics (ICHI) 2017 Aug;:pages 380–385. doi: 10.1109/ICHI.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu Aniruddh, Komorowski Matthieu, Ahmed Imran, Celi Leo A., Szolovits Peter, Ghassemi Marzyeh. Deep reinforcement learning for sepsis treatment. CoRR. 2017 [Google Scholar]
- 12.Escandell-Montero P, Chermisi M, Martínez-Martínez JM, Gómez-Sanchis J, Barbieri C, Soria-Olivas E, et al. Optimization of anemia treatment in hemodialysis patients via reinforcement learning. Artif Intell Med. 2014 Sep;62((1)):47–60. doi: 10.1016/j.artmed.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Martín-Guerrero JD, Gomez F, Soria-Olivas E, Schmidhuber J, Climente-Martí M, Jiménez- Torres NV. A reinforcement learning approach for individualizing erythropoietin dosages in hemodialysis patients. Expert Syst Appl. 2009;36((6)):9737–9742. [Google Scholar]