Abstract
Physical activities during and after cancer treatment have favorable psychosocial effects. Increasingly, yoga has become a popular approach to improving the quality of life (QoL) of women with breast cancer. However, the extant synthetic evidence on yoga has not used other exercise comparison conditions. This meta-analysis aimed to systematically assess yoga-specific effects relative to any other physical exercise intervention (eg, aerobics) for women with breast cancer. QoL was the primary outcome of interest. Eight randomized controlled trials with 545 participants were included. The sample-weighted synthesis at immediate postintervention revealed marginally statistically and modest practically significant differences suggesting yoga’s potentially greater effectiveness: d = 0.14, P = .10. However, at longer term follow-up, no statistically or practically significant between-group difference was observed. This meta-analysis preliminarily demonstrated that yoga is probably as effective as other exercise modalities in improving the QoL of women with breast cancer. Both interventions were associated with clinically significant improvements in QoL. Nearly all of the yoga intervention programs, however, were very poorly resourced. Larger and better controlled trials of well-endowed yoga programs are needed.
Keywords: anxiety, breast cancer, complementary therapy, depression, evidence-based practice, exercise, fatigue, mental health, meta-analysis, quality of life, systematic review, yoga
Breast cancer is the most prevalent cancer among women worldwide. Each year more than 1.7 million women are diagnosed and more than 500 000 die from breast cancer, making it the leading cause of cancer death among women globally.1 But oncologic advancements in detection and treatment have contributed to remarkable increases in breast cancer survival. However, survivors may experience numerous adverse treatment effects that can occur during treatment or may be delayed and even persist for years after initial treatment.
Breast cancer survivors prevalently experience psychosocial distress associated with pain, fatigue, anxiety (fear of recurrence), depressive symptoms and challenges in diverse life space domains.2,3 Clearly, such psychosocial distress can adversely affect one’s quality of life (QoL). QoL refers to an individual’s overall well-being across a variety of psychosocial and associated physical domains of functioning.4 Thus, QoL may be thought a sentinel indicator of high-quality breast cancer care. Beyond mere survivorship such measures provide a window into the quality of that survival, that is, into women’s capacities to fully participate in and enjoy their lives during and after breast cancer treatments.
Fatigue seems a major contributor to reduced QoL. Prevalence studies of breast cancer-related fatigue have found that nearly all women with breast cancer reported experiencing some level of fatigue during their cancer treatments, and that more than half of them rated their levels of fatigue as moderate to severe.5 Furthermore, previous longer term studies of breast cancer survivors found that many women continued to experience persistent cancer-related fatigue that decreased their activity levels.5 And it has been shown that breast cancer survivors who experience diminished physical activity levels are at substantially increased risk of death.6
Physical activity has beneficial psychosocial effects at any stage of cancer treatment.7 Physical activity has been proposed as a nonpharmacologic intervention to improve psychological well-being and physical functioning and reduce fatigue across diverse cancers. Furthermore, physical activity after breast cancer treatment has been strongly associated with significant QoL improvements.8,9 A recent systematic review and meta-analysis of 60 randomized controlled trials (RCTs) found significant QoL improvements across diverse exercise regimes among women with breast cancer.10 It allowed for the aggregated estimate that three-quarters of the women in any exercise group had better postintervention QoLs than typically did otherwise similar women in usual care control groups.
Another synthesis of 14 RCTs found physical activity to be a safe and relatively low-cost alternative to traditional, psychosocial, and/or pharmacological, interventions in the management of depressive symptoms among women with breast cancer.11 However, cancer-related pain and discomfort can be significant barriers to participation in exercise programs among women with breast cancer.12 Contrary to many forms of exercise, yoga is a highly adaptable activity. Specifically, yoga postures can be readily modified to accommodate various physical limitations unique to breast cancer patients and so may serve as a most favorable or best-fitting physical activity intervention for this population.6,13
Yoga refers to the spiritual, physical, and mental disciplines rooted in ancient Indian philosophy.14 Yoga was initially systemized in the Yoga Sutras of Patanjali, a collection of spiritual texts containing 8 interrelated components of yoga. These interrelated components outline behaviors that promote an ethical lifestyle. Yoga practice emphasizes the unity of the mind and body to encourage a state of equanimity and presence.15 Yoga practice is typically a combination of “asana” or physical postures done with controlled breathing and/or meditations. There are various traditional styles of yoga that involve physical postures, including Ashtanga, Hatha, Iyengar, and Sivananda. Contemporary yoga has been influenced by traditional yoga styles. However, they are typically practiced as a physical exercise regime meant to develop strength, flexibility and to promote relaxation and a sense well-being.
Yoga is one of the most prevalent complementary therapies used in breast cancer care.16 Yoga interventions have been commonly identified as positive adjuvant therapies for breast cancer. Furthermore, a growing body of evidence indicates that yoga substantially improves the QoL of women with breast cancer. A recent meta-analysis inferred that yoga interventions for women with breast cancer were highly effective in alleviating anxiety and depression, but especially in improving their overall QoL.17 Integrating the findings of these 16 RCTs, 8 of every 10 women with breast cancer who participated in a yoga intervention were estimated to have better overall health-related QoLs than comparable women in a control group. This synthetic finding was consistent with several previous primary studies and reviews of yoga’s effectiveness with women with breast cancer. Together they have suggested yoga practices to be low-risk, cost-effective ways to improve QoL as proxied by various interrelated psychosocial measures such as fatigue, anxiety and depression.18–21
Previous Research Syntheses of Yoga in Breast Cancer Care
Our initial overview of this field found multiple, previous systematic reviews and meta-analyses. One of the earliest of these synthesized 6 RCTs that compared yoga intervention groups with nonactive control groups.21 It estimated a modest positive effect of yoga on health-related QoL. Another contemporaneous synthesis of 12 trials compared yoga interventions with psychoeducational interventions or to no intervention.19 It estimated moderate to very large complementary effects of yoga on health-related QoL as well as on related alleviations of anxiety and depressive symptoms. In aggregate, 19 of every 20 of its yoga intervention participants scored better on standardized measures of anxiety and depression at follow-up than did its typical control group participant. Three more contemporaneous or more recent systematic reviews and or meta-analyses of 48 more RCTs essentially reached the same conclusions of moderately large to very large positive effects of yoga on health-related QoL, including the central interrelated measures of fatigue, anxiety, and depression.16,17,20 The clear majority of primary studies included in these syntheses used nonactive control conditions such as waiting lists or usual care conditions. Most typically, for example, women in such control groups received guideline-based breast cancer care without any complementary interventions. This field’s aggregate synthetic evidence, therefore, allows for the inference that the complementary effect of yoga is quite large in enhancing the QoL of women with breast cancer. But that is like the synthetic evidence on physical exercise more generally. Such exercise regimes, ranging from weight training to aerobic exercises, have also been observed to largely enhance the QoL of women with breast cancer.
Need for This Research Synthesis
The synthetic question about the yoga-specific enhancement of QoL among women with breast cancer remains unanswered. Given that diverse exercises, including yoga, are so beneficial for women with breast cancer, one may fairly wonder if yoga is any more beneficial than other types of exercise. This meta-analysis aims to answer that potentially, very practically, important question and advances the hypothesis that yoga is more effective than other complementary exercise interventions. Yuanqing Pan and her colleagues also, importantly we think, suggested that any such yoga effects are probably larger among women who had practiced yoga over longer periods of time.17 They suggested 3 months as a minimum such practice duration criterion. Beyond duration, we think that practice intensity and overall yoga program endowment (aggregate hours of practice) probably matter as well. The present meta-analysis, therefore, also aims to explore moderations of any observed yoga-specific effects in this field by the intensities and durations of yoga practices or their overall yoga program endowments.
Methods
Search Strategy
This study explored the hypothesis that yoga is more effective in enhancing the QoL of women with breast cancer than are other types of exercise. To do so we searched the following research databases for relevant studies until November 30, 2018: CINAHL, Cochrane Central Register of Controlled Trials, Google Scholar, Medline/PubMed, Proquest Dissertations and Theses, PsycINFO, Scopus, Social Services Abstracts, Social Work Abstracts, SPORTDiscus, and the Web of Science Conference Proceedings Indexes. Published peer-reviewed or so-called gray unpublished studies were eligible for inclusion to guard against publication bias.22,23 The following keyword search scheme was used: breast and (cancer or tumor or carcinoma or neoplasm) and (yoga or asana) and (psychosocial or quality of life or QoL or fatigue or anxiety or depression). We also searched the bibliographies of this field’s 5, previously cited, systematic reviews and or meta-analyses for eligible studies.
Data Extraction and Quality Assessment
Both reviewers abstracted study characteristics independently from full primary study articles. After discussion their agreement was 100%. The meta-analytic database included the following study characteristics: publication year and country; participants’ ages, breast cancer stages of patients or survivors, race/ethnicity and socioeconomic/health insurance statuses; intensity, duration and program endowment/total contact hours by type of yoga intervention and other exercise control groups; length of follow-up and attrition rates; type/validity of measure(s); and study analytic sample sizes and blinding. As for study quality and its potential relationship to study outcomes, to gain an individualized view of each, study quality items were assessed separately rather than computing study quality summary scores.24
Meta-Analysis
Cohen’s d-index was used as the main meta-analytic effect size statistic.25 In doing so, the primary studies’ diverse statistical outcomes were converted to a common metric to enable between-study comparisons. It can be calculated directly from study group means and standard deviations (d = M 1 − M 2 / ((SD 1 + SD 2) / 2) or can be derived from other parametric or nonparametric statistics.26 It should be noted that hypothetically supportive and counter-hypothetical effects were reported, respectively, as positive and negative ds. Primary study fixed effects (ds) were weighted by their inverse variances to ensure that larger, more precise studies influenced the synthesis more than smaller studies.27
As this turned out to be a near empty review of inadequately powered RCTs, combined statistical significance was estimated with 90% confidence intervals (CIs).26,28 A 90% CI that includes the null value of 0.00 indicates that the aggregate difference between all the 2 study groups was not statistically significant a P < .10. Each study contributed 2 data points in this meta-analysis; one each at immediate postintervention and at each primary study’s longest period of follow-up. Each effect distribution was then tested for heterogeneity with Cochran’s Q statistic.26,29 With a chi square (χ2) distribution, it tested if the variability of effects within each follow-up period was greater than could have been expected by sampling error alone. It should also be noted that we ensured that each study contributed only once to each meta-analysis: immediate postintervention and longer follow-up. When multiple outcomes based on different psychosocial concepts were reported, individual effects (ds) were calculated for each measure (eg, fatigue, depression and QoL) and then pooled to obtain a single effect on overall QoL for that study. Finally, we explored potential moderations of yoga’s effectiveness by yoga program endowments, relatively lesser versus better-endowed programs with Cochran’s Qb statistic. The meta-analytic analogue of the t test, it is a function of Q and, again, distributed as χ2. All meta-analytic calculations were cross-validated by both reviewers and with discussion, were replicated with 100% agreement.
To aid practical interpretations, significant sample-weighted ds were converted to Cohen’s U 3 statistics.25 A more intuitively appealing measure of practical significance, U 3 compares the scores of all the people in the aggregated intervention group with the score of the median or most typical person in the aggregated control group. In this way it tends to put the emphasis on people, rather than statistics. For instance, a hypothetically supportive U 3 of 75% resulting from a comparison of women with breast cancer who received a yoga intervention or another exercise intervention on a continuous measure of QoL at 6-month follow-up would be interpreted as follows. Three-quarters or 15 of every 20 of the women in the yoga group scored higher on the QoL measure at follow-up than did the typical, otherwise similar, women in the other exercise group.
Results
Study Selection
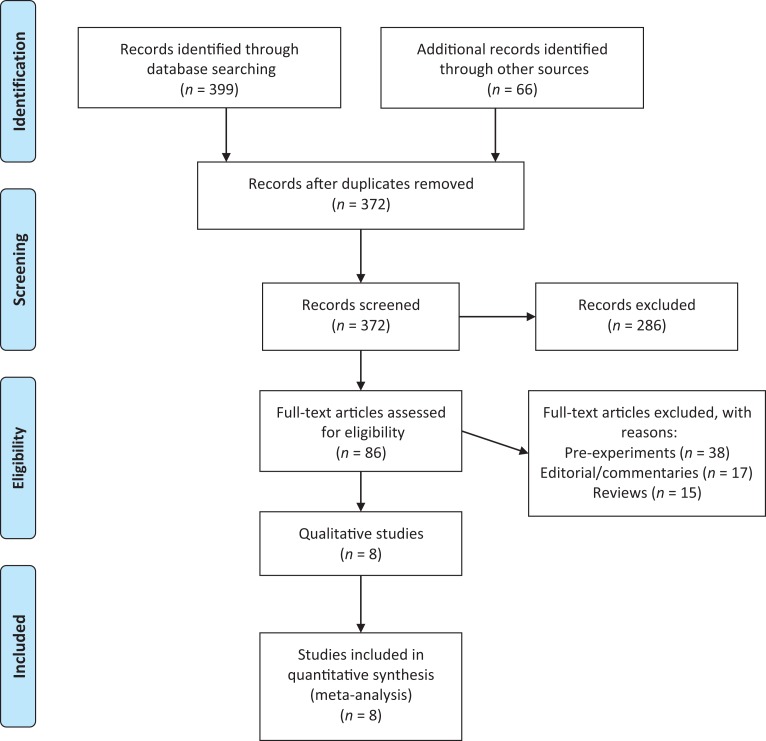
Eight RCTs that independently compared yoga with any other exercise intervention and used a validated outcome measure were retrieved for this meta-analysis.30–37 A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram outlining the study selection process that was cross-validated by both reviewers is displayed in Figure 1.38
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the systematic review process.
Study Characteristics
Descriptive characteristics and outcomes of the 8 RCTs at immediate postintervention and follow-up are, respectively, presented in Tables 1 and 2. They were published between 2008 and 2018 and accomplished in the United States (3), Turkey (2), and India, Germany, and the United Kingdom (1 each). The 545 participants, all women with nonmetastasized (not stage IV) breast cancer, ranged in age from 18 to 80 and seemed predominantly middle aged (mean ages ranged from 50 to 55 years). Five studied patients undergoing their first course of cancer treatment while 3 studied survivors, albeit relatively immediate survivors, all within the first 6 months of treatment; except for 1 study that required participants to be at least 3 years posttreatment. Very small total study samples ranged from 20 to 123 participants (median = 75) immediately postintervention. At that point participant attrition ranged from none to 44% (median = 12%). Five of the studies followed their participants for an additional 3 to 12 months (median = 6 months). Attrition at follow-up ranged from 15% to 64% (median = 30%). Aggregated attrition immediately postintervention and at follow-up did not differ significantly between the 2 study groups. With only 2 exceptions, the trials were largely devoid of sociodemographic information on the racial or ethnic group or socioeconomic status of their participants.
Table 1.
Characteristics and Outcomes of Studies Included in the Meta-Analysis: Immediate Postintervention.
| Authors, Year, and Country | Sample Characteristics | Treatment, n and Control Groups, n | Intensity and Duration | Follow-up Length Attrition Rates (%)a | Outcome Measures | d-Index (90% CI) |
|---|---|---|---|---|---|---|
| Chandwani et al, 2014, USA | Patients, stages 0-III ages 26 to 79 y | VYASA yoga, 49; stretching, 52 | Yoga studio 3 1-hour sessions/week, 6 weeks | 6 weeks, 7%, 7% | BFI, CES-D, and SF-36, Fatigue, Depression, and QoL | −0.80 (−1.14, −0.46) |
| Chaoul et al, 2018, USA | Patients, stages I-III, ages 18+ y, mean = 50 y | Tibetan yoga, 64; stretching, 59 | 1-on-1 format 7, 75- to 90-minute sessions, 12 weeks | 12 weeks, 13%, 13% | BFI, Fatigue | −0.21 (−0.09, 0.51) |
| Harder et al, 2015, UK | Patients, stages I-III, ages 18 to 80 y | Iyengar yoga, 40; standard exercise, 40 | Yoga DVD 1 1-hour session/week 10 weeks | 10 weeks, 11%, 11% | FACT-B QoL | 0.07 (−0.30, 0.44) |
| Lötzke et al, 2016, Germany | Patients, stages I-III, age mean = 51 y | Iyengar yoga, 43; physical exercise, 46 | Yoga studio 1 1-hour session/week 12 weeks | 12 weeks, 4%, 2% | CFS-D and EORTC QOL-C30 Fatigue and QoL | 0.23 (−0.12, 0.58) |
| Rao et al, 2008, India | Patients, stages II-III, ages 30-70 y | Integrated yoga, 33; shoulder exercise, 36 | Audiotape or leaflet, unclear, 4 weeks | 4 weeks, 26%, 32% | STAI, BDI, and FLIC Anxiety, Depression, and QoL | 0.86 (0.45, 1.27) |
| Stan et al, 2016, USA | Survivors, stages 0-II, ages 20-75 y | Hatha yoga, 14; strength training, 9 | Yoga DVD 3 90-minute sessions, 12 weeks | 12 weeks, 22%, 44% | MFSI-SF and FACT-B Fatigue, and QoL | 0.27 (−0.44, 0.98) |
| Yagli and Ulger, 2015, Turkey | Survivors, stages I-II, ages 65-70 y | Classic yoga, 10; physical exercise, 10 | Yoga studio 1 1-hour session/week 8 weeks | 8 weeks, 0%, 0% | VAS, BDI, and NHP Fatigue, Depression, and QoL | 0.85 (0.08, 1.62) |
| Yagli et al, 2015, Turkey | Survivors, stages I-II, ages 20-60 y | Yoga and aerobics, 19 aerobic exercise, 21 | Yoga studio 3 1-hour sessions/week 6 weeks | 6 weeks, 20%, 25% | FSS and EORTC QOL-C30 Fatigue and QoL | 0.50 (−0.02, 1.03) |
| Meta-analytic summary statistics: Sample-weighted d-index (90% CI) | 0.14 (0.00, 0.28) |
Abbreviations: BDI, Beck Depression Inventory; BFI, Brief Fatigue Inventory; CES-D, Centers for Epidemiological Studies-Depression; CFS-D, Cancer Fatigue Scale; CI, confidence interval; EORTC QoL-C30, European Organisation for Research and Treatment of Cancer, Health-Related Quality of Life (cancer-specific); FACT-B, Functional Assessment of Cancer Therapy-Breast; FLIC, Functional Living Index of Cancer; FSS, Fatigue Severity Scale; MFSI-SF, Multidimensional Fatigue Symptom Inventory–Short Form; NHP, Nottingham Health Profile; QoL, Quality of Life; SF-36, Medical Outcomes Study Short Form (mental health); STAI, State Trait Anxiety Inventory; VAS, visual analog scale (fatigue); VYASA, Vivekananda Yoga Anusandhana Samsthana.
aRespective treatment and control group attrition rates.
Table 2.
Characteristics and Outcomes of Studies Included in the Meta-Analysis: Longest Period of Follow-up.
| Authors, Year, Country | Sample Characteristics | Treatment, n and Control Groups, n | Intensity and Duration | Follow-up Length Attrition Rates (%)a | Outcome Measures | d-Index (90% CI) |
|---|---|---|---|---|---|---|
| Chandwani et al, 2014, USA | Patients, stages 0-III, ages 26-79 y | VYASA yoga, 43; stretching, 43 | Yoga studio 3 1-hour sessions/week for 6 weeks | 6 months, 19%, 23% | BFI, CES-D, and SF-36 Fatigue, Depression, and QoL | −1.80 (−2.22, −1.38) |
| Chaoul et al, 2018, USA | Patients, stages I-III, ages 18+ y, mean = 50 y | Tibetan yoga, 47; stretching, 39 | 1-on-1 format 7 75- to 90-minute sessions 12 weeks, 6-month booster | 12 months, 36%, 43% | BFI, Fatigue | −0.14 (−0.50, 0.22) |
| Harder et al, 2015, UK | Patients, stages I-III, ages 18-80 y | Iyengar yoga, 39; standard exercise, 39 | Yoga DVD 1 1-hour session/week 10 weeks | 6 months, 15%, 15% | FACT-B, QoL | 0.03 (−0.34, 0.40) |
| Lötzke et al, 2016, Germany | Patients, stages I-III, age mean = 51 y | Iyengar yoga, 16; physical exercise, 22 | Yoga studio 1 1-hour session/week, 12 weeks | 3 months, 64%, 53% | CFS-D and EORTC QOL-C30 Fatigue and QoL | 0.10 (−0.44, 0.64) |
| Rao et al, 2008, India | Patients, stages II-III, ages 30-70 y | Integrated yoga, 33; shoulder exercise, 36 | Audiotape or leaflet, unclear 4 weeks | 1 month, 26%, 32% | STAI, BDI, & FLIC Anxiety, Depression, and QoL | 0.86 (0.45, 1.27) |
| Stan et al, 2016, USA | Survivors, stages 0-II, ages 20-75 y | Hatha yoga, 14; strength training, 9 | Yoga DVD, 3 90-minute sessions, 12 weeks | 3 months, 22%, 44% | MFSI-SF and FACT-B Fatigue and QoL | 0.30 (−0.41, 1.01) |
| Yagli and Ulger, 2015, Turkey | Survivors, stages I-II, ages 65-70 y | Classic yoga, 10; physical exercise, 10 | Yoga studio 1 1-hour session/week 8 weeks | 2 months, 0%, 0% | VAS, BDI, and NHP Fatigue, Depression, and QoL | 0.85 (0.08, 1.62) |
| Yagli et al, 2015, Turkey | Survivors, stages I-II, ages 20-60 y | Yoga and aerobics, 19; aerobic exercise, 21 | Yoga studio 3 1-hour sessions/week, 6 weeks | 1.5 months, 20%, 25% | FSS and EORTC QOL-C30 Fatigue and QoL | 0.50 (−0.02, 1.03) |
| Meta-analytic summary statistics: Sample-weighted d-index (90% CI) | −0.05 (−0.21, 0.11) |
Abbreviations: BDI, Beck Depression Inventory; BFI, Brief Fatigue Inventory; CES-D, Centers for Epidemiological Studies-Depression; CFS-D, Cancer Fatigue Scale; CI, confidence interval; EORTC QoL-C30, European Organisation for Research and Treatment of Cancer, Health-Related Quality of Life (cancer-specific); FACT-B, Functional Assessment of Cancer Therapy-Breast; FLIC, Functional Living Index of Cancer; FSS, Fatigue Severity Scale; MFSI-SF, Multidimensional Fatigue Symptom Inventory-Short Form; NHP, Nottingham Health Profile; QoL, Quality of Life; SF-36, Medical Outcomes Study Short form (mental health); STAI, State Trait Anxiety Inventory; VAS, visual analog scale (fatigue); VYASA, Vivekananda Yoga Anusandhana Samsthana.
aRespective treatment and control group attrition rates.
Heterogeneous yoga interventions were used across the studies, including Hatha, Tibetan, Iyengar, VYASA, and integrated yoga programs. The typical yoga session proceeded as follows: preparatory warm-ups, breathing exercises, selected postures, cool-down postures, and concluded with a meditation or deep relaxation component. Similarly, the other exercise interventions varied and essentially included stretching, strength training and or aerobics. Participants in 5 studies received personalized yoga instruction in small group sessions (1 was individualized) led by certified yoga instructors, physiotherapists, and or breast cancer–informed instructors. The other 3 studies merely provided participants with DVDs, audiotapes, and or leaflets on yoga. Finally, the yoga interventions varied in duration from 4 to 12 weeks (median = 10 week) and in intensity from 1 to 3 times a week (median was once a week) for 60- to 90-minute sessions. Total yoga intervention “contact hours” ranged from 5 to 18 hours (median = 10 hours). The other exercise programs seemed similarly endowed.
Meta-Analytic Findings
Primary study outcomes are displayed in the far-right columns of both tables. At immediate postintervention, the sample-weighted d was 0.14. Though it was not statistically significant at P < .05, this synthetic finding did approach a more liberal statistical significance criterion (90% CI 0.00-0.28 or P = .10). But the corresponding U 3 was quite small (56%). Pooling the 8 trial findings, yoga demonstrated a very slight advantage. Only 11 of every 20 yoga participants did better on psychosocial measures of QoL than the typical other exercise participant. However, at the longest period of follow-up the sample-weighted d was essentially 0 to 0.05 (90% CI −0.21 to 0.11). Any marginal advantage of yoga seemed to have faded out. Finally, though the primary study effects were significantly heterogeneous at postintervention and follow-up, respectively, χ2(7) = 32.92 and 67.73, both P < .05, they were not moderated or explained by yoga program duration, intensity, or total “contact hours.” This is probably not surprising as the yoga intervention programs were homogeneously poorly endowed. Typically, yoga program participants met in small groups (3 had no personal contact with an instructor or other women with breast cancer) for 1 hour a week for 10 weeks. Finally, and again of no surprise given their rather homogenous lack of power and blinding, no other coded study characteristic significantly moderated study outcomes.
Discussion
An overview of 7 previous systematic reviews and or meta-analyses found exercise interventions, including yoga, to effectively complement breast cancer care. Their beneficial psychosocial effects were generally large, being associated with diminished fatigue, anxiety and depression, and so with an overall enhancement of QoL. In aggregate, they estimated that approximately 16 to 19 of every 20 exercise intervention participants with breast cancer assessed their QoL as higher than did otherwise similar women who did not participate in any complementary exercise intervention. However, questions about the specific effects of yoga, that is, its effectiveness relative to other types of exercise had not been systematically answered. In response, this meta-analysis synthesized the evidence from 8 RCTs that compared yoga to other types of exercise among breast cancer patients and survivors. Our hypothesis that yoga is more effective than other exercise modalities in improving the QoL of women with breast cancer was not supported. Immediately postintervention, an extremely small, probably clinically inconsequential, advantage of yoga was suggested. However, at longer follow-ups of 3 to 12 months, the apparent advantage of yoga had disappeared.
Some, yoga enthusiasts in particular, may lament these meta-analytic findings. Such “nonsignificant” findings, however, may ultimately be quite significant clinically. In fact, this synthetic evidence clearly affirms that yoga is as effective, and that is highly effective, as other exercise modalities such as strength training and aerobics for improving the QoL of women with breast cancer. In an era when more clinicians are combining their professions’ best practices with evidence-informed complementary therapies, such evidence may serve to expand clinical arsenals.39 Oncologists and allied mental health professionals working in traditional breast cancer care contexts can feel confident as they complement such care with innovations at the mind-body interface, including yoga and related mindfulness interventions.40
Physicians and other clinicians necessarily strive for good fits between patients’/clients’ characteristics and intervention methods. As in other domains of medical and psychotherapeutic practice, exercise interventions, including yoga, that consider an individual’s needs and preferences are more likely to be successful in facilitating the life space uptake and incorporation of such physical activities.41 In this way, yoga seems a natural good fit with breast cancer care. It may serve as a more advantageous exercise module because the postures can be graded and modified to accommodate various functional limitations, cancer-related fatigue and or pain, and can be easily adapted throughout the cancer recovery-survivorship trajectory. In the future, such yoga (and other exercise) programs in breast cancer care should continue to consider the interests and preferences of the women they aim to serve, to facilitate their optimal uptake of yoga practices and other physical activities.7 Toward this end it would help, we think, to know much more about the women who participated in these studies as well as about the yoga interventions themselves. Neither were described in much detail in the primary study reports. Recall that only 2 studies described the racialized/ethnic or socioeconomic status of their participants and neither of those studied their effects on the acceptability or effectiveness of various yoga practices. More case studies the likes of Rebecca Strauss and Terry Northcut’s39 as well as case series and qualitative research will be needed to facilitate the best patient/client-intervention fits in this field.
Other Limitations and Future Research Needs
A major methodological shortcoming of the 8 RCTs was their small sample sizes. Their typical or median yoga intervention analytic sample size was only 37 postintervention and only 26 at follow-up. This field seems grossly underpowered. Such small samples probably could not have ensured the kind of confident control for even unanticipated confounds that one expects to achieve through randomization. Future trials ought to be powered by ample samples sufficient to allow the detection of modest, but clinically significant, between-group differences with confidence. For example, using standard statistical criteria (1-tailed α = .05; power1 − β = .80), samples of between 150 and 300 yoga and exercise participants each would be required to detect differences characterized by ds of 0.20 to 0.30.38,42 Such larger trials also ought to be amply funded, allowing for the staffing, training, and procedural supports needed to ensure high follow-up rates and longer term, postintervention follow-up periods. A final prevalent limitation of the included trials was their apparent lack of blinding. It is true that all types of blinding are probably not possible, or even desirable, across all phases of research in this field. However, future trials ought to minimally protect against potential detection bias by ensuring that those who collect and analyze outcome data are blind to participants’ group status.
This field’s knowledge may be most limited by the apparent lack of resources it has invested in its yoga intervention programs. In short, its yoga interventions have been homogeneously poorly endowed. Most typically such yoga, often without personal professional guidance, was practiced for an hour a week over 10 weeks. One wonders if that is enough practice for participants to become competent and to derive yoga’s full hypothesized benefits. Other fields of practice, preschool interventions such as Head Start for example, have observed large initial benefits that then tended to faded-out over time. But the benefits were demonstrated not to fade out among well-endowed preschool programs with highly qualified teachers in classrooms with low teacher/student ratios and ample contact hours each week.43 Perhaps similar resource lacks can explain the seeming fade-out effect of yoga. One may further wonder if similarly well-endowed yoga programs would demonstrate much larger yoga-specific beneficial effects that do not fade-out over time. Such, we think, is a critically important hypothesis that remains for future research testing.
Ruling Out Publication Bias
Though its sampling frame included unpublished sources, this meta-analytic sample ultimately included only published studies. One may legitimately wonder if publication bias could be a potent alternative explanation for its findings. However, this seems improbable for the following reason. Ten of the 16 study outcomes reviewed were null and, consequently, the review’s key synthetic finding at longest follow-up was also null. Those are precisely the sorts of findings one would not readily expect to retrieve and derive from published studies if publication bias, that is, a preference to publish significant findings, was potent. This field’s editorial review boards seemed to have been quite open to publishing null findings.
Conclusion
Our meta-analysis synthesized preliminary evidence that yoga seems to be as effective as other exercise modalities for improving the QoL of women with breast cancer. However, because yoga methods can be modified to accommodate functional limitations unique to cancer patients, they may be more practically effective. No exemplary, truly well-resourced yoga intervention program has yet been tested in this field. Amply powered, well-controlled trials ought to do so.
Acknowledgments
We are grateful for the library reference support of Marg McCaffrey-Piche, Leddy Library, the internal review of Josée Jarry, Department of Psychology and the technical assistance of Amy Alberton, Derek Campbell, and Rachel R. Jewell, School of Social Work, University of Windsor.
Footnotes
Author Contributions: DE conceptualized the study and led the analysis and writing. KMG supervised the analysis and writing. Both authors designed the study, interpreted its findings and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Daline El-Hashimi  https://orcid.org/0000-0002-7069-6926
https://orcid.org/0000-0002-7069-6926
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Can J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt ME, Wiskemann J, Steindorf K. Quality of life, problems, and needs of disease-free breast cancer survivors 5 years after diagnosis. Qual Life Res. 2018;27:2077–2086. [DOI] [PubMed] [Google Scholar]
- 3. Koch L, Jansen L, Herrmann A, et al. Quality of life in long-term breast cancer survivor—a 10-year longitudinal population-based study. Acta Oncol. 2013;52:1119–1128. [DOI] [PubMed] [Google Scholar]
- 4. International Society for Quality of Life Research. What is health-related quality of life research? http://www.isoqol.org/about-isoqol/what-is-health-related-quality-of-life-research. Accessed January 17, 2019.
- 5. Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. [DOI] [PubMed] [Google Scholar]
- 6. Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buffart LM, Galvão DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40:27–40. [DOI] [PubMed] [Google Scholar]
- 8. Fairman CM, Focht BC, Lucas AR, Lustberg MB. Effects of exercise interventions during different treatments in breast cancer. J Community Support Oncol. 2016;14:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2986. [DOI] [PubMed] [Google Scholar]
- 10. Falcetta FS, de Träsel HAV, de Almeida FK, Falcetta MRR, Falavigna M, Rosa DD. Effects of physical exercise after treatment of early breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2018;170:455–476. [DOI] [PubMed] [Google Scholar]
- 11. Patsou ED, Alexias GD, Anagnostopoulos FG, Karamouzis MV. Effects of physical activity on depressive symptoms during breast cancer survivorship: a meta-analysis of randomised control trials. ESMO Open. 2017;2:e000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Courneya KS, McKenzie DC, Reid RD, et al. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med. 2008;35:116–122. [DOI] [PubMed] [Google Scholar]
- 13. Koulal MJ, Knight JM. Increasing provider awareness of and recommendations for yoga and meditation classes for cancer patients. Support Care Cancer. 2018;26:3635–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feuerstein G. The Yoga Tradition: Its History, Literature, Philosophy and Practice. Prescott, AZ: Hohm Press; 1998. [Google Scholar]
- 15. Iyengar BKS. Light on Yoga. New York, NY: Schocken Books; 1966. [Google Scholar]
- 16. Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pan Y, Yang K, Wang Y, Zhang L, Liang H. Could yoga practice improve treatment-related side effects and quality of life for women with breast cancer? A systematic review and meta-analysis. Asia Pac J Clin Oncol. 2015;13:e79–e95. [DOI] [PubMed] [Google Scholar]
- 18. Galliford M, Robinson S, Bridge P, Carmichael M. Salute to the sun: a new dawn in yoga therapy for breast cancer. J Med Radiat Sci. 2017;64:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: A systematic review and meta-analysis. BMC Cancer. 2012;12:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harder H, Parlour L, Jenkins V. Randomised controlled trials of yoga interventions for women with breast cancer: a systematic literature review. Support Care Cancer. 2012;20:3055–3064. [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Yang KH, Tian JH, Wang CM. Effects of yoga on psychologic function and quality of life in women with breast cancer: a meta-analysis of randomized controlled trials. J Altern Complement Med. 2012;18:994–1002. [DOI] [PubMed] [Google Scholar]
- 22. Grenier AM, Gorey KM. Effectiveness of social work with older people and their families: a meta-analysis of conference proceedings. Soc Work Res. 1998;22:60–64. [Google Scholar]
- 23. de Smidt GA, Gorey KM. Unpublished social work research: systematic replication of a recent meta-analysis of published intervention effectiveness research. Soc Work Res. 1997; 21:58–62. [Google Scholar]
- 24. Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463–471. [DOI] [PubMed] [Google Scholar]
- 25. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 26. Cooper H. Research Synthesis and Meta-Analysis: A Step-By-Step Approach. 5th ed Thousand Oaks, CA: Sage; 2017. [Google Scholar]
- 27. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 28. Yaffe J, Montgomery P, Hopewell S, Shepard LD. Empty reviews: a description and consideration of Cochrane systematic reviews with no included studies. PLoS One. 2012;7: e36626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd ed Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 30. Chaoul A, Milbury K, Spelman A, et al. Randomized trial of Tibetan yoga in patients with breast cancer undergoing chemotherapy. Cancer. 2018;124:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lötzke D, Wiedemann F, Recchia DR, et al. Iyengar-yoga compared to exercise as a therapeutic intervention during (neo)adjuvant therapy in women with stage I-III breast cancer: health-related quality of life, mindfulness, spirituality, life satisfaction, and cancer-related fatigue. Evid Based Complement Alternat Med. 2016;2016:5931816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24:4005–4015. [DOI] [PubMed] [Google Scholar]
- 33. Harder H, Langridge C, Solis-Trapala I, et al. Post-operative exercises after breast cancer surgery: results of a RCT evaluating standard care versus standard care plus additional yoga exercise. Eur J Integr Med. 2015;7:202–210. [Google Scholar]
- 34. Yaglı NV, Şener G, Arıkan H, et al. Do yoga and aerobic exercise training have impact on capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther. 2015;14:125–132. [DOI] [PubMed] [Google Scholar]
- 35. Yagli NV, Ulger O. The effects of yoga on the quality of life and depression in elderly breast cancer patients. Complement Ther Clin Pract. 2015;21:7–10. [DOI] [PubMed] [Google Scholar]
- 36. Chandwani DK, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rao RM, Nagendra HR, Raghuram N, et al. Influence of yoga on mood states, distress, quality of life and immune outcomes in early stage breast cancer patients undergoing surgery. Int J Yoga. 2008;1:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. A preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269. [DOI] [PubMed] [Google Scholar]
- 39. Strauss RJ, Northcut TB. Using yoga interventions to enhance clinical social work practices with young women with cancer. Clin Soc Work J. 2014;42:228–236. [Google Scholar]
- 40. Singh SK, Gorey KM. Relative effectiveness of mindfulness and cognitive behavioral interventions for anxiety disorders: meta-analytic review. Soc Work Mental Health. 2018; 16:238–251. [Google Scholar]
- 41. Ijsbrandy C, Ottevanger PB, Diogenia MT, Gerritsen WR, van Hartend WH, Hermensa RPMG. Review: effectiveness of implementation strategies to increase physical activity uptake during and after cancer treatment. Crit Rev Oncol Hematol. 2018;122:157–163. [DOI] [PubMed] [Google Scholar]
- 42. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 43. Gorey KM. Early childhood education: a meta-analytic affirmation of the short- and long-term benefits of educational opportunity. Sch Psychol Q. 2001;16:9–30. [Google Scholar]