Abstract
Background
Glaucoma is a leading cause of irreversible blindness. A number of minimally invasive surgical techniques have been introduced as a treatment to prevent glaucoma progressing. Among them, endoscopic cyclophotocoagulation (ECP) is a cyclodestructive procedure developed by Martin Uram in 1992.
Objectives
To evaluate the efficacy and safety of ECP in people with open angle glaucoma (OAG) and primary angle closure whose condition is inadequately controlled with drops.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2018, Issue 6); Ovid MEDLINE; Ovid Embase; the ISRCTN registry; ClinicalTrials.gov and the WHO ICTRP. The date of the search was 12 July 2018.
Selection criteria
We searched for randomised controlled trials (RCTs) of ECP compared to other surgical treatments (other minimally invasive glaucoma device techniques, trabeculectomy), laser treatment or medical treatment. We also planned to include trials where these devices were combined with phacoemulsification compared to phacoemulsification alone.
Data collection and analysis
Two review authors planned to independently extract data from reports of included studies using a data collection form and analyse data based on methods expected by Cochrane. Our primary outcome was proportion of participants who were drop‐free (not using eye drops). Secondary outcomes included mean change in IOP; proportion of participants who achieved an IOP of 21 mmHg or less, 17 mmHg or less or 14 mmHg or less; and proportion of participants experiencing intra‐ and postoperative complications, We planned to measure all outcomes in the short‐term (six to 18 months), medium‐term (18 to 36 months), and long‐term (36 months onwards).
Main results
We found one ongoing study that met our inclusion criteria (ChiCTR‐TRC‐14004233). The study compares combined phacoemulsification with ECP to phacoemulsification alone in people with primary angle closure glaucoma. The primary outcome is intraocular pressure (IOP) and number of IOP‐lowering drugs. A total of 50 people have been enrolled. The study started in February 2014 and the trialists have completed recruitment and are in the process of collecting data.
Authors' conclusions
There is currently no high‐quality evidence for the effects of ECP for OAG and primary angle closure. Properly designed RCTs are needed to assess the medium and long‐term efficacy and safety of this technique.
Plain language summary
Endoscopic cyclophotocoagulation for open angle glaucoma and primary angle closure
What was the aim of the review? The aim of this Cochrane Review was to find out if endoscopic cyclophotocoagulation (ECP) lowers the pressure in the eye for people with open angle glaucoma or angle closure. The Cochrane Review authors collected and analysed all relevant studies to answer this question and found no completed studies and one ongoing study.
Key messages There are no relevant published studies comparing ECP with other treatments.
What was studied in the review? Glaucoma is a common eye condition and can cause blindness if left untreated. In glaucoma, the optic nerve (which connects the eye to the brain) is damaged, often due to increased pressure in the eye due to build‐up of fluid. ECP is a type of surgery in which doctors use a laser to slow down the production of this fluid. This may lead to lower eye pressure and a lower chance of damage to the optic nerve. ECP may cause less damage to the eye than other types of glaucoma surgery. This could be safer and more comfortable and help recovery.
What were the main results of the review? The Cochrane Review authors did not find any completed studies that could be included in this review.
How up‐to‐date is the review? The Cochrane Review authors searched for studies published up to 12 July 2018.
Background
The protocol for this review (Tóth 2017) was based on the protocol from the published review on ab interno trabecular bypass surgery with Trabectome for open angle glaucoma (OAG) (Hu 2016).
Description of the condition
Glaucoma is a progressive optic neuropathy, affecting 3.5% of people aged 40 to 80 years (Tham 2014). It is the leading cause of irreversible blindness, affecting over 64 million people globally (Tham 2014). This figure is expected to increase to 110 million people by 2040. OAG is the most common type, accounting for 86% of cases (Tham 2014). In one large population cohort, one in six people with OAG became bilaterally blind (Peters 2013). Angle closure glaucoma is less common than OAG, but is more likely to result in bilateral blindness. The only proven way to prevent vision loss is to reduce the pressure inside the eye (intraocular pressure (IOP)) over the long term (AGIS 2000; CNTG Study Group 1998; Heijl 2002; Kass 2002). Approaches to reducing IOP include medical therapy, laser treatments and surgery. Because commercially available eye‐drop preparations have a short‐lasting effect, medical therapy requires eye‐drops to be instilled one or more times daily for life. Adherence is very poor, even if use is monitored (Friedman 2009; Okeke 2009). Conventional surgical techniques, such as trabeculectomy, are associated with significant risks, with more than 40% of people developing perioperative complications (Kirwan 2013; Lichter 2001), and reoperation being needed in 7% to 18% of people (Gedde 2012; Kirwan 2013). Therefore, they are often reserved for disease that is progressing despite other treatments (King 2013).
Description of the intervention
Several minimally invasive surgical techniques have been developed with the aim of achieving long‐term reduction of IOP with a better safety profile than conventional surgery (Francis 2011a). Among them, endoscopic cyclophotocoagulation (ECP) is a cyclodestructive procedure developed by Martin Uram in 1992 (Uram 1995).
How the intervention might work
The ciliary body is the site of aqueous humour production. In cyclodestructive procedures, the secretory epithelium of the ciliary epithelium is damaged, which leads to reduced aqueous humour secretion and lower IOP. ECP incorporates a diode laser, an aiming beam and videocamera imaging. Direct visualisation of the ciliary endothelium allows the delivering of energy precisely to the ciliary processes in a highly titratable fashion, while minimising collateral damage to the surrounding tissue.
Why it is important to do this review
Consultation with patients and healthcare professionals has identified a need for better treatments for glaucoma (James Lind Alliance 2013). Minimally invasive glaucoma surgeries (MIGS) carry the possibility of safe and effective long‐term reduction of IOP, removing concerns about permanent vision loss due to non‐adherence to eye‐drops. A single treatment may also be more acceptable to patients than daily and indefinite self‐administration of eye‐drops. Initial results of ECP were reported in 1992 by Uram (Uram 1992), where he treated 10 eyes of 10 people with neovascular glaucoma. Since then, several studies have demonstrated the IOP‐lowering effect of ECP in different glaucoma forms (Chen 1997; Francis 2011b; Lima 2004). In the light of the potential benefits for patients and the widespread uptake of the technique, it is important to critically evaluate the evidence for the efficacy and safety of ECP treatment. Importantly, ECP may be combined with phacoemulsification (cataract surgery), a sight‐restoring operation to remove the natural lens of the eye when it has lost clarity. Since phacoemulsification itself reduces IOP (Mansberger 2012), we specifically examined the evidence for the efficacy of ECP when combined with phacoemulsification in comparison to phacoemulsification alone. This Cochrane Review was conducted in parallel with other reviews undertaken by the Cochrane Eyes and Vision MIGS Consortium, which included MIGS techniques and devices such as the Trabectome (NeoMedix, Tustin, CA) (Hu 2016), Hydrus Schlemm's canal Microstent (Ivantis Inc., Irvine, CA) (Otarola 2017), XEN Glaucoma Implant (AqueSys Implant, Aliso Viejo, CA) (King 2018), and IStent or IStent inject (Glaukos Corporation, Laguna Hills, CA).
Objectives
To evaluate the efficacy and safety of ECP in people with open angle glaucoma (OAG), ocular hypertension and primary angle closure with or without glaucoma whose condition is inadequately controlled with drops.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) reported in any language, irrespective of their publication status.
Types of participants
Participants had OAG of any type, including primary and secondary OAG, or primary angle closure with raised IOP with or without glaucoma (PAC‐OHT or primary angle closure glaucoma). We excluded secondary forms of angle closure. As there are no universally accepted criteria by which glaucoma may be defined, we permitted studies to use their own definitions of glaucoma. In addition, we included participants with ocular hypertension, normal tension glaucoma or possible glaucoma (suspects for glaucoma). We applied no restrictions regarding location, setting or demographic factors.
Types of interventions
The intervention was ECP. Although it is possible to deliver variable degrees of treatment on the ciliary body with titratable power levels, we did not apply any particular inclusion or exclusion criteria around these or other treatment delivery parameters. There are two main approaches to reach ciliary body: via limbal or pars plana entry. As pars plana entry requires anterior vitrectomy, it cannot be considered as MIGS, and was not part of this review.
We planned to compare ECP:
in combination with phacoemulsification compared with phacoemulsification alone;
to laser treatment (selective laser trabeculoplasty or argon laser trabeculoplasty);
to other MIGS techniques;
to conventional glaucoma surgery (trabeculectomy);
to medical therapy.
We excluded all trials that compared ECP with aqueous shunts or another cyclodestructive procedure (including ECP) as these are covered by another Cochrane Review (Chen 2016). We also excluded trials that evaluated ECP using different delivery methods or parameters, as these trials are covered by another Cochrane Review (Michelessi 2018).
Types of outcome measures
We did not use the reporting of particular outcomes as a criterion for eligibility for the review. We did not exclude studies from review solely on the grounds of an outcome of interest not being reported.
We planned to report outcomes in the short‐term (six to 18 months), medium‐term (18 to 36 months) and long‐term (longer than 36 months).
Primary outcomes
Proportion of participants who were drop‐free (not using eye drops).
Several different glaucoma outcome measures have been specified as primary outcomes in other Cochrane Reviews and protocols (Ismail 2015). One study classified IOP, visual field, safety and anatomic outcomes as being highly important to glaucoma experts (Ismail 2016). A panel of patients from the Patient and Public Involvement Group of the National Institute for Health Research (NIHR) Biomedical Research Centre for Ophthalmology identified drop‐free disease control as a highly valued outcome (unpublished). We chose a participant‐centred primary outcome.
In assessing this outcome, we planned to report how prescribing of IOP‐lowering eye drops was determined during follow‐up. We planned to examine whether the people measuring IOP and healthcare professionals deciding upon the prescribing of IOP‐lowering eye drops were masked to treatment group.
Secondary outcomes
Mean change in IOP measured using Goldmann applanation tonometry.
Mean change in number of IOP‐lowering drops taken per day.
Proportion of participants achieved an IOP of 21 mmHg or less.
Proportion of participants achieved an IOP of 17 mmHg or less.
Proportion of participants achieved an IOP of 14 mmHg or less.
Proportion of participants required further glaucoma surgery, including laser, as recorded by the investigators of the included trials.
Mean change in health‐related quality of life (HRQoL).
Adverse effects
Proportion of participants with intraoperative and postoperative complications including, but not restricted to, the following:
loss of visual acuity (more than 2 Snellen lines or more than 0.3 logMAR, according to the method of recording visual acuity; or loss of light perception);
bleeding, as recorded by the investigators;
endophthalmitis, as recorded by the investigators;
IOP spikes (postoperative rise in IOP, measured using Goldmann applanation tonometry, of more than 10 mmHg compared to the previous assessment, including during the first postoperative month).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following electronic databases for RCTs and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 12 July 2018.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 12 July 2018) (Appendix 1).
MEDLINE Ovid (1946 to 12 July 2018) (Appendix 2).
Embase Ovid (1980 to 12 July 2018) (Appendix 3).
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 12 July 2018) (Appendix 4).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 12 July 2018) (Appendix 5).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 12 July 2018) (Appendix 6).
Searching other resources
We searched the reference lists of included studies for other possible studies. We contacted any individuals or organisations whom we believe were conducting relevant RCTs, but we received no answer. We checked manufacturer's website (Endo Optiks, Little Silver, NJ; endooptiks.com) to ascertain if any new trials were being undertaken but there were no details of any new studies currently being planned or conducted.
Data collection and analysis
Selection of studies
Two review authors (MT, AS) independently screened titles and abstracts of all articles identified by the search using web‐based online review management software (Covidence). If abstracts were not available, we planned to screen full‐text articles. We planned to obtain full‐text copies of all reports retained after this initial screening, and two review authors would have assessed them independently for inclusion in the review. If there was disagreement regarding eligibility, a third review author would have arbitrated. If any full‐text reports were rejected, we planned to record the reasons for this in the 'Characteristics of excluded studies' table. As we only found one ongoing trial and no completed RCTs for inclusion in our review, we were not able to complete the steps for data extraction or analysis. In future updates, if we find any RCTs that meet our inclusion criteria or if the ongoing trial is completed, results published and is eligible for inclusion, we will follow the process outlined below.
Data extraction and management
We planned to extract data from reports of included studies using a data collection form, which would have been developed and piloted on the first five studies included. Two review authors planned to work independently to extract study characteristics from reports of each study and enter the data into Review Manager 5 (RevMan 5) (Review Manager 2014). If there was disagreement, a third review author would have arbitrated.
We planned to present the data collected in Appendix 7 in the 'Characteristics of included studies' table. Where data on included studies were missing or unclear, we would have contacted the individuals or organisations involved to obtain clarification. We planned to collect and use the most detailed numerical data available to facilitate analyses of included studies. We would have attempted to obtain these data from individuals or organisations in preference to less precise methods such as extracting numeric data from graphs. If this was necessary, two review authors would have independently extracted the data and a third review author would have arbitrated in case of disagreement.
Assessment of risk of bias in included studies
We planned to use the latest version of the Cochrane 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias and assign judgements of this for included studies (Higgins 2017).
Measures of treatment effect
We planned to use the risk ratio as the measure of effect for the primary outcome (proportion of participants who are drop‐free).
We planned to use the mean difference as the measure of effect to report mean change in IOP. Secondary safety outcomes would have been reported as risk ratios. Health‐related quality of life outcomes would have been reported as mean differences for continuous or risk ratios for binary data.
Unit of analysis issues
We planned to assess whether included studies had included one or two eyes from each participant and whether or not randomisation was conducted at the level of the participant or the eye. There is a potential for medical treatments, such as topical beta blockers, used for one eye to influence the outcome in the other eye (Piltz 2000). Surgery to lower IOP in one eye may also affect the IOP of the fellow eye (Radcliffe 2010). Therefore, we planned to exclude studies that adopted a paired design.
Dealing with missing data
We planned to endeavour to minimise missing outcome data by contacting individuals and organisations to obtain them. If the data were unavailable, but the level of missing data in each group and reasons for missing data in each group were similar, we may simply have analysed available case data if an intention‐to‐treat (ITT) analysis had not been performed. If authors had conducted their own ITT analysis despite missing data, we planned to document whether they provided any justification for the method they used to deal with missing data and whether they compared their ITT result with an available case result.
Assessment of heterogeneity
We planned to assess heterogeneity between trials by careful examination of the study reports, assessing forest plots and examining of the I2 statistic. We intended to consider I2 values greater than 50% as indicative of substantial heterogeneity and, therefore, suggestive that meta‐analysis might not be wise; however, we planned to give consideration to the consistency of the effect estimates. If all estimates were in the same direction, we might have meta‐analysed even where heterogeneity was evident; we planned to comment on the heterogeneity.
Assessment of reporting biases
We planned to use a funnel plot to assess the risk of publication bias if there were more than 10 trials in our review.
Data synthesis
We planned to undertake a meta‐analysis where data appeared clinically, methodologically and statistically homogeneous. We intended to check that participants, interventions, comparators and outcomes were sufficiently similar to give a clinically meaningful result and that our I2 result indicated that a majority of the proportion of the variance in this plot did not reflect variation in true effects (i.e. I2 less than 50%). If all estimates were in the same direction, we might have meta‐analysed even where heterogeneity was evident, but we planned to comment on this. We intended to use a random‐effects model unless there were fewer than three eligible studies, in which case we would have use a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We do not plan to conduct subgroup analysis in future updates of the review.
Sensitivity analysis
We planned to assess the impact of excluding studies at high risk of bias for an outcome in one or more key domains.
'Summary of findings' table
We planned to prepare tables to summarise the findings of the review, including the assessment of the certainty of evidence for all outcomes using the GRADE approach (GRADEpro 2015).
We planned to report ECP compared to the following comparison groups described under Types of interventions:
in combination with phacoemulsification compared with phacoemulsification alone;
laser treatment;
other MIGS techniques;
conventional glaucoma surgery (trabeculectomy); or
medical therapy.
We planned to report the following outcomes at medium‐term follow‐up (18 to 36 months) in the 'Summary of findings' table.
Proportion of participants who were drop‐free (not using eye drops).
Mean change in IOP measured using Goldmann applanation tonometry.
Mean change in number of IOP‐lowering drops taken per day.
Proportion of participants who required further glaucoma surgery, including laser.
Mean change in health‐related quality of life.
Proportion of participants experiencing intraoperative complications.
Proportion of participants experiencing postoperative complications (any time point).
Results
Description of studies
Results of the search
The electronic searches yielded 452 references (Figure 1). After removing 59 duplicates, the Cochrane Information Specialist (CIS) screened 393 records and removed 351 references that were clearly not relevant to the scope of the review. We screened the remaining 42 references, rejected 41 and identified one ongoing study (ChiCTR‐TRC‐14004233) that met the inclusion criteria.
1.
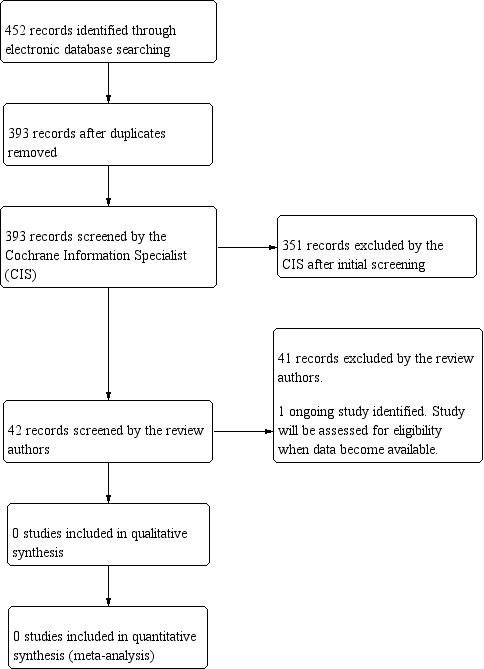
Study flow diagram.
Included studies
We found no completed RCTs that met our inclusion criteria.
Ongoing studies
We found one ongoing study that met our inclusion criteria (ChiCTR‐TRC‐14004233). The study compares combined phacoemulsification with ECP to phacoemulsification alone in people with primary angle closure glaucoma. The primary outcome is intraocular pressure (IOP) and number of IOP‐lowering drugs. The study was started in 2014 and a total of 50 people have been enrolled. See Characteristics of ongoing studies for further details.
Risk of bias in included studies
We included no published RCTs that met our inclusion criteria.
Effects of interventions
There were no completed RCTs reporting outcomes of ECP in open angle glaucoma or primary angle closure.
Discussion
We found no RCTs reporting the outcomes of ECP in open angle glaucoma or primary angle closure. We found one RCT in progress. We will report the outcomes of this trial when they become available.
Summary of main results
There are currently no RCTs reporting the outcomes of ECP in open angle glaucoma or primary angle closure.
Overall completeness and applicability of evidence
We performed a thorough search of available evidence as outlined in the published protocol (Tóth 2017).
Quality of the evidence
We found no trials for inclusion in this review.
Potential biases in the review process
While we performed a thorough search of the literature, it is possible that we missed relevant published or ongoing RCTs.
Agreements and disagreements with other studies or reviews
We found no reviews for comparison.
Authors' conclusions
Implications for practice.
There is currently no high‐quality evidence available for the efficacy or safety of endoscopic cyclophotocoagulation for open angle glaucoma or primary angle closure. Practitioners need to take this into consideration when reviewing the treatment options for open angle glaucoma and primary angle closure.
Implications for research.
Endoscopic cyclophotocoagulation has been available and used in the National Health System for several years. Properly designed randomised controlled trials are needed to assess the medium‐ and long‐term efficacy and safety of endoscopic cyclophotocoagulation compared to conventional medical, laser and surgical treatments for open angle glaucoma. The randomised controlled trials should report clinical outcomes, outcomes that are relevant to patients such as quality of life and outcomes important to service planning such as cost effectiveness.
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We thank Nitin Anand and Jennifer Evans for their comments on the published protocol that forms the template for this one (Hu 2016) and on the review itself. We thank Professor Tham Chee Yung Clement for responding to queries on his ongoing trial (ChiCTR‐TRC‐14004233).
We thank the members of the MIGS Consortium for their input in this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Glaucoma, Open‐Angle] explode all trees #2 MeSH descriptor: [Intraocular Pressure] explode all trees #3 MeSH descriptor: [Ocular Hypertension] explode all trees #4 OAG or POAG or IOP or OHT #5 simple near/3 glaucoma* #6 open near/2 angle near/2 glaucoma* #7 chronic near/2 glaucoma* #8 secondary near/2 glaucoma* #9 low near/2 tension near/2 glaucoma* #10 low near/2 pressure near/2 glaucoma* #11 normal near/2 tension near/2 glaucoma* #12 normal near/2 pressure near/2 glaucoma* #13 pigment near/2 glaucoma* #14 MeSH descriptor: [Exfoliation Syndrome] this term only #15 exfoliat* near/2 syndrome* #16 exfoliat* near/2 glaucoma* #17 pseudoexfoliat* near/2 syndrome* #18 pseudoexfoliat* near/2 glaucoma* #19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 MeSH descriptor: [Endoscopy] this term only #21 MeSH descriptor: [Lasers, Semiconductor] this term only #22 MeSH descriptor: [Light Coagulation] this term only #23 MeSH descriptor: [Laser Coagulation] this term only #24 endoscop* near/2 cyclophotocoagulat* #25 ECP #26 cycloablat* #27 #20 or #21 or #22 or #23 or #24 or #25 or #26 #28 #19 and #27
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp glaucoma open angle/ 14. exp intraocular pressure/ 15. ocular hypertension/ 16. (OAG or POAG or IOP or OHT).tw. 17. (simple$ adj3 glaucoma$).tw. 18. (open adj2 angle adj2 glaucoma$).tw. 19. (primary adj2 glaucoma$).tw. 20. (chronic adj2 glaucoma$).tw. 21. (secondary adj2 glaucoma$).tw. 22. (low adj2 tension adj2 glaucoma$).tw. 23. (low adj2 pressure adj2 glaucoma$).tw. 24. (normal adj2 tension adj2 glaucoma$).tw. 25. (normal adj2 pressure adj2 glaucoma$).tw. 26. (pigment$ adj2 glaucoma$).tw. 27. exfoliation syndrome/ 28. (exfoliat$ adj2 syndrome$).tw. 29. (exfoliat$ adj2 glaucoma$).tw. 30. (pseudoexfoliat$ adj2 syndrome$).tw. 31. (pseudoexfoliat$ adj2 glaucoma$).tw. 32. Glaucoma, Angle‐Closure/ 33. (angle$ adj3 (occlud$ or narrow$ or width or close$ or closure)).tw. 34. (glaucoma$ adj3 (occlud$ or narrow$ or width or close$ or closure)).tw. 35. (PAC or PACS or PACG or ACG).tw. 36. or/13‐35 37. Endoscopy/ 38. Lasers, Semiconductor/ 39. light coagulation/ 40. laser coagulation/ 41. (endoscop$ adj2 cyclophotocoagulat$).tw. 42. ECP.tw. 43. cycloablat$.tw. 44. or/37‐43 45. 36 and 44 46. 12 and 45
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. open angle glaucoma/ 34. intraocular pressure/ 35. intraocular hypertension/ 36. (OAG or POAG or IOP or OHT).tw. 37. (open adj2 angle adj2 glaucoma$).tw. 38. (primary adj2 glaucoma$).tw. 39. (chronic adj2 glaucoma$).tw. 40. (secondary adj2 glaucoma$).tw. 41. (low adj2 tension adj2 glaucoma$).tw. 42. (low adj2 pressure adj2 glaucoma$).tw. 43. (normal adj2 tension adj2 glaucoma$).tw. 44. (normal adj2 pressure adj2 glaucoma$).tw. 45. (pigment$ adj2 glaucoma$).tw. 46. exfoliation syndrome/ 47. (exfoliat$ adj2 syndrome$).tw. 48. (exfoliat$ adj2 glaucoma$).tw. 49. (pseudoexfoliat$ adj2 syndrome$).tw. 50. (pseudoexfoliat$ adj2 glaucoma$).tw. 51. closed angle glaucoma/ 52. glaucomatous optic neuropathy/ 53. neovascular glaucoma/ 54. secondary glaucoma/ 55. (angle$ adj3 (occlud$ or narrow$ or width or close$ or closure)).tw. 56. (glaucoma$ adj3 (occlud$ or narrow$ or width or close$ or closure)).tw. 57. (PAC or PACS or PACG or ACG).tw. 58. or/33‐57 59. laser coagulation/ 60. ophthalmic argon laser/ 61. (endoscop$ adj2 cyclophotocoagulat$).tw. 62. ECP.tw. 63. cycloablat$.tw. 64. or/59‐63 65. 58 and 64 66. 32 and 65
Appendix 4. ISRCTN search strategy
endoscopic cyclophotocoagulation OR ECP OR cycloablation
Appendix 5. ClinicalTrials.gov search strategy
(endoscopic cyclophotocoagulation OR ECP OR cycloablation)
Appendix 6. ICTRP search strategy
endoscopic cyclophotocoagulation OR cycloablation
Appendix 7. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
|
Number of study arms Method of randomisation Exclusions after randomisation Losses to follow‐up Number randomised/analysed Method of masking How were missing data handled? e.g. available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes Unit of randomisation/unit of analysis |
|
|
| Participants | ||
| Country | — | Setting Ethnic group Method of recruitment Participation rate Equivalence of baseline characteristics (Y/N) Diagnostic criteria |
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | — | |
| Exclusion criteria | — | |
| Interventions | ||
| Intervention (n = ) Comparator (n = ) |
|
ECP surgical parameters, e.g. degrees of ciliary epithelium treated, laser power Comparator parameters, e.g. dosage of drugs |
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports |
Adverse events reported (Y/N) |
Planned/actual length of follow‐up |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants month/year to month/year | Full study name: (if applicable) Date of publication Reported subgroup analyses (Y/N) Were trial investigators contacted? |
| Sources of funding | — | |
| Declaration of interest | — | |
| ECP: endoscopic cyclophotocoagulation; IOP: intraocular pressure; n: number of participants; N: no; RCT: randomised controlled trial; Y: yes. | ||
Characteristics of studies
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐14004233.
| Trial name or title | Combined phacoemulsification‐endoscopic cyclophotocoagulation versus phacoemulsification alone in primary angle‐closure glaucoma: a randomized controlled trial |
| Methods | Randomised parallel controlled trial, 2 study arms |
| Participants | Country: China Total number of participants: 50 Inclusion criteria: diagnosis of primary angle closure glaucoma; requiring ≥ 2 IOP‐lowering drugs to control IOP, or IOP > 21 mmHg on maximally tolerable drugs; able and willing to give informed consent, prior to randomisation; aged > 18 years Exclusion criteria: previous intraocular surgery or cyclophotocoagulation, with the exception of laser peripheral iridotomy and argon laser peripheral iridoplasty; secondary causes of angle closure including iridocorneal‐endothelial syndrome, neovascular glaucoma etc.; unable to co‐operate for reliable visual field and OCT examination |
| Interventions | Intervention: phacoemulsification + endoscopic cyclophotocoagulation, 25 participants Comparator: phacoemulsification alone, 25 participants |
| Outcomes |
Primary outcome: IOP and number of IOP‐lowering drugs Secondary outcome: best‐corrected visual acuity, surgical complications and need for additional surgical interventions |
| Starting date | 13 February 2014 |
| Contact information | email: clemtham@cuhk.edu.hk Name: Professor Tham Chee Yung Clement Address: Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, 3/F, Hong Kong Eye Hospital, 147K Argyle Street, Kowloon, Hong Kong Tel: +86 39535823 |
| Notes | Current status: recruitment complete, trialists are now rounding up the 2‐year follow‐up data (personal communication with Clement Tham, 1 August 2018). Trial Registry Number: ChiCTR‐TRC‐14004233 |
IOP: intraocular pressure; OCT: optical coherence tomography.
Differences between protocol and review
The follow‐up times for the outcomes were decided after the protocol was published.
An additional co‐author, A Shah joined the review team.
The protocol included combination therapy with phacoemulsification as a separate comparison and also for subgroup analysis. After discussion within the review team and MIGS Consortium, we opted to include it as a separate comparison as this is likely to be a different indication.
Contributions of authors
Protocol MT, KH and CB wrote the protocol. All authors reviewed and approved the protocol.
Review MT and AS screened the search results. MT extracted the data for the ongoing study and AS checked the data. MT wrote the review with edits from AS. KH, CB and GG commented on the draft.
Sources of support
Internal sources
-
National Institute for Health Research (NIHR), UK.
CB acknowledges financial support for her CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, National Health Service or the Department of Health.
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
The authors are seeking funding to address the subject of this review.
MT: none known AS: none known KH has lectured on 'Constructing clinical trials for MIGS – the lack of evidence and what to do about it' at the Moorfields International Glaucoma Symposium 2016, sponsored by Laboratoires Thea, which is contributing an educational grant to Moorfields Eye Hospital. CB: none known GG has since 2012 received travel funding and from his host organisation received both educational and unrestricted research funding from pharmaceutical and equipment manufacturers that are involved in the treatment of glaucoma but none that are otherwise related to the subject of this report.
New
References
References to ongoing studies
ChiCTR‐TRC‐14004233 {published data only}
- ChiCTR‐TRC‐14004233. Combined phacoemulsification‐endoscopic cyclophotocoagulation versus phacoemulsification alone in primary angle‐closure glaucoma: a randomized controlled trial. www.chictr.org.cn/showprojen.aspx?proj=5335 Date first received: 2 April 2014.
Additional references
AGIS 2000
- AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. American Journal of Ophthalmology 2000;130(4):429‐40. [DOI] [PubMed] [Google Scholar]
Chen 1997
- Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. American Journal of Ophthalmology 1997;124(6):787‐96. [DOI] [PubMed] [Google Scholar]
Chen 2016
- Chen MF, Kim CH, Coleman AL. Cyclodestructive procedures for refractory glaucoma. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012223] [DOI] [PMC free article] [PubMed] [Google Scholar]
CNTG Study Group 1998
- Collaborative Normal‐Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal‐tension glaucoma and patients with therapeutically reduced intraocular pressures. American Journal of Ophthalmology 1998;126(4):487‐97. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 14 July 2018. Melbourne, Australia: Veritas Health Innovation.
Francis 2011a
- Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 2011;118(7):1466‐80. [DOI] [PubMed] [Google Scholar]
Francis 2011b
- Francis BA, Kawji AS, Vo NT, Dustin L, Chopra V. Endoscopic cyclophotocoagulation (ECP) in the management of uncontrolled glaucoma with prior aqueous tube shunt. Journal of Glaucoma 2011;20(8):523‐7. [DOI] [PubMed] [Google Scholar]
Friedman 2009
- Friedman DS, Okeke CO, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology 2009;116(6):1097‐105. [DOI] [PubMed] [Google Scholar]
Gedde 2012
- Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow‐up. American Journal of Ophthalmology 2012;153(5):804‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 25 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Heijl 2002
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archives of Ophthalmology 2002;120(10):1268‐79. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hu 2016
- Hu K, Gazzard G, Bunce C, Wormald R. Ab interno trabecular bypass surgery with Trabectome for open angle glaucoma. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD011693.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 2015
- Ismail R, Azuara‐Blanco A, Ramsay CR. Outcome measures in glaucoma: a systematic review of Cochrane reviews and protocols. Journal of Glaucoma 2015;24(7):533‐8. [DOI] [PubMed] [Google Scholar]
Ismail 2016
- Ismail R, Azuara‐Blanco A, Ramsay CR. Consensus on outcome measures for glaucoma effectiveness trials: results from a delphi and nominal group technique approaches. Journal of Glaucoma 2016;25(6):539‐46. [DOI] [PubMed] [Google Scholar]
James Lind Alliance 2013
- James Lind Alliance Sight Loss, Vision Priority Setting Partnership. Setting priorities for eye research. www.sightlosspsp.org.uk (accessed 5 June 2017).
Kass 2002
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open‐angle glaucoma. Archives of Ophthalmology 2002;120(6):701‐13. [DOI] [PubMed] [Google Scholar]
King 2013
- King A, Azuara‐Blanco A, Tuulonen A. Glaucoma. BMJ 2013;346:f3518. [DOI] [PubMed] [Google Scholar]
King 2018
- King AJ, Shah A, Nikita E, Hu K, Mulvaney CA, Stead R, Azuara‐Blanco A. Subconjunctival draining minimally‐invasive glaucoma devices for medically uncontrolled glaucoma. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD012742.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirwan 2013
- Kirwan JF, Lockwood AJ, Shah P, Macleod A, Broadway DC, King AJ, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology 2013;120(12):2532‐9. [DOI] [PubMed] [Google Scholar]
Lichter 2001
- Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108(11):1943‐53. [DOI] [PubMed] [Google Scholar]
Lima 2004
- Lima FE, Magacho L, Carvalho DM, Susanna R Jr, Avila MP. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. Journal of Glaucoma 2004;13(3):233‐7. [DOI] [PubMed] [Google Scholar]
Mansberger 2012
- Mansberger SL, Gordon MO, Jampel H, Bhorade A, Brandt JD, Wilson B, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology 2012;119(9):1826‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michelessi 2018
- Michelessi M, Bicket AK, Lindsley K. Cyclodestructive procedures for non‐refractory glaucoma. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD009313.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okeke 2009
- Okeke CO, Quigley HA, Jampel HD, Ying GS, Plyler RJ, Jiang Y, et al. Adherence with topical glaucoma medication monitored electronically. The Travatan Dosing Aid study. Ophthalmology 2009;116(2):191‐9. [DOI] [PubMed] [Google Scholar]
Otarola 2017
- Otarola F, Hu K, Gazzard G, Bunce C. Ab interno trabecular bypass surgery with Schlemm's Canal Microstent (Hydrus) for open angle glaucoma. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 2013
- Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open‐angle glaucoma. American Journal of Ophthalmology 2013;156(4):724‐30. [DOI] [PubMed] [Google Scholar]
Piltz 2000
- Piltz J, Gross R, Shin DH, Beiser JA, Dorr DA, Kass MA, et al. Contralateral effect of topical beta‐adrenergic antagonists in initial one‐eyed trials in the ocular hypertension treatment study. American Journal of Ophthalmology 2000;130(4):441‐53. [DOI] [PubMed] [Google Scholar]
Radcliffe 2010
- Radcliffe NM, Musch DC, Niziol LM, Liebmann JM, Ritch R, Collaborative Initial Glaucoma Treatment Study Group. The effect of trabeculectomy on intraocular pressure of the untreated fellow eye in the collaborative initial glaucoma treatment study. Ophthalmology 2010;117(11):2055‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Tham 2014
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta‐analysis. Ophthalmology 2014;121(11):2081‐90. [DOI] [PubMed] [Google Scholar]
Uram 1992
- Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology 1992;99(12):1823‐8. [DOI] [PubMed] [Google Scholar]
Uram 1995
- Uram M. Endoscopic cyclophotocoagulation in glaucoma management. Current Opinion in Ophthalmology 1995;6(2):19‐29. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Tóth 2017
- Tóth M, Hu K, Bunce C, Gazzard G. Endoscopic cyclophotocoagulation (ECP) for open angle glaucoma and primary angle closure. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012741] [DOI] [PMC free article] [PubMed] [Google Scholar]


