Abstract
Purpose:
To compare the levels of gene expression for enzymes involved in production and elimination of reactive oxygen/nitrogen species (ROS/RNS) in normal human corneal cells (NL cells) with those in human corneal cells with keratoconus (KC cells) in vitro.
Methods:
Primary NL and KC stromal fibroblast cultures were incubated with apocynin (an inhibitor of NADPH oxidase) or N-nitro-L-arginine (N-LLA; an inhibitor of nitric oxide synthase). ROS/RNS levels were measured using an H2 DCFDA fluorescent assay. The RT2 Profiler™ PCR Array for Oxidative Stress and Antioxidant Defense was used for initial screening of the NL and KC cultures. Transcription levels for genes related to production or elimination of ROS/RNS were analyzed using quantitative PCR. Immunohistochemistry was performed on 10 intact human corneas using antibodies against SCARA3 and CPSF3.
Results:
Array screening of 84 antioxidant-related genes identified 12 genes that were differentially expressed between NL and KC cultures. Compared with NL cells, quantitative PCR showed that KC cells had decreased expression of antioxidant genes SCARA3 isoform 2 (0.59-fold, P = 0.02) and FOXM1 isoform 1 (0.61-fold, P = 0.03). KC cells also had downregulation of the antioxidant genes SOD1 (0.4-fold, P = 0.0001) and SOD3 (0.37-fold, P = 0.02) but increased expression of SOD2 (3.3-fold, P < 0.0001), PRDX6 (1.47-fold, P = 0.01), and CPSF3 (1.44-fold, P = 0.02).
Conclusion:
The difference in expression of antioxidant enzymes between KC and NL suggests that the oxidative stress imbalances found in KC are caused by defects in ROS/RNS removal rather than increased ROS/RNS production.
Keywords: CPSF3, FOXM1, Keratoconus, PRDX6, SCARA3, SOD
INTRODUCTION
Keratoconus (KC) is a progressive corneal thinning disorder that causes irregular astigmatism and reduced vision. It affects approximately 1 in 375 individuals and is an indication for corneal transplant surgery.[1,2,3,4] In about 6%–10% of cases, KC reportedly has an autosomal dominant inheritance pattern with incomplete penetrance.[5,6,7] The mechanism(s) causing KC are not known but are thought to involve a combination of genetic and environmental factors. The cornea is constantly exposed to ultraviolet light, which is a common source of free radicals and reactive oxygen/nitrogen species (ROS/RNS). Numerous molecular and biochemical studies have reported elevated levels of oxidative damage in corneas, cultured cells, and tears from eyes with KC.[8,9,10,11] Oxidative stress causes tissue degradation,[12] which may contribute to stromal thinning and loss of Bowman's layer in a cornea with KC.[1,2] Ex vivo corneas with KC contain biochemical markers for peroxynitrites and malondialdehydes, which are cytotoxic byproducts of the nitric oxide and lipid peroxidation pathways, respectively.[8] Peroxynitrites (ONOO-), formed by the combination of superoxides (O•-) with nitric oxides [Figure 1], can damage DNA, proteins, and lipids. An increase in oxidative stress is associated with inherited and acquired mitochondrial dysfunction.[13] Pathak et al identified mutations in complex 1 genes that are pathogenic and exclusive to KC.[14] Corneas with KC also have elevated levels of mitochondrial DNA (mtDNA) damage along with decreased levels of mitochondrial-encoded cytochrome c oxidase (complex IV subunit 1, MTCO1), consistent with mitochondrial dysfunction.[15]
Figure 1.
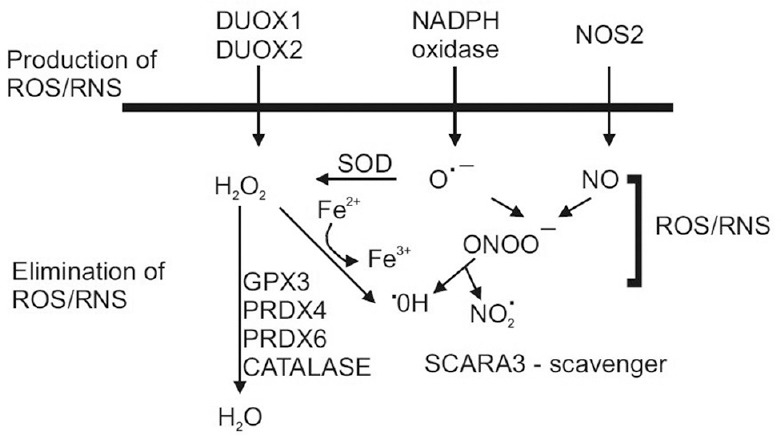
Schematic diagram of pathways involved in production and elimination of ROS/RNS. SOD, superoxide dismutase; NOS2, inducible nitric oxide synthase; H2O2, hydrogen peroxide; O•–, superoxide; ONOO– peroxynitrite; •OH, hydroxyl; NO2 •, Nitrogen dioxide. GPX3, glutathione peroxidase 3; PRDX4, peroxiredoxin 4; PRDX6, peroxiredoxin 6.
Evidence of the oxidative stress seen in corneas with KC has also been found in cultured KC cells. KC fibroblasts have higher levels of ROS/RNS and caspase-3/7 activity in vitro, along with altered levels of cytochrome c oxidase (complex IV subunit II, MTCO2), a decreased mitochondrial membrane potential, degraded mtDNA, and increased activation of the caspase-9 mitochondrial pathway.[9,16] There is normally a balance between the amounts of ROS and RNS produced and eliminated with antioxidants, but this balance is altered in patients with KC, even on a systemic level.[17,18]
The present study was designed to evaluate the key components involved in the balance of oxidative stress elements. On the production side, we measured gene expression levels for multiple subunits of NADPH oxidase, an enzyme responsible for production of superoxides, and for inducible nitric oxide synthase (NOS2), which produces nitric oxide [Figure 1]. On the elimination side, we measured the gene expression levels for nine antioxidant enzymes, along with SCARA3, which is a major ROS/RNS scavenger, and FOXM1, a transcription factor for antioxidant genes. Our findings suggest that oxidative stress likely results from a disruption of the elimination pathways rather than overproduction of ROS or RNS in corneas with KC. This finding may provide important guidance for future therapeutic reduction of oxidative damage in corneas with KC.
METHODS
Corneal Fibroblast Cultures
Six normal corneas from donors aged 24–59 (mean 40.2) years were received within 24 hours post mortem from the National Disease Research Interchange (Philadelphia, PA). Five corneas with KC from patients aged 25–46 (mean 34.8) years were received in McCarey-Kaufman medium on ice within 24 hours of penetrating keratoplasty. The donors and patients were matched for age and sex. The study was performed according to the tenets of the Declaration of Helsinki for research involving human subjects and a written informed consent was obtained from all study participants.
The diagnosis of KC was based on more than one of the following criteria: Munson's sign, Rizzuti phenomenon, slit-lamp findings (e.g., stromal thinning, Vogt's striae, Fleischer ring, and epithelial or subepithelial scarring); retroillumination signs (e.g., oil droplet sign, scissoring on retinoscopy); photokeratoscopy signs (e.g., infero-temporal, inferior, or central compression of mires); videokeratography signs (e.g., localized increased surface power and/or inferior-superior dioptric asymmetry), and corneal topography. NL and KC stromal cells were isolated and cultured as described elsewhere.[19] When corneal stromal cells are cultured in 10% fetal bovine serum, they differentiate into fibroblasts.[20,21] Our corneal cells stained with vimentin (a marker for fibroblasts) but did not stain with alpha smooth muscle actin (a marker for myofibroblasts). The morphology was consistent with fibroblasts and not corneal keratocytes.
Inhibitor Studies
Third passage fibroblasts were used for the individual experiments (n = 4). Cells were plated overnight in phenol red-free minimum essential medium with 10% fetal bovine serum (Omega Scientific Inc., Tarzana, CA). Some cells were cultured for 2 hours with either apocynin (30 μM, an inhibitor of NADPH oxidase [Sigma-Aldrich, St Louis, MO]) or N-nitro-L-arginine (N-LLA; 100 μM, an irreversible inhibitor of constitutive nitric oxide synthase [NOS]) and a reversible inhibitor of inducible NOS (iNOS; Sigma-Aldrich). ROS/RNS levels were measured using a fluorescent dye (2’-,7’-dichlorodihydrofluorescein diacetate) assay (Invitrogen, Waltham, MA) as described previously.[9] The statistical analyses were performed using the unpaired t-test.
Gene Expression Array Studies
The NL (n = 2) and KC (n = 2) corneal cell cultures were analyzed using the RT2 Profiler™ PCR Array for Oxidative Stress and Antioxidant Defense (SABiosciences, Frederick, MD). RNA was isolated using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) following the manufacturer's protocol. For the complementary DNA synthesis, 1 μg of RNA was used for the RT2 First Strand Kit (SABiosciences) according to the manufacturer's protocol. The complementary DNA was then processed at the UCLA Sequencing Core Facility (Los Angeles, CA). The analyses were performed using the SA Biosciences RT2 array analysis software.
Quantitative PCR Analyses
After our initial screen using the Oxidative Stress and Antioxidant Defense array, we performed quantitative polymerase chain reaction (Q-PCR) on enzymes associated with production and elimination of ROS/RNS in the NL (n = 6) and KC (n = 5) cultures. The NADPH oxidase family consists of 7 isoforms, each consisting of a core catalytic subunit (NOX and DUOX) with up to 5 regulatory subunits [Figure 2]: p22phox (CYBA), p67phox (NCF2), and p47phox (NCF1).[22] Gene expression analyses were also performed for antioxidant enzymes responsible for ROS/RNS elimination, i.e., CAT, GPX3, PRDX3, PRDX4, PRDX6, SOD1, SOD2, SOD3, and USP36. Isoforms for SCARA3, a ROS scavenger, were evaluated along with CPSF3, a processing endonuclease associated with SCARA3. The expression levels for FOXM1 (a transcription factor for antioxidant enzymes) were measured. The primers for the total FOXM1 and isoform 1 FOXM1 were available but not for isoforms 2 or 3. Q-PCR primers were purchased from Qiagen.
Figure 2.
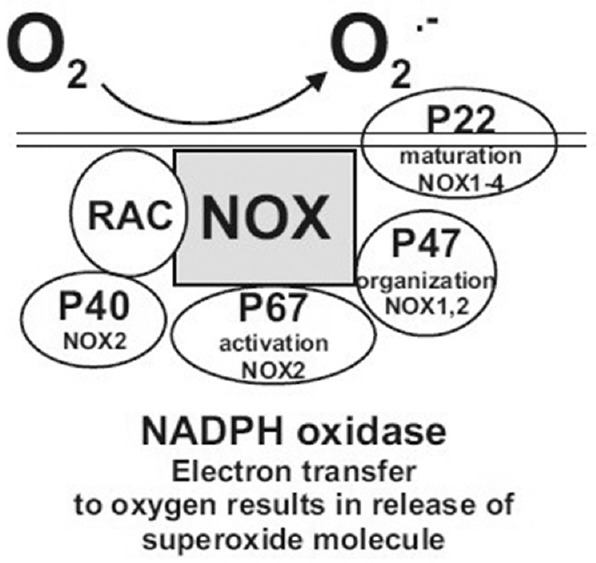
Schematic diagram of subunits associated with the NADPH oxidase enzyme. The p22phox subunit is utilized by NOX1-NOX4 but not the NOX5, DUOX1, or DUOX2 enzymes. The p47phox subunit is utilized by NOX1 and NOX2. The p67phox and p40phox subunits are only associated with NOX2.
RNA was extracted from the pellets of the cultured cells using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions and quantified on a Nanodrop 2000. Next, 500 ng of RNA was reverse-transcribed into complementary DNA using the QuantiTect Reverse Transcription Kit (Qiagen). The Q-PCR was carried out with the QuantiFast SYBR Green PCR Kit (Qiagen) according to the manufacturer's protocol, using β2-microglobulin primers (forward, CTCGCGCTACTCTCTCTTTCTG; reverse, GCTTACATGTCTCGATCCCACTT) and TATA box binding protein (Qiagen) as the housekeeping genes. The data were analyzed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA).
Immunohistochemistry Studies
Ten NL corneas and 10 KC corneas were examined by immunohistochemistry using the rabbit polyclonal antibody to Scavenger Receptor Class A, Member 3 protein (SCARA3, GeneTex, Irvine, CA) or the rabbit polyclonal antibody to Cleave and Polyadenyl Specific Factor 3 (CPSF3, GeneTex). The secondary antibody was the Alexa FluorÒ 555 goat anti-rabbit IgG (H + L; Invitrogen). The corneas were embedded in Tissue-Tek OCT compound (Sakura Finetek USA Inc., Torrance, CA) prior to freezing in liquid nitrogen. Tissue sections were cut, mounted onto microscope slides, and stored at −80°C. The tissues were counterstained with DAPI (Invitrogen). Slide images were captured with a Nikon E600 fluorescent microscope and MetaMorph software. The epithelial and stromal staining patterns were examined using a subjective 0–4+ scoring system (0, little or no staining; 1+, weak staining; 2+, moderate staining; 3+, intense staining; and 4+, very intense staining).
Statistical Analysis
The statistical analysis was performed using Simple Interactive Statistical Analysis (SISA) Internet software (Quantitative Skills, Amsterdam, the Netherlands) and the Student's t-test using GraphPad Prism software.
RESULTS
Pathways for Production of ROS/RNS
Studies were performed to determine if the ROS/RNS levels in NL and KC cultures could be altered by pre-treatment with apocynin, an inhibitor of NADPH oxidase, or N-LLA, an inhibitor of NOS. The KC cultures (n = 5) had a 1.8-fold increase in ROS/RNS levels compared to untreated NL cultures (P = 0.004), which could not be reversed by pre-treatment with apocynin (P = 0.9) or N-LLA (P = 0.8; Figure 3). These inhibitor studies indicated that the higher ROS/RNS levels found in KC cultures could not be reversed by blocking the NADPH oxidase or NOS pathways. The mechanism of action of apocynin involves inhibition of the association of the NADPH oxidase p47phox subunit with the membrane-bound heterodimer[22] and we reasoned that perhaps the p47phox subunit was diminished in human corneal fibroblasts. The gene expression patterns of various NADPH oxidase subunits were examined to ensure that our findings were not caused by reduced expression of the p47phox subunit in cultured KC cells. The p47phox subunit is associated with NOX1 and NOX2 but not with NOX3, NOX4, NOX5, DUOX1, or DUOX2. In addition to the p47phox subunit, the gene expression patterns of p22phox, which is associated with NOX1-NOX4, DUOX1 and DUOX2, were also analyzed. The mRNA expression of p67phox was measured because it is associated with NOX2 and expressed in human corneal cells.[23] Table 1 shows that the expression patterns for p47phox, p67phox, p22phox, DUOX1, DUOX2, and NOS2 were not changed in KC cells when compared with NL cells.
Figure 3.
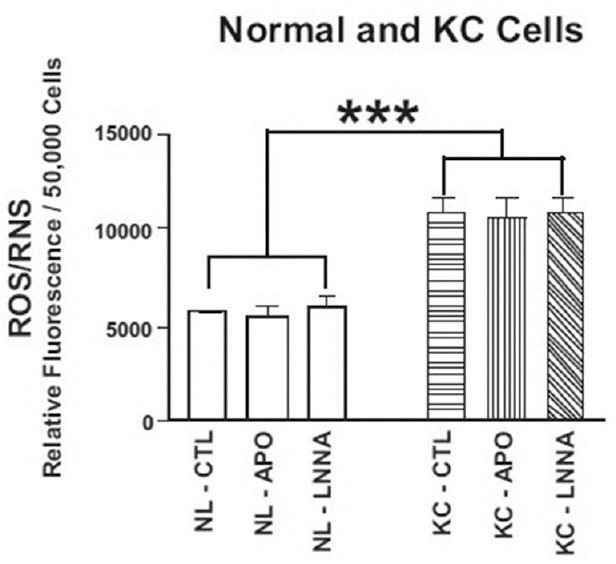
KC cells have significantly elevated ROS/RNS levels compared with NL cells (1.8-fold, ***P = 0.004). Pretreatment for 2 hours with 30 μM apocynin (an inhibitor of NADPH oxidase) or 100 μM N-nitro-L-arginine (an inhibitor of nitric oxide synthase) did not decrease the ROS/RNS levels to those in NL cells. KC, keratoconus; NL, normal; RNS, reactive nitrogen species; ROS, reactive oxygen species.
Table 1.
Differential Gene Expression in Normal Cells (n=6) Compared to KC Cells (n=5)
| Symbol | Gene Name | GeneBank Accession No. | Function | P | ΔΔCT | Fold |
|---|---|---|---|---|---|---|
| DUOX2, LNOX1 | Dual oxidase 2 | NM_014080 | Direct release of hydrogen peroxide | 0.06 | -0.81±0.41 | 0.57 |
| DUOX1, LNOX2 | Dual oxidase 2 | NM_175940 | Direct release of hydrogen peroxide | 0.59 | -0.41±0.75 | 0.75 |
| NM_017434 | ||||||
| NCF1, p47phox | Neutrolphil cytosolic factor 1 | NM_000265 | Organization subunit for NADPH oxidase | 0.13 | 0.94±0.6 | 1.9 |
| NCF2, p67phox | Neutrolphil cytosolic factor 2 | NM_000433 | Activation subunit for NADPH oxidase | 0.74 | 0.21±0.64 | 1.2 |
| NM_001127651 | ||||||
| NM_001190789 | ||||||
| NM_001190794 | ||||||
| CYBA, p22phox | Cytochrome b-245, alpha polypeptide | NM_000101 | Maturation subunit for NADPH oxidase | 0.09 | -0.58±0.34 | 0.67 |
| NOS2 | Nitric oxide synthase 2 | NM_153292 | Inducible NOS, production of nitric oxide | 0.11 | -0.46±0.28 | 0.73 |
| NM_000625 |
Fold values greater than 1 indicate up regulation of the gene compared to NL cells. Fold values less than 1 indicate down regulation of the gene compared to NL cells. NL cells are assigned a value of 1. Fold=2-ΔΔCT
Pathways for Elimination of ROS/RNS
Antioxidant genes associated with elimination of ROS/RNS were altered in KC cultures when compared with NL cultures [Table 2]. The KC cells had a significant reduction in gene expression for two antioxidant enzymes, i.e., SOD1 (NM_000454, P = 0.0001) and SOD3 (NM_003102, P = 0.02), while SOD2 (NM_00636, NM_001024465, P = 0.0001) and PRDX6 (NM_004905, P = 0.01) had increased expression. The levels for USP36, CAT, GPX3, and PRDX4 were similar in NL cells and KC cells.
Table 2.
Differential Expression of Antioxidant Enzyme Genes in Normal Cells (n=6) and KC Cells (n=5)
| Symbol | Gene Name | GeneBank accession no. | Function | P | ΔΔCT | Fold |
|---|---|---|---|---|---|---|
| CAT | Catalase | NM_001752 | Heme enzyme that converts reactive oxygen species to H2O and O2 | 0.139 | -0.47±0.31 | 0.72 |
| GPX3 | Glutathione peroxidase 3 (plasma) | NM_002084 | Detoxifies H2O2 | 0.069 | 1.03±0.55 | 2.05 |
| PRDX3 | Peroxiredoxin 3 | NM_006793 | Antioxidant enzyme localized in the mitochondria | 0.17 | 0.23±0.16 | 1.2 |
| PRDX4 | Peroxiredoxin 4, AOE37-2 | NM_006406 | Redox regulation, reduces H2O2 & hydroyperoxides | 0.08 | -0.63±0.35 | 0.65 |
| PRDX6 | Peroxiredoxin 6, 1-Cys, AOP2 | NM_004905 | Redox regulation, reduces H2O2 & hydroyperoxides | 0.01 | 0.56±0.21 | 1.47 |
| SOD1 | Superoxide dismutase 1, soluble | NM_000454 | Copper and zinc binding antioxidant enzyme that destroys free superoxide radicals | 0.0001 | -1.28±0.28 | 0.4 |
| SOD2 | Superoxide dismutase 2, mitochondrial | NM_000636 | Manganese binding antioxidant enzyme that converts superoxide byproducts of oxidative phosphorylation to H2O2 and O2 | <0.0001 | 1.74±0.3 | 3.3 |
| NM_001024465 | ||||||
| SOD3 | Superoxide dismutase 3, extracellular | NM_003102 | Antioxidant enzyme that catalyzes the dismutation of 2 superoxide radicals in to H2O2 and O2 | 0.02 | -1.43±0.57 | 0.37 |
| USP36 | Ubiquitin Specific Peptidase 36 | NM_025090 | Deubiquinating cysteine protease | 0.19 | 0.31±0.23 | 1.24 |
Fold values greater than 1 indicate up regulation of the gene compared to NL cells. Fold values less than 1 indicate down regulation of the gene compared to NL cells. NL cells are assigned a value of 1. Fold=2-ΔΔCT
SCARA3 (also known as CSR1 and MSR1) is a ROS scavenger that protects cells against ultraviolet light. The gene expression levels for total SCARA3 (NM_016240, NM_182826) and the full-length isoform, variant 1 (NM_016240), were not significantly changed in comparison with NL cells [Table 3]. The shorter SCARA3 variant 2 (NM_182826, see lower panel) showed a 0.59-fold decrease (P = 0.02) in the KC cells when compared with the NL cells. The gene expression levels for CPSF3, a pre-mRNA 3-prime end-processing endonuclease that is closely associated with SCARA3,[24] was significantly increased in the KC cells (1.44-fold, P = 0.0017).
Table 3.
Differential Gene Expression of SCARA3, a ROS Scavenger, in Normal Cells (n=6) and KC Cells (n=5)
| Symbol | Gene Name | GeneBank Accession No. | Function | P | ΔΔCT | Fold |
|---|---|---|---|---|---|---|
| SCARA3, Total | Scavenger receptor class A, member 3 | NM_016240 | Depletes ROS, protects cells from oxidative stress generated from UV light | 0.57 | 0.18±0.31 | 1.13 |
| NM_182826 | ||||||
| SCARA3, Isoform 1 | Scavenger receptor class A, member 3 | NM_016240 | Depletes ROS, protects cells from oxidative stress generated from UV light | 0.29 | -0.29±0.27 | 0.82 |
| SCARA3, Isoform 2 | Scavenger receptor class A, member 3 | NM_182826 | Depletes ROS, protects cells from oxidative stress generated from UV light | 0.02 | -0.75±0.3 | 0.59 |
| CPSF3 | Cleavage and polyadenylation specificity factory 3 | NM_016207 | Pre-mRNA 3-prime-end-processing endonuclease | 0.0017 | 0.53±0.15 | 1.44 |
Fold values greater than 1 indicate up regulation of the gene compared to NL cells. Fold values less than 1 indicate down regulation of the gene compared to NL cells. NL cells are assigned a value of 1. Fold=2-ΔΔCT
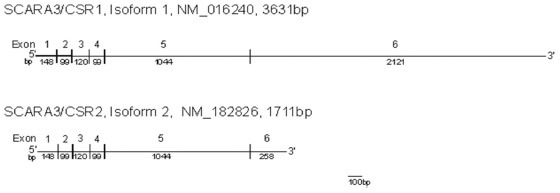
Given that SCARA3 and CPSF3 have not been previously identified in human corneas, immunohistochemistry was performed on NL and KC corneas with SCARA3-specific and CPSF3-specific antibodies [Figure 4]. The SCARA3 and CPSF3 proteins were present in stromal cells in both NL and KC corneas (box inserts). Surprisingly, we found the greatest staining for the SCARA3 protein in the NL epithelium (+4 in 4 samples and + 3 in 6 samples) with slightly less in the KC epithelial cells (+3 in 7 samples and +2 in 3 samples).
Figure 4.
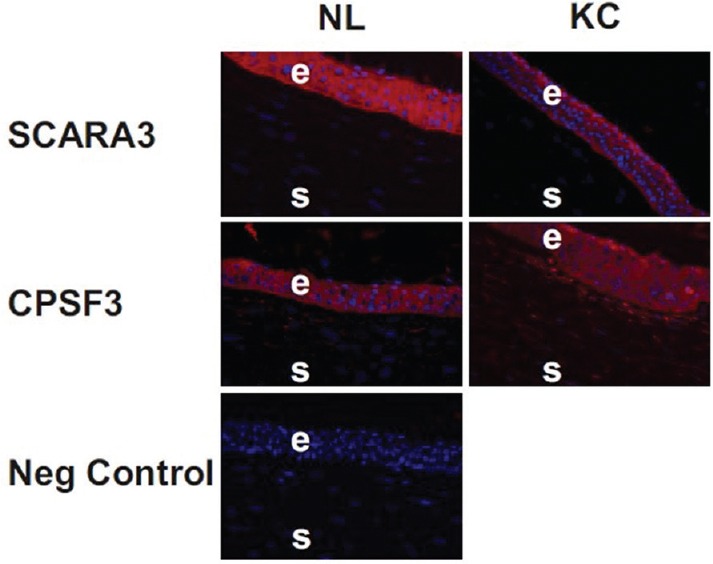
Immunohistochemistry shows SCARA3 staining in NL and KC corneas. The epithelial cells have a distribution of SCARA3 throughout the cytoplasm of the NL and KC corneas. The CPSF3 protein staining is found within the cytoplasm of the epithelial cells and stromal cells. No staining was observed when only the secondary antibody (IgG) was used on the tissue sections (negative control). NL, normal; KC, keratoconus, E, epithelial; S, stroma.
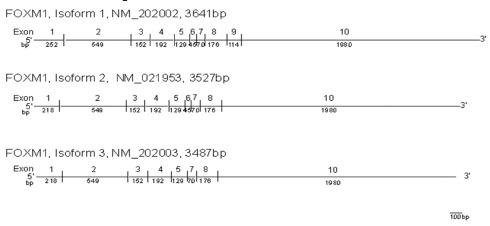
Expression for the FOXM1 isoform 1, an important transcription factor for antioxidant genes, was significantly decreased in KC cells [Table 4]. In these cells, expression of the FOXM1 isoform variant 1 (NM_202002) was decreased by 0.61-fold (P = 0.03). This full-length FOXM1 isoform has 10 exons and contains 3641 bp. Exon 9 is missing in isoform variant 2 of FOXM1 (NM_021953), while both exons 6 and 9 are missing in isoform variant 3 of FOXM1 (NM_202003).
Table 4.
Differential Gene Expression of FOXM1 Transcription Factor in Normal Cells (n=6) and KC Cells (n=5)
| Symbol | Gene Name | GeneBank Accession No. | Function | P | ΔΔCT | Fold |
|---|---|---|---|---|---|---|
| FOXM1, Total | Forkhead box M1 | NM_021953 | Transcription factor of antioxidant genes | 0.6 | -0.25±0.49 | 0.84 |
| NM_202002 | ||||||
| NM_202003 | ||||||
| FOXM1 Isoform 1 | Forkhead box M1 | NM_202002 | Transcription factor of antioxidant genes | 0.03 | -0.72±0.32 | 0.61 |
Fold values greater than 1 indicate up regulation of the gene compared to NL cells. Fold values less than 1 indicate down regulation of the gene compared to NL cells. NL cells are assigned a value of 1. Fold=2-ΔΔCT
DISCUSSION
Pathways for Production of ROS/RNS
The major source of superoxides in human corneal stromal fibroblasts has been reported to be through the NADPH oxidase pathway.[23] Our results support this finding and show that NL and KC human corneal fibroblasts have similar expression levels of p22phox, p47phox, and p67phox NADPH oxidase subunits. Analyses of other genes in the NADPH oxidase family showed expression of DUOX1 and DUOX2 in human NL and KC corneal cells. Unlike other NADPH oxidase family members that produce superoxides that are then converted into hydrogen peroxides, the DUOX1 and DUOX2 oxidases are NADPH oxidase homologues that directly produce hydrogen peroxide.[25,26] Although DUOX1 and DUOX2 proteins have been previously reported in thyroid cells, epithelial cells in the airway, prostate cells, salivary glands, and gastrointestinal cells,[27] their roles in human corneal cells are unclear at this time and warrant further investigation. No differences in any of the NADPH oxidase enzymes were found between NL and KC cells, so we concluded that it was unlikely that the elevated ROS/RNS levels in KC cells were the result of increased NADPH oxidase-related production.
While Buddi et al observed that KC stromal cells show increased accumulation of iNOS protein,[8] our KC and NL cell cultures showed similar expression levels of iNOS. Peroxynitrite and nitric oxide are toxic to corneal cells due to inducing gelatinase/matrix metalloproteinase (MMP)-2 activity and diminishing TIMP-1 levels, reflecting a direct relationship between elevated oxidative stress and tissue degradation.[12,28] Activation of MMPs, such as MMP-2 and proxidase, is important in the degradation and remodeling of collagen in the corneal stroma.[29] Oxidative stress-induced activation of MMPs and abnormal enzyme levels contribute to the stromal thinning and loss of Bowman's layer which are characteristics of KC.
Pathways for Elimination of ROS/RNS
An alternative mechanism for accumulation of ROS/RNS is decreased removal of the oxidants once they are formed. Screening of 84 antioxidant-related genes showed 12 genes that were differentially expressed between the NL and KC cultures. These were further analyzed with Q-PCR and showed depressed expression levels of SOD1 and SOD3, both of which are critical antioxidant enzymes that convert superoxide to hydrogen peroxide. The SOD1 gene (cytosolic copper-zinc SOD) is on chromosome 21 and has been linked to KC through a deletion within intron 2 that segregates in affected family members.[30,31] This deletion was recently reported in subjects with sporadic KC,[32] contrasting with other studies that did not find this defect in association with KC.[33] The enzyme activity for SOD3 (extracellular matrix SOD) was also lower in corneas with KC.[34] SOD1-null and SOD3-null mouse models showed elevated superoxide levels in the corneas, which could contribute to oxidative damage.[35] Furthermore, it is reasonable to propose that lower antioxidant enzyme levels in KC cells may lead to increased accumulation of ROS/RNS, as seen in our cultured KC cells.
SOD2 is a tetrameric enzyme that contains manganese and is located in the matrix of the mitochondria. Previously, it has been shown in an American population[15] and in a Chinese population[36] that corneas with KC have more mtDNA damage than normal corneas. While the Chinese study found that expression of SOD2 was lower in corneas with KC than in normal corneas,[36] it was 3.3-fold higher in KC cells than in NL cells in the present study. Interestingly, Ortak et al reported that subjects with KC had higher total plasma SOD levels than subjects without KC.[37] These conflicting findings point to a need for additional studies to explain this discrepancy and whether increased mtDNA damage has stimulating or inhibiting effects on transcription of SOD2. Based on the results of this study and other previous studies, the elimination patterns for ROS/RNS are significantly altered in subjects with KC and in KC cell cultures, which can contribute to oxidative damage and enzymatic matrix degradation, which are key features in corneas with KC.
PRDX6 is a redox regulation enzyme that reduces levels of hydrogen peroxide and phospholipid hydroperoxides and protects cells against oxidative injury and inflammation.[38] We found that expression levels of PRDX6 were higher in KC cells than in NL cells. Differential expression of peroxiredoxin isoforms has been associated with Fuchs’ endothelial dystrophy.[39] In human ovarian cancer cells, its overexpression reduces ROS levels and caspase activity and stops cells from undergoing apoptosis.[40] Given the altered levels of numerous antioxidant enzymes, corneas with KC would have altered levels of superoxides and hydrogen peroxide, which could dramatically change cell signaling cascades, cytokine levels, and degradative enzyme activity.
The KC cells showed a significant decrease in isoform 2 SCARA3 gene expression when compared to the NL cells. SCARA3, located at chromosome 8p21, is a critical scavenger protein that depletes ROS and thereby protects cells from oxidative stress. When in the unstressed state, SCARA3 is localized to the Golgi apparatus in the endoplasmic reticulum; however, upon exposure to hydrogen peroxide, SCARA3 scavenger proteins diffuse into the cytoplasm[41] where they transport free radicals to lysosomes for processing and removal. SCARA3 has two alternatively spliced variants. The shorter SCARA3 isoform (CSR2, NM_182826), which is present at lower levels in KC cells, lacks the collagen-like domain, has exon 6 shortened to 258 bp with a shorter C-terminal tail, and codes for a 466-amino acid protein. SCARA3/CSR1 isoform 1 (NM_01240) contains 3631 bp with a full-size exon 6 and a long poly(A)3’ tail. The predicted 606-amino acid protein has a leucine zipper motif that overlaps at its N-terminus with a transmembrane domain and two collagen-like domains. The protein has numerous phosphorylation and N-glycosylation sites, a putative heme-binding site, and microbody-targeting signals.
Normal skin fibroblasts exposed to ultraviolet light or hydrogen peroxide show significantly elevated CSR/SCARA3 expression, which can be reversed by pretreatment with antioxidant compounds.[41] Cells overexpressing CSR1/SCARA3 or CSR2/SCARA3 have increased resistance to damage caused by ultraviolet light.[41] This feature is an important property for corneal cells that are constantly being exposed to sunlight. The collagenous domains of the full-length CSR1/SCARA3 isoform can bind lipids and other negatively charged molecules, such as fibrinogen and laminins. The shorter splice variant CSR2/SCARA3 lacks these collagenous domains, which could contribute to functional behavior different from that of the full-length CSR1/SCARA3. The significance of decreased SCARA3 in KC cells is not known, but one could speculate that lower levels of this important scavenger would result in elevated levels of ROS/RNS and the increased apoptosis of anterior stromal cells found in corneas with KC.[42] Another mechanism by which CSR/SCARA3 may affect KC cells is via its association with CPSF3, an enzyme necessary for polyadenylation of RNA.[43,44] The CPSF3 protein is a critical pre-mRNA 3-prime end-processing endonuclease that converts heteronuclear RNA to mRNA.[24] Increased levels of CPSF3, as seen in KC cells, potentially alter patterns of cell death and other cell functions. Zhu et al showed that the C-terminus of CSR1/SCARA3 interacts with CPSF3, which is then translocated from its location in the nucleus to a cytosolic distribution.[45] In KC cells, there were significantly higher expression levels of SCARA3 isoform 2, which has a shortened C-terminus. This altered expression could potentially affect pre-mRNA processing within the cells and might account for the numerous altered genes reportedly found in corneas with KC.[46,47,48]
Altered Gene Regulation
Our data, along with those of others, suggest that KC cells have abnormalities in multiple antioxidant pathways that contributes to the pathology of KC. The present study showed that KC cells had a 0.61-fold decline in FOXM1 isoform 1 (NM_202002). FOXM1 is in the Forkhead Box transcription factor family that has a conserved winged helix DNA domain and is a transcription factor for antioxidant enzymes. It has dual functions involving cell cycle progression and also tumor cell migration/invasion. FOXM1 promotes cell growth by regulating genes involved in DNA repair, mitosis, chromatin assembly, and protein metabolism.[49,50] In tumors, high FOXM1B expression levels are correlated with a poor prognosis.[49] FOXM1 plays an essential role in regulation of oxidative stress[51] and modulates intracellular ROS levels by upregulating expression levels of antioxidant genes that protect cells from oxidative damage. Fibroblasts with FOXM1 silenced by siRNA transfection become significantly less viable after treatment with hydrogen peroxide.[51] Low levels of FOXM1 in KC cells result in diminished expression of antioxidant genes and potential accumulation of ROS/RNS within these cells. This would render KC cells more susceptible to oxidative damage and less able to survive exposure to stressors, such as hydrogen peroxide or low pH.[9,16]
In summary, our findings suggest that the elevated ROS/RNS levels found in KC cells are more likely to reflect less efficient elimination of these oxidants rather than overproduction. The antioxidant quercetin is a potential therapy that may address both the elimination of oxidants as well as regulation of metabolism in corneas with KC, considering that excessive lactate production has been associated with oxidative stress and KC.[52,53,54] Future studies should focus on therapeutic agents that can bring the ROS/RNS balance into equilibrium and reduce oxidative damage in corneas with KC.
Financial Support and Sponsorship
This study was supported by Schoellerman Charitable Foundation, the Discovery Eye Foundation, Departmental Unrestricted Grant from Research to Prevent Blindness, and the National Keratoconus Foundation.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
We want to thank all of the keratoconus patients and their families for participating in this study.
REFERENCES
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Zadnik K, Barr JT, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea. 1996;15:139–146. doi: 10.1097/00003226-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Godefrooij D, de Wit G, Uiterwaal C, Imhof S, Wisse R. Age-specific incidence and prevalence of keratoconus: A Nationwide Registration Study. Am J Ophthalmol. 2017;175:169–172. doi: 10.1016/j.ajo.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Ihalainen A. Clinical and epidemiological features of keratoconus genetic and external factors in the pathogenesis of the disease. Acta Ophthalmol Suppl. 1986;178:1–64. [PubMed] [Google Scholar]
- 6.Gonzalez V, McDonnell P. Computer-assisted corneal topography in parents of patients with keratoconus. Arch Ophthalmol. 1992;110:1413–1414. doi: 10.1001/archopht.1992.01080220074024. [DOI] [PubMed] [Google Scholar]
- 7.Bisceglia L, Ciaschetti M, De Bonis P, Campo PA, Pizzicoli C, Scala C, et al. VSX1 mutational analysis in a series of Italian patients affected by keratoconus: Detection of a novel mutation. Invest Ophthalmol Vis Sci. 2005;46:39–45. doi: 10.1167/iovs.04-0533. [DOI] [PubMed] [Google Scholar]
- 8.Buddi R, Lin B, Atilano SR, Zorapapel NC, Kenney MC, Brown DJ. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002;50:341–351. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- 9.Chwa M, Atilano SR, Reddy V, Jordan N, Kim DW, Kenney MC. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:1902–1910. doi: 10.1167/iovs.05-0828. [DOI] [PubMed] [Google Scholar]
- 10.Saijyothi AV, Fowjana J, Madhumathi S, Rajeshwari M, Thennarasu M, Prema P, et al. Tear fluid small molecular antioxidants profiling shows lowered glutathione in keratoconus. Exp Eye Res. 2012;103:41–46. doi: 10.1016/j.exer.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Arnal E, Peris-Martinez C, Menezo JL, Johnsen-Soriano S, Romero FJ. Oxidative stress in keratoconus? Invest Ophthalmol Vis Sci. 2011;52:8592–8597. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- 12.Brown DJ, Lin B, Chwa M, Atilano SR, Kim DW, Kenney MC. Elements of the nitric oxide pathway can degrade TIMP-1 and increase gelatinase activity. Mol Vis. 2004;10:281–288. [PubMed] [Google Scholar]
- 13.Vallabh NA, Romano V, Willoughby CE. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion. 2017;36:103–113. doi: 10.1016/j.mito.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Pathak D, Nayak B, Singh M, Sharma N, Tandon R, Sinha R, et al. Mitochondrial complex 1 gene analysis in keratoconus. Mol Vis. 2011;17:1514–1525. [PMC free article] [PubMed] [Google Scholar]
- 15.Atilano SR, Coskun P, Chwa M, Jordan N, Reddy V, Le K, et al. Accumulation of mitochondrial DNA damage in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1256–1263. doi: 10.1167/iovs.04-1395. [DOI] [PubMed] [Google Scholar]
- 16.Chwa M, Atilano SR, Hertzog D, Zheng H, Langberg J, Kim DW, et al. Hypersensitive response to oxidative stress in keratoconus corneal fibroblasts. Invest Ophthalmol Vis Sci. 2008;49:4361–4369. doi: 10.1167/iovs.08-1969. [DOI] [PubMed] [Google Scholar]
- 17.Toprak I, Kucukatay V, Yildirim C, Kilic-Toprak E, Kilic-Erkek O. Increased systemic oxidative stress in patients with keratoconus. Eye (Lond) 2014;28:285–289. doi: 10.1038/eye.2013.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilic R, Cavunt A, Bayraktar S, Kurt A, Kavutcu M. Evaluation of serum superoxide dismutase activity, malondialdehyde, and zinc and copper levels in patients with keratoconus. Cornea. 2016;35:1512–1515. doi: 10.1097/ICO.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 19.Kenney MC, Chwa M, Opbroek AJ, Brown DJ. Increased gelatinolytic activity in keratoconus keratocyte cultures. Cornea. 1994;13:114–124. doi: 10.1097/00003226-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hassel JR, Schrecengost PK, Rada JA, SundarRaj N, Sassi G, Thoft RA. Biosynthesis of stromol motrix proteoglycans and basement membrane components by human corneal fibroblasts. Invest Opthalmol Vis Sci. 1992;33:547–557. [PubMed] [Google Scholar]
- 21.Jester JV, Huang J, Fisher S, Spiekerman J, Chang JH, Wright WE, et al. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- 22.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien WJ, Heimann T, Rizvi F. NADPH oxidase expression and production of superoxide by human corneal stromal cells. Mol Vis. 2009;15:2535–2543. [PMC free article] [PubMed] [Google Scholar]
- 24.Mandel CR, Kaneko S, Zhang H, Gebauer D, Vethantham V, Manley JL, et al. Polyadenylation factor CPSF-73 is the pre-mRNA 3’-end-processing endonuclease. Nature. 2006;444:953–956. doi: 10.1038/nature05363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 28.Bourges JL, Torriglia A, Valamanesh F, Benezra D, Renard G, Behar-Cohen FF. nitrosative stress and corneal transplant endothelial cell death during acute graft rejection. Transplantation. 2007;84:415–423. doi: 10.1097/01.tp.0000275378.45133.82. [DOI] [PubMed] [Google Scholar]
- 29.Kilic R, Cumurcu T, Sancaktar E, Evliyaoglu O, Sezer H. Systemic prolidase activity and oxidative stress in keratoconus. Curr Eye Res. 2016;41:28–33. doi: 10.3109/02713683.2015.1004717. [DOI] [PubMed] [Google Scholar]
- 30.Udar N, Atilano SR, Brown DJ, Holguin B, Small K, Nesburn AB, et al. SOD1: A candidate gene for keratoconus. Invest Ophthalmol Vis Sci. 2006;47:3345–3351. doi: 10.1167/iovs.05-1500. [DOI] [PubMed] [Google Scholar]
- 31.Udar N, Atilano SR, Small K, Nesburn AB, Kenney MC. SOD1 haplotypes in familial keratoconus. Cornea. 2009;28:902–907. doi: 10.1097/ICO.0b013e3181983a0c. [DOI] [PubMed] [Google Scholar]
- 32.De Bonis P, Laborante A, Pizzicoli C, Stallone R, Barbano R, Longo C, et al. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol Vis. 2011;17:2482–2494. [PMC free article] [PubMed] [Google Scholar]
- 33.Stabuc-Silih M, Strazisar M, Hawlina M, Glavac D. Absence of pathogenic mutations in VSX1 and SOD1 genes in patients with keratoconus. Cornea. 2010;29:172–176. doi: 10.1097/ICO.0b013e3181aebf7a. [DOI] [PubMed] [Google Scholar]
- 34.Behndig A, Karlsson K, Johansson BO, Brannstrom T, Marklund SI. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Invest Ophthalmol Vis Sci. 2001;42:2293–2296. [PubMed] [Google Scholar]
- 35.Behndig A. Corneal endothelial integrity in aging mice lacking superoxide dismutase-1 and/or superoxide dismutase-3. Mol Vis. 2008;14:2025–2030. [PMC free article] [PubMed] [Google Scholar]
- 36.Hao XD, Chen ZL, Qu ML, Zhao XW, Li SX, Chen P. Decreased integrity, content, and increased transcript level of mitochondrial DNA are associated with keratoconus. PLoS One. 2016;11:e0165580. doi: 10.1371/journal.pone.0165580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortak H, Sogut E, Tas U, Mesci C, Mendil D. The relation between keratoconus and plasma levels of MMP-2, zinc, and SOD. Cornea. 2012;31:1048–1051. doi: 10.1097/ICO.0b013e318254c028. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, Yu HJ, Wang HY, Wang WT, Jin SH, Zhu P, et al. Topical administration of peroxiredoxin-6 on the cornea suppresses inflammation and neovascularization induced by ultraviolet radiation. Invest Ophthalmol Vis Sci. 2012;53:8016–8028. doi: 10.1167/iovs.12-10064. [DOI] [PubMed] [Google Scholar]
- 39.Jurkunas UV, Rawe I, Bitar MS, Zhu C, Harris DL, Colby K, et al. Decreased expression of peroxiredoxins in fuchs’ endothelial dystrophy. Invest Ophthalmol Vis Sci. 2008;49:2956–2963. doi: 10.1167/iovs.07-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pak JH, Choi WH, Lee HM, Joo WD, Kim JH, Kim YT, et al. Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Invest. 2011;29:21–28. doi: 10.3109/07357907.2010.535056. [DOI] [PubMed] [Google Scholar]
- 41.Han HJ, Tokino T, Nakamura Y. CSR, a scavenger receptor-like protein with a protective role against cellular damage caused by UV irradiation and oxidative stress. Hum Mol Genet. 1998;7:1039–1046. doi: 10.1093/hmg/7.6.1039. [DOI] [PubMed] [Google Scholar]
- 42.Kim WJ, Rabinowitz YS, Meisler DM, Wilson SE. Keratocyte apoptosis associated with keratoconus. Exp Eye Res. 1999;69:475–481. doi: 10.1006/exer.1999.0719. [DOI] [PubMed] [Google Scholar]
- 43.Ryan K. Evidence that polyadenylation factor CPSF-73 is the mRNA 3’ processing endonuclease. RNA. 2004;10:565–573. doi: 10.1261/rna.5214404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominski Z, Yang XC, Purdy M, Wagner EJ, Marzluff WF. A CPSF-73 homologue is required for cell cycle progression but not cell growth and interacts with a protein having features of CPSF-100. Mol Cell Biol. 2005;25:1489–1500. doi: 10.1128/MCB.25.4.1489-1500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu ZH, Yu YP, Shi YK, Nelson JB, Luo JH. CSR1 induces cell death through inactivation of CPSF3. Oncogene. 2009;28:41–51. doi: 10.1038/onc.2008.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph R, Srivastava OP, Pfister RR. Differential epithelial and stromal protein profiles in keratoconus and normal human corneas. Exp Eye Res. 2011;92:282–298. doi: 10.1016/j.exer.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen K, Birkenkamp-Demtro¨der K, Ehlers N, Orntoft TF. Identification of differentially expressed genes in keratoconus epithelium analyzed on microarrays. Invest Ophthalmol Vis Sci. 2003;44:2466. doi: 10.1167/iovs.02-0671. [DOI] [PubMed] [Google Scholar]
- 48.Mace M, Galiacy SD, Erraud A, Mejía JE, Etchevers H, Allouche M, et al. Comparative transcriptome and network biology analyses demonstrate antiproliferative and hyperapoptotic phenotypes in human keratoconus corneas. Invest Ophthalmol Vis Sci. 2011;52:6181–6191. doi: 10.1167/iovs.10-70981. [DOI] [PubMed] [Google Scholar]
- 49.Wang IC, Chen YJ, Hughes DE, Ackerson T, Major ML, Kalinichenko VV, et al. FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. J Biol Chem. 2008;283:20770–20778. doi: 10.1074/jbc.M709892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrovic V, Costa RH, Lau LF, Raychaudhuri P, Tyner AL. Negative regulation of the oncogenic transcription factor FoxM1 by thiazolidinediones and mithramycin. Cancer Biol Ther. 2010;9:1008–1016. doi: 10.4161/cbt.9.12.11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, et al. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908–2918. doi: 10.1038/emboj.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKay TB, Lyon D, Sarker-Nag A, Priyadarsini S, Asara JM, Karamichos D. Quercetin attenuates lactate production and extracellular matrix secretion in keratoconus. Sci Rep. 2015;5:9003. doi: 10.1038/srep09003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKay TB, Sarker-Nag A, Lyon D, Asara JM, Karamichos D. Quercetin modulates keratoconus metabolism in vitro . Cell Biochem Funct. 2015;33:341–350. doi: 10.1002/cbf.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKay TB, Karamichos D. Quercetin and the ocular surface: What we know and where we are going. Exp Biol Med (Maywood) 2017;242:565–572. doi: 10.1177/1535370216685187. [DOI] [PMC free article] [PubMed] [Google Scholar]


