Abstract
Purpose:
To assess the effect of a novel intense pulsed light (IPL) therapy on tear proteins and lipids in eyes with Meibomian gland dysfunction (MGD).
Methods:
Twenty-four eyes of 12 patients with MGD were recruited and received five overlapping flashes (565-1400 nm) directed at the lower eyelid. The IPL parameters include intensity: 2.5 to 6.5 J/cm2, voltage: 100 to 240 V, frequency: 50 to 60 Hz, input: 16 W, maximum optical energy: 23 J, pulse duration: <2.0 ms, and repetition time: 1-3.5 s. Tear samples were evaluated immediately before and 2 weeks after IPL therapy and included measurements of protein concentration, electrophoretic mobility by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, lipid profile assessments, and thin-layer chromatography (TLC) for phospholipids.
Results:
Significant improvements were observed in tear protein concentrations and molecular weight after IPL therapy. The most pronounced effect was in the molecular weight of tear lysozyme, lactoferrin, and albumin. Tear lipids showed an improvement in the concentrations of total lipids, triglycerides, cholesterol, and phospholipids. On TLC, the tears in patients with MGD had significantly lower amounts of anionic phosphatidylethanolamine, phosphatidylinositol, and phosphatidylserine but amounts zwitterionic neutral phospholipid phosphatidylcholine were normal. These anionic phospholipids showed obvious recovery after IPL therapy.
Conclusion:
IPL therapy is effective in eyes with MGD. It improved tear protein and lipid content and composition. The anionic phospholipids were more responsive to IPL therapy than were the other zwitterionic phospholipids.
Keywords: Intense Pulsed Light, Lipids, Meibomian Gland Dysfunction, Protein, Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis, Tears, Thin-layer Chromatography
INTRODUCTION
Meibomian gland dysfunction (MGD) is one of the most common causes of dry eye.[1] It is a diffuse malfunction of the Meibomian glands, whose terminal duct is completely or partially obstructed. The glandular secretion changes in quality or/and quantity, resulting in an unstable tear film.[1] Its main signs range from dryness, eye irritation, foreign body sensation, burning, watering, and fatigue.[2] Its occurrence fluctuates worldwide, from 3.5% to nearly 70%, and this makes scientists and clinical doctors anxious.[3]
MGD pathogenesis starts with ductal epithelium hyperkeratinization and increased meibum viscosity. The obstruction occurs when the terminal duct is filled with thickened meibum, which comprises keratinized cell material, resulting in intraglandular cystic dilation, gland failure, and low secretion.[1] Decreased meibum outflow increases the production and release of fatty acids, monoglycerides, and diglycerides by commensal bacteria into the tear film, thereby triggering irritation.[4,5]
Previous treatments for MGD varied from artificial tears, warm compression, Meibomian gland expression, and omega-3 supplementation to topical cyclosporine and corticosteroids, and oral antibiotics; all of which provide only short-term symptom relief.[6,7,8,9,10,11] This indicates the need for more treatment options, including intense pulsed light (IPL) therapy. The early application of IPL was initiated in patients with dry eye disease (DED), when a patient with rosacea showed improvement of dry eye symptoms after receiving IPL therapy. IPL therapy was first authorized by the U.S. Food and Drug Administration in 1995 for the treatment of lower-limb telangiectasias. It is usually used in the cosmetic industry to treat vascular abnormalities, such as benign venous malformations, cavernous hemangiomas, telangiectasias, port-wine stains, rosacea, and acne.[12,13,14,15,16] This polychromatic, noncoherent, and broad-spectrum pulsed light source applies a xenon flash lamp to emit wavelengths of light ranging from 400 to 1200 nm, which simultaneously targets numerous chromophores (such as hemoglobin and melanin). Numerous studies showed that hemoglobin primarily absorbs at a wavelength of 580 nm, causing the blood cells in the abnormal telangiectasias to absorb the light, coagulate, close the blood vessels, and thus reduce vascularization.[17] Moreover, IPL therapy has been employed in dermatology for treating hypertrichosis, facial rhytids, and pigmented lesions.[18] IPL therapy is an effective and safe monotherapy for managing inflammatory acne vulgaris, improving skin elasticity, and decreasing the amount and depth of wrinkles with a low complication risk.[19,20] Subsequent studies using IPL therapy for treating DED caused by MGD have regularly shown its benefits.[15,16,21,22,23] This study aimed to assess the effect of a single session IPL therapy on tear proteins and lipids in patients with MGD.
METHODS
Patients
Patients (mean age, 50 ± 10 years) diagnosed with MGD were selected from the outpatient ophthalmic unit of our institute. The patients complained of one of the following symptoms: dryness, foreign body sensation, burning, and tearing for more than 3 months. In addition, clinical examination revealed redness or thickening of the eyelid margin, telangiectasia, reduced or no secretions, poor tear break up time, and gland capping. The control group was selected from the workplace and previously diagnosed as not suffering from any eye disease.
Treatment Procedure
IPL therapy was applied as described by Toyos et al after obtaining written informed consent from all the patients.[24] The study was conducted according to the principles of the Declaration of Helsinki and was approved by the Human Research and Ethics Committee of our institute.
Using an IPL device (Philips Lumea SC2007/60; Philips, Netherlands), we applied five overlapping flashes directed at the skin below the lower eyelid of the patients with no pressure. The emitted light wavelength ranged from 565 to 1400 nm, and the light intensity ranged from 2.5 to 6.5 J/cm2 according to Fitzpatrick skin type grading.[23] The operating voltage was 100-240 V; frequency, 50-60 Hz; input, 16 W; maximum optical energy, 23 J; pulse duration <2.0 ms; and repetition time, 1-3.5 s. During the IPL therapy, the treatment areas were identical for different patients. Ultrasound gel was applied on the patient's face from the tragus to tragus to conduct the light, aid in spreading the energy recurrently, and offer a degree of safety.
Tear Sample Collection
Tear samples (25-100 μl) were collected from control subjects and from patients with MGD before and 2 weeks after IPL therapy. Reflex tear secretion was stimulated by directing a jet of pressurized air onto the cornea. Mechanical or chemical stimulation was avoided because it could lead to an elevated level of albumin originating from the serum. Tear fluid was collected using 50-μl glass capillaries and stored in polyethylene tubes at -20°C until analysis.[25]
Protein Analysis
Total protein content was obtained by measuring the absorbance at 750 nm by using a spectrophotometer (Evolution 600 PC UV-Vis; Thermo Fisher Scientific, Madison, WI). The standard curve was generated by plotting the absorbance at 750 nm of different standard protein concentrations of bovine serum albumin, and was used as a reference to determine the concentration of the unknown tear samples.[26] Protein composition of the tears was analyzed according to the molecular weight using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) employing 10% separating gel and 3% stacking gel.[27]
Lipid Analysis
Total lipid content was measured by reacting the lipids with sulfuric and phosphoric acids and vanillin to form a pink colored complex. The absorbance of the samples and standard was measured against that of the blank at 545 nm.[28] Cholesterol content was determined using an enzymatic colorimetric method after enzymatic hydrolysis and oxidation, by measuring the absorbance of the standard and tear samples against that of the blank at 500 nm.[29] Triglyceride content was determined using an enzymatic colorimetric method, and the absorbance of the samples and standard was measured against that of the blank at 505 nm.[30] Phospholipids were precipitated using trichloroacetic acid and oxidized to phosphate with sulfuric acid and perchloric acid. Inorganic phosphorus present as phosphate forms a phosphomolybdate complex with molybdic acid. The complex is reduced by stannous chloride to a blue colored complex, which can be measured colorimetrically at 650 nm.[31]
Thin-layer chromatography (TLC) was performed for quantitative analysis of phospholipids using a thick layer of silica gel on glass plates.[32] Four reference phospholipids of the highest purity, ranging in quantities from 30 to 60 μg, were pooled together. Phosphatidylinositol, phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine were used for this study and applied together with 50-μl tear samples. All reagents were analytical-reagent grade, purchased from Sigma-Aldrich (St. Louis, MO). The data on tear protein, lipid, and phospholipid contents, measured before and after treatment, were compared using Student's t-test. All values were expressed as the mean ± SD, and P < 0.05 was considered statistically significant.
RESULTS
Demographic Data
The participated subjects (24 subjects) in this study were divided into two groups: (a) control group (12 subject, 50%, 24 eyes) was selected from the work place in our institute and (b) MGD group (12 subject, 50%, 24 eyes). The participants were of 50 ± 10 years of age. There were 58.3% males and 41.7% females for both groups.
Total Protein Content
Figure 1 illustrates the concentration of tear proteins in the controls, patients before IPL therapy, and patients 2 weeks after therapy. The total protein concentration was 53.65 ± 1.2 mg/ml and 72.13 ± 1.98 mg/ml in controls and patients with MGD, respectively (P < 0.001). Two weeks after IPL therapy, tear protein content in the patients showed an obvious improvement with a value of 56.23 ± 2.19 mg/ml, which was comparable to that in the controls (P < 0.05).
Figure 1.
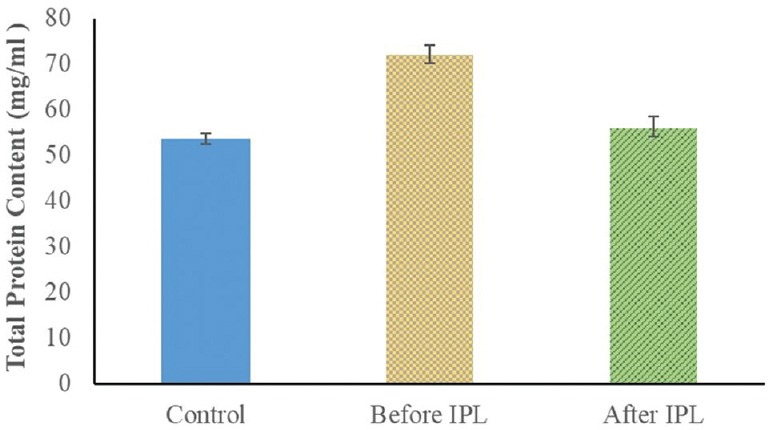
Total protein content in tear samples from the controls and eyes of patients with Meibomian gland dysfunction (MGD) before treatment with intense pulsed light (IPL).
SDS-PAGE
The SDS-PAGE scanning patterns of tear proteins of the controls and patients before IPL therapy are illustrated in Figure 2. The control pattern was characterized by nine fractions representing the different tear protein fractions with varying molecular weights, broadening, and intensities. The most obvious fractions in the pattern were those of lysozyme (14 kDa), serum albumin (64 kDa), and lactoferrin (85 kDa). The tear protein pattern of patients with MGD showed decreased intensity of lactoferrin and lysozyme and increased intensity of serum albumin. Moreover, the other protein fractions at 108, 200, and 285 kDa showed increased intensity, and the fraction at 183 kDa shifted towards the low molecular weight region at 161 kDa. The scanning pattern of tear proteins after IPL therapy [Figure 3] showed obvious improvement in the lysozyme, serum albumin, lactoferrin, and all other fractions; however, the fraction at 108 kDa shifted to the high molecular weight region at 133 kDa.
Figure 2.
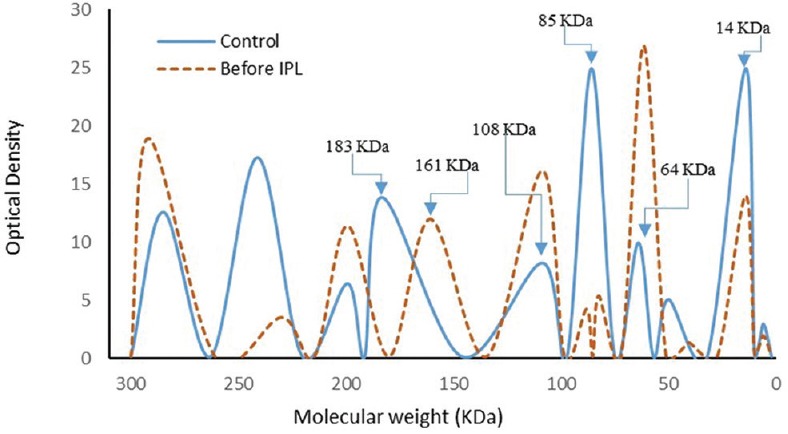
Electrophoretic pattern of tear proteins in the controls and patients before treatment with intense pulsed light (IPL).
Figure 3.
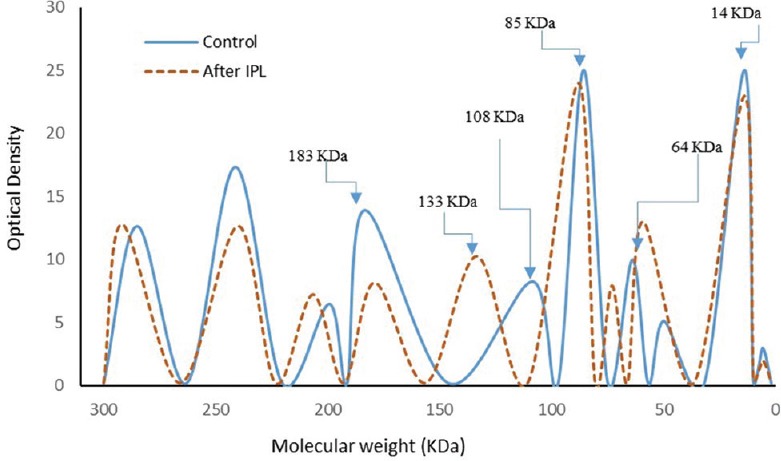
Electrophoretic pattern of tear proteins in the controls and patients after treatment with intense pulsed light (IPL).
Measurement of Lipid Profiles
Figure 4 illustrates the change in total lipid, cholesterol, triglyceride, and phospholipid concentrations in the controls, patients before IPL therapy, and patients 2 weeks after therapy. Total tear lipids showed obvious improvement after IPL therapy. The total lipid concentrations were 7.87 ± 0.28, 4.41 ± 0.30 in the controls before IPL therapy (P < 0.001), and 6.60 ± 0.38 mg/ml for patients after IPL therapy (P < 0.05). Moreover, tear cholesterol concentration was 3.99 ± 0.54 mg/ml in the controls and significantly reduced to 3.27 ± 0.06 (P < 0.01) and 3.65 ± 0.05 mg/ml (P < 0.05) in patients before and after IPL therapy, respectively, with percentage decreases of 18.11% and 8.41% compared to the controls. Additionally, tear triglyceride concentrations showed remarkable improvement in patients after IPL therapy (P < 0.001) compared to pretreatment values. The tear triglyceride values were 2.8 ± 0.16, 2.46 ± 0.23, and 2.65 ± 0.23 mg/ml in the controls, patients before IPL therapy, and patients after therapy, respectively. The phospholipid concentration in the controls was 1.81 ± 0.12 mg/ml, which decreased to 1.05 ± 0.13 mg/ml in patients with MGD (percentage decrease, 42%; P < 0.001) and showed noticeable improvement after IPL therapy (1.53 ± 0.24 mg/ml) with a percentage decrease of 15.6% (P < 0.01).
Figure 4.
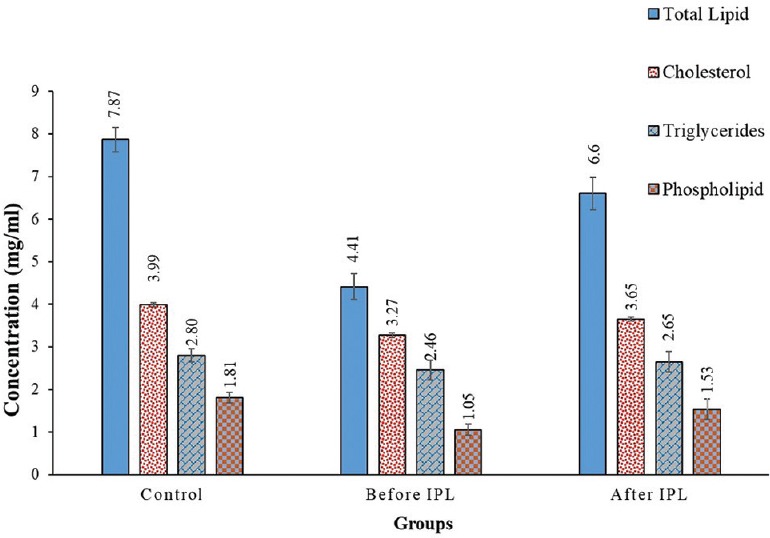
The concentrations of total lipids, cholesterol, triglycerides, and phospholipids in tear samples from the controls, patients before intense pulsed light (IPL) therapy, and patients after IPL therapy.
TLC for Phospholipids
Figure 5 illustrates the concentrations of different polar phospholipids separated by TLC. Phosphatidylserine concentration in the control was 3.88 ± 0.01 μg/ml and then decreased to 2.53 ± 0.4 μg/ml in patients with MGD (P < 0.001) and increased to 3.7 ± 0.05 μg/ml in patients after IPL therapy. Phosphatidylinositol concentration in patients with MGD decreased significantly (P < 0.001) and improved after IPL therapy (P < 0.05) with values of 5.4 ± 0.01, 3.76 ± 0.29, and 4.95 ± 0.01 μg/ml in the controls, patients before IPL therapy, and patients after therapy, respectively. Moreover, phosphatidylethanolamine concentration in patients with MGD decreased significantly and improved completely after IPL therapy (P > 0.05) with values of 4.46 ± 0.02, 3.13 ± 0.02, and 4.45 ± 0.04 μg/ml in the controls, patients before IPL therapy, and patients after therapy, respectively. Furthermore, phosphatidylcholine concentration in patients with MGD showed a non-significant decrease (P > 0.05) compared to that in the controls, and the concentration in the controls was 11.06 ± 0.05 and decreased to 10.65 ± 0.04 μg/ml in MGD patients. After 2 weeks, IPL therapy induced an increase in phosphatidylcholine concentration (12.88 ± 0.1 μg/ml; P < 0.05).
Figure 5.
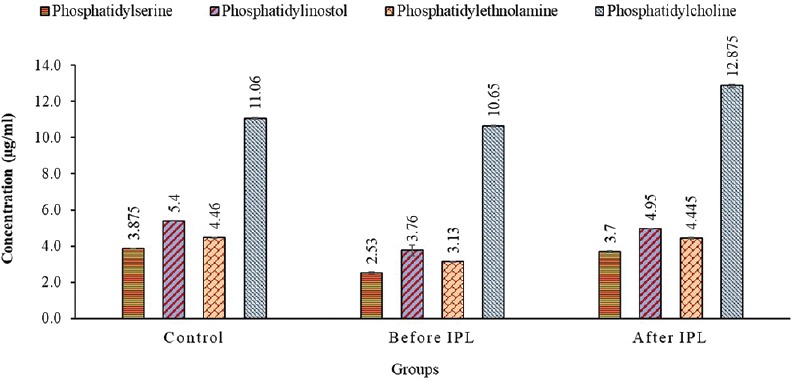
The concentrations of different phospholipids obtained using thin-layer chromatography in tear samples from the controls, patients before intense pulsed light (IPL) therapy, and patients after IPL therapy.
DISCUSSION
MGD is a widespread ocular surface disease. The efficacy of previous treatments for MGD remains temporary and unsatisfactory, suggesting the need for new therapeutic approaches. The tear film has an extremely ordered structure on the ocular surface, consisting of three layers: an outer lipid layer, a middle aqueous layer (which contains electrolytes, proteins, various metabolites, and some small organic molecules), and an inner mucin layer.[33] The consistency and function of the tear film depend on its biochemical structure.[34] It is a unique fluid with numerous functions including lubrication and protection of the cornea and ocular surfaces from infection. Additionally, it plays a vital role in the optical properties of the eye, provides vital nutrients and oxygen to the avascular cornea, transports fragments and cellular waste, and preserves a clear fluid to lubricate and moisturize the ocular surfaces. Tear film instability can produce variations in bacterial flora in the eyelids and conjunctiva, leading to the production of endotoxins, lipopolysaccharides and/or lipase activation, and causing eyelid inflammation and lipid changes.[35,36]
The effect of IPL therapy on DED was first recognized when patients being treated for acne, rosacea, or other skin complications reported improvements in their dry eye symptoms. The obtained data were promising and helped further modify and develop an IPL device aimed at the treatment of DED caused by MGD.[17]
In this study, a single IPL session was applied using a xenon flash lamp to the skin around the eyes of 12 patients with MGD. Thereafter, we evaluated the tear protein content, SDS-PAGE patterns, lipid profiles, and TLC concentrations of various phospholipids.
The results revealed significant improvements in the tear protein content of patients with MGD after IPL therapy. The decrease in the protein content of tear in eyes with MGD and the improvement after IPL therapy may directly affect the composition of tear proteins. In this study, SDS-PAGE provided a good analysis of the main tear proteins, such as lactoferrin, lysozyme, albumin, 20- to 60-kDa proteins, and immunoglobulins.[37] Lactoferrin is a multifunctional single-chain polypeptide with antioxidant, bacteriostatic, and anti-inflammatory properties. It is a metal-binding protein present in high concentrations in human tears. Lysozyme is considered a glycolytic enzyme with an antimicrobial activity.[38]
SDS-PAGE of the control tear samples revealed lactoferrin and lysozyme on the electrophoretic pattern, with molecular weights of 83 and 14 kDa, respectively.[37] The decrease in lactoferrin and lysozyme levels, as well as the increase in tear albumin level, in patients with MGD may have resulted from an inflammatory reaction.[37] Moreover, the decrease in lysozyme and lactoferrin levels can indicate the low antimicrobial defense capacity of tears, a high tendency for ocular infections, and offer useful information about the existence of local oxidative stress, which is intensified by the inflammatory reaction.[37,39] Furthermore, elevated levels of albumin and immunoglobulins, reported in several studies, indicate both inflammatory and foreign body reactions.[39] The electrophoretic pattern in Figure 3 also shows a significant improvement in all fractions after the IPL therapy, except for the fraction at 108 kDa. This improvement in lysozyme and lactoferrin levels is the best indicator of the efficacy of IPL therapy, and the shift in the molecular weight from 108 to 133 kDa may be due to their irreversible nature or the need for more IPL sessions.
The alteration in quality of Meibomian gland secretion is the main characteristic of eyes with MGD. Hence, the change in lipid composition can result in lipid layer instability, loss of functionality, and finally disease signs. The obstruction of the Meibomian gland ducts affects the production of lipids required for the lipid layer of the tear film.[40] Thinning or loss of the lipid layer at the tear surface leads to increased tear evaporation, resulting in dry eye.[41] The tear film lipid layer mainly contains nonpolar, hydrophobic lipids such as cholesteryl esters, wax esters, monoglycerides, diglycerides, and triglycerides.[42,43] Additionally, smaller amounts of hydrophilic polar lipids, such as phospholipids, are present.[44] Polar lipids in the tear film lipid layer are necessary because nonpolar lipids are unable to cover the whole tear film and lack the elasticity to respond to changing surface tension.
In the present study, quantitative measurements of total lipids, cholesterol, triglycerides, and phospholipids were performed. Our data indicate significant decreases in the concentrations of total lipids, cholesterol, triglycerides, and phospholipids in the eyes of patients with MGD before IPL therapy. These results showed that the most significant changes occur in the composition of polar lipids (phospholipids) rather than in that of nonpolar lipids (cholesterol and triglycerides) as previously described.[45] Additionally, an analysis of the lipid components in patients with MGD showed a significant decrease in triglycerides and cholesterol. Decreased unsaturation of the nonpolar fatty acids tends to increase their melting point, thereby leading to the thickening of the meibum within the central duct and abnormal structure of the gland.[46]
The estimated amount of polar lipids in the tear ranges from 5 to 20 mol % of all lipids.[45,46] Regardless of their small proportion relative to that of nonpolar lipids, polar lipids seem to critically impact the function of the tear film lipid layer, the surface active properties of the tear film lipid layer compositions, and, consequently, the health of the ocular surface.[47,48] Furthermore, the decrease in total lipid content in eyes with MGD, found in this study, indicated a disturbance in the surface activity of the polar lipids because it depends on the actual amount of lipids at the interface. Even with a very high content of polar lipids, no surface activity is detected if the total amount of lipids is too low to form a lipid layer.
Phospholipid structures are equally important in providing a thicker, more stable lipid layer in the tear. Phospholipids have basically zwitterionic and anionic types, forming the bi-layer of the cell wall.[49] All phospholipids contain a negative charge on the oxygen attached to the phosphorous group. Phospholipids that have polar groups at R3 and a net negative charge, such as phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol, are defined as “anionic phospholipids.” If the polar groups at R3 contain a positive charge, such as in phosphatidylcholine, the overall net charge of the system is neutral, and such phospholipids are called “zwitterionic phospholipids”.
In this regard, an analysis of polar phospholipids using TLC showed a highly significant decrease (P < 0.001) in the concentrations of the anionic phospholipids phosphatidylserine, phosphatidylinositol, and phosphatidylethanolamine in eyes with MGD. Their concentrations improved after IPL therapy, providing a constant interface between non-polar lipids at the surface and the hydrophilic aqueous layer. These molecules enhance the lipid layer thickness and reduce the dry eye symptoms. Furthermore, the concentration of phosphatidylcholine in the tear film was comparable between patients with MGD and controls. This is in agreement with the finding of a previous study that reported low levels of anionic phosphatidylethanolamine, but not zwitterionic phospholipid phosphatidylcholine, were associated with evaporative dry eye.[50]
These results suggest some possible mechanisms whereby IPL therapy could treat the symptoms of eyes with MGD. First, the light from the IPL device is directly applied to the skin; this could result in the production of heat higher than the body temperature, which is enough to melt the pathological secretions, prevent inflammatory mediator secretion, and decrease bacterial overgrowth.[23,24] Previous studies showed that the melting point of Meibomian gland secretions in patients with MGD was 3°C higher than that in normal eyes, and thermal therapies such as IPL could melt the pathologically dysfunctional lipids and relieve the ocular surface symptoms associated with MGD.[3,11]
Second, the IPL device we used emits energy in a band ranging from the base of the visible spectrum (565 nm) to near infrared (1400 nm). Its basic principle depends on the absorption of photons by endogenous or exogenous chromophores within the skin, such as melanin, hemoglobin, and water. This energy, delivered to the target structures, produces heat by inducing a photothermal reaction and subsequent destruction of the target through a process called selective photothermolysis. This process seems to liquefy the abnormal viscous meibum, dilate the glands, and stimulate the secretion of normal meibum.[51] The wavelength should be selected (using a filter, available from 500 to 755 nm) depending on the absorption peak of the target chromophore, and the pulse duration should last less than the thermal relaxation time to limit the diffusion of heat and damage to surrounding structures. In addition, a varied range of treatment parameters, including pulse duration, pulse sequence, and pulse delay time, can be adapted, thus giving users better versatility and precision.[17,52]
Third, IPL therapy may exert an effect on relieving inflammation pain and neurogenic pain.[53] It acts as a powerful warm compress that liquefies the toothpaste like secretion plugging the gland, improves eyelid apposition, and thus the pumping mechanism of the Meibomian gland during blinking. Moreover, IPL therapy closes the microvasculature feeding the inflammatory mediators to the gland, hence inhibiting their abnormal function.[22]
In conclusion, this study highlights the efficacy of using IPL as a therapeutic option for MGD, albeit during a short-term follow-up period (2 weeks), and shows significant improvement in tear composition. A study with a larger sample size and longer follow-up period would be helpful for better assessing both the effectiveness of the technique as well as any adverse events.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Nichols KK. The international workshop on Meibomian gland dysfunction: Introduction. Invest Ophthalmol Vis Sci. 2011;52:1917–1921. doi: 10.1167/iovs.10-6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on Meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. doi: 10.1167/iovs.10-6997e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borchman D, Foulks GN, Yappert MC, Milliner SE. Differences in human meibum lipid composition with Meibomian gland dysfunction using NMR and principal component analysis. Invest Ophthalmol Vis Sci. 2012;53:337–347. doi: 10.1167/iovs.11-8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham JE, Moore JE, Jiru X, Moore JE, Goodall EA, Dooley JS, et al. Ocular pathogen or commensal: A PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 2007;48:5616–5623. doi: 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- 6.Korb DR, Blackie CA. Restoration of Meibomian gland functionality with novel thermodynamic treatment device – a case report. Cornea. 2010;29:930–933. doi: 10.1097/ICO.0b013e3181ca36d6. [DOI] [PubMed] [Google Scholar]
- 7.Korb DR, Blackie CA. Meibomian gland therapeutic expression: Quantifying the applied pressure and the limitation of resulting pain. Eye Contact Lens. 2011;37:298–301. doi: 10.1097/ICL.0b013e31821bc7c5. [DOI] [PubMed] [Google Scholar]
- 8.Oleñik A, Mahillo-Fernández I, Alejandre-Alba N, Fernández-Sanz G, Alarcón Pérez M, Luxan S, et al. Benefits of omega-3 fatty acid dietary supplementation on health-related quality of life in patients with Meibomian gland dysfunction. Clin Ophthalmol. 2014;8:831–836. doi: 10.2147/OPTH.S62470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Yang Z, Yu H, Song H. Acupuncture therapy is more effective than artificial tears for dry eye syndrome: Evidence based on a meta-analysis? Evid Based Complement Alternat Med. 2015;2015:143858. doi: 10.1155/2015/143858. doi: 10.1155/2015/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobolewska B, Doycheva D, Deuter C, Pfeffer I, Schaller M, Zierhut M. Treatment of ocular rosacea with once-daily low-dose doxycycline. Cornea. 2014;33:257–260. doi: 10.1097/ICO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 11.Goto E, Monden Y, Takano Y, Mori A, Shimmura S, Shimazaki J, et al. Treatment of noninflamed obstructive Meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol. 2002;86:1403–1407. doi: 10.1136/bjo.86.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negishi K, Tezuka Y, Kushikata N, Wakamatsu S. Photorejuvenation for Asian skin by intense pulsed light. Dermatol Surg. 2001;27:627–632. doi: 10.1046/j.1524-4725.2001.01002.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldman MP, Weiss RA, Weiss MA. Intense pulsed light as a non-ablative approach to photoaging. Dermatol Surg. 2005;31:1179–1187. doi: 10.1111/j.1524-4725.2005.31924. [DOI] [PubMed] [Google Scholar]
- 14.Papageorgiou P, Clayton W, Norwood S, Chopra S, Rustin M. Treatment of rosacea with intense pulsed light: Significant improvement and long-lasting results. Br J Dermatol. 2008;159:628–632. doi: 10.1111/j.1365-2133.2008.08702.x. [DOI] [PubMed] [Google Scholar]
- 15.Haedersdal M, Beerwerth F, Nash JF. Laser and intense pulsed light hair removal technologies: From professional to home use. Br J Dermatol. 2011;165:31–36. doi: 10.1111/j.1365-2133.2011.10736.x. [DOI] [PubMed] [Google Scholar]
- 16.Patriota RC, Rodrigues RC, Cuc′e LC. Intense pulsed light in photoaging: A clinical, histopathological and immunohistochemical evaluation. An Bras Dermatol. 2011;86:1129–1133. doi: 10.1590/s0365-05962011000600010. [DOI] [PubMed] [Google Scholar]
- 17.Fabi SG, Goldman MP. The safety and efficacy of combining poly-L-lactic acid with intense pulsed light in facial rejuvenation: A retrospective study of 90 patients. Dermatol Surg. 2012;38:1208–1216. doi: 10.1111/j.1524-4725.2012.02476.x. [DOI] [PubMed] [Google Scholar]
- 18.Raulin C, Greve B, Grema H. IPL technology: A review. Lasers Surg Med. 2003;32:78–87. doi: 10.1002/lsm.10145. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Luo X, Lu J, Chen J, Zuo C, Xiang Y, et al. IPL irradiation rejuvenates skin collagen via the bidirectional regulation of MMP-1 and TGF-β1 mediated by MAPKs in fibroblasts. Lasers Med Sci. 2011;26:381–387. doi: 10.1007/s10103-010-0870-1. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande AJ. Efficacy and safety evaluation of high-density intense pulsed light in the treatment of grades II and IV acne vulgaris as monotherapy in dark-skinned women of child bearing age. J Clin Aesthet Dermatol. 2018;11:43–48. [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg DJ. Current trends in intense pulsed light. J Clin Aesthet Dermatol. 2012;6:45–53. [PMC free article] [PubMed] [Google Scholar]
- 22.Toyos R. Intense, pulsed light for dry eye syndrome. Cataract Refrac Surg Today. 2009:71–73. [Google Scholar]
- 23.Craig JP, Chen YH, Turnbull PRK. Prospective trial of intense pulsed light for the treatment of Meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2015;56:1965–1970. doi: 10.1167/iovs.14-15764. [DOI] [PubMed] [Google Scholar]
- 24.Toyos R, Mc Gill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to Meibomian gland dysfunction: A 3-year retrospective study. Photomed Laser Surg. 2015;33:41–46. doi: 10.1089/pho.2014.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labbé A, Brignole-Baudouin F, Baudouin C. Ocular surface investigations in dry eye. J Fr Ophtalmol. 2007;30:76–97. doi: 10.1016/s0181-5512(07)89557-x. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Zollner N, Kirsch K. Uber die quantitative bestimmung yon lipoiden (mikromethode) mittels der vielen natiirliehen lipoiden (allen bekannten Plasmalipoiden) gemeinsamen Sulfophosphovanillin-Reaktion. Z Gesamte Exp Med. 1962;135:545–561. [Google Scholar]
- 29.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 30.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- 31.Connerty HV, Briggs AR, Eaton EH., Jr Serum phospholipid estimation. Clin Chem. 1961;7:580–587. [PubMed] [Google Scholar]
- 32.Skipski VP, Peterson RF, Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964;90:374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiffany JM. Tears and conjunctiva. In: Harding JJ, editor. Biochemistry of the Eye. London: Chapman & Hall; 1997. pp. 45–78. [Google Scholar]
- 34.Puinhas A, Sampaio P, Castanheira EMS, Real Oliveira MECD, Lira M. Comparison of IgA, TNF-α and surface tension of the tear film in two different times of the day. Cont Lens Anterior Eye. 2013;36:140–145. doi: 10.1016/j.clae.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Stahl U, Wilcox M, Stapleton F. Osmolality and tear film dynamics. Clin Exp Optom. 2012;95:3–11. doi: 10.1111/j.1444-0938.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- 36.Baudouin C. A new approach for better comprehension of diseases of the ocular surface. J Fr Ophtalmol. 2007;30:239–246. doi: 10.1016/s0181-5512(07)89584-2. [DOI] [PubMed] [Google Scholar]
- 37.Chiva A. Electrophoresis of tear proteins as a new diagnostic tool for two high risk groups for dry eye. Computer users and contact lens wearers. J Med Life. 2011;4:228–233. [PMC free article] [PubMed] [Google Scholar]
- 38.Versura P, Nanni P, Bavelloni A, Blalock WL, Piazzi M, Roda A, et al. Tear proteomics in evaporative dry eye disease. Eye. 2010;24:1396–1402. doi: 10.1038/eye.2010.7. [DOI] [PubMed] [Google Scholar]
- 39.Sariri R, Khamedi A. Variations in electrophoretic tear protein pattern due to contact lens wear. J Chromatogr A. 2007;1161:64–66. doi: 10.1016/j.chroma.2007.05.108. [DOI] [PubMed] [Google Scholar]
- 40.Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243–251. [PubMed] [Google Scholar]
- 41.Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45. doi: 10.1016/s0014-4835(61)80006-7. [DOI] [PubMed] [Google Scholar]
- 42.Yamada M, Mochizuki H, Kawai M, Tsubota K, Bryce TJ. Decreased tear lipocalin concentration in patients with Meibomian gland dysfunction. Br J Ophthalmol. 2005;89:803–805. doi: 10.1136/bjo.2004.055822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butovich IA, Arciniega JC, Lu H, Molai M. Evaluation and quantitation of intact wax esters of human meibum by gas liquid chromatography-ion trap mass spectrometry. Invest Ophthalmol Vis Sci. 2012;53:3766–3781. doi: 10.1167/iovs.11-9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55:289–298. doi: 10.1194/jlr.M044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mc Culley JP, Shine WE. The lipid layer of tears: Dependent on Meibomian gland function. Exp Eye Res. 2004;78:361–365. doi: 10.1016/s0014-4835(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 46.Mc Culley JP, Shine WE. Changing concepts in the diagnosis and management of blepharitis. Cornea. 2000;19:650–658. doi: 10.1097/00003226-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Pucker AD, Haworth KM. The presence and significance of polar meibum and tear lipids. Ocul Surf. 2015;13:26–42. doi: 10.1016/j.jtos.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Rantamäki AH, Holopainen JM. The effect of phospholipids on tear film lipid layer surface activity. Invest Ophthalmol Vis Sci. 2017;58:149–154. doi: 10.1167/iovs.16-20468. [DOI] [PubMed] [Google Scholar]
- 49.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 4th ed. New York: Garland Science; 2002. Molecular Biology of the Cell; p. 62. [Google Scholar]
- 50.Shine W, Mc Culley JP. Keratoconjunctivitis sicca associated with Meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852. doi: 10.1001/archopht.116.7.849. [DOI] [PubMed] [Google Scholar]
- 51.Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O’brien T, et al. The international workshop on Meibomian gland dysfunction: Report of the subcommittee on management and treatment of Meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2050–2064. doi: 10.1167/iovs.10-6997g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciocon DH, Boker A, Goldberg DJ. Intense pulsed light: What works, what's new, what's next. Facial Plast Surg. 2009;25:290–300. doi: 10.1055/s-0029-1243077. [DOI] [PubMed] [Google Scholar]
- 53.de Godoy CH, Silva PF, de Araujo DS, Motta LJ, Biasotto-Gonzalez DA, Politti F, et al. Evaluation of effect of low-level laser therapy on adolescents with temporomandibular disorder: Study protocol for a randomized controlled trial. Trials. 2013;14:229. doi: 10.1186/1745-6215-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]


