Abstract
Purpose:
To compare histologic abnormalities of tear film and tear osmolarity between normal eyes and eyes with pterygium.
Methods:
This was a prospective, hospital-based, case–control study involving 95 patients (65 men, 30 women) with unilateral pterygium. The tear meniscus height (TMH), Schirmer's test-1 (SCH-1) score, Rose Bengal staining (RBS) score, tear film breakup time (TBUT), tear osmolarity (TO), and conjunctival impression cytology (CIC) were assessed in both eyes. The Chi-square and Student's t-tests were used to compare the results between the two groups. P values <0.05 were considered statistically significant.
Results:
The mean patient age was 50.9 years, with the largest age group being the 45–55 year-old bracket across both genders. Most patients (82.1%) had nasal pterygium, and 80% were involved in outside activities. The mean assessment values in the case and control groups were as follows: TMH, 0.21 vs. 0.24 mm; SCH-1, 13.2 vs. 17.8 mm; RBS, 4.38 vs. 2.51 points; TBUT, 8.7 vs. 13.2 seconds; TO, 306 vs. 299 mOsm/L (P < 0.001 in all cases). The proportions of abnormal assessment values in the case and control groups were as follows: TMH, 82.1% vs. 3.16%; SCH-1, 20% vs. 2.1%; RBS, 30.53% vs. 4.22%; TBUT, 61.05% vs. 6.3%; TO, 10.52% vs. 1.05%; CIC, 33.7% vs. 7.37% (P < 0.05 for all comparisons).
Conclusion:
This study showed that the quantity and quality of tear film, as well as the number of goblet cells, decreased, but the tear osmolarity increased in eyes with pterygium. Furthermore, the TMH, RBS results, TBUT, and CIC have more precise state of the patient's tear condition with the disease of the pterygium.
Keywords: Conjunctival Impression Cytology, Pterygium, Rose Bengal Staining, Schirmer's Test, Tear Breakup Time, Tear Meniscus Height, Tear Osmolarity
INTRODUCTION
Pterygium is a fibro-vascular growth of the conjunctiva that extends across the limbus and invades the cornea.[1,2] Its exact cause is unknown, although too much exposure to ultraviolet (UV) light may lead to these growths.[2,3] Pterygium occurs more often among people who live in warm climates and spend a lot of time outdoors in sunny or windy environments. Common symptoms include redness, blurred vision, and eye irritation,[1,2,3] and affected patients frequently have dry eye symptoms.[4,5] Various clinical tests are used to evaluate tear film and ocular surface. Schirmer's (SCH) test and the tear meniscus height (TMH) test measure the quantity of the tear film. Patients diagnosed with aqueous tear deficiency generally have an SCH score of less than 10 mm and a decreased TMH.[4,5,6] Relatedly, the tear film breakup time (TBUT) test measures the quality of the tear film. A rapid TBUT (<10 seconds) is frequently observed in patients with Meibomian gland dysfunction. However, a similar result occurs in patients with goblet cell loss and/or mucin deficiency.[5,7] The Rose Bengal staining (RBS) indirectly ascertains the presence of reduced tear volume by detecting damaged epithelial cells. The extent of staining is generally correlated with the severity of the aqueous deficiency.[7,8] Tear osmolarity (TO) measurement has proven highly reliable in confirming clinical diagnoses of keratoconjunctivitis sicca or dry eye.[9,10] Conjunctival impression cytology (CIC) is an innovative technique for studying conjunctival viability. It comprises a non-invasive or minimally invasive biopsy of the ocular surface epithelium, with no side effects or contraindications.[11] It is still unclear whether tear dysfunction is a precursor to pterygium growth or rather whether pterygium causes tear dysfunction. Therefore, the results of the present study may increase our understanding of the qualitative and quantitative changes in the tear film in patients with pterygium.
METHODS
This study was conducted in accordance with the tenets of the World Medical Association of Helsinki from October 2015 to October 2016. Informed consent was obtained from all participants after the purpose and possible consequences of the study were explained. The inclusion criteria were as follows: unilateral pterygium of any grade, lack of any systemic disease, and age between 25 and 75 years. The presence of fibrovascular tissue extending from the bulbar conjunctiva onto the cornea either nasally or temporally was identified as pterygium. The exclusion criteria were as follows: any corneal disease or scar, contact lens use in the previous 3 months, cicatricial ocular surface disease, other comorbid ocular diseases such as ocular allergies, continuous use of topical ocular medications, and history of ocular surgery or ocular injury. This prospective study included 95 patients (95 eyes) with unilateral pterygium. The eye with pterygium was taken as the case, while the fellow normal eye was taken as the control in each case. The mean patient age was 50.9 ± 8.2 years (range: 25–75 years). All patients were examined to rule out any coexisting ocular disease, such as blepharitis, disorders of the lacrimal system, or ocular surface abnormality. Histories of diabetes mellitus, hypertension, collagen-vascular diseases, use of topical or systemic drugs, or ocular surgery led to patient exclusion from the study. Tear secretion and stability were evaluated in both eyes of patients with unilateral pterygium using tear function tests (TO and CIC). The SCH-1 was performed without topical anesthesia by placing a standard SCH test filter strip in the midlateral portion of the lower fornix. After 5 minutes, the strips were removed and the extent of wetting was recorded in millimeters. Results of less than 10 mm were considered abnormal. Before the RBS test was performed, a drop of topical anesthetic was applied, and the excess was washed out using saline. A slightly moistened strip of Rose Bengal filter paper was then applied to the inferior bulbar conjunctiva. The patient was examined under a slit lamp using red-free illumination (green filter). The Van-Bijsterveld scoring system was used to classify the staining as normal or abnormal. In accordance with this grading scale, the ocular surface was divided into three zones (nasal bulbar conjunctiva, temporal bulbar conjunctiva, cornea). The density of staining was evaluated in each zone and given a score from 0 to 3, for a maximum possible score of nine. The staining dots were scored as follows: single (1 point), scattered (2 points), confluent (3 points). The score from each part of the ocular surface was added up, and a score of 3 or greater was considered abnormal. The TMH was evaluated using Fourier domain optical coherence tomography (FD-OCT; RTVue-100, Optovue Inc., Freemont, CA, USA). Vertical 2-mm scan images of the middle of the lower eyelid were obtained three times in each eye. Tear meniscus height were defined as the height of the triangular-shaped cross section between the lower eyelid margin and the cornea, and were measured with RTVue-100 image analysis software.
The TMH test was classified as follows: normal, ≥0.22 mm; abnormal <0.22 mm. Before the TBUT was recorded, the precorneal tear film was stained with fluorescein. The patient was then examined under a slit lamp using red-free illumination (blue filter). The time interval between the opening of the eye lids and the appearance of the first dry spots on the cornea was recorded using a stop watch. The average of three recordings was recorded as the TBUT. A TBUT value less than 10 seconds was considered abnormal. To prevent excessive reflex secretion of tears, contact with cornea was avoided.
The TO was measured using the TearLab Osmolarity System (TearLab Corp., San Diego, CA, USA) at least 30 minutes after the tear function test. When the system was ready, the patient was asked to look up, and a handled pen with a chip test card mounted on its tip that served as a laboratory assay was touched to the inferior tear meniscus above the lower eyelid. After the green light on the pen disappeared, indicating the conclusion of the tear-collection process, the pen was placed on the TearLab Reader. The code on the chip test card was entered into the TearLab Reader, and the results of the measurement process were obtained within a maximum of 30 seconds. A TO value >308 mOsm/L was considered abnormal. The CIC was evaluated using cellulose acetate paper strips (3 × 10 mm with a diagonal edge). The eyes were topically anaesthetized using 4% xylocaine drops. A speculum was inserted, and the lacrimal lake at the inner canthus was dried with a swab. Blunt, smooth-edged forceps were used to grasp the filter paper strip at one end, and the paper was applied to the temporal bulbar conjunctiva. A smooth glass rod held in the other hand was used to press the paper gently. The paper strip was then removed with a peeling motion after 2–3 seconds. The strip was dropped into a bottle containing fixative solution (ethyl alcohol, formaldehyde, and glacial acetic acid at a 20:1:1 volume ratio) and transferred to the laboratory for staining. The slides were examined under a light microscope. Impression cytology specimens were graded as normal or abnormal based on epithelial cell morphology or goblet cell density. Normal impressions from the controls showed sheets of polygonal epithelial cells interspersed with numerous goblet cells. The abnormal impressions showed squamous metaplasia of the epithelial cells and altered goblet cell densities. Data were analyzed using either MS Excel or SPSS version 17.0 for Windows statistical software. Quantitative and qualitative data were presented as a percentage (%) or mean ± SD, as appropriate. The two study groups were compared using Student's t-test or the Chi-square test. All P values < 0.05 were considered statistically significant, and P values < 0.01 were considered highly significant.
RESULTS
The present study was conducted over a period of 1 year and involved 95 patients with unilateral pterygium. Of these, 65 (68.4%) were men and 30 (31.6%) were women, with the age group being the 45–55 year-old bracket across both sexes [Table 1]. As shown in Figure 1, 82.1% (78 eyes) of the patients had nasal pterygium, 15.8% (15 eyes) had temporal pterygium, and 2.1% (2 eyes) had double-head pterygium (both nasal and temporal). As shown in Table 2, 80% (76 eyes) of patients with unilateral pterygium worked in an outdoor environment, while 20% (19 eyes) worked indoors. The mean TMH values in the pterygium and control eyes were 0.21 ± 0.07 mm and 0.24 ± 0.06 mm, respectively, constituting a highly significant difference (P < 0.001). Abnormal TMH (<0.22 mm) was observed in 78 out of 95 eyes (82.1%) with pterygium and in three out of 95 (3.16%) control eyes. The Chi-square test showed that this difference was highly significant (chi square = 65.96; P < 0.001; [Table 3]). The mean SCH-1 values in the pterygium and control eyes were 13.2 ± 4.1 mm and 17.8 ± 4.1 mm, respectively, constituting a highly significant difference (P < 0.001; [Figure 2]). In addition, 20% of the eyes with pterygium showed abnormal SCH-1 (<10 mm), while 2.1% of the control eyes did, constituting a significant difference (Chi-square = 5.411; P = 0.019; [Table 4]). As shown in Figure 3, the mean RBS score in the pterygium eyes was 4.38 ± 1.95, while that in the control eyes was 2.51 ± 1.27, constituting a difference of 1.87 that was highly significant (P < 0.001). According to Table 5, 30.53% of the pterygium eyes and 4.22% of control eyes had an abnormal RBS (score ≥3), constituting a highly significant difference (Chi-square = 9.226; P < 0.001). The mean values of TBUT in the pterygium and control eyes were 8.7 ± 1.6 s and 13.2 ± 2.1 s, respectively, constituting a highly significant difference (P < 0.001). The TBUT was abnormal (<10 sec) in 61.05% of pterygium eyes and 6.3% of control eyes, constituting a highly significant difference (Chi-square = 63.77; P < 0.001; [Table 6]). The mean TO value in the eyes with pterygium was 306 ± 9.2 mOsm/L, while that in control eyes was 299 ± 8.0 mOsm/L, constituting a highly significant difference (P < 0.001). As demonstrated in Table 7, the TO value was abnormal (>308 mOsm/L) in 10.52% of pterygium eyes and 1.05% of control eyes, constituting a significant difference (Chi-square = 4.978; P = 0.024). As presented in Table 8, the CIC was abnormal in 33.7% of pterygium eyes and 7.37% of control eyes, constituting a highly significant difference (Chi-square = 9.865; P < 0.001).
Table 1.
Distribution of age and sex
| Age range (years) | Males (n=65) | Females (n=30) | Total (n=95) | |||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| 25-35 | 5 | 5.26% | 7 | 7.37% | 12 | 12.63% |
| 35-45 | 19 | 20% | 6 | 6.31% | 25 | 26.31% |
| 45-55 | 25 | 26.31% | 11 | 11.58% | 36 | 37.89% |
| 55-65 | 9 | 9.47% | 4 | 4.21% | 13 | 13.68% |
| 65-75 | 7 | 7.37% | 2 | 2.1% | 9 | 9.47% |
Figure 1.
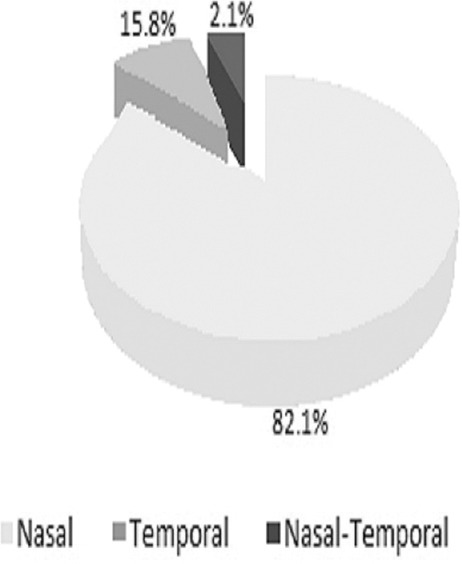
Percentage distribution of pterygium based on location.
Table 2.
Percentage distribution of pterygium based on work environment
| Activity environment | Males (n=65) | Females (n=30) | Total (n=95) | |||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| Outdoor | 57 | 87.7% | 19 | 63.3% | 76 | 80% |
| Indoor | 8 | 12.3% | 11 | 36.7% | 19 | 20% |
Table 3.
Inferior tear meniscus height in pterygium and control eyes
| Test | Cases (n=95) | Controls (n=95) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| TMH ≥0.22 mm (Normal) | 17 | 17.9% | 92 | 96.84% |
| TMH <0.22 mm (Abnormal) | 78 | 82.1% | 3 | 3.16% |
Cases, eyes with pterygium; Controls, healthy eyes; TMH, tear meniscus height; mm, millimeters
Figure 2.
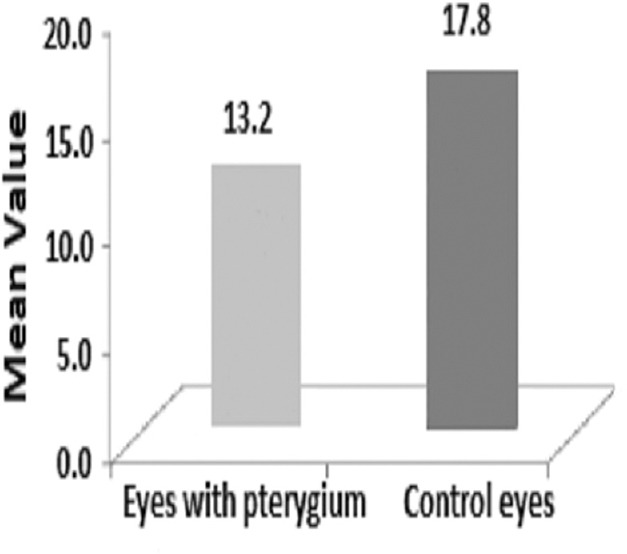
Comparison of Schirmer's test-1 results in pterygium and control eyes.
Table 4.
Schirmer’s test-1 in pterygium and control eyes
| Test | Cases (n=95) | Controls (n=95) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| SCH-1 ≥10 mm (Normal) | 76 | 80% | 93 | 97.9% |
| SCH-1 <10 mm (Abnormal) | 19 | 20% | 2 | 2.1% |
Cases, eyes with pterygium; Controls, healthy eyes; SCH-1, Schirmer’s test-1; mm, millimeters
Figure 3.
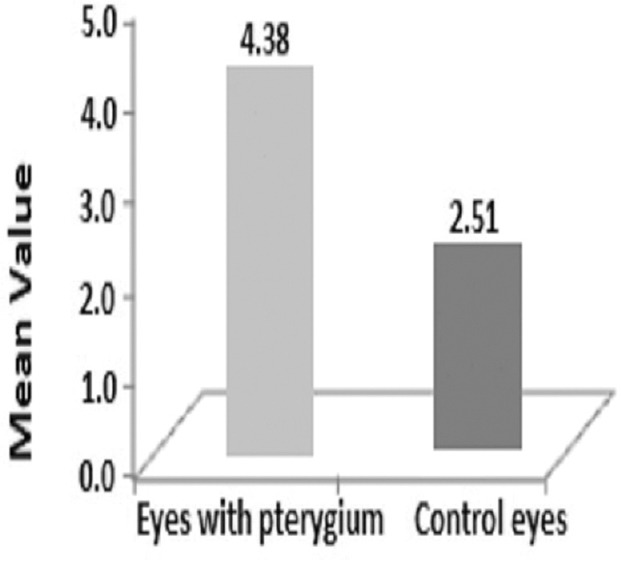
Comparison of Rose Bengal staining score in the pterygium and control eyes.
Table 5.
Rose Bengal staining in pterygium and control eyes
| Test | Cases (n=95) | Controls (n=95) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| RBS <3 scores (Normal) | 66 | 69.47% | 91 | 95.78% |
| RBS ≥3 scores (Abnormal) | 29 | 30.53% | 4 | 4.22% |
RBS, Rose Bengal staining; Cases, eyes with pterygium; Controls, healthy eyes
Table 6.
Tear breakup time in pterygium and control eyes
| Test | Cases (n=95) | Controls (n=95) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| TBUT ≥10 sec (Normal) | 37 | 38.95 | 89 | 93.7% |
| TBUT <10 sec (Abnormal) | 58 | 61.05% | 6 | 6.3% |
Cases, eyes with pterygium; Controls, healthy eyes; TBUT, tear breakup time; sec, seconds
Table 7.
Tear osmolarity in pterygium and control eyes
| Test | Cases (n=95) | Controls (n=95) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| TO ≤308 mOsm/L (Normal) | 85 | 89.48% | 94 | 98.95% |
| TO >308 mOsm/L (Abnormal) | 10 | 10.52% | 1 | 1.05% |
Cases, eyes with pterygium; Controls, healthy eyes; TO, tear osmolarity; mOsm/L, milliosmoles per liter
Table 8.
Conjunctival impression cytology in pterygium and control eyes
| Conjunctival impression cytology | Eyes with pterygium (n=95) | Control eyes (n=95) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Normal | 63 | 66.3% | 88 | 92.63% |
| Abnormal | 32 | 33.7% | 7 | 7.37% |
DISCUSSION
Pterygium is one of the most common conjunctival disorders, with the highest prevalence in the fourth to sixth decades of life in the present study, corroborating previous studies.[3,12,13,14] Furthermore, our results indicate that pterygium was more common in men than in women which is in line with results of the previous study.[15,16] This finding is attributable to the fact that men spend more time outdoors and are more exposed to the damaging effects of UV sunlight. Previous studies have shown that pterygium occurs predominantly at the nasal limbus.[13,17] In the current study, 82.1% of the patients had nasal pterygium. Furthermore, 80% of the patients were outdoor workers. Outdoor activities are the most common risk factor for pterygium,[1,3,12] because they expose the eyes to heat, dust, wind, and solar radiation. Relatedly, several studies have shown that there is no association between pterygium and the nature of the work itself.[18]
In laboratory studies, fibroblast cells cultured from pterygium tissue have shown upregulation of matrix metalloproteinases (MMPs) when exposed to UV stimulation. Moreover, alteration or deregulation of stem–microenvironmental networking provoked disease development.[19] Some researchers have postulated that pterygium is associated with a limbal microenvironmental anomaly in which resident epithelial cells become hyperproliferative.[20]
In the present study, the mean TMH value, measured using OCT, was significantly lower in eyes with pterygium than in control eyes. Furthermore, the distribution percentage of TMH was normal (≥0.22 mm) in 96.84% of healthy eyes. On the other hand, 82.1% of the pterygium eyes had an abnormal TMH (<0.22 mm). It follows that the tear film quantitation is changed in the pterygium eyes. Onkar et al[21] also demonstrated that the TMH, measured using OCT, was significantly lower in eyes with pterygium than in control eyes. They reported that 90.2% of pterygium eyes showed abnormal TMH, which was a higher proportion than in our study, although they considered a TMH value <0.3 mm, rather than <0.22 mm, to be abnormal, so the different result was likely due to the cutoffs used to define abnormal TMH.
In the current study, the mean SCH-1 value in pterygium eyes was significantly lower than that in control eyes, and a higher proportion of pterygium eyes showed abnormal SCH-1, corroborating previous studies.[14,22,23] It follows that there is a correlation between pterygium and inadequacy of tear film. The average RBS score was 1.87 points higher in pterygium eyes than in control eyes, and a higher proportion of pterygium eyes showed abnormal RBS (≥3 points). Our study corroborates another by Oh et al,[24] who found that the average RBS score was significantly higher in pterygium eyes than in control eyes. This higher average RBS score indicates that the tear film is unstable in pterygium eyes.
In the present study, the mean TBUT values in pterygium and control eyes were 8.7 ± 1.6 s and 13.2 ± 2.1 s, respectively. Similarly, Moreno JC et al[25] found that TBUT values were significantly reduced in eyes with pterygium. Roka et al[26] found that the mean TBUT value was 10.56 seconds in patients with pterygium and 16.52 seconds in control patients, constituting a significant difference between the groups. El-Sersy et al[27] found that the mean TBUT was 11.70 ± 2.16 seconds in normal healthy eyes, with a range of 8.5–16.0 seconds. In eyes with pterygium, this value was markedly lower, at 5.91 ± 1.95 seconds. Relatedly, the present study demonstrated that the TBUT was abnormal (<10 seconds) in 61.05% of pterygium eyes and 6.3% of control eyes. In a study by Rahman et al,[14] the TBUT was abnormal in 75.6% of pterygium eyes and 9.3% of healthy eyes, while Lemp et al[28] reported an unstable TBUT in 54% of pterygium eyes and 26.27% of healthy eyes. This abnormal TBUT, which was found more frequently in eyes with pterygium than in eyes without pterygium, may suggest that mucin abnormalities may be a predisposing factor for pterygium, or that pterygium itself causes mucin abnormalities.[4] Reduced TBUT can, in turn, be caused by several mechanisms. For instance, normal blinking may be compromised in eyes with pterygium, which may lead to desiccated epithelium and therefore shorter TBUT. Furthermore, irregularities in the surface epithelium of eyes with pterygium may compromise the surface tension and stability of tears.[29] Tear hyperosmolarity has been identified as an important factor in the pathogenesis of dry eye syndrome and has recently been included as a part of the definition of dry eye.[30,31]
In the current study, the difference in tear osmolarity between pterygium eyes and control eyes was significant. Furthermore, we found that tear osmolarity was abnormal in 10.52% of pterygium eyes and in 1.05% of control eyes. The present study is consistent with that by Julio et al,[31] who found that tear osmolarity in pterygium eyes was significantly higher than that in control eyes. Several studies have reported that UV-mediated genetic alteration can affect the expression of cytokines, such as interleukin (IL)-6 and IL-8, in patients with pterygium. IL-6 and IL-8 can induce the production of MMPs, which tend to be localized at the advancing edges of pterygium. The release of IL-6, IL-8, and MMPs into the tear film may lead to ocular surface damage and tear film instability, ultimately resulting in epithelial cell apoptosis, goblet cell loss, reduced mucus secretion, and tear hyperosmolarity.[28,29,30,31,32] Therefore, we posit that tear hyperosmolarity and abnormal tear film function are associated with pterygium.
Impression cytology is a fast, cost-effective, and non-invasive tool for diagnosis and follow-up of ocular surface disorders.[11] The present study demonstrated that the CIC was abnormal in 33.7% of pterygium eyes and 7.37% of control eyes, which was a significant difference, and that there was significant squamous metaplasia and altered goblet cell density in pterygium eyes. In this regard, the present study is consistent with that by Bandyopadhyay et al,[33] who reported that 36% of pterygium eyes and 6% of healthy eyes had abnormal CIC. In agreement with a study by Shreya et al,[34] we believe that ocular surface lesion caused by pterygium is associated an encroachment of altered bulbar conjunctiva onto the cornea. As in the present study, decreased goblet cell density and altered epithelial cell morphology were more common in pterygium eyes than in control eyes.
In the current study, the tear function, TO, and CIC tests demonstrated significant changes in eyes with pterygium compared with healthy eyes, including decreased tear secretion, instability of the tear film, increased evaporation, deterioration of epithelial cells, elevated tear osmolarity, and reduced goblet cell density. This indicates that pterygium leads to abnormal tear film and dry eye. Moreover, a higher proportion of eyes with pterygium showed abnormal results across various dry eye tests. It follows that assessing the quantitative and qualitative status of the tear film using the TMH, RBS, and TBUT tests is important for detecting tear film dysfunction, and that goblet cell counts using CIC are clinically necessary in pterygium eyes.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Moukoury Nyolo E, Epee E, Nsangou JFI, Noa Noa Tina B. Pterygiun in a tropical region: Analysis of 344 cases in Cameroon. Bull Soc Belge Ophtalmol. 2009;311:11–15. [PubMed] [Google Scholar]
- 2.Shiroma H, Higa A, Sawaguchi S, Iwase A, Tomidokoro A, Amano S. Prevalence and risk factors of pterygium in a southwestern island of Japan: The Kumejima Study. Am J Ophthalmol. 2009;148:766–771. doi: 10.1016/j.ajo.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Cajucom-Uy H, Tong L, Wong TY, Tay WT, Saw SM. The prevalence of and risk factors for pterygium in an urban Malay population: The Singapore Malay Eye Study (SiMES) Br J Ophthalmol. 2010;94:977–981. doi: 10.1136/bjo.2008.150847. [DOI] [PubMed] [Google Scholar]
- 4.Rajab AY. Evaluation of tear film stability in pterygium and pingueculae. Ann Coll Med Mosul. 2013;39:132–135. [Google Scholar]
- 5.Ganeshpuri AS, Kamble BS, Patil P, Wadgaonkar SP. A comparative study of tear film stability and secretion in pterygium patients-Diabetic vs. nondiabetic. Int J Health Sci Res. 2014;4:86–97. [Google Scholar]
- 6.Anguria P, Ntuli S, Carmichael T. Relationships of heredity and dry eye with pterygia in black African patients. SAMJ. 2011;101:110. doi: 10.7196/samj.4416. [DOI] [PubMed] [Google Scholar]
- 7.Yanoff, Duker. Ophthalmology. (4th edition chapter) 2013;23:274–6. ISBN 978-1455-7398-44. [Google Scholar]
- 8.Wang S, Jiang B, Gu Y. Changes of tear film function after pterygium operation. Ophthalmic Res. 2011;45:210–215. doi: 10.1159/000321531. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S, Nichols KK. Collecting tear osmolarity measurements in the diagnosis of dry eye. Exp Rev Ophthalmol. 2009;4:451–453. [Google Scholar]
- 10.Ozsutcu M, Arslan B, Erdur SK, Gulkilik G, Kocabora SM, Muftuoglu O. Tear osmolarity and tear film parameters in patients with unilateral pterygium. Cornea. 2014;33:1174–1178. doi: 10.1097/ICO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. Br J Ophthalmol. 2005;89:1655–1659. doi: 10.1136/bjo.2005.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viso E, Gude F, Rodríguez-Ares MT. Prevalence of pinguecula and pterygium in a general population in Spain. Eye (Lond) 2011;25:350–357. doi: 10.1038/eye.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antony AT, Mini PA, Dalia S. Pterygium and Dry Eye- A Clinical Correlation. J Med Scie Clin Res. 2017;5:23654–23659. [Google Scholar]
- 14.Rahman A, Yahya K, Fasih U, Huda W, Shaikh A. Comparison of Schirmer's test and tear film breakup time test to detect tear film abnormalities in patients with Pterygium. J Pak Med Assoc. 2012;6:1214–1216. [PubMed] [Google Scholar]
- 15.Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, Tan DT. Prevalence and risk factors associated with dry eye symptoms: A population based study in Indonesia. Br J Ophthalmol. 2002;86:1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. Prevalence and risk factors of pterygium and pinguecula: The Tehran Eye Study. Eye (Lond) 2009;23:1125–1129. doi: 10.1038/eye.2008.200. [DOI] [PubMed] [Google Scholar]
- 17.Dolezalova V. Is the occurrence of a temporal pterygium really so rare? Ophthalmologica. 1977;174:88–91. doi: 10.1159/000308583. [DOI] [PubMed] [Google Scholar]
- 18.Asokan R, Venkatasubbu RS, Velumuri L, Lingam V, George R. Prevalence and associated factors for pterygium and pinguecula in a South Indian population. Ophthalmic Physiol Opt. 2012;32:39–44. doi: 10.1111/j.1475-1313.2011.00882.x. [DOI] [PubMed] [Google Scholar]
- 19.Jang SY, Lee SY, Yoon JS. Meibomian gland dysfunction in longstanding prosthetic eye wearers. Br J Ophthalmol. 2013;97:398–402. doi: 10.1136/bjophthalmol-2012-302404. [DOI] [PubMed] [Google Scholar]
- 20.Das P, Gokani A, Bagchi K, Bhaduri G, Chaudhuri S, Law S. Limbal epithelial stem-microenvironmental alteration leads to pterygium development. Mol Cell Biochem. 2015;402:123–139. doi: 10.1007/s11010-014-2320-z. [DOI] [PubMed] [Google Scholar]
- 21.Onkar A, Pandey DJ, Bist HK, Sen S. Tear and pterygium: A clinico-pathological study of conjunctiva for tear film anomaly in pterygium. J Eye Cataract Surg. 2017;3:24. [Google Scholar]
- 22.Bekibele CO, Baiyeroju AM, Ajaiyeoba A, Akang EE, Ajayi BG. Case control study of dry eye and related ocular surface abnormalities in Ibadan, Nigeria. Int Ophthalmol. 2010;30:7–13. doi: 10.1007/s10792-008-9281-8. [DOI] [PubMed] [Google Scholar]
- 23.Rajiv, Mithal S, Sood AK. Pterygium and dry eye-A clinical correlation. Indian J Ophthalmol. 1991;39:15–16. [PubMed] [Google Scholar]
- 24.Oh HJ, Park YG, Yoon KC. Changes of ocular surface and tear film in patients with pinguecula and pterygium. J Korean Ophthalmol Soc. 2006;47:717–724. [Google Scholar]
- 25.Moreno JC, Garcia VG, Garcia L. Evaluation of tear film in patients with Pterygium. Eur J Ophthalmol. 2011;00(00) [Google Scholar]
- 26.Roka N, Shrestha SP. Assessment of tear secretion and tear film instability in cases with pterygium and normal subjects. Nepal J Ophthalmol. 2013;5:16–23. doi: 10.3126/nepjoph.v5i1.7816. [DOI] [PubMed] [Google Scholar]
- 27.El-Sersy TH. Role of pterygium in ocular dryness. J Egypt Ophthalmol Soc. 2014;107:205–208. [Google Scholar]
- 28.Lemp MA, Baudouin C, Baum J. The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international Dry Eye WorkShop. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 29.Manhas A, Gupta D, Gupta A, Kumar D, Manhas RS, Manhas GS. Clinical correlation between dry eye and pterygium: A study done at government medical college Jammu, Jammu and Kashmir, North India. Int J Res Med Sci. 2017;5:3087–3094. [Google Scholar]
- 30.Lemp MA, Bron AJ, Baudouin C. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792–798. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Julio G, Lluch S, Pujol P, Alonso S, Merindano D. Tear osmolarity and ocular changes in pterygium. Cornea. 2012;31:1417–1421. doi: 10.1097/ICO.0b013e318259c934. [DOI] [PubMed] [Google Scholar]
- 32.Detorakis ET, Zaravinos A, Spandidos DA. “Growth factor expression in ophthalmic pterygia and normal conjunctiva”. Int J Mol Med. 2010;25:513–516. doi: 10.3892/ijmm_00000371. [DOI] [PubMed] [Google Scholar]
- 33.Bandyopadhyay R, Nag D, Mondal SK, Gangopadhyay S, Bagchi K, Bhaduri G. Ocular surface disorder in pterygium: Role of conjunctival impression cytology. Indian J Pathol Microbiol. 2010;53:692–695. doi: 10.4103/0377-4929.72036. [DOI] [PubMed] [Google Scholar]
- 34.Shreya T, Poorvi G. Role of impression cytology in detecting gobletcell damage in various ocular surface disorder. Austin J Clin Ophthalmol. 2016;3:1065. [Google Scholar]


