Abstract
Age-related macular degeneration (AMD) is a major cause of vision loss in the developed world and its pathogenesis is a topic of active research. To date, much study has been focused on the role of the retinal pigment epithelium (RPE) and Bruch's membrane (BrM) in AMD pathogenesis, but the role of the choroid has also been investigated. In this review, we focus on recent advancements in research in the role of the choroid in AMD, beginning with an exploration of the histopathologic, cellular and molecular changes that occur in the choroid in AMD and concluding by discussing new choroidal imaging techniques and patterns seen on fluorescein angiography, indocyanine green angiography, spectral-domain optical coherence tomography and optical coherence tomography angiography. Exploring these domains will lead to a better understanding of the factors at play beyond the outer retina in this important disease.
Keywords: Age-related Macular Degeneration, Choroid, Histopathology, Imaging
INTRODUCTION
Age-related macular degeneration (AMD) is a progressive, sight-threatening disease and the major cause of permanent vision loss among elderly people in the developed world.[1] The prevalence of AMD is increasing as the population ages, with an expected 3 million individuals affected by the year 2020.[2] Its pathogenesis is complex and remains an area of active research.
Multiple risk factors for AMD have been identified, with age being the largest non-modifiable risk factor and cigarette smoking being the most strongly associated modifiable risk factor.[3] Several genetic associations have also been identified. Genome-wide association studies (GWAS) have identified an association between the Y402H polymorphism in complement factor H (CFH) with an increased risk of AMD, indicating dysregulation of the complement cascade in the pathogenesis of the disease.[4,5] Other commonly implicated loci are the ARMS2/HTRA1 genes,[6,7,8,9,10] though the mechanism of this association is still under investigation.
While the bulk of research focuses on the role of the retinal pigment epithelium (RPE) in the pathogenesis of AMD, the role of the choroid cannot be overlooked. There is a growing idea that pathologic changes in outer retinal perfusion may be a significant early contributing factor to AMD. In this review, we will focus on new advancements in research in the role of the choroid in the pathogenesis of AMD, beginning with an exploration of the histopathologic, cellular and molecular changes that occur throughout the natural history and concluding by discussing new choroidal imaging techniques and the patterns seen on fluorescein angiography, indocyanine green angiography, optical coherence tomography and optical coherence tomography angiography. Understanding the classification and histopathologic characteristics of AMD, as well as the function and anatomy of the choroid, will give us a better insight into the pathologic changes that occur in the choroid throughout the course of the disease.
METHODS
A literature review was conducted using the terms “choroid AMD,” “choroidal histopathology AMD” and “choroid imaging AMD” using the PubMed database from the year 2008 through October 2018.
Choroid in AMD
The hallmark characteristics of AMD are drusen and RPE changes. According to the Age-Related Eye Disease Study (AREDS) classification [Figure 1],[11] a few small drusen or no drusen are considered category 1, or no AMD; several small drusen or a few medium sized drusen in one or both eyes are classified as category 2 or early AMD. Intermediate AMD is category 3, which involves many medium sized drusen or one or more large drusen in one or both eyes. Atrophy of light sensitive cells including the RPE, outer retina and supporting tissue on the central retinal area resulting in geographic atrophy (GA), or choroidal neovascularization (CNV), the presence of abnormal and fragile blood vessels in the sub-RPE or sub-retinal space in neovascular AMD (NVAMD), are considered the advanced form of AMD, AREDS category 4.[11] Progression from early to late AMD is associated with severe and irreversible vision loss.
Figure 1.
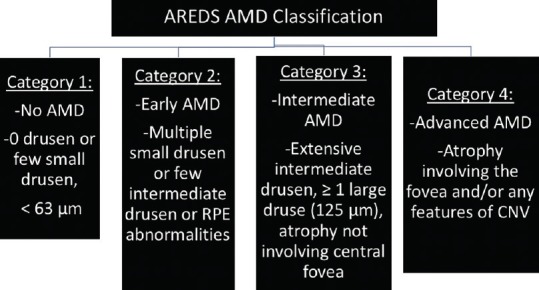
AREDS classification of AMD.[11]
Histopathology
Chronological age-related changes in Bruch's membrane (BrM) begin with thickening of the outer collagenous layer of BrM and accumulation of thin deposits between the RPE cells and their basement membrane (basal laminar deposits (BlamD)).[12] When BlamD thicken and accumulate long spacing collagen, lipoproteins, and inflammatory proteins, they are associated with AMD. Accumulation of lipid-containing deposits and membranous deposits within the inner collagenous layer are called basal linear deposits (BlinD) [Figure 2].[12] Both types of BrM deposits, especially BlinD, correspond to drusen.[13,14] Oxidative damage related to environmental exposures may result in accumulation of RPE waste products, which contributes to the pathogenesis of AMD.[15,16]
Figure 2.
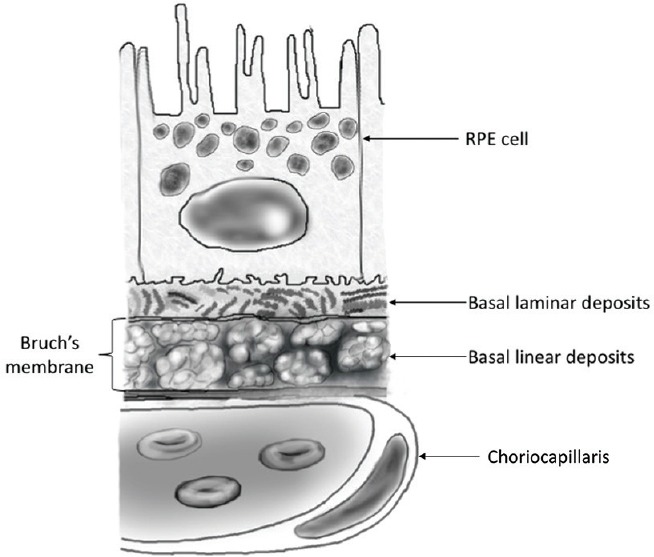
Location of subretinal deposits. Basal laminar deposits (BlamD) are found between the RPE and its basement membrane, while basal linear deposits (BlinD) are located within Bruch's membrane. The presence of BlinD is strongly correlated with drusen and AMD.
Normal Anatomy of the Choroid
The choroid is composed of three vascular layers: the inner choriocapillaris (CC) and middle and outer layers of vessels (Sattler's and Haller's layers). The choriocapillaris is a continuous layer of capillaries with surrounding pericytes, lying below the RPE and BrM. While the inner retina derives its nutrition from the retinal vascular system, the choroid nourishes the outer retina, and the RPE depends on the choriocapillaris for survival. Additionally, the choroid supplies the central 250 to 600 μm of the macula (foveal avascular zone), which lacks retinal blood vessels.[17,18] The endothelia of CC vessels have multiple fenestrations, especially on the side facing the retina, allowing for directional flow of oxygen and filtered materials from the choroid to the RPE, while the larger middle and outer choroidal vessels lack endothelial fenestrations.[17,19]
Blood flow through the choroid is high, with choroidal flow consuming over 70% of the blood flow in the eye.[20] Regulation of flow is controlled by the autonomic nervous system, via both sympathetic and parasympathetic pathways.[21] Due to the rapid rate of blood flow through the choroidal circulation, the arterio-venous O2 difference is only 2-3%.[22] In addition to blood vessels, normal choroidal stroma contains melanocytes, macrophages, lymphocytes, mast cells and plasma cells, as well as collagen fibers and nerve fibers.[23]
Choroidal Histopathology in AMD
Progressive involutional changes occur in the choriocapillaris with normal aging.[23] While some atrophy of the choriocapillaris is expected with age,[23,24] CC atrophy in AMD exceeds the amount attributable to normal aging.[25,26] Even at early stages of AMD, CC dropout has been observed.[25] Using Ulex europaeus agglutinin lectin and confocal microscopy, Seddon et al observed that in all early AMD samples, CC dropout was present in the absence of RPE loss.[25] Adjacent to regions of CC dropout, they observed neovascular buds, perhaps representing a precursor to NVAMD in early AMD.[25]
It has been suggested that the degree of choriocapillaris pruning is related to the number of drusen present.[27] In the work of Mullins et al, lectin histochemistry with Ulex europaeus agglutinin-I was used to label CC endothelium.[27] They observed an inverse relationship between choroidal thickness and the density of deposits. In addition, CC density under drusen was 45% lower than in regions without drusen.[27]
Vascular dropout appears to be linearly related to the progression of AMD. Histopathologic analysis by Seddon et al using Ulex europaeus agglutinin lectin revealed higher CC vascular loss for all stages of AMD compared to controls.[25] In early and intermediate AMD, 20.5% and 12.5% loss of vascular area was seen, respectively. A 39.0% loss was seen in GA, and a 38.2% reduction in cases of neovascularization without RPE detachment.[25]
“Ghost vessels” represent the remains of previously healthy CC vessels that have lost their endothelia. Using endothelial cell (EC)-binding lectin from Ulex europaeus to differentiate live from ghost vessels, Mullins et al showed that in early AMD (AREDS grades 2 and 3), as the number of ghost vessels increases, CC density decreases, suggesting that CC dropout is due to endothelial loss and precedes RPE atrophy.[27]
In GA, atrophy of the RPE and photoreceptor cells occurs and the choriocapillaris thins.[27] McLeod et al performed a postmortem analysis of choroid wholemounts from subjects with GA.[28] Their measurement of the RPE and CC areas in those wholemounts showed 50% attenuation of the choriocapillaris and reduced branching at the area of RPE atrophy, while the CC appeared morphologically normal in areas with no RPE atrophy.[28] In addition, mean capillary diameters in GA eyes showed significant reduction at, but not beyond, the area of atrophy. Ultrastructural analysis of GA cases showed less endothelial fenestration of the choriocapillaris compared to controls and thickening of the capillary endothelium.[28]
In neovascular AMD, new, aberrant vessels grow from the choroid through BrM to the sub-RPE and sub-neurosensory retina, leading to exudation and visual impairment. McLeod et al demonstrated CC dropout in CNV.[28] In regions of CNV in AMD, the decrease in choroidal vascularity was present, even in the absence of RPE atrophy. Lumenal architecture and diameter of the choriocapillaris vessels appeared unchanged in subjects with neovascular AMD.[28] McLeod et al suggested that the primary event in GA occurs at the RPE level, with changes in the choriocapillaris occurring secondarily.[28] On the other hand, in CNV, it appears that CC dropout occurs primarily, causing hypoxia, vascular endothelial growth factor (VEGF) production and neovascularization.[27] These findings have been supported by electron microscopy, where CC loss has been shown in all stages of AMD.[29] CC loss was present in both neovascular and non-neovascular AMD, but as in the previous studies, attenuation was independent of RPE damage in CNV, while it was limited to the area of atrophy in GA.[29]
Cellular Characteristics
The presence of inflammatory cells, including macrophages, lymphocytes and mast cells, has been reported in the normal human choroid.[30] Mast cells (MCs) reside in vascularized connective tissues close to blood vessels, where, upon activation, they degranulate and release cytokines, proteases and a number of vasoactive substances [Figure 3a].[31] In normal elderly subjects, mast cells have been observed in large numbers near blood vessels in the outer two layers of the choroid [Figure 3b].[30] Bhutto et al found that the number of mast cells and degranulated mast cells was significantly increased in early AMD, exudative AMD, and GA compared to age-matched controls.[32] While MCs were distributed in Sattler's and Haller's layers of the choroid in controls, in subjects with GA and CNV, they were found in close proximity to the choriocapillaris [Figure 3c]. Areas with the greatest numbers of degranulated MCs showed loss of the choriocapillaris, potentially due to release of proteolytic substances on degranulation. Formation of CNV may be related to release of angiogenic MC granule contents.[32]
Figure 3.
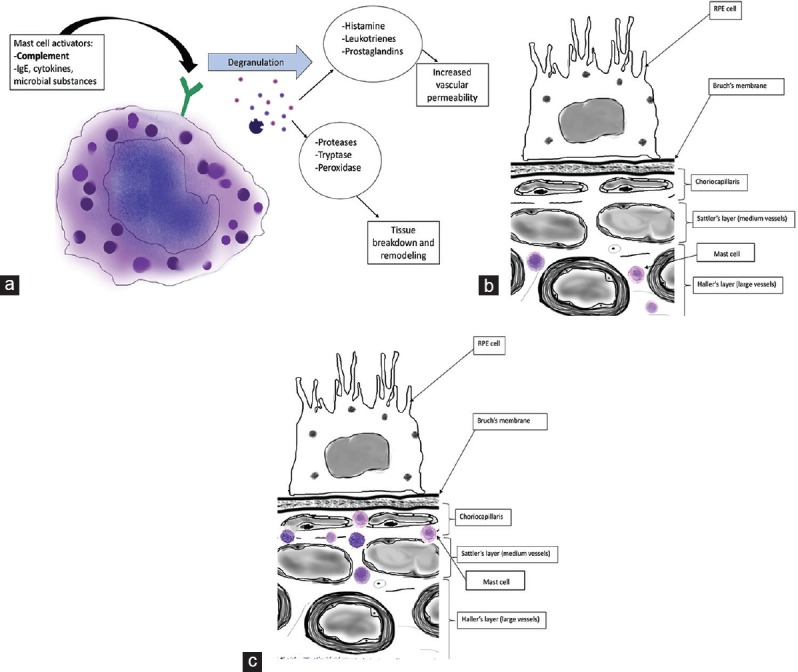
(a) Schematic of mast cell activation and degranulation. MCs are activated by complement, cytokines, etc., Granule contents include proteases that cause tissue breakdown and remodeling and vasoactive inflammatory mediators such as histamine and prostaglandins. MC activation in the choriocapillaris may contribute to CNV.[32] (b) Mast cell location in choroid of normal eye. MCs remain in Sattler's and Haller's layers.[30] (c) Mast cell location in the choroid of an eye with AMD. MCs migrate to the choriocapillaris.[32]
Choroidal antigen presenting cells, particularly macrophages, have also been studied in association with AMD. Macrophages serve as important phagocytes and mediators of inflammation and are found in outer levels of the choroidal stroma in normal eyes.[33] Using immunohistochemical techniques, McLeod et al found a significant increase in activated macrophages (IBA1+/HLA-DR+ cells) in the submaculae of early and intermediate AMD compared to age-matched controls.[34] In regions of CNV in eyes with neovascular AMD, significantly more activated macrophages were found. Overall, the mean macrophage cell volume was lower and sphericity was higher in all AMD groups compared to controls, indicating that macrophage activation and proliferation is present in higher levels in subjects with AMD, particularly the neovascular type.[34] Interestingly, subjects with the CFH Y204H polymorphism have been found to have elevated numbers of choroidal macrophages.[35]
Molecular Characteristics
Inflammation seems to play a key role in the pathogenesis of AMD, and the part of the complement pathway is a well-established concept. Single nucleotide polymorphisms (SNPs) in complement regulatory factor H, are associated with increased risk of AMD.[4,36,37,38,39,40] In particular, significant association with AMD was seen in a tyrosine-402→histidine-402 polymorphism at the locus coding for complement factor H.[36]
The complement cascade is a component of the innate immune system. It leads to initiation of the inflammatory response via one of three pathways, which converge on a final common pathway leading to production of the membrane attack complex (MAC). Complement regulatory proteins exist, which prevent cell lysis and inflammation caused by the MAC by inactivation of C3b.[41,42]
Accumulated complement complexes and increased levels of C-reactive protein have been demonstrated in the BrM, CC and drusen of eyes with AMD and are associated with CC endothelial cell lysis and CC thinning.[8,37,42,43,44,45] Specifically, drusen have been found to contain complement activators and fragments, implicating activation of the complement cascade in drusen formation.[37] Not only has complement complex accumulation been identified in AMD, but the severity seems to be Y402H allele-specific.[42] Mullins et al showed that elevated levels of MAC are found in the RPE and choroid of subjects homozygous for the Y402H high risk histidine allele compared to donors homozygous for the low risk tyrosine allele.[42] This finding lends support to the theory that loss-of-function mutations in CFH lead to unregulated MAC formation, causing choroidal endothelial loss in AMD.
To further evaluate the molecular basis of choroidal thinning, Sohn et al performed a proteomic analysis of eyes with thick and thin choroids in AMD and age-matched controls.[26] They found that thin choroids showed reduced levels of serine protease inhibitor A3 (SERPINA3), which inhibits proteolysis. Less intuitively, they found increased levels of tissue inhibitor of metalloproteinases-3 (TIMP3) in thin choroids, which they hypothesized may lead to abnormal CC vessel turnover. This imbalance between proteases and protease inhibitors is likely involved in choroidal thinning in AMD.[26]
The role of autophagy in AMD pathogenesis has also been explored. Autophagy is the process by which cells dispose of damaged organelles and other debris.[46] Dysfunction of this pathway leads to accumulation of cellular waste products, potentially leading to inflammation and oxidative damage to the RPE and choroidal endothelium.[46] In the retina, extracellular deposition of amyloid beta (Aβ) and phosphorylated tau has been seen in normal aging photoreceptor outer segments and BrM.[47,48,49,50] Deposition of (Aβ) and phosphorylated tau and their role in small blood vessel damage is widely studied in dementia and neurodegenerative disease. Aβ causes formation of free radicals, leading to vasoconstriction, reduced blood flow and endothelial cell apoptosis,[50] while phosphorylated tau can induce mitochondrial dysfunction.[50] Aboelnour et al demonstrated increased deposition of Aβ and phosphorylated tau in the choroidal vessels of complement factor H knockout (Cfh_/_) mice, suggesting a relationship of overactive complement to Aβ and tau deposition.[50] While both knockout mice and controls showed deposition of Aβ and phosphorylated tau in the choroidal vessels, the increased levels in knockout mice may suggest a role in endothelial cell damage, capillary loss and low perfusion in early AMD.[50] The presence of Aβ in drusen may also be related to prolonged inflammasome activation in the choroid, leading to increased apoptosis and pyroptosis of RPE cells in AMD.[51]
Choroid in AMD by Imaging Techniques
Fluorescein angiography and indocyanine green angiography
Fluorescein angiography (FA) is the gold standard for analysis of CNV and has been used to quantify areas of both CNV and GA.[52] However, fluorescein dye leaks from blood vessels, making it less ideal for visualization of details in the choroidal circulation.[53]
Early in the course of AMD, hard drusen can cause “window defects”, or early hyperfluorescence on FA due to thinning of the overlying RPE, through which choroidal vessels can be seen.[54] FA has demonstrated slow choroidal filling in early AMD.[54] Because fluorescein dye is not highly bound to plasma proteins, it allows for visualization of patterns of leakage when choroidal neovascularization is present, defined as occult or classic.[55]
Although FA is useful for visualization of CNV, retinal-choroidal anastomoses and other choroidal vascular abnormalities are better visualized using indocyanine green angiography (ICGA). In contrast to fluorescein, ICG dye binds to plasma proteins, remains within CC vessels, and allows for more detailed visualization of choroidal vascular patterns.[56] In early AMD, ICGA has demonstrated reduced fluorescence of the choroid, suggesting slow choroidal filling in the early stages of disease. In particular, a prolonged choroidal filling phase is associated with a higher density of confluent hard drusen, which appear hyperfluorescent on ICGA.[57] In geographic atrophy, ICGA shows hypofluorescent areas with loss of background fluorescence from degeneration of the underlying choriocapillaris.[58]
Choroidal appearance on ICGA can be particularly helpful in distinguishing advanced GA from Stargardt disease (STGD), which can appear clinically similar. Giani et al found that “dark atrophy,” or late hypocyanescence on ICGA was significantly more common in STGD than in AMD.[58] As STGD is a hereditary macular dystrophy, they hypothesized that dark atrophy may be due to more extensive atrophy in STGD compared to GA.[58]
ICGA's utility is highest in evaluation of neovascular AMD, where several types of choroidal vascular anomalies have been identified. Among these patterns are retinal-choroidal anastomoses (RCA), polypoidal, retinal angiomatous proliferation (RAP) and deep retinal vascular anomalous complexes (RVACs).[59] The work of Kim et al showed that in NVAMD, the presence of punctate hyperfluorescent spots on ICGA correlates with choroidal vascular hyperpermeability (CVH).[60]
Optical coherence tomography
Enhanced depth imaging optical coherence tomography (EDI-OCT) technology has shown that the choroid thins with normal aging.[24] While some degree of thinning is expected with age, multiple OCT studies have demonstrated that choroidal thinning occurs disproportionately in AMD, even at early stages of disease [Figure 4].[61,62,63,64,65,66,67,68]
Figure 4.
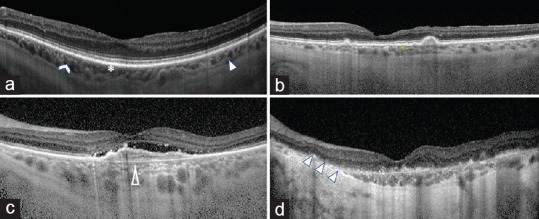
Representative SD-OCT imaging of the choroid in AMD progression. (a) normal control with all three choroidal layers visible: choriocapillaris (asterisk), Sattler's layer (chevron) and Haller's layer (solid arrow head). (b) Intermediate AMD with drusen and thinning of the choroid compared with normal control. (c) Late AMD with CNV and atrophy of the choriocapillaris (hollow arrow head). (d) Late AMD with GA and severe atrophy of the choroid (triple arrow heads).
Choroidal thinning identified on spectral domain OCT (SD-OCT) in early AMD may be associated with an increased risk of progression to more advanced disease.[63] In patients with early AMD, several studies have shown a significantly higher degree of choroidal thinning in eyes with reticular pseudodrusen (RPD) compared to those without.[69,70,71] Using SD-OCT, Lu et al showed significant progressive thinning of the subfoveal choroid in AMD.[62] Mean thickness in intermediate AMD was 215.6 μm, while it was 200.4 μm in late NVAMD.(62) Other OCT studies revealed similar significant choroidal thinning in advanced stages of dry AMD.[61,72] In agreement with the histopathologic findings of McLeodet al,[28] Govetto et al found that the amount of choroidal thinning was more significant in eyes with neovascular AMD compared to non-neovascular AMD fellow eyes.[72] Additionally, a relationship has been identified between chronic cigarette smoke exposure and choroidal thinning in subjects with early AMD.[73]
Contradictory to reports of CC thinning in intermediate AMD, Almeida et al found that choriocapillaris-equivalent thickness (CCET) was increased in patients with intermediate AMD compared to age-matched controls, perhaps due to increased VEGF and accumulation of ghost vessels at earlier stages of disease.[74] Using EDI-OCT and the Iowa Reference Algorithms for objective segmentation, they determined that there was also less CC thinning in response to positional change (from sitting to supine) in patients with intermediate AMD compared to controls. The decreased response to positional change may be related to a loss of CC elasticity.[74]
Three patterns of CNV have been identified with OCT. Type 1 refers to sub-RPE neovascularization, type 2 is subretinal, and type 3 is intraretinal proliferation.[75] Another choroidal entity identified on OCT is the “pachychoroid spectrum” of diseases, which involves abnormal, dilated vessels in the deep choroid (pachyvessels), accompanied by atrophy of Sattler's layer and the choriocapillaris.[76] The pachychoroid spectrum encompasses a number of diseases, including central serous chorioretinopathy (CSC), polypoidal choroidal vasculopathy (PCV), pachychoroid neovasculopathy and pachychoroid pigment epitheliopathy (PPE).[76,77] The term “pachychoroid pigment epitheliopathy (PPE)” was instituted by Warrow et al to describe pathologic changes seen in the RPE overlying dilated choroidal vessels.[77] Yanagi et al found that PPE was strongly associated with underlying non-exudative neovascularization in AMD.[78] They hypothesize that pachyvessels may compress the inner choroid (i.e. CC), causing disturbances in choroidal circulation.[78]
Further investigating pachyvessel morphology, Baek et al used swept-source en face OCT to evaluate changes in the choroidal vasculature in neovascular and non-neovascular AMD, CSC and PCV.[79] They found pachyvessels in 46% of eyes with typical NVAMD and demonstrated that morphologic changes are not limited to the CC, but also involve the deeper choroidal vessels. The choroid was globally thin, with focal areas of dilation of deeper vessels.[79]
Takahashi et al further characterized changes in the large choroidal vessel layer in NVAMD.[80] Using swept-source OCT, they demonstrated that NVAMD eyes with CVH on ICGA had higher mean subfoveal choroidal thickness, larger choroidal vessel lumenal areas, expanded choroidal stroma, and a thicker large choroidal vessel layer, likely due to leakage of fluid into the interstitium.[80]
OCT angiography
OCT angiography (OCTA) allows for three-dimensional visualization of blood flow in the retina and choroid and is an excellent tool for assessing CC vessel density. Multiple OCTA studies have demonstrated choroidal thinning in all stages of AMD, with particularly significant thinning associated with the presence of reticular pseudodrusen (RPD) and drusen.[81,82,83,84,85] The vessel density of the CC, Sattler's and Haller's layers are reduced in all groups of patients with non-neovascular AMD compared to controls.[81]
In patients with GA, OCTA has demonstrated persistence, but dramatic thinning, of the choriocapillaris at the area of atrophy. This area corresponded to hyperfluorescence seen on FA.[86] Using OCTA, Pellegrini et al demonstrated that, when compared with STGD eyes, subjects with GA showed less dramatic attenuation of the CC, while those affected by STGD showed dramatic CC loss with additional loss of the Sattler layer.[86] They hypothesized that residual RPE cells in GA may preserve the underlying choroid, while in STGD, the loss of the RPE is more profound, leading to disappearance of the CC.[86] Thus, OCTA has led to a better understanding of the dark atrophy initially seen with ICGA.
OCTA has been especially helpful in better characterizing choroidal morphology. In early AMD, OCTA has shown alterations in choriocapillaris composition, with fibrosis and an increased ratio of stroma to vessels.[82] Wirth et al investigated OCTA characteristics of active and quiescent CNV.[87] In active CNV, a “peripheral halo” was seen around the lesion, suggesting reduced flow to the surrounding capillaries. Inactive lesions were characterized on OCTA by a main trunk vessel with thinning of intralesional capillaries and loss of the peripheral halo. They found that CNV lesions do not fully involute after treatment, but rather regress in depth and persist.[87] OCTA has allowed for better visualization of choroidal morphology in AMD and has further corroborated the choroidal attenuation seen on histopathology and SD-OCT.
CONCLUSION
The pathogenesis of AMD is complicated, multifactorial and incompletely characterized. Given the inherent challenges in viewing the choroid, its role in AMD has been difficult to assess. With the recent advent of SD-OCT and OCTA, it is now possible to visualize and measure the choroid, which was previously challenging. Histopathologic studies have demonstrated that choroidal vascular depletion is present in early AMD and is progressive in both wet and dry late AMD. The order in which CC dropout occurs may be different in GA versus CNV, with vascular dysfunction and subsequent ischemia perhaps being the inciting event in neovascular disease. The choroidal vascular dropout seen on histopathology has been borne out in both SD-OCT and OCTA studies, with significant thinning seen in AMD versus controls, particularly in neovascular AMD. The choroid has also been implicated in the investigation of inflammation and complement activation in AMD, with disproportionate accumulation of the membrane attack complex in the choriocapillaris, as well as mast cell and macrophage activation.
One potential mechanism by which neovascular AMD develops might begin with choroidal vascular dysregulation. This could manifest as pachychoroid changes and/or vascular depletion. With inefficient clearance of RPE waste products and defective oxygen transport to the RPE, lipid and other waste may build up in the subretinal space as drusen and drusenoid deposits. In individuals predisposed to pro-inflammatory states, such as those with mutations in complement inhibitors, those with defective autophagy, or those who smoke, these deposits may induce oxidative damage and cause inflammatory mediators such as mast cells and macrophages to migrate from the deeper layers of the choroid to the choriocapillaris. At the site of action, these cells may activate complement and release proteases and vasoactive substances such as VEGF, causing damage to the overlying RPE and proliferation of new, aberrant blood vessels in an attempt to correct the relative state of ischemia. Thickening of Bruch's membrane due to deposition of collagen, lipid and oxidized waste would further worsen the ischemia, causing release of more VEGF and more neovascularization. Available imaging modalities have demonstrated that attenuation and disruption of the choriocapillaris architecture can be present at the earliest stages of AMD.
In GA, choroidal dropout and other CC vascular abnormalities do not appear to extend beyond the area of atrophy, suggesting that atrophy of the RPE and photoreceptors may occur prior to vascular thinning in non-neovascular AMD. This suggests a trophic relationship between the outer retina and choroid and also indicates that the pathogenesis of wet versus dry AMD is potentially different.
Age-related macular degeneration is likely a heterogeneous group of diseases, with many etiologies and risk factors leading to a group of similar phenotypes. More research is required to further elucidate the inciting factors that lead to choroidal dysregulation and atrophy. Additionally, a better understanding of the time course in which vascular changes occur with respect to retinal changes may be helpful in establishing differences in the pathogenesis of GA and CNV, as well as identifying early features of disease that could lead to earlier intervention and better patient outcomes.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: A review of association. Eye (Lond) 2005;19:935–944. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- 4.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao S, Ko A, Partanen M, Pakzad-Vaezi K, Merkur AB, Albiani DA, et al. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. Am J Ophthalmol. 2013;156:1176–1183. doi: 10.1016/j.ajo.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Spencer KL, Court BL, Olson LM, Scott WK, Haines JL, et al. Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria. Invest Ophthalmol Vis Sci. 2009;50:3084–3090. doi: 10.1167/iovs.08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iejima D, Nakayama M, Iwata T. HTRA1 Overexpression induces the exudative form of age-related macular degeneration. J Stem Cells. 2015;10:193–203. [PubMed] [Google Scholar]
- 8.Jones A, Kumar S, Zhang N, Tong Z, Yang JH, Watt C, et al. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc Natl Acad Sci U S A. 2011;108:14578–14583. doi: 10.1073/pnas.1102853108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama M, Iejima D, Akahori M, Kamei J, Goto A, Iwata T. Overexpression of HtrA1 and exposure to mainstream cigarette smoke leads to choroidal neovascularization and subretinal deposits in aged mice. Invest Ophthalmol Vis Sci. 2014;55:6514–6523. doi: 10.1167/iovs.14-14453. [DOI] [PubMed] [Google Scholar]
- 10.Vierkotten S, Muether PS, Fauser S. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch's membrane via cleavage of extracellular matrix components. PLoS One. 2011;6:e22959. doi: 10.1371/journal.pone.0022959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahimi KB, Handa JT. Lipids, lipoproteins, and age-related macular degeneration. J Lipids 2011. 2011:802059. doi: 10.1155/2011/802059. doi: 10.1155/2011/802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- 14.Lommatzsch A, Hermans P, Muller KD, Bornfeld N, Bird AC, Pauleikhoff D. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes Arch Clin Exp Ophthalmol. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- 15.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 16.Spaide RF, Armstrong D, Browne R. Continuing medical education review: Choroidal neovascularization in age-related macular degeneration--what is the cause? Retina. 2003;23:595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Torcynznski E. Chroid and suprachoroid. In Jakobiec FA (editor) Ocular Anatomy, Embryology, and Teratology. Harper & Row, Philadelphia. 1982:553–85. [Google Scholar]
- 18.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Mrejen S, Spaide RF. Optical coherence tomography: Imaging of the choroid and beyond. Surv Ophthalmol. 2013;58:387–429. doi: 10.1016/j.survophthal.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Parver LM, Auker C, Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol. 1980;89:641–646. doi: 10.1016/0002-9394(80)90280-9. [DOI] [PubMed] [Google Scholar]
- 21.Scholfield CN, McGeown JG, Curtis TM. Cellular physiology of retinal and choroidal arteriolar smooth muscle cells. Microcirculation. 2007;14:11–24. doi: 10.1080/10739680601072115. [DOI] [PubMed] [Google Scholar]
- 22.Alm A, Bill A. Blood flow and oxygen extraction in the cat uvea at normal and high intraocular pressures. Acta Physiol Scand. 1970;80:19–28. doi: 10.1111/j.1748-1716.1970.tb04765.x. [DOI] [PubMed] [Google Scholar]
- 23.Chirco KR, Sohn EH, Stone EM, Tucker BA, Mullins RF. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye (Lond) 2017;31:10–25. doi: 10.1038/eye.2016.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakatsuki Y, Shinojima A, Kawamura A, Yuzawa M. Correlation of aging and segmental choroidal thickness measurement using swept source optical coherence tomography in healthy eyes. PLoS One. 2015;10:e0144156. doi: 10.1371/journal.pone.0144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seddon JM, McLeod DS, Bhutto IA, Villalonga MB, Silver RE, Wenick AS, et al. Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol. 2016;134:1272–1280. doi: 10.1001/jamaophthalmol.2016.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohn EH, Khanna A, Tucker BA, Abramoff MD, Stone EM, Mullins RF. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014;55:1352–1360. doi: 10.1167/iovs.13-13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35:2562–2573. doi: 10.1016/j.neurobiolaging.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 30.May CA. Mast cell heterogeneity in the human uvea. Histochem Cell Biol. 1999;112:381–386. doi: 10.1007/s004180050420. [DOI] [PubMed] [Google Scholar]
- 31.Dvorak AM. New aspects of mast cell biology. Int Arch Allergy Immunol. 1997;114:1–9. doi: 10.1159/000237635. [DOI] [PubMed] [Google Scholar]
- 32.Bhutto IA, McLeod DS, Jing T, Sunness JS, Seddon JM, Lutty GA. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br J Ophthalmol. 2016;100:720–726. doi: 10.1136/bjophthalmol-2015-308290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94:918–25. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 34.McLeod DS, Bhutto I, Edwards MM, Silver RE, Seddon JM, Lutty GA. Distribution and quantification of choroidal macrophages in human eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57:5843–5855. doi: 10.1167/iovs.16-20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang JC, Cao S, Wang A, To E, Law G, Gao J, et al. CFH Y402H polymorphism is associated with elevated vitreal GM-CSF and choroidal macrophages in the postmortem human eye. Mol Vis. 2015;21:264–272. [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 37.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 39.Lundh von Leithner P, Kam JH, Bainbridge J, Catchpole I, Gough G, Coffey P, et al. Complement factor h is critical in the maintenance of retinal perfusion. Am J Pathol. 2009;175:412–421. doi: 10.2353/ajpath.2009.080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes AE, Bridgett S, Meng W, Li M, Curcio CA, Stambolian D, et al. Sequence and expression of complement factor H gene cluster variants and their roles in age-related macular degeneration risk. Invest Ophthalmol Vis Sci. 2016;57:2763–2769. doi: 10.1167/iovs.15-18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liszewski MK, Atkinson JP. Complement regulators in human disease: Lessons from modern genetics. J Intern Med. 2015;277:294–305. doi: 10.1111/joim.12338. [DOI] [PubMed] [Google Scholar]
- 42.Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011;93:565–567. doi: 10.1016/j.exer.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullins RF, Schoo DP, Sohn EH, Flamme-Wiese MJ, Workamelahu G, Johnston RM, et al. The membrane attack complex in aging human choriocapillaris: Relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184:3142–3153. doi: 10.1016/j.ajpath.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng S, Whitmore SS, Sohn EH, Riker MJ, Wiley LA, Scheetz TE, et al. Molecular response of chorioretinal endothelial cells to complement injury: Implications for macular degeneration. J Pathol. 2016;238:446–456. doi: 10.1002/path.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chirco KR, Flamme-Wiese MJ, Wiley JS, Potempa LA, Stone EM, Tucker BA, et al. Evaluation of serum and ocular levels of membrane attack complex and C-reactive protein in CFH-genotyped human donors. Eye (Lond) 2018;32:1740–1742. doi: 10.1038/s41433-018-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Bai Y, Huang L, Qi Y, Zhang Q, Li S, et al. Protective effect of autophagy on human retinal pigment epithelial cells against lipofuscin fluorophore A2E: Implications for age-related macular degeneration. Cell Death Dis. 2015;6:e1972. doi: 10.1038/cddis.2015.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer's A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoh Kam J, Lenassi E, Malik TH, Pickering MC, Jeffery G. Complement component C3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. Am J Pathol. 2013;183:480–492. doi: 10.1016/j.ajpath.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Hoh Kam J, Lenassi E, Jeffery G. Viewing ageing eyes: Diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013127. Epub 2010/10/20. doi: 10.1371/journal.pone. 0013127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aboelnour A, Kam JH, Elnasharty MA, Sayed-Ahmed A, Jeffery G. Amyloid beta deposition and phosphorylated tau accumulation are key features in aged choroidal vessels in the complement factor H knock out model of retinal degeneration. Exp Eye Res. 2016;147:138–143. doi: 10.1016/j.exer.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Gao J, Cui JZ, To E, Cao S, Matsubara JA. Evidence for the activation of pyroptotic and apoptotic pathways in RPE cells associated with NLRP3 inflammasome in the rodent eye. J Neuroinflammation. 2018;15:15. doi: 10.1186/s12974-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24:82–86. doi: 10.1161/01.cir.24.1.82. [DOI] [PubMed] [Google Scholar]
- 53.Bloome MA. Fluorescein angiography: Risks. Vision Res. 1980;20:1083–1097. doi: 10.1016/0042-6989(80)90045-0. [DOI] [PubMed] [Google Scholar]
- 54.Pauleikhoff D, Spital G, Radermacher M, Brumm GA, Lommatzsch A, Bird AC. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117:1353–1358. doi: 10.1001/archopht.117.10.1353. [DOI] [PubMed] [Google Scholar]
- 55.Gong J, Yu S, Gong Y, Wang F, Sun X. The diagnostic accuracy of optical coherence tomography angiography for neovascular age-related macular degeneration: A comparison with fundus fluorescein angiography. J Ophthalmol. 2016;2016:7521478. doi: 10.1155/2016/7521478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flower RW, Hochheimer BF. Clinical infrared absorption angiography of the choroid. Am J Ophthalmol. 1972;73:458–459. doi: 10.1016/0002-9394(72)90079-7. [DOI] [PubMed] [Google Scholar]
- 57.Arnold JJ, Quaranta M, Soubrane G, Sarks SH, Coscas G. Indocyanine green angiography of drusen. Am J Ophthalmol. 1997;124:344–356. doi: 10.1016/s0002-9394(14)70826-8. [DOI] [PubMed] [Google Scholar]
- 58.Giani A, Pellegrini M, Carini E, Peroglio Deiro A, Bottoni F, et al. The dark atrophy with indocyanine green angiography in Stargardt disease. Invest Ophthalmol Vis Sci. 2012;53:3999–4004. doi: 10.1167/iovs.11-9258. [DOI] [PubMed] [Google Scholar]
- 59.Axer-Siegel R, Bourla D, Priel E, Yassur Y, Weinberger D. Angiographic and flow patterns of retinal choroidal anastomoses in age-related macular degeneration with occult choroidal neovascularization. Ophthalmology. 2002;109:1726–1736. doi: 10.1016/s0161-6420(02)01149-1. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, Chang YS, Lee TG, Kim CG. Choroidal vascular hyperpermeability and punctate hyperfluorescent spot in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015;56:1909–1915. doi: 10.1167/iovs.14-16000. [DOI] [PubMed] [Google Scholar]
- 61.Zheng F, Gregori G, Schaal KB, Legarreta AD, Miller AR, Roisman L, et al. Choroidal thickness and choroidal vessel density in nonexudative age-related macular degeneration using swept-source optical coherence tomography imaging. Invest Ophthalmol Vis Sci. 2016;57:6256–6264. doi: 10.1167/iovs.16-20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu L, Xu S, He F, Liu Y, Zhang Y, Wang J, et al. Assessment of choroidal microstructure and subfoveal thickness change in eyes with different stages of age-related macular degeneration. Medicine (Baltimore) 2016;95:e2967. doi: 10.1097/MD.0000000000002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrara D, Silver RE, Louzada RN, Novais EA, Collins GK, Seddon JM. Optical coherence tomography features preceding the onset of advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:3519–3529. doi: 10.1167/iovs.17-21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yiu G, Chiu SJ, Petrou PA, Stinnett S, Sarin N, Farsiu S, et al. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol. 2015;159:617–626. doi: 10.1016/j.ajo.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Lindner M, Bezatis A, Czauderna J, Becker E, Brinkmann CK, Schmitz-Valckenberg S, et al. Choroidal thickness in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:875–882. doi: 10.1167/iovs.14-14933. [DOI] [PubMed] [Google Scholar]
- 66.Koh LHL, Agrawal R, Khandelwal N, Sai Charan L, Chhablani J. Choroidal vascular changes in age-related macular degeneration. Acta Ophthalmol. 2017;95:e597–e601. doi: 10.1111/aos.13399. [DOI] [PubMed] [Google Scholar]
- 67.Esmaeelpour M, Ansari-Shahrezaei S, Glittenberg C, Nemetz S, Kraus MF, Hornegger J, et al. Choroid, Haller’s, and Sattler's layer thickness in intermediate age-related macular degeneration with and without fellow neovascular eyes. Invest Ophthalmol Vis Sci. 2014;55:5074–5080. doi: 10.1167/iovs.14-14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fein JG, Branchini LA, Manjunath V, Regatieri CV, Fujimoto JG, Duker JS. Analysis of short-term change in subfoveal choroidal thickness in eyes with age-related macular degeneration using optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2014;45:32–37. doi: 10.3928/23258160-20131220-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garg A, Oll M, Yzer S, Chang S, Barile GR, Merriam JC, et al. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013;54:7075–7081. doi: 10.1167/iovs.13-12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yun C, Ahn J, Kim M, Hwang SY, Kim SW, Oh J. Ocular perfusion pressure and choroidal thickness in early age-related macular degeneration patients with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2016;57:6604–6609. doi: 10.1167/iovs.16-19989. [DOI] [PubMed] [Google Scholar]
- 71.Yun C, Oh J, Ahn SE, Hwang SY, Kim SW, Huh K. Peripapillary choroidal thickness in patients with early age-related macular degeneration and reticular pseudodrusen. Graefes Arch Clin Exp Ophthalmol. 2016;254:427–435. doi: 10.1007/s00417-015-3054-7. [DOI] [PubMed] [Google Scholar]
- 72.Govetto A, Sarraf D, Figueroa MS, Pierro L, Ippolito M, Risser G, et al. Choroidal thickness in non-neovascular versus neovascular age-related macular degeneration: A fellow eye comparative study. Br J Ophthalmol. 2017;101:764–769. doi: 10.1136/bjophthalmol-2016-309281. [DOI] [PubMed] [Google Scholar]
- 73.Sigler EJ, Randolph JC, Calzada JI, Charles S. Smoking and choroidal thickness in patients over 65 with early-atrophic age-related macular degeneration and normals. Eye (Lond) 2014;28:838–846. doi: 10.1038/eye.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almeida DR, Zhang L, Chin EK, Mullins RF, Kucukevcilioglu M, Critser DB, et al. Comparison of retinal and choriocapillaris thicknesses following sitting to supine transition in healthy individuals and patients with age-related macular degeneration. JAMA Ophthalmol. 2015;133:297–303. doi: 10.1001/jamaophthalmol.2014.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30:1333–1349. doi: 10.1097/IAE.0b013e3181e7976b. [DOI] [PubMed] [Google Scholar]
- 76.Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016;36:499–516. doi: 10.1097/IAE.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 77.Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33:1659–1672. doi: 10.1097/IAE.0b013e3182953df4. [DOI] [PubMed] [Google Scholar]
- 78.Yanagi Y, Mohla A, Lee WK, Lee SY, Mathur R, Chan CM, et al. Prevalence and risk factors for nonexudative neovascularization in fellow eyes of patients with unilateral age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2017;58:3488–3495. doi: 10.1167/iovs.16-21167. [DOI] [PubMed] [Google Scholar]
- 79.Baek J, Lee JH, Jung BJ, Kook L, Lee WK. Morphologic features of large choroidal vessel layer: Age-related macular degeneration, polypoidal choroidal vasculopathy, and central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2018;256:2309–2317. doi: 10.1007/s00417-018-4143-1. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi Y, Koizumi H, Hasegawa T, Izumi T, Maruko I, Sonoda S, et al. Comparison of subfoveal choroidal structures in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2018;62:576–583. doi: 10.1007/s10384-018-0615-4. [DOI] [PubMed] [Google Scholar]
- 81.Ahn SM, Lee SY, Hwang SY, Kim SW, Oh J, Yun C. Retinal vascular flow and choroidal thickness in eyes with early age-related macular degeneration with reticular pseudodrusen. BMC Ophthalmol. 2018;18:184. doi: 10.1186/s12886-018-0866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cicinelli MV, Rabiolo A, Marchese A, de Vitis L, Carnevali A, Querques L, et al. Choroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiography. Br J Ophthalmol. 2017;101:1193–1200. doi: 10.1136/bjophthalmol-2016-309481. [DOI] [PubMed] [Google Scholar]
- 83.Ueda-Arakawa N, Ooto S, Ellabban AA, Takahashi A, Oishi A, Tamura H, et al. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am J Ophthalmol. 2014;157:994–1004. doi: 10.1016/j.ajo.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 84.Toto L, Borrelli E, Di Antonio L, Carpineto P, Mastropasqua R. Retinal vascular plexuses’ changes in dry age-related macular degeneration, evaluated by means of optical coherence tomography angiography. Retina. 2016;36:1566–1572. doi: 10.1097/IAE.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 85.Nesper PL, Soetikno BT, Fawzi AA. Choriocapillaris nonperfusion is associated with poor visual acuity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2017;174:42–55. doi: 10.1016/j.ajo.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pellegrini M, Acquistapace A, Oldani M, Cereda MG, Giani A, Cozzi M, et al. Dark atrophy: An optical coherence tomography angiography study. Ophthalmology. 2016;123:1879–1886. doi: 10.1016/j.ophtha.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 87.Wirth MA, Freiberg F, Pfau M, Wons J, Becker MD, Michels S. Optical coherence tomography angiography in age-related macular degeneration: Persistence of vascular network in quiescent choroidal neovascularization. Acta Ophthalmol. 2017;95:428–430. doi: 10.1111/aos.13226. [DOI] [PubMed] [Google Scholar]


