Introduction
The recent development of immunotherapy has advanced the treatment of various cancers. The anti-PD-1 antibody nivolumab was designated by the FDA as a breakthrough therapy in September 2014. Subsequently, the anti-PD-1 antibody pembrolizumab received the same designation. Nivolumab is currently approved as a drug with good efficacy against a range of specific malignancies including malignant melanoma, non-small cell lung cancer, kidney cancer, stomach cancer, Hodgkin's lymphoma, and urothelial carcinoma. In addition, it is being investigated for the treatment of other types of cancer in promising clinical studies. Pembrolizumab was also approved by the FDA for similar indications in addition to the microsatellite instability-high or mismatch repair-deficient solid tumors.

Prof. M. Kudo Editor Liver Cancer
Hepatocellular carcinoma (HCC) is considerably more heterogeneous than other solid tumors or hematologic malignancies. Because of the lack of major driver mutations in HCC, treating patients with drugs that deplete hepatic functional reserve is challenging. Accordingly, researchers are investigating therapeutic approaches that differ from those used for other cancers. Among such therapies, the anti-PD-1 antibody nivolumab showed promise as a therapeutic agent for HCC in the CheckMate040 study [1]. Furthermore, numerous pharmaceutical companies have initiated phase III and/or earlier clinical trials of anti-PD-1 or anti-PD-L1 antibodies and anti-CTLA-4 antibodies for HCC (Table 1).
Table 1.
Immune checkpoint inhibitors
| Target cell | Molecule | Develop. code | Drug name | TM name | Antibody class | Company |
|---|---|---|---|---|---|---|
| T cell | PD-1 | BMS-36558 ONO-4538 |
Nivolumab | Opdivo | Fully human IgG4 antibody |
ONO/BMS |
| PD-1 | MK-4375 | Pembrolizumab | Keytruda | Humanized IgG4 antibody |
Merck | |
| PD-1 | BGB-A317 | Tislelizumab | Not approved | Humanized IgG4 antibody |
BeiGene | |
| PD-1 | SHR-1210 | Camrelizumab | Not approved | Humanized IgG4 antibody |
Jiangsu HengRui Medicine |
|
| Tumor cell | PD-L1 | MPDL3280A | Atezolizumab | Tecentriq | Fully human IgG1 antibody |
Roche |
| PD-L1 | MEDI4736 | Durvalumab | Imfinzi | Humanized IgG1 antibody |
AstraZeneca | |
| PD-L1 | MSB-0010718C | Avelumab | Barencio | Humanized IgG1 antibody |
Merck-Serono | |
| T cell | CTLA-4 | BMS-734016 | Ipilimumab | Yervoy | Fully humanized IgG1 antibody |
BMS Medarex |
| CTLA-4 | MEDI1123 | Tremelimumab | Not approved | Fully humanized IgG2 antibody |
AstraZeneca MedImmune |
|
Single-agent HCC therapies with anti-PD-1 antibody have yielded a higher response rates ranging from 17 to 20% with durable response than previously reported molecular targeted agents (MTAs) [1, 2]. Therefore, nivolumab was approved in September 22, 2017, as second-line therapy for HCC patients after progression on sorafenib therapy based on the phase I/II study only. For similar reasons, pembrolizumab was also approved by the FDA (November 9, 2018) as second-line therapy for patients with HCC who progress after sorafenib therapy.
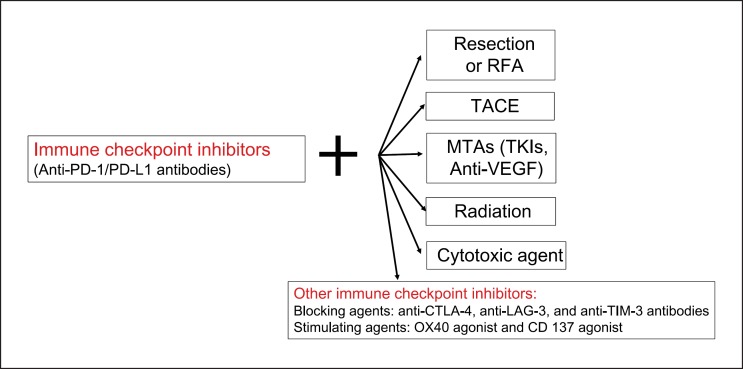
However, these agents show limited benefit from monotherapy of immune checkpoint inhibitors in approximately 30–40% of patients with progressive disease or those with stable disease developing into progressive disease. Researchers are therefore actively seeking therapeutic approaches for HCC that combine immune checkpoint inhibitors and other agents, similar to the therapeutic strategies commonly used in other cancers. Representative examples include immune checkpoint inhibitor combinations (PD-1/PD-L1 antibodies plus CTLA-4 antibodies), PD-1/PD-L1 antibodies plus MTAs, and PD-1/PD-L1 antibodies plus existing local therapeutic agents (Fig. 1).
Fig. 1.
Treatment strategy using immune checkpoint inhibitors. Future direction: combination therapy. RFA, radiofrequency ablation; TACE, transarterial chemoembolization; MTAs, molecular targeted agents; TKIs, tyrosine kinase inhibitors; VEGF, vascular endothelial growth factor.
Immune Checkpoint Inhibitor Combination Therapies
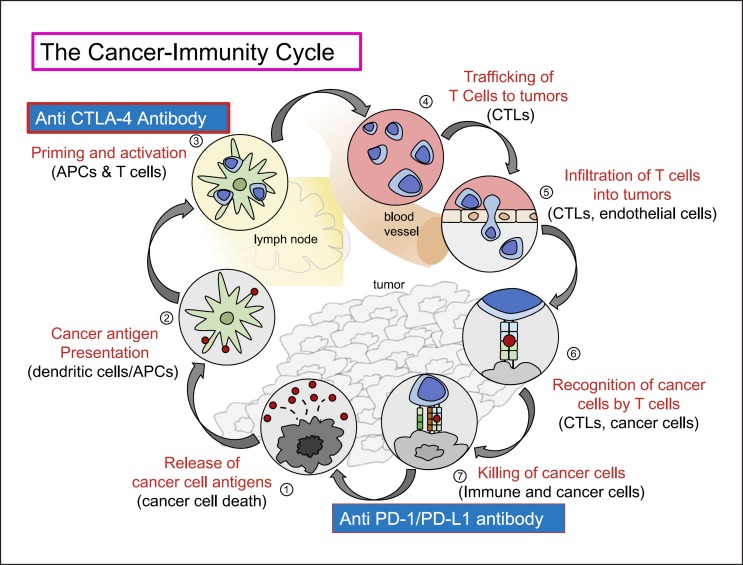
In the field of immunotherapy, the results of combination therapy with anti-PD-1/anti-PD-L1 antibodies and anti-CTLA-4 antibodies in HCC are awaited with much anticipation (Fig. 1). Concomitant nivolumab and ipilimumab show a superior therapeutic effect over monotherapies for melanoma [3, 4], although the efficacy of this combination in HCC is under investigation [5, 6]. The rationale for combination therapies with anti-PD-1/anti-PD-L1 and anti-CTLA-4 antibodies is as follows: In the absence of CD8+ T cells, stimulation of tumor immunity cannot be expected because these essential lymphocytes are absent from the tumor site, regardless of whether the PD-1/PD-L1 pathway is blocked. However, the anti-tumor effect is increased by the concomitant administration of anti-CTLA-4 antibodies that block the B7-CTLA-4 pathway, as this block leads to an increase in activated CD8+ T cells in lymph nodes followed by an increase in activated CD8+ T cells infiltrating the tumor tissue [7] (Fig. 2). Furthermore, CTLA-4 is expressed on regulatory T cells (Treg cells) that infiltrate into tumors to play a role as suppressors of anti-tumor immunity in the tumor microenvironment [8]. Theoretically, anti-CTLA-4 antibodies should enhance CD8+ T cell immune activation by inhibiting CTLA-4 expressed on Treg cells. This is the rationale for the ongoing clinical trials of combination therapies of anti-CTLA-4 antibodies with anti-PD-1/PD-L1 antibodies for HCC (Fig. 2).
Fig. 2.
The cancer immunity cycle. Modified from Chen and Mellman [7].
Combination therapy with nivolumab and the anti-CTLA-4 antibody ipilimumab at a range of doses and dose intervals is under investigation in the CheckMate040 study. Another therapeutic regimen currently under investigation is the combination of the anti-PD-L1 antibody durvalumab and the anti-CTLA-4 antibody tremelimumab, and the efficacy and safety of this combination are being evaluated in comparison with monotherapies in a phase I/II clinical study. The phase I part of this study was reported at the 2017 annual meeting of the American Society of Clinical Oncology (ASCO) [5]. Durvalumab plus tremelimumab combination therapy showed an objective response rate (ORR) of 25% in 40 HCC patients, yielding a more favorable outcome than durvalumab monotherapy [5]. These results were the basis for the initiation of a currently ongoing phase III clinical study (HIMALAYA study).
Combination Therapies with Immune Checkpoint Inhibitors and MTAs
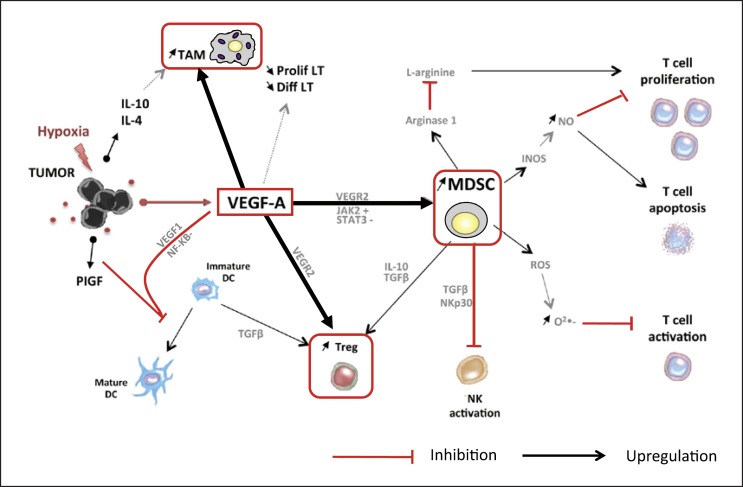
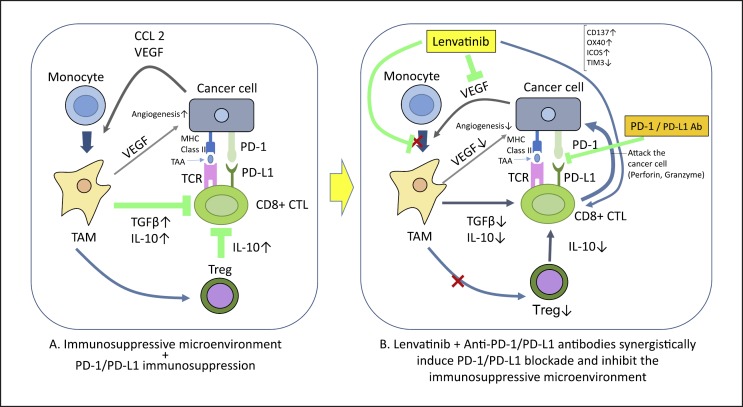
Immune checkpoint inhibitor-MTA combination therapies have raised high expectations in recent years. These therapies affect vascular endothelial growth factor (VEGF)-A, which is released from tumor cells and the vascular endothelium in the region of the cancer. VEGF-A induces a wide range of immune suppressor cells, including tumor-associated macrophages (TAMs), Treg cells, and myeloid-derived suppressor cells (MDSCs), creating a microenvironment that suppresses tumor immunity (Fig. 3). These cells prevent the maturation of dendritic cells and inhibit the activation of NK cells and T cells by releasing immunosuppressive cytokines (IL-10 or TGF-β) [9]. Stromal cells in the liver (e.g., Kupffer cells, dendritic cells, hepatic endothelial cells, and hepatic stellate cells) are also implicated in the immunosuppressive environment of HCC [10]. On this theoretical basis, VEGF activity could be suppressed by anti-VEGF antibodies and multikinase (including VEGF)-inhibiting MTAs in the following ways: (1) antigen presentation to dendritic cells is increased; (2) T cell activation is promoted in the priming phase; (3) tumor vessels are normalized, thereby improving trafficking and infiltration of T cells from lymph nodes to the region of the tumor; (4) the Treg cells, TAMs, and MDSCs mentioned above are suppressed, and the humoral factors TGF-β and IL-10 decrease at the tumor site, leading to improved immunity in the tumor microenvironment; (5) the inhibition of checkpoint molecules by anti-PD-1/PD-L1 antibodies activates the cancer immunity cycle against the tumor [7]. Furthermore, activated T cells can then effectively attack the tumor, inducing necrosis of tumor cells (Fig. 2). Pembrolizumab and lenvatinib combination therapy suppresses TAMs and Treg cells, counteracting their immunosuppressive effect on the microenvironment. This decreases TGF-β and IL-10, downregulates the expression of PD-1 and T cell immunoglobulin and mucin domain 3 (TIM-3), and promotes the immune-activating cytokine IL-12, resulting in an anti-tumor immunity-promoting effect, as shown in a preclinical study [11] (Fig. 4). A number of clinical trials of immune checkpoint inhibitor-MTA combination therapies have been initiated based on this hypothesis (Table 2). Pembrolizumab plus lenvatinib combination therapy shows high response rates of 50–70% and durable responses in renal cancer, endometrial cancer, and head and neck cancer according to results reported at the 2018 annual meeting of the European Society of Medical Oncology (ESMO), and the 2017 and 2018 ASCO meetings. A number of similar, early-phase clinical studies of HCC therapies are currently ongoing, including the pembrolizumab plus lenvatinib trial and atezolizumab plus bevacizumab trial (Table 2).
Fig. 3.
Immunosuppressive microenvironment produced by VEGF. MDSC, myeloid-derived suppressor cell; DC, dendritic cell; TAM, tumor-associated macrophage; T-reg, T-regulatory cell. Modified from Voron et al. [9].
Fig. 4.
Mechanism of synergistic effects of lenvatinib and anti-PD-1/PD-L1 antibodies.
Table 2.
Immune checkpoint inhibitors in combination with MTAs in HCC
| Phase | Target | Agent | Company | Trial # |
|---|---|---|---|---|
| 1–2 | PD-1 + TGFβ receptor I | Nivolumab + galunisertib | Eli Lilly | NCT02423343 |
| 1 | PD-L1 + VEGFR-2 | Durvalumab + ramucirumab | Eli Lilly | NCT02572687 |
| 1 | PD-1 + multikinase | Pembrolizumab + nintedanib | Gustave Roussy |
NCT02856425 |
| 1 | PD-1 + multikinase | PDR001 + sorafenib | Novartis | NCT02988440 |
| 1–2 | PD-1 + c-Met | PDR001 + capmatinib (INC280) | Novartis | NCT02795429 |
| 1–2 | PD-1; CTLA-4 + MET/VEGFR2 | Nivolumab + ipilimumab + cabozantinib | BMS | NCT01658878 |
| 1 | PD-1 + multikinase | Nivolumab + lenvatinib | Ono | NCT03418922 |
| 1–2 | PD-L1 + multikinase | Averumab + axitinib | Pfizer | NCT03289533 |
| 1 | PD-1 + multikinase | SHR-1210 + apatinib | Jiangsu HengRui Medicine |
NCT02942329 |
| 1 | PD-1 + multikinase | Pembrolizumab + lenvatinib | Eisai | NCT03006926 |
| 1–2 | PD-L1 + anti-VEGF | Atezolizumab + bevacizumab | Roche | NCT03170960 |
| 3 | PD-L1 + anti-VEGF | Atezolizumab + bevacizumab | Roche | NCT03434379 |
The results of three anti-PD-1/PD-L1 antibody plus MTA combination immunotherapies for HCC were presented simultaneously at the 2018 annual ASCO meeting. The first of these reports involved a phase Ib clinical trial of pembrolizumab plus lenvatinib combination therapy. In that study, safety and efficacy evaluations included only 30 and 26 patients, respectively; however, the ORR was 42.3%, and the disease control rate was 100%, with no cases of progressive disease. Outcomes were favorable, and progression-free survival was 9.7 months [12] (Table 3).
Table 3.
Results of immune checkpoint inhibitors and combination therapy
| Nivolumab (n = 214) | Pembrolizumab (n = 104) | Pembrolizumab plus lenvatinib (n = 26) | Atezolizumab plus bevacizumaba (n = 73) | SHR-1210 plus apatinib (n = 18) | Durvalumab plus tremelimumab (n = 40) | |
|---|---|---|---|---|---|---|
| ORR, % | 20 (15–26)b | 17 (11–26)b | 42.3 (23.4–63.1)c | 34c | 38.9c | 25b |
| DCR, % | 64 (58–71) | 62 (52–71) | 100 | 75 | 83.3 | 57.5 (>16 weeks) |
| PFS, months | 4.0 (2.9–5.4) | 4.9 (3.4–7.2) | 9.7 (5.6-NE) | 7.5 (0.4–23.9) | 7.2 (2.6-NE) | NA |
| OS, months | NR (9 M, 74%) | 12.9 (9.7–15.5) | NR | NR | NR | NA |
| DOR, months | 9.9 (8.3-NE) | ≤9 (77%) | NE | NR | NE | NA |
Figures in parentheses indicate 95% CI unless otherwise stated. ORR, objective response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; DOR, duration of response; NR, not reached; NE, not estimable; NA, not available.
Independent Review Facility (IRF) assessment.
RECIST 1.1.
Modified RECIST.
The second of these reports involved combination therapy with the anti-PD-L1 antibody atezolizumab and the anti-VEGF antibody bevacizumab. The results generated considerable interest in the field of HCC therapy because of the high ORR of 61% based on RECIST 1.1 and according to an investigator assessment of 23 patients, and 65% according to an independent review [13].
The third of these reports involved the anti-PD-1 antibody SHR1210 combined with apatinib. This study involved 18 patients and yielded fair outcomes, with a response rate of 38.9%, disease control rate of 83.3%, and progression-free survival of 7.2 months [14] (Table 3).
The highest response rate reported at ASCO 2018 in June was for the clinical study of atezolizumab plus bevacizumab. However, updated results (including 73 patients) were reported at the 2018 annual ESMO meeting in October, and the response rates were substantially reduced: 32% according to RECIST 1.1 by investigator assessment and 27% according to RECIST 1.1 by independent assessment [15] (Tables 4, 5). The ORR of 27% according to independent review that was reported at ESMO 2018 was greatly reduced from the corresponding ORR of 65% reported at ASCO 2018. The ORR of this combination trial was 34% according to the modified RECIST by independent review assessment (Table 5).
Table 4.
Comparison between data at ASCO 2018 and ESMO 2018 [15]
| ASCO 2018 Atezo + Bev (n = 23) | ESMO 2018 Atezo + Bev (n = 73) | |
|---|---|---|
| ORR, n (%) | 14 (61) | 23 (32) |
| CR | 0 (0) | 1 (1) |
| PR | 14 (61) | 22 (30) |
| SD | 5 (22) | 33 (45) |
| PD | 4 (17) | 13 (18) |
| DCR (CR + PR + SD), n (%) | 19 (83) | 56 (78) |
Investigator-assessed results (per RECIST v1.1). Seventy-three patients were evaluable for efficacy with a minimum follow-up of 16 weeks. ORR, objective response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate.
Table 5.
Comparison between data at ASCO 2018 and ESMO 2018 [15]
| ASCO 2018 Atezo + Bev (n = 23)a | ESMO 2018 Atezo + Bev (n = 73)a | ESMO 2018 Atezo + Bev (n = 73)b | |
|---|---|---|---|
| ORR, n (%) | 15 (65) | 20 (27) | 25 (34) |
| CR | 1 (4) | 4 (5) | 8 (11) |
| PR | 14 (61) | 16 (22) | 17 (23) |
| SD | 7 (30) | 35 (48) | 30 (41) |
| PD | 1 (4) | 14 (19) | 14 (19) |
| DCR (CR + PR + SD), n (%) | 22 (96) | 55 (75) | 55 (75) |
Seventy-three patients were evaluable for efficacy with a minimum follow-up of 16 weeks. ORR, objective response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate.
Independent review facility-assessed results (per RECIST v1.1).
Independent review facility-assessed results (per mRECIST).
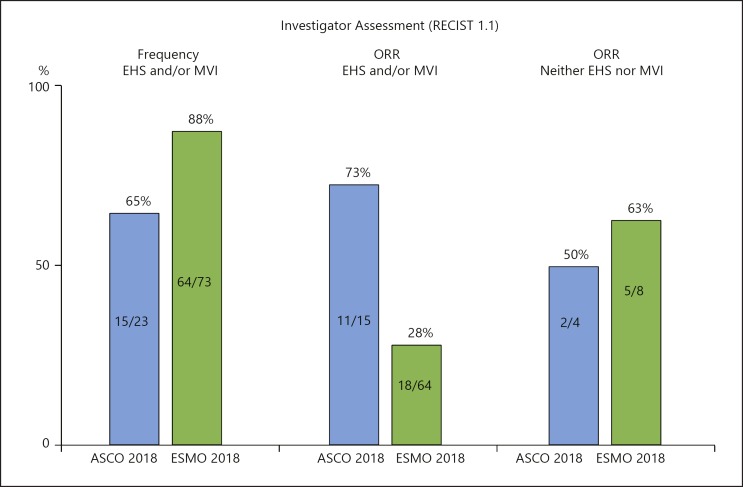
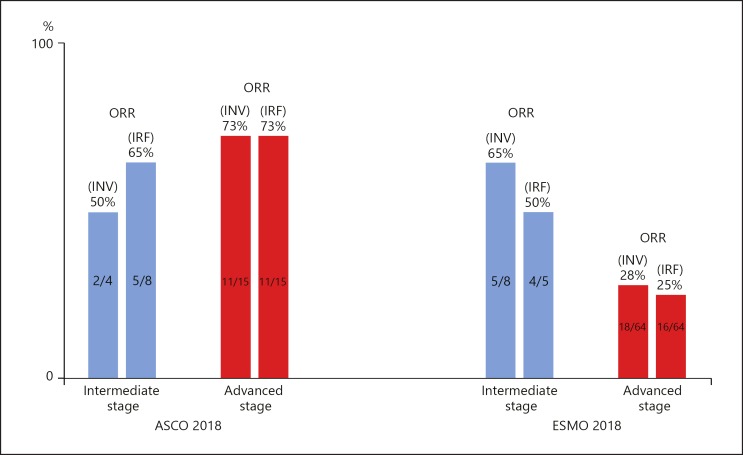
A major factor in the reduction of response rate was the increase in the proportion of patients with macrovascular invasion (MVI) and/or extrahepatic spread (EHS) from 65% (ASCO 2018) to 88% (ESMO 2018) (Table 6; Fig. 5). The ESMO data showed that the response rate was 28% in patients with MVI and/or EHS (advanced stage HCC), compared with 63% in patients with neither MVI nor EHS (intermediate stage HCC). Advanced-stage HCC is a subgroup that responds poorly to systemic therapy compared with intermediate-stage HCC (Table 6; Fig. 6). According to the ASCO results, the response rate was 73% in patients with MVI and/or EHS (advanced stage HCC) versus 50% in patients with neither MVI nor EHS (intermediate stage HCC). These figures show an opposite pattern to that of the ESMO results (Fig. 6). The ORR reported at ESMO 2018 appears to be consistent with the pattern in the previous systemic therapy, as the ORR of a Japanese subpopulation from the REFLECT trial was 61.3% (n = 31) for intermediate-stage HCC and 38.0% (n = 50) for advanced-stage HCC in the lenvatinib arm [16].
Table 6.
Phase 1b of atezolizumab + bevacizumab: baseline characteristics and ORR
| ASCO 2018 (n = 23) | ESMO 2018 (n = 73) | |||
|---|---|---|---|---|
| n (%) | ORR | n (%) | ORR | |
| EHS | 11 (48) | NA | 51 (70) | NA |
| MVI | 6 (23) | NA | 41 (56) | NA |
| EHS and/or MVI | 15 (65) | 11/15 (73) | 64 (88) | 18/64 (28) |
| Neither EHS nor MVI | 4 (17) | 2/4 (50) | 8 (11) | 5/8 (63) |
Investigator-assessed results (RECIST 1.1). ORR, objective response rate; NA, not available; EHS, extrahepatic spread; MVI, macrovascular invasion.
Fig. 5.
Phase 1b of atezolizumab + bevacizumab combination therapy. Extrahepatic spread (EHS) and/or macrovascular invasion (MVI).
Fig. 6.
Phase 1b of atezolizumab + bevacizumab combination therapy. Comparison of ORR between intermediate and advanced HCC. ORR, objective response rate; INV, investigator assessment; IRF, independent review facility assessment. Intermediate stage: neither extrahepatic spread nor macrovascular invasion. Advanced stage: extrahepatic spread and/or macrovascular invasion.
It is easily speculated that progressive disease is more frequently observed, and tumor shrinkage is more difficult to achieve in advanced-stage HCC because of the more frequently observed phenotype change, such as epithelial-mesenchymal transition, and/or higher tumor burden/vascular invasion than in intermediate-stage HCC. The higher response rates in advanced-stage HCC than those in intermediate-stage HCC reported at ASCO 2018 could be attributed to the smaller number of patients evaluated.
The currently available results for combination immunotherapies are shown in Table 3. It may be important to note that the ORR may change, either improve or decrease, with further increases in the number of patients evaluated from an experience of the above-mentioned combination study. A phase III study of atezolizumab plus bevacizumab (IMbrave150 study) is ongoing, as is the phase III study of durvalumab plus tremelimumab (HIMALAYA study). The results of these studies are eagerly awaited.
Conclusion
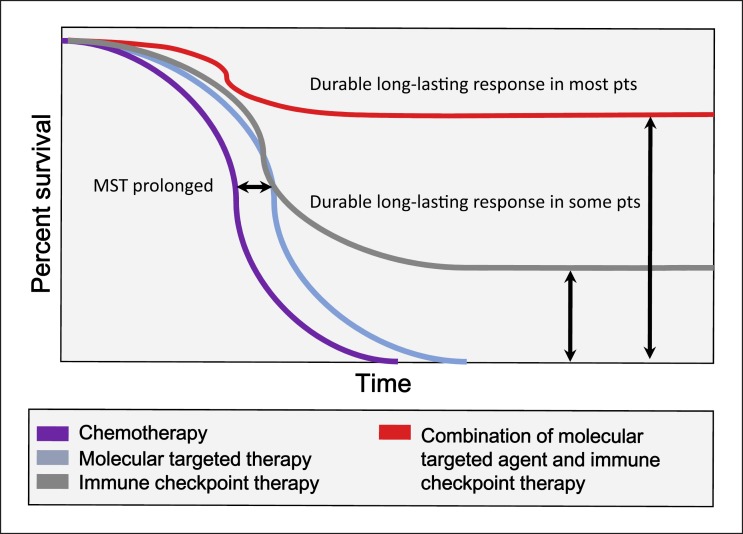
Prolonged survival and cure may be expected for patients who respond to immune checkpoint inhibitors, as described by Sharma and Allison [17, 18]. These authors propose that a durable long-lasting response may be achieved with combination immunotherapies in majority of the patients (Fig. 7) [17]. Combination therapies of anti-PD-1/anti-PD-L1 antibodies with other MTAs or with other immune checkpoint inhibitors such as anti-CTLA-4 agents may bring a much better survival in HCC patients and lead to a paradigm shift in the treatment strategy for this disease. The recent development of immune checkpoint inhibitors [19], multiple MTAs [20], and combination therapies as described above [21] may lead to major innovations in the treatment of HCC in the very near future. Further drug development based on an improved understanding of the mechanisms of action of combination therapy should substantially improve the prognosis of patients with unresectable HCC.
Fig. 7.
Improved overall survival as a result of combination therapy: immune checkpoint inhibitors and molecular targeted therapy. Modified from Sharma and Allison [18].
Disclosure Statement
Masatoshi Kudo received lecture fees from Bayer, Eisai, MSD, and Ajinomoto, research grants from Chugai, Otsuka, Takeda, Taiho, Sumitomo Dainippon, Daiichi Sankyo, MSD, Eisai, Bayer, AbbVie, Medico's Hirata, Astellas Pharma, and Bristol-Myers Squibb, and advisory consulting fees from Kowa, MSD, Bristol-Myers Squibb, Bayer, Chugai, Taiho, Eisai, and Ono Pharmaceutical.
References
- 1.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017 Jun;389((10088)):2492–502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. KEYNOTE-224 investigators Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018 Jul;19((7)):940–52. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015 May;372((21)):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013 Jul;369((2)):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Borad MJ, Yong WP, Morse M, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma(HCC): Phase I safety and efficacy analyses. J Clin Oncolol. 2017 May;35((15 suppl)):4073. [Google Scholar]
- 6.Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017 Feb;6((2)):101–12. doi: 10.1159/000452138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DS, Mellman I. Oncology meets immunology the cancer-immunity cycle. Immunity. 2013 Jul 25;39((1)):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008 Oct;322((5899)):271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 9.Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014 Apr;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010 Feb;34((1)):1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Bao X, Macgrath S, Tabata K, Hori Y, Tachino S, Matijevici M, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. (2016);27((suppl_6)):2PD. [Google Scholar]
- 12.Ikeda M, Sung MW, Kudo M, Kobayashi M, Baron AD, Finn RS. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients (pts) with unresectable hepatocellular carcinoma (uHCC) J Clin Oncol. 2018;36((15 suppl)):4076. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein S, Pishvaian MJ, Lee MS, et al. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC) J Clin Oncol. 2018;36((15 suppl)):4074. [Google Scholar]
- 14.Xu JM, Zhang Y, Jia R, Wang Y, Liu R, Zhang G, Zhao C, et al. Anti-programmed death-1 antibody SHR-1210 (S) combined with apatinib (A) for advanced hepatocellular carcinoma (HCC), gastric cancer (GC) or esophagogastric junction (EGJ) cancer refractory to standard therapy: A phase 1 trial. J Clin Oncol. 2018;36((15 suppl)):4075. [Google Scholar]
- 15.Pishvaian MJ, Lee MS, Ryoo BY, Stein S, Lee KH, Verret W, Spahn J, et al. Updated safety and clinical activity results from a Phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC) ESMO. 2018 Oct-Dec; Munich; LBA26. [Google Scholar]
- 16.Yamashita T, Kudo M, Ikeda K, Izumi N, Ikeda M, Okusaka T, et al. Analysis of Japanese subpopulation from reflect trial. The 18th annual meeting of Japan Association of Molecular Targeted Therapy for HCC. 2018 July 14; Tokyo. [Google Scholar]
- 17.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015 Apr;348((6230)):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015 Apr;161((2)):205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver Cancer. 2016 Nov;6((1)):1–12. doi: 10.1159/000449342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M. Combination Cancer Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2018 Mar;7((1)):20–7. doi: 10.1159/000486487. [DOI] [PMC free article] [PubMed] [Google Scholar]