Abstract
Background
Paracetamol (acetaminophen) is vastly recommended as the first‐line analgesic for osteoarthritis of the hip or knee. However, there has been controversy about this recommendation given recent studies have revealed small effects of paracetamol when compared with placebo. Nonetheless, past studies have not systematically reviewed and appraised the literature to investigate the effects of this drug on specific osteoarthritis sites, that is, hip or knee, or on the dose used.
Objectives
To assess the benefits and harms of paracetamol compared with placebo in the treatment of osteoarthritis of the hip or knee.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, AMED, CINAHL, Web of Science, LILACS, and International Pharmaceutical Abstracts to 3 October 2017, and ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP) portal on 20 October 2017.
Selection criteria
We included randomised controlled trials comparing paracetamol with placebo in adults with osteoarthritis of the hip or knee. Major outcomes were pain, function, quality of life, adverse events and withdrawals due to adverse events, serious adverse events, and abnormal liver function tests.
Data collection and analysis
Two review authors used standard Cochrane methods to collect data, and assess risk of bias and quality of the evidence. For pooling purposes, we converted pain and physical function (Western Ontario and McMaster Universities Osteoarthritis Index function) scores to a common 0 (no pain or disability) to 100 (worst possible pain or disability) scale.
Main results
We identified 10 randomised placebo‐controlled trials involving 3541 participants with hip or knee osteoarthritis. The paracetamol dose varied from 1.95 g/day to 4 g/day, and the majority of trials followed participants for three months only. Most trials did not clearly report randomisation and concealment methods and were at unclear risk of selection bias. Trials were at low risk of performance, detection, and reporting bias.
At 3 weeks' to 3 months' follow‐up, there was high‐quality evidence that paracetamol provided no clinically important improvements in pain and physical function. Mean reduction in pain was 23 points (0 to 100 scale, lower scores indicated less pain) with placebo and 3.23 points better (5.43 better to 1.02 better) with paracetamol, an absolute reduction of 3% (1% better to 5% better, minimal clinical important difference 9%) and relative reduction of 5% (2% better to 8% better) (seven trials, 2355 participants). Physical function improved by 12 points on a 0 to 100 scale (lower scores indicated better function) with placebo and was 2.9 points better (0.95 better to 4.89 better) with paracetamol, an absolute improvement of 3% (1% better to 5% better, minimal clinical important difference 10%) and relative improvement of 5% (2% better to 9% better) (7 trials, 2354 participants).
High‐quality evidence from eight trials indicated that the incidence of adverse events was similar between groups: 515/1586 (325 per 1000) in the placebo group versus 537/1666 (328 per 1000, range 299 to 360) in the paracetamol group (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.92 to 1.11). There was less certainty (moderate‐quality evidence) around the risk of serious adverse events, withdrawals due to adverse events, and the rate of abnormal liver function tests, due to wide CIs or small event rates, indicating imprecision. Seventeen of 1480 (11 per 1000) people treated with placebo and 28/1729 (16 per 1000, range 8 to 29) people treated with paracetamol experienced serious adverse events (RR 1.36, 95% CI 0.73 to 2.53; 6 trials). The incidence of withdrawals due to adverse events was 65/1000 participants in with placebo and 77/1000 (range 59 to 100) participants with paracetamol (RR 1.19, 95% CI 0.91 to 1.55; 7 trials). Abnormal liver function occurred in 18/1000 participants treated with placebo and 70/1000 participants treated with paracetamol (RR 3.79, 95% CI 1.94 to 7.39), but the clinical importance of this effect was uncertain. None of the trials reported quality of life.
Subgroup analyses indicated that the effects of paracetamol on pain and function did not differ according to the dose of paracetamol (3.0 g/day or less versus 3.9 g/day or greater).
Authors' conclusions
Based on high‐quality evidence this review confirms that paracetamol provides only minimal improvements in pain and function for people with hip or knee osteoarthritis, with no increased risk of adverse events overall. Subgroup analysis indicates that the effects on pain and function do not differ according to the dose of paracetamol. Due to the small number of events, we are less certain if paracetamol use increases the risk of serious adverse events, withdrawals due to adverse events, and rate of abnormal liver function tests.
Current clinical guidelines consistently recommend paracetamol as the first‐line analgesic medication for hip or knee osteoarthritis, given its low absolute frequency of substantive harm. However, our results call for reconsideration of these recommendations.
Keywords: Aged; Humans; Middle Aged; Acetaminophen; Acetaminophen/administration & dosage; Acetaminophen/adverse effects; Acetaminophen/therapeutic use; Analgesics, Non-Narcotic; Analgesics, Non-Narcotic/administration & dosage; Analgesics, Non-Narcotic/adverse effects; Analgesics, Non-Narcotic/therapeutic use; Arthralgia; Arthralgia/drug therapy; Arthralgia/etiology; Liver; Liver/drug effects; Osteoarthritis, Hip; Osteoarthritis, Hip/drug therapy; Osteoarthritis, Knee; Osteoarthritis, Knee/drug therapy; Pain Measurement; Randomized Controlled Trials as Topic
Plain language summary
Paracetamol for treating people with hip or knee osteoarthritis
Background
Osteoarthritis of the hip or knee is a progressive disabling disease affecting many people worldwide. Although paracetamol is widely used as a treatment option for this condition, recent studies have called into question how effective this pain relief medication is.
Search date
This review includes all trials published up to 3 October 2017.
Study characteristics
We included randomised clinical trials (where people are randomly put into one of two treatment groups) looking at the effects of paracetamol for people with hip or knee pain due to osteoarthritis against a placebo (a 'sugar tablet' that contains nothing that could act as a medicine). We found 10 trials with 3541 participants. On average, participants in the study were aged between 55 and 70 years, and most presented with knee osteoarthritis. The treatment dose ranged from 1.95 g/day to 4 g/day of paracetamol and participants were followed up between one and 12 weeks in all but one study, which followed people up for 24 weeks. Six trials were funded by companies that produced paracetamol.
Key results
Compared with placebo tablets, paracetamol resulted in little benefit at 12 weeks.
Pain (lower scores mean less pain)
Improved by 3% (1% better to 5% better), or 3.2 points (1 better to 5.4 better) on a 0‐ to 100‐point scale.
• People who took paracetamol reported that their pain improved by 26 points.
• People who took placebo reported that their pain improved by 23 points.
Physical function (lower scores mean better function)
Improved by 3% (1% better to 5% better), or 2.9 points (1.0 better to 4.9 better) on a 0‐ to 100‐point scale.
• People who took paracetamol reported that their function improved by 15 points.
• People who had placebo reported that their function improved by 12 points.
Side effects (up to 12 to 24 weeks)
No more people had side effects with paracetamol (3% less to 3% more), or 0 more people out of 100.
• 33 out of 100 people reported a side effect with paracetamol.
• 33 out of 100 people reported a side effect with placebo.
Serious side effects (up to 12 to 24 weeks)
1% more people had serious side effects with paracetamol (0% less to 1% more), or one more person out of 100.
• Two out of 100 people reported a serious side effect with paracetamol.
• One out of 100 people reported a serious side effect with placebo.
Withdrawals due to adverse events (up to 12 to 24 weeks)
1% more people withdrew from treatment with paracetamol (1% less to 3% more), or one more person out of 100.
• Eight out of 100 people withdrew from paracetamol treatment.
• Seven out of 100 people withdrew from placebo treatment.
Abnormal liver function tests (up to 12 to 24 weeks):
5% more people had abnormal liver function tests (meaning there was some inflammation or damage to the liver) with paracetamol (1% more to 10% more), or five more people out of 100.
• Seven out of 100 people had an abnormal liver function test with paracetamol.
• Two out of 100 people had an abnormal liver function test with placebo.
Quality of the evidence
High‐quality evidence indicated that paracetamol provided only minimal improvements in pain and function for people with hip or knee osteoarthritis, with no increased risk of adverse events overall. None of the studies measured quality of life. Due to the small number of events, we were less certain if paracetamol use increased the risk of serious side effects, increased withdrawals due to side effects, and changed the rate of abnormal liver function tests. However, although there may be more abnormal liver function tests with paracetamol, the clinical implications are unknown.
Summary of findings
Summary of findings for the main comparison. Paracetamol versus placebo for hip or knee osteoarthritis.
| Paracetamol versus placebo for hip or knee osteoarthritis | ||||||
|
Population: people with hip or knee osteoarthritis Settings: various rehabilitation, orthopaedic, or rheumatology clinics Intervention: paracetamol Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | What happens | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Paracetamol | |||||
|
Mean change in pain (0–100 scale) Short term (3–12 weeks), where 0 = no pain |
The mean change in pain score in the placebo group was –23a | The mean change in pain score in the paracetamol group was 3.2 points lower (1.0 lower to 5.4 lower) |
MD –3.23 (–5.43 to –1.02) |
2355 (7) | ⊕⊕⊕⊕ High | Clinically unimportant improvement; absolute change 3% better (5% better to 1% better); relative change 5% better (2% better to 8% better)b |
|
Physical function (WOMAC function 0–100) 3–12 weeks, 0 = better function |
The mean change in physical function score in the placebo group was –12a | The mean physical function score in the paracetamol group was 2.9 points lower (4.9 lower to 1.0 lower) |
MD –2.92 (–4.89 to –0.95) |
2354 (7) | ⊕⊕⊕⊕ High | Clinically unimportant improvement; absolute change 3% better (5% better to 1% better); relative change 5% better (2% better to 9% better)b. |
|
Quality of life Not measured |
See comment | See comment | — | — | See comment | Not measured |
|
Withdrawals due to adverse events 24 weeks |
65 per 1000 |
77 per 1000 (59 to 100) |
RR 1.19 (0.91 to 1.55) | 3023 (7) | ⊕⊕⊕⊝ Moderatec | The difference was not statistically or clinically significant; absolute change 1% more withdrew with paracetamol (1% less to 3% more); relative change 19% more (9% less to 55% more). |
|
Total adverse events: number experiencing 24 weeks |
325 per 1000 | 328 per 1000 (299 to 360) |
RR 1.01 (0.92 to 1.11) |
3252 (8) | ⊕⊕⊕⊕ High | The difference was not statistically or clinically significant; absolute change: no more events with paracetamol (3% less to 3% more); relative change 1% more (8% less to 11% more). |
|
Liver toxicity: number experiencing abnormal liver function tests 24 weeks |
18 per 1000 | 70 per 1000 (36 to 136) |
RR 3.79 (1.94to 7.39) |
1237 (3) | ⊕⊕⊕⊝ Moderatec | The clinical impact of the higher risk of abnormal liver function tests was unclear; absolute change 5% more abnormal tests with paracetamol (1% more to 10% more); relative change 279% more (94% more to 639% more). |
|
Serious adverse events: number experiencing 24 weeks |
11 per 1000 | 16 per 1000 (8 to 29) |
RR 1.36 (0.73 to 2.53) |
3209 (6) | ⊕⊕⊕⊝ Moderatec | Absolute change: no more events with paracetamol (up to 1% more); relative change 36% more (27% less to 153% more). |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference;RR: risk ratio; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aBased on score in placebo group at 3 months' follow‐up as reported in Miceli‐Richard 2004.
bRelative changes calculated as absolute change (mean difference) divided by mean at baseline in the placebo group from Miceli‐Richard 2004 (mean values were: 69.0 (standard deviation 17) on 0‐ to 100‐point visual analogue pain scale; and 54.0 (standard deviation 15) on 0‐ to 100‐point WOMAC function subscale).
cDowngraded by one level due to imprecision (small number of events, or the 95% confidence intervals do not exclude a clinically important change).
Background
Osteoarthritis of the hip or knee is the 11th highest contributor to global disability, when disability is measured by years lived with disability (Vos 2012), and its global prevalence is estimated at 4% (Cross 2014; Hoy 2014). In this context, it is important to manage osteoarthritis in an effective and efficient way (Barten 2015), and the control of pain symptoms is an important component of treatment (Prior 2014). The use of paracetamol (acetaminophen) is consistently recommended as the first‐line analgesic for this condition (Hochberg 2012; Zhang 2005). However, there has been controversy about using paracetamol in international clinical guidelines, mainly because previous studies have reported small effects for the medication compared with placebo (Towheed 2006; Zhang 2004; Zhang 2010). The perceived safety profile of paracetamol over other analgesics such as non‐steroidal anti‐inflammatory drugs (NSAIDs), has led to the increase of its use, resulting in paracetamol being among the most common non‐prescription medications used for osteoarthritis. However, there is evidence linking paracetamol with increased risk of gastrointestinal, cardiovascular, and kidney diseases, and mortality (Roberts 2016). A Cochrane Review of placebo‐controlled trials investigating the benefits of paracetamol for hip or knee osteoarthritis could provide credible information for clinical decision‐making based on the highest standard of evidence. This review is an update of a meta‐analysis on paracetamol for spinal pain or osteoarthritis (Machado 2015).
Description of the condition
Osteoarthritis is a complex and progressive joint disease characterised by focal cartilage loss, accompanied by subchondral bone changes and involvement of all joint tissues as deterioration of tendons and ligaments, and various degrees of inflammation of the synovium resulting from biomechanical and systemic effects (Hochberg 2012; Zhang 2010). This prevalent disease is associated with significant pain, stiffness, functional impairment, and reduced quality of life (Buckwalter 2006; Woolf 2003), resulting in a large societal and economic burden (Barten 2015).
Description of the intervention
Paracetamol is an antipyretic and analgesic medication that is not thought to have significant anti‐inflammatory properties. Although its mechanism of inducing analgesia is not yet completely understood, the drug is thought to work in part by decreasing production of prostaglandins through inhibitory effects involving cyclo‐oxygenase‐2 (COX‐2) (Crofford 2001; Graham 2002; Graham 2013). Clinical practice guidelines for osteoarthritis management recommend paracetamol as the first choice when pain medication for osteoarthritis is needed, because of its safer profile compared with NSAIDs, particularly in people who are at risk of gastrointestinal ulcer (Zhang 2005; Zhang 2007). If paracetamol does not provide sufficient pain relief, then NSAIDs or other physical treatments may be considered (Bartels 2016; Derry 2016; Jüni 2015; Puljak 2017; Regnaux 2015; Smink 2011).
How the intervention might work
The details of the mechanism of action of paracetamol are now becoming clearer after its use for more than a century (Graham 2013). It is agreed that paracetamol inhibits the production of prostaglandins, which are mediators of fever, pain, and inflammation, by blocking the cyclo‐oxygenase pathways (Graham 2013). Paracetamol may have more central than peripheral analgesic effects and has similar but weaker anti‐inflammatory actions than NSAIDs, such as ibuprofen (Graham 2003; Graham 2013). Indeed, its pharmacological profile is of a weak selective COX‐2 inhibitor (Hinz 2008). One feature of paracetamol is its effectiveness in low levels of inflammation such as seen with tooth extraction. However, in contrast to selective COX‐2 inhibitors including celecoxib, paracetamol does not decrease intense inflammatory states such as rheumatoid arthritis or gout. COX‐1 and COX‐2 are bifunctional enzymes with cyclo‐oxygenase and peroxidase functions. Paracetamol is an inhibitor of the peroxidase function of COX‐1 and COX‐2 enzymes. From this process, it is effective in reducing prostaglandin synthesis when there are low levels of arachidonic acid, the precursor of prostaglandins. The analgesic effect of paracetamol is dependent upon several neurotransmitter systems in the central nervous system, including serotonin, opiate, and endogenous cannabinoid systems. In experimental studies, inhibitors of these systems block the analgesic effects of paracetamol (Graham 2013).
Why it is important to do this review
Pain is the most common symptom of osteoarthritis, and as pain levels rise, people experience a reduced range of motion and impaired physical function. The pain and functional limitations substantially reduce the quality of life of people with osteoarthritis. Paracetamol is a widely used analgesic for hip or knee osteoarthritis but the evidence on its benefits and harms remains controversial (Towheed 2006; Zhang 2004; Zhang 2010). The current review was conducted according to the guidelines recommended by the Cochrane Musculoskeletal Group Editorial Board (Ghogomu 2014), and is an update of the Machado 2015 review published in BMJ on 31 March 2015. This Cochrane Review has focused on data for hip and knee osteoarthritis and has included subgroup analysis on the dosage of paracetamol (i.e. 3.0 g/day or less or 3.9 g/day or more). Our updated search focusing on osteoarthritis trials identified 726 additional records; however, none of these records were eligible to be included in the review according to our inclusion criteria.
Objectives
To assess the benefits and harms of paracetamol (acetaminophen) compared with placebo in the treatment of osteoarthritis of the hip or knee.
Methods
Criteria for considering studies for this review
Types of studies
We included only published (full reports in a peer‐reviewed journal) randomised controlled trials comparing the benefits and harms of paracetamol with placebo for osteoarthritis of the hip or knee. Trials with quasi‐random allocation procedures were not included to avoid biased estimates of treatment effects. There were no restrictions for language or publication date.
Types of participants
We included only people with osteoarthritis of the hip or knee (intensity and duration of symptoms were not restricted). Both clinical and imaging‐based diagnoses of osteoarthritis were included in the review. People of any level of healthcare could be included: primary, secondary, or tertiary. We excluded trials evaluating analgesia in the immediate postoperative period, although studies in which participants had previous hip, or knee surgery were eligible, studies with mixed populations of participants with rheumatoid arthritis and osteoarthritis were also excluded, unless separate data were provided for osteoarthritis.
Types of interventions
Randomised controlled trials comparing the benefits of any dose regimen or administration mode of paracetamol versus placebo for hip or knee osteoarthritis. We have not included any study assessing the benefit and harm of the combination of paracetamol with any other type of medication.
Types of outcome measures
Major outcomes
The major outcomes were pain intensity, physical function, and quality of life ‐ as currently recommended for osteoarthritis trials (Altman 1996; Pham 2004), adverse events, serious adverse events, withdrawals due to adverse events, and liver toxicity. If data for more than one pain scale were provided in a trial, we referred to a previously described hierarchy of pain‐related outcomes (Juhl 2012; Jüni 2006; Reichenbach 2007), and extracted data on the pain scale that was highest on this list:
-
Pain intensity
pain overall;
pain on walking;
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale;
pain on activities other than walking;
WOMAC global scale;
Lequesne Osteoarthritis Index global score;
other algofunctional scale;
patient’s global assessment;
physician's global assessment;
other outcome;
no continuous outcome reported.
If a trial provided data on more than one physical function impairment scale, we extracted data according to the following hierarchy:
-
Physical function
global disability score;
walking disability;
WOMAC disability subscore;
composite disability scores other than WOMAC;
disability other than walking;
Lequesne Osteoarthritis Index global score;
other algofunctional scale.
Quality of life.
Withdrawals due to adverse events.
Total adverse events.
Liver toxicity (positive alterations in liver function tests).
Serious adverse events.
Minor outcomes
Walking disability.
Rescue medication (rate of participants using rescue medication).
Patient adherence (rate of participants who adhered to at least 85% of prescribed number of tablets per day).
Long‐term toxicity (rate of participants reporting long‐term toxicity).
Radiographic joint structure changes (e.g. joint space width) were not included in this review, as it is unlikely paracetamol will have an effect on this outcome, thus we substituted liver toxicity as a major outcome.
Search methods for identification of studies
Electronic searches
We updated the search (3 October 2017) on the following electronic databases, without restrictions on language:
Cochrane Central Register of Controlled Trials (CENTRAL): via OvidSP, 1991 to October 2017;
MEDLINE: via OvidSP, 1946 to September Week 3 2017;
Embase: via OvidSP, 1974 to September 2017;
Allied and Complementary Medicine Database (AMED): via OvidSP, 1985 to September 2017;
Cumulative Index to Nursing and Allied Health Literature (CINAHL): via EBSCO, 1982 to September 2017;
Web of Science: via Thomson Reuters, 1900 to September 2017;
Latin American and Caribbean Literature in Health Sciences (LILACS): via BIREME, 1986 to September 2017;
International Pharmaceutical Abstracts (IPA): via OvidSP, 1970 to September 2017.
We used a combination of relevant keywords to construct the search strategy including paracetamol, acetaminophen, osteoarthritis, osteoarthrosis, placebo, randomised, and controlled trial. Two review authors (GCM and MBP) conducted the first screening of potentially relevant records based on titles and abstract, and three review authors (GCM, MBP, and AAO for the update) independently performed the final selection of included trials based on full‐text evaluation. We performed citation tracking on included studies and relevant systematic reviews, and searched relevant websites and clinical trials registries for unpublished studies. We resolved disagreements by consensus between the three review authors. The search strategy for each database is presented in the following appendices: Appendix 1 (CENTRAL), Appendix 2 (MEDLINE), Appendix 3 (Embase), Appendix 4 (AMED), Appendix 5 (CINAHL), Appendix 6 (Web of Sciences), Appendix 7 (LILACS), and Appendix 8 (IPA).
Searching other resources
The review authors had previously contacted the pharmaceutical manufacturers for details of unpublished and ongoing trials. We searched and identified the relevant trials or reviews in reference lists. We also searched clinical trial registries (ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on 20 October 2017, and asked personal contacts about ongoing and unpublished studies. There were no language or publication restrictions. See search strategy for unpublished and ongoing trials in Appendix 9.
Data collection and analysis
Selection of studies
Three review authors (GCM, MBP, and AAO) independently screened the titles and abstracts of studies identified by the updated searches using a standardised data extraction form to eliminate those that clearly did not satisfy the inclusion criteria. We obtained full reports for the remaining studies to determine inclusion in the review. A fourth review author (MLF) resolved any disagreements. If multiple reports described the same trial, we considered all. In case of multiple reports of the same study, these were collated, so that each study, rather than each report, was the unit of interest in the review.
Data extraction and management
Three review authors (GCM, MBP, and AAO) independently extracted trial information using a standardised data collection form. Review authors were not blinded to the authors' names and institutions, journal of publication, or study results at any stage of the review. We resolved disagreements through discussion. One review author (AAO) entered data suitable for meta‐analysis into Review Manager 5 (Review Manager 2012), and another review author (GCM) double checked entries. We extracted the following data:
bibliometric data: authors, affiliations, language, and year of publication;
characteristics of included trials: trial design, sample size, details of participants, inclusion and exclusion criteria, characteristics of the experimental intervention, type of control used, dosage, frequency, duration of treatment interventions, duration of follow‐up, and primary and secondary outcomes;
characteristics of included participants: age, sex, type of diagnosis for hip and knee osteoarthritis, types of joints affected (knee, hip, or both), and duration of symptoms;
statistical data: means, standard deviations (SD), and sample sizes for major outcomes. We extracted mean estimates in the following hierarchical order: change scores, and final values.
For our minor outcomes, we extracted the number of cases and the total sample size. The safety outcomes extracted from included trials were the number of participants reporting any adverse effect, number of participants reporting any serious adverse effect (as defined by each study), number of participants who withdrew from the study because of adverse effects, and number of participants with abnormal results on liver function tests (hepatic enzyme activity 1.5 times the upper limit of the reference range or greater).
Assessment of risk of bias in included studies
Two review authors (GCM and MBP) independently assessed 'Risk of bias' for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed each included study within the following 'Risk of bias' domains: adequacy of sequence generation, allocation concealment and blinding, how incomplete outcome data (dropouts) were addressed, evidence of selective outcome reporting, and whether trials were funded by companies that produced paracetamol. We resolved disagreements by consensus. We used Review Manager 5 to generate figures and summaries (Review Manager 2012).
Measures of treatment effect
We used risk ratio (RR) and 95% confidence intervals (CI) for dichotomous data based on the number of events in the control and intervention groups of each study. For continuous data, we calculated mean differences (MD) and 95% CI between paracetamol and placebo groups. Pain and physical function (WOMAC function) scores were converted to a common 0 (no pain or disability) to 100 (worst possible pain or disability) scale before meta‐analysis. We considered a minimal clinically important difference (MCID) of 9 points on a 100‐point scale for pain and physical function outcomes based upon the practice in the osteoarthritis field (Wandel 2010).
In the Comments column of the 'Summary of findings' table, we reported the absolute percent difference, the relative percent change from baseline, and the number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH) (NNTB and NNTH were provided only when the outcome showed a clinically important difference between treatment groups).
For dichotomous outcomes, such as adverse events, we calculated the NNTB or NNTH from the control group event rate and the relative risk using the Visual Rx NNT calculator (Cates 2008). We calculated the NNTB for continuous measures using the Wells calculator (available at the CMSG Editorial office, musculoskeletal.cochrane.org/). We used the MCID to interpret results, and for input into the Wells calculator. We assumed an MCID of 9 points in a 10‐point scale for pain (Wandel 2010); and 10 points on a 100‐point scale for function or disability.
For dichotomous outcomes, we calculated the absolute risk difference using the Risk Difference statistic in Review Manager 5 and expressed the result as a percentage. For continuous outcomes, we calculated the absolute benefit as the improvement in the intervention group minus the improvement in the control group (MD), in the original units, and expressed it as a percentage.
We calculated the relative percent change for dichotomous data as the RR – 1 and expressed it as a percentage. For continuous outcomes, we calculated the relative difference as the absolute benefit divided by the baseline mean of the control group, expressed as a percentage.
Unit of analysis issues
Seven included studies were traditional randomised parallel‐group trials and three were crossover trials (Pincus 2004a; Pincus 2004b; Amadio 1983). The unit of analysis was between‐person for all trials. We stratified all outcomes into four time points of assessment for the primary analyses: immediate term (two weeks or less), short term (more than two weeks but three months or less), intermediate term (more than three months but 12 months or less), and long term (more than 12 months). When studies reported multiple time points within each category, we used the time point closest to one week for immediate term, eight weeks for short term, six months for intermediate term, and 12 months for long term (although no studies reported long‐term outcomes and only one reported intermediate‐term outcomes). For studies reporting on multiple intervention groups (e.g. groups of different dosages) and included in the same pooled analysis, we halved the sample size of the placebo group to avoid unit of analysis issues.
Dealing with missing data
We contacted the authors of the studies to obtain data that were missing or insufficient in their report that we needed to assess eligibility of the studies or as input for meta‐analysis, or both. If statistics were missing, such as SD, we calculated them from other available statistics (e.g. 95% CIs), according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We examined heterogeneity using visual inspection of effect size distribution and overlap of the CIs in forest plots, as well as using results of the Chi² test and the I² statistic as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered studies to be statistically heterogeneous if the I² statistic was greater than 50%.
Assessment of reporting biases
We planned to use funnel plots to assess publication bias but we had fewer than 10 trials included in any particular pooled analysis.
Data synthesis
We entered all quantitative results into Review Manager 5 (Review Manager 2012). We pooled data (statistical pooling with meta‐analysis) for the comparison paracetamol versus placebo for each outcome for which data were reported in trials. We used a random‐effects model for all analyses.
'Summary of findings' table
We created Table 1 for the following outcomes: pain, physical function, quality of life, any adverse events, serious adverse events, withdrawals due to adverse events, and liver toxicity (positive for abnormal results on liver function tests). We included data from the short‐term follow‐up times (three to 12 weeks) for pain and function and data from the last follow‐up time point (between 12 and 24 weeks) for adverse events and withdrawals.
We used the GRADE system to assess the quality of the evidence for each pooled analysis (Guyatt 2008), defined as the extent of confidence into the estimates of treatment benefits and harms, with outcomes of interest being ranked according to their relevance for clinical decision making as of limited importance, important, or critical (Guyatt 2011). The overall quality of the evidence for each outcome was based on 'Risk of bias,' publication bias, inconsistency of results, indirectness, and imprecision (Guyatt 2008; Higgins 2011). For 'Risk of bias,' we downgraded the quality of the evidence if more than 25% of participants were from studies with an overall high 'Risk of bias.' We aimed to assess publication bias by visual inspection of funnel plots (scatterplot of the effect of estimates from individual studies against its standard error) and also by the results of Egger's test (small‐study effects) (Egger 1997). If the Egger's test result was significant (two tailed P < 0.1), we downgraded the quality of evidence (GRADE) by one level for publication bias for all meta‐analyses (Guyatt 2011). Results were downgraded for inconsistency if there was significant heterogeneity present by visual inspection or if the I² value was greater than 50%. We downgraded the evidence for imprecision if the limits of the 95% CI crossed the MCID for continuous outcomes, or there were fewer than 200 events for dichotomous outcomes. The GRADE Working Group recommends four levels of evidence:
high‐quality evidence: further research is very unlikely to change confidence in estimate of effect;
moderate‐quality evidence: further research is likely to have an important impact on confidence in estimate of effect and may change the estimate;
low‐quality evidence: further research is very likely to have an important impact on confidence in estimate of effect and is likely to change the estimate;
very low‐quality evidence: very little confidence in the effect estimate.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to assess if pain and function differed between people with hip or knee osteoarthritis, and with the dose of paracetamol (3.0 g/day or less versus 3.9 g/day or more).
Sensitivity analysis
We conducted no sensitivity analyses for this review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
The updated search resulted in 1033 additional records with duplicates: 127 additional references from CENTRAL, 242 from MEDLINE, 243 from Embase, 15 from AMED, 250 from CINAHL, 113 from Web of Sciences, 17 from LILACS, and 26 from IPA. After excluding 307 duplicates, we screened 726 titles and abstracts, and eight additional potentially relevant papers for full‐text evaluation. There were no new studies for inclusion in this update, therefore, we analysed the original nine records, or 10 randomised controlled trials (Altman 2007; Amadio 1983; Case 2003; Golden 2004; Herrero‐Beaumont 2007; Miceli‐Richard 2004; Pincus 2004a; Pincus 2004b; Prior 2014; Zoppi 1995). We presented the flow chart of the original and updated search in Figure 1.
1.
Prisma flow diagram of trials investigating efficacy of paracetamol versus placebo for osteoarthritis.
Included studies
The review includes nine records reporting 10 randomised controlled trials and yielded a pooled sample size of 3541 participants. One paper reported on the results of two separate randomised trials (Pincus 2004a; Pincus 2004b). Details of included studies are listed in the Characteristics of included studies table. Years of publication ranged from 1983 to 2014 and all studies were double‐blind randomised placebo‐controlled trials. The follow‐up time ranged from one to 24 weeks. Paracetamol was administered orally (as tablets or capsules). The total oral dose and dose regimens for paracetamol varied across trials. Doses ranged from 1.95 g/day to 4 g/day, but no trials used paracetamol doses between 3.0 to 3.9 g/day. One trial used a three‐arm design (Altman 2007), and all three treatment groups were included in the meta‐analyses following the recommendation in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The washout period before treatment started varied across trials, ranging from one day to six months. The washout periods were 12 weeks for corticosteroids, six weeks for intra‐articular steroids, and three days to two weeks for NSAIDs. Participants stopped taking simple analgesics from one to 10 days. One trial reported that the washout for glucosamine drugs was six months, and two trials used "five half lives" to define this period. Nine trials used the diagnosis of osteoarthritis based on image evidence and clinical assessment, whereas one trial based the diagnosis solely on image evidence.
Excluded studies
For this update, after retrieving the full text for final assessment, the review authors excluded eight studies: three were not randomised controlled trials (Papou 2015; van Tunen 2016; Zheng 2015), one study did not investigate paracetamol and lacked a placebo control group (Skou 2015), one study did not include people with hip or knee osteoarthritis (Park 2015), two trials did not investigate paracetamol (Ha 2016; Lao 2015), and one trial lacked a placebo control group (Verkleij 2015). See Characteristics of excluded studies table.
Ongoing studies
An additional search for protocols or unpublished trials identified 74 records. Five ongoing studies that fit the inclusion criteria were found on ClinicalTrials.gov and WHO ICTRP. One registered clinical trial was withdrawn (NCT01420666), and two had no results posted online (ACTRN12613000840785; NCT02845271). Two records provided results posted online, but not in a peer‐reviewed journal (NCT01105936; NCT02311881).
Risk of bias in included studies
The results from the 'Risk of bias' assessment are summarised in Figure 2 and Figure 3.
2.
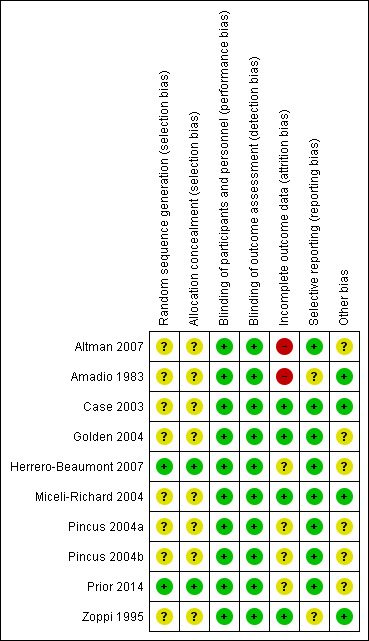
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
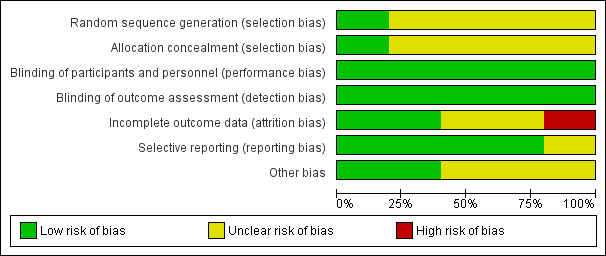
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In general, included trials did not describe their randomisation and allocation processes adequately and were at unclear risk of selection bias. Only two trials adopted appropriate methods of sequence generation and allocation concealment and were at low risk of selection bias (Herrero‐Beaumont 2007; Prior 2014).
Blinding
All included trials successfully reported blinding of participants, personnel, and outcome assessors and were at low risk of performance and detection bias.
Incomplete outcome data
Four studies had follow‐up rates of 90% or higher were at low risk of attrition bias (Case 2003; Golden 2004; Miceli‐Richard 2004; Zoppi 1995). Two trials were at high risk of attrition bias due to high dropout rates (Altman 2007; Amadio 1983), and the remaining four trials were at unclear risk, since there were no significant differences in the reasons for dropout between the groups despite the large dropout rate.
Selective reporting
One trial did not include measures of physical function (Zoppi 1995), and one trial did not report pain outcome measures (Amadio 1983), and were at unclear risk of reporting bias.
Other potential sources of bias
Six trials were funded by companies that produced paracetamol and were at unclear risk of bias for the other sources of bias domain (Altman 2007; Golden 2004; Herrero‐Beaumont 2007; Pincus 2004a; Pincus 2004b; Prior 2014).
Effects of interventions
See: Table 1
Results are presented separately according to outcome measures and follow‐up time points. No studies measured results after six months, thus we reported pain and physical function outcomes at immediate term (two weeks or less) and short term (more than two weeks to three months or less) only.
Major outcomes
Pain intensity
There was high‐quality evidence that paracetamol provided a small, likely clinically unimportant improvement in pain in the immediate term (MD –3.32, 95% CI –5.83 to –0.81; Analysis 1.1; Figure 4) and the short term (MD –3.23, 95% CI –5.43 to –1.02; Analysis 1.1; Figure 4; Table 1).
1.1. Analysis.
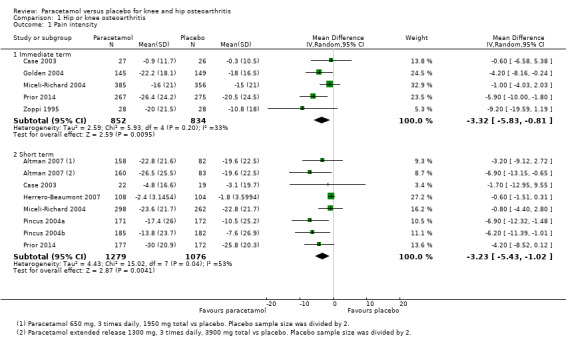
Comparison 1 Hip or knee osteoarthritis, Outcome 1 Pain intensity.
4.
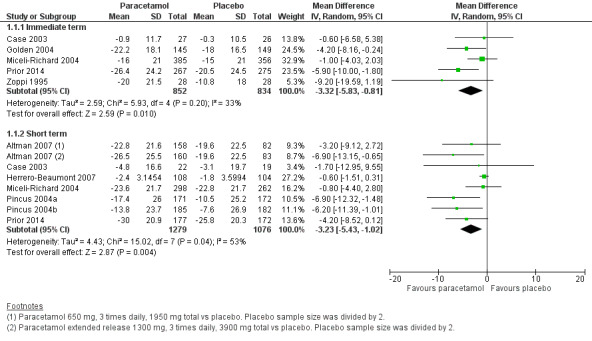
Forest plot of comparison: 1 Hip or knee osteoarthritis, outcome: 1.1 Pain. Pain scores are expressed on scale of 0 to 100. Immediate term = follow‐up two weeks or less; short term = follow‐up more than two weeks but three months or less.
Physical function
Moderate‐quality evidence (downgraded for inconsistency) indicating there was probably no difference between groups in physical function in the immediate term (MD –1.83, 95% CI –6.41 to 2.75; Analysis 1.2; Figure 5), and high‐quality evidence indicated a small clinically unimportant improvement with paracetamol in the short term (MD –2.92, 95% CI –4.89 to –0.95; Analysis 1.2; Figure 5; Table 1).
1.2. Analysis.
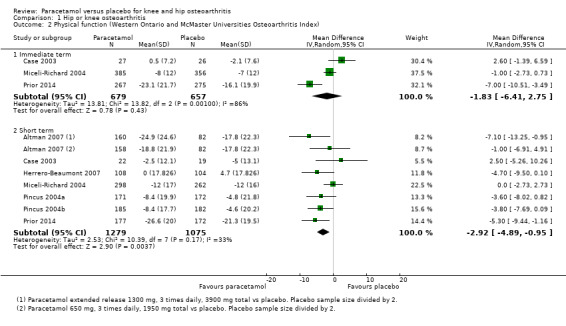
Comparison 1 Hip or knee osteoarthritis, Outcome 2 Physical function (Western Ontario and McMaster Universities Osteoarthritis Index).
5.
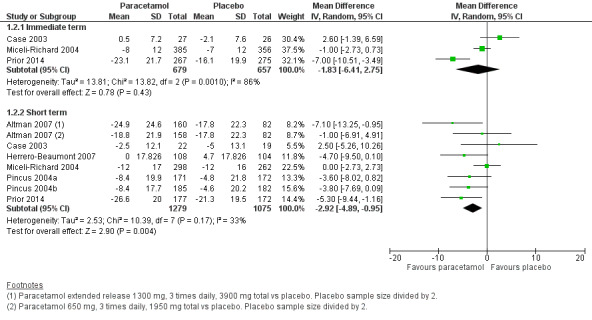
Forest plot of comparison: 1 Hip or knee osteoarthritis, outcome: 1.2 Physical function (WOMAC). Physical function scores expressed on scale of 0 to 100. Immediate term = follow‐up two weeks or less; short term = follow‐up two weeks or greater but three months or less.
Quality of life
None of the studies measured quality of life.
Withdrawals due to adverse events
There was less certainty due to small event rates, indicating imprecision (moderate‐quality evidence), around the risk of withdrawals due to adverse events with paracetamol (RR 1.19, 95% CI 0.91 to 1.55).
Total adverse events
There was high‐quality evidence indicating that the risk of any adverse event was similar between paracetamol and placebo treatment groups (RR 1.01, 95% CI 0.92 to 1.11; Analysis 1.3; Figure 6; Table 1).
1.3. Analysis.
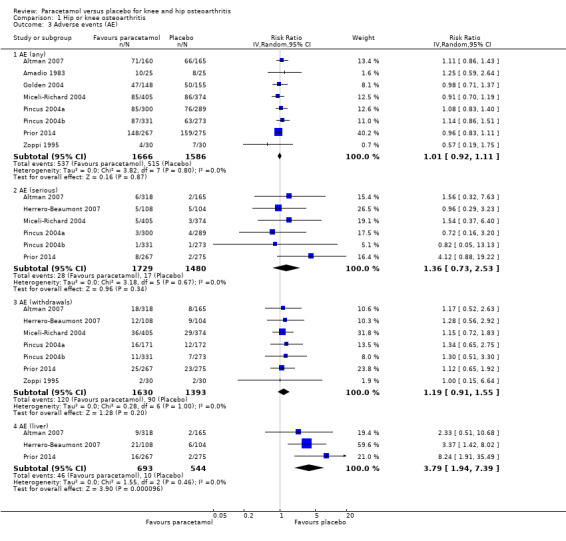
Comparison 1 Hip or knee osteoarthritis, Outcome 3 Adverse events (AE).
6.
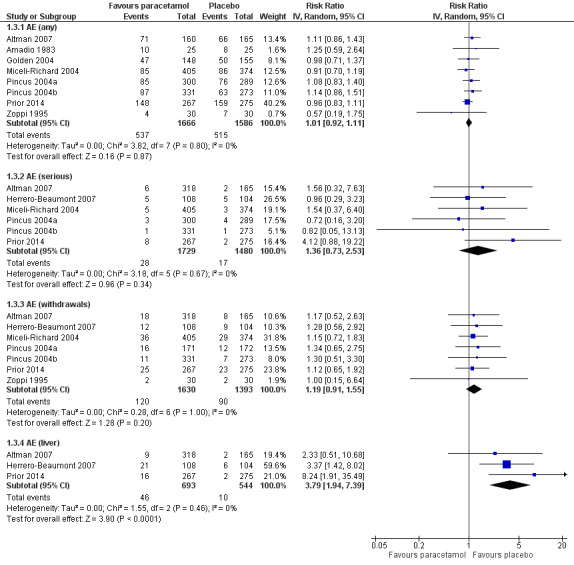
Forest plot of comparison: 1 Hip or knee osteoarthritis, outcome: 1.4 Adverse events (AE). Any = number of participants reporting any AE; serious = number of participants reporting any serious AE (as defined by each study); withdrawals = number of participants withdrawn from study because of AEs; liver = number of participants with abnormal results on liver function tests.
Liver toxicity
Abnormal liver function tests were more likely to occur with paracetamol (RR 3.79, 95% CI 1.94 to 7.39), but the evidence was downgraded due to wide CIs (imprecision), indicating uncertainty around these effect estimates.
Serious adverse events
There was less certainty due to small event rates, indicating imprecision (moderate‐quality evidence), around the risk of serious adverse events with paracetamol (RR 1.36, 95% CI 0.73 to 2.53).
Minor outcomes
Low‐quality evidence for minor outcomes was available from single studies only with potential risk of selection or attrition bias (downgraded for risk of bias and imprecision).
Walking disability
It was uncertain if walking disability (measured using the 50‐foot walking test) differed between paracetamol and placebo groups in the immediate term (RR –0.55, 95% CI –2.09 to 0.99) and short term (RR 0.06, 95% CI –2.44 to 2.56; Analysis 1.4).
1.4. Analysis.
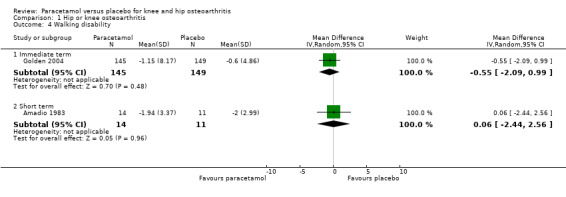
Comparison 1 Hip or knee osteoarthritis, Outcome 4 Walking disability.
Rescue medication
it was uncertain if the use of rescue analgesic medication differed between paracetamol and placebo groups (RR 0.86, 95% CI 0.77 to 0.97; Analysis 1.5).
1.5. Analysis.
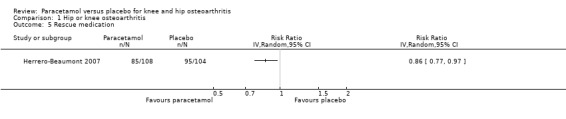
Comparison 1 Hip or knee osteoarthritis, Outcome 5 Rescue medication.
Patient adherence
it is uncertain if adherence to treatment differed between paracetamol and placebo groups adherence to treatment (RR 0.95, 95% CI 0.87 to 1.03; Analysis 1.6).
1.6. Analysis.
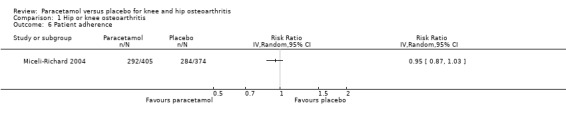
Comparison 1 Hip or knee osteoarthritis, Outcome 6 Patient adherence.
Long‐term toxicity
None of the studies measured long‐term toxicity.
Subgroup analyses
We planned subgroup analyses to assess for potential differences in outcomes in participants with knee osteoarthritis and those with hip osteoarthritis, but data for participants with hip and knee osteoarthritis were not presented separately in the included studies. A subset of trials included participants with knee osteoarthritis only (Amadio 1983; Case 2003; Golden 2004; Herrero‐Beaumont 2007; Miceli‐Richard 2004), and four trials reported pain and function (Case 2003; Golden 2004; Herrero‐Beaumont 2007; Miceli‐Richard 2004). Moderate‐quality evidence (downgraded for imprecision) indicated that paracetamol provided no important improvement in pain at immediate follow‐up (MD –1.96, 95% CI –4.19 to 0.27) or short‐term follow‐up (MD –1.37, 95% CI –4.24 to 1.49) (Analysis 2.1). There were similar results for physical function at immediate follow‐up (MD 0.33, 95% CI –3.07 to 3.74) and short‐term follow‐up (MD –1.08, 95% CI –4.66 to 2.50). See: Analysis 2.2; Analysis 2.3.
2.1. Analysis.
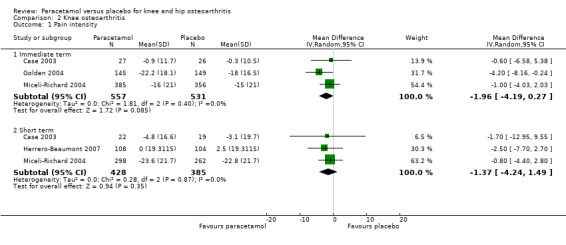
Comparison 2 Knee osteoarthritis, Outcome 1 Pain intensity.
2.2. Analysis.
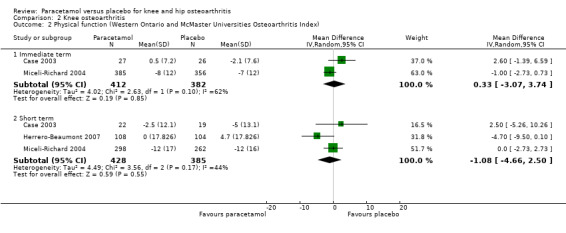
Comparison 2 Knee osteoarthritis, Outcome 2 Physical function (Western Ontario and McMaster Universities Osteoarthritis Index).
2.3. Analysis.
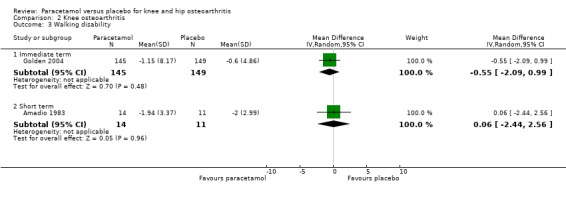
Comparison 2 Knee osteoarthritis, Outcome 3 Walking disability.
Up to nine trials presented data for the subgroup analysis comparing lower and higher dose of paracetamol (3.0 g/day or less versus 3.9 g/day or more) (Altman 2007; Case 2003; Golden 2004; Herrero‐Beaumont 2007; Miceli‐Richard 2004; Pincus 2004a; Pincus 2004b; Prior 2014; Zoppi 1995). There were no important differences in outcomes for low‐dose versus high‐dose paracetamol with respect to pain in the immediate term (Analysis 3.1) or short term (Analysis 3.2), or physical function in the short term (Analysis 3.4). We could not perform a subgroup analysis in the immediate term for physical function as no studies using lower‐dose paracetamol reported this outcome at this time point.
3.1. Analysis.
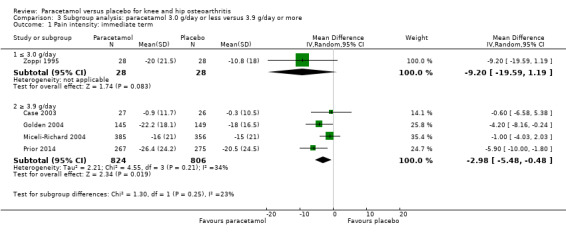
Comparison 3 Subgroup analysis: paracetamol 3.0 g/day or less versus 3.9 g/day or more, Outcome 1 Pain intensity: immediate term.
3.2. Analysis.
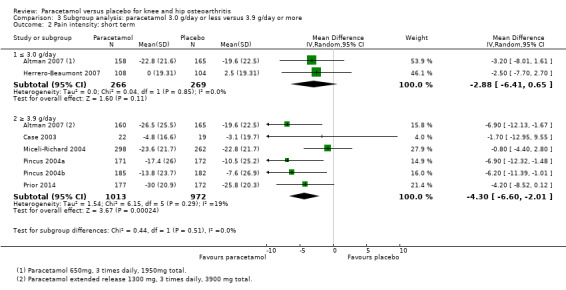
Comparison 3 Subgroup analysis: paracetamol 3.0 g/day or less versus 3.9 g/day or more, Outcome 2 Pain intensity: short term.
3.4. Analysis.
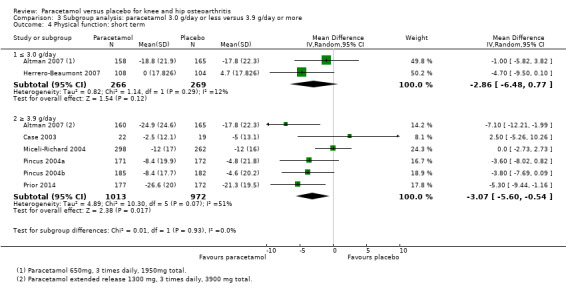
Comparison 3 Subgroup analysis: paracetamol 3.0 g/day or less versus 3.9 g/day or more, Outcome 4 Physical function: short term.
Discussion
Summary of main results
In this update of the systematic review and meta‐analysis by Machado 2015, we included nine eligible records reporting 10 randomised placebo‐controlled trials (3541 participants) on the effects of paracetamol and placebo for people with hip or knee osteoarthritis. Thresholds for clinical importance were chosen a priori and included 9 points for pain and physical function (on a 100‐point scale). In general, there was moderate‐ to high‐quality evidence that treatment effects of paracetamol compared with placebo on both pain and physical function outcomes were, at best, too small to be of clinical relevance for this population. Although the risk of total adverse events likely did not differ between the paracetamol and placebo groups (high‐quality evidence), the use of paracetamol may have increased the risk of abnormal liver test results (moderate‐quality evidence, downgraded for imprecision). However, we acknowledge that the clinical importance of this finding was uncertain. Due to the small number of events, we were less certain if paracetamol use increased the risk of serious adverse events or withdrawals due to adverse events. Our subgroup analyses indicated that the effects on pain and function did not differ according to the dose of paracetamol (3.0 g/day or less versus 3.9 g/day or more). We did not include a responder analysis in this review, as thresholds used to define responders in this field are still not well defined. It is possible, however, that the results of a responder analysis would yield different conclusions.
Overall completeness and applicability of evidence
Paracetamol is the most commonly used non‐prescription medication for musculoskeletal pain, including osteoarthritis (Brand 2014), but this systematic review has shown that there is moderate‐ to high‐quality evidence that the effects of paracetamol compared with placebo are too small to be of clinically importance for people with hip or knee osteoarthritis. Given only one trial included a follow‐up longer than 12 weeks (Herrero‐Beaumont 2007), the results of this review are mostly restricted to immediate‐ and short‐term follow‐ups.
Previous research has highlighted that the focus on pharmacological interventions for knee and hip osteoarthritis is in disconnect with the best evidence‐based recommendations for care of this condition (Brand 2008; Brand 2014). For instance, there is a very important component of lifestyle‐based management, such as obesity control and physical activity uptake that is not addressed with pharmacological approaches (Basedow 2015; Brand 2008). In fact, evidence has established that physical programmes (e.g. weight loss, exercise including lower limb strengthening exercises) are associated with large effect sizes (greater than 20) on pain reduction in this population (Uthman 2013). Our results showed that pharmacological management of hip or knee osteoarthritis through simple analgesics only offers small and clinically unimportant effects on pain and physical function.
Quality of the evidence
Overall, the quality of evidence for the outcomes measured up to 12 weeks considered critical for clinical decision making (pain, function, and overall adverse events) was high according to the GRADE system. According to the GRADE Working Group, a grading of high quality of evidence indicates that further research is unlikely to change the effect estimates substantially. For serious adverse events (short term), withdrawals due to adverse events and liver toxicity (indicated by abnormal liver function tests), evidence was downgraded from high to moderate due to imprecision (the total number of events was small). According to the GRADE Working Group grades of evidence, a grading of moderate quality of evidence indicates that further research may have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
None of the trials measured quality of life or long‐term toxicity outcomes.
Potential biases in the review process
We based our review on an extensive electronic literature search, citation tracking, and search for unpublished trials, so it seems unlikely that we missed relevant trials, provided that they were published as full‐text articles or accessible in conference proceedings or trial registries (Egger 2003). Two review authors independently performed selection of trials, data extraction, and 'Risk of bias' assessment to reduce bias and transcription errors (Gøtzsche 2007). Therefore, we are confident that potential biases during the review process were minimised. However, the review presented some limitations. The number of studies in each meta‐analysis was relatively small and that prevented us from conducting a small‐study effect analysis using funnel plots. Previous exposure of participants to paracetamol was also frequently reported by included trials, suggesting that a proportion of 'non‐responders' to the medication were possibly included in the analyses. We acknowledge this could have influenced the final results.
Agreements and disagreements with other studies or reviews
The previous non‐Cochrane review on paracetamol for hip or knee osteoarthritis was conducted in 2004 (Zhang 2004), after which, six additional randomised placebo‐controlled trials were published. The authors of this previous review included trials of osteoarthritis affecting multiple joints and not just the hip or knee. Moreover, they included analyses comparing NSAIDs versus paracetamol and NSAIDs versus placebo. Zhang 2004 concluded that paracetamol was an effective analgesic medication for pain relief due to osteoarthritis (standardised mean difference 0.21, 95% CI 0.02 to 0.41). However, in this update of Machado 2015, we included subgroup analyses to verify possible differences between the two body regions (hip and knee) and dose of paracetamol (3 g/day or 4 g/day) on pain and physical function. Our review has provided moderate‐ to high‐quality evidence that the effects of paracetamol on pain and function were not different when comparing hip or knee osteoarthritis or different doses used, and were too small to be considered clinically relevant.
Authors' conclusions
Implications for practice.
Despite guidelines for the treatment of non‐traumatic knee complaints recommending paracetamol as the first‐choice analgesic in treating pain due to osteoarthritis (Jordan 2003; Zhang 2007), this updated review confirms previous findings that paracetamol provides minimal, probably clinically unimportant benefits in the immediate and short term for people with hip or knee osteoarthritis. Moreover, paracetamol does not provide statistically significant or clinically important effects for people with knee osteoarthritis only.
Implications for research.
The use of drugs as an intervention has been previously associated with improvements on pain and physical function in people with osteoarthritis (Barthel 2010; Schein 2008). However, this Cochrane systematic review including only randomised placebo‐controlled trials investigating the benefits and harms of paracetamol compared with placebo for hip and knee osteoarthritis revealed that paracetamol alone does not offer clinically important benefits for this population. These results were consistent irrespective of the dose of paracetamol or pain location. Future research should focus on developing and implementing models of care that are focused on evidence‐based non‐pharmacological approaches for the care of knee and hip osteoarthritis.
What's new
| Date | Event | Description |
|---|---|---|
| 27 August 2019 | Amended | Typographical error corrected in the plain language summary |
Acknowledgements
This research received no funding from the public, commercial, or not‐for‐profit sectors. AOL is supported by CNPQ (Conselho Nacional de Desenvolvimento Científico e Tecnologico), Brazil. DJH holds a National Health and Medical Research Council Practitioner Fellowship. MLF holds a fellowship from Sydney Medical Foundation/Sydney Medical School.
Appendices
Appendix 1. CENTRAL search strategy
acetaminophen.mp. OR exp Acetaminophen/
Analgesics, Non‐Narcotic/tu
(paracetamol OR tylenol OR panadol).mp.
OR/1‐3
exp Osteoarthritis, Hip/ OR exp Osteoarthritis/ OR exp Osteoarthritis, Spine/ OR exp Osteoarthritis, Knee/
low back pain.mp. OR exp Low Back Pain/
neck pain.mp. OR exp Neck Pain/
("low back pain" OR "back pain" OR "neck pain" OR backache OR lumbago OR "neck ache" OR "spin* pain" OR "knee pain" OR "hip pain").mp.
OR/5‐8
4 and 9
limit 10 to yr="2015 ‐Current"
Appendix 2. MEDLINE search strategy
acetaminophen.mp. OR exp Acetaminophen/
paracetamol.mp
analgesic*.ab,ti. OR Analgesics, Non‐Narcotic/tu, th
(aceta OR actimin OR anacin OR apacet OR "aspirin free anacin" OR acamol OR acetalgin OR adol OR aldolOR OR alvedon OR apiretal OR atamel OR atasol OR benuron OR biogesic OR "biogesic kiddielets" OR buscapina OR banesin OR "ben u ron" OR calpol OR captin OR cemol OR coldex OR cotibin OR crocin OR dafalgan OR daleron OR "dawa ya magi" OR depon OR dexamol OR dolex OR dolgesic OR doliprane OR dolorol OR dolprone OR "duiyixian anjifen pian" OR dapa OR dolo OR datril OR duatrol OR dayquil OR efferalgan OR enelfa OR europain OR febrectal OR febricet OR febridol OR fensum OR feverall OR fibi OR "fibi plus" OR gelocatil OR gripin OR gesic OR genapap OR genebs OR hedex OR hedanol OR herron OR influbene OR kafa OR kitadol OR lekadol OR lupocet OR lemsip OR liquiprin OR pyrigesic OR mexalen OR milidon OR minoset OR momentum OR napa OR "neo kiddielets" OR neopap OR "oraphen pd" OR pyrigesic OR pacol OR pamol OR parol OR panado OR panadol OR panamax OR panda OR panodil OR pyrigesic OR paracet OR paracetamol OR paracitol OR paralen OR paramed OR paramol OR parol OR perdolan OR perfalgan OR pinex OR "pyongsu cetamol" OR pyrenol OR pyrigesic OR plicet OR panadrex OR paratabs OR paralgin OR phenaphen OR revanin OR rokamol OR rubophen OR redutemp OR sara OR scanol OR "sinpro n" OR "snaplets fr" OR suppap OR tachipirin OR tachipirina OR tafirol OR tapsin OR termalgin OR tempra OR thomapyrin OR tipol OR "togal classic duo" OR treuphadol OR triaminic OR tylenol OR tamen OR tapanol OR tipol OR uphamol OR vermidon OR vitamol OR valorin OR xumadol OR zolben).tw.
OR/1‐4
osteoarthritis.mp. OR exp Osteoarthritis/
exp Low Back Pain/
exp Back Pain/
exp Neck Pain/
("low back pain" OR "back pain" OR "neck pain" OR backache OR lumbago OR "neck ache" OR "spin* pain" OR "knee pain" OR "hip pain").mp.
OR/6‐10
randomized controlled trial.pt. OR exp Randomized Controlled Trial/
"randomized controlled trial".mp.
exp Random Allocation/
placebo.mp. OR exp Placebos/ OR exp Placebo Effect/
(random* adj3 trial).ab,ti.
"controlled clinical trial".mp. OR exp Controlled Clinical Trial/
random*.ab,ti.
OR/12‐18
5 AND 11 AND 19
(2015* 2016* or 2017*).dp. or (2015* or 2016* or 2017*).ed.
20 AND 21
limit 22 to humans
Appendix 3. Embase search strategy
acetaminophen.mp. OR paracetamol.mp. OR Paracetamol/
(aceta OR actimin OR anacin OR apacet OR "aspirin free anacin" OR acamol OR acetalgin OR adol OR aldolOR OR alvedon OR apiretal OR atamel OR atasol OR benuron OR biogesic OR "biogesic kiddielets" OR buscapina OR banesin OR "ben u ron" OR calpol OR captin OR cemol OR coldex OR cotibin OR crocin OR dafalgan OR daleron OR "dawa ya magi" OR depon OR dexamol OR dolex OR dolgesic OR doliprane OR dolorol OR dolprone OR "duiyixian anjifen pian" OR dapa OR dolo OR datril OR duatrol OR dayquil OR efferalgan OR enelfa OR europain OR febrectal OR febricet OR febridol OR fensum OR feverall OR fibi OR "fibi plus" OR gelocatil OR gripin OR gesic OR genapap OR genebs OR hedex OR hedanol OR herron OR influbene OR kafa OR kitadol OR lekadol OR lupocet OR lemsip OR liquiprin OR pyrigesic OR mexalen OR milidon OR minoset OR momentum OR napa OR "neo kiddielets" OR neopap OR "oraphen pd" OR pyrigesic OR pacol OR pamol OR parol OR panado OR panadol OR panamax OR panda OR panodil OR pyrigesic OR paracet OR paracetamol OR paracitol OR paralen OR paramed OR paramol OR parol OR perdolan OR perfalgan OR pinex OR "pyongsu cetamol" OR pyrenol OR pyrigesic OR plicet OR panadrex OR paratabs OR paralgin OR phenaphen OR revanin OR rokamol OR rubophen OR redutemp OR sara OR scanol OR "sinpro n" OR "snaplets fr" OR suppap OR tachipirin OR tachipirina OR tafirol OR tapsin OR termalgin OR tempra OR thomapyrin OR tipol OR "togal classic duo" OR treuphadol OR triaminic OR tylenol OR tamen OR tapanol OR tipol OR uphamol OR vermidon OR vitamol OR valorin OR xumadol OR zolben).mp.
OR/1‐2
osteoarthritis.mp. OR Osteoarthritis/
low back pain.mp. OR Low Back Pain/
backache.mp. OR Backache/
neck pain.mp. OR Neck Pain/
("low back pain" OR "back pain" OR "neck pain" OR backache OR lumbago OR "neck ache" OR "spin* pain" OR "knee pain" OR "hip pain").mp.
OR/4‐8
randomized controlled trial.mp. OR Randomized Controlled Trial/
randomization.mp. OR Randomization/
placebo.mp. OR Placebo/
randomized.ti,ab
placebo.ti,ab
randomly.ti,ab
OR/10‐15
3 AND 9 AND 16
(2015* or 2016* or 2017*).dd. or (2015* or 2016* or 2017*).dp.
17 AND 18
limit 19 to human
Appendix 4. AMED search strategy
acetaminophen.mp. OR exp Acetaminophen/
analgesics.mp. OR exp Analgesics/
drug therapy.mp. OR exp Drug Therapy/
analgesic*.ab,ti.
(aceta OR actimin OR anacin OR apacet OR "aspirin free anacin" OR acamol OR acetalgin OR adol OR aldolor OR alvedon OR apiretal OR atamel OR atasol OR benuron OR biogesic OR "biogesic kiddielets" OR buscapina OR banesin OR "ben u ron" OR calpol OR captin OR cemol OR coldex OR cotibin OR crocin OR dafalgan OR daleron OR "dawa ya magi" OR depon OR dexamol OR dolex OR dolgesic OR doliprane OR dolorol OR dolprone OR "duiyixian anjifen pian" OR dapa OR dolo OR datril OR duatrol OR dayquil OR efferalgan OR enelfa OR europain OR febrectal OR febricet OR febridol OR fensum OR feverall OR fibi OR "fibi plus" OR gelocatil OR gripin OR gesic OR genapap OR genebs OR hedex OR hedanol OR herron OR influbene OR kafa OR kitadol OR lekadol OR lupocet OR lemsip OR liquiprin OR pyrigesic OR mexalen OR milidon OR minoset OR momentum OR napa OR "neo kiddielets" OR neopap OR "oraphen pd" OR pyrigesic OR pacol OR pamol OR parol OR panado OR panadol OR panamax OR panda OR panodil OR pyrigesic OR paracet OR paracetamol OR paracitol OR paralen OR paramed OR paramol OR parol OR perdolan OR perfalgan OR pinex OR "pyongsu cetamol" OR pyrenol OR pyrigesic OR plicet OR panadrex OR paratabs OR paralgin OR phenaphen OR revanin OR rokamol OR rubophen OR redutemp OR sara OR scanol OR "sinpro n" OR "snaplets fr" OR suppap OR tachipirin OR tachipirina OR tafirol OR tapsin OR termalgin OR tempra OR thomapyrin OR tipol OR "togal classic duo" OR treuphadol OR triaminic OR tylenol OR tamen OR tapanol OR tipol OR uphamol OR vermidon OR vitamol OR valorin OR xumadol OR zolben).tw.
OR/1‐5
osteoarthritis.mp. OR exp Osteoarthritis/
low back pain.mp. OR exp Low Back Pain/
back pain.mp. OR exp Backache/
neck pain.mp. OR exp Neck Pain/
("low back pain" OR "back pain" OR "neck pain" OR backache OR lumbago OR "neck ache" OR "spin* pain" OR "knee pain" OR "hip pain").mp.
OR/7‐11
randomized controlled trial.mp. OR exp Randomized Controlled Trials/
randomized controlled trial.pt.
random allocation.mp. OR exp Random Allocation/
placebo.mp. OR exp Placebos/
(random* adj3 trial).ab,ti.
random*.ab,ti.
OR/13‐18
6 AND 12 AND 19
limit 20 to yr="2015 ‐Current"
Appendix 5. CINAHL search strategy
(MH "Acetaminophen") OR "acetaminophen"
(MH "Analgesics+/TU")
"analgesic$"
"paracetamol"
"tylenol"
"panadol"
OR/1‐6
(MH "Osteoarthritis+") OR "osteoarthritis" OR (MH "Osteoarthritis, Spine+") OR (MH "Osteoarthritis, Knee") OR (MH "Osteoarthritis, Hip")
(MH "Low Back Pain") OR "low back pain" OR (MH "Back Pain+")
(MH "Neck Pain") OR "neck pain"
(MH "Knee Pain+") OR "knee pain"
"hip pain"
"backache"
OR/8‐13
7 AND 14
EM 201501‐
DT 2015‐
OR/16‐17
15 AND 18
Appendix 6. Web of Sciences search strategy
acetaminophen
Paracetamol OR tylenol OR panadol
OR/1‐2
osteoarthritis
back pain
neck pain
(spin* pain" OR "knee pain" OR "hip pain")
OR/4‐7
3 AND 8
randomized controlled trial
random allocation
placebo
controlled clinical trial
Random*
OR/10‐14
9 AND 15 (Timespan=2015‐2017)
Appendix 7. LILACS search strategy
((acetaminophen OR paracetamol OR tylenol OR panadol) AND (osteoarthritis OR back pain OR lumbago OR backache OR neck pain)) AND (instance:"regional") AND (db:("LILACS") AND year_cluster:("2015" OR "2016" OR "2017"))
Appendix 8. IPA search strategy
acetaminophen.mp.
(aceta or actimin or anacin or apacet or "aspirin free anacin" or acamol or acetalgin or adol or aldolOR or alvedon or apiretal or atamel or atasol or benuron or biogesic or "biogesic kiddielets" or buscapina or banesin or "ben u ron" or calpol or captin or cemol or coldex or cotibin or crocin or dafalgan or daleron or "dawa ya magi" or depon or dexamol or dolex or dolgesic or doliprane or dolorol or dolprone or "duiyixian anjifen pian" or dapa or dolo or datril or duatrol or dayquil or efferalgan or enelfa or europain or febrectal or febricet or febridol or fensum or feverall or fibi or "fibi plus" or gelocatil or gripin or gesic or genapap or genebs or hedex or hedanol or herron or influbene or kafa or kitadol or lekadol or lupocet or lemsip or liquiprin or pyrigesic or mexalen or milidon or minoset or momentum or napa or "neo kiddielets" or neopap or "oraphen pd" or pyrigesic or pacol or pamol or parol or panado or panadol or panamax or panda or panodil or pyrigesic or paracet or paracetamol or paracitol or paralen or paramed or paramol or parol or perdolan or perfalgan or pinex or "pyongsu cetamol" or pyrenol or pyrigesic or plicet or panadrex or paratabs or paralgin or phenaphen or revanin or rokamol or rubophen or redutemp or sara or scanol or "sinpro n" or "snaplets fr" or suppap or tachipirin or tachipirina or tafirol or tapsin or termalgin or tempra or thomapyrin or tipol or "togal classic duo" or treuphadol or triaminic or tylenol or tamen or tapanol or tipol or uphamol or vermidon or vitamol or valorin or xumadol or zolben).tw.
OR/1‐2
osteoarthritis.mp.
low back pain.mp.
back pain.mp.
neck pain.mp.
("low back pain" or "back pain" or "neck pain" or backache or lumbago or "neck ache" or "spin* pain" or "knee pain" or "hip pain").mp.
OR/4‐8
3 AND 9
(2015* or 2016* or 2017*).em.
10 AND 11
Appendix 9. ClinicalTrials.gov and WHO ICTRP search strategy
ClinicalTrials.gov: Search: (paracetamol OR acetaminophen) AND placebo AND Condition: osteoarthritis.
WHO ICTRP: Title: (paracetamol OR acetaminophen) AND placebo AND Condition: osteoarthritis.
Data and analyses
Comparison 1. Hip or knee osteoarthritis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediate term | 5 | 1686 | Mean Difference (IV, Random, 95% CI) | ‐3.32 [‐5.83, ‐0.81] |
| 1.2 Short term | 7 | 2355 | Mean Difference (IV, Random, 95% CI) | ‐3.23 [‐5.43, ‐1.02] |
| 2 Physical function (Western Ontario and McMaster Universities Osteoarthritis Index) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Immediate term | 3 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐6.41, 2.75] |
| 2.2 Short term | 7 | 2354 | Mean Difference (IV, Random, 95% CI) | ‐2.92 [‐4.89, ‐0.95] |
| 3 Adverse events (AE) | 9 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 AE (any) | 8 | 3252 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 3.2 AE (serious) | 6 | 3209 | Risk Ratio (IV, Random, 95% CI) | 1.36 [0.73, 2.53] |
| 3.3 AE (withdrawals) | 7 | 3023 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.91, 1.55] |
| 3.4 AE (liver) | 3 | 1237 | Risk Ratio (IV, Random, 95% CI) | 3.79 [1.94, 7.39] |
| 4 Walking disability | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Immediate term | 1 | 294 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐2.09, 0.99] |
| 4.2 Short term | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐2.44, 2.56] |
| 5 Rescue medication | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6 Patient adherence | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Knee osteoarthritis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediate term | 3 | 1088 | Mean Difference (IV, Random, 95% CI) | ‐1.96 [‐4.19, 0.27] |
| 1.2 Short term | 3 | 813 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐4.24, 1.49] |
| 2 Physical function (Western Ontario and McMaster Universities Osteoarthritis Index) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Immediate term | 2 | 794 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐3.07, 3.74] |
| 2.2 Short term | 3 | 813 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐4.66, 2.50] |
| 3 Walking disability | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Immediate term | 1 | 294 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐2.09, 0.99] |
| 3.2 Short term | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐2.44, 2.56] |
Comparison 3. Subgroup analysis: paracetamol 3.0 g/day or less versus 3.9 g/day or more.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity: immediate term | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 ≤ 3.0 g/day | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐9.2 [‐19.59, 1.19] |
| 1.2 ≥ 3.9 g/day | 4 | 1630 | Mean Difference (IV, Random, 95% CI) | ‐2.98 [‐5.48, ‐0.48] |
| 2 Pain intensity: short term | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 ≤ 3.0 g/day | 2 | 535 | Mean Difference (IV, Random, 95% CI) | ‐2.88 [‐6.41, 0.65] |
| 2.2 ≥ 3.9 g/day | 6 | 1985 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐6.60, ‐2.01] |
| 3 Physical function: immediate term | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
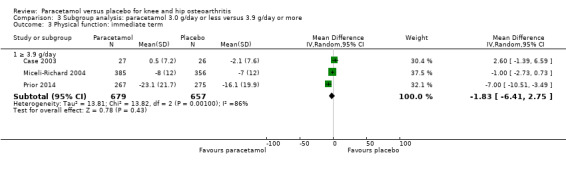
| 3.1 ≥ 3.9 g/day | 3 | 1336 | Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐6.41, 2.75] |
| 4 Physical function: short term | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ≤ 3.0 g/day | 2 | 535 | Mean Difference (IV, Random, 95% CI) | ‐2.86 [‐6.48, 0.77] |
| 4.2 ≥ 3.9 g/day | 6 | 1985 | Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐5.60, ‐0.54] |
3.3. Analysis.
Comparison 3 Subgroup analysis: paracetamol 3.0 g/day or less versus 3.9 g/day or more, Outcome 3 Physical function: immediate term.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altman 2007.
| Methods | Multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants |
Population: 483 participants with osteoarthritis of the hip or knee Setting: 47 investigational sites in the US Age: mean 62.2 years, range 40–90 years Numbers: paracetamol 3900 mg/day group: 160; paracetamol 1950 mg/day group: 158; placebo group: 165 Inclusion criteria: presence of symptomatic idiopathic osteoarthritis of the hip or knee for a minimum of 6 months with a history of hip or knee pain requiring the use of NSAIDs, paracetamol, or other analgesic on a regular basis (≥ 3 days/week) for ≥ 3 months before the screening visit. History of positive therapeutic benefit with paracetamol use for osteoarthritis pain. Participants must have reported maximum osteoarthritis pain intensity experienced during the 24 hours prior to the baseline visit at a pain level of moderate or moderately severe on a 5‐point Likert scale. Exclusion criteria: taking analgesic therapy for other indications; taking anticoagulants, psychotherapeutic agents, aspirin in daily doses > 325 mg, or statin hypolipidaemic agents in doses that had not been stabilised within 3 months of the screening visit; taking glucosamine, chondroitin sulphate, or shark cartilage in doses that had not been stabilised within 6 months of the screening visit; known alcohol abuse, intravenous drug use, drug dependency, or history of significant psychiatric illness in the previous 12 months; received oral corticosteroids within 2 months of screening or intra‐articular or periarticular corticosteroid or hyaluronan injections into the study joint within 6 months of screening; history of gastrointestinal or hepatic disease; clinically apparent inflammation of the study knee joint, secondary osteoarthritis of the study joint, history of acute inflammatory arthritis or pseudogout of the study joint, or medical history, physical examination, or radiographic evidence suggestive of other types of arthritis, collagen vascular disease, or fibromyalgia. |
|
| Interventions | Participants randomly assigned to 1 of 3 treatment groups:
Participants instructed to take the assigned study medication every 8 hours for 12 weeks or until study discontinuation. Washout: during the washout period, participants could not take any prescription or non‐prescription NSAID, paracetamol, aspirin, or analgesic in any form. |
|
| Outcomes |
Primary efficacy end points were mean change from baseline through 12 weeks. |
|
| Notes | Source of funding: company that produced paracetamol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to one of three treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "double‐blind;" "placebo‐controlled study;" "the placebo caplets were (...) similar in colour, size, and shape to the acetaminophen (...) caplets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 82/318 withdrawn from paracetamol groups (27 due to "lack of efficacy"); 54/165 withdrawn from control group (34 due to "lack of efficacy"). No description of reasons for dropout given. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all outcomes that were of interest in the study were reported. |
| Other bias | Unclear risk | The trial was supported by a pharmaceutical company. |
Amadio 1983.
| Methods | Cross‐over, randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Population: 25 participants with knee osteoarthritis diagnosis by image and clinical symptoms Age: median 64 years, range 43–80 years Inclusion criteria: X‐ray findings of typical bilateral osteoarthritis of the knee (i.e. narrowing of the joint space and osteophyte formation) within 3 months of entry into the study. Required to have ≥ 1 of: pain at rest, tenderness on pressure, swelling, and heat. Exclusion criteria: any concomitant illness that could affect the knees, such as rheumatoid arthritis, rheumatic fever, positive rheumatoid factor > 1 to 40, very high sedimentation rates, gouty arthritis, periarteritis nodosa, dermatomyositis, scleroderma, disseminated lupus erythematosus (preferably with negative antinuclear antibodies), psoriasis, syphilitic neuropathy, ochronosis, or significant primary metabolic bone disease; pregnant women; known allergy to paracetamol; and severe mechanical instability of, or evidence of recent acute trauma to, the knees. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: to minimise the effects of previous medication upon the assessment of paracetamol therapy, all possibly interfering drug regimens were discontinued prior to the study. Steroid therapy (including intra‐articular injection) was terminated at 6 weeks, NSAIDs ≥ 2 weeks, and salicylates ≥ 48 hours prior to study entry. Follow‐up: 4 weeks. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "By randomization, half of the patients received acetaminophen [paracetamol] and half placebo." |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind;" "placebo‐crossover study;" "identically‐appearing placebo." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind crossover study." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/25 participants completed the full 3‐week follow‐up. Number of participants who discontinued placebo treatment early was significantly greater than the number of participants who discontinued paracetamol early. |
| Selective reporting (reporting bias) | Unclear risk | No pain outcome measures reported. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Case 2003.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Population: 57 participants with knee osteoarthritis diagnosis by image and clinical assessment Setting: Rush Presbyterian‐St Luke's Medical Center, Chicago Age (mean): paracetamol group: 62.1 years; placebo group: 61.7 years; range of all included participants 40 to 75 years Number: paracetamol group: 29; placebo group: 28. Inclusion criteria: presence of radiographic osteoarthritis (modified Kellgren‐Lawrence grade > 1), medial joint space narrowing or osteophytes; clinical criteria included presence of pre‐enrolment ambulatory pain, moderate pain, or increased pain during a 2‐week washout period following discontinuation of pre‐existing analgesic or anti‐inflammatory medications (or both); capable of independent ambulation without the aid of a stick or walker. Exclusion criteria: prior intolerance to either of study medications or history of an NSAID allergy or intolerance; functional class I or IV; history of peptic ulcer disease or of significant other gastrointestinal disease; significant hepatic abnormality; renal insufficiency; haematological disease; presence of joint disease other than osteoarthritis; presence of joint replacements in the lower extremity; use of substances that could interfere with pain perception (tranquillisers, hypnotic agents, or excessive alcohol intake); and anticoagulation therapy. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: 2‐week washout period following discontinuation of pre‐existing analgesic or anti‐inflammatory osteoarthritis medications (or both) was applied. Follow‐up: 2 and 12 weeks. |
|
| Outcomes |
|
|
| Notes | We converted WOMAC pain to 0–100, and function to 0–100. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized to treatment." Comment: unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "double‐blind;" "placebo‐controlled study;" "The placebos were supplied by the respective manufacturers of the active medications and were identical in appearance to the active medications." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At week 2: 2/29 withdrawn from the paracetamol group; 2/28 withdrawn from the placebo group. No difference of withdraw reasons between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were of interest in the study were reported. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Golden 2004.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Population: 303 participants with knee osteoarthritis diagnosis by image and clinical assessment Age (mean): paracetamol group: 61.1 years; placebo group: 60.3 years Numbers: paracetamol group: 148; placebo group: 155 Inclusion criteria: diagnosed with osteoarthritis that showed ≥ 1 of the following radiographic changes: subchondral sclerosis, joint space narrowing, presence of osteophytes or marginal lipping, or cyst formation in the knee joint of osteoarthritis stage I–III, as documented in the previous 3 years; at least moderate pain in the knee on weight bearing as measured on a categorical scale; episodic flares of osteoarthritis. Exclusion criteria: stage IV osteoarthritis;, moderate‐to‐severe chronic low back pain; inflammatory joint diseases including rheumatoid arthritis, gout, mixed connective tissue disease, seronegative spondyloarthropathy, psoriatic arthritis, or systemic lupus erythematosus; had been on a daily regimen of prescription NSAIDs for arthritis pain for the past 3 months; had recent traumatic injury, history of hypersensitivity or intolerance to any of the study medications; history of peptic ulceration within the previous 9 months, gastrointestinal surgery, complaints, or dysfunction that could interfere with drug absorption; or any other significant medical condition. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: all participants had completed the required washout period. Follow‐up: 1 week |
|
| Outcomes |
|
|
| Notes | Converted 1–4 Likert scale to 0–100 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned in equal numbers." Comment: unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind;" "placebo‐controlled;" "All study medication was in the form of equally sized capsules in identical blister packs and packaged in boxes." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/148 withdrawn from analysis in the paracetamol group; 6/155 withdrawn from analysis in the placebo group. The reasons were similar across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were of interest in the study were reported. |
| Other bias | Unclear risk | Received funds from a company that produced paracetamol. |
Herrero‐Beaumont 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Population: 212 participants with knee osteoarthritis diagnosis by image and clinical assessment criteria according to the American College of Rheumatology Setting: Spain and Portugal Age (mean): paracetamol group: 63.8 years; placebo group: 64.9 years Numbers: paracetamol group: 108; placebo group: 104 Inclusion criteria: Grade II or III Kellgren/Lawrence radiographic system Exclusion criteria: obese people |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: recommended duration of washout prior to randomisation ≥ 3 months for corticosteroids and 6 months for glucosamine or other drugs considered specific for osteoarthritis. Follow‐up: 6 months |
|
| Outcomes |
|
|
| Notes | Converted 95% CI to SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomly assigned;" "A block randomization list was generated by computer." Comment: adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization (...) was maintained by individuals who had no contact with investigators who assigned patients to their randomized treatments;" "the individual code was kept in single‐sealed, opaque envelopes." Comment: adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, double‐dummy;" "placebo‐controlled;" "double‐dummy placebo formulations were identical in appearance to the active medications." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 28/108 participants did not present complete data according to protocol (5 due to "lack of efficacy") in the paracetamol group; 34/104 participants did not present complete data according to protocol (8 due to "lack of efficacy") in the placebo group. There was no description of the reasons for dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were of interest in the study were reported. |
| Other bias | Unclear risk | Study received funds from a company that produced paracetamol. |
Miceli‐Richard 2004.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Population: 779 participants with knee osteoarthritis diagnosed according to the Lequesne criteria Age (mean): 70 years Numbers: paracetamol group: 405; placebo group: 374 Inclusion criteria: symptomatic osteoarthritis of the knee for ≥ 3 months; global pain intensity of the knee during physical activities for the past 24 hours ≥ 30 mm on a 100‐mm VAS. Exclusion criteria: had a prosthesis or recent (< 1 year) surgery of the studied knee; history of allergy to paracetamol; history of hepatitis, severe hepatic or kidney failure; steroids during the past 4 weeks; current treatment with enzymatic inductors or inhibitors; history of asthma or allergy potentially requiring concomitant treatment during the study; pregnancy, lactation, or inefficacious contraception; history of drug abuse or alcoholism. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: duration of washout of paracetamol or NSAIDs ≥ 24 hours Follow‐up: 1 and 6 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned." Comment: unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind;" "placebo controlled;" "matched placebo." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/374 participants discontinued from the paracetamol group (7 due to "lack of efficacy"); 20/405 participants discontinued from the placebo group (7 due to "lack of efficacy"). None of the participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were of interest in the study were reported. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
Pincus 2004a.
| Methods | Cross‐over, randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Population: 524 participants with hip or knee osteoarthritis diagnosis according to Lequesne criteria Age (mean): paracetamol group: 63.7 years; placebo group: 62.8 years Inclusion criteria: aged ≥ 45 years, radiographic Kellgren‐Lawrence grade II–IV, pain score 40–90 mm on a VAS, and designation by the treating physician that the participant was a candidate for long‐term treatment with a COX‐2 inhibitor drug or an analgesic drug. Exclusion criteria: significant medical comorbidities; rheumatoid arthritis or other inflammatory arthritis; acute joint trauma; chronic pain syndrome; expected need for surgery during the course of the study; oral or parenteral corticosteroids within 2 months, or intra‐articular injections of hyaluronic acid within 9 months; pregnant or lactating women, or not using contraception. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: 3–7 days Follow‐up: 6 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were assigned randomly." Comment: unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind, double dummy;" "placebo‐controlled." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: missing data were imputed using appropriate methods: the last observation carried forward procedure used to manage missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were of interest in the paper were reported. |
| Other bias | Unclear risk | Study received funds from a company that produced paracetamol. |
Pincus 2004b.
| Methods | Cross‐over, randomised, double‐blinded, placebo‐controlled trial | |
| Participants |
Population: 556 participants with hip or knee osteoarthritis diagnosis according to Lequesne criteria Age (mean): paracetamol group: 64.8 years; placebo group: 63.4 years Inclusion criteria: aged ≥ 45 years, radiographic Kellgren‐Lawrence grade II–IV, pain score 40–90 mm on a VAS, and designation by the treating physician that the participant was a candidate for long‐term treatment with a COX‐2 inhibitor drug or an analgesic drug. Exclusion criteria: significant medical comorbidities; rheumatoid arthritis or other inflammatory arthritis; acute joint trauma; chronic pain syndrome; expected need for surgery during the course of the study; oral or parenteral corticosteroids within 2 months, or intra‐articular injections of hyaluronic acid within 9 months; pregnant or lactating women, or not using contraception. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: 3–7 days Follow‐up: 6 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were assigned randomly." Comment: unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind, double dummy;" "placebo‐controlled." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: missing data were imputed using appropriate methods: the last observation carried forward procedure used to manage missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were of interest in the paper were reported. |
| Other bias | Unclear risk | Study received funds from a company that produced paracetamol. |
Prior 2014.
| Methods | Randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants |
Population: 542 participants with hip or knee osteoarthritis were assessed by physical examination and radiographic evaluation Setting: conducted by 60 investigators at 58 sites in the US Age (mean): paracetamol group: 61.7 years; placebo group: 61.7 years Numbers: paracetamol group: 267; placebo group: 275). Inclusion criteria: aged ≥ 40 years with symptomatic idiopathic osteoarthritis of the hip or knee for ≥ 6 months; osteoarthritis pain that required NSAIDs, paracetamol, or another analgesic agent on a regular basis for ≥ 3 months before the screening visit; history of positive therapeutic benefit with paracetamol used for osteoarthritis pain; history of moderate‐to‐severe osteoarthritis when not taking analgesic medication; had to have knee pain, radiographic osteophytes, and had to have met ≥ 1 of the following 3 criteria: morning stiffness (< 30 minutes' duration), crepitus on motion or 40 years of age or older; had to have hip pain, radiographic femoral, acetabular osteophytes, and radiographic joint space narrowing (or a combination) as established by the American College of Rheumatology for idiopathic osteoarthritis of the hip. Exclusion criteria: history of surgery; major trauma in the past 6 months; signs of clinically important active inflammation of the study knee joint; secondary osteoarthritis of the study joint; history of acute inflammatory arthritis or pseudogout of the study joint; history that suggested other types of arthritis or fibromyalgia; known alcohol abuse. intravenous drug use; drug dependency or history of significant psychiatric illness in the past 12 months; history of clinically important gastrointestinal or hepatic disease within the past 6 months; received analgesic therapy for chronic or recurrent pain conditions for indications other than osteoarthritis; received systemic corticosteroids within past 2 months; received intra‐articular or periarticular corticosteroid or hyaluronan injections of the study joint within past 6 months; received any analgesic or anti‐inflammatory drugs within 5 drug half‐lives plus an additional 48 hours before baseline; current use of aspirin, anticoagulants, or psychotherapeutic agents; undergoing non‐pharmacological therapy requiring direct supervision within the past month. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Washout: all potential participants underwent a washout period from their current osteoarthritis pain medication. Follow‐up: weeks 2, 4, 8, and 12 after baseline. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization." Comment: adequate. |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment assignments remained concealed from the patients, the investigators, and personnel directly involved in monitoring the study or reviewing the data until after study completion." Comment: adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind;" "placebo‐controlled;" "placebo caplets were the same shape, size, and color." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 90/267 participants did not complete the trial from the paracetamol group (43 due to "lack of efficacy"); 103/275 participants did not complete the trial from the placebo group (56 due to "lack of efficacy"). No clear description of the reasons for drop‐outs were given. |
| Selective reporting (reporting bias) | Low risk | Trial was registered and the protocol was reported in ClinicalTrials.gov. All outcomes that are of interest in the paper were reported. |
| Other bias | Unclear risk | Study conducted, analysed, and supported by the company that funded the study and produced paracetamol. All the authors were employees of the company that funded the study and produced paracetamol at the time of the trial. |
Zoppi 1995.
| Methods | Multicentre, randomised, placebo‐controlled, double‐blind clinical trial | |
| Participants |
Population: 60 participants with hip or knee osteoarthritis were assessed by radiographic evidence Age (mean): paracetamol group: 57.6 years; placebo group: 55.3 years Numbers: paracetamol group: 30; placebo group: 30 Inclusion criteria: either sex, aged > 18 years with arthritis of the knee or the hip in a steady phase for ≥ 2 months; presented with permanent residual pain refractory to management by symptomatic analgesic treatment; judged the pain over the previous 24 hours in the absence of symptomatic treatment to be greater than or equal to moderate pain on a verbal 5‐point scale; interrupted previous analgesic drug treatment during the study; gave their informed consent. Exclusion criteria: contraindication to paracetamol; use of an oxicam in the 24 hours preceding the start of the trial; intellectual level preventing full understanding of pain assessment scales and study procedure; had to undergo surgery or infiltration on synovectomy. |
|
| Interventions | Participants randomly assigned to 1 of 2 treatment groups:
Follow‐up: 1 week |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized study." Comment: unclear. |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind;" "placebo‐controlled." Comment: probably done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind." Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/30 participants withdrew from the paracetamol group due to adverse effects; 6/30 participants withdrew from the placebo group (2 due to adverse effects and 4 due to "lack of efficacy"). |
| Selective reporting (reporting bias) | Unclear risk | No physical function outcome measures reported. |
| Other bias | Low risk | Study appeared free of other sources of bias. |
CI: confidence interval; COX‐2: cyclo‐oxygenase‐2; ER: extended release; NSAID: non‐steroidal inflammatory drug; SD: standard deviation; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ha 2016 | Did not include the intervention of interest. |
| Lao 2015 | Did not include the intervention of interest. |
| Papou 2015 | Not a randomised controlled trial. |
| Park 2015 | Did not include people with hip or knee osteoarthritis. |
| Skou 2015 | Did not include the intervention of interest and lacked a placebo control group. |
| van Tunen 2016 | Not a randomised controlled trial. |
| Verkleij 2015 | Did not include a placebo control group. |
| Zheng 2015 | Not a randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000840785.
| Trial name or title | A randomized, blinded, comparator‐controlled trial investigating a 28‐day course of Lyrica in participants with knee osteoarthritis who exhibit neuropathic pain, compared with a 28‐day course of acetaminophen |
| Methods | Investigating whether pregabalin is more effective than standard paracetamol in relieving pain and widespread neuropathic‐type symptoms in people with mild to moderately painful knee osteoarthritis, who have been assessed for the additional presence of neuropathic pain. |
| Participants | Osteoarthritis of the knee > 6 months' duration diagnosed according to American College of Rheumatology clinical classification system, and based on radiological and clinical presentation; pain in the index knee ≥ 4 out of 10 with an additional 1–1.5 out of 10 increase in pain on walking when withdrawn from their usual analgesic medication; no additional clinically significant joint involvement and otherwise in good health; American Rheumatology Association functional Class I, II, or III; no recent arthroscopy, injections with glucocorticoid, or hyaluronan in last 6 months to index knee; no plan for joint replacement surgery during the study period. |
| Interventions | Lyrica Paracetamol Placebo |
| Outcomes | Primary outcome: cold detection and cold pain threshold at index knee to assess cold hyperalgesia Secondary outcomes: WOMAC pain subscale; physical function using the Aggregated Locomotor Function Score; quality of life measured with the SF‐36 |
| Starting date | 17 June 2013 |
| Contact information | Name: Prof Anthony Wright Address: Curtin University, School of Physiotherapy, Building 408, Kent Street, Bentley WA 6102 Australia Telephone: +61 8 9266 4644 Email: t.wright@curtin.edu.au |
| Notes | Date last updated: July 2013 |
NCT01105936.
| Trial name or title | A randomised, double‐blind, evaluation of the effects of paracetamol on the BOLD fMRI response to painful stimuli in subjects with osteoarthritis |
| Methods | Investigating if fMRI can detect the effects of a known pain medicine at over‐the‐counter doses in people with osteoarthritis of the knee. In this study, blood oxygen level‐dependent response to mechanical stimulation via pressure stimuli applied to the tibio‐femoral joint and patello‐femoral in participants with knee osteoarthritis following 4 treatment doses of any of 3 treatment will be compared. |
| Participants | Diagnosis of osteoarthritis of 1 or both knees for 3 months; men or women aged ≥ 45 years; score a minimum of 4 out of 10 on the numerical pain rating and a maximum of 8 at screening. |
| Interventions | Paracetamol Placebo |
| Outcomes | Primary outcome: blood oxygen level‐dependent response in the tibio‐femoral joint of knee osteoarthritis Secondary outcomes: subjective NRS response before stimulation; NRS response prior the fMRI scan; NRS response after the fMRI scan |
| Starting date | 1 September 2010 |
| Contact information | Name: GlaxoSmithKline Address: Hospital Del Mar, Barcelona, Spain |
| Notes | Last verified: May 2017 |
NCT01420666.
| Trial name or title | Maxi‐Analgesic OA Study: multicentre, double‐blind, placebo‐controlled, randomized, parallel group comparison of the effects of Maxigesic 325 with acetaminophen or ibuprofen on patients with pain from osteoarthritis |
| Methods | Determining whether the analgesic effects of Maxigesic are greater than paracetamol, ibuprofen, or placebo in people with painful osteoarthritis of the hip or knee. |
| Participants | Have symptoms of osteoarthritis of the knee or hip for ≥ 6 months that has required analgesic medication; have confirmed radiological evidence of osteoarthritis; aged 45–80 years inclusive; require long‐term medication for treatment of painful osteoarthritis; have painful osteoarthritis of the knee or hip with a pain score of ≥ 40 mm and ≤ 80 mm on the WOMAC VAS pain subscale at rest following a 3‐ to 7‐day washout of existing analgesics. |
| Interventions | Maxigesic 325 Paracetamol Ibuprofen Placebo |
| Outcomes | Primary outcome: WOMAC pain subscale Secondary outcomes: WOMAC physical function subscale, WOMAC stiffness subscale, use of rescue medication, safety, patient global assessment |
| Starting date | 22 August 2011 |
| Contact information | Name: John Moodie Address: 32 Kahikatea Drive, Hamilton, New Zealand |
| Notes | Last verified: January 2016 (the study is not ongoing as it has been withdrawn for an administrative reason) |
NCT02311881.
| Trial name or title | An efficacy and safety study of sustained‐release paracetamol in subjects with osteoarthritis |
| Methods | Determining whether paracetamol 1000 mg sustained‐release tablets administered orally, twice daily are effective and safe in the treatment of people with osteoarthritis of the knee or hip |
| Participants | Men or women aged 40–80 years; diagnosis of moderate‐to‐moderately severe osteoarthritis of either the knee or hip with respect to the following: pain in 1 knee/hip over 3 months immediately before screening visit; use of non‐steroidal anti‐inflammatory drugs, paracetamol, or any other analgesic for ≥ 3 days per week for ≥ 3 months prior to screening visit; clinical diagnosis of osteoarthritis of knee/hip for minimum 6‐month duration prior to screening visit; therapeutic benefit with paracetamol use with a score of ≥ 1 on 5‐point categorical scale; radiological evidence of ≥ Grade 2 osteoarthritis according to Kellgren‐Lawrence radiographic criteria; increased WOMAC pain subscale score of ≥ 20% following untreated run‐in period; moderate‐to‐moderately severe self‐reported pain on a 5‐point categorical scale following untreated run‐in period; historical self‐reported positive therapeutic benefit with paracetamol use for osteoarthritis pain relief. |
| Interventions | Paracetamol Placebo |
| Outcomes | Primary outcome: WOMAC pain subscale Secondary outcomes: WOMAC physical function subscale, WOMAC stiffness subscale, Global Patient Assessment of Arthritis scores, use of rescue medication, Chronic Pain Sleep Inventory scores, Patient Global Assessment of Response to Therapy scores |
| Starting date | 9 December 2014 |
| Contact information | Name: GlaxoSmithKline Address: not available |
| Notes | Last verified: February 2017 |
NCT02845271.
| Trial name or title | Proof‐of‐concept study to assess the efficacy, tolerability and safety of a single intraarticular dose of GZ389988 versus placebo in patients with painful osteoarthritis of the knee |
| Methods | Assessing the efficacy of a single intra‐articular dose of GZ389988 compared to placebo for relief of knee pain in patients with osteoarthritis of the knee |
| Participants | Men or women aged 40–80 years; diagnosis of primary knee osteoarthritis, based upon the following: fulfilling the American College of Rheumatology Clinical and Radiographic criteria for osteoarthritis (at least knee pain and osteophytes), with X‐ray evidence within the last 6 months for Kellgren‐Lawrence classification II–IV; WOMAC pain subscore (walking pain) over the last 48 hours ≥ 40 and ≤ 90 on VAS 0–100 in the target knee at screening with or without medication, and ≤ 30 on VAS 0–100 in the contralateral knee at screening with or without medication; WOMAC pain subscore (walking pain) between 50 and 90 using the VAS 0–100, corresponding to moderate‐to‐severe pain in the target knee at baseline; symptomatic for > 6 months; ambulatory with an active lifestyle and in good general health. |
| Interventions |
GZ389988 Paracetamol Paracetamol + codeine Paracetamol + tramadol hydrochloride Placebo |
| Outcomes | Primary outcome: WOMAC pain subscale Secondary outcomes: WOMAC physical function subscale, WOMAC stiffness subscale, Patient Global Assessment, Patient Global Impression of Change, Patient Global Assessment of Response to Therapy, use of rescue medication |
| Starting date | 27 July 2016 |
| Contact information | Name: Genzyme, a Sanofi Company Address: Berlin, Germany |
| Notes | Last verified: September 2017 |
fMRI: functional magnetic response imaging; NRS: numerical rating scale; SF‐36: 36‐item Short Form; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Differences between protocol and review
This is an update of a review published in the BMJ (Machado 2015). The study protocol was previously registered on PROSPERO (registration number CRD42013006367). We followed recommendations of the Cochrane Musculoskeletal Group in this review, which was not stated in the protocol or previous version of this review as it was not yet published. However, there were no substantial changes from the protocol or the previous version of this review.
Contributions of authors
AOL, GCM, and MLF: drafting the protocol, obtaining copies of trials and entering data into Review Manager 5.
All authors: study design and concept, and approving the final draft.
AOL and GCM: developing the search strategy, searching for trials and data extraction.
AOL, GCM, and MLF: selection of relevant trials and performed the analyses.
All authors: interpreting the results.
AOL and MLF: first draft of the review.
All authors: approving the final version.
All authors had access to the data and took responsibility for the integrity of the data and the accuracy of the data analysis.
MLF is guarantor.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
AOL: no conflict of interest.
GCM, MBP, PHF, ROD, AJM, and MLF: conducted a systematic review that investigated the benefits of paracetamol for patients with osteoarthritis and spinal pain published in the BMJ.
ROD and AJM: investigators on the PACE (Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies) trial of paracetamol in low back pain which was jointly funded by the National Health and Medical Research Council of Australia and GlaxoSmithKline Australia (a manufacturer of paracetamol).
AM: received research funding support from GlaxoSmithKline for a PhD scholarship.
DJH is a consultant for Flexion, Merck Serono, and Nestlé.
Edited (no change to conclusions)
References
References to studies included in this review
Altman 2007 {published data only}
- Altman RD, Zinsenheim JR, Temple AR, Schweinle JE. Three‐month efficacy and safety of acetaminophen extended‐release for osteoarthritis pain of the hip or knee: a randomized, double‐blind, placebo‐controlled study. Osteoarthritis and Cartilage 2007;15:454‐61. [DOI] [PubMed] [Google Scholar]
Amadio 1983 {published data only}
- Amadio P, Doyle M. Evaluation of acetaminophen in the management of osteoarthritis of the knee. Current Therapeutic Research – Clinical and Experimental 1983;34(1):59‐66. [Google Scholar]
Case 2003 {published data only}
- Case JP, Baliunas AJ, Block JA. Lack of efficacy of acetaminophen in treating symptomatic knee osteoarthritis: a randomized, double‐blind, placebo‐controlled comparison trial with diclofenac sodium. Archives of Internal Medicine 2003;163(2):169‐78. [DOI] [PubMed] [Google Scholar]
Golden 2004 {published data only}
- Golden HE, Moskowitz RW, Minic M. Analgesic efficacy and safety of nonprescription doses of naproxen sodium compared with acetaminophen in the treatment of osteoarthritis of the knee. American Journal of Therapeutics 2004;11:85‐94. [DOI] [PubMed] [Google Scholar]
Herrero‐Beaumont 2007 {published data only}
- Herrero‐Beaumont G, Ivorra JA, Carmen Trabado M, Blanco FJ, Benito P, Martín‐Mola E, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double‐blind, placebo‐controlled study using acetaminophen as a side comparator. Arthritis and Rheumatism 2007;56(2):555‐67. [DOI] [PubMed] [Google Scholar]
Miceli‐Richard 2004 {published data only}
- Miceli‐Richard C, Bars M, Schmidely N, Dougados M. Paracetamol in osteoarthritis of the knee. Annals of the Rheumatic Diseases 2004;63(8):923‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 2004a {published data only}
- Pincus T, Koch G, Lei H, Mangal B, Mangal B, Sokka T, Moskowitz R, et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Annals of the Rheumatic Diseases 2004;63(8):931‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pincus 2004b {published data only}
- Pincus T, Koch G, Lei H, Mangal B, Mangal B, Sokka T, Moskowitz R, et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Annals of the Rheumatic Diseases 2004;63(8):931‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prior 2014 {published data only}
- Prior MJ, Harrison DD, Frustaci ME. A randomized, double‐blind, placebo‐controlled 12 week trial of acetaminophen extended release for the treatment of signs and symptoms of osteoarthritis. Current Medical Research & Opinion 2014;30(11):2377‐87. [DOI] [PubMed] [Google Scholar]
Zoppi 1995 {published data only}
- Zoppi M, Peretti G, Boccard E. Placebo‐controlled study of the analgesic efficacy of an effervescent formulation of 500 mg paracetamol in arthritis of the knee or the hip. European Journal of Pain 1995;16:1‐2. [Google Scholar]
References to studies excluded from this review
Ha 2016 {published data only}
- Ha CW, Park YB, Min BW, Han SB, Lee JH, Won YY, et al. Prospective, randomized, double‐blinded, double‐dummy and multicenter phase IV clinical study comparing the efficacy and safety of PG201 (Layla) and SKI306X in patients with osteoarthritis. Journal of Ethnopharmacology 2016;181:1‐7. [DOI] [PubMed] [Google Scholar]
Lao 2015 {published data only}
- Lao L, Hochberg M, Lee DY, Gilpin AM, Fong HH, Langenberg P, et al. Huo‐Luo‐Xiao‐Ling (HLXL)‐Dan, a Traditional Chinese Medicine, for patients with osteoarthritis of the knee: a multi‐site, randomized, double‐blind, placebo‐controlled phase II clinical trial. Osteoarthritis and Cartilage 2015;23(12):2102‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papou 2015 {published data only}
- Papou A, Hussain S, McWilliams D, Zhang W, Doherty M. Sensitivity to change of SF‐36 health survey and patient generated index in people with chronic knee pain commenced on oral analgesia: analysis of data from a clinical trial. Annals of the Rheumatic Diseases 2015;74:363. [DOI] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park YB, Ha CW, Cho SD, Lee MC, Lee JH, Seo SS, et al. A randomized study to compare the efficacy and safety of extended‐release and immediate‐release tramadol HCl/acetaminophen in patients with acute pain following total knee replacement. Current Medical Research and Opinion 2015;31(1):75‐84. [DOI] [PubMed] [Google Scholar]
Skou 2015 {published data only}
- Skou ST, Rasmussen S, Laursen MB, Rathleff MS, Arendt‐Nielsen L, Simonsen O, et al. The efficacy of 12 weeks non‐surgical treatment for patients not eligible for total knee replacement: a randomized controlled trial with 1‐year follow‐up. Osteoarthritis and Cartilage 2015;23(9):1465‐75. [DOI] [PubMed] [Google Scholar]
van Tunen 2016 {published data only}
- Tunen JA, Leeden M, Bos WH, Cheung J, Esch M, Gerritsen M, et al. Optimization of analgesics for greater exercise therapy participation among patients with knee osteoarthritis and severe pain: a feasibility study. Arthritis Care and Research 2016;68(3):332‐40. [DOI] [PubMed] [Google Scholar]
Verkleij 2015 {published data only}
- Verkleij SP, Luijsterburg PA, Willemsen SP, Koes BW, Bohnen AM, Bierma‐Zeinstra SM. Effectiveness of diclofenac versus paracetamol in knee osteoarthritis: a randomised controlled trial in primary care. British Journal of General Practice 2015;65(637):530‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2015 {published data only}
- Zheng Y, Kostenbader K, Barrett T, Hisaw E, Giuliani MJ, Chen Y, Young JL. Tolerability of biphasic‐release hydrocodone bitartrate/acetaminophen tablets (MNK‐155): a phase III, multicenter, open‐label study in patients with osteoarthritis or chronic low back pain. Clinical Therapeutics 2015;1(37):1235‐47. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12613000840785 {published data only}
- A randomized, blinded, comparator‐controlled trial investigating a 28‐day course of Lyrica in participants with knee osteoarthritis who exhibit neuropathic pain, compared with a 28‐day course of acetaminophen. Ongoing study 17 June 2013.
NCT01105936 {published data only}
- A randomised, double‐blind, evaluation of the effects of paracetamol on the BOLD fMRI response to painful stimuli in subjects with osteoarthritis. Ongoing study 1 September 2010.
NCT01420666 {published data only}
- Maxi‐Analgesic OA Study: multicentre, double‐blind, placebo‐controlled, randomized, parallel group comparison of the effects of Maxigesic 325 with acetaminophen or ibuprofen on patients with pain from osteoarthritis. Ongoing study 22 August 2011.
NCT02311881 {published data only}
- An efficacy and safety study of sustained‐release paracetamol in subjects with osteoarthritis. Ongoing study 9 December 2014.
NCT02845271 {published data only}
- Proof‐of‐concept study to assess the efficacy, tolerability and safety of a single intraarticular dose of GZ389988 versus placebo in patients with painful osteoarthritis of the knee. Ongoing study 27 July 2016.
Additional references
Altman 1996
- Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage 1996;4(4):217‐43. [DOI] [PubMed] [Google Scholar]
Bartels 2016
- Bartels E M, Juhl C B, Christensen R, Hagen K B, Danneskiold‐Samsoe B, Dagfinrud H, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD005523.pub3] [DOI] [Google Scholar]
Barten 2015
- Barten DJ, Dorsman SA, Dekker J, Veenhof C, Bakker DH. Treatment of hip/knee osteoarthritis in Dutch general practice and physical therapy practice: an observational study. BMC Family Practice 2015;16(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barthel 2010
- Barthel HR, Peniston JH, Clark MB, Gold MS, Altman RD. Correlation of pain relief with physical function in hand osteoarthritis: randomized controlled trial post hoc analysis. Arthritis Research & Therapy 2010;12(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basedow 2015
- Basedow M, Williams H, Shanahan EM, Runciman WB, Esterman A. Australian GP management of osteoarthritis following the release of the RACGP guideline for the non‐surgical management of hip and knee osteoarthritis. BMC Research Notes 2015;5(8):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brand 2008
- Brand CA. The role of self‐management in designing care for people with osteoarthritis of the hip and knee. Medical Journal of Australia 2008;189(S):25‐8. [DOI] [PubMed] [Google Scholar]
Brand 2014
- Brand CA, Harrison C, Tropea J, Hinman RS, Britt H, Bennell K. Management of osteoarthritis in general practice in Australia. Arthritis Care and Research 2014;66:551‐8. [DOI] [PubMed] [Google Scholar]
Buckwalter 2006
- Buckwalter JA, Martin JA. Osteoarthritis. Advanced Drug Delivery Reviews 2006;20(58):150‐67. [DOI] [PubMed] [Google Scholar]
Crofford 2001
- Crofford LJ. Rational use of analgesic and antiinflammatory drugs. New England Journal of Medicine 2001;20(345):1844‐6. [DOI] [PubMed] [Google Scholar]
Cross 2014
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases 2014;73:1323‐30. [DOI] [PubMed] [Google Scholar]
Derry 2016
- Derry S, Conaghan P, Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No.: CD007400. DOI: 10.1002/14651858.CD007400.pub3. [DOI] [PMC free article] [PubMed]
Egger 1997
- Egger M, Zellweger‐Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;2(305):326‐9. [DOI] [PubMed] [Google Scholar]
Egger 2003
- Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment (Winchester, England) 2003;7(1):1‐76. [PubMed] [Google Scholar]
Ghogomu 2014
- Ghogomu EA, Maxwell LJ, Buchbinder R, Rader T, Pardo Pardo J, Johnston RV, et al. Updated method guidelines for Cochrane musculoskeletal group systematic reviews and metaanalyses. Journal of Rheumatology 2014;41(2):194‐205. [DOI] [PubMed] [Google Scholar]
Graham 2002
- Graham GG, Graham RI, Day RO. Comparative analgesia, cardiovascular and renal effects of celecoxib, rofecoxib and acetaminophen (paracetamol). Current Pharmaceutical Design 2002;8(12):1063‐75. [DOI] [PubMed] [Google Scholar]
Graham 2003
- Graham GG, Scott KF. Mechanisms of action of paracetamol and related analgesics. Inflammopharmacology 2003;11(4):401‐13. [DOI] [PubMed] [Google Scholar]
Graham 2013
- Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013;21(3):201‐32. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Cook DJ, Jaeschke R, Pauker SG, Schünemann HJ. Grades of recommendation for antithrombotic agents: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6):123S‐31S. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Gøtzsche 2007
- Gøtzsche PC, Hróbjartsson A, Maric K, Tendal B. Data extraction errors in meta‐analyses that use standardized mean differences. JAMA 2007;298(4):430‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hinz 2008
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase‐2 inhibitor in man. FASEB Journal 2008;22(2):383‐90. [DOI] [PubMed] [Google Scholar]
Hochberg 2012
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care and Research 2012;64:465‐74. [DOI] [PubMed] [Google Scholar]
Hoy 2014
- Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases 2014;73:968‐74. [DOI] [PubMed] [Google Scholar]
Jordan 2003
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Annals of the Rheumatic Diseases 2003;62(12):1145‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juhl 2012
- Juhl C, Lund H, Roos EM, Zhang W, Christensen R. A hierarchy of patient‐reported outcomes for meta‐analysis of knee osteoarthritis trials: empirical evidence from a survey of high impact journals. Arthritis 2012;2012:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2006
- Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Practice and Research 2006;20(4):720‐40. [DOI] [PubMed] [Google Scholar]
Jüni 2015
- Jüni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, et al. Intra‐articular corticosteroid for knee osteoarthritis. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD005328.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Machado 2015
- Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta‐analysis of randomised placebo controlled trials. BMJ 2015;350:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pham 2004
- Pham T, Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT‐OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis and Cartilage 2004;12(5):389‐99. [DOI] [PubMed] [Google Scholar]
Puljak 2017
- Puljak L, Marin A, Vrdoljak D, Markotic F, Utrobicic A, Tugwell P. Celecoxib for osteoarthritis. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No.: CD009865. DOI: 10.1002/14651858.CD009865.pub2. [DOI] [PMC free article] [PubMed]
Regnaux 2015
- Regnaux JP, Lefevre‐Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, et al. High‐intensity versus low‐intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD010203.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reichenbach 2007
- Reichenbach S, Sterchi R, Scherer M, Trelle S, Burgi E, Burgi U, et al. Meta‐analysis: chondroitin for osteoarthritis of the knee or hip. Annals of Internal Medicine 2007;146(8):580‐90. [DOI] [PubMed] [Google Scholar]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2016
- Roberts E, Delgado Nunes V, Buckner S, Latchem S, Constanti M, Miller P, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Annals of the Rheumatic Diseases 2016;75(3):552‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schein 2008
- Schein JR, Kosinski MR, Janagap‐Benson C, Gajria K, Lin P, Freedman JD. Functionality and health‐status benefits associated with reduction of osteoarthritis pain. Current Medical Research and Opinion 2008;24:1255‐65. [DOI] [PubMed] [Google Scholar]
Smink 2011
- Smink AJ, Ende CH, Vliet Vlieland TP, Swierstra BA, Kortland JH, Bijlsma JW, et al. "Beating osteoARThritis": development of a stepped care strategy to optimize utilization and timing of non‐surgical treatment modalities for patients with hip or knee osteoarthritis. Clinical Rheumatology 2011;30(12):1623‐9. [DOI] [PubMed] [Google Scholar]
Towheed 2006
- Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004257.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uthman 2013
- Uthman O, Windt D, Jordan JL, Dziedzic K, Healey E, Peat GM, et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta‐analysis. British Journal of Medicine 2013;347:f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wandel 2010
- Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta‐analysis. BMJ 2010;341(16):4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolf 2003
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization 2003;81(9):646‐56. [PMC free article] [PubMed] [Google Scholar]
Zhang 2004
- Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta‐analysis of randomised controlled trials. Annals of the Rheumatic Diseases 2004;63(8):901‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2005
- Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther KP, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the Rheumatic Diseases 2005;64:669‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2007
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis and Cartilage 2007;15(19):981‐1000. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang WG, Nuki RW, Moskowitz S, Abramson RD, Altman NK, Arden S, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis and Cartilage 2010;18(4):476‐99. [DOI] [PubMed] [Google Scholar]


