Abstract
Purpose of Review
Post-concussion syndrome (PCS), when the patient’s concussion symptoms last longer than 4–6 weeks, affects 10–30% of concussion patients. PCS presents a significant source of morbidity to patients and a management challenge to providers. In this review, we present the current evidence and best management approaches for pediatric PCS.
Recent Findings
There is limited high-quality evidence in pediatric PCS. There is some evidence supporting pharmaceutical management of post-traumatic headaches, cognitive symptoms, and emotional symptoms. Vestibular-ocular dysfunction should be evaluated and managed appropriately. Neuropsychological recovery is expected, but requires appropriate attention to Return to Learn. Emotional symptoms are common in PCS and the evidence supports treatment with cognitive behavioral therapy.
Summary
PCS presents a unique therapeutic challenge affecting multiple domains for patients—physical, sleep, cognitive, and emotional. Successful management of PCS requires a multi-disciplinary and individualized approach. There remains a significant need for further research, specifically looking into the outcomes and effective interventions in pediatric PCS.
Keywords: Post-concussion syndrome, Mild traumatic brain injury, Pediatric concussion, Post-traumatic headache, Cognitive behavioral therapy
Introduction
There exists an important subset of pediatric patients with acute concussions that can develop persistent symptoms after a concussion. In children, “persistent symptoms” is generally defined as concussion symptoms lasting more than 4 weeks and may continue for longer than 3 months [1]. Estimates of the prevalence of prolonged recovery can vary from 10 to 30% depending on the patient cohort and timeframe used to define “prolonged” [2••]. Although the terminology may vary, the term “post-concussion syndrome” (PCS) is most consistently used since it accurately reflects clinical recovery outside of the expected timeframe. PCS can profoundly affect a child’s function physically, cognitively, and emotionally and presents a significant source of morbidity to patients and a management challenge to providers [3–5].
Current evidence supports that both injury-related and noninjury-related factors affect an individual patient’s recovery and long-term prognosis after a concussion [6••, 7]. As such, predicting the course and long-term outcomes in children with mild traumatic brain injury (mTBI) is complex. In addition to the specific details of the injury, other demographic and premorbid factors such as neurological or psychiatric history, learning disabilities, prior academic performance, preinjury symptoms, and psychosocial stressors, such as school-related bullying, can contribute significantly to predicting the risk of developing PCS [6••, 7]. In addition to the standard post-concussion symptom scales, the clinician should also obtain a detailed headache profile (preinjury history, type, location, severity, and family history), premorbid sleep profile, prior drug and alcohol use, and a detailed psychiatric history and consider other psychosocial stressors [2••].
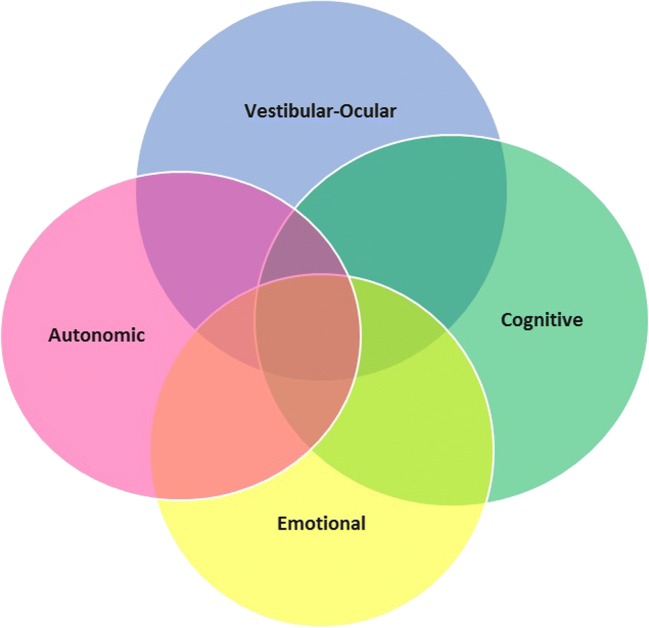
Concussion symptoms tend to cluster into four main groups. Individual patients may exhibit greater or fewer symptoms in each area (see Fig. 1). These include autonomic dysfunction (headache, sleep difficulties, light/sound sensitivity, postural hypotension), vestibular-ocular dysfunction (dizziness, poor balance, nausea, vision changes), cognitive symptoms (feeling “foggy” or slow, easily distracted, decreased memory, decreased concentration), and emotional symptoms (irritability, anxiety, moodiness, sadness) [8•]. These persistent symptoms after a concussion may also be reflective of coexisting and/or confounding pathologies that do not necessarily reflect continued physiological changes to the brain from the injury.
Fig. 1.
Clinical picture of PCS—symptoms tend to cluster in four groups
Most management approaches for PCS revolve around these four areas. Due to the variability in presentation, it can be helpful to classify different phenotypic clusters based on the involved domains (headaches, balance/vestibular dysfunction, neurocognitive, psychological) [2••]. Based on the concussion phenotype, a correlative plan can be developed, including medical management, behavioral modifications, and guidance for Return to Learn (RTL) and Return to Play (RTP).
Successful management of PCS often requires a multi-disciplinary, holistic approach focused on a neurobiopsychosocial model of mTBI. This approach is required to identify primary and secondary processes confounding recovery and to develop a corresponding, individualized treatment plan. Traditionally, management of persistent symptoms after a concussion has been an extension of the guidelines for acute injuries as there is a limited scope of evidence on treatment for PCS. The evidence and general recommendations for management of these different domains will now be reviewed.
Post-Traumatic Headaches
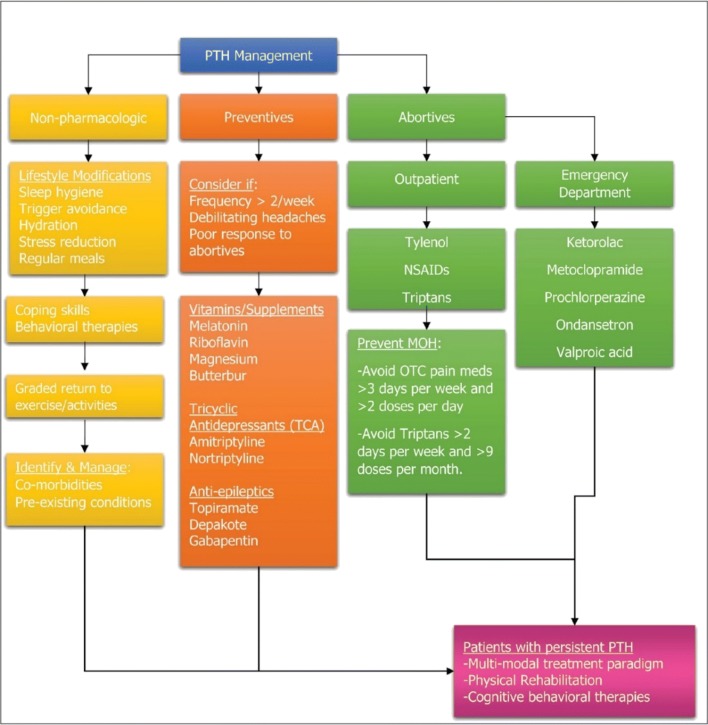
Although pharmacological interventions are widely used in the management of post-traumatic headaches (PTH) after a concussion, there is currently limited evidence supporting their use [2••]. A comprehensive summary treatment paradigm is in Fig. 2 [9••].
Fig. 2.
(Reprinted with permission from SLACK Incorporated [9••]) A multimodal treatment paradigm incorporating physical rehabilitation and cognitive behavioral therapies. NSAIDs non-steroidal anti-inflammatory drugs, PTH posttraumatic headache
PTH are one of the most common complaints in patients with persistent symptoms after an acute concussion. Persistent PTH are defined as headaches lasting more than 3 months post-injury and often present as migraine or tension-type headaches [9••, 10]. Pre-existing history or profile for migraines or mood disturbance may increase the risk of development of PTH. In addition to a detailed headache history, it is important to screen for medication overuse headaches (MOH). Up to 70% of adolescents with chronic PTH meet the criteria for MOH [11, 12]. There are no established guidelines for the management of pediatric PTH and no high-quality studies comparing medication efficacy in PTH. A systematic review published in 2012 found there was insufficient evidence to recommend any specific pharmacological intervention for PTH [13].
Management of PTH is typically extrapolated from the management of a primary headache disorder with lifestyle modifications focusing on sleep hygiene, hydration, exercise, avoidance of triggers, stress management, and use of preventative and abortive medications.
Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen or naproxen, are often initially used with variable results. If there is an insufficient response to NSAIDS and the headaches have migranous features, serotonin agonists (triptans) are often used and typically well tolerated in children [8•]. The American Academy of Neurology recommends consideration of preventative medications when headaches are occurring more frequently than twice per week.
In a retrospective review of adolescents at a regional concussion clinic, 82% of patients noted a benefit from the use of amitriptyline [14]. Some studies have shown good therapeutic benefits and long-term benefits from the use of a local anesthetic nerve block of the scalp [14–16]. There is emerging data on the use of melatonin for the treatment of persistent PTH [9••] as well as a migraine preventative [17]. Vitamin B2 (riboflavin) has been shown to be an effective prophylactic treatment for migraine headaches [18, 19], which has sometimes been extrapolated to PTH as well. Other nutraceuticals, including high-dose magnesium and coenzyme Q-10, have some evidence in prophylaxis of migraine headaches, but there are no properly designed and powered clinical trials in the pediatric concussion population [20–22]. Opiate use is not indicated for the treatment of pediatric headaches [11].
Vestibular-Ocular Dysfunction and Rehabilitation Therapy
A comprehensive visual motor assessment is a critical but often underutilized tool in the evaluation of PCS. Vestibular-ocular dysfunction is common in concussed athletes. In one study, vestibular-ocular dysfunction was found in 29% of acute sports-related concussion and in 63% of those with PCS. If vestibular-ocular dysfunction was seen in the acute phase, those children and adolescent athletes took twice as long to achieve clinical recovery and were four times as likely to develop PCS [23].
Vestibular rehabilitation therapy (VRT) can feasibly be started even in the acute phases of concussion with more and more evidence showing it can possibly reduce overall symptom duration [24•, 25]. Furthermore, VRT may be able to reduce physical and cognitive rest time and prevent the negative effects of prolonged inactivity. Female sex, preinjury depression, post-traumatic amnesia, dizziness, blurred vision, and difficulty focusing at the time of injury are independent risk factors for the development of vestibular-ocular dysfunction among children and adolescents with sports concussion [26••, 27].
To determine the need and direction of physical therapy and VRT with PCS, a thorough screening of each patient needs to be completed as cause of symptoms can vary significantly. Specifically, dizziness can be multifactorial and requires screening of all involved systems after a concussion. Certain tests may hold more value when trying to differentiate the cause of dizziness after a concussion and can provide guidance with differential diagnosis [28]. In patients who have persistent symptoms of dizziness, blurred vision, motion sensitivity, and/or balance impairments after a concussion with standard care, comprehensive screening and treatment of concussion require physical therapists with orthopedic, neurologic, and vestibular training.
Screening for vestibular-ocular dysfunction should include specific assessments including dynamic visual acuity, positional tests for peripheral vestibular dysfunction, postural stability/balance assessment, and head impulse test/head thrust. The vestibular-ocular motor screening (VOMS) allows for a comprehensive assessment of patient symptoms during performance of smooth pursuit, horizontal and vertical saccades and vestibular-ocular reflexes, normal point convergence, and visual motion sensitivity. VOMS has been found to identify athletes’ vestibular and ocular impairment from pre- to post-sports-related concussion [29]. In the pediatric population, identification of vestibular-ocular deficits is key for RTL and RTP, as children may not always be able to recognize these specific deficits and correlated symptoms. A formal balance assessment, such as the Balance Error Scoring System (BESS) or the more dynamic Functional Gait Assessment, should be part of every vestibular-ocular evaluation [30].
The cervical spine needs to be evaluated for cervicogenic causes of ongoing symptoms including dizziness, neck pain, and/or headache in PCS [31]. Even in the absence of neck pain or readily apparent cervicogenic symptoms, the neck should be screened as a cause of persistent post-concussive symptoms [32••]. These screening tests include range of motion, palpation of paraspinal muscles, segmental mobility, and stability, posture, neuromuscular control, strength of cervical muscles and scapular muscles, joint position error, vibratory testing, and other tests that look at changes in signs/symptoms when the neck position is changed or stressed.
Finally, screening should include exertional testing to identify impaired responses to physiologic stress or exertion (autonomic dysfunction). Exertional assessments can be used to track recovery after concussion and should use symptom presence and exacerbation as a guide [33•]. The Buffalo Concussion Treadmill Test is used to separate out symptoms caused by physiologic concussion (verses cervicogenic causes, for example) and may be more reliable than computerized neurocognitive testing at predicting the RTP process [34].
Treatment for PCS should specifically address exertional impairments as well as findings from the cervical spine, vestibular, and oculomotor exams and need to be tailored to each individual and guided by patient response to treatment. While the PCS examination can be standardized for patients, the corresponding treatment will vary from person to person [2••]. Referral to neuro-ophthalmology should be considered if symptoms of diplopia, severe issues with convergence/saccades/smooth pursuits and/or if ocular misalignment persists or if there is a vertical misalignment. Some visual deficits need time to heal, but if persisting longer than 4 weeks, it may be helpful to look into vision therapy (prism lenses may be necessary as well as specific vision therapy exercises).
VRT has been found to be more effective than continued cognitive and physical rest in reducing persistent symptoms of dizziness, unsteadiness, and imbalance in adolescents with persistent PCS [35]. Current evidence to quantify efficacy and optimal prescription of VRT after a concussion is limited [36••]. VRT combined with cervical spine physiotherapy was found to significantly decrease RTP time at 8 weeks compared to usual care [29]. Preliminary evidence shows that VRT may decrease perceived disability in patients with dizziness from PCS [37]. Another study found faster improvements in dizziness, balance problems, symptoms, and disability with VRT for individuals with mild-to-moderate TBI in comparison to a control group, although the effects were not significant between the groups 2 months after the intervention was stopped [38]. Although the long-term effects of early initiated VRT after concussion have not been well studied, VRT may provide earlier relief in the short term and may decrease the time until return to sport and activities.
Neurocognitive Symptoms and Return to Learn
It is firmly established within the neuropsychological literature that a single concussion does not produce long-term neuropsychological consequences in the vast majority of children and adults [39•]. The question of the long-term effects of multiple concussions on cognitive function is less clear, in part, because of a paucity of prospective studies with cohorts that are not professional contact-sport athletes. Despite that, the expectations for neuropsychological recovery following a concussion is quite positive. PCS can impact a child’s ability to engage optimally in settings of everyday life, including school [40]. Moreover, a concussion does not occur isolated from other social, health, cognitive, and behavioral challenges that exist for children and adolescents. Indeed, the risk factors for more enduring cognitive and behavioral changes following a concussion/mTBI generally reflect premorbid factors [41], such as low socioeconomic status, history of learning disability or attention-deficit hyperactivity disorder (ADHD), and mood disorders [42, 43].
Regarding specific cognitive challenges common in PCS, difficulties with attention/concentration and aspects of executive function, such as working memory (i.e., the ability to maintain, manipulate, and synthesize information held in mind) can affect academic functioning as the student transitions back into school. It should be emphasized that comprehensive neuropsychological evaluations are not necessary for the development of an RTL protocol. However, an evaluation by a neuropsychologist with expertise in concussions may help hasten the recovery process [44•]. Cognitive difficulties typically manifest in the context of other PCS symptoms, including alterations in vision, arousal, and emotion. The result can be significant, but usually time-limited in impacting academic function [40, 45]. Reassuringly, a recent population-level study in high school students did not reveal evidence of a long-term decline in academic performance over a period of a year following a concussion [46].
Although the effects on academic function after a concussion are limited, the RTL process to transition back into school remains an important focus within the broader field of pediatric concussion care. It is increasingly clear that long absences from school have a deleterious effect on children’s psychological and academic functioning. The recommendation is for earlier RTL with appropriate modifications to the environment and academic demands to provide the best likelihood of a successful transition. RTL is an individualized program that is based on various factors including symptom severity, the nature of the symptoms (e.g., vision deficit with fatigue vs. post-traumatic migraine with memory challenges), duration of the symptoms, and premorbid functioning. In addition, there is evidence that adolescents have greater challenge with RTL than younger children [47••].
Although the RTL process is less defined than RTP protocols, there are some general principles that guide the development of an RTL protocol. Effective coordination and communication among the student, the parents, school personnel, and the medical professional is critical. Often the medical professional will provide the RTL plan to the parents and one member from the school (e.g., a school nurse). The involvement of a school psychologist or social worker can also be beneficial for coordinating academic accommodations and assessing student’s adjustment especially for students with mood symptoms.
In terms of student’s readiness for attending school, it is generally expected that prior to returning to school, the injured student can manage a modest degree of cognitive activity/exertion, such as reading for 30 min. Once a student can tolerate a modest degree of cognitive stimulation, RTL emphasizes a progressive increase in the amount of time spent in school (i.e., environmental stimulation) and the amount of cognitive/learning activity required (i.e., cognitive stimulation). For example, students can initially return to half-days of school without requiring the student to complete homework or examinations and including rest breaks if needed. Additional adjustments or modifications to learning and/or environmental demands are tailored to each student based on their respective symptom presentation. As illustrated, students with vision and ocular-motor deficits typically need to avoid electronic screens, utilize regular breaks while completing visually based tasks, and benefit from audio-recorded lectures. Students with memory and attention issues may benefit from a reduction in overall work demands including homework and extended time to complete examinations until symptoms have improved. In general, only after a student has progressed to full academic participation (i.e., no modifications are in place), return to sports may be considered. Our experience, like that of others, is that students with higher symptom load and comorbid mood disorders progress more slowly to full academic participation [47••, 48•]. As the large majority of students transition back to full academic participation within a few months, considerations of more long-standing academic supports such as a 504 plan or individualized education plan (IEP) are rarely needed.
Pharmacological Treatment of Neurocognitive Symptoms
Cognitive deficits, especially in the domains of attention, concentration, and distractibility, are quite common in PCS. These symptoms do resemble those seen in ADHD and there has been some limited evidence showing the benefits of stimulants in PCS. A recent review of the literature found nine such studies with three of them focused on children. There was limited evidence that immediate release methylphenidate had some positive impact in the domains of attention, fatigue, and depression [49•]. Although not specifically a stimulant, amantadine is thought to potentiate dopamine, giving it a stimulant-like effect. One small cohort study of adolescents with PCS found that the use of amantadine improved symptoms and cognitive performance when compared to historical controls [50]. There is little to no evidence supporting the use of nutraceuticals for cognitive deficits in PCS.
Emotional Symptoms and Psychological Treatment
It has been proposed that persistent symptoms after a concussion are strongly linked to non-neurologic variables, notably issues such as anxiety and trauma, and that psychosocial interventions should therefore play a primary role in treatment [51, 52••]. Despite this, psychosocial treatments such as cognitive behavioral therapy (CBT) are under-researched and underutilized for this condition. It is hypothesized that this is due to mental health stigma, insurance constraints, overreliance on medication, lack of trained CBT providers, failure to diagnose comorbidities, and lack of physician referrals to CBT [53, 54]. However, if these psychosocial factors go untreated, youths with PCS are at risk for ongoing health problems into adulthood [39•].
There is a fund of research on the effectiveness of CBT for pediatric pain and injury for both youths and adults [55•, 56]. CBT has been shown to reduce pain frequency and severity, reduce associated stress, anxiety and depression, improve sleep, and improve functioning across domains, including school and physical activity [57, 58]. Recent research specifically supports the effectiveness of CBT for pediatric headache [59, 60]. As headaches are a predominant symptom in pediatric PCS, behavioral treatments may enhance or replace pharmacotherapy, with the advantage of eliminating dangerous side effects and reducing costs [61•]. As CBT targets both physical and emotional components of PCS, it is a particularly robust intervention, as premorbid and comorbid issues like anxiety and poor emotional awareness are shown to maintain and prolong PCS symptoms [2••, 62]. These confounding issues may create a psychological predisposition for the development of persistent PCS symptoms after an mTBI [63].
While research on CBT for PCS is limited, particularly for children, existing research is promising. Review articles have demonstrated that CBT had consistently positive results in PCS [64••, 65]. One study showed that CBT delivered soon after a concussion is well tolerated, may reduce PCS incidence, and can facilitate PCS recovery [66]. A randomized controlled trial comparing CBT to “usual care” for adolescent PCS patients determined that adolescents in the CBT group had clinically and statistically significant improvements in PCS symptoms, reduced depressive symptoms, and more functional gains at 6 months compared with the usual care group [67]. Other studies have shown that a CBT protocol in youth with PCS resulted in improved ability to tolerate physical exertion, decreased psychological distress and symptoms, and a return to functioning [68, 69].
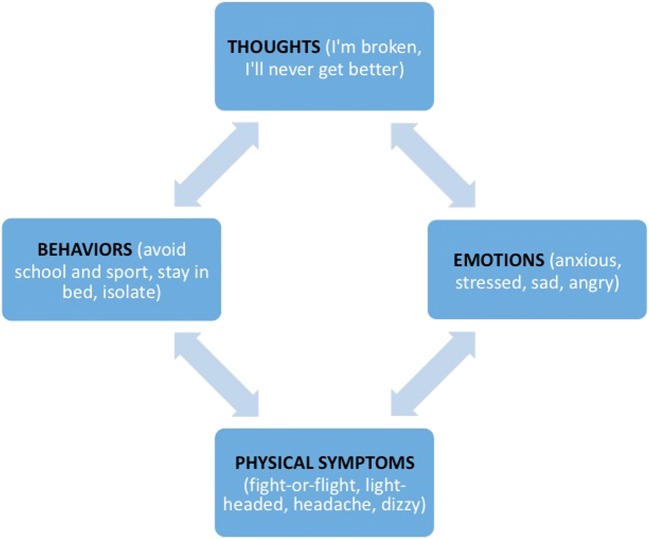
CBT is rooted in the premise that cognition, emotions, physical symptoms, and behaviors all affect one another in a multi-directional, interactive cycle (see Fig. 3). For example, injured youths often engage in catastrophic thoughts such as, “I’m broken, I’ll never get better.” Fear of permanent brain damage not only exacerbates and maintains acute symptoms [70], but these cognitive distortions also trigger depression, stress, and anxiety, which activate the “fight-or-flight” response. This results in physical symptoms such as light-headedness, dizziness, and headache, triggering and exacerbating existing PCS symptoms [59]. Negative thoughts and feelings subsequently result in fear-based coping behaviors, such as avoiding sport and physical activity, isolating, and missing school [71]. Avoidance predicts symptom severity, disability, and depression [72]. As youths fall behind, become isolated, and miss sport and social functions, they understandably become even more stressed, sad, and anxious, which triggers additional somatic symptoms and maladaptive coping behaviors [73]. Thus, the cycle continues until broken. It is therefore important to normalize, treat, and not stigmatize the emotional impact of PCS on youths.
Fig. 3.
The CBT cycle of pediatric PCS
CBT teaches youths to break the PCS cycle by reducing negative cognitions, transforming negative emotions, and implementing healthier coping skills. Standard PCS CBT protocols typically include psychoeducation (e.g., reassurance, prognosis) and affect education (e.g., learning the relationship between stress and physical symptoms), relaxation strategies, mindfulness, biofeedback, imagery, distraction strategies, cognitive restructuring (e.g., reattribution of symptoms to benign causes, reducing catastrophic predictions), and “pacing” for return to school and play [70, 74–76]. CBT should only be administered by trained therapists with appropriate educational backgrounds (MSW, MFT, PsyD, PhD). Parent sessions are also considered an important component of CBT for pediatric health conditions and may be considered as needed [77, 78].
One significant issue in the PCS literature is that there is no single, uniform CBT treatment protocol. Historically, various CBT manuals have been utilized in PCS studies with evidence of success [74, 79, 80]. However, clinical trials and outpatient CBT treatments continue to differ in the protocol, duration, and intensity of treatment, modality (group vs individual), therapist training and background, and skills taught. As such, the definition of “CBT treatment” varies from study to study and provider to provider. Nevertheless, the potential for CBT to target premorbid, comorbid, and active PCS symptoms—in concert with medical interventions—makes it an important candidate for continued utilization and research.
Pharmacological Treatment of Emotional Symptoms
In addition to appropriate psychotherapy including CBT, some patients may benefit from pharmacological management of mood symptoms. It is critical to gather a thorough psychiatric history including prior psychiatric diagnosis and treatments, family history, and current symptoms. It is also important to separate severity of symptoms from a normal response to a stressor, such as having a phobia of driving a car after a motor-vehicle accident to a psychiatric DSM diagnosis such as post-traumatic stress disorder. Tools, such as the Beck Depression Inventory-II, Patient Health Questionnaire, or Brief Symptom Inventory-18, may help to define prior history of depression, anxiety, and somatization affecting protracted recovery after a concussion [2].
Antidepressants, specifically selective serotonin reuptake inhibitors (SSRIs), have become a primary treatment for concussion-related mood symptoms because of perceived clinical efficacy and relatively few side effects. The evidence is fairly limited and mostly extrapolated from a more severe TBI population. There is some evidence that SSRIs reduced depression symptoms and cognitive impairments after mTBI [81, 82]. Other antidepressants, including those with a mixture of effects on serotonin, norepinephrine, and dopamine, have little to no evidence for treatment of post-concussion depression [83]. In addition to the prior-mentioned melatonin, Trazodone is often used in the brain-injured population to treat sleep disorders in the acute phase after TBI [84].
Conclusions
PCS presents a unique therapeutic challenge affecting multiple domains for patients—autonomic, vestibular-ocular, cognitive, and emotional. The most effective treatment for PCS is a multi-disciplinary, biopsychosocial approach in which three different but equally important domains are considered: biology (sex, age, genetics, etiology, medications, sleep, diet, etc.), psychological functioning (thoughts about injury and pain, emotional functioning, coping behaviors), and social functioning (effects of parenting, isolation vs support, school attendance, sports, etc.). Successful management of PCS requires a multi-disciplinary and individualized approach that focuses on the specific phenotype of symptoms whether predominantly autonomic dysfunction, vestibular-ocular dysfunction, cognitive difficulties, psychological symptoms, and, ideally, a combination of these clusters. In addition to medical management, patients may benefit from the inclusion of other disciplines including vestibular rehabilitation, neuropsychological supports and/or cognitive behavioral therapy. There remains a significant need for further research, specifically looking into outcomes and effective interventions including pharmaceutical options in PCS.
Conflict of Interest
Mitul Kapadia, Alison Scheid, Eric Fine, and Rachel Zoffness each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Concussion
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Babcock L, Byczkowski T, Wade SL, Ho M, Mookerjee S, Bazarian JJ. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013;167(2):156–161. doi: 10.1001/jamapediatrics.2013.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makdissi M, Schneider KJ, Feddermann-Demont N, Guskiewicz KM, Hinds S, Leddy JJ, McCrea M, Turner M, Johnston KM. Approach to investigation and treatment of persistent symptoms following sport-related concussion: a systematic review. Br J Sports Med. 2017;51(12):958–968. doi: 10.1136/bjsports-2016-097470. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma. 2008;25(9):1049–1056. doi: 10.1089/neu.2008.0566. [DOI] [PubMed] [Google Scholar]
- 4.Hessen E, Nestvold K. Indicators of complicated mild TBI predict MMPI-2 scores after 23 years. Brain Inj. 2009;23(3):234–242. doi: 10.1080/02699050902748349. [DOI] [PubMed] [Google Scholar]
- 5.Dikmen S, Machamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. J Int Neuropsychol Soc. 2010;16(3):401–411. doi: 10.1017/S1355617710000196. [DOI] [PubMed] [Google Scholar]
- 6.Lumba-Brown A, Yeates KO, Sarmiento K, Breiding MJ, Haegerich TM, Gioia GA, Turner M, Benzel EC, Suskauer SJ, Giza CC, Joseph M. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;4:e182853. doi: 10.1001/jamapediatrics.2018.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCrea M, Manley G. State of the science on pediatric mild traumatic brain injury: progress toward clinical translation. JAMA Pediatr. 2018;4:e182846. doi: 10.1001/jamapediatrics.2018.2846. [DOI] [PubMed] [Google Scholar]
- 8.Grady MF, Master CL. Return to school and learning after concussion: tips for pediatricians. Pediatr Ann. 2017;46(3):e93–e98. doi: 10.3928/19382359-20170220-04. [DOI] [PubMed] [Google Scholar]
- 9.Langdon R, Taraman S. Posttraumatic headache. Pediatr Ann. 2018;47(2):e61–e68. doi: 10.3928/19382359-20180131-01. [DOI] [PubMed] [Google Scholar]
- 10.Kuczynski A, Crawford S, Bodell L, Dewey D, Barlow KM. Characteristics of post-traumatic headaches in children following mild traumatic brain injury and their response to treatment: a prospective cohort. Dev Med Child Neurol. 2013;55(7):636–641. doi: 10.1111/dmcn.12152. [DOI] [PubMed] [Google Scholar]
- 11.Pinchefsky E, Dubrovsky AS, Friedman D, Shevell M. Part I—evaluation of pediatric post-traumatic headaches. Pediatr Neurol. 2015;52(3):263–269. doi: 10.1016/j.pediatrneurol.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Heyer GL, Idris SA. Does analgesic overuse contribute to chronic post-traumatic headaches in adolescent concussion patients? Pediatr Neurol. 2014;50(5):464–468. doi: 10.1016/j.pediatrneurol.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe TK, Bell KR, Walker WC, Schomer K. Systematic review of interventions for post-traumatic headache. PM&R. 2012;4(2):129–140. doi: 10.1016/j.pmrj.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Dubrovsky AS, Friedman D, Kocilowicz H. Pediatric post-traumatic headaches and peripheral nerve blocks of the scalp: a case series and patient satisfaction survey. Headache. 2014;54(5):878–887. doi: 10.1111/head.12334. [DOI] [PubMed] [Google Scholar]
- 15.Seeger TA, Orr S, Bodell L, Lockyer L, Rajapakse T, Barlow KM. Occipital nerve blocks for pediatric posttraumatic headache: a case series. J Child Neurol. 2015;30(9):1142–1146. doi: 10.1177/0883073814553973. [DOI] [PubMed] [Google Scholar]
- 16.Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Curran C, Ng K. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–1303. doi: 10.1542/peds.108.6.1297. [DOI] [PubMed] [Google Scholar]
- 17.Gonçalves AL, Ferreira AM, Ribeiro RT, Zukerman E, Cipolla-Neto J, Peres MF. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;10:jnnp-2016. doi: 10.1136/jnnp-2016-313458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson DF, Saluja HS. Prophylaxis of migraine headaches with riboflavin: a systematic review. J Clin Pharm Ther. 2017;42(4):394–403. doi: 10.1111/jcpt.12548. [DOI] [PubMed] [Google Scholar]
- 19.Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhäupl KM, Arnold G. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11(7):475–477. doi: 10.1111/j.1468-1331.2004.00813.x. [DOI] [PubMed] [Google Scholar]
- 20.Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence-based rationale? A systematic review. Headache. 2018;58(2):199–209. doi: 10.1111/head.13217. [DOI] [PubMed] [Google Scholar]
- 21.Shoeibi A, Olfati N, Sabi MS, Salehi M, Mali S, Oryani MA. Effectiveness of coenzyme Q10 in prophylactic treatment of migraine headache: an open-label, add-on, controlled trial. Acta Neurol Belg. 2017;117(1):103–109. doi: 10.1007/s13760-016-0697-z. [DOI] [PubMed] [Google Scholar]
- 22.Trojian TH, Wang DH, Leddy JJ. Nutritional supplements for the treatment and prevention of sports-related concussion—evidence still lacking. Curr Sports Med Rep. 2017;16(4):247–255. doi: 10.1249/JSR.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 23.Ellis MJ, Cordingley D, Vis S, Reimer K, Leiter J, Russell K. Vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr. 2015;16(3):248–255. doi: 10.3171/2015.1.PEDS14524. [DOI] [PubMed] [Google Scholar]
- 24.Lennon A, Hugentobler JA, Sroka MC, Nissen KS, Kurowski BG, Gagnon I, Quatman-Yates CC. An exploration of the impact of initial timing of physical therapy on safety and outcomes after concussion in adolescents. J Neurol Phys Ther. 2018;42(3):123–131. doi: 10.1097/NPT.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reneker JC, Hassen A, Phillips RS, Moughiman MC, Donaldson M, Moughiman J. Feasibility of early physical therapy for dizziness after a sports-related concussion: a randomized clinical trial. Scand J Med Sci Sports. 2017;27(12):2009–2018. doi: 10.1111/sms.12827. [DOI] [PubMed] [Google Scholar]
- 26.Ellis MJ, Cordingley DM, Vis S, Reimer KM, Leiter J, Russell K. Clinical predictors of vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr. 2017;19(1):38–45. doi: 10.3171/2016.7.PEDS16310. [DOI] [PubMed] [Google Scholar]
- 27.Teel EF, Marshall SW, Shankar V, McCrea M, Guskiewicz KM. Predicting recovery patterns after sport-related concussion. J Athl Train. 2017;52(3):288–298. doi: 10.4085/1062-6050-52.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reneker JC, Moughiman MC, Cook CE. The diagnostic utility of clinical tests for differentiating between cervicogenic and other causes of dizziness after a sports-related concussion: an international Delphi study. J Sci Med Sport. 2015;18(4):366–372. doi: 10.1016/j.jsams.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Schneider KJ, Meeuwisse WH, Nettel-Aguirre A, Barlow K, Boyd L, Kang J, Emery CA. Cervicovestibular rehabilitation in sport-related concussion: a randomised controlled trial. Br J Sports Med. 2014;48(17):1294–1298. doi: 10.1136/bjsports-2013-093267. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy E, Quinn D, Tumilty S, Chapple CM. Clinical characteristics and outcomes of treatment of the cervical spine in patients with persistent post-concussion symptoms: a retrospective analysis. Musculoskelet Sci Pract. 2017;29:91–98. doi: 10.1016/j.msksp.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Elbin RJ, Sufrinko A, Anderson MN, Mohler S, Schatz P, Covassin T, Mucha A, Collins MW, Kontos AP. Prospective changes in vestibular and ocular motor impairment after concussion. J Neurol Phys Ther. 2018;42(3):142–148. doi: 10.1097/NPT.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storey EP, Wiebe DJ, D’Alonzo BA, Nixon-Cave K, Jackson-Coty J, Goodman AM, Grady MF, Master CL. Vestibular rehabilitation is associated with visuovestibular improvement in pediatric concussion. J Neurol Phys Ther. 2018;42(3):134–141. doi: 10.1097/NPT.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 33.• Quatman-Yates C, Bailes A, Constand S, Sroka MC, Nissen K, Kurowski B, et al. Exertional tolerance assessments after mild traumatic brain injury: a systematic review. Archives of physical medicine and rehabilitation. 2017;23. A review of exertional assessments after concussion. [DOI] [PMC free article] [PubMed]
- 34.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Curr Sports Med Rep. 2013;12(6):370–376. doi: 10.1249/JSR.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 35.Park K, Ksiazek T, Olson B. Effectiveness of vestibular rehabilitation therapy for treatment of concussed adolescents with persistent symptoms of dizziness and imbalance. J Sport Rehabil. 2018;20(XX):1–6. doi: 10.1123/jsr.2016-0222. [DOI] [PubMed] [Google Scholar]
- 36.Murray DA, Meldrum D, Lennon O. Can vestibular rehabilitation exercises help patients with concussion? A systematic review of efficacy, prescription and progression patterns. Br J Sports Med. 2017;51(5):442–451. doi: 10.1136/bjsports-2016-096081. [DOI] [PubMed] [Google Scholar]
- 37.Nagib S, Linens SW. Vestibular rehabilitation therapy improves perceived disability associated with dizziness post-concussion. J Sport Rehabil. 2018:1–5. [DOI] [PubMed]
- 38.Kleffelgaard I, Soberg HL, Tamber AL, Bruusgaard KA, Pripp AH, Sandhaug M, Langhammer B. The effects of vestibular rehabilitation on dizziness and balance problems in patients after traumatic brain injury: a randomized controlled trial. Clin Rehabil. 2018;30:0269215518791274. doi: 10.1177/0269215518791274. [DOI] [PubMed] [Google Scholar]
- 39.Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M, Jordan BD, Randolph C, Dvořák J. A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med. 2017;51(12):969–977. doi: 10.1136/bjsports-2017-097791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak Z, Aglipay M, Barrowman N, Yeates KO, Beauchamp MH, Gravel J, Freedman SB, Gagnon I, Gioia G, Boutis K, Burns E. Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatr. 2016;170(12):e162900. doi: 10.1001/jamapediatrics.2016.2900. [DOI] [PubMed] [Google Scholar]
- 41.Babikian T, Merkley T, Savage RC, Giza CC, Levin H. Chronic aspects of pediatric traumatic brain injury: review of the literature. J Neurotrauma. 2015;32(23):1849–1860. doi: 10.1089/neu.2015.3971. [DOI] [PubMed] [Google Scholar]
- 42.Iverson GL, Silverberg ND, Mannix R, Maxwell BA, Atkins JE, Zafonte R, Berkner PD. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132–1140. doi: 10.1001/jamapediatrics.2015.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terry DP, Brassil M, Iverson GL, Panenka WJ, Silverberg ND. Effect of depression on cognition after mild traumatic brain injury in adults. Clin Neuropsychol. 2018;4:1–3. doi: 10.1080/13854046.2018.1459853. [DOI] [PubMed] [Google Scholar]
- 44.Kirkwood MW, Peterson RL, Connery AK, Baker DA, Forster J. A pilot study investigating neuropsychological consultation as an intervention for persistent postconcussive symptoms in a pediatric sample. J Pediatr. 2016;169:244–249. doi: 10.1016/j.jpeds.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Swanson MW, Weise KK, Dreer LE, Johnston J, Davis RD, Ferguson D, Hale MH, Gould SJ, Christy JB, Busettini C, Lee SD. Academic difficulty and vision symptoms in children with concussion. Optom Vis Sci. 2017;94(1):60–67. doi: 10.1097/OPX.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell K, Hutchison MG, Selci E, Leiter J, Chateau D, Ellis MJ. Academic outcomes in high-school students after a concussion: a retrospective population-based analysis. PLoS One. 2016;11(10):e0165116. doi: 10.1371/journal.pone.0165116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purcell LK, Davis GA, Gioia GA. What factors must be considered in ‘return to school’ following concussion and what strategies or accommodations should be followed? A systematic review. Br J Sports Med. 2018;2:bjsports-2017. doi: 10.1136/bjsports-2017-097853. [DOI] [PubMed] [Google Scholar]
- 48.Iverson GL, Gardner AJ, Terry DP, Ponsford JL, Sills AK, Broshek DK, Solomon GS. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941–948. doi: 10.1136/bjsports-2017-097729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laccarino MA, Philpotts LL, Zafonte R, Biederman J. Stimulant use in the management of mild traumatic brain injury: a qualitative literature review. J Attent Disord. 2018;1:1087054718759752. doi: 10.1177/1087054718759752. [DOI] [PubMed] [Google Scholar]
- 50.Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil. 2013;28(4):260–265. doi: 10.1097/HTR.0b013e318257fbc6. [DOI] [PubMed] [Google Scholar]
- 51.Brown EA, Kenardy JA, Dow BL. PTSD perpetuates pain in children with traumatic brain injury. J Pediatr Psychol. 2014;39(5):512–520. doi: 10.1093/jpepsy/jsu014. [DOI] [PubMed] [Google Scholar]
- 52.Jak A. The Primary role of mental health treatment in resolution of persistent post-concussive symptoms. Curr Treat Options Psychiatry. 2017;4(3):231–240. [Google Scholar]
- 53.Simons LE, Logan DE, Chastain L, Cerullo M. Engagement in multidisciplinary interventions for pediatric chronic pain: parental expectations, barriers, and child outcomes. Clin J Pain. 2010;26(4):291–299. doi: 10.1097/AJP.0b013e3181cf59fb. [DOI] [PubMed] [Google Scholar]
- 54.Wakefield EO, Jerson B. Social influences in the evaluation and treatment of biopsychosocial contributors to adolescent chronic pain. Topics Pain Manag. 2017;32(9):1–8. [Google Scholar]
- 55.Darnall BD, Carr DB, Schatman ME. Pain psychology and the biopsychosocial model of pain treatment: ethical imperatives and social responsibility. Pain Med. 2017;18(8):1413–1415. doi: 10.1093/pm/pnw166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eccleston C, Palermo TM, de C Williams AC, Holley AL, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2014;5(5):CD003968. doi: 10.1002/14651858.CD003968.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher E, Heathcote LC, Eccleston C, Simons LE, Palermo TM. Assessment of pain anxiety, pain catastrophizing, and fear of pain in children and adolescents with chronic pain: a systematic review and meta-analysis. J Pediatr Psychol. 2017;43(3):314–325. doi: 10.1093/jpepsy/jsx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coakley R, Wihak T. Evidence-based psychological interventions for the management of pediatric chronic pain: new directions in research and clinical practice. Child Aust. 2017;4(2):9. doi: 10.3390/children4020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bougea A, Spantideas N, Chrousos GP. Stress management for headaches in children and adolescents: a review and practical recommendations for health promotion programs and well-being. J Child Health Care. 2018;22(1):19–33. doi: 10.1177/1367493517738123. [DOI] [PubMed] [Google Scholar]
- 60.Powers SW, Kashikar-Zuck SM, Allen JR, LeCates SL, Slater SK, Zafar M, Kabbouche MA, O’Brien HL, Shenk CE, Rausch JR, Hershey AD. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA. 2013;310(24):2622–2630. doi: 10.1001/jama.2013.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faedda N, Cerutti R, Verdecchia P, Migliorini D, Arruda M, Guidetti V. Behavioral management of headache in children and adolescents. J Headache Pain. 2016;17(1):80. doi: 10.1186/s10194-016-0671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brent DA, Max J. Psychiatric sequelae of concussions. Curr Psychiatry Rep. 2017;19(12):108. doi: 10.1007/s11920-017-0862-y. [DOI] [PubMed] [Google Scholar]
- 63.Wood RL, O’Hagan G, Williams C, McCabe M, Chadwick N. Anxiety sensitivity and alexithymia as mediators of postconcussion syndrome following mild traumatic brain injury. J Head Trauma Rehabil. 2014;29(1):E9–17. doi: 10.1097/HTR.0b013e31827eabba. [DOI] [PubMed] [Google Scholar]
- 64.Thomas RE, Alves J, Mlis V, Marcus M, Magalhaes R. Therapy and rehabilitation of mild brain injury/concussion: systematic review. Restor Neurol Neurosci. 2017;35(6):643–666. doi: 10.3233/RNN-170761. [DOI] [PubMed] [Google Scholar]
- 65.Al Sayegh A, Sandford D, Carson AJ. Psychological approaches to treatment of postconcussion syndrome: a systematic review. J Neurol Neurosurg Psychiatry. 2010;81(10):1128–1134. doi: 10.1136/jnnp.2008.170092. [DOI] [PubMed] [Google Scholar]
- 66.Silverberg ND, Hallam BJ, Rose A, Underwood H, Whitfield K, Thornton AE, Whittal ML. Cognitive-behavioral prevention of postconcussion syndrome in at-risk patients: a pilot randomized controlled trial. J Head Trauma Rehabil. 2013;28(4):313–322. doi: 10.1097/HTR.0b013e3182915cb5. [DOI] [PubMed] [Google Scholar]
- 67.McCarty CA, Zatzick D, Stein E, Wang J, Hilt R, Rivara FP. Collaborative care for adolescents with persistent postconcussive symptoms: a randomized trial. Pediatrics. 2016;13:e20160459. doi: 10.1542/peds.2016-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh S, Polster D, Choe M, Giza C, Babikian T. Treating prolonged post-concussion symptoms in a pediatric patient with the simultaneous application of cognitive behavioral therapy and subthreshold exercise: a case study. Neurology. 2018;90(15 Supplement):6.318. [Google Scholar]
- 69.McNally KA, Patrick KE, LaFleur JE, Dykstra JB, Monahan K, Hoskinson KR. Brief cognitive behavioral intervention for children and adolescents with persistent post-concussive symptoms: a pilot study. Child Neuropsychol. 2018;24(3):396–412. doi: 10.1080/09297049.2017.1280143. [DOI] [PubMed] [Google Scholar]
- 70.Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829–836. doi: 10.1076/jcen.23.6.829.1022. [DOI] [PubMed] [Google Scholar]
- 71.Keightley ML, Côté P, Rumney P, Hung R, Carroll LJ, Cancelliere C, Cassidy JD. Psychosocial consequences of mild traumatic brain injury in children: results of a systematic review by the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95(3):S192–S200. doi: 10.1016/j.apmr.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 72.Silverberg ND, Panenka W, Iverson GL. Fear avoidance and clinical outcomes from mild traumatic brain injury. J Neurotrauma. 2018;18(ja). [DOI] [PubMed]
- 73.van der Naalt J, Timmerman ME, de Koning ME, van der Horn HJ, Scheenen ME, Jacobs B, Hageman G, Yilmaz T, Roks G, Spikman JM. Early predictors of outcome after mild traumatic brain injury (UPFRONT): an observational cohort study. Lancet Neurol. 2017;16(7):532–540. doi: 10.1016/S1474-4422(17)30117-5. [DOI] [PubMed] [Google Scholar]
- 74.Ferguson RJ, Mittenberg W. Sourcebook of psychological treatment manuals for adult disorders. Boston: Springer; 1996. Cognitive-behavioral treatment of postconcussion syndrome; pp. 615–655. [Google Scholar]
- 75.Palermo TM. Cognitive-behavioral therapy for chronic pain in children and adolescents: Oxford University Press; 2012.
- 76.Van Heugten C, Renaud I, Resch C. The role of early intervention in improving the level of activities and participation in youths after mild traumatic brain injury: a scoping review. Concussion. 2017;2(3):CNC38. doi: 10.2217/cnc-2016-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palermo TM, Valrie CR, Karlson CW. Family and parent influences on pediatric chronic pain: a developmental perspective. Am Psychol. 2014;69(2):142–152. doi: 10.1037/a0035216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rook S, Gauntlett-Gilbert J. Parent involvement in pediatric pain interventions. Pediatr Pain Lett. 2016;18:9–13. [Google Scholar]
- 79.Mittenberg W, Zielinski R, Fichera S. Recovery from mild head injury: a treatment manual for patients. Psychother Priv Pract. 1993;12(2):37–52. [Google Scholar]
- 80.Potter S, Brown RG. Cognitive behavioural therapy and persistent post-concussional symptoms: integrating conceptual issues and practical aspects in treatment. Neuropsychol Rehabil. 2012;22(1):1–25. doi: 10.1080/09602011.2011.630883. [DOI] [PubMed] [Google Scholar]
- 81.Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12(2):226–232. doi: 10.1176/jnp.12.2.226. [DOI] [PubMed] [Google Scholar]
- 82.Zasler ND. Mild traumatic brain injury: medical assessment and intervention. J Head Trauma Rehabil. 1993;8:13–29. [Google Scholar]
- 83.Willer B, Leddy JJ. Management of concussion and post-concussion syndrome. Curr Treat Options Neurol. 2006;8(5):415–426. doi: 10.1007/s11940-006-0031-9. [DOI] [PubMed] [Google Scholar]
- 84.Zafonte RD, Cullen N, Lexell J. Serotonin agents in the treatment of acquired brain injury. J Head Trauma Rehabil. 2002;17(4):322–334. doi: 10.1097/00001199-200208000-00006. [DOI] [PubMed] [Google Scholar]