Abstract
The American cancer survivor population is ever-growing, with necessary follow-up primarily accomplished in a high-touch fashion— adding to unsustainability and fragmentation of care. Given the complexities of the health care system processes needed to support survivorship, engineering approaches may best address performance deficits and facilitate the provision of patient-centered care. Such collaboration between health care and engineering is recommended for redesigning health care delivery systems. By using Systems Engineering Initiative for Patient Safety (SEIPS), a systems engineering model widely used to improve health care quality and delivery, the authors examine the work system to identify the barriers and facilitators to necessary care in the presence of a survivorship care plan and visit. Recommendations for future improvement include ensuring that care-planning processes are dynamic, clearly assigned, resilient, and integrated with electronic health record systems.
Keywords: cancer survivorship, care planning, human factors engineering, population health management, survivorship care plans (SCPs), Systems Engineering Initiative for Patient Safety (SEIPS)
BACKGROUND
By 2040, the population of American cancer survivors will have climbed to 26.1 million (based on the definition of an individual from the time of diagnosis through the balance of his or her life). Most of these individuals live 5 or more years after cancer diagnosis.1,2 Maintaining their health requires communication and coordination among and between specialists, primary care, and survivors to deliver necessary preventive and supportive care.3,4 Ongoing survivorship care can be fragmented by inadequate communication and care coordination processes.5,6 This fragmentation contributes to inferior health outcomes stemming from a failure to provide necessary care and the provision of unnecessary or duplicative services.7 To improve communication and coordination, the Institute of Medicine (IOM), previously recommended that oncology teams routinely prepare individualized survivorship care plans (SCPs) to guide care decisions beyond primary, active treatment.6
In the decade and more after the IOM recommendation, randomized trial evidence for the provision of SCPs has remained mixed, with both positive and negative outcomes,8–14 whereas the adoption of SCPs and care-planning processes remains low. Estimates vary, but most survivors and primary care providers (PCPs) report not receiving SCPs.15–17 Similarly, most oncology practices report not providing SCPs, although these data may not reflect the impact of changing national guidelines.15,16 Nevertheless, multiple professional and accrediting societies recommend SCP provision,18–20 whereas survivors and PCPs indicate a desire to receive the information within SCPs.21,22
At an individual level, care planning includes the coordination of necessary care between specialists and primary care with survivor engagement and empowerment, hopefully assisted by an SCP and a care-planning visit. At the population level, case managers, navigators, quality officers, and administrators may track outcomes across all survivors23 within and across health care systems.24 However, existing care-planning processes are fraught with health care system barriers to adoption, implementation, and maintenance.25,26 The IOM and the National Academy of Engineering recommend using systems engineering principles and tools to improve health care delivery, quality, and safety.27,28 For survivorship population health management, this recommendation means using systems engineering approaches to identify, develop, and sustain best practices informed by the needs of survivors, caregivers, clinicians, organizations, and communities.29 Ideally, these best practices start “upstream” at diagnosis to capture pertinent data in real time and include all survivors at a system or organizational level.
CURRENT PROCESSES: WHAT DO WE KNOW?
National accreditation standards are a major factor motivating many US hospitals and practices toward the development and delivery of SCPs,20 although meeting these standards has been challenging.30,31 Details are lacking about how organizations go about providing SCPs, care-planning visits, and other follow-up processes.32 Metrics regarding the impact of different processes are lacking, partly because of difficulties collecting and combining longitudinal follow-up from multiple sources, such as the electronic health record (EHR), billing or claims data, patient-reported outcomes, etc.
Currently, SCPs and their corresponding survivorship visits are delivered in diverse ways. Key data to include are well defined,33 but the tools for preparing SCPs differ. Some SCPs use manual abstraction and data transfer between the medical record into survivorship software (eg, Journey Forward [Canton, MA], Passport for Care),34,35 whereas others auto-populate SCPs with EHR data36 or extract such data to separate web platforms and return prepared SCPs into the EHR as static documents.37 More complexity arises because the individuals delivering SCP and follow-up care also vary. Individual(s) who prepare and deliver SCPs may not be member(s) of the care team or plan to participate in follow-up care.36 Care planning may be distinct, stand-alone visits (whether face-to-face vs phone) or incorporated into routine clinical visits.36,38 Finally, responsibilities for follow-up tasks are variously delineated, with potentially oncologists, primary care, survivor, or caregiver ensuring that the recommended care is accomplished. How can we use a systems engineering approach to re-engineer the survivorship care processes that are needed to provide comprehensive population management of cancer survivors?
MASTERING COMPLEXITY: A SYSTEMS ENGINEERING APPROACH
A particularly pertinent systems engineering approach to tackling health care complexity is the Systems Engineering Initiative for Patient Safety (SEIPS) model illustrated in Figure 1.39,40 SEIPS integrates the well known health care quality model of structure-process-outcome published by Donabedian to evaluate how the work system structure affects care processes and outcomes.41,42 SEIPS has been used by health care researchers, professionals, and educators in both inpatient and outpatient settings to improve health outcomes, such as patient safety, quality of care delivery, transitions of care and coordination, usability, and implementation of health information technology, as well as managing a variety of health care activities, such as infection control, surgical readmissions, primary care workflows, and decision support.43–51 The SEIPS model provides a framework for evaluating the complex interplay of work system factors that influence care processes and impact outcomes within the survivorship context. The work system can be conceptualized as a collection of elements: persons with roles and responsibilities (eg, survivors, oncologist, PCPs) performing tasks (eg, ordering a test) while using various tools/technologies (eg, EHR, decision support) in an environment (eg, office, clinician workroom, home) and an organizational context (eg, practice, health care system). For instance, a typical survivorship process includes an oncologist (person) searching for information (task) within the EHR (technology) in an outpatient oncology office (environment) that is part of a larger oncology practice (organization). Collectively, work system elements facilitate or impede the clinical processes of providing care for survivors, which subsequently affects patient and health care quality outcomes. By understanding the work system elements, the design and integration of tasks, technology, and clinical processes can be designed to better support the respective needs of individuals while optimizing system performance.36–40
Figure 1.
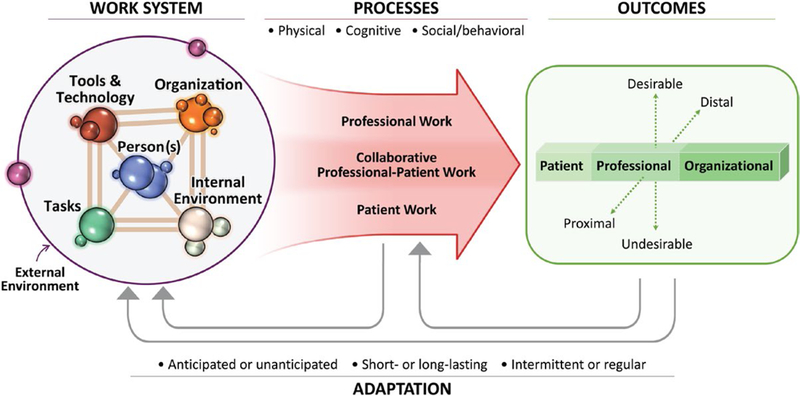
This is the Systems Engineering Initiative for Patient Safety (SEIPS) 2.0 Model. Reprinted with permission from: Holden RJ, Carayon P, Gurses AP et al SEIPS 2.0: a human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics. 2013;56:1669–1686.
To deliver efficient and effective survivorship care, work system barriers to care delivery processes must be identified and mitigated. Similarly, work system facilitators must be identified and supported. As applied to cancer survivorship, a key strength of SEIPS is the understanding that professional work, collaborative work, and patient work all contribute to survivorship care. Survivorship examples of these concepts of work include: 1) professional work (primary care using an SCP to inform clinical decision making), 2) shared work (communication between clinicians or shared decision making between clinician and survivor), and 3) survivor work (management of activity and diet as a contribution of health maintenance or undergoing recommended cancer screening). Work may need to be well delineated versus shared and equally actionably between multiple individuals. Health care organizations can use the SEIPS model as a foundation to further refine and adapt their individualized needs and resources to: 1) describe the work system elements; 2) identify barriers and facilitators that influence care processes; 3) integrate patient and organizational outcomes; and 4) provide feedback between the work system, processes, and measures of the desired outcomes.
Examining the Survivorship Work System
The survivorship vignette (Fig. 2) demonstrates the application of SEIPS. For this vignette, we will identify an element that is likely to have the greatest impact as a barrier or facilitator. However, a barrier or facilitator may be influenced by more than 1 element because of interactions between work systems.
Figure 2.

This is a survivorship vignette. PCP indicates primary care provider; SCP, survivorship care plan.
Person
A person, such as a clinician, survivor, or caregiver, is placed in the center of the work system in the SEIPS model. Ideally, the work system is designed to facilitate performance by this individual, whether survivor or clinician, as well as to minimize negative effects such as work stress, errors, or poor health outcomes. This focus on the person, or user-centeredness, can improve individual performance and subsequent outcomes.52 Because multiple individuals are doing work in the context of survivorship care planning, we need to examine the work system from each perspective. In this vignette, Ms. J was aware that she needed to share updated family history information with her providers ( facilitator). She assumed that, if she told her PCP, then her oncologist would receive the information, but the oncology and primary care teams do not share the same EHR (barrier), and the new family history did not seem urgent enough to warrant a call. The SCP does not contain information about anastrozole or bone-density screening, because this was an interceding change in care (barrier).
Tasks
Survivorship care processes comprise a series of tasks performed by clinicians, survivors, and/or caregivers, either individually or as a team. Work systems factors, such as the organization, environment, or technology, impact an individual’s or team’s ability to complete tasks and influence the workload required to complete tasks and achieve desired outcomes. Survivor engagement is vital: survivors should be at the center of the care team, with care personalized to the individual’s needs, values, and preferences to maximize this engagement. In this vignette, the oncologist could not monitor surveillance tasks without regularly seeing the survivor (barrier). Formal feedback loops to ensure completion of tests rarely exist across health care organizations (barrier). Even within an organization, screening tasks may rely on survivor prompting of clinicians ( facilitator) or clinician assessment during follow-up ( facilitator). Multiple clinicians on the oncology and primary care teams may spend time monitoring ( facilitator), using resources that may not be sufficient given projected workforce shortages (barrier). No feedback exists for the oncologist or survivor if the PCP declines a task, is unable to accomplish a task, or does not recognize that a task exists (barrier). The work system lacks resilience if individuals fail to remember tasks (barrier), if they are unaware that they were responsible for the task (barrier), or if survivors are lost to follow-up (barrier).
Tools/technology
Tools and technologies must support the provision of survivorship care while decreasing negative outcomes such as increasing workloads for the clinicians and survivors. It is noteworthy that tools and technologies must support shared work, which is highly dependent on both communication and individual work. Without tools/technologies to support communication and coordination for shared work, clinicians and survivors rely on high-touch processes (such as scheduling additional visits and the use of patient navigation to ensure care delivery). In this vignette, because the PCP and oncologist are in different organizations/EHRs, information is not easily shared (barrier). The SCP does not explicitly instruct the PCP regarding the next actions when the family history changes (barrier). In addition, the provider responsible for ordering and managing bone-density screening is not delineated (barrier). Even if roles and responsibilities are defined ( facilitator), organizations may not facilitate the acceptance by each clinician and/or survivor of their assigned role and responsibility; and acceptance does not guarantee53 that the accepting individual can manage with the appropriate competency (barrier).
Organization
The provision of survivorship care presents challenges for care coordination and communication, because activities may occur across multiple organizational systems and geographic areas. Temporal challenges may exist: various work schedules may pose barriers to communication (eg, if a survivor visits urgent care after hours, when the oncology team is not available). In this vignette, the oncologist and PCP did not have a ready means for communicating with each other and instead relied on the survivor (barrier). Organizations can support communication and care coordination across health systems through both expectation and clearly described criteria and pathways to facilitate such contact, and they can pressure EHR vendors to address technologic barriers to communications across EHRs.
Environment
Both internal and external environmental factors can influence the care planning and care provision processes. The internal environment, such as the clinic setting in which follow-up visits or care planning occur, can serve as a facilitator or a barrier (eg, when a shared EHR facilitates communication between clinicians). The external environment, such as the societal, economic, ecological, and policy factors influencing the work system, can serve as barrier or facilitator by having an impact on reimbursement, changes in national guidelines, etc. In this vignette, Ms. J lives 2 hours away from the cancer clinic (barrier). Telehealth visits are supported by the cancer center ( facilitator) but are not practical given the out-of-pocket cost without insurance coverage for telehealth (barrier).
Addressing Survivorship Barriers Using the SEIPS Model
Although it is not intended to be an exhaustive list, Ms. J’s case highlights challenges with long-term survivorship care. Preparing an SCP and providing a care-planning visit marks a moment in which information about the survivor, cancer diagnosis and treatment, and follow-up recommendations are synthesized together. The vignette demonstrates that a one-time synthesis may not fulfill the patient’s dynamically changing posttreatment needs. Although necessary care is reviewed at follow-up visits, information may not be retained or understood by survivors; other mechanisms for communication to other clinicians may likewise fail.54 Consequently, oncology and primary care teams may accomplish necessary follow-up care in a high-touch55 fashion, such as scheduling follow-up visits for the primary purpose of tracking care receipt, navigator or nursing phone calls to ascertain receipt of care, etc.56 High-touch may be desirable for certain patients or circumstances, but it also increases clinician workload at a time when both oncology and primary care face predicted workforce shortages,32,57–59 and health care resources may be strained.60 Figure 3A references the current disconnected state of information sharing.
Figure 3.
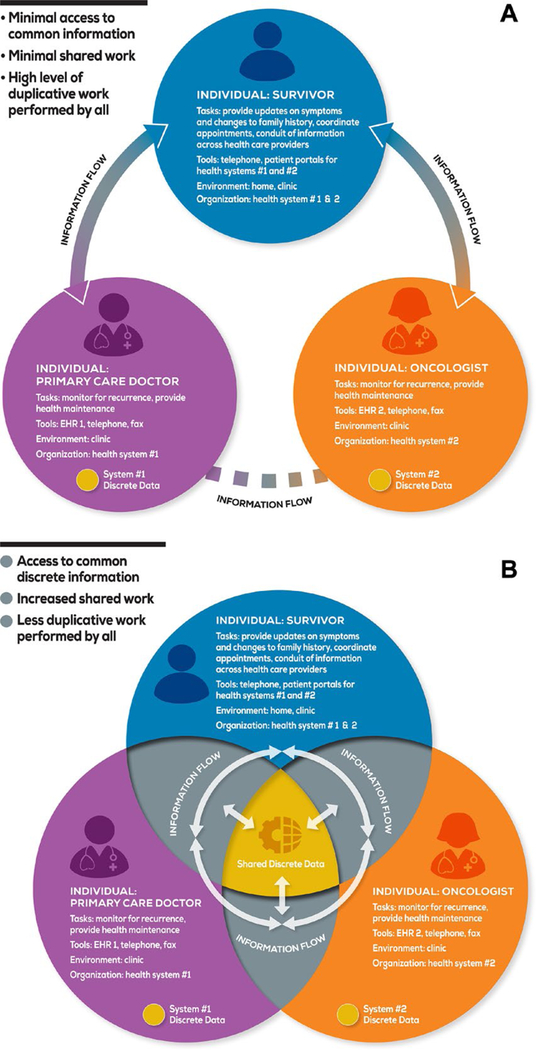
(A) The current work system and (B) the future/desired work system are illustrated. EHR indicates electronic health record.
By the end of 2017, 90% of office-based physicians will use EHRs61; thus, EHRs will play a central role in survivorship clinical activities. The use of health information technologies (HITs) has been identified as a critical component in the provision of high-quality cancer care.62 In survivorship, HIT processes can be leveraged to synthesize survivorship data upstream to coordinate primary, active cancer treatment and also can be compiled for SCPs, so that these data can work harder by assisting in outcome measurement as well as document preparation. Ideally, HITs will enable closed-loop communications to occur both reliably between survivors and their care teams and equitably across all populations.63,64 High-tech solutions might include EHR-based cancer registries capable of operating in real-time and defining populations, tracking desired care and prompting clinicians and survivors of needed testing outside of visits. Table 1 indicates how assessment of the work system using SEIPS can identify elements that contribute to a barrier highlighted in the vignette. Mitigating barriers and supporting the facilitators could mitigate some of the challenges caused by predicted workforce shortages by shifting from high-touch (clinician resource intensive) to high-tech processes and improving overall work system efficiency and effectiveness. The SEIPS model can be applied as a tool to design or re-engineer the work system to achieve the desired or needed changes.39,40 In the absence of continued visits, no standard pathway may exist for the oncology team to monitor clinical activities or communicate with survivors and PCPs—this was part of the rationale for the adoption of SCPs.6 Assuming that SCPs are not simply archived and forgotten by PCPs or survivors,17,65 there should be concern that SCPs are not typically updated. Users may abandon them because of outdated or inaccurate information or, worse, may rely on them to guide care despite outdated information. Formal handoffs to ensure that a survivor or PCP is comfortable managing survivorship care are not standardized.54,56 In the vignette, Ms. J did not undergo bone-density screening although she was receiving anastrozole: the SCP did not contain this recommendation (it was prepared several years prior, when Ms. J was still receiving tamoxifen), and other mechanisms failed (Ms. J’s memory, oncology clinic notes to PCP). An assessment using SEIPS, as demonstrated in Table 1, would indicate that work system elements do not support the process of creating and disseminating dynamic, updatable SCP content. Current tools and technology do not support updating SCP content, nor do current national/organizational standards require updates. Moreover, a defined individual who is to assume the task and take responsibility for updating SCPs may not exist.
TABLE 1.
Selected Barrier and Potential Changes or Solutions to Address Them
| Barrier | Assessment of Work System Elements Using SEIPS | Potential Desired Changes or Strategies |
|---|---|---|
| Reliance on static unidirectional care plan processes | Task: Updates not being performed | Task: Dynamic updates of survivorship information; design and implement workflows to support these tasks, including assignment of responsibilities |
| Organization: Poor transfer of information across health care systems | Organization: Improve transfer of information; greater EHR interoperability, better data-sharing agreements | |
| Persons: Lack of defined person responsible, no workflows | Persons: Identify individuals or group within organization responsible, design formal processes for persons to acknowledge responsibilities | |
| Tools/tech: Do not support updating SCP content without considerable personnel and time resources | Tools/tech: Clinical workflows to support discrete data capture, use of discrete HER data to support automatic updates, design processes that leverage EHR ability to check and process data in real-time | |
| Environment: National standards do not require updates | Environment: Standards adopted to suggest/require updates, clear assignment of responsibilities |
Abbreviations: EHR, electronic health record; SCP, survivorship care plan; SEIPS, Systems Engineering Initiative for Patient Safety.
To address these barriers, the oncology community might adapt current SCP processes to support entry of new data. However, doing so with manual data entry would be personnel-intensive and time-intensive. Ideally, processes would automatically update, and new recommendations would be pushed to survivors and clinicians while anticipating the potential for errors and/or failure. These processes could incorporate acknowledgments by survivors and PCPs that actions are needed and that responsibility is being accepted or declined for these actions. For example, imagine that emerging data suggest that survivors who received treatment with drug XYZ should receive a 1-time screening. In the current state, some survivors might be informed during a follow-up visit with oncology, some might reach out based on information obtained from social networks, and others might fall through the cracks. In a future state, we might identify all survivors who receive drug XYZ using EHR-based registries and push new recommendations to survivors through a portal message or paper letter. PCPs might receive a message through the EHR or by fax. Survivors and PCPs acknowledge receiving this recommendation electronically when possible, and case managers confirm receipt for remaining cases. The EHR tracks these acknowledgments and completion of the recommended screening. Clinicians are notified to pursue further if the recommended screening is not accomplished, intervening earlier if neither the survivor nor the PCP acknowledges. Such a dynamic system delivers reinforcing messages and feedback, with recovery mechanisms to detect, correct, and mitigate errors included in its design, and completes the necessary follow-up without requiring extensive manual data abstraction or survivor follow-up visits with oncology.
A challenge with the envisioned future state is that information about diagnosis and treatment often has poor extractability within EHRs.66,67 Current processes require significant time and personnel resources to extract this information for SCP creation68 but may not leverage the information beyond SCP delivery. When information about diagnosis and treatment is not discrete within an EHR, then the diagnosis and treatment information cannot be leveraged further without high-touch follow-up processes, even when summarized in an SCP. Within an EHR, discrete data typically indicate both measurable and reportable information at a low level of granularity.67,69 For instance, the concept received anthracycline chemotherapy may file to multiple discrete data elements (drug name, drug dose, number of doses, dates of doses, ordering provider, etc). Once data are discretely captured, they can potentially be leveraged to track and identify populations (using the EHR) and the presence or absence of necessary, redundant, or unneeded care. In the received anthracycline chemotherapy example, the data necessary to define this population discretely would have been reviewed for SCP creation and care-planning visit. Leveraging the EHR to retain key survivorship information discretely requires clinical behavior change, but the context is ripe for ensuring data capture. Figure 3B references a desired future state with increased data sharing facilitated by EHRs. The functionalities of EHRs can support repeated SCP updates and cancer survivor registries and can translate these into improved outcomes using medical logic modules, such as health maintenance and best practice advisories.70 However, we need willingness to adopt these functionalities and the new clinical behaviors and workflow they will require. We also need faster, better feedback to EHR vendors, who, in turn, may ask for more consensus and standardization regarding survivorship care delivery. Finally, as oncology and survivorship medical communities, we may benefit from greater coordination between those generating guidelines and those delivering survivorship care.
CONCLUSION
System issues in survivorship care planning prevent us from realizing the dream: a world in which every organization, clinician, survivor, and caregiver engages in real-time, facile, and dynamic management to deliver necessary (but not redundant or unneeded) survivorship care without overburdening oncology and primary care resources. No single solution works for every individual, organization, and situation; and the exact nature of the strategies and processes used to reach the end goals of improving survivorship care will vary.
The integration of oncology and engineering may lead to improved quality of care and clinical efficiency. By using the SEIPS model, numerous system barriers affecting survivor care coordination have been identified. Ongoing research should address system factors that negatively affect survivor care, such as problematic HIT usability, lack of integration with the EHR, care-planning activities that negatively affect clinical workflow, and inadequate information and decision support for clinician and survivor needs.
Acknowledgments
FUNDING SUPPORT
Amye J. Tevaarwerk and Mary E. Sesto received support from the National Cancer Institute (grants P30 CA014520 and P20 CA103697), Gijsberta J. van Londen received support from the National Cancer Institute (grant 5P30 CAO47904), and Jennifer R. Klemp Founder/CEO, Cancer Survivorship Training, Inc received support from the Centers for Disease Control and Prevention (grant CDC15–1501).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Amye J. Tevaarwerk reports that an immediate family member is an employee of Epic Systems. Gijsberta J. van Londen reports personal fees from Eisai. Jennifer R. Klemp, Bradford W. Hesse, and Mary E. Sesto made no disclosures.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesse BW, Arora NK, Klein WM. Communication science: connecting systems for health. In: Hesse BW, Ahern D, Beckjord E, eds. Oncology Informatics: Using Health Information Technology to Improve Processes and Outcomes in Cancer Care Boston, MA: Elsevier Inc; 2016:253–275. [Google Scholar]
- 4.Klabunde CN, Haggstrom D, Kahn KL, et al. Oncologists’ perspectives on post-cancer treatment communication and care coordination with primary care physicians [published online ahead of print January 10, 2017]. Eur J Cancer. Care (Engl) doi: 10.1111/ecc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland JH, Hewitt M, Ganz PA. Cancer survivorship: a new challenge in delivering quality cancer care. J Clin Oncol 2006;24:5101–5104. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt ME, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 7.Mansukhani RP, Bridgeman MB, Candelario D, et al. Exploring transitional care: evidence-based strategies for improving provider communication and reducing readmissions. P T 2015;40:690–694. [PMC free article] [PubMed] [Google Scholar]
- 8.de Rooij BH, Ezendam NPM, Nicolaije KAH, et al. Effects of survivorship care plans on patient reported outcomes in ovarian can- cer during 2-year follow-up—the ROGY care trial. Gynecol Oncol 2017;145:319–328. [DOI] [PubMed] [Google Scholar]
- 9.Boekhout AH, Maunsell E, Pond GR, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. J Cancer Surviv 2015;9:683–691. [DOI] [PubMed] [Google Scholar]
- 10.Hershman DL, Greenlee H, Awad D, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat 2013;138:795–806. [DOI] [PubMed] [Google Scholar]
- 11.Brothers BM, Easley A, Salani R, et al. Do survivorship care plans impact patients’ evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecol Oncol 2013;129: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kvale EA, Huang CS, Meneses KM, et al. Patient-centered support in the survivorship care transition: outcomes from the Patient-Owned Survivorship Care Plan Intervention. Cancer 2016;122:3232–3242. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaije KA, Ezendam NP, Vos MC, et al. Impact of an au- tomatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol 2015;33:3550–3559. [DOI] [PubMed] [Google Scholar]
- 14.Maly RC, Liang LJ, Liu Y, et al. Randomized controlled trial of survivorship care plans among low-income, predominantly Latina breast cancer survivors. J Clin Oncol 2017;35:1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanch-Hartigan D, Forsythe LP, Alfano CM, et al. Provision and discussion of survivorship care plans among cancer survivors: results of a nationally representative survey of oncologists and primary care physicians. J Clin Oncol 2014;32:1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsythe LP, Parry C, Alfano CM, et al. Use of survivorship care plans in the United States: associations with survivorship care. J Natl Cancer Inst 2013;105:1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ezendam NP, Nicolaije KA, Kruitwagen RF, et al. Survivorship care plans to inform the primary care physician: results from the ROGY care pragmatic cluster randomized controlled trial. J Cancer Surviv 2014;8:595–602. [DOI] [PubMed] [Google Scholar]
- 18.Denlinger CS, Sanft T, Baker KS, et al. Survivorship, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1140–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Society of Clinical Oncology. Cancer Survivorship: Next Steps for Patients and Their Families Alexandria, VA: American Society of Clinical Oncology; 2011. [Google Scholar]
- 20.American College of Surgeons. Cancer Program Standards: Ensuring Patient-Centered Care, 2016 Edition Chicago, IL: American College of Surgeons, Commission on Cancer; 2016. [Google Scholar]
- 21.Shalom MM, Hahn EE, Casillas J, et al. Do survivorship care plans make a difference? A primary care provider perspective. J Oncol Pract 2011;7:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faul LA, Rivers B, Shibata D, et al. Survivorship care planning in colorectal cancer: feedback from survivors and providers. J Psychosoc Oncol 2012;30:198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med 2010;363:501–504. [DOI] [PubMed] [Google Scholar]
- 24.Kindig D, Stoddart G. What is population health? Am J Public Health 2003;93:380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dulko D, Pace CM, Dittus KL, et al. Barriers and facilitators to implementing cancer survivorship care plans. Oncol Nurs Forum 2013;40:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin 2012;62:101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan G, Bo-Linn G, Carayon P, et al. Bringing a Systems Approach to Health [discussion paper] Washington, DC: Institute of Medicine and National Academy of Engineering; 2013. [Google Scholar]
- 28.National Academy of Engineering (US) and Institute of Medicine (US) Committee on Engineering and the Health Care System; Reid PP, Compton WD, Grossman JH, Fanjiang G, eds. Building a Better Delivery System: A New Engineering/Health Care Partnership. The National Academies Collection: Reports funded by National Institutes of Health Washington, DC: The National Academies Press; 2005. [PubMed] [Google Scholar]
- 29.Wetterneck T, Kelly MM, Carayon P, et al. Improving quality and safety through human factors collaborations with healthcare: the systems engineering initiative for patient safety. Proc Hum Factors Ergon Soc Annu Meeting 2014;58:728–732. [Google Scholar]
- 30.Stricker CT, O’Brien M. Implementing the Commission on Cancer standards for survivorship care plans. Clin J Oncol Nurs 2014;18:15–22. [DOI] [PubMed] [Google Scholar]
- 31.Arvey SR, Gayer C, Nutt S. Cancer programs’ readiness to implement patient-centered standards of care: an assessment of Commission on Cancer accredited programs Chicago, IL: American College of Surgeons’ Commission on Cancer; http://newsmanager.commpartners.com/acscoc/downloads/CoC%20Readiness%20Assessment%20Results%20050914.pdf [Google Scholar]
- 32.Halpern MT, Viswanathan M, Evans TS, et al. Models of cancer survivorship care: overview and summary of current evidence. J Oncol Pract 2015;11:e19–e27. [DOI] [PubMed] [Google Scholar]
- 33.Mayer DK, Nekhlyudov L, Snyder CF, et al. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract 2014;10:345–351. [DOI] [PubMed] [Google Scholar]
- 34.Hausman J, Ganz PA, Sellers TP, et al. Journey forward: the new face of cancer survivorship care. JOncol Pract 2011;7(3 suppl):e50s–e56s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poplack DG, Fordis M, Landier W, et al. Childhood cancer survi- vor care: development of the Passport for Care. Nat Rev Clin Oncol 2014;11:740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tevaarwerk AJ, Hocking WG, Zeal JL, et al. Accuracy and thoroughness of treatment summaries provided as part of survivorship care plans prepared by 2 cancer centers. J Oncol Pract 2017;13:e486–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill-Kayser CE, Jacobs LA, Gabriel P, et al. Feasibility study of an electronic interface between internet-based survivorship care plans and electronic medical records. J Oncol Pract 2016;12:e380–e387. [DOI] [PubMed] [Google Scholar]
- 38.Sprague BL, Dittus KL, Pace CM, et al. Patient satisfaction with breast and colorectal cancer survivorship care plans. Clin J Oncol Nurs 2013;17:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carayon P, Schoofs Hundt A, Karsh BT, et al. Work system de- sign for patient safety: the SEIPS model. Qual Saf Health Care 2006;15(suppl 1):i50–i58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holden RJ, Carayon P, Gurses AP, et al. SEIPS 2.0: a human factors framework for studying and improving the work of healthcare pro- fessionals and patients. Ergonomics 2013;56:1669–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donabedian A. The quality of medical care. Science 1978;200:856–864. [DOI] [PubMed] [Google Scholar]
- 42.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q 1966;44(suppl):166–206. [PubMed] [Google Scholar]
- 43.Carayon P. Sociotechnical systems approach to healthcare quality and patient safety. Work 2012;41(suppl 1):3850–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carayon P, Wetterneck TB, Rivera-Rodriguez AJ, et al. Human factors systems approach to healthcare quality and patient safety. Appl Ergon 2014;45:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carayon P, Bass E, Bellandi T, Gurses A, Hallbeck S, Mollo V. Socio-technical systems analysis in health care: a research agenda. IIE Trans Healthc Syst Eng 2011;1:145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kianfar S, Carayon P, Hundt AS, Hoonakker P. Understanding care coordination for chronically ill patients. Proc Hum Factors Ergon Soc Annu Meeting 2014;58:170–174. [Google Scholar]
- 47.Carayon P, Alyousef B, Hoonakker P, et al. Challenges to care coordination posed by the use of multiple health IT applications. Work 2012;41(suppl 1):4468–4473. [DOI] [PubMed] [Google Scholar]
- 48.Ngam C, Schoofs Hundt A, Haun N, et al. Barriers and facilitators to Clostridium difficile infection prevention: a nursing perspective. Am J Infect Control 2017;45:1363–1368. [DOI] [PubMed] [Google Scholar]
- 49.Carayon P, Xie A, Kianfar S. Human factors and ergonomics as a patient safety practice. BMJ Qual Saf 2014;23:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acher AW, LeCaire TJ, Hundt AS, et al. Using human factors and systems engineering to evaluate readmission after complex surgery. JAm Coll Surg 2015;221:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bisantz AM, Carayon P, Miller A, Khunlerkit A, Arbaje A, Xiao Y. Using human factors and systems engineering to improve care coordi- nation. Proc Hum Factors Ergon Soc Annu Meeting 2012;56:855–859. [Google Scholar]
- 52.Brunner J, Chuang E, Goldzweig C, et al. User-centered design to improve clinical decision support in primary care. Int J Med Inform 2017;104:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen MJ, Binz-Scharf M, D’Agostino T, et al. A mixed-methods examination of communication between oncologists and primary care providers among primary care physicians in underserved com- munities. Cancer 2015;121:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dossett LA, Hudson JN, Morris AM, et al. The primary care pro- vider (PCP)-cancer specialist relationship: a systematic review and mixed-methods meta-synthesis. CA Cancer J Clin 2017;67:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wunderlich NV, Wangenheim FV, Bitner MJ. High tech and high touch: a framework for understanding user attitudes and behaviors related to smart interactive services. J Ser Res 2013;16:3–20. [Google Scholar]
- 56.Neuman HB, Jacobs EA, Steffens NM, et al. Oncologists’ perceived barriers to an expanded role for primary care in breast cancer survi- vorship care. Cancer Med 2016;5:2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang W, Williams JH, Hogan PF, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract 2014;10:39–45. [DOI] [PubMed] [Google Scholar]
- 58.Colwill JM, Cultice JM, Kruse RL. Trends: will generalist physician supply meet demands of an increasing and aging population? Health Affairs 2008;27:w232–w241. [DOI] [PubMed] [Google Scholar]
- 59.Kline R, Adelson K, Kirshner JJ, et al. The oncology care model: perspectives from the Centers for Medicare & Medicaid Services and participating oncology practices in academia and the community. Am Soc Clin Oncol Educ Book 2017;37:460–466. [DOI] [PubMed] [Google Scholar]
- 60.Levit L, Balogh E, Nass S, et al. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis Challenges of an Aging Population Washington, DC: Board on Health Care Services, The National Academies Press; 2013. [PubMed] [Google Scholar]
- 61.Health IT Dashboard. Office-Based Physician Electronic Health Record Adoption Washington, DC: Office of the National Coordination for Health Information Technology, US Department of Health and Human Services; 2015. [Google Scholar]
- 62.Institute of Medicine. Delivering High Quality Cancer Care: Charting a New Course for a System in Crisis Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 63.Beckjord E, Van Londen GJ, Rechis R. Survivorship. In: Hesse BW, Ahern D, Beckjord E, eds. Oncology Informatics: Using Health Information Technology to Improve Processes and Outcomes in Cancer 1st ed Boston, MA, Elsevier, 2016:159–178. [Google Scholar]
- 64.Parry C, Kent EE, Forsythe LP, et al. Can’t see the forest for the care plan: a call to revisit the context of care planning. J Clin Oncol 2013;31:2651–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donohue S, Haine JE, Li Z, et al. The impact of a primary care education program regarding cancer survivorship care plans: results from an engineering, primary care, and oncology collaborative for survivorship health [published online ahead of print September 20, 2017]. J Cancer Educ doi: 10.1007/s13187-017-1281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donohue SM, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. JOncol Pract 2015;11:e329–e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abernethy AP, Gippetti J, Parulkar R, et al. Use of electronic health record data for quality reporting. J Oncol Pract 2017;13: 530–534. [DOI] [PubMed] [Google Scholar]
- 68.Birken SA, Ellis SD, Walker JS, et al. Guidelines for the use of survi- vorship care plans: a systematic quality appraisal using the AGREE II instrument [serial online]. Implement Sci 2015;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waegemann CP, Tessier C, Barbash A, et al. ; for the Consensus Workgroup on Health Information Capture and Report Generation. Healthcare Documentation: A Report on Information Capture and Report Generation Newton, MA: Medical Records Institute; 2002. [Google Scholar]
- 70.Kircher SM, Slocum M, Garcia SF, et al. Use of a best-practice advi- sory to increase survivorship clinic referrals [abstract]. J Clin Oncol 2017;35(5 suppl):53. [Google Scholar]


