Abstract
Accurate analyses of the delayed effects of acute radiation exposure (DEARE) in survivors of the hematopoietic acute radiation syndrome (H-ARS) are hampered by low numbers of mice for examination due to high lethality from the acute syndrome, increased morbidity and mortality in survivors, high cost of husbandry for long-term studies, biological variability, and inconsistencies of models from different laboratories complicating meta-analyses. To address this, a compilation of 38 similar H-ARS studies conducted over a seven-year period in the authors’ laboratory, comprising more than 1,500 irradiated young adult C57BL/6 mice and almost 600 day-30 survivors, was assessed for hematopoietic DEARE at various times up to 30 months of age. Significant loss of long-term repopulating potential of phenotypically-defined primitive hematopoietic stem cells (HSC) was documented in H-ARS survivors, as well as significant decreases in all hematopoietic lineages in peripheral blood, prominent myeloid skew, significantly decreased bone marrow cellularity and numbers of lineage-negative Sca-1+ cKit+ CD150+ cells (KSLCD150+; the phenotype known to be enriched for HSC), and increased cycling of KSLCD150+ cells. Studies interrogating the phenotype of bone marrow cells capable of initiation of suspension cultures and engraftment in competitive transplantation assays documented the phenotype of HSC in H-ARS survivors to be the same as that in non-irradiated age-matched controls. This compilation study adds rigor and validity to our initial findings of persistent hematopoietic dysfunction in H-ARS survivors that arises at the level of the HSC and which affects all classes of hematopoietic cells for the life of the survivor.
Keywords: Health effects (radiation effects), mice, whole body irradiation, bone marrow
INTRODUCTION
Exposure to potentially lethal high-dose radiation, from malicious activity or radiation accidents, results in the acute radiation syndromes (ARS). Bone marrow (BM) is the most sensitive tissue to radiation damage (Till and McCulloch 1961; Abramson et al. 1977; Visser et al. 1984; Jones et al. 1989; Jones et al. 1990; Keller and Snodgrass 1990; Hall 2000), owing to the highly proliferative nature of hematopoiesis driven by rare hematopoietic stem (HSC) and progenitor cells (HPC) that produce billions of blood cells daily to maintain homeostasis. HSC sustain life-long hematopoiesis and reconstitute normal BM functions following myelosuppression through two unique properties: 1) the ability to differentiate into all the formed elements of the blood, and 2) the ability to self-renew into daughter HSC (Till and McCulloch 1961; Visser et al. 1984; Jones et al. 1990; Keller and Snodgrass 1990). Whole-body exposures or significant partial-body exposures of 1–10 Gy in mice or up to approximately 6 Gy in humans result in the hematopoietic-ARS (H-ARS) due to loss of radiosensitive HSC/HPC (McCulloch and Till 1962; van Bekkum 1991; Inoue et al. 1995; Belkacemi et al. 2003) and death from infection and/or hemorrhage if left untreated (Coleman et al. 2003; Dainiak et al. 2003).
Survivors of the acute syndromes suffer later in life from the delayed effects of acute radiation exposure (DEARE) affecting multiple organ systems and which may result in fibrosis and loss of function in a stochastic dose-dependent manner. DEARE effects on the hematopoietic system are known as residual bone marrow damage (RBMD), a latent condition characterized by functionally compromised HSC and depressed hematopoiesis for years, most evident under stress (Botnick et al. 1979; Mauch et al. 1988; Chua et al. 2012; Chua et al. 2014) and hypothesized to be secondary to senescence (Hellman and Botnick 1977; Meng et al. 2003; Wang et al. 2006; Wang et al. 2006). Neupogen (granulocyte-colony stimulating factor, G-CSF) and Neulasta (pegylated-G-CSF) were recently approved by the FDA for treatment of H-ARS, but neither of these medical countermeasures (MCM), nor any other efficacious mitigator to our knowledge, have shown efficacy against DEARE (Chua et al. 2014) (and unpublished).
Efficacy studies for H-ARS and DEARE must adhere to the criteria of FDA Animal Rule (AR) (Crawford 2002) and the recent FDA Guidance document (FDACDER 2015), drafted to guide drug development when human efficacy studies are unethical to perform. Suitable animal models of ARS and DEARE are essential for development of effective MCM against radiation. Such models can also provide valuable information regarding the natural history of ARS and DEARE, information that can be potentially exploited for development of new MCM, as well as better understanding of radiation effects on organ systems and the impact that MCM treatment for ARS may have on the severity and breadth of DEARE.
The authors have developed, extensively characterized, and validated murine models of H-ARS in male and female C57BL/6 mice, and have used these models to study mechanisms and efficacy of MCM for FDA licensure under the Animal Rule for eventual stockpiling by the US government in the event of terrorist use of radiation (Plett et al. 2012; Plett et al. 2015). Using these models, more than 200 studies testing the survival efficacy and dose/schedule optimization of more than 70 MCM from the government and private industry have been conducted over the past 13 years (Shakhov et al. 2012; Hoggatt et al. 2013; Plett et al. 2014; Faller et al. 2017). Whenever possible, survivors of H-ARS are aged in our facility for up to 2.5 years of age and used to study the natural history of DEARE in radiation survivors, and the temporal occurrence of DEARE in different organs (Chua et al. 2012; Chua et al. 2014; Unthank et al. 2015). Initial findings published in 2012 & 2014 documented severe hematopoietic dysfunction and loss of long-term HSC repopulating potential that persisted for up to 16–19 months (mo.) after exposure (the latest time point analyzed therein) (Chua et al. 2012). Subsequent studies documented the kinetics of fibrosis & dysfunction in non-hematopoietic organs (heart, lung) in H-ARS C57BL/6 survivors up to 2 years of age (Unthank et al. 2015).
Ongoing H-ARS survival studies conducted over the past seven years using the authors’ consistent and reproducible murine model developed in young adult 3-month-old C57Bl/6 mice, and continued aging of survivors from these H-ARS studies, have provided a unique opportunity to combine data from multiple similar studies into a composite dataset. This dataset is comprised of 38 H-ARS studies involving more than 1,500 mice initially exposed to lethal radiation on day 0, and 585 “day 30” survivors that were then enrolled in DEARE studies represented in this paper. H-ARS survivors were then analyzed for multiple hematopoietic parameters using sophisticated assays over the course of their lifetime. The large number of mice per experimental group reduced the inherent biological variability in radiation survivors, smoothing data curves and adding experimental rigor. These data are novel in that they are derived from a substantial number of mice of consistent age, gender, exposure parameters, husbandry, and to our knowledge, are the only such dataset of this magnitude. The mega-mouse study conducted at the Argonne National Laboratory between 1972 to 1989 irradiated 49,000 mice, but those studies used a variety of exposure parameters, dose rates, types of radiation, geometries, and schedules (Wang et al. 2010; Haley et al. 2011), such that the number of mice in any particular group with consistent exposure conditions is much less than the 585 survivors mice in the current study. This publication further advances the understanding of and mechanisms surrounding hematopoietic DEARE and adds rigor and validity to our initial findings of persistent hematopoietic dysfunction in H-ARS survivors that arises at the level of the HSC / HPC and which affects all classes of hematopoietic cells for the life of the survivor.
MATERIALS AND METHODS
Mice and husbandry.
Specific pathogen-free C57BL/6 mice (50/50 male/female; Jackson Laboratory, Bar Harbor, Maine) were received at 10 weeks of age, an age analogous to a “young adult” human. All studies are performed on mice of the same age to avoid age-related changes in radiosensitivity (Grahn and Hamilton 1957; Grahn 1958; Yuhas and Storer 1967; Casarett 1968). Weights ranged from 12.6–25.1gm for females and 18.3–30.7gm for males, with more than 95% of study mice falling between 15.0–23.4gm and 19.6–30.7gm, respectively. Mice were uniquely identified by ear punch and/or tail marks, and acclimatized for 2 weeks prior to irradiation at 12 weeks of age. Husbandry and health status monitoring were carried out as previously described (Plett et al. 2012). Mice were housed in microisolator cages and provided with autoclaved acidified water in sipper tubes on days 1–30 post-irradiation and wet feed between days 4–30 post-irradiation. Irradiated mice were observed twice daily and scored on a scale of 0 to 3 for signs meeting the criteria for early euthanasia based on the severity of hunched posture, squinted/closed eyes, and activity (Plett et al. 2012). Mice with total scores of 8 or 9 underwent euthanasia by cervical dislocation or CO2 asphyxiation (Plett et al. 2012). All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Recipient mice and donors of competitor cells used in competitive transplantation assays were of the congenic PtrcaPep3b/BoyJ (B6.BoyJ; CD45.1+) or the F1 hybrid of C57Bl/6 and B6.BoyJ mice (CD45.2+CD45.1+) strains. These two strains were used interchangeably as transplant recipients and competitor cell donors, and their leukocytes were distinguished from C57Bl/6 cells in transplant recipients by expression of CD45.1. B6.BoyJ and F1 hybrid mice were bred in-house and used between 8–12 weeks of age.
Study design.
Murine survivors from 38 MCM screening studies performed over a period of 7 years (2010–2016) were analyzed for DEARE. All studies and experiments reported in this paper are listed in Table 1, along with the year the study was conducted, the expected and actual LDXX/30, and the number of surviving mice that were then enrolled in DEARE studies. Non-irradiated age-matched controls are not documented in this table. Mice were exposed to total-body irradiation (TBI, LD50/30 to LD90/30) and treated with vehicle in MCM screening studies (referred to herein as “TBI-Veh”). Mice were randomized to treatment groups so that TBI-Veh mice and MCM-treated mice resided in the same cage. Non-irradiated (NI) age- and sex-matched mice aged along with the TBI-Veh mice served as controls. Irradiated mice were assessed for health status for 30 days and euthanized when deemed moribund, as previously described(Plett et al. 2012). “Day 30” survivors were allowed to age in our animal facility for up to 30 mo. of age (27 mo. post-TBI). Subgroups of aging mice were euthanized at defined time points and assessed for residual bone marrow damage (RBMD) as described below. Some aging mice required humane euthanasia due to ulcerative dermatitis, labored breathing, severely hunched posture, inactivity, neurological symptoms or the presence of abnormal growths negatively affecting movement.
Table 1.
Year, radiation dose, expected and actual LDXX/30, number of mice, and parameters assessed in each study
| Year | Rad Dose (Gy) |
LDXX/30 (expected)a |
LDXX/30 (actual) |
No. of miceb |
Fig. 1A (Wt.) |
Figs. 1B-F (CBC) |
Fig. 2 (BM) |
Fig. 3 (HSC trpl.) |
Figs. 4A-E (Culture) |
Figs. 4F-H (CD150 trpl.) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 7.76 | LD50/30 | LD26/30 | 20 | X | |||||
| 7.96 | LD70/30 | LD26/30 | 8 | X | ||||||
| 7.96 | LD70/30 | LD50/30 | 58 | X | X | X | X | |||
| 2011 | 7.96 | LD70/30 | LD30/30 | 70 | X | |||||
| 7.86 | LD60/30 | LD20/30 | 16 | X | X | |||||
| 8.10 | LD80/30 | LD50/30 | 7 | X | X | X | ||||
| 8.29 | LD90/30 | LD30/30 | 27 | X | X | X | X | |||
| 7.92 | LD50/30 | LD35/30 | 13 | X | X | X | ||||
| 8.06 | LD70/30 | LD35/30 | 12 | X | X | X | X | |||
| 2012 | 8.53 | LD50/30 | LD77/30 | 4 | X | X | X | X | ||
| 2013 | 8.53 | LD50/30 | LD55/30 | 22 | X | X | X | X | ||
| 8.72 | LD70/30 | LD60/30 | 13 | X | X | X | X | |||
| 8.53 | LD50/30 | LD50/30 | 9 | X | X | X | X | |||
| 8.72 | LD70/30 | LD70/30 | 6 | X | X | X | X | |||
| 9.04 | LD90/30 | LD80/30 | 4 | X | X | X | X | |||
| 8.53 | LD50/30 | N/Dc | 6 | X | X | X | X | |||
| 8.53 | LD50/30 | LD40/30 | 11 | X | X | X | X | |||
| 8.72 | LD70/30 | LD90/30 | 2 | X | X | X | X | |||
| 8.53 | LD50/30 | LD49/30 | 42 | X | X | |||||
| 8.53 | LD50/30 | LD40/30 | 14 | X | X | |||||
| 8.72 | LD70/30 | LD73/30 | 10 | X | X | X | ||||
| 8.72 | LD70/30 | LD65/30 | 7 | X | X | X | ||||
| 9.27 | LD95/30 | LD95/30 | 1 | X | X | |||||
| 2014 | 8.53 | LD50/30 | LD65/30 | 12 | X | X | ||||
| 8.72 | LD70/30 | LD85/30 | 9 | X | X | X | X | |||
| 9.27 | LD95/30 | LD90/30 | 2 | X | X | X | ||||
| 8.53 | LD50/30 | LD40/30 | 12 | X | X | |||||
| 8.72 | LD70/30 | LD55/30 | 9 | X | X | X | ||||
| 8.53 | LD50/30 | LD50/30 | 25 | X | X | |||||
| 8.72 | LD70/30 | LD60/30 | 13 | X | X | |||||
| 2015 | 8.53 | LD50/30 | LD30/30 | 26 | X | |||||
| 8.72 | LD70/30 | LD80/30 | 10 | X | ||||||
| 8.72 | LD70/30 | LD75/30 | 10 | X | ||||||
| 8.72 | LD70/30 | LD81/30 | 15 | X | X | |||||
| 2016 | 9.04 | LD90/30 | LD90/30 | 14 | X | X | ||||
| 9.04 | LD90/30 | LD87/30 | 5 | X | X | X | ||||
| 8.72 | LD70/30 | LD78/30 | 9 | X | X | |||||
| 8.53 | LD50/30 | LD73/30 | 32 | X | ||||||
LDXX/30 were derived from three separate dose response relationship studies conducted in 2006, 2011, and 2012.
The total number of day 30 survivors utilized in studies in this paper was 585. These resulted from more than 1,500 mice irradiated on day 0.
A majority of mice underwent scheduled euthanasias prior to day 30, thus the actual LDXX/30 could not be determined.
Irradiation and dosimetry.
Mice were placed in single chambers of a Plexiglas irradiation apparatus and were exposed to a single uniform total-body dose of gamma radiation between7.76 and 9.27 Gy from a 137Cs radiation source (see Table 1 for estimated and actual LDXX/30 for all doses of radiation used in these studies). The dose rate over the 7 year period in which the studies were performed was 0.59 – 0.62 Gy min−1 for years 2010–2012 (GammaCell 40; Nordion International, Kanata, Ontario, Canada) or 0.93 – 1.03 Gy min−1 for years 2012–2016 (Mark 1 Irradiator, JL Shepherd, San Fernando, CA). For irradiations utilizing the GammaCell 40 irradiator, mice were exposed bilaterally on the dorsal and ventral sides, while in the Mark Irradiator, mice were rotated horizontally and exposed from a single source posteriorly and anteriorly.
In-house dosimetry verified that dose homogeneity in the exposure field of the mice was 0.0–4.3% of calculated central dose. To verify exposure doses, Landauer Inlight OSL nanodosimeters were placed in mouse phantoms and exposed along with the mice during all or select exposures in most studies. Nanodosimeters were read on a validated Landauer microStar reader calibrated with standard Dot dosimeters exposed with a NIST-traceable 137Cs source (Battelle Memorial Institute, WA). Reproducibility of individual dots was 3±1% with accuracy of 4±2%. Dose output checks (using farmer-type ion chambers and a validated electrometer), and dose field uniformity checks by exposing film, are performed periodically by an onsite medical physicist.
TBI-Vehicle mice.
TBI-Veh mice used in these studies previously served as controls for H-ARS MCM screening studies and were thus administered appropriate vehicle solutions using an identical administration route and schedule as that of the MCM under evaluation in each study. Volumes, routes, and composition of vehicle solutions used in studies presented herein are as follows: 50 uL for intramuscular and 94 to 128 uL for subcutaneous injections and oral gavages. Vehicle solutions consisted of: 20 mM Tris, pH 7.5, 200 mM NaCl, 10% glycerol; or 0.05% Tween-20; PlasmaLyte A solution; or 10 mM sodium acetate, pH 4.8, 140 mM NaCl; or 10 mM sodium acetate, 5% sorbitol, 0.003% polysorbate 20, pH 4.0; 160mmol/L sodium chloride solution (Normal saline; 0.9% NaCl, w/v); or 10 mM sodium phosphate, 4% mannitol, 1% sucrose pH 6.2; or Dextrose 5% in water (D5W); or 5% DMSO Polyethylene glycol (PEG)-200; or 10% EtOH Sesame oil: Cremophor RH40 (55:35 w/w); or 20 mM sodium phosphate monobasic; or 1% sucrose and 4% D-mannitol in Water for Injection, pH 6.5±0.1; or 50mmM Citrate buffer pH4-4.4; or empty liposomes; or 0.9% saline (w/v) + 0.1% bovine serum albumin (BSA) solution; or 5% (D)-Glucose solution; or sodium acetate (10 mM), mannitol (45 mg mL−1), polysorbate 20 (0.05 mg mL−1), pH 4.0; or 10 mM sodium phosphate, 4% mannitol, 1% sucrose pH 6.2.
Residual bone marrow damage studies.
TBI-Veh mice which survived H-ARS, along with non-TBI age-matched controls, were assessed at various times up to 30 mo. of age (27 mo. post-TBI) for body weight, complete blood count (CBC), bone marrow (BM) cellularity, hematopoietic progenitor number, primitive hematopoietic progenitor phenotypes, cell cycle analyses, and HSC multilineage reconstitution potential in competitive transplantation assays, all as previously described (Chua et al. 2012; Chua et al. 2014).
Suspension cultures were initiated with phenotypically-defined BM cells isolated from TBI-Veh mice at 4 mo. or 8 mo. post-LD70/30 (872 cGy). Briefly, BM cells were enumerated, stained with fluorescently conjugated antibodies, and isolated by flow cytometric cell sorting to yield lineage-negative (CD3, B220, Gr1) cells of the following subgroups: Sca-1+, Sca-1−, cKit+, cKit−, CD150+, and CD150−. Purified cells from 3 mice per treatment group (TBI-Veh or age-matched NI controls) were pooled and 900 to 3 × 105 cells per well used to initiate suspension cultures in 5% CO2 in 100%-humidified air. Media consisted of Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 20% fetal bovine serum (FBS), 59 uM 2-mercaptoethanol, 10 ng/mL murine stem cell factor (mSCF, PeproTech), 50 ng/mL murine FMS-related tyrosine kinase 3 ligand (mFlt3L, eBioscience), 20 ng/ml murine megakaryocyte-derived growth factor/thrombopoietin (mMDGF/TPO, Peprotech), 10 ng/ml murine interleukin-3 (mIL-3, STEMCELL Technologies), and 25 ng/mL human interleukin-6 (hIL-6, STEMCELL Technologies). Cytokines were delivered every other day and cells enumerated weekly. Cultures were visualized daily for confluence, and demi-depopulated as needed to maintain confluence below 90–100%.
Statistical analyses.
The Student’s unpaired t-test was used to compare the following parameters: body weight, CBC parameters, BM cellularity, percent and number of BM primitive hematopoietic cells per mouse, cell cycle status, and donor chimerism. While many parameters measured over time were also significantly different at individual time points, a single asterisk denoting significance comparing all mice over time was used rather than multiple asterices at individual time points, for the ease of the reader.
RESULTS
Body weights and CBC.
Body weights and CBC were documented for up to 30 mo. of age (27 mo. post-TBI) in H-ARS survivors from 38 studies conducted over the past 7 years. Data were pooled and in agreement with our earlier studies conducted with fewer mice (Chua et al. 2012), the compilation data showed significantly decreased body weight in H-ARS survivors compared with NI, which becomes more dissimilar as the mice age (Fig. 1A). The concentrations of white blood cells (WBC, Fig. 1B), neutrophils (NE, Fig. 1C), lymphocytes (LY, Fig. 1D), red blood cells (RBC, Fig. 1E), and platelets (PLT, Fig. 1F) of TBI-Veh mice were all significantly less than those in NI age-matched controls over time. The number of LY in TBI-Veh mice was approximately half the value of NI mice, and the most disparate of CBC values between TBI-Veh and NI. The larger difference in the number of LY between TBI-Veh and NI mice (Fig. 1D) compared to NE (Fig. 1C) illustrated the myeloid skew that occurred in irradiated survivors, as we and others have shown (Morrison et al. 1996; Kim et al. 2003; Chua et al. 2012).
Figure 1. Body weight and CBC profiles in TBI-Veh and NI mice.
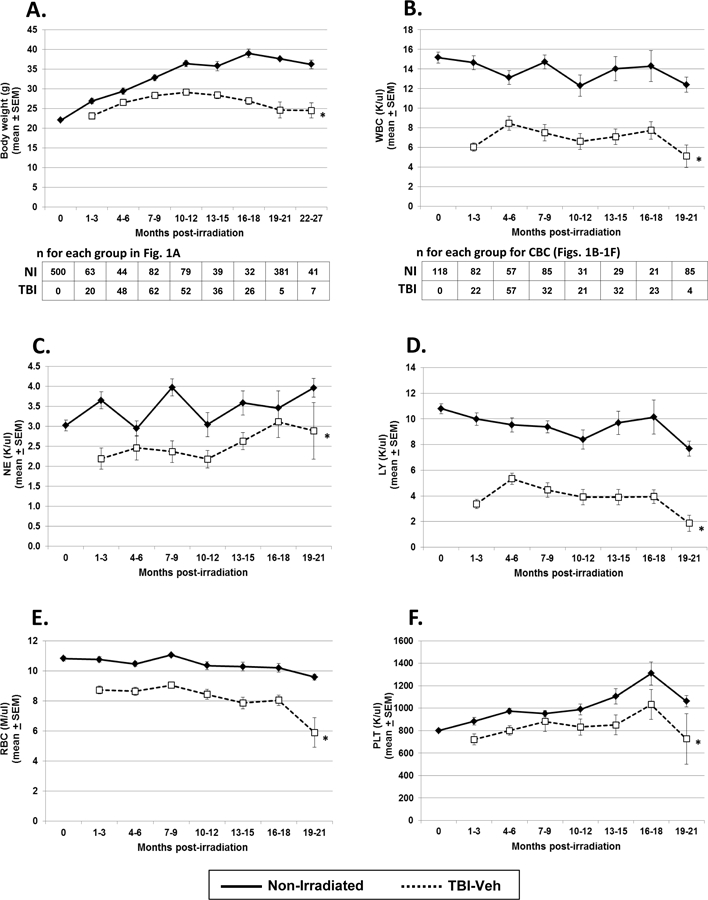
TBI-Veh and NI mice were assessed at various times up to 27 mo. post-irradiation for body weight (panel A), and up to 21 mo. post-irradiation for CBC, including white blood cells (WBC, panel B), neutrophils (NE, panel C), lymphocytes (LY, panel D), red blood cells (RBC, panel E), and platelets (PLT, panel F). Blood for CBC was obtained by tail snips and assayed using a Hemavet system (Drew Scientific, Waterbury, CT). Lines represent mean ± SEM; * p < 0.01 comparing TBI-Veh to NI age-matched controls. The number of mice analyzed for body weight and CBC at each time point are given below panels A and B, respectively.
Bone marrow (BM) cellularity and primitive hematopoietic phenotypes.
Similar to CBC, all classes of BM hematopoietic cells were also significantly decreased in TBI-Veh mice compared to NI (Fig. 2) in this comprehensive dataset. The total number of BM cells in TBI-Veh mice was significantly decreased compared to that in NI age-matched controls (Fig. 2A), similar to our previous data from a smaller set of studies (Chua et al. 2012). While the percentage and absolute number of KSL cells were significantly decreased in TBI-Veh mice (Figs. 2B and 2C), the percentage of KSL CD150+ cells was significantly increased over time (Fig. 2D), due to the increased expression of CD150 on hematopoietic cells after irradiation as we (Chua et al. 2012) and others (Simonnet et al. 2009) have reported. The increased expression of CD150 on KSL from TBI-Veh mice did not, however, result in increased number of KSL CD150+ in TBI-Veh mice (Fig. 2E) due to the significantly decreased numbers of the parent population, KSL.
Figure 2. Bone marrow (BM) cellularity, and primitive hematopoietic phenotypes in TBI-Veh and NI mice.
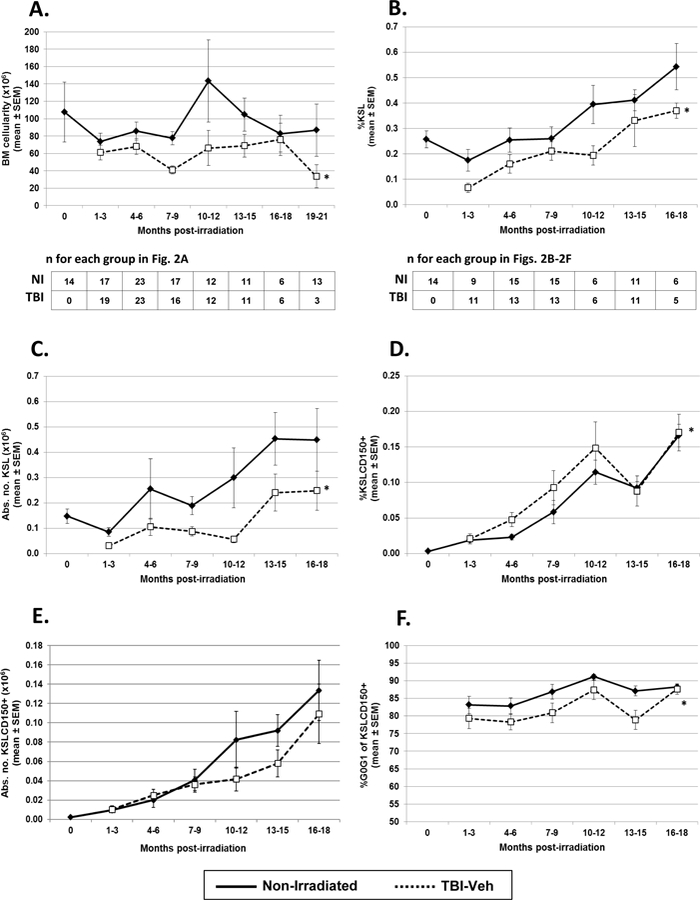
TBI-Veh and NI mice were assessed at various times up to 21 mo. post-irradiation for total number of BM cells / mouse (BM cellularity, panel A), and up to 18 mo. post-irradiation for primitive hematopoietic populations, including percentage of BM cKit+ Sca-1+ lin- cells (KSL cells, panel B), the absolute number of KSL cells / mouse (panel C), the percentage of KSL CD150+ cells (KSLCD150+, panel D), the absolute number of KSLCD150+ cells / mouse (panel E), and the cycling activity of KSLCD150+ cells (panel F). The absolute number of BM cells per mouse was calculated based on cell counts from LDBM or WBM samples. BM cells were stained for cell surface hematopoietic stem cell markers, Sca-1, cKit, lineage markers (CD3, B220, Gr1) and CD150, and analyzed by flow cytometry for primitive hematopoietic populations. BM cells that were surface stained for hematopoietic stem cell markers were fixed and then stained with the DNA stain DAPI and analyzed for cell cycle activity by flow cytometry. Lines represent mean ± SEM; * p < 0.05 comparing TBI-Veh to non-irradiated age-matched controls. The number of mice analyzed for BM cellularity are shown below panel A; the number of mice analyzed for all other parameters are shown below panel B.
Cell cycle analyses.
The authors previously demonstrated reduced hematopoietic stem cell engraftment potential and increased cell cycle activity in KSL CD150+ cells isolated from BM of TBI-Veh mice compared to similar cells from NI age-matched controls (Chua et al. 2012), indicating loss of key functional characteristics of HSC. These findings were further substantiated in this comprehensive analysis of 38 experiments conducted over the past 7 years. The percentage of TBI-Veh KSLCD150+ cells in G0/G1 at different times post-exposure was found to be significantly lower over time compared to NI KSLCD150+ cells (Fig. 2F), indicating significantly increased cell cycling and loss of mitotic quiescence.
HSC long-term engraftment potential.
This comprehensive analysis further demonstrated the inability of phenotypically-defined KSL CD150+ cells isolated from bone marrow of TBI-Veh mice to reconstitute multi-lineage hematopoiesis in competitive transplantation experiments, the only true test of HSC content (Fig. 3). Loss of HSC potential was evident as early as one month post-TBI all the way to 27 mo. post-TBI (Fig. 3). Significant loss of lymphoid repopulating potential in favor of myeloid reconstitution was observed in transplant recipients (data not shown), as the authors’ have previously shown (Chua et al. 2012; Chua et al. 2014). Fig. 3 also illustrates the loss in engraftment potential of HSC from naïve NI mice that occurred naturally with aging (Morrison et al. 1996; Kim et al. 2003; Kamminga et al. 2005; Nijnik et al. 2007; Rossi et al. 2007; Rube et al. 2011; Snoeck 2013; Behrens et al. 2014).
Figure 3. Long-term engraftment potential of primitive hematopoietic populations in TBI-Veh and NI mice in competitive transplantation assays.
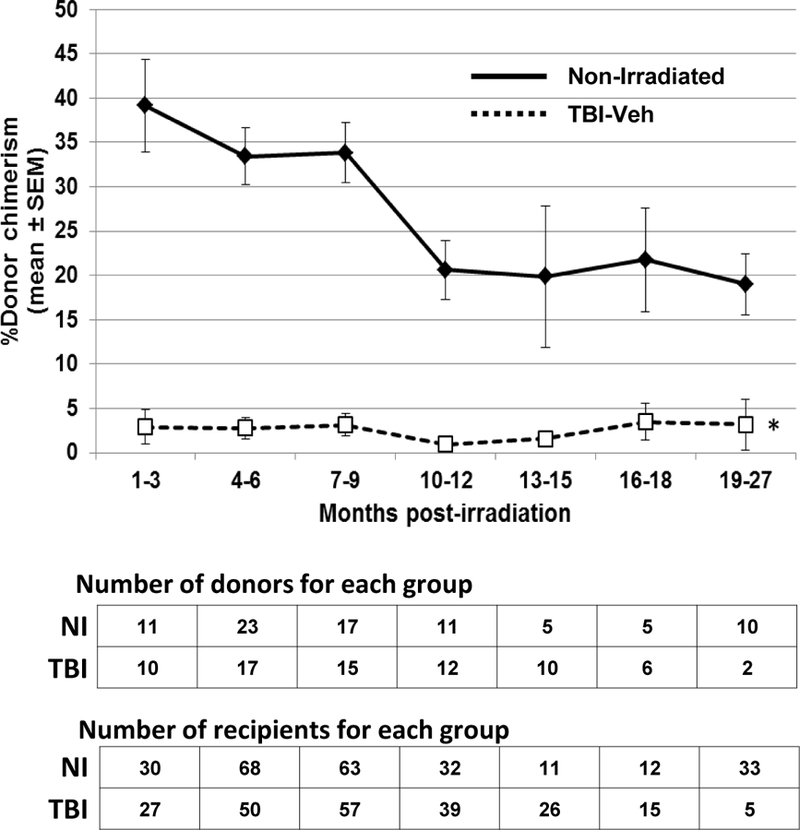
TBI-Veh and NI mice were assessed at various times up to 27 mo. post-irradiation for long-term engraftment potential of primitive KSLCD150+ cells. Lethally irradiated congenic murine recipients were transplanted with 150 KSLCD150+ donor cells, along with 1–2×105 LDBM competitor cells of congenic origin. Peripheral blood samples from tail-snips were obtained from transplanted recipients and analyzed by flow cytometry to determine donor chimerism using antibodies against CD45.1 and CD45.2. Lines represent mean ± SEM; * p < 0.01 comparing TBI-Veh to non-irradiated age-matched controls. The number of donor and recipient mice at each time point are given below the figure.
Retention of cell surface HSC phenotype in TBI-Veh mice.
A potential concern with regards to our observation of severely diminished HSC repopulation potential of phenotypically-defined KSL CD150+ cells from TBI-Veh mice was the possibility that the HSC cell surface phenotype may have changed after irradiation, such that cKit, Sca-1, or CD150 may no longer be expressed on HSC from H-ARS survivors. A precedent for this notion is the temporary loss of cKit on HSC isolated within days from irradiated mice (Shen et al. 2004). To define the cell surface phenotype of HSC from TBI-Veh mice, lineage-negative cells from TBI-Veh mice at 8 mo. post-TBI were subdivided into Sca-1+ and Sca-1-cells, cKit+ and cKit-cells, and CD150+ and CD150-cells by flow cytometric cell sorting. These 6 populations were assayed for proliferative potential (in comparison to similar cells isolated from age-matched NI controls) in cytokine-supplemented suspension cultures as a measure of primitive hematopoietic potential. For each of the three markers, cultures from both NI and TBI-Veh cells initiated with the phenotype known to be expressed on long-term HSC (i.e., lin− Sca-1+, lin− cKit+, lin− CD150+) produced more cells than cultures initiated with lin− Sca-1−, lin− cKit−, or lin− CD150− (Figs 4A, 4B, and 4C). Not surprisingly, proliferative potential of cells from NI mice was generally higher than similar populations isolated from TBI-Veh mice. The positive phenotype (lin− Sca-1+, lin− cKit+, and lin− CD150+) from both NI (Fig. 4D) and TBI-Veh (Fig. 4E) mice also produced a greater number of hematopoietic colonies than the corresponding negative phenotype.
Figure 4. Retention of steady state HSC cell surface markers in TBI-Veh mice.
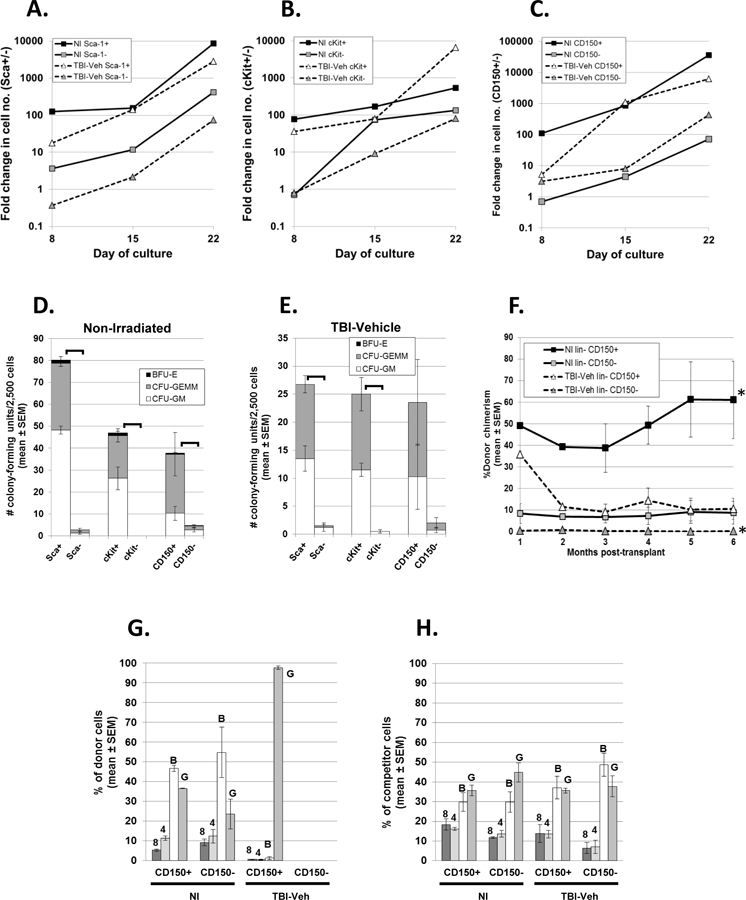
Sca-1+ and Sca-1− lineage-negative (lin-) cells (panel A), cKit+ and cKit− lin- cells (panel B), and CD150+ and CD150- lin- cells (panel C) were isolated from NI and TBI-Veh mice at 8 mo. post-TBI and used to initiate suspension cultures in IMDM plus 20% FBS, 59 uM 2-mercaptoethanol, mSCF, mFlt3L, mMDGF/TPO, mIL-3, and hIL-6. Cultures were demi-depopulated as needed and cells enumerated weekly. Data are fold-change in cell number from the initial cell number (900 to 3 × 105/well), used to initiate cultures on day 0, and are representative of two experiments with similar results (the 2nd experiment used cells from TBI-Veh mice at 4 mo. post-TBI). When data on day 8 from all 3 cultures are pooled together, cell number of the “positive” phenotype (Sca- 1+, cKit+, and CD150+) of the NI and the positive phenotypes of the TBI-Veh are significantly increased compared to their respective negative phenotypes (Sca-1-, cKit-, and CD150-); p≤0.0111. Cells from NI mice (panel D) and TBI-Veh mice (panel E) used to initiate cultures in panels A, B, and C were also examined for hematopoietic progenitor cells in standard methylcellulose assays. Data in panels D and E are the mean number of colonies per 2,500 cells from 2 experiments initiated with cells from TBI-Veh mice at 4 mo. and 8 mo. post-TBI. Brackets indicate significant differences (p<0.05) in total number of progenitors. Panel F shows monthly donor-derived chimerism in competitive transplantation assays in mice transplanted with lin- CD150+ cells or lin- CD150− cells isolated from NI mice or TBI-Veh mice 9.4 mo. post-8.72 Gy. 1.3 × 104 cells from a pool of 2 donors were transplanted into recipient mice (n=2 recipients for the NI CD150+ group; n=3 recipients for all other groups) along with 2 × 105 competitor cells. *p<0.01 comparing TBI-Veh lin- CD150+ with TBI-Veh lin- CD150-, and NI lin- CD150+ with NI lin- CD150-. Panel G shows donor lineage reconstitution in peripheral blood at 6 mo. post-transplant of the mice in panel F. Data in panel G represent the percentage of donor CD8+ (8), CD4+ (4), B220+ B cells (B), and Gr-1+ granulocytes (G), expressed as a percentage of total donor cells. Panel H shows lineage reconstitution by the congenic competitor cells (expressed as a percentage of total competitor cells) that were co-transplanted with cells from NI or TBI-Veh mice and which acts as an internal standard for normal lineage reconstitution.
To further validate these findings, the long-term term repopulating potential of lin- CD150+ and lin- CD150− isolated from TBI-Veh mice was examined in competitive transplantation assays in comparison to similar cells from NI mice. As expected, lin- CD150+ cells isolated from NI mice demonstrated predictably robust and sustained donor chimerism of between 40–60%, indicative of HSC activity (Fig. 4F). The TBI-Veh lin- CD150+ population showed initial donor chimerism at 1 month post-transplant of ~35%, however, this was not sustained and decreased to ~10% for the remainder of the 6 month period. Chimerism in mice transplanted with lin- CD150- cells from both NI and TBI-Veh mice was significantly less than chimerism in mice transplanted with lin- CD150+ cells (Fig. 4F). These data strongly suggested that the HSC phenotype, with respect to CD150 expression, in TBI-Veh mice was the same as in naïve non-irradiated mice, namely CD150+. The transient donor chimerism suggested the presence of short-term repopulating progenitors within the TBI-Veh lin- CD150+ population and lack of long-term engrafting HSC, in agreement with our previous findings (Chua et al. 2012).
We have also transplanted whole bone marrow (WBM) in comparison to purified KSL CD150+ cells from TBI-Veh mice and have shown similar diminished engraftment potential of WBM as that observed with KSL CD150+ cells in Fig. 3, further substantiating that there were very few cells of any phenotype with long-term engraftment potential in the marrow of TBI-Veh mice (data not shown).
Lineage reconstitution in recipients of TBI-Veh lin- CD150+ cells was skewed toward the myeloid lineage, similar to the myeloid skewing previously reported for KSLCD150+ cells isolated from TBI-Veh mice (Fig. 4G) (Chua et al. 2012). Lineage reconstitution data could not be obtained for mice transplanted with lin- CD150- cells, as the low donor chimerism did not allow lineage assessment. Interestingly, the NI lin- CD150- population showed a similar profile of multi-lineage reconstitution as observed for the NI lin- CD150+ population (Fig. 4G) and F1 competitor cells (Fig. 4H), indicating that while stem cell potential was low in NI lin- CD150- cells, multi-lineage reconstitution potential was retained, in agreement with Weksberg et. al. (Weksberg et al. 2008). Taken together, these data strongly suggest that HSC in TBI-Veh mice continue to express cKit, Sca-1, and CD150, just as HSC in NI mice, albeit severely depleted of functional HSC activity compared to NI controls.
DISCUSSION
The data reported herein represent a composite analysis of more than 38 H-ARS studies conducted over seven years, most of which were screening studies for survival efficacy of candidate medical countermeasures according to the FDA’s Animal Rule. These studies utilized more than 1,500 initial vehicle-treated control mice exposed to doses of radiation ranging from the LD50/30 to the LD90/30, and 585 day-30 survivors that were then aged in our animal facility for examination of the delayed effects of acute radiation exposure (DEARE) affecting the hematopoietic system. These data expand upon earlier publications generated with smaller group sizes and datasets (Chua et al. 2012; Chua et al. 2014), and provide increased power and rigor to our observation of a permanent defect in hematopoiesis originating at the level of the hematopoietic stem cell (HSC). Radiation-induced HSC dysfunction in survivors of lethal radiation exposure affected the two hallmarks properties of HSC, namely self-renewal and multilineage differentiation capability, manifesting as significantly reduced production of blood cells and premature prominent myeloid skewing.
The novel aspect of these data is the substantial dataset from which they were derived. Examinations of DEARE in survivors of lethal radiation exposure are difficult to conduct, primarily due to expense of long-term animal care and the need for large numbers of animals for statistically meaningful data. Retrospective analysis of unrelated studies from multiple investigators are often not a feasible option due to difficulty understanding the effect that differing exposure parameters and husbandry may have on DEARE outcomes. The mega-mouse experiments conducted at Argonne National Laboratory from 1972 to 1989 irradiated 49,000 mice (Wang et al. 2010; Haley et al. 2011), but differences in radiation geometry, fractionation schedules, type, and dose rate in subgroups prevented analyses of a dataset as large as the current study. Human data are even more difficult to examine. The most extensively studied human data are derived from survivors of the atomic bombings in Hiroshima and Nagasaki, but confounding factors such as age and nutritional status at time of exposure, range of doses received, radiation quality, and difficulties in determining exposure dose and exposure geometry make correlations of disease risk and radiation exposure difficult (Little 2009). In apparent contrast to our data, Kyoizumi et. al (Kyoizumi et al. 2016) showed seeming recovery of hematopoietic stem and progenitor cells in atomic bomb survivors. However, the doses received by the atomic bomb survivors were likely sub-lethal (albeit still damaging), allowing some recovery of hematopoietic stem and progenitor cells, while the doses of radiation received by mice in the current studies were in the lethal range (LD50/30-LD90/30). Other studies have shown possible reductions in self renewal capability of hematopoietic stem / progenitor cells secondary to dose-dependent DNA damage (Kajimura et al. 2016), in agreement with our studies showing loss of HSC repopulating potential in competitive transplantation assays which does not recover for the life of the mouse. Others have documented detriments in immune function in atomic bomb survivors (Kusunoki and Hayashi 2008), similar to our studies showing severe myeloid skew and a relative decrease in circulating lymphocytes much greater than that of other hematopoietic lineages in irradiated mice. The myeloid skew observed in irradiated mice illustrated the loss of multilineage differentiation potential of HSC from irradiated survivor mice.
A potential concern in our examinations of HSC multilineage engraftment potential of bone marrow KSL CD150+ cells from irradiated survivor mice is that, had the phenotype of HSC changed in irradiated survivors, then assaying KSL CD150+ cells as the “HSC phenotype” may have been in error. A precedent for a change in the HSC phenotype after irradiation lies in the observation of significant downregulation of cell surface cKit on HSC after irradiation (Shen et al. 2004) and (unpublished observations), likely due to local environmental factors in the irradiated bone marrow niche. While expression of cKit eventually returned on HSC exposed to an irradiated microenvironment, this did not preclude changes in other cell surface markers that may alter the phenotype of HSC from irradiated mice long after exposure. To this point, expression of CD150 is remarkably upregulated on KSL cells from irradiated survivor mice for life as we (Chua et al. 2012) and others (Simonnet et al. 2009) have documented. To ensure that interrogating KSL CD150+ cells for HSC potential was not pursuing the wrong HSC phenotype, we examined hematopoietic function of lineage-negative cells subdivided based on expression or lack thereof of Sca-1, cKit, or CD150. In agreement with the documented HSC phenotype of lineage-negative Sca-1+ cKit+ CD150+ in non-irradiated naïve mice, cells from irradiated survivors expressing these markers performed better in all hematopoietic assays compared to their counterparts lacking expression, substantiating the assumption that the definition of KSL CD150+ as the phenotype of long-term repopulating HSC does not change in survivors of lethal radiation exposure.
CONCLUSION
A compilation of 38 similar H-ARS studies conducted over a seven-year period in the authors’ laboratory, comprising more than 1,500 irradiated young adult C57BL/6 mice and nearly 600 day-30 survivors, was assessed for hematopoietic DEARE at various times up to 30 months of age. This large dataset adds rigor and validity to our findings of persistent hematopoietic dysfunction in H-ARS survivors that arises at the level of the hematopoietic stem cell and which adversely affects all classes of hematopoietic cells for the life of the survivor.
Acknowledgments
FUNDING:
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases (NIAID) under contracts HHSN266200500043C and HHSN272201000046C and grants 1U01AI107340-01 and 2R44 AI088288-03A1, and National Institute on Aging (NIA) under grant R01AG046246-01, National Institutes of Health, Department of Health and Human Services, and the Department of Defense under grants PR140896, PR141527, and PR140433P1.
LITERATURE
- (FDACDER) FaDACfDEaR (2015). Product development under the animal rule: Guidance for industry, FDA Silver Spring, MD. [Google Scholar]
- Abramson S, Miller RG and Phillips RA. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. Journal of Experimental Medicine 145: 1567; 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens AJM, Rudolph KL and Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol 16(3): 201–207; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkacemi Y, Bouchet S, Frick J, Huchet A, Pene F, Aigueperse J, Gourmelon P, Lopez M and Gorin NC. Monitoring of residual hematopoiesis after total body irradiation in humans as a model for accidental x-ray exposure: dose-effect and failure of ex vivo expansion of residual stem cells in view of autografting. Int J Radiat Oncol Biol Phys 57(2): 500–507.; 2003. [DOI] [PubMed] [Google Scholar]
- Botnick LE, Hannon EC and Hellman S. A long lasting proliferative defect in the hematopoietic stem cell compartment following cytotoxic agents. Int J Radiat Oncol Biol Phys 5(9): 1621–1625; 1979. [DOI] [PubMed] [Google Scholar]
- Casarett A (1968). Radiation biology Englewood, New Jersey, Prentice-Hall Inc. [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Joshi M, Tabbey R, Katz BP, MacVittie TJ and Orschell CM. Long-Term Hematopoietic Stem Cell Damage in a Murine Model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Physics 103(4): 356–366 310.1097/HP.1090b1013e3182666d3182666f; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HL, Plett PA, Sampson CH, Katz BP, Carnathan GW, MacVittie TJ, Lenden K and Orschell CM. Survival efficacy of the pegylated G-CSF Maxy-G34 and Neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Physics 106(1): 21–38; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, Moulder JE, Preston RJ, Seed TM, Stone HB, Tofilon PJ and Wong RS. Molecular and cellular biology of moderate- dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res 159(6): 812–834; 2003. [DOI] [PubMed] [Google Scholar]
- Crawford L (2002). New drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. U. D. o. H.a. H. S. Food and Drug Administration, Federal Register. 21 CFR parts 314 and 601, FDA, HHS; ACTION: Final Rule: 37988–37998 [PubMed]
- Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ and Farese AM. The hematologist and radiation casualties. Hematology: 473–496; 2003. [DOI] [PubMed] [Google Scholar]
- Faller DV, Castaneda SA, Zhou D, Vedamony M, Newburger PE, White GL, Kosanke S, Plett PA, Orschell CM, Boosalis MS and Perrine SP. An oral HemokineTM, α-methylhydrocinnamate, enhances myeloid and neutrophil recovery following irradiation in vivo. Blood Cells, Molecules, and Diseases 63: 1–8; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D Acute radiation response of mice from a cross between radiosensitive and radioresistant strains. Genetics 43: 835–843; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn D and Hamilton K. Genetic variation in the acute lethal response of four inbred mouse strains to whole body x-irradiation. Genetics 42(3): 189–198; 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B, Wang Q, Wanzer B, Vogt S, Finney L, Yang PL, Paunesku T and Woloschak G. Past and future work on radiobiology mega-studies: a case study at Argonne National Laboratory. Health Phys 100(6): 613–621; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E (2000). Acute effects of total-body irradiation. Radiobiolgy for the radiologist Hall E Philadelphia, PA, Lippincott Williams & Wilkins: 124–135. [Google Scholar]
- Hellman S and Botnick LE. Stem cell depletion: an explanation of the late effects of cytotoxins. Int J Radiat Oncol Biol Phys 2(1–2): 181–184; 1977. [DOI] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Stilger KN, Plett PA, Sampson CH, Chua HL, Orschell CM and Pelus LM. Recovery from hematopoietic injury by modulating prostaglandin E(2) signaling post-irradiation. Blood Cells Mol Dis 50(3): 147–153; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hirabayashi Y, Mitsui H, Sasaki H, Cronkite EP, Bullis JE Jr., Bond VP and Yoshida K Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction. Exp Hematol 23(12): 1296–1300.; 1995. [PubMed] [Google Scholar]
- Jones RJ, Celano P, Sharkis SJ and Sensenbrenner LL. Two phases of engraftment established by serial bone marrow transplantation in mice. Blood 73(2): 397–401; 1989. [PubMed] [Google Scholar]
- Jones RJ, Wagner JE, Celano P, Zicha MS and Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming units. Nature 347: 188–189; 1990. [DOI] [PubMed] [Google Scholar]
- Kajimura J, Kyoizumi S, Kubo Y, Misumi M, Yoshida K, Hayashi T, Imai K, Ohishi W, Nakachi K, Weng NP, Young LF, Shieh JH, Moore MA, van den Brink MR and Kusunoki Y. Relationship between spontaneous gammaH2AX foci formation and progenitor functions in circulating hematopoietic stem and progenitor cells among atomic-bomb survivors. Mutat Res Genet Toxicol Environ Mutagen 802: 59–65; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga LM, Van Os R, Ausema A, Noach EJK, Weersing E, Dontje B, Vellenga E and De Haan G. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells 23(1): 82–92; 2005. [DOI] [PubMed] [Google Scholar]
- Keller G and Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med 171(5): 1407–1418; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Moon H- B and Spangrude GJ. Major Age-Related Changes Of Mouse Hematopoietic Stem/Progenitor Cells. Annals of the New York Academy of Sciences 996(1): 195–208; 2003. [DOI] [PubMed] [Google Scholar]
- Kusunoki Y and Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol 84(1): 1–14; 2008. [DOI] [PubMed] [Google Scholar]
- Kyoizumi S, Kubo Y, Misumi M, Kajimura J, Yoshida K, Hayashi T, Imai K, Ohishi W, Nakachi K, Young LF, Shieh J-H, Moore MA, van den Brink MRM and Kusunoki Y. Circulating Hematopoietic Stem and Progenitor Cells in Aging Atomic Bomb Survivors. Radiation research 185(1): 69–76; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot 29(2a): A43–59; 2009. [DOI] [PubMed] [Google Scholar]
- Mauch P, Rosenblatt M and Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood 72(4): 1193–1196; 1988. [PubMed] [Google Scholar]
- McCulloch E and Till J. The sensitivity of cells from normal mouse bone marrow to radiation in vitro and vivo. Radiation Res 16: 822; 1962. [PubMed] [Google Scholar]
- Meng A, Wang Y, Van Zant G and Zhou D. Ionizing radiation and busulfan induce premature senescence in murine bone marrow hematopoietic cells. Cancer Res 63(17): 5414–5419; 2003. [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A and Weissman IL. The aging of hematopoietic stem cells. Nature Medicine 2(9): 1011–1016; 1996. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA and Cornall RJ. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447(7145): 686–690; 2007. [DOI] [PubMed] [Google Scholar]
- Plett PA, Chua HL, Sampson CH, Katz BP, Fam CM, Anderson LJ, Cox G and Orschell CM. PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Physics 106(1): 7–20; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Jackson W, Vemula S, Sellamuthu R, Fisher A, Feng H, Wu T, MacVittie TJ and Orschell CM. The H-ARS Dose Response Relationship (DRR): Validation and Variables. Health Physics 109(5): 391–398; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, Johnson CS, Katz BP, Farese AM, Parker J, MacVittie TJ and Orschell CM. Establishing a Murine Model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Physics 103(4): 343–355 310.1097/HP.1090b1013e3182667309; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J and Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447(7145): 725–729; 2007. [DOI] [PubMed] [Google Scholar]
- Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M and Rube C. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One 6(3): e17487; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, Bratanova-Toshkova TK, Shakhova VV, Young J, Weil MM, Panoskaltsis-Mortari A, Orschell CM, Baker PS, Gudkov A and Feinstein E. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). PLoS One 7(3): e33044; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Yu H, Yuan Y, Huang P and Cheng T. Rapid Downregulation of c-Kit Expression Is Associated with Reduced Repopulation Potential of Donor Hematopoietic Stem Cells in Recipients after Total Body Irradiation. Blood 104(11): 1197–1197 (meeting abstract); 2004. [Google Scholar]
- Simonnet AJ, Nehme J, Vaigot P, Barroca V, Leboulch P and Tronik-Le Roux D. Phenotypic and functional changes induced in hematopoietic stem/progenitor cells after gamma-ray radiation exposure. Stem Cells 27(6): 1400–1409; 2009. [DOI] [PubMed] [Google Scholar]
- Snoeck HW. Aging of the hematopoietic system. Curr Opin Hematol 20(4): 355–361; 2013. [DOI] [PubMed] [Google Scholar]
- Till JE and McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research 14: 213; 1961. [PubMed] [Google Scholar]
- Unthank JL, Miller SJ, Quickery AK, Ferguson EL, Wang M, Sampson CH, Chua HL, DiStasi MR, Feng H, Fisher A, Katz BP, Plett PA, Sandusky GE, Sellamuthu R, Vemula S, Cohen EP, MacVittie TJ and Orschell CM. Delayed Effects of Acute Radiation Exposure in a Murine Model of the H-ARS: Multiple-Organ Injury Consequent to <10 Gy Total Body Irradiation. Health Physics 109(5): 511–521; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bekkum DW. Radiation sensitivity of the hemopoietic stem cell. Radiat Res 128(1 Suppl): S4–8.; 1991. [PubMed] [Google Scholar]
- Visser JWM, Bauman JGJ, Mulder AH, Eliason JF and DeLeeuw AM. Isolation of murine pluripotent hematopoietic stem cells. Journal of Experimental Medicine 159: 1576; 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Paunesku T and Woloschak G. Tissue and data archives from irradiation experiments conducted at Argonne National Laboratory over a period of four decades. Radiat Environ Biophys 49(3): 317–324; 2010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Schulte BA, LaRue AC, Ogawa M and Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 107(1): 358–366; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Schulte BA and Zhou D. Hematopoietic stem cell senescence and long-term bone marrow injury. Cell Cycle 5(1): 35–38; 2006. [DOI] [PubMed] [Google Scholar]
- Weksberg DC, Chambers SM, Boles NC and Goodell MA. CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood 111(4): 2444–2451; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas JM and Storer JB. The effect of age on two modes of radiation death and on hematopoietic cell survival in the mouse. Radiat Res 32(3): 596–605.; 1967. [PubMed] [Google Scholar]


