Abstract
Understanding why breast cancer survivors are at an increased risk for cognitive and affective disorders is essential for developing targeted treatment plans and improving quality of life. Microglia priming results in chronic neuroinflammation and can contribute to neuronal degeneration and dysfunction, thereby offering a potential mechanism for altered brain function that persists after tumor removal. This study examined whether mammary tumors alter microglia and augment the inflammatory profile and behavior of mice. To test this, non-metastatic mammary tumor cells (67NR) were injected orthotopically into the mammary glands of BALB/c mice, allowed to grow for 16 days, and then the tumors were removed via mastectomy. Following a 14-day surgical recovery, the mice were challenged with lipopolysaccharide (LPS), and then central and peripheral inflammation, anxiety, and depressive-like behavior were evaluated. Here we show that major central and peripheral inflammatory markers were not altered by tumor growth nor mastectomy surgery alone. However, hippocampal mRNA expression of major proinflammatory cytokines IL-1β and TNFα was increased in tumor removal animals, persisting past surgical recovery. Nonetheless, the immune and behavioral responses following LPS administration were comparable among groups. In sum, these data demonstrate that the combination of tumor and mastectomy promotes neuroinflammation; however, immune challenge did not elucidate this inflammation as maladaptive for the host.
Keywords: Breast cancer, Mastectomy, Neuroinflammation, Microglia, Priming
1. Introduction
There were more than 250,000 new breast cancer diagnoses in the United States in 2017, and among those diagnosed nearly 90% are expected to survive 5 years (Howlader et al., 2017). Even after treating the tumors, many women experience mental health sequelae including depression and anxiety that can persist for years (Bower et al., 2008; Maass et al., 2015; Wang et al., 2014). The pathogenesis linking breast cancer with cognitive and affective deficits is unclear, but previous studies have implicated neuroinflammation triggered by the tumor itself (Norden et al., 2015; Pyter et al., 2009; Yang et al., 2014) and/or aggressive treatment modalities such as chemotherapy (Ahles and Saykin, 2002; Downie et al., 2006) as key players. Understanding the cause of these deficits is essential to developing more targeted treatment plans and improving quality of life for breast cancer survivors.
Microglia, innate immune cells of the central nervous system (CNS), receive and propagate inflammatory signals from peripheral tissues in addition to acting locally (Norden and Godbout, 2013). Thus, microglia are an integral component of the immune-brain interface and communicating peripheral immune stimuli to the brain. Microglia exhibit a ramified phenotype with many long processes, but in response to a pathologic insult undergo a morphologic change characterized by shorter, wider processes and increased expression of inflammatory markers (Streit et al., 1999; Wolf et al., 2017). In general, activated microglia and the resulting inflammation and sickness behavior are thought to protect the host through strategic physiological and behavioral changes (Hart, 1988; Perry et al., 2007). However, during certain processes such as aging, stress, or CNS injury “primed” microglia can arise. Primed microglia result in a heightened and prolonged response to homeostatic disturbances, thereby contributing to enhanced neuroinflammation and neurodegeneration (Fenn et al., 2015; Wolf et al., 2017). Further, pro-inflammatory cytokines including interleukin-1β (IL-1 β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNFα) not only induce sickness behaviors but also can promote potentially mala-daptive behaviors, such as anxiety and depression (Dantzer et al., 2008; Hou et al., 2017; Thomas et al., 2005; Tiemeier et al., 2003; Tuglu et al., 2003). It is unknown whether peripheral tumors are able to prime microglia, which could provide insight into a potential pathogenesis for the neurological disorders reported by breast cancer survivors.
The purpose of this study was to examine whether peripheral tumors, specifically mammary tumors, prime microglia and augment the inflammatory profile and behavior of mice. To test this, non-metastatic mammary tumor cells (67NR) were orthotopically injected into BALB/c mice, allowed to grow for 16 days, and then the tumors were removed via mastectomy surgery to model a breast cancer survivor. Following surgical recovery, the mice were challenged with lipopolysaccharide (LPS), and subsequent central and peripheral inflammation, anxiety, and depressive-like behavior were evaluated.
2. Results
2.1. Tumor burden
Mammary tumors were resected at day 16 of growth (n = 46), at which point they comprised a mean 0.398% ± 0.040 of body mass across experiments. Accounting for treatment groups, tumor burden in animals that received saline injection (0.360% ± 0.065 of body mass) was not different than in those that received LPS injection (0.425% ± 0.052 of body mass) (Fig. 1B, p > 0.05). Body weight change, deducting tumor weight, was also comparable for all mice following their respective surgery and treatment (p > 0.05, data not shown).
Fig. 1.
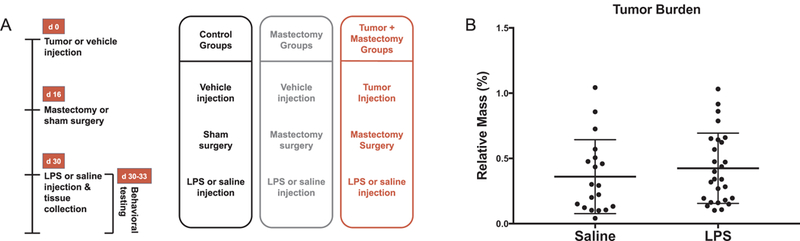
(A) Experimental timeline for control, mastectomy, and tumor + mastectomy treatment groups. (B) Tumor burden for experimental animals (percentage of relative body mass) expressed as treatment mean ± SEM. Mean tumor burden encompassing all experiments was comparable for LPS (n = 27) and saline (n = 19) treatment groups (p > 0.05).
2.2. No inflammation is present at the time of mastectomy
The first study assessed the central and peripheral inflammatory profile of mice at day 16 of mammary tumor growth, which corresponds with the time tumors are surgically removed in subsequent experiments. Specifically, this compared inflammation existing at the time of mastectomy surgery in tumor animals injected with 67NR cells to control animals injected with saline (n = 15/group). There were no differences in serum inflammation when comparing IL-1β, TNFα, IFNγ, IL-6, IL-5, IL-2, and IL-10 serum protein concentrations between tumor and control animals (Fig. 2A, p > 0.05). However, mice with tumors had increased serum CXCL1 (KC/GRO) concentrations relative to mice without tumors (Fig. 2A, t (26) = 4.03, p < 0.001). Likewise, there were no significant differences in hippocampal inflammation present with respect to IL-1β, TNFα, IFNγ, and IL-6 mRNA expression between tumor and control animals (Fig. 2C, p > 0.05). Moreover, serum corticosterone concentrations, which have the potential to alter inflammation, did not differ between tumor animals and control animals at the time of sampling (Fig. 2B, p > 0.05).
Fig. 2.
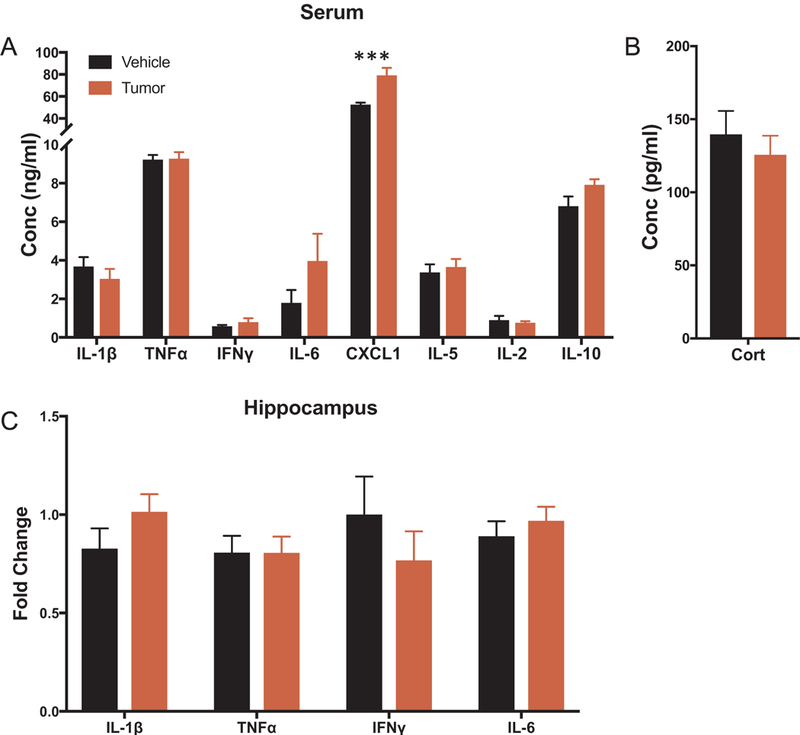
Inflammatory profile of mice at day 16 of tumor growth (n = 15/group). (A-B) Serum protein concentrations of IL-1β, TNFα, IFNγ, IL-6, CXCL1, IL-5, IL-2, IL-10 and corticosterone. (C) Hippocampal mRNA expression of IL-1β, TNFα, IFNγ, and IL-6 displayed as fold change from vehicle animals. Data is presented as treatment mean ± SEM, ***p < 0.001.
2.3. Mammary tumor removal attenuates several inflammatory markers and corticosterone in serum
For the second study, central and peripheral inflammatory markers were evaluated in mice from the following experimental groups: (1) control (no tumor + skin incision only), (2) mastectomy control (no tumor + mammary gland removal), and (3) tumor + mastectomy. This measured inflammation present following 14 d recovery from mastectomy surgery in tumor-bearing mice compared to the control and mastectomy control groups. A group consisting of sham surgery in tumor-bearing mice was not included because the tumor sizes would have reached removal criteria prior to completion of the study. Among the three surgical groups, half of the mice were challenged with a single intraperitoneal injection of 0.33 mg/kg LPS and the other half received volume-matched isotonic saline. Tissues were collected 4 h after injection (n = 20–25/group).
Among animals that were immune challenged (received LPS injection), tumor + mastectomy animals had lower levels of serum CXCL1, IL-2, and corticosterone than both controls and mastectomy controls (Fig. 3E, p < 0.05; Fig. 3F, p < 0.05; Fig. 3I, p < 0.01, respectively). When comparing animals that were not immune challenged (received saline injection), there were no differences in serum inflammation (IL-1β, TNFα, IL-6, CXCL1, IL-2, IL-5, or IL-10) or corticosterone between surgical groups (Fig. 3, p > 0.05), with the exception of IFNγ. Serum IFNγ was significantly lower in the tumor + mastectomy group than the control group (Fig. 3C, p < 0.05).
Fig. 3.
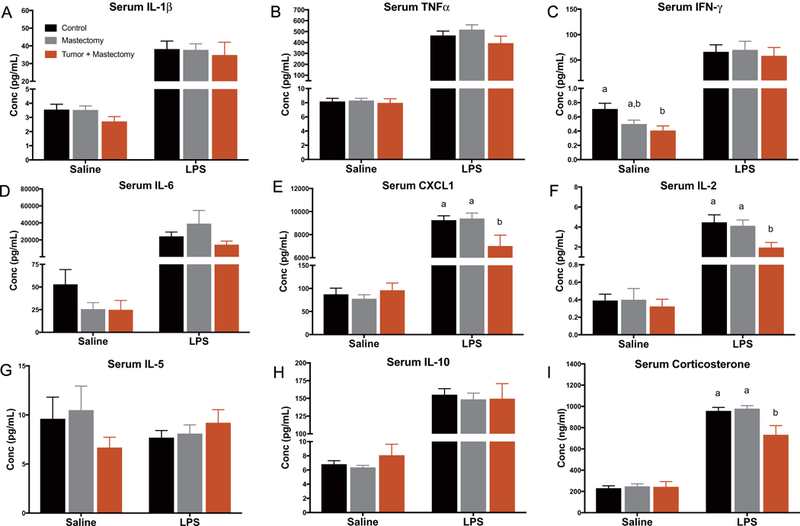
Circulating inflammatory protein markers in control, mastectomy, and tumor + mastectomy groups measured 4 h after receiving either LPS or saline injection (n = 20–25/group). (A-I) Serum protein concentrations of IL-1β, TNFα, IFNγ, IL-6, CXCL1, IL-2, IL-5, IL-10 and corticosterone expressed in all treatment groups. Data is presented as treatment mean ± SEM. Graph bars that do not share a letter are statistically significantly different at p < 0.05.
2.4. Mammary tumor removal increases hippocampal inflammation, but not following LPS
Also for the second study, central inflammation was evaluated 4 h following LPS or saline injection at the 14 day recovery time point. For mice that were not immune challenged, hippocampal mRNA expression of proinflammatory cytokines IL-1β and TNFα, microglial marker CD68, and anti-inflammatory cytokine receptor IL-4Rα was increased in the tumor + mastectomy group. Specifically, IL-1β, CD68, and IL-4Rα expression was increased in tumor + mastectomy animals compared to control animals (Fig. 4, p < 0.05), whereas TNFα was in-creased compared to both the control and mastectomy control animals (Fig. 4B, p < 0.01). However, when mice received LPS injection prior to tissue collection, there was no difference in inflammatory or priming marker mRNA expression between treatment groups (Fig. 4A–G, p > 0.05).
Fig. 4.
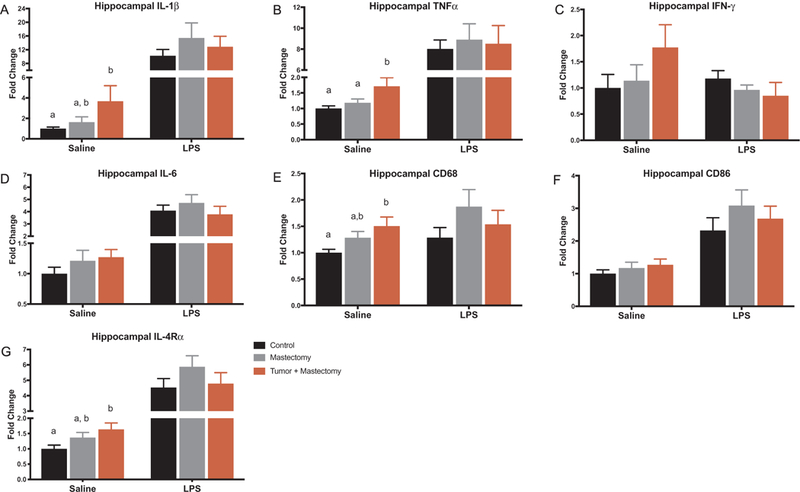
Hippocampal inflammatory gene expression in control, mastectomy, and tumor + mastectomy groups measured 4 h after receiving either LPS or saline injection (n = 20–25/group). (A-G saline) Hippocampal mRNA levels of IL-1β, TNFα, IFNγ, IL-6, CD68, CD86, and IL-4R α expressed in all treatment groups 14 days after surgery. IL-1β, CD68, IL-4R α expression was increased in mice that had mastectomy tumor removal surgery compared to control animals (p < 0.05). TNFα expression was increased in mice following mastectomy tumor removal compared to both the control and mastectomy control animals (p < 0.01). (A-G LPS) When mice were injected with LPS 4 h prior to tissue collection, there were no significant differences between surgical groups. All data is presented as treatment mean ± SEM. Graph bars that do not share a letter are statistically significantly different at p < 0.05.
2.5. Mammary tumor removal does not alter anxiety or depressive-like behavior
A third study was conducted to assess whether behavioral differences could be detected between treatment groups (control, mastectomy control, and tumor + mastectomy animals; n = 15–20/group). This subset of mice was sacrificed 72 h after intraperitoneal injection of either 0.33 mg/kg LPS or volume-matched saline on day 14 of surgical recovery.
There were no group differences in total locomotor activity as measured by total number of beam breaks and rears in the open field test (Fig. 5B, C, p > 0.05), nor anxiety-like behavior as measured by central tendency in the open field test (Fig. 5A, p > 0.05). Similarly, no differences in depressive-like behavior as measured by latency to float and floating duration during forced swim testing (Fig. 5D, E, p > 0.05) nor sucrose consumption assessing anhedonia were present between treatment groups (Fig. 5F, p > 0.05).
Fig. 5.
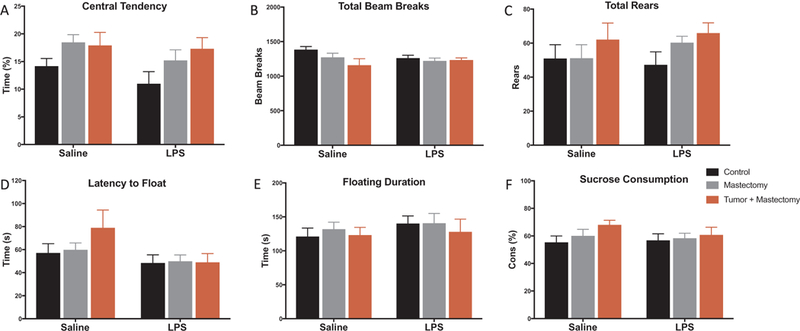
Behavioral testing was evaluated 72 h following LPS or saline injection for control, mastectomy, and mastectomy + tumor animals (n = 15–20/group). (A–C) Central tendency during first 5 min, total beam breaks, and total rears were evaluated during open field testing and were not different between treatment groups. (D–E) Latency to float and total floating duration were comparable between treatment groups during forced swim testing. (F) There was also no difference in sucrose consumption after 2 days of acclimation as a measure of anhedonia. All data is presented as treatment mean ± SEM. Graph bars that do not share a letter are statistically significantly different at p < 0.05.
3. Discussion
These studies were conducted to determine whether mammary tumors prime microglia, which would provide a potential pathogenesis for the anxiety and depression experienced by breast cancer survivors. Survivors have an increased risk of developing mental health disorders (Bower et al., 2008; Maass et al., 2015; Wang et al., 2014), despite prior treatment to physically remove the tumor. Because most women diagnosed with breast cancer undergo lumpectomy or mastectomy surgery as a mainstay of their treatment protocol (Fisher et al., 2002; Veronesi et al., 2002), studying mastectomized mice serves as an appropriate model for breast cancer survivorship (Pyter et al., 2017). Both a control (skin incision) and a surgical control (mastectomy without tumor) group were included in these experiments to assess surgical effects of the mastectomy procedure itself. We hypothesized mice that underwent mastectomy tumor removal would exhibit an exaggerated neuroinflammatory response following immune stimulation when compared to control animals. This would support the potential for peripheral tumors, explicitly mammary tumors, to prime microglia.
Within a subset of mice, we first measured the peripheral and central inflammation present after 16 days of 67NR mammary tumor growth. This determined the inflammatory profile of tumor-bearing mice at the time tumors are removed in subsequent experiments. We found that only CXCL1 (also known as KC/GRO) was increased in the serum of animals with tumors, which is consistent with previous studies utilizing this cell line (Walker et al., 2017). CXCL1 is an early signaling molecule which recruits support cells to promote angiogenesis, inflammation, and tumorigenesis (Acharyya et al., 2012; DeFilippo et al., 2013). Besides this early immune response in blood, all other inflammatory markers measured, including major proinflammatory cytokines IL-1 β, TNFα, IFNγ, and IL-6, were comparable between tumor-bearing and non-tumor-bearing animals in serum and hippocampal tissue. Thus, the mastectomy procedures in these experiments were performed before the tumors caused extensive peripheral inflammation, as observed in other studies at later time points after tumor induction (Walker et al., 2017).
Similar results were observed when examining the effects of mastectomy alone on inflammation. Following a 14-day recovery from mastectomy surgery, there were no differences in cytokine or chemokine levels in the blood or brain between control and mastectomy control animals. Both of these groups were non-tumor-bearing, but the surgical control mice underwent a mastectomy surgery mimicking the surgical time and invasiveness when performed to remove tumors. Together with Experiment 1, these data indicate that tumor growth alone nor mastectomy surgery alone significantly alter the central or peripheral inflammatory profile of mice.
Interestingly, there were significant changes to neuroinflammation when comparing tumor removal mice to controls. Specifically, there was increased hippocampal mRNA expression of IL-1 β, TNFα and CD68 in the tumor removal group after 14 days of surgical recovery. CD68 (macrosialin) is a scavenger receptor in monocytes that functions to clear cellular debris and induces microglial production of reactive oxygen species and inflammatory molecules (Wong et al., 2005). CD68 expression, along with other markers including CD86, has been used to identify active microglia (Norden and Godbout, 2013; Tanaka et al., 2013) and therefore were examined in the present studies. However, immunohistochemistry was not explicitly performed here, which is necessary for confirmation of microglial activation, as there are other cellular sources of IL-1β and TNFα. CNS damage from disease, trauma, or toxicity is associated with activation of microglia and astrocytes and the resulting cytokine and chemokine production including IL-1β and TNF α (Block and Hong, 2005; Sriram and O’Callaghan, 2007; Ubogu et al., 2006). We found that mammary tumor growth and removal, but not tumor growth or surgical removal alone, increases expression of major proinflammatory markers in brain tissue. This suggests a synergistic neuroinflammatory effect when mammary tumor growth is followed by mastectomy surgery and indicates that previous tumor growth may affect how the immune system reacts to surgery.
Neuroinflammation discussed herein is defined as the increased presence of proinflammatory cytokines within CNS tissue; accordingly, there was chronic neuroinflammation present in this mouse model of breast cancer survivorship. Not all neuroinflammation is functionally similar; there are varying degrees of neuroinflammation and, depending on the situation, it may result in positive or negative consequences for the host. For example, transient, controlled neuroinflammation is generally considered beneficial as these cytokines and chemokines recruit leukocytes to the brain and improve phagocyte efficacy (DiSabato et al., 2016). This leads to enhanced tissue repair and promotes host recovery. Furthermore, repeated subthreshold immune challenges prior to infection (termed euflammation) is associated with reduced sickness behavior and increased neuroprotective effects (Liu et al., 2016; Tarr et al., 2014). On the other hand, maladaptive immune responses including chronic or uncontrolled neuroinflammation is generally damaging to the CNS. Increased proinflammatory cytokines including IL-1β, TNFα, and IFNγ, chemokines, reactive oxygen species, and other inflammatory mediators can cause neuronal degeneration and cognitive deficits (Norden and Godbout, 2013). The mixed positive and negative consequences of neuroinflammation complicate inter-pretation of its presence, and thus each situation must be evaluated separately considering context.
In these experiments, the tumor + mastectomy treatment group, although exhibiting increased neuroinflammation, simultaneously had decreased serum IFNγ and displayed no observed behavioral changes (specifically anxiety-like or depressive-like) as measured through open field, forced swim, and sucrose anhedonia testing. Furthermore, expression of the receptor for IL-4 (IL-4R α) was increased in the tumor removal group. IL-4 is an antiinflammatory cytokine that reduces acute inflammation and promotes an M2 microglial phenotype which helps switch from damage to repair during the healing process (DiSabato et al., 2016). Upregulation of this receptor follows a normal homeostatic response to proinflammatory cytokines, a response that can be dampened or missing with microglial insults such as aging (Fenn et al., 2012). Taken together, these data suggest that the modest, controlled neuroinflammation observed following mastectomy tumor removal fits with an advantageous rather than harmful host response under these particular conditions.
The next experiments administered lipopolysaccharide (LPS) to the treatment groups to evaluate the response to an immune challenge. LPS is a bacterial endotoxin derived from the cell wall of Gram negative bacteria and is commonly used as an immune stimulus with well-documented physiologic and behavioral responses characterized as “sickness behavior” (Banasikowski et al., 2015). Primed microglia with a reactive phenotype have an exacerbated response to a single LPS in-jection, resulting in larger increases in central and peripheral inflammatory markers (e.g. IL-1β and IL-6) than controls (Godbout et al., 2005). Previous studies have examined the effects of tumor-induced inflammatory priming in tumor-bearing animals (Pyter et al., 2014; Vichaya et al., 2017). Indeed, administration of LPS to animals har-boring mammary tumors (Pyter et al., 2014) results in an exaggerated inflammatory response in the hippocampus (IκBα mRNA), cortex (CD11b mRNA), and hypothalamus (IDO1 mRNA) 4 h following LPS administration. Additionally, mice injected with oropharyngeal epithelial cells transfected with HPV E6/E7 oncogenes and hRAS demonstrate an exaggerated inflammatory response in the hippocampus (IL-1β and TNFα mRNA) and cortex (IL-1β mRNA), 24 h following LPS ad-ministration (Vichaya et al., 2017). However, in the present study, there were no exaggerated increases in the inflammatory response 4 h following LPS injection or prolonged behavior deficits 72 h after LPS administration observed in the tumor removal group consistent with microglial priming; in fact, serum IL-2 and CXCL1 levels were increased to a significantly lesser degree in the tumor removal group than both surgical control groups.
Additionally, the increased mRNA expression of hippocampal IL-1β, TNFα, and CD68 observed in the tumor removal group without immune challenge was not apparent following LPS injection. Moreover, no anxiety or depressive-like behavioral changes were measured through open field, forced swim, and sucrose anhedonia testing 72 h after LPS injection. At this later time point we would not expect to see behavioral changes from LPS injection in the control groups but would expect to capture prolonged sickness behavior if the tumor and its removal had in fact caused microglial priming. Ergo, because there were no group differences in behavior, we reject the hypothesis that microglial priming was likely to have occurred in response to the tumor and its removal. These collective data following immune challenge further support that the neuroinflammation present after mastectomy tumor removal may be providing more of a benefit than detriment to the host.
In these experiments we did not find convincing evidence that mammary tumor growth followed by mastectomy surgery primes microglia in mice. The LPS dose we used (0.33 mg/kg LPS injection) was based on previous studies, in which it was effective in demonstrating microglial priming (Fenn et al., 2015; Godbout et al., 2005). However, it is possible that either a different type of immune challenge or a different dose of LPS may have yielded an exaggerated inflammatory response or behavioral changes between treatment groups. Likewise, our analysis timing (serum and hippocampus at 4 h and behavior at 72 h following LPS injection) was based on prior priming studies (Fenn et al., 2015), but still provide a mere snapshot of potential priming effects. Lastly, the 67NR cell line was chosen for these experiments because it does not metastasize or leave the primary tumor site (Heppner et al., 2000). This was critical to eliminate confounding results such as neuroinflammation and behavioral changes due to spread of the tumor to other parts of the body including the CNS, rather than measuring the response to a single peripheral tumor. While possessing this ideal characteristic, the 67NR cell line does not induce as robust of an inflammatory response as other murine mammary tumor cell lines (Walker et al., 2017). Performing these experiments with other cell lines may provide evidence supporting microglial priming and observable behavioral changes.
In summary, the present study demonstrates that mammary tumorbearing followed by mastectomy surgery resulted in chronic neuroinflammation. Nonetheless, no exaggerated responses to immune challenge or behavioral changes were observed to substantiate that microglial priming arises from peripheral tumors. Instead, the resulting inflammation may have beneficial effects for the host under these experimental conditions. Further studies are needed to investigate the potential for microglial priming from other mammary tumor cell lines and peripheral tumors.
4. Materials and methods
4.1. Animals
Adult female BALB/cAnNCrl mice were obtained from Charles River Laboratories (Wilmington, MA) at 8 weeks of age. Mice were individually housed in microisolation cages (NextGen Mouse, Allentown Inc.) with 14:10 light/dark cycle and provided ad libitum access to irradiated food (Teklad 7912, Envigo) and chlorinated reverse-osmosis water. All mice were provided with 300 mL of ¼-inch corncob bedding (Bed-o’ Cobs, The Andersons, Maumee, OH) and a single 5 cm × 5 cm cotton nestlet (NES3600, Ancare) for environmental enrichment. Upon arrival, mice were acclimated for at least 7 days prior to experimental manipulations. All studies were approved by The Ohio State University IACUC and were conducted in AAALAC-accredited facilities.
4.2. Cell line
Murine mammary tumor cells (67NR cell line) were obtained from Barbara Ann Karmanos Cancer Institute (Detroit, MI). These cells were isolated from a spontaneous mammary tumor in a BALB/cfC3H mouse (Dexter et al., 1978). 67NR cells are highly tumorigenic, but non-metastatic and fail to leave their primary tumor site (Heppner et al., 2000). The cells were cultured using sterile technique in Dulbecco’s Modified Eagle Medium (Gibco 11965, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were incubated at 37 °C with 5% carbon dioxide in a hu-midified atmosphere. Cell passage remained constant throughout the experiment and cells tested negative for Mycoplasma contamination prior to injection (PlasmoTest kit, InvivoGen). An automated cell counter (Cell Countess II, Life Technologies) was used to verify number and viability of tumor cells. Cells were diluted with DMEM to a concentration of 1 × 106 cells per 10 µL for injection.
4.3. Tumor injections
Under general anesthesia, 67NR cells were injected into the right abdominal mammary gland (fourth mammary gland) using aseptic technique. Mice were induced using isoflurane gas and maintained in a surgical plane of anesthesia via precision vaporizer. A single 0.1 mg/kg dose of buprenorphine was administered subcutaneously. The hair around the mammary gland was removed via depilatory cream and the skin was aseptically prepared with alternating povidone-iodine and alcohol scrubs. An approximately 3 mm skin incision was made medial to the right abdominal nipple. Depending on study group, the mammary fat pad was visualized and injected with 100 µL of either 1 × 105 tumor cells or DMEM vehicle. The skin was apposed with tissue glue (Vetbond, 3 M, Maplewood, MN) and the mice were recovered from anesthesia. Once tumors were externally palpable, measurements were taken using a calipers every three days until removal. Mice with no visible tumor growth were excluded from data analysis.
4.4. Mastectomy surgery
Tumors grew for 16–17 days prior to surgical removal. Under general anesthesia, a skin-sparing mastectomy was performed to remove the right abdominal mammary gland and any visible tumor using aseptic technique. Mice were anesthetized and surgically prepared as described above, instead administering a single 1 mg/kg dose of sustained-release buprenorphine (Buprenorphine SR-LAB, ZooPharm, Windsor, CO) for postoperative analgesia. An approximately 5 mm skin incision was made medial to the right abdominal nipple. The tumor was excised using blunt dissection and electrocautery, keeping the tumor capsule intact. Any remaining visible fat pad was also removed. The skin incision was closed using polyglactin 910 suture (Vicryl, Ethicon Inc.) in a simple interrupted pattern.
Mastectomy surgery was also performed as described above on a subset of animals without tumors (“mastectomy control”). Alternatively, sham surgery animals only received a skin incision without mammary gland removal (“control”). Mice that had tumors regrow following mastectomy were excluded from analysis. All mice recovered from surgery for 14 days prior to LPS injection and sub-sequent tissue collection (Fig. 1A).
4.5. LPS injections
Mice were injected with lipopolysaccharides from Escherichia coli O127:B8 (Sigma-Aldrich, L5668) diluted with sterile saline. Mice received a 0.33 mg/kg dose of LPS intraperitoneally (IP) once either 4 h prior to tissue collection or 72 h prior to behavioral analysis and tissue collection depending on the experiment. Mice appear clinically normal after receiving this low dose of LPS.
4.6. Perfusion and tissue collection
Whole blood was collected from the facial vein, centrifuged at 2000×g for 25 min, and the serum supernatant was stored at −80 °C until assayed. Following blood collection, mice were injected with 270 mg/kg pentobarbital (Euthasol, Virbac, Fort Worth, TX) diluted with sterile saline IP. Mice were perfused with phosphate buffered saline immediately following loss of pedal reflex. Whole brain was collected in RNAlater Solution (Invitrogen, Fisher Scientific) over ice and stored at −80 °C until processed.
4.7. RNA extraction, cDNA, and PCR for inflammatory markers
The hippocampus was isolated from each brain and total RNA was extracted using Ambion TRIzol Reagent (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s specifications. A spectrophotometer (NanoDrop One, Thermo Scientific) verified the quality and quantity of RNA. M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) was used to synthesize cDNA. Probes for inflammatory genes of interest were obtained from Applied Biosystems (Life Technologies, Waltham, MA): IL-1β (Mm00434228_m1), IL-6 (Mm00446190m1), TNFα (Mm00443258_m1), IFNγ (Mm01168134_m1), CD68 (Mm03047343_m1), CD86 (Mm00444543_m1), and IL-4Rα (Mm01275139_m1).
4.8. Serum ELISA for inflammatory markers
Serum cytokine and chemokine levels were determined using V-PLEX Proinflammatory Panel 1 Mouse Kit (Meso Scale Discovery, Rockville, MD) according to manufacturer’s instructions. This kit measures mouse IFN-ɣ, IL-1β, IL-2, IL-4, IL-5, IL-6, KC/GRO (CXCL1), IL-10, IL-12p70, and TNF-α protein levels. The assay was read on a Meso QuickPlex SQ 120 machine and the results were analyzed using MSD Discovery Workbench 4.0 software. IL-4 and IL-12p70 failed to reach a detectable range in the serum of most of the mice, and therefore the data were not analyzed for any experiments.
4.9. Corticosterone testing
Serum corticosterone levels were measured in duplicate using DetectX Corticosterone Enzyme Immunoassay Kit (Arbor Assay, Ann Arbor, MI).
4.10. Sucrose anhedonia test
At the start of the dark cycle and immediately following LPS injection, mice were given 6 h ad lib access to normal drinking water in one bottle and 2% sucrose solution in a second bottle. Food was removed from the cage when the bottles were present. Access to the sucrose and regular water bottles repeated for 2 days additional days, switching the location of the sucrose each day to prevent positional bias. Consumption data was analyzed from the third day for exhibition of anhedonia and depressive-like behavior.
4.11. Open field test
All behavior testing beginning with open field test was completed during the animal’s active period, beginning with onset of the dark phase, and reflects previous studies (Walker et al., 2017). 72 h post-LPS injection, mice were placed in a 36 cm × 36 cm polypropylene open field arena with two stacked rows of infrared crossbeams. Beam breaks were recorded on each mouse for 10 min using Photobeam Activity System software (San Diego Instruments). Central tendency, number of rears, and locomotion activity were analyzed to assess anxiety-like behavior and locomotion.
4.12. Forced swim test
Following open field test, mice were placed in a 5000 mL glass beaker containing 3500 mL of water at approximately 27 °C for 5 min. The mice were videotaped then analyzed using Observer XT 8.0 software (Noldus Information Technology). Time spent swimming, time spent floating, and number of floating vs. swimming bouts were recorded to evaluate depressive-like behavior.
4.13. Statistical analysis
All data were analyzed using GraphPad Prism statistical software (Prism 7, GraphPad Software, La Jolla, CA). Outliers were identified using Grubb’s test and excluded prior to analyses. Student’s t-test (ex-periment 1) or standard one-way AVOVA (experiments 2 and 3) were used for statistical comparison. When appropriate, Tukey’s multiple comparisons test provided post hoc analyses. Results are reported as treatment means ± standard error of the mean. An alpha level of p ≤0.05 was considered significant.
HIGHLIGHTS.
Tumor growth and mastectomy alone did not alter inflammatory markers.
Tumor-bearing followed by mastectomy surgery resulted in chronic neuroinflammation.
Responses to immune challenge did not suggest microglial priming.
The inflammation present was likely beneficial rather than maladaptive.
Acknowledgments
The authors thank The Ohio State University for the exceptional animal care provided by University Laboratory Animal Resources. This research was supported by the National Institutes of Health [R01CA194924].
References
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J, 2012. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, 2002. Breast cancer chemotherapy-related cognitive dysfunction. Clin. Breast Cancer 3, 84–90. [DOI] [PubMed] [Google Scholar]
- Banasikowski TJ, Cloutier CJ, Ossenkopp KP, Kavaliers M, 2015. Repeated ex-posure of male mice to low doses of lipopolysaccharide: dose and time dependent development of behavioral sensitization and tolerance in an automated light-dark anxiety test. Behav. Brain Res 286, 241–248. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS, 2005. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol 76, 77–98. [DOI] [PubMed] [Google Scholar]
- Bower JE, 2008. Behavioral symptoms in breast cancer patients and survivors: fatigue, insomnia, depression, and cognitive disturbance. J. Clin. Oncol 26, 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, Van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N, 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121, 4930–4937. [DOI] [PubMed] [Google Scholar]
- Dexter DL, Kowalski HM, Blazer BA, Fligiel Z, Vogel R, Heppner GH, 1978. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res 38, 3174–3181. [PubMed] [Google Scholar]
- DiSabato DJ, Quan N, Godbout JP, 2016. Neuroinflammation: the devil is in the details. J. Neurochem 139, 136–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie FP, Mar Fan HG, Houede-Tchen N, Yi Q, Tannock IF, 2006. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psycho-Oncology 15, 921–930. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP, 2012. Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav. Immun 26, 766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP, 2015. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol. Psychiatry 76, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N, 2002. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of in-vasive breast cancer. New Engl. J. Med 347, 1233–1241. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW, 2005. Exaggerated neuroinflammation and sickness behavior in aged mice after activation of the peripheral innate immune system. FASEB J 19, 1329–1331. [DOI] [PubMed] [Google Scholar]
- Hart BL, 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev 12, 123–137. [DOI] [PubMed] [Google Scholar]
- Heppner GH, Miller FR, Shekhar PVM, 2000. Nontransgenic models of breast cancer. Breast Cancer Res 2, 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L, Baldwin DS, 2017. Peripheral inflammatory cytokines and immune balance in generalised anxiety disorder: case-controlled study. Brain Behav. Immun 62, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, 2017. SEER Cancer Statistics Review, 1975–2014 National Cancer Institute; https://seer.cancer.gov/csr/1975_2014/. [Google Scholar]
- Liu X, Nemeth DP, Tarr AJ, Belevych N, Syed ZW, Wang Y, Ismail AS, Reed NS, Sheridan JF, Yajnik AR, Disabato DJ, Zhu L, Quan N, 2016. Euflammation attenuates peripheral inflammation-induced neuroinflammation and mitigates immune-to-brain signaling. Brain Behav. Immun 54, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass SW, Roorda C, Berendsen AJ, Verhaak PFM, De Bock GH, 2015. The prevalence of long-term symptom of depression and anxiety after breast cancer treatment: a systematic review. Maturitas 82, 100–108. [DOI] [PubMed] [Google Scholar]
- Norden DM, Godbout JP, 2013. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol 39, 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Bicer S, Clark Y, Jing R, Henry CJ, Wold LE, Reiser PJ, Godbout JP, McCarthy DO, 2015. Tumor growth increases neuroinflammation, fatigue, and depressive-like behavior prior to alterations in muscle function. Brain Behav. Immun 43, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C, 2007. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol 7, 161–167. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Pineros V, Galang JA, McClintock MK, Prenergast BJ, 2009. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hy-pothalamic-pituitary-adrenal axis regulation. Proc. Natl. Acad. Sci 106, 9069–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Bih SEM, Sattar H, Prendergast BJ, 2014. Peripheral tumors alter neuroinflammatory responses to lipopolysaccharide in female rats. Brain Res 1552, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Suarez-Kelly LP, Carson WE, Kaur J, Bellisario J, Bever SR, 2017. Novel rodent model of breast cancer survival with persistent anxiety-like behavior and inflammation. Behav. Brain Res 330, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, O’Callaghan JP, 2007. Divergent roles for tumor necrosis factor-α in the brain. J. Neuroinnume Pharmacol 2, 140–153. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA, 1999. Reactive microgliosis. Prog. Neurobiol 57, 563–581. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Matsuwaki T, Yamanouchi K, Nishihara M, 2013. Exacerbated inflammatory response related to activated microglia after traumatic brain injury in progranulin-deficient mice. Neuroscience 231, 49–60. [DOI] [PubMed] [Google Scholar]
- Tarr AJ, Liu X, Reed NS, Quan N, 2014. Kinetic characteristics of euflammation: The induction of controlled inflammation without overt sickness behavior. Brain Behav. Immun 42, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT, 2005. Increase in interleukin-1beta in late-life depression. Am. J. Psychiatry 162, 175–177. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Hofman A, Van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MM, 2003. Inflammatory proteins and depression in the elderly. Epidemiology 14, 103–107. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E, 2003. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 170, 429–433. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM, 2006. The expression and function of che-mokines involved in CNS inflammation. Trends Pharmacol. Sci 27, 48–55. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E, 2002. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. New Engl. J. Med 347, 1227–1232. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Vermeer DW, Christian DL, Molkentine JM, Mason KA, Lee JH, Dantzer R, 2017. Neuroimmune mechanisms of behavioral alterations in a syn-geneic murine model of human papilloma virus-related head and neck cancer. Psychoneuroendocrinology 79, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Borniger JC, Surbhi, Zalenski AA, Muscarella SL, Fitzgerald JA, Zhang N, Gaudier-Diaz MM, DeVries CA, 2017. Mammary tumors induce central pro-inflammatory cytokine expression, but not behavioral deficits in balb/c mice. Sci. Rep 7, 8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Liu J, Liu L, Wang F, Ma Z, Gao D, Zhang Q, Yu Z, 2014. The status and correlates of depression and anxiety among breast cancer survivors in Eastern China: a population-based, cross-sectional case-control study. BMC Public Health 14, 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HW, Kettenmann H, 2017. Microglia in physiology and disease. Annu. Rev. Physiol 79, 619–643. [DOI] [PubMed] [Google Scholar]
- Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, De Beer MC, De Villiers WJ, Finch CE, 2005. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci. Lett 390, 76–80. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Jung U, Shin T, Wang H, Moon C, 2014. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav. Immun 36, 147–155. [DOI] [PubMed] [Google Scholar]


