Abstract
Substance P (SP), a neurotransmitter released after injury, has been linked to deregulated tissue repair and fibrosis in musculoskeletal tissues and other organs. Although SP inhibition is an effective treatment for nausea, it has not been previously considered as an anti-fibrotic therapy. Although there are extensive medical records of individuals who have used SP antagonists, our analysis of human registry data revealed that patients receiving these antagonists and arthroplasty are exceedingly rare, thus precluding a clinical evaluation of their potential effects in the context of arthrofibrosis. Therefore, we pursued in vivo studies to assess the effect of SP inhibition early after injury on pro-fibrotic gene expression and contractures in an animal model of post-traumatic joint stiffening. Skeletally mature rabbits (n=24) underwent surgically induced severe joint contracture, while injected with either fosaprepitant (a selective SP antagonist) or saline (control) early after surgery (3, 6, 12, and 24 hours). Biomechanical testing revealed that differences in mean contracture angles between the groups were not statistically significant (p=0.27), suggesting that the drug neither mitigates nor exacerbates joint contracture. However, microarray gene expression analysis revealed that mRNA levels for proteins related to cell signaling, pro-angiogenic, pro-inflammatory and collagen matrix production were significantly different between control and fosaprepitant treated rabbits (p<0.05). Hence, our study demonstrates that inhibition of SP alters expression of pro-fibrotic genes in vivo. This finding will motivate future studies to optimize interventions that target SP to reduce the formation of post-traumatic joint contractures.
Keywords: Post-traumatic joint contracture, microarray, animal model
Post-surgical stiffness continues to be a major adverse outcome of traumatic elbow joint injury [Morrey, 2005]. Patients that are recalcitrant to traditional treatment modalities for joint contractures may benefit from supplementary treatments with pharmacological agents. However, to date, drug-based therapies for chronic joint contracture are limited. Consequently, research into new treatment options for joint contractures is urgently needed. The transformation of fibroblasts into myofibroblasts, the key effector cells responsible for contracture formation, is thought to represent an essential step in the fibrotic process [Abdel et al., 2011; Broughton et al., 2006; Hildebrand et al., 2004]. Recent studies have shown that myofibroblasts are active early in the process and peak two weeks post injury [Abdel et al., 2011]. Furthermore, gene expression analyses by microarray profiling suggest that numerous inflammatory genes are upregulated very soon after injury in a rabbit model of joint contracture [Morrey, 2011]. Based on these findings, we propose that early pharmacologic intervention may be necessary to prevent the pathologic cascade responsible for myofibroblast transformation and subsequent joint contracture formation.
Neuropeptides, including Substance P (SP), may provide promising new targets for contracture intervention. SP is found predominantly in nerve tissues and is released from peripheral terminals of sensory nerve fibers in the skin, muscles and joints soon after tissue injury. However, other cell types including neutrophils, macrophages, lymphocytes, dendritic cells, and eosinophils have also been shown to produce and secrete SP in addition to upregulating the receptor for SP during inflammation [Marriott, 2001]. Increased nerve density and SP levels have been linked to hypertrophic scarring, abdominal adhesions, and other types of fibrosis [Cohen et al., 2007; Lindström et al., 2007; Scott et al., 2007]. More specifically, SP is directly involved in cell signaling, up-regulation of inflammatory genes, and tissue healing [Burssens et al., 2005; Delgado et al., 2005; Felderbauer et al., 2007; Grorud et al., 2007; Mantyh, 1991; Steyaert et al., 2006; Yamada et al., 2005]. The cellular target of SP is neurokinin-1 (NK-1), a 7 transmembrane G-protein receptor, thus inhibiting SP via this receptor soon after tissue injury should reduce long-term contracture formation by preventing myofibroblasts from producing excess collagen.
Our experimental approach selectively inhibits SP via its NK-1 receptor at early time points after injury. Rabbits given an inhibitor of SP early after injury should have altered mRNA expression of pro-fibrotic genes and less clinically severe contractures. Therefore, our primary goal was to apply a novel customized microarray (‘gene chip’) to determine if pro-fibrotic mRNA transcripts were altered in an established animal model of joint contracture. A secondary goal was to determine if SP inhibition would result in the formation of contractures less severe than in joints left untreated by the drug. Our results reveal gene expression levels consistent with a pattern of endogenous cells responding to the treatment, and this information may be leveraged to enable isolation of processes most important for the formation, prevention and treatment of post-traumatic joint contracture.
Materials & Methods
Human RNA-Seq Data
Human tissues were collected with approval of the Mayo Clinic Institutional Review Board (IRB-13–005619). More specifically, seven skeletal muscle and seven joint capsule tissues were harvested as discarded surgical waste after obtaining informed consent from each patient. Tissues were snap frozen in liquid nitrogen immediately after surgical removal, and stored in a −80°C freezer until RNA extraction. Genomic RNA was isolated using the miRNeasy Mini kit (Qiagen, Valencia, CA) as described in the manufacturer’s protocol with a total elution volume of 50 μL. As described in detail previously [Dudakovic et al., 2014; Lin et al., 2016], high-throughput next-generation RNA-seq data were collected by the Mayo Clinic Human Genome Core Facility (Rochester, MN). Similarly, reads were mapped to the human genome version 19 (hg19) by the Mayo Clinic Bioinformatics Core Facility (Rochester, MN) using an established bioinformatics pipeline that allows for the expression levels of mRNA to be viewed as reads per kilobase pair per million mapped reads (RPKM). Gene lists from RNA-seq data were further analyzed using FunRich [Pathan et al., 2015], STRING [Szklarczyk et al., 2017], and GeneMania [Warde-Farley et al., 2010].
Human Joint Registry Data
With institutional approval, Mayo Clinic electronic medical records from 2000–2015 were used to search human patient charts for any history of a SP antagonist. Patients who had a history of Aprepitant-Emend, fosaprepitant, netupitant, palonostetron, or rolapitant were identified. Using an institutional total joint registry and companion electronic medical records, these patients were then further searched for a history of arthrofibrosis in any joint or a history of any total joint arthroplasty performed at our institution.
Study Design
After obtaining Institutional Animal Review Committee approval, 24 skeletally mature New Zealand White (NZW) female rabbits weighing between 2.5 and 3.5 kg were divided equally into 4 groups of 6 rabbits. All animals underwent surgeries on their right knees to create contractures as previously described [Nesterenko et al., 2009]. Groups 1 and 3 each consisted of 6 rabbits injected with SP inhibitor (i.e., fosaprepitant, an NK-1 antagonist) at four time points after the initial surgery (3, 6, 12, and 24 hours). Groups 2 and 4 were injected with saline to serve as controls. Groups 1 and 2 were sacrificed at 72 hours and groups 3 and 4 were sacrificed at 24 weeks post-surgery. After sacrifice, all groups were analyzed by gene microarray expression profiling, bioinformatics, and biomechanical measurement.
Surgical Procedure
Briefly, rabbits underwent general anesthesia by inhalation. A 2–3 cm midline skin incision was made over the right knee, followed by a lateral parapatellar arthrotomy. Two 3-mm cortical windows were created at the non-articular cartilage portions of the medial and lateral femoral condyles. The anterior and posterior cruciate ligaments were sectioned. The knee was then hyperextended to −45° to disrupt the posterior capsule. Operative knees were then immobilized in flexion with a 1.6 mm threaded Kirschner wire (K-wire; Stryker, Minneapolis, MN) that had terminal threads to receive machined nuts for tibial fixation. The K-wire was drilled through the tibia, advanced subcutaneously, and bent around the femur under direct visualization. A 160° flexion angle was consistently observed with the bent end of the K-wire engaging the femur proximally and a threaded nut tightened over the K-wire at the tibial insertion. All skin incisions and the arthrotomy were closed in layers with running 4–0 Vicryl sutures (Ethicon, Johnson & Johnson, Somerville, NJ).
Injections
The right knees of rabbits in groups 1 and 3 (n = 12) were injected with fosaprepitant (Merck & Co., Inc.), a selective NK-1 antagonist, at 3, 6, 12 and 24 hours after surgery. Each rabbit was injected with four 0.25 mL injections, for a total of 1 mL (1 mg) of fosaprepitant. Groups 2 and 4 rabbits served as controls that received normal saline with the identical approach, timeframe, and volume of injections as treated animals. To accomplish the injections, a 25-gauge needle was introduced into the knee joint using a medial parapatellar approach to a depth of 5 mm. The injection placement technique was validated prior to initiation of the study using fluoroscopy-guided visualizations and contrast reagents to ensure the correct position and placement of the syringe. The 12 animals in groups 1 and 2 were sacrificed 72 hours after the initial surgery (k-wire placement). Groups 3 and 4 underwent a second surgical procedure 8 weeks after initial surgery and were sacrificed at 24 weeks. All animals underwent biomechanical testing immediately after sacrifice using a custom-made, standardized, validated joint measuring device to determine flexion contracture angles [Nesterenko et al., 2009]. Immediately after biomechanical testing, joint capsules were harvested and snap frozen in liquid nitrogen for molecular analysis.
Surgical K-Wire Removal and Remobilization
Eight weeks after the initial surgery, the 12 animals in groups 3 and 4 underwent a second operation on their right knees to have the K-wires removed. Bridging heterotopic ossification was manually disrupted. The initial 8-week timeframe from index surgery to K-wire removal represented the immobilization period. Animals were allowed free cage activity for an additional 16 weeks after K-wire removal. These animals were then sacrificed and sampled as described above. Left knees of each animal served as a non-operative internal control within each group. The right knee joint flexion angles in each of the groups were then measured and compared to the non-operated, internal control (left) limbs.
RNA Isolation
After specimens were collected and snap frozen, they were sectioned on a microtome for mRNA extraction as described previously [Hildebrand et al., 2006]. Briefly, we utilized a core facility to generate 20 μm sections by cryostat that maintains the tissue block and sections frozen at all times. Sections were collected into microcentrifuge tubes for RNA extraction using the 5 PRIME kit (Fischer Scientific, Pittsburgh, PA) and protocol. Total genomic RNA was first purified utilizing a lysis solution, followed by DNase treatment. All RNA was eluted using 50 μL of nanopure water through a chemical purification column capable of accommodating up to 40 mg of tissue per sample. RNA quantity and purity was measured using a NanoDrop Spectrophotometer (Thermo Scientific Fischer, Wilmington, DE). RNA quantity and integrity was further assessed using 2 μL aliquots entered into an Agilent 2100 Bioanalyzer (Santa Clara, CA).
Gene Microarray Expression Analysis
After mRNA extraction, a custom microarray was used to evaluate time-sensitive fold changes of 384 genes in the rabbit genome. A cDNA-mediated annealing, selection, extension and ligation (DASL) assay pool was designed and synthesized through the Illumina (San Diego, CA) custom DASL service that interrogates 384 Oryctolagus cuniculus mRNA targets. Analysis of mRNA was conducted according to manufacturer’s instructions for the DASL assay. Briefly, 100 ng of total RNA from fresh frozen tissues were reverse transcribed with biotinylated primers. The resulting cDNA was annealed to chimeric query oligonucleotides, which contain a gene-specific region and a universal primer sequence for PCR amplification, and then bound to streptavidin-conjugated paramagnetic particles. The gene-specific oligonucleotides were extended by second-strand cDNA synthesis and then ligated. Subsequently, the products were sequestered by magnetic separation, washed to remove unbound molecules, and amplified by PCR with fluorophore-labeled universal primers. The resulting PCR products were purified, applied to DASL Universal beadchips, incubated for 30 minutes at 60°C, and then hybridized for 16 hours at 45°C. The bead-chips were washed and scanned in a BeadArray Reader using BeadScan v3 software (Illumina, San Diego, CA). Quality assessment parameters were determined to be within normal ranges before proceeding to final data reduction. Quantities greater than 100 ng were obtained for spotting on the microarray. Quality control was ensured by RNA integrity number (RIN) measured greater than 8 for all samples on a quantitative scale of 1 (poor) to 10 (high).
Six samples were run per array and samples were randomly allocated to each array. Gene expression data was normalized using fastlo [Ballman et al., 2004]. The probe-level PM data for genes were summarized using the geometric mean to obtain a measure of expression for each corresponding gene. Differential expression between the operated and unoperated limbs, and differences among the groups were analyzed using the R-package limma [Gentleman et al., 2004; Smyth, 2005; Team, 2007]. A linear model was fit and the contrast for treatment relative to control was calculated for each probeset. Empirical Bayesian methods were used to rank genes in order of evidence for differential expression [Smyth, 2004]. This method obtains a modified t-statistic by shrinking the probe-wise sample variances towards a common value and augmenting the degrees of freedom. False Discovery Rates (FDR) were calculated based on the method of Benjamini and Hochberg [Benjamini et al., 2001]. Statistical comparisons were made between the following samples: (i) operated fosaprepitant injected knees versus unoperated control knees sacrificed at 72 hours, (ii) fosaprepitant injected knees versus unoperated control knees sacrificed at 24 weeks, and (iii) operated fosaprepitant injected knees sacrificed at 72 hours versus operated fosaprepitant injected knees sacrificed at 24 weeks. Fold-change thresholds were set to either 2.0 or 1.5 for filtering gene lists involving the 72-hour or 24-week sample groups, respectively. Alpha values of p=0.05 were enforced for carryover of any gene lists for downstream analysis by FunRich [Pathan et al., 2015], and STRING [Szklarczyk et al., 2017].
Biomechanical Statistical Analysis
Sample sizes for biomechanical analysis were calculated assuming a 5% type 1 error and 80% power to detect an effect size of 30 degrees with a standard deviation of 20 degrees. The knee joint flexion angles in each of the groups were measured with a standard load of 20N at a rate of 5 degrees/second and directly compared. Comparison was also made between operative and non-operative limbs. A two-sample t-test assuming unequal variances with a significance level set at p< 0.05 was used to determine statistical significance. Data are presented as average values ± standard deviation. All procedures and measurements were performed by the same individual in order to minimize experimental variability.
Results
Expression of Substance P related genes in joint tissues
Because we considered SP as potential target for anti-fibrotic drug therapy in joint tissues, we examined whether components of this pathway are actively expressed in tissues derived from joint capsules that were surgically removed during arthroplasty. Gene ontology information was used to query RNA-seq data from 14 human surgical specimens to generate a selected list of genes linked to SP (Supplementary Table 1). Network visualization of these genes (using the STRING analysis tool) revealed strong functional connections between members of the Tachykinin and Prostadaglandin genes (Fig. 1a). Hierarchical clustering of RNA-seq expression values for the SP-related genes in skeletal muscle tissues (n=7) and joint capsule tissues (n=7) resulted in two well-supported clades that grouped by tissue type. Similarly, gene expression values formed two distinct clusters for this select group of relevant molecular markers (Fig. 1b), although both clusters contained members of the three groups of genes (Tachykinins, Prostadaglandins, and Endothelin Converting Enzymes). Importantly, average RPKM values for Tachykinin receptors 1, 2 are elevated in samples derived from capsule tissues relative to mRNA derived from muscle tissue samples, while TACR3 displays the opposite pattern (Fig. 1c). Interestingly, the average RPKM level for Tachykinin 1 is greater in capsule tissue than muscle, whereas differences between sample types for TAC3 and TAC4 were less prominent (Fig. 1d). Taken together, components of the SP pathway are selectively expressed in joint tissues, suggesting that SP inhibitors have the potential to target this pathway locally in the joint environment.
Fig. 1:

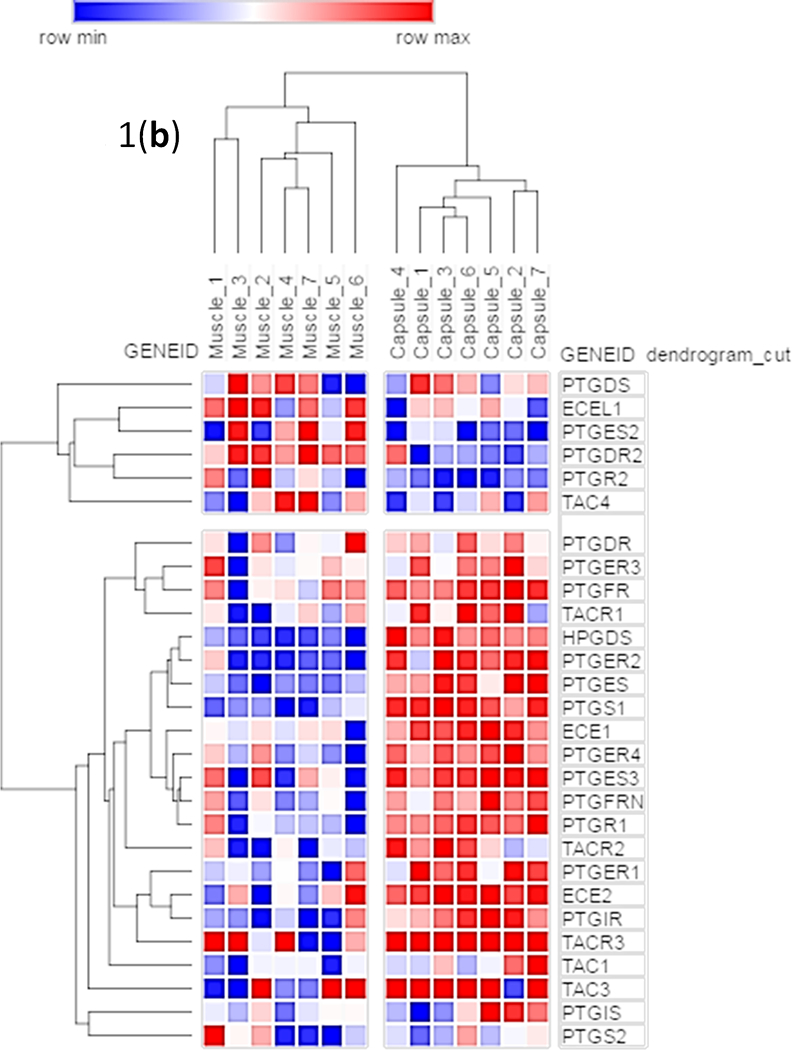
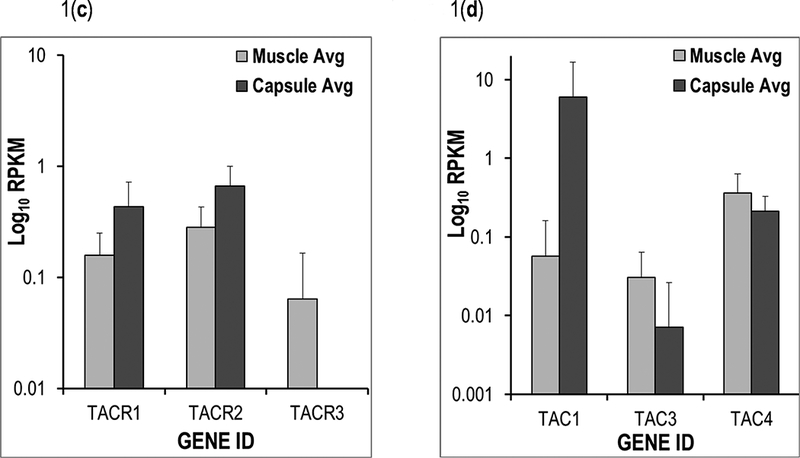
(a) A subset of genes from the Mayo Clinic Musculoskeletal RNA-seq Atlas were used to generate a STRING [Szklarczyk et al., 2017] figure of functionally interconnected elements derived from human skeletal muscle and joint capsule tissues. (b) The same tissues were used to make a hierarchical clustering heatmap in GeneMania [Warde-Farley et al., 2010]. The bar graph of average RPKM values compares (c) TACR1, TACR2, and TACR3; and (d) TAC1, TAC3, and TAC4 among human skeletal muscle and capsule tissues.
Analysis of Co-Occurrence of Substance P Use in Arthroplasty Patients with Arthrofibrosis
We analyzed human total joint registry data for patients treated with SP inhibitors as part of routine clinical care for nausea relief. For the period of 2000–2015, we identified 4802 patients with a history of using an SP antagonist. Additionally, 30,936 patients received total hip and total knee arthroplasty at Mayo Clinic during the same period. Using our simple search strategy, 326 patients developed a history of joint contracture/ arthrofibrosis, but only 118 of these THA and TKA patients also had a history of SP antagonist use. Eight patients had a history of total joint arthroplasty, arthrofibrosis, and SP antagonist use. Hence, our analysis of clinical records in the Mayo Clinic joint database reveals that co-occurrences of SP use and arthroplasty are rare (about 1 in 250 TJA patients), and that cases of arthrofibrosis and SP antagonist use are extremely rare (about 1 in 4000 TJA patients). Animal models were therefore considered to directly test for potential effects of this therapeutic reagent within the context of arthrofibrosis prevention and/or treatment.
Rabbit Surgeries and Biomechanical Analyses
Twenty four rabbits divided into four groups underwent successful surgeries to place k-wire immobilization devices that remained in place for 8 weeks. After k-wires were removed, animals were injected with fosaprepitant (treatment) or saline (control) and sacrificed at either 72 hours or 24 weeks (Fig. 2). No surgical complications were noted before, or during procedures. One animal in group 4 died before the scheduled endpoint and was therefore removed from the study. At 24 weeks post-surgery, the fosaprepitant injected knees had a mean residual contracture of 48.0° (± 16.0°), while saline injected knees had a mean residual contracture 44.5° (± 10.1°). Hence, there was no statistically significant difference (p=0.27) in flexion contracture angle between two groups measured after 24 weeks (Fig. 3).
Fig. 2:
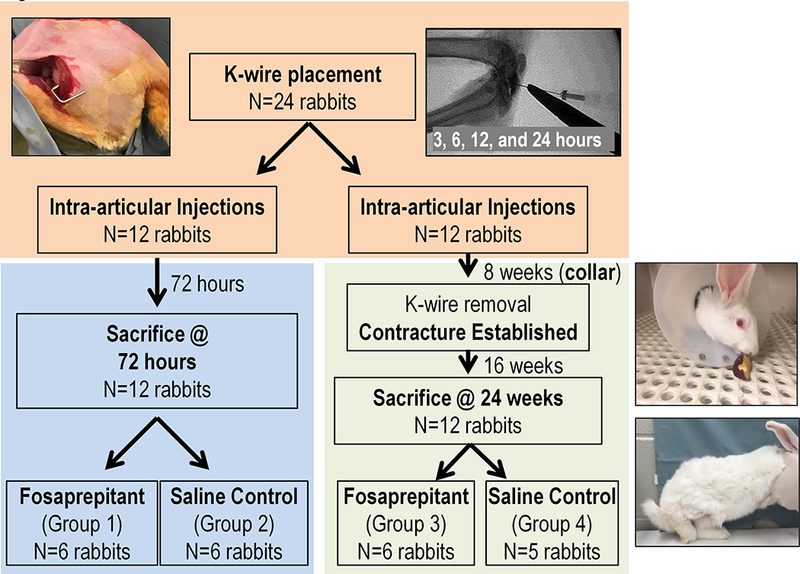
The experimental outline of this study including procedures (pictured: k-wire placement; collar and immobilization; contracture establishment; and validation of the injection location by fluoroscopic guidance), numbers of animals, and treatment groups used for the four study groups and two time courses.
Fig. 3:

Results of biomechanical measurements of flexion contracture angles in animals injected with either saline or fosaprepitant and analyzed at 24 weeks.
Ingenuity (IPA) Analysis of Microarray Data
The IPA analysis of samples from the 72 hour groups resulted in the definition of “skeletal and muscular system development and function” as the most highly scored functional group (score=42) that differed between the two groups (Fig. 4a). Interestingly, the secondary category highlighted connective tissues and disorders related to their function as a highly scored category as well (score=24). The functional significance of other categories was less obvious, yet the numbers of molecules related to each of the top biological functions included Genetic disorders (n=54), Cancer (n=45) as well as cellular growth and proliferation (n=41). Inflammatory response and inflammatory disease each involved top biological functions identified by the IPA analysis and yielded 33 and 37 molecules, respectively (Fig. 4a).
Fig. 4:
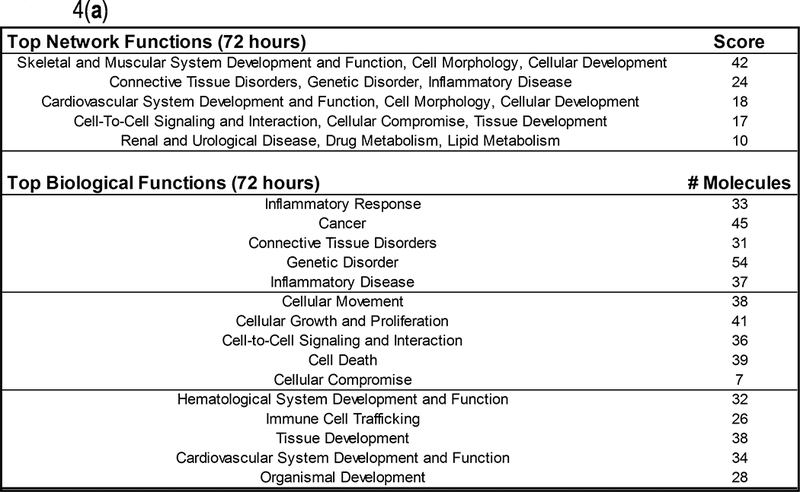

Ingenuity (IPA) analysis results for (a) the comparison between groups 1 and 2 (72 hours), and (b) the comparison between groups 3 and 4 (24 weeks).
Samples from 24 week groups, after analysis by IPA, were identified as being functionally different in the category of cell cycle, cell morphology, and cellular development, although the scores were lower for these estimates (score=21) than for 72 hour samples. Similarly, the numbers of molecules involved in top biological functions identified by IPA were cancer, as well as cellular growth and proliferation, each involving 18 genes (Fig. 4b). Gene ontology categories associated with proliferative expansion and cellular development are expected to be expressed in tissues undergoing repair after injury.
Genes of Interest and Functional Clustering
Genes of interest (defined by elevated fold change and significant p-value) from the microarray analysis comparing three sets of samples (a) 72 hour treated versus 24 week treated, (b) 72 hour treated versus control, and (c) 24 week treated versus control revealed 48 genes for further functional clustering (Fig. 5a). These genes were extracted and analyzed via STRING and found to be tightly connected, aside from nine markers, mostly transiently expressed (e.g., FGFBP2, TNMD). Of note, RNA expression levels were elevated for select types of interleukins, chemokines and cytokines (Fig. 5b). These changes in gene expression could perhaps result from a response to fosaprepitant treatment.
Fig. 5:


(a) A FunRich [Pathan et al., 2015] comparison of significantly different genes between the three groups of samples compared to reveal overlapping genes among the groups for further analysis by (b) STRING [Szklarczyk et al., 2017] (n=48).
Discussion
Post-traumatic joint contracture is challenging to overcome with either physical therapy or surgery [Keschner and Paksima, 2007; Lindenhovius and Jupiter, 2007; Morrey, 2005]. When surgical treatment is selected, intraoperative motion achieved at the time of release is difficult to maintain [Keschner and Paksima, 2007; Lindenhovius and Jupiter, 2007; Morrey, 2005]. Interestingly, the severity of a contracture may depend largely on the biologic response of the affected individual, as different patients develop varying degrees of fibrosis, even under similar operative conditions [Morrey, 1990]. As with other fibrotic diseases, pharmacologic interventions have the potential to minimize initiation of early-fibrosis related processes and thereby prevent and/or treat joint contractures.
Based on studies investigating the influence of neuropeptides on wound healing, it is reasonable to hypothesize that inhibition of SP might alter the fibrotic cascade and prevent, or reduce, the amount of joint contracture. Studies by Cohen et al found that a neurokinin-1 receptor antagonist reduced intra-abdominal adhesion formation and increased peritoneal matrix metalloproteinase activity following intra-abdominal injury, however beneficial effects of the treatment may be time-sensitive [Cohen et al., 2007]. Other investigators found that maximal inhibition by aprepitant (the solid form of fosaprepitant) was attained after 2 hours with occupied receptor levels decreasing by 4 hours [Lindström et al., 2007]. Notwithstanding, the inhibitory effects of this drug are fast-acting and short-lived, occurring within a few hours, rather than days.
Our study confirmed that SP is involved in the inflammatory response to injury, as a clear effect on the mRNA of several key genes was seen with inhibition. Specifically, SP inhibition appears to mediate a positive response by early up-regulation of several anti-fibrotic and anti-inflammatory genes, including, interferon inducible protein (IP-10), fibromodulin (FMOD), Caveolin-1 (CAV1), PPARγ (PPARG), Heme oxygenase (HO), regulator of G-protein signaling 4 (RGS4), and BMP-4. In particular, IP-10 may act as an early shut-off switch for the inflammatory cascade at 72 hrs [Broughton et al., 2006]. Likewise, PPARγ is interesting in that agonistic action helps to prevent inflammation and fibrosis in other pulmonary fibrosis models [Lin et al., 2010], while the effects of PPAR-related anti-inflammatory agonists are mediated by RGS4. Rosiglitazone, a PPARγ agonist inhibits lung fibroblast migration and proliferation, as well as myofibroblast differentiation [Lin et al., 2010].
Other components of the fibrotic phenotype were also affected by SP inhibition. For example, ECM transcripts were upregulated after SP inhibition. Among individuals with SP inhibition by fosaprepitant, there was significant down regulation of type IV collagen at 24 weeks and expression levels of expression for select TIMPs at 72 hours. Up-regulation of TIMPs is expected to attenuate MMP-mediated ECM degradation and could signal the activation of a feedback loop correlated with both pro-fibrotic and anti-fibrotic phenotypes. Using another model of joint contracture, Hildebrand et al have shown that TIMP1, TIMP2 and TIMP3 decreased, which correlated with their examination of human elbow capsular tissue [Hildebrand et al., 2008; Hildebrand et al., 2006]. However, TIMPs are known to increase in other fibrotic conditions including burn injuries and contractures resulting from Dupuytren’s disease [Ulrich et al., 2003a; Ulrich et al., 2003b; Ulrich et al., 2010]. Differential regulation TIMP’s may be time dependent, which would be anticipated for feedback loops that control ECM remodeling. Intuitively, protective effects would occur early in the process of inflammation to limit excessive destruction of normal cells and matrix. Later during scar formation, TIMPs may attenuate normal or natural breakdown of ECM and collagen to allow scar remodeling.
Although SP inhibition upregulates many anti-inflammatory mediators, some pro-inflammatory and pro-fibrotic genes also exhibit increased expression. For example, IL-1 and IL-8 which contribute to pro-fibrotic phenotypes in the uninhibited model were not upregulated in the SP inhibited group. Instead, the cytokine milieu was altered by elevations of IL-4 and IL-6, which may reflect feedback inhibition to silence inflammation and accommodate T-cell isotype switching, as has been shown in other fibrotic conditions (e.g., scleroderma) [Lakos et al., 2006]. Furthermore, pro-fibrotic genes TGFβ2, alpha 6 integrin (ITGA6), actinin alpha, collagen 3, fibronectin, MMP2, and TF were upregulated in SP inhibited vs controls at 72 hours. At 24 weeks IL-6, Versican and BCL anti-apoptotic genes were also up regulated. The differential up-regulation of pro-fibrotic and anti-fibrotic genes, as well as pro and anti-apoptotic signals highlights the considerable complexity of fibrosis formation, and suggests it involves many intersecting feedback loops that operate at different time-scales. For example, while anti-fibrotic genes are upregulated with the use of SP inhibition, alternative pathways may be upregulated to counteract this inhibition. Additionally, TGFβ2 was down-regulated in the saline control group. This finding corroborates previous studies showing that TGFβ1 isoform was upregulated 24 hrs post injury, but not at other time points [Morrey, 2011]. TGF-β is the primary signal for fibroblast proliferation at low levels, but inhibits fibroblast growth at high levels and promotes the myofibroblast phenotype. Recent data also revealed that myofibroblast numbers (transformed fibroblasts) peak 2 weeks post injury [Abdel et al., 2011]. These data highlight the importance of a time-sensitive alteration in signal type and signal strength that seems to dictate injury response.
Bioinformatics analysis of sampled collected after 24 weeks revealed up-regulation of several anti-fibrotic genes: BMP-7, PEDF, and PLAU, which have previously shown anti-fibrotic effects. Furthermore, PEDF is a known Wnt antagonist, and may alter differentiation of myofibroblasts by blocking the SMAD2 beta-Catenin pathway [Carthy et al., 2011; Mao et al., 2011; Park et al., 2011; Schmitz et al., 2011; Zhang et al., 2006]. Interestingly, apoptotic markers (e.g., Caspases 1 and 10) were also upregulated indicating possible death of myofibroblasts in contracted tissues. Also upregulated in our treatment group, PLAU positively affects wound healing, while its inhibitor PLAI is pro-fibrotic in lung models of fibrosis [Chang et al., 2010]. TNFα, a marker of inflammation, is present in many fibrotic conditions has also been shown to be anti-fibrotic in later stages of fibrosis by antagonizing the effects of TGFβ [Leask and Abraham, 2004]. Cutaneous scar formation functions as a balance between pro-fibrotic and anti-fibrotic gene products, and also programmed cell death at specific times that determine tissue healing [Moulin et al., 2004].
Despite encouraging results from several previous studies, as well as up-regulation of several key anti-fibrotic genes, our study did not show a biomechanical effect of SP inhibitor on joint contracture. Differences between biomechanical and molecular data may result from numerous factors including: (i) multiple pleiotropic pathways that function to inhibit or activate pro-fibrotic genes may go undetected [e.g., [Namazi et al., 2011]], (ii) alterations in gene expression may not influence tissue-level phenotypic patterns that reflect clinical fibrosis, and (iii) our dosing and sampled time points may not have captured the overarching pattern of disease progression and/or attenuation [e.g., [Marriott, 2001]]. However, we observed gross clinical differences between tissues from animals injected with fosaprepitant and controls suggesting a tissue-level response to the intervention. Specifically, scar tissues sampled from the fosaprepitant group appeared more gelatinous and less robust than tissues from control animals. Our study utilized a lower dose than Cohen et al. and may not have reached the critical level required for intracellular calcium signaling [Cohen et al., 2007]. Further refinement of drug dosing and delivery will likely advance the field of arthrofibrosis prevention and treatment, although our delivery method seems safe, reliable, and clinically translatable.
Our study presents several limitations. First, a subset of the injected material may have diffused away from the joint over time. Extensive troubleshooting of the injection system confirmed reproducibility of the needle placement, although monitoring fosaprepitant retention within the joint would require temporal tracking of contrast reagents. Second, the current animal model reliably produces a joint contracture after a long period of post-surgical immobilization. The fosaprepitant release kinetics (and homogeneity of the drug throughout the joint) may be mitigated by prolonged knee immobilization. Third, use of this delivery system and drug have not been published previously in rabbits, thus genomic and/or anatomical differences between humans and rabbits may limit the translatability of our findings. However, rabbit knees are commonly used for modeling orthopedic complications and often reveal statistically significant differences between treated and untreated groups for translatable measures that help guide clinical care.
Strengths of the current study include use of a custom microarray to identify new genes that have hitherto not been published in reference to joint contracture formation. Previous authors have analyzed some single gene transcripts and protein expression levels in an attempt to understand the pathophysiology of joint contracture [Hildebrand et al., 2005; Hildebrand et al., 2006; Hildebrand et al., 2007], although genome-wide screening methods were not employed. Our data clearly showed an effect of fosaprepitant on mRNA transcripts that are upregulated and down-regulated during joint contracture formation. Changes in gene expression did not correlate with a detectable biomechanical effect of fosaprepitant treatment. Additional culture-based experiments on the cellular effects of SP inhibition will be required before a potential tissue-level benefit of injected fosaprepitant to contracture release can be fully rejected, and by extension, maximizing the dose and delivery of intra-articular fosaprepitant will ensure greater testability of the complex biological system of joint contracture induction and/or release.
Supplementary Material
Supplementary Table 1. The gene subset used for intersection with human RNA-seq data.
Supplementary Table 2. All overlapping genes n=48 from the FunRich comparison of three sample comparisons that were further depicted in a STRING analysis.
Acknowledgments
We thank the members of the Abdel and van Wijnen Laboratories, including William Trousdale, Christopher Salib, Roman Thaler and Amel Dudakovic for stimulating discussions. This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01 AR049069 (to AJVW) and F32 AR068154 (to EAL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also appreciate the generous philanthropic support from the William H. and Karen J. Eby Foundation, as well as the Anna-Maria and Stephen Kellen Foundation. David Lewallen reports personal fees and other from Acuitive, Stryker, Pipeline Biomedical, Zimmer, and Ketai Medical Devices, as well as patents on selected hip and knee implants with royalties paid by Zimmer. He is also employed part time as the Medical Director for The American Joint Replacement Registry. Matthew Abdel has stock options with Imagen Technologies. No other authors have conflict of interest disclosures.
Contract grant sponsor: National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases; Contract grant numbers: R01 AR049069, F32 AR068154.
References
- Abdel MP, Morrey ME, Barlow JD, Kreofsky CR, An KN, Steinmann SP, Morrey BF, Sanchez-Sotelo J. 2011. Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture. J Orthop Res. [DOI] [PubMed] [Google Scholar]
- Ballman KV, Grill DE, Oberg AL, Therneau TM. 2004. Faster cyclic loess: normalizing RNA arrays via linear models. Bioinformatics 20:2778–86. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. 2001. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125:279–84. [DOI] [PubMed] [Google Scholar]
- Broughton G 2nd, Janis JE, Attinger CE. 2006. The basic science of wound healing. Plast Reconstr Surg 117:12S–34S. [DOI] [PubMed] [Google Scholar]
- Burssens P, Steyaert A, Forsyth R, van Ovost EJ, Depaepe Y, Verdonk R. 2005. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing Foot & ankle international. / American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society; 26:832–9. [DOI] [PubMed] [Google Scholar]
- Carthy JM, Garmaroudi FS, Luo Z, McManus BM. 2011. Wnt3a induces myofibroblast differentiation by upregulating TGF-beta signaling through SMAD2 in a beta-catenin-dependent manner. PLoS One 6:e19809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. 2010. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem 285:8196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PA, Gower AC, Stucchi AF, Leeman SE, Becker JM, Reed KL. 2007. A neurokinin-1 receptor antagonist that reduces intraabdominal adhesion formation increases peritoneal matrix metalloproteinase activity. Wound Repair Regen 15:800–8. [DOI] [PubMed] [Google Scholar]
- Delgado AV, McManus AT, Chambers JP. 2005. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med 230:271–80. [DOI] [PubMed] [Google Scholar]
- Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, Radel DJ, Anderson JM, Nair AA, Evans JM, Krych AJ, Smith J, Deyle DR, Stein JL, Stein GS, Im HJ, Cool SM, Westendorf JJ, Kakar S, Dietz AB, van Wijnen AJ. 2014. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. Journal of cellular biochemistry 115:1816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felderbauer P, Bulut K, Hoeck K, Deters S, Schmidt WE, Hoffmann P. 2007. Substance P induces intestinal wound healing via fibroblasts--evidence for a TGF-beta-dependent effect. Int J Colorectal Dis 22:1475–80. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grorud KW, Jensen KT, Provenzano PP, Vanderby R Jr. 2007. Adjuvant neuropeptides can improve neuropathic ligament healing in a rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 25:703–12. [DOI] [PubMed] [Google Scholar]
- Hildebrand KA, Sutherland C, Zhang M. 2004. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res 22:313–20. [DOI] [PubMed] [Google Scholar]
- Hildebrand KA, Zhang M, Germscheid NM, Wang C, Hart DA. 2008. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop 79:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand KA, Zhang M, Hart DA. 2005. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop Relat Res 439:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand KA, Zhang M, Hart DA. 2006. Joint capsule matrix turnover in a rabbit model of chronic joint contractures: Correlation with human contractures. J Orthop Res 24:1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand KA, Zhang M, Hart DA. 2007. Myofibroblast upregulators are elevated in joint capsules in posttraumatic contractures. Clin Orthop Relat Res 456:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keschner MT, Paksima N. 2007. The stiff elbow. Bull NYU Hosp Jt Dis 65:24–8. [PubMed] [Google Scholar]
- Lakos G, Melichian D, Wu M, Varga J. 2006. Increased bleomycin-induced skin fibrosis in mice lacking the Th1-specific transcription factor T-bet. Pathobiology 73:224–37. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. 2004. TGF-beta signaling and the fibrotic response. Faseb J 18:816–27. [DOI] [PubMed] [Google Scholar]
- Lin Q, Fang LP, Zhou WW, Liu XM. 2010. Rosiglitazone inhibits migration, proliferation, and phenotypic differentiation in cultured human lung fibroblasts. Exp Lung Res 36:120–8. [DOI] [PubMed] [Google Scholar]
- Lin Y, Lewallen EA, Camilleri ET, Bonin CA, Jones DL, Dudakovic A, Galeano-Garces C, Wang W, Karperien MJ, Larson AN. 2016. RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. Journal of orthopaedic research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenhovius AL, Jupiter JB. 2007. The posttraumatic stiff elbow: a review of the literature. J Hand Surg [Am] 32:1605–23. [DOI] [PubMed] [Google Scholar]
- Lindström E, von Mentzer B, Påhlman I, Ahlstedt I, Uvebrant A, Kristensson E, Martinsson R, Novén A, de Verdier J, Vauquelin G, efficacy. Nracbivriaiv, 15. S-EJ. 2007. Neurokinin 1 receptor antagonists: correlation between in vitro receptor interaction and in vivo efficacy. J Pharmacol Exp Ther 322:1286–93. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. 1991. Substance P and the inflammatory and immune response. Ann N Y Acad Sci 632:263–71. [DOI] [PubMed] [Google Scholar]
- Mao T, Gao L, Li H, Li J. 2011. Pigment epithelium-derived factor inhibits high glucose induced oxidative stress and fibrosis of cultured human glomerular mesangial cells. Saudi Med J 32:769–77. [PubMed] [Google Scholar]
- Marriott I, Bost JL. 2001. Substance P. Cytokine Reference.
- Morrey BF. 1990. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am 72:601–18. [PubMed] [Google Scholar]
- Morrey BF. 2005. The posttraumatic stiff elbow. Clin Orthop Relat Res:26–35. [PubMed] [Google Scholar]
- Morrey MEA MP; An KN; Grill D; Sanchez-Sotelo J; Morrey BF 2011. The Genetic Basis of Arthrofibrosis: Microarray and Bioinformatics Analysis of the Temporal Expression of 364 Genes During Contracture Formation. unpublished. [DOI] [PubMed]
- Moulin V, Larochelle S, Langlois C, Thibault I, Lopez-Valle CA, Roy M. 2004. Normal skin wound and hypertrophic scar myofibroblasts have differential responses to apoptotic inductors. J Cell Physiol 198:350–8. [DOI] [PubMed] [Google Scholar]
- Namazi MR, Fallahzadeh MK, Schwartz RA. 2011. Strategies for prevention of scars: what can we learn from fetal skin? Int J Dermatol 50:85–93. [DOI] [PubMed] [Google Scholar]
- Nesterenko S, Morrey ME, Abdel MP, An KN, Steinmann SP, Morrey BF, Sanchez-Sotelo J. 2009. New rabbit knee model of posttraumatic joint contracture: indirect capsular damage induces a severe contracture. J Orthop Res 27:1028–32. [DOI] [PubMed] [Google Scholar]
- Park K, Lee K, Zhang B, Zhou T, He X, Gao G, Murray AR, Ma JX. 2011. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol 31:3038–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan M, Keerthikumar S, Ang CS, Gangoda L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim A. 2015. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 15:2597–2601. [DOI] [PubMed] [Google Scholar]
- Schmitz JC, Protiva P, Gattu AK, Utsumi T, Iwakiri Y, Neto AG, Quinn M, Cornwell ML, Fitchev P, Lugea A, Crawford SE, Chung C. 2011. Pigment epithelium-derived factor regulates early pancreatic fibrotic responses and suppresses the profibrotic cytokine thrombospondin-1. Am J Pathol 179:2990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JR, Muangman P, Gibran NS. 2007. Making sense of hypertrophic scar: a role for nerves. Wound Repair Regen 15 Suppl 1:S27–31. [DOI] [PubMed] [Google Scholar]
- Smyth GK. 2004. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:Article3. [DOI] [PubMed] [Google Scholar]
- Smyth GK. 2005. Limma: linear models for microarray data In Gentleman R, Carey V, Dudoit S, Irizarry R,WH, editor^editors. Bioinformatics and Computational Biology Solutions using R and Bioconductor. . New York: Springer; p397–420. [Google Scholar]
- Steyaert AE, Burssens PJ, Vercruysse CW, Vanderstraeten GG, Verbeeck RM. 2006. The effects of substance P on the biomechanic properties of ruptured rat Achilles’ tendon. Arch Phys Med Rehabil 87:254–8. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P. 2017. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic acids research 45:D362–D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. 2007. R: A language and environment for statistical computingeditor^editors. R Foundation for Statistical Computing. Vienna, Austria. [Google Scholar]
- Ulrich D, Hrynyschyn K, Pallua N. 2003a. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in sera and tissue of patients with Dupuytren’s disease. Plast Reconstr Surg 112:1279–86. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Noah EM, von Heimburg D, Pallua N. 2003b. TIMP-1, MMP-2, MMP-9, and PIIINP as serum markers for skin fibrosis in patients following severe burn trauma. Plast Reconstr Surg 111:1423–31. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. 2010. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg 63:1015–21. [DOI] [PubMed] [Google Scholar]
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT. 2010. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic acids research 38:W214–W220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Yanai R, Inui M, Nishida T. 2005. Sensitizing effect of substance P on corneal epithelial migration induced by IGF-1, fibronectin, or interleukin-6. Invest Ophthalmol Vis Sci 46:833–9. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. 2006. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. Faseb J 20:323–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. The gene subset used for intersection with human RNA-seq data.
Supplementary Table 2. All overlapping genes n=48 from the FunRich comparison of three sample comparisons that were further depicted in a STRING analysis.


