Abstract
Central leptin administration can ameliorate hyperglycemia in insulin-deficient rodent models independently of insulin; however, the underlying neuronal mechanism are unclear. Here, we investigate the contribution of key elements within the central melanocortin system by examining whether central leptin injection can ameliorate hyperglycemia in total insulin-deficient mice that either lacked melanocortin 4 receptors (MC4Rs) in the whole body [knockout (KO); MC4R KO] or selectively, in single-minded homolog 1 (SIM1)–expressing neurons (SIM1ΔMC4R). We further investigated the contribution of leptin receptors (LEPRs) in agouti-related protein (AgRP)–expressing neurons (AgRP∆LEPR). Leptin injections into the cerebral ventricle attenuated mortality and elevated blood glucose in total insulin-deficient MC4R KO mice. Total insulin-deficient SIM1ΔMC4R mice exhibited the same magnitude reduction of blood glucose in response to leptin injections as MC4R KO mice, suggesting SIM1 neurons are key to MC4R-mediated, insulin-independent, glucose-lowering effects of leptin. Central leptin injection also partially rescued glucose levels in total insulin-deficient AgRP∆LEPR mice. In brain slice studies, basal discharge of AgRP neurons from mice with total insulin deficiency was increased and leptin partially reduced their firing rate without membrane potential hyperpolarization. Collectively, our findings indicate that, contrary to glucose-lowering effects of leptin in the presence of insulin or partial insulin deficiency, MC4Rs in SIM1 neurons and LEPRs in AgRP neurons are not solely responsible for glucose-lowering effects of leptin in total insulin deficiency. This indicates that the central melanocortin system operates with other neuronal systems to fully mediate glucose-lowering effects of leptin in an insulin-independent manner.
Leptin signaling in the hypothalamus can regulate glucose metabolism without insulin (1–4) by modulating several neuronal groups (2, 5–8); however, the precise neuronal population(s) underlying insulin-independent, glucose-lowering effects of central leptin remains to be fully clarified. Agouti-related protein (AgRP) and proopiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus (ARC) are part of an extended central melanocortin system, and available evidence indicates that leptin acts directly both on POMC and AgRP neurons that project to melanocortin 4 receptor (MC4R)–expressing neurons in the hypothalamic paraventricular nucleus (PVN) to regulate energy homeostasis through modulation of food intake and energy expenditure (9). Of special interest for the current study are observations that glucose-lowering effects of leptin in mice with partial insulin-deficiency or type 2 diabetes are blunted by global knockout of MC4Rs (10–12) and effectively abolished by genetic deletion of leptin receptors (LEPRs) in AgRP neurons (8, 10). Collectively, available evidence indicates that the central melanocortin system, including MC4Rs in the PVN and LEPRs upstream in AgRP neurons of the ARC, play pivotal roles in glucose-lowering effects of leptin in partial insulin deficiency.
Although this evidence indicates that elements of the central melanocortin system are key mediators of glucose-lowering effects of leptin, a recent study reported that mechanisms of glucose metabolism substantially differ between partial and total insulin deficiency (13). Briefly, partial insulin-deficiency by streptozocin (STZ) injections fails to elicit hyperglycemia in glucagon receptor knockout (KO) mice (14, 15). However, total insulin deficiency in a genetically engineered model does lead to hyperglycemia in glucagon receptor KO mice (13). Further, previous studies have demonstrated that mechanisms underlying glucose-lowering effects of leptin significantly differ depending on the absence or presence of insulin (2, 3). Of special importance is that previous studies (6, 8, 10, 16), except ours (2), which investigated mechanisms of glucose-lowering effects of leptin used models of partial insulin deficiency or type 2 diabetes. Because leptin can regulate glucose metabolism in both an insulin-dependent and insulin-independent manner (3, 17–19), a complete understanding of leptin’s mechanisms of action requires testing in the total absence of insulin signaling.
To accomplish this, we investigated the role of the central melanocortin system in glucose-lowering effects of central leptin using genetically engineered mice with total insulin deficiency. Specifically, we used mice lacking MC4Rs in the whole body as well as specifically in single-minded homolog 1 (SIM1) neurons resident mainly in the hypothalamic PVN. We also used mice lacking LEPRs only in AgRP neurons. To induce total insulin deficiency, we used Ins2 promoter [also known as rat insulin promoter (RIP)] diphtheria toxin (DT) receptor (RIP-DTR) transgenic mice, which allowed us to achieve total insulin deficiency with DT injections (2, 13, 20). Although previous studies have shown that MC4Rs and LEPRs in AgRP neurons are absolutely required for glucose-lowering effects of central leptin in the presence of normal insulin or its partial absence, we found these key receptors and neuronal systems to be only partially required for glucose-lowering effects of leptin in total insulin deficiency.
Materials and Methods
Genetically engineered mice
All genetically engineered mice, with the exception of RIP-DTR mice, were obtained from Dr. Joel Elmquist at the University of Texas Southwestern Medical Center, and are also available from the Jackson Laboratory (Bar Harbor, ME). To generate MC4R KO mice, we bred Mc4rTB/− mice (no. 006414) (21). Mc4rTB/TB mice recapitulate the phenotype of MC4R global KO mice (21). To generate mice lacking MC4Rs in SIM1 neurons, we bred Sim1-Cre (21) (no. 06395) with Mc4rflox/− mice (22) (no. 023720). To generate mice lacking LEPRs in AgRP neurons, we bred Agrp-IRES-Cre (23) (no. 012899) with Leprflox/− mice (24) (no. 008327). To induce total insulin deficiency, RIP-DTR mice (20) (obtained from Dr. Pedro Herrera at Geneva University) were bred with mice described previously. To perform in vitro electrophysiology and determine if DT injections affected the viability of AgRP neurons, we introduced the tdTomato allele (25) (no. 007909) to identify AgRP neurons under fluorescent microscopy. Mice used are as follows: Mc4rTB/TB∷RIP-DTR, Mc4r−/−∷RIP-DTR (control for Mc4rTB/TB∷RIP-DTR), Sim1-Cre∷Mc4rflox/flox∷RIP-DTR, Mc4rflox/flox∷RIP-DTR (control for Sim1-Cre∷Mc4rflox/flox∷RIP-DTR), Agrp-IRES-Cre∷Leprflox/flox∷RIP-DTR, Leprflox/flox∷RIP-DTR (control for Agrp-IRES-Cre∷Leprflox/flox∷RIP-DTR), and Agrp-IRES-Cre∷RIP-DTR∷Ai9TB/−. We used KAPA Mouse genotyping kits (KAPA Biosystems, Wilmington, MA) to determine genotypes. All genotyping primers and predicted band sizes are described in Table 1. We used 3- to 6-month-old male mice whose body weights (BWs) were >25 g. Care of mice was according to established National Institutes of Health guidelines, and all procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center and University of Texas Health San Antonio.
Table 1.
The Primer Sequences for Genotyping
| Genotype | Primers |
Expected Band Size, bp
|
|
|---|---|---|---|
| MC4R TB | GCAGTACAGCGAGTCTCAGG | wt | 500 |
| GTGCAAGTGCAGGTGCCAG | tg | 400 | |
| CTCCAACAGGCTTATGACACC | ∆ | ≥500 | |
| MC4R flox | GCACTAGGCTGCTCTGGCTC | wt | 300 |
| CAAGGTTCCACACTTGTTCTTTC | tg | 400 | |
| GTCTCGGGCAATGAACCATTCCTTC | |||
| Sim1-Cre | TTTTGGTTTTGGATGAGTCTGTGGAG | wt | 499 |
| TGGGATTAGCGTGTTTCAACTGAGC | tg | 266 | |
| CACGACCGGCAAACGGACAGAA | |||
| AgRP-IRES-Cre | GCT TCT TCA ATG CCT TTT GC | wt | 550 |
| AGG AAC TGC TTC CTT CAC GA | tg | 280 | |
| AGGTGGGCCCTAAGTTGAGT | |||
| Lepr flox | TGAACAGGCTTGAGAACATGAACAC | wt | 200 |
| AGAATGAAAAAGTTGTTTTGGGACGAT | LoxP | 250 | |
| Lepr ∆ | TGAACAGGCTTGAGAACATGAACAC | Δ | 250 |
| GGTGTCTGATTTGATAGATGGTCTT | wt | 648 | |
| RIP-DTR tg allele | ACG GCT GCT TCA TCT ACA AGG | tg | 230 |
| TTG GTG TCC ACG TAG TAG TAG | Internal control | 450 | |
| CCCTCCTCACCTTTCCCTTA | |||
| CTGTCCTCAGAAAGCCTTGG | |||
| RIP-DTR WT allele | CTC CGG AAA GCA GTG AGG TAA G | wt | 304 |
| GAG GGA GAA AAA TGC GGA | Internal control | 600 | |
| GGTCAGCCTAATTAGCTCTGT | |||
| GATCTCCAGCTCCTCCTCTGTC | |||
| tdTomato TB | AAG GGA GCT GCA GTG GAG TA | wt | 297 |
| CCG AAA ATC TGT GGG AAG TC | tg | 197 | |
| GGC ATT AAA GCA GCG TAT CC | |||
| CTG TTC CTG TAC GGC ATG G | |||
Abbreviations: tg, transgenic allele; wt; wild-type allele; Δ, germline deletion allele.
Assessment of basal metabolism before induction of insulin deficiency
We measured BW of MC4R KO mice at ∼12 weeks of age. We measured BW weekly after weaning (age 4 weeks) for mice lacking MC4Rs in SIM1 neurons (SIM1ΔMC4R) and mice lacking LEPRs in AgRP neurons (AgRPΔLEPR). Blood glucose and plasma insulin were measured at age 8 to 12 weeks before inducing insulin deficiency. Blood glucose was measured with a commercially available glucose monitor (Bayer Contour, Parsippany, NJ). Insulin was measured using a commercially available ELISA kit (Crystal Chem, Elk Grove Village, IL) (26).
Induction of insulin deficiency
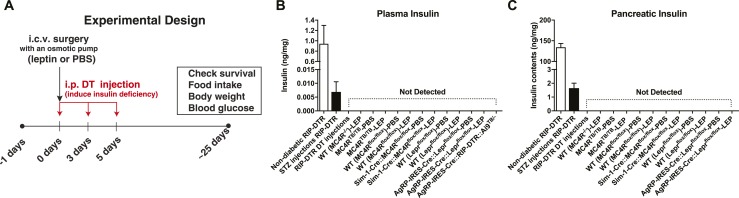
To induce insulin deficiency, mice were treated with DT (Sigma, St. Louis, MO). DT was dissolved in sterile 0.9% NaCl solution at a concentration of 150 µg/mL and kept at −80°C until use. Each concentrated DT aliquot was diluted to 0.075 µg/mL in sterile saline and delivered intraperitoneally in a dose of 0.5 µg/kg BW on days 0, 3, and 5 to ablate pancreatic β cells (Fig. 1A).
Figure 1.
DT injections ablate pancreatic β cells of mice expressing RIP-DTR. (A) The experimental design for Figs. 2–4. Insulin levels in the (B) plasma and (C) pancreas. Nondiabetic RIP-DTR mice were injected with sterile PBS. STZ was injected twice (150 mg/kg BW) at a 7-day intervals and the blood and pancreas from STZ-injected mice were collected 14 days after the first injection. All groups except nondiabetic RIP-DTR and STZ-injected RIP-DTR are described in the “Materials and Methods” and “Results” sections. (A) DT was administered as described in the “Materials and Methods” section (0.5 µg/kg BW on days 0, 3, and 5) except for injections of Agrp-IRES-Cre∷RIP-DTR∷Ai9TB/− mice (0.5 µg/kg BW on days 0, 1, and 2). n = 3 to 13. Values are mean ± SEM.
Leptin administration into the brain
Leptin (Peprotech, Rocky Hill, NJ; 25 ng/h/0.11 µL) was dissolved in sterile PBS (pH = 7.4; Invitrogen, Carlsbad, CA) and administered by intracerebroventricular (ICV) infusion using osmotic pumps (Alzet, Cupertino, CA), as previously described (1, 2). Briefly, a cannula (Brain infusion kit; Alzet) was stereotaxically positioned into a lateral cerebral ventricle [−0.34 mm from the bregma, 1 mm lateral (right side), −2.5 mm from the skull], and secured by cyanoacrylate adhesive gel (Loctite 454; Loctite, Düsseldorf, Germany). An osmotic minipump designed for use in mice (model 1004; Alzet) was implanted subcutaneously and attached via a catheter to the lateral ventricle cannula for ICV infusion. PBS was administered to the control group as a placebo treatment. We continuously administered leptin for up to 25 days because pumps are rated to deliver for approximately 28 days.
Measurement of metabolic parameters and survival
We measured glucose, BW, and food intake every 5 days as previously described (1, 2). Because previous studies have shown that central leptin signaling can reduce lethal effects of insulin deficiency independent of its actions on glucose metabolism (1, 2), we plotted survival to determine if MC4R, MC4R in SIM1 neurons, or LEPRs in AgRP neurons are required for leptin’s capacity to reduce lethality in insulin-deficient mice.
Measurement of pancreatic insulin levels
To confirm that DT injections ablated pancreatic β cells, we measured insulin from the pancreas of each mouse separately at the end of experiments, as previously described (1, 2). Briefly, the pancreas was removed, weighed, and submerged in 3 mL cold acid ethanol solution (AES; 0.18M HCl in 70% ethanol) and stored at −20°C. On assay day 1, pancreas tissue was homogenized, and the homogenizer was rinsed with another 3 mL cold AES. The AES wash solution was then added to the pancreas homogenate so that the total assay volume was ∼6 mL. At this point, the solution was kept at −20°C overnight. On assay day 2, supernatant was collected after the pancreatic homogenate was centrifuged (4000 rpm × 30 minutes at 4°C). Next, another 3 mL cold AES was added to the pancreatic precipitate and same steps repeated such that the total volume of supernatant was ∼12 mL. On assay day 3, we repeated the same procedure without the washing steps, and the total volume of supernatant was ∼15 mL; this was kept at −20°C. Pancreatic extract was then diluted 1:100 with sample dilution buffer supplied in the Insulin ELISA kit and insulin levels were measured.
Electrophysiology
Slice preparation
After mice were deeply anesthetized with isoflurane, brains were removed and placed in ice-cold cutting buffer containing (in millimoles): sucrose 261, KCl 2, MgSO4 2, NaH2PO4 1.25, CaCl2 1 MgCl2 1, HEPES 10, glucose 10, and ascorbic acid 0.4 (pH 7.4, OsM ∼298). Each brain was cut into 250 µm-thick coronal slices with a vibratome (Leica Microsystems, Wetzlar, Germany) and transferred to an oxygenated gassed incubation chamber filled with standard artificial cerebrospinal fluid containing (in millimoles): NaCl 140, KCl 2.5, CaCl2 2, MgCl2 1, HEPES 10, glucose 10, and ascorbic acid 0.4 (pH 7.4, OsM ∼298) at room temperature for at least 1 hour before recording commenced.
Slice recordings
Whole cell patch-clamp recordings from tdTomato-positive neurons were performed with the aid of infrared-differential interference contrast optics and a 16-bit EMCCD digital camera (Photometrics, Inc., Huntington Beach, CA). Patch electrodes were pulled (Flaming/Brown P-97; Sutter Instrument Co., Novato, CA) from borosilicate glass capillaries and polished to a tip resistance of 5 to 7 MΩ. Electrodes were filled with a solution containing (in millimoles): K-gluconate 135, MgCl2 1, HEPES 10, EGTA 1, KCl 10, Mg-ATP 2, and Na-GTP 0.5 (pH 7.2, OsM ∼287). Recordings were obtained using an Axopatch 200B amplifier and pCLAMP software (v10.5, Axon Instruments, San Jose, CA). Signals were filtered at 2 kHz, digitized 10 kHz (Digidata 1440A; Axon Instruments), and saved on a computer for offline analysis. Normal artificial cerebrospinal fluid was supplemented with 100 µM picrotoxin, 10 µM CNQX, and 50 µM DL-AP5 to block GABAA, AMPA, and NMDA receptors, respectively. In current-clamp experiments, leptin (100 nM) was bath applied for ∼4 minutes. Baseline discharge was recorded in current-clamp mode for 5 minutes. Slices were then bath perfused with leptin (100 nM) for ∼10 minutes. Leptin-modulated discharge was recorded during the last 5 minutes of exposure.
Assessment of mRNA
Mice were deeply anesthetized with isoflurane and mediobasal hypothalamus was quickly removed, frozen in liquid nitrogen, and subsequently stored at –80°C. RNA was extracted using STAT60 reagent (Amsbio, Cambridge, MA). Complementary DNA from 1 μg of input RNA was generated with the High Capacity cDNA Reverse Transcription Kits (Life Technologies, Carlsbad, CA). SYBR Green PCR master mix (Life Technologies) was used for the quantitative real time PCR analysis Sequences of deoxy-oligonucleotides primers were: 18S (5′ catgcagaacccacgacagta and 5′ cctcacgcagcttgttgtcta), Agrp (5′ cggccacgaacctctgtag and 5′ctcatcccctgcctttgc), and Ins2 (5′ tcaacatggccctgtggat and 5′ aaaggtgctgcttgaaaaagc).
Immunohistochemistry
Mice were deeply anesthetized with isoflurane and underwent transcardiac perfusion fixation with 4% paraformaldehyde, as previously described (27). After cryoprotection in 30% sucrose-sterile PBS solution, brains were cut in 25-μm sections using a freezing microtome. Brain sections were mounted on glass slides using antifade mounting medium with 4′,6-diamidino-2-phenylindole (H-1500; Vector Laboratory, Burlingame, CA). Images were captured by fluorescence microscopy (model BZ-X710; Keyence, Osaka, Japan). Neurons expressing tdTomato fluorescent and distributed in the ARC at the coronal section approximately −2.0 mm from the caudal to the bregma were manually counted.
Data analysis
Data are represented as the group mean ± SEM in each figure legend. Statistical significance was determined using GraphPad PRISM software (ver7, GraphPad, San Diego, CA) by unpaired t test, one-way ANOVA followed by Tukey multiple comparison test, two-way ANOVA followed by one-way ANOVA (Tukey multiple comparison test if the interaction was significant) or unpaired t test in the same factor (if the interaction was not significant), or repeated measures ANOVA followed by unpaired t test if the interaction was significant. For analysis of survival curves, log-rank (Mantel-Cox) testing was used. Because the number of mice surviving declined over time, we were prohibited from using repeated measures ANOVA (Fig. 2E, 2H, and 2K; Fig. 3E, 3H, and 3K; and Fig. 4E, 4H, and 4K). We, therefore, performed statistical analysis and showed each day individually in Figs. 2–4. For all tests, statistical significance was set at a critical value of P < 0.05.
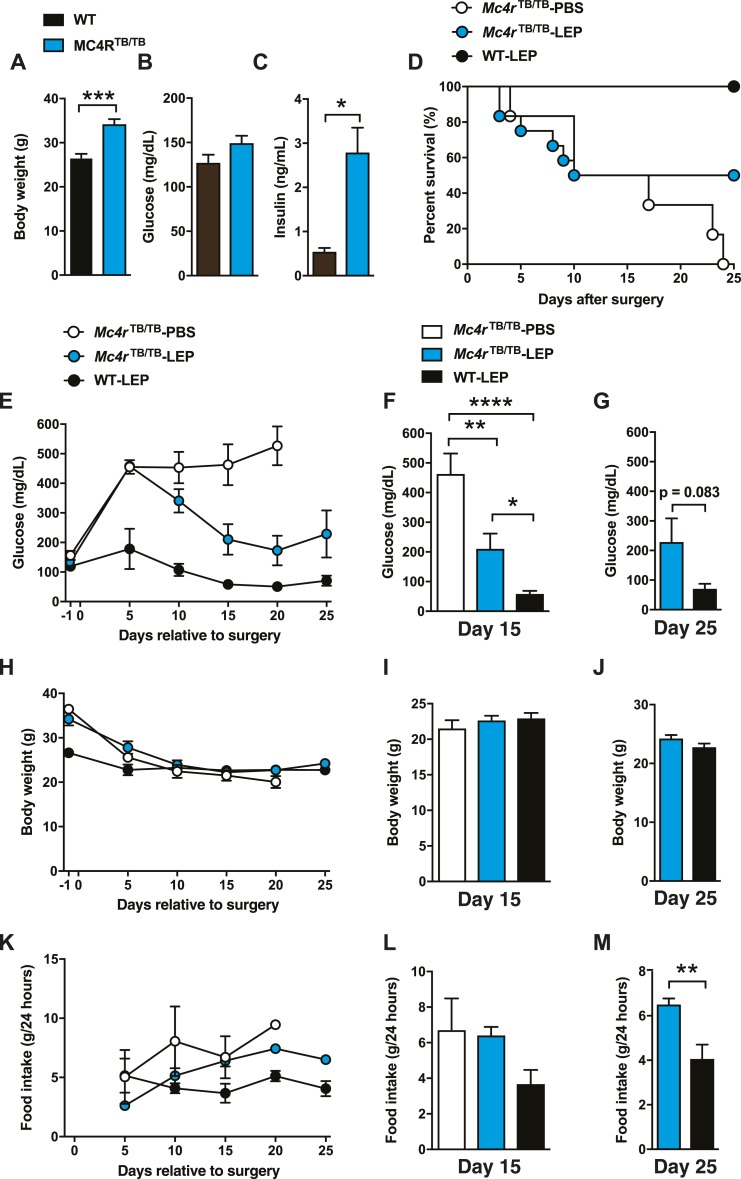
Figure 2.
MC4Rs are partially required for glucose-lowering effects of leptin in total insulin deficiency. (A) Body weight, (B) blood glucose, and (C) plasma insulin levels before inducing insulin deficiency in MC4R KO mice. Age was ∼3 months. (D) Survival plot, (E) time course of blood glucose and blood glucose levels at (F) day 15 and (G) day 25, (H) BW as a function of time in treatment and BW values at (I) day 15 and (J) day 25, (K) and the time course of food intake and food intake at (L) day 15 and (M) day 25 of total insulin-deficient Mc4rTB/TB (MC4R KO) mice with ICV leptin injections (Mc4rTB/TB-LEP). ICV leptin administration was started at day 0. DT was administered at days 0, 3, and 5. Administration control group (Mc4rTB/TB-PBS) was administered with sterile PBS, and WT control was used for genotype control group (WT-LEP). n = 1 to 12. Values are mean ± SEM. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05.
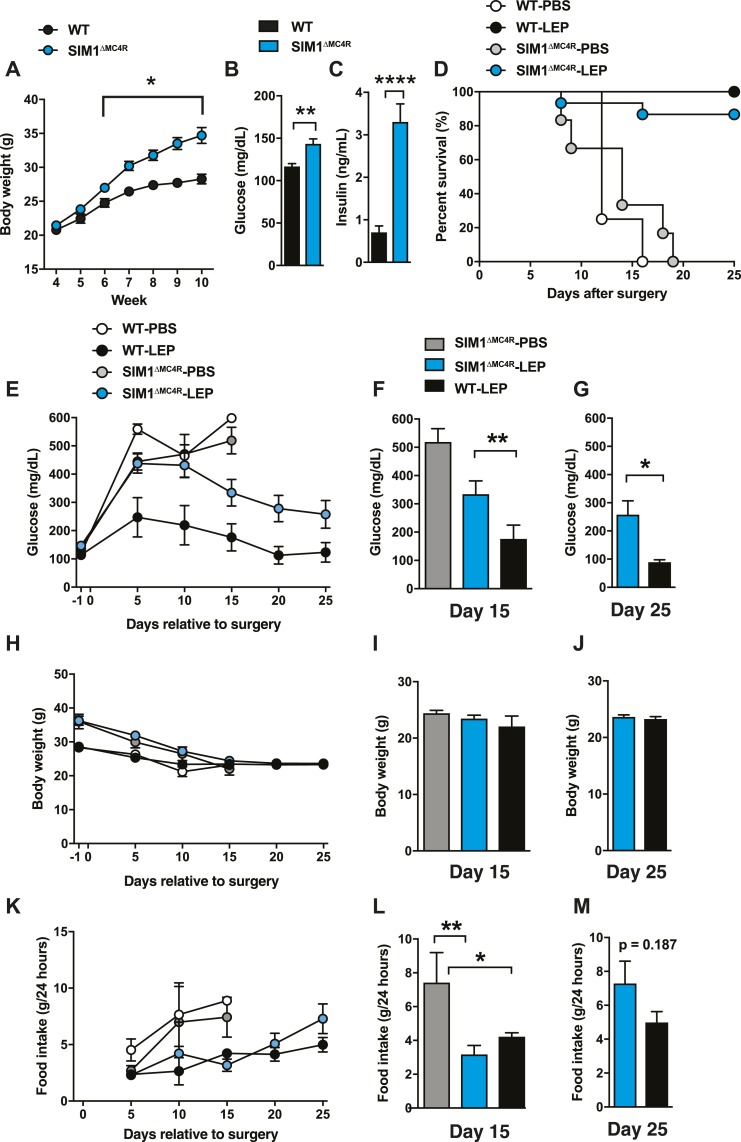
Figure 3.
MC4Rs in SIM1 neurons mediate MC4R-dependent glucose-lowering effects of leptin in total insulin deficiency. (A) Time course of BW, (B) blood glucose, and (C) plasma insulin levels before insulin deficiency in SIM1∆MC4R mice. [Age was ∼10 to 12 weeks in (B) and (C).] (D) Survival plot, (E) time course of blood glucose and blood glucose levels at (F) day 15 and (G) day 25, (H) time course of BW and BW at (I) day 15 and (J) day 25, (K) and time course of food intake and food intake at (L) day 15 and (M) day 25 of total insulin-deficient mice lacking SIM1∆MC4R mice with ICV leptin injections (SIM1∆MC4R-LEP). ICV leptin administration was started at day 0. DT was administered at days 0, 3, and 5. Administration control group (insulin-deficient SIM1∆MC4R mice that received ICV PBS) was administered with sterile PBS, and WT control was used for genotype control group (WT-LEP and WT-PBS). n = 1 to 19. Values are mean ± SEM. ****P < 0.0001; **P < 0.01; *P < 0.05.
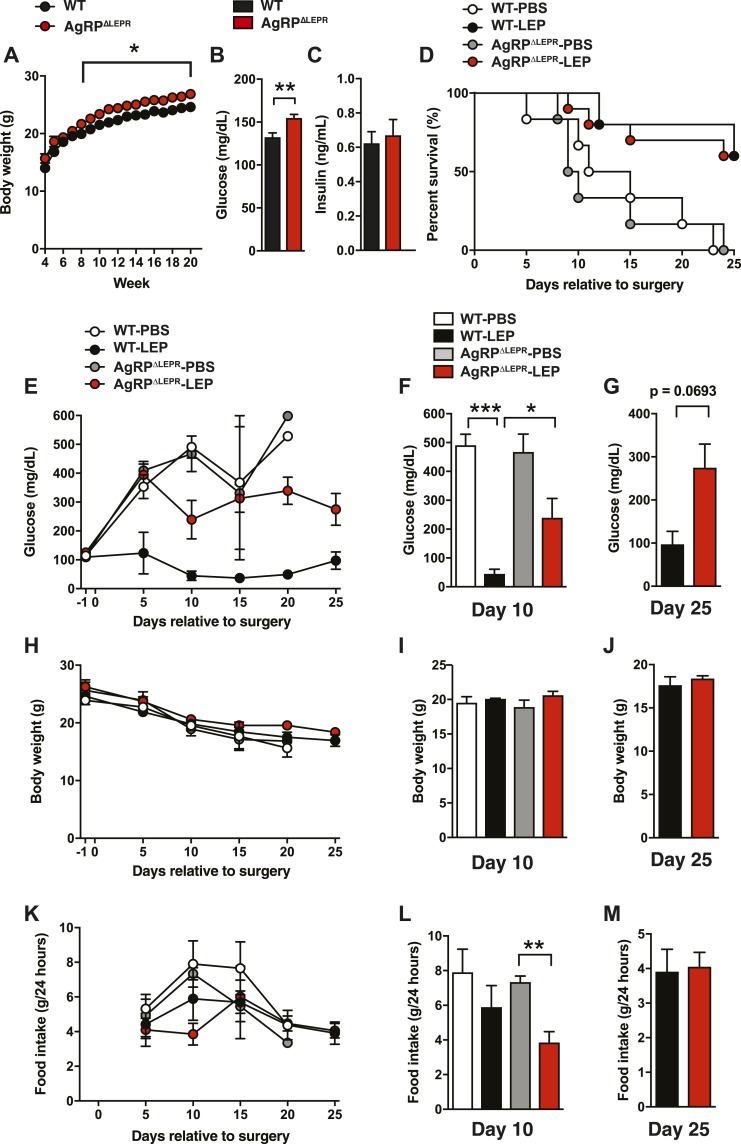
Figure 4.
LEPRs in AgRP neurons are partially required for glucose-lowering effects of leptin in total insulin deficiency. (A) Time course of BW, (B) blood glucose, and (C) plasma insulin levels before insulin deficiency. [Age was ∼8 to 12 weeks in (B) and (C).] (D) Survival plot, (E) time course of blood glucose and blood glucose levels at (F) day 10 and (G) day 25, (H) time course of BW and BW at (I) day 10 and (J) day 25, (K) and time course of food intake and food intake at (L) day 10 and (M) day 25 of total insulin-deficient mice lacking AgRP∆LEPR and mice with ICV leptin injections (AgRP∆LEPR-LEP). ICV leptin administration was started at day 0. DT was administered at days 0, 3, and 5. Administration control group (AgRP∆LEPR-PBS) was administered with sterile PBS, and WT control was used for genotype control group (WT-LEP and WT-PBS). n = 1 to 21. Values are mean ± SEM. ***P < 0.001; ***P < 0.001; **P < 0.01; *P < 0.05.
Results
MC4Rs are partially required for glucose-lowering effects of leptin in total insulin-deficient mice
DT injections can eliminate pancreatic β cells in mice expressing RIP-DTR (2, 13, 20). We confirmed that STZ injections [the same method we used previously (1)] into RIP-DTR mice did not completely eliminate pancreatic insulin, whereas DT injections did (Fig. 1B and 1C). Insulin levels were undetectable in all insulin-deficient groups described subsequently (Fig. 1B and 1C), indicating that the RIP-DTR approach did induce bona fide total insulin deficiency in all animal models used in this study.
Although MC4Rs have been reported to be crucial for glucose-lowering effects of leptin (6, 10, 16), this has not been tested in the setting of total insulin deficiency. We therefore determined if chronic ICV leptin injection reduced hyperglycemia in total insulin-deficient Mc4rTB/TB mice. As previously reported (21, 22), Mc4rTB/TB mice had significantly greater BW and higher blood insulin than wild-type (WT) control mice, although blood glucose levels were comparable to those in WT control mice before insulin deficiency (Fig. 2A–2C).
We had three groups: (i) insulin-deficient MC4R KO mice that received ICV PBS (Mc4rTB/TB-PBS); (ii) insulin-deficient MC4R KO mice that received ICV leptin (Mc4rTB/TB-LEP); and (iii) insulin-deficient WT control mice that received ICV leptin (WT-LEP). ICV leptin administration did not fully prevent the mortality in total insulin-deficient MC4R KO mice (Mc4rTB/TB-LEP vs WT-LEP; P = 0.0476, Fig. 2D), but it did improve the survival rate of Mc4rTB/TB-LEP compared with Mc4rTB/TB-PBS (50% vs 0% at day 25; Fig. 2D). We found that Mc4rTB/TB-LEP showed significantly lower blood glucose than Mc4rTB/TB-PBS, yet the glucose levels in Mc4rTB/TB-LEP were still higher than in WT-LEP (Fig. 2E–2G). BW was not significantly different across groups after 10 days (Fig. 2H–2J), demonstrating that glucose-lowering effects of leptin did not result from BW differences, which is consistent with previous reports (1, 2, 28, 29). After day 10, there was no difference in food intake between Mc4rTB/TB-LEP and Mc4rTB/TB-PBS (Fig. 2K–2L). On day 25, blood glucose levels in Mc4rTB/TB-LEP were higher than in WT-LEP (Fig. 2G), as was food intake (Fig. 2M). Previous studies have shown that reduced food intake does not play a major role in glucose-lowering effects of leptin in the partial absence of insulin (1, 29), suggesting that it is unlikely the difference of blood glucose between Mc4rTB/TB-LEP and WT-LEP resulted from the difference of food intake. Collectively, our data suggest that, whereas MC4Rs significantly contribute to glucose-lowering effects of leptin in the total absence of insulin, they are not fully required for the effects.
MC4Rs in SIM1 neurons are sufficient to account for MC4R-dependent leptin-mediated glucose-lowering effects in the total absence of insulin
Next, we determined the role of MC4Rs in SIM1 neuron in mediating glucose-lowering effects of leptin in total insulin-deficient mice. SIM1 is expressed in the PVN (21), and previous studies have shown MC4Rs in SIM1 neurons significantly contribute to regulation of glucose metabolism (21, 22). PVN-specific ablation of MC4Rs by the adeno-associated virus approach recapitulated metabolic phenotypes of ablating MC4Rs in SIM1 neurons (22), suggesting that SIM1 neurons specifically within the PVN are a key substrate for MC4R regulation of glucose metabolism. As previously reported (22), SIM1ΔMC4R mice exhibited significantly higher BW, blood glucose, and plasma insulin levels before insulin deficiency (Fig. 3A–3C). Our next studies made use of the following four groups: (i) insulin-deficient SIM1∆MC4R mice that received ICV PBS; (ii) insulin-deficient SIM1ΔMC4R mice that received ICV leptin (SIM1∆MC4R-LEP); (iii) insulin-deficient WT control mice that received ICV PBS (WT-PBS); and (iv) WT-LEP.
SIM1∆MC4R-LEP and WT-LEP showed comparable survival (P = 0.240; Fig. 3D); however, blood glucose in SIM1∆MC4R-LEP was higher than in WT-LEP (Fig. 2E–2G). There were no significant differences in BW after day 15 between SIM1∆MC4R-LEP and WT-LEP (Fig. 3H–3J). Food intake between WT-LEP and SIM1∆MC4R-LEP was comparable (Fig. 3K–3M). Interestingly, blood glucose at day 25 in SIM1∆MC4R-LEP was comparable to that in Mc4rTB/TB-LEP (257.8 ± 48.78 and 228.8 ± 79.83 mg/dL), suggesting that MC4Rs in SIM1 neurons may be responsible for MC4R-dependent glucose-lowering effects of central leptin when insulin is completely absent. Of note, although the glucose levels of Mc4rTB/TB-LEP and SIM1∆MC4R-LEP were comparable, the survival was not comparable (86.7% vs 50% at day 25; Fig. 3D). Because PVN neurons are likely crucial for the metabolic phenotype of mice lacking MC4Rs in SIM1 neurons (22), MC4Rs in PVN neurons may play a key role in glucose-lowering effects of central leptin in mice with total insulin deficiency.
LEPRs in AgRP neurons are partially required for glucose-lowering effects of leptin in the total absence of insulin
POMC and AgRP neurons that project to the PVN are vital participants in the central melanocortin system that regulates energy metabolism in the presence of insulin (9, 12). Previously, we determined that LEPRs in POMC neurons play only a minor role in glucose-lowering effects of leptin in total insulin deficiency (2). To determine the role of AgRP neurons in glucose-lowering effects of leptin in total insulin deficiency, we generated AgRPΔLEPR mice. Before insulin deficiency, AgRP∆LEPR mice showed greater BW, as previously reported (30–32), and higher blood glucose than WT control mice, although insulin levels in AgRP∆LEPR mice were comparable to WT control mice (Fig. 4A–4C). For our next studies, we used the following four groups: (i) insulin-deficient AgRP∆LEPR mice that received ICV PBS (AgRP∆LEPR-PBS); (ii) insulin-deficient AgRP∆LEPR mice that received ICV leptin (AgRP∆LEPR-LEP); (iii) WT-PBS; and (iv) WT-LEP.
AgRP∆LEPR-LEP showed comparable mortality compared with WT-LEP (Fig. 4D). Blood glucose in AgRP∆LEPR-LEP was significantly higher than in WT-LEP at day 10 (Fig. 4F–4G), although blood glucose in AgRP∆LEPR-LEP was lower than in AgRP∆LEPR-PBS (239.4 ± 66.77 vs 467.3 ± 61.8 mg/dL; P = 0.0759). There were no differences in BW and food intake after day 10 between WT-LEP and AgRP∆LEPR-LEP (Fig. 4H–4M). These data indicate that AgRP neurons are partially required for glucose-lowering effects of leptin in total insulin deficiency.
Total insulin deficiency hampers leptin’s actions on AgRP neuronal activity
Fasting dramatically increases AgRP neuronal activity and leptin can effectively silence this elevation of discharge (33). A previous paper demonstrated that partial insulin deficiency increased cFos expression throughout the brain, including AgRP neurons, and increased firing rates in AgRP neurons as well (8). These findings suggest that insulin deficiency can increase neuronal activity, which motivated us to ask if total insulin deficiency can alter neuronal activity of AgRP neurons and if leptin can still silence heightened AgRP neuronal discharge in total insulin-deficient mice.
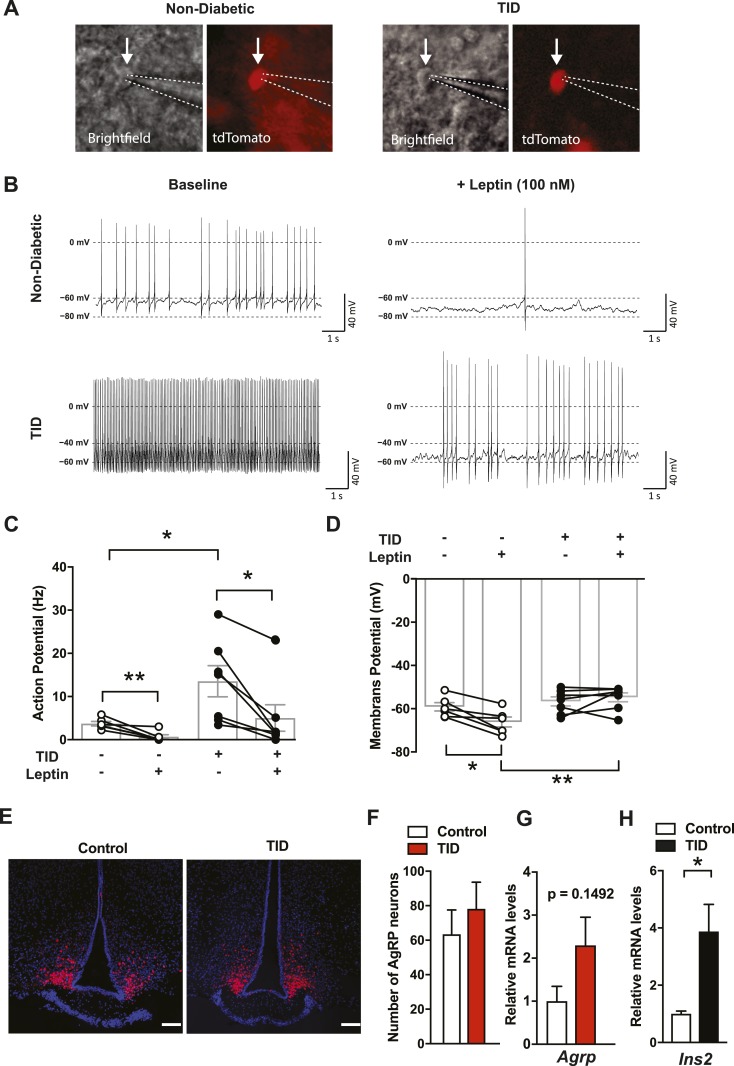
To visualize AgRP neurons in real time, we introduced tdTomato fluorescent reporter protein into Agrp-IRES-Cre∷RIP-DTR mice (Fig. 5A). We injected DT for 3 consecutive days (0.5 µg/kg BW) and carried out experiments 4 to 5 days after the final DT injection. Blood glucose levels were significantly higher than beforeinduction of insulin deficiency (116 ± 19.9 vs 432 ± 8.70 mg/dL, P < 0.0001) and plasma insulin was not detectable on the experimental day (Fig. 1B). Nondiabetic control groups were administered sterile saline instead of DT. Total insulin deficiency dramatically increased the frequency of action potentials in AgRP neurons (Fig. 5B and 5C). As previously reported (33), leptin significantly reduced the frequency of action potential (Fig. 5C) and decreased membrane potential in nondiabetic AgRP neurons (Fig. 5D). Leptin also significantly decreased the frequency of action potential discharge of AgRP neurons in total insulin deficiency (Fig. 5C and 5D). However, leptin did not hyperpolarize the membrane potential of AgRP neurons in total insulin deficiency. As a result, membrane potential during leptin exposure was significantly depolarized in AgRP neurons with total insulin deficiency compared with nondiabetic controls (Fig. 5D). Of note, DTR is also expressed in the central nervous system of RIP-DTR mice (2), which means that systemic DT injections have the potential to also ablate some AgRP neurons. However, our previous study demonstrates that the identical DT dosing regimen as used in the current study unlikely ablates the hypothalamic neurons (2). In line with that, we found that DT injections did not reduce the number of AgRP neurons and mRNA levels of Agrp and Ins2 (Fig. 5E–5H), suggesting that AgRP neurons in DT-injected RIP-DTR mice were fully intact. This, together with basic electrophysiological parameters of AgRP neurons (Fig. 5B–5D), indicates that our findings do not reflect loss or damage of AgRP neurons. Collectively, these data indicate that leptin in total insulin deficiency has diminished capacity to suppress elevated activities of AgRP neurons.
Figure 5.
Total insulin deficiency hampers leptin’s action on AgRP neuronal activities. (A) Brightfield and tdTomato fluorescent images of the targeted AgRP neurons in nondiabetic (left) and total insulin-deficient (TID) mice. Arrows indicate the targeted cells; dotted lines show the tip of the patch pipette. (B) Representative traces showing that baseline discharge (left) of an AgRP neuron from a mouse with T1D (bottom) and a nondiabetic control (top). Bath-applied leptin (right) normalized discharge frequency between groups by reducing firing rate more in the neurons from a T1D rather than a nondiabetic mouse. (C) Action potential discharge frequency (Hz) and (D) membrane potential (mV) among AgRP neurons from TID and nondiabetic mice with and without leptin. (E) Representative images (red, tdTomato fluorescent; blue, 4′,6-diamidino-2-phenylindole DNA staining) and (F) the number of AgRP neurons in Agrp-IRES-Cre∷RIP-DTR∷Ai9TB/− mice 10 days after induction of total insulin deficiency. DT was injected three times (days 0, 1, and 2). Control group was injected with sterile saline. Scale bars, 100 µm. (G) mRNA levels of Agrp in the hypothalamus of RIP-DTR mice 2 days after a single DT injection. (H) mRNA levels of Ins2 in the hypothalamus of RIP-DTR 10 days after induction of total insulin deficiency. Of note, our previous study demonstrated that 48 hours after a single DT injection, mRNA levels of preproinsulin in the pancreas were dramatically reduced (2). n = 4 to 6. Values are mean ± SEM. **P < 0.01; *P < 0.05.
Discussion
Our data demonstrate that MC4Rs and LEPRs in AgRP neurons are partially required for glucose-lowering effects of leptin in total insulin deficiency. MC4Rs expressed specifically in SIM1 neurons, likely MC4Rs in the PVN (21, 22), appear to play a substantial role in MC4R-dependent, leptin-mediated, glucose-lowering in total deficiency (Figs. 2 and 3). Considered together with previous findings (2), the current study indicates that the improvement of blood glucose by central leptin in insulin-deficient mice does not predict survival rate (Figs. 2–4), suggesting that multiple sites of action of leptin are involved in mediating its capacity to reduce mortality in total insulin-deficient mice.
Total insulin deficiency significantly increased the ongoing discharge of AgRP neurons, which is consistent with the associated loss of adiposity and hence blunted leptin-mediated suppression of discharge. In addition to elevating basal discharge, total insulin deficiency was accompanied by blunted inhibitory efficacy of leptin among identified AgRP neurons relative to nondiabetic controls (Fig. 5). This implies total insulin deficiency leads to ample neuroadaptations within AgRP neurons, and possibly other neurons (8). Diminished leptin inhibition of AgRP neuronal discharge in total insulin-deficient AgRP neurons also pinpoint that glucose-lowering effects of leptin without insulin could not solely rely on LEPRs in AgRP neurons.
Results of the current study indicate that glucose-lowering effects of leptin in total insulin-deficient rodents reflect complex modes of action involving potentially multiple cellular targets and/or processes. Our results appear to be in direct conflict with several previous studies in which MC4Rs and LEPRs on AgRP neurons have been reported to be absolutely required for central leptin-mediated lowering of blood glucose in the presence of normal or partial insulin signaling (8, 10, 16). These apparently conflicting results have several potential explanations, including (i) different degrees of insulin deficiency, (ii) associated differences in BW/adiposity during central leptin treatment, (iii) use of different doses and durations of central leptin administration, and (iv) different timing of gene manipulations.
It should be stressed that differences in BWs during treatment with central leptin could affect leptin’s capacity to lower blood glucose. Insulin is an anabolic and lipogenic hormone (34); as such, the degree of insulin deficiency produced in any particular animal model will result in different levels of BW loss. BW in control and MC4R KO mice in the current study were comparable up to 10 days after induction of total insulin deficiency (Fig. 2H). By contrast, MC4R KO rats with only partial insulin deficiency retain greater BW and hence likely have greater adiposity relative to their partial insulin-deficient MC4R intact controls (16). Because higher lipid substrates in blood can blunt or reverse glucose-lowering effects of leptin (35, 36), it is possible that higher blood lipid substrate from fat depots in partially insulin-deficient rodents could hamper glucose-lowering effects of leptin and might help explain greater dependence of central leptin on LEPR expressing AgRP neurons than was observed in the current study. Apparent conflict between findings of the present and previous studies could also result from an inhibitory interaction between insulin on leptin, as a previous study demonstrated that insulin can reverse leptin-induced depolarization of POMC neurons (37). Further studies are needed to clarify whether residual insulin or hyperinsulinemia can negatively affect leptin action on AgRP, MC4R, or other neurons, and whether such actions translate into diminished glucose-lowering effects of leptin in partial insulin-deficient (8) or type 2 diabetic mice (10).
Another difference between our results and those previously published relates to the dose of central leptin used to lower blood glucose in the setting of partial or total insulin deficiency. In a recent study, Xu et al. (8) used partial insulin-deficient mice and continuously delivered leptin ICV at a dose of ∼227 ng/h for 7 days. In the current study, we continuously delivered leptin ICV at a dose of ∼25 ng/h for up to 25 days. Comparing results over the initial 7 days of leptin infusion, both studies yielded very similar results, indicating that genetic deletion of LEPR exclusively from AgRP neurons is sufficient to largely prevent glucose lowering by central leptin in the initial phase of treatment, even though our dose was approximately ninefold less. Thus, different doses of ICV leptin alone do not appear to explain divergent results regarding the primacy of LEPR on AgRP neurons in mediating glucose-lowering effects of leptin in insulin-deficient models, regardless of whether deficiency is partial, as in the case of the study by Xu et al. (8), or total, as in the current study.
As noted previously, the duration of central leptin treatment has varied across studies (6, 8, 10, 16) and could contribute to different outcomes related to the sites and/or mechanisms of leptin action to lower blood glucose. Previous studies examined metabolic parameters for 7 to 12 days after beginning leptin administration (6, 8, 10, 16). Our data in totally insulin-deficient MC4R KO mice indicate that blood glucose is not lowered until days 10 to 15 of leptin administration. Although a previous study showed that deletion of LEPRs in AgRP neurons of adult mice fully prevented glucose-lowering effects of leptin in partially insulin-deficient mice up to 7 days, our data demonstrate that deletion of LEPRs in AgRP neurons initially abolished (∼10 days), but subsequently only blunted, glucose-lowering effects of leptin in mice with total insulin deficiency (Fig. 4). Our previous study in mice with total insulin deficiency showed a similar time course of leptin action after LEPRs were re-expressed in hypothalamic GABAergic neurons or POMC/hypothalamic GABAergic neurons (2). Thus, it is possible that a longer treatment period may be required for leptin to significantly lower blood glucose in rodents with partial insulin-deficiency. Further investigations will be needed to dissect this phenotypic discrepancy of glucose-lowering effects of leptin between partial and total insulin deficiency.
The timing of genetic manipulation could also be an important factor in the discrepant results of the present and previous studies. Whereas previous studies have shown that prenatal deletion of AgRP neurons does not affect food intake and BW after birth, deletion in adulthood causes anorexia and, eventually, death (38). These findings suggest there are drastically different metabolic consequences depending on the developmental stage or age at which manipulation of specific genes occurs. Another study showed that prenatal deletion of AgRP neurons causes obesity resulting from increased food intake and reduced energy expenditure (39). Thus, AgRP neurons appear to undergo developmental compensations when gene manipulations induce metabolic malfunctions that are life-threatening metabolic dysfunctions such as starvation (38) and do not undergo such compensatory adaptations when the results of genetic manipulations are not life-threatening, such as increased food intake/BW and decreased energy expenditure (i.e., metabolic consequences reflecting positive energy balance) (39, 40). It is still unclear what compensations occur for positive energy balance resulting from developmental ablation of LEPRs in AgRP neurons. Studies in total insulin-deficient mice in which LEPRs are deleted from AgRP neurons in adulthood are needed to further reveal the complex role LEPRs in AgRP neurons play in lowering blood glucose independently of insulin.
In summary, our result shows that mechanisms underlying insulin-independent, glucose-lowering effects of leptin are complicated by involvement of multiple sites of leptin actions, rather than a single site. Our data also imply that neuroadaptations, over time following induction of insulin deficiency, can explain the efficacy of glucose-lowering effects of leptin. Methodological differences exist between the current and previous studies regarding the time point of gene manipulation of LEPRs relative to the induction of insulin deficiency, underscoring the need to carefully consider these issues when designing experiments and interpreting their experimental outcomes.
Acknowledgments
We thank Drs. Joel Elmquist and Pedro Herrera for providing the transgenic animals.
Financial Support: This work was supported by the American Heart Association Scientist Development Grant 14SDG17950008 (to T.F.), University of Texas Rising STARs Award (to T.F.), and the National Institutes of Health Grant HL 088052 (to G.M.T).
Author Contributions: A.K.S. performed and analyzed experiments and edited the manuscript. J.Y. designed, performed, and analyzed experiments, and edited the manuscript. N.S.G. and N.A. performed experiments and edited the manuscript. G.M.T. supervised experiments and edited manuscript. T.F. designed, performed, supervised, and analyzed experiments, and wrote the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AES
acid ethanol solution
- AgRP
agouti-related protein
- AgRP∆LEP
mice lacking leptin receptors in agouti-related peptide-expressing neurons
- AgRP∆LEPR-LEP
insulin-deficient mice lacking leptin receptors in agouti-related peptide-expressing neurons that received intracerebroventricular leptin
- AgRP∆LEPR-PBS
insulin-deficient mice lacking leptin receptors in agouti-related peptide-expressing neurons that received intracerebroventricular PBS
- ARC
hypothalamic arcuate nucleus
- BW
body weight
- DT
diphtheria toxin
- ICV
intracerebroventricular
- KO
knockout
- LEPR
leptin receptor
- MC4R
melanocortin 4 receptor
- Mc4rTB/TB-LEP
insulin-deficient melanocortin 4 receptor knockout mice that received intracerebroventricular leptin
- Mc4rTB/TB-PBS
insulin-deficient melanocortin 4 receptor knockout mice that received intracerebroventricular PBS
- POMC
proopiomelanocortin
- PVN
paraventricular nucleus
- RIP-DTR
rat insulin promoter diphtheria toxin promoter
- SIM1
single-minded homolog 1
- SIM1ΔMC4R
mice lacking melanocortin 4 receptors in single-minded homolog 1–expressing neurons
- SIM1ΔMC4R-PBS
insulin-deficient mice lacking melanocortin 4 receptors in single-minded homolog 1–expressing neurons that received intracerebroventricular PBS
- SIM1∆MC4R-LEP
insulin-deficient mice lacking melanocortin 4 receptors in single-minded homolog 1–expressing neurons that received intracerebroventricular leptin
- SIM1ΔMC4R-PBS
insulin-deficient mice lacking melanocortin 4 receptors in single-minded homolog 1–expressing neurons that received intracerebroventricular PBS
- STZ
streptozocin
- WT
wild-type
- WT-LEP
wild-type control mice that received intracerebroventricular leptin
- WT-PBS
wild-type control mice that received intracerebroventricular PBS
References
- 1. Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci USA. 2010;107(40):17391–17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, Elmquist JK, Baldi P, Coppari R. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujikawa T, Coppari R. Living without insulin: the role of leptin signaling in the hypothalamus. Front Neurosci. 2015;9(108):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. German JP, Thaler JP, Wisse BE, Oh-I S, Sarruf DA, Matsen ME, Fischer JD, Taborsky GJ Jr, Schwartz MW, Morton GJ. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology. 2011;152(2):394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meek TH, Matsen ME, Dorfman MD, Guyenet SJ, Damian V, Nguyen HT, Taborsky GJ Jr, Morton GJ. Leptin action in the ventromedial hypothalamic nucleus is sufficient, but not necessary, to normalize diabetic hyperglycemia. Endocrinology. 2013;154(9):3067–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meek TH, Matsen ME, Damian V, Cubelo A, Chua SC Jr, Morton GJ. Role of melanocortin signaling in neuroendocrine and metabolic actions of leptin in male rats with uncontrolled diabetes. Endocrinology. 2014;155(11):4157–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meek TH, Matsen ME, Faber CL, Samstag CL, Damian V, Nguyen HT, Scarlett JM, Flak JN, Myers MG Jr, Morton GJ. In uncontrolled diabetes, hyperglucagonemia and ketosis result from deficient leptin action in the parabrachial nucleus. Endocrinology. 2018;159(4):1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, Kong D. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature. 2018;556(7702):505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci. 2016;19(2):206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonçalves GH, Li W, Garcia AV, Figueiredo MS, Bjørbæk C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Reports. 2014;7(4):1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mifflin S, Cunningham JT, Toney GM. Neurogenic mechanisms underlying the rapid onset of sympathetic responses to intermittent hypoxia. J Appl Physiol (1985).2015;119(12):1441–1448. [DOI] [PMC free article] [PubMed]
- 12. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. [DOI] [PubMed] [Google Scholar]
- 13. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, Herrera PL. Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. eLife. 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, Zhang BB. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50(1):142–150. [DOI] [PubMed] [Google Scholar]
- 15. Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, Burgess SC, Unger RH. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci U S A. 2012;109(37):14972–14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. da Silva AA, Spradley FT, Granger JP, Hall JE, do Carmo JM. Brain-mediated antidiabetic, anorexic, and cardiovascular actions of leptin require melanocortin-4 receptor signaling. J Neurophysiol. 2015;113(7):2786–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11(9):692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46(12):2119–2123. [DOI] [PubMed] [Google Scholar]
- 19. Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest. 1997;100(12):3105–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. [DOI] [PubMed] [Google Scholar]
- 22. Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, Yang Z, Fuller PM, Elmquist JK, Lowell BB. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci USA. 2014;111(36):13193–13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11(9):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–991. [DOI] [PubMed] [Google Scholar]
- 25. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. RRID: AB_2732074.
- 27. Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514(5):518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA. 2008;105(37):14070–14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107(11):4813–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egan OK, Inglis MA, Anderson GM. Leptin signaling in AgRP neurons modulates puberty onset and adult fertility in mice. J Neurosci. 2017;37(14):3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rupp AC, Allison MB, Jones JC, Patterson CM, Faber CL, Bozadjieva N, Heisler LK, Seeley RJ, Olson DP, Myers MG Jr. Specific subpopulations of hypothalamic leptin receptor-expressing neurons mediate the effects of early developmental leptin receptor deletion on energy balance. Mol Metab. 2018;14:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG Jr, Schwartz GJ, Chua SC Jr. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baver SB, Hope K, Guyot S, Bjørbaek C, Kaczorowski C, O’Connell KM. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci. 2014;34(16):5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21(6):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Denroche HC, Kwon MM, Quong WL, Neumann UH, Kulpa JE, Karunakaran S, Clee SM, Brownsey RW, Covey SD, Kieffer TJ. Leptin induces fasting hypoglycaemia in a mouse model of diabetes through the depletion of glycerol. Diabetologia. 2015;58(5):1100–1108. [DOI] [PubMed] [Google Scholar]
- 36. Perry RJ, Zhang XM, Zhang D, Kumashiro N, Camporez JP, Cline GW, Rothman DL, Shulman GI. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20(7):759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJ, Batterham RL, Ashford ML, Vanhaesebroeck B, Withers DJ. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10(5):343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. [DOI] [PubMed] [Google Scholar]
- 39. Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, Prola A, Flamment M, Foufelle F, Magnan C, Luquet S. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 2012;31(22):4276–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan K, Knight ZA, Friedman JM. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol Metab. 2014;3(7):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]